Abstract
Background
Eclampsia, the occurrence of a seizure (fit) in association with pre‐eclampsia, is rare but potentially life‐threatening. Magnesium sulphate is the drug of choice for treating eclampsia. This review assesses its use for preventing eclampsia.
Objectives
To assess the effects of magnesium sulphate, and other anticonvulsants, for prevention of eclampsia.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (4 June 2010), and the Cochrane Central Register of Controlled Trials Register (The Cochrane Library 2010, Issue 3).
Selection criteria
Randomised trials comparing anticonvulsants with placebo or no anticonvulsant, or comparisons of different drugs, for pre‐eclampsia.
Data collection and analysis
Two authors assessed trial quality and extracted data independently.
Main results
We included 15 trials. Six (11,444 women) compared magnesium sulphate with placebo or no anticonvulsant: magnesium sulphate more than a halved the risk of eclampsia (risk ratio (RR) 0.41, 95% confidence interval (CI) 0.29 to 0.58; number needed to treat for an additional beneficial outcome (NNTB) 100, 95% CI 50 to 100), with a non‐significant reduction in maternal death (RR 0.54, 95% CI 0.26 to 1.10) but no clear difference in serious maternal morbidity (RR 1.08, 95% CI 0.89 to 1.32). It reduced the risk of placental abruption (RR 0.64, 95% CI 0.50 to 0.83; NNTB 100, 95% CI 50 to 1000), and increased caesarean section (RR 1.05, 95% CI 1.01 to 1.10). There was no clear difference in stillbirth or neonatal death (RR 1.04, 95% CI 0.93 to 1.15). Side effects, primarily flushing, were more common with magnesium sulphate (24% versus 5%; RR 5.26, 95% CI 4.59 to 6.03; number need to treat for an additional harmful outcome (NNTH) 6, 95% CI 5 to 6).
Follow‐up was reported by one trial comparing magnesium sulphate with placebo: for 3375 women there was no clear difference in death (RR 1.79, 95% CI 0.71 to 4.53) or morbidity potentially related to pre‐eclampsia (RR 0.84, 95% CI 0.55 to 1.26) (median follow‐up 26 months); for 3283 children exposed in utero there was no clear difference in death (RR 1.02, 95% CI 0.57 to 1.84) or neurosensory disability (RR 0.77, 95% CI 0.38 to 1.58) at age 18 months.
Magnesium sulphate reduced eclampsia compared to phenytoin (three trials, 2291 women; RR 0.08, 95% CI 0.01 to 0.60) and nimodipine (one trial, 1650 women; RR 0.33, 95% CI 0.14 to 0.77).
Authors' conclusions
Magnesium sulphate more than halves the risk of eclampsia, and probably reduces maternal death. There is no clear effect on outcome after discharge from hospital. A quarter of women report side effects with magnesium sulphate.
Plain language summary
Magnesium sulphate and other anticonvulsants for women with pre‐eclampsia
Magnesium sulphate helps prevent eclamptic fits in pregnant women with pre‐eclampsia ('toxaemia').
Some women have high blood pressure with protein in their urine during pregnancy (pre‐eclampsia, or 'toxaemia'). Most women with pre‐eclampsia give birth without problems. However, severe pre‐eclampsia can cause problems such as stroke, kidney failure, liver failure, and blood clotting. A few women have seizures (eclampsia). These problems can lead to the baby being born too small or too soon. If the woman has eclampsia, both she and her baby are at high risk of death. Eclampsia is most common in low‐ and middle‐income countries.
This review of 15 studies showed magnesium sulphate reduced the number of women having eclampsia, but did not improve the babies' health. Magnesium sulphate had side effects for the mother, mostly flushing. Follow‐up of women and children is reassuring that there are no adverse effects later on.
Background
Description of the condition
Pre‐eclampsia is a multisystem disorder that is usually associated with raised blood pressure and proteinuria, but can also involve the woman's liver, kidneys, clotting system, or brain. If the placenta is involved this may led to growth restriction or premature birth. Pre‐eclampsia is a relatively common complication of pregnancy, and can occur at any time during the second half of pregnancy or in the first few weeks after delivery. Pre‐eclampsia is described in more detail in the generic protocol on interventions for treatment of pre‐eclampsia and its consequences (Duley 2009).
For many women who have pre‐eclampsia the outcome is good, but severe disease can lead to death or serious problems for the woman and/or her baby.
Eclampsia, defined as the occurrence of one or more seizures (fits) in association with the syndrome of pre‐eclampsia, is a rare but serious complication. In the UK it is associated with one in 2000 deliveries (Douglas 1994), while in low‐ and middle‐income countries it complicates between one in 100 and one in 1700 deliveries (WHO 1988). Eclampsia probably accounts for 50,000 deaths a year worldwide, which is about 10% of direct maternal deaths (Duley 1992). One aim of antenatal care is to detect pre‐eclampsia in the hope that the onset of serious complications (including eclampsia) can be delayed or prevented.
Magnesium sulphate is the anticonvulsant of choice for treatment of women with eclampsia, as it is better than diazepam (Duley 2003b), phenytoin (Duley 2003a) or lytic cocktail (Duley 2000).
Anticonvulsants were introduced for women with pre‐eclampsia in the belief that they would prevent the first seizure, and so improve outcome. Predicting who is at risk of an eclamptic seizure is difficult, as only around 1% to 2% of those with even severe pre‐eclampsia will have a seizure. This has contributed to the wide variation in policies for prophylactic anticonvulsants (Duley 1994). In the USA, for example, an estimated 5% of pregnant women receive magnesium sulphate before delivery (USA ‐ Texas 1995), whilst in the UK a quarter of obstetricians do not use any prophylactic anticonvulsant and only 40% report using magnesium sulphate (Gülmezoglu 1998).
Description of the intervention
The formulation used for clinical care is magnesium sulphate heptahydrate, usually provided as a 50% solution which is approximately 2 mmol magnesium/mL. Traditionally this is given as a loading dose followed by a maintenance regimen, which can be either intramuscular (Pritchard 1955; Pritchard 1984) or intravenous (Zuspan 1978).
The loading dose is usually 4 g given over a few minutes, either by intravenous injection or a short infusion. However, 6 g has been advocated (Sibai 1990) whilst others omit an intravenous loading dose when using the intramuscular maintenance regimen (Begum 2001). The traditional intramuscular regimen starts with 10 g by intramuscular injection (given as 5 g into each buttock) combined with the 4 g intravenous loading dose, and the maintenance regimen is then 5 g by intramuscular infection every four hours (Pritchard 1955; Pritchard 1984). For the intravenous regimen, the magnesium sulphate maintenance regimen is usually an infusion of 1 g/hour (Zuspan 1978, although 2 g/hour has been advocated (USA ‐ Memphis 1997; USA ‐ Tennessee 2001). Duration of therapy is usually 24 hours in total. Alternatives are to continue until 24 hours after delivery, or to stop treatment after 12 hours unless there is a specific indication to continue. Alternative regimens for magnesium sulphate are covered by a separate Cochrane review (Duley 2008).
There are potential hazards associated with the use of magnesium sulphate. Side effects include flushing, muscle weakness and nausea. Serious adverse effects such as respiratory and cardiac arrest are rare, but if they do occur are potentially life threatening. As magnesium sulphate is a smooth muscle relaxant it is used in some countries, such as the USA, as a tocolytic drug to prevent preterm birth for women in threatened preterm labour. Although this practice is not supported by evidence from randomised trials (Crowther 2003), if magnesium sulphate does have a tocolytic effect on uterine muscle this would be a potential adverse effect when used for women with pre‐eclampsia, as it might lead to an increase in caesarean section, postpartum haemorrhage or retained placenta.
Case‐controlled studies first suggested that exposure to magnesium sulphate whilst in utero was associated with a reduction in cerebral palsy or low birthweight children (Nelson 1995), and other studies suggested there may be an increase in the risk of paediatric death (Mittendorf 1998; Scudiero 2000). This hypothesis that in utero exposure to magnesium sulphate might be neuro protective for preterm infants has now been tested in randomised trials (Doyle 2009).
How the intervention might work
The aetiology of pre‐eclampsia and eclampsia remains unclear. It is therefore unsurprising that the mode of action for magnesium sulphate in prevention and treatment of eclamptic seizures is also not clearly understood. Although magnesium sulphate is not usually considered to be an 'anticonvulsant', it is better than traditional anticonvulsant drugs for control of eclamptic seizures. This anticonvulsant activity may be mediated by magnesium’s role as an N‐methyl‐D‐aspartate (NMDA) antagonist (Euser 2009). Stimulation of NMDA receptors by neurotransmitters such as glutamate may lead to seizures when neuronal networks are over‐activated. Magnesium may prevent and control eclamptic seizures by inhibiting NMDA receptors. The action of magnesium as an NMDA inhibitor may also block the neuronal damage associated with ischaemia (Goldman 1988; Sadeh 1989). Another possible mechanism is that magnesium sulphate may lead to cerebral vasodilatation, with subsequent reduction of cerebral ischaemia (Belfort 1992).
Magnesium is also a calcium antagonist, and a smooth muscle relaxant. It may therefore affect the cerebral endothelium which forms the blood brain barrier. Lowering intracellular calcium may limit paracellular transport of vascular contents, such as ions and proteins, effectively decreasing the factors which promote cerebral oedema and seizure activity (Euser 2009).
The calcium antagonist activity of magnesium sulphate has led to the belief that it also lowers systemic blood pressure, but this has not been supported by evidence from randomised trials (Magpie Trial 2002). Magnesium sulphate does not appear to be an effective antihypertensive drug (Abalos 2007).
The serious adverse effects of magnesium sulphate, such as respiratory depression and respiratory or cardiac arrest, are due to its action as a smooth muscle relaxant. However, the effect on smooth muscle follows a dose response: deep tendon reflexes are lost at a serum magnesium level of 10 mEq/L; respiratory depression begins at 15 mEq/L; and cardiac arrest is a risk at greater than15 mEq/L. This very specific dose response relationship means that careful clinical monitoring, with prompt action to stop or reduce the magnesium sulphate, will minimise serious adverse effects. As magnesium is excreted by the kidneys, women with impaired renal function may accumulate magnesium, and so be at risk of serious adverse effects if the dose is not reduced or stopped when tendon reflexes disappear. Clinical monitoring of tendon reflexes, respiration rate and urine output is therefore recommended during administration of magnesium sulphate (Duley 1996). Monitoring of serum magnesium levels is costly and unnecessary.
If toxicity does develop, calcium gluconate is an effective antidote.
Why it is important to do this review
The principal question is whether a policy of using an anticonvulsant drug for women with pre‐eclampsia does more good than harm, to both her and her baby, than a policy of not using an anticonvulsant. In the past a variety of anticonvulsant drugs have been suggested for the care of women with pre‐eclampsia. Magnesium sulphate is now the drug of choice for treatment of women with eclampsia (Collab Trial 1995; Duley 2000; Duley 2003a; Duley 2003b), however, and so it is also the drug of choice to evaluate for women with pre‐eclampsia.
As we do not have any reliable way of predicting who will develop eclampsia, the number of women potentially eligible for anticonvulsant therapy is large. As for any prophylactic therapy, for magnesium sulphate to be worthwhile for women with pre‐eclampsia, it must be safe for both the woman and the baby, as well as effective in preventing eclampsia.
Other aspects of the care of women with very high blood pressure or severe pre‐eclampsia are covered by other Cochrane reviews. These include drugs for very high blood pressure (Duley 2006), plasma volume expansion (Duley 1999) and timing of delivery (Churchill 2002).
Objectives
The primary aim was to assess the benefits and hazards for women and their babies of magnesium sulphate, or other anticonvulsant drug, when used for women with pre‐eclampsia. If magnesium sulphate or other anticonvulsant therapy is beneficial, secondary aims were to compare the differential effects of alternative agents, and to assess whether the effects differed with different levels of severity of pre‐eclampsia.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials of the administration of an anticonvulsant drug to women with pre‐eclampsia, including trials that compared an anticonvulsant with none or with placebo, and trials that compared one anticonvulsant drug with another. We excluded quasi‐randomised trials.
Types of participants
Any women with pre‐eclampsia, regardless of whether before or after delivery, whether a singleton or multiple pregnancy, or whether an anticonvulsant had been given before trial entry. If women with eclampsia had also been entered into the trial, we included only data for women with pre‐eclampsia in this review.
As sufficient data are now available, we have included the planned subgroup analysis by severity of pre‐eclampsia in the updated review. Severe pre‐eclampsia was defined wherever possible as women with two or more signs or symptoms of imminent eclampsia, or blood pressure of at least 170/110 mmHg and 3+ proteinuria or, if on antihypertensive agents, 150/100 mmHg and 2+ proteinuria If the definition of severe pre‐eclampsia was not specified, we still included women in this category if the authors described them as having severe pre‐eclampsia. We classified women who did not have any of these criteria as 'not severe pre‐eclampsia'.
Types of interventions
All randomised comparisons of an anticonvulsant drug, or other agents used specifically to prevent eclampsia, with placebo (or no anticonvulsant). Also, comparisons of one anticonvulsant drug with another.
Anticonvulsant drugs which have been used for pre‐eclampsia include magnesium sulphate, diazepam (Valium), phenytoin, nimodipine, and chlormethiazole.
Types of outcome measures
Primary outcomes
For women
Death;
eclampsia;
measures of serious maternal morbidity related to either pre‐eclampsia or anticonvulsant use (such as renal failure, cardiac arrest, liver failure, stroke, coagulopathy and respiratory depression).
For the baby
Death: stillbirths (death in utero at or after 20 weeks' gestation), perinatal deaths (stillbirths plus deaths in the first week of life), death before discharge from hospital, neonatal deaths (death in the first 28 days after birth), deaths after the first 28 days;
preterm birth, defined as birth before 37 completed weeks' gestation;
in a special care nursery for more than seven days.
Secondary outcomes
For women
Pulmonary edema;
pneumonia;
antihypertensive therapy;
progression from mild to severe pre‐eclampsia;
toxicity;
side effects;
dialysis;
ventilation;
admission to intensive care;
length of stay;
induction of labour;
length of labour;
placental abruption;
caesarean section;
retained placenta;
postpartum haemorrhage;
long‐term follow‐up (death after discharge from hospital, serious morbidity potentially related to pre‐eclampsia, stroke, renal failure, hypertension, depression).
For the baby
Severity of preterm birth: very preterm birth (before 32 to 34 completed weeks) and extremely preterm birth (before 26 to 28 completed weeks);
death before discharge from hospital or in a special care nursery for more than seven days;
respiratory distress syndrome;
infection;
necrotising enterocolitis;
retinopathy of prematurity;
intraventricular haemorrhage;
small‐for‐gestational age: defined as growth below the 3rd centile, or lowest centile reported;
Apgar score at five minutes: low (less than seven) and very low (less than four) or lowest reported;
use of hospital resources: admission to special care nursery, length of stay, endotracheal intubation, use of mechanical ventilation;
long‐term growth and development: blindness, deafness, seizures, poor growth, neurodevelopmental delay and cerebral palsy;
side effects associated with the intervention.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (4 June 2010).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched CENTRAL (The Cochrane Library 2010, Issue 3) using the search strategy detailed in Appendix 1.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 2. For this update we used the following methods when assessing the reports identified by the updated search.
Selection of studies
Two review authors independently assessed studies for eligibility (for this update D Chou (DC) and L Duley (LD)). We resolved differences and discrepancies by discussion with a third review author (for this update M Gulmezoglu (MG)). As LD had been involved in one trial potentially eligible for this review (Magpie Trial 2002), DC and MG assessed and extracted data for this study.
Data extraction and management
We designed a form to extract data from newly included papers. DC entered the data into Review Manager software (RevMan 2008) and LD or MG checked them for accuracy. We resolved discrepancies by discussion. If the two review authors could not agree, we consulted a third review author.
There was no blinding of authorship or results. Whenever possible, we sought unpublished data from investigators.
Assessment of risk of bias in included studies
(1) Sequence generation (checking for possible selection bias)
We describe for each included study whether the method used to generate the allocation sequence was described in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
adequate (any truly random process, e.g. random number table; computer random number generator);
inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We describe for each included study whether the method used to conceal the allocation sequence and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered studies to be at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the analyses which we undertook. We assessed methods as:
adequate;
inadequate;
unclear.
(5) Selective reporting bias
We describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
adequate (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We describe for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we present the results as mean difference with 95% confidence intervals. When pooling data across studies, we estimated the mean difference if the outcomes are measured in the same way between trials. We used the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not anticipate any cluster‐randomised trials on this topic. However, if we did find any, we would have included them using the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009).
Crossover trials
We excluded crossover trials, as for pre‐eclampsia they can only assess surrogate outcomes and markers. The important clinical outcomes are at birth, and beyond.
Dealing with missing data
We noted the levels of attrition for included studies. We explored, using sensitivity analysis, the impact of including studies with high levels of missing data in the overall assessment of treatment effect.
For all outcomes, we analysed the data, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing ('available case' analysis).
We analysed data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention. If in the original reports participants were not analysed in the group to which they were randomised, and there was sufficient information in the trial report, we would have attempted to restore them to the correct group.
Assessment of heterogeneity
We applied tests of heterogeneity between trials, if appropriate, using the I² statistic. If we identified heterogeneity among the trials (I² greater than 50%), we would have explored it by prespecified subgroup analysis and performing sensitivity analyses. We would have used a random‐effects meta‐analysis as an overall summary if this was considered appropriate.
Assessment of reporting biases
Where we suspected reporting bias (see 'Selective reporting bias' above), we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we have explored the impact of including such studies in the overall assessment of results by sensitivity analysis.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed‐effect inverse variance meta‐analysis for combining data where trials examined the same intervention, and the trials' populations and methods were judged sufficiently similar. Where we suspected clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects may differ among trials, we used random effects meta‐analysis.
If we identified substantial heterogeneity in a fixed‐effect meta‐analysis we noted this and repeated the analysis using a random‐effects method, when appropriate.
Subgroup analysis and investigation of heterogeneity
We performed statistical analyses using the Review Manager software (RevMan 2008), with results presented as risk ratios (RR)and risk difference (RD). From 1/RD, we calculated the number needed to treat for benefits or harm. For each measure we have provided the 95% confidence intervals. We used the fixed‐effect model for calculating the RR. If there was clear heterogeneity among the studies in any one outcome, we used a random‐effects model. We also explored possible factors in the heterogeneity, including study quality, clinical factors as determined by the pre‐specified subgroup analyses, and the play of chance.
We planned subgroup analyses for the main outcomes by:
severity of pre‐eclampsia at trial entry: severe pre‐eclampsia; not‐severe pre‐eclampsia; severity unclear, unspecified or mixed (see above for definitions);
whether delivered or not at trial entry: not delivered at trial entry; delivered; unclear, unspecified or mixed;
gestation at trial entry: 34 weeks' gestation or more at trial entry; below 34 weeks, unclear, unspecified, or mixed;
whether anticonvulsants had been given before trial entry: no anticonvulsant before trial entry; anticonvulsant before trial entry; unclear, unspecified, or mixed.
We used primary outcomes only for the subgroup analyses. We planned subgroups by dose and route of administration for magnesium sulphate for maternal death, eclampsia, baby deaths and side effects.
Sensitivity analysis
We carried out sensitivity analyses to explore the effect of trial quality for important outcomes in the review. Where there is risk of bias associated with a particular aspect of study quality (e.g. inadequate allocation concealment), we explored this by sensitivity analyses using the primary outcomes.
Results
Description of studies
For a full description of the characteristics of included studies, see table of Characteristics of included studies.
Results of the search
We have included 15 studies, and excluded 34.
Included studies
One international trial (Magpie Trial 2002) was considerably larger than any others, with 10,141 women recruited. Two other studies had more than 1000 women (Nimodipine SG 2003; USA ‐ Texas 1995); five had 100 to 1000 women (South Africa 1994; South Africa 1998; USA ‐ Maryland 1993; USA ‐ Memphis 1997; USA ‐ Tennessee 2001); and the remaining seven studies had fewer than 100 women (Denmark 2000; India 2008; Malaysia 1994; Mexico 1992; Mexico 1998; Taiwan 1995; USA ‐ Alabama 1995).
The trials were conducted in a mix of high‐income, middle‐income and low‐income countries. They were all conducted in hospitals. The small trials were largely in tertiary referral hospitals. However, the largest trial, with 175 centres in 33 countries, included a wide range of secondary level hospitals as well as tertiary level (Magpie Trial 2002).
