Abstract
Background
Alternative institutional settings have been established for the care of pregnant women who prefer little or no medical intervention. The settings may offer care throughout pregnancy and birth, or only during labour; they may be part of hospitals or freestanding entities. Specially designed labour rooms include bedroom‐like rooms, ambient rooms, and Snoezelen rooms.
Objectives
Primary: to assess the effects of care in an alternative institutional birth environment compared to care in a conventional setting. Secondary: to determine if the effects of birth settings are influenced by staffing, architectural features, organizational models or geographical location.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 March 2012).
Selection criteria
All randomized or quasi‐randomized controlled trials which compared the effects of an alternative institutional birth setting to a conventional setting.
Data collection and analysis
We used the standard methods of the Cochrane Collaboration Pregnancy and Childbirth Group. Two review authors evaluated methodological quality. We performed double data extraction and presented results using risk ratios (RR) and 95% confidence intervals (CI).
Main results
Ten trials involving 11,795 women met the inclusion criteria. We found no trials of freestanding birth centres or Snoezelen rooms. Allocation to an alternative setting increased the likelihood of: no intrapartum analgesia/anesthesia (six trials, n = 8953; RR 1.18, 95% CI 1.05 to 1.33); spontaneous vaginal birth (eight trials; n = 11,202; RR 1.03, 95% CI 1.01 to 1.05); breastfeeding at six to eight weeks (one trial, n = 1147; RR 1.04, 95% CI 1.02 to 1.06); and very positive views of care (two trials, n = 1207; RR 1.96, 95% CI 1.78 to 2.15). Allocation to an alternative setting decreased the likelihood of epidural analgesia (eight trials, n = 10.931; RR 0.80, 95% CI 0.74 to 0.87); oxytocin augmentation of labour (eight trials, n = 11,131; RR 0.77, 95% CI 0.67 to 0.88); instrumental vaginal birth (eight trials, n = 11,202; RR 0.89, 95% CI 0.79 to 0.99), and episiotomy (eight trials, n = 11,055; RR 0.83, 95% CI 0.77 to 0.90). There was no apparent effect on other adverse maternal or neonatal outcomes. Care by the same or separate staff had no apparent effects. No conclusions could be drawn regarding the effects of continuity of caregiver or architectural characteristics. In several of the trials included in this review, the design features of the alternative setting were confounded by important differences in the organizational models for care (separate staff for the alternative setting, offering more continuity of caregiver), and thus it is difficult to draw inferences about the independent effects of the physical birth environment.
Authors' conclusions
Hospital birth centres are associated with lower rates of medical interventions during labour and birth and higher levels of satisfaction, without increasing risk to mothers or babies.
Keywords: Female; Humans; Pregnancy; Analgesia, Epidural; Analgesia, Epidural/statistics & numerical data; Analgesia, Obstetrical; Analgesia, Obstetrical/statistics & numerical data; Birthing Centers; Birthing Centers/organization & administration; Birthing Centers/standards; Breast Feeding; Breast Feeding/statistics & numerical data; Confidence Intervals; Delivery Rooms; Interior Design and Furnishings; Odds Ratio; Randomized Controlled Trials as Topic
Plain language summary
Alternative versus conventional institutional settings for birth
In high‐ and moderate‐income countries, labour wards have become the settings for childbirth for the majority of childbearing women. Routine medical interventions have also increased steadily over time, leading to many questions about benefits, safety, and risk for healthy childbearing women. The design of conventional hospital labour rooms is similar to the design of other hospital sick rooms, i.e. the hospital bed is a central feature of the room, and medical equipment is in plain view. In an effort to support normal labour and birth for healthy childbearing women, a variety of institutional maternity care settings have been constructed. Some are 'home‐like' bedrooms within hospital labour wards. Others are 'home‐like' birthing units adjacent to the labour wards. Others are freestanding birth centres. More recently, 'ambient' and Snoezelen rooms have been constructed within labour wards; these rooms are not home‐like but contain a variety of sensory stimuli and furnishings designed to promote feelings of calmness, control, and freedom of movement.
The primary aim of this review is to evaluate the effects, on labour and birth outcomes, of care in an alternative institutional birth setting compared with care in a conventional hospital labour ward. We included ten trials involving 11,795 women. We found no trials of freestanding birth centres. When compared to conventional institutional settings, alternative settings were associated with reduced likelihood of medical interventions, increased likelihood of spontaneous vaginal birth, increased maternal satisfaction, and greater likelihood of continued breastfeeding at one to two months postpartum, with no apparent risks to mother or baby. Unfortunately, in several trials, the design features of the alternative setting were confounded by differences in the organizational models of care (including separate staff and more continuity of caregiver in the alternative setting), and thus it is not possible to draw conclusions about the independent effects of the design of the birth environment. We conclude that women and policy makers should be informed about the benefits of institutional settings which focus on supporting normal labour and birth.
Background
In high‐ and moderate‐income countries, labour wards have become the settings for labour and birth for the majority of childbearing women. Routine medical interventions have also increased steadily over time, leading to many questions about benefits, safety, and risk for healthy childbearing women. The design of conventional hospital labour rooms is similar to the design of other hospital patient rooms, i.e. the hospital bed is a central feature of the room, and medical equipment such as oxygen, suction, and intravenous equipment are in plain view.
As a critique of 'technological' approaches to childbirth, there has been a steady increase in interest in the impact of the care environment on the outcomes of labour and birth. Since the 1970s in many high‐income countries, many hospitals have endeavoured to make their labour rooms or birthing units 'home‐like,' although a more accurate term would be 'bedroom‐like,' since the hospital bed is still the prominent feature. Labour rooms are decorated and furnished to be like Western middle‐class bedrooms, with medical equipment concealed from view (Fannin 2003). The bedroom‐like rooms draw on notions of domesticity and the naturalness of birth, while hiding the technology behind curtains and wood cabinets (Fannin 2003). According to Fannin (Fannin 2003), these hybrid spaces send dual messages to birthing women and to the staff working in them, exemplifying the struggles over competing conceptualizations of safety, control, and family, and thus over the very meaning of birth itself (Fannin 2003). In a parallel trend, alternative locations for care which are geographically separate from the hospital labour and delivery unit have been gaining prominence in high‐income countries. These 'freestanding' birth centres have evolved both out of concerns that routine hospital policies and practices may have spillover effects on birth centre care, and as a means of providing an alternative to home birth. Freestanding units offer more scope for separation between 'technological' and 'social' models of birth, and recent observational evidence from The Birthplace study in England supports their efficacy and safety (Brocklehurst 2011).
In recent years, in recognition that a bedroom (home‐like or otherwise) may not be the optimum environment to support normal labour, other types of institutional birth settings have been constructed. While the new types of rooms share the same values (decreasing anxiety and fear, promoting mobility and personal control), they do not resemble home environments and do not contain hospital labour beds. One such room is the ambient room (Hodnett 2009), in which scenes from nature are projected on a wall, a variety of music is available, and other features encourage mobility during labour. Another type of room is the Snoezelen room, in which the user is exposed to multiple sensory stimulations including fibre‐optic lights, auditory stimuli, and aromatherapy. A qualitative study of women's labour experiences in a Snoezelen room found that the users would choose it again in a future labour (Hauck 2008).
Alternative settings vary in location and staffing models. While some alternative settings have arisen as a re‐configuration of previously existing facilities, others have been purpose built. Some in‐hospital birth centres are adjacent to conventional labour wards, or on another floor of the same hospital. Others are freestanding centres that are not physically part of a hospital but may or may not have administrative linkages to a hospital. The organizational models of care delivery in birth centres vary. The model of care may or may not involve continuity of care provider, in which the same staff provide antenatal as well as intrapartum care. While the core staff of birth centres are usually midwives or nurse‐midwives, they may be a separate staff or they may be part of the regular labour ward staff. If they are part of the regular labour ward staff, they provide care for women in the birthing centre as well as women in the traditional labour ward, necessitating a shift in philosophical orientation from one emphasizing normality and avoidance of interventions to one emphasizing detection/management of risk and use of routine interventions. Another common, though not universal, feature is that these units have no routine input by medical practitioners. In these cases, the core staff are usually midwives or nurse‐midwives, sometimes with the addition of trained but non‐professional assistants, and/or doulas.
The focus of this review is on alternative institutional environments for labour and birth. While the home‐like, ambient, and Snoezelen settings vary in whether they also include antenatal care, continuity of care, and in their structural characteristics, they share a philosophical orientation towards promoting normal birth. Their philosophies and guidelines value minimal intervention in labour and the promotion of enhanced freedom and control for women in labour, and booking is restricted to women deemed at low risk of obstetric emergency. All include labour rooms which do not look like hospital sick rooms. This review is complementary to two other Cochrane reviews, 'Midwifery‐led versus other models of care delivery for childbearing women' (Hatem 2008) and 'Home versus hospital birth' (Olsen 2004).
Objectives
The primary objective was to evaluate the effects, on labour and birth outcomes, of care in an alternative institutional birth setting compared with care in a conventional hospital labour ward.
Secondary objectives were to determine if the effects of care in alternative birth settings were influenced by: (a) whether the staff in the alternative setting were also part of the conventional maternity care staff; (b) whether care in the alternative setting included more continuity of care provider than women experienced in the conventional hospital setting; (c) whether the alternative setting was in a building that was geographically separate from the hospital; and (d) the architectural characteristics of the alternative setting.
Methods
Criteria for considering studies for this review
Types of studies
All randomized or quasi‐randomized controlled trials which compared the effects of an alternative institutional birth environment with conventional maternity ward care.
Types of participants
Pregnant women at low risk of obstetric complications.
Types of interventions
We included trials if the intervention included care during labour and birth in an alternative institutional birth setting. Antenatal and postnatal care may also have occurred in the alternative setting. Care may have been provided by the same group of caregivers, or by separate groups of caregivers in the alternative versus conventional settings. We excluded trials comparing home birth with institutional birth; they are the subject of another Cochrane review (Olsen 2004).
Types of outcome measures
We identified the following pre‐specified primary and secondary outcomes for mother and baby.
Primary outcomes
Mother
Spontaneous vaginal birth.
Maternal death or serious maternal morbidity, e.g. uterine rupture, admission to intensive care unit; septicemia.
No analgesia/anesthesia for labour or birth.
Labour augmentation with artificial oxytocics.
Very positive views of intrapartum care. (This was a composite outcome, defined as the highest category of ratings (such as "very satisfied"), in whatever measure was used by trial authors. If trial authors used more than one measure of women's views, we chose the one assessing satisfaction with intrapartum care.)
