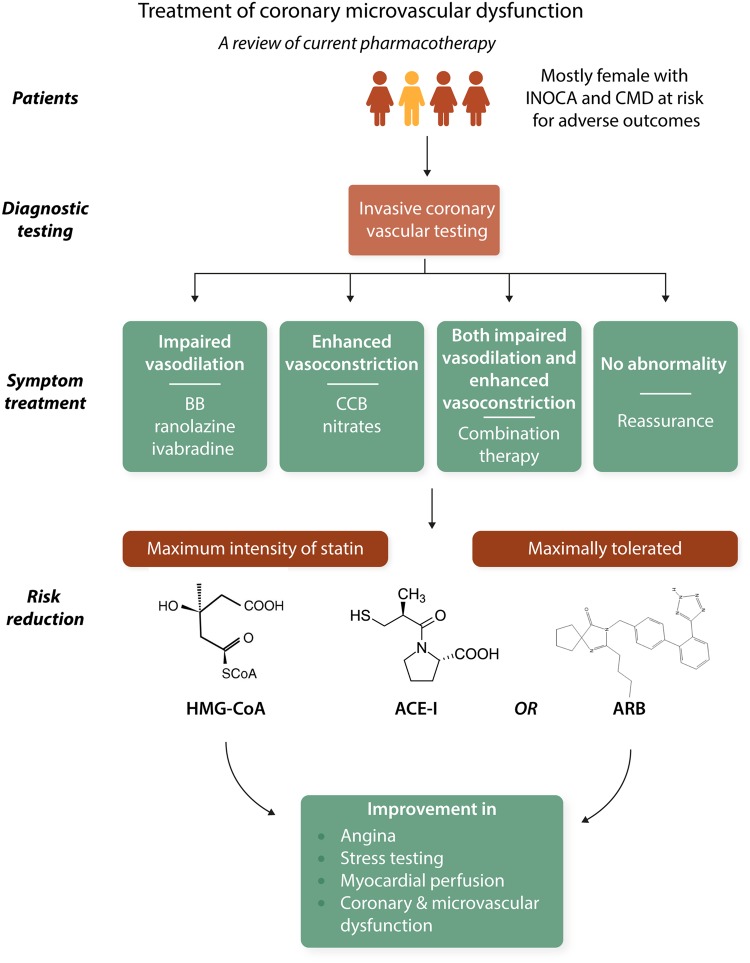
Abstract
Contemporary data indicate that patients with signs and symptoms of ischaemia and non-obstructive coronary artery disease (INOCA) often have coronary microvascular dysfunction (CMD) with elevated risk for adverse outcomes. Coronary endothelial (constriction with acetylcholine) and/or microvascular (limited coronary flow reserve with adenosine) dysfunction are well-documented, and extensive non-obstructive atherosclerosis is often present. Despite these data, patients with INOCA currently remain under-treated, in part, because existing management guidelines do not address this large, mostly female population due to the absence of evidence-based data. Relatively small sample-sized, short-term pilot studies of symptomatic mostly women, with INOCA, using intense medical therapies targeting endothelial, microvascular, and/or atherosclerosis mechanisms suggest symptom, ischaemia, and coronary vascular functional improvement, however, randomized, controlled outcome trials testing treatment strategies have not been completed. We review evidence regarding CMD pharmacotherapy. Potent statins in combination with angiotensin-converting enzyme inhibitor (ACE-I) or receptor blockers if intolerant, at maximally tolerated doses appear to improve angina, stress testing, myocardial perfusion, coronary endothelial function, and microvascular function. The Coronary Microvascular Angina trial supports invasive diagnostic testing with stratified therapy as an approach to improve symptoms and quality of life. The WARRIOR trial is testing intense medical therapy of high-intensity statin, maximally tolerated ACE-I plus aspirin on longer-term outcomes to provide evidence for guidelines. Novel treatments and those under development appear promising as the basis for future trial planning.
Keywords: INOCA, CMD, Women, Angina, Ischaemia
Graphical Abstract
Graphical Abstract.
This article is part of the Spotlight Issue on Coronary Microvascular Dysfunction.
1. Introduction
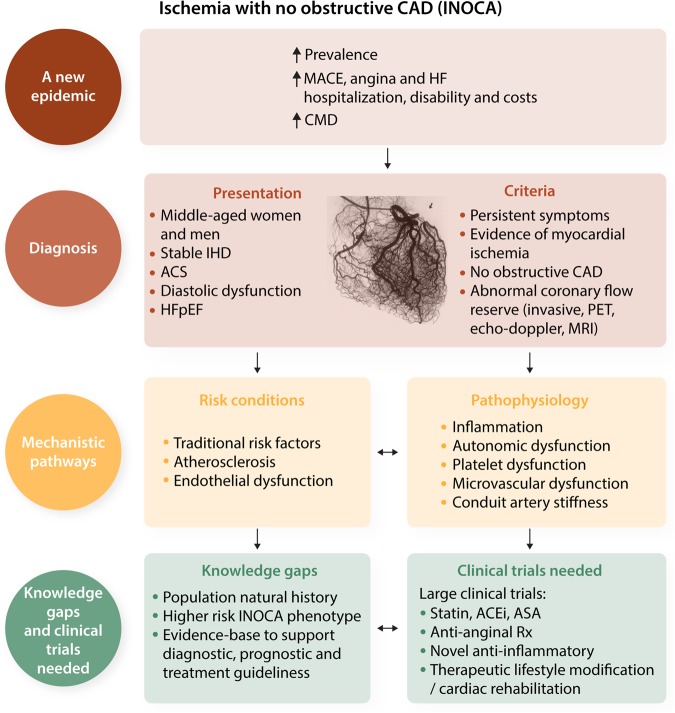
Older reports suggested the prognosis of angina with evidence of ischaemia and non-obstructive coronary artery disease (INOCA) was benign.1–3 Now, considerable contemporary data support the conclusion that these patients often have coronary microvascular dysfunction (CMD) and are at higher risk for adverse outcomes vs. reference subjects.4–9 Nevertheless, INOCA patients currently remain under-treated, because management guidelines do not address this population due to the absence of evidence-based data.10
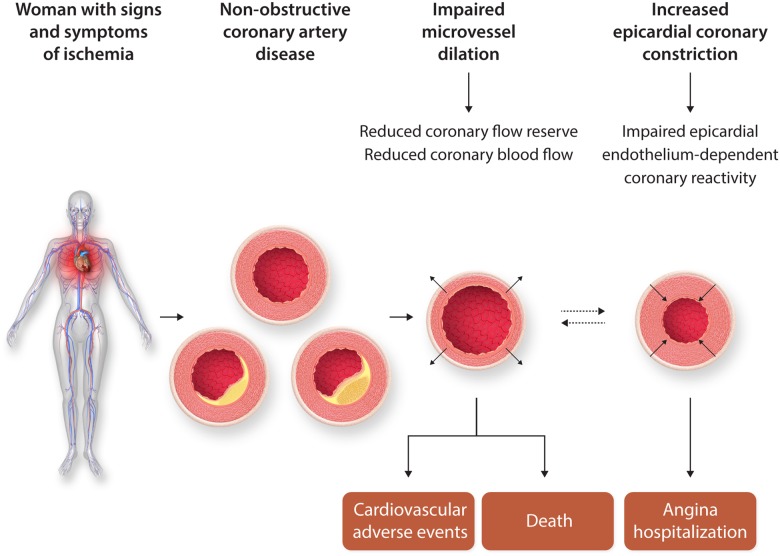
Mechanistically, coronary endothelial (constriction with acetylcholine) and/or microvascular [limited coronary flow reserve (CFR) with adenosine] dysfunction (Figure 1) are well-documented and associated with adverse prognosis.11 Furthermore, diffuse non-obstructive atherosclerosis with ‘compensatory’ coronary remodelling is well documented in women.12,13 Persistent angina, ischaemia on stress testing, multiple risk factors, and more extensive non-obstructive plaque (multi-vessel) predict higher risk for adverse events.14–17
Figure 1.
CMD reactivity testing and prognosis. Women with no obstructive coronary artery disease and the potential role of coronary reactive testing to identify those at higher risk for adverse events. Permission granted to reproduce figure from AlBadri et al.11
Relatively small sample-sized, short-term pilot studies of INOCA subjects, mostly women, using intense medical therapies targeting endothelial, microvascular, and/or atherosclerosis mechanisms suggest symptom, ischaemia, and coronary vascular functional improvement,18 however, randomized, controlled major outcome trials testing treatment strategies have not been completed. We review the evidence regarding the treatment of CMD.
2. Review of pharmacotherapy
2.1 Conventional therapy—anti-atherosclerosis treatment
Statins, angiotensin-converting enzyme inhibitor (ACE-I) or receptor blockers (ARB), and low-dose aspirin are secondary prevention anti-atherothrombosis treatments that beneficially impact many mechanisms summarized above that likely contribute to symptoms and outcomes in INOCA patients; they counteract oxidative stress, are anti-inflammatory, improve both endothelial and microvascular function,19,20 and rebalance sympathetic dysregulation (Table 1).19–22 These treatments improve angina, stress testing, myocardial perfusion, coronary endothelial function, and microvascular function in small-size trials. Statins reduce plaque lipid-rich core, inflammation, macrophage, and foam cell formation, promote fibrous cap thickening, and decrease platelet reactivity.23 Drug combinations (e.g. statins plus ACE-I) appear to amplify some benefits.20
Table 1.
