Abstract
Background
During pregnancy, a Rhesus negative (Rh‐negative) woman may develop antibodies when her fetus is Rhesus positive (Rh‐positive). These antibodies may harm Rh‐positive babies.
Objectives
To assess the effects of antenatal anti‐D immunoglobulin on the incidence of Rhesus D alloimmunisation when given to Rh‐negative women without anti‐D antibodies.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 May 2015) and reference lists of retrieved studies.
Selection criteria
Randomised trials in Rh‐negative women without anti‐D antibodies given anti‐D after 28 weeks of pregnancy, compared with no treatment, placebo or a different regimen of anti‐D.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy.
Main results
We included two trials involving over 4500 women, comparing anti‐D prophylaxis with no anti‐D during pregnancy in this review. Overall, the trials were judged to be at moderate to high risk of bias. The quality of the evidence for pre‐specified outcomes was also assessed using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach.
In regards to primary review outcomes, there did not appear to be a clear difference in the risks of immunisation when women who received anti‐D at 28 and 34 weeks' gestation were compared with women who were not given antenatal anti‐D: risk ratio (RR) for incidence of Rhesus D alloimmunisation during pregnancy was 0.42 (95% confidence interval (CI) 0.15 to 1.17, two trials, 3902 women; GRADE: low quality evidence); at birth of a Rh‐positive infant the RR was 0.42 (95% CI 0.15 to 1.17, two trials, 2297 women); and within 12 months after birth of a Rh‐positive infant the average RR was 0.39 (95% CI 0.10 to 1.62, two trials, 2048 women; Tau²: 0.47; I²: 39%; GRADE: low quality evidence). Neither of the trials reported on incidence of Rhesus D alloimmunisation in subsequent pregnancies.
Considering secondary outcomes, in one trial, women receiving anti‐D during pregnancy were shown to be less likely to register a positive Kleihauer test (which detects fetal cells in maternal blood) in pregnancy (at 32 to 25 weeks) (RR 0.60, 95% CI 0.41 to 0.88; 1884 women; GRADE: low quality evidence) and at the birth of a Rh‐positive infant (RR 0.60, 95% CI 0.46 to 0.79; 1189 women; GRADE: low quality evidence). No clear differences were seen for neonatal jaundice (RR 0.26, 95% CI 0.03 to 2.30; 1882 infants; GRADE: very low quality evidence). Neither of the trials reported on adverse effects associated with anti‐D treatment.
Authors' conclusions
Existing studies do not provide conclusive evidence that the use of anti‐D during pregnancy benefits either mother or baby in terms of incidence of Rhesus D alloimmunisation during the pregnancy or postpartum, or the incidence of neonatal morbidity (jaundice) (low to very low quality evidence). However women receiving anti‐D may be less likely to register a positive Kleihauer test in pregnancy and at the birth of a Rh‐positive infant (low quality evidence). Fewer women who receive anti‐D during pregnancy may have Rhesus D antibodies in a subsequent pregnancy, with benefits for the baby, however this needs to be tested in studies of robust design.
Plain language summary
Anti‐D administration in pregnancy for preventing Rhesus alloimmunisation
Women whose blood group is Rh‐negative sometimes form Rh‐antibodies when carrying a Rh‐positive baby, in response to the baby's different red blood cell make‐up. This sensitisation is more likely to happen during birth, but occasionally occurs in late pregnancy. These antibodies can cause anaemia, and sometimes death, for a Rh‐positive baby in a subsequent pregnancy. Giving the mother anti‐D after the first birth is known to reduce this problem. This review assessed two trials with moderate to high risk of bias and found that giving anti‐D during pregnancy may help as well, although more research is required to confirm these possible benefits and identify any possible harms.
Summary of findings
Summary of findings for the main comparison. Anti‐D administration in pregnancy compared with no treatment for pregnancy for preventing Rhesus alloimmunisation.
| Anti‐D administration in pregnancy compared with no treatment for pregnancy for preventing Rhesus alloimmunisation | ||||||
| Patient or population: Rh‐negative women without anti‐D antibodies Settings: obstetric/maternity units Intervention: anti‐D administration in pregnancy Comparison: no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Anti‐D administration in pregnancy | |||||
| Incidence of Rhesus D alloimmunisation during pregnancy | Study population | RR 0.42 (0.15 to 1.17) | 3902 (2 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | ||
| 6 per 1000 | 3 per 1000 (1 to 8) | |||||
| Incidence of Rhesus D alloimmunisation postpartum (at birth of Rh‐positive infant and at follow‐up (up to 12 months)) | Study population | RR 0.39 (0.10 to 1.62) | 2048 (2 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | ||
| 15 per 1000 | 6 per 1000 (2 to 24) | |||||
| Incidence of Rhesus D alloimmunisation in a subsequent pregnancy | See comment | See comment | Not estimable | 0 (0) | See comment | This outcome was not reported in the 2 included studies |
| Incidence of positive Kleihauer test (at 32 to 35 weeks' gestation) | Study population | RR 0.60 (0.41 to 0.88) | 1884 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | ||
| 70 per 1000 | 42 per 1000 (29 to 62) | |||||
| Incidence of positive Kleihauer test (at birth of a Rh‐positive infant) | Study population | RR 0.60 (0.46 to 0.79) | 1189 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | ||
| 202 per 1000 | 121 per 1000 (93 to 159) | |||||
| Neonatal jaundice | Study population | RR 0.26 (0.03 to 2.30) | 1882 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2,3 | ||
| 4 per 1000 | 1 per 1000 (0 to 10) | |||||
| Adverse events attributed to anti‐D treatment | See comment | See comment | Not estimable | 0 (0) | See comment | This outcome was not reported in the 2 included studies |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Both studies contributing data had design limitations (‐1).
2Wide confidence intervals crossing the line of no effect (‐1). We have not downgraded for few events or small sample size.
3One study with serious design limitations (‐2).
Background
Description of the condition
Rhesus incompatibility and haemolytic disease of the fetus/newborn
Haemolytic disease of the fetus and newborn can occur when the baby's red blood cells are destroyed. The most common cause is rhesus incompatibility, when antibodies from a Rh‐negative mother target and destroy 'foreign' red blood cells from a Rh‐positive fetus (Chilcott 2002). Haemolytic disease was a major cause of perinatal mortality, morbidity and long‐term disability until the 1970s.
Pathogenesis
Rh‐negative mothers carrying a Rh‐positive fetus may produce anti‐D antibodies (anti‐D) following small feto‐maternal haemorrhages at birth (Chown 1954; Chilcott 2002). The production of anti‐D antibodies occurs in response to the presence of fetal red blood cells in the maternal circulation; this maternal immune response towards the fetal Rhesus antigen is known as ‘sensitisation’ or immunisation. It is believed to take between five and 15 weeks for such antibodies to appear in the maternal circulation following a sensitising event such as birth (Gunson 1976). Sensitisation is believed to have no adverse health effects for the mother, and the first baby is usually not harmed, as the pregnancy is generally complete by the time that sensitisation has occurred. These maternal antibodies (directed against antigens inherited from the father) may, however cause haemolytic disease in subsequent pregnancies with Rh‐positive fetuses.
