Abstract
Obesity increases fall risk, and fall-related injuries in older adults. While prior work suggests obesity influences postural stability during standing, little is known about how obesity affects walking stability. Therefore, this study compared walking stability in older adults with and without obesity. Exploratory analyses were also conducted to evaluate the associations between measures of body habitus and gait stability as well as the association between prospective stumbles and falls and gait stability. A total of 34 older adults (17 with obesity, 17 with normal weight) walked on a treadmill at a self-selected speed. Walking stability was quantified as the local dynamic stability of the trunk in all three planes of motion. Participants also performed a series of functional tests, and were followed for a one-year period during which they reported falls and stumbles. Although participants with obesity performed significantly worse than participants without obesity on most functional tests, there were no differences in stability between groups in any direction (p=0.18 - 0.78; η2 =0.003 - 0.056), nor between those with and without a prospective fall or stumble (p = 0.18 - 0.93; η2 = 0.003-0.054). There were significant, albeit weak, correlations between BMI, waist circumference, and waist-to-height ratio and walking instability (p = 0.027 - 0.042; ρ = 0.36-0.39). Increased body mass, in absence of other obesity-related comorbidities, may have minimum impact on walking stability and in turn fall risk in older adults.
Keywords: walking, falls, body habitus, activity, function, dynamic, body mass
Introduction
More than one in four older adults fall each year (Bergen et al., 2016) and in 2017 alone, nearly 3 million reported serious fall-related injuries (The Centers for Disease Control and Prevention, 2017). Obesity in older adults increases the odds of falling and of a fall-related injury by up to 80% (Finkelstein et al., 2007). Following a fall, obesity increases the odds of disability in activities of daily living by up to 40% (Himes and Reynolds, 2012) and adults with obesity are more likely to suffer reduced quality of life following a fall (Fjeldstad et al., 2008). Given the increasing prevalence of obesity in older adults, it is critical to identify population-specific fall risk factors in order to develop targeted interventions and minimize the sequelae of falls.
Postural instability, a predictor of falls (Pajala et al., 2008; Piirtola and Era, 2006) may, in part, explain the relationship between obesity and fall risk. Indeed, older women with obesity demonstrate increased postural sway (instability) in comparison to those with healthy body mass index (BMI) (Dutil et al., 2013) and in a cohort of adult males with BMI of 17.4-63.8 kg/m2, body weight accounted for 52% of the variance in postural stability (Hue et al., 2006). Reduced postural stability with obesity reflects the deleterious effects of increased plantar surface pressure on both mechanoreceptor sensitivity (Wu and Madigan, 2014) and sensorimotor integrative processes (Lhomond et al., 2016). Accordingly, reducing plantar pressure through weight loss alone improves postural stability (Teasdale et al., 2007), and thus may reduce fall risk. Nonetheless, the majority of falls by older adults occur during gait (Robinovitch et al., 2013) and measures of postural stability do not account for the dynamic nature of walking.
One way to assess dynamic stability is to quantify the locomotor response to small “intrinsic” perturbations (i.e. neuromuscular noise) during walking (Dingwell et al., 2001). Using this approach, older adults (≥65 years) have been found to be more unstable than younger adults (Kang and Dingwell, 2008), with gait instability increasing well before the age of 65 (Terrier and Reynard, 2015). Although people with obesity display gait characteristics thought to represent compensatory strategies to manage instability (see review by Wearing et al (2006)), the extent to which obesity in older adults directly impacts gait stability is unknown. Given the impact of added weight and inertia on postural stability in healthy young subjects (Costello et al., 2012), it is logical that increased truncal fat and associated inertia can impact local dynamics stability in older adults with obesity. Evaluating the impact of obesity on gait stability is important given that gait instability, assessed using the aforementioned approach, has been associated with fall risk in older adults (Lockhart and Liu, 2008; Tajali et al., 2019; Toebes et al., 2012; van Schooten et al., 2015).
The purpose of this study was to compare walking stability between older adults with obesity (BMI ≥ 30 kg/m2) and older adults with normal weight (18.25 kg/m2 < BMI < 25 kg/m2). We hypothesized that older adults with obesity would be less stable than older adults with normal weight. We also explored the associations between measures of body habitus other than BMI (e.g., body fat distribution) and walking stability. Secondarily, we explored the association between prospective stumbles and falls and gait stability.
Methods
Participants
Participants were recruited over a three-year period from the North Chicago community in response to flyers as part of a larger study. Potential participants were screened via phone to ensure they met initial inclusion criteria: self-reported ability to walk one mile at any pace with minimum rest and a BMI = 18.5-24.9 kg/m2 or BMI ≥ 30 kg/m2. Participants were excluded for: use of an assistive device for walking; artificial joint replacement; self-reported history of diabetic peripheral neuropathy, osteoporosis or neurological conditions that interfere with gait; or BMI=25-29.9 kg/m2. Additional exclusion criteria assessed in person included: compromised range of motion in the lower limb or trunk, untreated hypertension or cardiovascular abnormalities, osteoporosis (T-score of ≤ 2.5 for the femoral neck7-8 assessed with dual energy x-ray absorbitometry (DEXA) scan), or any other pathophysiology that could compromise safety. A total of 26 people with obesity and 29 people with normal weight passed all screening and engaged in at least one aspect of data collection (Figure 1). Here, we analyzed data from 17 older adults with obesity (72.6 ± 5.4 yrs, 35.1 ± 4.2 kg/m2) who completed 10 minutes of treadmill walking and 17 age- and sex-matched older adults with normal weight (70.5 ± 4.2 yrs, 23.0 ± 1.7 kg/m2). All participants provided their written informed consent to participate in this study approved by the Rosalind Franklin University IRB.
