Abstract
Background
Misoprostol (Cytotec, Searle) is a prostaglandin E1 analogue widely used for off‐label indications such as induction of abortion and of labour. This is one of a series of reviews of methods of cervical ripening and labour induction using standardised methodology.
Objectives
To determine the effects of vaginal misoprostol for third trimester cervical ripening or induction of labour.
Search methods
The Cochrane Pregnancy and Childbirth Group's Trials Register (November 2008) and bibliographies of relevant papers. We updated this search on 15 February 2012 and added the results to the awaiting classification section.
Selection criteria
Clinical trials comparing vaginal misoprostol used for third trimester cervical ripening or labour induction with placebo/no treatment or other methods listed above it on a predefined list of labour induction methods.
Data collection and analysis
We developed a strategy to deal with the large volume and complexity of trial data relating to labour induction. This involved a two‐stage method of data extraction.
We used fixed‐effect Mantel‐Haenszel meta‐analysis for combining dichotomous data.
If we identified substantial heterogeneity (I² greater than 50%), we used a random‐effects method.
Main results
We included 121 trials. The risk of bias must be kept in mind as only 13 trials were double blind.
Compared to placebo, misoprostol was associated with reduced failure to achieve vaginal delivery within 24 hours (average relative risk (RR) 0.51, 95% confidence interval (CI) 0.37 to 0.71). Uterine hyperstimulation, without fetal heart rate (FHR) changes, was increased (RR 3.52 95% CI 1.78 to 6.99).
Compared with vaginal prostaglandin E2, intracervical prostaglandin E2 and oxytocin, vaginal misoprostol was associated with less epidural analgesia use, fewer failures to achieve vaginal delivery within 24 hours and more uterine hyperstimulation. Compared with vaginal or intracervical prostaglandin E2, oxytocin augmentation was less common with misoprostol and meconium‐stained liquor more common.
Lower doses of misoprostol compared to higher doses were associated with more need for oxytocin augmentation and less uterine hyperstimulation, with and without FHR changes.
We found no information on women's views.
Authors' conclusions
Vaginal misoprostol in doses above 25 mcg four‐hourly was more effective than conventional methods of labour induction, but with more uterine hyperstimulation. Lower doses (25 mcg four‐hourly or less) were similar to conventional methods in effectiveness and risks. The authors request information on cases of uterine rupture known to readers. The vaginal route should not be researched further as another Cochrane review has shown that the oral route of administration is preferable to the vaginal route. Professional and governmental bodies should agree guidelines for the use of misoprostol, based on the best available evidence and local circumstances.
[Note: The 27 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Keywords: Female; Humans; Pregnancy; Cervical Ripening; Labor, Induced; Administration, Intravaginal; Misoprostol; Misoprostol/administration & dosage; Oxytocics; Oxytocics/administration & dosage; Pregnancy Trimester, Third; Randomized Controlled Trials as Topic
Plain language summary
Vaginal misoprostol is effective in inducing labour but more research is needed on safety
Sometimes it is necessary to bring on labour artificially because of safety concerns for the mother or baby. Misoprostol is a hormone given by insertion through the vagina or rectum, or by mouth to ripen the cervix and bring on labour. The review of 121 trials found that larger doses of misoprostol are more effective than prostaglandin and that oxytocin is used in addition less often. However, misoprostol also increases hyperstimulation of the uterus. With smaller doses, the results are similar to other methods. The trials reviewed are too small to determine whether the risk of rupture of the uterus is increased. More research is needed into the safety and best dosages of misoprostol. Another Cochrane review has shown that the oral route of administration is preferable to the vaginal route.
Background
Sometimes it is necessary to bring on labour artificially because of safety concerns for the mother or baby. This review is one of a series of reviews of methods of labour induction using a standardised protocol. For more detailed information on the rationale for this methodological approach, please refer to the currently published 'generic' protocol (Hofmeyr 2009). The generic protocol describes how a number of standardised reviews will be combined to compare various methods of preparing the cervix of the uterus and inducing labour.
The main problems experienced during induction of labour are ineffective labour, and excessive uterine activity which may cause fetal distress. Both problems may lead to an increased risk of caesarean section. Methods of induction of labour include administration of oxytocin, prostaglandins, prostaglandin analogues and smooth muscle stimulants such as herbs or castor oil (Mitri 1987), or mechanical methods such as digital stretching of the cervix and sweeping of the membranes, hygroscopic cervical dilators, extra‐amniotic balloon catheters, artificial rupture of the membranes, and nipple stimulation.
Standardised 'scoring' of the cervix prior to labour induction has been recommended (Bishop 1964). Oxytocin has the disadvantage of a high failure rate when the cervix is unfavourable (low cervical score), and requiring monitored continuous intravenous infusion.
Artificial rupture of membranes is also less effective or may not be possible when the cervix is unfavourable. It may increase the risk of infection if labour does not proceed promptly. Rupture of membranes may also increase the vertical transmission of specific maternal infections such as HIV.
Unsuccessful labour induction is most likely when the cervix is unfavourable and, in this circumstance, prostaglandin preparations have proved to be beneficial (Keirse 1993; MacKenzie 1997). Those prostaglandins that have been registered for cervical ripening and labour induction are expensive and unstable, requiring refrigerated storage. Uterine hyperstimulation has been identified as a particular problem during labour induction with prostaglandins, and has been treated with tocolysis (Egarter 1990).
Misoprostol (Cytotec, Searle) is a methyl ester of prostaglandin E1 additionally methylated at C‐16 and is marketed for use in the prevention and treatment of peptic ulcer disease caused by prostaglandin synthetase inhibitors. It is inexpensive, easily stored at room temperature and has few systemic side effects. It is rapidly absorbed orally and vaginally. The reported mean peak serum misoprostol acid following oral administration was 227 pg/ml versus vaginal route 165 pg/ml; the times to peak levels were 34 versus 80 minutes. Vaginally absorbed serum levels are more prolonged (Zieman 1997). Irrespective of serum levels, vaginal misoprostol may have locally mediated effects.
Misoprostol has been shown in several studies to be an effective myometrial stimulant of the pregnant uterus, selectively binding to EP‐2/EP‐3 prostanoid receptors (Senior 1993).
Misoprostol has been used widely for obstetric and gynaecological indications despite the fact that it has not been registered for such use. It has therefore not undergone the systematic testing for appropriate dosage and safety required for registration.
Misoprostol is an effective abortifacient, both alone and following pretreatment with mifepristone (Norman 1991). Its widespread use in Brazil (Costa 1993) resulted in the identification of teratogenic effects (Fonseca 1991).
Use of misoprostol for second trimester termination of pregnancy has been associated with uterine rupture, particularly when combined with oxytocin infusion. In a report of 803 women admitted with abortion complications in Rio de Janeiro, 458 reported using misoprostol (Costa 1993). There was one maternal death from uterine rupture at 16 weeks' gestation following self‐medication with misoprostol.
Third trimester cervical ripening and labour induction with misoprostol have been reported using the oral, vaginal, rectal and buccal/sublingual routes. Clinical experience with misoprostol for labour induction has been reviewed by Wing (Wing 1999b).
Mariani Neto et al (Mariani Neto 1987) first reported using oral misoprostol 400 micrograms (mcg) four hourly for induction of labour following intrauterine death.
In a subsequent paper (Mariani Neto 1988), they described 'uterine tachysystole' with misoprostol use at term, which appeared unrelated to dosage. Since that time, several small studies have confirmed an increased incidence of uterine tachysystole (greater than five contractions per 10 minutes for at least 20 minutes), uterine hypersystole/hypertonus (a contraction of two minutes or more) and/or uterine hyperstimulation syndrome (uterine tachysystole or hypersystole with fetal heart rate (FHR) changes such as persistent decelerations, tachycardia or reduced short term variability). The conclusion from a meta‐analysis was that published data confirmed the safety of intravaginal misoprostol for cervical ripening and labour induction. The data showed an increased incidence of uterine tachysystole (odds ratio 2.70, 95% confidence intervals 1.80 to 4.04), but there was no statistically significant increase in adverse fetal outcome (Sanchez‐Ramos 1997). Wing et al (088 Wing 1995a; 044 Wing 1995b; 025 Wing 1996; 038 Wing 1997) have suggested that uterine hyperstimulation and meconium passage with vaginal misoprostol may be less frequent using a 25 microgram dose, six hourly.
Merrell and co‐workers (Merrell 1995) reported a series of 62 inductions of labour with vaginal misoprostol. There were two stillbirths, one apparently due to a tight nuchal cord, and one unexplained. They commented on rapid onset of contractions and described one woman with induction to delivery interval of only two hours. In a subsequent abstract (Merrell 1996), they described labour inductions with vaginal misoprostol in 345 women with live fetuses and 86 with intrauterine deaths. There was one unexplained maternal death; two uterine ruptures, one of which followed a previous caesarean section; eight caesarean sections for fetal distress and one for uterine hyperstimulation; and 10 perinatal deaths.
There have been several reports of uterine rupture following misoprostol labour induction with and without previous caesarean section (Bennett 1997; Sciscione 1998; Blanchette 1999; Matthews 1999; Khosla 2002). One unpublished case of uterine rupture occurred in a nulliparous woman following misoprostol use (EM Smith, personal communication). At term plus 12 days she received misoprostol 100 mcg vaginally. After six hours her cervix was found to be 7 cm dilated, and she progressed to full dilatation within a further 70 minutes. Fetal distress was suspected. Ventouse application produced no descent, so delivery was effected by caesarean section. The infant showed no signs of life at birth. After resuscitation, life was sustained for a few hours only. A posterior uterine tear arising from the cervix and spiraling up the posterior aspect of the uterus was discovered and repaired. Because such uterine tears are rare in nulliparous women without prolonged labour or syntocinon use, a causal relationship with the use of misoprostol must be considered.
One trial of misoprostol for labour induction in women with prior caesarean section has been terminated prematurely because of disruption of the uterine incision in two of the first 17 misoprostol‐treated women (025 Wing 1998a). The dosage of misoprostol used was conservative (25 µg six hourly to a maximum of four doses). Two and three doses were used respectively in the two cases of ruptured uterus.
In a retrospective review, uterine rupture occurred in 5/89 (5.6%) of women with previous caesarean delivery who had labour induced with misoprostol, compared with 1/423 (0.2%) of those who did not (Plaut 1999). In another retrospective review of labour induction in 575 women with previous caesarean section, the rate of uterine rupture was 5/172 (2.9%) for prostaglandin E2 gel; 1/129 (0.76%) for intracervical Foley catheter; and 3/474 (0.74%) for induction not requiring cervical ripening; compared with 7/1544 (0.45%) for spontaneous trial of labour (Ravasia 2000). In a third retrospective review, no uterine ruptures were detected among 48 women with previous caesarean section whose labour was induced with misoprostol 50 mcg vaginally four hourly (Choy‐Hee 2001).
Personal discussion with colleagues has revealed several cases of rupture of an unscarred uterus following misoprostol usage, possibly related to higher dosages than have been used in the trials reviewed. These cases are usually not reported. We call on readers to send us details of any such cases known to them, including if possible age, parity, any previous uterine surgery, dosage of misoprostol and details of the uterine rupture. This will enable us to compile a register of such problems.
This review will focus on the effectiveness and safety of misoprostol administered vaginally for cervical ripening and labour induction in the third trimester of pregnancy.
The use of oral (Alfirevic 2006) and buccal/sublingual (Muzonzini 2004) misoprostol for cervical priming and labour induction, compared with other methods including misoprostol administered vaginally, are reviewed separately.
Objectives
To determine, from the best available evidence, the effectiveness and safety of misoprostol administered vaginally for third trimester cervical ripening and induction of labour.
Methods
Criteria for considering studies for this review
Types of studies
Clinical trials comparing misoprostol administered vaginally for cervical ripening or labour induction, with placebo/no treatment or other methods listed above it on a predefined list of methods of labour induction (seeMethods); the trials included some form of random allocation to either group; they reported one or more of the pre‐stated outcomes; reasonable measures were taken to ensure allocation concealment; and violations of allocated management were not sufficient to materially affect outcomes. We have not included quasi‐randomised trials.
Types of participants
Pregnant women due for third trimester induction of labour. We have not excluded multiple pregnancies. Predefined sub‐group analyses (see list below): previous caesarean section or not; nulliparity or multiparity; membranes intact or ruptured, and cervix unfavourable, favourable or undefined. Only those outcomes with data appear in the analysis tables.
Types of interventions
Vaginal administration of misoprostol compared with placebo/no treatment or any other method above it on a predefined list of methods of labour induction.
Primary comparisons
Misoprostol versus placebo/no treatment Misoprostol versus oxytocin Misoprostol versus vaginal prostaglandins Misoprostol versus intracervical prostaglandins Low dosage misoprostol regimens versus higher dosage regimens Misoprostol gel versus tablets
In all the studies of misoprostol versus prostaglandins, the prostaglandin used was dinoprostone intravaginally as a gel, tablet or slow‐release pessary, or intracervically as a gel. In most of the studies, oxytocin was used with similar protocols for both the misoprostol and the prostaglandin group, except that in one study (175 Kadanali 1996) oxytocin was started if indicated after six hours in the dinoprostone group and only after 24 hours in the misoprostol group. The effective comparison in this trial is therefore misoprostol versus dinoprostone plus early oxytocin. The results are in keeping with those of other studies.
Types of outcome measures
Two authors of labour induction reviews (Justus Hofmeyr and Zarko Alfirevic) have prespecified clinically relevant outcomes for trials of methods of cervical ripening/labour induction. We have settled differences by discussion.
We chose five primary outcomes as being most representative of the clinically important measures of effectiveness and complications. We limited sub‐group analyses to the primary outcomes: (1) vaginal delivery not achieved within 24 hours; (2) uterine hyperstimulation with FHR changes; (3) caesarean section; (4) serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood); (5) serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicaemia).
Perinatal and maternal morbidity and mortality are composite outcomes. This is not an ideal solution because some components are clearly less severe than others. It is possible for one intervention to cause more deaths but less severe morbidity. However, in the context of labour induction at term this is unlikely. All these events will be rare, and a modest change in their incidence will be easier to detect if composite outcomes are presented. We have explored the incidence of individual components as secondary outcomes (see below).
Secondary outcomes relate to measures of effectiveness, complications and satisfaction.
Measures of effectiveness
(6) Cervix unfavourable/unchanged after 12 to 24 hours; (7) oxytocin augmentation.
Complications
(8) Uterine hyperstimulation without FHR changes; (9) uterine rupture; (10) epidural analgesia; (11) instrumental vaginal delivery; (12) meconium‐stained liquor; (13) Apgar score less than seven at five minutes; (14) neonatal intensive care unit admission; (15) neonatal encephalopathy; (16) perinatal death; (17) disability in childhood; (18) maternal side effects (all); (19) maternal nausea; (20) maternal vomiting; (21) maternal diarrhoea; (22) other maternal side effects; (23) postpartum haemorrhage (as defined by the trial authors); (24) serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture); (25) maternal death.
Measures of satisfaction
(26) Woman not satisfied; (27) caregiver not satisfied.
'Uterine rupture' will include all clinically significant ruptures of unscarred or scarred uteri. We will exclude trivial scar dehiscence noted incidentally at the time of surgery.
While we sought all the above outcomes, we have included only those with data in the analysis tables.
The terminology of uterine hyperstimulation is problematic (Curtis 1987). In this review we have used the term 'uterine hyperstimulation without FHR changes' to include uterine tachysystole (greater than five contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonus (a contraction lasting at least two minutes) and 'uterine hyperstimulation with FHR changes' to denote uterine hyperstimulation syndrome (tachysystole or hypersystole with FHR changes such as persistent decelerations, tachycardia or decreased short‐term variability). However, due to varied reporting there is the possibility of subjective bias in interpretation of these outcomes. Also, it is not always clear from trials if these outcomes are reported in a mutually exclusive manner.
We included outcomes in the analysis if reasonable measures were taken to minimise observer bias; missing data were insufficient to materially influence conclusions; and data were available for analysis according to original allocation.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (November 2008). We updated this search on 15 February 2012 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We hand searched the reference lists of trial reports and reviews.
We screened and assessed all Chinese papers according to the review protocol by a first‐language Chinese speaker (Linan Cheng) and excluded all due to serious methodological limitations.
We did not apply any language restrictions.
Data collection and analysis
To avoid duplication of data, the authors of induction of labour reviews agreed a specific order for labour induction methods, from one to 27. Each primary review included comparisons between one of the methods (from two to 27) with only those methods above it on the list. Thus, this review of intravenous oxytocin (4) included only comparisons with intracervical prostaglandins (3), vaginal prostaglandins (2) or placebo/no treatment (1). The current list is as follows:
(1) placebo/no treatment; (2) vaginal prostaglandins (Kelly 2003); (3) intracervical prostaglandins (Boulvain 2008); (4) intravenous oxytocin (Kelly 2001a); (5) amniotomy (Bricker 2000); (6) intravenous oxytocin with amniotomy (Howarth 2001); (7) vaginal misoprostol; (8) oral misoprostol (Alfirevic 2006); (9) mechanical methods including extra‐amniotic Foley catheter (Boulvain 2001); (10) membrane sweeping (Boulvain 2005); (11) extra‐amniotic prostaglandins (Hutton 2001); (12) intravenous prostaglandins (Luckas 2000); (13) oral prostaglandins (French 2001); (14) mifepristone (Hapangama 2009); (15) oestrogens with or without amniotomy (Thomas 2001); (16) corticosteroids (Kavanagh 2006a); (17) relaxin (Kelly 2001b); (18) hyaluronidase (Kavanagh 2006b); (19) castor oil, bath, and/or enema (Kelly 2001c); (20) acupuncture (Smith 2004); (21) breast stimulation (Kavanagh 2005); (22) sexual intercourse (Kavanagh 2001); (23) homoeopathic methods (Smith 2003); (24) nitric oxide donors (Kelly 2008); (25) buccal or sublingual misoprostol (Muzonzini 2004); (26) hypnosis; (27) other methods for induction of labour.
The reviews were analysed by the following clinical categories of participants:
previous caesarean section or not;
nulliparity or multiparity;
membranes intact or ruptured;
cervix favourable, unfavourable or undefined.
For most reviews, the initial data extraction process was conducted centrally. This was co‐ordinated from the Clinical Effectiveness Support Unit (CESU) at the Royal College of Obstetricians and Gynaecologists, UK, in co‐operation with the Pregnancy and Childbirth Group of The Cochrane Collaboration. This process allowed the data extraction process to be standardised across all the reviews. From 2001, the data extraction was no longer conducted centrally.
The trials were initially reviewed on eligibility criteria, using a standardised form and the basic selection criteria specified above. Following this, a standardised data extraction form was developed and then piloted for consistency and completeness. This pilot process involved the researchers at the CESU and previous review authors in the area of induction of labour. For a description of the methods used to carry out the initial reviews, seeAppendix 1.
Due to the large number of trials, double data extraction was not feasible and agreement between the three data extractors was therefore assessed on a random sample of trials to update in 2003. For the same reason, in the 2009 update, the data extraction was checked on around 50% of the trials in a random sample selection.
In 2008, the methods and software for carrying out reviews were updated, as a result of which new reviews and updates, where appropriate, use these new methods (Higgins 2008a; RevMan 2008), which are described in the Methods section of all the individual new and updated reviews.
For this update, we used the following methods when assessing the new trials identified by the updated search.
Selection of studies
One review author (Cynthia Pileggi (CP)) assessed for inclusion all the potential studies we identified as a result of the search strategy. We discussed studies for which there was any uncertainty with a second author (Justus Hofmeyr (GJH)).
Data extraction and management
We designed a form to extract data. For eligible studies, one review author (CP) extracted the data using the agreed form. GJH independently repeated selection of studies and data extraction on a random sample of studies. We resolved discrepancies through discussion or, if required, we would have consulted the third author. We entered the data into Review Manager software (RevMan 2008) and checked them for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
One review author (CP) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008a).
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3) Blinding (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We judged studies at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low risk of bias, high risk of bias or unclear risk of bias for participants;
low risk of bias, high risk of bias or unclear risk of bias for personnel;
low risk of bias, high risk of bias or unclear risk of bias for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we would re‐include missing data in the analyses. We assessed methods as:
low risk of bias (less than 5% loss to follow up);
high risk of bias;
unclear risk of bias.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2008a). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as summary risk ratio with 95% confidence intervals.
Continuous data
This systematic review did not include continuous data.
Unit of analysis issues
Cluster‐randomised trials
We would include cluster‐randomised trials in the analyses along with individually randomised trials. We would adjust their sample sizes using the methods described in the Handbook (Higgins 2008b) using an estimate of the intra cluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used,we planned to report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify cluster‐randomised trials in addition to the individually‐randomised trials, we plan to synthesise the relevant information. We would consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We also planned to acknowledge heterogeneity in the randomisation unit and perform a separate meta‐analysis.
Dealing with missing data
For included studies, we have noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we have carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial is the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity (I² greater than 50%), we explored it by pre‐specified subgroup analysis.
Assessment of reporting biases
Where we suspected reporting bias (see ‘Selective reporting bias’ above), we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed‐effect Mantel‐Haenszel meta‐analysis for combining dichotomous data where trials were examining the same intervention, and we judged the trials’ populations and methods sufficiently similar. Where we suspected clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects may differ between trials, we used a random‐effects meta‐analysis.
If we identified substantial heterogeneity, we noted this and performed the analysis using a random‐effects method.
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses:
previous caesarean section or not;
nulliparity or multiparity;
membranes intact or ruptured;
cervix favourable, unfavourable or undefined.
We used primary outcomes only in subgroup analysis.
For fixed‐effect meta‐analyses we conducted planned subgroup analyses classifying whole trials by interaction tests as described by Deeks 2001. For random‐effects meta‐analyses we assessed differences between subgroups by inspection of the subgroups’ confidence intervals; non‐overlapping confidence intervals indicate a statistically significant difference in treatment effect between the subgroups.
Sensitivity analysis
We plan to carry out sensitivity analysis by excluding trials with greater risk of bias, particularly with respect to allocation concealment, in a future update of this review.
Results
Description of studies
Included studies
We have included 121 studies in this review. See table of Characteristics of included studies for details. (Twenty‐seven reports from an updated search in February 2012 have been added toStudies awaiting classification.) Because a wide range of misoprostol dosages has been used, we have coded the included studies with a prefix to reflect roughly the dosage of vaginal misoprostol received in the first six hours, calculated as follows: initial dose + (s x (6 ‐ i)/4), where 's' is a subsequent dose within six hours, and 'i' is the interval in hours. This is based on the approximation that vaginal misoprostol is absorbed uniformly over a four‐hour period. Where a subsequent oral dose was used, the oral dose value was halved. Use of a gel preparation is indicated by the letter 'G'. This coding allows approximate ranking of the trials by misoprostol dosage, and enables readers to assess the effect of dosage on results. We detected no discrepancies in the sample of data extraction performed in duplicate.
Excluded studies
For details of excluded studies, see table of Characteristics of excluded studies.
Risk of bias in included studies
With the exception of 13 double‐blind trials (100G Fletcher 1993; 050 El‐Azeem 1997; 043 Farah 1997; 050 Surbek 1997; 050 Gotschall 1998; 025G Srisomboon 1998; 043 Diro 1999; 100 Montealegre 1999; 025 Stitely 2000; 048 Khoury 2001; 058 Ferguson 2002; 038 Meydanli 2003; 050 Ramsey 2003 ‐ blinded low versus high dose misoprostol comparison only), allocation was by means of sealed envelopes or unspecified, and treatment was not blinded. There is therefore a real possibility of bias affecting both the clinical management of the women (e.g. decisions to undertake caesarean section) and the assessment of outcomes. Such biases might operate in either direction (for example, a clinician enthusiastic about the potential of misoprostol might be less likely to perform caesarean section in the misoprostol group, while one anxious about the experimental nature of misoprostol might be more likely to perform caesarean section in this group).
We performed limited sensitivity analysis excluding non‐blinded studies for primary outcomes with significant heterogeneity and 10 or more trials included. For the comparison misoprostol versus vaginal prostaglandins, all women: the outcome vaginal delivery not achieved in 24 hours was unchanged; the outcome uterine hyperstimulation with FHR changes was no longer statistically significant (small numbers). For the comparison misoprostol versus oxytocin: the outcomes vaginal delivery not achieved in 24 hours and caesarean section were no longer statistically significant (small numbers remaining in the analysis).
The possibility of bias must be kept in mind in the interpretation of the results.
In the study of 050 Le Roux 2002, 93 of 573 enrolled women were excluded for 'protocol violations'. There did not appear to be a selective loss from any group, and the baseline data were similar between groups.
In 050 Pandis 2001, 235/670 were excluded after randomisation, mainly for spontaneous delivery before induction or induction by amniotomy for cervical score seven or more.
In 075 Ghidini 2001, seven of 65 enrolled women were excluded due to emergence of exclusion criteria. The groups were somewhat unbalanced (32 received 50 mcg and 26 received 100 mcg)
In 150 De la Torre 2001, 50 of 410 enrolled were withdrawn for protocol deviation (16), patient withdrawal (7), or missing data (27). The final groups differed in numbers (misoprostol 168, oxytocin 192). This raises the possibility of selective withdrawal from the misoprostol group.
In 088 Garry 2003, 14 women of 200 enrolled were withdrawn for physician request(10), used wrong medication (2), patient request (1) and unknown breech presentation (1). It suggests deviation from the intention to treat analysis.
The 2009 update used the new methodology of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008a) regarding the evaluation of sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, report of incomplete outcome data, selective outcome reporting bias and other sources. We have presented the quality evaluation of each included study in the corresponding risk of bias table.
This update includes 54 comparisons with more than 10 study results in the pooled analyses, 19 of them in primary outcomes. Five out of these 19 comparisons present asymmetrical funnel plots suggesting potential publication bias.
Effects of interventions
We have included 121 studies in this review. We sought all the outcomes listed under 'Types of outcome measures', and sub‐groups defined in 'Types of participants'. Only those with data appear in the analysis tables.
Vaginal misoprostol versus placebo
Primary outcomes
The 10 studies (1141 women) included in this part of the review (100G Fletcher 1993; 100G Srisomboon 1996; 025 Stitely 2000; 050 Thomas 2000; 025 Incerpi 2001; 050 Ortiz 2002; 025 McKenna 2004; 050 Gelisen 2005; 025 Krupa 2005; 025 Oboro 2005) showed a trend towards failure to deliver within 24 hours (five trials, 735 women, average relative risk (RR) 0.56, 95% CI 0.31 to 1.03).
Secondary outcomes
We found a clear effect of misoprostol on cervical ripening (two trials, average RR of unchanged cervix at 12 to 24 hours 0.09, 95% confidence interval (CI) 0.03 to 0.24).
Uterine hyperstimulation without FHR changes was increased (six trials, 794 women RR 3.52, 95% CI 1.78 to 6.99). Six trials (100G Fletcher 1993;025 Stitely 2000; 050 Thomas 2000; 050 Ortiz 2002; 050 Gelisen 2005; 025 Krupa 2005) with 814 women showed an unexpected reduction of meconium‐stained liquor with the use of misoprostol for labour induction.The numbers studied were too small to assess the impact on obstetric management and maternal and neonatal complications.
Vaginal misoprostol versus vaginal prostaglandins
There were 38 included trials with 7022 participants.
Primary outcomes
Failure to achieve vaginal delivery within 24 hours (22 trials, average RR 0.77, 95% CI 0.66 to 0.89) was reduced overall, but not in the two trials using less than 50 mcg misoprostol in the first six hours (038 Wing 1997; 048 Khoury 2001).
Uterine hyperstimulation with FHR changes was variable between trials, but overall tended to be more common with misoprostol (31 trials, average RR 1.43, 95% CI 0.97 to 2.09).
Caesarean sections were variable between trials, with a trend to be reduced with vaginal misoprostol (34 trials RR 0.95, 95%CI 0.87 to 1.03).
Secondary outcomes
Oxytocin augmentation was reduced with misoprostol (36 trials, average RR 0.68, 95% CI 0.60 to 0.76).
Uterine hyperstimulation without FHR changes was more common with misoprostol (26 trials, average RR1.99, 95% CI 1.41 to 2.79).
Epidural analgesia was used less frequently with misoprostol (eight trials, RR 0.92, 95% CI 0.85 to 0.99).
Meconium‐stained liquor was more common with misoprostol (18 trials, RR 1.35, 95% CI 1.13 to 1.61). There were no statistically significant differences in perinatal or maternal outcomes.
Results were similar for the sub‐groups of women with unfavourable cervices and those with intact membranes and unfavourable cervices.
There were similar trends for women with intact membranes and variable or undefined cervices, but the numbers were too small for clear outcomes.
For subgroups of primiparous or multiparous women, the numbers were small and no differences in any outcomes were shown, except that for all primiparous women, misoprostol shows reduced caesarean section (RR 0.82, 95% CI 0.68 to 0.99), and a trend to reduced vaginal delivery not achieved in 24h (average RR 0.70, 95% CI 0.46 to 1.05).
The results of 048 Khoury 2001 differed somewhat from other studies. This may be because a gel preparation of misoprostol was used, and it is possible that some activity is lost in the preparation or administration (see results of misoprostol gel versus tablets below).
Vaginal misoprostol versus intracervical prostaglandins
There were 27 included trials with 3311 participants.
Primary outcomes
Failure to achieve vaginal delivery within 24 hours was consistently reduced with misoprostol (13 trials, RR 0.63, 95% CI 0.56 to 0.71).
Uterine hyperstimulation with associated FHR changes was variable between trials, but all were consistent with the pooled result showing an increase with misoprostol (20 trials, RR 2.32, 95% CI 1.64 to 3.28). The latter result was similar for the sub‐group of trials studying women with intact membranes and unfavourable cervices.
Caesarean sections were variable between trials, with no significant differences overall.
Secondary outcomes
Only one trial reported the outcome 'failure to achieve cervical ripening within 12 hours' (075 Buser 1997); this was reduced with misoprostol (RR 0.68, 95% CI 0.52 to 0.88).
Oxytocin augmentation was used less often with misoprostol (20 trials, average RR 0.55, 95% CI 0.48 to 0.64).
Uterine hyperstimulation without FHR changes was more common with misoprostol (17 trials, RR 1.95, 95% CI 1.57 to 2.42).
The rates of vaginal instrumental delivery were variable between trials. Epidural analgesia was used less frequently with misoprostol (two trials, RR 0.64, 95% CI 0.48 to 0.86). Meconium‐stained liquor was increased with misoprostol (14 trials, RR 1.29, 95% CI 1.04 to 1.59). There were no statistically significant differences in perinatal or maternal outcomes.
Most of the trials studied women with unfavourable cervices, for whom the results were similar to the overall results. Results were also similar for women with intact membranes and unfavourable cervices. The two trials with intact membranes and variable or undefined cervix also showed a similar pattern of results.
Vaginal misoprostol versus oxytocin
There were 25 trials with 3074 participants.
Primary outcomes
Misoprostol, in the doses used in these trials, was more effective than oxytocin for labour induction (10 trials, average RR of failure to achieve vaginal delivery within 24 hours 0.65, 95% CI 0.47 to 0.90). Two trials using less than 50 mcg misoprostol showed no reduction (025 Wing 1998b; 025 Haghighi 2006).
Twenty‐five studies showed a reduction in caesarean section risk with the use of misoprostol (average RR 0.76, 95% CI 0.60 to 0.96).
Secondary outcomes
Uterine hyperstimulation without FHR changes was more common with misoprostol (15 trials, RR 2.24 95% CI 1.82 to 2.77 respectively). There was a trend to reduced epidural analgesia with misoprostol (three trials, RR 0.82, 95% CI 0.67 to 1.00). Vaginal instrumental delivery was reduced in the misoprostol group (13 trials, RR 0.74, 95% CI 0.56 to 0.99).
Apgar score less than 7 at five minutes, with 13 studies and 1906 participants in the general group, was substantially reduced with misoprostol use (RR 0.56, 95% CI 0.34 to 0.92). Four studies with 334 participants showed increased risk of maternal side effects (RR 5.04, 95% CI 1.51 to 16.86). There were no differences in other perinatal or maternal outcomes.
One trial in women with previous caesarean section was stopped when uterine rupture occurred in two of the first 17 women who received misoprostol (025 Wing 1998b) and in another study one uterine rupture occurred in 34 women in the misoprostol group (all women with unfavourable cervix, 050 Abdul 2007).
Misoprostol lower dosage regimen versus higher dose
There were 21 trials with 2913 participants. The dosages compared are as follows.
| Number of studies | Misoprostol low dosage | Misoprostol high dosage | Interval of use |
| 2 | 12.5 mcg | 25 mcg | 4 to 6 hours |
| 11 | 25 mcg | 50 mcg | 3 to 6 hours |
| 1 | 35 mcg | 50 mcg | 4.5 hours |
| 6 | 50 mcg | 100 mcg | 4 to 6 hours |
| Note: 006 Ewert 2006 was not included because of the mode of misoprostol administration. | |||
Primary outcomes
There was no significant difference in the risk of failures to achieve delivery within 24 hours. There was less uterine hyperstimulation with FHR changes in the lower dose groups (16 trials, RR 0.51, 95% CI 0.37 to 0.69).
Serious maternal complications were reported in one study (025 Wing 1996): one maternal death occurred in a primiparous woman, nine hours after a single misoprostol dose and shortly after amnioinfusion and epidural analgesia, from amniotic fluid embolisation. Two caesarean hysterectomies were performed for atonic uterine haemorrhage, 13 and 30 hours after single doses of misoprostol, in one primiparous woman with uncomplicated labour, and in one nulliparous woman who developed chorioamnionitis following prolonged labour induction attempts by oxytocin augmentation. It is not clear whether these three women were allocated to the low (25 mcg) or the higher (50 mcg) dosage regimen misoprostol group.
Secondary outcomes
There was significantly more use of oxytocin (18 trials, average RR 1.30, 95% CI 1.14 to 1.49). This effect was due to the trials with a lower range of doses, and was not seen in the trials in which the lower dosage was 50 mcg. There were no differences in mode of delivery, meconium‐stained liquor or maternal side effects. There was less uterine hyperstimulation without FHR changes (14 trials, RR 0.57, 95% CI 0.46 to 0.69). There was a trend to fewer babies being admitted to the neonatal intensive care unit (9 trials, RR 0.82, 95% CI 0.64 to 1.05), particularly in the higher dose ranges.
Five perinatal deaths were reported (019 Filho 2007; 050 Majoko 2002a). There was one uterine rupture (038 Has 2002) with the use of low dose of misoprostol and two with the use of higher dose of misoprostol (050 Majoko 2002a; 075 Reyna‐Villasmil 2005). However, most studies have not specifically reported these outcomes. We have included only those specified in the reports in the data tables.
Misoprostol gel versus tablets
Primary outcomes
In one trial with 467 participants reviewed (050G Carlan 1997), uterine hyperstimulation with FHR changes was reduced with the gel preparation (RR 0.49, 95% CI 0.29 to 0.83).
Secondary outcomes
The use of oxytocin (RR 1.26, 95% CI 1.13 to 1.41) and epidural analgesia (RR 1.19, 95% CI 1.03 to 1.38) were increased. It is possible that in the process of gel preparation some potency is lost or that absorption is reduced.
One study showed no benefit from moistening misoprostol prior to insertion with 11 ml 3% acetic acid, versus dry tablets (Sanchez Ramos 2002).
A cost analysis in a high‐income country showed that the reduced cost in the misoprostol group (Sterling mean 2134, SD 574 versus 2202, SD 595 per case) was insignificant in relation to the overall cost of labour induction (050 Rozenberg 2001).
Discussion
Overall, this systematic review found that vaginal misoprostol is the more effective option for induction of labour and cervical ripening compared with oxytocin, dinoprostone and placebo. It also found that higher doses of vaginal misoprostol have no comparative advantages to the lower doses. There is, in general, considerable consistency between trials, except with respect to caesarean section rates and to the low misoprostol dosage regimens. The trials show that vaginal misoprostol in dosages ranging from 25 mcg two to three hourly, to 50 mcg four hourly (most studies), to 100 mcg six to 12 hourly, appear to be more effective than oxytocin or dinoprostone in the usual recommended doses for induction of labour, but with increased rates of uterine hyperstimulation both without and with associated FHR changes. The rates of caesarean section were inconsistent, tending to be reduced with misoprostol. The indication for caesarean section was not a prespecified outcome in this review. However, there was a consistent pattern of more operations for fetal distress and fewer for poor labour progress in the misoprostol groups (see 'Characteristics of included studies' table under 'Outcomes').
No differences in perinatal or maternal outcome were shown. However, the trials were not sufficiently large to assess the likelihood of uncommon, serious adverse perinatal and maternal complications. Of particular concern are several reports of uterine rupture following misoprostol use in women with and without previous caesarean section. One maternal death from amniotic fluid embolism following misoprostol induction was reported.
The possibility of inadvertent bias because of the unblinded nature of these studies should be kept in mind.
Lower dosage regimens of misoprostol were not less effective than higher doses in terms of failure to achieve vaginal birth within 24 hours. Adverse effects were reduced, with lower rates of uterine hyperstimulation and a trend to fewer admissions to neonatal intensive care unit.
The finding of a significantly more meconium‐stained liquor with misoprostol versus vaginal or intracervical prostaglandins is of interest. Wing et al (088 Wing 1995a) suggested the possibility of meconium passage in response to uterine hyperstimulation or a direct effect of absorbed misoprostol metabolites on the fetal gastrointestinal tract. We have previously observed an increased rate of meconium‐stained liquor in women who have ingested castor oil, though causality was not proven, and suggested a possible direct effect of the castor oil metabolites on fetal bowel (Mitri 1987). It is unlikely that the small amount of hydrogenated castor oil found in misoprostol tablets (075 Chuck 1995) would have any pharmacological effect, but the possibility that misoprostol metabolites may directly stimulate fetal bowel is of interest. We have shown an in vitro effect of misoprostol on isolated rat ileum (as well as myometrium) (Matonhodze 2002).
In countries in which misoprostol is being used for non‐registered obstetric indications, there is a need for health authorities and professional organisations to clarify the medicolegal implications. Particularly in countries in which conventional prostaglandins are unaffordable, health authorities need to decide whether misoprostol should be used in specific circumstances and, if so, take steps to legalise and regulate such use.
The trials reviewed lacked information on women's views with respect to this method of labour induction.
Authors' conclusions
Implications for practice.
The comparison between oral and vaginal misoprostol is dealt with in a separate Cochrane review (Alfirevic 2006). That review suggests that the optimal route for administration of misoprostol for labour induction is oral, not vaginal. The reasons for this are as follows.
Safety. The oral route is associated with a reduction in Apgar score of less than seven at five minutes (RR 0.65, 95% CI 0.44 to 0.97); and uterine hyperstimulation without FHR changes (RR 0.58, 95%CI 0.35 to 0.96, significant heterogeneity).
Convenience and comfort for the woman.
Because of a short half‐life, the oral dose can be titrated against the uterine response, commencing with a low dose such as 25 mcg two‐hourly, and increasing if necessary in nulliparous women to a maximum dose of 50 mcg two‐hourly.
Accuracy of dosage. In many countries, misoprostol is available only as 200 mcg or 100 mcg tablets. Breaking these tablets into small fragments for vaginal administration carries the risk of inappropriate dosage. Accurate oral dosage can be achieved by dissolving misoprostol in tap water, shaking well and administering as a solution. Left over solution should be discarded 24 hours after preparation.
The relative disadvantages of oral versus vaginal misoprostol are greater need for oxytocin augmentation (RR 1.19, 95% CI 1.06 to 1.34, significant heterogeneity).
The results of this review are therefore of limited practical importance: in dosages of 25 mcg three hourly or more, vaginal misoprostol is more effective than conventional methods of cervical ripening and labour induction. However, uterine hyperstimulation with FHR changes are increased. Although no differences in perinatal outcome were shown, the studies were not sufficiently large to exclude the possibility of uncommon serious adverse effects. The increase in meconium‐stained liquor is also of concern. Anecdotal reports of uterine rupture following labour induction with misoprostol are cause for concern (Gherman 1999; Daisley 2000; Hill 2000; Majoko 2002b).
The limited information on lower dosage regimens (25 mcg four hourly or less) suggests that they may be as effective as other prostaglandins, without increased uterine hyperstimulation.
Though misoprostol shows promise as a highly effective, inexpensive and convenient agent for labour induction, the lack of registration for this purpose, and thus of well‐established regimens, is problematic.
In most countries misoprostol is not registered for use for labour induction. In countries in which its use is considered advantageous, it is important that health authorities provide guidelines for practitioners to ensure the greatest possible level of safety in its use.
Implications for research.
While this review assessed the efficacy and safety of vaginal misoprostol (including its different regimens) based on data from its comparison to oral misoprostol, the vaginal route should not be researched further.
Because of the potential economic and clinical advantages of misoprostol, there is the need for further trials to establish its safety, particularly the relative safety of various dosages of oral administration. On the basis of this review, such trials should have the following features.
(1) Randomised, double blind.
(2) Oral or sublingual route of administration.
(3) Sample size sufficient to detect moderate differences in important uncommon complications such as serious perinatal morbidity/mortality.
(4) Meconium‐stained liquor included as an outcome measure.
(5) Women's views included as an outcome.
Randomised trials sufficiently large to assess rare events such as uterine rupture are not feasible. Alternative research methods are necessary such as case‐control studies and prospective audits of complications in services in which misoprostol is used routinely for labour induction.
We would be grateful to receive reports of rare serious complications such as uterine rupture in order to compile a register of such incidents.
[Note: The 27 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Feedback
Mwanza, July 2002
Summary
From my experience of induction of labour I agree that the risk of failure is far less with misoprostol than with prostaglandin E2. If women are carefully selected and started with the lower dose (we used 50 micrograms in one hospital in Zambia) the complications of hyperstimulation and occasional excessive vomiting would be significantly reduced.
The low cost of misoprostol and its effectiveness support its use in low‐middle income countries.
[Summary of comment from Moses Mabimba Mwanza, July 2002]
Reply
We agree with Dr Mwanza that if misoprostol is used for labour induction, the dosage should be kept to a minimum. Our findings suggest that the vaginal dosage should not exceed 25 mcg 4‐hourly.
[Reply from Justus Hofmeyr, August 2002]
Contributors
Moses Mabimba Mwanza
What's new
| Date | Event | Description |
|---|---|---|
| 15 February 2012 | Amended | Search updated. Twenty‐seven reports added to Studies awaiting classification (Ayaz 2010; Balci 2010; Balci 2011; Begum 2009; Brennan 2011; Chaudhuri 2011; Chen 2000a; Ezechi 2008; Girija 2009; Girija 2011; Gupta 2010; Hosli 2008; Joo 2000; Kim 2000; Mahendru 2011; Norzilawati 2010; Pevzner 2011; Pezvner 2011; Powers 2011; Rolland 2011; Saeed 2011; Shakya 2010; Shanmugham 2011; Stephenson 2011; Tan 2010; Wing 2011; Yang 2000a). |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 1, 1998
| Date | Event | Description |
|---|---|---|
| 10 September 2009 | New search has been performed | We included 51 additional studies from an updated search in November 2008. We updated the search in April 2010 and added the results to Studies awaiting classification for consideration in the next update. |
| 10 September 2009 | New citation required but conclusions have not changed | New author updated review with an additional 51 studies, which have provided more precise and robust conclusions. |
| 1 October 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Clinical Effectiveness Support Unit, Royal College of Obstetricians and Gynaecologists (Tony Kelly, Josephine Kavanagh, Jane Thomas), for primary data extraction. Zarko Alfirevic, Jim Neilson and Sonja Henderson, Tony Kelly and Josephine Kavanagh for contributions to development of the generic protocol for reviews of labour induction. Edwin S‐Y Chan and Ken Gu of the NMRC Clinical Trials and Epidemiology Unit, Ministry of Health, Singapore for translating two Chinese papers. Linan Cheng for retrieving, translating and assessing 28 Chinese papers.
Appendices
Appendix 1. Methods used to assess trials included in previous versions of this review
A strategy has been developed to deal with the large volume and complexity of trial data relating to labour induction. Many methods have been studied, in many different categories of women undergoing labour induction. Most trials are intervention‐driven, comparing two or more methods in various categories of women. Clinicians and parents need the data arranged by category of woman, to be able to choose which method is best for a particular clinical scenario. To extract these data from several hundred trial reports in a single step would be very difficult. We have therefore developed a two‐stage method of data extraction. The initial data extraction is done in a series of primary reviews arranged by methods of induction of labour, following a standardised methodology. The data will then be extracted from the primary reviews into a series of secondary reviews, arranged by category of woman.
To avoid duplication of data in the primary reviews, the labour induction methods have been listed in a specific order, from one to 25. Each primary review includes comparisons between one of the methods (from two to 25) with only those methods above it on the list. Thus, the review of intravenous oxytocin (4) will include only comparisons with intracervical prostaglandins (3), vaginal prostaglandins (2) or placebo (1). Methods identified in the future will be added to the end of the list. The current list is as follows:
(1) placebo/no treatment; (2) vaginal prostaglandins; (3) intracervical prostaglandins; (4) intravenous oxytocin; (5) amniotomy; (6) intravenous oxytocin with amniotomy; (7) vaginal misoprostol; (8) oral misoprostol; (9) mechanical methods including extra‐amniotic Foley catheter; (10) membrane sweeping; (11) extra‐amniotic prostaglandins; (12) intravenous prostaglandins; (13) oral prostaglandins; (14) mifepristone; (15) estrogens; (16) corticosteroids; (17) relaxin; (18) hyaluronidase; (19) castor oil, bath, and/or enema; (20) acupuncture; (21) breast stimulation; (22) sexual intercourse; (23) homoeopathic methods; (24) nitric oxide; (25) buccal or sublingual misoprostol.
The primary reviews are analysed by the following subgroups: (1) previous caesarean section or not; (2) nulliparity or multiparity; (3) membranes intact or ruptured; (4) cervix favourable, unfavourable or undefined.
The secondary reviews will include all methods of labour induction for each of the categories of women for which subgroup analysis has been done in the primary reviews, and will include only five primary outcome measures. There will thus be six secondary reviews of methods of labour induction in the following groups of women:
(1) nulliparous, intact membranes (unfavourable cervix, favourable cervix, cervix not defined); (2) nulliparous, ruptured membranes (unfavourable cervix, favourable cervix, cervix not defined); (3) multiparous, intact membranes (unfavourable cervix, favourable cervix, cervix not defined); (4) multiparous, ruptured membranes (unfavourable cervix, favourable cervix, cervix not defined); (5) previous caesarean section, intact membranes (unfavourable cervix, favourable cervix, cervix not defined); (6) previous caesarean section, ruptured membranes (unfavourable cervix, favourable cervix, cervix not defined).
Each time a primary review is updated with new data, those secondary reviews which include data which have changed, will also be updated.
The trials included in the primary reviews were extracted from an initial set of trials covering all interventions used in induction of labour (see above for details of search strategy). The data extraction process was conducted centrally. This was co‐ordinated from the Clinical Effectiveness Support Unit (CESU) at the Royal College of Obstetricians and Gynaecologists, UK, in co‐operation with The Pregnancy and Childbirth Group of The Cochrane Collaboration in 2000. This process has allowed the data extraction process to be standardised across all the reviews.
The trials are initially reviewed on eligibility criteria, using a standardised form and the basic selection criteria specified above. Following this, data are extracted to a standardised data extraction form which was piloted for consistency and completeness. The pilot process involved the researchers at the CESU and previous reviewers in the area of induction of labour.
Information is extracted regarding the methodological quality of trials on a number of levels. This process is completed without consideration of trial results. Assessment of selection bias examines the process involved in the generation of the random sequence and the method of allocation concealment separately. These are then judged as adequate or inadequate using the criteria described in Table 01 for the purpose of the reviews.
Performance bias is examined with regards to whom was blinded in the trials i.e. patient, caregiver, outcome assessor or analyst. In many trials the caregiver, assessor and analyst were the same party. Details of the feasibility and appropriateness of blinding at all levels is sought.
Individual outcome data are included in the analysis if they meet the pre stated criteria in 'Types of outcome measures'. Included trial data are processed as described in the Cochrane Collaboration Handbook (Clarke 2000). Data extracted from the trials are analysed on an intention to treat basis (when this was not done in the original report, re‐analysis is performed if possible). Where data are missing, clarification is sought from the original authors. If the attrition was such that it might significantly affect the results, these data are excluded from the analysis. This decision rests with the reviewers of primary reviews and is clearly documented. Once missing data become available, they will be included in the analyses.
Data are extracted from all eligible trials to examine how issues of quality influence effect size in a sensitivity analysis. In trials where reporting is poor, methodological issues are reported as unclear or clarification sought.
Due to the large number of trials, double data extraction was not feasible and agreement between the three data extractors was therefore assessed on a random sample of trials.
Once the data had been extracted, they were distributed to individual reviewers for entry onto the Review Manager computer software (RevMan 1999), checked for accuracy, and analysed as above using the RevMan software. For dichotomous data, relative risks and 95% confidence intervals are calculated, and in the absence of heterogeneity, results are pooled using a fixed effects model.
The predefined criteria for sensitivity analysis include all aspects of quality assessment as mentioned above, including aspects of selection, performance and attrition bias.
Primary analysis is limited to the prespecified outcomes and sub‐group analyses. In the event of differences in unspecified outcomes or sub‐groups being found, these are analysed post hoc, but clearly identified as such to avoid drawing unjustified conclusions.
Data and analyses
Comparison 1. Misoprostol versus placebo/no treatment: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 5 | 769 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.31, 1.03] |
| 2 Uterine hyperstimulation with FHR changes | 5 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.95, 5.99] |
| 3 Caesarean section | 10 | 1141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.63, 1.05] |
| 4 Neonatal encephalopathy | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.64] |
| 6 Oxytocin augmentation | 5 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.38, 1.02] |
| 7 Uterine hyperstimulation without FHR changes | 6 | 794 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.52 [1.78, 6.99] |
| 8 Uterine rupture | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Instrumental vaginal delivery | 3 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.65, 1.77] |
| 10 Meconium‐stained liquor | 6 | 814 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.35, 0.87] |
| 11 Apgar score < 7 at 5 minutes | 4 | 717 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.34, 11.80] |
| 12 Neonatal intensive care unit admission | 6 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.60, 1.48] |
| 13 Perinatal death | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.14] |
| 14 Maternal side effects | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [0.12, 66.62] |
| 15 Postpartum haemorrhage | 3 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.19, 4.62] |
| 16 Serious maternal complication | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.12, 3.87] |
| 17 Maternal death | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.

Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
1.2. Analysis.

Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
1.3. Analysis.

Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 3 Caesarean section.
1.4. Analysis.

Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 4 Neonatal encephalopathy.
1.5. Analysis.

Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
1.6. Analysis.

Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 6 Oxytocin augmentation.
1.7. Analysis.

Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 7 Uterine hyperstimulation without FHR changes.
1.8. Analysis.

Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 8 Uterine rupture.
1.9. Analysis.

Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 9 Instrumental vaginal delivery.
1.10. Analysis.

Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 10 Meconium‐stained liquor.
1.11. Analysis.

Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 11 Apgar score < 7 at 5 minutes.
1.12. Analysis.
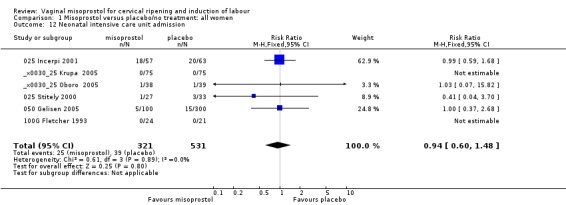
Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 12 Neonatal intensive care unit admission.
1.13. Analysis.

Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 13 Perinatal death.
1.14. Analysis.

Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 14 Maternal side effects.
1.15. Analysis.

Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 15 Postpartum haemorrhage.
1.16. Analysis.

Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 16 Serious maternal complication.
1.17. Analysis.
Comparison 1 Misoprostol versus placebo/no treatment: all women, Outcome 17 Maternal death.
Comparison 2. Misoprostol versus placebo/no treatment: all women, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 4 | 619 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.23, 2.15] |
| 2 Uterine hyperstimulation with FHR changes | 4 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.73, 5.71] |
| 3 Caesarean section | 7 | 862 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.69, 1.30] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.64] |
| 5 Oxytocin augmentation | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.26, 0.58] |
| 6 Uterine hyperstimulation without FHR changes | 5 | 714 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.47 [1.63, 7.38] |
| 7 Uterine rupture | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Instrumental vaginal delivery | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.50, 2.12] |
| 9 Meconium‐stained liquor | 4 | 612 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.89] |
| 10 Apgar score < 7 at 5 minutes | 3 | 567 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.34, 11.80] |
| 11 Neonatal intensive care unit admission | 3 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.35, 2.05] |
| 12 Perinatal death | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal side effects | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [0.12, 66.62] |
| 14 Postpartum haemorrhage | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.13, 6.37] |
| 15 Serious maternal complication | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal death | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.

Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 1 Vaginal delivery not achieved in 24 hours.
2.2. Analysis.

Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
2.3. Analysis.

Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 3 Caesarean section.
2.4. Analysis.

Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.
2.5. Analysis.

Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 5 Oxytocin augmentation.
2.6. Analysis.
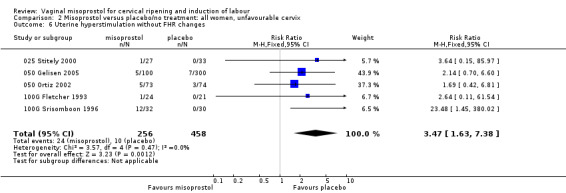
Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 6 Uterine hyperstimulation without FHR changes.
2.7. Analysis.

Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 7 Uterine rupture.
2.8. Analysis.
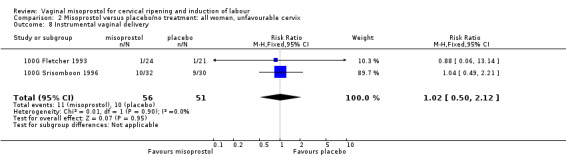
Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 8 Instrumental vaginal delivery.
2.9. Analysis.

Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 9 Meconium‐stained liquor.
2.10. Analysis.

Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 10 Apgar score < 7 at 5 minutes.
2.11. Analysis.

Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 11 Neonatal intensive care unit admission.
2.12. Analysis.

Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 12 Perinatal death.
2.13. Analysis.

Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 13 Maternal side effects.
2.14. Analysis.

Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 14 Postpartum haemorrhage.
2.15. Analysis.

Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 15 Serious maternal complication.
2.16. Analysis.

Comparison 2 Misoprostol versus placebo/no treatment: all women, unfavourable cervix, Outcome 16 Maternal death.
Comparison 3. Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 2 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.22, 0.70] |
| 2 Uterine hyperstimulation with FHR changes | 3 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.52, 10.16] |
| 3 Caesarean section | 5 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.75, 1.79] |
| 4 Epidural analgesia | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.26] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.64] |
| 6 Oxytocin augmentation | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.26, 0.58] |
| 7 Uterine hyperstimulation without FHR changes | 3 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.11 [1.91, 53.60] |
| 8 Uterine rupture | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Instrumental vaginal delivery | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.50, 2.12] |
| 10 Meconium‐stained liquor | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.28, 1.77] |
| 11 Apgar score < 7 at 5 minutes | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Neonatal intensive care unit admission | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.04, 3.70] |
| 13 Perinatal death | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal side effects | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [0.12, 66.62] |
| 15 Postpartum haemorrhage | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.13, 6.37] |
| 16 Serious maternal complication | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Maternal death | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.

Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 1 Vaginal delivery not achieved in 24 hours.
3.2. Analysis.

Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
3.3. Analysis.

Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 3 Caesarean section.
3.4. Analysis.
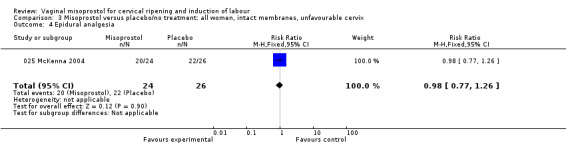
Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 4 Epidural analgesia.
3.5. Analysis.

Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
3.6. Analysis.
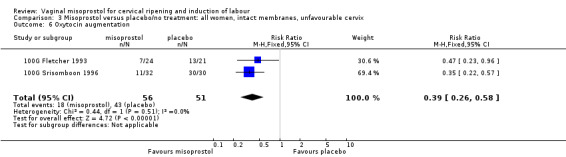
Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 6 Oxytocin augmentation.
3.7. Analysis.

Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 7 Uterine hyperstimulation without FHR changes.
3.8. Analysis.

Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 8 Uterine rupture.
3.9. Analysis.

Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 9 Instrumental vaginal delivery.
3.10. Analysis.

Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 10 Meconium‐stained liquor.
3.11. Analysis.

Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 11 Apgar score < 7 at 5 minutes.
3.12. Analysis.
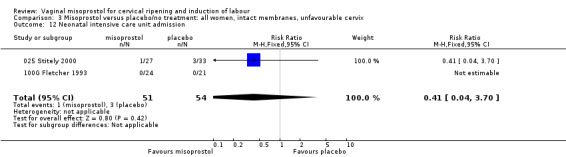
Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 12 Neonatal intensive care unit admission.
3.13. Analysis.

Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 13 Perinatal death.
3.14. Analysis.

Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 14 Maternal side effects.
3.15. Analysis.

Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 15 Postpartum haemorrhage.
3.16. Analysis.

Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 16 Serious maternal complication.
3.17. Analysis.

Comparison 3 Misoprostol versus placebo/no treatment: all women, intact membranes, unfavourable cervix, Outcome 17 Maternal death.
Comparison 4. Misoprostol versus placebo/no treatment: all women, ruptured membranes, variable or undefined cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.46, 34.96] |
| 2 Vaginal delivery not achieved in 24 hours | 2 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.31, 0.73] |
| 3 Caesarean section | 4 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.36, 0.91] |
| 4 Apgar score < 7 at 5 minutes | 2 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Neonatal intensive care unit admission | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.82] |
| 6 Neonatal encephalopathy | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Oxytocin augmentation | 2 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.29, 1.32] |
| 8 Serious maternal complications | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.12, 3.87] |
| 9 Perinatal death | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.14] |
| 10 Instrumental delivery | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.56, 2.22] |
| 11 Postpartum haemorrhage | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.82] |
| 12 Meconium‐stained liquor | 3 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.38, 1.81] |
| 13 Uterine hyperstimulation without FHR changes | 1 | 107 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.17, 3.38] |
4.1. Analysis.

Comparison 4 Misoprostol versus placebo/no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 1 Uterine hyperstimulation with FHR changes.
4.2. Analysis.
Comparison 4 Misoprostol versus placebo/no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 2 Vaginal delivery not achieved in 24 hours.
4.3. Analysis.

Comparison 4 Misoprostol versus placebo/no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 3 Caesarean section.
4.4. Analysis.

Comparison 4 Misoprostol versus placebo/no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 4 Apgar score < 7 at 5 minutes.
4.5. Analysis.

Comparison 4 Misoprostol versus placebo/no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 5 Neonatal intensive care unit admission.
4.6. Analysis.

Comparison 4 Misoprostol versus placebo/no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 6 Neonatal encephalopathy.
4.7. Analysis.

Comparison 4 Misoprostol versus placebo/no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 7 Oxytocin augmentation.
4.8. Analysis.

Comparison 4 Misoprostol versus placebo/no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 8 Serious maternal complications.
4.9. Analysis.

Comparison 4 Misoprostol versus placebo/no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 9 Perinatal death.
4.10. Analysis.

Comparison 4 Misoprostol versus placebo/no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 10 Instrumental delivery.
4.11. Analysis.

Comparison 4 Misoprostol versus placebo/no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 11 Postpartum haemorrhage.
4.12. Analysis.

Comparison 4 Misoprostol versus placebo/no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 12 Meconium‐stained liquor.
4.13. Analysis.

Comparison 4 Misoprostol versus placebo/no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 13 Uterine hyperstimulation without FHR changes.
Comparison 5. Misoprostol versus placebo/no treatment: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.49, 2.41] |
5.1. Analysis.

Comparison 5 Misoprostol versus placebo/no treatment: all primiparae, Outcome 1 Caesarean section.
Comparison 6. Misoprostol versus placebo/no treatment: all primiparae and unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.49, 2.41] |
6.1. Analysis.

Comparison 6 Misoprostol versus placebo/no treatment: all primiparae and unfavourable cervix, Outcome 1 Caesarean section.
Comparison 7. Misoprostol versus placebo/no treatment: all primiparae, intact membranes and unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.49, 2.41] |
7.1. Analysis.

Comparison 7 Misoprostol versus placebo/no treatment: all primiparae, intact membranes and unfavourable cervix, Outcome 1 Caesarean section.
Comparison 9. Misoprostol versus placebo/no treatment: all multiparous and unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.06, 6.05] |
9.1. Analysis.

Comparison 9 Misoprostol versus placebo/no treatment: all multiparous and unfavourable cervix, Outcome 1 Caesarean section.
Comparison 10. Misoprostol versus placebo/no treatment: all multiparous, intact membranes and unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.06, 6.05] |
10.1. Analysis.

Comparison 10 Misoprostol versus placebo/no treatment: all multiparous, intact membranes and unfavourable cervix, Outcome 1 Caesarean section.
Comparison 11. Misoprostol versus vaginal prostaglandin: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 22 | 5229 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.66, 0.89] |
| 2 Uterine hyperstimulation with FHR changes | 31 | 5830 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.97, 2.09] |
| 3 Caesarean section | 34 | 6855 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.87, 1.03] |
| 4 Serious neonatal morbidity or perinatal death | 3 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.98 [0.25, 145.59] |
| 5 Serious maternal morbidity or death | 2 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 2 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.44] |
| 7 Oxytocin augmentation | 38 | 7022 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.61, 0.76] |
| 8 Uterine hyperstimulation without FHR changes | 26 | 4804 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.41, 2.79] |
| 9 Uterine rupture | 5 | 1464 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.88] |
| 10 Epidural analgesia | 8 | 2141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 0.99] |
| 11 Instrumental vaginal delivery | 19 | 3593 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.89, 1.18] |
| 12 Meconium‐stained liquor | 18 | 3991 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.13, 1.61] |
| 13 Apgar score < 7 at 5 minutes | 17 | 3969 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.72, 1.41] |
| 14 Neonatal intensive care unit admission | 20 | 4530 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.15] |
| 15 Neonatal encephalopathy | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.98 [0.25, 145.59] |
| 16 Perinatal death | 6 | 1315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.24, 3.00] |
| 17 Serious maternal complications | 2 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.39] |
| 18 Maternal side effects (eg nausea, vomiting, diarrhoea) | 10 | 2698 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| 19 Postpartum haemorrhage | 8 | 1385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.21] |
11.1. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
11.2. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
11.3. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 3 Caesarean section.
11.4. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 4 Serious neonatal morbidity or perinatal death.
11.5. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 5 Serious maternal morbidity or death.
11.6. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
11.7. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 7 Oxytocin augmentation.
11.8. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 8 Uterine hyperstimulation without FHR changes.
11.9. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 9 Uterine rupture.
11.10. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 10 Epidural analgesia.
11.11. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 11 Instrumental vaginal delivery.
11.12. Analysis.
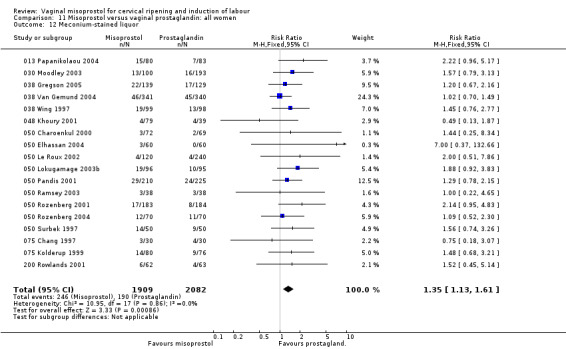
Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 12 Meconium‐stained liquor.
11.13. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 13 Apgar score < 7 at 5 minutes.
11.14. Analysis.
Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 14 Neonatal intensive care unit admission.
11.15. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 15 Neonatal encephalopathy.
11.16. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 16 Perinatal death.
11.17. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 17 Serious maternal complications.
11.18. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 18 Maternal side effects (eg nausea, vomiting, diarrhoea).
11.19. Analysis.

Comparison 11 Misoprostol versus vaginal prostaglandin: all women, Outcome 19 Postpartum haemorrhage.
Comparison 12. Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 18 | 4491 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.67, 0.89] |
| 2 Uterine hyperstimulation with FHR changes | 26 | 5010 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.17, 1.87] |
| 3 Caesarean section | 28 | 5832 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.05] |
| 4 Serious neonatal morbidity or perinatal death | 5 | 1344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.23, 3.70] |
| 5 Uterine rupture | 2 | 844 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.88] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.27, 0.98] |
| 7 Oxytocin augmentation | 21 | 4476 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.57, 0.79] |
| 8 Uterine hyperstimulation without FHR changes | 16 | 2683 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.21, 3.36] |
| 9 Serious maternal morbidity or death | 2 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 3 | 992 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.71, 1.11] |
| 11 Instrumental vaginal delivery | 12 | 2053 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.91, 1.34] |
| 12 Meconium‐stained liquor | 11 | 2346 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.05, 1.65] |
| 13 Apgar score < 7 at 5 minutes | 10 | 2399 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.69, 1.45] |
| 14 Neonatal intensive care unit admission | 10 | 2348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.73, 1.07] |
| 15 Neonatal encephalopathy | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.98 [0.25, 145.59] |
| 16 Perinatal death | 3 | 887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.17, 5.66] |
| 17 Serious maternal complications | 2 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.39] |
| 18 Maternal side effects (eg nausea, vomiting, diarrhoea) | 9 | 2344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| 19 Postpartum haemorrhage | 6 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.37, 1.91] |
12.1. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
12.2. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
12.3. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 3 Caesarean section.
12.4. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 4 Serious neonatal morbidity or perinatal death.
12.5. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 5 Uterine rupture.
12.6. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
12.7. Analysis.
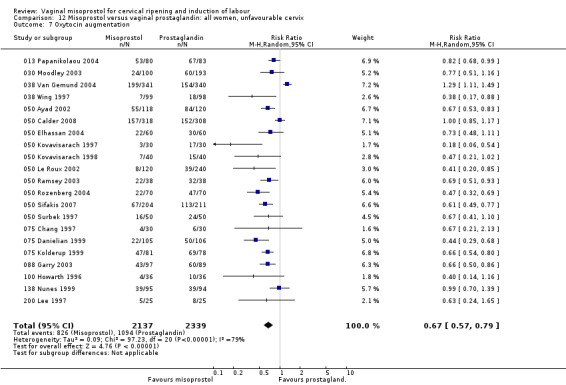
Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 7 Oxytocin augmentation.
12.8. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
12.9. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 9 Serious maternal morbidity or death.
12.10. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 10 Epidural analgesia.
12.11. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 11 Instrumental vaginal delivery.
12.12. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 12 Meconium‐stained liquor.
12.13. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 13 Apgar score < 7 at 5 minutes.
12.14. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 14 Neonatal intensive care unit admission.
12.15. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 15 Neonatal encephalopathy.
12.16. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 16 Perinatal death.
12.17. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 17 Serious maternal complications.
12.18. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 18 Maternal side effects (eg nausea, vomiting, diarrhoea).
12.19. Analysis.

Comparison 12 Misoprostol versus vaginal prostaglandin: all women, unfavourable cervix, Outcome 19 Postpartum haemorrhage.
Comparison 13. Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 8 | 1995 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.67, 0.91] |
| 2 Uterine hyperstimulation with FHR changes | 13 | 2309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.29, 2.72] |
| 3 Caesarean section | 14 | 3011 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88, 1.15] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.98 [0.25, 145.59] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.27, 0.98] |
| 6 Oxytocin augmentation | 10 | 2276 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.56, 0.86] |
| 7 Uterine hyperstimulation without FHR changes | 8 | 1590 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [1.02, 3.82] |
| 8 Uterine rupture | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Epidural analgesia | 2 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.57, 1.15] |
| 10 Instrumental vaginal delivery | 5 | 1156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.81, 1.36] |
| 11 Meconium‐stained liquor | 6 | 949 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.98, 2.04] |
| 12 Apgar score < 7 at 5 minutes | 4 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.61, 1.63] |
| 13 Neonatal intensive care unit admission | 6 | 1100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.89, 1.81] |
| 14 Neonatal encephalopathy | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.98 [0.25, 145.59] |
| 15 Perinatal death | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 68.95] |
| 16 Maternal side effects (eg nausea, vomiting, diarrhoea) | 3 | 883 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.53, 1.99] |
| 17 Postpartum haemorrhage | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.91] |
13.1. Analysis.

Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
13.2. Analysis.

Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
13.3. Analysis.

Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 3 Caesarean section.
13.4. Analysis.
Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 4 Serious neonatal morbidity or perinatal death.
13.5. Analysis.

Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
13.6. Analysis.

Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 6 Oxytocin augmentation.
13.7. Analysis.

Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 7 Uterine hyperstimulation without FHR changes.
13.8. Analysis.

Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 8 Uterine rupture.
13.9. Analysis.

Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 9 Epidural analgesia.
13.10. Analysis.

Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 10 Instrumental vaginal delivery.
13.11. Analysis.

Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 11 Meconium‐stained liquor.
13.12. Analysis.

Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 12 Apgar score < 7 at 5 minutes.
13.13. Analysis.
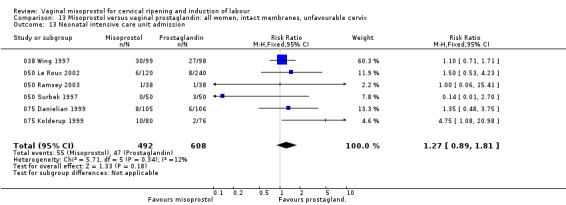
Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 13 Neonatal intensive care unit admission.
13.14. Analysis.

Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 14 Neonatal encephalopathy.
13.15. Analysis.

Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 15 Perinatal death.
13.16. Analysis.

Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 16 Maternal side effects (eg nausea, vomiting, diarrhoea).
13.17. Analysis.

Comparison 13 Misoprostol versus vaginal prostaglandin: all women, intact membranes, unfavourable cervix, Outcome 17 Postpartum haemorrhage.
Comparison 14. Misoprostol versus vaginal prostaglandin: all women, intact membranes, variable or undefined cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.28, 3.37] |
| 2 Caesarean section | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.27, 1.32] |
| 3 Oxytocin augmentation | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.51, 0.97] |
| 4 Uterine hyperstimulation without FHR changes | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.84 [0.74, 46.21] |
| 5 Epidural analgesia | 2 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.57, 1.15] |
| 6 Instrumental vaginal delivery | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.73 [0.49, 155.62] |
| 7 Perinatal death | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
14.1. Analysis.

Comparison 14 Misoprostol versus vaginal prostaglandin: all women, intact membranes, variable or undefined cervix, Outcome 1 Uterine hyperstimulation with FHR changes.
14.2. Analysis.

Comparison 14 Misoprostol versus vaginal prostaglandin: all women, intact membranes, variable or undefined cervix, Outcome 2 Caesarean section.
14.3. Analysis.
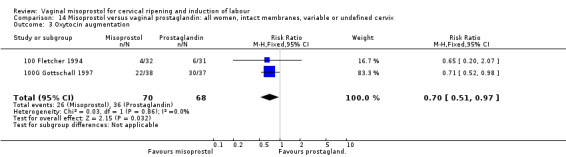
Comparison 14 Misoprostol versus vaginal prostaglandin: all women, intact membranes, variable or undefined cervix, Outcome 3 Oxytocin augmentation.
14.4. Analysis.

Comparison 14 Misoprostol versus vaginal prostaglandin: all women, intact membranes, variable or undefined cervix, Outcome 4 Uterine hyperstimulation without FHR changes.
14.5. Analysis.

Comparison 14 Misoprostol versus vaginal prostaglandin: all women, intact membranes, variable or undefined cervix, Outcome 5 Epidural analgesia.
14.6. Analysis.

Comparison 14 Misoprostol versus vaginal prostaglandin: all women, intact membranes, variable or undefined cervix, Outcome 6 Instrumental vaginal delivery.
14.7. Analysis.

Comparison 14 Misoprostol versus vaginal prostaglandin: all women, intact membranes, variable or undefined cervix, Outcome 7 Perinatal death.
Comparison 15. Misoprostol versus vaginal prostaglandin: all women with ruptured membranes and unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.98, 2.59] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.08 [0.60, 42.87] |
| 3 Uterine hyperstimulation without FHR changes | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.84 [1.93, 7.65] |
| 4 Instrumental vaginal delivery | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.82, 3.17] |
| 5 Apgar score < 7 at 5 minutes | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.44, 2.35] |
| 6 Neonatal intensive care unit admission | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.51, 1.31] |
| 7 Oxytocin augmentation | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.22, 0.64] |
15.1. Analysis.

Comparison 15 Misoprostol versus vaginal prostaglandin: all women with ruptured membranes and unfavourable cervix, Outcome 1 Caesarean section.
15.2. Analysis.

Comparison 15 Misoprostol versus vaginal prostaglandin: all women with ruptured membranes and unfavourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
15.3. Analysis.

Comparison 15 Misoprostol versus vaginal prostaglandin: all women with ruptured membranes and unfavourable cervix, Outcome 3 Uterine hyperstimulation without FHR changes.
15.4. Analysis.

Comparison 15 Misoprostol versus vaginal prostaglandin: all women with ruptured membranes and unfavourable cervix, Outcome 4 Instrumental vaginal delivery.
15.5. Analysis.

Comparison 15 Misoprostol versus vaginal prostaglandin: all women with ruptured membranes and unfavourable cervix, Outcome 5 Apgar score < 7 at 5 minutes.
15.6. Analysis.

Comparison 15 Misoprostol versus vaginal prostaglandin: all women with ruptured membranes and unfavourable cervix, Outcome 6 Neonatal intensive care unit admission.
15.7. Analysis.

Comparison 15 Misoprostol versus vaginal prostaglandin: all women with ruptured membranes and unfavourable cervix, Outcome 7 Oxytocin augmentation.
Comparison 16. Misoprostol versus vaginal prostaglandin: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 5 | 736 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.46, 1.05] |
| 2 Uterine hyperstimulation with FHR changes | 7 | 879 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.00, 2.91] |
| 3 Caesarean section | 8 | 1279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.68, 0.99] |
| 4 Uterine hyperstimulation without FHR changes | 4 | 646 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.80, 2.82] |
| 5 Apgar score < 7 at 5 minutes | 4 | 894 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.62, 3.20] |
| 6 Neonatal intensive care unit admission | 3 | 731 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.47, 2.25] |
| 7 Oxytocin augmentation | 7 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 0.99] |
| 8 Serious neonatal morbidity | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Perinatal death | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.36] |
| 10 Serious maternal morbidity or death | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 4 | 527 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.96, 1.76] |
| 12 Meconium‐stained liquor | 4 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.02, 2.33] |
| 13 Uterine rupture | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Postpartum haemorrhage | 2 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.39, 3.63] |
| 15 Serious maternal complications | 2 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.39] |
| 16 Epidural analgesia | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.07] |
| 17 Maternal side effects (eg nausea, vomiting, diarrhoea) | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.31, 1.09] |
16.1. Analysis.

Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 1 Vaginal delivery not achieved within 24 hours.
16.2. Analysis.

Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 2 Uterine hyperstimulation with FHR changes.
16.3. Analysis.

Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 3 Caesarean section.
16.4. Analysis.
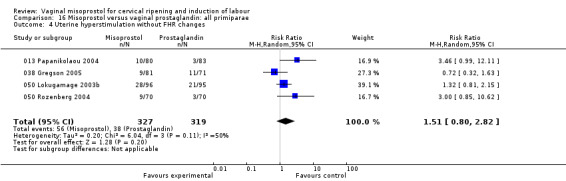
Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 4 Uterine hyperstimulation without FHR changes.
16.5. Analysis.

Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 5 Apgar score < 7 at 5 minutes.
16.6. Analysis.

Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 6 Neonatal intensive care unit admission.
16.7. Analysis.

Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 7 Oxytocin augmentation.
16.8. Analysis.
Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 8 Serious neonatal morbidity.
16.9. Analysis.

Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 9 Perinatal death.
16.10. Analysis.

Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 10 Serious maternal morbidity or death.
16.11. Analysis.

Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 11 Instrumental vaginal delivery.
16.12. Analysis.

Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 12 Meconium‐stained liquor.
16.13. Analysis.

Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 13 Uterine rupture.
16.14. Analysis.

Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 14 Postpartum haemorrhage.
16.15. Analysis.

Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 15 Serious maternal complications.
16.16. Analysis.

Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 16 Epidural analgesia.
16.17. Analysis.

Comparison 16 Misoprostol versus vaginal prostaglandin: all primiparae, Outcome 17 Maternal side effects (eg nausea, vomiting, diarrhoea).
Comparison 17. Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.29, 1.40] |
| 2 Uterine hyperstimulation with FHR changes | 7 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.13 [1.66, 10.28] |
| 3 Caesarean section | 5 | 888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.59, 1.00] |
| 4 Apgar score < 7 at 5 minutes | 3 | 703 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.55, 3.51] |
| 5 Serious neonatal morbidity | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal intensive care admission | 2 | 540 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.62, 1.22] |
| 7 Oxytocin augmentation | 2 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.38, 0.69] |
| 8 Perinatal death | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.36] |
| 9 Serious maternal morbidity or death | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Uterine hyperstimulation without FHR changes | 2 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.23 [1.33, 7.85] |
| 11 Instrumental vaginal delivery | 3 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.97, 2.11] |
| 12 Meconium‐stained liquor | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.83, 2.31] |
| 13 Uterine rupture | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Postpartum haemorrhage | 2 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.39, 3.63] |
| 15 Serious maternal complications | 2 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.39] |
| 16 Epidural analgesia | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.07] |
| 17 Maternal side effects (eg nausea, vomiting, diarrhoea) | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.31, 1.09] |
17.1. Analysis.

Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
17.2. Analysis.

Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
17.3. Analysis.

Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 3 Caesarean section.
17.4. Analysis.

Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 4 Apgar score < 7 at 5 minutes.
17.5. Analysis.

Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 5 Serious neonatal morbidity.
17.6. Analysis.

Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 6 Neonatal intensive care admission.
17.7. Analysis.

Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 7 Oxytocin augmentation.
17.8. Analysis.

Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 8 Perinatal death.
17.9. Analysis.

Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 9 Serious maternal morbidity or death.
17.10. Analysis.
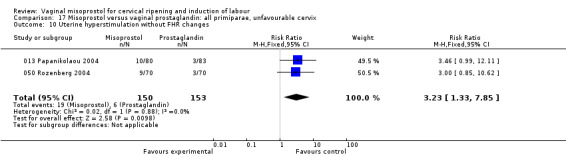
Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 10 Uterine hyperstimulation without FHR changes.
17.11. Analysis.
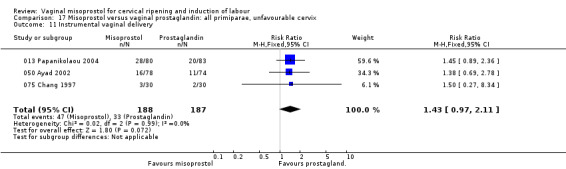
Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 11 Instrumental vaginal delivery.
17.12. Analysis.

Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 12 Meconium‐stained liquor.
17.13. Analysis.

Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 13 Uterine rupture.
17.14. Analysis.

Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 14 Postpartum haemorrhage.
17.15. Analysis.

Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 15 Serious maternal complications.
17.16. Analysis.

Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 16 Epidural analgesia.
17.17. Analysis.

Comparison 17 Misoprostol versus vaginal prostaglandin: all primiparae, unfavourable cervix, Outcome 17 Maternal side effects (eg nausea, vomiting, diarrhoea).
Comparison 18. Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.29, 1.40] |
| 2 Uterine hyperstimulation with FHR changes | 4 | 488 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.13 [1.66, 10.28] |
| 3 Caesarean section | 4 | 488 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.53, 1.18] |
| 4 Uterine hyperstimulation without FHR changes | 2 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.23 [1.33, 7.85] |
| 5 Apgar score < 7 at 5 minutes | 2 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.36, 15.76] |
| 6 Neonatal intensive care unit admission | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.14, 1.38] |
| 7 Oxytocin augmentation | 3 | 363 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.39, 1.05] |
| 8 Postpartum haemorrhage | 2 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.39, 3.63] |
| 9 Serious maternal complication | 2 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.39] |
| 10 Epidural analgesia | 1 | 140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.21, 2.26] |
| 11 Meconium‐stained liquor | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.83, 2.31] |
| 12 Serious maternal morbidity or death | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Serious neonatal morbidity | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Perinatal death | 1 | 163 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.51] |
| 15 Uterine rupture | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal side effects (eg nausea, vomiting, diarrhoea) | 3 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.31, 1.09] |
18.1. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
18.2. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
18.3. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 3 Caesarean section.
18.4. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 4 Uterine hyperstimulation without FHR changes.
18.5. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 5 Apgar score < 7 at 5 minutes.
18.6. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 6 Neonatal intensive care unit admission.
18.7. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 7 Oxytocin augmentation.
18.8. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 8 Postpartum haemorrhage.
18.9. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 9 Serious maternal complication.
18.10. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 10 Epidural analgesia.
18.11. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 11 Meconium‐stained liquor.
18.12. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 12 Serious maternal morbidity or death.
18.13. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 13 Serious neonatal morbidity.
18.14. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 14 Perinatal death.
18.15. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 15 Uterine rupture.
18.16. Analysis.

Comparison 18 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, unfavourable cervix, Outcome 16 Maternal side effects (eg nausea, vomiting, diarrhoea).
Comparison 19. Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, variable or undefined cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.35, 1.66] |
19.1. Analysis.

Comparison 19 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, variable or undefined cervix, Outcome 1 Uterine hyperstimulation with FHR changes.
19.2. Analysis.

Comparison 19 Misoprostol versus vaginal prostaglandin: all primiparae, intact membranes, variable or undefined cervix, Outcome 2 Caesarean section.
Comparison 20. Misoprostol versus vaginal prostaglandin: all primiparae with ruptured membranes and unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oxytocin augmentation | 1 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.08, 0.35] |
| 2 Instrumental vaginal delivery | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.69, 2.78] |
20.1. Analysis.

Comparison 20 Misoprostol versus vaginal prostaglandin: all primiparae with ruptured membranes and unfavourable cervix, Outcome 1 Oxytocin augmentation.
20.2. Analysis.

Comparison 20 Misoprostol versus vaginal prostaglandin: all primiparae with ruptured membranes and unfavourable cervix, Outcome 2 Instrumental vaginal delivery.
Comparison 21. Misoprostol versus vaginal prostaglandin: all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.69, 1.96] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.08, 2.57] |
| 3 Caesarean section | 3 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.38, 1.43] |
| 4 Oxytocin augmentation | 2 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.19, 2.09] |
| 5 Uterine hyperstimulation without FHR changes | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.37, 1.61] |
| 6 Instrumental delivery | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.20, 2.24] |
| 7 Apgar score < 7 at 5 minutes | 1 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.49] |
| 8 Neonatal intensive care unit admission | 1 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.32, 0.83] |
21.1. Analysis.

Comparison 21 Misoprostol versus vaginal prostaglandin: all multiparae, Outcome 1 Vaginal delivery not achieved within 24 hours.
21.2. Analysis.

Comparison 21 Misoprostol versus vaginal prostaglandin: all multiparae, Outcome 2 Uterine hyperstimulation with FHR changes.
21.3. Analysis.

Comparison 21 Misoprostol versus vaginal prostaglandin: all multiparae, Outcome 3 Caesarean section.
21.4. Analysis.

Comparison 21 Misoprostol versus vaginal prostaglandin: all multiparae, Outcome 4 Oxytocin augmentation.
21.5. Analysis.

Comparison 21 Misoprostol versus vaginal prostaglandin: all multiparae, Outcome 5 Uterine hyperstimulation without FHR changes.
21.6. Analysis.

Comparison 21 Misoprostol versus vaginal prostaglandin: all multiparae, Outcome 6 Instrumental delivery.
21.7. Analysis.

Comparison 21 Misoprostol versus vaginal prostaglandin: all multiparae, Outcome 7 Apgar score < 7 at 5 minutes.
21.8. Analysis.

Comparison 21 Misoprostol versus vaginal prostaglandin: all multiparae, Outcome 8 Neonatal intensive care unit admission.
Comparison 22. Misoprostol versus vaginal prostaglandin: all multiparae, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.68, 2.31] |
| 2 Caesarean section | 1 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.37, 1.54] |
| 3 Apgar score < 7 at 5 minutes | 1 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.49] |
| 4 Neonatal intensive care unit admission | 1 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.32, 0.83] |
| 5 Oxytocin augmentation | 5 | 915 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.48, 1.03] |
22.1. Analysis.

Comparison 22 Misoprostol versus vaginal prostaglandin: all multiparae, unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
22.2. Analysis.

Comparison 22 Misoprostol versus vaginal prostaglandin: all multiparae, unfavourable cervix, Outcome 2 Caesarean section.
22.3. Analysis.

Comparison 22 Misoprostol versus vaginal prostaglandin: all multiparae, unfavourable cervix, Outcome 3 Apgar score < 7 at 5 minutes.
22.4. Analysis.

Comparison 22 Misoprostol versus vaginal prostaglandin: all multiparae, unfavourable cervix, Outcome 4 Neonatal intensive care unit admission.
22.5. Analysis.

Comparison 22 Misoprostol versus vaginal prostaglandin: all multiparae, unfavourable cervix, Outcome 5 Oxytocin augmentation.
Comparison 23. Misoprostol versus vaginal prostaglandin: all multiparae, intact membranes, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.68, 2.31] |
| 2 Instrumental vaginal delivery | 2 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.91, 2.33] |
23.1. Analysis.

Comparison 23 Misoprostol versus vaginal prostaglandin: all multiparae, intact membranes, unfavourable cervix, Outcome 1 Vaginal delivery not achieved in 24 hours.
23.2. Analysis.

Comparison 23 Misoprostol versus vaginal prostaglandin: all multiparae, intact membranes, unfavourable cervix, Outcome 2 Instrumental vaginal delivery.
Comparison 24. Misoprostol versus vaginal prostaglandin: all multiparae, intact membranes, variable or undefined cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.11, 54.97] |
| 2 Caesarean section | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.06, 6.05] |
24.1. Analysis.

Comparison 24 Misoprostol versus vaginal prostaglandin: all multiparae, intact membranes, variable or undefined cervix, Outcome 1 Uterine hyperstimulation with FHR changes.
24.2. Analysis.

Comparison 24 Misoprostol versus vaginal prostaglandin: all multiparae, intact membranes, variable or undefined cervix, Outcome 2 Caesarean section.
24.3. Analysis.

Comparison 24 Misoprostol versus vaginal prostaglandin: all multiparae, intact membranes, variable or undefined cervix, Outcome 3 Caesarean section.
Comparison 25. Misoprostol versus intracervical prostaglandin: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 13 | 1627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.56, 0.71] |
| 2 Uterine hyperstimulation with FHR changes | 20 | 2224 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.64, 3.28] |
| 3 Caesarean section | 27 | 3311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.11] |
| 4 Serious neonatal morbidity/perinatal death | 2 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.09, 1.87] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.52, 0.88] |
| 6 Oxytocin augmentation | 20 | 2316 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.48, 0.64] |
| 7 Uterine hyperstimulation without FHR changes | 17 | 2178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.57, 2.42] |
| 8 Uterine rupture | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Epidural analgesia | 2 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.48, 0.86] |
| 10 Instrumental vaginal delivery | 13 | 1900 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.84, 1.32] |
| 11 Meconium‐stained liquor | 14 | 2018 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.04, 1.59] |
| 12 Apgar score < 7 at 5 minutes | 15 | 2114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.51, 1.70] |
| 13 Neonatal intensive care unit admission | 13 | 1773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.81, 1.52] |
| 14 Perinatal death | 2 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 68.95] |
| 15 Maternal side effects (eg nausea, vomiting, diarrhoea) | 7 | 731 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.68, 1.95] |
| 16 Postpartum haemorrhage | 3 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.22, 4.24] |
25.1. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
25.2. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
25.3. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 3 Caesarean section.
25.4. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 4 Serious neonatal morbidity/perinatal death.
25.5. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
25.6. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 6 Oxytocin augmentation.
25.7. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 7 Uterine hyperstimulation without FHR changes.
25.8. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 8 Uterine rupture.
25.9. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 9 Epidural analgesia.
25.10. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 10 Instrumental vaginal delivery.
25.11. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 11 Meconium‐stained liquor.
25.12. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 12 Apgar score < 7 at 5 minutes.
25.13. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 13 Neonatal intensive care unit admission.
25.14. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 14 Perinatal death.
25.15. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 15 Maternal side effects (eg nausea, vomiting, diarrhoea).
25.16. Analysis.

Comparison 25 Misoprostol versus intracervical prostaglandin: all women, Outcome 16 Postpartum haemorrhage.
Comparison 26. Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 10 | 1287 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.54, 0.69] |
| 2 Uterine hyperstimulation with FHR changes | 19 | 2124 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.64, 3.30] |
| 3 Caesarean section | 21 | 2499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.13] |
| 4 Serious neonatal morbidity | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.09, 1.87] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.52, 0.88] |
| 6 Oxytocin augmentation | 16 | 1733 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.50, 0.67] |
| 7 Uterine hyperstimulation without FHR changes | 16 | 1978 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.60, 2.47] |
| 8 Uterine rupture | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Epidural analgesia | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.41, 1.25] |
| 10 Instrumental vaginal delivery | 11 | 1478 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.67, 1.13] |
| 11 Meconium‐stained liquor | 11 | 1496 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.98, 1.61] |
| 12 Apgar score < 7 at 5 minutes | 10 | 1331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.36, 1.64] |
| 13 Neonatal intensive care unit admission | 10 | 1273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.70, 1.41] |
| 14 Perinatal death | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 68.95] |
| 15 Maternal side effects (eg nausea, vomiting, diarrhoea) | 6 | 561 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.41, 1.15] |
| 16 Postpartum haemorrhage | 3 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.39, 8.33] |
26.1. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
26.2. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
26.3. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 3 Caesarean section.
26.4. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 4 Serious neonatal morbidity.
26.5. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
26.6. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 6 Oxytocin augmentation.
26.7. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 7 Uterine hyperstimulation without FHR changes.
26.8. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 8 Uterine rupture.
26.9. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 9 Epidural analgesia.
26.10. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 10 Instrumental vaginal delivery.
26.11. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 11 Meconium‐stained liquor.
26.12. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 12 Apgar score < 7 at 5 minutes.
26.13. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 13 Neonatal intensive care unit admission.
26.14. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 14 Perinatal death.
26.15. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 15 Maternal side effects (eg nausea, vomiting, diarrhoea).
26.16. Analysis.

Comparison 26 Misoprostol versus intracervical prostaglandin: all women with unfavourable cervix, Outcome 16 Postpartum haemorrhage.
Comparison 27. Misoprostol versus intracervical prostaglandin: all women with intact membranes and unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 8 | 1088 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.56, 0.73] |
| 2 Uterine hyperstimulation with FHR changes | 13 | 1433 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.62 [2.22, 5.90] |
| 3 Caesarean section | 12 | 1433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.20] |
| 4 Maternal side effects | 3 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.34, 1.21] |
| 5 Serious neonatal morbidity | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.09, 1.87] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.52, 0.88] |
| 7 Oxytocin augmentation | 10 | 1075 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.43, 0.67] |
| 8 Uterine hyperstimulation without FHR changes | 12 | 1422 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.88, 3.23] |
| 9 Instrumental vaginal delivery | 8 | 1045 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.64, 1.14] |
| 10 Meconium‐stained liquor | 7 | 989 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.05, 1.89] |
| 11 Apgar score < 7 at 5 minutes | 6 | 814 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.24, 1.84] |
| 12 Neonatal intensive care unit admission | 8 | 1074 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.69, 1.42] |
| 13 Perinatal death | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 68.95] |
| 14 Postpartum haemorrhage | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.12, 65.82] |
27.1. Analysis.

Comparison 27 Misoprostol versus intracervical prostaglandin: all women with intact membranes and unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
27.2. Analysis.

Comparison 27 Misoprostol versus intracervical prostaglandin: all women with intact membranes and unfavourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
27.3. Analysis.

Comparison 27 Misoprostol versus intracervical prostaglandin: all women with intact membranes and unfavourable cervix, Outcome 3 Caesarean section.
27.4. Analysis.

Comparison 27 Misoprostol versus intracervical prostaglandin: all women with intact membranes and unfavourable cervix, Outcome 4 Maternal side effects.
27.5. Analysis.

Comparison 27 Misoprostol versus intracervical prostaglandin: all women with intact membranes and unfavourable cervix, Outcome 5 Serious neonatal morbidity.
27.6. Analysis.

Comparison 27 Misoprostol versus intracervical prostaglandin: all women with intact membranes and unfavourable cervix, Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
27.7. Analysis.

Comparison 27 Misoprostol versus intracervical prostaglandin: all women with intact membranes and unfavourable cervix, Outcome 7 Oxytocin augmentation.
27.8. Analysis.

Comparison 27 Misoprostol versus intracervical prostaglandin: all women with intact membranes and unfavourable cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
27.9. Analysis.

Comparison 27 Misoprostol versus intracervical prostaglandin: all women with intact membranes and unfavourable cervix, Outcome 9 Instrumental vaginal delivery.
27.10. Analysis.

Comparison 27 Misoprostol versus intracervical prostaglandin: all women with intact membranes and unfavourable cervix, Outcome 10 Meconium‐stained liquor.
27.11. Analysis.

Comparison 27 Misoprostol versus intracervical prostaglandin: all women with intact membranes and unfavourable cervix, Outcome 11 Apgar score < 7 at 5 minutes.
27.12. Analysis.

Comparison 27 Misoprostol versus intracervical prostaglandin: all women with intact membranes and unfavourable cervix, Outcome 12 Neonatal intensive care unit admission.
27.13. Analysis.

Comparison 27 Misoprostol versus intracervical prostaglandin: all women with intact membranes and unfavourable cervix, Outcome 13 Perinatal death.
27.14. Analysis.

Comparison 27 Misoprostol versus intracervical prostaglandin: all women with intact membranes and unfavourable cervix, Outcome 14 Postpartum haemorrhage.
Comparison 28. Misoprostol versus intracervical prostaglandin: all women with intact membranes and variable or undefined cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.38, 0.96] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.36] |
| 3 Caesarean section | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.65, 2.05] |
| 4 Serious neonatal morbidity/perinatal death | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Neonatal intensive care unit admission | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 6 Oxytocin augmentation | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.34, 0.71] |
| 7 Epidural analgesia | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.44, 0.87] |
| 8 Instrumental vaginal delivery | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.23, 4.06] |
| 9 Meconium‐stained liquor | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.84, 1.95] |
| 10 Apgar score < 7 at 5 minutes | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.35, 15.60] |
| 11 Perinatal death | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
28.1. Analysis.

Comparison 28 Misoprostol versus intracervical prostaglandin: all women with intact membranes and variable or undefined cervix, Outcome 1 Vaginal delivery not achieved in 24 hours.
28.2. Analysis.
Comparison 28 Misoprostol versus intracervical prostaglandin: all women with intact membranes and variable or undefined cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
28.3. Analysis.

Comparison 28 Misoprostol versus intracervical prostaglandin: all women with intact membranes and variable or undefined cervix, Outcome 3 Caesarean section.
28.4. Analysis.

Comparison 28 Misoprostol versus intracervical prostaglandin: all women with intact membranes and variable or undefined cervix, Outcome 4 Serious neonatal morbidity/perinatal death.
28.5. Analysis.

Comparison 28 Misoprostol versus intracervical prostaglandin: all women with intact membranes and variable or undefined cervix, Outcome 5 Neonatal intensive care unit admission.
28.6. Analysis.

Comparison 28 Misoprostol versus intracervical prostaglandin: all women with intact membranes and variable or undefined cervix, Outcome 6 Oxytocin augmentation.
28.7. Analysis.

Comparison 28 Misoprostol versus intracervical prostaglandin: all women with intact membranes and variable or undefined cervix, Outcome 7 Epidural analgesia.
28.8. Analysis.

Comparison 28 Misoprostol versus intracervical prostaglandin: all women with intact membranes and variable or undefined cervix, Outcome 8 Instrumental vaginal delivery.
28.9. Analysis.

Comparison 28 Misoprostol versus intracervical prostaglandin: all women with intact membranes and variable or undefined cervix, Outcome 9 Meconium‐stained liquor.
28.10. Analysis.

Comparison 28 Misoprostol versus intracervical prostaglandin: all women with intact membranes and variable or undefined cervix, Outcome 10 Apgar score < 7 at 5 minutes.
28.11. Analysis.

Comparison 28 Misoprostol versus intracervical prostaglandin: all women with intact membranes and variable or undefined cervix, Outcome 11 Perinatal death.
Comparison 29. Misoprostol versus oxytocin: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 10 | 1397 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.47, 0.90] |
| 2 Uterine hyperstimulation with FHR changes | 9 | 1419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.20, 2.91] |
| 3 Caesarean section | 25 | 3074 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.60, 0.96] |
| 4 Serious neonatal morbidity | 2 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.57, 1.60] |
| 5 Serious maternal morbidity or death | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.11 [0.31, 119.33] |
| 6 Maternal side effects (eg nausea, vomiting, diarrhoea) | 4 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.04 [1.51, 16.86] |
| 7 Oxytocin augmentation | 3 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.28, 0.95] |
| 8 Uterine hyperstimulation without FHR changes | 15 | 2050 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.24 [1.82, 2.77] |
| 9 Uterine rupture | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.12 [0.50, 34.25] |
| 10 Epidural analgesia | 3 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.67, 1.00] |
| 11 Instrumental vaginal delivery | 13 | 1639 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.56, 0.99] |
| 12 Meconium‐stained liquor | 12 | 1694 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.82, 1.35] |
| 13 Apgar score < 7 at 5 minutes | 13 | 1906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.34, 0.92] |
| 14 Neonatal intensive care unit admission | 11 | 1491 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.13] |
| 15 Serious maternal complications | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.15, 2.38] |
| 16 Perinatal death | 3 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.14, 4.39] |
| 17 Postpartum haemorrhage | 6 | 568 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.23, 1.23] |
29.1. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
29.2. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
29.3. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 3 Caesarean section.
29.4. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 4 Serious neonatal morbidity.
29.5. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 5 Serious maternal morbidity or death.
29.6. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 6 Maternal side effects (eg nausea, vomiting, diarrhoea).
29.7. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 7 Oxytocin augmentation.
29.8. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 8 Uterine hyperstimulation without FHR changes.
29.9. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 9 Uterine rupture.
29.10. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 10 Epidural analgesia.
29.11. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 11 Instrumental vaginal delivery.
29.12. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 12 Meconium‐stained liquor.
29.13. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 13 Apgar score < 7 at 5 minutes.
29.14. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 14 Neonatal intensive care unit admission.
29.15. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 15 Serious maternal complications.
29.16. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 16 Perinatal death.
29.17. Analysis.

Comparison 29 Misoprostol versus oxytocin: all women, Outcome 17 Postpartum haemorrhage.
Comparison 30. Misoprostol versus oxytocin: all women with unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 5 | 660 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.56, 1.35] |
| 2 Uterine hyperstimulation with FHR changes | 3 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.32, 2.39] |
| 3 Caesarean section | 14 | 1598 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.61, 1.08] |
| 4 Perinatal death | 2 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.22, 6.88] |
| 5 Serious maternal morbidity or death | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.11 [0.31, 119.33] |
| 6 Maternal side effects | 4 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.04 [1.51, 16.86] |
| 7 Postpartum haemorrhage | 2 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.52] |
| 8 Uterine hyperstimulation without FHR changes | 10 | 1192 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.66, 3.58] |
| 9 Uterine rupture | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.12 [0.50, 34.25] |
| 10 Serious maternal complications | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.15, 2.38] |
| 11 Instrumental vaginal delivery | 8 | 853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.25, 0.70] |
| 12 Meconium‐stained liquor | 8 | 904 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.66, 1.29] |
| 13 Apgar score < 7 at 5 minutes | 6 | 738 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.69, 4.27] |
| 14 Neonatal intensive care unit admission | 7 | 707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.77, 2.04] |
| 15 Serious neonatal morbidity | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.67] |
30.1. Analysis.

Comparison 30 Misoprostol versus oxytocin: all women with unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
30.2. Analysis.
Comparison 30 Misoprostol versus oxytocin: all women with unfavourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
30.3. Analysis.

Comparison 30 Misoprostol versus oxytocin: all women with unfavourable cervix, Outcome 3 Caesarean section.
30.4. Analysis.

Comparison 30 Misoprostol versus oxytocin: all women with unfavourable cervix, Outcome 4 Perinatal death.
30.5. Analysis.

Comparison 30 Misoprostol versus oxytocin: all women with unfavourable cervix, Outcome 5 Serious maternal morbidity or death.
30.6. Analysis.

Comparison 30 Misoprostol versus oxytocin: all women with unfavourable cervix, Outcome 6 Maternal side effects.
30.7. Analysis.

Comparison 30 Misoprostol versus oxytocin: all women with unfavourable cervix, Outcome 7 Postpartum haemorrhage.
30.8. Analysis.

Comparison 30 Misoprostol versus oxytocin: all women with unfavourable cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
30.9. Analysis.

Comparison 30 Misoprostol versus oxytocin: all women with unfavourable cervix, Outcome 9 Uterine rupture.
30.10. Analysis.

Comparison 30 Misoprostol versus oxytocin: all women with unfavourable cervix, Outcome 10 Serious maternal complications.
30.11. Analysis.
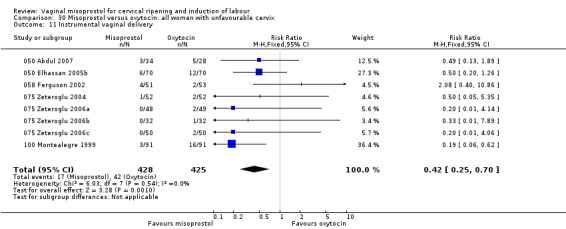
Comparison 30 Misoprostol versus oxytocin: all women with unfavourable cervix, Outcome 11 Instrumental vaginal delivery.
30.12. Analysis.

Comparison 30 Misoprostol versus oxytocin: all women with unfavourable cervix, Outcome 12 Meconium‐stained liquor.
30.13. Analysis.
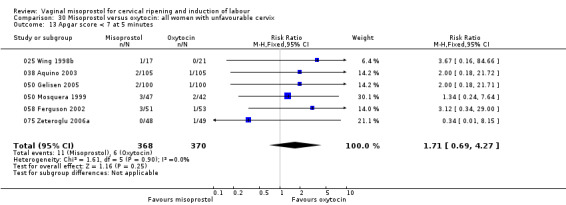
Comparison 30 Misoprostol versus oxytocin: all women with unfavourable cervix, Outcome 13 Apgar score < 7 at 5 minutes.
30.14. Analysis.

Comparison 30 Misoprostol versus oxytocin: all women with unfavourable cervix, Outcome 14 Neonatal intensive care unit admission.
30.15. Analysis.