In addition to feto‐maternal haemorrhage at birth, events during pregnancy may lead to the production of maternal anti‐D antibodies, and thus sensitisation may also occur during the antenatal period. As there is a direct, proportional relationship between the volume of fetal Rh‐positive red blood cells to which a Rh‐negative mother is exposed and the incidence of immunisation (Zipursky 1967; Jones 2004), sensitising events (in addition to birth) may include termination of pregnancy, miscarriage, and some invasive investigative procedures (Bowman 1996). The majority of sensitisations are however, thought to be caused by occult or 'silent' transplacental haemorrhages (Chilcott 2002).
Health consequences for the fetus or newborn
In a Rh‐negative mother, maternal anti‐D antibodies may cross the placenta and lead to the immune‐mediated destruction of fetal red blood cells. This more rapid destruction of the fetal red blood cells than normal, known as haemolytic disease of the fetus or newborn, can lead to anaemia and jaundice and in very severe cases, kernicterus (a form of brain damage caused by very high levels of bilirubin), or even death. A survey of 124 sensitised women showed that about 70% of their pregnancies were affected by some degree of haemolytic disease (Craig 1998). It has been estimated that approximately half of newborn infants with haemolytic disease are mildly affected, requiring no treatment. Of the remainder, half will become hydropic in utero, and half will be born apparently healthy but without treatment may die of kernicterus or be left severely disabled (Bowman 1965).
Description of the intervention
Anti‐D administration for preventing Rhesus alloimmunisation
In the 1960s, Stern found that sensitisation to Rh‐positive blood could be prevented by administering anti‐D (Stern 1961). Anti‐D gammaglobulin is a sterile solution containing anti‐D immunoglobulin G (IgG) antibodies manufactured from a pooled source of plasma of males and post‐menopausal women. The donors must be Rh‐negative and can be immunised to stimulate their immune system to produce anti‐D or to increase their anti‐D titre.
When anti‐D gammaglobulin became available in the early 1970s, deaths from haemolytic disease dramatically reduced, with postpartum administration effectively protecting against Rhesus alloimmunisation when properly used (Gravenhorst 1989; Crowther 1997). Postpartum prophylaxis has been shown to be effective in reducing the incidence of alloimmunisation six months after administration and in a subsequent pregnancy in a Cochrane review of six randomised controlled trials (Crowther 1997). The benefits were seen when anti‐D was given within 72 hours of birth, regardless of the ABO blood group status of the mother and baby. Higher doses were more effective than lower doses (Crowther 1997).
However, as sensitising events may also occur during pregnancy, postpartum anti‐D will not prevent Rhesus alloimmunisation which occurs in the antenatal period. Although Zipursky and Israels (Zipursky 1967) proposed that anti‐D could reduce the incidence of Rhesus alloimmunisation during pregnancy in Rh‐negative mothers nearly forty years ago, it may still occur, either because insufficient anti‐D is given after known sensitising events during pregnancy (or after birth), it is not given soon enough (within 72 hours), or due to silent feto‐maternal haemorrhage.
A transplacental haemorrhage from fetus to mother can be detected by the Kleihauer test (which detects the presence and estimates the amount of fetal cells in maternal blood). Injection of anti‐D will destroy these fetal cells and thus prevent sensitisation of the mother. The Kleihauer test will indicate how much anti‐D is likely to be required.
Antenatal prophylaxis ‐ routine or universal anti‐D administration in pregnancy
As occult or ‘silent’ sensitising events are thought to constitute the majority of sensitisations (Chilcott 2002), routine anti‐D prophylaxis during pregnancy for Rh‐negative mothers has been proposed and implemented in many countries (Engelfriet 2003). This is intended to supplement the practices of postpartum administration of anti‐D, and of offering anti‐D prophylaxis to Rh‐negative women who experience a known potentially sensitising event (such as miscarriage or threatened miscarriage) during their pregnancy. A recent meta‐analysis, including studies with historical controls in addition to those with concurrent controls suggested that there is strong evidence for the effectiveness of routine antenatal anti‐D prophylaxis for preventing sensitisation, in support of offering routine prophylaxis to all non‐sensitised pregnant Rh‐negative women (Turner 2012).
About 10% of all pregnancies involve a Rh‐negative mother with a Rh‐positive fetus; and in a first pregnancy, about 60% of Rh‐negative women will have a Rh‐positive baby (Chilcott 2002). Clearly if the father is known to be Rh‐negative, the baby will also be Rh‐negative and therefore anti‐D would not be needed. However, for antenatal prophylaxis, the Rhesus status of the fetus is usually not yet known, and so all non‐sensitised Rh‐negative mothers would generally need to be offered routine anti‐D. This means that approximately 40% of women carrying Rh‐negative babies would have anti‐D unnecessarily.
Routine antenatal anti‐D prophylaxis is usually not administered until 28 weeks’ gestation, since transplacental haemorrhages large enough to cause sensitisation do not usually occur until the third trimester (Contreras 1998) and thus Rhesus antibodies usually develop after the 28th week of gestation (Davey 1979). The half‐life of anti‐D antibodies is estimated to be, on average, 17 to 22 days (Bishler 2003). The two main approaches, a single 1500 international units (IU) dose at 28 weeks and 500 to 625 IU doses at 28 and 32 weeks, each theoretically ensure that 12 weeks after administration there is enough anti‐D to protect against 1 mL of red blood cells or 2 mL of whole blood (Mackenzie 2006). It is considered extremely unlikely that the volume of an antenatal transplacental haemorrhage would exceed 1 mL of fetal red blood (Mackenzie 2006).
Anti‐D administration has been widely regarded as a safe prophylactic intervention. Numerous studies have suggested that while small amounts of passive anti‐D may cross the placenta, the antenatal administration of anti‐D IgG does not have adverse consequences for the fetus (Liumbruno 2010). However, since anti‐D is derived from pooled donor plasma, there is a potential, or at least theoretical, risk of transmission of blood‐borne diseases (National Blood 2003).
Some countries experience problems in obtaining sufficient supplies of anti‐D, and so antenatal prophylaxis may be restricted to Rh‐negative women expecting their first baby (partial rather than universal prophylaxis).
The effects of offering routine or universal antenatal anti‐D prophylaxis to non‐sensitised Rh‐negative women is the focus of this systematic review.
How the intervention might work
The precise mechanism whereby administration of anti‐D immunoglobulin prevents alloimmunisation remains unclear (Kumpel 2001). Passive anti‐D causes rapid and non‐inflammatory clearance of passive anti‐D coated red blood cells, which stops the inflammatory destruction of fetal red blood cells, evoking a natural immune response (Coopamah 2003). In addition, antibody‐mediated immune suppression is believed to lead to the down‐regulation of maternal immature dendritic cells or anti‐D‐specific B cells before the anti‐D response develops (Kumpel 2002; Boruchov 2005).
It is considered unlikely that epitope masking (coating the fetal red blood cells with passive anti‐D to allow them to evade detection by the maternal immune system), plays a significant role in the prevention of an anti‐D response, as a large number of Rhesus D antigen sites on fetal red blood cells in the maternal circulation are not bound by passive anti‐D (Kumpel 2002).