Participants
Most trials in the review recruited women with severe, or relatively severe, pre‐eclampsia. Two small USA trials (USA ‐ Memphis 1997; USA ‐ Tennessee 2001) included women with mild pre‐eclampsia. Thirteen trials included only women who had not yet given birth. Two trials included women recruited postpartum: in one large trial 13% of women were randomised in the 24 hours after delivery (Magpie Trial 2002); in the other small trial postpartum women were included, but the numbers of women randomised before and after delivery are not reported (India 2008).
Interventions
Six trials (11,444 women) compared magnesium sulphate with placebo or no anticonvulsant (Magpie Trial 2002; South Africa 1994; South Africa 1998; Taiwan 1995; USA ‐ Memphis 1997; USA ‐ Tennessee 2001). In the largest of these studies (Magpie Trial 2002, 10,141 women) around half the women recruited had magnesium sulphate maintenance therapy by the intravenous route (1 g/hour), and half by the intramuscular route. In one study maintenance therapy was by the intramuscular route (South Africa 1994). In the other four studies maintenance was by the intravenous route (South Africa 1998;Taiwan 1995; USA ‐ Memphis 1997; USA ‐ Tennessee 2001); in two of these the dose was 1 g/hour (South Africa 1998; Taiwan 1995) and in the other two it was 2 g/hour (USA ‐ Memphis 1997; USA ‐ Tennessee 2001).
For most of the women in these six trials, the magnesium sulphate regimen was administered by local hospital staff within their normal clinical practice. Clinical monitoring of respiration, tendon reflexes and urine output was reported to have been used for most trials. None of the six studies reported using serum monitoring for magnesium sulphate.
Magnesium sulphate was also compared with phenytoin (four trials, 2345 women) (India 2008; USA ‐ Alabama 1995; USA ‐ Maryland 1993; USA ‐ Texas 1995); diazepam (two trials, 66 women) (Malaysia 1994; Mexico 1992); nimodipine (one trial, 1750 women) (Nimodipine SG 2003); and isosorbide (one trial, 36 women) (Mexico 1998). For three of these studies, maintenance magnesium sulphate therapy was by the intramuscular route (India 2008; Malaysia 1994; USA ‐ Texas 1995). The other studies used the intravenous route; in one the dose was 1 g/hour (Mexico 1992), in the remainder it was 2 g/hour. One small trial (33 women) compared magnesium chloride with methyl dopa (Denmark 2000).
Long‐term follow‐up
Only one trial has reported long‐term follow‐up for the women and children (Magpie Trial 2002). This study recruited 10,141 women in 175 centres in 33 countries. As follow‐up was not possible in all centres, 125 centres in 19 countries participated in the follow‐up study. At these 125 centres, 4782 women were selected for follow‐up, of whom 3375 (71%) were traced; and 4483 children were selected for follow‐up, of whom 3283 (73%) were traced.
The median time from delivery to follow up for women was 26 months (range from within 12 months to greater than 48 months from delivery, interquartile range 19 to 36 months). Outcome for children was assessed when they were 18 months old (corrected for gestational age at birth).
Excluded studies
We have excluded 35 studies. For details, seeCharacteristics of excluded studies.
Risk of bias in included studies
Allocation
For the six trials comparing magnesium sulphate with placebo/no anticonvulsant, in the largest study concealment of allocation was secure and completeness of follow‐up was 99% (Magpie Trial 2002). Adequate concealment of allocation was described for three trials (South Africa 1994; USA ‐ Memphis 1997; USA ‐ Tennessee 2001), in one another concealment of allocation was not adequate (South Africa 1998), and for two studies concealment of allocation was unclear (India 2008; Taiwan 1995).
For the trials comparing magnesium sulphate with another anticonvulsant, in the two large trials the procedure used to conceal allocation was not described in one (USA ‐ Texas 1995) and for the other it is unclear whether concealment of allocation was secure (Nimodipine SG 2003). Of the remaining trials, one described adequate concealment of allocation (Denmark 2000), but for the remainder concealment of allocation was unclear.
Blinding
Of the six trials comparing magnesium sulphate with placebo or no anticonvulsant, three used a placebo (Magpie Trial 2002; South Africa 1994; Taiwan 1995). For the comparisons of one drug with another, blinding of the intervention was not possible.
If blinding in the assessment of outcome was not mentioned, it was assumed not to have been done, and this was the case for all the trials that were not placebo controlled. Data coding and review of follow‐up data for women and babies in the Magpie Trial follow‐up study was blinded to treatment allocation (Magpie 2004).
Incomplete outcome data
In one trial, 17% of women were lost to follow‐up (South Africa 1998). This study also recruited over a long time period, 13+ years.
For the long‐term follow‐up of women and children in the Magpie Trial, data were available for 71% and 73% respectively. Losses to follow‐up were higher in countries with high perinatal mortality. Data were available for 49% women in high perinatal mortality countries, 74% in middle perinatal mortality countries, and 95% in low perinatal mortality countries. For the children, 92 centres reported 100% follow‐up, and 106 centres reported at least 90% follow‐up of the children born to women randomised prior to delivery. We excluded centres with less than 20% follow‐up.
Effects of interventions
1. Magnesium sulphate versus placebo or no anticonvulsant
Six trials (11,444 women) compared magnesium sulphate with placebo or no anticonvulsant.
For the woman
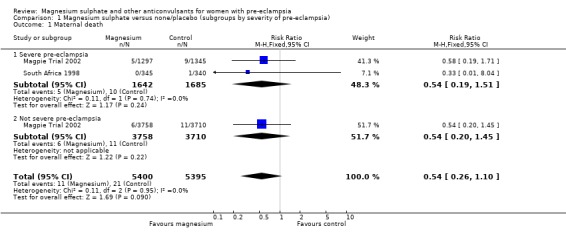
Maternal death
Two trials (10,795 women) reported maternal deaths. The risk of dying was reduced by 46% for women allocated magnesium sulphate rather than placebo or no anticonvulsant, although this did not achieve statistical significance (risk ratio (RR) 0.54, 95% confidence interval (CI) 0.26 to 1.10). This effect was consistent regardless of severity of pre‐eclampsia, whether antepartum at trial entry, gestation at trial entry or whether an anticonvulsant had been given before trial entry.
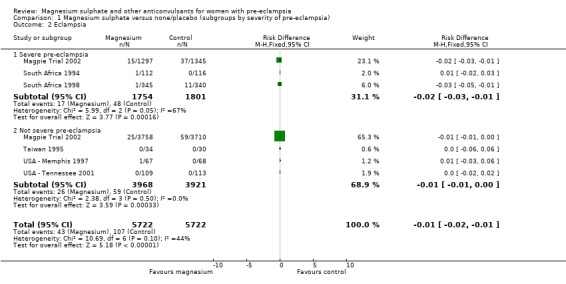
Eclampsia
Eclampsia was reported in six trials (11,444 women). There was more than a halving in the risk of eclampsia associated with the use of magnesium sulphate (RR 0.41, 95% confidence interval (CI) 0.29 to 0.58; risk difference (RD) ‐0.01, 95% CI ‐0.02 to ‐0.01; number needed to treat (NNTB) 100, 95% CI 50 to 100) rather than placebo or no anticonvulsant.
This effect is consistent regardless of whether or not women had severe pre‐eclampsia at trial entry: for women with severe pre‐eclampsia (RR 0.37, 95% CI 0.22 to 0.64; RD ‐0.02, 95% CI ‐0.03 to ‐0.01; NNTB 50, 95% CI 34 to 100); for women with not severe pre‐eclampsia (RR 0.44, 95% CI 0.28 to 0.69; RD ‐0.01, 95% CI ‐0.01 to ‐0.00; NNTB 100, 95% CI 100 to 500). It is also consistent regardless of whether the women were antepartum at trial entry, and irrespective of gestation at trial entry. The only exception is the small subgroup of women who had another anticonvulsant before trial entry, and this result probably reflects the play of chance as the numbers were small.
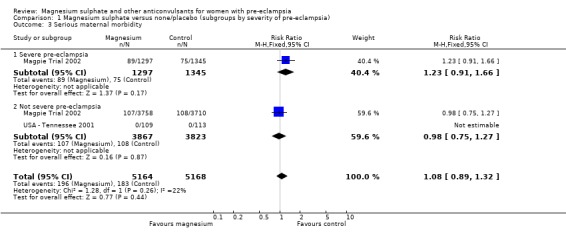
Serious maternal morbidity
For the two trials (10,332 women) reporting a composite outcome of serious maternal morbidity, there was no clear difference between the two groups (RR 1.08, 95% CI 0.89 to 1.32). This result was consistent across the subgroups. For the individual measures of serious morbidity, such as pneumonia, renal failure and liver failure, there was also no clear evidence of an overall difference in effect between the two groups.
Two trials (10,795 women) reported use of antihypertensive therapy after trial entry. There was a small (3%) reduction in the need for antihypertensive therapy associated with the use of magnesium sulphate rather than placebo or no anticonvulsant (RR 0.97, 95% CI 0.95 to 0.99).
Side effects and toxicity
Side effects were reported by women in both treatment groups (24% versus 5%), although the risk of any side effect was much higher for those allocated magnesium sulphate (RR 5.26, 95% CI 4.59 to 6.03; RD 0.19, 95% CI 0.18 to 0.21; NNTH 6, 95% CI 5 to 6) rather than placebo or no anticonvulsant. By far the most common side effect was flushing (20% versus 2%). Although other side effects were reported less frequently, all were increased for women allocated magnesium sulphate. These included nausea and/or vomiting, slurred speech, muscle weakness, hypotension (low blood pressure), dizziness, drowsiness or confusion, and headache.
Problems at the injection site were also more common for women allocated magnesium sulphate rather than placebo. Intramuscular administration of both magnesium sulphate and placebo was associated with more problems at the injection site (12% versus 8%) than intravenous use (5% versus 2%). For intramuscular use the risk of problems was increased for women allocated magnesium sulphate (RR 1.49, 95% CI 1.25 to 1.79; RD 0.04, 95% CI 0.02 to 0.06; NNTH 25, 95% CI 17 to 50) rather than placebo. Similarly it was also increased for intravenous use (RR 3.05, 95% CI 2.15 to 4.32; RD 0.03, 95% CI 0.02 to 0.04; NNTH 34, 95% CI 25 to 50).
Toxicity (absent or reduced tendon reflexes and/or respiratory depression) was uncommon, occurring in around 1% of women allocated magnesium sulphate and 0.5% of those allocated placebo. There was no clear evidence of an overall difference in the risk of absent or reduced tendon reflexes (RR 1.00, 95% CI 0.70 to 1.42). Although respiratory depression, or other respiratory problems, were rare, there was an increased risk for women allocated magnesium sulphate (RR 1.98, 95% CI 1.24 to 3.15; RD 0.0049, 95% CI 0.000 to 0.01; NNTH 206, 95% CI 100 to 1000) rather than placebo. There was no clear difference between the groups in whether the women received calcium gluconate (RR 1.35, 95% CI 0.63 to 2.88).
Complications of pregnancy, labour and delivery
The risk of placental abruption was reduced for women allocated magnesium sulphate (RR 0.64, 95% CI 0.50 to 0.83; RD ‐0.01, 95% CI ‐0.02 to 0.00; NNTB 100, 95% CI 50 to 1000) rather than placebo or no anticonvulsant. The risk of caesarean section was high in both groups (50% versus 47%). Women allocated magnesium sulphate had a small (5%) increased risk of caesarean section (six trials, 10,096 women) (RR 1.05, 95% CI 1.01 to 1.10; RD 0.03, 95% CI 0.01 to 0.04; NNTH 34, 95% CI 25 to 100) compared to those allocated placebo or no anticonvulsant.
There was no clear difference between the groups in the risk of induction of labour (one trial, 8771 women; RR 0.99, 95% CI 0.94 to 1.04), postpartum haemorrhage (RR 0.96, 95% CI 0.88 to 1.05), or manual removal of placenta (RR 0.90, 95% CI 0.72 to 1.12).
Follow‐up for women after discharge from hospital
At a median follow‐up time of two years, for the women there was no clear difference between the groups in the risk of death (RR 1.79, 95% CI 0.71 to 4.53) or of serious morbidity: serious renal problems (RR 0.65, 95% CI 0.21 to 1.99), persistent hypertension (RR 0.78, 95% CI 0.51 to 1.20) or depression (RR 0.90, 95% CI 0.66 to 1.22).
For the child
Stillbirth and neonatal death
There was no overall difference in the risk of stillbirth or neonatal death (three trials, 9961 babies; RR 1.04, 95% CI 0.93 to 1.15). This result is consistent regardless of whether or not the woman had severe pre‐eclampsia, and irrespective of gestation at trial entry.
For the composite outcome of death or in special care baby unit, there is no clear evidence of a clinically important difference (RR 1.02, 95% CI 0.95 to 1.08).
Neonatal morbidity
There was no clear difference in neonatal morbidity between the two groups: admission to special care baby unit (RR 1.01, 95% CI 0.96 to 1.06); admission to special care baby unit for more than seven days (RR 1.02, 95% CI 0.93 to 1.11); intubation at the place of delivery (RR 1.01, 95% CI 0.82 to 1.24).
Follow‐up for the children after discharge from hospital
Follow‐up at 18 months of age (corrected for gestation at birth) was reported by one trial (3283 children). There was no clear difference between the groups in the risk of death (RR 1.02, 95% CI 0.57 to 1.84), neurosensory disability (RR 0.77, 95% CI 0.38 to 1.58), cerebral palsy (RR 0.34, 95% CI 0.09 to 1.26), or the composite outcome of death or neurosensory disability (RR 1.06, 95% CI 0.90 to 1.25).
2. Magnesium sulphate versus phenytoin
Four trials (2343 women) compared magnesium sulphate with phenytoin. Magnesium sulphate reduced the risk of eclampsia compared with phenytoin (three trials, 2291 women RR 0.08, 95% CI 0.01 to 0.60; NNTB 97, 95% CI 62 to 221), although the number of events is small (0 versus 12).
Magnesium sulphate also increased the risk of caesarean section (two trials, 2192 women; RR 1.21, 95% CI 1.04 to 1.40; RD 0.048, 95% CI 0.012 to 0.084; NNTH 21 95% CI 12 to 83) compared with phenytoin. There is no information on other important measures of maternal morbidity. One trial (2165 babies) reported stillbirths (RR 0.62, 95% CI 0.27 to 1.41) and neonatal deaths (RR 0.26, 95% CI 0.03 to 2.31), with no clear difference between the two groups. There are no clear differences between the groups in the other reported measures of neonatal morbidity: Apgar at five minutes (RR 0.58, 95% CI 0.26 to 1.30) and admission to neonatal care (RR 1.00, 95% CI 0.63 to 1.59).
3. Magnesium sulphate versus diazepam
The two trials (66 women) comparing magnesium sulphate with diazepam are too small for any reliable conclusions about their differential effects.
4. Magnesium sulphate versus nimodipine
One trial compared magnesium sulphate with nimodipine (1650 women). The risk of eclampsia was lower for women allocated magnesium sulphate rather than nimodipine (0.8% versus 2.6%; RR 0.33, 95% CI 0.14 to 0.77; RD ‐0.02, 95% CI ‐0.03 to ‐0.00; NNTB 50, 95% CI 34 to 1000). The only other clear differences were an increase in respiratory problems associated with magnesium sulphate compared to nimodipine (1.3% versus 0.4%; RR 3.61, 95% CI 1.01 to 12.91), and a greater need for additional antihypertensive drugs associated with magnesium sulphate rather than nimodipine (54% versus 46%, RR 1.19, 95% CI 1.08 to 1.31).
Mortality for the baby is not reported. The only measures of neonatal morbidity reported were respiratory distress, hypotonia, intubation and hypotension; there were no clear differences between the groups for any of these four outcomes.
5. Magnesium chloride versus methyl dopa
One trial compared magnesium chloride with methyl dopa (31 women) and it was too small for any reliable conclusions about potential differential effects.
6. Magnesium sulphate versus nitrates
One trial (36 women) compared magnesium sulphate with isosorbide, a nitrate. Eclampsia was the only clinical outcome reported, and the study was too small for any reliable conclusions.
Discussion
Summary of main results
More than 11,000 women have been randomised into trials comparing magnesium sulphate with placebo or no anticonvulsant. Most other trials have compared magnesium sulphate with an alternative drug. The use of magnesium sulphate, rather than placebo or no anticonvulsant, for women with pre‐eclampsia is associated with a halving in the risk of eclampsia, and it seems likely that there is also a clinically important reduction in the risk of maternal death. There is no clear evidence that these benefits are reflected in any reduction in other measures of serious maternal morbidity. The only other effects are that there appears to be a reduction in the risk of placental abruption associated with magnesium sulphate, and a small increase (5%) in the risk of caesarean section. This review provides reasonable reassurance that there is no substantive effect on stillbirth or neonatal mortality associated with the use of magnesium sulphate. Nevertheless, a small increase or decrease in mortality for the baby has not been excluded. Additional reassurance about the safety of magnesium sulphate when used for women with pre‐eclampsia is that there is also no substantive effect on the composite outcome of death or in special care nursery for more than seven days.
The reduction in placental abruption is not reflected in any overall effect on mortality or morbidity for the baby. This is not surprising, as the number of women who had a placental abruption in these trials was small. The difference between the groups in the number with a placental abruption was 49 women. Even a moderate impact, for example a 15% reduction in mortality, would therefore only represent seven deaths and so be unlikely to influence the overall risk.
About a quarter of women report side effects associated with magnesium sulphate, the vast majority being flushing. Almost all the data on side effects and safety come from studies that used either the intramuscular regimen for maintenance therapy, or the intravenous route with 1 g per hour, and for around 24 hours. The use of higher doses and longer duration cannot be supported by these data. In particular, the reassurance about safety and lack of serious side effects cannot be extrapolated to higher doses or longer duration of therapy.
Follow‐up data from one large trial are now available. These data show that the reduction in risk of eclampsia following prophylaxis with magnesium sulphate is not associated with an excess of death or disability for the women after two to three years, and that exposure to magnesium sulphate whilst in utero is not associated with any clear difference in the risk of death or neurosensory disability for the children at 18 months of age. This provides reassurance about the longer term safety of magnesium sulphate when used for women with pre‐eclampsia.
In the comparisons of magnesium sulphate with other anticonvulsant drugs, magnesium sulphate was better than either phenytoin or nimodipine for prevention of eclampsia. There were insufficient data for reliable conclusions about the differential effects in the other comparisons.
Overall completeness and applicability of evidence
The halving in risk of eclampsia is consistent across the subgroups. In particular the reduction in risk ratio is similar regardless of severity of pre‐eclampsia. As eclampsia is more common amongst women with severe pre‐eclampsia than amongst those with moderate or mild pre‐eclampsia, the number of women who would need to be treated to prevent one case of eclampsia is greater for non‐severe pre‐eclampsia. Few women in this review had mild pre‐eclampsia, as the non‐severe category primarily includes women with moderate disease.
Although few women in this review had mild pre‐eclampsia, it would seem plausible that magnesium sulphate would also reduce the risk of eclampsia for these women. As the risk of eclampsia is low for these women, however, the number needed to treat would be high. The cost‐effectiveness of magnesium sulphate is best when its use is restricted to women with severe pre‐eclampsia (Simon 2006).
Women in these trials have been recruited in a wide range of countries and settings. The trials were all conducted in hospitals, at either secondary or tertiary level, in high‐, middle‐ and low‐income countries. All trials comparing magnesium sulphate with placebo or no anticonvulsant used clinical monitoring. Serum monitoring is not required. These results are therefore widely applicable to hospital settings throughout the world.
In high‐income countries, the intravenous maintenance regimen is usually used for magnesium sulphate. In low‐ and middle‐income countries, if hospitals do not have the resources to administer and monitor an intravenous infusion, intramuscular administration is preferable despite the higher incidence of problems at the injection site.
The benefits from use of magnesium sulphate for women with pre‐eclampsia must also be balanced against the costs associated with treatment. Using data from the Magpie Trial, the overall financial healthcare cost, adjusted for 2001 US dollars, to prevent a single case of eclampsia is $21,202 in high‐, $2473 in middle‐, and $456 in low‐income countries (Simon 2006). The cost effectiveness of magnesium sulphate is improved if it is used only for women with severe pre‐eclampsia, and in low‐income countries if the cost of purchasing the drug is reduced.
Quality of the evidence
The quality of the studies included in this review ranged from excellent to poor. However, most of the poor quality studies were small. The large study comparing magnesium sulphate with placebo was of high quality (Magpie Trial 2002).