Comparison 30 Misoprostol versus oxytocin: all women with unfavourable cervix, Outcome 15 Serious neonatal morbidity.
Comparison 31. Misoprostol versus oxytocin: all women with intact membranes and unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Instrumental delivery | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.20, 1.26] |
| 2 Vaginal delivery not achieved in 24 hours | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.33, 0.84] |
| 3 Caesarean section | 5 | 736 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.37, 0.90] |
| 4 Uterine hyperstimulation with FHR changes | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.27] |
| 5 Uterine hyperstimulation without FHR changes | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [1.48, 6.07] |
| 6 Meconium‐stained liquor | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.65, 3.01] |
| 7 Serious neonatal morbidity | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.56, 1.63] |
| 8 Apgar score < 7 at 5 minutes | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.72] |
31.1. Analysis.

Comparison 31 Misoprostol versus oxytocin: all women with intact membranes and unfavourable cervix, Outcome 1 Instrumental delivery.
31.2. Analysis.
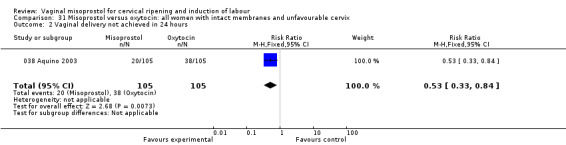
Comparison 31 Misoprostol versus oxytocin: all women with intact membranes and unfavourable cervix, Outcome 2 Vaginal delivery not achieved in 24 hours.
31.3. Analysis.

Comparison 31 Misoprostol versus oxytocin: all women with intact membranes and unfavourable cervix, Outcome 3 Caesarean section.
31.4. Analysis.

Comparison 31 Misoprostol versus oxytocin: all women with intact membranes and unfavourable cervix, Outcome 4 Uterine hyperstimulation with FHR changes.
31.5. Analysis.

Comparison 31 Misoprostol versus oxytocin: all women with intact membranes and unfavourable cervix, Outcome 5 Uterine hyperstimulation without FHR changes.
31.6. Analysis.

Comparison 31 Misoprostol versus oxytocin: all women with intact membranes and unfavourable cervix, Outcome 6 Meconium‐stained liquor.
31.7. Analysis.

Comparison 31 Misoprostol versus oxytocin: all women with intact membranes and unfavourable cervix, Outcome 7 Serious neonatal morbidity.
31.8. Analysis.

Comparison 31 Misoprostol versus oxytocin: all women with intact membranes and unfavourable cervix, Outcome 8 Apgar score < 7 at 5 minutes.
Comparison 32. Misoprostol versus oxytocin: all women with intact membranes and variable or undefined cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.30, 0.68] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [1.66, 7.08] |
| 3 Caesarean section | 3 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.13, 1.45] |
| 4 Serious maternal morbidity or death | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Uterine hyperstimulation without FHR changes | 2 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.74, 3.11] |
| 6 Epidural analgesia | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.51, 0.92] |
| 7 Instrumental vaginal delivery | 2 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.36, 2.06] |
| 8 Meconium‐stained liquor | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.71, 2.00] |
| 9 Apgar score < 7 at 5 minutes | 2 | 512 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.06, 15.26] |
| 10 Neonatal intensive care unit admission | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.65, 2.69] |
| 11 Perinatal death | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
32.1. Analysis.

Comparison 32 Misoprostol versus oxytocin: all women with intact membranes and variable or undefined cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
32.2. Analysis.

Comparison 32 Misoprostol versus oxytocin: all women with intact membranes and variable or undefined cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
32.3. Analysis.
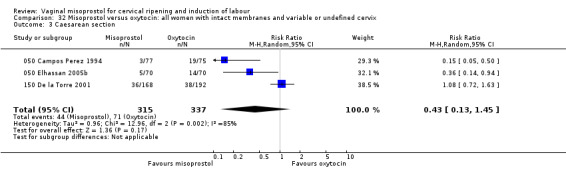
Comparison 32 Misoprostol versus oxytocin: all women with intact membranes and variable or undefined cervix, Outcome 3 Caesarean section.
32.4. Analysis.

Comparison 32 Misoprostol versus oxytocin: all women with intact membranes and variable or undefined cervix, Outcome 4 Serious maternal morbidity or death.
32.5. Analysis.

Comparison 32 Misoprostol versus oxytocin: all women with intact membranes and variable or undefined cervix, Outcome 5 Uterine hyperstimulation without FHR changes.
32.6. Analysis.

Comparison 32 Misoprostol versus oxytocin: all women with intact membranes and variable or undefined cervix, Outcome 6 Epidural analgesia.
32.7. Analysis.

Comparison 32 Misoprostol versus oxytocin: all women with intact membranes and variable or undefined cervix, Outcome 7 Instrumental vaginal delivery.
32.8. Analysis.

Comparison 32 Misoprostol versus oxytocin: all women with intact membranes and variable or undefined cervix, Outcome 8 Meconium‐stained liquor.
32.9. Analysis.

Comparison 32 Misoprostol versus oxytocin: all women with intact membranes and variable or undefined cervix, Outcome 9 Apgar score < 7 at 5 minutes.
32.10. Analysis.
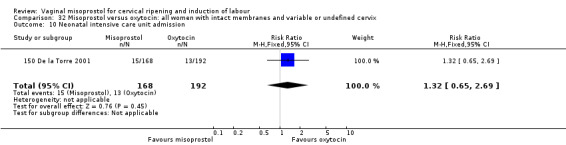
Comparison 32 Misoprostol versus oxytocin: all women with intact membranes and variable or undefined cervix, Outcome 10 Neonatal intensive care unit admission.
32.11. Analysis.

Comparison 32 Misoprostol versus oxytocin: all women with intact membranes and variable or undefined cervix, Outcome 11 Perinatal death.
Comparison 33. Misoprostol versus oxytocin: all women with ruptured membranes and unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.73, 5.46] |
| 2 Uterine hyperstimulation without FHR changes | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 2 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.20, 2.60] |
| 4 Maternal side effects | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.62, 194.17] |
33.1. Analysis.

Comparison 33 Misoprostol versus oxytocin: all women with ruptured membranes and unfavourable cervix, Outcome 1 Vaginal delivery not achieved in 24 hours.
33.2. Analysis.

Comparison 33 Misoprostol versus oxytocin: all women with ruptured membranes and unfavourable cervix, Outcome 2 Uterine hyperstimulation without FHR changes.
33.3. Analysis.

Comparison 33 Misoprostol versus oxytocin: all women with ruptured membranes and unfavourable cervix, Outcome 3 Caesarean section.
33.4. Analysis.

Comparison 33 Misoprostol versus oxytocin: all women with ruptured membranes and unfavourable cervix, Outcome 4 Maternal side effects.
Comparison 34. Misoprostol versus oxytocin: all women with ruptured membranes and variable or undefined cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 3 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.41, 3.41] |
| 2 Caesarean section | 4 | 520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.60, 1.51] |
| 3 Oxytocin augmentation | 2 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.30, 0.49] |
| 4 Uterine hyperstimulation without FHR changes | 3 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.04, 2.67] |
| 5 Epidural analgesia | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.12, 3.88] |
| 6 Instrumental vaginal delivery | 3 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.45, 1.27] |
| 7 Meconium‐stained liquor | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.36, 2.23] |
| 8 Apgar score < 7 at 5 minutes | 4 | 520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.17, 2.43] |
| 9 Neonatal intensive care unit admission | 3 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.52, 1.18] |
| 10 Postpartum haemorrhage | 2 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.40, 8.43] |
| 11 Vaginal delivery not achieved within 24 hours | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.55, 1.45] |
34.1. Analysis.

Comparison 34 Misoprostol versus oxytocin: all women with ruptured membranes and variable or undefined cervix, Outcome 1 Uterine hyperstimulation with FHR changes.
34.2. Analysis.

Comparison 34 Misoprostol versus oxytocin: all women with ruptured membranes and variable or undefined cervix, Outcome 2 Caesarean section.
34.3. Analysis.

Comparison 34 Misoprostol versus oxytocin: all women with ruptured membranes and variable or undefined cervix, Outcome 3 Oxytocin augmentation.
34.4. Analysis.

Comparison 34 Misoprostol versus oxytocin: all women with ruptured membranes and variable or undefined cervix, Outcome 4 Uterine hyperstimulation without FHR changes.
34.5. Analysis.

Comparison 34 Misoprostol versus oxytocin: all women with ruptured membranes and variable or undefined cervix, Outcome 5 Epidural analgesia.
34.6. Analysis.

Comparison 34 Misoprostol versus oxytocin: all women with ruptured membranes and variable or undefined cervix, Outcome 6 Instrumental vaginal delivery.
34.7. Analysis.

Comparison 34 Misoprostol versus oxytocin: all women with ruptured membranes and variable or undefined cervix, Outcome 7 Meconium‐stained liquor.
34.8. Analysis.

Comparison 34 Misoprostol versus oxytocin: all women with ruptured membranes and variable or undefined cervix, Outcome 8 Apgar score < 7 at 5 minutes.
34.9. Analysis.

Comparison 34 Misoprostol versus oxytocin: all women with ruptured membranes and variable or undefined cervix, Outcome 9 Neonatal intensive care unit admission.
34.10. Analysis.

Comparison 34 Misoprostol versus oxytocin: all women with ruptured membranes and variable or undefined cervix, Outcome 10 Postpartum haemorrhage.
34.11. Analysis.

Comparison 34 Misoprostol versus oxytocin: all women with ruptured membranes and variable or undefined cervix, Outcome 11 Vaginal delivery not achieved within 24 hours.
Comparison 35. Misoprostol versus oxytocin: all women with previous caesarean section.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.81, 2.24] |
| 2 Caesarean section | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.71, 3.83] |
| 3 Serious maternal morbidity or death | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.11 [0.31, 119.33] |
| 4 Uterine hyperstimulation without FHR changes | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.11 [0.31, 119.33] |
| 5 Uterine rupture | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.11 [0.31, 119.33] |
| 6 Meconium‐stained liquor | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.09, 1.79] |
| 7 Apgar score < 7 at 5 minutes | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.67 [0.16, 84.66] |
| 8 Neonatal intensive care unit admission | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.62, 5.52] |
35.1. Analysis.

Comparison 35 Misoprostol versus oxytocin: all women with previous caesarean section, Outcome 1 Vaginal delivery not achieved within 24 hours.
35.2. Analysis.

Comparison 35 Misoprostol versus oxytocin: all women with previous caesarean section, Outcome 2 Caesarean section.
35.3. Analysis.

Comparison 35 Misoprostol versus oxytocin: all women with previous caesarean section, Outcome 3 Serious maternal morbidity or death.
35.4. Analysis.

Comparison 35 Misoprostol versus oxytocin: all women with previous caesarean section, Outcome 4 Uterine hyperstimulation without FHR changes.
35.5. Analysis.

Comparison 35 Misoprostol versus oxytocin: all women with previous caesarean section, Outcome 5 Uterine rupture.
35.6. Analysis.

Comparison 35 Misoprostol versus oxytocin: all women with previous caesarean section, Outcome 6 Meconium‐stained liquor.
35.7. Analysis.

Comparison 35 Misoprostol versus oxytocin: all women with previous caesarean section, Outcome 7 Apgar score < 7 at 5 minutes.
35.8. Analysis.

Comparison 35 Misoprostol versus oxytocin: all women with previous caesarean section, Outcome 8 Neonatal intensive care unit admission.
Comparison 36. Misoprostol versus oxytocin: all women with previous caesarean section and unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.81, 2.24] |
| 2 Caesarean section | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.71, 3.83] |
| 3 Serious maternal morbidity or death | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.11 [0.31, 119.33] |
| 4 Uterine hyperstimulation without FHR changes | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.11 [0.31, 119.33] |
| 5 Uterine rupture | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.11 [0.31, 119.33] |
| 6 Meconium‐stained liquor | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.09, 1.79] |
| 7 Apgar score < 7 at 5 minutes | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.67 [0.16, 84.66] |
| 8 Neonatal intensive care unit admission | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.62, 5.52] |
36.1. Analysis.

Comparison 36 Misoprostol versus oxytocin: all women with previous caesarean section and unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
36.2. Analysis.

Comparison 36 Misoprostol versus oxytocin: all women with previous caesarean section and unfavourable cervix, Outcome 2 Caesarean section.
36.3. Analysis.

Comparison 36 Misoprostol versus oxytocin: all women with previous caesarean section and unfavourable cervix, Outcome 3 Serious maternal morbidity or death.
36.4. Analysis.

Comparison 36 Misoprostol versus oxytocin: all women with previous caesarean section and unfavourable cervix, Outcome 4 Uterine hyperstimulation without FHR changes.
36.5. Analysis.

Comparison 36 Misoprostol versus oxytocin: all women with previous caesarean section and unfavourable cervix, Outcome 5 Uterine rupture.
36.6. Analysis.
Comparison 36 Misoprostol versus oxytocin: all women with previous caesarean section and unfavourable cervix, Outcome 6 Meconium‐stained liquor.
36.7. Analysis.

Comparison 36 Misoprostol versus oxytocin: all women with previous caesarean section and unfavourable cervix, Outcome 7 Apgar score < 7 at 5 minutes.
36.8. Analysis.

Comparison 36 Misoprostol versus oxytocin: all women with previous caesarean section and unfavourable cervix, Outcome 8 Neonatal intensive care unit admission.
Comparison 37. Misoprostol versus oxytocin: all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.44, 1.31] |
| 2 Serious neonatal morbidity | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.67] |
| 3 Uterine hyperstimulation without FHR changes | 2 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.41, 6.74] |
| 4 Instrumental delivery | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.62] |
| 5 Meconium‐stained liquor | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.59, 1.88] |
| 6 Neonatal intensive care unit admission | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.40, 3.37] |
| 7 Perinatal death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal side effects (eg nausea, vomiting, diarrhoea) | 2 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [0.75, 24.89] |
| 9 Postpartum haemorrhage | 2 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.19] |
| 10 Serious maternal complications | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.15, 2.38] |
37.1. Analysis.

Comparison 37 Misoprostol versus oxytocin: all multiparae, Outcome 1 Caesarean section.
37.2. Analysis.

Comparison 37 Misoprostol versus oxytocin: all multiparae, Outcome 2 Serious neonatal morbidity.
37.3. Analysis.

Comparison 37 Misoprostol versus oxytocin: all multiparae, Outcome 3 Uterine hyperstimulation without FHR changes.
37.4. Analysis.

Comparison 37 Misoprostol versus oxytocin: all multiparae, Outcome 4 Instrumental delivery.
37.5. Analysis.

Comparison 37 Misoprostol versus oxytocin: all multiparae, Outcome 5 Meconium‐stained liquor.
37.6. Analysis.

Comparison 37 Misoprostol versus oxytocin: all multiparae, Outcome 6 Neonatal intensive care unit admission.
37.7. Analysis.
Comparison 37 Misoprostol versus oxytocin: all multiparae, Outcome 7 Perinatal death.
37.8. Analysis.

Comparison 37 Misoprostol versus oxytocin: all multiparae, Outcome 8 Maternal side effects (eg nausea, vomiting, diarrhoea).
37.9. Analysis.

Comparison 37 Misoprostol versus oxytocin: all multiparae, Outcome 9 Postpartum haemorrhage.
37.10. Analysis.

Comparison 37 Misoprostol versus oxytocin: all multiparae, Outcome 10 Serious maternal complications.
Comparison 38. Misoprostol versus oxytocin: all multiparae with unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.44, 1.31] |
| 2 Serious neonatal morbidity | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.67] |
| 3 Uterine hyperstimulation without FHR changes | 2 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.41, 6.74] |
| 4 Instrumental delivery | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.62] |
| 5 Neonatal intensive care unit admission | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.40, 3.37] |
| 6 Maternal side effects | 2 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [0.75, 24.89] |
| 7 Postpartum haemorrhage | 2 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.19] |
| 8 Meconium‐stained liquor | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.59, 1.88] |
| 9 Perinatal death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Serious maternal complications | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.15, 2.38] |
38.1. Analysis.

Comparison 38 Misoprostol versus oxytocin: all multiparae with unfavourable cervix, Outcome 1 Caesarean section.
38.2. Analysis.
Comparison 38 Misoprostol versus oxytocin: all multiparae with unfavourable cervix, Outcome 2 Serious neonatal morbidity.
38.3. Analysis.
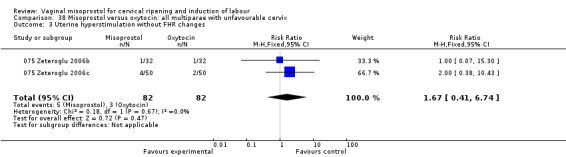
Comparison 38 Misoprostol versus oxytocin: all multiparae with unfavourable cervix, Outcome 3 Uterine hyperstimulation without FHR changes.
38.4. Analysis.

Comparison 38 Misoprostol versus oxytocin: all multiparae with unfavourable cervix, Outcome 4 Instrumental delivery.
38.5. Analysis.
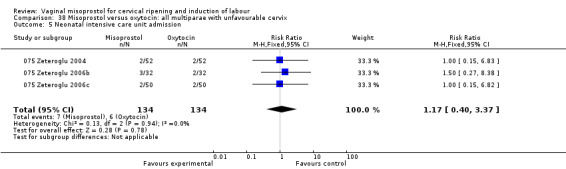
Comparison 38 Misoprostol versus oxytocin: all multiparae with unfavourable cervix, Outcome 5 Neonatal intensive care unit admission.
38.6. Analysis.

Comparison 38 Misoprostol versus oxytocin: all multiparae with unfavourable cervix, Outcome 6 Maternal side effects.
38.7. Analysis.

Comparison 38 Misoprostol versus oxytocin: all multiparae with unfavourable cervix, Outcome 7 Postpartum haemorrhage.
38.8. Analysis.

Comparison 38 Misoprostol versus oxytocin: all multiparae with unfavourable cervix, Outcome 8 Meconium‐stained liquor.
38.9. Analysis.

Comparison 38 Misoprostol versus oxytocin: all multiparae with unfavourable cervix, Outcome 9 Perinatal death.
38.10. Analysis.

Comparison 38 Misoprostol versus oxytocin: all multiparae with unfavourable cervix, Outcome 10 Serious maternal complications.
Comparison 39. Misoprostol lower versus higher dose: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 12 | 1887 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.96, 1.21] |
| 2 Uterine hyperstimulation with FHR changes | 16 | 2540 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.37, 0.69] |
| 3 Caesarean section | 21 | 2913 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.12] |
| 4 Serious maternal morbidity or death | 4 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.62, 1.39] |
| 6 Oxytocin augmentation | 18 | 2753 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.14, 1.49] |
| 7 Uterine hyperstimulation without FHR changes | 14 | 2085 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.46, 0.69] |
| 8 Uterine rupture | 3 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.11, 3.87] |
| 9 Epidural | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.63, 1.72] |
| 10 Instrumental vaginal delivery | 14 | 2116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.82, 1.44] |
| 11 Meconium‐stained liquor | 13 | 1673 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.69, 1.19] |
| 12 Apgar score < 7 at 5 minutes | 13 | 2045 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.44, 1.37] |
| 13 Neonatal intensive care unit admission | 9 | 1795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.64, 1.05] |
| 14 Perinatal death | 2 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.34] |
| 15 Maternal side effects (eg nausea, vomiting, diarrhoea) | 9 | 1653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.58, 1.24] |
| 16 Postpartum haemorrhage | 5 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.43, 1.35] |
39.1. Analysis.

Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
39.2. Analysis.

Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
39.3. Analysis.

Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 3 Caesarean section.
39.4. Analysis.
Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 4 Serious maternal morbidity or death.
39.5. Analysis.

Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
39.6. Analysis.

Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 6 Oxytocin augmentation.
39.7. Analysis.

Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 7 Uterine hyperstimulation without FHR changes.
39.8. Analysis.

Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 8 Uterine rupture.
39.9. Analysis.

Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 9 Epidural.
39.10. Analysis.

Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 10 Instrumental vaginal delivery.
39.11. Analysis.

Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 11 Meconium‐stained liquor.
39.12. Analysis.

Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 12 Apgar score < 7 at 5 minutes.
39.13. Analysis.

Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 13 Neonatal intensive care unit admission.
39.14. Analysis.

Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 14 Perinatal death.
39.15. Analysis.

Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 15 Maternal side effects (eg nausea, vomiting, diarrhoea).
39.16. Analysis.

Comparison 39 Misoprostol lower versus higher dose: all women, Outcome 16 Postpartum haemorrhage.
Comparison 40. Misoprostol lower versus higher dose: all women with unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 8 | 1563 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.96, 1.22] |
| 2 Uterine hyperstimulation with FHR changes | 13 | 2174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.38, 0.73] |
| 3 Caesarean section | 15 | 2214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.81, 1.13] |
| 4 Uterine rupture | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 4 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Perinatal death | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.87] |
| 7 Oxytocin augmentation | 11 | 2049 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.15, 1.67] |
| 8 Uterine hyperstimulation without FHR changes | 8 | 1569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.47, 0.76] |
| 9 Epidural | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.63, 1.72] |
| 10 Instrumental vaginal delivery | 9 | 1703 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.39] |
| 11 Meconium‐stained liquor | 7 | 1115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.66, 1.33] |
| 12 Apgar score < 7 at 5 minutes | 9 | 1609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.40, 1.58] |
| 13 Neonatal intensive care unit admission | 5 | 1290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.62, 1.10] |
| 14 Maternal side effects (eg nausea, vomiting, diarrhoea) | 4 | 1143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.45, 1.30] |
| 15 Postpartum haemorrhage | 1 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.27, 4.25] |
40.1. Analysis.

Comparison 40 Misoprostol lower versus higher dose: all women with unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
40.2. Analysis.

Comparison 40 Misoprostol lower versus higher dose: all women with unfavourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
40.3. Analysis.

Comparison 40 Misoprostol lower versus higher dose: all women with unfavourable cervix, Outcome 3 Caesarean section.
40.4. Analysis.

Comparison 40 Misoprostol lower versus higher dose: all women with unfavourable cervix, Outcome 4 Uterine rupture.
40.5. Analysis.

Comparison 40 Misoprostol lower versus higher dose: all women with unfavourable cervix, Outcome 5 Serious maternal morbidity or death.
40.6. Analysis.
Comparison 40 Misoprostol lower versus higher dose: all women with unfavourable cervix, Outcome 6 Perinatal death.
40.7. Analysis.

Comparison 40 Misoprostol lower versus higher dose: all women with unfavourable cervix, Outcome 7 Oxytocin augmentation.
40.8. Analysis.

Comparison 40 Misoprostol lower versus higher dose: all women with unfavourable cervix, Outcome 8 Uterine hyperstimulation without FHR changes.
40.9. Analysis.

Comparison 40 Misoprostol lower versus higher dose: all women with unfavourable cervix, Outcome 9 Epidural.
40.10. Analysis.

Comparison 40 Misoprostol lower versus higher dose: all women with unfavourable cervix, Outcome 10 Instrumental vaginal delivery.
40.11. Analysis.

Comparison 40 Misoprostol lower versus higher dose: all women with unfavourable cervix, Outcome 11 Meconium‐stained liquor.
40.12. Analysis.

Comparison 40 Misoprostol lower versus higher dose: all women with unfavourable cervix, Outcome 12 Apgar score < 7 at 5 minutes.
40.13. Analysis.

Comparison 40 Misoprostol lower versus higher dose: all women with unfavourable cervix, Outcome 13 Neonatal intensive care unit admission.
40.14. Analysis.

Comparison 40 Misoprostol lower versus higher dose: all women with unfavourable cervix, Outcome 14 Maternal side effects (eg nausea, vomiting, diarrhoea).
40.15. Analysis.

Comparison 40 Misoprostol lower versus higher dose: all women with unfavourable cervix, Outcome 15 Postpartum haemorrhage.
Comparison 41. Misoprostol lower versus higher dose: all women with intact membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.05, 11.72] |
| 2 Caesarean section | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.23, 1.84] |
| 3 Uterine hyperstimulation without FHR changes | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.23] |
| 4 Meconium‐stained liquor | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.12, 5.07] |
41.1. Analysis.

Comparison 41 Misoprostol lower versus higher dose: all women with intact membranes, Outcome 1 Vaginal delivery not achieved in 24 hours.
41.2. Analysis.

Comparison 41 Misoprostol lower versus higher dose: all women with intact membranes, Outcome 2 Caesarean section.
41.3. Analysis.

Comparison 41 Misoprostol lower versus higher dose: all women with intact membranes, Outcome 3 Uterine hyperstimulation without FHR changes.
41.4. Analysis.

Comparison 41 Misoprostol lower versus higher dose: all women with intact membranes, Outcome 4 Meconium‐stained liquor.
Comparison 42. Misoprostol lower versus higher dose: all women with intact membranes and unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 4 | 779 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.97, 1.30] |
| 2 Uterine hyperstimulation with FHR changes | 7 | 1035 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.31, 0.95] |
| 3 Caesarean section | 8 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.86, 1.39] |
| 4 Serious maternal morbidity or death | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Oxytocin augmentation | 5 | 929 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.96, 1.33] |
| 6 Uterine hyperstimulation without FHR changes | 3 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.51, 1.18] |
| 7 Instrumental vaginal delivery | 5 | 929 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.60, 1.57] |
| 8 Meconium‐stained liquor | 4 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.67, 1.51] |
| 9 Apgar score < 7 at 5 minutes | 4 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.42, 3.12] |
| 10 Neonatal intensive care unit admission | 2 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.23] |
| 11 Maternal side effects (eg nausea, vomiting, diarrhoea) | 3 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.36, 1.46] |
42.1. Analysis.

Comparison 42 Misoprostol lower versus higher dose: all women with intact membranes and unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
42.2. Analysis.

Comparison 42 Misoprostol lower versus higher dose: all women with intact membranes and unfavourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
42.3. Analysis.

Comparison 42 Misoprostol lower versus higher dose: all women with intact membranes and unfavourable cervix, Outcome 3 Caesarean section.
42.4. Analysis.

Comparison 42 Misoprostol lower versus higher dose: all women with intact membranes and unfavourable cervix, Outcome 4 Serious maternal morbidity or death.
42.5. Analysis.

Comparison 42 Misoprostol lower versus higher dose: all women with intact membranes and unfavourable cervix, Outcome 5 Oxytocin augmentation.
42.6. Analysis.
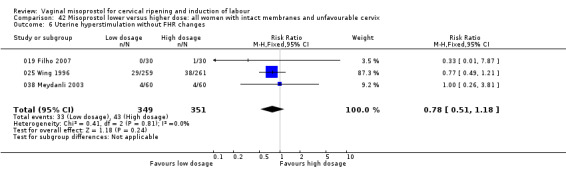
Comparison 42 Misoprostol lower versus higher dose: all women with intact membranes and unfavourable cervix, Outcome 6 Uterine hyperstimulation without FHR changes.
42.7. Analysis.

Comparison 42 Misoprostol lower versus higher dose: all women with intact membranes and unfavourable cervix, Outcome 7 Instrumental vaginal delivery.
42.8. Analysis.

Comparison 42 Misoprostol lower versus higher dose: all women with intact membranes and unfavourable cervix, Outcome 8 Meconium‐stained liquor.
42.9. Analysis.

Comparison 42 Misoprostol lower versus higher dose: all women with intact membranes and unfavourable cervix, Outcome 9 Apgar score < 7 at 5 minutes.
42.10. Analysis.

Comparison 42 Misoprostol lower versus higher dose: all women with intact membranes and unfavourable cervix, Outcome 10 Neonatal intensive care unit admission.
42.11. Analysis.

Comparison 42 Misoprostol lower versus higher dose: all women with intact membranes and unfavourable cervix, Outcome 11 Maternal side effects (eg nausea, vomiting, diarrhoea).
Comparison 43. Misoprostol lower versus higher dose: all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.19 [0.26, 105.59] |
| 2 Caesarean section | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.66, 14.74] |
| 3 Oxytocin augmentation | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 7.27 [0.93, 57.06] |
| 4 Uterine hyperstimulation without FHR changes | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.63, 3.26] |
| 5 Epidural | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.63, 1.72] |
| 6 Instrumental vaginal delivery | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.08, 2.05] |
| 7 Meconium‐stained liquor | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.25, 2.19] |
| 8 Apgar score < 7 at 5 minutes | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.13, 74.76] |
| 9 Neonatal intensive care unit admission | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.44, 6.88] |
| 10 Maternal side effects (eg nausea, vomiting, diarrhoea) | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.44, 2.03] |
43.1. Analysis.
Comparison 43 Misoprostol lower versus higher dose: all multiparae, Outcome 1 Uterine hyperstimulation with FHR changes.
43.2. Analysis.
Comparison 43 Misoprostol lower versus higher dose: all multiparae, Outcome 2 Caesarean section.
43.3. Analysis.

Comparison 43 Misoprostol lower versus higher dose: all multiparae, Outcome 3 Oxytocin augmentation.
43.4. Analysis.

Comparison 43 Misoprostol lower versus higher dose: all multiparae, Outcome 4 Uterine hyperstimulation without FHR changes.
43.5. Analysis.
Comparison 43 Misoprostol lower versus higher dose: all multiparae, Outcome 5 Epidural.
43.6. Analysis.

Comparison 43 Misoprostol lower versus higher dose: all multiparae, Outcome 6 Instrumental vaginal delivery.
43.7. Analysis.

Comparison 43 Misoprostol lower versus higher dose: all multiparae, Outcome 7 Meconium‐stained liquor.
43.8. Analysis.

Comparison 43 Misoprostol lower versus higher dose: all multiparae, Outcome 8 Apgar score < 7 at 5 minutes.
43.9. Analysis.

Comparison 43 Misoprostol lower versus higher dose: all multiparae, Outcome 9 Neonatal intensive care unit admission.
43.10. Analysis.

Comparison 43 Misoprostol lower versus higher dose: all multiparae, Outcome 10 Maternal side effects (eg nausea, vomiting, diarrhoea).
Comparison 44. Misoprostol lower versus higher dose: all multiparae with unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.98, 2.84] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.04, 20.36] |
| 3 Caesarean section | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.64, 5.31] |
| 4 Oxytocin augmentation | 2 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.84, 8.04] |
| 5 Uterine hyperstimulation without FHR changes | 2 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.07, 5.08] |
| 6 Epidural | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.63, 1.72] |
| 7 Instrumental vaginal delivery | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.13, 2.00] |
| 8 Meconium‐stained liquor | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.38, 1.89] |
| 9 Apgar score < 7 at 5 minutes | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.32, 28.96] |
| 10 Neonatal intensive care unit admission | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.44, 6.88] |
| 11 Postpartum haemorrhage | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.31, 2.41] |
| 12 Maternal side effects (eg nausea, vomiting, diarrhoea) | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.64, 1.98] |
44.1. Analysis.

Comparison 44 Misoprostol lower versus higher dose: all multiparae with unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
44.2. Analysis.

Comparison 44 Misoprostol lower versus higher dose: all multiparae with unfavourable cervix, Outcome 2 Uterine hyperstimulation with FHR changes.
44.3. Analysis.

Comparison 44 Misoprostol lower versus higher dose: all multiparae with unfavourable cervix, Outcome 3 Caesarean section.
44.4. Analysis.

Comparison 44 Misoprostol lower versus higher dose: all multiparae with unfavourable cervix, Outcome 4 Oxytocin augmentation.
44.5. Analysis.

Comparison 44 Misoprostol lower versus higher dose: all multiparae with unfavourable cervix, Outcome 5 Uterine hyperstimulation without FHR changes.
44.6. Analysis.

Comparison 44 Misoprostol lower versus higher dose: all multiparae with unfavourable cervix, Outcome 6 Epidural.
44.7. Analysis.

Comparison 44 Misoprostol lower versus higher dose: all multiparae with unfavourable cervix, Outcome 7 Instrumental vaginal delivery.
44.8. Analysis.

Comparison 44 Misoprostol lower versus higher dose: all multiparae with unfavourable cervix, Outcome 8 Meconium‐stained liquor.
44.9. Analysis.

Comparison 44 Misoprostol lower versus higher dose: all multiparae with unfavourable cervix, Outcome 9 Apgar score < 7 at 5 minutes.
44.10. Analysis.

Comparison 44 Misoprostol lower versus higher dose: all multiparae with unfavourable cervix, Outcome 10 Neonatal intensive care unit admission.
44.11. Analysis.

Comparison 44 Misoprostol lower versus higher dose: all multiparae with unfavourable cervix, Outcome 11 Postpartum haemorrhage.
44.12. Analysis.

Comparison 44 Misoprostol lower versus higher dose: all multiparae with unfavourable cervix, Outcome 12 Maternal side effects (eg nausea, vomiting, diarrhoea).
Comparison 45. Misoprostol gel versus tablet: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.29, 0.83] |
| 2 Caesarean section | 1 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.79, 1.45] |
| 3 Oxytocin augmentation | 1 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.13, 1.41] |
| 4 Epidural analgesia | 1 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.03, 1.38] |
| 5 Instrumental vaginal delivery | 1 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.74, 1.70] |
| 6 Apgar score < 7 at 5 minutes | 1 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.56, 2.38] |
| 7 Neonatal intensive care unit admission | 1 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.44, 1.24] |
45.1. Analysis.

Comparison 45 Misoprostol gel versus tablet: all women, Outcome 1 Uterine hyperstimulation with FHR changes.
45.2. Analysis.

Comparison 45 Misoprostol gel versus tablet: all women, Outcome 2 Caesarean section.
45.3. Analysis.
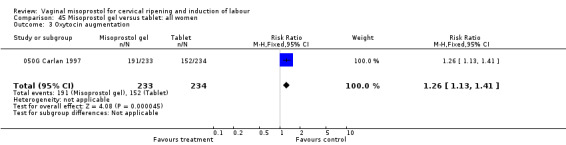
Comparison 45 Misoprostol gel versus tablet: all women, Outcome 3 Oxytocin augmentation.
45.4. Analysis.

Comparison 45 Misoprostol gel versus tablet: all women, Outcome 4 Epidural analgesia.
45.5. Analysis.

Comparison 45 Misoprostol gel versus tablet: all women, Outcome 5 Instrumental vaginal delivery.
45.6. Analysis.

Comparison 45 Misoprostol gel versus tablet: all women, Outcome 6 Apgar score < 7 at 5 minutes.
45.7. Analysis.

Comparison 45 Misoprostol gel versus tablet: all women, Outcome 7 Neonatal intensive care unit admission.
Comparison 46. Misoprostol gel versus tablet: all women with unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.29, 0.83] |
| 2 Caesarean section | 1 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.79, 1.45] |
| 3 Oxytocin augmentation | 1 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.13, 1.41] |
| 4 Epidural analgesia | 1 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.03, 1.38] |
| 5 Instrumental vaginal delivery | 1 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.74, 1.70] |
| 6 Apgar score < 7 at 5 minutes | 1 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.56, 2.38] |
| 7 Neonatal intensive care unit admission | 1 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.44, 1.24] |
46.1. Analysis.

Comparison 46 Misoprostol gel versus tablet: all women with unfavourable cervix, Outcome 1 Uterine hyperstimulation with FHR changes.
46.2. Analysis.

Comparison 46 Misoprostol gel versus tablet: all women with unfavourable cervix, Outcome 2 Caesarean section.
46.3. Analysis.

Comparison 46 Misoprostol gel versus tablet: all women with unfavourable cervix, Outcome 3 Oxytocin augmentation.
46.4. Analysis.

Comparison 46 Misoprostol gel versus tablet: all women with unfavourable cervix, Outcome 4 Epidural analgesia.
46.5. Analysis.

Comparison 46 Misoprostol gel versus tablet: all women with unfavourable cervix, Outcome 5 Instrumental vaginal delivery.
46.6. Analysis.

Comparison 46 Misoprostol gel versus tablet: all women with unfavourable cervix, Outcome 6 Apgar score < 7 at 5 minutes.
46.7. Analysis.