Pharmacotherapy of coronary microvascular dysfunction mechanistic trials
| Conventional treatments | Intermediate outcome impact |
|---|---|
| Anti-atherosclerosis treatment | |
| Statins | + |
| ACE-I, angiotensin renin blockers | + |
| Low-dose aspirin | + |
| Anti-angina treatments | |
| Calcium antagonists | + |
| Alpha beta-blockers | + |
| Beta-blockers | + |
| Nitrates | + |
| Ranolazine | +/− |
| Exercise | + |
| Enhanced external counter-pulsation | + |
| Imipramine, amitriptyline, nortriptyline | + |
| L-arginine | + |
| Spinal cord stimulation | + |
| Novel treatments | |
| RAAS active agents | |
| ACE-I quinapril | + |
| Mineralocorticoid inhibition-eplerenone | − |
| PDE | |
| PDE type 3 inhibition—cilostazol | + |
| PDE type 5 inhibition—sildenafil | + |
| Calcium channel antagonism—benidpine | + |
| Selective If-channel blockade—ivabradine | +/− |
| Rho-kinase inhibition—fasudil | + |
| Endothelin receptor antagonists—darusentan and atrasentan | + |
| Adenosine active agents—dipyridamole, caffeine, aminophylline, paraxanthine, pentoxifylline, theobromine, and theophylline | +/− |
| Myocardial metabolic active agents | |
| Dichloroacetate | − |
| Carnitine analogues—acetyl-L-carnitine, propionyl-L-carnitine, and L-carnitine | − |
| Perhexiline | − |
| Trimetazidine | + |
| Metformin | + |
| Amiodarone/dronedarone | + |
| Anti-inflammation agents | |
| IL-1b inhibition-canakinumab, rilonacept | − |
| Methotrexate | − |
| Hormone therapy | − |
| IL-1a-anakinra | − |
| IL-6-Tocilizumab | − |
| TNF-α inhibitors | + |
| Glycaemic active agents—SGLT 1 and 2 inhibitors, GLP-1 agonists | − |
| Androgen analogues—testosterone (T), low-dose T (transdermal patch), and T undecanoate injection | + |
| Oestrogen analogues—norethindrone/ethinyl oestradiol, CEOs and medroxyprogesterone acetate, and CEO | +/− |
| Nervous system active agents—neuropeptide Y and selective serotonin reuptake inhibitors (escitalopram) | +/− |
| Gene and cell-based therapies—autologous CD34 cells | − |
| Small-molecule targets | − |
+, benefit; +/−, no benefit; −, mixed benefit and no benefit; ACE-I, angiotensin-converting enzyme inhibitor; CEO, conjugated equine oestrogen; GLP-1, glucagon-like peptide 1; PDE, phosphodiesterase inhibition.
Thromboxane A2 (TXA2) inhibitors (low-dose aspirin and P2Y12 platelet inhibitors) are also likely useful in preventing adverse outcomes of INOCA patients.12 They minimize platelet-rich microemboli and related downstream events. Activation of thromboxane A2 synthase (TXAS)/TXA2/thromboxane prostanoid (TP) receptors leads to arterial constriction, platelet aggregation, and vascular injury. Microvascular dysfunction was characterized in ischaemia/reperfusion injury using genetically modified knock-out (TXAS−/−, TP−/−, and TXAS−/−TP−/−) mice.24 Activation of TXAS/TXA2/TP receptors led to microvascular constriction, platelet aggregation, and vascular injury; aspirin reduced endothelial platelet adhesion. Thus, inhibiting TXAS/TXA2/TP signalling confers microvascular protection against oxidative injury in the microcirculation.
2.2 Conventional therapy—anti-anginal treatment
Calcium antagonists fail to ameliorate CFR limitations in CMD patients25 but can prevent epicardial coronary vasospasm. Few controlled long-acting nitrate studies have been reported and beta-blockers are effective only for some.26 Notably, a highly selective beta-1 blocker with vasodilatory effects via nitric oxide (NO) production, nebivolol, has effects on angina and exercise capacity and is being studied in women with CMD.27 Intracoronary nebivolol increases CFR either due to reduction in resting flow (controls) or increase in maximal coronary flow [coronary artery disease (CAD) patients] as collateral flow index decreased parallel to reduction in myocardial oxygen consumption.28 Importantly, nebivolol improved left ventricular (LV) filling pressure and CFR in uncomplicated arterial hypertension suggesting myocardial stimulation of NO release and improvement in coronary microvascular function.
Ranolazine is an antianginal that inhibits the late sodium channel and reduces intracellular calcium in cardiomyocytes, leading to improved ventricular relaxation which should facilitate microvascular function.29 Results on CMD are conflicting. One pilot study showed improved symptoms in women with INOCA, and patients with low CFR improved CFR with treatment.30 A similar sized study showed some symptom improvement but no effect on CFR.31 A large randomized-crossover trial of ranolazine vs. placebo found no difference in symptoms or cardiac magnetic resonance imaging (cMRI)-myocardial perfusion reserve.32 But stratification by baseline invasive CFR suggested that those with reduced CFR improved with ranolazine.33
Other treatments include exercise training, which beneficially modulates adrenergic and NO pathways,34 but training trials are problematic for patient compliance and expense. Enhanced external counter-pulsation therapy can improve angina.35 Tricyclics (e.g. imipramine, amitriptyline, and nortriptyline) block multiple receptors, including muscarinic acetylcholine receptors, H1 and H2-histamine receptors, sodium and calcium channel receptors, and inhibit serotonin and norepinephrine reuptake. In a randomized, placebo-controlled trial of patients with INOCA, imipramine improved angina, possibly through visceral analgesic effects36 but not quality of life (QoL).37 Specific mechanism(s) for improvement require(s) additional investigation. L-arginine improved angina and vascular function,38 but increased myocardial infarction in obstructive CAD patients. Hormone therapy improved chest pain symptoms, menopausal symptoms, and quality of life, but did not improve ischaemia or endothelial dysfunction.39 Spinal cord stimulation normalizes abnormal pain perception,40 improves angina, and increases exercise tolerance.41
2.3 Novel therapies
RAAS active agents including ACE-I in a randomized, double-blind, placebo-controlled trial, of women with INOCA and CFR <3.0, found quinapril (80 mg/d) significantly improved CFR over placebo. Both ACE-I treatment and CFR increased significantly contributing to angina improvement. Furthermore, effects on CFR concentrated among women with CFRs <2.5, consistent with the overall WISE hypothesis.42
Other randomized controlled trials of ACE-I in CMD using intracoronary Doppler coronary flow measurements confirmed benefit with enalapril.21 CFR improved with ACE-I in subjects with diabetes,43 and coronary microvascular function improved with ACE-I using positron emission tomography imaging in patients with hypertension,44 although no effect was detected in two very small studies.45,46 Several non-randomized studies using different methods to assess coronary microvascular function in small patient samples, further confirmed ACE-I improved coronary microvascular function.47–50 Finally, ACE-I, with either a calcium antagonist or indapamide, also appeared beneficial.51,52 A small study including patients with diabetes also showed an effect.53
Whether the effect of ACE-I on coronary microvascular function is indirectly mediated via blood pressure (BP) reduction is uncertain, but it is unlikely the only mechanism of benefit given the critical role of angiotensin II in vascular function. Additional evidence suggests ACE-I is associated with improvement in markers of endothelial function, and several circulating biomarkers.25,26,54–56
Mineralocorticoid inhibition (eplerenone) added to ACE-I, in another randomized double-blind, placebo-controlled trial of women with INOCA, provided no additional angina reduction or improvement in CFR.57
2.4 Phosphodiesterase inhibition
Phosphodiesterase (PDE) type 3 inhibition-(cilostazol) is used for intermittent claudication and prevention after stroke, coronary stent restenosis, and percutaneous coronary intervention (PCI). PDE-3 receptors are subdivided into PDE-3As and PDE-3Bs; the former in platelets, vascular smooth muscle cells, and cardiomyocytes; the latter in adipocytes, hepatocytes, pancreatic β-cells, and macrophages. Inhibiting PDE increases intracellular cyclic adenosine monophosphate with anti-platelet, anti-inflammatory, and vasodilatory effects. Benefits in renal and retinal microvascular complications of diabetes were suggested.58 Cilostazol reduces superoxide anion, improves the production of local hepatocyte growth factor, and motility and morphogenesis of epithelial and endothelial cells.
Effects of cilostazol were assessed on coronary responses in patients with vasospastic angina (VSA, acetylcholine provocation). CFR, coronary volumetric flow, and diameter changes by N(G)-monomethyl-L-arginine were all significantly increased with cilostazol.59 In a prospective, multicentre study of patients with spontaneous or ergonovine-provoked VSA refractory to calcium antagonists and nitrates, addition of cilostazol appeared effective.60 In a multicentre randomized, double-blind, placebo-controlled trial of VSA patients refractory to amlodipine, cilostazol reduced angina frequency and intensity without serious adverse effects.61
PDE type 5 inhibition (sildenafil) improved perfusion of hypoperfused myocardium during exercise in a canine model.62 In CAD patients, a single dose of sildenafil, without a placebo comparison, dilated coronary arteries and improved endothelium-dependent vasodilatation.63 Others suggested continuous sildenafil infusion, in double-blinded placebo-controlled crossover design, did not reverse endothelial dysfunction in obstructive CAD patients.64
In women with INOCA, sildenafil acutely improved CFR using open, repeated measures design.65 The observed improvement concentrated among women with CFR <2.5 (i.e. CMD).
These encouraging preliminary findings support the need for additional larger, randomized, double-blind trials with these agents, particularly the longer-acting preparation vardenafil.
Calcium Channel Antagonism is widely used for VSA. Benidipine, a long-acting dihydropyridine, showed a beneficial prognostic impact in VSA patients.56 Additionally, with the hope of improving reduced vasodilator capacity of the coronary microcirculation and reducing cardiac afterload, calcium antagonists are often used for patients with CMD. However, they have shown variable results in trials of INOCA patients.25,26,54,55 It has been reported that intracoronary diltiazem does not improve CFR in patients with microvascular angina (MVA).25 Furthermore, no significant improvement in angina was observed with amlodipine in INOCA patients,55 and verapamil failed to reduce spontaneous episodes of ischaemic ST-segment changes in another study.26 Patients with abnormal vasodilator reserve can have improved symptoms, less nitrate use, and improved exercise tolerance with verapamil or nifedipine.54 Furthermore, long-acting nifedipine also exerted cardiovascular protective effects through inhibition of vascular inflammation and improvement of endothelial function in INOCA patients. Thus, long-acting L-type calcium channel blockers appear to be more favourable for the coronary microcirculation compared with short-acting ones.