Figure 1.
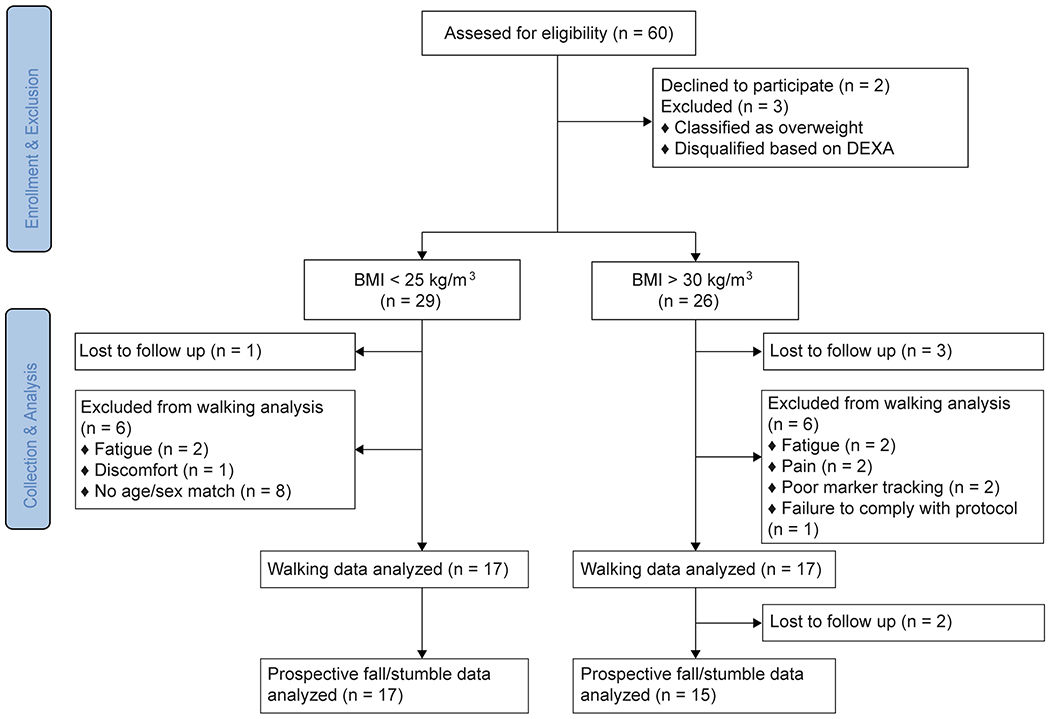
Flow diagram illustrating the flow of participants through this cross-sectional study.
Experimental Protocol
Participants first received a whole body DEXA scan to quantify percent of total, leg, and trunk fat. We also measured waist circumference (at the midpoint of the last rib and the iliac crest), hip circumference (at the level of the greater trochanters) and thigh circumference (at the left gluteal fold). Circumferences were taken twice and averaged (Table 1).
Table 1:
Participant demographics and measures of body habitus.
| Normal-weight Mean (SD) |
Obesity Class I-III Mean (SD) |
p-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 70.5 (4.2) | 72.6 (5.4) | 0.198 |
| Sex | 8M / 9F | 8M / 9F | - |
| Body Habitus | |||
| Height (cm) | 168.5 (9.1) | 169.1 (8.6) | 0.834 |
| Weight (kg) | 65.5 (1.7) | 100.5 (14.3) | <0.001* |
| BMI (kg/m2) | 23.0 (1.7) | 35.1 (4.1) | <0.001* |
| Waist Circumference (cm) | 81.7 (9.3) | 116.1 (10.7) | <0.001* |
| Hip Circumference (cm) | 94.5 (6.6) | 118.7 (8.2) | <0.001* |
| Waist-to-Hip Ratio | 0.86 (0.12) | 0.98 (0.09) | 0.004* |
| Waist-to-Height Ratio | 0.48 (0.05) | 0.68 (0.06) | <0.001* |
| Trunk Fat (%) | 21.6 (5.7) | 35.2 (4.4) | <0.001* |
| Leg Fat (%) | 31.5 (7.6) | 37.9 (5.8) | 0.017* |
| Total Fat (%) | 26.7 (6.1) | 38.4 (5.3) | <0.001* |
p < 0.05
Next, participants performed multiple functional tests. The 10-meter walk test was performed four times at maximal speed and the average of the last three trials was used in analysis. The Figure-8 walk test was performed once. The TUG was also performed once after a single practice trial. The four-square step test was performed twice after a practice trial and the fastest time was recorded. For the single leg stance test, participants stood on one limb with hands on hips for up to 30 seconds. Participants were able to choose which limb to stand on and the maximum time from 3 trials was recorded. Participants also walked several times across an 8 m walkway while the motion of passive reflective markers were tracked as part of the larger study. We quantified self-selected overground walking speed as the average speed of a marker on the T10 over all trials. We compared this to treadmill walking as previous research has suggested differences in self-selected speed overground and on a treadmill (Rosenblatt and Grabiner, 2010). Finally participants completed the Survey of Activities and Fear of Falling in the Elderly (SAFE) (Lachman et al., 1998). The scale asks participants whether they perform a series of 11 activities of daily living and, if so, their level of fear of falling during the activity (Fear of Falling subscale). A separate subscale (Activity Restriction subscale) asks participants to rate the extent to which they currently engage in each activity relative to five years ago.