Potential biases in the review process
The evidence for this review is derived from studies identified in a detailed search process. It is possible (but unlikely) that additional trials comparing the use of magnesium sulphate versus other medications in the treatment of pre‐eclampsia have been published but not identified. It is also possible that other studies have been conducted but are not yet published. Should any such studies be identified, we will include them in future updates of this review.
As one of the review authors was principal investigator for the Magpie Trial, two other review authors (M Gulmezoglu and D Chou) performed data extraction.
Agreements and disagreements with other studies or reviews
This review presents strong evidence that magnesium sulphate is the drug of choice for prevention eclampsia, and that this benefit in the short term is not associated with any clear evidence of an effect on long‐term outcome for either the women or children.
Other Cochrane reviews confirm that magnesium sulphate is also the treatment of choice for women with eclampsia (Duley 2000; Duley 2003a; Duley 2003b). Magnesium sulphate is therefore widely recommended for both prevention and treatment of eclampsia (Langer 2008; RCOG 2006; Sheth 2002). Alternative regimens for administration of magnesium sulphate for women with eclampsia and pre‐eclampsia are compared elsewhere (Duley 2008).
For women at risk of preterm birth, the use of magnesium sulphate also reduces the risk of their child having cerebral palsy (Doyle 2009).
Authors' conclusions
Implications for practice.
Magnesium sulphate should be available in all hospitals providing care for women with eclampsia and pre‐eclampsia. Its use should be considered for women with pre‐eclampsia for whom there is concern about the risk of eclampsia. Cost effectiveness is improved if its use is restricted to women with severe pre‐eclampsia. As magnesium sulphate is an inexpensive drug, it is appropriate for low‐income countries. For low‐income countries, cost effectiveness is also improved if the purchase price for magnesium sulphate is low.
Intravenous administration for maintenance therapy is preferable, where there are appropriate resources, as this is associated with fewer side effects and injection site problems than intramuscular use. Duration of treatment should not normally exceed 24 hours, and if the intravenous route is used for maintenance therapy the dose should not exceed 1 g/hour. Data on side effects, serious adverse effects and long‐term outcome presented in this review may not apply to use of higher doses. Administration and clinical monitoring of magnesium sulphate can be done by medical, midwifery or nursing staff, provided they are appropriately trained. Serum monitoring is not necessary.
Implications for research.
The trials in this review included women only after admission to hospital. Whether a loading dose of magnesium sulphate should be used for women at primary care level before they are transferred to hospital is unclear. Other factors in this decision are likely to include how long it will take to get the woman to hospital, the support that is available during transfer, and severity of her pre‐eclampsia.
Other questions that require further research are what is the minimum effective dose of magnesium sulphate; what is the optimal duration of therapy; and when is the optimal time to give it.
Any new agents for eclampsia prophylaxis should be compared with magnesium sulphate in large randomised trials. These studies should include long‐term follow‐up of women and children, and assessment of cost effectiveness.
Feedback
Alford, February 2004
Summary
Pre‐eclampsia can be prevented if the mother is checked for reverse T3 hypothyroidism and treated properly with liothyronine (Cytomel‐‐T3). It normalizes the incubator temperature and prevents the hypotension that leads to the pre‐eclampsia.
Reply
Although there is an association between subclinical hypothyroidism and pre‐eclampsia, it is unclear whether hypothyroidism influences the risk of pre‐eclampsia (North 2009). There is no clear evidence that treatment of subclinical hypothyroidism influences the risk of women developing pre‐eclampsia.
Contributors
Comment received from RM Alford, February 2004. Reply by L Duley and D Chou, May 2010.
What's new
| Date | Event | Description |
|---|---|---|
| 24 June 2010 | New search has been performed | Updated search: two new studies included (India 2008; Mexico 1998), 27 new studies excluded and a new ongoing trial identified. Longer term follow‐up is now included. Methods updated according to the new Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). |
| 24 June 2010 | New citation required but conclusions have not changed | New author (D Chou) joined the review team. |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 3, 1996
| Date | Event | Description |
|---|---|---|
| 11 September 2008 | Amended | Review converted to new format. |
| 25 February 2003 | New citation required and conclusions have changed | Substantive amendment. The search strategy has been updated. Subgroup analyses are now included in the comparisons tables. New included trials identified: Denmark 2000; Magpie Trial 2002; USA ‐ Tennessee 2001. Also new excluded trials. |
Acknowledgements
We thank Rebecca Gainey for translating Vargas 1998; Xiaojing Lai for translating Li 1998; Maria Kalousi for translating Neto 2000; Ussanee Sangkomkamhang for translating Tongsong 1992; Maria Tenorio for translating Rodriquez 1990; and Laura Woodhouse for translating Walss 1992.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Hypertension, Pregnancy‐Induced explode all trees #2 hypertens* near pregnan* #3 eclamp* #4 preeclamp* or pre next eclamp* #5 magnesium #6 diazepam #7 lytic next cocktail #8 phenytoin #9 chlorpromazine #10 meperidine or pethidine #11 MeSH descriptor Anticonvulsants explode all trees #12 anticonvulsant* or anti next convulsant* #13 (#1 OR #2 OR #3 OR #4) #14 (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) #15 (#13 AND #14)
Appendix 2. Methods used in previous version of the review
Data collection and analysis
Two reviewers independently assessed for eligibility. Two reviewers independently extracted and double entered data. Discrepancies were resolved by discussion. If the two reviewers could not agree, the third reviewer was consulted. There was no blinding of authorship or results. Whenever possible, we sought unpublished data from investigators. We assigned a quality score for concealment of allocation to each trial, using the following criteria:
(A) adequate concealment of allocation; (B) unclear whether adequate concealment of allocation; (C) inadequate concealment of allocation.
We excluded quasi‐randomised trials, for example those using alternate allocation.
In addition, we assigned to each reported outcome quality scores for completeness of follow‐up and blinding of the assessment of outcome using the following criteria:
For completeness of follow‐up:
(A) less than 3% of participants excluded; (B) 3% to 9.9% of participants excluded; (C) 10% to 19.9% of participants excluded.
Excluded: If not possible to enter data based on intention to treat, and/or 20% of participants were excluded from that outcome.
For blinding of assessment of outcome:
(A) Double blind, neither caregiver nor participant knew or were likely to guess the allocated treatment. (B) Single blind, either the caregiver or the participant knew the allocation. Or, the trial is described as double blind, but side effects of one or other treatment mean that it is likely that for a substantial proportion of participants (greater than 40%) the allocation could be correctly identified. (C) No blinding, both caregiver and participant knew (or were likely to guess) the allocated treatment; or, blinding not mentioned.
Excluded: no blinding, and the outcomes were very subjective.
We performed statistical analyses using the Review Manager software (RevMan 2000), with results presented as risk ratio (RR) and risk difference (RD). From 1/RD the number needed to treat for for an additional beneficial outcome (NNTB) or number need to treat for an additional harmful outcome (NNTH) were calculated. For each measure the 95% confidence intervals are given. The fixed‐effects model was used for calculating the risk. If there was clear heterogeneity between the studies in any one outcome, a random effects model was used. Possible factors in the heterogeneity were also explored, including study quality, clinical factors as determined by the prespecified subgroup analyses, and the play of chance.
Subgroup analyses for the main outcomes were planned by severity of pre‐eclampsia at trial entry (see above for definition), whether delivered or not, gestation at trial entry (above or below 34 weeks), and whether anticonvulsants had already been given. The main outcomes were maternal death, eclampsia, severe maternal morbidity, total fetal and neonatal deaths, and death or serious morbidity for the baby (such as greater than seven days in a special care baby unit). Subgroups by dose and route of administration for magnesium sulphate were planned for maternal death, eclampsia, baby deaths and side effects.
Data and analyses
Comparison 1. Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 2 | 10795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.26, 1.10] |
| 1.1 Severe pre‐eclampsia | 2 | 3327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.19, 1.51] |
| 1.2 Not severe pre‐eclampsia | 1 | 7468 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.20, 1.45] |
| 2 Eclampsia | 6 | 11444 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.02, ‐0.01] |
| 2.1 Severe pre‐eclampsia | 3 | 3555 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.03, ‐0.01] |
| 2.2 Not severe pre‐eclampsia | 4 | 7889 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.01, ‐0.00] |
| 3 Serious maternal morbidity | 2 | 10332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.32] |
| 3.1 Severe pre‐eclampsia | 1 | 2642 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.91, 1.66] |
| 3.2 Not severe pre‐eclampsia | 2 | 7690 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.75, 1.27] |
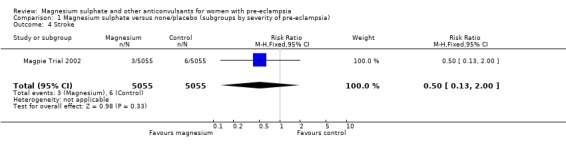
| 4 Stroke | 1 | 10110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.13, 2.00] |
| 5 Pulmonary oedema | 3 | 10560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.60, 1.57] |
| 6 Pneumonia | 1 | 10110 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.90, 6.07] |
| 7 Renal failure | 1 | 10110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.55, 1.17] |
| 8 Renal dialysis | 2 | 10338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.21, 2.32] |
| 9 Liver failure | 1 | 10110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.11] |
| 10 Coagulopathy | 1 | 10110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.16] |
| 11 Cardiac arrest | 1 | 10110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.21, 2.98] |
| 12 Respiratory arrest | 1 | 10110 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.49, 12.88] |
| 13 Any antihypertensive therapy | 2 | 10795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.95, 0.99] |
| 14 Rapid acting antihypertensives | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Intravenous or intramuscular hydralazine | 2 | 10338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 1.00] |
| 14.2 Oral nifedipine | 2 | 10276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.88, 0.99] |
| 15 Progression from mild to severe pre‐eclampsia | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.53, 1.55] |
| 16 Toxicity | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Absent or reduced tendon reflexes | 2 | 10677 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.70, 1.42] |
| 16.2 Respiratory depression, or other respiratory problem | 2 | 10677 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.24, 3.15] |
| 16.3 Respiratory depression and absent tendon reflexes | 3 | 10899 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.96 [0.72, 49.40] |
| 17 Given calcium gluconate | 2 | 10795 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.63, 2.88] |
| 18 Side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Feeling warm/flushed | 2 | 10127 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.38 [7.74, 11.37] |
| 18.2 Nausea and/or vomiting | 1 | 9992 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.88 [5.46, 14.43] |
| 18.3 Slurred speech | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.13, 73.42] |
| 18.4 Muscle weakness | 1 | 9992 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.99 [5.22, 27.54] |
| 18.5 Hypotension | 1 | 9992 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.11, 3.26] |
| 18.6 Dizziness | 1 | 9992 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.70 [1.84, 7.42] |
| 18.7 Drowsiness or confusion | 1 | 9992 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.01, 4.87] |
| 18.8 Headache | 1 | 9992 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.19, 3.76] |
| 18.9 Any reported side effects | 1 | 9992 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.26 [4.59, 6.03] |
| 19 Problems at injection site | 1 | 9992 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.52, 2.08] |
| 19.1 Intramuscular injection | 1 | 4553 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.25, 1.79] |
| 19.2 Intravenous injection | 1 | 5439 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [2.15, 4.32] |
| 20 Placental abruption | 2 | 8838 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.50, 0.83] |
| 21 Caesarean section | 6 | 10096 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [1.01, 1.10] |
| 22 Induction of labour | 1 | 8774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.94, 1.04] |
| 23 Postpartum haemorrhage | 2 | 8909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.05] |
| 24 Manual removal of retained placenta | 1 | 8774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.72, 1.12] |
| 25 In special care baby unit > 7 days | 1 | 8260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.11] |
| 26 Outcome for the women 2 years after the birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 Death after discharge from hospital | 1 | 3359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.71, 4.53] |
| 26.2 Morbidity potentially related to pre‐eclampsia | 1 | 3375 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.55, 1.26] |
| 26.3 Hypertension | 1 | 3375 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.51, 1.20] |
| 26.4 Stroke | 1 | 3375 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.89] |
| 26.5 Renal problems | 1 | 3375 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.21, 1.99] |
| 26.6 Depression | 1 | 3340 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.66, 1.22] |
| 27 Stillbirths and neonatal deaths | 3 | 9961 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.93, 1.15] |
| 27.1 Severe pre‐eclampsia | 3 | 3341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.88, 1.18] |
| 27.2 Not severe pre‐eclampsia | 1 | 6620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.91, 1.21] |
| 28 Mortality for the fetus or infant (by time of death) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 Stillbirth | 3 | 9961 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.87, 1.12] |
| 28.2 Perinatal death | 2 | 9259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.88, 1.10] |
| 28.3 Neonatal death | 1 | 8260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.94, 1.42] |
| 28.4 Infant death (from 28 days to 1 year) | 1 | 8260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.52, 2.12] |
| 29 Death or in special care baby unit > 7 days | 1 | 9024 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.08] |
| 29.1 Severe pre‐eclampsia | 1 | 2404 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.85, 1.02] |
| 29.2 Not severe pre‐eclampsia | 1 | 6620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.99, 1.17] |
| 30 Apgar score < 7 at 5 minutes | 1 | 8260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.22] |
| 31 Intubated at place of birth | 1 | 8260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.82, 1.24] |
| 32 Admission to special care baby unit | 1 | 8260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.96, 1.06] |
| 33 Blood transfusion | 1 | 8774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.77, 1.09] |
| 34 Outcome for the children at 18 months (corrected for gestation at birth) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 34.1 Death after discharge | 1 | 2895 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.57, 1.84] |
| 34.2 Blind | 1 | 2895 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.21, 5.06] |
| 34.3 Deaf | 1 | 2895 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.19, 22.54] |
| 34.4 Severe cerebral palsy | 1 | 2895 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.09, 1.26] |
| 34.5 Developmental delay | 1 | 2895 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.35, 1.63] |
| 34.6 Neurosensory disability | 1 | 3283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.38, 1.58] |
| 34.7 Death or neurosensory disability | 1 | 3283 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.90, 1.25] |
1.1. Analysis.
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 1 Maternal death.
1.2. Analysis.
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 2 Eclampsia.
1.3. Analysis.
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 3 Serious maternal morbidity.
1.4. Analysis.
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 4 Stroke.
1.5. Analysis.
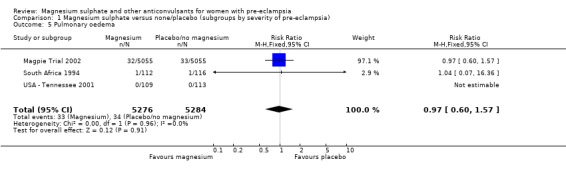
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 5 Pulmonary oedema.
1.6. Analysis.
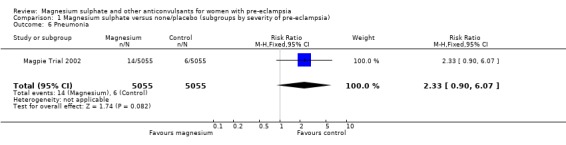
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 6 Pneumonia.
1.7. Analysis.
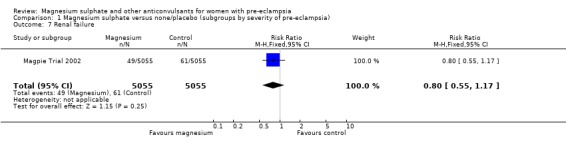
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 7 Renal failure.
1.8. Analysis.
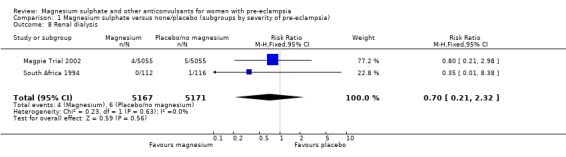
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 8 Renal dialysis.
1.9. Analysis.
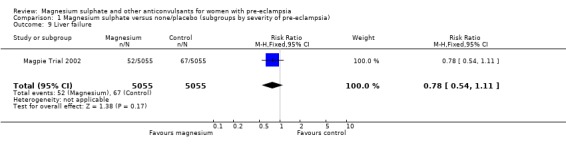
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 9 Liver failure.
1.10. Analysis.
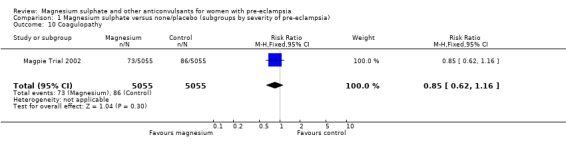
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 10 Coagulopathy.
1.11. Analysis.
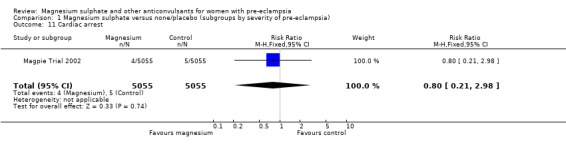
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 11 Cardiac arrest.
1.12. Analysis.
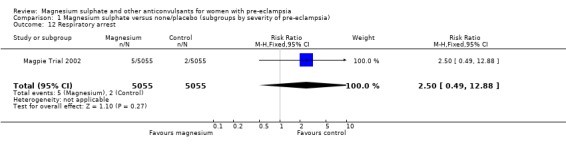
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 12 Respiratory arrest.
1.13. Analysis.
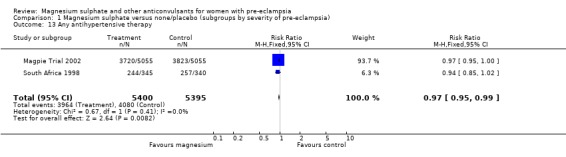
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 13 Any antihypertensive therapy.
1.14. Analysis.
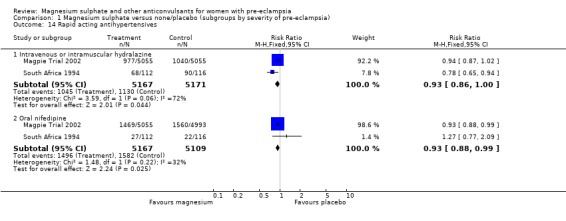
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 14 Rapid acting antihypertensives.
1.15. Analysis.
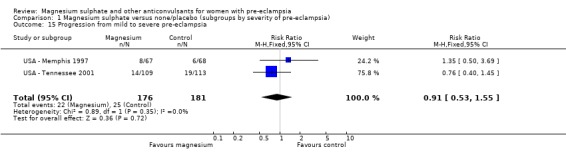
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 15 Progression from mild to severe pre‐eclampsia.
1.16. Analysis.
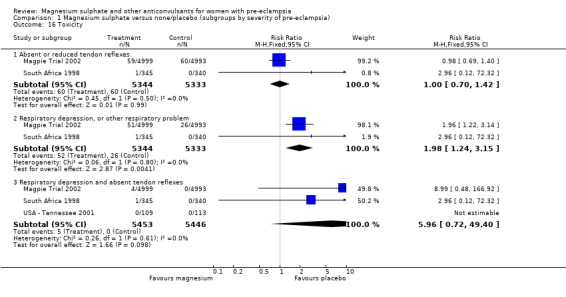
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 16 Toxicity.
1.17. Analysis.
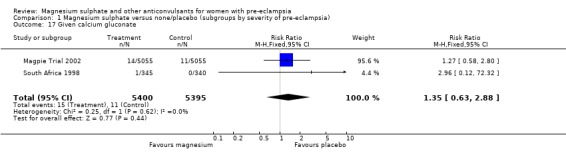
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 17 Given calcium gluconate.
1.18. Analysis.
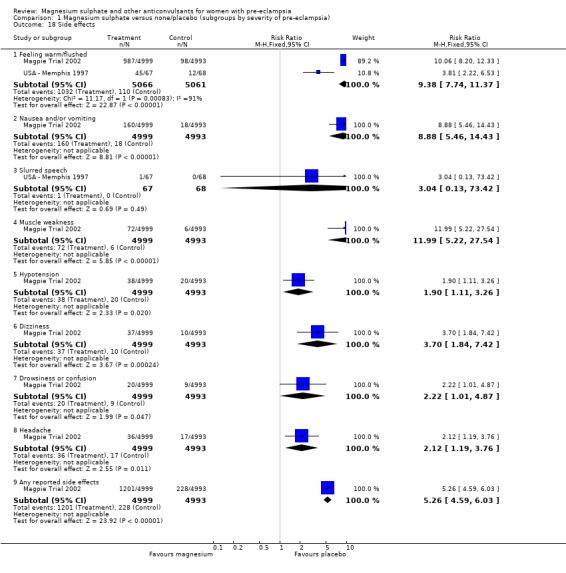
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 18 Side effects.