Selective If-channel blockade using ivabradine is a specific bradycardic agent that selectively reduces sinus node activity through inhibition of the If-current.66 In contrast to β-blockers, ivabradine does not cause vasoconstriction or negative inotropic effects.66 Beneficial effects of ivabradine in ischaemic heart disease (IHD) are mediated by indirect effects to improve exercise tolerance, prolong time to ischaemia during exercise, and reduce angina severity and frequency vs. other antianginal agents in patients with stable angina.66,67 Ivabradine improved angina in patients with MVA but coronary microvascular function did not change, suggesting that symptomatic improvement could be attributed to heart-rate-lowering effect.31 However, others have found that ivabradine improves CFR in non-obstructed coronary arteries of patients with stable CAD both at the intrinsic heart rate and at paced heart rate identical to that before treatment.68 Thus, ivabradine improves CFR in patients with stable CAD. These effects persist even after heart rate correction indicating improved microvascular function.69 Thus, it is possible that ivabradine and/or perhaps some of the several other If-channel inhibitors have a role in CMD patients, although further studies are needed.
Rho-kinase inhibition impacts small guanosine triphosphate-binding protein Rho that enhances myosin light chain phosphorylation through inhibition of myosin-binding subunit of myosin phosphatase, leading to vascular smooth muscle hypercontraction.70 Rho-kinase has a key role in the pathogenesis of coronary vasomotion abnormalities and associated VSA where its activity in circulating neutrophils is a biomarker for diagnosis, assessment of activity, and long-term prognosis.71 Fasudil, a specific Rho-kinase inhibitor, is highly effective in preventing acetylcholine-induced coronary spasm and resultant myocardial ischaemia.72 The Rho-kinase pathway has been shown to be substantially involved in endothelial dysfunction, vascular smooth muscle hypercontraction, and vasospasm as well as inflammatory cell accumulation in blood vessel adventitia,73 and a pathogenetic mechanism in patients with chest pain and non-obstructive CAD.74 Intracoronary fasudil is effective not only for patients with epicardial coronary spasm72 but also for approximately two-thirds of patients with MVA.75 Fasudil may be effective in CMD when Rho-kinase plays a role of the disorder. In models, myocardial hypertrophy induced by pressure overload leads to myocardial dysfunction, CMD, and ischaemia possibly due to oxidative stress, enhanced vasoconstriction to endothelin‐1 (ET-1), and compromised endothelial NO function via elevated Rho-kinase signalling.76
Endothelin receptor antagonists (ERA) address ET-1, which increases peripheral and coronary vascular tone via ETA‐activation.77–79 ET‐1 contributes to coronary endothelial dysfunction78 and its tonic inhibitory effect on myocardial perfusion is related to atherosclerosis risk factor burden.80 In patients with MVA, ET‐1 is increased and linked with reduced time to onset exercise angina.81 Additionally, some suggest increased ET-1 activity is associated with reduced CFR in women.82 An abnormal pattern of diffuse, heterogeneous myocardial perfusion was associated with ET-1 in CMD83 and the ERA antagonist, darusentan, improved myocardial perfusion and increased the homogeneity perfusion. These findings imply that in patients with CMD, ET‐1 caused regional reductions in myocardial perfusion that can be improved with ERA receptor blockade. In a randomized placebo‐controlled trial of the ERA antagonist, atrasentan for 6 months, in patients with CMD, chronic ERA antagonism improved microvascular coronary endothelial function.84 This was accompanied by reductions in BP and plasma glucose. The Coronary Microvascular Angina (CorMicA) investigators found peripheral arterioles from patients with INOCA showed enhanced constriction to ET‐1 and the thromboxane agonist U46619, compared with reference subjects.85 These findings support the hypothesis that patients with INOCA are at risk of developing more generalized small vessel dysfunction. Two small randomized trials of an ET‐1 receptor antagonist in MVA suggested benefit83,84 and larger trials are needed. To this end, the Precision Medicine With Zibotentan in Microvascular Angina (PRIZE) study is a randomized, double-blind, placebo-controlled, crossover trial of zibotentan, an oral endothelin A receptor-selective antagonist, in MVA (ClinicalTrials.gov Identifier: NCT04097314). A total of 356 patients will be enrolled and at least 100 will be randomized in multiple centres across the UK. The primary outcome is exercise duration on the Bruce treadmill protocol. Secondary outcomes include patient-reported outcome measures, and a cMRI sub-study will provide information on the effects of treatment on myocardial blood flow.
Adenosine active agents include dipyridamole which was proposed to increase CBF by selective coronary vasodilatation without systemic haemodynamic effects,86 and is useful to evaluate CMD in patients without obstructive coronary stenoses.87,88 However, it is limited as an antianginal agent.89 Although additional studies are needed, dipyridamole may be an effective in CMD patients without coronary obstruction. Xanthine derivatives (e.g. caffeine, aminophylline, paraxanthine, pentoxifylline, theobromine, and theophylline) may have beneficial effects for CMD patients related to several mechanisms.90,91 They inhibit arteriolar dilation of adenosine by inhibition of vascular smooth adenosine-A2 receptors, which may redistribute CBF in CMD patients towards ischaemic regions where adenosine release is increased.90 Xanthines may exert an analgesic effect, antagonizing stimulation of cardiac nerve pain fibres by adenosine, a major mediator of ischaemic chest pain.91 However, in a randomized trial, oral aminophylline did not improve exercise-related ischaemic electrocardiogram (ECG) changes in MVA subjects, although angina improved.92 In another study of INOCA patients,93 intravenous aminophylline (total dose 6 mg/kg within 15 min) demonstrated benefit for time to onset ischaemia and exercise-induced chest pain. Disparate effects on symptoms and ST-segment changes of ischaemia warrant additional study as it is possible that xanthines may improve exercise tolerance in MVA, especially if pain sensitivity is the issue.
Myocardial metabolic active agents improve the efficiency of myocardial metabolism and substrate handling. Dichloroacetate (DCA), inhibits all isoforms of pyruvate dehydrogenase (PDH) kinase (PDK), the ‘gate-keeping’ enzyme in glucose oxidation. Myocardial metabolic reprogramming in ischaemia, infarction, and hypertrophy shares similarities with chronic hypoxia. Activation of PDH with DCA during chronic hypoxia maintains myocardial adenosine triphosphate (ATP) and glycogen improving hypoxic tolerance. In a type 2 diabetes model, DCA restored PDH activity, reversed LV diastolic dysfunction and normalized blood glucose.94 A first-in-human trial of DCA in idiopathic pulmonary arterial hypertension (iPAH) suggests that PDK is ‘a druggable target’ for microvascular improvement in ‘genetically susceptible’ patients.95 iPAH is a progressive vascular disease with occlusive vascular remodelling due to pro-proliferative and antiapoptotic environment in the wall of resistance pulmonary arteries. If similar changes occur in coronary resistance vessels in CMD, these findings could lead the way for novel precision medicine approaches to CMD. These findings suggest that PDK activation can improve myocardial function during hypoxic conditions such as ischaemia due to CMD and clinical trials appear warranted.
Carnitine analogues include acetyl-L-carnitine (ALC), propionyl-L-carnitine (PLC), and L-carnitine (LC) which are naturally occurring carnitine derivates formed by carnitine acetyltransferase. Beneficial cardiovascular (CV) effects of ALC and PLC have been extensively evaluated in animal models and humans. Multiple clinical trials have suggested ALC and PLC as potential strategies in management of peripheral arterial disease, myocardial ischaemia, cerebral ischaemia, and heart failure as well as type 2 diabetes, an independent risk factor for cardiovascular disease development. However, as attractive as these carnitine analogues appear, no adequately powered randomized, controlled studies are available in English language searches that focus on INOCA patients or CMD.
Perhexiline, classed as a coronary vasodilator, binds to mitochondrial carnitine palmitoyltransferase (CPT)-1 and CPT-2 to shift myocardial substrate utilization from long-chain fatty acids to carbohydrates through inhibition of CPT-1 and, to a lesser extent, CPT-2. This increases glucose and lactate utilization, per unit oxygen consumption and results in an oxygen‐sparing effect to explain remarkable antianginal efficacy.96 This increases ATP production for the same O2 consumption and potentiates platelet responsiveness to NO. Most recently, this agent has been shown to be a KLF14 activator independent of its function in mitochondria.97 Perhexiline reduces leucocyte-endothelial adhesion in vivo and this is a KLF14-dependent protective effect on endothelial cell inflammatory activation.98 Due to hepatic and peripheral nerve toxicity in poor metabolizers (∼10% of exposed), development was discontinued in the USA, but it is used elsewhere with dose modification in poorly metabolizing patients identified through plasma monitoring that can eliminate significant side effects.96 Data with perhexiline from patients with hypertrophic cardiomyopathy, where CMD is prevalent, are encouraging.99
Trimetazidine (TMZ), monotherapy and in combination with other antianginal agents, improved angina and exercise time in 12-double-blind, randomized trials of patients with stable angina.100 In INOCA patients, TMZ improved angina and stress testing results vs. conventional therapy. Moreover, there was an improvement in silent ischaemia, nitrate consumption, functional class, myocardial perfusion, and endothelial function probably related to reduced ET-1 and increased antioxidant status.101 A single TMZ dose increased exercise duration, total work, and improved ECG signs of ischaemia. An ongoing, randomized trial could provide definitive evidence of metabolic cardioprotection for chronic stable angina or acute coronary syndrome in patients post-PCI.102
Metformin prevents diabetes and may reduce adverse outcomes, but effects on microvascular function and angina are unclear. A double-blind, randomized, placebo-controlled study103 of metformin in non-diabetic women with INOCA found metformin reduced weight and insulin resistance, improved endothelium-dependent microvascular function and responses to acetylcholine, exercise-induced ST-segment depression, Duke treadmill score, and chest pain. However, the mechanism is unclear and larger controlled trials of longer duration are warranted.