Participants then walked on a treadmill for 10 minutes at a self-selected walking speed. We determined this by first increasing speed until the participant felt they could comfortably maintain the speed for 10 minutes. We then started with a faster speed and gradually decreased speed until the participant was again comfortable. The self-selected treadmill speed was the average of these two values. An eight-camera motion capture system (Vicon, Oxford Metrics, Oxford, UK) tracked the motion of a passive reflective marker placed on the 10th thoracic vertebra (T10) at 100 Hz. This location was chosen for analyses based on the importance of trunk stability in maintenance of human locomotion and on prior studies that evaluated trunk stability when considering falls (Lockhart and Liu, 2008; Tajali et al., 2019; Toebes et al., 2012; van Schooten et al., 2015). We also tracked motion of the sacrum and left and right heels. Data was collected in separate, ~30 s segments, with trials manually initiated and stopped by the researcher; 10 minutes of walking did not necessarily correspond to 20 separate trials of 30 s.
Finally, every two weeks for one year following the laboratory visit, participants were emailed a survey that asked if they fell or stumbled, and, if so, the cause (Rosenblatt et al., 2017). Of the 34 participants, 32 returned these surveys (Fig 1). We eliminated any fall or stumble that did not clearly occur during locomotion (e.g. fell while standing or sitting). This eliminated 9 falls and 44 stumbles.
Data Analysis
Kinematic data were filtered using a fifth order low-pass Butterworth filter with a cutoff frequency of 20 Hz, and then velocity was calculated using a 3-point difference formula. We identified heel strikes using a velocity detection algorithm and the position of the heel and sacral markers (Zeni et al., 2008).
We assessed local dynamic stability as the quantitative response of the system’s state variables to small perturbations (Dingwell et al., 2001). The T10 velocity was used to reconstruct a multi-dimensional state space, separately for each direction of movement, according to:
where Q(t) was the dE-dimensional state space, q(t) was the original time series, T was the time delay, and dE was the embedding dimension. For each trial we found the first minimum of the average mutual information function (Wing et al., 1986). We then averaged this across all trials for each participant to obtain their subject-specific time delay, T (van Schooten et al., 2013). dE was set to 5 for all calculations based on global false nearest neighbors analysis (Kennel et al., 1992).
Local stability analysis is sensitive to the number of cycles (i.e. strides) (Bruijn et al., 2009) and data points (Granata and England, 2006). To standardize cycles, we analyzed 288 strides for each participant. This number was chosen as all participants had at least 16 trials and a minimum of 18 strides per trial (16 x 18 = 288). This number of strides is more than sufficient to obtain a reliable measure of local dynamic stability (van Schooten et al., 2014). We also verified that stability measures in our sample converged prior to 288 strides (see Appendix). All trials of 18 strides were resampled to 2700 points (~150 data points/stride). Thus the trials were consistent in length but the inter-stride variability in timing was maintained. We then calculated the mean local divergence of nearest neighbor trajectories (divergence curves) using Rosenstein’s algorithm (Rosenstein et al., 1993). Since subjects walked at different speeds, the time axes of these curves were rescaled by dividing by the average stride time. The short-term exponents (λs*) was the slope of a line fit to the divergence curves between 0 and 0.5 strides. The λs* were averaged across all 16 trials to obtain a single value for each direction. Larger positive exponents indicate greater local instability (i.e., neighboring paths diverge more quickly).
Statistical Analyses
All measures were first checked for normality and outliers. We compared self-selected overground (OG) and treadmill (TM) speeds using a mixed model ANOVA with condition (TM/OG) and group (Obese, Normal Weight) as within- and between-subjects effects, respectively. We then used t-tests for between-group comparisons of measures of body habitus, and all other functional measures, bar single leg stance. A log-rank test was used to compare bounded s single leg stance time between groups. In each plane, we compared local stability (λs*) between groups using Mann-Whitney U tests. We secondarily ran these tests separating the cohort into those who did and did not report a prospective fall (fallers vs. non-fallers) and those that did and did not report a prospective stumble (stumblers vs. non-stumblers).
Given that BMI may not well capture body composition (Ashwell and Gibson, 2009; Rothman, 2008), we ran a series of correlations (Pearson’s or Spearman’s) to explore the association between stability and body habitus (BMI, percent fat, body proportions). All statistical analyses were performed in IBM SPSS v 24 (IBM, Armonk, NY) with a significance level of 0.05.
Results
There were significant differences between the group with obesity and with normal weight for all measures of body habitus except height (Table 1). There was a significant effect of group (p = 0.006), condition (p = 0.004) and significant group × condition interaction (p = 0.048) for self-selected speed. Regardless of condition, obese participants walked slower than the normal-weight participants. While both groups decreased their speed on the treadmill, the reduction was greater for obese older adults (Table 1). The group with obesity also walked on the treadmill with a wider step width and shorter stride length (Table 1) and performed poorer on a majority of functional tests (Table 2). Compared to the group with normal weight, the group with obesity had a significantly longer TUG (p < 0.004) and figure eight walk (p = 0.015), slower 10-m walk speeds (p = 0.014), and were unable to stand on one leg as long (p = 0.001). There was no difference in time to complete the four-square step between the groups (p = 0.078, η2 = 0.091), nor the SAFE (Fear of Falling: p = 0.11, η2 = 0.081; Activity Restriction: p= 0.338; η2 = 0.032).