Baby
Perinatal death or serious perinatal morbidity. (Serious perinatal morbidity was a composite outcome which included birth asphyxia defined by trialists, neonatal encephalopathy, severe respiratory distress syndrome, and other conditions threatening life or predictive of long‐term disability.)
Perinatal and maternal morbidity are composite outcomes. This is not an ideal solution because some components are clearly less severe than others. It is possible for one intervention to cause more deaths but fewer babies with severe morbidity. All these outcomes are likely to be rare, and a modest change in their incidence is easier to detect if composite outcomes are presented.
Secondary outcomes
Mother
Instrumental vaginal birth (forceps or vacuum).
Caesarean delivery.
Postpartum hemorrhage.
Epidural analgesia.
Episiotomy.
Baby
Admission to neonatal intensive care unit.
Five‐minute Apgar score less than seven.
Perinatal mortality.
Any breastfeeding at six to eight weeks of age.
Search methods for identification of studies
Electronic searches
The Trials Search Coordinator searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (30 March 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference list of retrieved studies.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
In the prior update, two review authors (E Hodnett (EH), J Weston (JW)) independently assessed the studies for eligibility, except for one trial (Hodnett 2009), which was assessed by two other review authors (S Downe (SD), D Walsh (DW)). For the current update, all authors assessed the new trials. We would have resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors (EH and JW or EH and SD) extracted the data using the agreed form. A third review author (DW) extracted data for Hodnett 2009. We resolved discrepancies through discussion. We entered data into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In the prior update, one author (DW) assessed the risk of bias for Hodnett 2009. We would have resolved any disagreement by discussion or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
adequate (any truly random process, e.g. random number table; computer random number generator);
inadequate (any non random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
adequate (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We described for each included study the methods used, if any, to blind personnel from knowledge of which intervention a participant received. Since women and care providers cannot be blinded to type of institutional birth environment, we considered blinding adequate if outcomes were recorded by outcome assessors who had no knowledge of the woman's group assignment. We judged studies at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. To be included in the review, data on a given outcome had to be available for at least 80% of those who were originally randomized. Where sufficient information was reported, or could be supplied by the trial authors, we included missing data in the analyses. We assessed methods as:
adequate;
inadequate:
unclear.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
adequate (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias, including, for example, whether the trial was stopped early due to a data‐dependent process, there was evidence of extreme baseline imbalance, or there have been claims of fraud.
We assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
One trial (Bernitz 2011) enrolled women to one of three groups: a midwife‐managed, in‐hospital birth centre (MU); a "Normal Unit" (NU) which had access to epidural analgesia, oxytocics in first and second stage labour, and operative delivery; and a "Special Unit" (SU) designed for women in need of additional surveillance. With the assent of the trial authors, for analysis purposes we combined the data from the NU and SU.
Dichotomous data
For dichotomous data, we present results as summary risk ratio with 95% confidence intervals.
Continuous data
All pre‐specified outcomes were dichotomous.
Unit of analysis issues
Cluster‐randomized trials
We planned to include cluster‐randomized trials in the analyses along with individually‐randomized trials. For cluster‐randomized trials, we would have adjusted their sample sizes or standard errors using the methods described in the Handbook (Section 16.3.4 or 16.3.6) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If we had used ICCs from other sources, we would have reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. If we had identified both cluster‐randomized trials and individually‐randomized trials, we planned to synthesize the relevant information. We would have considered it reasonable to combine the results from both if there were little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit was considered to be unlikely. We would also have acknowledged heterogeneity in the randomization unit and performed a separate meta‐analysis.
Dealing with missing data
For included studies, we noted levels of attrition. We included data for a given outcome only if the data were available for at least 80% of those originally randomized.
For all outcomes we have carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomized to each group in the analyses. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the T² was greater than zero and either the I² was greater than 30% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Where we suspected reporting bias (see ‘Selective reporting bias’ above), we planned to contact study authors to ask them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we planned to not include the outcome data from that trial.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect inverse variance meta‐analysis for combining data. Had we suspected clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects may differ between trials, we would have used random‐effects meta‐analysis.
If we identified substantial heterogeneity in a fixed‐effect meta‐analysis according to our pre‐specified criteria, we repeated the analysis using a random‐effects method. In such instances, we reported whether the two methods of analysis yielded important differences, and we reported the T² and I².
We excluded from analyses data for any outcome in which data were missing for more than 20% of those originally randomized.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses:
type of alternative institutional setting (bedroom‐like, ambient, Snoezelen);
location of alternative setting (in‐hospital or freestanding birth centre);
staffing model (separate staff for alternative setting or same staff who work in conventional labour ward setting);
whether continuity of caregiver was a component of the care in the alternative setting.
The outcomes which we used in subgroup analyses were chosen from the primary outcomes, on the basis of their importance from the perspective of parents, care providers, and policy makers. They were: spontaneous vaginal birth, serious maternal morbidity/mortality, serious perinatal morbidity/mortality, and very positive views of intrapartum care.
For fixed‐effect meta‐analyses, we conducted planned subgroup analyses classifying whole trials by interaction tests as described by Deeks 2001. For random‐effects meta‐analyses we assessed differences between subgroups by inspection of the subgroups’ confidence intervals; non‐overlapping confidence intervals indicate a statistically significant difference in treatment effect between the subgroups.
When we identified substantial heterogeneity, we investigated it using visual inspection of the forest plots and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it, and we reported the T² and I².
Sensitivity analysis
We conducted sensitivity analyses based on two conditions. We compared the results when studies with a high risk of bias were included versus excluded, and we compared fixed‐effect versus random‐effects analyses when evidence of statistical heterogeneity was present. We defined statistical heterogeneity as a) an I² value greater than 30% and b) inconsistency between trials in the direction or magnitude of effects (judged visually).
Results
Description of studies
See table of Characteristics of included studies. Ten trials involving 11,795 women met inclusion criteria for the review, although nine trials involving 11,503 women provided data for this review. One trial (Abdullahi 1990) (n = 292) reported no data relevant to the review's pre‐specified outcomes. If in the future the trial authors supply usable data, we will incorporate the data into the review. One trial (Hodnett 2009) was a small randomized controlled trial to assess feasibility and acceptability of an ambient labour room. We found no trials of freestanding units, Snoezelen rooms or other alternative labour room designs. All other trials compared bedroom‐like settings with conventional institutional labour wards. Five trials included at least some antenatal care in the alternative setting (Begley 2009; Byrne 2000; Hundley 1994; MacVicar 1993; Waldenstrom 1997).
All of the alternative settings were characterized by a philosophical orientation towards labour and birth as fundamentally normal experiences. All restricted access to women who were experiencing normal pregnancies. However, there were differences in the scope of the interventions. The Stockholm trial (Waldenstrom 1997) enrolled 1860 women in an evaluation of care during pregnancy, childbirth, and the postpartum period by a team of 10 midwives at a hospital birth centre, compared with standard care by different midwives during the antenatal, intrapartum and postnatal periods, in which intrapartum care was in a conventional labour ward in the same hospital. The Aberdeen trial (Hundley 1994) enrolled 2844 women in an evaluation of care in an alternative, midwife‐managed delivery unit compared with care in a consultant‐led labour ward; the same midwives also worked in both intrapartum settings. In the Norwegian trial (Bernitz 2011), which enrolled 1111 in spontaneous labour, separate staff worked in the alternative and conventional settings. The London (Chapman 1986), Montreal (Klein 1984), Danish (Abdullahi 1990) and Toronto (Hodnett 2009) trials were smaller trials which compared care in alternative birth rooms within standard labour wards; the same staff cared for women in both groups. The Leicester trial (MacVicar 1993) enrolled 3510 women in an evaluation of intrapartum care in an alternative, midwife‐managed unit compared with care in a standard labour ward in the same hospital; women allocated to the former group had up to three antenatal visits in a clinic run by the midwives in the birth centre, with the remainder of their antenatal care by their general practitioner or community midwife. The Australian trial (Byrne 2000) enrolled 201 women at 20 to 36 weeks' gestation. Birth centre care was provided by midwives who were 'committed to the normality of the birth process' and involved antenatal, intrapartum, and up to 12 hours of postnatal care. The Irish trial (Begley 2009) enrolled 1653 women prior to 24 weeks' gestation, in an evaluation of midwifery‐led versus consultant‐led care. One component of midwifery‐led care was the setting for intrapartum and postpartum care ‐ a home‐like unit adjacent to the conventional labour ward.
Thus the trials varied considerably in the scope of the intervention (study groups which differed solely in intrapartum care versus study groups in which there were differences in antenatal and/or postnatal care as well as intrapartum care), and the length of time between randomization and onset of 'treatment', but all trials shared common aspects of the intervention: intrapartum care in a setting that did not look like a conventional hospital patient room and did not offer medical interventions such as epidural analgesia or intrapartum oxytocics. All trials but one (Bernitz 2011), in which care in the alternative birth setting was by separate midwifery staff also involved increased continuity of caregiver.
We found no randomized trials which compared care in a freestanding birth centre with hospital‐based birth centres or conventional hospital care.
Response rates to questionnaires seeking information about women's satisfaction with their birth experiences were at least 80% in only three trials (Hodnett 2009; Hundley 1994; Waldenstrom 1997). Although 1860 women were enrolled in the Stockholm trial (Waldenstrom 1997), data on maternal satisfaction outcomes were sought from, and reported on, the first 1230 women who were enrolled. Postpartum questionnaire data were only available for 22% of those enrolled in the Irish trial (Begley 2009). Of the three trials with usable outcome data, two (Hodnett 2009; Waldenstrom 1997), employed the same measure of satisfaction (interest in the same birth setting in the future), while the third (Hundley 1994) reported on whether the woman's labour and delivery were managed as she liked.
Substantial numbers of women allocated to alternative settings were transferred to standard care either before or during labour, because they no longer met eligibility criteria for the alternative setting. The most common reasons for intrapartum transfer were: failure to progress in labour, fetal distress, and desire for pharmacologic analgesia. In the Australian trial (Byrne 2000), only 23/100 women allocated to birth centre care actually gave birth in the birth centre. In two UK trials (Hundley 1994; MacVicar 1993), 46% of women randomized to the birth centres actually gave birth in them. Thirty‐four per cent of women in the Stockholm trial (Waldenstrom 1997) were transferred to standard care antenatally or intrapartum for medical reasons, and an additional 3% withdrew from birth centre care at their own request. In the Montreal trial (Klein 1984), 63% of nulliparous women and 19% of multiparous women were transferred intrapartum to standard care, for an overall transfer rate of 43%. In the London trial (Chapman 1986), 29% of 76 women were transferred from birth room to standard care. Transfers in the Toronto trial (Hodnett 2009) were for women's preference (n = 1) and at delivery if a caesarean delivery was to be performed (13% of 30 women). Permanent transfers from midwifery‐led care in the Irish trial (Begley 2009) included 505 women antenatally (most commonly for induction of labour), 144 during the intrapartum period (most commonly for complications or slow progress), and five postnatally, for an overall transfer rate of 59%. However, wherever possible, the woman's midwife remained with her after an intrapartum transfer of care. In the Norwegian trial (Bernitz 2011), the transfer rate from the alternative setting was 28.4% (117 of 412).