1.19. Analysis.
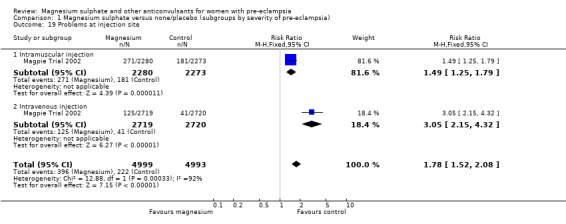
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 19 Problems at injection site.
1.20. Analysis.
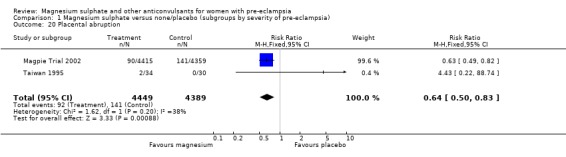
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 20 Placental abruption.
1.21. Analysis.
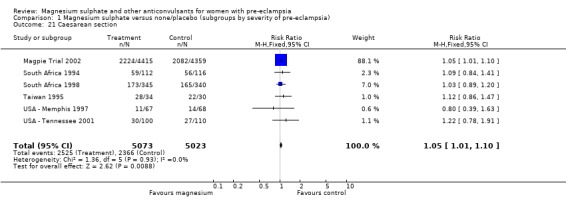
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 21 Caesarean section.
1.22. Analysis.
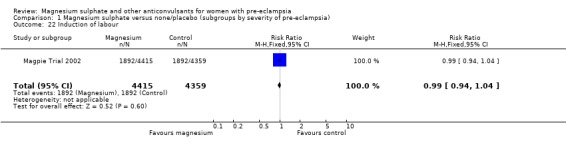
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 22 Induction of labour.
1.23. Analysis.
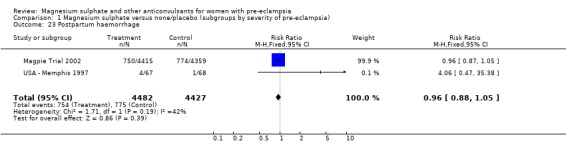
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 23 Postpartum haemorrhage.
1.24. Analysis.
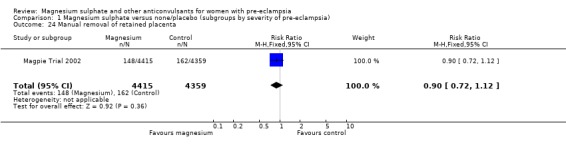
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 24 Manual removal of retained placenta.
1.25. Analysis.
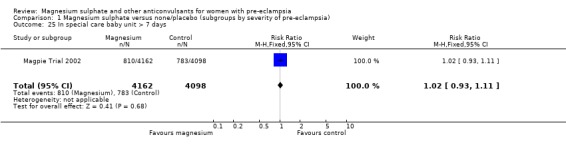
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 25 In special care baby unit > 7 days.
1.26. Analysis.
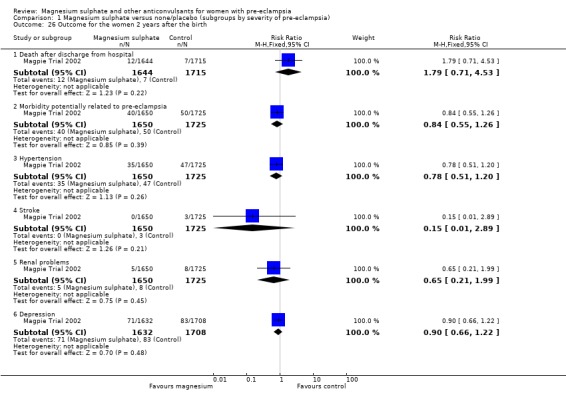
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 26 Outcome for the women 2 years after the birth.
1.27. Analysis.
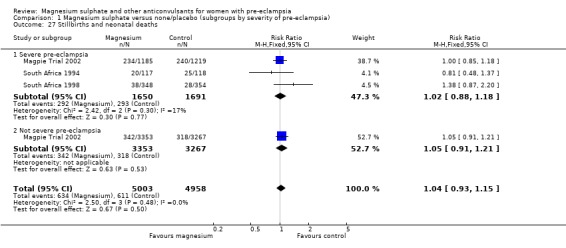
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 27 Stillbirths and neonatal deaths.
1.28. Analysis.
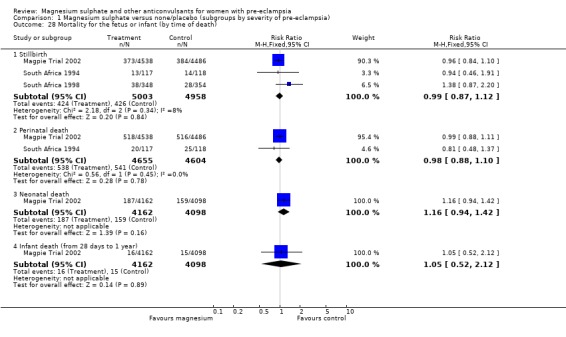
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 28 Mortality for the fetus or infant (by time of death).
1.29. Analysis.
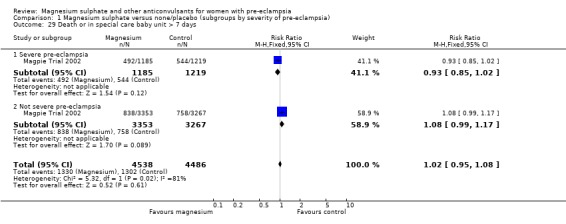
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 29 Death or in special care baby unit > 7 days.
1.30. Analysis.
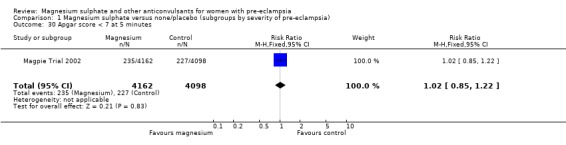
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 30 Apgar score < 7 at 5 minutes.
1.31. Analysis.
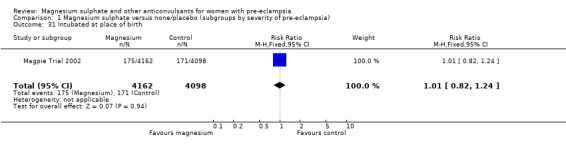
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 31 Intubated at place of birth.
1.32. Analysis.
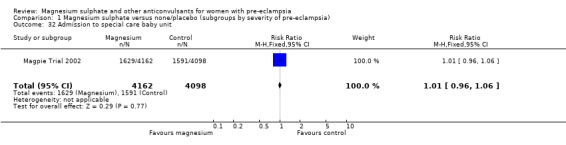
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 32 Admission to special care baby unit.
1.33. Analysis.
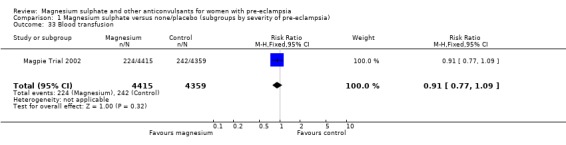
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 33 Blood transfusion.
1.34. Analysis.
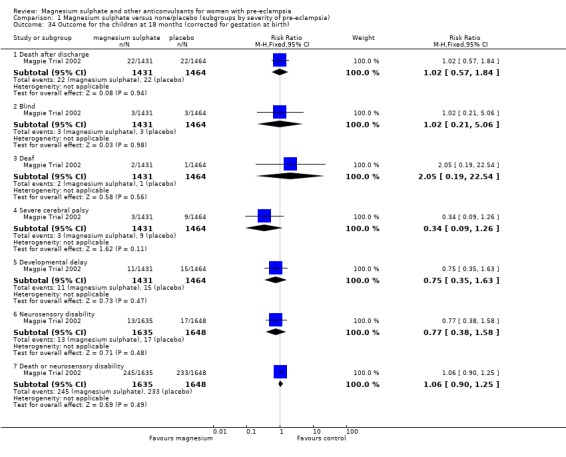
Comparison 1 Magnesium sulphate versus none/placebo (subgroups by severity of pre‐eclampsia), Outcome 34 Outcome for the children at 18 months (corrected for gestation at birth).
Comparison 2. Magnesium sulphate versus none/placebo (subgroups by whether delivered at trial entry).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 2 | 10795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.26, 1.09] |
| 1.1 Antepartum at trial entry | 2 | 9460 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.26, 1.19] |
| 1.2 Postpartum at trial entry | 1 | 1335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.48] |
| 2 Eclampsia | 6 | 11444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.29, 0.58] |
| 2.1 Antepartum at trial entry | 6 | 10109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.27, 0.57] |
| 2.2 Postpartum at trial entry | 1 | 1335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.16, 1.80] |
| 3 Serious maternal morbidity | 1 | 10110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.88, 1.30] |
| 3.1 Antepartum at trial entry | 1 | 8775 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.33] |
| 3.2 Postpartum at trial entry | 1 | 1335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.48, 1.77] |
2.1. Analysis.
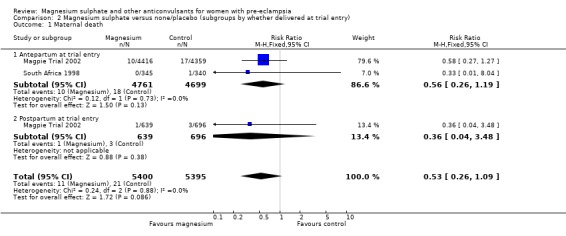
Comparison 2 Magnesium sulphate versus none/placebo (subgroups by whether delivered at trial entry), Outcome 1 Maternal death.
2.2. Analysis.
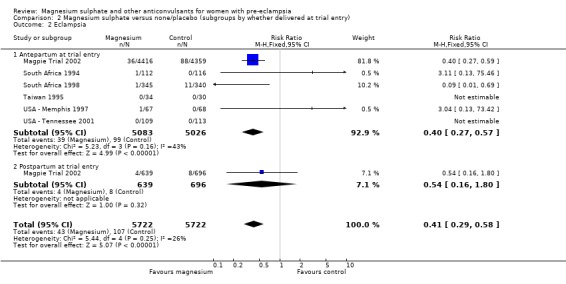
Comparison 2 Magnesium sulphate versus none/placebo (subgroups by whether delivered at trial entry), Outcome 2 Eclampsia.
2.3. Analysis.
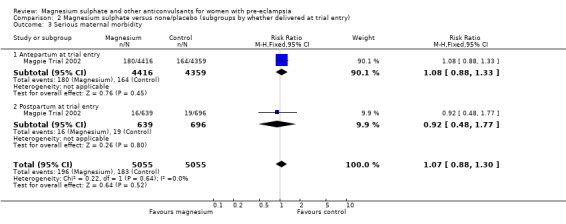
Comparison 2 Magnesium sulphate versus none/placebo (subgroups by whether delivered at trial entry), Outcome 3 Serious maternal morbidity.
Comparison 3. Magnesium sulphate versus none/placebo (subgroups by gestation at trial entry).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 2 | 9460 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.26, 1.20] |
| 1.1 < 34 weeks | 1 | 2412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.21, 1.91] |
| 1.2 >/= 34 weeks | 1 | 6363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.18, 1.63] |
| 1.3 Gestation not specified | 1 | 685 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.04] |
| 2 Eclampsia | 6 | 10109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.27, 0.57] |
| 2.1 < 34 weeks | 1 | 2412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.28, 1.06] |
| 2.2 >/= 34 weeks | 2 | 6498 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.24, 0.59] |
| 2.3 Gestation not specified | 4 | 1199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.06, 0.84] |
| 3 Serious maternal morbidity | 1 | 8775 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.89, 1.34] |
| 3.1 < 34 weeks | 1 | 2412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.68, 1.18] |
| 3.2 >/= 34 weeks | 1 | 6363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.00, 1.86] |
| 3.3 Gestation not specified | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Stillbirths and neonatal deaths | 3 | 9961 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.14] |
| 4.1 < 34 weeks | 1 | 2443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.92, 1.14] |
| 4.2 >/= 34 weeks | 1 | 6581 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.85, 1.33] |
| 4.3 Gestation not specified | 2 | 937 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.78, 1.57] |
| 5 Death or in special care baby unit > 7 days | 1 | 9024 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.97, 1.07] |
| 5.1 < 34 weeks | 1 | 2443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.97, 1.07] |
| 5.2 >/= 34 weeks | 1 | 6581 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.17] |
| 5.3 Gestation not specified | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
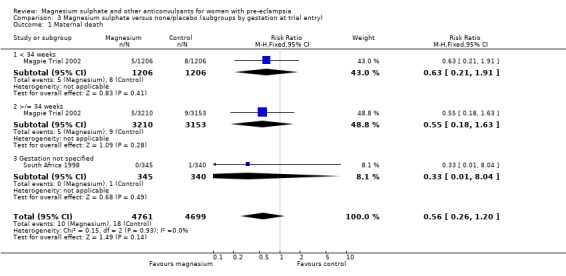
Comparison 3 Magnesium sulphate versus none/placebo (subgroups by gestation at trial entry), Outcome 1 Maternal death.
3.2. Analysis.
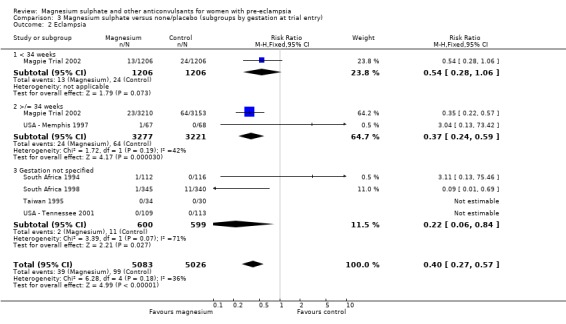
Comparison 3 Magnesium sulphate versus none/placebo (subgroups by gestation at trial entry), Outcome 2 Eclampsia.
3.3. Analysis.
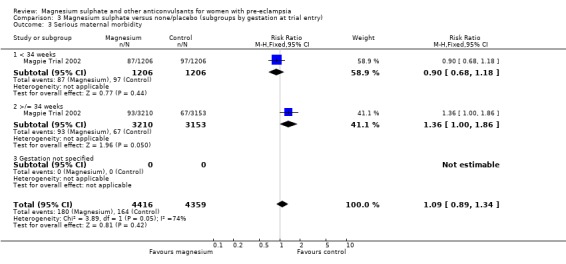
Comparison 3 Magnesium sulphate versus none/placebo (subgroups by gestation at trial entry), Outcome 3 Serious maternal morbidity.
3.4. Analysis.
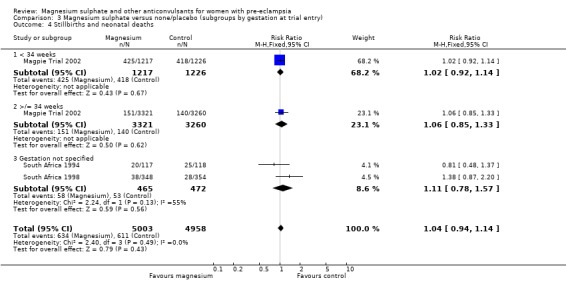
Comparison 3 Magnesium sulphate versus none/placebo (subgroups by gestation at trial entry), Outcome 4 Stillbirths and neonatal deaths.
3.5. Analysis.
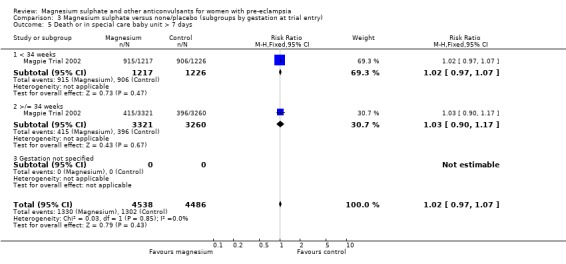
Comparison 3 Magnesium sulphate versus none/placebo (subgroups by gestation at trial entry), Outcome 5 Death or in special care baby unit > 7 days.
Comparison 4. Magnesium sulphate versus none/placebo (subgroups by whether anticonvulsant before trial entry).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 2 | 10732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.26, 1.09] |
| 1.1 Anticonvulsant before trial entry | 1 | 874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.20, 4.88] |
| 1.2 No anticonvulsant before trial entry | 2 | 9858 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.20, 1.03] |
| 2 Eclampsia | 6 | 11381 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.29, 0.58] |
| 2.1 Anticonvulsant before trial entry | 1 | 874 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.49, 3.11] |
| 2.2 No anticonvulsant before trial entry | 3 | 10086 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.22, 0.48] |
| 2.3 Unclear whether anticonvulsant before trial entry | 3 | 421 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.13, 73.42] |
| 3 Serious maternal morbidity | 1 | 10047 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.87, 1.29] |
| 3.1 Anticonvulsant before trial entry | 1 | 874 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.69, 1.85] |
| 3.2 No anticonvulsant before trial entry | 1 | 9173 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.85, 1.30] |
| 4 Stillbirths and neonatal deaths | 3 | 9901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.93, 1.14] |
| 4.1 Anticonvulsant before trial entry | 1 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.11, 2.00] |
| 4.2 No anticonvulsant before trial entry | 3 | 9097 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.87, 1.09] |
| 5 Death or in special care baby unit > 7 days | 1 | 8965 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.08] |
| 5.1 Anticonvulsant before trial entry | 1 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.00, 1.39] |
| 5.2 No anticonvulsant before trial entry | 1 | 8161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.92, 1.06] |
4.1. Analysis.
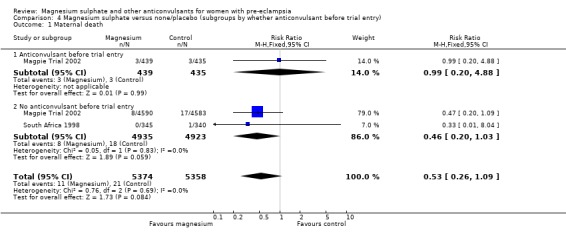
Comparison 4 Magnesium sulphate versus none/placebo (subgroups by whether anticonvulsant before trial entry), Outcome 1 Maternal death.
4.2. Analysis.
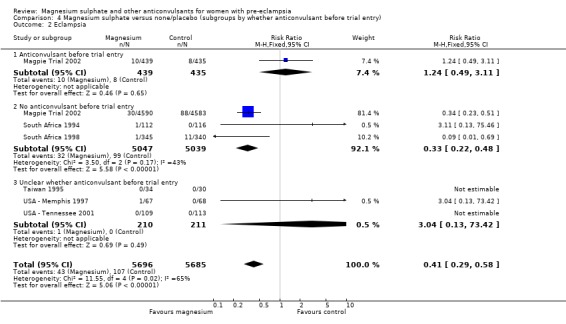
Comparison 4 Magnesium sulphate versus none/placebo (subgroups by whether anticonvulsant before trial entry), Outcome 2 Eclampsia.
4.3. Analysis.
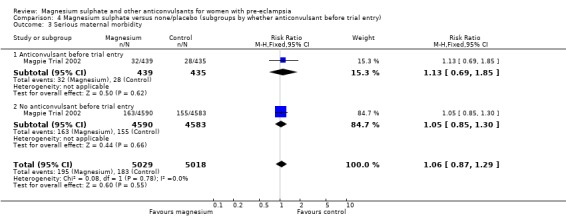
Comparison 4 Magnesium sulphate versus none/placebo (subgroups by whether anticonvulsant before trial entry), Outcome 3 Serious maternal morbidity.
4.4. Analysis.
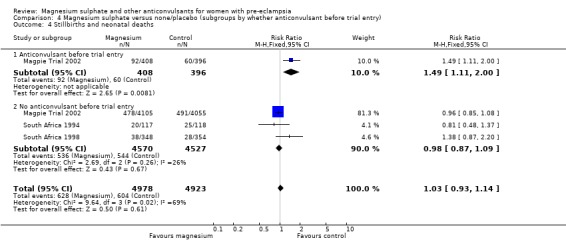
Comparison 4 Magnesium sulphate versus none/placebo (subgroups by whether anticonvulsant before trial entry), Outcome 4 Stillbirths and neonatal deaths.
4.5. Analysis.
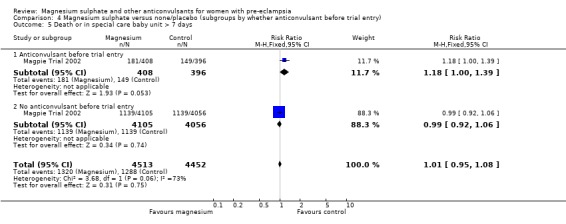
Comparison 4 Magnesium sulphate versus none/placebo (subgroups by whether anticonvulsant before trial entry), Outcome 5 Death or in special care baby unit > 7 days.
