Introduction
Ischaemic heart disease (IHD) persists as the leading cause of premature death and disability worldwide.1 IHD may present as acute myocardial infarction (MI) or a chronic coronary syndrome.2 IHD is increasingly recognized as a concomitant problem in systemic health problems, such as rheumatoid arthritis. Coronary atherosclerosis is a major cause of IHD. The historical primacy of coronary artery disease (CAD) leads some clinicians to view coronary heart disease (CHD) and IHD as synonymous, interchangeable terms. Emerging clinical evidence indicates this is far from being the case and a major reappraisal is warranted.3 CHD represents a subset of IHD, and these terms should not be used interchangeably.
Under-recognition of coronary microvascular disease: time for a reappraisal
Coronary microvascular dysfunction (CMD) has, historically, been under-recognized, not least since the microvessels are invisible to currently available clinical imaging techniques. This simple issue has underpinned key misconceptions about IHD and major knowledge gaps relating to CMD.3 Atherosclerosis is the major cause of CHD and the pathogenesis, prognosis, and treatment of these problems are well-established.2 In recent years, new insights into the causes and consequences of IHD have called into question the CAD stenosis-centred/CHD paradigm.
Most recently, the ISCHEMIA trial results were reported at the Scientific Sessions of the American Heart Association (16 November 2019) (https://professional.heart.org/professional/ScienceNews/UCM_505226_ISCHEMIA-Clinical-Trial-Details.jsp).4 The central hypothesis of the ISCHEMIA trial was that in patients with angina and moderate–severe myocardial ischaemia, compared with initial non-invasive, medical management, a routine invasive strategy with cardiac catheterization followed by coronary revascularization plus optimal medical therapy, would improve prognosis. After 3.3 years of follow-up, there was no difference in the primary endpoint between the randomized groups.4 This trial did have limitations. Under-recruitment and a lower than expected event rate reduced the statistical power for analysis of the primary outcome that ultimately led to a belated, yet prespecified change in the primary composite outcome. Longer-term follow-up with accrual of more events may provide new insights. Nonetheless, ISCHEMIA is the largest study of its kind, and the results call into question the benefits of coronary revascularization in patients with myocardial ischaemia. Crucially, it questions the stenosis-centred pathophysiology of IHD and warrants reassessment of the role of CMD in IHD.
Clinical relevance of coronary microvascular dysfunction
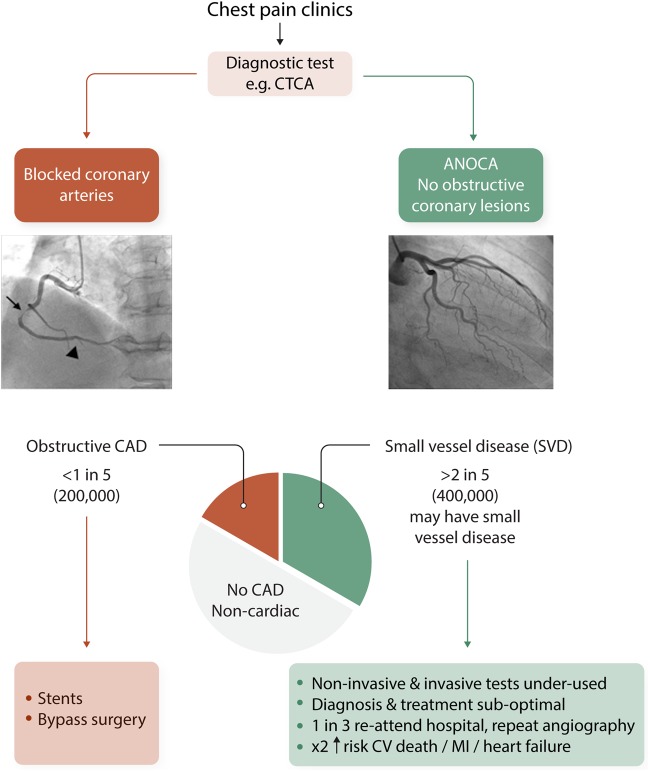
CMD is increasingly implicated as a relevant cause of IHD.4 Angina secondary to myocardial ischaemia may occur in patients with no obstructive CAD (INOCA). In fact, fewer than one in five patients presenting with known or suspected angina have obstructive CAD, as revealed by anatomical imaging with computed tomography coronary angiography (CTCA)5 (Figure 1). In the clinic, the cause of the angina is uncertain in the majority of affected patients, most of whom are women.5,6 This becomes all the more relevant given that CTCA-guided management leads to worse angina and quality of life overall, contrary to what might be anticipated.7 The Coronary Microvascular Function and CT Coronary Angiogram (CorCTCA) study is currently examining the prevalence and clinical significance of CMD in patients with