Risk of bias in included studies
With the exception of the quasi‐random method (alternation) used in the smallest trial (Klein 1984), all trials used adequate methods of sequence generation. Concealment was inadequate in two trials ((Chapman 1986; Klein 1984) and unclear in one trial ( Abdullahi 1990). Selective reporting was a problem in three trials: Abdullahi 1990 only reported outcomes for primiparous women (55% of those randomized); Chapman 1986 , with the exception of caesarean birth, had high losses to follow‐up for all outcomes and mailed questionnaires only to those who had not been dropped from study analyses (61%); and Klein 1984 did not report results of postpartum questionnaires. Figure 1 and Figure 2 illustrate that the trials were of variable quality.
1.
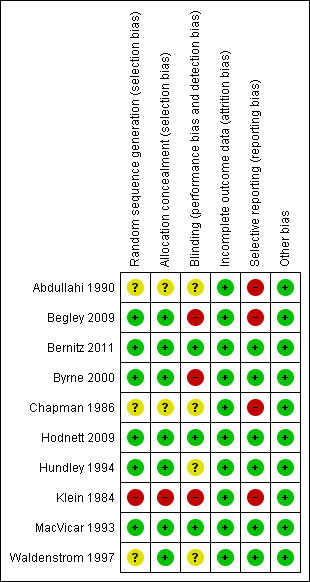
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
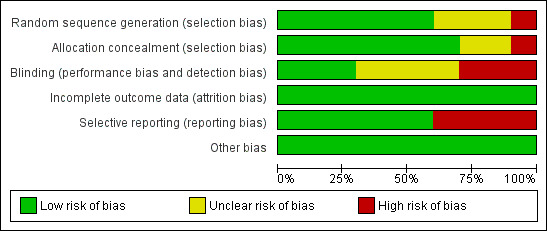
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Effects of interventions
Main comparisons: alternative versus conventional institutional settings for birth
Primary outcomes
Women who were randomized to receive care in an alternative birth setting were more likely to labour and give birth without analgesia/anesthesia (six trials, n = 8953; risk ratio (RR) 1.18, 95% confidence interval (CI) 1.05 to 1.33) (Analysis 1.1), and more likely to have a spontaneous vaginal birth (eight trials, n = 11,202; RR 1.03, 95% CI 1.01 to 1.05) (Analysis 1.6). Allocation to care in an alternative setting had no apparent effect on serious maternal morbidity/mortality (four trials, n = 6334; RR 1.11, 95% CI 0.23 to 5.36) (Analysis 1.13). In oxytocin augmentation of labour (Analysis 1.2), the I² was 61%, τ² was 0.02, and the P value for the Chi² test of heterogeneity was 0.01. The majority of the observations came from very large trials, and visual inspection of the forest plots did not suggest inconsistency in the direction of effects. A sensitivity analysis, in which we removed the methodologically weakest trial (Klein 1984), had no effect on heterogeneity. We have therefore reported the results of random‐effects analyses. Women allocated to an alternative birth setting were less likely to have oxytocin augmentation of labour (eight trials, n = 11,131; RR 0.77, 95% CI 0.67 to 0.88) (Analysis 1.2).
1.1. Analysis.
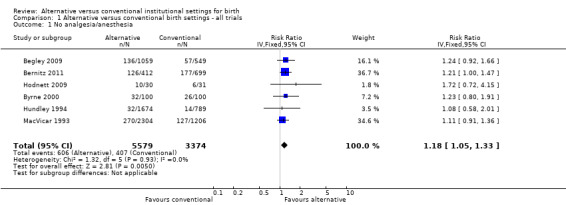
Comparison 1 Alternative versus conventional birth settings ‐ all trials, Outcome 1 No analgesia/anesthesia.
1.6. Analysis.
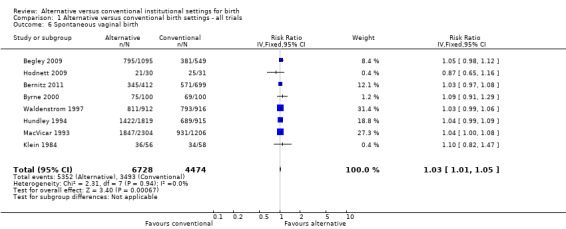
Comparison 1 Alternative versus conventional birth settings ‐ all trials, Outcome 6 Spontaneous vaginal birth.
1.13. Analysis.
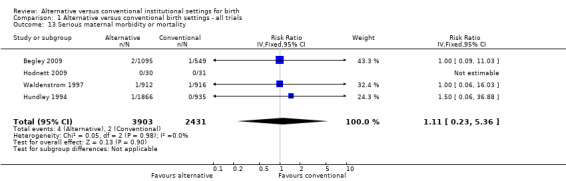
Comparison 1 Alternative versus conventional birth settings ‐ all trials, Outcome 13 Serious maternal morbidity or mortality.
1.2. Analysis.
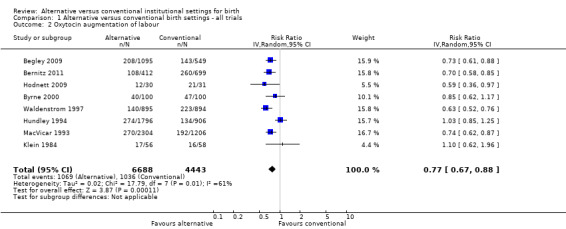
Comparison 1 Alternative versus conventional birth settings ‐ all trials, Outcome 2 Oxytocin augmentation of labour.
Three trials which measured women's views of their care had at least 80% follow‐up (Hodnett 2009; Hundley 1994; Waldenstrom 1997), as noted above under Description of studies; none of the questions in one trial (Hundley 1994) were conceptually similar to those used in the other two. Thus the meta‐analysis results include only two trials (Hodnett 2009; Waldenstrom 1997), and the measure used was whether the woman would prefer the same setting for a subsequent birth, (two trials, n = 1207; RR 1.96, 95% CI 1.78 to 2.15) (Analysis 1.15). Other measures of satisfaction with the childbirth experience reported in the trials included involvement in the process of birth, freedom to express feelings, support from midwives, and indicators of involvement in decision‐making; all results either favoured those allocated to an alternative birth setting or suggested no differences.
1.15. Analysis.
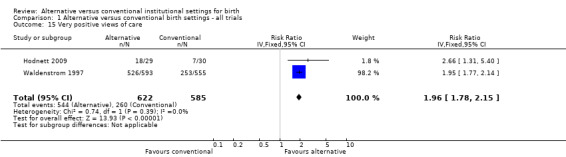
Comparison 1 Alternative versus conventional birth settings ‐ all trials, Outcome 15 Very positive views of care.
Only five trials reported both perinatal mortality and serious perinatal morbidity (Begley 2009; Hodnett 2009; Hundley 1994; Klein 1984; Waldenstrom 1997). We noted evidence of substantial heterogeneity: the τ² was 0.35, I² was 66%, and the P value for the Chi² test for heterogeneity was 0.05. We did not perform a sensitivity analysis, since the only trial which was methodologically weak (Klein 1984) contributed no adverse outcomes to the meta‐analysis. Visual inspection of the forest plot showed that one large trial (Waldenstrom 1997) had effects in opposite directions to the other trials. Close examination of the trial reports failed to identify a reason why their results should not be combined. Comparison of results using a fixed‐effect and random‐effects model indicated results were comparable. We report the results of the random‐effects model (five trials, n = 6385; RR 1.17, 95% CI 0.51 to 2.67) (Analysis 1.11).
1.11. Analysis.
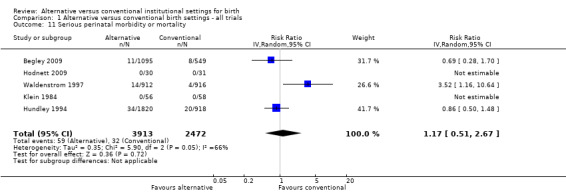
Comparison 1 Alternative versus conventional birth settings ‐ all trials, Outcome 11 Serious perinatal morbidity or mortality.
Secondary outcomes
Women allocated to alternative settings were less likely to have epidural analgesia (eight trials, n = 10,931; RR 0.80, 95% CI 0.74 to 0.87) (Analysis 1.3), instrumental vaginal birth (eight trials, n = 11,202; RR 0.89, 95% CI 0.79 to 0.99) (Analysis 1.4) and episiotomy (eight trials, n = 11,055; RR 0.83, 95% CI 0.77 to 0.90) (Analysis 1.7). For caesarean birth (nine trials, n = 11,350), the RR was 0.88, 95% CI 0.78 to 1.00 (Analysis 1.5). Allocation to an alternative setting had no apparent effect on postpartum hemorrhage (six trials, n = 10,712; RR 0.94, 95% CI 0.82 to 1.08) (Analysis 1.8).
1.3. Analysis.
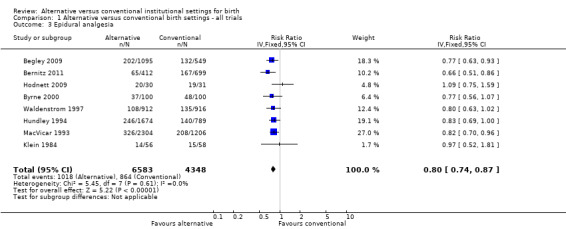
Comparison 1 Alternative versus conventional birth settings ‐ all trials, Outcome 3 Epidural analgesia.
1.4. Analysis.
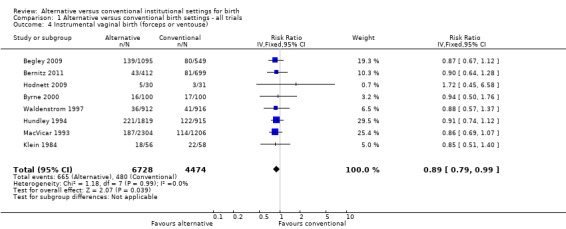
Comparison 1 Alternative versus conventional birth settings ‐ all trials, Outcome 4 Instrumental vaginal birth (forceps or ventouse).