Table 2:
Participant gait and functional measures, survey results, and prospective falls and stumbles.
| Normal-weight (N=17) Mean (SD) |
Obesity Class I-III (N=15) Mean (SD) |
p-value | |
|---|---|---|---|
| Gait Measures | |||
| Self-selected treadmill speed (m/s) | 1.05 (0.24) | 0.80 (0.22) | 0.004*^ |
| Self-selected overground speed (m/s) | 1.08 (0.17) | 0.98 (0.15) | 0.126^ |
| Step Width (cm) | 9.60 (3.73) | 13.55 (4.85) | 0.012* |
| Stride Length (m) | 1.10 (0.16) | 0.88 (0.23) | 0.003* |
| Functional Measures | |||
| Timed Up and Go (s) | 7.83 (0.93) | 9.05 (1.33) | 0.004* |
| 10-m Walk Test (m/s) | 1.97 (0.26) | 1.74 (0.24) | 0.014* |
| Figure-8 Walk Time (s) | 6.73 (1.07) | 7.73 (1.21) | 0.015* |
| Figure-8 Walk Steps | 12.53 (1.28) | 15.06 (2.05) | <0.001* |
| Four Square Step Test (s) | 7.93 (1.40) | 9.37 (2.90) | 0.078 |
| Single Leg Support Test (s) † | 30 [13.79, 30] | 11.86 [1.25, 30] | 0.001* |
| SAFE Survey Results | |||
| Fear of Falling † | 0.11 [0, 0.44] | 0.25 [0, 0.63] | 0.119 |
| Activity Restriction † | 0.5 [0, 7] | 1 [0, 7] | 0.338 |
| Prospective Falls and Stumbles | |||
| Number of “fallers” | 11 | 5 | 0.079^^ |
| Falls per “faller” † | 1 [1, 12] | 2 [1, 3] | 0.088 |
| Number of “stumblers” | 13 | 12 | 0.809^^ |
| Stumbles per “stumbler” † | 5 [1, 45] | 4 [1,22] | 0.241 |
p < 0.05
p-value obtained from post-hoc tests within a mixed-model ANOVA; all other p-values are obtained from t-tests
p-value obtained from Ch-squared test
Data reported as Median [Min, Max]
There were no significant differences between groups for walking stability in the mediolateral (ML) direction (p = 0.182, η2 = 0.056), anterioposterior (AP) direction (p = 0.786, η2 = 0.003), or vertical (VT) direction (p = 0.357, η2 = 0.027) (Fig 2). Only three of the correlations between measures of body habitus and walking stability (Appendix) were statistically significant: ML instability was positively correlated with BMI (ρ = 0.374; p = 0.029); and VT instability was positively correlated with waist circumference (r = 0.385; p = 0.027) and with waist-to-height ratio (ρ = 0.356; p = 0.042; Fig 3).
Figure 2.
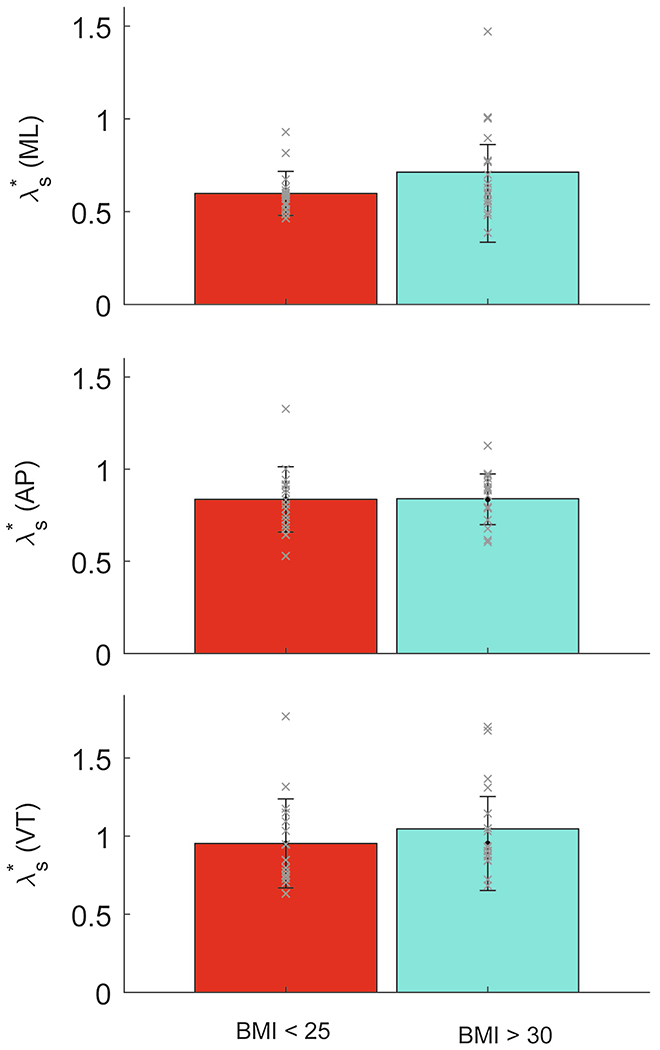
Mean values of λs* for participants without obesity (BMI: 18.5-24.9 kg/m2) and participants with obesity (BMI: ≥30 kg/m2) are shown for the medial-lateral (ML: top), anterior-posterior (AP: middle), and vertical (VT; bottom) directions. Individual data are shown as ‘x’. Error bars are one standard deviation across subjects about the mean. There were no significant differences in stability between the normal-weight and obese groups in any planes of motion.
Figure 3.