angina but no obstructive CAD, as defined by CTCA.8 The recent Coronary Microvascular Angina (CorMicA trial) served evidence that undertaking tests of coronary vascular function during clinically indicated coronary angiography identifies relevant endotypes (microvascular angina, vasospastic angina, and non-cardiac chest pain) and targeted therapy was associated with improvements in angina and quality of life at 6-9 and 12 months.10 Considering acute MI, about 1 in 10 patients presenting with MI have no obstructive coronary arteries (MINOCA).11 Microvascular and vasospastic disease are also implicated. Considering the natural history, INOCA and MINOCA may underlie the development of heart failure with preserved ejection fraction12 which is an increasingly recognized, prevalent cause of heart failure.
Figure 1.
Ischaemic heart disease endotypes. ANOCA, angina with no obstructive coronary arteries; CAD, coronary artery disease; CTCA, computed tomography coronary angiography; CV, cardiovascular; MI, myocardial infarction.
Coronary microvascular disease may be part of a systemic continuum of microvascular disease, with multiple affected organ beds.13 Small vessel disease in the heart and brain links INOCA with vascular dementia.13 The deleterious effects of vascular risk factors, such as hypertension, obesity, smoking, and diabetes are relevant, and genetic associations,14 notably leading to increased exposure to endothelin-115 are also implicated. CMD is causally implicated in multiple systemic conditions including the cardiotoxicity of chemotherapy, systemic inflammatory conditions, such as rheumatoid arthritis, heart failure, and pregnancy.16 Sex associations are also relevant.17 Obstructive CAD typically associates with male sex, whereas small vessel disease associates with female sex.5,6,8,9 Since anatomical imaging with CTCA is diagnostically most useful for identifying and excluding CAD and compared with ischaemia testing, least useful for the diagnosis of CMD, an all-comers strategy based on CTCA introduces a sex bias.3 Under-recognition and under-treatment of heart disease in women is a hot topic16,17 and more research seems warranted.
Some of the persisting, clinically relevant questions are: (i) In INOCA and MINOCA, is myocardial ischaemia the consequence and/or cause of microvascular dysfunction? (ii) Is chronic myocardial ischaemia therapeutically modifiable? (iii) Is microvascular dysfunction a common problem after successful revascularization? (iv) If so, what are the mechanisms underlying microvascular dysfunction, what treatments might be disease-modifying and beneficial to patients? (v) Is CMD a modifiable therapeutic target? (vi) What is the natural history of CMD? The clinical relevance of microvascular dysfunction in patients with flow-limiting CAD is being investigated in the DEFINE-FLOW study18 due to be reported in 2020. The Changes in Ischemia and Angina Over 1 Year Among ISCHEMIA Trial Screen Failures With no Obstructive CAD on Coronary CT Angiography (CIAO) substudy will also be informative.19
Coronary microvascular dysfunction in the spotlight
Accordingly, CMD has generated substantial interest in the clinical and basic science communities in recent years. This Spotlight Issue brings together internationally leading thought-leaders, researchers, and their trainees. The authors have a broad range of backgrounds including basic science, translational research, and clinical studies. Their remit is to focus on ‘hot topics’ in CMD and give perspectives on the science.