1.7. Analysis.
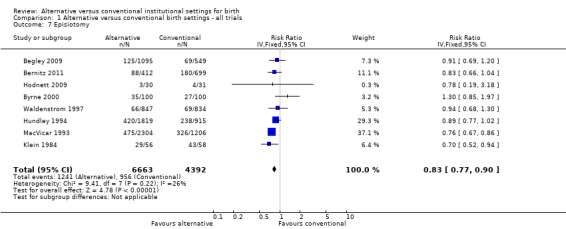
Comparison 1 Alternative versus conventional birth settings ‐ all trials, Outcome 7 Episiotomy.
1.5. Analysis.
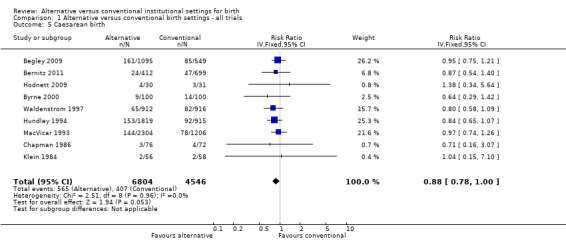
Comparison 1 Alternative versus conventional birth settings ‐ all trials, Outcome 5 Caesarean birth.
1.8. Analysis.
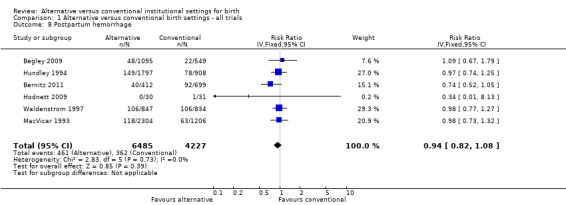
Comparison 1 Alternative versus conventional birth settings ‐ all trials, Outcome 8 Postpartum hemorrhage.
Allocation to alternative birth settings had no apparent effect on babies' five‐minute Apgar scores less than seven (seven trials, n = 7665; RR 0.98, 95% CI 0.70 to 1.38) (Analysis 1.9); admission to a neonatal intensive care unit (seven trials, n = 10,978; RR 1.09, 95% CI 0.94 to 1.26) (Analysis 1.10); or perinatal deaths (eight trials, n = 11,206; RR 1.67, 95% CI 0.93 to 3.00) (Analysis 1.12).
1.9. Analysis.
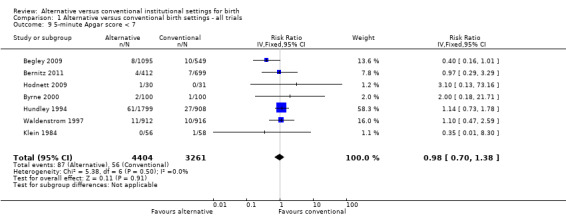
Comparison 1 Alternative versus conventional birth settings ‐ all trials, Outcome 9 5‐minute Apgar score < 7.
1.10. Analysis.
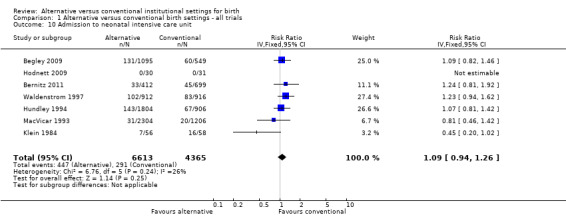
Comparison 1 Alternative versus conventional birth settings ‐ all trials, Outcome 10 Admission to neonatal intensive care unit.
1.12. Analysis.
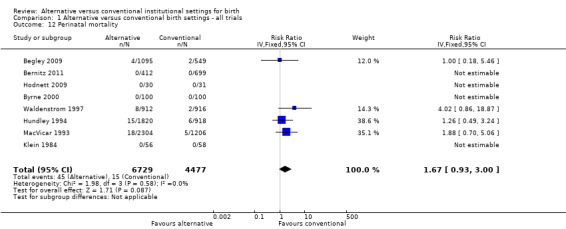
Comparison 1 Alternative versus conventional birth settings ‐ all trials, Outcome 12 Perinatal mortality.
The results of one trial indicate that babies of women allocated to alternative settings were more likely to be breastfed at six to eight weeks (one trial; n = 1147; RR 1.04, 95% CI 1.02 to 1.06) (Analysis 1.14).
1.14. Analysis.
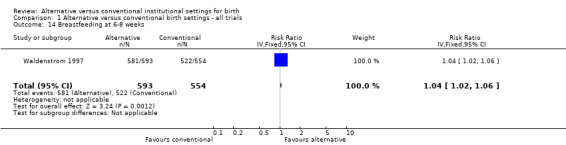
Comparison 1 Alternative versus conventional birth settings ‐ all trials, Outcome 14 Breastfeeding at 6‐8 weeks.
Subgroup analyses: care by the same versus separate staff
All but one trial (Bernitz 2011), in which the staff of the alternative setting were separate from the staff of the conventional setting, also involved more continuity of caregiver in the alternative setting. We found no trials of freestanding birth centre care compared with conventional institutional settings, no trials of Snoezelen rooms, only one small (n = 62) trial (Hodnett 2009) of an ambient room, and no trials of other architectural designs for labour rooms. Therefore, the subgroup analyses were confined to comparisons of trials in which either the same or separate midwifery/nursing staff provided care in the two settings.
In the four outcomes of interest in the subgroup analysis, there were no apparent effects, based on whether the same or separate staff cared for women in the two settings, on the likelihood of spontaneous vaginal birth (Analysis 2.1), Chi² test for subgroup differences 0.00, P = 0.94), serious perinatal morbidity/mortality (Analysis 2.2), Chi² test for subgroup differences 0.92, P = 0.34), or serious maternal morbidity/mortality (Analysis 2.3), Chi² test for subgroup differences 0.05, P = 0.83). It was not possible to draw conclusions in regard to women's views of their care, since the comparison involved one small trial (n = 62) in which the staff were the same (Hodnett 2009) with a large trial (n = 1927) in which there was separate staffing of the two units (Waldenstrom 1997).
2.1. Analysis.
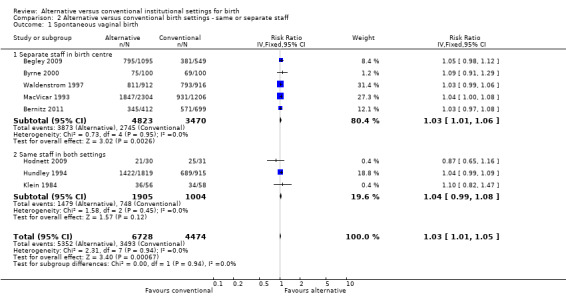
Comparison 2 Alternative versus conventional birth settings ‐ same or separate staff, Outcome 1 Spontaneous vaginal birth.
2.2. Analysis.
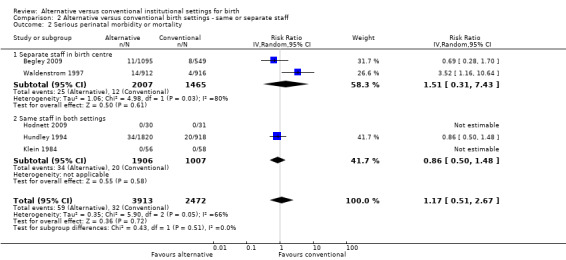
Comparison 2 Alternative versus conventional birth settings ‐ same or separate staff, Outcome 2 Serious perinatal morbidity or mortality.
2.3. Analysis.
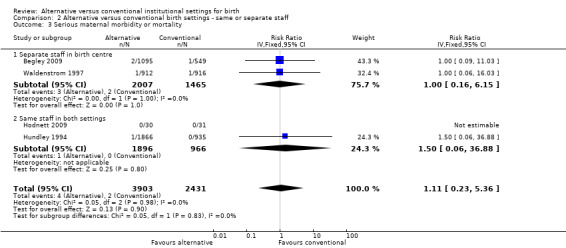
Comparison 2 Alternative versus conventional birth settings ‐ same or separate staff, Outcome 3 Serious maternal morbidity or mortality.
Discussion
Summary of main results
The benefits of alternative institutional settings for birth include increased likelihood of spontaneous vaginal birth, labour and birth without analgesia/anesthesia, breastfeeding at six to eight weeks postpartum, satisfaction with care, and decreased likelihood of oxytocin augmentation, assisted vaginal birth, caesarean birth, and episiotomy. The results are consistent with a growing body of research which has demonstrated the independent effects of physical attributes of the hospital room on caregivers’ behaviour and patients’ health outcomes, including postsurgical complications and length of stay (Ulrich 2004). However, in several of the trials included in this review, the design features of the alternative setting were confounded by important differences in the organizational models for care (separate staff for the alternative setting, offering more continuity of caregiver), and thus it is difficult to draw inferences about the independent effects of the physical birth environment. Furthermore, the effects of an alternative environment may be overpowered by routine institutional policies and practices (Fannin 2003).
There were only three trials in which losses to follow‐up on questionnaires were low enough to permit inclusion of the data in this review (Hodnett 2009; Hundley 1994; Waldenstrom 1997), but similar results are reported in the other trials that measured satisfaction with care. Different measures were used in the trials, but the results pertaining to women's ratings of their birth experiences consistently favoured the group allocated to the alternative setting. Given the generally high rates of transfer from alternative settings to the conventional ward for intrapartum care, which presumably would create disappointment, these results strongly suggest higher levels of satisfaction in those allocated to alternative birth settings.
Overall completeness and applicability of evidence
Although more than 11,000 women have participated in randomized trials of alternative birth settings, the low number of women allocated to alternative settings who actually gave birth in their allocated setting serves to dilute both the potential benefits and risks of alternative settings. Other important factors that complicate interpretation of the results are the variations in organizational models of care in the trials, including the potential impact of antenatal care, continuity of caregiver, and midwifery‐led versus consultant‐led care.
Authors' conclusions
Implications for practice.
Pregnant women should be informed that hospital birth centres are associated with lower rates of medical interventions during labour and birth and higher levels of satisfaction, without increasing risk to themselves or their babies. Decision‐makers who wish to decrease rates of medical interventions for women experiencing normal pregnancies should consider developing birthing units with policies and practices to support normal labour and birth.