Why it is important to do this review
The benefit of postpartum anti‐D prophylaxis in reducing the incidence of alloimmunisation after administration and in a subsequent pregnancy has been established (Crowther 1997). As occult or 'silent' sensitising events are thought to constitute the majority of sensitisations, it is important to assess whether routine or universal antenatal anti‐D prophylaxis (to non‐sensitised Rh‐negative women) is effective in preventing Rhesus alloimmunisation and the potential adverse health consequences for the fetus and infant in the current pregnancy and/or in subsequent pregnancies.
Objectives
To assess the effects of administering anti‐D immunoglobulin at 28 weeks or more of pregnancy on the incidence of Rhesus D alloimmunisation during pregnancy (and/or in subsequent pregnancies) when given to Rh‐negative women without anti‐D antibodies.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomised, quasi‐randomised and cluster‐randomised trials. We will include studies published as abstracts only. We will exclude cross‐over trials.
Types of participants
Rh‐negative women without anti‐D antibodies at 28 weeks' gestation.
Types of interventions
Anti‐D immunoglobulin at 28 weeks or more of gestation (regardless of timing, dose and route of administration), compared with no treatment or a placebo; or comparisons of different anti‐D regimens.
Types of outcome measures
Primary outcomes
Incidence of Rhesus D alloimmunisation (during pregnancy, postpartum, and in subsequent pregnancies)
Secondary outcomes
Incidence of positive Kleihauer test (a test that detects fetal cells in the maternal blood)
Neonatal morbidity (e.g. neonatal jaundice, anaemia and kernicterus) in current or subsequent pregnancies
Adverse events attributed to anti‐D treatment
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 May 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of retrieved studies
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeCrowther 2013.
For this update, the following methods were used, including to assess the one report that was identified as a result of the updated search. These methods are based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Assessment of the quality of the evidence
For this update we assessed the quality of the evidence using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison.
Incidence of Rhesus D alloimmunisation during pregnancy
Incidence of Rhesus D alloimmunisation postpartum
Incidence of Rhesus D alloimmunisation in subsequent pregnancies
Incidence of positive Kleihauer test (at 32 to 35 weeks and at birth of a Rh‐positive infant)
Neonatal morbidity (e.g. neonatal jaundice)
Adverse events attributed to anti‐D treatment
We used GRADE profiler (GRADE 2014) to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We planned to use the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
In future updates of this review, if we identify any eligible cluster‐randomised trials. we plan to include them in the analyses along with individually‐randomised trials. We plan to adjust their sample sizes using the methods described in the Handbook (Section 16.3.4) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we plan to report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We also plan to acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We considered cross‐over trials inappropriate for this review question.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect had not been clinically meaningful, we would not have combined trials. Where we used random‐effects analyses, the results have been presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We planned to consider whether an overall summary was meaningful, and if it was, use random‐effects analysis to produce it.
If possible, we planned to carry out subgroup analysis considering aspects of the regimen for administration.
Timing of administration (e.g. single 1500 IU dose at 28 weeks versus 500 to 625 IU doses at 28 and 32 weeks)
Route of administration (intramuscular versus intravenous)
Dose administered
We planned to use only the primary outcomes in subgroup analysis. We were able to perform subgroup analysis for primary outcomes by dose administered only.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). In future updates, if more trials are included, we will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of adequacy of allocation concealment (including quasi‐randomisation) (selection bias) and other risk of bias components (including high attrition rates and attrition bias), by excluding those studies rated as 'high risk of bias' for these components. We would have restricted this to the primary outcomes. However, due to the paucity of data, sensitivity analyses were not possible in this version of the review.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
The updated search of the Cochrane Pregnancy and Childbirth Group's Trials Register found one ongoing trial (ACTRN12613000661774).
We previously included two trials (Huchet 1987; Lee 1995), excluded one paper (Ismail 2002), and found one ongoing trial (CTRI/2008/091/000157).
ACTRN12613000661774 intends to recruit 300 women and compare a single dose (1500 international units (IU) Rh(D)) at 28 weeks' gestation with routine treatment (625 IU Rh(D) Immunoglobin at 28 and 34 weeks) and was scheduled to start recruiting in May 2013, while CTRI/2008/091/000157 is recorded as not yet recruiting but intends to compare a single dose of 1500 IU Rh(D) with no intervention in 100 women.
Included studies
Two trials of anti‐D immunoglobulin met our inclusion criteria, one from France (Huchet 1987), and one from the UK (Lee 1995). Both trials compared routine antenatal anti‐D prophylaxis with no anti‐D prophylaxis, and neither study used a placebo.
Huchet 1987 recruited 1969 Rh‐negative pregnant women without anti‐D antibodies who attended antenatal clinics in the Paris region. Of the 1882 women with results available, 1450 were primigravid and 432 were multigravid. These women gave birth to 599 Rh‐positive babies in the anti‐D group and 590 Rh‐positive babies in the control group.
Lee 1995 recruited 2541 Rh‐negative primigravidae; 1273 to the control group and 1268 to the treatment group. No further data were available for 469 women (205 in the control group and 264 in the treatment group). A further 52 women allocated to the treatment group did not receive both doses of anti‐D, leaving 2020 women with results available for analysis. Of these women, 1108 gave birth to Rh‐positive infants and were tested at the time of birth (595 in the control group and 513 in the treatment group); and 72 women with Rh‐positive babies were not tested for anti‐D at the time of the birth.
Huchet 1987 administered 100 µg (500 IU) anti‐D at 28 and 34 weeks' gestation (total dose of 200 µg). Lee 1995 administered 50 µg (250 IU) anti‐D at 28 and 34 weeks' gestation (total dose of 100 µg).
For further details of the two included studies, see Characteristics of included studies.
Excluded studies
One paper was excluded (this was a plan for a trial, which is not proceeding at this stage, see Characteristics of excluded studies).
Risk of bias in included studies
Summaries for the risk of bias of the included studies are given in Figure 1 and Figure 2.
1.
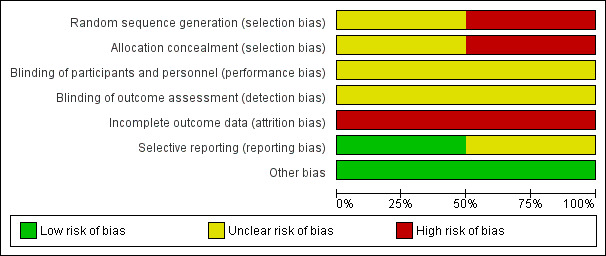
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
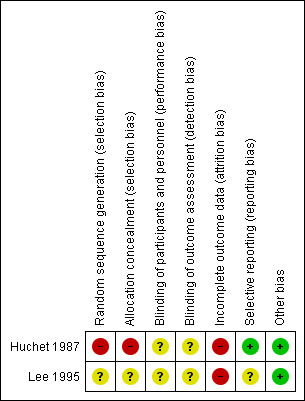
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In the Huchet 1987 trial, women were allocated to the two treatment groups by even or uneven year of birth, and thus the trial was judged to be at a high risk of selection bias. In Lee 1995, no detail was provided regarding the random sequence generation, and whilst sealed envelopes were used to conceal allocation, no detail was provided regarding how the envelopes were numbered (i.e. if they were numbered consecutively), and if they were opaque, and thus selection bias was judged to be unclear.