The Spotlight Issue begins with a Position Paper, ‘Coronary Microvascular Dysfunction in Cardiovascular Disease’, from the European Society of Cardiology (ESC) Working Group on Coronary Pathophysiology and the Microcirculation.20 The Position Paper by Drs Padro, Manfrini, Badimon, and coauthors highlights, firstly, updated evidence on the pathophysiological consequences of microvascular dysfunction in the heart. Secondly, they focus on the relevance of cardiovascular risk factors and comorbid conditions for microcirculatory dysfunction. Thirdly, they highlight the clinical consequences of CMD, which is not a benign problem. They conclude that clinical strategies should prioritize detection of CMD which in turn will help in the stratification of cardiovascular in support of precision medicine.
The first review article focuses on experimental models of CMD. Sorop et al.21 discuss the benefits and pitfalls of existing small and large animal models of CMD, with a specific focus on metabolic disturbances which may be experimentally induced or spontaneous. They provide a comprehensive description of relevant experimental research involving a range of species. They also highlight the value of experimental models for identifying novel therapeutic targets and for the subsequent development and testing of novel therapeutic interventions.
The next article focuses on ‘Diagnosis of coronary microvascular dysfunction in the clinic’. Ong et al.22 cover the diagnosis of CMD in an article that discusses the invasive and non-invasive methods for the assessment of CMD in humans. They highlight an integrative approach for assessing coronary vascular function using a diagnostic guidewire initially and then pharmacological reactivity testing using intracoronary administration of acetylcholine (ACh). They highlight the Interventional Diagnostic Procedure developed by Berry and Ford9 as the current gold standard for assessing coronary vascular function. A review from Bairey Merz et al.23 on ‘Treatment of CMD’, provides a comprehensive overview of pharmacotherapies with potential efficacy in alleviating CMD. The article highlights pivotal clinical trials in CMD, such as CorMicA9 and WARRIOR (ClinicalTrials.gov Identifier: NCT03417388). In addition, they highlight novel therapeutics, including gene and cell-based therapies.
The Spotlight also includes articles on CMD in different cardiovascular disease settings. Sechtem et al.24 focus on CMD in stable IHD, including INOCA and obstructive CAD. They focus on challenging concepts including CMD in the absence of atherosclerosis, CMD detection, microvascular spasm, collateral connections, and the prognostic importance of global coronary flow reserve. Konijnenberg et al.25 focus on the pathophysiology and diagnosis of CMD in acute MI. The authors state that the current standard of care, primary percutaneous coronary intervention, successfully restores coronary blood flow in the vast majority of patients yet most also have evidence of failed myocardial perfusion, revealed as microvascular obstruction (MVO) using magnetic resonance imaging. MVO confers an adverse prognosis and in spite of multiple therapeutic trials, MVO has no evidence-based treatment and has an unmet therapeutic need. The manuscript also discusses pre-clinical models. Camici et al.26 discuss the mechanisms by which CMD is a contributing factor to the transition from left ventricular hypertrophy to heart failure with either a reduced or preserved ejection fraction. Relevant mechanisms are discussed. CMD in genetic cardiomyopathy is also described. Konst et al.27 describe the pathogenic role of CMD in the setting of other cardiac or systemic conditions. They highlight diabetes mellitus, obesity, and vascular inflammation as relevant causes of CMD.
A further disease modifier of CMD pathology is sex.16,17 Women who are under investigation for myocardial ischaemia are more likely to have non-obstructive CAD on coronary angiography and CMD is relevant. In the final article of this Spotlight Issue, Waheed et al.28 explore sex associations of INOCA, MINOCA, symptoms, risk factors and, intriguingly, sex-specific factors such as inflammation, mental stress, autonomic, and neuro-endocrine dysfunction that may cause women—relative to men—to be more likely to develop CMD. Sex differences have major implications for both diagnosis and treatment of cardiovascular disease.