It was not possible to examine the separate influences of types of alternative hospital settings or continuity of caregiver, and there were no trials of freestanding birth centres. There were no apparent differences in effects, based on whether the same or separate staff provided care in the alternative and conventional units. Thus those who wish to develop a alternative birth setting, and those who wish to use them, have little to go on when making decisions about the autonomy of the setting or its architectural features. These issues are critically important, in light of women's reports of greater satisfaction with alternative institutional birth settings, and the lower rates of interventions associated with alternative settings.
Implications for research.
Future trials should measure and report serious perinatal morbidity as well as perinatal mortality. It would also be helpful to consider the importance of ensuring high‐quality inter‐professional working relationships, with clear protocols for consultation and transfer of care. Future trials should also address the potential confounding effects of differences in the extent of continuity of caregiver in the alternative versus conventional birth settings. It would be helpful to have full descriptions of both the alternative and usual care interventions. And trials should include evidence‐based approaches to encourage high response rates to postal questionnaires, as well as cost‐effectiveness analyses.
Given the growing awareness of the importance of the birth environment, the escalating caesarean delivery rates in many high‐income countries, and the favourable results of large observational studies of freestanding birth centres (e.g.Brocklehurst 2011), randomized trials of freestanding birth centres are warranted. Similarly, adequately‐powered trials are needed, of architectural designs which promote freedom of mobility and enhance feelings of calmness and control.
Questions have arisen about: the impact of competing philosophical, political, and administrative pressures on the operation of alternative settings (Annandale 1987); these questions require qualitative investigation. Qualitative studies, examining what happens when women are transferred from alternative to conventional birth settings, would shed light on the impact of transfer on women, care providers, and decision‐making processes regarding the need for intervention. Questions which can be answered quantitatively include: the effects of alternative settings on birth outcomes, women's preferences for traditional labour ward care compared to birth centre care, the pros and cons of freestanding versus hospital‐based birth centres, and the optimum organizational models of birth centre care. Evidence from both qualitative and quantitative sources is needed, to provide a complete picture of the nature, benefits, and risks of birth centre care.
Feedback
Fahy, January 2007
Summary
The review authors comment about a "trend towards higher rates of perinatal mortality in the alternative settings" has been reported elsewhere in support of claims that birth centres are less safe than conventional settings for labour (1). A possible explanation for any real increase in perinatal mortality could be delayed transfer from the birth centre. We examined reports of the six trials included in the review and, as in the Cochrane review, found 41 perinatal deaths amongst women allocated birth centres. Only six of these deaths, however, were of normally formed babies who reached term. It is only these babies who were eligible to be born in a birth centre. Three of these six deaths were of women who had been allocated birth centre care but actually received standard labour care.
This raises questions about the validity of the underlying randomised trials. These studies have an experimental design where researcher control should ensure that people receive the specific treatment that was planned for them (2). The Cochrane Handbook gives no guidance on how to evaluate either the quality of the researchers' definition of the planned treatments, or the agreement between what treatment was actually provided and what the researcher planned (3). For the majority of the trials in this review the treatments are not adequately defined. Nor did they adequately control the treatments actually provided to each allocated group. It is not clear how any birth centre trial can sensibly be considered to have been scientifically controlled. The reviewers attempted to deal with this crucial point by claiming that they were only looking at the effect of the 'setting', but their objectives clearly state that they were examining the effect of "care within a setting".
In conclusion, this review is scientifically weak because of the weaknesses of the underlying trials.
References 1.Pesce A. (2005). Media Transcript: Discussion of Midwife‐led birthing units ‐ AMA's Dr Andrew Pesce on the Today Show. Retrieved July 9th, 2006, from http://www.ama.com.au/web.nsf/doc/WEEN‐6FS3EU 2.Borelli B, Sepinwall D, Ernst D, Bellg A, Czajkowski S, Berger R. A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research. Journal of Consulting and Clinical Psychology 2005 73:852‐860. 3. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 4.2.5. 2005. Retrieved 10th July, 2006, from http://www.cochrane.org/resources/handbook/
(Summary of feedback from Kathleen Fahy and Sally Tracy, January 2007)
Reply
We acknowledge in our Review that place of birth is a complex amalgamation of setting and care (and, indeed, philosophies of care) within that setting. We did not, and never intended to, distinguish 'setting' from 'care within the setting'. On the contrary, the two are indistinguishable, involving not only architectural differences but also different policies and procedures (and frequently, different care providers), compared to a conventional hospital labour ward.
We agree that one reason for excess perinatal mortality (if indeed it is a "true" excess) could be delayed transfer. But there may be other factors, such as those we raise in our Discussion. Systematic reviews, and randomized trials, report what happened but cannot tell one why it happened. The fact that some of the babies who died were antenatal transfers and not at term does not invalidate the Review, as it included the outcomes of women and babies from the point of trial entry, which, in some of the included studies, was early in pregnancy. Intention‐to‐treat captures all the outcomes consequent on the initial place of booking, as those are the outcomes that are likely to pertain for women in 'real life' who make similar booking decisions.
We point out in the Discussion that the high rates of transfer out of alternative settings serve to dilute both the potential benefits and risks. The issue here is not one of validity, but of the precision of the results. It would be unethical to keep women in their allocated form of care, regardless of subsequent changes in risk factors or preferences. The important question for women and providers is whether choosing a alternative setting is likely to be better or worse than choosing a conventional hospital setting. Making this judgment will include an assessment of the rates of transfer between settings, and of fetal and infant wellbeing at various stages of gestation. The package of care provided in alternative settings needs to be examined in conjunction with that delivered in the referral unit(s), since this is what women are potentially signing up for when they make their booking decision.
In our view, none of the points raised threaten the internal validity of the Review, but they do illustrate the turmoil that can arise when results are used to support the arguments of one faction or another without regard to the full context of a study, especially in a highly contested area like place of birth. We acknowledge the difficulties faced by both those who want to maximize choice for childbearing women, and those who are concerned about safety issues. We hope that a close reading of our introduction, methods, and discussion will reveal that we share some of the concerns of both groups of protagonists, and we hope that the design of future research in this area can benefit from on‐going debates like the one we are addressing here.
(Response from Ellen Hodnett and Soo Downe, May 2007)
Contributors
Kathleen Fahy and Sally Tracy
What's new
| Date | Event | Description |
|---|---|---|
| 16 May 2012 | New search has been performed | Search updated on 30 March 2012. One new trial (Bernitz 2011) included and two trials (Chambliss 1992; Law 1999) excluded. Minor edits to text and tables. |
| 16 May 2012 | New citation required but conclusions have not changed | Minor changes to Results which did not alter Conclusions. |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 3, 1996
| Date | Event | Description |
|---|---|---|
| 13 July 2010 | New citation required and conclusions have changed | This update has expanded the focus of the review to a variety of types of alternative institutional birth settings. The title has been changed to reflect the expansion of the focus. |
| 13 July 2010 | New search has been performed | Search updated. Three new trials identified and included (Abdullahi 1990; Begley 2009; Hodnett 2009). Revision to every aspect of the Review, to expand the focus to incorporate new types of alternative birth settings, to bring it up‐to‐date in terms of current methodological guidelines, and to incorporate the three new trials. |
| 12 May 2008 | Amended | Converted to new review format. |
| 12 November 2004 | New search has been performed | New search conducted in May 2004. We did not identify additional studies. Revisions to entire review, including background, objectives, methods, results, discussion, implications, and tables have been made. |
Acknowledgements
We thank Angela Cooke for the translation of the Danish trial (Abdullahi 1990), Declane Devane and Stine Bernitz for providing additional information about the Irish trial (Begley 2009) and Norwegian trial (Bernitz 2011) respectively, and Nadine Edwards and Julie Weston for their co‐authorship of previous versions of the review.
Data and analyses
Comparison 1. Alternative versus conventional birth settings ‐ all trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No analgesia/anesthesia | 6 | 8953 | Risk Ratio (IV, Fixed, 95% CI) | 1.18 [1.05, 1.33] |
| 2 Oxytocin augmentation of labour | 8 | 11131 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.67, 0.88] |
| 3 Epidural analgesia | 8 | 10931 | Risk Ratio (IV, Fixed, 95% CI) | 0.80 [0.74, 0.87] |
| 4 Instrumental vaginal birth (forceps or ventouse) | 8 | 11202 | Risk Ratio (IV, Fixed, 95% CI) | 0.89 [0.79, 0.99] |
| 5 Caesarean birth | 9 | 11350 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.78, 1.00] |
| 6 Spontaneous vaginal birth | 8 | 11202 | Risk Ratio (IV, Fixed, 95% CI) | 1.03 [1.01, 1.05] |
| 7 Episiotomy | 8 | 11055 | Risk Ratio (IV, Fixed, 95% CI) | 0.83 [0.77, 0.90] |
| 8 Postpartum hemorrhage | 6 | 10712 | Risk Ratio (IV, Fixed, 95% CI) | 0.94 [0.82, 1.08] |
| 9 5‐minute Apgar score < 7 | 7 | 7665 | Risk Ratio (IV, Fixed, 95% CI) | 0.98 [0.70, 1.38] |
| 10 Admission to neonatal intensive care unit | 7 | 10978 | Risk Ratio (IV, Fixed, 95% CI) | 1.09 [0.94, 1.26] |
| 11 Serious perinatal morbidity or mortality | 5 | 6385 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.51, 2.67] |
| 12 Perinatal mortality | 8 | 11206 | Risk Ratio (IV, Fixed, 95% CI) | 1.67 [0.93, 3.00] |
| 13 Serious maternal morbidity or mortality | 4 | 6334 | Risk Ratio (IV, Fixed, 95% CI) | 1.11 [0.23, 5.36] |
| 14 Breastfeeding at 6‐8 weeks | 1 | 1147 | Risk Ratio (IV, Fixed, 95% CI) | 1.04 [1.02, 1.06] |
| 15 Very positive views of care | 2 | 1207 | Risk Ratio (IV, Fixed, 95% CI) | 1.96 [1.78, 2.15] |
Comparison 2. Alternative versus conventional birth settings ‐ same or separate staff.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous vaginal birth | 8 | 11202 | Risk Ratio (IV, Fixed, 95% CI) | 1.03 [1.01, 1.05] |
| 1.1 Separate staff in birth centre | 5 | 8293 | Risk Ratio (IV, Fixed, 95% CI) | 1.03 [1.01, 1.06] |
| 1.2 Same staff in both settings | 3 | 2909 | Risk Ratio (IV, Fixed, 95% CI) | 1.04 [0.99, 1.08] |
| 2 Serious perinatal morbidity or mortality | 5 | 6385 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.51, 2.67] |
| 2.1 Separate staff in birth centre | 2 | 3472 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.31, 7.43] |
| 2.2 Same staff in both settings | 3 | 2913 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.50, 1.48] |
| 3 Serious maternal morbidity or mortality | 4 | 6334 | Risk Ratio (IV, Fixed, 95% CI) | 1.11 [0.23, 5.36] |
| 3.1 Separate staff in birth centre | 2 | 3472 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.16, 6.15] |
| 3.2 Same staff in both settings | 2 | 2862 | Risk Ratio (IV, Fixed, 95% CI) | 1.50 [0.06, 36.88] |
| 4 Very positive views of intrapartum care | 2 | 1207 | Risk Ratio (IV, Fixed, 95% CI) | 1.96 [1.78, 2.15] |
| 4.1 Separate staff in birth centre | 1 | 1148 | Risk Ratio (IV, Fixed, 95% CI) | 1.95 [1.77, 2.14] |
| 4.2 Same staff in both settings | 1 | 59 | Risk Ratio (IV, Fixed, 95% CI) | 2.66 [1.31, 5.40] |
2.4. Analysis.
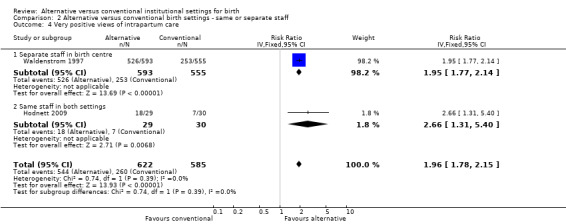
Comparison 2 Alternative versus conventional birth settings ‐ same or separate staff, Outcome 4 Very positive views of intrapartum care.