Comparison 5. Magnesium sulphate versus none/placebo (subgroups by dose and route of administration for maintenance therapy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 2 | 10795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.26, 1.09] |
| 1.1 Intramuscular maintenance regimen | 1 | 4593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.25, 1.48] |
| 1.2 Intravenous maintenance regimen ‐ 1 g/hour | 2 | 6202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.12, 1.43] |
| 1.3 Intravenous maintenance regimen ‐ 2 g/hour | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Eclampsia | 6 | 11444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.29, 0.58] |
| 2.1 Intramuscular maintenance regimen | 2 | 4821 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.24, 0.65] |
| 2.2 Intravenous maintenance regimen ‐ 1 g/hour | 3 | 6266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.24, 0.66] |
| 2.3 Intravenous maintenance regimen ‐ 2 g/hour | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.13, 73.42] |
| 3 Stillbirths and neonatal deaths | 3 | 9961 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.93, 1.14] |
| 3.1 Intramuscular maintenance regimen | 2 | 4565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.89, 1.15] |
| 3.2 Intravenous maintenance regimen ‐ 1 g/hour | 2 | 5396 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.89, 1.27] |
| 3.3 Intravenous maintenance regimen ‐ 2 g/hour | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Any reported side effects | 2 | 10127 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.16 [4.52, 5.89] |
| 4.1 Intramuscular maintenance regimen | 1 | 4553 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.84 [4.80, 7.09] |
| 4.2 Intravenous maintenance regimen ‐ 1 g/hour | 1 | 5439 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.67 [3.86, 5.66] |
| 4.3 Intravenous maintenance regimen ‐ 2 g/hour | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.81 [2.22, 6.53] |
5.1. Analysis.
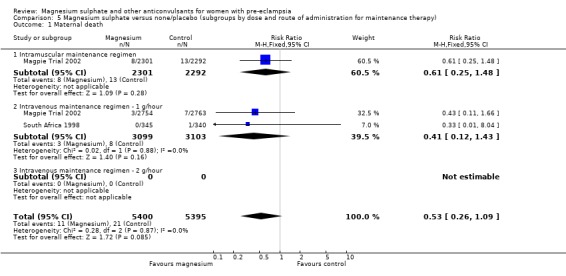
Comparison 5 Magnesium sulphate versus none/placebo (subgroups by dose and route of administration for maintenance therapy), Outcome 1 Maternal death.
5.2. Analysis.
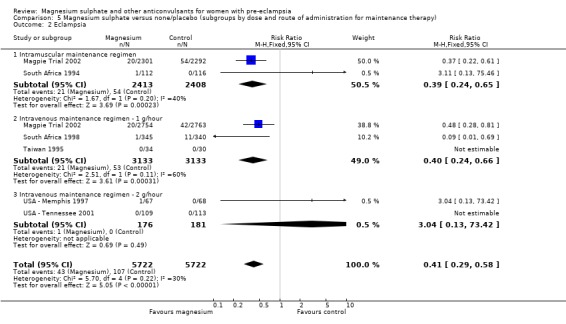
Comparison 5 Magnesium sulphate versus none/placebo (subgroups by dose and route of administration for maintenance therapy), Outcome 2 Eclampsia.
5.3. Analysis.
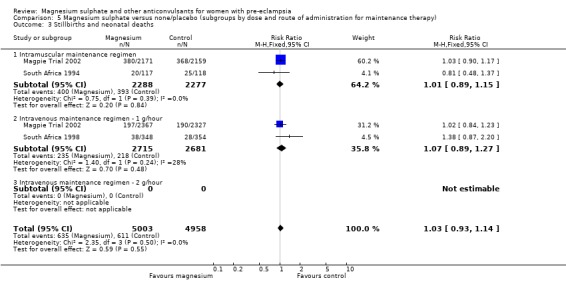
Comparison 5 Magnesium sulphate versus none/placebo (subgroups by dose and route of administration for maintenance therapy), Outcome 3 Stillbirths and neonatal deaths.
5.4. Analysis.
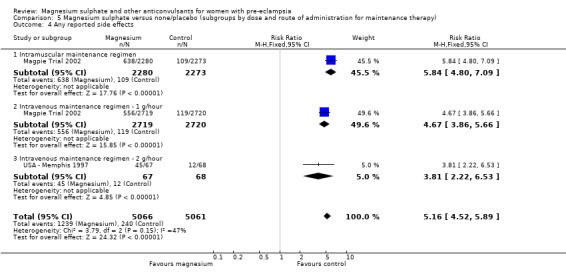
Comparison 5 Magnesium sulphate versus none/placebo (subgroups by dose and route of administration for maintenance therapy), Outcome 4 Any reported side effects.
Comparison 6. Magnesium sulphate versus phenytoin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eclampsia | 3 | 2291 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.60] |
| 2 Complications of labour | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Induction of labour | 1 | 2138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.82, 1.05] |
| 2.2 Augmentation of labour | 1 | 2138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 3 Caesarean section | 2 | 2192 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.04, 1.40] |
| 4 Mortality for the fetus or infant (by time of death) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Stillbirth | 1 | 2165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.41] |
| 4.2 Neonatal death | 1 | 2165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.31] |
| 5 Infant morbidity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Low Apgar at 5 minutes | 1 | 2141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.26, 1.30] |
| 5.2 Admission to special care baby unit | 1 | 2141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.63, 1.59] |
6.1. Analysis.
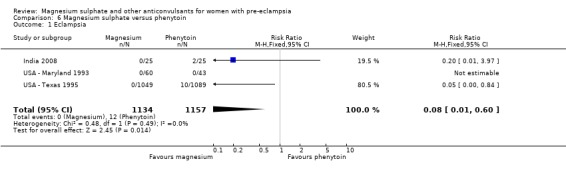
Comparison 6 Magnesium sulphate versus phenytoin, Outcome 1 Eclampsia.
6.2. Analysis.
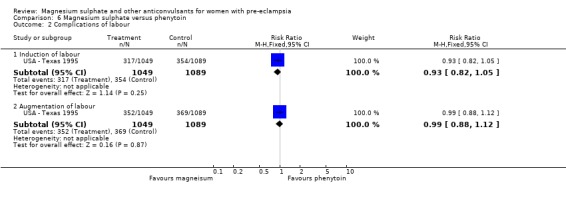
Comparison 6 Magnesium sulphate versus phenytoin, Outcome 2 Complications of labour.
6.3. Analysis.
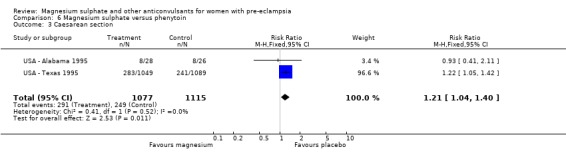
Comparison 6 Magnesium sulphate versus phenytoin, Outcome 3 Caesarean section.
6.4. Analysis.
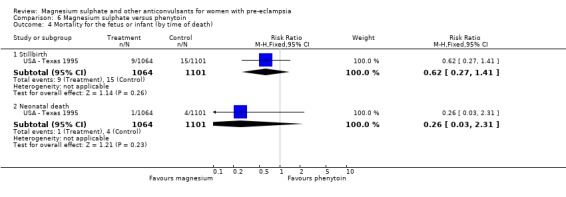
Comparison 6 Magnesium sulphate versus phenytoin, Outcome 4 Mortality for the fetus or infant (by time of death).
6.5. Analysis.
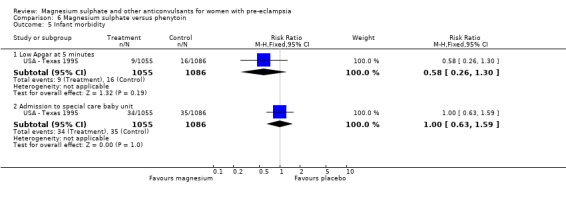
Comparison 6 Magnesium sulphate versus phenytoin, Outcome 5 Infant morbidity.
Comparison 7. Magnesium sulphate versus diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eclampsia | 2 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.31] |
| 2 Caesarean section | 2 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.76, 1.27] |
| 3 Stillbirths and neonatal deaths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Stillbirth | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Serinatal death | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.

Comparison 7 Magnesium sulphate versus diazepam, Outcome 1 Eclampsia.
7.2. Analysis.
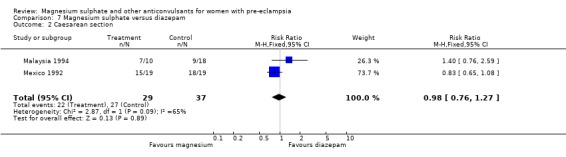
Comparison 7 Magnesium sulphate versus diazepam, Outcome 2 Caesarean section.
7.3. Analysis.
Comparison 7 Magnesium sulphate versus diazepam, Outcome 3 Stillbirths and neonatal deaths.
Comparison 8. Magnesium sulphate versus nimodipine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eclampsia | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.14, 0.77] |
| 2 Stroke | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Coagulopathy | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.14, 2.47] |
| 4 Respiratory problems | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.61 [1.01, 12.91] |
| 5 Cardiac failure | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.93 [0.24, 102.49] |
| 6 Respiratory depression | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.61 [1.01, 12.91] |
| 7 Antihypertensive drug | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.08, 1.31] |
| 8 Oliguria | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.79, 1.68] |
| 9 Side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Flushing | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.47 [2.47, 8.09] |
| 9.2 Nausea/vomiting | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.81, 1.69] |
| 9.3 Headache | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.63, 1.40] |
| 9.4 Hypotension | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.44, 4.33] |
| 10 Placental abruption | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.46, 3.77] |
| 11 Caesarean section | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.94, 1.13] |
| 12 Postpartum haemorrhage | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.09, 5.56] |
| 13 Respiratory distress syndrome | 1 | 1564 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.84, 1.81] |
| 14 Neonatal hypotonia | 1 | 1564 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.91, 3.46] |
| 15 Baby intubated | 1 | 1564 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.91, 2.05] |
| 16 Neonatal hypotension | 1 | 1564 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.06, 1.58] |
8.1. Analysis.
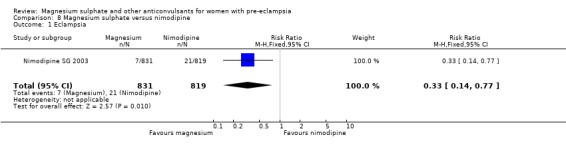
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 1 Eclampsia.
8.2. Analysis.
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 2 Stroke.
8.3. Analysis.
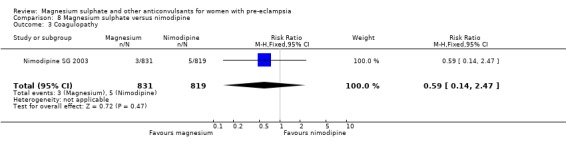
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 3 Coagulopathy.
8.4. Analysis.
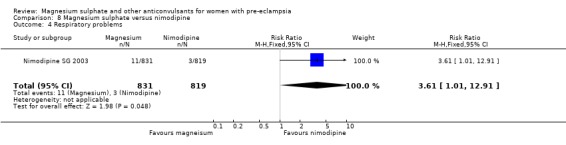
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 4 Respiratory problems.
8.5. Analysis.
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 5 Cardiac failure.
8.6. Analysis.
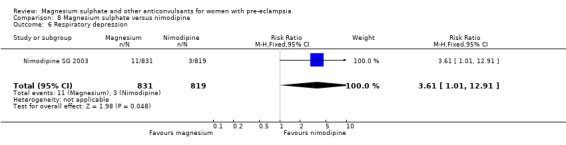
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 6 Respiratory depression.
8.7. Analysis.
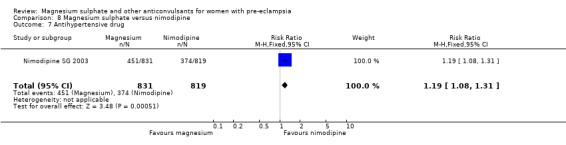
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 7 Antihypertensive drug.
8.8. Analysis.
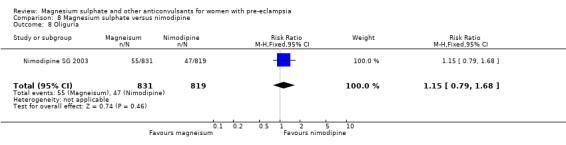
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 8 Oliguria.
8.9. Analysis.
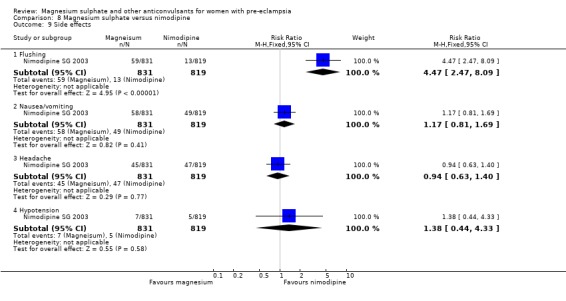
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 9 Side effects.
8.10. Analysis.
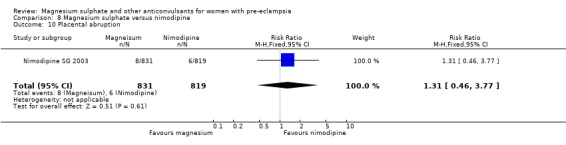
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 10 Placental abruption.
8.11. Analysis.
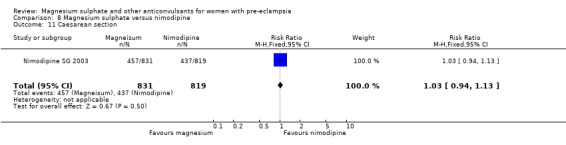
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 11 Caesarean section.
8.12. Analysis.
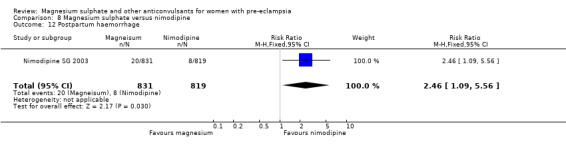
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 12 Postpartum haemorrhage.
8.13. Analysis.
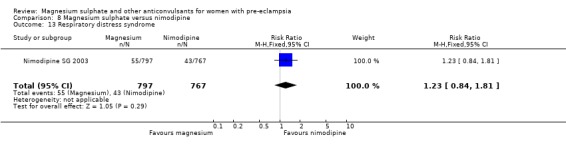
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 13 Respiratory distress syndrome.
8.14. Analysis.
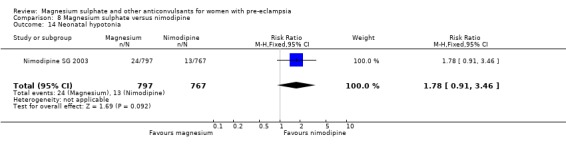
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 14 Neonatal hypotonia.
8.15. Analysis.
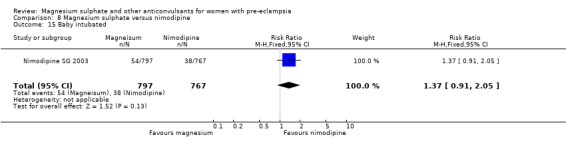
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 15 Baby intubated.
8.16. Analysis.
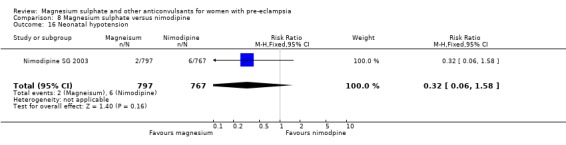
Comparison 8 Magnesium sulphate versus nimodipine, Outcome 16 Neonatal hypotension.
Comparison 9. Magnesium chloride versus methyl dopa.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Other antihypertensive therapy | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.61, 1.43] |
| 2 Admission to special care baby unit | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.08, 17.71] |
9.1. Analysis.
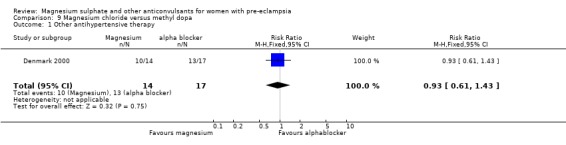
Comparison 9 Magnesium chloride versus methyl dopa, Outcome 1 Other antihypertensive therapy.
9.2. Analysis.
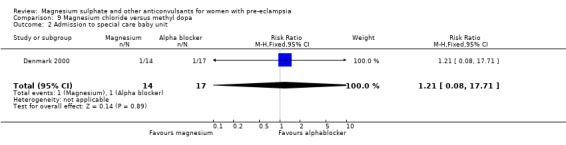
Comparison 9 Magnesium chloride versus methyl dopa, Outcome 2 Admission to special care baby unit.
Comparison 10. Magnesium sulphate versus nitrate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eclampsia | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
10.1. Analysis.
Comparison 10 Magnesium sulphate versus nitrate, Outcome 1 Eclampsia.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Denmark 2000.
| Methods | Please see details below. | |
| Participants | 33 nulliparous women with singleton pregnancy and BP > 140/90 mmHg x 2 over 3 hr. Excluded: pre‐existing HT, cardiac or renal disease, BP > 180/120 mmHg after hydralazine. | |
| Interventions | MgCl2: 80 mmol IV in first 24 hr, then 40 mmol in next 24 hr. Then 15 mmol/day MgOH2 orally until 3 days after delivery. Methyl dopa: 250 mg x 4/day. Day after delivery reduced by 250 mg/day. | |
| Outcomes | Women: additional antihypertensive. Baby: admission to SCBU. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Computer generated." |
| Allocation concealment? | Low risk | "Numbered sealed opaque envelopes opened if the patients accepted participation and gave a signed consent." |
| Blinding? All outcomes | Unclear risk | Code was kept in the hospital dispensary but treatment regimens different, so unlikely to be blinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | 2 exclusions from MgSO4 group (1 withdrawal, 1 had methyl dopa). |
India 2008.
| Methods | Please see details below. | |
| Participants | 100 women, 50 with eclampsia and 50 severe pre‐eclampsia, regardless of age, parity, gestational age, singleton or multiple pregnancy, or whether delivered. Diagnostic criteria for severe pre‐eclampsia not given. | |
| Interventions | MgSO4: loading dose 4 g IV over 20‐30 minutes and 10 gm IM (5 g into each buttock), maintenance 5 g IM every 4 hours. Phenytoin: loading dose 1000 mg in 200 ml normal saline over 1 hour, maintenance 500 mg orally 10 hours later. Both regimens continued for 24 hours postpartum. |
|
| Outcomes | Woman: eclampsia. Baby: none reported. |
|
| Notes | Only the 50 women with pre‐eclampsia are included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Randomly subdivided"; no other information. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? All outcomes | High risk | Blinding is not mentioned, but as the interventions are quite different blinding of clinicians and women would not have been possible. |
| Incomplete outcome data addressed? All outcomes | High risk | No losses to follow‐up are reported. The only outcome reported is eclampsia. No other outcomes are listed in methods, but it would seem surprising if no other data were collected. |
Magpie Trial 2002.
| Methods | Please see details below. | |
| Participants | 10141 women with uncertainty about whether to use MgSO4, before birth or 24 hours postpartum, DBP ≥90 mmHg, SBP ≥140 mmHg x 2 30‐30 min apart, ≥1+ proteinuria.
Excluded: hypersensitivity to Mg, hepatic coma with risk of renal failure, myasthenia gravis. For follow‐up: 4782 women selected, and 3375 (71%) traced; 4483 children selected, and 3283 (73%) traced. |
|
| Interventions | MgSO4: 4 g IV bolus. Then either 1 g/hr IV infusion or 10 g IM with bolus followed by 5 g every 4 hr. Continued for 24 hr.