Comparison 46 Misoprostol gel versus tablet: all women with unfavourable cervix, Outcome 7 Neonatal intensive care unit admission.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
006 Ewert 2006.
| Methods | Double‐blinded randomised multicenter study. Randomisation and allocation concealment were computer generated. | |
| Participants | Inclusion criteria: pregnant women were aged 18 years or older, 37 to 42 weeks of gestation, requiring cervical ripening and labour induction and at least 1 previous delivery with gestation of 37 weeks or more. The current pregnancy had to be a singleton fetus with cephalic presentation and the women had to be classified as a pregnant uncomplicated with Bishop score less than 6. Exclusion criteria: women with plus than 4 terms full deliveries and those with caesarean delivery. Spontaneous labour, tocolytic agents used within 7 days before induction, the use of any other cervical‐ripening or labour‐inducing agent before enrolment, suspected cephalopelvic disproportion, fetal distress, use of non steroidal anti‐inflammatory drugs 4 hours before the drug studied treatment, pyrexia, unexplained genital bleeding after 24 weeks of this pregnancy, pelvic inflammatory disease, placenta previa and known or suspected allergy to misoprostol or other prostaglandins. |
|
| Interventions | A single misoprostol vaginal insert was administered high into the posterior fornix and positioned transversally behind the cervix. Each insert was loaded with 1 of the 4 dose reservoirs of misoprostol being investigated: 25, 50, 100 and 200 mcg. The vaginal insert was designed to release misoprostol at a rate of approximately 1, 2, 4 and 8 mcg per hour respectively. | |
| Outcomes | The median time to vaginal delivery was 27.5, 19.1, 13.1 and 10.6 hours for the 25‐, 50‐, 100‐ and 200‐mcg doses, respectively. The percentage of women who delivered within 12 hours was 9%, 14%, 47% and 53% (P < .001 using the 25 mcg group as the comparator) and within 24 hours was 42%, 79%, 81% and 70%(P = .003). Uterine hyperstimulation syndrome occurred in 1 women who received the 25 mcg, 2 women who received the 100 mcg, and 3 women who received 200 mcg dose reservoirs. | |
| Notes | This trial was conducted at 6 sites in the United Kingdom. At this review the comparison of doses were made grouping lower 2 doses vs higher 2 doses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded only for the outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
013 Papanikolaou 2004.
| Methods | Randomised unblinded trial. Allocation not stated. | |
| Participants | Inclusion criteria: women with > 39 completed weeks singleton gestation. Exclusion criteria: previous uterine scar, malpresentation, cervix dilated > 3cm, uterine contraction > 3/10 minutes, any contraindication to vaginal delivery, hypersensitivity to prostaglandins, parity > 5, abnormal antepartum testing, cephalopelvic disproportion, premature rupture of membranes and maternal illnesses. |
|
| Interventions | The intervention group received 100 mcg 6 hourly and the comparison group received 50 mcg 4 hourly of vaginal misoprostol until labour. | |
| Outcomes | In two groups the dose used of misoprostol were similar. There was no difference between two groups in mean time to delivery, caesarean rate, Apgar of 5 minutes and meconium passage. | |
| Notes | Department of Obstetrics and Gynaecology, Isparta, Turkey. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
013 Tedesco 2002.
| Methods | Single blind randomised trial with computer‐generated allocation. | |
| Participants | Inclusion criteria: indication for induction of labour; intact membranes; live fetus; cephalic presentation; cervical score < 6; gestation 37+ weeks. Exclusion criteria: caesarean section or other uterine surgery; antepartum haemorrhage; pyrexia; anaemia; mental incapacity; contraindication to labour; fetal anomaly. | |
| Interventions | Vaginal misoprostol 12.5 vs 25 mcg in absorbable capsules, repeated once after 6 hours if not in labour. | |
| Outcomes | Time from first dose to delivery (12.5 mcg: 28.7 +/‐ 19.6 vs 25 mcg: 23.6 +/‐10.3 hours); mode of delivery; cardiotocograph changes; meconium staining; Apgar scores; uterine hyperstimulation; analgesia. | |
| Notes | June 2000 to July 2001. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
019 Filho 2007.
| Methods | Double‐blinded randomised controlled clinical trial. Allocation by sequentially numbered sealed opaque envelopes. | |
| Participants | Inclusion criteria: pregnant women with indication to induction of labour, singleton, gestational age from 37 to 42 weeks, reactive non‐stress test, vertex presentation and no rupture of membranes or labour. Exclusion criteria: previous uterine scar, premature rupture of membranes, preterm birth, contraindication to vaginal delivery, any vaginal bleeding, doubts in the gestational age and known allergy to prostaglandins. |
|
| Interventions | The patients received 12.5 or 25 mcg vaginal misoprostol 4 hourly until effective labour. | |
| Outcomes | The two groups did not differ in the mean time from induction to delivery, in the frequency of vaginal delivery, Apgar score and tachysystole frequency. | |
| Notes | Maternity Sant'Anna of Santa Casa de Misericórdia de Sobral, Fortaleza, Ceara, Brazil. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded only for the outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
025 Elhassan 2005a.
| Methods | "An open randomised controlled clinical trial". The sequence generation and allocation concealment were not stated. | |
| Participants | Inclusion criteria: pregnant women with single babies, unripe cervices and intact membranes. Exclusion criteria: previous uterine surgery, antepartum haemorrhage, asthma, heart disease and grand multiparity. |
|
| Interventions | The intervention group received 25 mcg of misoprostol 6 hourly up to 4 doses. The comparison group received 50 mcg of misoprostol 6 hourly up to 4 doses. | |
| Outcomes | The induction‐delivery interval was significantly longer in the 25 mcg group vs. 50 mcg group (21.9 h ± 4.3 h vs 9.6 h ± 2.2 h, p = 0.04). More women in the 25 mcg group received oxytocin (61.3% vs. 56.3% p > 0.05). Significantly fewer patients delivered vaginally in the 25 mcg group (61.3% vs. 90.6%, p = 0.05). There were no differences between the two groups at neonatal outcomes. | |
| Notes | This study was conducted at the labour ward of Wad Medani Hospital at Sudan, from January to July 2004. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
025 Haghighi 2006.
| Methods | Randomised trial. Allocation concealment made by sequential sealed opaque envelope. | |
| Participants | Inclusion criteria: women with singleton pregnancy, gestational age from 29 to 36 weeks, PPROM, parity of 2 or less, Bishop score ≤ 4, Normal FHR reactivity, absence of uterine contractions and vertex presentation. Exclusion criteria: previous uterine scar, fetal growth retardation, pre‐eclampsia, evidence of cephalopelvic disproportion, chorioamnionitis and contraindication of prostaglandin treatment. |
|
| Interventions | The intervention group received 1 25 mcg dose of misoprostol. If the contractions were not adequate in 3 hours, oxytocin augmentation was started. The comparison group received oxytocin infusion (started with 2 mcU/min and increased every 15 minutes until adequate uterine contraction). | |
| Outcomes | There was a statistically significant difference in the interval between admittance and vaginal delivery (507.68 min ± 248.01 min in the misoprostol group vs 596.66 min ± 246.38 min in the control group; P < .005) and also in the incidence of caesarean section due to failed labour induction (9.2% in the misoprostol group vs 18.5% in the control group). | |
| Notes | Akbarabadi Hospital in Tehran, Iran. The study run from December 2002 to May 2004. The numbers of baseline data and the neonatal outcomes were not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | High risk | Inadequate |
| Other bias | High risk | Inadequate |
025 Incerpi 2001.
| Methods | Misoprostol or placebo dispensed by pharmacy according to computer‐generated randomisation schedule. | |
| Participants | Inclusion criteria: women with pre‐gestational or gestational diabetes; good control of blood glucose levels; singleton gestation; intact membranes; cervical score < 5; uterine contractions < 8/hour; cephalic presentation; gestational age 38.5 weeks or more; normal amniotic fluid index; reactive fetal heart rate pattern; good compliance. Exclusion criteria: multiple pregnancy; estimated fetal weight > 4500 or < 2000 g; ruptured membranes; placenta praevia; vaginal bleeding; active genital herpes; glaucoma; hypersensitivity to prostaglandins; renal, hepatic or cardiovascular disease; severe asthma; parity > 5. | |
| Interventions | Misoprostol 25 mcg or placebo vaginally on day 1; if not in labour after 4 hours, discharged home; repeated on day 4 if enrolment criteria still met; if cervical score > 8, labour induced with amniotomy and oxytocin infusion; if not delivered by day 7, labour induced with amniotomy and oxytocin, or misoprostol 25 mcg 4‐hourly. | |
| Outcomes | Primary outcome: delivery within 7 days (misoprostol 31/57 vs placebo 36/63). | |
| Notes | Los Angeles County ‐ University of Southern California Women's and Children's Hospital and Good Samaritan Hospital, August 1996 to November 2000. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
025 Krupa 2005.
| Methods | Open randomised controlled trial with allocation concealment using consecutively numbered sealed opaque envelopes. | |
| Participants | Inclusion criteria: pregnant women with PROM confirmed up to 6 hours after occurrence, gestational age ≥ 37 weeks, cephalic presentation and a live fetus showing no signs of fetal compromise as evaluated by cardiotography. Exclusion criteria: previous caesarean section or uterine surgery, being in labour at admission, presence of fetal malformation of incompatible with life, twin pregnancy or strongly suspected or confirmed chorioamnionitis. |
|
| Interventions | The intervention group received 25 mcg of misoprostol 6 hourly up to 4 doses. The comparison group received vital signs monitorisation during 24 hours. After 24 hours, if the women did not initiate labour, oxytocin was started. | |
| Outcomes | The misoprostol group had a significantly shorter latency period (9.4 h vs 15.8 h), a shorter time interval from recruitment to delivery (18.9 h vs 27.5 h), a shorter period of maternal hospitalisation and a slightly higher proportion of alterations of contractility when compared to expectant group. Caesarean section was higher in the observational group. The complications were similar in the groups. Within 24 hours, 44% of the women had delivered in the expectant group against 73% in the misoprostol group. | |
| Notes | The study was conducted between January 2000 and May 2003. Faculty of Medical Sciences, Universidade Estadual de Campinas ‐ UNICAMP, São Paulo, Brazil. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
025 Kumar 2001.
| Methods | "Patients were randomly assigned..." Sequence generation and allocation concealment were not stated. | |
| Participants | Inclusion criteria: obstetric indications of labour induction including hypertensive disorders of pregnancy, intrauterine growth retardation of fetus and postdatism; medical complications including diabetes and renal disease; no history of previous caesarean section or uterine surgery; absence of active labour or fetal distress and singleton pregnancy with vertex presentation and no contraindication of vaginal delivery. | |
| Interventions | The intervention group received 25 mcg dose of misoprostol six hourly until adequate uterine contractions were achieved. The patients in active phase of labour with arrest of dilatation received oxytocin for augmentation. The comparison group received dinoprostone gel 0.5 mg intra cervically. If there was absence of uterine contraction after 10 hours of dinoprostone, the patient started to receive oxytocin infusion. | |
| Outcomes | The average interval from start to induction of vaginal delivery was shorter in misoprostol group (1315 min ± 811min) compared to dinoprostone/oxytocin group (1512 min ± 712 min) (p < 0.01). There were no significant difference in route of delivery, incidence of uterine hyperstimulation and perinatal outcome. | |
| Notes | The study was conducted in INHS Asvini from January 1999 to January 2000. Colba, Mubai. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unblinded for personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
025 Majoko 2001.
| Methods | "Non blinded randomized controlled trial", did not provide information on allocation methods | |
| Participants | Women admitted for induction of labour, with singleton pregnancy and cephalic presentation | |
| Interventions | Vaginal misoprostol 50 mcg versus prostaglandin F2 alpha 2 times 8 hourly if needed. | |
| Outcomes | Need of oxytocin, caesarian section rate and delivery interval. | |
| Notes | Only abstract available. This trial did not contribute with any data because were provided only percentages. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
025 Majoko 2002c.
| Methods | Randomised controlled trial. Randomisation by computer generated table and allocation recorded on a card placed in a sealed opaque envelope . | |
| Participants | Inclusion criteria: women for IOL after 37 weeks with singleton pregnancy in cephalic presentation. Exclusion criteria: previous uterine surgery, abnormal FHR pattern, contra‐indication to vaginal delivery and known sensitivity to prostaglandin or misoprostol. |
|
| Interventions | Intervention group received vaginal misoprostol 50 mcg at maximum of 2 doses each 8 hours. There were 3 comparison groups: 1. Suspension of oral misoprostol 4 hourly. 2. Prostaglandin F2α gel 5 mg intra cervically in 2 doses (8 hours interval) if the Foley was well located. 3. Prostaglandin E2 pessary 3 mg, vaginally repeated after 8 hours if necessary. |
|
| Outcomes | There was no difference in mode of delivery. There was significantly reduced risk of caesarean section in the intervention group (OR 0.20; 95% CI 0.22 to 0.78). The need of oxytocin augmentation was reduced in the group of prostaglandin E2 pessary, vaginal misoprostol and oral misoprostol. The vaginal misoprostol reduced the interval from induction to delivery, but the 2 misoprostol groups increased the admissions at the neonatal unit. | |
| Notes | Harare Maternity Hospital, Zimbabwe. The vaginal misoprostol was compared with the vaginal prostaglandin. The other groups were evaluated in the specific review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Inadequate |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
025 McKenna 2004.
| Methods | Randomised, double blinded, placebo‐controlled trial. Allocation by a centre of randomisation. | |
| Participants | Inclusion criteria: pregnant women older than 18 years old, at list 40 weeks of pregnancy. Bishop < 9. Well dated pregnancy. Exclusion criteria: oligohydramnios, rupture of membranes, malpresentation, vaginal bleeding, multiple gestation, labour and history of prior caesarean section. |
|
| Interventions | The intervention group received a 1 time 25 mcg intravaginal. The comparison group received an inert substance as placebo, both in gel capsules. | |
| Outcomes | The mean interval to delivery was significantly lower in the misoprostol group, 4.2 days ± 4.1 days compared with 6.1 days ± 3.6 days, P = .04 after receiving the insert. The interval to delivery was significantly less in the misoprostol group only for nulliparous women. The survival curves for the interval from intervention to delivery were significantly different (P = .04); for misoprostol the median interval was 4.1 days to delivery compared with 9.2 days for placebo. | |
| Notes | Wright‐Paterson US Air Force Base Medical Center. 1 patient of the intervention group and 3 of the comparison group were excluded from the trial after randomisation. They did not received the medication of the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Adequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
025 Meyer 2005.
| Methods | Randomised unblinded controlled trial. Allocation by sequential numbered opaque envelope. | |
| Participants | Inclusion criteria: gestation at term, vertex presentation, Bishop score 6, singleton pregnancies, intact membranes, cephalic presentation and non‐stress test previous. Exclusion criteria: Bishop score > 6, labour, any uterine scar and rupture of membranes. |
|
| Interventions | The intervention group received 25 mcg of vaginal misoprostol single dose. The comparison group received an intra cervically dose of 0.5 mg of dinoprostone. | |
| Outcomes | Single dose of misoprostol significantly decreased the cumulative dose of oxytocin, the cumulative dose of time of oxytocin administration, and the dose intensity of time oxytocin (dose divided by time). | |
| Notes | The study was conducted from November 1999 to December 2001 at the University of Vermont. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Inadequate only for personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
025 Oboro 2005.
| Methods | Randomised controlled trial. Allocation by card drawn from a consecutive series of sealed envelopes. | |
| Participants | Inclusion criteria: women with singleton gestation in cephalic presentation, Bishop score ≤ 8, 40 weeks of gestational age. Exclusion criteria: patients with rupture of membranes, vaginal bleeding, a prior uterine incision, non‐reactive non‐stress test and an estimated fetal weight of > 4500g. |
|
| Interventions | The intervention group received one dose of 25 mcg misoprostol vaginally and the comparison group received no treatment. | |
| Outcomes | Misoprostol was associated with significant decrease of time to delivery, earlier gestational age at delivery, shorter duration of active labour, without changes at the neonatal outcome. | |
| Notes | The trial was conducted from August 1, 2000 to October 31, 2001 at Zonal General Hospital, Kwale, of Delta state of Nigeria. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
025 Sheela 2007.
| Methods | "A total of 150 women were randomised". The sequence generation and the allocation concealment were not stated. | |
| Participants | Inclusion criteria: all women at term, singleton pregnancies, live fetus, cephalic presentation, obstetric or medical indication for induction of labour, intact membranes, Bishop score ≤ 4, and reassuring non‐stress test. Exclusion criteria: grand multiparas, previous caesarean section, other uterine scar, medical contraindication for prostaglandins and those with maternal or fetal compromise. |
|
| Interventions | One group received 0.5 mg intracervical dinoprostone 12 hourly (maximum 3 doses) and the other two groups received 25 or 50 mcg vaginal misoprostol 6 hourly (maximum 5 doses). The patients could receive oxytocin augmentation. | |
| Outcomes | There were no differences between the groups in mode of delivery, neonatal outcomes, caesarean section rate, failed induction, failed induction and hyperstimulation. The women from the misoprostol group required fewer oxytocin augmentation and present shorter interval from induction to delivery. | |
| Notes | Data from oral misoprostol were accessed in a specific review. Department of Obstetrics and Gynaecology, St. John's Medical College Hospital, Karnataka, India. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Unclear risk | Unclear |
025 Stitely 2000.
| Methods | Double‐blind, placebo‐controlled in computer generated sequence, permuted block design. Medications prepared independently by pharmacy. | |
| Participants | Inclusion criteria: gestational age 41 weeks to 41 weeks 6 days; singleton pregnancy; intact membranes; cervical score 4 or less; uterine contractions < 8 per hour; amniotic fluid index > 5 cm; reactive cardiotocography; maternal age 18 to 50 years. Exclusion criteria: fetal malpresentation; estimated fetal weight > 4500 g or < 2000 g; placenta praevia; vasa praevia; unexplained vaginal bleeding; active herpes simplex; hypersensitivity to prostaglandins; prior uterine surgery or caesarean section; evidence of intra‐amniotic infection; severe asthma or cardiovascular disease; renal or hepatic dysfunction. | |
| Interventions | 1. Misoprostol 25 mcg (1/4 tablet). 2. Placebo (dicalcium phosphate 1/5 tablet). Medication placed in posterior vaginal fornix. FHR and uterine activity monitored continuously for 4 hours, then discharged. Repeated after 24 hours if cervical score < 9 and other original criteria unchanged. After a further 24 hours, labour induced with oxytocin if cervical score > 6, pre‐induction vaginal misoprostol 25 mcg if < 6. | |
| Outcomes | Primary: number of inpatient inductions needed by study day 3 (3/27 vs 28/33). Secondary: mode of delivery; Apgar score < 7 at 5 minutes; meconium passage; neonatal ICU admission; number of medication doses (1.41 sem 0.1 vs 1.85 sem 0.1); dosing required on study day 2 (15/27 vs 30/33); change in cervical score day 1‐2 (2.7 sem 0.8 vs 0.96 sem 0.008); first dose to delivery (36.9 sem 3.8 vs 61.3 sem 3.8 hours). | |
| Notes | Naval Medical Center, Portsmouth VA and National Naval Medical Center, Bethesda, MD; December 7 1997 to December 10 1999. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
025 Wang 1998.
| Methods | This study described as random allocation but there were no details in methods. | |
| Participants | Term pregnant women, singleton pregnancy, vertex presentation and intact membranes. There were no exclusion criteria. |
|
| Interventions | Patients received 50 mcg or 25 mcg vaginal misoprostol. | |
| Outcomes | The study did not show differences between these 2 dosages of misoprostol. | |
| Notes | The study was enrolled from November 96 to November 97. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | Adequate |
025 Wing 1996.
| Methods | Allocation by sequentially numbered sealed opaque envelopes, maintained by the primary investigator. Sequence from a computerised random number generator. | |
| Participants | Membranes were intact, cervical status unfavourable. Inclusion criteria: women requiring induction of labour for medical or obstetric reasons; singleton pregnancy; cephalic presentation; intact membranes; cervical score < 5; reactive nonstress test; < 8 uterine contractions per hour. Exclusion criteria: estimated fetal weight > 4500 g; evidence of cephalopelvic disproportion; placenta praevia; unexplained vaginal bleeding; vasa praevia; active herpes simplex infection; contraindication to receiving prostaglandins; renal or hepatic dysfunction; suspected chorioamnionitis; previous uterine surgery; parity > 5. Indications for labour induction: oligohydramnios (6‐hourly misoprostol 131, 3‐hourly 126); pre‐eclampsia (47, 43); post‐term pregnancy (32, 35); macrosomia (8, 17); diabetes mellitus (17, 9); abnormal antepartum testing (12, 13); other (18, 12). |
|
| Interventions | Misoprostol 25 mcg inserted into the posterior vaginal fornix 6‐hourly (n = 259) versus 3‐hourly (n = 261), until 3 uterine contractions per 10 minutes, cervical score > 7, cervical dilation > 3 cm or spontaneous rupture of membranes (maximum 24 hours). Artificial rupture of membranes usually performed when the cervix was 80% effaced and 3 cm dilated, or 4 cm dilated. Oxytocin augmentation was used for lack of contractions after maximum dosage or spontaneous rupture of membranes, or for arrested cervical dilation, > 3 hours after the last misoprostol dose. Uterine hyperstimulation was treated in some cases by tocolytic therapy. |
|
| Outcomes | Oxytocin augmentation; uterine tachysystole (> 5 contractions per 10 minutes); uterine hypertonus (contraction > 2 minutes) (6‐hourly misoprostol 12/259, 3‐hourly 11/261); hyperstimulation syndrome (tachysystole or hypertonus with fetal heart rate abnormalities); induction to delivery interval; mode of delivery; vaginal delivery within 24 hours; terbutaline used for hyperstimulation (18/259, 26/261); neonatal resuscitation (83/259, 90/261); days in NICU (mean 9.2 (SD 8.6), 9.8 (12.8)); meconium aspiration syndrome (3/259, 2/261); hyperbilirubinaemia (14/259, 16/261). Tachysystole occurred after the first dose of misoprostol in 43/520 (8.3%) of women. One maternal death occurred in a primiparous woman, 9 hours after a single misoprostol dose and shortly after amnioinfusion and epidural analgesia, from amniotic fluid embolization. Two caesarean hysterectomies were performed for atonic uterine haemorrhage 13 and 30 hours after single doses of misoprostol, in one primiparous woman with uncomplicated labour, and one nulliparous woman following prolonged induction attempt with oxytocin augmentation and chorioamnionitis. |
|
| Notes | Los Angeles, California, USA. October 1994 to June 1995. 522/535 women agreed to participate. No women withdrew from the protocol. Two women allocated to misoprostol 6‐hourly were excluded from the analysis because of deviation from the study protocol. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
025 Wing 1998a.
| Methods | Randomised clinical trial with allocation concealment by consecutive numbered sealed opaque envelopes. | |
| Participants | Inclusion criteria: women with spontaneous ruptured membranes, singleton pregnancies with cephalic presentation, reactive FHR pattern beyond 36 weeks' gestational age, not in labour. Exclusion criteria: women with cervical dilatation in > 3cm, in labour, estimated fetal weight > 4500g or evidence of cephalopelvic disproportion, estimated fetal weight < 1800g, placenta previa, unexplained vaginal bleeding, active herpes simplex, scared uterus, clinical signal of maternal infection, parity > 5, moderate or severe pre‐existing medical disease or any contraindication for ose of prostaglandin. |
|
| Interventions | 1. Misoprostol 25 mcg placed in the posterior vaginal fornix, repeated after 6 hours if necessary, n = 98. 2. Intravenous incremental oxytocin infusion to maximum dose of 22 mU/minute, n = 99. | |
| Outcomes | Induction to delivery interval (misoprostol 811.5 +/‐ 511.4 vs oxytocin 747.0 +/‐ 448.0 minutes, p = 0.65); caesarean section; abnormal FHR tracing (29/98 vs 29/99); chorioamnionitis (28/98 vs 26/99); meconium‐stained liquor; Apgar score < 7 at 1 and 5 minutes; neonatal resuscitation (24/98 vs 27/99); neonatal ICU admission. | |
| Notes | 3 withdrawals not accounted for. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
025 Wing 1998b.
| Methods | Women 'assigned by means of a computerised random number generator' using numbered, sealed opaque envelopes. | |
| Participants | 38 of 40 women requiring induction of labour for medical or obstetric indications with one immediate prior caesarean delivery agreed to participate.
Inclusion criteria: singleton pregnancy; cephalic presentation; intact membranes; cervical score < 6; reactive nonstress test; < 8 uterine contractions per hour. Exclusion criteria: estimated fetal weight > 4500g; evidence of cephalopelvic disproportion; placenta praevia; unexplained vaginal bleeding; vasa praevia; active herpes simplex infection; contraindication to receiving prostaglandins; renal or hepatic dysfunction; suspected chorioamnionitis; previous uterine surgery; parity > 5, prior classical caesarean section. |
|
| Interventions | 1. Misoprostol 25 mcg vaginally every 6 hours until 3 contractions per 10 minutes, cervical score 8 or more, or cervix 3 cm dilated (maximum 4 doses). Amniotomy and oxytocin augmentation used when necessary (n = 17).
2. Intravenous oxytocin 1 mU per minute, increased every 30 minutes of necessary (maximum 22 mU per minute) (n = 21). Management included active amniotomy and continuous fetal heart rate and uterine activity monitoring. |
|
| Outcomes | Vaginal delivery within 24 hours; oxytocin augmentation; route of delivery; uterine dehiscence (asymptomatic uterine scar disruption); uterine rupture (separation of uterine scar requiring emergency laparotomy). | |
| Notes | October 28, 1995 to November 18, 1996. Women's Hospital, Los Angeles, USA. Calculated sample size 160. Trial terminated prematurely because of disruption of uterine scar in two women who received misoprostol. Further outcome details received on request from authors. One woman withdrew from protocol requesting caesarean section (misoprostol group) and included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
025G Srisomboon 1998.
| Methods | Allocated by 'block randomisation'. | |
| Participants | Pregnant women eligible for labour induction. Inclusion criteria: singleton pregnancy; parity < 4; vertex presentation; obstetric or medical indication for labour induction; intact membranes; cervical score < 5; gestational age > 35 weeks. Exclusion criteria: labour; fetal distress; previous caesarian delivery or other uterine surgery; definite cephalopelvic disproportion; contraindication to the use of prostaglandins. |
|
| Interventions | Vaginal misoprostol 25 mcg versus 50 mcg in carboxymethylcellulose gel, 6‐hourly until adequate contraction, cervical score > 6, cervical dilation > 3, or spontaneous rupture of membranes (maximum 4 doses). If cervix favourable, amniotomy performed and oxytocin infused if necessary. Continuous cardiotocography was used. | |
| Outcomes | Tachysystole (> 5 contractions per 10 minutes; hypertonus (contraction > 90 seconds); hyperstimulation syndrome. | |
| Notes | November 1995 to May 1996, Chiang Mai University Hospital, Thailand. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
030 Moodley 2003.
| Methods | Allocation by sequentially numbered opaque envelopes with computer‐generated sequence. Not blinded. | |
| Participants | Inclusion criteria: viable term or near‐term pregnancy; indication for induction of labour. Exclusion criteria: previous caesarean section; malpresentation; non‐reassuring fetal heart rate pattern; cervical score > 5; parity > 4. | |
| Interventions | Misoprostol 25 mcg into posterior vaginal fornix; if cervix unchanged and not in labour after 4 hours, oral misoprostol solution (200 mcg in 200 ml water), 20 mcg 2‐hourly until adequate contractions, up to 3 doses; versus dinoprostone 1 mg into the posterior vaginal fornix 6‐hourly for up to 3 doses if contractions inadequate. | |
| Outcomes | Delivery within 24 hours; induction to delivery time; meconium staining of liquor; non‐reassuring fetal heart rate pattern; hyperstimulation syndrome (hypersystole and/or tachysystole with abnormal fetal heart rate pattern); mode of delivery; perinatal mortality; average dose requirements. | |
| Notes | King Edward VIII Hospital, Durban, South Africa. A third arm (oral misoprostol) is for consideration in the oral misoprostol for labour induction review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
038 Aquino 2003.
| Methods | This was a randomised, by computer generation, controlled clinical trial with a sequential numbered envelope used for allocation. | |
| Participants | Inclusion criteria: patients with medical indication for induction of labour, singleton gestation, gestational age greater than 36 weeks, vertex presentation, intact membranes, Bishop score < 6, no labour occurring and normal fetal heart rate. Exclusion criteria: pelvic dystocia, estimate fetal weight greater than 4 kg, evidence of cephalopelvic disproportion, placenta previa, any unexplained vaginal bleeding, parity > 5, fetal malformation, previous uterine scar, any situation when vaginal delivery war not indicated or contraindication to use misoprostol. |
|
| Interventions | The intervention group used 25 mcg of vaginal misoprostol 4 hourly (maximum 200 mcg) and the comparison group received oxytocin alone. | |
| Outcomes | The caesarean section rate and the time to induction from delivery was significantly shorter in the misoprostol group. Tachysystole was more frequent in the intervention group. There were no differences in the neonatal outcomes between the groups. | |
| Notes | Maternity Hospital Leonor Mendes de Barros, São Paulo, Brazil, from November 1998 to December 2000. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear for personnel, but blinded for participants and outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
038 Cecatti 2000.
| Methods | 'Randomised controlled trial'. | |
| Participants | Term pregnant women with indication for labour induction; intact membranes. | |
| Interventions | Vaginal misoprostol 25 mcg 4‐hourly (maximum 8 doses versus oxytocin infusion. | |
| Outcomes | Latent period; induction to vaginal delivery time; route of delivery; hyperstimulation syndrome. | |
| Notes | Hospital‐Maternity Leonor Mendes de Barros, Sao Paulo, Brazil. November 1998 to August 1999. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
038 Clark 1998.
| Methods | Method of women being 'randomised' not described. | |
| Participants | Inclusion criteria: obstetric or medical indication for labour induction; singleton pregnancy; vertex presentation; reassuring fetal status; cervical score +/< 5. Exclusion criteria: abnormal FHR pattern; placenta praevia; active herpes infection; history of asthma, glaucoma, cardiac or hepatic disease; chorioamnionitis; previous uterine scar; parity > 5. |
|
| Interventions | 1. Misoprostol 25 µg to the posterior vaginal fornix every 4 hours (maximum 4 doses) until adequate contraction pattern or active labour, vs 2. dinoprostone gel 0.5 mg intracervically every 4 hours (maximum 4 doses) until adequate contractions or cervical score > 5. Oxytocin used if labour not established after 4 doses, or labour protracted. Continuous FHR and uterine activity monitoring were used. | |
| Outcomes | Oxytocin use; induction to delivery interval (misoprostol 1181 +/‐ 566 vs dinoprostone 1403 +/‐ 566 minutes, p = 0.02); caesarean section (15% vs 31%); hyperstimulation syndrome. | |
| Notes | Caesarean section (percentages only given as E1 15% and E2 31%. However previous sentence and relative risk figure given correspond to 31% for E1, 15% for E2). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
038 El‐Sherbiny 2001.
| Methods | Randomised using random number tables. | |
| Participants | Inclusion criteria: singleton; live fetus; 37 or more weeks' gestation; cephalic presentation; intact membranes. Exclusion criteria: parity > 5; previous uterine surgery. | |
| Interventions | Vaginal misoprostol gel 25 vs 50 mcg 4‐hourly (maximum 6 doses). Prepared by mixing one 200 mcg tablet with 8 or 4 ml hydroxyethyl gel. | |
| Outcomes | Tachysystole; hypersystole; uterine hyperstimulation; oxytocin used; induction‐delivery time; caesarean section; meconium‐stained liquor; neonatal outcomes. | |
| Notes | El‐Sherbiny Hospital and El‐Salaam General hospital, Egypt, May 1997 to April 1999. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
038 Eroglu 2007.
| Methods | Randomised trial with a sequence generation not stated. The allocation concealment was made by sequential envelopes pulled by hospital residents. | |
| Participants | Inclusion criteria: women with gestational age ≥ 37 weeks, singleton pregnancy, Bishop score ≥ 6, vertex presentation, no contraindication to vaginal delivery, and obstetric indication for induction of labour. Exclusion criteria: Bishop score > 6, prior caesarean delivery, placenta previa, unexplained vaginal bleeding and contraindication or allergic reaction to the use of prostaglandins. |
|
| Interventions | The study groups used 50 mcg or 25 mcg of vaginal misoprostol 4 hourly (maximum 6 doses). The amniotomy was performed after 2 ‐ 3 hours without labour progress. The patients could receive oxytocin augmentation. | |
| Outcomes | The mean time from induction to delivery was shorter in the 50 mcg group. The use of oxytocin was smaller in the 50 mcg group. The incidence of tachysystole was higher in the 50 mcg group. There were no differences between the two groups in the caesarean section rate and neonatal outcomes. | |
| Notes | The study was conducted from June 2004 and March 2006. Department of Obstetrics and Gynaecology, Baskent University, Ankara, Turkey. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
038 Gregson 2005.
| Methods | Randomised controlled trial. Allocation concealment was made by consecutively numbered, sealed, opaque envelope. | |
| Participants | Inclusion criteria: singleton pregnancy women, cephalic presentation, gestational age at term (37‐42 weeks), parity < 4 times and no contraindication to prostaglandin. Exclusion criteria: significant maternal or fetal medical condition, previous uterine surgery and significant uterine activity. |
|
| Interventions | The intervention group received 25 mcg dose of misoprostol 4 hourly until 6 doses. The comparison group received dinoprostone vaginal gel 1‐2 mg dose 6 hourly repeated at maximum of 3 mg in 24 hours. | |
| Outcomes | Primary: induction to vaginal delivery interval. Secondary: requirements of oxytocin, mode of delivery, number of women delivering < 24 hours, incidence of uterine contraction abnormalities, incidence of abnormal cardiotocography recordings, 5‐minute Apgar scores, umbilical cord pH recordings, analgesia requirements, admission to NICU and blood loss at delivery. | |
| Notes | The study took place at Queen Mary's Sidcup NHS trust between 18th July and 2nd December 2003. Florence Nightingale School of Nursing and Midwifery, King's College, UK. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear for participants, but blinded for personel and outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
038 Has 2002.
| Methods | Tablets in bottles 'A' and 'B' prepared by the pharmacist. Placed in paper packets according to a table, stratified for age and parity. The first allocation in each group by lots, then alternation. | |
| Participants | Inclusion criteria: singleton pregnancy; 37 or more weeks; cervical score 4 or less; vertex presentation; estimated fetal weight 4500 g or more; four or fewer contractions per hour. Exclusion criteria: prior uterine surgery; contraindication to prostaglandins; poor fetal surveillance scores; active genital herpes infection; placenta praevia; abruptio placentae; vasa praevia; unexplained vaginal bleeding. |
|
| Interventions | 25 mcg versus 50 mcg in tablets reconstituted by the pharmacy, 4‐hourly (maximum 6 doses). Oxytocin infusion if not in labour 4 hours after the third dose. | |
| Outcomes | Primary: caesarean section; oxytocin use; tachysystole (6 or more contractions per 10 minutes, for 20 minutes); hypersystole (a contraction lasting 2 minutes); hyperstimulation (tachysystole or hypersystole and fetal heart rate abnormality); fetal distress requiring delivery. Secondary: caesarean section for fetal distress (low dose 6/58 vs high dose 16/56); umbilical artery pH < 7 (0/58 vs 2/56). | |
| Notes | June 1998 to November 2000, Istanbul Medical School, Turkey. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
038 Kidanto 2006.
| Methods | Randomised trial. Allocation concealment made by sealed opaque envelope with sequence generation by computer. | |
| Participants | Inclusion criteria: pregnant women with singleton vertex presentation and gestational age > 36 weeks. Exclusion criteria: previous miomectomy, uteroplasty and caesarean section. |
|
| Interventions | The intervention group received 25 mcg of misoprostol 4 hourly until four doses. The comparison group received oxytocin infusion and amniotomy. | |
| Outcomes | The interval from induction to delivery was shorter in the misoprostol group (10.86 h) compared to oxytocin group (15.45 h). There were fewer caesarean sections and neonatal ward in the misoprostol group. The induction with misoprostol was effective, safe and cheaper than oxytocin for IOL. | |
| Notes | Muhinbili National Hospital, Dar es Salaam Tanzania. The study was performed between June to December of 2004. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unclear for participants and for outcome assessors. No blinding for personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Unclear risk | Unclear |
038 Krithika 2008.
| Methods | "Prospective randomized study" with no reference for allocation concealment methods. | |
| Participants | Inclusion criteria: women with singleton pregnancy at more than 34 weeks with Bishop score less than 4 admitted for induction of labour. Exclusion criteria: scared uterus, active medical disorder, antepartum haemorrhage, abnormal fetal heart rate pattern, contracted pelvis, cephalopelvic disproportion, suspected chorioamnionitis and known uterine abnormalities. |
|
| Interventions | The intervention group received vaginal misoprostol 25 mcg 4 hourly until 6 doses. The comparison group received 0.5 mg of endocervical prostaglandin E2 and if necessary a second dose 12 hours later. | |
| Outcomes | Induction to delivery interval was significantly shorter in the misoprostol group. The rate of complications were comparable in between groups. | |
| Notes | A total of 100 women were enrolled in this trial. Study developed in the Department of Obstetrics and Gynaecology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
038 Meydanli 2003.
| Methods | Double‐blind study. Random allocation by sealed consecutively numbered opaque envelopes in computer‐generated random sequence in varying blocks. | |
| Participants | Inclusion criteria: singleton live gestation; vertex presentation; intact membranes; gestational age > 41 weeks; cervical score < 5; < 4 spontaneous uterine contractions per hour; estimated fetal weight < 4500 g; reactive cardiotocography. Exclusion criteria: hypersensitivity to prostaglandins; previous caesarean section or uterine surgery; body mass index =/> 30 before pregnancy; previous labour induction attempt in the current pregnancy; hypertensive disorders of pregnancy; fetal growth restriction; diabetes. | |
| Interventions | Misoprostol 25 mcg (1/4 100 mcg tablet) compared with 50 mcg (1/4 200 mcg tablet). Labour induction with misoprostol 4‐hourly placed in the posterior vaginal fornix, up to 6 doses. Vaginal examination and cardiotocography prior to each dose. No further misoprostol when uterine contractions of 3 per 10 minutes. When in labour or cervical score 8 or more, artificial rupture of membranes. Oxytocin used for poor progress more than 4 hours after last dose of misoprostol. No epidural analgesia or intravenous sedation used. | |
| Outcomes | Time from first dose to delivery; delivery with 12 hours and 24 hours; mode of delivery; emergency caesarean section for fetal hear rate abnormality; misoprostol doses, oxytocin requirement; tachysystole (> 5 contractions per 10 minutes for 20 minutes; uterine hyperstimulation (tachysystole with fetal tachycardia, late decelerations or reduced variability); birthweight; meconium‐stained amniotic fluid; arterial cord pH < 7.16; neonatal intensive care unit admission. | |
| Notes | July 2001 to June 2002; two tertiary training centres in Turkey. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
038 Murthy 2006.
| Methods | "The women enrolled into the study were randomized". The sequence generation was not stated and the allocation was made by sealed envelopes. | |
| Participants | Inclusion criteria: singleton gestation, gestational age between 37 ‐ 42 weeks, live uterine fetus, intact membranes, cephalic presentation and Bishop score ≤ 5. Exclusion criteria: multiple pregnancy, malpresentation, abnormal fetal heart rate pattern, cephalopelvic disproportion, rupture of membranes, previous caesarean section, parity more than 5 and history of hypersensitivity to prostaglandins. |
|
| Interventions | The intervention group received 25mcg vaginal misoprostol 4 hourly (maximum 200 mcg/patient). The comparison group used 0.5 mg intracervical dinoprostone associated with oxytocin after 6 hours. | |
| Outcomes | The intervention group has shorter interval from induction to delivery. Fetal distress was more common in the intervention group. Neonatal outcomes were similar. The cost of the therapy was significantly less in the misoprostol group. | |
| Notes | The study was from December 2003 to May 2004. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
038 Van Gemund 2004.
| Methods | Randomised multicentre trial using a random number table. The allocation was by sequentially numbered, opaque and sealed envelope. | |
| Participants | Inclusion criteria: singleton pregnancy, cephalic or breech presentation, gestational age at least 36 completed weeks and Bishop score < 6. Exclusion criteria: contraindications to use prostaglandins, previous caesarean section or uterine incision and no informed consent. |
|
| Interventions | The intervention group used 25 mcg of vaginal misoprostol 4 hourly (maximum 3 doses per day until 2 days). The comparison group used 1 mg of vaginal dinoprostone 4 hourly until 3 doses per day. | |
| Outcomes | The median induction‐delivery interval was longer in the misoprostol group compared to the dinoprostone group (25 versus 19 hours , P = 0.008). Significantly fewer neonates were admitted at the NICU in the group of misoprostol intervention. | |
| Notes | The study was carried out between 1 November 1999 and 31 December 2002 in the Netherlands. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
038 Wing 1997.
| Methods | Allocation by sequentially numbered sealed opaque envelopes, maintained by the primary investigator. Sequence from a computerised random number generator. | |
| Participants | Membranes were intact, cervical status unfavourable. Inclusion criteria: women requiring induction of labour for medical or obstetric reasons; singleton pregnancy; cephalic presentation; intact membranes; cervical score < 5; reactive fetal heart rate pattern; < 8 uterine contractions per hour. Exclusion criteria: abnormal fetal heart rate pattern; malpresentation; estimated fetal weight > 4500g; suspected cephalopelvic disproportion; ruptured membranes; placenta praevia; unexplained vaginal bleeding; vasa praevia; active herpes simplex infection; contraindication to receiving prostaglandins; renal or hepatic dysfunction; suspected chorioamnionitis; previous uterine surgery; parity > 5. Indications for labour induction: oligohydramnios (misoprostol 46, dinoprostone 39); pre‐eclampsia (21, 28); post‐term pregnancy (16, 10); macrosomia (4, 8); diabetes mellitus (7, 8); impaired fetal growth (4, 3); other (1, 2). |
|
| Interventions | Misoprostol 25 mcg inserted into the posterior vaginal fornix 4‐hourly, maximum 6 doses or 24 hours (n = 99) versus dinoprostone 10 mg vaginal insert, delivering 0.3 mg per hour (n = 98), until 3 uterine contractions per 10 minutes, cervical score > 7, cervical dilation > 2 cm or spontaneous rupture of membranes. The dinoprostone insert was removed if the above occurred or if there were uterine contraction or fetal heart rate abnormalities, or 24 hours had elapsed. Artificial rupture of membranes usually performed when the cervix was 80% effaced and 3 cm dilated, or 4 cm dilated. Oxytocin augmentation was used for lack of contractions after maximum dosage or spontaneous rupture of membranes, or for arrested cervical dilation, > 2 hours after last misoprostol dose or dinoprostone removal. Continuous fetal heart rate and uterine activity monitoring was used. Uterine hyperstimulation was treated in some cases with terbutaline 250 mcg intravenously or subcutaneously. |
|
| Outcomes | Oxytocin augmentation; uterine tachysystole (> 5 contractions per 10 minutes); uterine hypertonus (contraction > 2 minutes); hyperstimulation syndrome (tachysystole or hypertonus with fetal heart rate abnormalities); abnormal fetal heart rate patterns (23/99 vs 35/98); induction to delivery interval (misoprostol mean 1429, SD 793 vs dinoprostone 1484, 866 minutes); mode of delivery; vaginal delivery within 24 hours; terbutaline used for hyperstimulation (3/99 vs 3/98); neonatal resuscitation (29/99 vs 25/98); days in NICU (mean 7.4, SD 5.1 vs 10.0, 13.4); meconium aspiration syndrome (1/99 vs 1/98); hyperbilirubinaemia (13/99 vs 7/98). Frequent uterine contractions occurred in 9 women after a single misoprostol dose, the onset occurring after mean 5.1 (SD 2.4) hours, and 11 after a second dose. |
|
| Notes | Los Angeles, California, USA. October 1 995 to June 1996. 200/206 women agreed to participate. No women withdrew from the protocol. 3/101 in the dinoprostone group were excluded from the data analysis because of deviation from the study protocol. In the abstract report all 200 women are included in the analysis, and the induction delivery times are somewhat different. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
043 Diro 1999.
| Methods | Double‐blind study. Quartered 100 or 200 mcg misoprostol tablets were supplied by the research pharmacy according to random number tables. | |
| Participants | Women undergoing induction of labour for maternal or fetal reasons. Inclusion criteria: cervical score < 5. Exclusion criteria: cardiopulmonary disease; HIV infection; malpresentation; multiple gestation; placenta praevia; previous uterine incision; 2 or more regular contractions in 10 minutes; sickle cell disease. |
|
| Interventions | Misoprostol 25 versus 50 mcg to posterior vaginal fornix 3‐hourly till the onset of labour (maximum 8 doses). Membranes ruptured as soon as safe and feasible. Women not in labour after 24 hours received PGE2 gel intravaginally or oxytocin infusion. | |
| Outcomes | Induction to onset of active labour; induction to delivery (low dose 1194 SD 785 vs high dose 933 SD 555 minutes); duration of first and second stages of labour; oxytocin augmentation and total dose; tachysystole, hyperstimulation; mode of delivery; perinatal outcome. | |
| Notes | University of Miami/Jackson Memorial Centre, USA, September 1995 to April 1997. Of 284 women randomised, 33 withdrawn: 19 because of increased cervical score between randomisation and start of study; 12 randomised in violation of exclusion criteria; and 2 because of missing data (delivered vaginally). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
043 Farah 1997.
| Methods | Allocation by computer generated randomisation table. Pharmacy supplied one‐fourth of a 200 µg or 100 µg tablet, which appeared identical. | |
| Participants | Of 430 women enrolled, 29 excluded after randomisation and 2 after administration of misoprostol, all because enrolment criteria were not met.
Inclusion criteria: indication for induction of labour; cervical score < 5; singleton third trimester pregnancy; vertex presentation. Exclusion criteria: active labour; fetal distress; previous uterine surgery; contraindication to vaginal delivery; contraindication to use of prostaglandins. |
|
| Interventions | Misoprostol 25 µg or 50 µg into the posterior vaginal fornix 3 hourly until adequate labour achieved (3 contractions per 10 minutes). Maximum 8 applications. Continuous fetal heart rate monitoring. As soon as cervical dilation permitted, amniotomy performed and intrauterine pressure catheter and scalp electrode applied. Oxytocin used if not in labour after 8 doses or for active phase of labour arrest. Epidural analgesia or intravenous sedation offered. Hyperstimulation syndrome was managed with change in position, oxygen by face mask and in some cases subcutaneous terbutaline or vaginal lavage to remove the misoprostol tablet. | |
| Outcomes | Mode of delivery; delivery intervals (25 µg 970 +/‐ 684 vs 50 µg 826 +/‐ 554 minutes); occurrence of active labour (180/192 vs 191/207); vaginal delivery after one dose (48/192 vs 79/207); maternal and perinatal outcomes; cord pH < 7.16 (13/192 vs 27/207); blind assessment of cardiotocograph for tachysystole, hypertonus and hyperstimulation syndrome. | |
| Notes | University Medical Centre, Jacksonville and Shands Hospital, Gainesville, July 1994 to September 1995. Apparent transposition of results for delivery < 12 hours and delivery < 24 hours in table IV (see text page 366). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
044 Chen 2005.
| Methods | Randomised clinical trial with allocation by sealed sequential envelopes. | |
| Participants | Singleton pregnant women with gestational age from 36 to 41 weeks. | |
| Interventions | Vaginal misoprostol 25 mcg 3 hourly versus no intervention. | |
| Outcomes | Measure of urinary cGMP/creatinine during labour and duration of latent phase. | |
| Notes | This trial did not contribute any data to this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
044 Nanda 2007.
| Methods | "The patients were divided randomly by drawing lots and were balanced in blocks of 6" and "The investigators were blinded to the allocation", but the methods were not specified. | |
| Participants | Intervention group: women between 25 ‐ 35 years old, single fetus, cephalic presentation, intact membranes and Bishop score ≤ 6. Exclusion criteria: hypersensitivity to prostaglandin, previous caesarean section, asthma, epilepsy, grand multiparae (4 or more gestations), placenta previa, cephalopelvic disproportion and malpresentation. |
|
| Interventions | The intervention group received 25 mcg 3 hourly (maximum 200 mcg). The comparison group used 0.5 mg of dinoprostone intra cervically repeated 6 hourly until 3 doses in 24 hours. | |
| Outcomes | The use of misoprostol in pregnant woman for labour induction showed successes compared with dinoprostone. The labour induction with misoprostol showed less need for oxytocin augmentation. | |
| Notes | Departament of Obstetrics and Gynecology, Sharma Post Graduate Institute of Medical Sciences, India. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Inadequate |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear for outcome assessors, but blinded for participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
044 Wing 1995b.
| Methods | Allocation by sequentially numbered sealed opaque envelopes, maintained by the primary investigator. Sequence from random number table in blocks of 6. | |
| Participants | Membranes were intact, cervical status unfavourable. Inclusion criteria: women requiring induction of labour for medical or obstetric reasons; singleton pregnancy; cephalic presentation; intact membranes; cervical score < 5; reactive nonstress test; < 4 uterine contractions per hour. Exclusion criteria: estimated fetal weight > 4500 g; evidence of cephalopelvic disproportion; placenta praevia; unexplained vaginal bleeding; vasa praevia; active herpes simplex infection; contraindication to receiving prostaglandins; renal or hepatic dysfunction; suspected chorioamnionitis; previous uterine surgery; parity > 5. Indications for labour induction: oligohydramnios (misoprostol 53, dinoprostone 57); pre‐eclampsia (39, 25); post‐term pregnancy (19, 24); macrosomia (12, 15); diabetes mellitus (7, 6); abnormal antepartum testing (3, 3); other (5, 7). |
|
| Interventions | Misoprostol 25 mcg inserted into the posterior vaginal fornix 3‐hourly, maximum 8 doses or 24 hours (n = 138) versus dinoprostone gel 0.5 mg intracervically 6‐hourly, maximum 3 doses (n = 137), until 3 uterine contractions per 10 minutes, cervical score > 7, cervical dilation > 3 or spontaneous rupture of membranes. Artificial rupture of membranes usually performed when the cervix was 80% effaced and 3 cm dilated. Oxytocin augmentation was used for lack of contractions after maximum dosage or spontaneous rupture of membranes, or for arrested cervical dilation, > 3 hours after misoprostol and > 6 hours after dinoprostone. Uterine hyperstimulation was treated in some cases with tocolytic therapy. |
|
| Outcomes | Oxytocin augmentation; uterine tachysystole (> 5 contractions per 10 minutes); uterine hypertonus (contraction > 2 minutes) (misoprostol 0/138, dinoprostone 5/137); hyperstimulation syndrome (tachysystole or hypertonus with fetal heart rate abnormalities); induction to delivery interval; mode of delivery; vaginal delivery within 24 hours; terbutaline used for hyperstimulation (9/138, 6/137); neonatal resuscitation (44/138, 43/137); days in NICU (mean 7.9 (SD 6.1), 9.9 (7.5)); meconium aspiration syndrome (1/138, 3/137); hyperbilirubinaemia (10/138, 13/137). Frequent uterine contractions occurred in 9 women after a single misoprostol dose, the onset occurring after mean 5.1 (SD 2.4) hours, and 11 after a second dose. |
|
| Notes | Los Angeles, California, USA. February to June 1994. 276/287 women agreed to participate. No women withdrew from the protocol. One woman allocated to dinoprostone, inadvertently received misoprostol, and was excluded from the analysis. The authors postulate that the increased rate of meconium‐stained amniotic fluid may be due to the increased incidence of uterine tachysystole, or to a direct effect of misoprostol on the fetal gastrointestinal tract. The authors do not recommend this dosage for induction of labour. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