Amiodarone was introduced as an antianginal in 1967 and used in Europe for stable, unstable, and variant angina.104 Combining amiodarone with multiple conventional antianginal drugs was well-tolerated. In addition to decreased myocardial oxygen demand, amiodarone increased myocardial oxygen supply, improved CFR and prevented coronary constriction.105,106 Intracoronary amiodarone confirmed potent coronary vasodilation with decreased coronary resistance and increased coronary flow.107 In a placebo-controlled trial108 of stable angina patients receiving ‘triple’ antianginal therapy, amiodarone increased exercise duration, rate-pressure-product and reduced ST-segment depression.108 Its iodine-free derivative, dronedarone with better side-effect profile and multichannel blockade, has favourable coronary microcirculatory, myocardial protectant, and remodelling effects in experimental models.109 Dronedarone’s good safety profile and decreased angina hospitalizations support testing in INOCA patients without heart failure.
Anti-inflammation agents block associated endothelial dysfunction that plays a key role in CMD pathogenesis. Specific approaches to modify inflammation and assess-related effects on CMD are difficult since essentially all effective anti-ischaemic and anti-atherosclerosis agents modify inflammation, to some degree.110 Interleukin (IL)-1β is central to the inflammatory response that drives IL-6 signalling. There is considerable interest to test anti-inflammatory agents in CMD. Low-dose methotrexate was ineffective in the Cardiovascular Inflammation Reduction Trial among subjects with obstructive CAD. Colchicine in the LoDoCo2 and COLCOT trials, as well as proposed trials involving other modulators of IL-1, IL-6, and the NLRP3 inflammasome offer promise. IL-1a-anakinra, in rheumatoid arthritis (RA) patients improved myocardial contractility and relaxation, CFR, and brachial fibromuscular dysplasia (FMD).111 IL-6-tocilizumab, an IL-6 receptor antagonist, improved brachial FMD and aortic stiffness in RA.112 Whether these arterial changes are direct effects of IL-6/IL-6 receptor pathway inhibition, maintained over-time, and translate into better outcomes warrants further studies. In revascularized NSTEMI patients, tocilizumab attenuated inflammation and cardiac troponin T release but did not influence coronary microvascular or endothelial function.113
IL-1b inhibition—rilonacept and canakinumab bind IL-beta before it binds to its receptor complex. Canakinumab, a monoclonal antibody targeting IL-1β, reduced CV events independent of lipid-lowering and other treatments in the CANTOS trial.114 Another report suggested beneficial effects on carotid intimal media thickness and arterial stiffness.115 In chronic kidney disease patients, not on dialysis, rilonacept was well-tolerated and improved brachial FMD without changing conduit vessel stiffness as it reduced indices of systemic inflammation.116 Multiple studies suggest that anti-tumour necrosis factor alpha (TNF-α) treatment improves endothelial function in patients with RA, psoriasis and psoriatic arthritis.117,118
SGLT2 channel inhibition—multiple studies demonstrate that blocking endothelial sodium-glucose cotransporter-2 improves endothelial function in diabetes.119 If this improves ischaemia and outcomes in patients with CMD remains to be determined. Also, the effect of SGLT1 and 2 inhibition is unknown. Because these agents improve cardiovascular outcomes in diabetes trials, they offer promise for CMD.
Hormones—androgen analogues—in men with stable angina low-dose testosterone (T, transdermal patch) increased androgen levels two-fold as time to exercise-induced ischaemia increased.120 A placebo-controlled trial in men with angina and low plasma T found T undecanoate injection improved exercise time.80 Larger and longer studies are needed to clarify testosterone treatment in INOCA.100 Oestrogen analogues—post-menopausal oestrogen may be effective121 and in a placebo-controlled trial, there was less-frequent angina with 1 mg norethindrone/10 μg ethinyl oestradiol, fewer hot flashes/night sweats and less avoidance of intimacy.39 But no differences in ischaemia, brachial FMD, compliance, or adverse events between treatments were observed. However, recruitment was limited by safety concerns prompted with Women’s Health Initiative (WHI) hormone trial early results. However, recent WHI data concluded conjugated equine oestrogens (CEO, 0.625 mg/day) plus medroxyprogesterone acetate (2.5 mg/day) for 5.6 years or CEO alone for 7.2 years was not associated with increased all-cause, CV, or cancer mortality during 18 years follow-up.122 These safety data may make it possible to better clarify the role of oestrogen in post-menopausal-women with CMD.
Nervous system active agents are considered since INOCA patients may experience ischaemia with mental stress and transient coronary constriction, which is also reflected in the peripheral microvasculature.123 Catecholamines, acetylcholine, histamine, serotonin, neuropeptide Y (NPY), and other molecules have been proposed. NPY is co-stored with norepinephrine in perivascular sympathetic nerves and released after sympathetic stimulation. This potent vasoconstrictor and inhibitor of cardiac vagal activity induces transient ischaemia by microvascular constriction, and increases during exercise in angina patients.124 A randomized study found NPY inhibition lowered systolic BP during and after exercise,125 however, did not affect exercise-induced ischaemia in CAD patients. NPY also stimulates endothelial differentiation of mesenchymal stem cells and may contribute to myocardial repair by increasing differentiated cardiomyocytes in ischaemia-damaged myocardium.126 This provides a novel possibility for cell-based cardiac repair and remodelling with CMD and after myocardial injury. Further studies are needed to elucidate dose–responses and effects of NPY inhibitors in coronary microvasculature. Selective serotonin reuptake inhibitors—escitalopram reduced platelet serotonin receptor transporters and altered transporter binding affinity density of 5-hydroxytriptan uptake sites and LV diastolic dysfunction and measures of psychological functioning improved without effecting exercise-induced ischaemia.127
Gene and cell-based therapies—including cellular injections with bone-marrow-derived CD34+ cells have shown promise, in refractory angina, some of which is likely related to CMD. Increasing evidence indicates that paracrine factors, or growth and differentiation factors, from transplanted cells and/or reactive immune cells modify healing processes including neovascularization, inflammation, fibrosis, contractility, bioenergetics, and endogenous repair.128 This knowledge, should foster development of novel small-molecule pharmacological strategies derived from these paracrine factors to stimulate endogenous mechanisms and provide alternatives to current therapies for CMD.
Small molecules directly target agents that decrease BP and heart rate, oxidative metabolism, antioxidant, and antiapoptotic activities. These small molecules need to be tested in INOCA experimental models. One such small molecule, fluorine substituent (TT-10), a fluorine-containing biologically active compound, promotes cardiomyocyte proliferation with antiapoptotic and antioxidant effects in vitro and in vivo. In mice with experimental myocardial ischaemia and infarction, TT-10 improved cardiac function attenuating infarct size and fibrosis.129 If these small molecules modified experimental IHD in animal models and showed acceptable safety and effectiveness in acute and chronic CMD, clinical trials could advance this field.
3. Diagnostic Testing Management
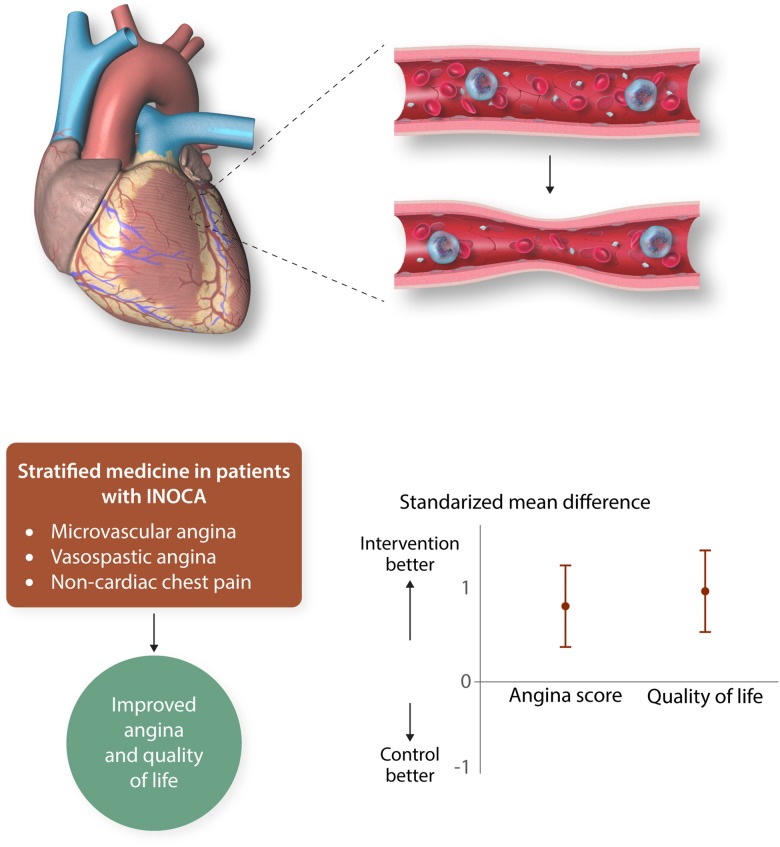
Much is known about the diagnostic value of invasive tests of coronary vascular function, but these tests are rarely used in clinical practice. Due to lack of evidence for patient benefit, therapy should be altered based on coronary function testing. The CorMicA trial130 (Figure 2) addressed this evidence-gap hypothesizing that stratified medicine, combining an interventional diagnostic procedure (IDP) with guideline-linked medical therapy, would be feasible and lead to improvements in angina and QoL in INOCA patients. Accordingly, patients with INOCA (∼3/4 women, age 61) had an IDP, which measured CFR, index of microcirculatory resistance, and fractional flow reserve (intravenous adenosine and intracoronary acetylcholine according to guidelines131) as an adjunctive procedure following angiogram acquisition. Patients were then randomized to either ‘IDP-with results disclosed and ESC guideline-based management’ or ‘sham-with results not disclosed and standard of care’. Among those randomized to IDP recommendations included, for ‘CMD’—a beta-blocker (nebivolol), life-style changes, consider ACE-I and statin; for ‘coronary vasospasm’—calcium antagonist with or without long-acting nitrate, life-style changes; and for ‘non-cardiac chest pain’—stop antianginal drugs, discharge from cardiology, consider non-cardiac investigation. At 6 months, the IDP patients had less angina (P = 0.001), less often and less severe, with improved QoL (P < 0.001) and none required treatment change. No serious adverse events occurred during testing and there were no differences in major adverse cardiac events at 6 months.