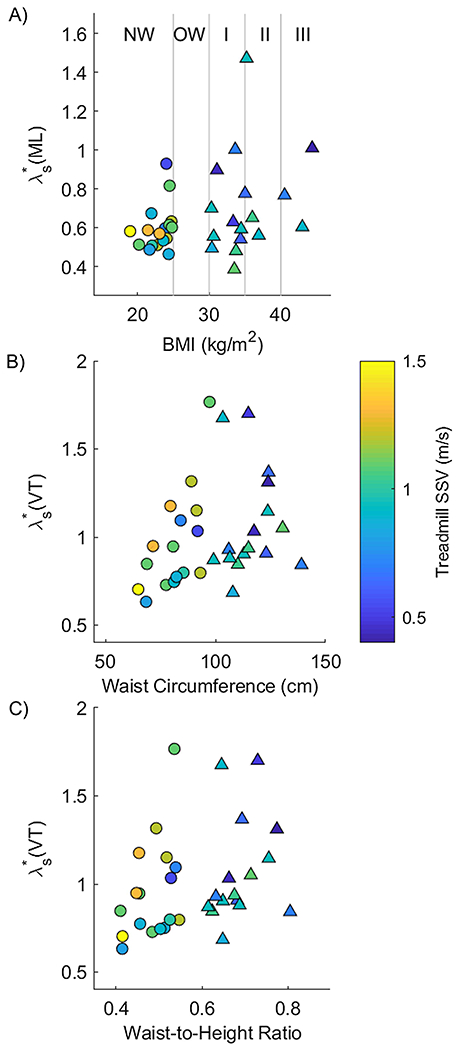
Significant correlations of λs* and measures of body habitus are shown with points shaded according to the individual’s self-selected treadmill velocity. Symbols are shown as ‘o’ for participants without obesity and ‘Δ’ for participants with obesity. A) Medial-lateral instability (λs*) is shown as a function of body mass index (BMI). Vertical lines delineate the different categories of BMI corresponding to healthy weight (HW), overweight (OW), and Class I-III obesity (I, II, and III). There was a correlation between medial-lateral instability and BMI (ρ = 0.374, p = 0.029). B) VT stability as a function of waist circumference (ρ = 0.385, p = 0.027). C) VT stability as a function of waist-to-height ratio (ρ = 0.356, p = 0.042).
Across both groups, there were 48 falls and 239 stumbles. A total of 13 participants with normal weight and 12 with obesity stumbled while 11 participants with normal weight and 5 with obesity fell. There were no significant differences between groups in the rate of falls or stumbles (Table 2). When we divided our entire cohort based on prospective falls, we found no significant differences in stability between those who fell and those who did not, in either the ML direction (p = 0.927, η2 = 0.003), AP direction (p = 0.547, η2 = 0.039), or VT direction (p = 0.412, η2 = 0.013; Fig 4). Similarly, there were no significant differences in stability between those who prospectively stumbled and those who did not (ML: p = 0.360, η2 = 0.011; AP: AP: p = 0.917, η2 = 0.006; VT: p = 0.188, η2 = 0.054; Fig 4).
Figure 4.
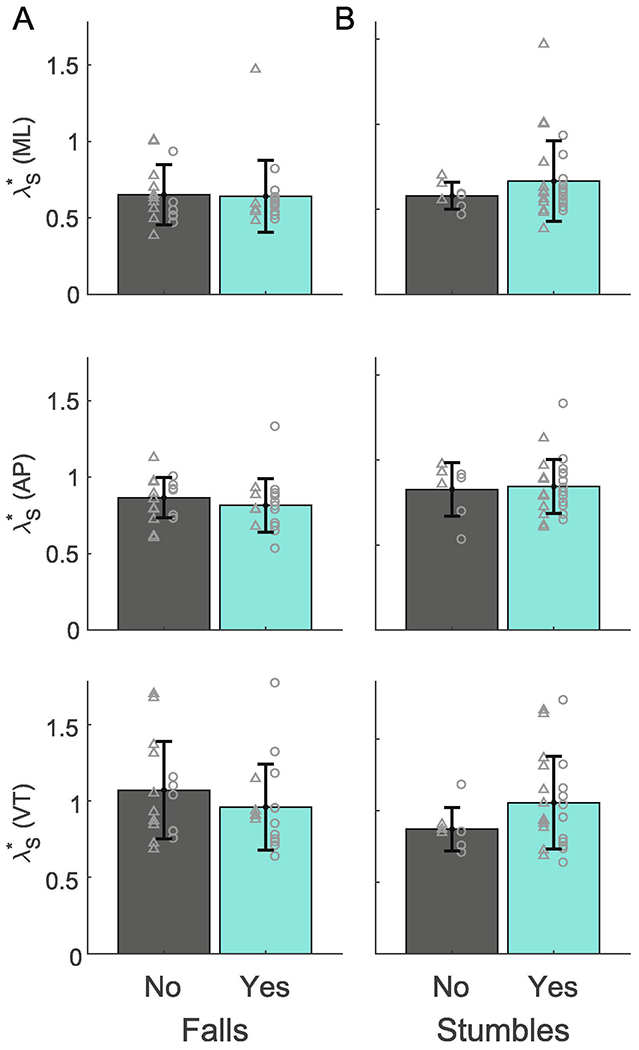
Mean values of λs* for people with prospective falls and those without prospective falls (A) and stumblers and non-stumblers (B) are shown for the medial-lateral (ML: top), anterior-posterior (AP: middle), and vertical (VT: bottom) directions. Error bars are one standard deviation across subjects about the mean. There were no significant differences in stability between groups.