Comparison 3. Alternative versus conventional birth settings ‐ variations in continuity of caregiver.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous vaginal birth | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Greater continuity of caregiver in birth centre | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 No difference in extent of continuity of caregiver | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious perinatal morbidity or mortality | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Greater continuity of caregiver in birth centre | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 No difference in extent of continuity of caregiver | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Serious maternal morbidity or mortality | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Greater continuity of caregiver in birth centre | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 No difference in extent of continuity of caregiver | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Very positive views of intrapartum care | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Greater continuity of caregiver in birth centre | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 No difference in extent of continuity of caregiver | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Alternative versus conventional birth settings ‐ freestanding versus in‐hospital.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous vaginal birth | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Freestanding | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 In‐hospital | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious perinatal morbidity or mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Freestanding | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 In‐hospital | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Serious maternal morbidity or mortality | 0 | 0 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Very positive views of intrapartum care | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Freestanding | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 In‐hospital | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Variations in alternative settings ‐ bedroom‐like, ambient, Snoezelen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous vaginal birth | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Bedroom‐like | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Ambient | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Snoezelen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious perinatal morbidity or mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Bedroom‐like | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Ambient | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Snoezelen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Serious maternal morbidity or mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Bedroom‐like | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Ambient | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Snoezelen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Very positive views of intrapartum care | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Bedroom‐like | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Ambient | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Snoezelen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdullahi 1990.
| Methods | Randomized controlled trial; women consented at 33 weeks' gestation and were randomized when they arrived in labour. | |
| Participants | 292 nulliparous and multiparous low‐risk women at term (38‐42 weeks' gestation) (147 in experimental group and 145 in control group) at a hospital in Denmark. Exclusion criteria were: non‐cephalic position. | |
| Interventions | The experimental group was cared for in the 'green room'; an 'environmental' delivery room that included a large bed (2 m x 2 m), bath tub, curtains, plants, artwork, tape recorder and wall bar. The control group was cared for in the 'white room' which was the normal hospital labour room. Both rooms were in the same physical location and so it is assumed that the same staff cared for women in both study groups. | |
| Outcomes | Spontaneous onset of labour, vacuum delivery, caesarean delivery, episiotomy, intervention rates, 5‐minute Apgar score < 10, umbilical cord pH. | |
| Notes | No information available at this time as to how many women remained in the 'green room' for delivery. Attempt to contact author met with no response. No data from the trial were usable (see 'Risk of bias' Table). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'randomization‐grouping' with no further details. Not stratified by parity. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals from data collection were noted. However, all outcomes were not reported for all cases. |
| Selective reporting (reporting bias) | High risk | Results are reported only for subgroups, e.g. those whose labour onset was spontaneous, or primiparous women. The risk of reporting bias made all data unusable. |
| Other bias | Low risk | No other potential sources of bias were noted. |
Begley 2009.
| Methods | Randomized controlled trial. Eligibile and consenting women were randomized at the time of booking for antenatal care. | |
| Participants | 1653 nulliparous and multiparous women (randomized in a 2:1 ratio ‐‐ 1102 in the midwifery‐led care group and 551 to consultant‐led care), booked for delivery at 2 hospitals in Ireland. Participants were < 24 weeks' gestation, judged to be low in obstetrical risk, aged 16‐40 years, with singleton pregnancies. | |
| Interventions | Midwifery‐led care: shared antenatal care between midwives and family doctors. Intrapartum care by midwives, who (whenever possible) remained with women who were transferred to consultant‐led care in the standard labour ward. Antenatal and intrapartum care were provided in a refurbished unit with a separate entrance, adjacent to the conventional labour ward. The unit contained two birthing rooms with home‐like decor and a birthing pool. Medical equipment was concealed from view. Consultant‐led care: the organization and delivery of care, from initial booking through the postnatal period, was led by a consultant‐obstetrician, within either a public or private system of maternity care. General practitioners may also have been involved in antenatal care. Intrapartum care was provided by midwives, but consultants may have been present for the birth. No details about the conventional hospital labour wards were provided. |
|
| Outcomes | 10 primary outcomes: induction of labour, continuous electronic fetal monitoring, augmentation of labour, episiotomy, caesarean birth, instrumental birth, postpartum hemorrhage, Apgar score < 7, initiation of breastfeeding; and umbilical cord pH. An extensive list of secondary outcomes (n > 60) included serious maternal and perinatal morbidity and mortality, healthcare costs, and medical interventions during pregnancy, childbirth, and the postnatal period. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized random number generator. |
| Allocation concealment (selection bias) | Low risk | Central telephone randomization service. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Neither women nor care providers could be blinded. Data collectors were unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Medical record data were available for all but 9 of the1653 who were randomized. Usable postpartum questionnaire data was only obtained for 22% of those originally randomized. |
| Selective reporting (reporting bias) | High risk | Pre‐specified neonatal outcomes included hypoxic ischemic encephalopathy, neonatal seizures, meconium aspiration, serious neonatal trauma but no outcome data were provided. Results were listed for all study outcomes. |
| Other bias | Low risk | No other potential sources of bias were noted. |
Bernitz 2011.
| Methods | Randomized controlled trial, stratified by parity (nullipara or not). Participants were randomly assigned to one of 3 in‐hospital units for labour and birth: MU, NU, or SU. | |
| Participants | 1111 women, admitted for delivery at a hospital in Norway. Eligibility criteria included: low risk, spontaneous labour, at term, single fetus in cephalic presentation. Consent was sought at 18‐20 weeks gestation and confirmed after eligibility was confirmed at the time of admission for labour. | |
| Interventions | Each unit has its own separate staff. The MU was geared to care for women expecting minimal intervention during labour and birth. Midwives managed the unit, and neither epidural analgesia nor oxytocin in first or second stage labour were available. If interventions or a higher level of care were needed, women were transferred to the NU or SU. The NU was also geared to care for women expecting normal births, but had access to epidural analgesia and operative vaginal delivery, inductions, and oxytocin augmentation. The SU was organized for women in need of special surveillance prior to, during, and after birth. Midwives were responsible for all normal births in all 3 units. | |
| Outcomes | Mode of delivery, dystocia, oxytocin augmentation, pharmacologic analgesia, acupuncture, postpartum hemorrhage, episiotomy and lacerations, 5‐minute Apgar Score < 7, NICU care, intrapartum transfer. | |
| Notes | After consultation with the trial authors, data from women allocated to the NU and SU were combined and compared with data from women allocated to the MU. 117/412 (28.4%) allocated to the MU were transferred intrapartum to a higher level of care. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Digital randomization database was developed by the clinical research unit of a University hospital. Randomization was pre‐specified to allocated 37.5%, 37.5% and 25.0% to the NU, MU and SU, respectively. |
| Allocation concealment (selection bias) | Low risk | Midwife who administered the randomization entered the woman's name and checked for eligibility before receiving the randomization number and allocation from the database. "Allocation was concealed." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Women and providers could not be blinded to group allocation. All data were entered by the midwife in charge of the electronic records for the department. A midwife at each unit monitored the entries. All participants' data were checked by a midwife not working on any of the 3 units. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were reported for all trial participants. Two demographic variables ‐ education and social status ‐ were unknown for < 2% of those enrolled. |
| Selective reporting (reporting bias) | Low risk | Outcomes were pre‐specified and all were reported. |
| Other bias | Low risk | No other risks of bias were noted. |
Byrne 2000.
| Methods | Randomized controlled trial; women who consented were randomized between 20‐36 weeks' gestation. | |
| Participants | 201 nulliparous and multiparous women booked for delivery at a hospital in Adelaide, Australia (100 in experimental group and 101 in control group). All were experiencing normal, uncomplicated pregnancies. | |
| Interventions | Those allocated to birth centre care had antenatal, intrapartum, and up to 12 hours of intrapartum care from a staff of midwives who were "committed to the normality of the birth process". Intrapartum care may have been by midwives who were not known to the women. The women were also encouraged to attend 2 classes about the birthing centre. The birthing centre consisted of 2 bedroom‐like rooms adjacent to the delivery suite, staffed by midwives. The control group received usual care antenatal care and their intrapartum care was in the conventional delivery suite; they were under the care of the staff there which included both a midwife and doctor. | |
| Outcomes | Maternal satisfaction, intervention rates, method of infant feeding at 6 weeks postpartum, and costs. | |
| Notes | Experimental group: 13 allocated to birth centre care did not receive it because of staffing problems, and 64 were transferred to delivery suite care for medical reasons. Control: 1 woman was lost to follow‐up, and 1 transferred to birthing centre at her request. The author has been contacted for additional information about perinatal morbidity, but no reply has been received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization scheme in balanced, variable‐sized blocks prepared by off‐site clerical officer not otherwise involved in the study. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes were opened by the off‐site clerical officer. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Both baseline and outcome medical record data were collected by the researcher, and the researcher telephoned the clerical officer to obtain the participant's allocation. Participants placed their questionnaires in sealed envelopes, which were collected by staff and delivered to the researcher. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant was lost to follow‐up when she moved and delivered at another hospital. Postpartum questionnaire data were obtained from < 80% of the participants and thus not used. |
| Selective reporting (reporting bias) | Low risk | Results were listed for all study outcomes. |
| Other bias | Low risk | No other potential sources of bias were noted. |
Chapman 1986.