Figure 2.
CorMicA. Stratified medical therpay guided by an interventional diagnostic procedure (IDP) improves health status of patients with symptoms and/or sings of angina but no ischaemia and non-obstructive coronary artery disease (INOCA). Permission granted to reproduce figure from Ford et al.130
This ‘first trial’ to investigate a coronary function testing strategy for INOCA, found the majority of INOCA patients have an underlying diagnosis amenable to diagnosis in the catheter laboratory and angina improved when therapy was tailored for a specific coronary disorder. Future trials are anticipated to determine the wider external validity of this approach.
4. Current clinical guidelines
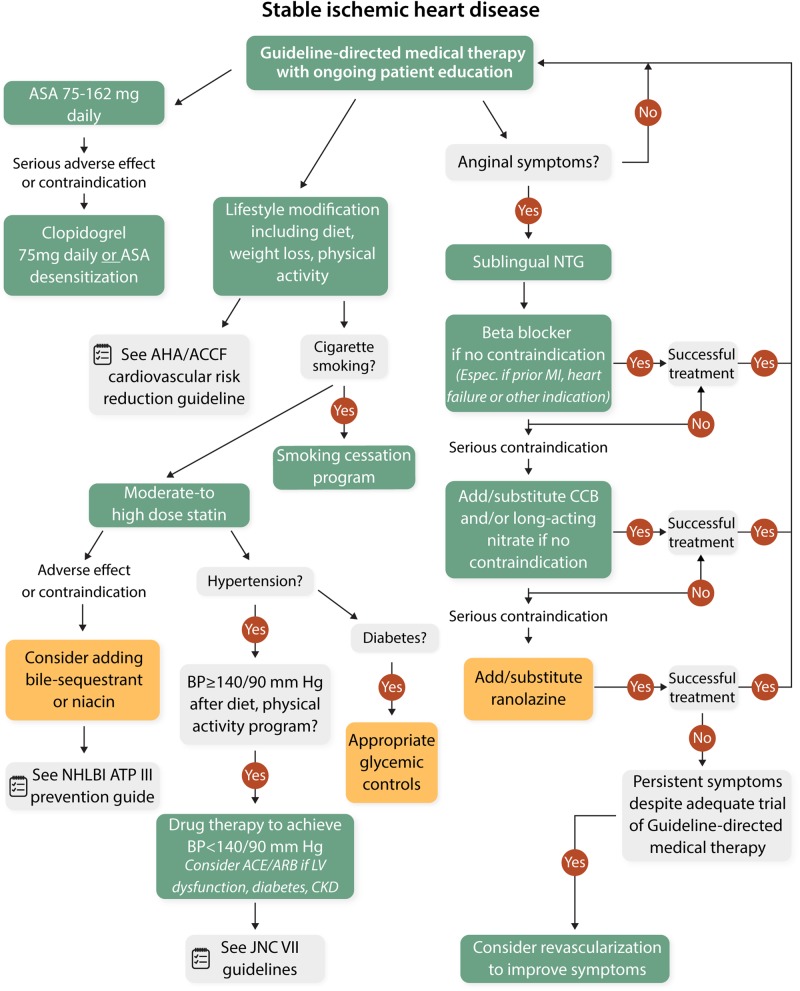
The European Society of Cardiology specifies treatment of MVA and coronary vasospasm (Table 2),132 while the Japanese Circulation Society outlines treatment of VSA.133 Both guidelines confirm relatively low levels of evidence for treatment and no large randomized outcome trials. Recent USA stable IHD guidelines do not directly address angina treatment,134 while prior guidelines do not specifically address CMD or VSA (Figure 3),135 thus are potentially relevant/useful as evidenced in the CorMicA trial.130
Table 2.
Adapted European Society of Cardiology microvascular angina guidelines
| Recommendations | Classa | Levelb |
|---|---|---|
| Guidewire-based CFR and/or microcirculatory resistance measurements should be considered in patients with persistent symptoms, but coronary arteries that are either angiographically normal or have moderate stenoses with preserved iwFR/FFR | IIa | B |
| Intracoronary acetylcholine with ECG monitoring may be considered during angiography, if coronary arteries are either angiographically normal or have moderate stenoses with preserved iwFR/FFR, to assess microvascular spasm | IIb | B |
| Transthoracic Doppler of the LAD, CMR, and PET may be considered for non-invasive assessment of CFR | IIb | B |
Adapted from Bairey Merz et al.32
CFR, coronary flow reserve; CMR, cardiac magnetic resonance; ECG, electrocardiogram; FFR, fractional flow reserve; iwFR, instantaneous wave-free ratio; LAD, left anterior descending; PET, positron emission tomography.
Class of recommendation.
Level of evidence.
Figure 3.
ACCF/AHA/ACP/AATS/PCNA/SCAI/STS stable ischaemic heart disease guidelines. Colours respond to the class of recommendations in the ACCF/AHA. The algorithms do not represent a comprehensive list of recommendations. The use of bile acid sequestrant is relatively contraindicated when triglycerides are ≥200 mg/dL and is contraindicated when triglycerides are ≥500 mg/dL. Dietary supplement niacin must not be used as a substitute for prescription niacin. ACCF, American College of Cardiology Foundation; ACE-I, angiotensin-converting enzyme inhibitor; AHA, American Heart Association; ARB, angiotensin-receptor blocker; ASA, aspirin; ATP III, Adult Treatment Panel 3; BP, blood pressure; CCB, calcium channel blocker; CKD, chronic kidney disease; HDL-C, high density lipoprotein cholesterol; JNC VII, Seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure; LDL-C, low density lipoprotein cholesterol; LV, left ventricular; MI, myocardial infarction; NHLBI, National Heart, Lung and Blood Institute; NTG, nitroglycerine.
5. Knowledge gaps and evidence needed for guidelines
Several knowledge gaps should be addressed to further our understanding of CMD and develop treatment strategies (Figure 4). Population natural history for identification of higher risk phenotypes can provide evidence for at-risk populations to enrol in trials, as well to develop treatment targets. Evidence regarding which antianginal treatments are most effective for angina in INOCA with CMD is needed. Investigation regarding relations between reduction in angina and reductions in adverse cardiovascular events and recurrent hospitalization should be sought.
Figure 4.
CMD and INOCA knowledge gaps.
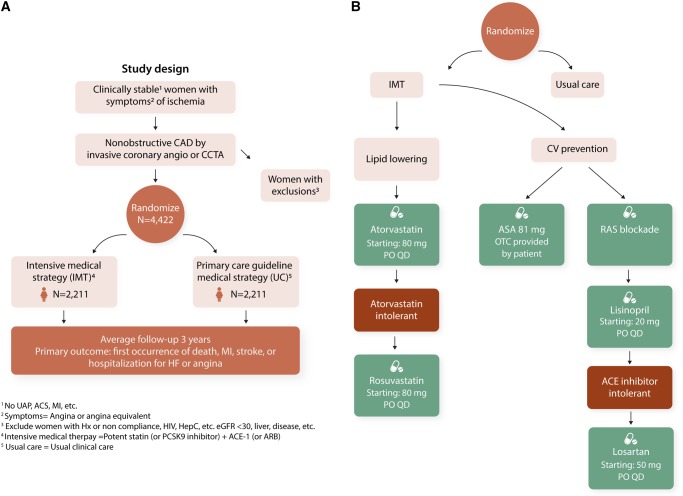
Multiple, relatively small, intermediate outcome trials in subjects using conventional secondary prevention and novel treatments offer promising targets for major outcome trials. There is need for larger randomized outcome trials given the CMD prevalence and lack of evidenced-based guidelines. A large randomized multicentre, prospective, randomized, blinded outcome study evaluating intensive medical therapy (high-intensity statin, ACE-Is or ARBs, and aspirin) vs. usual care in 4422 symptomatic women with INOCA is underway [Women’s Ischaemia Treatment Reduces Events In Non-Obstructive CAD (WARRIOR)-NCT03417388] (Figure 5).
Figure 5.
WARRIOR trial.
6. Summary and conclusions
Currently, treatment for patients with INOCA centres on symptom and primary risk factor therapy and the prevailing practice is largely reassurance, often with repeated testing. This increasingly appears unacceptable because of the documented presence of CMD in most patients, the ischaemia-related symptoms that frequently limit functional status, the relatively high major adverse cardiovascular event rates, and healthcare resource consumption. Prior, small sample size, short-term pharmacologic probe studies in similar subjects suggest symptom/functional improvement. Specifically, potent statins in combination with ACE-Is or if intolerant ARBs, at maximally tolerated doses appear effective for improved angina, stress testing, myocardial perfusion, coronary endothelial function, and microvascular function. Several novel therapies also appear promising and serve the basis for future trial planning. Recent data support benefit of invasive diagnostic testing and specific therapy to improve symptoms and QoL. The ongoing WARRIOR trial (Figure 5) is testing intense medical therapy of high-intensity statin, maximally tolerated ACE-I, and aspirin on longer-term outcomes to gather evidence for CMD management guidelines.
Conflict of interest: C.N.B.M. receives funding from Abbott Diagnostics, Sanofi (paid through CSMC) and serves as Board of Director for iRhythm. C.B. is employed by The University of Glasgow which holds research and consultancy agreements for work done in the course of his employment with the following companies: AstraZeneca, Abbott Vascular, GSK, Heartflow, Menarini, Novartis, Philips and Siemens Healthcare. H.S. is employed by The Tohoku University which holds research and consultancy agreements for his work with the following companies: Bayer Yakuhin, Dai-ichi Sankyo, and Japan Heart Foundation. C.P. receives research grants from GE Healthcare, Merck, Sanofi, CLS Behring, Biocardia, McJunkin Family Foundation, Brigham & Women's Hospital, Gatorade Trust through the University of Florida Department of Medicine, Athersys Inc., AMI MultiStem, and Mesoblast, Inc.; has received consultant fees/honoraria from Verily Life Sciences LLC Project Baseline OSMB (Google), Ironwood, XyloCor, Slack Inc., Imbria Pharmaceuticals, Milestone Pharmaceuticals Inc., Ventrix, Inc., AstraZeneca Pharmaceuticals, and Sanofi-Aventis.