048 Khoury 2001.
| Methods | "Computer‐generated" assignment. Examiner blinded to the dose of misoprostol but not to whether misoprostol or dinoprostone was used. | |
| Participants | Inclusion criteria: indication for labour induction; singleton pregnancy; gestational age 37 weeks or more; vertex presentation; intact membranes; cervical score 4 or less; reassuring fetal heart rate pattern. Exclusion criteria: unexplained third trimester vaginal bleeding; placenta praevia; active herpes simplex infection; history of asthma or glaucoma; suspected cephalopelvic disproportion; parity > 5; uterine scar; estimated fetal weight > 4500 g or > 4000 g in a diabetic mother. |
|
| Interventions | Misoprostol 35 mcg versus 50 mcg suppositories in fatty base vaginally 4.5 hourly (maximum 6 doses) versus dinoprostone 10 mg vaginal insert, left for 22.5 hours. Insert removed or misoprostol stopped for active labour or cervical score > 7 or cervical dilatation > 2 or spontaneous rupture of membranes or uterine contraction abnormalities or abnormal fetal heart rate pattern. Syntocinon infusion commenced one hour after the last dose of misoprostol or removal of the insert, if not in labour. For misoprostol vs dinoprostone comparison, the two misoprostol regimens have been combined. | |
| Outcomes | Tachysystole (6 contractions per 10 minutes for 20 minutes); hypertonus (a contraction lasting 2 minutes); uterine hyperstimulation (tachysystole or hypertonus plus fetal heart rate abnormality). | |
| Notes | Inova Fairfax Hospital, VA, USA, June 1998 to June 1999. Cardiotocograph tracings were evaluated blind to the allocation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
050 Abdul 2007.
| Methods | Randomised clinical trial. Random sequence generated by computer. Allocation by numbered sealed opaque envelopes. | |
| Participants | Inclusion criteria: consecutive patients with medical indication to induction of labour. Exclusion criteria: patients with scared uterus and multiple gestation. |
|
| Interventions | The intervention group used 50 mcg of vaginal misoprostol 6 hourly during 24 hours. The comparison group used oxytocin alone. | |
| Outcomes | There were no differences between the groups about mean time to delivery, Apgar score and perinatal mortality rate. | |
| Notes | The study period was from November 2005 to October 2006. Labour Unit of Federal Medical Center of Azare, Nigeria. One patient of the misoprostol group had a ruptured uterus. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
050 Agarwal 2003.
| Methods | Assigned by "computerised random numbers". Not blinded. | |
| Participants | Inclusion criteria: singleton pregnancy; cephalic presentation; intact membranes; cervical score < 7; reactive fetal heart rate testing. Exclusion criteria: previous caesarean section; contraindication to prostaglandin; chorioamnionitis. | |
| Interventions | Misoprostol 50 mcg vaginally versus dinoprostone 0.5 mg intracervically; repeated after 6 hours if cervical score < 7, cervical dilation < 3 cm, and no adequate uterien contractions (3 or more for 40 seconds or more per 10 minutes); maximum 3 doses. Artificial rupture of membranes once cervix 3 or more cm dilated. Intravenous oxytocin if no adequate labour after 3 doses or rupture of membranes. | |
| Outcomes | Tachysystole (6 or more contractions per 10 minutes for 20 minutes); hypertonus (contraction for 2 minutes or more); hyperstimulation (tachysystole or hypertonus plus abnormal fetal heart rate pattern); change in cervical score after 6 hours (2.98 (SD 2.57) vs 2.05 (SD 1.83)); route of delivery; oxytocin augmentation; passage of meconium; fetal heart rate deceleration; single insertion (54/60 vs 52/60); time to onset of contractions (2.03 (SD 1.3) vs 2.04 (SD 1.0) hours); induction to delivery interval (12.89 (SD 6.5) vs 18.01 (SD 8.4) hours); delivery within 12 hours (52.5% vs 35%). | |
| Notes | June 2001 to February 2002, New Delhi. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
050 Ayad 2002.
| Methods | "There were 238 patients randomly assigned". The sequence generation and the allocation concealment were not stated. | |
| Participants | Inclusion criteria: women with gestational age > 36 weeks, singleton pregnancy, premature rupture of membranes < 24 hours, Bishop score < 8, vertex presentation and fewer contraction than 12 per hour. Exclusion criteria: prior caesarean delivery, parity > 5 and no reassuring fetal monitoring. |
|
| Interventions | The intervention group received 50 mcg vaginal misoprostol and the comparison group received 0.5 mg intra cervically dinoprostone. Both groups used the oxytocin if there was inadequate labour. | |
| Outcomes | Mean time from induction to delivery and the need of oxytocin were significantly fewer in the misoprostol group. There were no difference between the groups in spontaneous labour rate, type of delivery and perinatal outcome. | |
| Notes | The study was performed from February 1999 to February 2000. Prince Rashid Ibn Al‐Hassan Hospital, Ramtha, Jordan. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
050 Bounyasong 2000.
| Methods | "This prospective randomised study" has no stated sequence generation and allocation concealment. | |
| Participants | Inclusion criteria: singleton pregnancy, gestational age of 38 weeks, vertex presentation, intact membranes and no evidence of fetal distress. Exclusion criteria: abnormal lie, premature rupture of membranes, oligohydramnios, prior uterine scar, uterine contraction, obstetrical complication, contraindication to prostaglandins and severe medical diseases. |
|
| Interventions | The patients were randomised to receiving 50 mcg or 25 mcg of vaginal misoprostol 6 hourly each group. Amniotomy was performed when the cervical dilatation was achieved 3‐4cm. Oxytocin augmentation was used if indicated in both groups. | |
| Outcomes | There were no differences between the groups in caesarean section rate. The neonatal asphyxia and the uterine hyperstimulation were greater in the 50 mcg group (P = 0.15 and 0.0315). | |
| Notes | Department of Obstetrics and Gynaecology of Srisangwal Hospital, Thailand. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Inadequate |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
050 Calder 2008.
| Methods | "This randomized, open" with not stated allocation methods. | |
| Participants | Inclusion criteria: all women with singleton pregnancy, with 18 years or older, with at least 37 weeks of pregnancy and unfavourable cervix. Exclusion criteria: women with multiple pregnancy, insulin dependency for diabetes control, multiple pregnancy, PROM, ascending infection, systemic infection, placenta praevia, placental abruption, unexplained vaginal bleeding, active cardiac, pulmonary, renal or hepatic disease a contraindication to vaginal delivery or known allergy to prostaglandins. |
|
| Interventions | The intervention group received vaginal misoprostol 25 mcg (or 50 mcg in nulliparous women) followed by further administration of 25 mcg 4 hourly until a total of 3 doses. Comparioson group: vaginal dinoprostone 3 mg followed by a second dose of 3 mg (6 hours later). | |
| Outcomes | A total of 626 women were enrolled in this trial. The rate of vaginal deliveries achieved within 24 hours of induction did not significantly differ between the misoprostol and dinoprostone groups. Those treatments were comparable for other efficacy measures and for presence of maternal or fetal averse events. | |
| Notes | Eighteen NHS study centers were enrolled in this trial. Reprint of BJOG | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Inadequate |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
050 Campos Perez 1994.
| Methods | Open randomised study. Computer‐generated randomisation. | |
| Participants | Membranes were intact, cervical status variable. Pregnant women with single fetus. Bishop score < 5 in 35/78 misoprostol and 32/75 oxytocin. Pregnancy complications: hypertension, misoprostol 28 vs oxytocin 26; intrahepatic cholestasis 13 vs 16; impaired fetal growth 6 vs 5; prolonged pregnancy 25 vs 27; others 6 vs 4. | |
| Interventions | Misoprostol 50 mcg tablet intravaginally (n = 78) vs intravenous oxytocin 2‐32 mU/minute (n = 75). | |
| Outcomes | Delivery within 24 hours; induction to delivery interval; uterine hyperstimulation (> 5 contractions in 10 minutes); caesarean section; Apgar scores. Intrauterine pressures recorded in a subset of 10 women in each group: intensity of contractions as mean (standard deviation): misoprostol 48.7 (10.3) vs oxytocin 56.2 (14.5) mmHg; uterine tone 10.5 (3.6) vs 9.6 (4.8). Differences not statistically significant. |
|
| Notes | Valdivia, Chile. One woman excluded from the misoprostol group because of accidental removal of the misoprostol tablet during a vaginal examination 180 minutes after initiation of the induction. Authors conclude misoprostol safe for induction of labour. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
050 Charoenkul 2000.
| Methods | 'Randomised' drugs inserted by a resident not involved in the outcome assessment. Randomisation stratified by parity. | |
| Participants | Pregnant women with indications for labour induction; singleton pregnancy; 37 or more weeks of gestation; cervical score < 7. Exclusion criteria: suspected cephalo‐pelvic disproportion; estimated fetal weight > 4000 g; parity > 5; previous caesarean section and other uterine surgery; suspected chorioamnionitis; contraindications to vaginal delivery; contraindications to the use of prostaglandins; moderate to severe medical disease. | |
| Interventions | Misoprostol 50 mcg or dinoprostone 3 mg vaginally. | |
| Outcomes | Primary: change of cervical score at 24 hours. Secondary: uterine tachysystole (5 or more contractions per 10 minutes for 20 minutes); hypertonus (a contraction lasting 90 seconds); hyperstimulation syndrome (tachysystole or hypersystole and fetal heart rate changes); vaginal delivery in 24 hours; caesarean section; caesarean section for fetal distress (misoprostol 6/72 vs dinoprostone 3/71). | |
| Notes | Vajira Hospital, Bangkok, Thailand, November 1998 to December 1999. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
050 Denguezli 2007.
| Methods | Randomised controlled trial with a computer sequence generation and allocation concealment from a predetermined random table. | |
| Participants | Women with obstetrical indication for cervical ripening and induction of labour. Inclusion criteria: obstetrical indication, medical complication, absence of active labour or fetal distress, no previous caesarean section delivery or other type of uterine surgery, singleton pregnancy with vertex presentation and no contraindication to vaginal delivery. Exclusion criteria: less than 18 years, gestational age less than 36 weeks, PROM, history of dystocia or forceps delivery, history of more than one episode of surgical interruption of pregnancy. |
|
| Interventions | The intervention group received 50 mcg of misoprostol 6 hourly until Bishop favourable (maximum 200 mcg). The comparison group received intracervical prostaglandin 0.5 mg 6 hourly until induction of labour (maximum 2 mg). | |
| Outcomes | The proportion of vaginal delivery within 24 hours was significantly higher in the misoprostol group (75%) than in the dinoprostone group (53.8%) (RR = 1.40, 95% CI (1.07‐1.45), p = 0.02).There was no difference between the mean time interval of delivery in the misoprostol group and the dinoprostone group (14.9 vs. 15.8h) (p = 0.51). Secondary outcomes were evolution of Bishop score during the labour, rate of caesarean delivery, tachysystole, hyperstimulation syndrome, maternal side effects and neonatal outcomes. | |
| Notes | University Hospital Medical Centre The patients were included from 1 August 2003 to 30 April 2004. No blinding trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear for participants and outcome assessors and open for personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
050 El‐Azeem 1997.
| Methods | Double blind randomised trial. | |
| Participants | 29 women with indication for induction of labour. | |
| Interventions | Misoprostol 50 µg in an applicator plus placebo gel in a syringe (n = 15), versus 0.5 mg dinoprostone in a syringe plus placebo applicator (n = 14), repeated after 6 hours if necessary. Oxytocin started 6 hours after first or second dose as indicated. | |
| Outcomes | Mean change in cervical score after 6 hours (misoprostol 4.2 +/‐ 6.3 versus dinoprostone 2.3+/‐3.3, p < 0.05); induction to delivery interval (17.2+/‐10.1 versus 32.9+/‐18.6, p = 0.03); delivery with prostaglandin alone (46.2% versus 16.7%); oxytocin dosage and duration. No data in format suitable for review. | |
| Notes | 1994 to 1995. The percentages could not be converted to proportions (16.7% of 14 is 2.3). Full report of trial awaited for incorporation of data. Not clear whether vaginal or intracervical dinoprostone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
050 Elhassan 2004.
| Methods | "Prospective randomised controlled clinical trial". The sequence generation and allocation concealment were not stated. | |
| Participants | Inclusion criteria: women admitted to the labour ward with singleton pregnancy and unripe cervix. Exclusion criteria: previous uterine surgery, antepartum haemorrhage, glaucoma, asthma, heart disease and grand multiparity. |
|
| Interventions | The intervention group received 50 mcg of misoprostol 6 hourly until 200 mcg. The comparison group received vaginal prostaglandin 0.5 mg 6 hourly until 2 mg. | |
| Outcomes | The induction‐to‐delivery interval (mean ± S.D.) was 17.5 h ± 7.6h with misoprostol and 19.15 h ± 6.9 h with dinoprostone(p > 0.05). Secondary outcomes were route of delivery, need of oxytocin augmentation and neonatal outcomes. | |
| Notes | This study was conducted at the labour ward of Wad Medani Hospital at Sudan, from March to December 2001. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unblinded for personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
050 Elhassan 2005b.
| Methods | "An open randomised controlled clinical trial". The sequence generation and allocation concealment were not stated. | |
| Participants | Included criteria: pregnant women with single babies, favourable cervix and intact membranes. Exclusion criteria: previous uterine surgery, antepartum haemorrhage, asthma, heart disease and grand multiparity. |
|
| Interventions | The intervention group received 50 mcg of misoprostol 6 hourly until 200 mcg. The comparison group received oxytocin alone (they started with 2 mcU/min, which was doubled at 30‐min interval until labour). | |
| Outcomes | The induction‐delivery interval was significantly shorter in misoprostol group ( 8.2 h ± 1.1 h vs 12.04 h ± 1.5 h) vs oxytocin group. The secondary outcomes were rate of instrumental vaginal delivery, caesarean section and neonatal outcomes. | |
| Notes | This study was conducted at the labour ward of Wad Medani Hospital at Sudan, from August 2004 to February 2005. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Unclear risk | Unclear |
050 Frohn 2002.
| Methods | Allocation by computer‐generated random number sequence in sealed opaque envelopes, in balanced blocks of 50. | |
| Participants | Inclusion criteria: ruptured membranes; gestation 34 weeks or more; singleton gestation; reassuring fetal heart rate pattern; cephalic presentation; not in labour; cervical dilation < 3 cm; effacement no more than 80%; no contraindication to labour and vaginal delivery. Exclusion criteria: estimated fetal weight > 4500 g; intrauterine infection; contraindication to use of prostaglandins. Women with one previous lower transverse caesarean section were not excluded. | |
| Interventions | Misoprostol 50 mcg vaginally, repeated after 6 hours if not in labour, versus dinoprostone gel 2.5 mg vaginally 6‐hourly. If not in labour 12 hours after the first dose, oxytocin infusion started. Continuous cardiotocography was used. | |
| Outcomes | Tachysystole (6 or more contractions per 10 minutes for 20 minutes); hyperstimulation (tachysystole and FHR changes); chorioamnionitis; postpartum endometritis; neonatal sepsis. | |
| Notes | Arnold Palmer Hospital for Women and Children, Florida, USA. January 1995 to December 2000. Not blinded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
050 Gelisen 2005.
| Methods | Randomised controlled trial. The investigators were not involved at the clinical care of the patients and they carried out the randomisation by opening sealed opaque envelope. | |
| Participants | Inclusion criteria: singleton live pregnancy with vertex presentation and intact membranes, gestational age plus than 41 completed weeks, Bishop score < 5, absence of spontaneous uterine contractions, estimated fetal body weight < 4500g, reactive non‐stress test and amniotic fluid index ≥ 5cm. Exclusion criteria: known hypersensitivity to the use of prostaglandins, previous caesarean delivery or other uterine surgery, non‐cephalic presentation, body mass index ≥ 30 before conception, any previous attempt at induction of labour during the current pregnancy and low‐lying placenta. |
|
| Interventions | The intervention group received 50 mcg of misoprostol 6 hourly until 24 hours of labour. The first comparison group received oxytocin alone (the initial doses started with 1 mU/min, increased by 1mU/min every 15 minutes until contractions of 200 ‐ 250 Montevideo units were achieved.) The second comparison group received a Foley catheter balloon inserted to above the cervical OS (inflated with 50 ml of sterile saline). After the expulsion of the catheter the oxytocin was started with the same regime described above. The third comparison group was the spontaneous follow up until 42 weeks of pregnancy. If the women complete 42 weeks, the IOL started with 50 mcg of vaginal misoprostol 6 hourly until 24 h. If the delivery was not achieved, the caesarean section was performed. |
|
| Outcomes | The abdominal delivery rate was 19.3% in the intervention, first and second comparison groups, and 22% in the spontaneous follow up. The meconium‐stained amniotic fluid and meconium aspiration were significantly higher in the follow‐up group. Rates of emergency abdominal delivery, NICU admission and low umbilical artery pH were similar at the groups. | |
| Notes | The trial was performed in a tertiary training centre in Turkey. SSK Ankara Maternity and Women's Health Teaching Hospital, Kocaeli, Turkey. For the analyses of data at this review the mechanical methods to IOL were not included. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unclear for participants, inadequate for personnel and blinded for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
050 Gotschall 1998.
| Methods | Medications prepared by hospital pharmacy in a randomised, double‐blind fashion. | |
| Participants | Women presenting for induction of labour. | |
| Interventions | Misoprostol 50 vs 100 mcg intravaginally as a single dose. If not in labour after 6 hours, oxytocin was commenced. | |
| Outcomes | Primary: induction to delivery time. Secondary: need for oxytocin; delivery mode; maternal symptoms; uterine hyperstimulation. | |
| Notes | Three women excluded for failed induction. Not indicated which group(s) they belonged to. Data not included, pending further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
050 Kovavisarach 1997.
| Methods | "All patients were randomized". The sequence generation and allocation concealment were not stated. | |
| Participants | Inclusion criteria: women with gestational age ≥ 37 weeks, indication for induction of labour, Bishop score < 7 and no contraindication to use prostaglandins. No exclusion criteria. |
|
| Interventions | The intervention group used 100 mcg 12/12 hours (maximum 200 mcg). The intervention group used vaginal dinoprostone 3 mg two times. | |
| Outcomes | Bishop score and tachysystole were significantly higher in the misoprostol group. Induction to delivery interval was significantly lower in misoprostol group (p < 0.05). Mode of delivery, Apgar score, hyperstimulation, neonatal and maternal complications were not different in the group. | |
| Notes | The study enrolled patients from March 15 to September 15, 1995. Department of Obstetrics and Gynaecology, Rajavith Hospital, Ministry of Public Health, Bangkok, Thailand. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unblinded for personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
050 Kovavisarach 1998.
| Methods | "The woman were randomized in two groups". The sequence generation and the allocation concealment were not stated. | |
| Participants | The pregnant women were included if the gestational age was higher than 37 weeks, Bishop score < 7 and no contraindication to use prostaglandin. | |
| Interventions | The intervention group used 50 mcg vaginal misoprostol 6 hourly (maximum 150 mcg). The comparison group used 3 mg vaginal dinoprostone 6 hourly (3 times) and both groups could receive oxytocin augmentation. | |
| Outcomes | Misoprostol group presented significantly higher tachysystole (p < 0.05) and lower induction to delivery interval, induction to oxytocin stimulation interval and oxytocin stimulation to delivery interval. There were no difference of mode of delivery, Apgar score, hyperstimulation syndrome, neonatal and maternal complications. | |
| Notes | The study was enrolled from December 1, 1996 to August 30,1996. Department of Obstetrics and Gynaecology, Rajavith Hospital, Ministry of Public Health, Bangkok, Thailand. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Paucity of information mainly in the neonatal side |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
050 Le Roux 2002.
| Methods | "Computer randomisation" to vaginal misoprostol, oral misoprostol or dinoprostone in ration 1:1:2. Stratified by delivery site. Allocations sealed in opaque envelopes opened by labour ward staff. Cervical assessment and cardiotocography before enrolment. | |
| Participants | Women having labour induced with singleton cephalic fetus; 34 weeks or more gestation; intact membranes; no fetal distress on cardiotocography; no painful contractions; age 18 years or more. Exclusion criteria: previous caesarean section; parity > 4; fetal anomaly; fetal death; cervical score > 7 where amniotomy without prostaglandin preparation was possible. |
|
| Interventions | Vaginal misoprostol 50 mcg (maximum 4 doses) versus oral misoprostol 50 mcg (maximum 4 doses) versus dinoprostone gel 1 mg vaginally (maximum 2 doses), 6‐hourly. Artificial rupture of membranes and incremental oxytocin infusion were used for poor progress in established labour, not for labour induction. Continuous cardiotocography was used. If tachysystole occurred, the next dose was delayed until the tachysystole had resolved. For this review only vaginal misoprostol vs dinoprostone compared. | |
| Outcomes | Primary: vaginal delivery within 24 hours; secondary: mode of delivery; indication for caesarean section; caesarean section for fetal distress (vaginal misoprostol 33/120 vs dinoprostone 33/240); placental abruption (vaginal misoprostol 4/120 vs dinoprostone 6/240); fetal complications (thick meconium‐stained liquor; 5 minute Apgar score < 7; admission to neonatal intensive care unit; hypoxic ischaemic encephalopathy); tachysystole (5 or more uterine contractions in 10 minutes, in two 10‐minute windows; fetal distress (a fetal heart rate tracing that justified immediate delivery). | |
| Notes | One tertiary and one secondary academic hospital in Cape Town, South Africa. 93/573 enrolled women were excluded for clerical errors (65), exclusion criteria ignored (18), patient withdrew (1), incorrect dosage (5), underage (2). No blinding of clinicians or of the cardiotocographic evaluation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
050 Lokugamage 2003a.
| Methods | Allocation by opaque numbered sealed envelopes in computer‐generated sequence. Not blinded. | |
| Participants | Inclusion criteria: multiparous women; > 36 weeks' gestation. Exclusion criteria: previous caesarean section; uterine surgery; parity > 5; abnormal FHR pattern; malpresentation; estimated fetal weight > 4500 g; other evidence of cephalopelvic disproportion; placenta praevia; contraindication to prostaglandin therapy. | |
| Interventions | Vaginal misoprostol 50 mcg single dose versus two doses, the second after 6 hours if no uterine activity and amniotomy not possible. After 24 hours, dinoprostone used if needed. Oxytocin used after rupture of membranes if needed. | |
| Outcomes | Induction to delivery time (789 (SD 539) vs 576 (SD 331) minutes); delivery within 12 and 24 hours; oxytocin augmentation; tachysystole (6 or more contractions in 10 minutes); hyperstimulation (abnormal FHR pattern related to excessive uterine activity); mode of delivery; failed induction of labour; Apgar score; umbilical venous blood pH (7.33 (7.12‐7.49) vs 7.29 (7.11‐7.49)); admission to NICU. | |
| Notes | University College Hospital, London, UK, January 1998 to December 1999. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
050 Lokugamage 2003b.
| Methods | Randomised unblinded trial with allocation concealment in opaque sealed numbered, opened consecutively. | |
| Participants | Inclusion criteria: nulliparous women at term (37 to 42 weeks of gestation). Exclusion criteria: previous uterine surgery, abnormal FHR, malpresentation, estimated fetal body weight > 4.500 g, or other evidence of cephalopelvic disproportion, placenta previa and any contraindication to prostaglandin therapy. |
|
| Interventions | The intervention group received 50 mcg of misoprostol and then if it was required another dose of 50 mcg after 6 hours. The comparison group received 2 mg of dinoprostone vaginal, amniotomy and additional dose of 1 mg before 24 hours. If there was no success within 24 hours the regimen was repeated for a second day. | |
| Outcomes | The induction to delivery interval (1047 min vs1355 min, p = 0.01), delivery within 12 hours (35.4% vs 18.9%, p = 0.02) and delivery within 24 hours (83.3% vs 63.3%, p = 0.82) were all shorter in the misoprostol arm. There was no differences in rates of oxytocin augmentation, tachysystole and hyperstimulation syndrome. There was no difference in neonatal outcomes. | |
| Notes | The study run from January 1998 to December 1999. Royal Free & University College London Medical School, London, UK. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
050 Majoko 2002a.
| Methods | Single‐blinded randomised controlled trial. Researcher knew group allocation. Allocated by cards in numbered sealed opaque cards, randomised by random number tables. | |
| Participants | Inclusion criteria: women with obstetric indication for induction of labour; singleton live fetus; cephalic presentation; 37 weeks' gestation or more. Exclusion criteria: previous uterine surgery; vaginal delivery contraindicated. | |
| Interventions | Half versus quarter 200 µg misoprostol tablet inserted into posterior vaginal fornix. Repeated after 8 hours if cervical score < 10 (maximum 2 doses). | |
| Outcomes | Induction to delivery interval; mode of delivery; augmentation with oxytocin; postpartum haemorrhage; uterine trauma; fetal outcomes. | |
| Notes | Harare Maternity Hospital, Zimbabwe, June to September 1998. Terminated early because of complications in the 100 µg group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
050 Mosquera 1999.
| Methods | Allocation by computer‐generated sequence using sealed, opaque, numbered envelopes. | |
| Participants | Inclusion criteria: gestation 42 or more weeks; single fetus, alive, cephalic. Exclusion criteria: uterine contractions; ruptured membranes; placenta praevia, placental abruption, breech presentation, dystocia; previous uterine surgery; fetal anomaly; multiple pregnancy. |
|
| Interventions | Misoprostol 50 microgram doses dissolved in 0.5 ml to 1 ml saline intravaginally, versus oxytocin intravenous infusion. | |
| Outcomes | Induction to delivery (misoprostol 15.4 +/‐ 1.5, oxytocin 18.6 +/‐ 2.0). | |
| Notes | University Hospital Del Valle, Cali, Colombia, 2 April 1993 to 2 April 1996. Four from the misoprostol group were withdrawn. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
050 Neiger 2001.
| Methods | "Randomly assigned". | |
| Participants | Inclusion criteria: indication for labour induction; cervical score < 5; singleton pregnancy; cephalic presentation; term; intact membranes; reactive FHR pattern. Exclusion criteria: contraindication to vaginal delivery; previous uterine surgery; labour; vaginal bleeding; asthma; hypersensitivity to prostaglandins. |
|
| Interventions | Misoprostol 50 mcg vaginally versus dinoprostone 0.5 mg intracervically, maximum 2 doses 6 hours apart; continuous cardiotocography; oxytocin infusion when cervical score > 8 or at 2 hours, if not in labour; amniotomy when cervix 3‐4 cm dilated. | |
| Outcomes | Number of doses; change in cervical score; induction interval; oxytocin use; caesarean section; uterine hyperstimulation (tachysystole or hypertonus with abnormal FHR pattern). | |
| Notes | University of Tennessee Medical Center, August 1995 to December 1996. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
050 Ortiz 2002.
| Methods | Randomised trial with sequence generation and allocation not stated. | |
| Participants | Inclusion criteria: gestation at term, singleton pregnancy, cephalic presentation, rupture of membranes and Bishop score ≤ 4. Exclusion criteria: fetal distress, estimated fetal weight > 4 kg, placenta previa, prolapsed cord, fever and diarrhoea. |
|
| Interventions | The patients were divided in three groups: I. patients received 50 mcg vaginal misoprostol associated with oxytocin EV until labour; II. patients received intravaginal placebo associated with oxytocin; III. patients received 50 mcg vaginal misoprostol associated with placebo EV until labour. |
|
| Outcomes | The frequency of tachysystole, mode of delivery and perinatal outcomes were similar among the three groups. The mean time from induction to delivery was different in the three groups: Group I: 48.75 minutes, Group II: 537.05, Group III: 474.54 (p < 0.05). | |
| Notes | The analysis data will include groups I and II considering oxytocin as a co‐intervention. Hospital Civil de Culiacán, Universidade Autónoma de Sinaloa. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear for outcome assessors and blinded for participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
050 Pandis 2001.
| Methods | Computer‐generated random sequence in balanced blocks of 10. Allocation by contacting the lead researcher. | |
| Participants | Inclusion criteria: age > 16 years; singleton pregnancy; gestational age 37‐42 weeks; live fetus; cephalic presentation; intact membranes. Exclusion criteria: history of antepartum haemorrhage; previous uterine surgery; allergy to prostaglandins. |
|
| Interventions | Ultrasound and clinical vaginal assessment before induction. Misoprostol 50 mcg vaginally versus dinoprostone (nulliparae with cervical score < 5: 2 mg; score 5‐6 and all multipara 1 mg). In both groups: dose repeated after 6 hours, and process repeated after 24 hours if necessary; score 7 or more had artificial rupture of membranes as well; cardiotocography after the medication and throughout labour; oxytocin augmentation if needed 6 or more hours after last prostaglandin dose. | |
| Outcomes | Primary: vaginal delivery in 24 hours. Secondary: hyperstimulation syndrome (tachysystole, > 5 contractions per 10 minutes for 20 minutes or hypersystole, a contraction > 2 minutes with FHR abnormalities); caesarean section; adverse maternal and neonatal outcome. | |
| Notes | September 2000 to September 2001. Universitats‐Frauenklinik, Kantonsspital, Basel, Switzerland; King George, Harold Wood and Southend Hospitals, Essex, UK. 235/67 excluded after randomisation, mainly for spontaneous delivery before induction or induction by amniotomy for cervical score 7 or more. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
050 Ramsey 1998.
| Methods | Described as a "prospective randomized blinded phase III clinical trial". | |
| Participants | No reference to gestational age. | |
| Interventions | Vaginal misoprostol 50 mcg 6 hourly 2 times versus vaginal Prepidil® or Cervidil® 10mg one dose. | |
| Outcomes | Analisys of costs for induction of labour. | |
| Notes | This study did not contribute with any data to this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Adequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
050 Ramsey 2003.
| Methods | Prospective randomised blinded phase III clinical trial. Randomisation by pharmacy using dynamic allocation with stratification by parity and initial cervical score (0‐2 vs > 2). | |
| Participants | Inclusion criteria: indication for labour induction; cervical score < 6; singleton vertex pregnancy; no contraindication to vaginal delivery; < 4 uterine contractions per hour; reactive cardiotocography. Exclusion criteria: hypersensitivity to prostaglandins; ruptured membranes; suspected chorioamnionitis; parity > 5; previous caesarean delivery or uterine surgery; previous attempted labour induction for this pregnancy. | |
| Interventions | 1. Misoprostol 50 µg intravaginally 6‐hourly for 2 doses, vs 2. dinoprostone pessary 10 mg intravaginally vs 3. dinoprostone gel 0.5 mg intracervically 6‐hourly for 2 doses. Second doses omitted if > 3 uterine contractions in 10 minutes, fetal distress or ruptured membranes. After 12 hours the cervix was reassessed and oxytocin commenced if necessary. Continuous cardiotocography was used. If no cervical change in 24 hours, treatment repeated. | |
| Outcomes | Mean change in cervical score over 12 hours (misoprostol 5.2 +/‐ 3.1 vs dinoprostone pessary 3.2 +/‐ 2.3 vs dinoprostone gel 2.2 +/‐ 1.3); cervical score > 5 after 12 hours (30/38 vs 23/38 vs 14/35); time to vaginal delivery (23.9 +/‐ 11.1 vs 31.5 +/‐ 13.5 vs 31.1 +/‐ 14.2 hours); mean cost ($2.37 +/‐ 0.65 vs $168.23 +/‐ 0 vs 203.43 +/‐ 21.84); complete cervical dilation within 24 hours (26/38 vs 19/38 vs 18/35); time to delivery (24.0 +/‐ 10.8 vs 32.2 +/‐ 14.7 vs 31.6 +/‐ 13.4 hours); delivery within 48 hours (37/38 vs 28/38 vs 26/35); additional cervical ripening (4/38 vs 8/38 vs 10/35); caesarean section; obstetric outcomes; neonatal outcomes. | |
| Notes | Mayo Medical Centre, University of Alabama at Birmingham, April 1996 to August 1997. Two earlier abstracts Ramsay 1998 and 2001) assumed to be reports of the same study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
050 Ramsey 2005.
| Methods | "Randomized clinical trial" with method of allocation and sequence generation not stated. | |
| Participants | Inclusion criteria: Bishop score ≤ 5, singleton pregnancy with vertex presentation and no contraindication to vaginal delivery, absence of spontaneous contractions and reactive non‐stress test. Exclusion criteria: known hypersensitivity to prostaglandins, ruptures of membranes, suspected chorioamnionitis, parity more than five, previous caesarean delivery or uterine surgery and previous attempted induction of labour for this pregnancy. |
|
| Interventions | The intervention group received 50 mcg of vaginal misoprostol repeated dosing 6 hours later and there are 2 comparison groups. The first comparison group received 0.5 mg of dinoprostone intra cervically repeated 6 hours late and the second one received 10 mg of dinoprostone intravaginally repeated 12 hours late. | |
| Outcomes | The 55% of the misoprostol treated women demonstrated abnormal tracing cardiotocography event during first 24 hours of induction compared with 21.1% and 31.4% respectively the comparison group. | |
| Notes | Data collected at Mayo Medical Center from April 1996 to August 1997. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Adequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
050 Rozenberg 2001.
| Methods | Computer‐generated randomisation in balanced blocks of 4‐16, using sealed opaque envelopes. Midwife who administered induction agent asked not to divulge allocation to woman or other staff. | |
| Participants | Inclusion criteria: singleton; cephalic; cervical score > 6; < 4 uterine contractions per hour. Exclusion criteria: age < 18 years; fetal distress; feto‐pelvic disproportion; placental praevia; previous caesarean section; allergy to prostaglandins. | |
| Interventions | Misoprostol 50 mcg versus dinoprostone gel 2 mg, vaginally and repeated after 6 hours if not in labour; if not in labour after 24 hours, misoprostol or dinoprostone (2 mg, 1 mg, 1 mg) repeated 4‐hourly for 3 doses; amniotomy after 2‐3 cm cervical dilation; oxytocin augmentation for poor progress of labour. If not in labour after 48 hours, amniotomy and syntocinon if cervical score > 6, or caesarean section. | |
| Outcomes | Primary: vaginal delivery within 24 hours. Secondary: vaginal delivery within 12 hours; time from randomisation to delivery; cervical score after 12 hours; caesarean section; caesarean section for fetal distress (misoprostol 24/184 vs dinoprostone 13/185); forceps delivery; thick meconium‐stained liquor; fetal distress; hypertonus (a contraction of 2 minutes); hyperstimulation (tachysystole or hypertonus with FHR abnormality); uterine tachysystole (> 5 contractions/10 minutes for 20 minutes); Apgar scores; arterial cord pH; meconium aspiration (3/184 vs 3/185); NICU admission; poor neonatal outcome (6/184 vs 9/185); vaginal pain (3/184 vs 34/185); maternal complications; cost (Sterling 2134, SD 574 vs 2202, SD 595). | |
| Notes | Poissy Hospital, France, July 1997 to April 1999. One withdrawal after enrolment in the misoprostol group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not used |
050 Rozenberg 2004.
| Methods | Randomised clinical trial. Sequence generation by computer with list permuted blocks of 4 blinded to the investigators and allocation by sealed opaque envelopes. | |
| Participants | Inclusion criteria: singleton pregnancy, cephalic presentation, Bishop score < 5 and < 4 spontaneous uterine contraction per hour. Exclusion criteria: age < 18 years old, fetal distress before induction, fetal pelvic disproportion, placenta previa, premature rupture of membranes, breech or transverse lie, previous caesarean delivery and known allergy to prostaglandin. |
|
| Interventions | The intervention group received 50 mcg of vaginally misoprostol 6 hourly until 250 mcg in 24 hours. The comparison group received 10 mg of vaginal dinoprostone repeated 12 hours later or until 48 hours after the primary dose. | |
| Outcomes | Neonatal tolerance was similar in the 2 groups, with no difference in the caesarean delivery rate for fetal distress or in the incidence of meconium‐stained amniotic fluid. Time to vaginal delivery was shortened by misoprostol. | |
| Notes | Data collected from February 2000 and February 2001. Poissy Hospital, University Versailles‐St Quentin, France. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinded for participants, unblinded for personnel and unclear for the outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | High risk | Inadequate |
| Other bias | Low risk | Adequate |
050 Sahu 2004.
| Methods | "The women were randomly allocated". The sequence generation and the allocation concealment were not stated. | |
| Participants | Inclusion criteria: singleton pregnancy, vertex presentation, Bishop score < 5 and intact membranes. Exclusion criteria: multiple pregnancy, parity > 4, breech presentation, previous uterine scar, hypersensibility to prostaglandins, probable cephalopelvic disproportion, vaginal bleeding in second pattern and vaginal or cervical infection. |
|
| Interventions | The intervention group received 50 mcg vaginal misoprostol repeated if no cervical ripening after 6 hours. The comparison group received 0.5 mg intracervical dinoprostone repeated after 12 hours if necessary. Both groups could use oxytocin augmentation. | |
| Outcomes | There were no differences between the groups in time interval from induction to delivery, in the incidence of tachysystole, hypersystole and caesarean section rate. The incidence of delivery before 12 hours was higher in the misoprostol group (p < 0.02). | |
| Notes | The study was carried from January to April 2002. Rajah Muthaih Medical College and Hospital, Annamalai University, Tamil Nadu. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinded only for personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Unclear risk | Unclear |
050 Sifakis 2007.
| Methods | "A sequence from a computerized random number generator was used for the allocation of patients to each group." | |
| Participants | Inclusion criteria: singleton pregnancy with vertex presentation, intact membranes, Bishop score ≤ 4, reactive non‐stress test and absence of labour. Exclusion criteria: cephalopelvic disproportion, suspected fetal distress, renal or hepatic dysfunction, contraindication to prostaglandin administration, previous caesarean delivery or uterine surgery and parity greater than 5. |
|
| Interventions | The intervention group received 50 mcg of vaginal misoprostol 6 hourly at the maximum of 3 doses. The comparison group received 3 mg of dinoprostone intravaginally at the maximum of 3 doses. |
|
| Outcomes | The mean interval from labour induction to delivery and the average use of oxytocin was smaller in the misoprostol group. | |
| Notes | Department of Obstetrics and Gynecology, University of Crete, Greece. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | High risk | Inadequate |
050 Surbek 1997.
| Methods | Random number‐generated table used by hospital pharmacy to allocate identical‐looking vaginal suppositories with quick‐release, white gelatin capsules filled with the commercially available drugs. The code was not broken till study closure. | |
| Participants | Of 103 enrolled, 3 excluded for protocol violations. Inclusion criteria: viable term pregnancy; induction of labor required; willing to participate; cervical score < 6; reactive cardiotocography; singleton vertex presentation; labour absent. Misoprostol group had significantly lower cervical scores. Exclusion criteria: malpresentation; previous uterine surgery; contraindication to receive prostaglandins. |
|
| Interventions | Misoprostol 50 µg vs prostaglandin E2 3 mg into the posterior vaginal fornix, repeated if not in labour after 6, 24 and 30 hours; if not in labour after 48 hours, intravenous oxytocin was given; the cervix was assessed before each administration; oxytocin augmentation (1.25 to 20 mU per minute) was used at the discretion of the attending midwives and residents; preferably late artificial rupture of membranes was performed. External cardiotocographyc monitoring was used. Analgesia included spasmolytic and epidural analgesia. | |
| Outcomes | Primary: delivery within 24 hours. Secondary: intrapartum complications, maternal side effects (vomiting 2/50 vs 3/50, diarrhoea 1/50 vs 1/50, fever 2/50 vs 4/50), fetal outcome. Tachysystole (6 or more contractions per 10 minutes); hyperstimulation syndrome (tachysystole plus FHR abnormalities), both assessed blind; uterine contraction pain in the latent or early active phase of the first stage of labour, assessed 12 hours after delivery using a pain analogue scale (7.4 (3‐10) vs 7.7 (1‐10); FHR anomalies (16/50 vs 17/50); epidural analgesia (22/50 vs 25/50); spasmolytic therapy (27/50 vs 30/50). | |
| Notes | Basel University Hospital, January to November 1995. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
050 Thomas 2000.
| Methods | "Randomized", method not stated. | |
| Participants | Women with term pre‐labour spontaneous rupture of membranes; reassuring fetal status; vertex presentation. | |
| Interventions | Intravaginal misoprostol 50 mcg vaginally, repeated if necessary after 6 hours, versus placebo. Oxytocin used if not in labour after 12 hours. | |
| Outcomes | Induction to delivery interval (misoprostol 15.5 SD 7.3 vs placebo 19.0 SD 6.7 hours); oxytocin; oxytocin dose (7.6 SD 7.9 vs 11.6 SD 9.5 mU/minute); caesarean section; chorioamnionitis (2/27 vs 5/25); fetal meconium passage. | |
| Notes | Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
050G Carlan 1997.
| Methods | Opaque sealed envelopes in computer‐generated random sequence. | |
| Participants | Inclusion criteria:
singleton pregnancy; cervical score < 7; estimated fetal weight < 4500 g; live fetus; gestational age > 26 weeks. Exclusion criteria: vaginal bleeding; non‐reassuring FHR pattern; breech presentation; uterine contractions 4 or more per 20 minutes; contraindication to vaginal birth. Women with previous caesarean section or ruptured membranes were not excluded. |
|
| Interventions | Misoprostol gel (50 mcg mixed with 1 ml saline and 4 ml hydroxy ethylcellulose gel) vs tablets (moistened with 4‐8 drops of saline); inserted vaginally 8‐hourly in dosages of 50 mcg x 2 then 100 mcg, until labour, cervical score 7 or more, intervention required or 6 doses given. | |
| Outcomes | Hyperstimulation; time from administration to labour or start of induction (gel 18.2 SD 16.6 vs tablet 13.8 SD 11.4); and delivery (29.0 SD 19 vs 22.4 SD 15); caesarean section and oxytocin use. | |
| Notes | Arnold Palmer Hospital for women and children, Tampa, Florida, USA. August 1 1995 to February 1 1997. The physicians were not blinded to the treatment allocation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
058 Ferguson 2002.
| Methods | Double‐masked, allocation by random number tables. | |
| Participants | Inclusion criteria: membranes intact, modified cervical score < 5, singleton vertex presentation. Exclusion criteria: > 5 contractions per hour; active cardiovascular disease; vaginal bleeding; glaucoma; asthma; hypersensitivity to prostaglandins or beta‐adrenergic agents; abnormal FHR pattern; any other contraindication to vaginal delivery; (after 1998): previous caesarean section. | |
| Interventions | Vaginal misoprostol 50 mcg stat then 25 mcg 4‐hourly, versus syntocinon infusion 1 mU/minute, increased hourly ‐2 ‐4 or reduced if > 3 contractions/10 minutes; continuous electronic FHR monitoring; induction of labour after 16 hours, or sooner if modified cervical score > 6 or spontaneous rupture of membranes; early amniotomy and intrauterine pressure catheter monitoring. | |
| Outcomes | Uterine tachysystole (> 5 contractions in 10 minutes for 20 minutes); hypertonia (contraction lasting > 2 minutes); hyperstimulation (above plus abnormal FHR pattern, managed with lateral position, oxygen and terbutaline. | |
| Notes | Recruitment 1996 to 2000. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
063 Varaklis 1995.
| Methods | Open, randomised trial. Allocation by numbered, sealed opaque envelopes, in sequence from random number tables. | |
| Participants | Membranes were intact, cervical status unfavourable. Women at term with a medical indication for induction of labour. Exclusion criteria: severe oligohydramnios (< 2 cm pocket); non‐reactive non‐stress test; prior uterine surgery; malpresentation; multiple pregnancy; 3 or more contractions in 10 minutes; cervical score > 5. |
|
| Interventions | Misoprostol 25 mcg 2‐hourly administered vaginally, maximum 6 doses (n = 36), versus prostaglandin E2 0.5 mg intracervically 6‐hourly, maximum 2 doses (n = 32); administration stopped when uterine contractions reached 3 in 10 minutes; membranes ruptured when cervix 3 cm dilated, and scalp electrode and intrauterine pressure catheter placed; oxytocin infusion commenced 12 hours after the first dose if not in progressive labour. | |
| Outcomes | Frequent uterine contractions (> 5 per 10 minutes over 20 minutes); hyperstimulation (frequent contractions or prolonged contraction > 2 minutes, with fetal tachycardia, late decelerations or loss of short‐term variability); times to labour onset, rupture of membranes, full cervical dilation and delivery; use of oxytocin; mode of delivery; neonatal outcome. | |
| Notes | Maine, USA. After enrolment, 11/80 (14%) excluded because of protocol violations (5), receiving prostaglandin E2 vaginal gel rather than the trial preparation (2), prematurity (1), HELLP syndrome (1), spontaneous labour (2). The 2 cases of uterine hyperstimulation occurred after the 2nd and 3rd misoprostol doses, and the one of frequent contractions after the 3rd dose. The authors recommend further study of the dosing interval. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
075 Buser 1997.
| Methods | Allocation by numbered opaque sealed envelopes. Sequence taken from random number table in blocks of 6. | |
| Participants | Membranes were intact, cervical status unfavourable. Inclusion criteria: women requiring induction of labour; singleton pregnancy at term, cephalic presentation; reassuring FHR tracing; cervical score < 6. Indications for labour induction: post‐dates pregnancy (misoprostol 31, dinoprostone 23); pre‐eclampsia (21, 23); decreased amniotic fluid (9, 6); macrosomia (3, 12); gestational diabetes (2, 2); fetal growth restriction (3, 2); other (6, 11). Exclusion criteria: ruptured membranes; low‐lying placenta; previous caesarean section; parity > 5; suspected feto pelvic disproportion; history of asthma, glaucoma or cardiac disease. |
|
| Interventions | Misoprostol 50 mcg placed in the posterior vaginal fornix 4‐hourly (n = 76), versus dinoprostone gel 0.5 mg intracervically 6‐hourly (n = 79), unless adequate uterine contractions (3 per 10 minutes lasting > 40 seconds), cervix 3 cm dilated and 100% effaced, or spontaneous rupture of membranes, maximum 3 doses. Oxytocin was used when necessary for augmentation of labour, commencing at least 4 hours after misoprostol or 6 hours after dinoprostone. If the cervix was unchanged and no adequate uterine contractions occurred after 3 doses, the women were offered the option of returning another day for attempted labour induction. For the purposes of analysis, they remained in the group originally allocated. Artificial rupture of membranes was generally performed when the cervix was 3‐4 cm dilated and at least 80% effaced. A fetal scalp electrode and intrauterine pressure transducer were placed at the discretion of the attending physician. |
|
| Outcomes | Cervical ripening (score improved by > 3) (misoprostol 38/76, dinoprostone 21/79); active labour; caesarean delivery; induction‐delivery interval; oxytocin augmentation; instrumental vaginal delivery; dystocia; non‐reassuring FHR patterns; uterine tachysystole; uterine hypersystole; uterine hyperstimulation; Apgar score < 6 at 5 minutes; maternal and neonatal complications. | |
| Notes | St Louis, Missouri, USA. July 1994 to December 1995. Private community hospital with more than 35 attending obstetricians. The authors suggest that the high rate of caesarean section in the misoprostol group may be due to the tendency for the obstetricians to react to unfamiliar situations such as uterine hyperstimulation with an experimental drug, by performing caesarean section, rather than pharmacological management as has been described in other studies (eg Wing 1995a, Wing 1995b, Wing 1996). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
075 Chang 1997.
| Methods | "Randomly divided into two groups". | |
| Participants | Membranes were intact, cervical status variable, nulliparous women. Inclusion criteria: term singleton pregnancy; scheduled for induction of labour. Exclusion criteria: known contraindications to vaginal prostaglandins; previous uterine surgery; antepartum haemorrhage; fetal distress; premature rupture of membranes; abnormal lie; cephalopelvic disproportion; maternal illness for which induction of labour was inappropriate. |
|
| Interventions | Misoprostol 50 mcg into posterior vaginal fornix 4‐hourly, maximum 600 mcg (n = 30) versus dinoprostone 3 mg vaginal tablets 6 hourly, maximum 9 mg (n = 30). Cervical scores were evaluated every 4 hours. If cervical score was 9 or more and uterine contractions inadequate, oxytocin infusion was started. | |
| Outcomes | Maternal temperature, pulse, blood pressure and side effects; cervical scores after 12 hours (misoprostol mean 9.7, standard deviation 3.1 vs dinoprostone 7.3, 2.5, p < 0.05); induction to delivery interval (16.5, 2.7 vs 25.7, 3.8 hours, p < 0.001); occurrence of spontaneous labour (86% vs 77%); uterine hyperstimulation (13.4% vs 8.9%); meconium staining (10% vs 13%); mode of delivery; maternal complications (none); umbilical artery blood flow velocity waveforms (all normal); cord arterial blood gases (7.29, 0.73 vs 7.32, 0.91); Apgar scores at 1 and 5 minutes ('the same'); neonatal complications. | |
| Notes | Tainan, Taiwan. July 1994 to June 1995. Some data reported as percentages only. Not clear what denominator was used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
075 Chuck 1995.
| Methods | Open, randomised trial. Computer‐generated random allocation using opaque envelopes. | |
| Participants | Membranes were intact or ruptured, cervical status unfavourable. Pregnant women at 35 to 42 weeks' gestation, admitted for induction of labour. Exclusion criteria: non‐vertex presentation; uterine scar other than prior low transverse caesarean section; ominous FHR tracing; multiple gestation; complete cervical effacement. Reasons for induction (misoprostol, dinoprostone): post‐dates pregnancy (6, 12); oligohydramnios (7, 3); hypertensive disorders (4, 6); growth impairment (1, 4); premature rupture of membranes (16, 11); diabetes mellitus (10, 7); non‐reassuring antepartum surveillance (4, 4); other (1, 3). Other characteristics: initial cervical score 3 or less (26, 26); nulliparous (23, 21); prior caesarean section (5, 10). |
|
| Interventions | Application 4‐hourly if uterine contractions not adequate for up to 5 doses of misoprostol 50 mcg into the posterior vaginal fornix (n = 49), vs dinoprostone 0.5 mg intracervically (n = 50); oxytocin started after 5th dose if progress of labour unsatisfactory; membranes ruptured when cervix 3 cm dilated and fully effaced. | |
| Outcomes | Induction to delivery interval; number of doses (misoprostol mean 1.8 (SD 1.1) vs dinoprostone 2.5 (1.4)); number of women delivered after a single dose (20/49 vs 11/50); oxytocin augmentation; mode of delivery; fever > 100 degrees Fahrenheit (2/49 vs 4/50); gastrointestinal symptoms; uterine tachysystole (> 5 contractions per 10 minutes without FHR changes); uterine hyperstimulation (frequent or prolonged contractions with FHR decelerations or tachycardia); postpartum haemorrhage; Apgar scores < 7; NICU admission. | |
| Notes | Los Angeles, USA. September 1993 to January 1994. Of 103 women enrolled, 4 (3.9%) were excluded because of protocol violations. Authors conclude misoprostol is "apparently safe ... and may become the drug of choice for induction of labor". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
075 Danielian 1999.
| Methods | Random numbers allocated by pharmacy. | |
| Participants | Inclusion criteria: 37 to 42 weeks; singleton cephalic presentation; intact membranes. Exclusion criteria: > 1 caesarean section; uterine surgery; significant fetal or maternal medical condition; known prostaglandin hypersensitivity; cervical score 8 or more. |
|
| Interventions | Misoprostol 50 mcg vaginally 4‐hourly for maximum 4 doses (n = 105), compared with dinoprostone 1 mg vaginally 6‐hourly, maximum 3 doses) (n = 106); given until cervical score > 7 or labour ensued or membranes ruptured. Amniotomy when cervical score 8 or more. Oxytocin augmentation if indicated. | |
| Outcomes | Median induction ‐ delivery interval misoprostol 14.4 vs dinoprostone 22.9 hours. Delivered after single dose (81/105 vs 52/106). No vaginal delivery in 12 hours (67/105 vs 91/106). Median visual analogue pain score (n = 18 vs 24) at induction (20 vs 16), before analgesia given (84 vs 66), during second stage (90 vs 77) and at delivery (79 vs 75). | |
| Notes | Clinicians not blinded. No post‐randomisation exclusions or withdrawals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
075 Escudero 1997.
| Methods | Open trial. Assigned by means of a randomised table. No further details given. | |
| Participants | Membranes were intact or ruptured, cervical status variable. Pregnant women who required labour induction for obstetric or medical reasons. Inclusion criteria: singleton pregnancy; vertex presentation. Exclusion criteria: labour; fetal distress; previous uterine surgery; contraindication to vaginal delivery. Indications for labour induction: post‐dates pregnancy (misoprostol 15, oxytocin 15); pre‐eclampsia (22, 30); ruptured membranes (15, 15); fetal demise (4, 0); anencephaly (0, 1); other (1, 2). Unfavourable cervix was not specified as a criterion, though the mean cervical score was low (2.6, SD 1.5). |