| Methods | Randomized controlled trial; women enrolled at or before 30 weeks' gestation. | |
| Participants | 148 multiparous women booked for delivery at London, UK hospital were enrolled (76 in experimental group and 72 in control group). All were multiparous, had normal pregnancies and deliveries with previous babies, had asked for early discharge and lived within 5 miles of the hospital. | |
| Interventions | All participants had routine antenatal care. During labour and birth the experimental group was cared for in an alternative birth room close to the labour ward. The control group was admitted to the labour ward. The same group of community midwives cared for both the experimental and control groups during labour. | |
| Outcomes | Reason for withdrawal, perineal trauma, meconium staining, forceps delivery, caesarean delivery, breastfeeding, effect on relationship with baby, preferred birth setting for future pregnancies. | |
| Notes | 22 (29%) in the experimental group were withdrawn, 11 before labour. 13 (18%) were withdrawn in the standard care group, 10 in the antenatal period. Only caesarean birth was reported for the complete sample; thus caesarean birth was the only usable outcome in this Review. No additional information was available when the author was contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | "Random envelope selection." No mention if opaque or consecutively‐numbered or centrally controlled. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only mode of delivery data were usable as it was reported for the complete sample. The large numbers of missing data for those originally randomized made all other data unusable. |
| Selective reporting (reporting bias) | High risk | Questionnaires were only mailed to those "remaining in the study" (61%). |
| Other bias | Low risk | No other evidence of bias. |
Hodnett 2009.
| Methods | Randomized controlled trial; women who consented were randomized on arrival at the hospital before admission to labour and delivery. | |
| Participants | 62 nulliparous and multiparous women were enrolled (31 in the experimental group and 31 in the control group) at 2 hospitals in Toronto, Canada. Inclusion criteria were: spontaneous onset of labour; about to be admitted to a labour room; singleton vertex fetus; no contraindications to vaginal birth. Exclusion criteria were: medical indications (such as complications or need for intravenous infusion) or preferences (such as desire for immediate epidural) that would limit mobility. | |
| Interventions | Experimental: an ambient room which contained a double bed size mattress in the corner of the room (in place of the hospital bed); multiple pillows; dimmed lighting; projection of beaches and waterfalls on the wall; a closed door with a 'do not disturb' sign; iPods with a variety of music options; and, equipment to promote upright positioning (chair, poster). In the ambient room ascultation was used to monitor the fetal heart, medical equipment was hidden but within easy reach and there was limited use of technologies unless medically indicated. The hospital bed was returned to the room at the request of the patient or physician. Control: the usual labour room which included the labour bed as the main focus; a lounge chair; routine continuous fetal heart rate monitoring; visible medical equipment; bright overhead lighting and normal hospital noises.The same staff cared for women in both study groups. | |
| Outcomes | Participant and staff evaluation of room; use of hospital bed; time hospital bed was in the room; use of ambient equipment; mode of delivery; rate of interventions; duration of labour events; Apgar score. | |
| Notes | This was a pilot trial to assess the feasibility and acceptability of the ambient room for women and caregivers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Centralized touch tone phone randomization service. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Research assistant was blinded to group allocation when collecting outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman withdrew consent for medical record data to be collected. An in‐hospital questionnaire was completed by 29/31 women in the experimental group and 30/31 in the control group. |
| Selective reporting (reporting bias) | Low risk | Results were listed for all study outcomes. |
| Other bias | Low risk | No other potential sources of bias were noted. |
Hundley 1994.
| Methods | Randomized controlled trial, women were enrolled early in pregnancy at first booking appointment. | |
| Participants | 2844 nulliparous and multiparous women (1900 in experimental group and 944 in control group) who were low‐risk at booking at a hospital in Aberdeen, Scotland. Exclusion criteria were: age > 35, height < 150 cm, pre‐existing maternal disease, history of infertility or prior obstetric complications, multiple pregnancy. | |
| Interventions | Experimental: antenatal care and delivery in a midwife‐managed, alternative unit 20 yards from the hospital's delivery suite. Strict protocols were in place for booking, admission, and transfer of women. Labour was managed with minimal intervention and fetal monitoring by intermittent auscultation. Control: care in the consultant‐led delivery suite. The midwives' unit was staffed by hospital midwives who also worked in the delivery suite. | |
| Outcomes | Number transferred from care in the midwives' unit and reason for transfer, type of fetal heart rate monitoring, fetal distress, meconium staining in labour, shoulder dystocia, undiagnosed malpresentation, induction of labour, augmentation of labour, delay in 1st and 2nd stage labour, intrapartum analgesia, mobility in labour, perineal trauma, mode of delivery, neonatal resuscitation, neonatal intensive care unit admission, stillbirths and neonatal deaths, satisfaction with care, costs. | |
| Notes | 54% of those allocated to the experimental group were not delivered in the midwives' unit. 1.5% were lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated as 'simple unstratified'. 2:1 randomization scheme favouring the experimental group. |
| Allocation concealment (selection bias) | Low risk | Consecutively‐numbered, sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated if data collectors were blinded to allocation group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data collected on 96% of those randomized. Questionnaires completed by 87% of those randomized. |
| Selective reporting (reporting bias) | Low risk | Results were listed for all study outcomes. |
| Other bias | Low risk | No other potential sources of bias were noted. |
Klein 1984.
| Methods | A quasi‐random study (see below), women were enrolled in labour on arrival at hospital. | |
| Participants | 114 nulliparous and multiparous women (56 allocated to the birth room and 58 to conventional care), at low risk for obstetric complications. | |
| Interventions | Intrapartum care in a alternative birth room was compared to standard care in an adjacent labour ward in a tertiary care hospital in Montreal, Canada. The same medical and nursing staff provided care in both settings. | |
| Outcomes | Oxytocin augmentation of labour, epidural analgesia, forceps delivery, episiotomy, perineal trauma, caesarean delivery, 1‐minute Apgar score < 7, admission to special care nursery. | |
| Notes | In the experimental group, transfer from the birth room for labour or delivery occurred in 39 of 62 (63%) primiparas and 11 of 59 (19%) multiparas. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Strict alternation". Only 1 alternative birth room was available. When an eligible woman arrived, if the room was available, the nurse telephoned the trial coordinator, who gave out the next allocation. |
| Allocation concealment (selection bias) | High risk | Centrally controlled but not randomized. Because of shift changes and low numbers of women enrolled, the authors felt the staff could not predict the next treatment allocation. But without randomization it was possible. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Data collection was carried out by a research assistant, who was present during active labour and thus knew study group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Medical record outcomes were reported for all those originally enrolled in the study. |
| Selective reporting (reporting bias) | High risk | In‐hospital postpartum questionnaire data were not reported. |
| Other bias | Low risk | No other evidence of bias. |
MacVicar 1993.
| Methods | Randomized controlled trial. Envelopes containing random assignment were attached to the records of pregnant women by the secretary when they arrived for their first prenatal appointment. Their eligibility for the study was assessed during the appointment and the envelope was opened for those considered eligible. The Zelen method was used (i.e. women were randomized prior to seeking consent to participate, and consent was sought only from the experimental group). Envelopes were attached to the records of 7906 women and 3510 (44%) of those were considered eligible for the study. 8% of those randomized to the experimental group refused to participate in the trial. | |
| Participants | 3510 nulliparous and multiparous women (2304 in experimental group and 1206 in control group) in Leicester, UK. Exclusion criteria: previous caesarean delivery, maternal illness such as diabetes, epilepsy, and renal disease, previous stillbirth or neonatal death, previous small for gestational age baby, multiple pregnancy, Rhesus antibodies, and elevated serum alpha‐feto protein level on 2 occasions. | |
| Interventions | Experimental: antenatal care that included routine care by the general practitioner or community midwife except for 3 scheduled visits to the clinic staffed by hospital midwives, and intrapartum care in a 3‐room, alternative unit adjacent to the delivery suite, staffed by 10 staff midwives who were not normally involved with the care of women in the delivery suite. Control: routine antenatal care and care in the delivery suite. The majority had antenatal care shared between a consultant and general practitioner or community midwife; a small number had antenatal care from the general practitioner and community midwife. | |
| Outcomes | Induction of labour, augmentation of labour, intrapartum bleeding, meconium staining, electronic fetal monitoring, fetal heart rate abnormality, delay in 1st or 2nd stage labour, intrapartum analgesia, mode of delivery, perineal trauma, postpartum hemorrhage, neonatal resuscitation, prolonged neonatal hospital stay, stillbirths, neonatal deaths, numbers of, and reasons for, transfers from the experimental form of care, woman's satisfaction. | |
| Notes | In the experimental group 45% of the women were transferred to specialist care in the delivery suite (23% during the antenatal period, 18% during first stage labour, and 4% in the second or third stage or after delivery). We contacted one of the authors for details about neonatal morbidity but none is available. The primary author is retired. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated the allocation was done by 'random sequence' and envelopes where produced by the statistician who was not involved in the enrolment process. 2:1 randomization scheme favouring the experimental group. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed, opaque envelopes containing random assignment were attached to the records of pregnant women at booking. For those considered eligible for the study the envelopes were opened. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Data collection done by research assistant blinded to the group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were obtained on 95% of those randomized. Completion rate for the 6‐week maternal questionnaire was 71% and thus not used. |
| Selective reporting (reporting bias) | Low risk | Results were listed for all study outcomes. |
| Other bias | Low risk | No other potential sources of bias were noted. |
Waldenstrom 1997.