Blinding
In both trials (Huchet 1987; Lee 1995), no placebo was used in the control group, and no further details were provided regarding blinding of the trial personnel or outcome assessors. It was considered unclear as to whether the objectively measured outcomes would have been affected by a lack of blinding. Therefore, both trials were judged to be at an unclear risk of performance bias and detection bias.
Incomplete outcome data
Outcome data at the time of birth were not available for 4.4% (87/1969) of women who entered the French trial (Huchet 1987) and for more than 23% (593/2541) of women in the UK trial (Lee 1995); with more losses to follow‐up in the treatment group (335/1268; 26%) than the control group (258/1273; 20%). In the Huchet 1987 trial, additional women were lost to follow‐up at two to 12 months, leaving only 940 of the 1189 women who gave birth to a Rh‐positive baby available for analysis for these outcomes.
In Huchet 1987 an intention‐to‐treat analysis was possible for some outcomes, but this was not the case for Lee 1995. Both trials were judged to be at high risk of attrition bias due to incomplete outcome data.
Selective reporting
All outcome measures reported appear to have been pre‐specified in the Huchet 1987 trial, and it was thus judged to be at a low risk of selective reporting. In Lee 1995, a number of outcomes that may have been expected were not reported, including for example, positive Kleihauer during pregnancy/delivery/postpartum and neonatal morbidity, and thus the trial was judged to be at an unclear risk of selective reporting. Neither trial reported adverse effects related to treatment.
Other potential sources of bias
No other obvious source of bias for the included studies was apparent.
Overall risk of bias was judged to be high for Huchet 1987 and unclear for Lee 1995.
Effects of interventions
See: Table 1
See: Table 1
Primary outcomes
Incidence of Rhesus D alloimmunisation during pregnancy; postpartum; and in subsequent pregnancies
When women received anti‐D at 28 and 34 weeks' gestation, the data pooled over both trials (Huchet 1987; Lee 1995) did not reveal a clear difference between anti‐D and no anti‐D in the incidence of Rheus D alloimmunisation, during pregnancy (risk ratio (RR) 0.42, 95% confidence Interval (CI) 0.15 to 1.17; two trials, 3902 women; GRADE: low quality evidence) (Analysis 1.1), after the birth of a Rh‐positive infant (RR 0.42, 95% CI 0.15 to 1.17; two trials, 2297 women) (Analysis 1.2), or within 12 months after birth of a Rh‐positive infant (average RR 0.39, 95% CI 0.10 to 1.62; two trials, 2048 women; GRADE: low quality evidence) (Analysis 1.3). The moderate heterogeneity (I² = 39%) in the latter result may partly reflect the different doses used in the two randomised controlled trials, although the interaction test did not reveal a clear subgroup difference based on dose of anti‐D (see below). Huchet 1987 also reported on the incidence of Rhesus D alloimmunisation at two to 12 months following birth for primigravidae only, and did not find a clear difference between groups (RR: 0.11, 95% CI 0.01 to 2.04; 722 women) (Analysis 1.4)
1.1. Analysis.
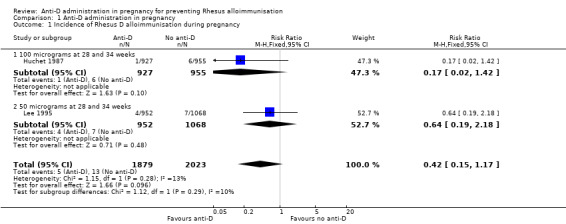
Comparison 1 Anti‐D administration in pregnancy, Outcome 1 Incidence of Rhesus D alloimmunisation during pregnancy.
1.2. Analysis.
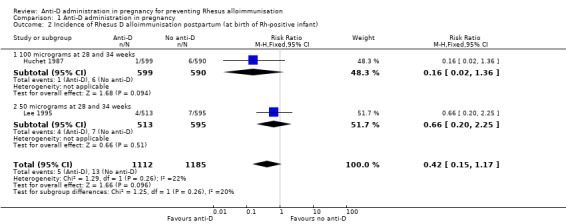
Comparison 1 Anti‐D administration in pregnancy, Outcome 2 Incidence of Rhesus D alloimmunisation postpartum (at birth of Rh‐positive infant).
1.3. Analysis.
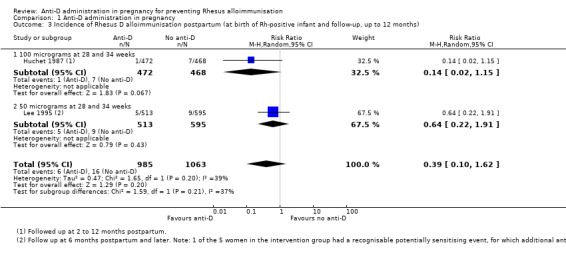
Comparison 1 Anti‐D administration in pregnancy, Outcome 3 Incidence of Rhesus D alloimmunisation postpartum (at birth of Rh‐positive infant and follow‐up, up to 12 months).
1.4. Analysis.
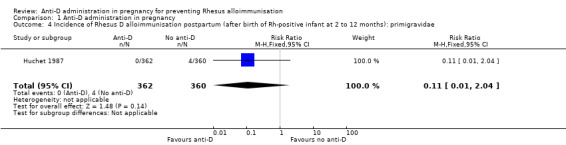
Comparison 1 Anti‐D administration in pregnancy, Outcome 4 Incidence of Rhesus D alloimmunisation postpartum (after birth of Rh‐positive infant at 2 to 12 months): primigravidae.
Neither of the two trials reported Rhesus D alloimmunisation in a subsequent pregnancy.
Subgroup analyses
The subgroup analyses for dose of anti‐D treatment have been integrated into the main structure of the graphs (Analysis 1) and comments relating to these subgroups have been made below.
The interaction tests, considering dose of anti‐D treatment administered (100 µg (Huchet 1987) versus 50 µg at 28 and 34 weeks (Lee 1995)) did not reveal any clear subgroup differences for any of the primary outcomes: immunisation during pregnancy (Chi²: 1.12; P: 0.29; I²: 10.4%) (Analysis 1.1), after birth of a Rh‐positive infant (Chi ²: 1.25; P: 0.26; I²:20.3%) (Analysis 1.2) or within 12 months after birth of a Rh‐positive infant (Chi²: 1.59; P: 0.21; I²: 37.0%) (Analysis 1.3).
We were unable to perform other planned subgroup analyses (based on route of administration or timing of administration) or sensitivity analyses due to paucity of data.
Secondary outcomes
Incidence of positive Kleihauer test
In Huchet 1987, a positive Kleihauer result was found less commonly during pregnancy at 32 to 35 weeks (RR 0.60, 95% CI 0.41 to 0.88; 1884 women; GRADE: low quality evidence) (Analysis 1.5), and after the birth of a Rh‐positive infant (RR 0.60, 95% CI 0.46 to 0.79; 1189; GRADE: low quality evidence) (Analysis 1.6) in the group of women treated at 28 and 34 weeks' gestation with anti‐D, compared with the group of women who received no anti‐D. After the birth of a Rh‐positive infant, no clear difference between groups was shown however for the number of women with a Kleihauer result greater than one in 10,000 (RR 0.95, 95% CI 0.59 to 1.54; 1189 women) (Analysis 1.7).