We recognize and thank experts from the COVADIS (Coronary Vasomotor Disorders International Study Group) and ESC Working Group on Coronary Pathophysiology and Microcirculation for their collaboration. The Editors hope that by bringing this collection of articles together, the Spotlight will enhance interest for research in CMD. This problem pervades human disease, mechanisms are poorly understood and specific treatments are lacking. CMD presents an exciting field for discovery and translation to reduce the unmet therapeutic need.
Conflict of interest: C.B. is employed by the University of Glasgow which holds consultancy and research agreements with companies that have commercial interests in the diagnosis and treatment of ischaemic heart disease. The companies include Abbott Vascular, AstraZeneca, Boehringer Ingelheim, GSK, HeartFlow, Menarini, Novartis, and Siemens Healthcare. These companies had no involvement in this manuscript. D.J.D. serves ad hoc as a consultant for Medtronic and Sanofi-Aventis. These companies had no involvement in this manuscript.
Funding
C.B. has research funding from the British Heart Foundation (FS/17/26/32744; RE/18/6134217) and the Medical Research Council (MR/S018905/1). D.J.D. has research funding from the Netherlands CardioVascular Research Initiative: an initiative with support of the Dutch Heart Foundation [(RECONNECT)].
References
- 1.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 3. Berry C. Stable coronary syndromes: the case for consolidating the nomenclature of stable ischemic heart disease. Circulation 2017;136:437–439. [DOI] [PubMed] [Google Scholar]
- 4. Ford TJ, Corcoran D, Berry C.. Stable coronary syndromes: pathophysiology, diagnostic advances and therapeutic need. Heart 2018;104:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–2391. [DOI] [PubMed] [Google Scholar]
- 6. Mangion K, Adamson PD, Williams MC, Hunter A, Pawade T, Shah ASV, Lewis S, Boon NA, Flather M, Forbes J, McLean S, Roditi G, van Beek EJR, Timmis AD, Newby DE, McAllister DA, Berry C.. Sex associations and computed tomography coronary angiography-guided management in patients with stable chest pain. Eur Heart J 2019;doi:10.1093/eurheartj/ehz903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williams MC, Hunter A, Shah A, Assi V, Lewis S, Mangion K, Berry C, Boon NA, Clark E, Flather M, Forbes J, McLean S, Roditi G, van Beek EJ, Timmis AD, Newby DE; Scottish COmputed Tomography of the HEART (SCOT-HEART) Trial Investigators. Symptoms and quality of life in patients with suspected angina undergoing CT coronary angiography: a randomised controlled trial. Heart 2017;103:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sidik NP, McEntegart M, Roditi G, Ford TJ, McDermott M, Morrow A, Byrne J, Adams J, Hargreaves A, Oldroyd KG, Stobo D, Wu O, Messow CM, McConnachie A, Berry C.. Rationale and design of the British Heart Foundation (BHF) Coronary Microvascular Function and CT Coronary Angiogram (CorCTCA) study. Am Heart J 2020;221:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sidik N, McCartney P, Corcoran D, Collison D, Rush C, McConnachie A, Touyz RM, Oldroyd KG, Berry C.. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol 2018;72:2841–2855. [DOI] [PubMed] [Google Scholar]
- 10. Ford TJ, Stanley B, Sidik N, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, McCartney P, Corcoran D, Collison D, Rush C, Sattar N, McConnachie A, Touyz RM, Oldroyd KG, Berry C.. 1-year outcomes of angina management guided by invasive coronary function testing (CorMicA). JACC Cardiovasc Interv 2020;13:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, De Caterina R, Zimarino M, Roffi M, Kjeldsen K, Atar D, Kaski JC, Sechtem U, Tornvall P; WG on Cardiovascular Pharmacotherapy. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2017;38:143–153. [DOI] [PubMed] [Google Scholar]
- 12. Crea F, Bairey Merz CN, Beltrame JF, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Camici PG; Coronary Vasomotion Disorders International Study Group (COVADIS). The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J 2017;38:473–477. [DOI] [PubMed] [Google Scholar]
- 13. Berry C, Sidik N, Pereira AC, Ford TJ, Touyz RM, Kaski JC, Hainsworth AH.. Small-vessel disease in the heart and brain: current knowledge, unmet therapeutic need, and future directions. J Am Heart Assoc 2019;8:e011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA). https://clinicaltrials.gov/ct2/show/NCT01471522 (17 February 2020, date last accessed).