|
| Interventions | Misoprostol 50 mcg placed in the posterior vaginal fornix 4‐hourly until 3 uterine contractions of at least 40 seconds per 10 minutes, maximum 600 mcg; artificial rupture of membranes as soon as possible; arrest in cervical dilation at 5 or more cm cervical dilation managed with oxytocin infusion (n = 57); versus labour induction with oxytocin according to standard protocol of the centre (n = 63). Continuous electronic FHR monitoring in all women. Cervical assessments were repeated every 4 hours. Uterine hyperstimulation was managed by left lateral positioning, nasal oxygen administration, nifedipine 10 mg sublingually and flushing the misoprostol from the vagina with saline or stopping the oxytocin infusion. Of 22 women with unsuccessful oxytocin labour induction after 24 hours, 9 had labour induced with misoprostol and all had vaginal deliveries without complications. |
|
| Outcomes | Cervical score 4 hours after application of the drug (misoprostol mean 5.3, SD 3.6 vs oxytocin 5.5, 3.9); time from induction to delivery (11.3, 6.9 h vs 8.4, 4.1 h); oxytocin use; route of delivery; Apgar scores (9.1, 0.9 vs 9.0, 1.3 at 5 minutes); complications during labour induction and after delivery; uterine hyperstimulation with and without FHR changes. | |
| Notes | Lima, Peru. September 1994 to March 1995. Of 63 women enrolled in the misoprostol group, 3 (4.8%) were excluded because of protocol violations. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
075 Fuchs 2006.
| Methods | "Prospective double‐blind, controlled trial" with no reference to allocation concealment methods. | |
| Participants | Inclusion criteria: singleton pregnancies undergoing induction of labour. Exclusion criteria: women with Bishop score < 5, PROM, in labour women, non‐reassuring fetal testing and scared uterus. |
|
| Interventions | The intervention group received vaginal prostaglandin E1 gel 50 mcg, 4 hourly and continuous infusion of placebo solution intra venally. Control group: intravaginal placebo gel, 4 hourly and oxytocin titrated to a maximum infusion of 30 mU per minute. |
|
| Outcomes | Time to delivery was not significantly different in between groups. | |
| Notes | Only abstract available. No data included in this systematic review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear for data assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Inadequate |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
075 Ghidini 2001.
| Methods | Allocated by random number generated at the hospital pharmacy. | |
| Participants | Women admitted for induction of labour; singleton pregnancy; 37 weeks' gestation. Exclusion criteria: non‐vertex presentation; uterine scar other than from previous lower transverse caesarean section; non‐reassuring cardiotocography; cervix dilated > 3 cm; uterine contractions > 3/10 minutes; contraindication for vaginal delivery; hypersensitivity to prostaglandins; parity < 5. |
|
| Interventions | Vaginal misoprostol 50 mcg 4‐hourly versus 100 mcg 6‐hourly until adequate contraction pattern or dilatation > 3 cm or forewater amniotomy or signs of uterine hyperstimulation. Intravenous oxytocin infusion after 24 hours. | |
| Outcomes | Primary: caesarean section. Secondary: vaginal delivery < 24 hours; induction to delivery interval; oxytocin augmentation; fetal and neonatal morbidity; hyperstimulation (tachysystole of 6 contractions/10 minutes for 20 minutes, or hypertonus of a contraction lasting for 2 minutes, with changes in the FHR; caesarean section for fetal distress (2/32 vs 3/26) umbilical artery pH < 7.1 (50 mcg 0/32 vs 100 mcg 1/26). | |
| Notes | Inova Alexandria Hospital, Virginia, USA. Seven of 65 enrolled women excluded due to emergence of exclusion criteria. The groups were somewhat unbalanced (32 50 mcg and 26 100 mcg). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Women were assigned by means of a random number generated at the Hospital Pharmacy to receive either misoprostol 100 μg every 6 hours or 50 μg every 4 hours. |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7 of 65 women withdrawn because misoprostol was not administered for clinical reasons. |
075 Kolderup 1999.
| Methods | Assignment in envelopes according to random number tables. | |
| Participants | Inclusion criteria: singleton pregnancy 31 or more weeks; cervical score < 6; vertex presentation; < 12 contractions per hour. Exclusion criteria: prior uterine surgery; ruptured membranes; contraindication for vaginal delivery; parity > 5; non‐reassuring fetal surveillance. |
|
| Interventions | 1. Misoprostol 50 mcg vaginally, repeated 4‐hourly if necessary to maximum of 6 doses (81 women); 2. dinoprostone gel 0.5 mg intracervically, repeated 6‐hourly if necessary, to a maximum of 4 doses (78 women). Fetal and uterine monitoring for at least 1 hour after treatment. Amniotomy attempted when cervix 3‐4 cm dilated. If not in adequate labour 4 hours after last dose or arrest of cervical dilation for more than 2 hours after 4 cm dilation, oxytocin infusion commenced or the woman was crossed over to the other group. | |
| Outcomes | Primary: caesarean delivery, induction to delivery time (misoprostol 19.8 hours SD 11.5 vs dinoprostone 28.9 SD 14.8, p = 0.005), oxytocin use, hyperstimulation syndrome (tachysystole with fetal bradycardia, or FHR pattern requiring treatment), fetal distress requiring delivery. Secondary outcomes: late decelerations and fetal bradycardia; meconium stained liquor; tachysystole; number of doses used (1.4 SD 1.0 vs 2.2 SD 1.3, p = 0.0005). | |
| Notes | 3 hospitals in California. January 1994 to December 1996. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
075 Lemancewicz 1999.
| Methods | Computer‐generated randomisation table. | |
| Participants | Inclusion criteria: indication for induction of labour; singleton; cephalic; reactive cardiotocograph; 41 weeks of pregnancy. Exclusion criteria: estimated fetal weight > 4500 g; evidence of cephalopelvic disproportion; placenta praevia; unexplained vaginal bleeding; vasa praevia; renal or hepatic dysfunction; contraindication to prostaglandins; suspected chorioamnionitis; previous uterine surgery. |
|
| Interventions | Misoprostol 50 mcg in posterior vaginal fornix 4‐hourly till progressive labour, contractions 3 per 10 minutes, ruptured membranes or delivery (maximum 300 mcg, n = 44); versus intravenous oxytocin at 1 mU per minute, increase every 30 minutes by 2 mU per minute till uterine activity adequate (maximum 17 mU per minute n = 47). | |
| Outcomes | Doppler velocimetry of umbilical, uterine and arcuate arteries before and 2‐3 hours after induction (no significant differences between groups). Induction to delivery (misoprostol 615, SD 65 vs oxytocin 711 SD 70 minutes); caesarean section; FHR changes; meconium. | |
| Notes | Bialystok University Hospital, Poland. 3‐way study. Misoprostol vs intracervical dinoprostone published as Urban 2003. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
075 Magtibay 1998.
| Methods | Method of randomisation not specified. | |
| Participants | Pregnant women with an indication for labour induction. Inclusion criteria: singleton; cephalic; live fetus; cervical score < 5. Exclusion criteria: previous caesarean section. |
|
| Interventions | Intravaginal misoprostol 50 mcg 4‐hourly (n = 17) compared with intracervical dinoprostone 0.5 mg and oxytocin infusion (n = 19). | |
| Outcomes | Change in cervical score after 12 hours (misoprostol median 4 versus dinoprostone 1); delivery within 36 hours (15/17 vs 9/19); time to complete dilatation (n = 30; 17 h vs 24 h); caesarean section. | |
| Notes | Two women allocated to the dinoprostone group refused treatment. Outcome measures assessed blind to allocation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
075 Megalo 2004.
| Methods | Randomised non‐blinded trial with allocation concealment through sealed opaque envelopes. | |
| Participants | Inclusion criteria: pregnant women between 36 ‐ 42 weeks of gestation that presents indication for cervical ripening, with singleton pregnant, cephalic presentation, normal FHR tracing, unscarred uterus, absence of fetal anomalies, no contraindication for vaginal delivery, absence of chorioamnionitis and no hypersensitivity to prostaglandin. | |
| Interventions | The intervention group received 50 mcg of vaginal misoprostol 4 hourly (maximum 5 times) and the comparison group received 0.5 mcg of dinoprostone intracervical 6 hourly up to 3 times. If necessary they received 3 mg of dinoprostone intravaginal (maximum 2 times). | |
| Outcomes | Time induction‐to‐delivery and the need of oxytocin were reduced with misoprostol (p < 0.05). Pathological CTG tracing was more frequent in the misoprostol treated group (p > 0.001). | |
| Notes | The study was conducted during 18 months in the Department of Obstetrics and Gynaecology, Lausanne University Hospital (CHUV). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Only blinded for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
075 Mundle 1996.
| Methods | Open, randomised trial. Allocation by sequentially numbered opaque sealed envelopes, prepared from randomisation tables in blocks of four. Caregivers were unaware of the blocking. | |
| Participants | Membranes were intact, cervical status variable. Inclusion criteria: indication for labour induction; single live fetus; gestation greater than 37 weeks; cephalic presentation; intact membranes. Exclusion criteria: non‐reassuring FHR tracing; prior uterine surgery; known hypersensitivity to prostaglandins; contraindications to vaginal birth. |
|
| Interventions | Misoprostol 50 mcg in the upper vagina 4 hourly until progressive labour, contraction frequency of 3 per 10 minutes, membranes ruptured, non‐reassuring FHR tracing, delivery or maximum of 16 doses (n = 111); versus physician‐chosen combinations of dinoprostone 0.5 mg intracervically for cervical ripening or 1‐2 mg intravaginal gel for induction, and oxytocin infusion (n = 111). Artificial rupture of membranes in both groups was at the discretion of the attending physician. After membrane rupture, augmentation of labour was by oxytocin infusion. Oxytocin was not allowed within 4 hours of the last misoprostol or 6 hours of the last dinoprostone dose. Continuous electronic FHR and uterine contraction monitoring were used. |
|
| Outcomes | Labour induction to vaginal delivery time (misoprostol mean 753, standard deviation 588 vs dinoprostone/oxytocin 941, 506 minutes); oxytocin use (22/111 vs 46/111); method of delivery; epidural use (34/111 vs 55/111); no analgesia (13/111 vs 9/111); meconium; scalp pH done (12/111 vs 9/111); intact perineum (17/111 vs 18/111). Neonatal assessments blind to group allocation: Apgar scores; cord pH (mean 7.28, standard deviation 0.09 vs 7.28, 0.10); neurological and general physical assessment; birth asphyxia (profound metabolic or mixed acidaemia, 5 minutes Apgar score 3 or less, neonatal neurologic abnormality and dysfunction of one other major body system). Oxytocin use has not been included in the review outcomes because oxytocin use was an option for induction of labour in only the dinoprostone group, and would therefore be expected to be used more frequently in that group. |
|
| Notes | St John's, Newfoundland. Canada. March to September 1994. There were no losses to follow up. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
075 Reyna‐Villasmil 2005.
| Methods | Randomised trial with allocation method and sequence generation not stated. | |
| Participants | Inclusion criteria: singleton pregnancy, gestational age > 37 weeks, Bishop score < 4, cephalic presentation, vertex and estimated fetal weight < 4 kg. Exclusion criteria: uterine surgery, placenta previa, contraindication to use prostaglandins, fetal distress, herpes simplex active genital infection and abruptio placentae. |
|
| Interventions | The intervention group received 50 mcg or 100 mcg vaginal misoprostol 4 hourly (maximum 6 doses each group). Oxytocin augmentation could be used if there were no labour after third dose of misoprostol (4 hours after third dose). | |
| Outcomes | The interval from induction to delivery was shorter in the 100 mcg group (p < 0.05).The need of oxytocin augmentation was higher in the 50 mcg group. The caesarean section was double in the 100 mcg group (p < 0.05). | |
| Notes | The study was enrolled from June 2002 to November 2003 at the Service of Obstetrics from Central Hospital "Dr. Urquinaona", Maracaibo, Venezuela. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unblinded for personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
075 S‐Ramos 1997.
| Methods | Open randomised trial. Allocations in numbered, sealed envelopes, in computer‐generated random sequence. | |
| Participants | Membranes were ruptured, cervical status variable. Inclusion criteria: women admitted for induction of labour for term premature rupture of membranes; pregnancy 36 weeks or more; singleton vertex presentation; no evidence of labour; cervical dilation < 2 cm and effacement no more than 80%; reassuring FHR tracing; spontaneous rupture of membranes. Exclusion criteria: intrauterine infection; contraindication to labour or vaginal delivery; previous uterine surgery. |
|
| Interventions | Misoprostol 50 mcg into the posterior vaginal fornix 4‐hourly until labour established (at least 3 contractions in 10 minutes), maximum 12 doses, augmented with oxytocin for active labour arrest (n = 70); versus oxytocin infusion commenced at 1‐2 mU per minute (n = 71). Continuous FHR monitoring in all women. Intrauterine pressure catheter and scalp electrode applied as soon as possible. Episodes of hyperstimulation were treated with position change, oxygen therapy and tocolysis with terbutaline 250 mcg subcutaneously. Fetal scalp blood sampling was performed when indicated. |
|
| Outcomes | Delivery within 24 hours; uterine tachysystole, hypertonus and hyperstimulation; chorioamnionitis (misoprostol 4/70, oxytocin 5/71); postpartum endometritis; retained placenta (1/70, 2/71). | |
| Notes | Jacksonville, Florida, USA. November 1992 to October 1993. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
075 Sahin 2002.
| Methods | Computer‐generated random number table, in numbered, sealed envelopes. | |
| Participants | Inclusion criteria: women with pre‐eclampsia/eclampsia in whom induction of labour was anticipated; cervical score < 5. Exclusion criteria: abnormal lie; non‐cephalic presentation; abnormal FHR pattern; abnormal umbilical diastolic velocities; multiple pregnancy; previous caesarean section; antepartum haemorrhage; premature rupture of membranes; expected cephalopelvic disproportion; maternal illness which contraindicated induction with prostaglandins. | |
| Interventions | Misoprostol 50 mcg vaginally 4‐hourly (maximum 4 doses), versus oxytocin infusion at 1‐30 mU per minute. | |
| Outcomes | Cervical score at 12 hours; induction to delivery time, Apgar scores; in labour within 12 hours; caesarean sections; admission to NICU. | |
| Notes | Women not in labour after 12 hours were excluded from further analysis in the report. As this would introduce bias, only the caesarean section rates, which could be re‐calculated for all women, have been included for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
075 Saleen 2006.
| Methods | "The study designed as a randomised trial" with sequence generation and allocation concealment methods not stated. | |
| Participants | Women with singleton alive pregnancies and Bishop score 5 requiring induction of labour between 37 to 42 weeks gestational. | |
| Interventions | There were 3 groups: A. 50 mcg vaginal misoprostol 4 hourly (maximum 200 mcg); B. 3 mg vaginal dinoprostone pessary 6 hourly (2 times); C. supra‐cervical Foley catheter. Each patient could use oxytocin augmentation. |
|
| Outcomes | There were no significant difference in outcomes between those groups. | |
| Notes | The mechanical method are accessed in a specific review. The trial was conducted at Hamdard University Hospital and Patel Hospital from July 2004 to June 2005. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
075 Tabor 1995.
| Methods | "Prospective randomised trial". | |
| Participants | Membranes were intact or ruptured, cervical status unfavourable. Women with cervical score < 5. | |
| Interventions | Misoprostol 50 mcg administered vaginally 4‐hourly, maximum 12 doses (n = 68) vs prostaglandin E2 gel 0.5 mg intracervically 6‐hourly, maximum 3 doses (n = 59). Oxytocin commenced when cervix favourable or having > 3 uterine contractions per 10 minutes. |
|
| Outcomes | Induction to delivery times; oxytocin use; caesarean sections; maternal and neonatal morbidity. | |
| Notes | Fort Worth, Texas, USA. Authors conclude that misoprostol shortens induction time without increased morbidity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
075 Urban 2003.
| Methods | Allocation by computer‐generated randomisation table, consecutively numbered sealed opaque envelopes. | |
| Participants | Inclusion criteria: singleton pregnancy; reassuring FHR tracing; 41 weeks' gestation; cervical score < 6. Exclusion criteria: multiple pregnancy; malpresentation; estimated fetal eight > 4500 g; evidence of cephalopelvic disproportion; placenta praevia; unexplained vaginal bleeding; vasa praevia; renal or hepatic dysfunction; allergy to prostaglandins; suspected chorioamnionitis; previous caesarean delivery; uterine surgery. | |
| Interventions | Misoprostol 50 mcg vaginally 4‐hourly (maximum 300 mcg) versus dinoprostone 0.5 mg intracervically 6‐hourly (maximum dose 1 mg), until progressive labour or contraction frequency of 3 in 10 minutes, or ruptured membranes or delivery. Oxytocin infusion was used for failure to progress in labour after adequate cervical ripening. Continuous FHR monitoring was used. | |
| Outcomes | Doppler velocimetry of umbilical, uterine and arcuate arteries; mean time to delivery (misoprostol 615 (SEM 65) versus dinoprostone 772 (82) minutes, p = 0.14); oxytocin augmentation; caesarean section; umbilical artery pH; abnormal FHR patterns; uterine hyperstimulation. | |
| Notes | 3‐way trial, data on misoprostol versus oxytocin published previously as Lemancewiecz 1999. No mention in 1999 paper that it was a three‐way trial, but communication with first author of 2003 report confirmed that was the case. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
075 Webb 1997.
| Methods | "Randomised", no details given (abstract report). 100 enrolled women reviewed. | |
| Participants | Inclusion criteria: indication for labour induction in third trimester; cervical score < 6. Exclusion criteria: contraction frequency > 6 per hour; multiple pregnancy; breech; uterine scar other than single lower transverse; parity > 4. |
|
| Interventions | Prostaglandin E2 4 mg vaginal suppositories 4 mg to posterior fornix vs dinoprostone 0.5 mg intracervical gel vs misoprostol 50 µg to posterior fornix; 4‐hourly until cervix 2 cm dilated, contraction frequency 3 per 10 minutes or membrane rupture associated with contractions. Oxytocin used when indicated, only after discontinuation of the study drug. | |
| Outcomes | Time to 4 cm cervical dilation: prostaglandin E2 19.4 hours vs dinoprostone 17.7 vs misoprostol 10.8; time to delivery 27 hours vs 30.5 vs 15.3; oxytocin requirement 86% vs 82% vs 56%; caesarean section 35% vs 41% vs 23%. Dinoprostone use was associated with significantly fewer episodes of terbutaline administration and stated hyperstimulation. Misoprostol use was associated with significantly less fever. | |
| Notes | No comment on exclusions. Data in abstract inadequate for inclusion in tables. Further data awaited for inclusion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
075 Zeteroglu 2004.
| Methods | Randomised trial. Allocation method and sequence generation not stated. | |
| Participants | Inclusion criteria: grand multiparas, Bishop score < 6 and gestational age ≥ 35 weeks. Exclusion criteria: Abnormal lie, non‐cephalic presentation, abnormal heart rate patterns, abnormal umbilical diastolic velocities, gestational age < 35 weeks, history of previous caesarean section, antepartum haemorrhage, expectation of cephalopelvic disproportion and any maternal illness or contraindication for prostaglandins. |
|
| Interventions | The intervention group received 50 mcg of vaginal misoprostol 4 hourly (maximum 4 doses) and the comparison group received oxytocin alone. | |
| Outcomes | The mean time from induction to delivery was significantly shorter in the misoprostol group (p = 0.02). | |
| Notes | University of Yünzücü Yil, Medical Faculty, Van, Turkey. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
075 Zeteroglu 2006a.
| Methods | Randomised trial with allocation not stated. Computer generation of random assignment. | |
| Participants | Inclusion criteria: singleton pregnancies, gestational age > 37 weeks, no contraindication for induction of labour, premature rupture of membranes > 24 hours. Exclusion criteria: previous labour induction failure, non‐cephalic presentation, chorioamnionitis, prior uterine surgery, contraindication to prostaglandins. |
|
| Interventions | The intervention group received 50 mcg of vaginal misoprostol 4 hourly (maximum 200 mcg) and the comparison group received oxytocin alone. | |
| Outcomes | The mean time from induction to delivery was shorter in the misoprostol group (p = 0.063). | |
| Notes | The trial was conducted during 2 years. University of Yünzücü Yil, Medical Faculty, Van, Turkey. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
075 Zeteroglu 2006b.
| Methods | Randomised trial with allocation by sealed opaque numbered envelopes. Sequence generation made by computer. | |
| Participants | Inclusion criteria: grand multiparas pregnancies (≥ 10 pregnancies), admission for whom after 35 weeks to induction of labour, well dated with US (< 22 weeks) and Bishop ≤ 6. Exclusion criteria: abnormal position, non cephalic presentation, abnormal heart rate pattern, < 35 gestational weeks, multiple pregnancies, history of caesarean section, antepartum haemorrhage, expectation of cephalopelvic disproportion or any maternal illness or which induction was contraindicated with prostaglandins. |
|
| Interventions | The intervention group received 50 mcg vaginal misoprostol with oxytocin augmentation if necessary. The comparison group received oxytocin associated with amniotomy. | |
| Outcomes | There was no difference between the groups in rates of vaginal delivery and mean time from induction to delivery. | |
| Notes | University of Yünzücü Yil, Medical Faculty, Van, Turkey. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
075 Zeteroglu 2006c.
| Methods | Randomised trial. Computer generated random number table. Allocation method not described. | |
| Participants | Inclusion criteria: advanced maternal age (> 35 years), Bishop score < 6, indication for induction of labour at 35 weeks or more, singleton and vertex presentation. Exclusion criteria: abnormal lie, non‐cephalic presentation, abnormal umbilical diastolic velocities, age below 35 weeks, multiple pregnancies, previous caesarean section, antepartum haemorrhage, expectation of cephalopelvic disproportion, or any maternal contraindication to use prostaglandins. |
|
| Interventions | The intervention group received 50 mcg vaginal misoprostol 4 hourly (maximum: 200 mcg) and the comparison group received oxytocin alone. | |
| Outcomes | The induction to delivery interval was significantly shorter in the misoprostol group. The vaginal delivery rate was higher in the misoprostol group. | |
| Notes | University of Yünzücü Yil, Medical Faculty, Van, Turkey. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unclear only for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Inadequate |
| Selective reporting (reporting bias) | High risk | Inadequate |
| Other bias | High risk | Inadequate |
088 Garry 2003.
| Methods | Randomised trial with allocation concealment made according to a computer‐generated schedule prepared by a hospital pharmacy. | |
| Participants | Inclusion criteria: pregnant women with obstetric indication for IOL, Bishop ≤ 4, cephalic presentation and reassuring fetus status. Exclusion criteria: known fetal structural or chromosomal anomalies, fetal death, refusal to participate, multiple gestation and any contraindication to vaginal delivery. |
|
| Interventions | The intervention group received 50 mcg of misoprostol 3 hourly until 400 mcg. The comparison group received dinoprostone 10 mg vaginal repeated if was necessary after 12 hours. | |
| Outcomes | The interval from start of induction to vaginal delivery was significantly shorter in the misoprostol group (794.5 ± 408 minutes vs 1005.3 ± 523 minutes; p < 0.02). A non reassuring FHR tracing was the indication for 71.4% of caesarean deliveries in the misoprostol group, compared to 40% in the dinoprostone group (p = 0.03). There were no significant differences in neonatal outcomes. | |
| Notes | Winthrop University Hospital, Mineola, NY, USA. The study ran from 1 April 1998 to 6 June 1999. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Only blinded for personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Inadequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
088 Pi 1999.
| Methods | Randomised by computer sequence generation. Allocation methods not stated. | |
| Participants | Inclusion criteria: term pregnancy, low platelets, premature rupture of membranes and women needed to IOL. Exclusion criteria: scarred uterus, antepartum haemorrhage, abnormal cardiotocography, transverse lie, fetus not engaged, patients with asthma and with cardiac problem. |
|
| Interventions | Interventions groups used 50 mcg rectal or vaginal misoprostol 3 hourly until effective contraction. Comparison group used oxytocin alone. | |
| Outcomes | There was no difference on use of vaginal or rectal misoprostol for labour induction. The results indicate that misoprostol had a higher rate of successful induction for labour than oxytocin. | |
| Notes | October 1997 to May 1998. Department of Obstetrics and Gynaecology. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Inadequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | High risk | Inadequate |
088 Saggaf 2001.
| Methods | Sealed envelopes with computer‐generated randomisation. | |
| Participants | Inclusion criteria: women admitted for induction of labour; cephalic presentation; singleton; intact membranes; cervical score < 6; reassuring cardiotocography. Exclusion criteria: previous uterine surgery; antepartum haemorrhage; ruptured membranes; malpresentation; estimated fetal weight > 4500 g; chorioamnionitis; known hypersensitivity to prostaglandins. | |
| Interventions | Misoprostol 50 mcg vaginally every 3 hours, maximum 6 doses, versus dinoprostone 3 mg vaginally 6‐hourly, maximum 3 doses. If no labour, oxytocin started 3 hours after last misoprostol or 6 hours after last dinoprostone dose. There was continuous cardiotocography during labour. | |
| Outcomes | Mode of delivery; time from insertion to labour (misoprostol 6.3 SD 3.4 vs dinoprostone 10.3 SD 7.1 hours; time from labour to delivery (4.9 SD 2.6 vs 5.6 SD 4.9 hours); tachysystole (6 or more contractions per 10 minutes for 20 minutes); hypertonus (a contraction lasting 2 or more minutes; hyperstimulation (tachysystole or hypersystole with non‐reassuring cardiotocograph); Apgar scores (5 min 9.4 SD 0.6 vs 8.3 SD 1.9). | |
| Notes | King Fahad Military Hospital, Saudi Arabia, April 1999 to August 2000. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
088 Sanchez Ramos 1998.
| Methods | Computer‐generated randomisation table, maintained in the research section of the hospital pharmacy. Physicians were not blinded to allocation. Review of labour tracings was not stated to have been performed blindly. Analysis was according to intention to treat. | |
| Participants | Inclusion criteria: obstetric or medical indications for labour induction; no active labour; no fetal distress; no previous uterine surgery; singleton pregnancy; vertex presentation; no contraindication to vaginal delivery; informed consent (n = 223). | |
| Interventions | 1. Misoprostol 50 µg to the posterior vaginal fornix 3‐hourly until adequate uterine contractions (maximum 8 doses); oxytocin for arrest in the active phase of labour, versus 2. dinoprostone 10 mg vaginal insert in the posterior fornix, which was removed when adequate uterine contractions or hyperstimulation occurred, or after 12 hours. Continuous electronic FHR and uterine activity monitoring. As soon as cervical dilation permitted, artificial rupture of the membranes was performed and an intrauterine pressure catheter and scalp electrode applied. If not in labour after 24 hours, oxytocin was used. Uterine hyperstimulation was managed with left lateral positioning, removal of the tablet or insert, oxygen administration and subcutaneous terbutaline 250 µg. | |
| Outcomes | Induction to vaginal delivery interval (misoprostol median 698, interquartile range 395‐1053 vs dinoprostone 1041, 792‐1531, p < .001); induction to delivery (699, 395‐1053 vs 1053, 780‐1590, p < .001; successful induction (98/108 vs 77/115, p < .001; vaginal delivery within 12 hours (44/108 vs 22/115, p < .001); vaginal delivery within 24 hours; need for oxytocin; maximum oxytocin rate (8, 4‐13.2 vs 13, 6‐20 mU/minute, p < .001); uterine tachysystole; hyperstimulation; need for scalp pH sampling (4/108 vs 5/115); assisted delivery; caesarean section; cord pH (mean 7.29 +/‐ 0.09 vs 7.30 +/‐ 0.08); cord pH < 7.16 (9/97 vs 4/99); Apgar score < 7 at 1 minute (11/108 vs 8/115) and 5 minutes; admission to NICU; maternal side effects (none noted in either group); average costs of induction ($85 vs $606). | |
| Notes | University of Florida Health Sciences Centre, Jacksonville. February to October 1996. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
088 Wing 1995a.
| Methods | Allocation by sequentially numbered sealed opaque envelopes, maintained by the primary investigator. Sequence from random number table in blocks of 6. | |
| Participants | Membranes were intact, cervical status unfavourable. Inclusion criteria: women requiring induction of labour for medical or obstetric reasons; singleton pregnancy; cephalic presentation; intact membranes; cervical score < 5; reactive nonstress test; < 4 uterine contractions per hour. Exclusion criteria: estimated fetal weight > 4500 g; evidence of cephalopelvic disproportion; placenta praevia; unexplained vaginal bleeding; vasa praevia; active herpes simplex infection; contraindication to receiving prostaglandins; renal or hepatic dysfunction; suspected chorioamnionitis; previous uterine surgery; parity > 5. Indications for labour induction: oligohydramnios (misoprostol 37, dinoprostone 41); pre‐eclampsia (14, 5); post‐term pregnancy (5, 9); macrosomia (4, 5); abnormal antepartum testing (3, 1); other (5, 6). |
|
| Interventions | Misoprostol 50 mcg inserted into the posterior vaginal fornix 3‐hourly, maximum 6 doses (n = 68) versus dinoprostone gel 0.5 mg intracervically 6‐hourly, maximum 3 doses (n = 67), until 3 uterine contractions per 10 minutes, cervical score > 7, cervical dilation > 3 or spontaneous rupture of membranes. Artificial rupture of membranes usually performed when the cervix was 80% effaced and 3 cm dilated. Oxytocin augmentation was used for lack of contractions after maximum dosage or spontaneous rupture of membranes, or for arrested cervical dilation, > 3 hours after misoprostol and > 6 hours after dinoprostone. Uterine hyperstimulation was treated in some cases by tocolytic therapy. |
|
| Outcomes | Oxytocin augmentation; uterine tachysystole (> 5 contractions per 10 minutes); uterine hypertonus (contraction > 2 minutes) (misoprostol 1/68, dinoprostone 2/67); hyperstimulation syndrome (tachysystole or hypertonus with FHR abnormalities); induction to delivery interval; mode of delivery; vaginal delivery within 24 hours; terbutaline used for hyperstimulation (6/68, 3/67); diarrhoea (2/68, 2/67); fever (2/68, 0/67); neonatal resuscitation (15/68, 5/67); days in NICU (mean 11.2 (SD 8.1), 5.8 (2.4)); meconium aspiration syndrome (3/68, 1/67); hyperbilirubinaemia (8/68, 2/67). | |
| Notes | Los Angeles, California, USA. October to November 1993. 135/140 women agreed to participate. There were no withdrawals from the protocol. The authors postulate that the increased rate of meconium‐stained amniotic fluid may be due to the increased incidence of uterine tachysystole, or to a direct effect of misoprostol on the fetal gastrointestinal tract. The authors do not recommend this dosage for induction of labour. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
100 Fletcher 1994.
| Methods | Randomised by pulling sealed odd and even numbers from a box. | |
| Participants | Membranes were intact, cervical status variable. Women scheduled for induction of labour. Exclusion criteria: contraindications to vaginal prostaglandins (including uterine scar); antepartum haemorrhage; fetal distress; premature rupture of the membranes; abnormal lie; cephalopelvic disproportion; maternal illness for which induction of labour was contraindicated. Indications for induction of labour: hypertension (misoprostol 11, dinoprostone 13); postdates (10, 11); diabetes (4, 3); other (7, 4). |
|
| Interventions | Insertion into the posterior vaginal fornix of misoprostol 100 mcg (n = 32) vs dinoprostone 3 mg (n = 31). If not in labour after 12 hours, oxytocin was commenced (sometimes delayed because of staff shortage). | |
| Outcomes | Cervical score 12 hours after drug insertion (misoprostol mean 9.1 (SD 1.1) vs dinoprostone 7.7 (2.4)); insertion to delivery interval; spontaneous labour; uterine hyperstimulation (> 5 contractions per 10 minutes with fetal bradycardia); mode of delivery; maternal complications; Apgar scores (5 minutes mean 8.8 vs 9.1); perinatal death (excluding one induction of labour for intrauterine death). | |
| Notes | Kingston, Jamaica. September to October 1992. One woman (1/32, 3%) excluded from dinoprostone group because of inadvertently receiving misoprostol. The authors conclude that larger studies are needed to confirm safety. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
100 Herabutya 1997.
| Methods | "Blocked randomisation". | |
| Participants | Membranes were intact or ruptured, cervical status unfavourable. Women with indications for labour induction and unfavourable cervices. | |
| Interventions | Misoprostol 100 mcg placed in the posterior vaginal fornix (n = 60), versus prostaglandin E2 1.5 mg in gel placed in the endocervix (n = 50). If not in labour after 24 hours, labour induced with amniotomy and oxytocin. | |
| Outcomes | Induction to delivery interval (misoprostol mean 19.1, SD 10.6 vs prostaglandin E2 21.4, 13.1 hours); induction of labour after 24 hours (5/60 vs 13/50); oxytocin augmentation; caesarean section; uterine hyperstimulation; Apgar scores; admission to NICU. | |
| Notes | Bangkok, Thailand. Data from abstract; full report awaited. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
100 Howarth 1996.
| Methods | Consecutively numbered opaque sealed envelopes in computer‐generated random sequence. The trial medication was administered by an investigator not involved in ongoing care of the women. Labour ward staff were blind to the allocation. | |
| Participants | Membranes were intact, cervical status unfavourable. Women due for induction of labour; singleton pregnancy; cephalic presentation; no fetal distress; estimated fetal weight > 2000 g; intact membranes; unfavourable cervix. Exclusion criteria: contraindication to vaginal delivery; previous caesarean section; parity > 4; contraindication to prostaglandin administration including asthma or glaucoma. Indications for induction of labour: hypertension (misoprostol 16, dinoprostone 18); post‐dates pregnancy (15, 9); other (5, 9). |
|
| Interventions | Administration into posterior vaginal fornix (and repeated after 6 hours if the cervix remained unfavourable) of misoprostol 100 mcg vs dinoprostone 1 mg. | |
| Outcomes | Frequent uterine contractions (> 5 per 10 minutes); uterine hyperstimulation (frequent contractions with suspicious or ominous changes in the FHR pattern); analgesia use (misoprostol 24/36 vs dinoprostone 26/36); oxytocin augmentation; delivery within 6 (12/36 vs 3/36) and 12 hours (30/36 vs 13/36); not in labour within 12 hours; induction to delivery interval; caesarean section; Apgar score at 5 minutes. FHR tracings were examined blind to the group allocation. |
|
| Notes | Pretoria, South Africa. April to June 1995. The authors conclude that larger trials are needed to address dosage and safety. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
100 Montealegre 1999.
| Methods | Double blind, double placebo randomised trial. | |
| Participants | Inclusion criteria: indication for labour induction (pre labour rupture of membranes, hypertension, prolonged pregnancy); cervical score 6 or less; consent. Exclusion criteria: previous caesarean section or other uterine surgery; chorioamnionitis; dystocia; fetal distress; placenta praevia; medical and surgical contraindications to labour; multiple pregnancy, parity > 5. |
|
| Interventions | Misoprostol 100 mcg vaginally plus intravenous lactated Ringer solution at 2 mU per minute, increasing 2 mU every 20 minutes (maximum 30 mU or 8 hours), versus oxytocin solution plus placebo tablet. | |
| Outcomes | Time to delivery; method of delivery; uterine hypersystole, fetal distress. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
100 Ozsoy 2004.
| Methods | Randomised unblinded trial. Allocation not stated. | |
| Participants | Inclusion criteria: women with > 39 completed weeks singleton gestation. Exclusion criteria: previous uterine scar, malpresentation, cervix dilated > 3cm, uterine contraction > 3/10 minutes, any contraindication to vaginal delivery, hypersensitivity to prostaglandins, parity > 5, abnormal antepartum testing, cephalopelvic disproportion, premature rupture of membranes and maternal illnesses. |
|
| Interventions | The intervention group received 100 mcg 6 hourly and the comparison group received 50 mcg 4 hourly of vaginal misoprostol until labour. | |
| Outcomes | In two groups the dose used of misoprostol were similar. There was no difference between two groups in mean time to delivery, caesarean rate, Apgar of 5 minutes and meconium passage. | |
| Notes | Department of Obstetrics and Gynaecology, Isparta, Turkey. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Inadequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Unclear risk | Unclear |
100G Fletcher 1993.
| Methods | Randomised, double blind trial. Coded sachets of powdered medication used. Code broken at end of study. | |
| Participants | Membranes were intact, cervical status unfavourable. Women due for induction of labour in the third trimester with unfavourable cervix; no contraindication to prostaglandin use. Indications for induction of labour: post dates pregnancy (misoprostol 12, placebo 11); pre‐eclampsia (7, 5); pre‐eclampsia with intrauterine death (1, 1); diabetes mellitus (1, 2); other (3, 2). |
|
| Interventions | Powdered misoprostol 100 mcg (n = 24) vs ethinyl estradiol 0.05 mg ('placebo') (n = 21), each mixed with hydroxyethyl gel 2.7 mg, administered with a syringe into the posterior vaginal fornix; if not in labour after 12 hours, oxytocin induction was commenced or planned. | |
| Outcomes | Results were as follows (misoprostol vs placebo, mean values (standard deviation) or proportions): insertion to delivery in hours (15.6 (12.5) vs 43.2 (20.5)); no improvement in cervical score (3/24 vs 13/21); oxytocin used (7/24 vs 13/21), all significant p < 0.05. Complications: instrumental vaginal delivery (1/24 vs 1/21); caesarean section (2/24 vs 3/21); meconium‐stained liquor (2/24 vs 0/21); fetal tachycardia (0/24 vs 2/21); uterine hyperstimulation (1/24 vs 0/21); postpartum haemorrhage (1/24 vs 0/21). | |
| Notes | Kingston, Jamaica. Three women allocated to the placebo group excluded because of damage to the sachets. The authors conclude that misoprostol is effective and safe for cervical ripening. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
100G Gottschall 1997.
| Methods | Allocated by sequentially numbered, sealed opaque envelopes. Used random number tables in permuted blocks of 6. | |
| Participants | Inclusion criteria: intended pre‐induction cervical ripening or labour induction; singleton fetus, cephalic presentation; intact membranes; reactive FHR tracing; no contraindications to vaginal delivery. Out of 262 women for induction of labour, 75 enrolled. Exclusion criteria: uterine scar; known allergy to prostaglandins. |
|
| Interventions | Misoprostol 100 µg or prostaglandin E2 (dinoprostone) gel prepared by the hospital pharmacy 5 mg, into the posterior vaginal fornix. After 6 hours if in labour, amniotomy; if not, oxytocin 0.6 to 20 mU per minute. | |
| Outcomes | Primary outcome: time to delivery (misoprostol 14.7 +/‐ 6.4 hours vs prostaglandin E2 20.4 +/‐ 10.2). Secondary outcomes: need for oxytocin; change in cervical score at 6 hours; uterine tachysystole (6 per 10 minutes); uterine hypertonus (> 2 minutes); hyperstimulation syndrome. | |
| Notes | New Britain General Hospital, Connecticut, November 1995 to August 1996. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
100G Srisomboon 1996.
| Methods | Allocation by "blocked randomisation". | |
| Participants | Inclusion criteria: obstetric or medical indication for labour induction; singleton pregnancy; parity 3 or less; vertex presentation; cervical score 4 or less; gestational age > 35 weeks. Exclusion criteria: labour; fetal distress; previous uterine surgery; definite cephalopelvic disproportion; history of 2nd or 3rd trimester n = haemorrhage; contraindication to use of prostaglandins. |
|
| Interventions | 1. Misoprostol 100 mcg crushed and mixed with 5 ml hydroxyethyl cellulose gel into the posterior vaginal fornix (32). 2. Gel alone (30). All women received continuous cardiotocography. Oxytocin was started after 12 hours if not in labour. | |
| Outcomes | Change in cervical length measured by transvaginal ultrasound (24 (SD 9.1) vs 2.2 (4.3), p < .001); cervical score (Bishop 1964) 12 hours after gel insertion (8.1 (2.7) vs 0.9 (1.2), p < .001); time to vaginal delivery (h) (12.0 (8.3) vs 25.5 (6.7), p < 0.001); analgesia (23/32 vs 22/30, NS); fetal distress (2/32 vs 2/30, NS); 5‐minute Apgar score (9.9 (0.4) vs 9.9 (0.4), NS); side effects; uterine hyperstimulation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
100G Srisomboon 1997.
| Methods | Allocation by "blocked randomisation". | |
| Participants | Membranes were intact, cervical status unfavourable. Inclusion criteria: singleton pregnancy; parity > 4; vertex presentation; obstetric or medical indication for labour induction; intact membranes with no prior stripping; cervical score > 5; gestation > 35 weeks. Exclusion criteria: labour; fetal distress; previous uterine surgery; evidence of cephalopelvic disproportion; placenta praevia; cord presentation; vasa praevia; contraindication to the use of prostaglandins. Indications for labour induction: gestational hypertension/pre‐eclampsia (intravaginal 19, intracervical 12); impaired fetal growth (13, 13); post‐term pregnancy (14, 20); other (4, 5). |
|
| Interventions | Misoprostol gel (100 mcg crushed and mixed with 3 ml hydroxyethyl cellulose gel in a 10 ml syringe fitted with a nylon feeding tube, 8" 5 FR), instilled under vision into the posterior vaginal fornix (n = 50) versus within the endocervical canal during slow withdrawal of the feeding tube (n = 50). Continuous external cardiotocography in all women. No oxytocin, pelvic examination or amniotomy within 12 hours of instillation. After 12 hours, if the cervix remained unfavourable, misoprostol was repeated; if the cervical score was > 6, an amniotomy was performed and oxytocin infusion instituted if necessary, starting at 1‐2 mU per minute. Oxytocin was also used if there were no cervical changes or regular uterine contractions after the second dose of misoprostol. The definition of hypertonus was a uterine contraction > 90 rather than 120 seconds. Uterine hyperstimulation with FHR changes was treated with left lateral positioning, oxygen by nasal catheter, and terbutaline 250 mcg intravenously or subcutaneously. |
|
| Outcomes | Cervical score 12 hours after misoprostol administration (intravaginal mean 10.1, SD 2.7, intracervical 9.9, 2.9) insertion to vaginal delivery (16.4, 8.6 vs 17.0, 8.6); vaginal delivery in 24 hours; uterine hyperstimulation without FHR changes; oxytocin use; analgesia (40/5 vs 38/50). | |
| Notes | Chiang Mai, Thailand. August 1994 to September 1995. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
138 Nunes 1999.
| Methods | Randomisation using sealed, opaque envelopes in computer‐generated sequence. | |
| Participants | Inclusion criteria: term singleton pregnancy, cervical score < 5, reassuring FHR pattern; exclusion criteria: previous caesarean section or other uterine scar, vaginal bleeding, parity > 4, polyhydramnios, cephalopelvic disproportion, previous fetal death, fetal growth restriction. | |
| Interventions | Vaginal misoprostol 100 mcg, if necessary 50 or 100 mcg after 3 hours, 50 mcg after a further 6 hours (n = 95); versus vaginal dinoprostone 2 mg, if necessary 2 or 1 or 0.5 mg after 6 hours, 1 mg after a further 6 hours (n = 94). | |
| Outcomes | Change in cervical score (no significant difference), caesarean deliveries; interval from initial dose to the active phase of labor (9.8 +/‐ 5.8 and 14.2 +/‐ 10.2 hours, p < .01), interval from initial dose to delivery (15.3 +/‐ 9.8 and 19.1 +/‐ 13.2 hours, p = .027) for the misoprostol and dinoprostone groups, respectively. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
150 De la Torre 2001.
| Methods | Allocation by hospital pharmacy using computer‐generated randomisation schedule. Attendants not blinded after randomisation. FHR patterns were analysed blind to allocation. | |
| Participants | Inclusion criteria: medical indication for labour induction; single live fetus; cephalic presentation. Exclusion criteria: abnormal FHR tracing; uterine contractions every 5 minutes or less; previous uterine surgery; uterine overdistension; intra amniotic infection; hypersensitivity to prostaglandins; medical contraindications; contraindication to vaginal delivery. Use of fetal scalp sampling, and tocolytic therapy in response to non‐reassuring FHR tracings was left to the discretion of the managing physician. Indications for induction of labour: pre‐eclampsia (misoprostol 23, oxytocin 29); post‐term pregnancy (21, 15); diabetes mellitus (1, 1); oligohydramnios (8, 6); other (7, 15). |
|
| Interventions | Misoprostol 100 mcg in the posterior vaginal fornix every 4 hours until adequate uterine contractions achieved (maximum 5 doses), versus intravenous oxytocin commencing at 1 mU per minute, increase by 1 mU every 30 minutes to achieve adequate uterine activity (> 200 Montevideo units), maximum 36 mU per minute. Women in the misoprostol group received oxytocin augmentation if required, more than 4 hours after the last dose of misoprostol. Analysis was by intention to treat. All women were monitored continuously with electronic tocodynamometry, intrauterine pressure monitoring in 52%. Amniotomy was generally performed when cervical dilation was about 3‐4 cm. |
|
| Outcomes | Primary: caesarean section. Secondary: vaginal delivery in 24 hours (data from Kramer 1997 used); epidural analgesia (data from Kramer 1997 used); induction to delivery time; maternal and neonatal outcomes; uterine tachysystole (> 5 contractions/10 minutes for 20 minutes); hypertonus (a contraction lasting 2 minutes); hyperstimulation syndrome (tachysystole or hypertonus with FHR abnormality); caesarean section for fetal distress (misoprostol 23/168 vs oxytocin 15/192); use of terbutaline (Kramer 1997: misoprostol 12/60, oxytocin 2/66); abnormal FHR patterns; meconium stained amniotic fluid; epidural analgesia; Apgar scores; admission to NICU. | |
| Notes | University of New Mexico Health Sciences Centre, June 1995 to July 1998. Of 410 enrolled, 50 withdrawn for protocol deviation (16), patient withdrawal (7), or missing data (27). The final groups differed in numbers (misoprostol 168, oxytocin 192). This raises the possibility of selective withdrawal from the misoprostol group. In the abstract report, 58 women in the misoprostol group are reported on rather than 60. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
150 Kulshreshtha 2007.
| Methods | "A total of 40 women were randomly selected". The sequence generation and the allocation concealment were not stated. | |
| Participants | Inclusion criteria: primigravida and multigravida, gestational age ≥ 34 weeks, singleton gestation, cephalic presentation with indication for induction of labour. Exclusion criteria: abnormal FHR, multigravida ( more than 3), cephalopelvic disproportion, multiple pregnancy, unexplained vaginal bleeding, previous uterine surgery, malpresentation and contraindication to use prostaglandin. |
|
| Interventions | The intervention group received 100 mcg vaginal misoprostol 6 hourly (maximum 6 doses). The comparison group received 0.5 mg intra‐cervically dinoprostone 4 hourly (maximum 6 doses). | |
| Outcomes | The induction delivery interval was shorter in the misoprostol group (p < 0.05). There were very few maternal side effects in this study and no differences between the groups in neonatal outcomes. | |
| Notes | Department of Obstetrics & Gynaecology and Pharmacology of S. N. Medical College and Hospital, Agra. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unblinded only for personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
150 Ngai 2000.
| Methods | Allocation using sealed envelopes. | |
| Participants | Inclusion criteria: singleton pregnancy; > 7 weeks; cephalic presentation; ruptured membranes. Exclusion criteria: previous caesarean section; meconium‐stained liquor; allergy to prostaglandins. |
|
| Interventions | Misoprostol 100 mcg 4‐hourly (maximum 3 doses; if no contractions after 12 hours or poor progress, syntocinon used; versus oxytocin 1 mU per minute, increasing every 15 minutes (maximum 32 mU per minute). | |
| Outcomes | See analyses. | |
| Notes | 86 women enrolled. One excluded (undiagnosed breech presentation). Cervical scores missing on 5 women. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
175 Kadanali 1996.
| Methods | "Random" allocation by sealed envelopes. | |
| Participants | Membranes were intact or ruptured, cervical status unfavourable. Women with medical or obstetric indications for labour induction. Inclusion criteria: singleton vertex presentation; 37 to 42 weeks' gestation. Exclusion criteria: active labour; fetal distress; cervical score > 5. Indications for labour induction: post‐dates pregnancy (misoprostol 42, oxytocin 50); pre‐eclampsia (28, 22); premature rupture of membranes (10, 14); diabetes (6, 4); impaired fetal growth (8, 6); other (18, 16). |
|
| Interventions | Misoprostol 100 mcg into the posterior vaginal fornix, repeated orally every 2 hours until labour established (3 contractions in 10 minutes); oxytocin if not in active labour after 24 hours (n = 112); versus dinoprostone (Cerviprost) instilled into the cervix; oxytocin commenced if after 6 hours if indicated according to a uniform protocol. All women had continuous electronic FHR monitoring. Uterine hyperstimulation was managed by changing the mother's position to left lateral, oxygen by nasal catheter and intravenous ritodrine at 0.3 mg/minute. | |
| Outcomes | Labour induction to delivery interval (misoprostol mean 9.2, SD 2.4 vs dinoprostone/oxytocin 15.2. 3.2 hours); cervical score after 6 hours (6.5, 3.2 vs 6.0,3.6); 5 cm cervical dilation to delivery interval (1.6, 1.2 vs 7.8, 2.4 hours); fetal distress (4/112 vs 4/112); delivered within 12 hours (72/112 vs 28/112); uterine hyperstimulation with and without FHR changes; vacuum delivery; caesarean section; Apgar score < 5 at 5 minutes (2/112 vs 2/112); cord pH < 7.16 (8/112 vs 10/112). | |
| Notes | Erzurum, Turkey. March to August 1995. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
200 Lee 1997.
| Methods | Allocated by coded numbers in sealed envelopes. An independent clinician administered the allocated vaginal medication according to a master list. The rest of the staff were kept blind to the treatment given. | |
| Participants | Inclusion criteria: para 3 or less; singleton pregnancy; cephalic presentation; no previous caesarean section; no contraindication to prostaglandin therapy; uncomplicated pregnancy; cervical score 6 or less. | |
| Interventions | Misoprostol 200 mcg tablet versus dinoprostone 3 mg, inserted vaginally 6‐hourly, maximum 2 doses. The cervix was assessed every 6 hours. When 'ready for labour', transferred to the labour ward. If no labour ensued, oxytocin given. If the cervix remained unfavourable after 24 hours, caesarean section was performed. | |
| Outcomes | Established labour (misoprostol 23/25 vs dinoprostone 16/25); induction to delivery (676 SD 411 vs 875 SD 406 minutes); cervical score increase over 6 hours (3.3 SD 2 vs 2.0 SD 2); delivered within 6 hours (5/25 vs 3/25); delivered within 12 hours (18/25 vs 7/25); second dose of prostaglandin used (10/25 vs 17/25); estimated blood loss (180 SD 48 vs 246 SD 336 ml). | |
| Notes | Kuantan General Hospital, Malaysia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
200 Rowlands 2001.
| Methods | Random allocation using consecutively numbered sealed opaque envelopes. | |
| Participants | Inclusion criteria: nulliparous; singleton; cephalic; gestation > 37 weeks; no known contraindication to vaginal delivery; cervical score < 6. Exclusion criteria: known medical complications in pregnancy; fetal compromise; previous uterine surgery; active labour; rupture of membranes; previous attempt at induction of labour; contraindication to prostaglandins. |
|
| Interventions | Misoprostol 200 mcg vs dinoprostone 2 mg pessary vaginally; repeated if necessary after 24 and 30 hours. When cervical score 6 or more, membranes artificially ruptured and oxytocin administered. Continuous cardiotocography. | |
| Outcomes | Primary: induction to vaginal delivery interval (misoprostol 926 SD 569 vs dinoprostone 1578 SD 791); vaginal delivery < 12 hours (45/49 vs 36/47); duration of active labour to vaginal delivery (354 SD 221 vs 497 SD 266). Secondary outcomes: repeated doses (1/49 vs 16/47); artificial rupture of membranes; oxytocin augmentation; mode of delivery; non‐reassuring cardiotocograph; side effects; Apgar score; admission to NICU; epidural analgesia (21/49 vs 29/47); blood loss at delivery (263 SD 168 vs 268 SD 138). | |
| Notes | January 1996 to November 1998. Three Australian obstetric units. Women not blinded. Obstetrician responsible for labour care not informed of group allocation. One woman excluded from the misoprostol group after enrolment because cervical score 7. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
CTG: cardiotocograph FHR: fetal heart rate HELLP: haemolysis, elevated liver enzymes and low platelets ICU: intensive care unit IOL: induction of labour mcg: micrograms mU: milliunit NICU: neonatal intensive care unit NS: non‐stress para: paragraph sem: standard error of the mean SD: standard deviation vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adair 1998 | Comparison of oral versus vaginal misoprostol. For consideration for inclusion in the review of oral misoprostol for labour induction. |
| Aggarwal 2006 | Excluded because of methodological inconsistencies. |
| Arrieta 2000 | Randomised comparison of misoprostol in escalating dosages with oxytocin for labour induction. Excluded because the first dose of misoprostol was intracervical, not vaginal. |
| Azeem 2006 | Excluded because of methodological inconsistencies. |
| Balintona 2001 | Provisionally excluded pending full report, because data not available in usable format (abstract only). Misoprostol 50 mcg 6‐hourly x 2 doses vs Prepidil 0.5 mg 6‐hourly x 2 doses vs Cervidil 10 mg insert for 12 hours, intravaginally. Adverse cardiotocographic event in 50% vs 14 % vs 11.1 %). |
| Belfrage 2000 | Excluded because of exclusions from analysis. 4/110 women were excluded from the misoprostol group and 10/100 from the dinoprostone group due to lack of compliance with the protocol, and a further 8 in the dinoprostone group were excluded from further analysis because of failure of cervical ripening after 24 hours. |