2 centres in Bangladesh used 5 g IM then 2.5 g every 4 hr. Placebo: by identical regimen. Dose halved if oliguria. Clinical monitoring alone for all women. |
|
| Outcomes | Woman: death, eclampsia, respiratory depression, pneumonia, cardiac arrest, renal failure, coagulopathy, liver failure, pulmonary oedema, stroke, side effects, caesarean section, postpartum haemorrhage, transfusion, admission to high care. For long‐term follow‐up: death after discharge, stoke, serious renal problems, severe hypertension. Baby: death, gestation at birth, Apgar < 7 at 5 min, intubation at place of delivery, ventilation, admission SCBU, death or SCBU > 7 days. For long‐term follow‐up: death or neurosensory disability (defined as 1 or more of blind, deaf, severe cerebral palsy, or developmental delay), cerebral palsy, blind, deaf, developmental delay (> 2 sd below mean), other disability. |
|
| Notes | Multicentre trial, 175 centres in 33 countries. 85% recruitment in low‐ and middle‐income countries. Trial stopped early after the Data Monitoring Committee revealed data to the Trial Steering Committee. Outcome at delivery and for the baby only included for women randomised before birth. Follow‐up In a subset of 125 centres in 19 countries. For the women median time from birth to follow‐up was 26 months (range from within 12 months to greater than 48 months from delivery, interquartile range 19‐36 months). Women were assessed by a brief questionnaire, available in English and Spanish. It was either sent by post, administered in clinic or during a home visit, or completed over the telephone. Children were assessed at 18 months (corrected for gestational age at birth) using the Ages and Stage Questionnaire (ASQ) (Squires 1999). The ASQ was available in English and Spanish, and either sent by post, administered in the clinic or during a home visit, or completed over the telephone. It has 30 questions in 5 domains: communication, gross motor, fine motor, problem solving, and personal‐social. To pass, a child must pass all 5 domains. An additional section asked about parental concerns, and was not scored. For some centres the ASQ was not thought feasible for cultural or language reasons, and a short ASQ was developed with 3 questions in 3 domains (gross motor, communication, problem solving). If the family could not be contacted, information about whether the child was 'alive and well' was collected whenever possible. Children who failed the ASQ were invited for clinical and neurodevelopmental assessment using the Bayley Scales of Infant Development (BSID‐II), the Griffiths Tests, or a similar alternative. Where these tests were not available, assessment was by clinical history and examination based on the Health Status Questionnaire (Jones 2002). In the UK, the Office of National Statistics provided date and cause of any deaths. For surviving women and children an additional questionnaire was to the general practitioner 18 months after delivery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated allocation sequence. |
| Allocation concealment? | Low risk | Via central telephone service (2037 women), or consecutively numbered, sealed treatment packs, stratified by centre (8104 women). |
| Blinding? All outcomes | Low risk | Solutions of MgSO4 and placebo looked identical in pre‐prepared treatment packs. Clinicians were asked not to measure serum magnesium concentrations, unless clinically necessary, and to report if measurements were taken. In follow‐up studies, those involved in finding participants and assessing their outcomes were blinded to treatment group allocation. |
| Incomplete outcome data addressed? All outcomes | Low risk | 5 women excluded: 2 each group no data available, 1 in MgSO4 group entered into wrong trial. Compliance 99% (MgSO4 4999/5055; placebo 4993/5055). Follow‐up 7927 women randomised at the 125 centres in 19 countries participating in follow‐up. Of these, 2544 were not included in follow‐up for logistic reasons (such as giving birth outside a pre‐specified time frame, or in a pre‐defined geographic area). A further 601 women were excluded: 109 were from centres excluded as < 20% women contacted; 466 were discharged without a surviving child; and 26 opted out. Of the 4782 women included in follow‐up, data are available for 3375 (71%): 1650 allocated MgSO4and 1725 allocated placebo. 6922 children were born to women randomised at the 125 centres in 19 countries participating in follow‐up. Of these 2271 were not included for logistic reasons (such as giving birth outside a pre‐specified time frame, or in a pre‐defined geographic area). A further 168 children were excluded: 101 were from centres excluded as < 20% families contacted; 40 whose death or disability was due to a problem at conception or embryogenesis, and 27 whose parents opted out. Of the 4483 children included in follow‐up, data are available for 3283 (73%): 1635 born to women allocated magnesium sulphate and 1648 to women allocated placebo. |
Malaysia 1994.
| Methods | Please see details below. | |
| Participants | 28 women with PE (DBP > 110mmHg + proteinuria) and 11 women with eclampsia (data not included in this review). | |
| Interventions | MgSO4: "Pritchard's regimen", no other information. Diazepam: not stated. |
|
| Outcomes | Woman: death, eclampsia, caesarean section. Baby: death. |
|
| Notes | Interim data on an ongoing study, published in abstract form only. Additional information from verbal presentation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Randomly allocated," nor further information given. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? All outcomes | High risk | Blinding not described. |
| Incomplete outcome data addressed? All outcomes | High risk | Interim data for a trial ongoing at the time the abstract was published. No data from the completed trial. |
Mexico 1992.
| Methods | Please see details below. | |
| Participants | 38 women > 28 weeks' gestation with SBP >/= 150 mmHg, DBP >/= 110 mmHg, proteinuria 2+, no previous treatment, at least 1 symptom (of headache, blurred vision, epigastric pain) and no epilepsy. | |
| Interventions | MgSO4: 4 g IV over 15 min, then 1 g/hr infusion. Diazepam: 30 mg in 500 ml 5% glucose IV at 60 microg/hr. If convulsions, bolus of 10 mg IV. |
|
| Outcomes | Woman: eclampsia, caesarean section. Baby: mean Apgar scores (1 and 5 min). |
|
| Notes | Nifedipine for BP control. Translated from the Spanish. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Permuted block randomisation" using random number tables. |
| Allocation concealment? | Unclear risk | Allocation was by sealed, numbered envelopes. |
| Blinding? All outcomes | Unclear risk | Not described. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | The total number of women eligible for trial entry is not described. |
Mexico 1998.
| Methods | Please see details below. | |
| Participants | 36 women with severe pre‐eclampsia who were over 36 weeks' gestation. Excluded: women with mild pre‐eclampsia, less than 36 weeks' gestation, or who received anti‐hypertensive treatment less than 1 week prior, as well as women a history of diabetes, high blood pressure, alcohol or drug abuse. |
|
| Interventions | MgSO4: 4 g IV for first hour, then 1 g/hour for 5 hours. Isosorbide gas: as a spray, 1.25 mg repeated in 10 minutes if the average blood pressure reduction was less than 15%. |
|
| Outcomes | Woman: blood pressure, proteinuria, maternal heart rate, mode of delivery. Baby: Apgar. |
|
| Notes | Translated from the Spanish. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Randomly divided equally," no further information given. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? All outcomes | High risk | Blinding is not mentioned, but the interventions are different and so blinding of participants and clinicians would have been unlikely. |
| Incomplete outcome data addressed? All outcomes | High risk | No losses to follow‐up were reported. Outcomes were only recorded for the duration of the intervention ‐ 5 hours. |
Nimodipine SG 2003.
| Methods | Please see details below. | |
| Participants | 1750 women with PE, planned delivery and no previous MgSO4. BP ≥140/90 mmHg and 1+ proteinuria plus 1 of: headache, clonus, visual disturbance, epigastric pain, oliguria, pulmonary oedema, raised liver enzymes, haemolysis, oligohydramnios, IUGR. | |
| Interventions | MgSO4: according to local protocol. Either 4 g IV then 1 g/hr, or 6 g IV then 2 g/hr. Nimodipine: 60 mg 4 hourly, orally. Both regimens continued either for 24 hours total, or until 24 hours after delivery. Serum monitoring not required. |
|
| Outcomes | Woman: eclampsia, stroke, coagulopathy, respiratory problems, cardiac failure, antihypertensive drugs, side effects, abruption, caesarean section, PPH. Baby: RDS, hypotonia, intubation, hypotension. |
|
| Notes | Recruitment at 14 hospitals in 8 countries. Data for stillbirths and neonatal deaths not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomisation stratified by centre, blocks of 6. |
| Allocation concealment? | Unclear risk | Sealed opaque envelopes. |
| Blinding? All outcomes | High risk | Unblinded trial, but primary outcome was described as "binary, objective, and not subject to measurement bias". |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Recruitment 1995‐2000. 100 women (6%) excluded from analysis: 99 did not get allocated treatment, 1 withdrawn. Recruitment stopped early following interim analysis. |
South Africa 1994.
| Methods | Please see details below. | |
| Participants | 228 women with severe PE: DBP ≥110 mmHg for 4‐6 hours, proteinuria +, and delivery imminent. Excluded if prior anticonvulsant or antihypertensive. | |
| Interventions | MgSO4: 4 g IV over 20 min and 10 g IM (5 g into each buttock), then 5 g 4 hourly for 24 hours. Control: no anticonvulsant. Women being transferred were given phenobarbitone IM before transfer. |
|
| Outcomes | Woman: eclampsia, pulmonary oedema, renal failure, caesarean section. Baby: death (stillbirth, neonatal death). |
|
| Notes | For both groups, immediate BP control with dihydralazine (69%) or nifedipine (25%). Most women had phenobarbitone before entry. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "randomly distributed." |
| Allocation concealment? | Low risk | Consecutively numbered, sealed, opaque envelopes. Envelopes prepared by 1 author not involved in recruitment. |
| Blinding? All outcomes | High risk | Designed as open trial. |
| Incomplete outcome data addressed? All outcomes | Low risk | Data were complete. |
South Africa 1998.
| Methods | Please see details below. | |
| Participants | 822 women with severe PE: at least 2 of DBP >/= 110 mmHg, significant proteinuria, symptoms of imminent eclampsia. Also, > 16 years, no previous anticonvulsant. | |
| Interventions | MgSO4: 4 g IV in 200 ml saline over 20 min, then 1 g/hr (200 ml over 4 hr) until 24 hr after delivery.
Placebo: 200 ml over 20 min, then 200 ml over 4 hours until 24 hr after delivery. Treatment stopped if urine output < 30 ml/hr. Serum monitoring not required. Women transferred from outlying clinics received 1 g clonazepam IV before transfer. Women not transferred were given 1 g clonazepam IV after randomisation. |
|
| Outcomes | Woman: death, eclampsia, toxicity, antihypertensive therapy, caesarean section. Child: stillbirths. | |
| Notes | All women given clonazepam 1 mg. Recruitment over 13 years, 1982‐95. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. |
| Allocation concealment? | High risk | Allocation by sealed opaque envelopes containing card marked solution A or B. Cards, but not envelopes, consecutively numbered. Envelopes distributed in batches of 20, with equal numbers of A and B. Solutions prepared by pharmacy, and identity of A and B changed periodically. |
| Blinding? All outcomes | Low risk | Investigators blinded to identity of solutions. |
| Incomplete outcome data addressed? All outcomes | Low risk | 123 excluded as envelopes and data sheets lost. Review of hospital records suggests no eclampsia amongst these women. Further 14 post randomisation exclusions (4 delivered before treatment, 3 no solution available, 4 MgSO4 before entry, 2 no consent, 1 anuric). None had eclampsia. |
Taiwan 1995.
| Methods | "Randomised trial". | |
| Participants | 64 women with BP > 150/100 mmHg, plus at least one of 11 listed features of severe PE. Excluded if intrauterine death, chronic hypertension or eclampsia. | |
| Interventions | MgSO4: 4 g IV over 10 min, then 1 g/hr until 24 hours after delivery. Control: no anticonvulsant. |
|
| Outcomes | Women: eclampsia, caesarean section, abruption. Baby: Apgar score (1 min). |
|
| Notes | 8 women excluded, probably before randomisation but this is not completely clear. Women less than 34 weeks (32/64) managed conservatively, and duration of MgSO4 therapy not clear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Randomised", no further details given. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? All outcomes | Unclear risk | Not described. |
| Incomplete outcome data addressed? All outcomes | Low risk | 72 women eligible, excluded women with intrauterine fetal demise (2), chronic hypertension with superimposed pre‐eclampsia (2), and eclampsia (4). |
USA ‐ Alabama 1995.
| Methods | Please see details below. | |
| Participants | 54 women with singleton pregnancy requiring medical induction of labour for PIH, and with an unfavourable cervix. | |
| Interventions | MgSO4: 4 g IV and then an infusion of 2 g/hr. Phenytoin: 15 mg/kg IV over 2 hours, then 200 mg IV every 8 hours. |
|
| Outcomes | Woman: caesarean section, mean length of labour. Baby: mean Apgar scores, cord pH. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Blinded computer‐generated random number tables". |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? All outcomes | High risk | Nursing staff recorded "patient generated complaints or side effects noted, and date and time of cervical examinations". Magesium sulphate and phenytoin therapy was adjusted at the discretion of the attending physician. |
| Incomplete outcome data addressed? All outcomes | High risk | No losses to follow‐up are reported, but data are only available for a limited number of outcomes. |
USA ‐ Maryland 1993.
| Methods | Please see details below. | |
| Participants | 103 women with BP >/= 140/90 mmHg or rise in SBP of >/= 30 mmHg, or rise in DBP of >/= 15 mmHg, plus either >/= + proteinuria , or significant oedema, or eclampsia. Also, 2 women with eclampsia (data not included in this review). Excluded if MgSO4 before admission, history of seizure disorder, cardiac arrhythmia, phenytoin sensitivity or myasthenia gravis. | |
| Interventions | MgSO4: 6 g IV, then infusion of 2 g/hr. Mg levels every 6 hours. Phenytoin: 1000, 1250 or 1500 mg, depending on weight. Serum levels 1‐2 hours later to determine next dose (0‐500 mg), once stable checked every 12 hours. Both regimens continued for 24 hours after delivery. |
|
| Outcomes | Woman: eclampsia. No other outcomes reported separately for women with pre‐eclampsia and eclampsia. Baby: none reported. |
|
| Notes | Data for the 2 women with eclampsia not included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Sequence generated from random number table. |
| Allocation concealment? | Unclear risk | Sealed opaque envelopes |
| Blinding? All outcomes | High risk | Treatment not blinded, but interview for "subjective maternal side effects conducted by 1 author blinded to identity of drug patient received". |
| Incomplete outcome data addressed? All outcomes | Low risk | 12 post randomisation exclusions because twin pregnancy (8 women), no medical record (1), and lost envelopes (3). |
USA ‐ Memphis 1997.
| Methods | Please see details below. | |
| Participants | 135 women who were at least 37 weeks' gestation with recent onset PE (BP >/= 140/90 mmHg and proteinuria >/= 300 mg in 24 hr). Excluded if severe PE, fetal malpresentation, congenital anomalies, non‐reassuring fetal testing, contraindication to trial of labour. | |
| Interventions | MgSO4: 6 g IV bolus over 15‐20 min, then infusion of 2 g/hr. Continued until 12 hr postpartum. Placebo: saline solution administered by an identical regimen. | |
| Outcomes | Woman: duration of labour, use of oxytocin, caesarean, postpartum haemorrhage, infection, side effects, severe pre‐eclampsia. Baby: Apgar. | |
| Notes | 64% of women had labour induced, and 91% had an epidural. The trial was ended after interim analysis performed at 69% of planned enrolment. Power calculation (with use of actual labour data from control group) indicated that a sample size of 37 in each group would be required to detect 33% increase in length of labour. Sample size was sufficient to evaluate primary outcome. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Computer generated tables of random numbers. |
| Allocation concealment? | Low risk | Sealed, sequentially numbered opaque envelopes. |
| Blinding? All outcomes | Low risk | Double blinding of the allocated treatment. |
| Incomplete outcome data addressed? All outcomes | Low risk | |
USA ‐ Tennessee 2001.
| Methods | Please see details below. | |
| Participants | 222 women with mild pre‐eclampsia during labour. Excluded: chronic HT, severe PE. |
|
| Interventions | MgSO4: 6 g IV, then infusion of 2 g/hr.
Placebo: matching regimen. Clinical monitoring. |
|
| Outcomes | Women: progression to severe PE, eclampsia, HELLP, caesarean section, toxicity. Baby: meconium. | |
| Notes | Abstract only. 33 women who progressed to severe pre‐eclampsia were unblinded and given MgSO4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated group assignment was devised by simple randomisation sequence. |
| Allocation concealment? | Low risk | "Sealed, consecutively numbered, opaque envelopes." |
| Blinding? All outcomes | Low risk | Designed as a double blind study, but if the woman developed severe pre‐eclampsia after randomisation, group assignment was revealed. If the woman had been assigned to placebo, she was subsequently treated with magnesium. Data were collected by blinded chart abstraction, except for women who developed severe pre‐eclampsia after randomisation, data collection was unblinded in that circumstance. |
| Incomplete outcome data addressed? All outcomes | High risk | Published as abstract only, there is no information about other outcome data collected but not reported. |
USA ‐ Texas 1995.