- 15. Ford TJ, Corcoran D, Padmanabhan S, Aman A, Rocchiccioli P, Good R, McEntegart M, Maguire JJ, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sattar N, Hsu LY, Arai AE, Oldroyd KG, Touyz RM, Davenport AP, Berry C.. Genetic dysregulation of endothelin-1 is implicated in coronary microvascular dysfunction. Eur Heart J 2020;doi:10.1093/eurheartj/ehz915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker-Kleiner D, Kaye DM, Ky B, Santema BT, Sliwa K, Voors AA.. Sex differences in heart failure. Eur Heart J 2019;40:3859–3868c. [DOI] [PubMed] [Google Scholar]
- 17. Mehta PK, Bess C, Elias-Smale S, Vaccarino V, Quyyumi A, Pepine CJ, Bairey Merz CN.. Gender in cardiovascular medicine: chest pain and coronary artery disease. Eur Heart J 2019;40:3819–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Piek JJ. Combined Pressure and Flow Measurements to Guide Treatment of Coronary Stenoses (DEFINE-FLOW). https://clinicaltrials.gov/ct2/show/NCT02328820 (17 February 2020, date last accessed). [DOI] [PubMed]
- 19. Reynolds H. The Changes in Ischemia and Angina Over 1 Year Among ISCHEMIA Trial Screen Failures With no Obstructive CAD on Coronary CT Angiography (CIAO) substudy. https://clinicaltrials.gov/ct2/show/NCT02347215 (17 February 2020, date last accessed).
- 20. Padro B, Manfrini O, Bugiardini R, Canty J, Cenko E, De Luca G, Duncker DJ, Eringa EC, Koller A, Tousoulis D, Trifunovic D, Vavlukis M, de Wit C, Badimon L ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on ‘coronary microvascular dysfunction in cardiovascular disease'. Cardiovasc Res 2020;116:741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sorop O, van de Wouw J, Chandler S, Ohanyan V, Tune JD, Chilian WM, Merkus D, Bender SB, Duncker DJ.. Experimental animal models of coronary microvascular dysfunction. Cardiovasc Res 2020;116:756–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ong P, Safdar B, Seitz A, Hubert A, Beltrame J, Prescott E.. Diagnosis of coronary microvascular dysfunction in the clinic. Cardiovasc Res 2020;116:841–855. [DOI] [PubMed] [Google Scholar]
- 23. Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C.. Treatment of coronary microvascular dysfunction. Cardiovasc Res 2020;116:856–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sechtem U, Brown DL, Godo S, Lanza GA, Shimokawa H, Sidik N.. Coronary microvascular dysfunction in stable ischaemic heart disease (NOCAD and OCAD). Cardiovasc Res 2020;116:771–786. [DOI] [PubMed] [Google Scholar]
- 25. Konijnenberg LSF, Damman P, Duncker DJ, Kloner RA, Nijveldt R, van Geuns RJ, Berry C, Riksen NP, Escaned J, van Royen N.. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc Res 2020;116:787–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Camici PG, Tschöpe C, Di Carli MF, Rimoldi O, Van Linthout S.. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc Res 2020;116:806–816. [DOI] [PubMed] [Google Scholar]
- 27. Konst RE, Guzik TJ, Kaski JC, Maas A, Elias-Smale SE.. The pathogenic role of coronary microvascular dysfunction in the setting of other cardiac or systemic conditions. Cardiovasc Res 2020;116:817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Waheed N, Elias-Smale S, Malas W, Maas AH, Sedlak TL, Tremmel J, Mehta PK.. Sex differences in non-obstructive coronary artery disease. Cardiovasc Res 2020;116:829–840. [DOI] [PubMed] [Google Scholar]