| Bi 2000 | Excluded because data analysis was not based on the intention to treat. The randomisation and concealment were not reported in the text, only in the abstract. |
| Bolnick 2002a | Excluded because misoprostol 25 mcg 4‐hourly was compared with oxytocin, with concurrent dinoprostone (Cervidil) in both groups. There were no differences between the groups. |
| Bugalho 1995 | Allocation not random. A comparison of vaginal misoprostol and oxytocin for induction of labour. |
| Butler 2004 | For consideration in the oral misoprostol for induction of labour. |
| Cecatti 2001 | Abstract only. Brazilian 25 mcg tablet (Prostokos) compared with 1/8 200 mcg Cytotec tablet, 6‐hourly during the day for 48 hours. No differences in effectiveness. |
| Cecatti 2006 | The study compares 2 presentations of misoprostol (the vaginal specific 25 mcg presentation and the oral tablet divided in small parts:1/8 of oral presentation 200 mcg). Both groups used the same regimen and doses. |
| Cetin 1997 | Excluded because data not in prespecified format. Random allocation to misoprostol 100 mcg (n = 34) versus dinoprostone 0.5 mg intracervically (n = 34). Induction was 'successful' in 31/34 vs 28/34. Induction delivery interval was shorter with misoprostol (8.57 SD 4.03 vs 11.12 SD 2.07 hours). Uterine hyperstimulation occurred in 2/34 vs 1/34 respectively. |
| Chang 2003 | Excluded because misoprostol was administered intracervically, not vaginally. |
| Chen 2000 | Excluded because data analysis was not based on the intention to treat. |
| Chen 2001 | Excluded because data analysis was not based on the intention to treat. The randomisation and concealment were not reported in the text, only in the abstract. |
| Chen 2003 | Excluded because data analysis was not based on the intention to treat. The randomisation and concealment were not reported in the text, only in the abstract. |
| Chen 2004 | For consideration in the review of augmentation methods. |
| Cui 2001 | Excluded because data analysis was not based on the intention to treat. The randomisation and concealment were not reported in the text, only in the abstract. |
| Dai 2005 | Excluded because data analysis was not based on the intention to treat. |
| Delaney 2001 | Excluded provisionally, pending full report (insufficient information in abstract). |
| Ding 2001 | Excluded because data analysis was not based on the intention to treat. |
| Ding 2005 | Excluded because it is a brief communication with a lack of information on methods. No information concerning randomisation. |
| Ding 2006 | Excluded because data analysis was not based on the intention to treat. |
| Du 2000 | Excluded because data analysis was not based on the intention to treat. The randomisation and concealment were not reported in the text, only in the abstract. |
| Dundas 2000 | Excluded because no prespecified outcomes given in this abstract publication. To await fuller report. Misoprostol 50 mcg, followed after 12 hours by 50 mcg 6‐hourly (maximum 4 doses), versus dinoprostone 2 mg repeated if necessary after 12 hours and 1 mg after a further 6 hours. Allocation 'at random'. 11/257 withdrawn because of incomplete data. |
| Duru 1997 | Excluded because pregnancy termination in the second trimester was studied. |
| Echeverria 1995 | 'Randomised comparative study' ‐ excluded because method of randomisation not specified ‐ ?alternation. Inclusion criteria: postdates pregnancy (41 to 42 weeks, singleton, cephalic presentation, no cephalopelvic disproportion. Exclusion criteria: previous uterine surgery, placental insufficiency diagnosed by fetal monitoring and amniotic fluid volume). Interventions: vaginal misoprostol 100 mcg vs syntocinon infusion 2 to 32 mU per minute. Outcomes: delivery within 24 hours, time from induction to delivery, polysystole. Setting: Alto Riesgo Obstetric Clinic, 1 December 1994 to 30 April 1995. |
| Eftekhavi 2002 | Comparison of vaginal misoprostol with oxytocin for labour induction. Excluded because only percentages given in published abstract. Awaiting full report. |
| El‐Din 2000 | Excluded provisionally, pending full report (insufficient information in abstract). |
| Escalante 1993 | Excluded because does not fit the pre‐stated comparisons of this review. Labour was induced 'randomly' by either vaginal (n = 68) or intracervical (n = 32) misoprostol 100 mcg, repeated if necessary (in 4 women) after 24 hours. No statistically significant differences regarding cervical ripening and pregnancy outcome were found, though most of the data presented are for the whole group of 100 women. Maternal side effects occurred in 4 women. Uterine hyperstimulation occurred in 11 women, of whom 1 developed fetal distress which resolved with tocolytic therapy. There were 12 caesarean sections. |
| Fonseca 2007 | This study was excluded because of the inclusion of preterm pregnancies which were induced. |
| Girija 2006 | Only abstract available. There is no information on inclusion criteria, randomisation and allocation concealment. |
| Gorzelac 1999 | Excluded because there are no methodological details. |
| Gorzelac 2001 | Excluded because there is no information on randomisation. |
| Harms 2001 | Excluded provisionally, pending full report (insufficient information in abstract). |
| Hoesli 2003 | Abstract report only. Random allocation to misoprostol or dinoprostone for labour induction. Ultrasound measurement of cervical length. Vaginal delivery within 24 hours was compared for those with cervical length 0‐28 mm vs 29‐50 mm. Unable to use data as percentages only given. Awaiting full report. |
| How 2001 | Abstract only. Excluded because the comparison group was oral misoprostol. For consideration in the oral misoprostol review |
| Hu 2005 | Excluded because data analysis was not based on the intention to treat. |
| Jackson 1999 | Excluded provisionally because results not available in the abstract. |
| Jazayeri 2003 | Excluded because the inclusion/exclusion criteria are unclear and the results are presented using only percentages. Information on the size of each group is not stated. |
| Jouatte 2000 | Excluded because a retrospective study. |
| Kwon 1999 | For consideration for inclusion in the review of oral misoprostol. |
| Li 2003 | Excluded because data analysis was not based on the intention to treat. |
| Li XQ 2003 | Excluded because data analysis was not based on the intention to treat. The randomisation and concealment were not reported in the text, only in the abstract. |
| Liu 1998 | Excluded because data analysis was not based on the intention to treat. The randomisation and concealment were not reported in the text, only in the abstract. |
| Liu 2004 | Excluded because data analysis was not based on the intention to treat. |
| Lulu 1999 | Randomisation and concealment are not reported in the text, only in the abstract. |
| Luo 2000 | Excluded because data analysis was not based on the intention to treat. The randomisation and concealment were not reported in the text, only in the abstract. |
| Majoko 2002b | For consideration in the review of mechanical methods of labour induction. |
| Megalo 1998 | Abstract only. Excluded because the comparison group included both intracervical and intravaginal prostaglandins. There was an unexplained imbalance between the group size (89 vs 73). Full publication awaited. |
| Megalo 1999 | Excluded because no clinical outcomes reported. Comparison of cardiotocogram findings following labour induction with misoprostol or PGE2. |
| Molina 2000 | Excluded provisionally, pending full report (insufficient information in abstract). |
| Ngai 1996 | Excluded because misoprostol administered orally, not vaginally. To be considered for inclusion in the review 'Oral misoprostol for induction of labour'. |
| Nuthalapaty 2005 | Women between 14 and 24 weeks of pregnancy were included in this trial. |
| Ozgur 1997 | Excluded because 'randomisation' was according to odd and even hospital numbers. Large risk of allocation bias. |
| Patel 2000 | Excluded because misoprostol used for augmentation, not induction of labour. |
| Perry 1998 | Excluded because comparison was between vaginal misoprostol and a combination of an intracervical balloon catheter and dinoprostone. |
| Perry 1999 | Excluded because categorical data not given in abstract. Awaiting full report. |
| Porojanova 2005 | Excluded because there no reference to methods and to primary outcomes. |
| Roy 2003 | Study on the medical termination of pregnancies between 15 and 23 weeks of pregnancy. |
| Rust 1999 | For consideration for inclusion in the review of mechanical methods of labour induction. |
| Rust 2000 | For consideration for inclusion in the review of mechanical methods of labour induction. |
| Sabra 2000 | Excluded provisionally, pending full report (insufficient information in abstract). |
| Sanchez Ramos 1993 | Excluded because the intervention oxytocin preceded by prostaglandins when necessary was not a pre‐stated comparison. Random allocation by consecutively numbered, sealed opaque envelopes. Sequence generated by coin toss. Participants: membranes were intact or ruptured, cervical status variable. Women with obstetric or medical indications for induction of labour; singleton pregnancy; vertex presentation. Indications for labour induction: post‐dates pregnancy (misoprostol 14, oxytocin 10); pre‐eclampsia (22, 22); diabetes (3, 6); abnormal fetal testing (3, 9); ruptured membranes (9, 8); other (13, 10). Exclusion criteria: active labour; fetal distress; previous uterine surgery; contraindication to vaginal delivery. Interventions: misoprostol 50 mcg introduced into the posterior vaginal fornix 4‐hourly until adequate labour was achieved (maximum 12 doses, maximum actually used 4 doses); arrest of labour progress at 5 or more cm cervical dilation was managed with oxytocin augmentation; compared with oxytocin infusion commencing at 1‐2 mU per minute (preceded by cervical ripening with prostaglandin E2 gel if cervical score < 5, in 29/65 women). In both groups, artificial rupture of membranes was performed and intrauterine pressure monitoring and scalp electrode monitoring applied as soon as cervical dilation permitted. Fetal scalp sampling was performed for persistent FHR changes. Outcomes: undelivered 24 hours after initiating misoprostol or oxytocin; frequent uterine contractions (> 5 per 10 minutes); hyperstimulation (frequent contractions or prolonged contraction of 2 or more minutes, with fetal tachycardia, late decelerations or reduced short‐term variability). Jacksonville, Florida, USA. January to August 1992. Of 130 women enrolled, 1 (0.8%) was excluded after randomisation because of breech presentation. The authors conclude that the trial corroborates the effectiveness and apparent safety of misoprostol for labour induction. |
| Sanchez Ramos 2002 | Excluded because not a pre‐specified comparison. Misoprostol moistened with 1 ml 3% acetic acid compared with dry misoprostol, 50 mcg 4‐hourly vaginally, maximum 6 doses. Data on 162/177 randomised women given. No significant differences in time from start to vaginal delivery (moistened mean 1004, SD 636 versus dry 1130, 636 minutes); oxytocin (45/82 vs 48/80); no vaginal delivery within 24 hours 23/82 vs 32/80); hyperstimulation (6/82 vs 8/80); Caesarean section (12/82 vs 12/80); Apgar <7 at 5 minutes (1/82 vs 1/80); NICU admission (13/82 vs 14/80). |
| Sharma 2005 | 2 women undergoing caesarean section before the second dose of misoprostol excluded from the analysis. |
| Sheela 2006 | Excluded because no information was provided on the randomisation status, eligibility criteria or allocation strategy. |
| Shen 2003 | Excluded because data analysis was not based on the intention to treat. |
| Shi 2003a | Excluded because data analysis was not based on the intention to treat. The randomisation and concealment were not reported in the text, only in the abstract. |
| Shi 2003b | Excluded because data analysis was not based on the intention to treat. The randomisation and concealment were not reported in the text, only in the abstract. |
| Su 1998 | Excluded because data analysis was not based on the intention to treat. The randomisation and concealment were not reported in the text, only in the abstract. |
| Su 2003 | Excluded because data analysis was not based on the intention to treat. |
| Thach 2000 | Excluded provisionally, pending full report (insufficient information in abstract). |
| Tian 2003 | Excluded because data analysis was not based on the intention to treat. |
| Toppozada 1997 | For consideration for inclusion in review 'Oral misoprostol for induction of labour'. Women with cervical score < 5 were randomly allocated to induction of labour with vaginal misoprostol 100 mcg 3‐hourly, increasing to 200 mcg after 2 doses (maximum 1 mg), versus oral misoprostol 100 mcg 3‐hourly, increased to 200 mcg after the first dose. The induction to delivery interval was: vaginal mean 7.15 (standard deviation 4.39) hours vs oral 9.93 (3.68); side effects (nausea and vomiting) 2/20 vs 4/20; uterine hyperstimulation 8/20 vs 0/20; FHR changes 10/20 vs 1/20; caesarean section: 2/20 vs 4/20; instrumental vaginal delivery: 4/20 vs 2/20. |
| Varaklis 1994 | Excluded provisionally because no results available in the abstract. |
| Wang 2000 | Excluded because data analysis was not based on the intention to treat. |
| Wang 1997a | Excluded because the comparison group management does not fit into any of the pre‐defined comparison categories. Women with cervical score 4 or below, intact membranes and requiring labour induction were 'randomly divided' into 2 groups of 30. The study group received misoprostol 50 mcg vaginally 3‐hourly until labour was established (maximum 3 doses). The control group received 40 ml ricinus oil and 5 fried egg yolks; if not in labour after 12 hours, oxytocin was used; if the induction failed (not in labour after 24 hours), misoprostol was used. The number of women in labour with cervix 2 cm or more dilated after 24 hours was 28/30 for the study and 23/30 for the control group. The time from induction to vaginal delivery was 12.2 +/‐3.5 vs 18.1 +/‐ 3.2 hours respectively. Oxytocin during labour was used in 4/30 vs 12/30. Caesarean section was performed in 6/30 vs 6/30. Uterine hyperstimulation occurred in 5/30 vs 1/30. Diarrhoea or vomiting occurred in 2/30 vs 4/30, and low‐grade fever in 2/30 vs 0/30. |
| Wang 1997b | No predefined outcomes for this review reported. Vaginal misoprostol 100 mcg (n = 43) compared with placebo (n = 42). Oxytocin used after 12 hours if not in labour. The changes in cervical score at 12 hours were 4.4 +/‐ 2.2 vs 1.0 +/‐ 0.9; labour within 12 hours 29/43 vs 6/42; and medication to labour time 17.2 +/‐ 21.1 vs 40.6 +/‐ 26.0 hours respectively. No changes were noted in fetal blood flow indices. There were no differences in histology of 13 and 5 placentae studied. Uterine hyperstimulation occurred in 4/43 women in the misoprostol group. |
| Wang 1997c | Excluded because data analysis was not based on the intention to treat. |
| Wang 2005 | Excluded because data analysis was not based on the intention to treat. |
| Wicker 1995 | Study reported as an abstract only. Data are not available in a format suitable for analysis. The first author has been written to for further information. Intravaginal misoprostol gel 25 mcg 6 hourly was compared with 0.5 mg intracervical dinoprostone gel 6 hourly (maximum 3 doses), in 117 women with cervical scores of 5 or less and reassuring antenatal testing. Women receiving misoprostol had higher cervical scores after the first dose, shorter time from induction to oxytocin use and lower number of doses needed. No differences in complications were noted. |
| Wilk 2001 | Excluded because the analysis was not based on intention to threat. |
| Windrim 1997 | Excluded because misoprostol administered orally, not vaginally. To be considered for inclusion in the review 'Oral misoprostol for induction of labour'. |
| Wing 1999 | For consideration for inclusion in the 'oral misoprostol' review. |
| Yang 2000 | Excluded because data analysis was not based on the intention to treat. |
| Young 2001 | Excluded provisionally, pending full report (insufficient information in abstract). |
| Zang 1997 | Excluded because data analysis was not based on the intention to treat. |
| Zang 2003 | Excluded because data analysis was not based on the intention to treat. |
| Zhao 2003 | Excluded because data analysis was not based on the intention to treat. |
| Zhu 1998 | Excluded because data analysis was not based on the intention to treat. |
| Zhuang 2000 | Excluded because allocation not randomised. |
vs: versus
Characteristics of studies awaiting assessment [ordered by study ID]
Abedi‐Asl 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Ayaz 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Balci 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Balci 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Bebbington 2003a.
| Methods | Randomised controlled trial. |
| Participants | No description, only abstract available. |
| Interventions | Vaginal misoprostol 50 mcg 4 hourly until 3 doses vs dinoprostone 10 mg 4 hourly until 3 doses. |
| Outcomes | Presence of tachysystole, no references to primary outcomes of the review. |
| Notes | Awaiting full report. |
Bebbington 2003b.
| Methods | Randomised controlled trial. |
| Participants | No description, only abstract available. |
| Interventions | Vaginal misoprostol 50 mcg 4 hourly until 3 doses vs dinoprostone 10 mg 4 hourly until 3 doses. |
| Outcomes | Interval to active labour, need of oxytocin augmentation, mean of infused dose, route of delivery, Apgar score and NICU admissions. |
| Notes | Awaiting full report. |
Begum 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Bolnick 2002b.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Brennan 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Bricker 2007.
| Methods | "Randomized" study. |
| Participants | Women with > 34 weeks of pregnancy with PROM. |
| Interventions | "Misoprostol regimen" versus "vaginal dinoprostone and/ or intravenous oxytocin". |
| Outcomes | Rates of caesarian section and vaginal delivery not archived in 24h. |
| Notes | Awaiting full report for more precise methodological and intervention descriptions. |
Bricker 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Chaudhuri 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Chen 2000a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Deng 1999.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
ElSedeek 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Ezechi 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Girija 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Girija 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Gupta 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Gupta 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Hosli 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Joo 2000.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Kim 2000.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Li 2000.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Lughmani 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Mahendru 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Milchev 2003.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Moodley 2003.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Nigam 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Norzilawati 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Ozkan 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Pevzner 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Pevzner 2009a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Pevzner 2009b.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Pevzner 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Pezvner 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Powers 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Prager 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Rolland 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Saeed 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Shakya 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Shanmugham 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Stephenson 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Tabasi 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Tan 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Wang 2000.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Wing 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Wing 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Yang 2000a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Yin 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Botero 1998.
| Trial name or title | Oxytocin versus misoprostol for labour induction: a double blind randomised controlled trial. |
| Methods | |
| Participants | Pregnant women, aged between 15 and 45 years old. Indications for induction of labour: premature rupture of membranes at term; pregnancy‐induced hypertension at term; prolonged pregnancy; normal FHR tracing. |
| Interventions | Intravenous oxytocin, continuous infusion, 2 mU per minute (maximum 16 mU per minute) versus intravaginal misoprostol 100 mcg single dose. |
| Outcomes | Cesarean section, time to delivery, Apgar, fetal distress, tachysystole. |
| Starting date | July 1995. |
| Contact information | Luis Botero ‐ lfbotero@jkavercol.javeriana.edu.co. |
| Notes |
Gregson 2003.
| Trial name or title | To compare the safety and efficacy of low dose vaginal misoprostol and dinoprostone vaginal gel for induction of labour at term |
| Methods | Randomised trial. |
| Participants | Women at term (37 ‐ 42 completed weeks of gestation), single fetus, cephalic presentation, membrane may be intact or ruptured and reactive fetal heart tracing. |
| Interventions | IOL with vaginal misoprostol. |
| Outcomes | Uterine tachysystole, hyperstimulation, meconium stained liquor, Apgar score at 5 minutes, umbilical arterial pH and base deficit, neonatal unit admission, induction‐delivery interval, method of delivery, Bishop score at onset of labour, oxytocin requirements, mode of delivery and analgesia requirements in labour. |
| Starting date | 01 of July 2000 to 31 of December of 2003. |
| Contact information | Ms Sarah Gregson ‐ Queen Mary's Sidcoup NHS Trust. |
| Notes | Awaiting full publication. |
Jackson 2000.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
FHR: fetal heart rate IOL: induction of labour mU:
Contributions of authors
GJ Hofmeyr prepared the original version, and is responsible for maintaining the review. AM Gulmezoglu quality‐checked and revised the review.
C Pileggi critically appraised the studies and performed data extraction for the 2010 update of this review and contributed to the manuscript.
Sources of support
Internal sources
University of the Witwatersrand, South Africa.
University of Fort Hare, Eastern Cape, South Africa.
External sources
South African Medical Research Council, South Africa.
UNDP/UNFPA/WHO/World Bank (HRP), Switzerland.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
006 Ewert 2006 {published data only}
- Ewert K, Powers B, Robertson S, Alfirevic Z. Controlled‐release misoprostol vaginal insert in parous woman for labor induction. Obstetrics & Gynecology 2006;108:1130‐7. [DOI] [PubMed] [Google Scholar]
013 Papanikolaou 2004 {published data only}
- Papanikolaou EG, Plachouras N, Drougia A, Andronikou S, Vlachou C, Theodoros S, et al. Comparison of misoprostol and dinoprostone for elective induction of labour in nulliparous women at full term: a randomized prospective study. Reproductive Biology and Endocrinology 2004;2:70‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
013 Tedesco 2002 {published data only}
- Tedesco RP, Cecatti JG, Lourenco N, Filho M. Effectiveness of two different doses of vaginal misoprostol for cervical ripening and labor induction [Efetividade de duas diferentes doses de misoprostol por via vaginal para preparo cervical e inducao do parto]. Revista Brasileira de Ginecologia e Obstetricia 2002;24(10):614‐6. [Google Scholar]
019 Filho 2007 {published data only}
- Filho FAR, Alencar Junior CA, Feitosa FE, Arcanjo FCN. Low‐dose vaginal misoprostol (12.5 versus 25mcg) for induction of labor at term [Baixas doses de misoprostol vaginal (12.5 versus 25mcg) para indução de parto a termo]. Revista Brasileira de Ginecologia e Obstetrícia 2007;29(12):639‐46. [Google Scholar]
025 Elhassan 2005a {published data only}
- Elhassan EM, Mirghani OA, Adam I. Cervical ripening and labor induction with 25mcg versus 50mcg of intravaginal misoprostol. International Journal of Gynecology & Obstetrics 2005;90:234‐35. [DOI] [PubMed] [Google Scholar]
025G Srisomboon 1998 {published data only}
- Srisomboon J, Singchai S. A comparison between 25 micrograms and 50 micrograms of intravaginal misoprostol for labor induction. Journal of the Medical Association of Thailand 1998;81(10):779‐82. [PubMed] [Google Scholar]
025 Haghighi 2006 {published data only}
- Haghighi L. Intravaginal misoprostol in preterm premature rupture of membranes with low Bishop scores. International Journal of Gynecology & Obstetrics 2006;94(2):121‐2. [DOI] [PubMed] [Google Scholar]
025 Incerpi 2001 {published data only}
- Incerpi M, Fassett M, Kjos S, Tran S, Wing D. Vaginally administered misoprostol for outpatient labor induction in pregnancies with diabetes mellitus [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S120. [DOI] [PubMed] [Google Scholar]
- Incerpi MH, Fassett MJ, Kjos SL, Tran SH, Wing DA. Vaginally administered misoprostol for outpatient cervical ripening in pregnancies complicated by diabetes mellitus. American Journal of Obstetrics and Gynecology 2001;185:916‐9. [DOI] [PubMed] [Google Scholar]
025 Krupa 2005 {published data only}
- Graca Krupa F, Cecatti JG, Castro Surita FG, Milanez HM, Parpinelli MA. Misoprostol versus expectant management in premature rupture of membranes at term. BJOG: an international journal of obstetrics and gynaecology 2005;112(9):1284‐90. [DOI] [PubMed] [Google Scholar]
025 Kumar 2001 {published data only}
- Kumar S, Awasthi RT, Kapur A, Srinivas S, Parikh H, Sarkar S. Induction of labour with misoprostol ‐ a prostaglandin E1 analogue. Medical Journal Armed Forces of India 2001;57:107‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
025 Majoko 2001 {published data only}
- Majoko F, Zwizwai M, Lindmark G, Nystrom L. A randomised controlled trial of labour induction with misoprostol and prostaglandin F2α gel. 20th Conference on Priorities in Perinatal Care in Southern Africa; 2001 March 6‐9; KwaZulu‐Natal, South Africa. 2001.
025 Majoko 2002c {published data only}
- Majoko F, Zwizwai M, Nystrom L, Lindmark G. Vaginal misoprostol for induction of labour: a more effective agent than prostaglandin F2α gel and prostaglandin E2 pessary. Central African Journal of Medicine 2002;48(11‐12):123‐8. [PubMed] [Google Scholar]
025 McKenna 2004 {published data only}
- McKenna DS, Ester JB, Poffitt M, Waddell KR. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstetrics & Gynecology 2004;104(3):579‐84. [DOI] [PubMed] [Google Scholar]
025 Meyer 2005 {published data only}
- Meyer M, Pflum J. Outpatient administration of misoprostol decreases induction time. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S167. [Google Scholar]
- Meyer M, Pflum J, Howard D. Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstetrics & Gynecology 2005;105:466‐72. [DOI] [PubMed] [Google Scholar]
025 Oboro 2005 {published data only}
- Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent induction of labor to prevent post‐term pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2005;84(7):628‐31. [DOI] [PubMed] [Google Scholar]
025 Sheela 2007 {published data only}
- Sheela CN, Mhaskar A, George S. Comparison of vaginal misoprostol and oral misoprostol with intracervical dinoprostone gel for labour induction at term. Journal of Obstetrics and Gynaecology of India 2007;57(4):327‐30. [Google Scholar]
025 Stitely 2000 {published data only}
- Stitely ML, Browning J, Fowler M, Gendron RT, Gherman RB. Outpatient cervical ripening with intravaginal misoprostol. Obstetrics & Gynecology 2000;96:684‐8. [DOI] [PubMed] [Google Scholar]
025 Wang 1998 {published data only}
- Wang H, Li L, Pu L. The effect of 25mcg misoprostol on induction of labour in late pregnancy. Chinese Journal of Obstetrics and Gynecology 1998;33:469‐71. [PubMed] [Google Scholar]
025 Wing 1996 {published data only}
- Wing DA, Paul RH. A comparison of differing dosing regimens of vaginally administered misoprostol for preinduction cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1996;175:158‐64. [DOI] [PubMed] [Google Scholar]
025 Wing 1998a {published data only}
- Wing DA, Paul RH. Induction of labor with misoprostol for premature rupture of membranes beyond 36 weeks gestation. American Journal of Obstetrics and Gynaecology 1998;179(1):94‐9. [DOI] [PubMed] [Google Scholar]
- Wing DA, Paul RH. Induction of labor with misoprostol for premature rupture of membranes beyond 36 weeks gestation. American Journal of Obstetrics and Gynecology 1998;178:S93. [DOI] [PubMed] [Google Scholar]
025 Wing 1998b {published and unpublished data}
- Wing DA, Lovett K, Paul RH. Disruption of prior uterine incision following misoprostol for labour induction in women with previous cesarean delivery. Obstetrics & Gynecology 1998;91(5 Pt 2):828‐30. [DOI] [PubMed] [Google Scholar]
030 Moodley 2003 {published data only}
- Moodley J, Venkatachalam S, Songca P. Misoprostol for cervical ripening at and near term ‐ a comparative study. South African Medical Journal 2003;93:371‐4. [PubMed] [Google Scholar]
038 Aquino 2003 {published data only}
- Aquino MM, Cecatti JG. Misoprostol versus oxytocin for labor induction in term and post‐term pregnancy: randomized controlled trial. Sao Paulo Medical Journal 2003;121(3):102‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
038 Cecatti 2000 {published data only}
- Cecatti JG, Aquino MMA, Garcia GM, Rodrigues TMC. Misoprostol versus oxytocin for labor induction: randomized controlled trial. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 4). 2000:28.
038 Clark 1998 {published data only}
- Clark A, Cook V, Hill P, Spinnato J. Cervical ripening and labor induction: misoprostol vs dinoprostone. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S30. [Google Scholar]
038 El‐Sherbiny 2001 {published data only}
- El‐Sherbiny M. Vaginal misoprostol for labor induction 25ug versus 50ug dose regimens. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 4). 2000:30.
- El‐Sherbiny MT, El‐Gharieb IH, Gewley HA. Vaginal misoprostol for induction of labor: 25 vs. 50 ug dose regimen. International Journal of Gynecology & Obstetrics 2001;72:25‐30. [DOI] [PubMed] [Google Scholar]
038 Eroglu 2007 {published data only}
- Eroglu D, Oktem M, Yanik F, Kuscu E. Labor induction at term: a comparison of the effects of 50 microg and 25 microg vaginal misoprostol. Clinical and Experimental Obstetrics and Gynecology 2007;34(2):102‐5. [PubMed] [Google Scholar]
038 Gregson 2005 {published data only}
- Gregson S, Waterstone M, Norman I, Murrells T. A randomised controlled trial comparing low dose vaginal misoprostol and dinoprostone vaginal gel for inducing labour at term. BJOG: an international journal of obstetrics and gynaecology 2005;112:438‐44. [DOI] [PubMed] [Google Scholar]
038 Has 2002 {published data only}
- Has R, Batukan C, Ermis H, Cevher E, Araman A, Kilic G, et al. Comparison of 25 and 50 mcg vaginally administered misoprostol for preinduction of cervical ripening and labor induction. Gynecologic and Obstetric Investigation 2002;53:16‐21. [DOI] [PubMed] [Google Scholar]
038 Kidanto 2006 {published data only}
- Kidanto HL, Kaguta MM, Roosmalen J. Induction of labor with misoprostol or oxytocin in Tanzania. International Journal of Gynecology & Obstetrics 2006;96(1):30‐1. [DOI] [PubMed] [Google Scholar]
038 Krithika 2008 {published data only}
- Krithika KS, Aggarwal N, Suri V. Prospective randomised controlled trial to compare safety and efficacy of intravaginal misoprostol with intracervical cerviprime for induction of labour with unfavourable cervix. Journal of Obstetrics and Gynaecology 2008;28(3):294‐7. [DOI] [PubMed] [Google Scholar]
038 Meydanli 2003 {published data only}
- Meydanli MM, Caliskan E, Burak F, Narin MA, Atmaca R. Labor induction post‐term with 25 micrograms vs 50 micrograms of intravaginal misoprostol. International Journal of Gynecology & Obstetrics 2003;81:249‐55. [DOI] [PubMed] [Google Scholar]
038 Murthy 2006 {published data only}
- Murthy BK, Arkalgud MS. Misoprostol alone versus a combination of dinoprostone and oxytocin for induction of labour. Journal of Obstetrics and Gynaecology of India 2006;56(5):411‐6. [Google Scholar]
038 Van Gemund 2004 {published data only}
- Gemund N, Scherjon S, LeCessie S, Leeuwen JH, Roosmalen J, Kanhai HH. A randomised trial comparing low dose vaginal misoprostol and dinoprostone for labour induction. BJOG: an international journal of obstetrics and gynaecology 2004;111:42‐9. [DOI] [PubMed] [Google Scholar]
038 Wing 1997 {published data only}
- Wing DA, Ortiz‐Omphroy G, Paul RH. A comparison of intermittent vaginal administration of misoprostol with continuous dinoprostone for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1997;177:612‐8. [DOI] [PubMed] [Google Scholar]
- Wing DA, Paul RH. Vaginally administered misoprostol (Cytotec) versus the dinoprostone vaginal insert (Cervidil) for preinduction cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1997;176:S113. [DOI] [PubMed] [Google Scholar]
043 Diro 1999 {published data only}
- Diro M, Adra A, Gilles JM, Nassar A, Rodriguez A, Salamat SM, et al. A double‐blind randomized trial of two dose regimens of misoprostol for cervical ripening and labor induction. Journal of Maternal‐Fetal Medicine 1999;8(3):114‐8. [DOI] [PubMed] [Google Scholar]
043 Farah 1997 {published data only}
- Farah LA, Sanchez‐Ramos L, Rosa C, Valle GO, Gaudier FL, Delke I, et al. Randomised trial of two doses of the prostaglandin E1 analog misoprostol for labor induction. American Journal of Obstetrics and Gynecology 1997;177:364‐9. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Ramos L, Farah L, Rosa C, Johnson J, Delke I, Valle G. Comparative study of a two dose schedule of the PGE1 analogue misoprostol for labor induction in patients with an unfavorable cervix. American Journal of Obstetrics and Gynecology 1996;171(1 Pt 2):319. [Google Scholar]
044 Chen 2005 {published data only}
- Chen DC, Yuan SS, Su HY, Lo SC, Ren SS, Wu GJ. Urinary cyclic guanosine 3',5'‐monophosphate and cyclic adenosine 3',5'‐monophosphate changes in spontaneous and induced onset active labor. Acta Obstetricia et Gynecologica Scandinavica 2005;84:1081‐6. [DOI] [PubMed] [Google Scholar]
044 Nanda 2007 {published data only}
- Nanda S, Singhal SR, Papneja A. Induction of labour with intravaginal misoprostol and prostaglandin E2 gel: a comparative study. Tropical Doctor 2007;37(1):21‐4. [DOI] [PubMed] [Google Scholar]
044 Wing 1995b {published data only}
- Wing DA, Rahall A, Jones MM, Goodwin TM, Paul RH. Misoprostol: an effective agent for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1995;172:1811‐6. [DOI] [PubMed] [Google Scholar]
048 Khoury 2001 {published data only}
- Khoury A, Zhou Q, Gorenberg D, Nies B, Manley G, Mecklenburg F. A randomized clinical trial comparing misoprostol suppositories with continuous dinoprostone for cervical ripening and labor induction [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S118. [DOI] [PubMed] [Google Scholar]
- Khoury AN, Zhou Q‐P, Gorenberg DM, Nies BM, Manley GE, Mecklenburg FE. A comparison of intermittent vaginal administration of two different doses of misoprostol suppositories with continuous dinoprostone for cervical ripening and labor induction. Journal of Maternal Fetal Medicine 2001;10:186‐92. [DOI] [PubMed] [Google Scholar]
050 Abdul 2007 {published data only}
- Abdul MA, Ibrahim UN, Yusuf MD, Musa H. Efficacy and safety of misoprostol in induction of labour in a Nigerian tertiary hospital [L'efficacité et la sûreté de misoprosotl dans l'induction de travail dans un Hôpital Tertiaire Nigerian]. West African Journal of Medicine 2007;26(3):213‐6. [DOI] [PubMed] [Google Scholar]
050 Agarwal 2003 {published data only}
- Agarwal N, Gupta A, Kriplani A, Bhatla N, Parul N. Six hourly vaginal misoprostol versus intracervical dinoprostone for cervical ripening and labor induction. Journal of Obstetrics and Gynaecology Research 2003;29(3):147‐51. [DOI] [PubMed] [Google Scholar]
050 Ayad 2002 {published data only}
- Ayad IAA. Vaginal misoprostol in managing premature rupture of membranes. Eastern Mediterranean Health Journal 2002;8(4 & 5):515‐20. [PubMed] [Google Scholar]
050 Bounyasong 2000 {published data only}
- Bounyasong S. A randomized comparison between 25 microgram misoprostol gel and 50 microgram vaginal misoprostol tablet for induction of labour. Thai Journal of Obstetrics and Gynecology 2000;12(1):21‐5. [Google Scholar]
050 Calder 2008 {published data only}
- Calder AA, Loughney AD, Weir CJ, Barber JW. Induction of labour in nulliparous and multiparous women: a UK, multicentre, open‐label study of intravaginal misoprostol in comparison with dinoprostone. BJOG: an international journal of obstetrics and gynaecology 2008;115(10):1279‐88. [DOI] [PubMed] [Google Scholar]
- Calder AA, Loughney AJ, Denison F, Polson D, Erskine K, Dally JJ, et al. A randomised, open‐label comparison of intravaginal (APL202) and dinoprostone for cervical ripening and labour induction in nulliparae. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):58‐9. [DOI] [PubMed] [Google Scholar]
050 Campos Perez 1994 {published data only}
- Campos GA, Guzman S, Rodriguez JG, Voto LS, Margulies M. Misoprostol‐‐a PGE1 analog for induction of labor at term: comparative and randomized study with oxytocin [Misoprostol‐‐un analogo de la PGE1‐‐para la induccion de parto a termino: estudio comparativo y randomizado con oxitocina]. Revista Chilena de Obstetricia y Ginecologia 1994;59:190‐6. [PubMed] [Google Scholar]
- Campos Perez GA, Margulies M, Ortega I, Voto LS. Induction of labor with misoprostol, a PGE1 analog. Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30‐May 3; The Hague, Netherlands. 1991:97.
- Margulies M, Campos Perez G, Voto LS. Misoprostol to induce labour [letter]. Lancet 1992;339:64. [DOI] [PubMed] [Google Scholar]
050 Charoenkul 2000 {published data only}
- Charoenkul S, Sripramote M. A randomized comparison of one single dose of vaginal 50 mcg misoprostol with 3 mg dinoprostone in pre‐induction cervical ripening. Journal of the Medical Association of Thailand 2000;83:1026‐34. [PubMed] [Google Scholar]
050 Denguezli 2007 {published data only}
- Denguezli W, Trimech A, Haddad A, Hajjaji A, Saidani Z, Faleh R, et al. Eficacy and safety of six hourly vaginal misoprostol versus intracervical dinoprostone: a randomised controlled trial. Archives of Gynecology and Obstetrics 2007;276(2):119‐24. [DOI] [PubMed] [Google Scholar]
050 El‐Azeem 1997 {published data only}
- El‐Azeem S, Samuels P, Welch G, Staisch K. Term labor induction with PGE1 misoprostol versus PGE2 dinoprostone. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S113. [Google Scholar]
050 Elhassan 2004 {published data only}
- Elhassan M, Mirghani OA, Adam I. Intravaginal misoprostol versus dinoprostone as cervical ripening and labour inducing agents. International Journal of Gynecology & Obstetrics 2004;85:285‐6. [DOI] [PubMed] [Google Scholar]
050 Elhassan 2005b {published data only}
- Elhassan EM, Mirghani OA, Adam I. Misoprostol versus oxytocin for induction of labor. International Journal of Gynecology & Obstetrics 2005;91:254‐5. [DOI] [PubMed] [Google Scholar]
050 Frohn 2002 {published data only}
- Frohn WE, Simmons S, Carlan SJ. Prostaglandin E2 gel versus misoprostol for cervical ripening in patients with premature rupture of membranes after 34 weeks. Obstetrics & Gynecology 2002;99(2):206‐10. [DOI] [PubMed] [Google Scholar]
050G Carlan 1997 {published data only}
- Carlan SJ, Bouldin S, O'Brien WF. Extemporaneous preparation of misoprostol gel for cervical ripening: a randomized trial. Obstetrics & Gynecology 1997;90(6):911‐5. [DOI] [PubMed] [Google Scholar]
050 Gelisen 2005 {published data only}
- Gelisen O, Caliskan E, Dilbaz S, Ozdas E, Dilbaz B, Ozdas E, et al. Induction of labor with three different techniques at 41 weeks of gestation or spontaneous follow up until 42 weeks in women with definitely unfavorable cervical scores. European Journal of Obstetrics & Gynecology and Reprodutive Biology 2005;120(2):164‐9. [DOI] [PubMed] [Google Scholar]
050 Gotschall 1998 {published data only}
- Gottschall D, Borgida AF, Feldman DM, Alberti W, Rodis JF. Preinduction cervical ripening comparing 50 and 100 mcg of misoprostol. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S93. [DOI] [PubMed] [Google Scholar]
050 Kovavisarach 1997 {published data only}
- Kovavisarach E, Wattanasiri S. Comparison of intravaginal misoprostol and dinoprostone for cervical ripening and labour induction at term with unfavorable cervix: a randomized controlled study. Thai Journal of Obstetrics and Gynaecology 1997;9(3):175‐81. [Google Scholar]
050 Kovavisarach 1998 {published data only}
- Kovavisarach E, Worachet W. Randomized controlled trial of intravaginal 50 mcg misoprostol and 3 mg dinoprostone for cervical ripening and labour induction at term with unfavorable cervix. Thai Journal of Obstetrics and Gynaecology 1998;10(1):27‐32. [Google Scholar]
050 Le Roux 2002 {published data only}
- Roux PA, Olarogun JO, Penny J, Anthony J. Oral and vaginal misoprostol compared with dinoprostone for induction of labor: a randomized controlled trial. Obstetrics & Gynecology 2002;99(2):201‐5. [DOI] [PubMed] [Google Scholar]
050 Lokugamage 2003a {published data only}
- Lokugamage AU, Forsyth SF, Sullivan KR, Refaey H, Rodeck CH. Randomized trial in multiparous patients: investigating a singe vs. two‐dose regimen of intravaginal misoprostol for induction of labor. Acta Obstetricia et Gynecologica Scandinavica 2003;82:138‐42. [DOI] [PubMed] [Google Scholar]
050 Lokugamage 2003b {published data only}
- Lokugamage AU, Forsyth SF, Sullivan KR, Refaey H, Rodeck CH. Dinoprostone versus misoprostol: a randomized study of nulliparous women undergoing induction of labour. Acta Obstetricia et Gynecologica Scandinavica 2003;82:133‐7. [DOI] [PubMed] [Google Scholar]
050 Majoko 2002a {published data only}
- Majoko F, Nystrom L, Lindmark G. No benefit, but increased harm from high dose (100ug) misoprostol for induction of labour: a randomised trial of high vs. low (50ug) dose misoprostol. Journal of Obstetrics and Gynaecology 2002;22(6):614‐7. [DOI] [PubMed] [Google Scholar]
050 Mosquera 1999 {published data only}
- Mosquera J, Mesa JC, Navarro H, Cobo E, Neira C, Zuniga J. Study of the efficacy of misoprostol compared with oxytocin for labor induction in women with prolonged amenorrhea [Estudio de la eficacia de misoprostol comparado con oxitocina, en la induccion del parto en la amenorrea prolongada]. Revista Colombiana de Obstetricia y Ginecologia 1999;50(1):7‐12. [Google Scholar]
050 Neiger 2001 {published data only}
- Neiger R, Greaves PC. Comparison between vaginal misoprostol and cervical dinoprostone for cervical ripening and labor induction. Tennessee Medicine 2001;94(1):25‐7. [PubMed] [Google Scholar]
050 Ortiz 2002 {published data only}
- Morgan‐Ortiz F, Castro EQ, Martinez CBC, Barraza JB, Ramirez IO. Misoprostol and oxytocin for induction of cervical ripening and labor in patients with term pregnancy and premature membrane rupture [Misoprostol y oxitocina para induccion de madurez cervical y trabajo de parto en pacientes con embarazo a termino y ruptura prematura de membranas]. Ginecologia y Obstetricia de Mexico 2002;70:469‐76. [PubMed] [Google Scholar]
050 Pandis 2001 {published data only}
- Pandis GK, Papageorghiou AT, Otigbah CM, Howard RJ, Nikolaides KH. Randomized study of vaginal misoprostol (PGE1) and dinoprostone gel (PGE2) for induction of labour at term. Ultrasound in Obstetrics and Gynaecology 2001;18:629‐35. [DOI] [PubMed] [Google Scholar]
050 Ramsey 1998 {published data only}
- Ramsey PS, Harris DY, Ogburn PL, Heise RH, Magtibay PM, Ramin KD. Comparative cost analysis of prostaglandin analogues dinoprostone and misoprostol as labor preinduction agents [abstract]. Primary Care Update for Ob/Gyns 1998;5(4):182. [DOI] [PubMed] [Google Scholar]
050 Ramsey 2003 {published data only}
- Ramsey P, Harris D, Ogburn P, Heise R, Magtibay P, Ramin K. Comparative efficacy of prostaglandin analogues dinoprostone and misoprostol as labor preinduction agents. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S94. [Google Scholar]
- Ramsey P, Meyer L, Harris D, Ogburn P, Ramin K. Characterization of cardiotocographic abnormalities associated with dinoprostone and misoprostol cervical ripening/labor induction. American Journal of Obstetrics and Gynecology 2001;184(1):S115. [DOI] [PubMed] [Google Scholar]
- Ramsey PS, Harris DY, Ogburn PL, Heise RH, Magtibay PM, Ramin KD. Comparative efficacy and cost of the prostaglandin analogs dinoprostone and misoprostol as labor preinduction agents. American Journal of Obstetrics and Gynecology 2003;188(2):560‐5. [DOI] [PubMed] [Google Scholar]
050 Ramsey 2005 {published data only}
- Ramsey PS, Meyer L, Walkes BA, Harris D, Ogburn Jr PL, Heise RH, et al. Cardiotocographic abnormalities associated with dinoprostone and misoprostol cervical ripening. Obstetrics & Gynecology 2005;105(1):85‐90. [DOI] [PubMed] [Google Scholar]
050 Rozenberg 2001 {published data only}
- Rozenberg P, Chevret S, Goffinet F, Durand‐Zaleski I, Ville Y, Vayssiere C, et al. Induction of labour with a viable infant: a randomised clinical trial comparing intravaginal misoprostol and intravaginal dinoprostone. BJOG: an international journal of obstetrics and gynaecology 2001;108:1255‐62. [DOI] [PubMed] [Google Scholar]
050 Rozenberg 2004 {published data only}
- Rozenberg P, Chevret S, Senat MV, Bretelle F, Bonnal AP, Ville Y. A randomized trial that compared intravaginal misoprostol and dinoprostone vaginal insert in pregnancies at high risks of fetal distress. American Journal of Obsterics and Gynecology 2004;191:247‐53. [DOI] [PubMed] [Google Scholar]
050 Sahu 2004 {published data only}
- Sahu L, Chakravertty B. Comparison of prostaglandin E1 (misoprostol) with prostaglandin E2 (dinoprostone) for labour induction. Journal of Obsterics and Gynecology of India 2004;54(2):139‐42. [Google Scholar]
050 Sifakis 2007 {published data only}
- Sifakis S, Angelakis E, Avgoustinaks E, Fragouli Y, Mantas N, Koukoura O, et al. A randomized comparison between intravaginal misoprostol and prostaglandin E2 for labour induction. Archives of Gynecology and Obstetrics 2007;275:263‐7. [DOI] [PubMed] [Google Scholar]
050 Surbek 1997 {published data only}
- Surbek DV, Boesiger H, Hoesli I, Pavic N, Holzgreve W. A double‐blind comparison of the safety and efficacy of intravaginal misoprostol and prostaglandin E2 to induce labor. American Journal of Obstetrics and Gynecology 1997;177(5):1018‐23. [DOI] [PubMed] [Google Scholar]
- Surbek DV, Bosiger H, Hosli I, Pavic N, Holzgreve W. Cervical priming and labor induction with intravaginal misoprostol versus PGE2: a double‐blind randomized trial. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S112. [DOI] [PubMed] [Google Scholar]
- Surbek DV, Bosiger H, Pavic N, Hosli I, Stoz F, Holzgreve W. The safety of misoprostol for labor induction. Acta Obstetricia et Gynecologica Scandinavica 1997;76(167):36. [Google Scholar]
- Surbek DV, Bosiger H, Pavic N, Stoz F, Holzgreve W. Misoprostol (Cytotec) for labor induction in term pregnancies [Misoprostol (Cytotec) zur geburtseinleitung am termin]. 20th Congress of the Swiss Society of Gynecology and Obstetrics; 1997 June; Lugano, Switzerland. 1997:11.
050 Thomas 2000 {published data only}
- Thomas N, Longo SA, Rumney PJ, Nageotte MP, Asrat T. Intravaginal misoprostol in prelabor rupture of membranes at term. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S136. [Google Scholar]
058 Ferguson 2002 {published data only}
- Ferguson J, Head B, Frank F, Frank M, Singer J, Stefos T, et al. Misoprostol versus low‐dose oxytocin for cervical ripening: a prospective, randomized, double‐masked trial. American Journal of Obstetrics and Gynecology 2002;187:273‐80. [DOI] [PubMed] [Google Scholar]
063 Varaklis 1995 {published data only}
- Varaklis K, Gumina R, Stubblefield PG. Randomized controlled trial of vaginal misoprostol and intracervical prostaglandin E2 gel for induction of labor at term. Obstetrics & Gynecology 1995;86:541‐4. [DOI] [PubMed] [Google Scholar]
075 Buser 1997 {published data only}
- Arias F, Buser D, Mora G. Randomized comparison of misoprostol vs dinoprostone for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S141. [DOI] [PubMed] [Google Scholar]
- Buser D, Mora G, Arias F. A randomized comparison between misoprostol and dinoprostone for cervical ripening and labor induction in patients with unfavorable cervices. Obstetrics & Gynecology 1997;89(4):581‐5. [DOI] [PubMed] [Google Scholar]
075 Chang 1997 {published data only}
- Chang CH, Chang FM. Randomized comparison of misoprostol and dinoprostone for preinduction cervical ripening and labor induction. Journal of the Formosan Medical Association 1997;96:366‐9. [PubMed] [Google Scholar]
075 Chuck 1995 {published data only}
- Chuck F, Huffaker J. Labor induction with intravaginal prostaglandin E1 (PGE1) (Misoprostol, Cytotec) vs intracervical prostaglandin E2 (PGE2) (Dinoprostone, Prepidil gel): a randomized comparison. American Journal of Obstetrics and Gynecology 1995;172:424. [DOI] [PubMed] [Google Scholar]
- Chuck FJ, Huffaker BJ. Labor induction with intravaginal misoprostol versus intracervical prostaglandin E2 gel (Prepidil gel): randomized comparison. American Journal of Obstetrics and Gynecology 1995;173:1137‐42. [DOI] [PubMed] [Google Scholar]
075 Danielian 1999 {published data only}
- Danielian P, Porter B, Ferri N, Summers J, Templeton A. Misoprostol for induction of labour at term: a more effective agent than dinoprostone vaginal gel. British Journal of Obstetrics and Gynaecology 1999;106(8):793‐7. [DOI] [PubMed] [Google Scholar]
- Danielian PJ, Porter B. Induction of labour with misoprostol. Journal of Obstetrics and Gynaecology 1998;18(Suppl 1):S18‐S19. [Google Scholar]
075 Escudero 1997 {published data only}
- Escudero F, Contreras H. A comparative trial of labor induction with misoprostol versus oxytocin. International Journal of Gynecology & Obstetrics 1997;57:139‐43. [DOI] [PubMed] [Google Scholar]
075 Fuchs 2006 {published data only}
- Fuchs K, Brard L, Hodgman D, Silver H. Prostaglandin E1 gel vs. oxytocin for induction of labor at term. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S101. [Google Scholar]
075 Ghidini 2001 {published data only}
- Ghidini A, Spong CY, Korker V, Mariani E. Randomized controlled trial of 50 and 100 mcg of misoprostol for induction of labor at term. Archives of Gynecology and Obstetrics 2001;265:128‐30. [DOI] [PubMed] [Google Scholar]
075 Kolderup 1999 {published data only}
- Kolderup L, McLean L, Grullon K, Safford K, Kilpatrick SJ. Misoprostol is more efficacious for labor induction than prostaglandin E2, but is it associated with more risk?. American Journal of Obstetrics and Gynecology 1999;180(6 Pt 1):1543‐50. [DOI] [PubMed] [Google Scholar]
075 Lemancewicz 1999 {published data only}
- Lemancewicz A, Urban R, Scotnicki MZ, Karpiuk A, Urban J. Uterine and fetal Doppler flow changes after misoprostol and oxytocin therapy for induction of labor in post‐term pregnancies. International Journal of Gynecology & Obstetrics 1999;67:139‐45. [DOI] [PubMed] [Google Scholar]
075 Magtibay 1998 {published data only}
- Magtibay P, Ogburn P, Harris D, Suman V, Ramin K. Misoprostol as a labor induction agent: a pilot study comparing efficacy, safety and cost. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):327. [Google Scholar]
- Magtibay PM, Ramin KD, Harris DY, Ramsey PS, Ogburn PL. Misoprostol as a labor induction agent. Journal of Maternal Fetal Medicine 1998;7(1):15‐8. [DOI] [PubMed] [Google Scholar]
075 Megalo 2004 {published data only}
- Megalo A, Petignat P, Hohlfeld P. Influence of misoprostol or prostaglandin E2 for induction of labor on the incidence of pathological CTG tracing: a randomized trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;116:34‐8. [DOI] [PubMed] [Google Scholar]
075 Mundle 1996 {published data only}
- Mundle WR, Young DC. Vaginal misoprostol for induction of labor: a randomized controlled trial. Obstetrics & Gynecology 1996;88(4 Pt 1):521‐5. [DOI] [PubMed] [Google Scholar]
075 Reyna‐Villasmil 2005 {published data only}
- Reyna‐Villasmil E, Guerra‐Velasquez M, Torres‐Montilla M, Reyna‐Villasmil N, Mejia‐Montilla J, Labarca‐Vincero N. Comparative study of intravaginal misoprostol effect at 50 and 100 mcg in cervical ripening and labour induction [Estudio comparativo del efecto del misoprostol intravaginal a dosis de 50 y 100 mcg en la maturación cervical y la inducción del parto]. Investigacíon Clínica 2005;46(2):179‐86. [PubMed] [Google Scholar]
075 Sahin 2002 {published data only}
- Sahin HG, Sahin HA, Kocer M. Induction of labor in toxemia with misoprostol. Acta Obstetricia et Gynecologica Scandinavica 2002;81:252‐7. [DOI] [PubMed] [Google Scholar]
075 Saleen 2006 {published data only}
- Saleem S. Efficacy of dinoprostone, intracervical foleys and misoprostol in labour induction. Journal of the College of Physicians and Surgeons Pakistan 2006;16(4):276‐9. [PubMed] [Google Scholar]
075 S‐Ramos 1997 {published data only}
- Sanchez‐Ramos L, Chen A, Briones D, Valle GO, Gaudier FL, Delke I. Premature rupture of membranes at term: induction of labor with intravaginal misoprostol tablets (PGE1) or intravenous oxytocin. American Journal of Obstetrics and Gynecology 1994;170:377. [Google Scholar]
- Sanchez‐Ramos L, Chen AH, Kaunitz AM, Gaudier FL, Delke I. Labor induction with intravaginal misoprostol in term premature rupture of membranes: a randomized study. Obstetrics & Gynecology 1997;89:909‐12. [DOI] [PubMed] [Google Scholar]
075 Tabor 1995 {published data only}
- Tabor B, Anderson J, Stettler B, Wetwiska N, Howard T. Misoprostol vs prostaglandin E2 gel for cervical ripening. American Journal of Obstetrics and Gynecology 1995;172:425. [Google Scholar]
075 Urban 2003 {published data only}
- Urban R, Lemancewicz A, Urban J, Skotnicki MZ, Kretowska M. Misoprostol and dinoprostone therapy for labor induction: a Doppler comparison of uterine and fetal hemodynamic effects. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;106:20‐4. [DOI] [PubMed] [Google Scholar]
075 Webb 1997 {published data only}
- Webb GW, Raynor BD, Huddleston JF, Fandall HW. Induction of labor with an unfavorable cervix: a randomized prospective trial. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S22. [Google Scholar]
075 Zeteroglu 2004 {published data only}
- Zeteroglu S, Sahin HG, Sahin HA. Induction of labour with misoprostol in grand multiparous patients. International Journal of Gynecology & Obstetrics 2004;87:155‐6. [DOI] [PubMed] [Google Scholar]
075 Zeteroglu 2006a {published data only}
- Zeteroglu S, Engin‐Üstün Y, Üstün Y, Güvercinçi M, Sahin G, Kamaci M. A prospective randomized study comparing misoprostol and oxytocin for premature rupture of membranes at term. Journal of Maternal‐Fetal and Neonatal Medicine 2006;19(5):283‐7. [DOI] [PubMed] [Google Scholar]
075 Zeteroglu 2006b {published data only}
- Zeteroglu S, Sahin HG, Sahin HA. Induction of labour in great multipara with misoprostol. European Journal of Obstetrics & Gynecology and Reproductive Biology 2006;126(1):27‐32. [DOI] [PubMed] [Google Scholar]
075 Zeteroglu 2006c {published data only}
- Zeteroglu S, Sahin GH, Sahin HA. Induction of labor with misoprostol in pregnancies with advanced maternal age.. European Journal of Obstetrics & Gynecology and Reproductive Health 2006;129(2):140‐4. [DOI] [PubMed] [Google Scholar]
088 Garry 2003 {published data only}
- Garry D, Figueroa R, Kalish RB, Catalano CJ, Maulik D. Randomized controlled trial of vaginal misoprostol versus dinoprostone vaginal insert for labor induction. Journal of Maternal‐Fetal and Neonatal Medicine 2003;13:254‐9. [DOI] [PubMed] [Google Scholar]
088 Pi 1999 {published data only}
- Pi P, Zhu F. Clinical observation of misoprostol on induction in late pregnancy. Bulletin of Humam Medical University 1999;24:195‐7. [PubMed] [Google Scholar]