Funding
This work was supported by an unrestricted research grant from Gilead Sciences, and contracts from the National Heart, Lung and Blood Institutes (nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, K23HL105787, T32HL69751, R01 HL090957, 1R03AG032631), the National Institute on Aging, GCRC (MO1-RR00425), the National Center for Research Resources, the National Center for Advancing Translational Sciences (UL1TR000124), the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, the Erika Glazer Women’s Heart Health Project, and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, California. C.J.P. receives support from National Heart, Lung and Blood Institutes through grants HL087366 (University of Florida Regional Clinical Center for the Cardiovascular Cell Therapy Research Network), HL132448 (Brain-Gut Microbiome-Immune Axis in Hypertension), and HL033610 (Angiotensin and Neuroimmune Activation in Hypertension); the Gatorade Trust through funds distributed by the University of Florida, Department of Medicine; National Center for Advancing Translational Sciences—University of Florida Clinical and Translational Science UL1TR001427; PCORnet-OneFlorida Clinical Research Consortium CDRN-1501-26692; and US Dept. of Defense PR161603 (WARRIOR). H.S. is supported by the Japan Heart Foundation, the Japan Society for Promotion of Science (JSPS). C.B. acknowledges research support from the British Heart Foundation (PG/17/2532884; RE/18/6134217) and Medical Research Council (MR/S005714/1).
References
- 1. Kaski JC, Rosano GM, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA.. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol 1995;25:807–814. [DOI] [PubMed] [Google Scholar]
- 2. Kemp HG, Kronmal RA, Vlietstra RE, Frye RL.. Seven year survival of patients with normal or near normal coronary arteriograms: a CASS registry study. J Am Coll Cardiol 1986;7:479–483. [DOI] [PubMed] [Google Scholar]
- 3. Lichtlen PR, Bargheer K, Wenzlaff P.. Long-term prognosis of patients with anginalike chest pain and normal coronary angiographic findings. J Am Coll Cardiol 1995;25:1013–1018. [DOI] [PubMed] [Google Scholar]
- 4. Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CNB.. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med 2009;169:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang F-Y, Huang B-T, Lv W-Y, Liu W, Peng Y, Xia T-L, Wang P-J, Zuo Z-L, Liu R-S, Zhang C, Gui Y-Y, Liao Y-B, Chen M, Zhu Y.. The prognosis of patients with nonobstructive coronary artery disease versus normal arteries determined by invasive coronary angiography or computed tomography coronary angiography: a systematic review. Medicine 2016;95:e3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson BD, Shaw LJ, Buchthal SD, Bairey Merz CN, Kim H-W, Scott KN, Doyle M, Olson MB, Pepine CJ, den Hollander J, Sharaf B, Rogers WJ, Mankad S, Forder JR, Kelsey SF, Pohost GM.. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:2993–2999. [DOI] [PubMed] [Google Scholar]
- 7. Lin FY, Shaw LJ, Dunning AM, LaBounty TM, Choi J-H, Weinsaft JW, Koduru S, Gomez MJ, Delago AJ, Callister TQ, Berman DS, Min JK.. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol 2011;58:510–519. [DOI] [PubMed] [Google Scholar]
- 8. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN.. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mohandas R, Segal MS, Huo T, Handberg EM, Petersen JW, Johnson BD, Sopko G, Bairey Merz CN, Pepine CJ.. Renal function and coronary microvascular dysfunction in women with symptoms/signs of ischemia. PLoS One 2015;10:e0125374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 11. AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, Shaw LJ, Pepine CJ, Ahmed B.. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J Am Coll Cardiol 2019;73:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khuddus MA, Pepine CJ, Handberg EM, Bairey Merz CN, Sopko G, Bavry AA, Denardo SJ, McGORRAY SP, Smith KM, Sharaf BL, Nicholls SJ, Nissen SE, Anderson RD.. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Interv Cardiol 2010;23:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee B-K, Lim H-S, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC, Tremmel JA.. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation 2015;131:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E.. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 15. Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, Leon B, Bhatt DL, Fihn SD, Rumsfeld JS.. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 2014;312:1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF.. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SAJ, Voskuil M, Henriques JPS, Koch KT, de Winter RJ, Spaan JAE, Siebes M, Tijssen JGP, Meuwissen M, Piek JJ.. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv 2014;7:301–311. [DOI] [PubMed] [Google Scholar]
- 18. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL, Camici PG, Chilian WM, Clayton JA, Cooper LS, Crea F, Di Carli M, Douglas PS, Galis ZS, Gurbel P, Handberg EM, Hasan A, Hill JA, Hochman JS, Iturriaga E, Kirby R, Levine GN, Libby P, Lima J, Mehta P, Desvigne-Nickens P, Olive M, Pearson GD, Quyyumi AA, Reynolds H, Robinson B, Sopko G, Taqueti V, Wei J, Wenger N.. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kayikcioglu M, Payzin S, Yavuzgil O, Kultursay H, Can LH, Soydan I.. Benefits of statin treatment in cardiac syndrome-X1. Eur Heart J 2003;24:1999–2005. [DOI] [PubMed] [Google Scholar]
- 20. Pizzi C, Manfrini O, Fontana F, Bugiardini R.. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme A reductase in cardiac Syndrome X: role of superoxide dismutase activity. Circulation 2004;109:53–58. [DOI] [PubMed] [Google Scholar]
- 21. Chen JW, Hsu NW, Wu TC, Lin SJ, Chang MS.. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am J Cardiol 2002;90:974–982. [DOI] [PubMed] [Google Scholar]
- 22. Hamasaki S, Higano ST, Suwaidi JA, Nishimura RA, Miyauchi K, Holmes DR, Lerman A.. Cholesterol-lowering treatment is associated with improvement in coronary vascular remodeling and endothelial function in patients with normal or mildly diseased coronary arteries. Arterioscler Thromb Vasc Biol 2000;20:737–743. [DOI] [PubMed] [Google Scholar]
- 23. Vaughan CJ, Gotto AM Jr, Basson CT.. The evolving role of statins in the management of atherosclerosis. J Am Coll Cardiol 2000;35:1–10. [DOI] [PubMed] [Google Scholar]
- 24. Chiang CY, Chien CY, Qiou WY.. Genetic depletion of thromboxane A2/thromboxane-prostanoid receptor signalling prevents microvascular dysfunction in ischaemia/reperfusion injury. Thromb Haemost 2018;118:1982–1996. [DOI] [PubMed] [Google Scholar]
- 25. Sutsch G, Oechslin E, Mayer I, Hess OM.. Effect of diltiazem on coronary flow reserve in patients with microvascular angina. Int J Cardiol 1995;52:135–143. [DOI] [PubMed] [Google Scholar]
- 26. Bugiardini R, Borghi A, Biagetti L, Puddu P.. Comparison of verapamil versus propranolol therapy in syndrome X. Am J Cardiol 1989;63:286–290. [DOI] [PubMed] [Google Scholar]
- 27.Study to Evaluate Effect of Nebivolol on Angina in Women With Microvascular Disease (NIRVANA). https://clinicaltrials.gov/ct2/show/NCT01665508 (16 January 2019, date last accessed).