Discussion
Our hypothesis that older adults with obesity would be less stable than older adults with normal-weight was not supported. This agrees with prior work that found no effect of obesity on gait stability in young adults, albeit using a different stability measure (Liu and Yang, 2017). In addition our population was relatively healthy. Participants needed to be able to walk continuously for 10 minutes and lacked comorbidities (e.g., joint replacements or untreated hypertension) often associated with obesity (Kopelman, 2006) that are known to mediate the relationship between obesity and fall risk (Mitchell et al., 2015). Any impact of obesity on stability may not easily be observed in a relatively healthy cohort. Interestingly, despite their relative health and similar stability, the group with obesity performed significantly worse on all functional tests. However, these significant differences may not be clinically meaningful or indicative of falls. For example, both groups had TUG times well below the established cutoff for increase fall risk of 13.5 s (Shumway-Cook et al., 2000). Larger functional differences may be necessary to observe changes in walking stability. Alternatively, functional tests and gait stability might represent different constructs.
Although we found no significant differences in stability between the group with and without obesity, we cannot rule out the possibility that increased body mass impacts walking stability. First, we found a medium sized effect of obesity on ML instability. We also found significant correlations between several measures of body habitus and walking stability (Fig 3) which support prior studies demonstrating that central adiposity is a stronger predictor of falls in older adults with obesity than BMI (Cho et al., 2018; Lin et al., 2011). Nonetheless, all correlations were weak, potentially due to the small sample size.
The fact that obese older adults were no more unstable than normal weight older adults could reflect compensatory strategies adopted by the former. For example, our obese older adults walked slower, with greater step widths, and with smaller step lengths than those with normal weight, which may represent strategies to enhance stability (Wearing et al., 2006). Conversely, while slower walking speeds are thought to compensate for local dynamic instability (Kang and Dingwell, 2008), we observed greater instability those with slower walking speeds (Fig 3a). This may stem from the methodology we employed to calculate stability, as the relationship between speed and stability is method dependent (Stenum et al., 2014). Additional work is needed to fully understand the relationship between walking speed and gait stability, particularly in obese older adults.
Several prior studies demonstrated a relationship between local dynamic stability and falls in older adults. In one study, four older adults who fell in responses to a laboratory-induced slip were less locally stable than four who did not fall (Lockhart and Liu, 2008). In another study, local stability of the trunk explained 12% of the variance in fall history (Toebes et al., 2012). Two additional studies reported that trunk instability significantly increased the odds of a prospective fall in adults with multiple sclerosis (Tajali et al., 2019; van Schooten et al., 2015). Here, we found no significant difference in local dynamic stability between prospective fallers and non-fallers. However, differences in sensor location and recorded signals, as well as in the process used to reconstruct the state space can affect λs*. For example, van Schooten et al. placed an accelerometer at the L5 level to measure trunk movement during daily life, and calculated state-space based on acceleration in each direction over 10s bouts (T=10 samples, dE=7); Tajali et al. calculated their state space from time-delayed copies of trunk linear and angular velocities (T=25 samples, dE=12) obtained from a marker cluster placed on the T7 vertebrae. Differences in study locations and participant demographics also could have impacted results by affecting the distribution of fall types. Whereas our participants lived in the Chicago-land area, the study by Tajali took place in a warm climate where slipping on ice is less likely. In addition, our participants may have been healthier and higher functioning than those in the study by Tajali et al., which considered only participants with multiple sclerosis, or those in the study of van Schooten et al, which included some institutionalized older adults, as well as some who used walkers many of whom were older than our cohort. Accordingly, our participants may have been more likely to experience outdoor (versus indoor) falls (Kelsey et al., 2010), most of which (~60% versus 39%) occur during walking or vigorous activity with only 6% (vs 25%) occurring when “transitioning/not moving” (Kelsey et al., 2012).
It is somewhat surprising that we observed similar fall rates between older adults with and without obesity given that obesity is known to increase fall risk. It is possible that our older adults with obesity were less active, as previously reported (Bell et al., 2015), and were therefore exposed to fewer extrinsic fall-risk factors during gait, although sedentary behavior is known to mediate the effect of obesity on fall-risk (Mitchell et al., 2015). It is also possible that between-group differences in stability could emerge if we considered stability of a different body segment, e.g. the foot. Indeed, inferior body segments may be more sensitive to small perturbations (Kang and Dingwell, 2009). On the other hand, superior segments, e.g., trunk, may be more sensitive to between-group differences such as those due to age (Kang and Dingwell, 2009), or obesity.
We might expect that local dynamic stability during walking would be more strongly related to stumbling (loss of stability) than to falls. Nonetheless stability was not significantly different between those who did and did not report stumbling. Due to small sample size we did not differentiate those with multiple stumbles, despite the fact that multiple missteps (stumbles) increase the risk of fall over three-fold (Srygley et al., 2009). Moreover the definition of a stumble (“loss of balance that does not result in a fall”) is more ambiguous than a fall which may cause under- or over-reporting and impact findings. Indeed several participants reported multiple stumbles a week and some reported no stumbles over the course of a year.
A primary limitation of the current study is that we measured walking stability on a treadmill. While this is common, prior research has shown that young adults are locally more stable during treadmill versus overground walking in (Dingwell et al., 2001; Terrier and Deriaz, 2011). Unlike these young adults, our participants may have been uncomfortable walking on a treadmill, which may have impacted their gait. This, combined with the instruction to “choose a speed that you feel you could comfortably maintain for ten minutes.” may have led participants to be more conservative with their walking speed. Indeed both groups decreased their walking speed on the treadmill, with a greater decrease in the group with obesity. Differences in walking speed may impact treadmill function, with slower walking speeds resulting in less_stride-to-stride variation in treadmill belt speed (Tielke et al., 2019). On the other hand, speed-related alterations in treadmill function may be counteracted by greater body mass, which increases stride-to-stride variation in treadmill belt speed (Tielke et al., 2019). While between-group differences in treadmill function could confound results, it is unclear how local stability would be affected by small changes in belt speed. In addition to treadmill limitations, the capacity of the safety harness prevented us from recruiting individuals over 300 lb. Our results may not generalize to those beyond this range.