| Methods | Please see details below. | |
| Participants | 2138 women with BP >/= 140/90 mmHg. Excluded if postpartum or delivery imminent, epilepsy, or eclampsia. | |
| Interventions | MgSO4: 10 g (50% solution) IM (5 g in each buttock), then 5 g IM every 4 hours. If severe pre‐eclampsia, an additional 4 g IV (20% solution) before the first IM dose. Phenytoin: 1000 mg IV over 1 hour. 10 hours later, 500 mg orally. If eclampsia developed, all women received MgSO4. |
|
| Outcomes | Woman: eclampsia, caesarean section, induction of labour. Baby: death (stillbirth, neonatal death), Apgar score, admission to special care baby unit. |
|
| Notes | Only 18% of women had 2+ or more proteinuria, and 4% received an antihypertensive. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Randomly assigned," no further information given. |
| Allocation concealment? | Unclear risk | Numbered opaque envelopes, no other information. |
| Blinding? All outcomes | High risk | |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Of the 1049 women allocated phenytoin, 17 also received MgSO4, and 139 did not receive it because of 'logistic' problems (not clear if these women had MgSO4 instead). No reporting of compliance for those allocated MgSO4. |
BP: blood pressure DBP: diastolic BP HELLP: haemolysis elevated liver enzymes and low platelets hr: hour hrly: hourly HT: hypertension IM: intramuscular IUGR: intrauterine growth restriction IV: intravenous Mg: magnesium MgCl2: magnesium chloride MgSO4: magnesium sulphate min: minute mmHg: millimeters of mercury mmol: millimole PE: pre‐eclampsia PIH: pregnancy‐induced hypertension SBP: systolic BP SCBU: special care baby unit sd: standard deviation PPH: postpartum haemorrhage RDS: respiratory distress syndrome
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbade 2006 | Trial of 29 women to compare magnesium sulphate serum concentrations after treatment with 2 different magnesium sulphate regimens, Zuspan's vs an alternative scheme. |
| Antartani 2006 | Trial of 113 women with eclampsia to compare efficacy of MgSO4 versus phenytoin for prevention of recurrence of seizures. Unlikely to be a randomised study given imbalance in treatment groups (86 MgSo4 and 27 phenytoin). Published in abstract form only. |
| Begum 2002 | Trial of 401 women with eclampsia, comparing outcomes in women treated with loading dose of magnesium sulphate versus standard regimen. |
| Begum 2009 | All women with eclampsia (100 women). Comparision of a single IV loading dose of magnesium sulphate with a single combined IV and IM loading dose. |
| China 2000 | No clinical outcomes reported. Participants: 84 women with PE at 34‐42 weeks. Interventions: magnesium sulphate vs phentolamine. |
| Chissell 1994 | Trial of 17 women with severe pre‐eclampsia randomised to magnesium sulphate by IM or IV routes. |
| Chowdhury 2009 | Not a randomised trial. 630 women with eclampsia, allocated by unit to either IM magnesium sulphate or IV magnesium sulphate. |
| Ehrenberg 2006 | Trial of 200 women with mild pre‐eclampsia randomised to 12 or 24 hours of magnesium sulphate. |
| Fontenot 2005 | Trial of 98 women with severe pre‐eclampsia to assess the use of diuresis as a clinical parameter to determine duration of postpartum therapy (24 hours vs continued therapy until onset of diuresis). |
| Ghahiri 2005 | Trial of 66 women with mild pre‐eclampsia between 27 and 38 weeks' gestation to compare serum levels after IV magnesium sulphate to PO magnesium chloride. |
| Hangarga 2001 | Described as a series of 90 women with eclampsia. 32 received phenytoin, 34 received (modified) Menon's regimen, and 24 magnesium sulphate. The study is unlikely to be a randomised trial. |
| Laiteerapong 1999 | Trial of 34 women with severe pre‐eclampsia randomised to magnesium maintenance dose of 1 g/hr vs 2 g/hr to compare serum magnesium levels. Published as abstract, only. |
| Li 1998 | Study of 28 women with pregnancy‐induced hypertension to compare retinal blood flow following treatment with magnesium sulphate vs nimodipine. None of the pre‐specified outcomes for this review were reported in this study, which cannot be clearly identified as a randomised trial. Pertinent portions of the paper translated from the Chinese prior to review. |
| Maheshwari 1989 | Trial of 74 women with eclampsia. Comparison of magnesium sulphate with diazepam and phenytoin. |
| Muhammad 2009 | Trial of 39 women with eclampsia. Comparison of a low dose magnesium sulphate regimen with the standard regimen. |
| Mundle 2009 | Comparison of alternative regimens for magnesium sulphate: use of a pump to deliver intravenous therapy compared with manual IV loading dose and intramuscular maintenance. Women with pre‐eclampsia. |
| Nagar 1988 | Trial of 199 women with eclampsia randomised to magnesium sulphate or lytic cocktail. |
| Neto 2000 | Trial of 77 women with eclampsia randomised to magnesium sulphate or phenytoin. Translated from the Spanish prior to review. |
| Ola 2004 | Trial of 60 women with eclampsia randomised to magnesium sulphate or diazepam. |
| Rodriquez 1990 | Trial of 44 women with pre‐eclampsia or eclampsia randomised to magnesium sulphate or diazepam. One women diagnosed with eclampsia, 43 with pre‐eclampsia. Data were not analysed separately by treatment regimen for outcomes of interest. Pertinent portions of the paper translated from the Spanish prior to review. |
| Rudnicki 1990 | Trial comparing light and electron microscope changes in the placenta and umbilical cord of 28 women with pregnancy‐induced hypertension randomised to magnesium sulphate or placebo. None of the pre‐specified outcomes for this review were reported. |
| Rudnicki 1991 | Trial of 58 women with pregnancy‐induced hypertension to determine effects of magnesium vs placebo on maternal blood pressure. None of the pre‐specified outcomes for this review were reported. |
| Samal 2001 | Retrospective analysis of 120 women with eclampsia. Comparision of magnesium sulphate plus nifedipine with magnesium plus sedation (pethidine or diazepam). |
| Shilva 2007 | Trial of 50 women with antepartum eclampsia randomised to low dose (Dhaka maintenance regimen, 2.5 g IM every 4 hours) regimen or higher dose of magnesium sulphate. |
| Smith 2005 | Trial of 115 women with pre‐eclampsia to determine rates of hypotension in women treated with magnesium vs labetalol. None of the pre‐specified outcomes for this review were reported. Published in abstract form only. |
| South Africa 1996 | No clinical outcomes reported. Outcome for women with eclampsia not reported separately to pre‐eclampsia. Participants: 24 women with eclampsia (also in Collaborative Eclampsia Trial) and 18 with pre‐eclampsia. Interventions: magnesium sulphate versus phenytoin. |
| Suneja 2008 | Trial comparing alternative magnesium sulphate regimens postpartum for 60 women with severe pre‐eclampsia. Comparision of individuals criteria for determining duration of treatment with standard 24 hours. |
| Tanzania 1994 | Quasi randomised, alternate allocation. Participants: 59 women >/= 26 weeks' gestation, with DBP 90 mmHg or more, and proteinuria. Excluded if seizures in this pregnancy, any anticonvulsant drugs, or epilepsy. Interventions: diazepam 10 mg orally, then 5 mg 8 hourly vs no anticonvulsant. Outcomes: death, fits, cerebrovascular accident, cardiac failure. None reported for baby. All women received nifedipine for BP control, with methyl dopa if required. |
| Tongsong 1992 | Trial of 49 women with severe pre‐eclampsia or eclampsia randomised to magnesium maintenance dose of 1 g/hr vs 2 g/hr to compare serum magnesium levels. Pertinent portions of the paper translated from the Thai prior to review. |
| UK 1989 | Trial abandoned due to excessive maternal sedation with clonazepam, and neonatal feeding difficulties, jitteriness and drowsiness. No data available. Interventions: phenytoin versus clonazepam. |
| USA ‐ Texas 1991 | Data for the woman with eclampsia not reported separately from the women with pre‐eclampsia. Methods: "prospectively randomised". Participants: 1 woman with eclampsia, 11 with severe PE, 38 with mild PIH. Interventions: magnesium sulphate versus phenytoin. Outcomes: fits. |
| USA ‐ Texas 1992 | No clinical outcomes reported. Participants: 12 women with pre‐eclampsia. Intervention: magnesium sulphate vs placebo. |
| Varma 1972 | Case control study of 140 women with pre‐eclampsia treated with chlormethiazole (sedative/hypnotic) or barbiturates. |
| Wang 2008 | Trial to study the effects of magnesium sulphate on prostacyclin and thromboxane levels in women with severe pre‐eclampsia. In all, 50 women were randomised to continuous infusion of magnesium sulphate for 24 hours postpartum vs discontinuation of magnesium infusion when urinary output was > 100 ml/hr for 2 consecutive hours. |
BP: blood pressure DBP: diastolic blood pressure IM: intramuscular IV: intravenous MgSO4: magnesium sulphate PE: pre‐eclampsia PIH: pregnancy‐induced hypertension PO: per os (by mouth, orally) vs: versus
Characteristics of ongoing studies [ordered by study ID]
USA ‐ Utah.
| Trial name or title | Labetalol versus MgSO4 for the prevention of eclampsia trial (LAMPET). |
| Methods | Multicenter, randomised, controlled, clinical trial comparing the anti‐seizure effect of parenteral and/or oral labetalol versus parenteral (intravenous or intramuscular) magnesium sulphate with mild or severe pre‐eclampsia who are deemed to be at sufficient risk to warrant seizure prophylaxis with magnesium sulphate. |
| Participants | Patients with pre‐eclampsia (BP > 140 systolic and/or > 90 mmHg diastolic with 1+ or more proteinuria [or a 24 hour specimen with > 300 mg/day]), chronic hypertension (or superimposed pre‐eclampsia), or gestational hypertension deemed to be at risk for eclamptic convulsions and who would routinely be treated in the participating institution with some form of anti‐seizure prophylaxis during labour and delivery. |
| Interventions | Labetalol: either, 20 mg intravenous loading dose IV followed by 200 mg oral dose every 6 hours from the time of inclusion in the study until 24 hours postpartum or 200 mg oral dose every 6 hours from inclusion. Magnesium sulphate: intravenous magnesium sulphate as a 4 or 6 gram loading dose over 20 minutes followed by a 1 or 2 g/h continuous infusion until 24 hours postpartum. |
| Outcomes | Primary: occurrence of eclamptic seizure(s) after enrolment in the study. Secondary outcome measures Maternal: blood pressure, pulse pressure and heart rate changesNeed for additional antihypertensive medication (defined by need to control BP > 160 mmHg systolic and/or 110 mmHg). Subjective assessment of side effects by the patient. Objective assessment of new onset complications and/or side effects by the treating clinicians. Labour and delivery parameters including; induction to delivery interval, rate of cervical dilatation, oxytocin dosages, length of labour, delivery route, blood loss at delivery, and postpartum course. Type of anaesthesia administered (none, local infiltration for delivery only, epidural for labour, epidural for delivery, spinal for delivery, combined/spinal epidural for labour and delivery, general anaesthesia for delivery). Fetal and neonatal: occurrence of newly diagnosed fetal distress during labour necessitating emergent delivery. Umbilical cord gases at delivery (if available in institution); Apgar scores. Neonatal outcome as defined by NICU admission, hospital survival to discharge, length of hospital survival, days in NICU, need for blood transfusion, need for pressor agents, need for mechanical ventilation, neonatal cardiac dysrhythmias, neonatal cardiac failure, necrotising enterocolitis, sudden infant death, neonatal sepsis, neonatal hypoglycaemia, and there will be an other block for recording any other identified complications not mentioned. |
| Starting date | May 2003. |
| Contact information | Michael A Belfort, MD, PhD, Shalece Kofford, RN, MPH. |
| Notes | Estimated completion date December 2012. |
BP: blood pressure IV: intravenous NICU: neonatal intensive care unit
Contributions of authors
All review authors contributed to developing the methods. The initial review was written by L Duley (LD), D Henderson‐Smart (DHS), and M Gülmezoglu (MG). For this updated review, MG and D Chou (DC) extracted and double checked the newly included data. The text of the updated review was drafted by DC with the assistance of LD and contributions from MG and DHS.
Sources of support
Internal sources
Centre for Perinatal Health Services Research, University of Sydney, Australia.
HRP‐UNDP/UNFPA/WHO/World Bank Special Programme in Human Reproduction, Geneva, Switzerland.
External sources
Department for International Development, UK.
Medical Research Council, UK.
Declarations of interest
Lelia Duley is a principal investigator for the Magpie Trial, which compared magnesium sulphate with placebo and was, therefore, not involved in the assessment or data extraction of this trial.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Denmark 2000 {published data only}
- Rudnicki M, Frolich A, Pilsgaard K, Nyrnberg L, Moller M, Sanchez M, et al. Comparison of magnesium and methyldopa for the control of blood pressure in pregnancies complicated with hypertension. Gynecologic and Obstetric Investigation 2000;49:231‐5. [DOI] [PubMed] [Google Scholar]
India 2008 {published data only}
- Sharma R, Mir S, Rizvi M, Akthar S. Efficacy of magnesium sulphate versus phenytoin in seizure control and prophylaxis in patients of eclampsia and severe pre‐eclampsia. JK Science 2008;10(4):181‐5. [Google Scholar]
Magpie Trial 2002 {published and unpublished data}
- Bricker L, Magpie Trial Collaborative Group. The Magpie Trial: magnesium sulphate versus placebo for women with pre‐eclampsia. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA. 2000:47.
- Duley L. The Magpie follow up study: outcome after discharge from hospital for women and children recruited to a trial comparing magnesium sulphate with placebo for pre‐eclampsia. BMC Pregnancy and Childbirth 2004;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duley L, Campbell L. The Magpie Trial: magnesium sulphate for pre‐eclampsia, evaluating the effects on women and their babies. MIDIRS Midwifery Digest 1999;9:48‐51. [Google Scholar]
- Duley L, Magpie Trial Collaborative Group. The Magpie Trial: magnesium sulphate versus placebo for women with pre‐eclampsia. Hypertension in Pregnancy 2000;19(Suppl 1):63. [Google Scholar]
- Duley L, Neilson JP. Magnesium sulphate and pre‐eclampsia. Trial needed to see whether it's as valuable in pre‐eclampsia as in eclampsia. BMJ 1999;319:3‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duley L, Watkins K. Magnesium sulphate for treatment of pre‐eclampsia: a trial to evaluate the effects on women and their babies. Contemporary Reviews in Obstetrics and Gynaecology 1998;10(4):267‐74. [Google Scholar]
- Magpie Trial Collaborative Group. Do women with pre‐eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo‐controlled trial. Lancet 2002;359:1877‐90. [DOI] [PubMed] [Google Scholar]
- Magpie Trial Follow‐Up Study Collaborative Group. The Magpie trial: a randomised trial comparing magnesium sulphate with placebo for pre‐eclampsia. Outcome for children at 18 months. BJOG: an international journal of obstetrics and gynaecology 2007;114(3):289‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magpie Trial Follow‐Up Study Collaborative Group. The Magpie trial: a randomised trial comparing magnesium sulphate with placebo for pre‐eclampsia. Outcome for women at 2 years. BJOG: an international journal of obstetrics and gynaecology 2007;114(3):300‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley J, Magpie Trial Collaborative Group. The Magpie Trial: magnesium sulphate versus placebo for women with pre‐eclampsia. Women's Health ‐ into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town, South Africa. 1999:25.
- Simon J, Gray A, Duley L, on behalf of the Magpie Trial Collaborative Group. Cost‐effectiveness of prophylactic magnesium sulphate for 9996 women with pre‐eclampsia from 33 countries: economic evaluation of the Magpie Trial. BJOG: An International Journal of Obstetrics and Gynaecology 2006;113(2):144‐51. [DOI] [PubMed] [Google Scholar]
- Smyth RM, Spark P, Armstrong N, Duley L. Magpie Trial in the UK: methods and additional data for women and children at 2 years following pregnancy complicated by pre‐eclampsia. BMC Pregnancy and Childbirth 2009;9:15. [PUBMED: 19366459] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. The use of magnesium sulphate in pre‐eclampsia [abstract]. Singapore Journal of Obstetrics & Gynaecology 2003;34(Suppl 1):21. [Google Scholar]
Malaysia 1994 {published and unpublished data}
- Adeeb N, Hatta AZ, Shariff J. Comparing magnesium sulphate to diazepam in managing severe pre‐eclampsia and eclampsia. 10th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1996 August 4‐8; Seattle, Washington, USA. 1996:246.
- Adeeb N, Ho CM. Comparing magnesium sulphate versus diazepam in the management of severe pre‐eclampsia and eclampsia. 9th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1994 March 15‐18; Sydney, Australia. 1994:38.
Mexico 1992 {published data only}
- Walss Rodriguez RJ, Levario AR. Anticonvulsant treatment of severe pre‐eclampsia: comparison of diazepam and magnesium sulfate. Ginecologia y Obstetricia de Mexico 1992;60:331‐5. [PubMed] [Google Scholar]
Mexico 1998 {published data only}
- Vargas AG, Salmeron PI, Sanchez GAR, Jiminez AL, Rubio GAF. Efficancy of isosorbide in aerosol in the management of hypertensive crisis of severe preeclampsia [Eficacia del isosorbide en aerosol en el manejo de la crisis hipertensiva de la preeclampsia]. Ginecologia y Obstetricia De Mexico 1998;66:316‐9. [PubMed] [Google Scholar]
Nimodipine SG 2003 {published data only}
- Belfort M, Anthony J, Saade G, Nimodipine Study Group. Interim report of the nimodipine vs. magnesium sulfate for seizure prophylaxis in severe preeclampsia study: an international randomized controlled trial. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S7. [Google Scholar]
- Belfort M, Saade G, Yared M, Abedejos P, Dorman K. Change in estimated cerebral perfusion pressure following nimodipine or magnesium sulfate in patients with severe preeclampsia. American Journal of Obstetrics and Gynecology 1998;178:S114. [DOI] [PubMed] [Google Scholar]
- Belfort MA, Anthony J, Saade GR, Allen JC, for the Nimodipine Study Group. A comparison of magnesium sulfate and nimodipine for the prevention of eclampsia. New England Journal of Medicine 2003;348:304‐11. [DOI] [PubMed] [Google Scholar]
- Belfort MA, Saade GR, Yared M, Grunewald C, Herd A, Varner MA, et al. Change in estimated cerebral perfusion pressure after treatment with nimodipine or magnesium sulfate in patients with pre‐eclampsia. American Journal of Obstetrics and Gynecology 1999;181:402‐7. [DOI] [PubMed] [Google Scholar]
- Hollenberg NK. A comparison of magnesium sulfate and nimodipine for the prevention of eclampsia. Current Hypertension Reports 2003;5(4):288‐9. [PubMed] [Google Scholar]
South Africa 1994 {published data only}
- Moodley J, Moodley J. Prophylactic anticonvulsant therapy in hypertensive crises of pregnancy ‐ the need for a large randomized trial. Hypertension in Pregnancy 1994;13:245‐52. [Google Scholar]
South Africa 1998 {published data only}
- Anthony J, Rush R. A randomised controlled trial of intravenous magnesium sulphate versus placebo. British Journal of Obstetrics and Gynaecology 1998;105:809‐10. [DOI] [PubMed] [Google Scholar]
- Coetzee E, Dommisse J, Anthony J. A randomised controlled trial of intravenous magnesium sulphate versus placebo in the management of women with severe pre‐eclampsia. British Journal of Obstetrics and Gynaecology 1998;105:300‐3. [DOI] [PubMed] [Google Scholar]
- Coetzee E, Dommisse J, Anthony J. Are anticonvulsants necessary in the management of severe gestational proteinuric hypertension. International Journal of Gynecology & Obstetrics 1994;46:121. [Google Scholar]
- Coetzee E, Dommisse J, Anthony J. The prediction and prevention of seizures in 571 patients with severe pre‐eclampsia. 10th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1996 August 4‐8; Seattle, Washington, USA, 1996:124. Seattle, 1996:124.
- Coetzee EJ, Anthony J, Dommisse AJ. Does magnesium sulphate prevent eclampsia in severe gestational proteinuria hypertension. Prenatal and Neonatal Medicine 1996;1(Suppl 1):9. [Google Scholar]
- Coetzee EJ, Dommisse AJ. Eclampsia: not a preventable disease in the South African context. Proceedings of the 13th conference on Prioirities in Perinatal Care; 1994; South Africa, 1994:3‐5. 1994:3‐5.
- Moodley J, Pattinson RC, Hofmeyr GJ. A randomised controlled trial of intravenous magnesium sulphate versus placebo in the management of women with severe pre‐eclampsia [letter; comment]. British Journal of Obstetrics and Gynaecology 1999;106(3):289‐90. [DOI] [PubMed] [Google Scholar]
- Woods D, Anthony J, Dommisse J, Coetzee E. Can magnesium sulphate before delivery reduce the risk of hypoxic‐ischaemic encephalopathy. Proceedings of the 15th Conference on Priorities in Perinatal Care in South Africa; March 5‐8; Goudini Spa, South Africa. 1996.
Taiwan 1995 {published data only}
- Chen FP, Chang SD, Chu KK. Expectant management in severe preeclampsia: does magnesium sulfate prevent the development of eclampsia. Acta Obstetricia et Gynecologica Scandinavica 1995;74:181‐5. [DOI] [PubMed] [Google Scholar]
USA ‐ Alabama 1995 {published data only}
- Atkinson MW, Guinn D, Owen J, Hauth JC. Does magnesium sulfate affect the length of labor induction in women with pregnancy‐associated hypertension?. American Journal of Obstetrics and Gynecology 1995;173:1219‐22. [DOI] [PubMed] [Google Scholar]
- Atkinson MW, Guinn D, Owen J, Hauth JC. Does magnesium sulfate prolong labor induction in women with gestational hypertension?. American Journal of Obstetrics and Gynecology 1995;172:384. [DOI] [PubMed] [Google Scholar]
USA ‐ Maryland 1993 {published data only}
- Friedman SA, Lim KH, Baker C, Repke JT. Phenytoin vs magnesium sulfate in preeclampsia: a pilot study. American Journal of Perinatology 1993;10:233‐8. [DOI] [PubMed] [Google Scholar]
- Friedman SA, Lim KH, Baker CA, Repke JT. A comparison of phenytoin infusion versus magnesium sulphate infusion in preeclampsia. Proceedings of the 10th Annual Meeting of the Society of Perinatal Obstetricians; 1990 Jan 23‐27; Houston, Texas, USA. 1990:16.
- Repke JT, Friedman SA, Lim KH, Baker CA. Magnesium sulfate vs phenytoin in preeclampsia: preliminary results from a randomized clinical trial. Proceedings of 9th Annual Meeting of the Society of Perinatal Obstetricians; 1989 Feb 1‐4; New Orleans, Louisiana, USA. 1989:123.
USA ‐ Memphis 1997 {published data only}
- Witlin A, Friedman S, Sibai B. The effect of magnesium sulfate therapy on the duration of labor in women with mild preeclampsia at term: a randomized double‐blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S15. [DOI] [PubMed] [Google Scholar]
- Witlin AG, Friedman SA, Sibai BM. The effect of magnesium sulfate therapy on the duration of labor in women with mild preeclampsia at term: a randomized, double‐blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 1997;76:623‐7. [DOI] [PubMed] [Google Scholar]
USA ‐ Tennessee 2001 {published data only}
- Livingston J, Livingston L, Mabie B, Sibai B. The efficacy of magnesium sulfate in women with mild preeclampsia: a double blinded placebo controlled trial. Hypertension in Pregnancy 2002;20(Suppl 1):42. [Google Scholar]
- Livingston J, Livingston L, Ramsey R, Kao L, Mabie B, Sibai B. Magnesium sulfate in women with mild preeclampsia: a double blind placebo controlled trial. American Journal of Obstetrics and Gynecology 2001;185(Suppl 6):S75. [Google Scholar]
- Livingston JC, Livingston LW, Ramsey R, Mabie BC, Sibai BM. Magnesium sulfate in women with mild preeclampsia: a randomised controlled trial. Obstetrics and Gynecology 2003;101(2):217‐20. [DOI] [PubMed] [Google Scholar]
USA ‐ Texas 1995 {published data only}
- Leveno KJ, Alexander JM, McIntire DD, Lucas MJ. Does magnesium sulfate given for the prevention of eclampsia affect the outcome of labor?. American Journal of Obstetrics and Gynecology 1998;178:707‐12. [DOI] [PubMed] [Google Scholar]
- Lucas MJ, Leveno KJ, Cunningham MD. A comparison of magnesium sulfate with phenytoin for the prevention of eclampsia. New England Journal of Medicine 1995;333:201‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbade 2006 {published data only}
- Abbade JF, Costa RAA, Martins AMVC, Rudge MVC, Peracoli JC. Zuspan's scheme versus alternative scheme of magnesium sulphate to prevent or to treat eclampsia: comparison of magnesium serum concentrations. Hypertension in Pregnancy 2006;25(Suppl 1):152. [Google Scholar]
Antartani 2006 {published data only}
- Antartani RC, Kiran A. A comparative study of magnesium sulfate vs phenytoin for prevention of recurrence of seizures in eclampsia. 49th All India Congress of Obstetrics and Gynaecology; 2006 Jan 6‐9; Cochin, Kerala State, India. 2006:104.