- 28. Togni M, Vigorito F, Windecker S, Abrecht L, Wenaweser P, Cook S, Billinger M, Meier B, Hess OM.. Does the beta-blocker nebivolol increase coronary flow reserve? Cardiovasc Drugs Ther 2007;21:99–108. [DOI] [PubMed] [Google Scholar]
- 29. Hasenfuss G, Maier LS.. Mechanism of action of the new anti-ischemia drug ranolazine. Clin Res Cardiol 2008;97:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mehta PK, Goykhman P, Thomson LEJ, Shufelt C, Wei J, Yang Y, Gill E, Minissian M, Shaw LJ, Slomka PJ, Slivka M, Berman DS, Bairey Merz CN.. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging 2011;4:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Villano A, Di Franco A, Nerla R, Sestito A, Tarzia P, Lamendola P, Di Monaco A, Sarullo FM, Lanza GA, Crea F.. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol 2013;112:8–13. [DOI] [PubMed] [Google Scholar]
- 32. Bairey Merz CN, Handberg EM, Shufelt CL, Mehta PK, Minissian MB, Wei J, Thomson LEJ, Berman DS, Shaw LJ, Petersen JW, Brown GH, Anderson RD, Shuster JJ, Cook-Wiens G, Rogatko A, Pepine CJ.. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J 2016;37:1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rambarat CA, Elgendy IY, Handberg EM, Bairey Merz CN, Wei J, Minissian MB, Nelson MD, Thomson LEJ, Berman DS, Shaw LJ, Cook-Wiens G, Pepine CJ.. Late sodium channel blockade improves angina and myocardial perfusion in patients with severe coronary microvascular dysfunction: Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction ancillary study. Int J Cardiol 2019;276:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eriksson BE, Tyni-Lennè R, Svedenhag J, Hallin R, Jensen-Urstad K, Jensen-Urstad M, Bergman K, Sylvén C.. Physical training in syndrome X: physical training counteracts deconditioning and pain in syndrome X. J Am Coll Cardiol 2000;36:1619–1625. [DOI] [PubMed] [Google Scholar]
- 35. Kronhaus KD, Lawson WE.. Enhanced external counterpulsation is an effective treatment for syndrome X. Int J Cardiol 2009;135:256–257. [DOI] [PubMed] [Google Scholar]
- 36. Cannon RO, Quyyumi AA, Mincemoyer R, Stine AM, Gracely RH, Smith WB, Geraci MF, Black BC, Uhde TW, Waclawiw MA, Maher K, Benjamin SB.. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med 1994;330:1411–1417. [DOI] [PubMed] [Google Scholar]
- 37. Cox ID, Hann CM, Kaski JC.. Low dose imipramine improves chest pain but not quality of life in patients with angina and normal coronary angiograms. Eur Heart J 1998;19:250–254. [DOI] [PubMed] [Google Scholar]
- 38. Lerman A, Burnett JC Jr, Higano ST, McKinley LJ, Holmes DR Jr. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation 1998;97:2123–2128. [DOI] [PubMed] [Google Scholar]
- 39. Merz CN, Olson MB, McClure C, Yang YC, Symons J, Sopko G, Kelsey SF, Handberg E, Johnson BD, Cooper-DeHoff RM, Sharaf B, Rogers WJ, Pepine CJ.. A randomized controlled trial of low-dose hormone therapy on myocardial ischemia in postmenopausal women with no obstructive coronary artery disease: results from the National Institutes of Health/National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J 2010;159:987–e981–e987.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sestito A, Lanza GA, Le Pera D, De Armas L, Sgueglia GA, Infusino F, Miliucci R, Tonali PA, Crea F, Valeriani M.. Spinal cord stimulation normalizes abnormal cortical pain processing in patients with cardiac syndrome X. Pain 2008;139:82–89. [DOI] [PubMed] [Google Scholar]
- 41. Lanza G A, Sestito A, Sandric S, Cioni B, Tamburrini G, Barollo A, Crea F, De Seta F, Meglio M, Bellocci F, Maseri A,. Spinal cord stimulation in patients with refractory anginal pain and normal coronary arteries. Ital Heart J .2001;2:25–30. [PubMed] [Google Scholar]
- 42. Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper-DeHoff RM, Sopko G, Sharaf BM, Kelsey SF, Merz CN, Pepine CJ.. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J 2011;162:678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kawata T, Daimon M, Hasegawa R, Teramoto K, Toyoda T, Sekine T, Yamamoto K, Uchida D, Himi T, Yoshida K, Komuro I.. Effect on coronary flow velocity reserve in patients with type 2 diabetes mellitus: comparison between angiotensin-converting enzyme inhibitor and angiotensin II type 1 receptor antagonist. Am Heart J 2006;151:798.e9–798.e15. [DOI] [PubMed] [Google Scholar]
- 44. Akinboboye OO, Chou RL, Bergmann SR.. Augmentation of myocardial blood flow in hypertensive heart disease by angiotensin antagonists: a comparison of lisinopril and losartan. J Am Coll Cardiol 2002;40:703–709. [DOI] [PubMed] [Google Scholar]
- 45. Buus NH, Bøttcher M, Jørgensen CG, Christensen KL, Thygesen K, Nielsen TT, Mulvany MJ.. Myocardial perfusion during long-term angiotensin-converting enzyme inhibition or beta-blockade in patients with essential hypertension. Hypertension (Dallas, Tex: 1979) 2004;44:465–470. [DOI] [PubMed] [Google Scholar]
- 46. Parodi O, Neglia D, Palombo C, Sambuceti G, Giorgetti A, Marabotti C, Gallopin M, Simonetti I, L’Abbate A.. Comparative effects of enalapril and verapamil on myocardial blood flow in systemic hypertension. Circulation 1997;96:864–873. [DOI] [PubMed] [Google Scholar]
- 47. Masuda D, Nohara R, Tamaki N, Hosokawa R, Inada H, Hirai T, Li-Guang C, Tadamura E, Kudou T, Konishi J, Fujita M, Sasayama S.. Evaluation of coronary blood flow reserve by 13N-NH3 positron emission computed tomography (PET) with dipyridamole in the treatment of hypertension with the ACE inhibitor (Cilazapril). Ann Nucl Med 2000;14:353–360. [DOI] [PubMed] [Google Scholar]
- 48. Schwartzkopff B, Brehm M, Mundhenke M, Strauer BE.. Repair of coronary arterioles after treatment with perindopril in hypertensive heart disease. Hypertension (Dallas, Tex: 1979) 2000;36:220–225. [DOI] [PubMed] [Google Scholar]
- 49. Stamatelopoulos K, Bramos D, Manios E, Alexaki E, Kaladaridou A, Georgiopoulos G, Koroboki E, Kolyviras A, Stellos K, Zakopoulos N, Toumanidis S.. Pleiotropic effects of the acute and chronic inhibition of the renin-angiotensin system in hypertensives. J Hum Hypertens 2014;28:378–383. [DOI] [PubMed] [Google Scholar]
- 50. Motz W, Strauer BE.. Improvement of coronary flow reserve after long-term therapy with enalapril. Hypertension (Dallas, Tex: 1979) 1996;27:1031–1038. [DOI] [PubMed] [Google Scholar]
- 51. Engholm M, Mulvany MJ, Eftekhari A, Mathiassen ON, Buus NH, Christensen KL.. Positive effects of aggressive vasodilator treatment of well-treated essential hypertensive patients. J Hum Hypertens 2016;30:690–696. [DOI] [PubMed] [Google Scholar]
- 52. Neglia D, Fommei E, Varela-Carver A, Mancini M, Ghione S, Lombardi M, Pisani P, Parker H, D'amati G, Donato L, Camici PG.. Perindopril and indapamide reverse coronary microvascular remodelling and improve flow in arterial hypertension. J Hypertens 2011;29:364–372. [DOI] [PubMed] [Google Scholar]
- 53. Kawata T, Daimon M, Hasegawa R, Teramoto K, Toyoda T, Sekine T, Yamamoto K, Uchida D, Himi T, Yoshida K, Komuro I.. Effect of angiotensin-converting enzyme inhibitor on serum asymmetric dimethylarginine and coronary circulation in patients with type 2 diabetes mellitus. Int J Cardiol 2009;132:286–288. [DOI] [PubMed] [Google Scholar]
- 54. Cannon RO 3rd, Watson RM, Rosing DR, Epstein SE.. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. Am J Cardiol 1985;56:242–246. [DOI] [PubMed] [Google Scholar]
- 55. Lanza GA, Colonna G, Pasceri V, Maseri A.. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am J Cardiol 1999;84:854–856, a858. [DOI] [PubMed] [Google Scholar]
- 56. Nishigaki K, Inoue Y, Yamanouchi Y, Fukumoto Y, Yasuda S, Sueda S, Urata H, Shimokawa H, Minatoguchi S.. Prognostic effects of calcium channel blockers in patients with vasospastic angina—a meta-analysis. Circ J 2010;74:1943–1950. [DOI] [PubMed] [Google Scholar]
- 57. Bavry AA, Handberg EM, Huo T, Lerman A, Quyyumi AA, Shufelt C, Sharaf B, Merz CNB, Cooper-DeHoff RM, Sopko G, Pepine CJ.. Aldosterone inhibition and coronary endothelial function in women without obstructive coronary artery disease: an ancillary study of the national heart, lung, and blood institute-sponsored women’s ischemia syndrome evaluation. Am Heart J 2014;167:826–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Asal NJ, Wojciak KA.. Effect of cilostazol in treating diabetes-associated microvascular complications. Endocrine 2017;56:240–244. [DOI] [PubMed] [Google Scholar]
- 59. Watanabe K, Ikeda S, Komatsu J, Inaba S, Suzuki J, Sueda S, Funada J-I, Kitakaze M, Sekiya M.. Effect of cilostazol on vasomotor reactivity in patients with vasospastic angina pectoris. Am J Cardiol 2003;92:21–25. [DOI] [PubMed] [Google Scholar]
- 60. Yoo S-Y, Song S-G, Lee J-H, Shin E-S, Kim J-S, Park Y-H, Kim J, Chun K-J, Kim J-H.. Efficacy of cilostazol on uncontrolled coronary vasospastic angina: a pilot study. Cardiovasc Ther 2013;31:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shin ES, Lee JH, Yoo SY, Park Y, Hong YJ, Kim MH, Lee JY, Nam CW, Tahk SJ, Kim JS, Jeong YH, Lee CW, Shin HK, Kim JH.. A randomised, multicentre, double blind, placebo controlled trial to evaluate the efficacy and safety of cilostazol in patients with vasospastic angina. Heart (British Cardiac Society) 2014;100:1531–1536. [DOI] [PubMed] [Google Scholar]
- 62. Traverse JH, Chen YJ, Du R, Bache RJ.. Cyclic nucleotide phosphodiesterase type 5 activity limits blood flow to hypoperfused myocardium during exercise. Circulation 2000;102:2997–3002. [DOI] [PubMed] [Google Scholar]
- 63. Halcox JPJ, Nour KRA, Zalos G, Mincemoyer R, Waclawiw MA, Rivera CE, Willie G, Ellahham S, Quyyumi AA.. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol 2002;40:1232–1240. [DOI] [PubMed] [Google Scholar]