1.5. Analysis.
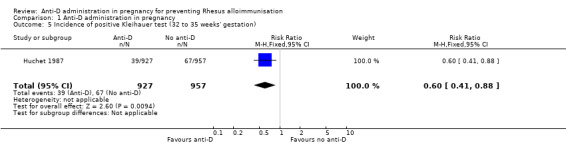
Comparison 1 Anti‐D administration in pregnancy, Outcome 5 Incidence of positive Kleihauer test (32 to 35 weeks' gestation).
1.6. Analysis.
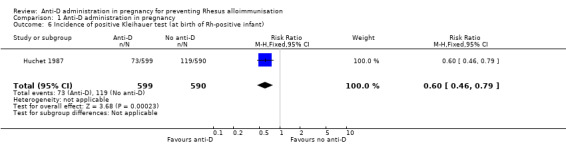
Comparison 1 Anti‐D administration in pregnancy, Outcome 6 Incidence of positive Kleihauer test (at birth of Rh‐positive infant).
1.7. Analysis.
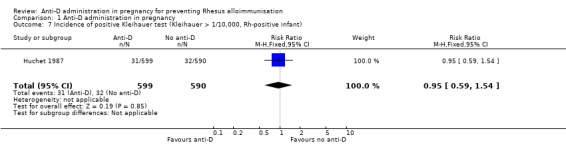
Comparison 1 Anti‐D administration in pregnancy, Outcome 7 Incidence of positive Kleihauer test (Kleihauer > 1/10,000, Rh‐positive infant).
Neonatal morbidity in current or subsequent pregnancies
Huchet 1987 reported on neonatal jaundice. There was only one case in the group of neonates whose mothers had received anti‐D, and three cases in the group of neonates born to mothers who received no anti‐D (RR 0.26, 95% CI 0.03 to 2.30; 1882 neonates; GRADE: very low quality evidence) (Analysis 1.8).
1.8. Analysis.
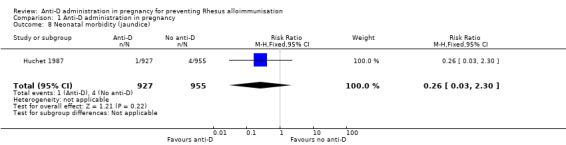
Comparison 1 Anti‐D administration in pregnancy, Outcome 8 Neonatal morbidity (jaundice).
Neither of the trials reported on any other outcomes relating to neonatal morbidity in current or subsequent pregnancies.
Adverse events attributed to anti‐D treatment
Neither of the two trials reported on adverse effects related to treatment.
Discussion
Summary of main results
While a policy of routine antenatal prophylaxis with anti‐D is unlikely to confer benefit or improve outcome in the current pregnancy, fewer women are likely to have Rhesus D antibodies in a subsequent pregnancy. As Chilcott 2002 points out, the clinical benefit sought is the avoidance of haemolytic disease in subsequent babies; if the mother "has a RhD‐positive infant and she would have been sensitised, and she goes on to have a further infant who is also Rh‐D positive".
The quantity of available evidence to answer such an important question of policy was disappointingly low and there was a moderate to high risk of bias in the included studies. We included only two studies in this review, with a total of over 4500 women; one trial was quasi‐randomised.
The reduction in the risk of alloimmunisation seen in this review in the Huchet 1987 trial for Rh‐negative women with a Rh‐positive baby from about 1% to 0.2% with anti‐D, however, is consistent with the findings of two non‐randomised community studies reported in Chilcott 2002 where the sensitisation rate was reduced from 0.95% to 0.35%. From these figures, Chilcott has calculated that 278 women would need to be treated antenatally with anti‐D to avoid one case of sensitisation (based on all Rh‐negative women; all will require treatment, since the 60% of women with Rh‐positive babies would not yet be identified). Based on the findings of this review, the number needed to treat to benefit would be slightly lower, at 213 women.
Use of a smaller dose of anti‐D (50 µg or 250 international units (IU)) in Lee 1995 at similar gestational ages failed to show any benefit. Women in the intervention and the control groups in both trials also received anti‐D after the birth of a Rh‐positive baby.
Anti‐D does not appear to be harmful to the fetus, although there is a theoretical risk of passive anti‐D in the mother causing fetal anaemia (Chilcott 2002). In 1994, batches of anti‐D used in Ireland in 1977 and 1978 were found to be contaminated with hepatitis C virus, but additional safety features were introduced and no further instances of transmission of infectious disease have been reported (National Blood 2003). Neonatal jaundice was the only outcome relating to neonatal morbidity reported in the Huchet 1987 trial, with no difference observed between groups. Neither of the trials reported on adverse effects related to anti‐D treatment.
The costs of prophylaxis need to be considered against the cost of antenatal monitoring and treatment of any affected infant whose mother develops antibodies. The National Institute for Health and Clinical Excellence (NICE) in the UK has suggested that universal routine antenatal anti‐D prophylaxis to prevent sensitisation, fetal loss and fetal morbidity is a cost‐effective approach (NHS 2011).
Although the evidence for postpartum prophylaxis is stronger (as more studies of higher quality have been completed), antenatal prophylaxis is also likely to decrease the number of sensitisations, without adverse effects and may be considered to be complementary to postpartum prophylaxis.
The decision to implement a policy of antenatal prophylaxis may also be influenced by the availability of anti‐D. In some countries, supplies of anti‐D gammaglobulin are limited and, on occasions, temporarily exhausted. Before local adoption of a programme of anti‐D prophylaxis in pregnancy, consideration would need to be given as to how to maintain an adequate supply of anti‐D gammaglobulin for women in more urgent need. In many countries, including the UK and Australia, guidelines now advise routine universal antenatal anti‐D prophylaxis (RCOG 2011; RANZCOG 2004).
Overall completeness and applicability of evidence
This review is limited with the inclusion of only two trials (Huchet 1987; Lee 1995), that did not report on immunisation in subsequent pregnancies, important secondary review outcomes including neonatal morbidity (Huchet 1987 reported only neonatal jaundice), or maternal adverse effects related to the anti‐D treatment. We were unable to perform subgroup analyses based on timing, number of treatments required, and on route of administration, due to the paucity of data.
Quality of the evidence
The two trials included in the review (with over 4500 women) were judged to be at a moderate to high risk of bias overall. The Huchet 1987 trial was quasi‐randomised, and thus at high risk of selection bias; the Lee 1995 trial did not clearly detail its selection methods. Neither trial used a placebo, and both trials had high rates of attrition.
We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach to assess evidence for pre‐specified outcomes; results ranged from very low quality (neonatal jaundice) to low quality (immunisation during pregnancy; immunisation up to 12 months after birth of a Rh‐positive infant; positive Kleihauer test at 32 to 35 weeks, and at the birth of a Rh‐positive infant). There was no available evidence regarding incidence of Rhesus D alloimmunisation in subsequent pregnancies, or regarding adverse effects associated with anti‐D treatment. Please see Table 1.