In conclusion, older adults with obesity and those with normal weight had similar trunk stability during treadmill walking and similar self-reported falls and stumbles. Increased body mass alone, in absence of comorbidities or clinically meaningful functional impairments, may not influence stability during unperturbed treadmill walking. These experimental findings confirm prior epidemiological findings. Future work including individuals with a wider range of BMI who walk at a variety of fixed speeds may be needed to more fully understand the effect of obesity on gait stability.
Supplementary Material
Acknowledgements
This research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases award number 1R03AR066326-01A1. The funding source had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
None declared.
References
- Ashwell M, Gibson S, 2009. Waist to height ratio is a simple and effective obesity screening tool for cardiovascular risk factors: analysis of data from the British National Diet and Nutrition Survey of adults aged 19–64 years. Obesity facts 2, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JA, Hamer M, van Hees VT, Singh-Manoux A, Kivimaki M, Sabia S, 2015. Healthy obesity and objective physical activity. Am J Clin Nutr 102, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen G, Stevens MR, Burns ER, 2016. Falls and Fall Injuries Among Adults Aged ≥65 Years — United States, 2014. Morbidity and Mortality Weekly Report 65. [DOI] [PubMed] [Google Scholar]
- Bruijn SM, van Dieen JH, Meijer OG, Beek PJ, 2009. Statistical precision and sensitivity of measures of dynamic gait stability. J Neurosci Methods 178, 327–333. [DOI] [PubMed] [Google Scholar]
- Cho BY, Seo DC, Lin HC, Lohrmann DK, Chomistek AK, 2018. BMI and Central Obesity With Falls Among Community-Dwelling Older Adults. Am J Prev Med 54, e59–e66. [DOI] [PubMed] [Google Scholar]
- Costello KE, Matrangola SL, Madigan ML, 2012. Independent effects of adding weight and inertia on balance during quiet standing. Biomed Eng Online 11, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwell JB, Cusumano JP, Cavanagh PR, Sternad D, 2001. Local dynamic stability versus kinematic variability of continuous overground and treadmill walking. J Biomech Eng 123,27–32. [DOI] [PubMed] [Google Scholar]
- Dutil M, Handrigan GA, Corbeil P, Cantin V, Simoneau M, Teasdale N, Hue O, 2013. The impact of obesity on balance control in community-dwelling older women. American Aging Association 35, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein EA, Chen H, Prabhu M, Trogdon JG, Corso PS, 2007. The relationship between obesity and injuries among US adults. American Journal of Health Promotion 21, 460–468. [DOI] [PubMed] [Google Scholar]
- Fjeldstad C, Fjeldstad AS, Acree LS, Nickel KJ, Gardner AW, 2008. The influence of obesity on falls and quality of life. Dyn Med 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata KP, England SA, 2006. Stability of dynamic trunk movement. Spine 31, E271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes CL, Reynolds SL, 2012. Effect of obesity on falls, injury, and disability. J Am Geriatr Soc 60, 124–129. [DOI] [PubMed] [Google Scholar]
- Hue O, Simoneau M, Marcotte J, berrigan F, Dore J, Marceau P, Marceau S, Tremblay A, Teasdale N, 2006. Body weight is a strong predictor of postural stability. Gait & Posture 26, 7. [DOI] [PubMed] [Google Scholar]
- Kang HG, Dingwell JB, 2008. Effects of walking speed, strength and range of motion on gait stability in healthy older adults. J Biomech 41, 2899–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HG, Dingwell JB, 2009. Dynamic stability of superior vs. inferior segments during walking in young and older adults. Gait Posture 30, 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey JL, Berry SD, Procter-Gray E, Quach L, Nguyen US, Li W, Kiel DP, Lipsitz LA, Hannan MT, 2010. Indoor and outdoor falls in older adults are different: the maintenance of balance, independent living, intellect, and Zest in the Elderly of Boston Study. J Am Geriatr Soc 58, 2135–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey JL, Procter-Gray E, Hannan MT, Li W, 2012. Heterogeneity of falls among older adults: implications for public health prevention. Am J Public Health 102, 2149–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennel MB, Brown R, Abarbanel HD, 1992. Determining embedding dimension for phase-space reconstruction using a geometrical construction. Phys Rev A 45, 3403–3411. [DOI] [PubMed] [Google Scholar]
- Kopelman P, 2006. Health risks associated with overweight and obesity. Obesity reviews 8, 5. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Howland J, Tennstedt S, Jette A, Assmann S, Peterson EW, 1998. Fear of falling and activity restriction: the survey of activities and fear of falling in the elderly (SAFE). J Gerontol B Psychol Sci Soc Sci 53, P43–50. [DOI] [PubMed] [Google Scholar]