088 Saggaf 2001 {published data only}
- Saggaf A, Rouzi AA, Radhan B, Alshehry S, Yamani T, Abduljabbar H. Misoprostol for preinduction cervical ripening and induction of labor: a randomized controlled trial. Saudi Journal of Obstetrics and Gynecology 2001;1(2):89‐93. [Google Scholar]
088 Sanchez Ramos 1998 {published data only}
- Sanchez‐Ramos L, Peterson DE, Delke I, Gaudier FL, Kaunitz AM. Labor induction with prostaglandin E1 misoprostol compared with dinoprostone vaginal insert: a randomized trial. Obstetrics & Gynecology 1998;91(3):401‐5. [DOI] [PubMed] [Google Scholar]
088 Wing 1995a {published data only}
- Wing DA, Jones MM, Rahall A, Goodwin TM, Paul RH. A comparison of misoprostol and prostaglandin E2 gel for preinduction cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1995;172:1804‐10. [DOI] [PubMed] [Google Scholar]
100 Fletcher 1994 {published data only}
- Fletcher H, Mitchell S, Frederick J, Simeon D, Brown D. Intravaginal misoprostol versus dinoprostone as cervical ripening and labor‐inducing agents. Obstetrics & Gynecology 1994;83:244‐7. [PubMed] [Google Scholar]
100G Fletcher 1993 {published data only}
- Fletcher HM, Mitchell S, Simeon D, Frederick J, Brown D. Intravaginal misoprostol as a cervical ripening agent. British Journal of Obstetrics and Gynaecology 1993;100:641‐4. [DOI] [PubMed] [Google Scholar]
100G Gottschall 1997 {published data only}
- Gottschall D, Borgida AF, Mihalek JJ, Sauer F, Rodis JF. Misoprostol versus prostin E2 gel for preinduction cervical ripening. American Journal of Obstetrics and Gynecology 1997;176:S141. [DOI] [PubMed] [Google Scholar]
- Gottschall DS, Borgida AF, Mihalek JJ, Sauer F, Rodis JF. A randomized clinical trial comparing misoprostol with prostaglandin E2 gel for preinduction cervical ripening. American Journal of Obstetrics and Gynecology 1997;177(5):1067‐70. [DOI] [PubMed] [Google Scholar]
100G Srisomboon 1996 {published data only}
- Srisomboon J, Tongsong T, Tosiri V. Preinduction cervical ripening with intravaginal prostaglandin E1 analogue misoprostol: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 1996;22(2):119‐24. [DOI] [PubMed] [Google Scholar]
100G Srisomboon 1997 {published data only}
- Srisomboon J, Piyamongkol W, Aiewsakul P. Comparison of intracervical and intravaginal misoprostol for cervical ripening and labour induction in patients with an unfavourable cervix. Journal of the Medical Association of Thailand 1997;80(3):189‐94. [PubMed] [Google Scholar]
100 Herabutya 1997 {published data only}
- Herabutya Y, O‐Prasertsawat P, Pokirom J. A comparison of intravaginal misoprostol and intracervical prostaglandin E2 gel for ripening of unfavourable cervix and labor induction. Journal of Obstetrics and Gynaecology Researc 1997;23:369‐74. [DOI] [PubMed] [Google Scholar]
100 Howarth 1996 {published data only}
- Howarth GR, Funk M, Steytler P, Pistorius L, Makin J, Pattinson RC. A randomised controlled trial comparing vaginally administered misoprostol to vaginal dinoprostone gel in labour induction. Journal of Obstetrics and Gynaecology 1996;16:474‐8. [Google Scholar]
- Steytler P, Howarth G, Makin J. Cervical ripening and labour induction. Randomised controlled trial comparing misoprostol and dinoprostone vaginal gel. Proceedings of the 14th Conference on Priorities in Perinatal Care in South Africa; 1995 March 7‐10; South Africa. 1995:167‐70.
- Steytler P, Howarth GR, Funk M, Pistorius L, Makin J, Pattinson RC. A randomised control trial comparing vaginally administered misoprostol to vaginal dinoprostone gel in labour induction. 15th Conference on Priorities in Perinatal Care in Southern Africa; 1996 March 5‐8; Goudini Spa, South Africa. 1996:13‐4.
100 Montealegre 1999 {published data only}
- Montealegre JA, Botero LF, Sabogal G. Labor induction with unfavorable cervix: randomized controlled trial double blind method. Oxitocyn vs. misoprosto [Induccion de trabajo de parto con cervix desfavorable experimento clinico aleatorizado doble ciego de oxiotocina vs microprostol]. Revista Colombiana de Obstetricia y Ginecologia 1999;50(3):133‐7. [Google Scholar]
100 Ozsoy 2004 {published data only}
- Ozsoy M, Ozsoy D. Induction of labor with 50 and 100mcg of misoprostol: comparison of maternal and fetal outcomes. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;113:41‐4. [DOI] [PubMed] [Google Scholar]
138 Nunes 1999 {published data only}
- Nunes F, Rodrigues R, Meirinho M. Randomised comparison between intravaginal misoprostol and dinoprostone for cervical ripening and induction of labour. American Journal of Obstetrics and Gynecology 1999;181(3):626‐9. [DOI] [PubMed] [Google Scholar]
150 De la Torre 2001 {published data only}
- Torre S, Gilson GJ, Flores S, Curet LB, Qualls CE, Rayburn WF. Is high‐dose misoprostol able to lower the incidence of caesarean section? A randomized controlled trial. Journal of Maternal Fetal Medicine 2001;10(2):85‐90. [DOI] [PubMed] [Google Scholar]
- Kramer RL, Gilson G, Morrison DS, Martin D, Gonzales JL, Curet LB. A randomized trial of misoprostol and oxytocin for induction of labor: safety and efficacy. Obstetrics & Gynecology 1997;176(1 Pt 2):S111. [DOI] [PubMed] [Google Scholar]
- Kramer RL, Gilson GJ, Morrison DS, Martin D, Gonzales JL, Qualls CR. A randomized trial of misoprostol and oxytocin for induction of labor: safety and efficacy. Obstetrics & Gynecology 1997;89(3):387‐91. [DOI] [PubMed] [Google Scholar]
150 Kulshreshtha 2007 {published data only}
- Kulshreshtha S, Sharma P, Mohan G, Singh S, Singh S. Comparative study of misoprostol versus dinoprostone for induction of labour. Indian Journal of Physiology and Pharmacology 2007;51(1):55‐61. [PubMed] [Google Scholar]
150 Ngai 2000 {published data only}
- Ngai SW, Chan YM, Lam SW, Loa TT. Labour characteristics and uterine activity: misoprostol compared with oxytocin in women at term with prelabour rupture of membranes. BJOG: an international journal of obstetrics and gynaecology 2000;107(2):222‐7. [DOI] [PubMed] [Google Scholar]
175 Kadanali 1996 {published data only}
- Kadanali S, Kucukozkan T, Zor N, Kumtepe Y. Comparison of labor induction with misoprostol vs. oxytocin/prostaglandin E2 in term pregnancy. International Journal of Gynecology & Obstetrics 1996;55:99‐104. [DOI] [PubMed] [Google Scholar]
200 Lee 1997 {published data only}
- Lee HY. A randomised double‐blind study of vaginal misoprostol vs dinoprostone for cervical ripening and labour induction in prolonged pregnancy. Singapore Medical Journal 1997;38(7):292‐4. [PubMed] [Google Scholar]
200 Rowlands 2001 {published data only}
- Rowlands S, Bell R, Donath S, Morrow S, Trudinger BJ. Misoprostol versus dinoprostone for cervical priming prior to induction of labour in term pregnancy: a randomised control trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2001;41(2):145‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adair 1998 {published data only}
- Adair CD, Weeks JW, Barrilleaux PS, Philibert L, Edwards MS, Lewis DF. Labor induction with oral versus vaginal misoprostol: a randomized, double‐blind trial. American Journal of Obstetrics and Gynecology 1998;178:S93. [DOI] [PubMed] [Google Scholar]
Aggarwal 2006 {published data only}
- Aggarwal N, Kirthika KS, Suri V, Malhotra S. Comparative evaluation of vaginal PGE‐1 analogue (misoprostol) and intracervical PGE‐2 gel for cervical ripening and induction of labor. 49th All India Congress of Obstetrics and Gynaecology; 2006 Jan 6‐9; Cochin, Kerala State, India. 2006:95.
Arrieta 2000 {published data only}
- Arrieta OB, Yances BR, Ciodaro CM, Penaranda WA, Aguilera JB. Induction of labor at term with misoprostol vs oxytocin [Induccion de trabajo de parto con microprostol vs. oxitocina]. Revista Colombiana de Obstetricia y Ginecologia 2000;51(1):8‐11. [Google Scholar]
Azeem 2006 {published data only}
- Azeem S. Buccal vs intravaginal misoprostol administration for cervical ripening in induction of labor. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:95.
Balintona 2001 {published data only}
- Ballintona J, Meyer L, Ramin K, Vasdev G, Ramsey P. Cardiotocographic abnormalities associated with labor induction. Anesthesiology 2001; Vol. 94, issue 1A:A67.
Belfrage 2000 {published data only}
- Belfrage P, Smedvig E, Gjessing L, Eggebo T, Okland I. A randomized prospective study of misoprostol and dinoproston for induction of labour. Acta Obstetricia et Gynecologica Scandinavica 2000;79:1065‐8. [PubMed] [Google Scholar]
Bi 2000 {published data only}
- Bi SH, Xu KH, Xing AY, Liu Y. Labour induced by low dose misoprostol in late gestation: a randomized controlled trial. Journal of West China University of Medical Science 2000;31(4):518‐9. [Google Scholar]
Bolnick 2002a {published data only}
- Bolnick J, Velazquez M, Gonzalez J, Leslie K, Rappoport V, McIlwane G, et al. Randomized trial of sustained‐release vaginal dinoprostone (pge2) with concurrent oxytocin versus vaginal misoprostol (pg1) for induction of labor at term. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S175. [Google Scholar]
Bugalho 1995 {published data only}
- Bugalho A, Bique C, Machungo F, Bergstrom S. Vaginal misoprostol as an alternative to oxytocin for induction of labor in women with late fetal death. Acta Obstetricia et Gynecologica Scandinavica 1995;74:194‐8. [DOI] [PubMed] [Google Scholar]
Butler 2004 {published data only}
- Butler B, Crane J, Delaney T. Induction of labour with misoprostol in women at term with an unfavorable cervix: a randomized comparison of oral and vaginal administration [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S190. [Google Scholar]
Cecatti 2001 {published data only}
- Cecatti JG, Faundes A, Pires HMB, Calderon IMP. Labor induction in women with unripe cervix using two products containing misoprostol. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 1):283. [Google Scholar]
Cecatti 2006 {published data only}
- Cecatti JG, Tedesco RP, Pires HMB, Calderon IM, Faúndes A. Effectiveness and safety of a new vaginal misoprostol product specifically labeled for cervical ripening and labor induction. Acta Obstetricia et Gynecologica 2006;85:706‐11. [DOI] [PubMed] [Google Scholar]
Cetin 1997 {published data only}
- Cetin A, Cetin M, Taskurt A, Izgik E. Misoprostol versus dinoprostone for labor induction in term pregnancies [Term gebeliklerde dogum induksiyonunda dinoproston yerine misoprostol kullanimi]. Jinekoloji ve Obstetrik Dergisi 1997;11:51‐4. [Google Scholar]
Chang 2003 {published data only}
- Chang YK, Chen WH, Yu MH, Liu HS. Intracervical misoprostol and prostaglandin E2 for labor induction. International Journal of Gynecology & Obstetrics 2003;80:23‐8. [DOI] [PubMed] [Google Scholar]
Chen 2000 {published data only}
- Chen LY. Comparison between the oral and vaginal administration of misoprostol for labour induction at term. Journal of Shanghai Medical University 2000;27(1):77‐8. [Google Scholar]
Chen 2001 {published data only}
- Chen P, Zhai GR, Liu M. Clinical study of labour induced by low dose of misoprostol. Journal of Henan Medical College for Staff and Workers 2001;13(2):132. [Google Scholar]
Chen 2003 {published data only}
- Chen JT. A comparison between two dose of vaginal administration of misoprostol for labor induction at term. Xian Dai Shi Yong Yi Xue 2003;15(10):639. [Google Scholar]
Chen 2004 {published data only}
- Chen DC, Ku CH, Chen CH, Wu GJ. Urinary nitric oxide metabolite changes in spontaneous and induced onset active labor. Acta Obstetricia et Gynecologica Scandinavica 2004;83:641‐6. [DOI] [PubMed] [Google Scholar]
Cui 2001 {published data only}
- Cui XJ. Possibility and safety of application of misoprostol in the induction of pregnant women with pre‐eclampsia. Progress in Obstetetric and Gynecology 2001;10(2):126‐8. [Google Scholar]
Dai 2005 {published data only}
- Dai JR. Analysis of three methods for induction of labor in 330 cases. Chinese Journal of Maternity and Child Health 2005;20(24):3029. [Google Scholar]
Delaney 2001 {published data only}
- Delaney T, Crane J, Hutchens D, Fanning C, Young D. Induction of labor with intravaginal misoprostol: a comparison of dosing intervals. American Journal of Obstetrics and Gynecology 2001; Vol. 185, issue 6 Suppl:S202.
Ding 2001 {published data only}
- Ding HF, Xang XJ. Clinical study of labour induced by low dose misoprostol in late gestation. Journal of Wan Nan Medical College 2001;20(1):31‐2. [Google Scholar]
Ding 2005 {published data only}
- Ding DC, Hsu S, Su HY. Low dose intravaginal misoprostol for induction of labor at term. International Journal of Gynecology and Obstetrics 2005;90(1):71‐3. [DOI] [PubMed] [Google Scholar]
Ding 2006 {published data only}
- Ding YH, Zhan HQ. Clinical observation on three induced labour methods for late pregnancy. Clinical Medicine 2006;26(6):9‐10. [Google Scholar]
Du 2000 {published data only}
- Du M. Clinical study of labour induced by low dose misoprostol in late gestation. Chinese Journal Coal Industry Medicine 2000;3(4):301‐2. [Google Scholar]
Dundas 2000 {published data only}
- Dundas K, Howe D, Hughes R. Misoprostol for induction of labour in primigravidae [abstract]. Journal of Obstetrics and Gynaecology 2000;20(Suppl 1):S50. [Google Scholar]
Duru 1997 {published data only}
- Duru N, Atay V, Pabuccu R, Ergun A, Tokac G, Aydin BA. Vaginal misoprostol versus oxytocin‐prostaglandin e2 gel in severe preeclampsia remote from term. Acta Obstetricia et Gynecologica Scandinavica 1997;76(176):37. [Google Scholar]
Echeverria 1995 {published data only}
- Echeverria E, Rocha M. Randomised comparative study of induced labor with oxytocin and misoprostol in prolonged pregnancies. Revista Chilena de Obstetricia y Ginecologia 1995;60:108‐11. [PubMed] [Google Scholar]
Eftekhavi 2002 {published data only}
- Eftekhavi N. A comparison of vaginal misoprostol with intravenous oxytocin for cervical ripening and labor induction [abstract]. Journal of Obstetrics and Gynaecology Research 2002;28(1):47‐8. [Google Scholar]
El‐Din 2000 {published data only}
- El‐Din NMN, Moghazt DAM. Cervical ripening and induction of labour with misoprostol, prostaglandin E2 or prostaglandin E2 gel: a randomized comparative clinical trial [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 4). 2000:29.
Escalante 1993 {published data only}
- Escalante G, Ribas D, Esquivel A, Moya R, Sanchez LO, Pena YC. Misoprostol intracervical or vaginal. Clinical characteristics in delivery induction [Misoprostol intracervical vs. vaginal: caracteristicas clinicas en la induccion del parto]. Revista Costarricense de Ciencias Medicas 1993;14(3):43‐50. [Google Scholar]
Fonseca 2007 {published data only}
- Fonseca L, Lucas M, Wood H, Phat'Ak D, Susan R, Gilstrap L, et al. RCT of misoprostol pre‐induction ripening vs oxytocin induction. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S106, Abstract no: 348. [Google Scholar]
- Fonseca L, Wood HC, Lucas MJ, Ramin SM, Patak D, Gilstrap III LC, et al. Randomized trial of preinduction cervical ripening: misoprostol vs oxytocin. American Journal of Obstetrics and Gynecology 2008;199:305‐e1‐305‐e5. [DOI] [PubMed] [Google Scholar]
Girija 2006 {published data only}
- Girija S, Manjunath AP. Randomized controlled trial of vaginal misoprostol: single 50 mcg dose versus multiple 25 mcg dose for labour induction. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:40.
Gorzelac 1999 {published data only}
- Leszczynska‐Gorzelak B, Laskowska M, Oleszczuk J. Using of misoprostol for preinduction and induction in term pregnancy. Ginekologia Polska 1999;70(12):881‐9. [PubMed] [Google Scholar]
Gorzelac 2001 {published data only}
- Leszczynska‐Gorzelak B, Laskowska M, Oleszczuk J. Comparative analysis of the effectiveness of misoprostol and prostaglandin E2 in the pre‐induction and induction of labor. Medical Science Monitor 2001;7(5):1023‐8. [PubMed] [Google Scholar]
Harms 2001 {published data only}
- Harms K, Nguyen C, Toy EC, Baker B. Intravaginal misoprostol versus cervidil for cervical ripening in term pregnancies [abstract]. Obstetrics & Gynecology 2001; Vol. 97, issue 4 Suppl:36S.
Hoesli 2003 {published data only}
- Hoesli I, Gairing A, Lapaire O, Tercanli S, Holzgreve W. Induction of labour: cervical length measurement beside misoprostol or dinoproston ‐ is it a reliable factor both for patients and their obstetrical team?. Ultrasound in Obstetrics & Gynecology 2003;22(Suppl 1):149. [Google Scholar]
How 2001 {published data only}
- How H, Leaseburge L, Khoury J, Siddiqi T, Sibai B. Is there an ideal route of misoprostol administration for cervical ripening and labor indoction?. American Journal of Obstetrics and Gynecology 2001;184(1):S118. [DOI] [PubMed] [Google Scholar]
Hu 2005 {published data only}
- Hu JL, Liu ML, Zhang MX. Study on the medication of misoprostol to induce premature rupture of membranes at term. Practical Clinical Medicine 2005;6(8):71‐4. [Google Scholar]
Jackson 1999 {published data only}
- Jackson N, Irvine R, Edmonds D, Paterson‐Brown S. Random allocation controlled trial of intravaginal misoprostol versus intravaginal dinoprostone for the induction of labour. Journal of Obstetrics and Gynaecology 2000;20(Suppl 1):S52. [Google Scholar]
- Jackson NV, Irvine R, Paterson‐Brown S. Randomised, controlled trial of intravaginal misoprostol versus intravaginal dinoprostone gel in the induction of labour at term. Womens Health ‐ into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 Oct 3‐6; Cape Town, South Africa. 1999:32.
- Jackson NV, Terzidou V, Irvine RV, Edmonds DK, Paterson‐Brown S. Randomised controlled trial of intravaginal misoprostol versus intravaginal dinoprostone for the induction of labour. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 4). 2000:29‐30.
Jazayeri 2003 {published data only}
- Jazayeri A, Jazayeri M, Jamal A, Eslamian L, Maoorsi V, Borna S. Prospective randomized clinical trial of cervical ripening with misoprostol for either 8 or 24 hours. American Journal of Obstetrics and Gynecology 2003;6 Suppl 1:S70. [Google Scholar]
Jouatte 2000 {published data only}
- Jouatte F, Subtil D, Marquis P, Plennevaux JL, Puech F. Medical indications of labor induction: a comparison between intravaginal misoprostol and intravenous dinoprostone [Declenchement du travail d'indication medicale: comparaison du misoprostol intravaginal avec une prostaglandine E2 administree par voie intraveineuse]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction (Paris) 2000;29:763‐71. [PubMed] [Google Scholar]
Kwon 1999 {published data only}
- Kwon JS, MacKenzie VP, Davies GAL. A comparison of oral and vaginal misoprostol for induction of labour at term: a randomized trial. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S128. [DOI] [PubMed] [Google Scholar]
Li 2003 {published data only}
- Li SJ. Clinical study of different dose of misoprostol for induction of labour. China Clinical Medicine Research Journal 2003;94:9375‐6. [Google Scholar]
Liu 1998 {published data only}
- Liu D, Wang X. Clinical study of labour induced by low dose misoprostol in late gestation. Journal of Changzhi Medical College 1998;12(4)(4):282‐4. [Google Scholar]
Liu 2004 {published data only}
- Liu YJ. Clinical study on the induction of labour by low dose misoprostol in 100 cases. Chinese Journal of Today's Medicine 2004;4(3):33‐4. [Google Scholar]
Li XQ 2003 {published data only}
- Li XQ 2003. Clinical study of labour induced by low dose misoprostol in late gestation. China Clinical Medicine Research Journal 2003;96:9632‐3. [Google Scholar]
Lulu 1999 {published data only}
- Lulu D, Zaiju H. Observation on the efficacy of intravaginal misoprostol for cervical ripening in the third trimester of pregnancy and nursing care. Journal of Nursing Science 1999;14:1‐4. [Google Scholar]
Luo 2000 {published data only}
- Luo LM, Wu QK, Tao MF, Shen GF. Study on increasing safety of misoprostol for induction of labour in the third trimester of pregnancy. Shanghai Sheng Wu Yi Xue Gong Cheng Zha Zhi 2000;21(1):50‐3. [Google Scholar]
Majoko 2002b {published data only}
- Majoko F, Zwizwai M, Lindmark G, Nystrom L. Labor induction with vaginal misoprostol and extra‐amniotic prostaglandin F2 alpha gel. International Journal of Gynecology & Obstetrics 2002;76:127‐33. [DOI] [PubMed] [Google Scholar]
Megalo 1998 {published data only}
- Megalo A, Hohlfeld P. Misoprostol (PGE1) as an alternative to PGE2 for pre‐induction cervical ripening and labour induction. 21st Conference of the Swiss Society of Gynecology and Obstetrics. 1998:19.
Megalo 1999 {published data only}
- Megalo A, Hohlfield P. Cervical ripening and induction of labour with misoprostol (PG1): effects on the cardiotocogram [Maturation du col et induction du travail par le misoprostol (PGE1): effets sur le cardiotocogramme]. Gynakologisch‐Geburtshilfliche Rundschau 1999;39:165. [Google Scholar]
Molina 2000 {published data only}
- Molina M, Perez R, Fraenkel K, Vergara X. Oxitocin and misoprostol in the inducement of the delivery work of full‐term pregnant women. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 1). 2000:95.
Ngai 1996 {published data only}
- Ngai SW, To WK, Lao T, Ho PC. Cervical priming with oral misoprostol in pre‐labor rupture of membranes at term. Obstetrics & Gynecology 1996;87:923‐6. [DOI] [PubMed] [Google Scholar]
Nuthalapaty 2005 {published data only}
- Nuthalapaty FS, Ramsey PS, Biggio JR, Owen J. High‐dose vaginal misoprostol versus concentrated oxytocin plus low dose vaginal misoprostol for midtrimester labor induction: a randomized trial. American Journal of Obstetrics and Gynecology 2005;193:1065‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozgur 1997 {published data only}
- Ozgur K, Kizilates A, Uner M, Erman O, Trak B. Induction of labor with intravaginal misoprostol versus intracervical dinoprostone. Archives of Gynecology and Obstetrics 1997;261:9‐13. [DOI] [PubMed] [Google Scholar]
Patel 2000 {published data only}
- Patel A, Gilles JM, Moffett D, Mahram R, Diro M, Burkett G. Can misoprostol be interchanged with oxytocin for augmentation of labor?. Obstetrics & Gynecology 2000;95(4 Suppl):10S. [Google Scholar]
Perry 1998 {published data only}
- Perry KG, Larmon JE, May WL, Robinette LG, Martin RW. Cervical ripening: a randomized comparison between intravaginal misoprostol and intracervical balloon catheter combined with intravaginal dinoprostone. American Journal of Obstetrics and Gynecology 1998;178:1333‐40. [DOI] [PubMed] [Google Scholar]
Perry 1999 {published data only}
- Perry KG, Larmon JE, Rinehart BK, Gebhart LD, May WL, Martin RW. Cervical ripening: a randomized clinical trial of an intracervical balloon catheter combined with either intravaginal dinoprostone or misoprostol. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S127. [DOI] [PubMed] [Google Scholar]
Porojanova 2005 {published data only}
- Porojanova V, Sampath J, Porojanova K. Misoprostol and induction of labour. Akusherstvo i Ginekologiia 2005;44(5):27‐30. [PubMed] [Google Scholar]
Roy 2003 {published data only}
- Roy G, Ferreira E, Hudon L, Marquette G. The efficacy of oral versus vaginal misoprostol for second‐trimester termination of pregnancy: a double‐blind, randomized placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):S70. [Google Scholar]
Rust 1999 {published data only}
- Rust OA, Greybush M, Singleton C, Atlas RO, Baldicci J. A comparison of preinduction cervical ripening techniques. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S126. [Google Scholar]
Rust 2000 {published data only}
- Rust O, Greybush M, Atlas R, Balducci J, Jones K. Does combination pharmacological and mechanical pre‐induction cervical ripening improve ripening to delivery interval?. American Journal of Obstetrics and Gynecology 1999;182(1):S136. [Google Scholar]
Sabra 2000 {published data only}
- Sabra A, Abdel‐Aleem H, Abdel‐Aleem A, Shaheen A. Misoprostol versus oxytocin safety and efficacy in induction of labor. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 1). 2000:95‐6.
Sanchez Ramos 1993 {published data only}
- Sanchez Ramos L, Kaunitz AM, Valle GO, Delke I, Schroeder PA, Briones DK. Labor induction with the prostaglandin E1 methyl analogue misoprostol versus oxytocin: a randomized trial. Obstetrics & Gynecology 1993;81:332‐6. [PubMed] [Google Scholar]
- Sanchez‐Ramos L, Kaunitz AM, Valle GO, Delke I, Schroeder PA, Briones DK. Labor induction with the prostaglandin E1 methyl analog misoprostol vs oxytocin: a randomized trial. International Journal of Gynecology & Obstetrics 1993;43:229. [PubMed] [Google Scholar]
Sanchez Ramos 2002 {published data only}
- Sanchez‐Ramos L, Danner CJ, Delke I, Kaunitz AM. The effect of tablet moistening on labor induction with intravaginal misoprostol: a randomized trial. Obstetrics & Gynecology 2002;99(6):1080‐4. [DOI] [PubMed] [Google Scholar]
Sharma 2005 {published data only}
- Sharma Y, Kumar S, Mittal S, Misra R, Dadhwal V. Evaluation of glyceryl trinitrate, misoprostol, and prostaglandin E2 gel for preinduction cervical ripening in term pregnancy. Journal of Obstetric and Gynaecology Research 2005;31(3):210‐5. [DOI] [PubMed] [Google Scholar]
Sheela 2006 {published data only}
- Sheela SR, Swamy MN, Ambika V. Induction of labour with 25 mcg versus 100 mcg of misoprostol. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:54.
Shen 2003 {published data only}
- Shen JX. Clinical study of labour induced by low dose misoprostol in prolonged pregnancy. Chinese Journal of Today's Medicine 2003;3(8):42‐3. [Google Scholar]
Shi 2003a {published data only}
- Shi XH, Shang LP, Tang LP. Clinical study of labour induced by low dose misoprostol. Journal Yiyang Medical College 2003;22(5):294‐5. [Google Scholar]
Shi 2003b {published data only}
- Shi XH, Ding AY, Zhang LP. Clinical study of labour induced by low dose misoprostol. Journal Yiyang Medical College 2003;22(5)(5):294‐5. [Google Scholar]
Su 1998 {published data only}
- Su MM, Shen YX. Clinical study of labour induced by low dose misoprostol in late gestation. Journal of Ningbo Medicine 1998;10(6):270. [Google Scholar]
Su 2003 {published data only}
- Su FM, Yang B, Xu HL, Zhang XM. A comparison between the oral and vaginal administration of misoprostol for labour induction at term. Journal of Xianning College (Medical sciences) 2003;17(1):43‐6. [Google Scholar]
Thach 2000 {published data only}
- Thach TS, Jamulitrat S, Chongsuviwatong V, Geater A, Pham TD. Misoprostol: an effective alternative to oxytocin for labour induction in term premature rupture of membrane and unfavourable cervix. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 3). 2000:53.
Tian 2003 {published data only}
- Tian LJ, Li YG, Xiao J, Wu SF. Clinical study of labour induced by low dose misoprostol and oxytocin. Chinese Journal of Today's Medicine 2003;3(10):27‐8. [Google Scholar]
Toppozada 1997 {published data only}
- Toppozada MK, Anwar MY, Hassan HA, el‐Gazaerly WS. Oral or vaginal misoprostol for induction of labor. International Journal of Gynecology & Obstetrics 1997;56:135‐9. [DOI] [PubMed] [Google Scholar]
Varaklis 1994 {published data only}
- Varaklis K, Cuming R, Stubblefield P. Misoprostol: a prostaglandin E1 analogue. International Journal of Gynecology & Obstetrics 1994;46:105. [Google Scholar]
Wang 1997a {published data only}
- Wang L, Shi C, Yang GZ, Li YY. A comparison of misoprostol and ricinus oil meal for cervical ripening and labor induction. Chung Hua Fu Chan Ko Tsa Chih 1997;32(11):666‐8. [PubMed] [Google Scholar]
Wang 1997b {published data only}
- Wang Z, Li W, Ouyang W, Ding Y, Wang F, Xu L, et al. Cervical ripening in the third trimester of pregnancy with intravaginal misoprostol: a double‐blind, randomized, placebo‐controlled study. Journal of Tongji Medical University 1998;18(3):183‐6. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li W, Ouyang W, Ding Y, Wang F, Xu L, et al. Safety and efficacy of intravaginal misoprostol for cervical ripening in the third trimester of pregnancy. Chung Hua Fu Chan Ko Tsa Chih 1997;32(6):326‐8. [PubMed] [Google Scholar]
Wang 1997c {published data only}
- Wang XG. Clinical study of labour induced by low dose misoprostol ‐ 83 cases. Journal of Jiangxi Medical College 1997;4:2009. [Google Scholar]
Wang 2000 {published data only}
- Wang YX, Zheng YZ, Yang L. Clinical study of labour induced by low dose misoprostol. World Journal of Medicine Today 2000;1(4):335‐6. [Google Scholar]
Wang 2005 {published data only}
- Wang ZH. Clinical study of labour induced by low dose misoprostol in late gestation. Chinese Medicine Hygiene 2005;6(14):32‐3. [Google Scholar]
Wicker 1995 {published data only}
- Wicker R, Albert J, Laurent S, Bellitt P. Evaluation of misoprostol and dinoprostone in cervical ripening. American Journal of Obstetrics and Gynecology 1995;172:424. [Google Scholar]
Wilk 2001 {published data only}
- Wilk M, Jureczko T, Preba R, Spinski A. Misoprostol versus oxytocin in induction of labor in post‐term pregnancy ‐ safety and effectiveness comparison. Wiadomosci Lekarskie 2001;54(11‐12):662‐7. [PubMed] [Google Scholar]
Windrim 1997 {published data only}
- Windrim R, Bennett K, Mundle W, Young DC. Oral administration of misoprostol for labor induction: a randomized controlled trial. Obstetrics & Gynecology 1997;89:392‐7. [DOI] [PubMed] [Google Scholar]
Wing 1999 {published data only}
- Wing DA, Ham D, Paul RH. A comparison of orally administered misoprostol to vaginally administered misoprostol for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S127. [DOI] [PubMed] [Google Scholar]
Yang 2000 {published data only}
- Yang R. Clinical study of labour induced by low dose misoprostol in late gestation. Continuing Medical Education 2000;14(2):43‐4. [Google Scholar]
Young 2001 {published data only}
- Young D, Delaney T, Armson T, Fanning C. Lower dose vaginal and oral misoprostol in labor induction ‐ rct [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S203. [Google Scholar]
Zang 1997 {published data only}
- Zhang LJ, Qu LP, Guo WX, Liu ZH. Clinical study of labour induced by low dose misoprostol. Journal of Ningxia Medicine 1997;19(6):362. [Google Scholar]
Zang 2003 {published data only}
- Zang CY. Clinical study of different doses of misoprostol for the induction of labour. Chinese Clinical Medicine Journal 2003;59:9839‐40. [Google Scholar]
Zhao 2003 {published data only}
- Zhao YL. Clinical study of labour induced by low dose misoprostol in late gestation. China Clinical Medicine Research Journal 2003;81:134‐42. [Google Scholar]
Zhu 1998 {published data only}
- Zhu HX, Hu LY, Luo Y, Li XQ. Clinical study on labour induction by low dose misoprostol. Jiang Shu Clinical Medical Journal 1998;2(3):255‐6. [Google Scholar]
Zhuang 2000 {published data only}
- Zhuang G, Su Q, Zhang J, Xie J, Zhu J, Cheng L. Study on misoprostol suspension per os for inducing term labor. Chinese Journal of Obstetric and Gynaecological Practice 2000;16(8):481‐3. [Google Scholar]
References to studies awaiting assessment
Abedi‐Asl 2007 {published data only}
- Abedi‐Asl Z, Farrokhi M, Rajaee M. Comparative efficacy of misoprostol and oxytocin as labor preinduction agents: a prospective randomized trial. Acta Medica Iranica 2007;45(6):443‐8. [Google Scholar]
Ayaz 2010 {published data only}
- Ayaz A, Shaukat S, Farooq MU, Mehmood K, Ahmad I, Ali Bahoo ML. Induction of labor: a comparative study of intravaginal misoprostol and dinoprostone. Taiwanese Journal of Obstetrics & Gynecology 2010;49(2):151‐5. [DOI] [PubMed] [Google Scholar]
Balci 2010 {published data only}
- Balci O, Mahmoud AS, Ozdemir S, Acar A. Induction of labor with vaginal misoprostol plus oxytocin versus oxytocin alone. International Journal of Gynecology & Obstetrics 2010;110(1):64‐7. [DOI] [PubMed] [Google Scholar]
Balci 2011 {published data only}
- Balci O, Mahmoud AS, Acar A, Colakoglu MC. Comparison of induction of labor with vaginal misoprostol plus oxytocin versus oxytocin alone in term primigravidae. Journal of Maternal‐Fetal and Neonatal Medicine 2011;24(9):1084‐7. [DOI] [PubMed] [Google Scholar]
Bebbington 2003a {published data only}
- Bebbington M, Pevzner L, Schmuel E, Bernstein P, Dayal A, Barnhard J, et al. Uterine tachysystole and hyperstimulation during induction of labor [abstract]. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):S211. [Google Scholar]
Bebbington 2003b {published data only}
- Bebbington M, Schmuel E, Pevzner L, Bernstein P, Dayal A, Barnhard J, et al. Misoprostol versus dinoprostone for labor induction at term: a randomized controlled trial [abstract]. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):S211. [Google Scholar]
Begum 2009 {published data only}
- Begum A, Butt RM, Kouser S, Naqvi S. Comparison of dinoprostone (pge2) and misoprostol (pge1) for induction of labor. Journal of Allama Iqbal Medical College 2009;7(4):20‐4. [Google Scholar]
Bolnick 2002b {published data only}
- Bolnick JM, Velazquez MD, Gonzalez JL, Rappaport VJ, McIlwain‐Dunivan G, Rayburn WF. Randomized trial between two active labor management protocols in the presence of an unfavorable cervix. American Journal of Obstetrics and Gynecology 2004;190(1):124‐8. [DOI] [PubMed] [Google Scholar]
Brennan 2011 {published data only}
- Brennan MC, Pevzner L, Wing DA, Powers BL, Rayburn WF. Retention of dinoprostone vaginal insert beyond 12 hours for induction of labor. American Journal of Perinatology 2011;28(6):479‐84. [DOI] [PubMed] [Google Scholar]
Bricker 2007 {published data only}
- Bricker L, Peden H, Alfirevic Z. The prommis trial: a multicentre randomised trial to evaluate a low dose misoprostol regimen for induction of labour in the presence of prelabour rupture of the amniotic membranes. Journal of Obstetrics and Gynaecology 2007;27(Suppl 1):S22‐S23. [Google Scholar]
Bricker 2008 {published data only}
- Bricker L, Peden H, Tomlinson AJ, Al‐Hussaini TK, Idama T, Candelier C, et al. Titrated low‐dose vaginal and/or oral misoprostol to induce labour for prelabour membrane rupture: a randomised trial. BJOG: an international journal of obstetrics and gynaecology 2008;115(12):1503‐11. [DOI] [PubMed] [Google Scholar]
Chaudhuri 2011 {published data only}
- Chaudhuri S, Mitra SN, Banerjee PK, Biswas PK, Bhattacharyya S. Comparison of vaginal misoprostol tablets and prostaglandin E2 gel for the induction of labor in premature rupture of membranes at term: a randomized comparative trial. Journal of Obstetrics and Gynaecology Research 2011;37(11):1564‐71. [DOI] [PubMed] [Google Scholar]
Chen 2000a {published data only}
- Chen TM. Clinical analysis of misoprostol on induction of labor in term pregnancy. Journal of Zhenjiang Medical College 2000, (4):652‐3. [Google Scholar]
Deng 1999 {published data only}
- Deng LL, Huang ZJ. Observation on the efficacy of intravaginal misoprostol for cervical ripening in the third trimester of pregnancy. Journal of Nursing Science 1999;14(2):67‐8. [Google Scholar]
ElSedeek 2009 {published data only}
- ElSedeek M, Awad EE, ElSebaey SM. Evaluation of postpartum blood loss after misoprostol‐induced labour. BJOG: an international journal of obstetrics and gynaecology 2009;116(3):431‐5. [DOI] [PubMed] [Google Scholar]
Ezechi 2008 {published data only}
- Ezechi OC, Loto OM, Ezeobi PM, Okogbo FO, Gbajabiamila T, Nwokoro CA. Safety and efficacy of misoprostol in induction of labour in prelabour rupture of fetal membrane in Nigerian women: a multicenter study. Iranian Journal of Reproductive Medicine 2008;6(2):83‐7. [Google Scholar]
Girija 2009 {published data only}
- Girija S, Manjunath AP. Comparison of two dosing regimens of vaginal misoprostol for labour induction: a randomised controlled trial. Journal of the Turkish German Gynecology Association Artemis 2009;10(4):220‐5. [PMC free article] [PubMed] [Google Scholar]
Girija 2011 {published data only}
- Girija S, Manjunath AP. A randomized controlled trial comparing low dose vaginal misoprostol and dinoprostone gel for labor induction. Journal of Obstetrics and Gynecology of India 2011;61(2):153‐60. [Google Scholar]
Gupta 2006 {published data only}
- Gupta N, Mishra SL, Shradha J. A randomized clinical trial comparing misoprostol and dinoprostone for cervical ripening and labor induction. Journal of Obstetrics and Gynecology of India 2006;56(2):149‐51. [Google Scholar]
Gupta 2010 {published data only}
- Gupta HP, Singh U, Mehrotra S. Comparative evaluation of 25 ug and 50ug of intravaginal misoprostol for induction of labor. Journal of Obstetrics and Gynecology of India 2010;60(1):51‐4. [Google Scholar]
Hosli 2008 {published data only}
- Hosli I, Zanetti‐Daellenbach R, Gairing A, Holzgreve W, Lapaire O. Selection of appropriate prostaglandin for the induction of labor at term is more predictive for the achievement of delivery within 24 hours than pre‐assessed cervical parameters ‐ a prospective, randomized trial. Geburtshilfe und Frauenheilkunde 2008;68(2):147‐51. [Google Scholar]
Joo 2000 {published data only}
- Joo SH, Hur EJ, Park JW, Lee WK. A comparison of the safety and efficacy of intravaginal prostaglandin e1 ( misoprostol ) and prostaglandin e2 ( dinoprostone ) to induce labor. Korean Journal of Obstetrics and Gynecology 2000;43(3):444‐50. [Google Scholar]
Kim 2000 {published data only}
- Kim JH, Yang HS. A comparison of intravaginal misoprostol and dinoprostone for cervical ripening and labor inducton in term pregnancy with unfavorable cervix. Korean Journal of Obstetrics and Gynecology 2000;43(2):243‐7. [Google Scholar]
Li 2000 {published data only}
- Li FM. A study of misoprostol on induction of labor in term pregnancy. Journal of Practical Obstetrics and Gynecology 2000;16(3):139‐41. [Google Scholar]
Lughmani 2009 {published data only}
- Lughmani S. Vaginal misoprostol versus oxytocin infusion for labour induction in great grand multipara. A randomized controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S250. [Google Scholar]
Mahendru 2011 {published data only}
- Mahendru R, Yadav S. Shortening the induction delivery interval with prostaglandins: a randomized controlled trial of solo or in combination. Journal of the Turkish German Gynecology Association 2011;12(2):80‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milchev 2003 {published data only}
- Milchev N, Kuzmanov B, Terzhumanov R. Cytotec: an effective drug for the induction of labor [Cytotec: efektivno sredstvo za induktsiia na razhdaneto]. Akusherstvo i Ginekologiia 2003;42(3):9‐11. [PubMed] [Google Scholar]
Moodley 2003 {published data only}
- Moodley J, Venkatachalam S, Songca P. Misoprostol for cervical ripening at and near term ‐ a comparative study. South African Journal of Obstetrics and Gynaecology 2003;9(2):34‐7. [PubMed] [Google Scholar]
Nigam 2010 {published data only}
- Nigam A, Madan M, Puri M, Agarwal S, Trivedi SS. Labour induction with 25 micrograms versus 50 micrograms intravaginal misoprostol in full term pregnancies. Tropical Doctor 2010;40(1):53‐5. [DOI] [PubMed] [Google Scholar]
Norzilawati 2010 {published data only}
- Norzilawati MN, Mashita MK, Shuhaila A, Zaleha AM. Vaginal misoprostol versus dinoprostone for induction of labor. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(S1):244. [Google Scholar]
Ozkan 2009 {published data only}
- Ozkan S, Caliskan E, Doger E, Yucesoy I, Ozeren S, Vural B. Comparative efficacy and safety of vaginal misoprostol versus dinoprostone vaginal insert in labor induction at term: a randomized trial. Archives of Gynecology and Obstetrics 2009;280(1):19‐24. [DOI] [PubMed] [Google Scholar]
Pevzner 2008 {published data only}
- Pevzner L, Rumney P, Petersen R, Wing D. Predicting a successful induction of labor: a secondary analysis of misoprostol vaginal insert trial. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S72. [Google Scholar]
Pevzner 2009a {published data only}
- Pevzner L, Alfirevic Z, Powers B, Wing D. Cardiotocographic abnormalities associated with misoprostol and dinoprostone cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S124. [DOI] [PubMed] [Google Scholar]
Pevzner 2009b {published data only}
- Pevzner L, Rayburn WF, Rumney P, Wing DA. Factors predicting successful labor induction with dinoprostone and misoprostol vaginal inserts. Obstetrics & Gynecology 2009;114(2 Pt 1):261‐7. [DOI] [PubMed] [Google Scholar]
Pevzner 2011 {published data only}
- Pevzner L, Alfirevic Z, Powers BL, Wing DA. Cardiotocographic abnormalities associated with misoprostol and dinoprostone cervical ripening and labor induction. European Journal of Obstetrics & Gynecology and Reproductive Biology 2011;156(2):144‐8. [DOI] [PubMed] [Google Scholar]
Pezvner 2011 {published data only}
- Pezvner L, Powers BL, Wing DA. Factors predicting successful induction of labor with misoprostol vaginal insert. Reproductive Sciences 2011;18(3 Suppl 1):182A‐3A. [Google Scholar]
Powers 2011 {published data only}
- Powers B, Parker L, Miller H, Wing DA, Rayburn W. A double‐blind, randomized, multicenter, dose‐ranging phase II study of the misoprostol vaginal insert. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S48. [Google Scholar]
Prager 2008 {published data only}
- Prager M, Eneroth‐Grimfors E, Edlund M, Marions L. A randomised controlled trial of intravaginal dinoprostone, intravaginal misoprostol and transcervical balloon catheter for labour induction. BJOG: an international journal of obstetrics and gynaecology 2008;115(11):1443‐50. [DOI] [PubMed] [Google Scholar]
Rolland 2011 {published data only}
- Rolland Souza A. [Titrated oral suspension compared with vaginal misoprostol for labor induction: a randomized controlled trial] [Misoprostol em solução oral titulada escalonada versus via vaginal para indução do trabalho de parto: ensaio clínico randomizado]. Revista Brazileira de Ginecologia e Obstetricia 2011;33(9):270. [Google Scholar]
Saeed 2011 {published data only}
- Saeed GA, Fakhar S, Nisar N, Alam AY. Misoprostol for term labor induction: a randomized controlled trial. Taiwanese Journal of Obstetrics & Gynecology 2011;50(1):15‐9. [DOI] [PubMed] [Google Scholar]
Shakya 2010 {published data only}
- Shakya R, Shrestha J, Thapa P. Safety and efficacy of misoprostol and dinoprostone as cervical ripening agents. JNMA, Journal of the Nepal Medical Association 2010;49(177):33‐7. [PubMed] [Google Scholar]
Shanmugham 2011 {published data only}
- Shanmugham D, Behera AK. Comparison of prostaglandin E1 (misoprostol) with prostaglandin E2 (Cerviprime gel) for induction of labour in primigravidae. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:112.
Stephenson 2011 {published data only}
- Stephenson ML, Pevzner L, Powers BL, Wing DA. Race/ethnicity differences in labor outcomes with misoprostol and dinoprostone vaginal inserts. Reproductive Sciences 2011;18(3 Suppl 1):183A. [Google Scholar]
Tabasi 2007 {published data only}
- Tabasi Z, Behrashi M, Mahdian M. Vaginal misoprostol versus high dose of oxytocin for labor induction: a comparative study. Pakistan Journal of Biological Sciences 2007;10(6):920‐3. [DOI] [PubMed] [Google Scholar]
Tan 2010 {published data only}
- Tan TC, Yan SY, Chua TM, Biswas A, Chong YS. A randomised controlled trial of low‐dose misoprostol and dinoprostone vaginal pessaries for cervical priming. BJOG: an international journal of obstetrics & gynaecology 2010;117(10):1270‐7. [DOI] [PubMed] [Google Scholar]
Wang 2000 {published data only}
- Wang YZ, Zhu BL, Li M, Bu YG. Clinical observation of the effects of accelerating the maturation of the cervix and induction by various doses of misoprostol. Anhui Medical Journal 2000;21(2):23‐4. [Google Scholar]
Wing 2008 {published data only}
- Wing DA, for the Misoprostol Vaginal Insert Consortium. Misoprostol vaginal insert compared with dinoprostone vaginal insert: a randomized controlled trial. Obstetrics & Gynecology 2008;112(4):801‐12. [DOI] [PubMed] [Google Scholar]
Wing 2011 {published data only}
- Wing DA, Miller H, Parker L, Powers BL, Rayburn WF, for theMVIM‐O‐204I. Misoprostol vaginal insert for successful labor induction. A randomized controlled trial. Obstetrics & Gynecology 2011;117(3):533‐41. [DOI] [PubMed] [Google Scholar]
Yang 2000a {published data only}
- Yang Z, Wang YL. A comparison of misoprostol and prostaglandin e2 gel for cervical ripening and induction of labour: a clinical study on efficacy of two different dosages gemfibrozil administered in hyperlipidemic patients. Journal of Jinzhou Medical College 2000;21(1):10‐2. [Google Scholar]
Yin 2006 {published data only}
- Yin CY, Zhou JZ, Wang BP, Lu XY. Effect and risk analysis of misoprostol in stimulating cervical maturity for post‐term pregnancy. Nan Fang Yi Ke Da Xue Xue Bao//Journal of Southern Medical University 2006;26(2):182‐4; 188. [PubMed] [Google Scholar]
References to ongoing studies
Botero 1998 {unpublished data only}
- Botero L. Labor induction with unfavourable cervix. Randomized controlled trial. Double blind method. Oxytocin versus misoprostol. Personal communication 1998.
Gregson 2003 {published data only}
- Gregson S. To compare the safety and efficacy of 'low dose' vaginal misoprostol and dinoprostone vaginal gel for induction of labour at term. Current Controlled Trials (www.controlled‐trials.com/mrct) (accessed 15 September 2004).
Jackson 2000 {published data only}
- Jackson N, Paterson‐Brown S. Labour characteristics and uterine activity: misoprostol compared with oxytocin in women at term with prelabour rupture of the membranes [letter]. BJOG: an international journal of obstetrics and gynaecology 2000;107(9):1181‐2. [DOI] [PubMed] [Google Scholar]
Additional references
Alfirevic 2006
- Alfirevic Z, Weeks A. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD001338.pub2] [DOI] [PubMed] [Google Scholar]
Bennett 1997
- Bennett BB. Uterine rupture during induction of labor at term with intravaginal misoprostol. Obstetrics & Gynecology 1997;89(5 Pt 2):832‐3. [DOI] [PubMed] [Google Scholar]
Bishop 1964
- Bishop EH. Pelvic scoring for elective induction. Obstetrics & Gynecology 1964;24:266‐8. [PubMed] [Google Scholar]
Blanchette 1999
- Blanchette HA, Nayak S, Erasmus S. Comparison of the safety and efficacy of intravaginal misoprostol (prostaglandin E1) with those of dinoprostone (prostaglandin E2) for cervical ripening and induction of labor in a community hospital. American Journal of Obstetrics and Gynecology 1999;180:1551‐9. [DOI] [PubMed] [Google Scholar]
Boulvain 2001
- Boulvain M, Kelly AJ, Lohse C, Stan CM, Irion O. Mechanical methods for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001233] [DOI] [PubMed] [Google Scholar]
Boulvain 2005
- Boulvain M, Stan CM, Irion O. Membrane sweeping for induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD000451.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulvain 2008
- Boulvain M, Kelly AJ, Irion O. Intracervical prostaglandins for induction of labour. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006971] [DOI] [PubMed] [Google Scholar]
Bricker 2000
- Bricker L, Luckas M. Amniotomy alone for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002862] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choy‐Hee 2001
- Choy‐Hee L, Raynor BD. Misoprostol induction of labor among women with a history of cesarean delivery. American Journal of Obstetrics and Gynecology 2001;184:1115‐7. [DOI] [PubMed] [Google Scholar]
Clarke 2000
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.1 [updated June 2000]. In: Review Manager (RevMan) [Computer program]. Version 4.1 Oxford, England: The Cochrane Collaboration, 2000.
Costa 1993
- Costa SH, Vessey MP. Misoprostol and illegal abortion in Rio de Janeiro, Brazil. Lancet 1993;341:1258‐61. [DOI] [PubMed] [Google Scholar]
Curtis 1987
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91‐5. [PubMed] [Google Scholar]
Daisley 2000
- Daisley H. Maternal mortality following the use of misoprostol. Medicine, Science and the Law 2000;40(1):78‐82. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. London: BMJ Books, 2001. [Google Scholar]
Egarter 1990
- Egarter CH, Husslein PW, Rayburn WF. Uterine hyperstimulation after low‐dose prostaglandin E2 therapy: tocolytic treatment in 181 cases. American Journal of Obstetrics and Gynecology 1990;163:794‐6. [DOI] [PubMed] [Google Scholar]
Fonseca 1991
- Fonseca W, Alencar AJC, Mota FSB, Coelho HLL. Misoprostol and congenital malformations. Lancet 1991;338:56. [DOI] [PubMed] [Google Scholar]
French 2001
- French L. Oral prostaglandin E2 for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003098] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gherman 1999
- Gherman RB, McBrayer S, Browning J. Uterine rupture associated with vaginal birth after cesarean section: a complication of intravaginal misoprostol?. Gynecologic and Obstetric Investigation 2000;50:212‐3. [DOI] [PubMed] [Google Scholar]
Hapangama 2009
- Hapangama D, Neilson JP. Mifepristone for induction of labour. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002865.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008a
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Higgins 2008b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008.. Available from www.cochrane‐handbook.org.
Hill 2000
- Hill DA, Chez RA, Quinlan J, Fuentes A, LaCombe J. Uterine rupture and dehiscence associated with intravaginal misoprostol cervical ripening. Journal of Reproductive Medicine 2000;45:823‐6. [PubMed] [Google Scholar]
Hofmeyr 2009
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Neilson JP, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002074.pub2] [DOI] [Google Scholar]
Howarth 2001
- Howarth G, Botha DJ. Amniotomy plus intravenous oxytocin for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003250] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2001
- Hutton EK, Mozurkewich EL. Extra‐amniotic prostaglandin for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003092] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2001
- Kavanagh J, Kelly AJ, Thomas J. Sexual intercourse for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003093] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2005
- Kavanagh J, Kelly AJ, Thomas J. Breast stimulation for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003392.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2006a
- Kavanagh J, Kelly AJ, Thomas J. Corticosteroids for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003100.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2006b
- Kavanagh J, Kelly AJ, Thomas J. Hyaluronidase for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003097.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keirse 1993
- Keirse MJNC. Prostaglandins in preinduction cervical ripening: meta‐analysis of worldwide clinical experience. Journal of Reproductive Medicine 1993;38 Suppl:89‐98. [PubMed] [Google Scholar]
Kelly 2001a
- Kelly AJ, Tan BP. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003246] [DOI] [PubMed] [Google Scholar]
Kelly 2001b
- Kelly AJ, Kavanagh J, Thomas J. Relaxin for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003103] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2001c
- Kelly AJ, Kavanagh J, Thomas J. Castor oil, bath and/or enema for cervical priming and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003099] [DOI] [PubMed] [Google Scholar]
Kelly 2003
- Kelly AJ, Kavanagh J, Thomas J. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003101] [DOI] [PubMed] [Google Scholar]
Kelly 2008
- Kelly AJ, Kavanagh J. Nitric oxide donors for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006901] [DOI] [Google Scholar]
Khosla 2002
- Khosla AH, Sirohiwal D, Sangwan K. A still birth and uterine rupture during induction of labour with oral misoprostol. Australia and New Zealand Journal of Obstetrics and Gynaecology 2002;42(4):412. [DOI] [PubMed] [Google Scholar]
Luckas 2000
- Luckas M, Bricker L. Intravenous prostaglandin for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002864] [DOI] [PMC free article] [PubMed] [Google Scholar]
MacKenzie 1997
- MacKenzie IZ, Burns E. Randomised trial of one versus two doses of prostaglandin E2 for induction of labour: 1. Clinical outcome. British Journal of Obstetrics and Gynaecology 1997;104:1062‐7. [DOI] [PubMed] [Google Scholar]
Mariani Neto 1987
- Mariani Neto C, Leao EJ, Baretto EM, Kenj G, Aquino MM. Use of misoprostol for labour induction in stillbirths. Revista Paulista de Medicina 1987;105:325‐8. [PubMed] [Google Scholar]
Mariani Neto 1988
- Mariani Neto C, Delbin AL, Val RD. Tocographic pattern caused by misoprostol [Padrao tocografico desencadeado pelo misoprostol]. Revista Paulista de Medicina 1988;106:205‐8. [PubMed] [Google Scholar]
Matonhodze 2002
- Matonhodze BB, Katsoulis LC, Hofmeyr GJ. Labor induction and meconium: in vitro effects of oxytocin, dinoprostone and misoprostol on rat ileum relative to myometrium. Journal of Perinatal Medicine 2002;30(5):405‐10. [DOI] [PubMed] [Google Scholar]
Matthews 1999
- Mathews JE, Mathai M, George A. Uterine rupture in a multiparous woman during labor induction with oral misoprostol. American Journal of Obstetrics and Gynecology 1999;181(3):626‐9. [DOI] [PubMed] [Google Scholar]
Merrell 1995
- Merell DA, Koch MAT. Induction of labour with misoprostol in the second and third trimesters of pregnancy. South African Medical Journal 1995;85:1088‐90. [PubMed] [Google Scholar]
Merrell 1996
- Merrell DA, Koch MAT, Thomas PC. Experience with vaginal and rectal misoprostol as an oxytocic agent in pregnancy.. PAFMACH Conference; 1996; Johannesburg, South Africa. 1996.
Mitri 1987
- Mitri F, Hofmeyr GJ, Gelderen CJ. Meconium during labour: self‐medication and other associations. South African Medical Journal 1987;71:431‐3. [PubMed] [Google Scholar]
Muzonzini 2004
- Muzonzini G, Hofmeyr GJ. Buccal or sublingual misoprostol for cervical ripening and labour induction. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004221.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Norman 1991
- Norman JE, Thong KJ, Baird DT. Uterine contractility and induction of abortion in early pregnancy by misoprostol and mifepristone. Lancet 1991;338:1233‐6. [DOI] [PubMed] [Google Scholar]
Plaut 1999
- Plaut MM, Schwartz ML, Lubarsky SL. Uterine rupture associated with the use of misoprostol in the gravid patient with a previous cesarean section. American Journal of Obstetrics and Gynecology 1999;180(6 pt 1):1532‐42. [DOI] [PubMed] [Google Scholar]
Ravasia 2000
- Ravasia DJ, Wood SL, Pollard JK. Uterine rupture during induced trial of labor among women with previous cesarean delivery. American Journal of Obstetrics and Gynecology 2000;183:1176‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Sanchez‐Ramos 1997
- Sanchez‐Ramos L, Kaunitz AM, Wears RL, Delke I, Gaudier FL. Misoprostol for cervical ripening and labor induction: a meta‐analysis. Obstetrics & Gynecology 1997;89:633‐42. [DOI] [PubMed] [Google Scholar]
Sciscione 1998
- Sciscione AC, Nguyen L, Manley JS, Shlossman PA, Colmorgen GH. Uterine rupture during preinduction cervical ripening with misoprostol in a patient with a previous caesarean delivery. Australian and New Zealand Journal of Obstetrics and Gynaecology 1998;38(1):96‐7. [PubMed] [Google Scholar]
Senior 1993
- Senior J, Marshall K, Sangha R, Clayton JK. In vitro characterisation of prostanoid receptors on human myometrium at term pregnancy. British Journal of Pharmacology 1993;108:501‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2003
- Smith CA. Homoeopathy for induction of labour. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003399] [DOI] [PubMed] [Google Scholar]
Smith 2004
- Smith CA, Crowther CA. Acupuncture for induction of labour. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002962.pub2] [DOI] [PubMed] [Google Scholar]
Thomas 2001
- Thomas J, Kelly AJ, Kavanagh J. Oestrogens alone or with amniotomy for cervical ripening or induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003393] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wing 1999b
- Wing DA. Labor induction with misoprostol. American Journal of Obstetrics and Gynecology 1999;181(2):339‐45. [DOI] [PubMed] [Google Scholar]
Zieman 1997
- Zieman M, Fong SK, Benowitz NL, Banskter D, Darney PD. Absorption kinetics of misoprostol with oral or vaginal administration. Obstetrics & Gynecology 1997;90:88‐92. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
CDSR 1999
- Hofmeyr GJ. Misoprostol administered vaginally for cervical ripening and labour induction in the third trimester (Cochrane Review). Cochrane Database of Systematic Reviews 1998, Issue 3. [DOI] [PubMed] [Google Scholar]
CDSR 2000
- Hofmeyr GJ, Gulmezoglu AM. Vaginal misoprostol for cervical ripening and labour induction in late pregnancy (Cochrane Review). Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI] [PubMed] [Google Scholar]
CDSR 2004
- Hofmeyr GJ, Gülmezoglu AM. Vaginal misoprostol for cervical ripening and induction of labour (Cochrane Review). Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI] [PubMed] [Google Scholar]