Potential biases in the review process
The evidence for this review has been derived from trials identified through a detailed search process. It is possible (but unlikely) that additional trials assessing routine anti‐D prophylaxis in pregnancy have been published but not identified. It is also possible that other studies have been conducted but not published. Should such studies be identified, we will include them in future updates of this review. Data from Huchet 1987 was obtained from a translation of the paper.
Authors' conclusions
Implications for practice.
In Rh‐negative women carrying a Rh‐positive baby, the risk of Rhesus D alloimmunisation during or immediately after a first pregnancy is about 1%. Administration of 100 µg (500 international units (IU)) anti‐D at 28 and 34 weeks' gestation to women in their first pregnancy may reduce this risk to about 0.2% without, to date, any observed adverse effects. Although such a policy generally will not confer benefit or improve outcome in the current pregnancy, fewer women are likely to have Rhesus D antibodies in any subsequent pregnancy.
In adopting such a policy, costs of prophylaxis versus the costs of care for women who become sensitised and their affected infants need to be considered, along with local adequacy of supply of anti‐D gammaglobulin.
Another Cochrane review has shown that postpartum administration of anti‐D gammaglobulin is effective as prophylaxis against Rhesus D alloimmunisation (see review on anti‐D prophylaxis postpartum, Crowther 1997).
Implications for research.
Further trials are warranted to determine the optimal timing, number of treatments, and effective dosage of anti‐D administration in pregnancy. The cost‐effectiveness of such a policy requires further evaluation. However, one of the most important areas to investigate is the effect of antenatal anti‐D on subsequent pregnancies. The move towards more universal antenatal anti‐D policies may provide an environment for more robust research to be carried out.
What's new
| Date | Event | Description |
|---|---|---|
| 31 May 2015 | New search has been performed | Search updated and one study added to 'Ongoing studies'. Addition of GRADE table. |
| 31 May 2015 | New citation required but conclusions have not changed | New search for studies and content updated (no change to conclusions). |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 2, 1996
| Date | Event | Description |
|---|---|---|
| 14 November 2012 | New citation required but conclusions have not changed | One trial added to ongoing studies (CTRI/2008/091/000157). |
| 30 September 2012 | New search has been performed | Format of review updated, including background format, methods, and results and discussion format. Characteristics of studies and 'Risk of bias' tables updated. Search updated and one report identified. |
| 10 November 2008 | Amended | Contact details updated. |
| 6 March 2008 | Amended | Converted to new review format. |
| 26 June 2007 | New search has been performed | Search updated. No new trials identified. |
| 30 April 2004 | New search has been performed | No new studies found in current update. One paper placed in 'excluded studies' (plan for a trial unlikely to proceed). Odds ratio changed to relative risk. Text expanded (e.g. 'Background'). |
| 31 August 2000 | New search has been performed | New search for trials conducted but none found. |
| 21 January 1999 | New search has been performed | Search updated. |
Acknowledgements
We thank Emily Bain for her assistance with this most recent update of the review.
For the first version of this review, we thank Professor Marc Keirse for his contributions, and Gill Gyte for her very helpful comments. We also thank Lynn Hampson and Denise Atherton for their help with the updates.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Anti‐D administration in pregnancy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of Rhesus D alloimmunisation during pregnancy | 2 | 3902 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.15, 1.17] |
| 1.1 100 micrograms at 28 and 34 weeks | 1 | 1882 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.42] |
| 1.2 50 micrograms at 28 and 34 weeks | 1 | 2020 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.19, 2.18] |
| 2 Incidence of Rhesus D alloimmunisation postpartum (at birth of Rh‐positive infant) | 2 | 2297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.15, 1.17] |
| 2.1 100 micrograms at 28 and 34 weeks | 1 | 1189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.36] |
| 2.2 50 micrograms at 28 and 34 weeks | 1 | 1108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.20, 2.25] |
| 3 Incidence of Rhesus D alloimmunisation postpartum (at birth of Rh‐positive infant and follow‐up, up to 12 months) | 2 | 2048 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.10, 1.62] |
| 3.1 100 micrograms at 28 and 34 weeks | 1 | 940 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.15] |
| 3.2 50 micrograms at 28 and 34 weeks | 1 | 1108 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.22, 1.91] |
| 4 Incidence of Rhesus D alloimmunisation postpartum (after birth of Rh‐positive infant at 2 to 12 months): primigravidae | 1 | 722 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.04] |
| 5 Incidence of positive Kleihauer test (32 to 35 weeks' gestation) | 1 | 1884 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.41, 0.88] |
| 6 Incidence of positive Kleihauer test (at birth of Rh‐positive infant) | 1 | 1189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.46, 0.79] |
| 7 Incidence of positive Kleihauer test (Kleihauer > 1/10,000, Rh‐positive infant) | 1 | 1189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.59, 1.54] |
| 8 Neonatal morbidity (jaundice) | 1 | 1882 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Huchet 1987.
| Methods | Quasi‐randomised trial. | |
| Participants | 1969 women were randomised from January 1983 to June 1984. Setting: women were recruited from 23 maternity units in the Paris region, France. Inclusion criteria: women who were primipara and were Rh‐negative. Exclusion criteria: none detailed. |
|
| Interventions |
Treatment group (Anti‐D) (n = 927) Women received 2 anti‐D immunoglobulin injections (100 µg by intramuscular injection (500 IU)) at 28 and 34 weeks' gestation, after blood samples had been taken. Control group (no Anti‐D) (n = 955) No placebo was given. In both groups, women who gave birth to a Rh‐positive baby were administered postpartum (intravenously in almost all cases) anti‐D immunoglobulin (100 µg), with possible re‐treatment following review of fetal red blood cell test results. 1450 women were primigravid and 432 were multigravid. |
|
| Outcomes | Incidence of immunisation during pregnancy, immunisation at 2 to 12 months following pregnancy, positive Kleihauer during pregnancy, at delivery, or postpartum. Cost‐effectiveness data also provided. | |
| Notes | From the translation received for this manuscript, 1969 women began the study, with 1882 monitored until they went into labour. The blood groups ABO and Rhesus D were determined using standard techniques. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not detailed ‐ women were allocated to groups on the basis of their birth year (even/odd). |
| Allocation concealment (selection bias) | High risk | As above. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not detailed, however considered unlikely in view of the intervention. The lack of blinding, however, may be considered unlikely to affect the objectively measured outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As above. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | From the translation received ‐ 1969 women began the study, of those 1882 were monitored until they were in labour (the 87 women not followed up to birth were not accounted for). In the control group, 2/957 women were excluded due to fetal‐maternal haemorrhage, leaving 955 who were monitored until labour. Of these women, 590/955 gave birth to a Rh‐positive baby, however 2 died at birth; 468 women were followed up postpartum (no reasons given for the 122 women not followed up postpartum). In the treatment group, 599/927 gave birth to a Rh‐positive baby; 472 women were followed up postpartum (no reasons given for the 127 women not followed up postpartum). |
| Selective reporting (reporting bias) | Low risk | No clear evidence of selective reporting ‐ outcome measures reported appear to have been pre‐specified. |
| Other bias | Low risk | No obvious risk of other bias. |
Lee 1995.