| Methods | Randomized controlled trial; women enrolled at first visit (mean 20 weeks' gestation). | |
| Participants | 1860 nulliparous and multiparous women (928 in the experimental group and 932 controls) who were: residents of Greater Stockholm, Sweden, and did not have any disease that might complicate the birth or jeopardize the baby's health, including diabetes, multiple pregnancy, pre‐eclampsia, drug abuse, or smoking during the current pregnancy. A history of low birthweight, preterm birth, perinatal death or a difficult vaginal birth were not exclusion criteria. Women were enrolled between October 1989 and June 1993. The 1230 women who gave birth between October 1989 and January 1992 comprised the sample to assess birth satisfaction and breastfeeding. | |
| Interventions | Experimental: antenatal, intrapartum, and postnatal care in a alternative birth centre located 1 floor below the ordinary labour ward at a Stockholm hospital, with 1:1 midwife‐woman ratio during labour, and discharge within 24 hours of the birth. Control: antenatal care at neighbourhood antenatal clinics, intrapartum care in the hospital labour delivery suite (usually each midwife caring for more than 1 woman), and postnatal care for 3‐4 days in the hospital postnatal ward. Staff working in the alternative birth centre did not work in the delivery suite. | |
| Outcomes | Transfers to and reasons for standard care, intrapartum medical interventions, operative delivery, postpartum hemorrhage, 5‐minute Apgar score < 7, transfer to NICU, perinatal mortality, serious perinatal morbidity, at least 1 postnatal home visit, breastfeeding, stopped breastfeeding within 2 months, sore nipples, engorgement, milk stasis, mastitis, satisfaction with care. | |
| Notes | 34% of birth centre group were transferred to standard care either antenatally or intrapartally, and an additional 2% were transferred in the postpartum period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of the process of sequence generation. 100 envelopes prepared at a time, with a 50/50 split between groups. Envelopes were "mingled" in a box. A new batch of envelopes was added when "a few" remained. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes were used and participants picked their own from the box. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not noted whether data collectors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 were lost to follow‐up for the main study outcomes. > 90% follow‐up in both groups for the postpartum questionnaire at 2 months. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | Earlier reports were of an n of 1230, because funding ended. Subsquently, additional funding permitted additional enrolment to increase statistical power for medical outcomes. |
MU: the midwife‐led unit NICU: neonatal intensive care unit NU: the normal unit SU: the special unit
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chambliss 1992 | Compared midwifery‐led and physician‐led care within the same conventional labour ward setting. |
| Law 1999 | Compared midwifery‐led and physician‐led care within the same conventional labour ward setting. |
Differences between protocol and review
The prior update expanded the focus of the review to a variety of types of alternative institutional birth settings and included an altered title to reflect the expansion of the focus. The prior update also included revisions to align it with current methodological guidelines of the Pregnancy and Childbirth Group. This involved pre‐specifying a limited number of primary and secondary outcomes, completing 'Risk of bias' tables, and a number of other methodological improvements.
Contributions of authors
All three review authors assessed the new trial reports for eligibility.
Ellen Hodnett: all aspects of preparation of revised review, including data extraction and data entry of the new trial. Soo Downe: participated in all decisions about the revised review and performed the second data extraction for the new trial. Denis Walsh: participated in all decisions about the revised review and made revisions to the Background and Discussion.
Sources of support
Internal sources
University of Toronto, Canada.
University of Central Lancashire, UK.
External sources
No sources of support supplied
Declarations of interest
Ellen Hodnett has given talks and has written about the importance of the birth environment, and she was the Principal Investigator for one of the trials included in the review. Soo Downe and Denis Walsh have completed qualitative and quantitative reviews of birth settings.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abdullahi 1990 {published data only (unpublished sought but not used)}
- Abdullahi L, Kongsgaard E, Mogensen K, Sass L, Bock JE. The significance of the environment for delivery at a special department [Miljoets betydning for fodsler pa specialafdeling]. Ugeskrift for Laeger 1990;152:732‐4. [PubMed] [Google Scholar]
Begley 2009 {published and unpublished data}
- Begley C, Devane D, Clarke M. An evaluation of midwifery‐led care in the Health Service Executive ‐ North Eastern Area: the report of the MidU study. Report to the Health Service Executive‐North Eastern Area. November 2009:316 pp.
Bernitz 2011 {published and unpublished data}
- Bernitz S, Rolland R, Blix E, Jacobsen M, Sjoborg K, Oian P. Is the operative delivery rate in low‐risk women dependent on the level of birth care? A randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2011;118(11):1357‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Byrne 2000 {published data only (unpublished sought but not used)}
- Byrne JP, Crowther CA, Moss JR. A randomised controlled trial comparing birthing centre care with delivery suite care in Adelaide, Australia. Australian and New Zealand Journal of Obstetrics and Gynaecology 2000;40(3):268‐74. [DOI] [PubMed] [Google Scholar]
Chapman 1986 {published data only (unpublished sought but not used)}
- Chapman MG, Jones M, Spring JE, Swiet M, Chamberlain GVP. The use of a birthroom: a randomized controlled trial comparing delivery with that in the labour ward. British Journal of Obstetrics and Gynaecology 1986;93:182‐7. [DOI] [PubMed] [Google Scholar]
Hodnett 2009 {published and unpublished data}
- Hodnett ED, Stremler R, Weston JA, Mckeever P. Re‐conceptualizing the hospital labor room: the Place (Pregnant and Laboring in an Ambient Clinical Environment) pilot trial. Birth 2009;36(2):159‐66. [DOI] [PubMed] [Google Scholar]
Hundley 1994 {published and unpublished data}
- Hundley VA, Cruickshank FM, Lang GD, Glazener CMA, Milne JM, Turner M, et al. Midwife managed delivery unit: a randomised controlled comparison with consultant led care. BMJ 1994;309:1400‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundley VA, Cruickshank FM, Milne JM, Glazener CM, Lang GD, Turner M, et al. Satisfaction and continuity of care: staff views of care in a midwife‐managed delivery unit. Midwifery 1995;11(4):163‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hundley VA, Donaldson C, Lang GD, Cruickshank FM, Glazener CMA, Milne JM, et al. Costs of intrapartum care in a midwife‐managed delivery unit and a consultant‐led labour ward. Midwifery 1995;11:103‐9. [DOI] [PubMed] [Google Scholar]
- Hundley VA, Milne JM, Glazener CM, Mollison J. Satisfaction and the three C's: continuity, choice, and control. Women's views from a randomised controlled trial of midwife‐led care. British Journal of Obstetrics and Gynaecology 1997;104:1273‐80. [DOI] [PubMed] [Google Scholar]
Klein 1984 {published data only}
- Klein M, Papageorgiou A, Westreich R, Spector‐Dunsky L, Elkins V, Kramer M, et al. Care in a birth room versus a conventional setting: a controlled trial. Canadian Medical Association Journal 1984;131:1461‐6. [PMC free article] [PubMed] [Google Scholar]
- Westreich R, Spector‐Dunsky L, Klein M, Papageorgiou A, Kramer M, Gelfand M. The influence of birth setting on the father's behavior toward his partner and infant. Birth 1991;18:198‐202. [DOI] [PubMed] [Google Scholar]
MacVicar 1993 {published data only (unpublished sought but not used)}
- MacVicar J, Dobbie G, Owen‐Johnstone L, Jagger C, Hopkins M, Kennedy J. Simulated home delivery in hospital: a randomised controlled trial. British Journal of Obstetrics and Gynaecology 1993;100:316‐23. [DOI] [PubMed] [Google Scholar]
Waldenstrom 1997 {published data only}
- Gottvall K, Waldenstrom U. Does birth center care during a woman's first pregnancy have any impact on her future reproduction?. Birth 2002;29(3):177‐81. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U. Effects of birth centre care on fathers' satisfaction with care, experience of the birth, and adaptation to fatherhood. Journal of Reproductive and Infant Psychology 1999;17(4):357‐68. [Google Scholar]
- Waldenstrom U, Nilsson CA. A randomized controlled study of birth center care versus standard maternity care: effects on women's health. Birth 1997;24:17‐26. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U, Nilsson CA. Experience of childbirth in birth center care: a randomized controlled study. Acta Obstetricia et Gynecologica Scandinavica 1994;73:547‐54. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U, Nilsson CA. No effect of birth centre care on either duration or experience of breast feeding, but more complications: findings from a randomised controlled trial. Midwifery 1994;10:8‐17. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U, Nilsson CA. Women's satisfaction with birth center care: a randomized, controlled study. Birth 1993;20:3‐13. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U, Nilsson CA, Winbladh B. The Stockholm birth centre trial: maternal and infant outcome. British Journal of Obstetrics and Gynaecology 1997;104:410‐8. [DOI] [PubMed] [Google Scholar]
- Wilhelmson B. First results from a randomized study: ABC for alternative childbirth [Forsta resultat fran randomiserad studie: ABC for alternativ forlossning]. Lakartidningen 1993;90:180‐2. [PubMed] [Google Scholar]
References to studies excluded from this review
Chambliss 1992 {published data only}
- Chambliss LR, Daly C, Medearis AL, Ames M, Krayne M, Paul RT. The role of selection bias in comparing cesarean birth rates between physician and midwifery management. Obstetrics & Gynecology 1992;80(2):161‐5. [PubMed] [Google Scholar]
Law 1999 {published data only}
- Law YYH, Lam KY. A randomized controlled trial comparing midwife‐managed care and obstetrician‐managed care for women assessed to be at low risk in the initial intrapartum period. Journal of Obstetrics and Gynaecology Research 1999;25(2):107‐12. [DOI] [PubMed] [Google Scholar]
Additional references
Annandale 1987
- Annandale EC. Dimensions of patient control in a freestanding birth center. Social Science and Medicine 1987;25(11):1235‐48. [DOI] [PubMed] [Google Scholar]
Brocklehurst 2011
- Birthplace in England Collaborative Group. Perinatal and maternal outcomes by planned place of birth for healthy women with low risk pregnancies: the Birthplace in England national prospective cohort study. BMJ 2011;343:d7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews of health care: meta‐analysis in context. London: BMJ Books, 2001. [Google Scholar]
Fannin 2003
- Fannin M. Domesticating birth in the hospital: "Family‐centered" birth and the emergence of "homelike" birthing rooms. Antipode 2003;35(3):513‐35. [Google Scholar]
Hatem 2008
- Hatem M, Sandall J, Devane D, Soltani H, Gates S. Midwife‐led versus other models of care for childbearing women. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004667.pub2] [DOI] [PubMed] [Google Scholar]
Hauck 2008
- Hauck E, Rivers C, Doherty K. Women's experience of using a Snoezelen room during labour in Western Australia. Midwifery 2008;24:460‐70. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Olsen 2004
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ulrich 2004
- Ulrich R, Quan X, Zimring C, Joseph A, Choudhary R. The role of the physical environment in the hospital of the 21st century: A once‐in‐a‐lifetime opportunity. http://www.healthdesign.org/research/reports/physical_environ.php (accessed 2004).
References to other published versions of this review
Hodnett 2005
- Hodnett ED, Downe S, Edwards N, Walsh D. Home‐like versus conventional institutional settings for birth. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD000012.pub2] [DOI] [PubMed] [Google Scholar]
Hodnett 2010
- Hodnett ED, Downe S, Walsh D, Weston J. Alternative versus conventional institutional settings for birth. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD000012.pub3] [DOI] [PubMed] [Google Scholar]