Begum 2002 {published data only}
- Begum A. Loading dose vs standard regime of magnesium sulphate in the management of eclampsia ‐ a randomized trial. XVI FIGO World Congress of Obstetrics & Gynecology (Book 2); 2000 Sept 3‐8; Washington DC, USA. 2000:47.
- Begum MR, Begum A, Quadir E. Loading dose versus standard regime of magnesium sulfate in the management of eclampsia: a randomized trial. Journal of Obstetrics and Gynaecology Research 2002;28:154‐9. [DOI] [PubMed] [Google Scholar]
Begum 2009 {published data only}
- Begum K. A lower dose of magnesium sulphate for control of convulsion in eclamptic women of Bangladesh. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S124. [Google Scholar]
China 2000 {published data only}
- Dianrong S, Lirong Y, Yinglin L. A comparison of phentolamine and magnesium sulphate in pre‐eclampsia. International Journal of Gynecology & Obstetrics 2000;68:259‐60. [DOI] [PubMed] [Google Scholar]
Chissell 1994 {published data only}
- Chissell S, Botha JH, Moodley J, McFadyen L. Intravenous and intramuscular magnesium sulphate regimens in severe pre‐eclampsia. South African Medical Journal 1994;84:607‐10. [PubMed] [Google Scholar]
Chowdhury 2009 {published data only}
- Chowdhury JR, Chaudhuri S, Bhattacharyya N, Biswas PK, Panpalia M. Comparison of intramuscular magnesium sulfate with low dose intravenous magnesium sulfate regimen for treatment of eclampsia. Journal of Obstetrics and Gynaecology Research 2009;35(1):119‐25. [DOI] [PubMed] [Google Scholar]
Ehrenberg 2006 {published data only}
- Ehrenberg H, Mercer B. Abbreviated post‐partum magnesium sulfate therapy for women with mild preeclampsia. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S73. [DOI] [PubMed] [Google Scholar]
- Ehrenberg HM, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for women with mild preeclampsia: a randomized controlled trial. Obstetrics & Gynecology 2006;108(4):833‐8. [DOI] [PubMed] [Google Scholar]
Fontenot 2005 {published data only}
- Fontenot MT, Lewis DF, Frederick JB, Wang Y, DeFranco EA, Groome LJ, et al. A prospective randomized trial of magnesium sulfate in severe preeclampsia: use of diuresis as a clinical parameter to determine the duration of postpartum therapy. American Journal of Obstetrics and Gynecology 2005;192:1788‐94. [DOI] [PubMed] [Google Scholar]
Ghahiri 2005 {published data only}
- Ghahiri A, Berjis K. A comparison between intravenous magnesium sulfate and oral magnesium chloride in mild preeclampsia. Journal of Research in Medical Sciences 2005;10(1):6‐9. [Google Scholar]
Hangarga 2001 {published data only}
- Hangarga US, Pragya S. A comparative study of phenytoin sodium with magnesium sulphate and Menon's regime in the treatment of eclampsia. Journal of Obstetrics and Gynecology of India 2001;51(3):68‐70. [Google Scholar]
Laiteerapong 1999 {published data only}
- Laiteerapong U, Leelahakorn S. Comparative study of serum magnesium levels attained from magnesium sulfate therapy for severe pre‐eclamptic patients between 1 gm/hr and 2 gm/hr regimen. Thai Journal of Obstetrics and Gynaecology 1999;11(4):281. [Google Scholar]
Li 1998 {published data only}
- Li M, Yang M, Qiu X. The effect of nimodipine on retinal blood flow in pregnancy induced hypertension. Zhonghua Fu Chan Ke Za Zh 1998; Vol. 33, issue 7:397‐9. [PubMed]
Maheshwari 1989 {published data only}
- Maheshwari JR, Desai SV, Hansotia MD, Walvekar VR. Anti‐convulsant therapy in eclampsia. Journal of Postgraduate Medicine 1989;35(2):66‐9. [PubMed] [Google Scholar]
Muhammad 2009 {published data only}
- Muhammad A, Ibrahim U, Nighat K, Muhammad Y, Abdullahi A. Low dose magnesium sulfate in the control of eclamptic fits: a randomized control trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S277‐8. [Google Scholar]
Mundle 2009 {published data only}
- Mundle S, Regi A, Biswas B, Bracken H, Easterling T, Winikoff B. Preeclampsia in low‐resource settings: a randomized trial of IV MgSO4 via flow controlled pump. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S278. [Google Scholar]
Nagar 1988 {published data only}
- Nagar S, Jain S, Kumari S, Ahuja L. Reassessment of therapy of eclampsia: comparison of mortality and morbidity of mother and fetus with parenteral magnesium sulphate and lytic cocktail therapy. Journal of Obstetrics and Gynaecology of India 1988;38(3):250‐5. [Google Scholar]
Neto 2000 {published data only}
- Neto JDV, Bertini AM, Taborda WC, Parente JV. Treatment of eclampsia: comparative study on the use of magnesium sulfate and phenytoin [Tratamento da eclampsia:estudio comparativo entre sulfato de magnesio e a fenitoina]. Revista Brasileira de Ginecologia e Obstetricia 2000;22(9):543‐9. [Google Scholar]
Ola 2004 {published data only}
- Ola RE, Odeneye OT, Abudu OO. Eclampsia: a randomized double blind trial of magnesium sulphate and diazepam in Lagos, Nigeria. Tropical Journal of Obstetrics and Gynaecology 2004;21(2):143‐7. [Google Scholar]
Rodriquez 1990 {published data only}
- Rodriquez Cruz CM, Suero Holguin HJ, Pena Acosta CA, Cots Martinez JM, Garcia CA, Camilo MA. Magnesium sulfate versus diazepam in the treatment of severe preeclampsia [Sulfato de magnesio versus diazepam en el manejo de la pre‐eclampsia severa]. Acta Medica Dominicana 1990;12(5):171‐5. [Google Scholar]
Rudnicki 1990 {published data only}
- Rudnicki M, Junge J, Frolich A, Ornvold K, Fischer‐Rasmussen W. Magnesium supplement in pregnancy‐induced hypertension. A clinicopathological study. APMIS 1990;98:1123‐7. [DOI] [PubMed] [Google Scholar]
Rudnicki 1991 {published data only}
- Rudnicki M, Frolich A, Rasmussen WF, McNair P. The effect of magnesium on maternal blood pressure in pregnancy‐induced hypertension. Acta Obstetricia et Gynecologica Scandinavica 1991;70:445‐50. [DOI] [PubMed] [Google Scholar]
Samal 2001 {published data only}
- Samal S, Gupta U, Agarwal P. Management of eclampsia with magnesium sulphate and nifedipine. Journal of Obstetrics and Gynecology of India 2001;51(3):71‐4. [Google Scholar]
Shilva 2007 {published data only}
- Shilva, Saha SC, Kalra J, Prasad R. Safety and efficacy of low‐dose MgSO4 in the treatment of eclampsia. International Journal of Gynecology & Obstetrics 2007;97(2):150‐1. [DOI] [PubMed] [Google Scholar]
Smith 2005 {published data only}
- Smith D, Warren J, Saade G, Clark S, Belfort M. Oral labetalol given to treated non hypertensive patients with preeclampsia is no more likely to cause hypotension than magnesium sulfate [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S78. [Google Scholar]
South Africa 1996 {published data only}
- Payne AJ, Naidu S, Moodley J, Hoffmann M, Gouws F. Non‐invasive assessment of the maternal cerebral circulation by transcranial doppler ultrasound in the hypertensive crises of pregnancy. American Journal of Obstetrics and Gynecology 1996;174:320. [Google Scholar]
Suneja 2008 {published data only}
- Suneja A, Sinha S, Vaid N, Ahuja S. A prospective randomized controlled trial to individualize the duration of post partum magnesium sulfate therapy. Hypertension in Pregnancy 2008;27(4):504. [Google Scholar]
Tanzania 1994 {published data only}
- Ramsay M, Rimoy G, Rubin P. Are anticonvulsants necessary to prevent eclampsia?. Proceedings of the 9th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1994 March 15‐18; Sydney, Australia. 1994:279.
- Ramsay MM, Rimoy GH, Rubin PC. Are anticonvulsants necessary to prevent eclampsia. Lancet 1994;343:540‐1. [DOI] [PubMed] [Google Scholar]
Tongsong 1992 {published data only}
- Tongsong T, Dejkijwikrom W, Tansuwannont W, Jantachai U, Siangpura U. A comparison of intravenous magnesium sulfate regimens in preeclampsia‐eclampsia between rate 1 gram and 2 grams per hour. Siriraj Hospital Gazette 1992;44(7):509‐16. [Google Scholar]
UK 1989 {published data only}
- Slater RM, Wilcox FL, Smith WD, Maresh M. Phenytoin in pre‐eclampsia. Lancet 1989;ii:1224‐5. [DOI] [PubMed] [Google Scholar]
USA ‐ Texas 1991 {published data only}
- Appleton M, Kuehl T, Raebel M, Adams H, Pickens J, Koops B, et al. Magnesium sulfate vs. phenytoin in pregnancy‐induced hypertension. American Journal of Obstetrics and Gynecology 1991;164:273. [DOI] [PubMed] [Google Scholar]
- Appleton MP, Kuehl TJ, Raebel MA, Adams HR, Knight AB, Gold WR. Magnesium sulfate versus phenytoin for seizure prophylaxis in pregnancy‐induced hypertension. American Journal of Obstetrics and Gynecology 1991;165:907‐13. [DOI] [PubMed] [Google Scholar]
USA ‐ Texas 1992 {published data only}
- Belfort MA, Moise KJ. Effect of magnesium sulfate on maternal brain blood flow in preeclampsia: a randomized, placebo‐controlled study. American Journal of Obstetrics and Gynecology 1992;167:661‐6. [DOI] [PubMed] [Google Scholar]
- Belfort MA, Moise KJ, Saade G. The effect of magnesium sulfate on maternal retinal blood flow in pregnancy‐induced hypertension: a randomized placebo‐controlled study. Proceedings of 39th Annual Meeting of the Society for Gynecologic Investigation; 1992 March 18‐21; San Antonio, USA. 1992:233.
- Belfort MA, Saade GR, Moise KJ. The effect of magnesium sulfate on maternal retinal blood flow in preeclampsia: a randomized placebo‐controlled study. American Journal of Obstetrics and Gynecology 1992;167:1548‐53. [DOI] [PubMed] [Google Scholar]
Varma 1972 {published data only}
- Varma T. Chlormethiazole in the treatment of pre‐eclampsia. Journal of Obstetrics and Gynaecology of the British Commonwealth 1972;79:513‐7. [DOI] [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang Y, Philibert L, Zhang Y, Gu Y, Tynes K, Lewis DF. Effects of magnesium sulfate on prostacyclin and thromboxane in women with severe preeclampsia. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):S92. [Google Scholar]
- Wang Y, Zhang Y, Canzoneri BJ, Gu Y, Philibert L, Lewis DF. Prostacyclin and thromboxane levels in women with severe preeclampsia undergoing magnesium sulfate therapy during antepartum and postpartum periods. Hypertension in Pregnancy 2008;27(1):17‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
USA ‐ Utah {published data only}
- Belfort MA. Labetalol versus MgSO4 for the prevention of eclampsia trial (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006).
- Warren J, Lacoursiere Y, Varner M, Silver R, Anthony J, Belfort M. First interim report on the labetalol versus magnesium sulfate for the prevention of eclampsia trial (LAMPET). Hypertension in Pregnancy 2004;23(Suppl 1):9. [Google Scholar]
Additional references
Abalos 2007
- Abalos E, Duley L, Steyn DW, Henderson‐Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD002252.pub2] [DOI] [PubMed] [Google Scholar]
Begum 2001
- Begum R, Begum A, Johanson R, Ali MN, Akhter S. A low dose ("Dhaka") magnesium sulphate regime for eclampsia. Acta Obstetricia et Gynecologica Scandinavica 2001;80(11):998‐1002. [PubMed] [Google Scholar]
Belfort 1992
- Belfort MA, Moise KJ. Effect of magnesium sulfate on maternal brain blood flow in preeclampsia: a randomized, placebo‐controlled study. American Journal of Obstetrics and Gynecology 1992;167(3):661‐6. [PUBMED: 1530019] [DOI] [PubMed] [Google Scholar]
Churchill 2002
- Churchill D, Duley L. Interventionist versus expectant care for severe pre‐eclampsia before term. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003106] [DOI] [PubMed] [Google Scholar]
Collab Trial 1995
- The Eclampsia Trial Collaborative Group. Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet 1995;345:1455‐63. [PubMed] [Google Scholar]
Crowther 2003
- Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD001060] [DOI] [PubMed] [Google Scholar]
Douglas 1994
- Douglas KA, Redman CWG. Eclampsia in the United Kingdom. BMJ 1994;309:1395‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2009
- Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004661.pub3] [DOI] [PubMed] [Google Scholar]
Duley 1992
- Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. British Journal of Obstetrics and Gynaecology 1992;99:547‐53. [DOI] [PubMed] [Google Scholar]
Duley 1994
- Duley L, Johanson R. Magnesium sulphate for pre‐eclampsia and eclampsia: the evidence so far. British Journal of Obstetrics and Gynaecology 1994;101:565‐7. [DOI] [PubMed] [Google Scholar]
Duley 1996
- Duley L. Magnesium sulphate regimens for women with eclampsia: messages from the Collaborative Eclampsia Trial. British Journal of Obstetrics and Gynaecology 1996;103(2):103‐5. [DOI] [PubMed] [Google Scholar]
Duley 1999
- Duley L, Williams J, Henderson‐Smart DJ. Plasma volume expansion for treatment of pre‐eclampsia. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001805] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2000
- Duley L, Gülmezoglu AM. Magnesium sulphate versus lytic cocktail for eclampsia. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD002960] [DOI] [PubMed] [Google Scholar]
Duley 2003a
- Duley L, Henderson‐Smart D. Magnesium sulphate versus phenytoin for eclampsia. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000128] [DOI] [PubMed] [Google Scholar]
Duley 2003b
- Duley L, Henderson‐Smart D. Magnesium sulphate versus diazepam for eclampsia. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000127] [DOI] [PubMed] [Google Scholar]
Duley 2006
- Duley L, Henderson‐Smart DJ, Meher S. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001449.pub2] [DOI] [PubMed] [Google Scholar]
Duley 2008
- Duley L, Matar HE, Almerie MQ, Hall DR. Alternative magnesium sulphate regimens for women with pre‐eclampsia and eclampsia. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007388] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2009
- Duley L, Henderson‐Smart DJ, Walker GJA. Interventions for treating pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007756] [DOI] [Google Scholar]
Euser 2009
- Euser AG, Cipolla MJ. Magnesium sulfate for the treatment of eclampsia: a brief review. Stroke; a journal of cerebral circulation 2009;40(4):1169‐75. [PUBMED: 19211496] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldman 1988
Gülmezoglu 1998
- Gülmezoglu M, Duley L. Use of anticonvulsants in eclampsia and pre‐eclampsia: survey of obstetricians in the United Kingdom and the Republic of Ireland. BMJ 1998;316:975‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Jones 2002
- Jones HP, Guildea ZE, Stewart JH, Cartlidge PH. The Health Status Questionnaire: achieving concordance with published disability criteria. Archives of Disease in Childhood 2002;86(1):15‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langer 2008
- Langer A, Villar J, Tell K, Kim T, Kennedy S. Reducing eclampsia‐related deaths‐‐a call to action. Lancet 2008;371(9614):705‐6. [DOI] [PubMed] [Google Scholar]
Magpie 2004
- Magpie Trial Follow Up Study Management Group, Magpie Trial Follow Up Study Collaborative Group. The Magpie Trial follow up study: outcome after discharge from hospital for women and children recruited to a trial comparing magnesium sulphate with placebo for pre‐eclampsia. BMC Pregnancy and Childbirth 2004;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mittendorf 1998
- Mittendorf R, Covert R, Boman J, Khoshnood B, Kwang‐Sun L, Siegler M. Is tocolytic magnesium sulphate associated with increased total paediatric mortality?. Lancet 1997;350:1517‐8. [DOI] [PubMed] [Google Scholar]
Nelson 1995
- Nelson KB, Grether JK. Can magnesium sulfate reduce the risk of cerebral palsy in very low birthweight infants?. Pediatrics 1995;95:263‐9. [PubMed] [Google Scholar]
North 2009
- North RA, Taylor RN. Subclinical hypothyroidism after pre‐eclampsia. BMJ 2009;339:b5183. [DOI] [PubMed] [Google Scholar]
Pritchard 1955
- Pritchard JA. The use of the magnesium ion in the management of eclamptogenic toxemias. Surgery, Gynecology and Obstetrics 1955;100(2):131‐40. [PubMed] [Google Scholar]
Pritchard 1984
- Pritchard JA, Cunningham FG, Pritchard SA. The Parkland Memorial Hospital protocol for treatment of eclampsia: evaluation of 245 cases. American Journal of Obstetrics and Gynecology 1984;148(7):951‐63. [DOI] [PubMed] [Google Scholar]
RCOG 2006
- Royal College of Obstetricians and Gynaecologists. RCOG Green‐top Guidelines. The Management of Severe Pre‐Eclampsia/Eclampsia. London: RCOG, 2006. [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Sadeh 1989
- Sadeh M. Action of magnesium sulfate in the treatment of preeclampsia‐eclampsia. Stroke; a journal of cerebral circulation 1989;20(9):1273‐5. [PUBMED: 2672428] [DOI] [PubMed] [Google Scholar]
Scudiero 2000
- Scudiero R, Khoshnood B, Pryde PG, Lee KS, Wall S. Perinatal death and tocolytic magnesium sulfate. Obstetrics & Gynecology 2000;96:178‐82. [DOI] [PubMed] [Google Scholar]
Sheth 2002
- Sheth SS, Chalmers I. Magnesium for preventing and treating eclampsia: time for international action. Lancet 2002;359(9321):1872‐3. [DOI] [PubMed] [Google Scholar]
Sibai 1990
- Sibai BM. Magnesium sulfate is the ideal anticonvulsant in preeclampsia‐eclampsia. American Journal of Obstetrics and Gynecology 1990;162(5):1141‐5. [DOI] [PubMed] [Google Scholar]
Simon 2006
- Simon J, Gray A, Duley L, on behalf of the Magpie Trial Collaborative Group. Cost‐effectiveness of prophylactic magnesium sulphate for 9996 women with pre‐eclampsia from 33 countries: economic evaluation of the Magpie Trial. BJOG: an international journal of obstetrics and gynaecology 2006;113(2):144‐51. [DOI] [PubMed] [Google Scholar]
Squires 1999
- Squires J, Potter L, Bricker D. The ASQ user's guide for the Ages and Stages Questionnaires: a parent completed, child monitoring system. 2nd Edition. Baltimore: Paul Brookes Publishing Co, 1999. [Google Scholar]
Vargas 1998
- Vargas AG, Salmeron PI, Sanchez GAR, Jiminez AL, Rubio GAF. Efficancy of isosorbide in aerosol in the management of hypertensive crisis of severe preeclampsia [Eficacia del isosorbide en aerosol en el manejo de la crisis hipertensiva de la preeclampsia]. Ginecologia y Obstetricia De Mexico 1998;66:316‐9. [PubMed] [Google Scholar]
Walss 1992
- Walss Rodriguez RJ, Levario AR. Anticonvulsant treatment of severe pre‐eclampsia: comparison of diazepam and magnesium sulfate. Ginecologia y Obstetricia de Mexico 1992;60:331‐5. [PubMed] [Google Scholar]
WHO 1988
- World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. American Journal of Obstetrics and Gynecology 1988;158:80‐3. [PubMed] [Google Scholar]
Zuspan 1978
- Zuspan FP. Problems encountered in the treatment of pregnancy‐induced hypertension. A point of view. American Journal of Obstetrics and Gynecology 1978;131(6):591‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Duley 2003
- Duley L, Gülmezoglu AM, Henderson‐Smart DJ. Anticonvulsants for women with pre‐eclampsia. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD000025] [DOI] [PubMed] [Google Scholar]