| Methods | Randomised controlled trial. | |
| Participants | 2541 women were randomised. Setting: obstetric units throughout the UK. Inclusion criteria: Rh‐negative primigravidae before 28 weeks' gestation. Exclusion criteria: any woman with anti‐D other than passive found at a 28‐week blood sample was excluded from the trial. Women who had already received anti‐D to cover a potentially sensitising event were not excluded ‐ where such an event took place after 28 weeks, the patient received anti‐D in the usual way. |
|
| Interventions |
Treatment group (Anti‐D) (n = 1268) Women in the treatment group received 50 µg (250 IU) anti‐D intramuscularly at 28 and 34 weeks' gestation (n = 952). Control group (no Anti‐D) (n = 1273) No placebo was given. Women in both groups "were considered for anti‐D Ig in the normal way at delivery". |
|
| Outcomes | Presence of anti‐D at birth and 6 months postpartum (repeated if equivocal); also reported "potentially sensitizing events." | |
| Notes | Sample size needed to detect 5‐fold reduction in sensitisation: 5200 women. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generation of random sequence was not detailed. |
| Allocation concealment (selection bias) | Unclear risk | Quote ‐ "sealed envelopes" were used; no further detail provided regarding how the envelopes were numbered, or whether they were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not detailed, however considered unlikely in view of the intervention. The lack of blinding, however, may be considered unlikely to affect the objectively measured outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As above. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1273 women were controls and 1268 were in the treatment group; no data were provided for 205 controls and 264 women from the treatment group (no details provided). In the control group, 649 women gave birth to Rh‐positive infants (398 infants were Rh‐negative; unknown for 21 infants). 1 additional woman was excluded from the control group after she was found to have immune anti‐D at randomisation with a history of threatened abortion. Therefore, 648 women from the control group were included in the analysis. In the treatment group 532 women and infants were included in the analysis (393 infants were Rh‐negative, and 52 women did not receive both doses of anti‐D and these women were excluded from further analyses ‐ unknown whether infants were Rh‐positive/negative). Not an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | Trial reported only presence of anti‐D at birth and 6 months postpartum; a number of outcomes that may have been expected, such as positive Kleihauer during pregnancy/delivery/postpartum, or neonatal morbidity were not reported. |
| Other bias | Low risk | No other obvious sources of bias identified. |
IU: international units
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ismail 2002 | This is only the plan for a trial. Trial not proceeding at this stage (Z Alfirevic, personal communication March 2004). |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12613000661774.
| Trial name or title | Detectability of anti‐D and compliance in two regimens. |
| Methods | Randomised controlled trial. |
| Participants | Recruitment target: 300 women. Setting: King Edward Memorial Hospital, WA, Australia. Inclusion criteria: female, pregnant, aged over 18 years, with a negative antibody screen and no contraindication for anti‐D intramuscular injection, such as previous anaphylaxis to immunoglobulin, isolated immunoglobulin A deficiency, or previously recorded endogenous anti‐D antibodies. Exclusion criteria: aged less than 18 years at recruitment, non‐pregnant, Rh‐positive, allergy/adverse reaction to constituents of anti‐D as per product information. |
| Interventions |
Treatment group: 1500 IU Rh(D) immunoglobulin‐VF at 28 weeks' gestation. Control group: 625 IU Rh(D) immunoglobulin‐VF at 28 and 34 weeks' gestation. |
| Outcomes |
Primary outcomes: detectability of anti‐D at delivery via standard detection practices employed at King Edward Memorial Hospital and proportion of women receiving doses at correct gestation via analysis of number of enrolments compared with the number and timing of doses delivered in each arm of the study. Secondary outcomes: risk factors for no detectable antibody at delivery via questionnaire and review of medical records, complication rates (obstetric and neonatal) via review of medical records and the total amount of anti‐D used per participant. |
| Starting date | 3/5/13 |
| Contact information | A/Prof Craig E Pennell School of Womens' and Infants' Health University of Western Australia 35 Stirling Highway, Crawley, WA 6009 Phone: +61893401326 Email: craig.pennell@uwa.edu.au |
| Notes |
CTRI/2008/091/000157.
| Trial name or title | A clinical trial to study the effect of injection of anti‐D administered during pregnancy for Rh‐negative mothers. |
| Methods | Randomised controlled trial. |
| Participants | Recruitment target: 100 women. Setting: Dr TMA Pai Rotary Hospital, Karkala, India. Inclusion criteria: all Rh‐negative and indirect agglutinin test negative primigravida and un‐sensitised multigravida who are willing to participate in the study. Exclusion criteria: all Rh‐negative mothers with Rh‐negative husbands. Indirect agglutination test positive. |
| Interventions |
Treatment group: antenatal administration of 300 µg (1500 IU) of Rh‐D immunoglobulin. Control group: no intervention. |
| Outcomes |
Primary outcomes: incidence of immunisation during pregnancy at term, at delivery and at 6 months. Secondary outcomes: incidence of neonatal hyperbilirubinaemia, need for exchange transfusion, and need for phototherapy. |
| Starting date | 1/12/2008 on the trial registry however, the trial is listed as "not yet recruiting". |
| Contact information |
Scientific queries: Dr AP Manjunath Associate Professor Department of Obstetrics and Gynaecology 576104, India Phone: 09845913140 Fax: 080257061 Email: manjunanth.ap@manipal.edu |
| Notes |
IU: international units
Differences between protocol and review
In this update, we have updated the methods to include the use of GRADE profiler (GRADE 2014), which was used to import data from Review Manager 5.3 (RevMan 2014) in order to create a 'Summary of findings' table.
In the previous update of this review, we updated the methods including the assessment of risk of bias. We separated the outcomes into primary and secondary outcomes, and added adverse effects attributed to treatment as a secondary outcome. We clarified that cluster trials, and cross‐over trials would be excluded, that comparisons will be with no treatment or placebo or a different anti‐D regimen.
Contributions of authors
Caroline A Crowther contributed to the development of the protocol, identification and selection of trials for inclusion and the preparation of the text of the first publication of the review.
Rosie McBain, Caroline A Crowther and Philippa Middleton contributed to the earlier update and this update.
Sources of support
Internal sources
ARCH: Australian Research Centre for Health of Women and Babies, Robinson Institute, Discipline of Obstetrics and Gynaecology, The University of Adelaide, Australia.
External sources
Australian Department of Health and Ageing, Australia.
National Health and Medical Research Council to the Australian and New Zealand Satellite of the Pregnancy and Childbirth Cochrane Review Group, Australia.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Huchet 1987 {published data only}
- Huchet J, Dallemagne S, Huchet C, Brossard Y, Larsen M, Parnet‐Mathieu F. The antepartum use of anti‐D immunoglobulin in rhesus negative women. Parallel evaluation of fetal blood cells passing through the placenta. The results of a multi‐centre study carried out in the region of Paris. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1987;16:101‐11. [PubMed] [Google Scholar]
Lee 1995 {published data only}
- Lee D, Rawlinson VI. Multicentre trial of antepartum low‐dose anti‐D immunoglobulin. Transfusion Medicine 1995;5:15‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ismail 2002 {published and unpublished data}
- Ismail K, Kilby M, Alfirevic Z, Khan K, Whittle M. Prospective randomised trial of alloimmunisation management (PRAM). Journal of Obstetrics and Gynaecology 2002;22(2 Suppl):S33. [Google Scholar]
References to ongoing studies
ACTRN12613000661774 {published data only}
- ACTRN12613000661774. Detectability of anti‐D and compliance in two regimens. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613000661774 (accessed 6 May 2015).