- Lhomond O, Teasdale N, Simoneau M, Mouchnino L, 2016. Neural Consequences of Increasing Body Weight: Evidence from Somatosensory Evoked Potentials and the Frequency-Specificity of Brain Oscillations. Front Hum Neurosci 10, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Liao KC, Pu SJ, Chen YC, Liu MS, 2011. Associated factors for falls among the community-dwelling older people assessed by annual geriatric health examinations. PLoS One 6, e18976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZQ, Yang F, 2017. Obesity May Not Induce Dynamic Stability Disadvantage during Overground Walking among Young Adults. PLoS One 12, e0169766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart TE, Liu J, 2008. Differentiating fall-prone and healthy adults using local dynamic stability. Ergonomics 51, 1860–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RJ, Lord SR, Harvey LA, Close JC, 2015. Obesity and falls in older people: mediating effects of disease, sedentary behavior, mood, pain and medication use. Arch Gerontol Geriatr 60, 52–58. [DOI] [PubMed] [Google Scholar]
- Pajala S, Era P, Koskenvuo M, Kaprio J, Tormakangas T, Rantanen T, 2008. Force platform balance measures as predictors of indoor and outdoor falls in community-dwelling women aged 63–76 years. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 63, 171–178. [DOI] [PubMed] [Google Scholar]
- Piirtola M, Era P, 2006. Force platform measurements as predictors of falls among older people - a review. Gerontology 52, 1–16. [DOI] [PubMed] [Google Scholar]
- Robinovitch SN, Feldman F, Yang Y, Schonnop R, Leung PM, Sarraf T, Sims-Gould J, Loughin M, 2013. Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study. Lancet 381, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt NJ, Bauer A, Grabiner MD, 2017. Relating minimum toe clearance to prospective, self-reported, trip-related stumbles in the community. Prosthet Orthot Int 41, 387–392. [DOI] [PubMed] [Google Scholar]
- Rosenblatt NJ, Grabiner MD, 2010. Measures of frontal plane stability during treadmill and overground walking. Gait Posture 31, 380–384. [DOI] [PubMed] [Google Scholar]
- Rosenstein MT, Collins JJ, De Luca CJ, 1993. A practical method for calculating largest Lyapunov exponents from small data sets. Physica D 65, 18. [Google Scholar]
- Rothman KJ, 2008. BMI-related errors in the measurement of obesity. International journal of obesity 32, S56. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Brauer S, Woollacott M, 2000. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther 80, 896–903. [PubMed] [Google Scholar]
- Srygley JM, Herman T, Giladi N, Hausdorff JM, 2009. Self-report of missteps in older adults: a valid proxy of fall risk? Arch Phys Med Rehabil 90, 786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenum J, Bruijn SM, Jensen BR, 2014. The effect of walking speed on local dynamic stability is sensitive to calculation methods. J Biomech 47, 3776–3779. [DOI] [PubMed] [Google Scholar]
- Tajali S, Mehravar M, Negahban H, van Dieen JH, Shaterzadeh-Yazdi MJ, Mofateh R, 2019. Impaired local dynamic stability during treadmill walking predicts future falls in patients with multiple sclerosis: A prospective cohort study. Clin Biomech (Bristol, Avon) 67, 197–201. [DOI] [PubMed] [Google Scholar]
- Teasdale N, Hue O, Marcotte J, Berrigan F, Simoneau M, Dore J, Marceau P, Marceau S, Tremblay A, 2007. Reducing weight increases postural stability in obese and morbid obese men. Int J Obes (Lond) 31, 153–160. [DOI] [PubMed] [Google Scholar]
- Terrier P, Deriaz O, 2011. Kinematic variability, fractal dynamics and local dynamic stability of treadmill walking. J Neuroeng Rehabil 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrier P, Reynard F, 2015. Effect of age on the variability and stability of gait: A cross-sectional treadmill study in healthy individuals between 20 and 69 years of age. Gait & Posture 41, 5. [DOI] [PubMed] [Google Scholar]
- The Centers for Disease Control and Prevention, C., 2017. 10 Leading Causes of Nonfatal Injury, United States. [Google Scholar]
- Tielke A, Ahn J, Lee H, 2019. Non-ideal behavior of a treadmill depends on gait phase, speed, and weight. Sci Rep 9, 12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toebes MJ, Hoozemans MJ, Furrer R, Dekker J, van Dieen JH, 2012. Local dynamic stability and variability of gait are associated with fall history in elderly subjects. Gait Posture 36, 527–531. [DOI] [PubMed] [Google Scholar]
- van Schooten KS, Pijnappels M, Rispens SM, Elders PJ, Lips P, van Dieen JH, 2015. Ambulatory fall-risk assessment: amount and quality of daily-life gait predict falls in older adults. J Gerontol A Biol Sci Med Sci 70, 608–615. [DOI] [PubMed] [Google Scholar]
- van Schooten KS, Rispens SM, Elders PJM, van Dieën JH, Pijnappels M, 2014. Toward ambulatory balance assessment: Estimating variability and stability from short bouts of gait. Gait & Posture 39, 695–699. [DOI] [PubMed] [Google Scholar]
- van Schooten KS, Rispens SM, Pijnappels M, Daffertshofer A, van Dieen JH, 2013. Assessing gait stability: the influence of state space reconstruction on inter- and intra-day reliability of local dynamic stability during over-ground walking. J Biomech 46, 137–141. [DOI] [PubMed] [Google Scholar]
- Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP, 2006. The biomechanics of restricted movement in adult obesity. Obes Rev 7, 13–24. [DOI] [PubMed] [Google Scholar]
- Wing AM, Turton A, Fraser C, 1986. Grasp size and accuracy of approach in reaching. Journal of motor behavior 18, 245–260. [DOI] [PubMed] [Google Scholar]
- Wu X, Madigan ML, 2014. Impaired plantar sensitivity among the obese is associated with increased postural sway. Neurosci Lett 583, 49–54. [DOI] [PubMed] [Google Scholar]
- Zeni JA Jr., Richards JG, Higginson JS, 2008. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture 27, 710–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