CTRI/2008/091/000157 {published data only}
- CTRI/2008/091/000157. A clinical trial to study the effect of injection anti D administered during pregnancy for Rh negative mothers. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=172 (accessed 6 May 2015).
Additional references
Bishler 2003
- Bishler J, Schondorfer G, Pabst G, Andresen I. Pharmacokinetics of anti‐D IgG in pregnant Rh‐D negative women. British Journal of Obstetrics and Gynaecology 2003;110:39‐45. [PubMed] [Google Scholar]
Boruchov 2005
- Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. Journal of Clinical Investigation 2005;115:2914‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowman 1965
- Bowman JM, Pollock JM. Amniotic fluid spectrophotometry and early delivery in the management of erythroblastosis fetalis. Pediatrics 1965;35:815. [PubMed] [Google Scholar]
Bowman 1996
- Bowman JM. Hemolytic disease of the newborn. Vox Sanguinis 1996;70:62‐7. [Google Scholar]
Chilcott 2002
- Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C. A review of the clinical effectiveness and cost effectiveness of routine anti‐D prophylaxis for pregnant women who are Rhesus (RhD) negative. London: National Institute of Clinical Excellence, 2002. [DOI] [PubMed] [Google Scholar]
Chown 1954
- Chown B. Anaemia from bleeding of the fetus into the mother’s circulation. Lancet 1954;263(6824):1213. [DOI] [PubMed] [Google Scholar]
Contreras 1998
- Contreras M. The prevention of Rh haemolytic disease of the fetus and newborn ‐ general background. British Journal of Obstetrics and Gynaecology 1998;105:7‐10. [DOI] [PubMed] [Google Scholar]
Coopamah 2003
- Coopamah MD, Freeman J, Semple JW. Anti‐D initially stimulates an Fc‐dependant leukocyte oxidative burst and subsequently suppresses erythrophagocytosis via interleukin‐1 receptor antagonist. Blood 2003;102:2862‐7. [DOI] [PubMed] [Google Scholar]
Craig 1998
- Craig JS, McClure BG, Tubman TRJ. Services should be centralised for pregnancies affected by RhD haemolytic disease. BMJ 1998;316:1611. [PubMed] [Google Scholar]
Crowther 1997
- Crowther C, Middleton P. Anti‐D administration after childbirth for preventing Rhesus alloimmunisation. Cochrane Database of Systematic Reviews 1997, Issue 2. [DOI: 10.1002/14651858.CD000021] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davey 1979
- Davey MG, Zipursky A. Proceedings of the McMaster Rh Conference. Vox Sanguinis 1979;36:50‐64. [Google Scholar]
Engelfriet 2003
- Engelfriet CP, Reesink HW, Judd WJ, Ulander VM, Kuosmanen M, Koskinen S, et al. Current status of immunoprophylaxis with anti‐D immunoglobin. Vox Sanguinis 2003;85(4):328‐37. [DOI] [PubMed] [Google Scholar]
GRADE 2014
- McMaster University.GRADEpro. [Computer program on www.gradepro.org]. Version [2014]. McMaster University, 2014..
Gravenhorst 1989
- Gravenhorst JB. Rhesus isoimmunisation. In: Chalmers I, Enkin MW, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1989:565‐77. [Google Scholar]
Gunson 1976
- Gunson HH, Stratton F, Philips PK. The primary Rho(D) immune response in male volunteers. British Journal of Haematology 1976;32:317‐29. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jones 2004
- Jones ML, Wray J, Wright J, Chilcott J, Forman K, Tappenden P, et al. A review of the clinical effectiveness of routine antenatal anti‐D prophylaxis for rhesus‐negative women who are pregnant. British Journal of Obstetrics and Gynaecology 2004;111:892‐902. [DOI] [PubMed] [Google Scholar]
Kumpel 2001
- Kumpel BM, Elson CJ. Mechanism of anti‐D mediated immune suppression ‐ a paradox awaiting resolution?. Trends in Immunology 2001;22:26‐31. [DOI] [PubMed] [Google Scholar]
Kumpel 2002
- Kumpel BM. On the mechanism of tolerance to the Rh D antigen mediated by passive anti‐D (Rh D prophylaxis). Immunology Letters 2002;82:67‐73. [DOI] [PubMed] [Google Scholar]
Liumbruno 2010
- Liumbruno GM, D’Alessandro A, Rea F, Piccinini V, Catalano L, Calizzani G, et al. The role of antenatal immunoprophylaxis in the prevention of maternal‐foetal anti‐Rh(D) alloimmunisation. Blood Transfusion 2010;8(1):8‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mackenzie 2006
- Mackenzie IZ, Roseman F, Findlay J, Thompson K, Jackson E, Scott J, et al. The kinetics of routine antenatal prophylactic intramuscular injections of polyclonal anti‐D immunoglobin. British Journal of Obstetrics and Gynaecology 2006;113:97‐101. [DOI] [PubMed] [Google Scholar]
National Blood 2003
- National Blood Authority. Guidelines on the prophylactic use of Rh D immunoglobulin (anti‐D) in obstetrics. Canberra, ACT: NHMRC, 2003. [Google Scholar]
NHS 2011
- NHS National Institute for Health and Clinical Excellece. Routine antenatal anti‐D prophylaxis for women who are rhesus D negative: Review of NICE technology appraisal guidance 41. http://www.nice.org.uk/ (accessed 14 November 2012).
RANZCOG 2004
- Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Guidelines for the use of RhD immunoglobulin (Anti‐D) in obstetrics in Australia. www.ranzcog.edu.au/ (accessed 2 November 2012).
RCOG 2011
- Royal College of Obstetricians and Gynaecologists. The use of anti‐D immunoglobulin for rhesus D prophylaxis. Green‐top guideline. http://www.rcog.org.uk/ (accessed 14 November 2012).
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Stern 1961
- Stern K, Goodman HS, Berger M. Experimental isoimmunisation to hemoantigens in man. Journal of Immunology 1961;87:189. [Google Scholar]
Turner 2012
- Turner RM, Lloyd‐Jones M, Anumba DO, Smith GC, Spiegelhalter DJ, Squires H, et al. Routine antenatal anti‐d prophylaxis in women who are rh(d) negative: meta‐analyses adjusted for differences in study design and quality. PLoS ONE 2012;7(2):e30711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zipursky 1967
- Zipursky A, Israels LG. The pathogenesis and prevention of Rh immunization. Canadian Medical Association Journal 1967;97:1245‐57. [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Crowther 1999
- Crowther CA, Middleton P. Anti‐D administration in pregnancy for preventing Rhesus alloimmunisation. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD000020] [DOI] [Google Scholar]
Crowther 2013
- Crowther CA, Middleton P, McBain RD. Anti‐D administration in pregnancy for preventing Rhesus alloimmunisation. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD000020.pub2] [DOI] [PubMed] [Google Scholar]