- 64. Robinson SD, Ludlam CA, Boon NA, Newby DE.. Phosphodiesterase type 5 inhibition does not reverse endothelial dysfunction in patients with coronary heart disease. Heart (British Cardiac Society) 2006;92:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Denardo SJ, Wen X, Handberg EM, Bairey Merz CN, Sopko GS, Cooper-DeHoff RM, Pepine CJ.. Effect of phosphodiesterase type 5 inhibition on microvascular coronary dysfunction in women: a Women’s Ischemia Syndrome Evaluation (WISE) ancillary study. Clin Cardiol 2011;34:483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Borer JS, Fox K, Jaillon P, Lerebours G; Ivabradine Investigators Group. Antianginal and antiischemic effects of ivabradine, an I(f) inhibitor, in stable angina: a randomized, double-blind, multicentered, placebo-controlled trial. Circulation 2003;107:817–823. [DOI] [PubMed] [Google Scholar]
- 67. Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K, Investigators I.. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J 2005;26:2529–2536. [DOI] [PubMed] [Google Scholar]
- 68. Skalidis EI, Hamilos MI, Chlouverakis G, Zacharis EA, Vardas PE.. Ivabradine improves coronary flow reserve in patients with stable coronary artery disease. Atherosclerosis 2011;215:160–165. [DOI] [PubMed] [Google Scholar]
- 69. Camici PG, Gloekler S, Levy BI, Skalidis E, Tagliamonte E, Vardas P, Heusch G.. Ivabradine in chronic stable angina: effects by and beyond heart rate reduction. Int J Cardiol 2016;215:1–6. [DOI] [PubMed] [Google Scholar]
- 70. Shimokawa H, Sunamura S, Satoh K.. RhoA/Rho-kinase in the cardiovascular system. Circ Res 2016;118:352–366. [DOI] [PubMed] [Google Scholar]
- 71. Nihei T, Takahashi J, Hao K, Kikuchi Y, Odaka Y, Tsuburaya R, Nishimiya K, Matsumoto Y, Ito K, Miyata S, Sakata Y, Shimokawa H.. Prognostic impacts of Rho-kinase activity in circulating leucocytes in patients with vasospastic angina. Eur Heart J 2018;39:952–959. [DOI] [PubMed] [Google Scholar]
- 72. Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A.. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation 2002;105:1545–1547. [DOI] [PubMed] [Google Scholar]
- 73. Ohyama K, Matsumoto Y, Takanami K, Ota H, Nishimiya K, Sugisawa J, Tsuchiya S, Amamizu H, Uzuka H, Suda A, Shindo T, Kikuchi Y, Hao K, Tsuburaya R, Takahashi J, Miyata S, Sakata Y, Takase K, Shimokawa H.. Coronary adventitial and perivascular adipose tissue inflammation in patients with vasospastic angina. J Am Coll Cardiol 2018;71:414–425. [DOI] [PubMed] [Google Scholar]
- 74. Suda A, Takahashi J, Hao K, Kikuchi Y, Shindo T, Ikeda S, Sato K, Sugisawa J, Matsumoto Y, Miyata S, Sakata Y, Shimokawa H.. Coronary functional abnormalities in patients with angina and nonobstructive coronary artery disease. J Am Coll Cardiol 2019;74:2350–2360. [DOI] [PubMed] [Google Scholar]
- 75. Mohri M, Shimokawa H, Hirakawa Y, Masumoto A, Takeshita A.. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol 2003;41:15–19. [DOI] [PubMed] [Google Scholar]
- 76. Tsai SH, Lu G, Xu X, Ren Y, Hein TW, Kuo L.. Enhanced endothelin-1/Rho-kinase signalling and coronary microvascular dysfunction in hypertensive myocardial hypertrophy. Cardiovasc Res 2017;113:1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Halcox JP, Nour KR, Zalos G, Quyyumi AA.. Coronary vasodilation and improvement in endothelial dysfunction with endothelin ET(A) receptor blockade. Circ Res 2001;89:969–976. [DOI] [PubMed] [Google Scholar]
- 78. MacCarthy PA, Pegge NC, Prendergast BD, Shah AM, Groves PH.. The physiological role of endogenous endothelin in the regulation of human coronary vasomotor tone. J Am Coll Cardiol 2001;37:137–143. [DOI] [PubMed] [Google Scholar]
- 79. Verhaar MC, Strachan FE, Newby DE, Cruden NL, Koomans HA, Rabelink TJ, Webb DJ.. Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation 1998;97:752–756. [DOI] [PubMed] [Google Scholar]
- 80. Mather KJ, Lteif AA, Veeneman E, Fain R, Giger S, Perry K, Hutchins GD.. Role of endogenous ET-1 in the regulation of myocardial blood flow in lean and obese humans. Obesity (Silver Spring) .2010;18:63–70. [DOI] [PubMed] [Google Scholar]
- 81. Kaski JC, Elliott PM, Salomone O, Dickinson K, Gordon D, Hann C, Holt DW.. Concentration of circulating plasma endothelin in patients with angina and normal coronary angiograms. Br Heart J 1995;74:620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cox ID, Botker HE, Bagger JP, Sonne HS, Kristensen BO, Kaski JC.. Elevated endothelin concentrations are associated with reduced coronary vasomotor responses in patients with chest pain and normal coronary arteriograms. J Am Coll Cardiol 1999;34:455–460. [DOI] [PubMed] [Google Scholar]
- 83. Johnson NP, Gould KL.. Physiology of endothelin in producing myocardial perfusion heterogeneity: a mechanistic study using darusentan and positron emission tomography. J Nucl Cardiol 2013;20:835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Reriani M, Raichlin E, Prasad A, Mathew V, Pumper GM, Nelson RE, Lennon R, Rihal C, Lerman LO, Lerman A.. Long-term administration of endothelin receptor antagonist improves coronary endothelial function in patients with early atherosclerosis. Circulation 2010;122:958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ford TJ, Rocchiccioli P, Good R, McEntegart M, Eteiba H, Watkins S, Shaukat A, Lindsay M, Robertson K, Hood S, Yii E, Sidik N, Harvey A, Montezano AC, Beattie E, Haddow L, Oldroyd KG, Touyz RM, Berry C.. Systemic microvascular dysfunction in microvascular and vasospastic angina. Eur Heart J 2018;39:4086–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Marchant E, Pichard A, Rodriguez JA, Casanegra P.. Acute effect of systemic versus intracoronary dipyridamole on coronary circulation. Am J Cardiol 1986;57:1401–1404. [DOI] [PubMed] [Google Scholar]
- 87. Mandoli GE, Cameli M, Minardi S, Crudele F, Lunghetti S, Mondillo S.. Layer-specific strain in dipyridamole stress echo: a new tool for the diagnosis of microvascular angina. Echocardiography 2018;35:2005–2013. [DOI] [PubMed] [Google Scholar]
- 88. Michelsen MM, Pena A, Mygind ND, Bech J, Gustafsson I, Kastrup J, Hansen HS, Høst N, Hansen PR, Prescott E.. Coronary microvascular dysfunction and myocardial contractile reserve in women with angina and no obstructive coronary artery disease. Echocardiography 2018;35:196–203. [DOI] [PubMed] [Google Scholar]
- 89. Picano E; Pisa study group. Dipyridamole in chronic stable angina pectoris: a randomized, double blind, placebo-controlled, parallel group study. Eur Heart J 2001;22:1785–1793. [DOI] [PubMed] [Google Scholar]
- 90. Crea F, Gaspardone A, Araujo L, Da Silva R, Kaski JC, Davies G, Maseri A.. Effects of aminophylline on cardiac function and regional myocardial perfusion: implications regarding its antiischemic action. Am Heart J 1994;127:817–824. [DOI] [PubMed] [Google Scholar]
- 91. Crea F, Pupita G, Galassi AR, el-Tamimi H, Kaski JC, Davies G, Maseri A.. Role of adenosine in pathogenesis of anginal pain. Circulation 1990;81:164–172. [DOI] [PubMed] [Google Scholar]
- 92. Elliott PM, Krzyzowska-Dickinson K, Calvino R, Hann C, Kaski JC.. Effect of oral aminophylline in patients with angina and normal coronary arteriograms (cardiac syndrome X). Heart (British Cardiac Society) 1997;77:523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yeşildağ O, Yazici M, Yilmaz O, Uçar R, Sağkan O.. The effect of aminophylline infusion on the exercise capacity in patients with syndrome X. Acta Cardiol 1999;54:335–337. [PubMed] [Google Scholar]
- 94. Le Page LM, Rider OJ, Lewis AJ, Ball V, Clarke K, Johansson E, Carr CA, Heather LC, Tyler DJ.. Increasing pyruvate dehydrogenase flux as a treatment for diabetic cardiomyopathy: a combined 13C hyperpolarized magnetic resonance and echocardiography study. Diabetes 2015;64:2735–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Michelakis ED, Gurtu V, Webster L, Barnes G, Watson G, Howard L, Cupitt J, Paterson I, Thompson RB, Chow K, O'Regan DP, Zhao L, Wharton J, Kiely DG, Kinnaird A, Boukouris AE, White C, Nagendran J, Freed DH, Wort SJ, Gibbs JSR, Wilkins MR.. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med 2017;9:eaao4583. [DOI] [PubMed] [Google Scholar]
- 96. Ashrafian H, Horowitz JD, Frenneaux MP.. Perhexiline. Cardiovasc Drug Rev 2007;25:76–97. [DOI] [PubMed] [Google Scholar]
- 97. Guo Y, Fan Y, Zhang J, Lomberk GA, Zhou Z, Sun L, Mathison AJ, Garcia-Barrio MT, Zhang J, Zeng L, Li L, Pennathur S, Willer CJ, Rader DJ, Urrutia R, Chen YE.. Perhexiline activates KLF14 and reduces atherosclerosis by modulating ApoA-I production. J Clin Invest 2015;125:3819–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hu W, Lu H, Zhang J, Fan Y, Chang Z, Liang W, Wang H, Zhu T, Garcia-Barrio MT, Peng D, Chen YE, Guo Y.. Kruppel-like factor 14, a coronary artery disease associated transcription factor, inhibits endothelial inflammation via NF-kappaB signaling pathway. Atherosclerosis 2018;278:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cooper RM, Raphael CE, Liebregts M, Anavekar NS, Veselka J.. New developments in hypertrophic cardiomyopathy. Can J Cardiol 2017;33:1254–1265. [DOI] [PubMed] [Google Scholar]
- 100. Marzilli M, Klein WW.. Efficacy and tolerability of trimetazidine in stable angina: a meta-analysis of randomized, double-blind, controlled trials. Coron Artery Dis 2003;14:171–179. [DOI] [PubMed] [Google Scholar]
- 101. Leonova IA, Boldeuva S, Zakharova O, Gaykovaya L.. Trimetazidine improves symptoms and reduces microvascular dysfunction in patients with microvascular angina. Eur Heart J 2017;38(Suppl. 1):ehx501P887. [Google Scholar]
- 102. Ferrari R, Ford I, Fox K, Marzilli M, Tendera M, Widimský P, Challeton JP, Danchin N.. A randomized, double-blind, placebo-controlled trial to assess the efficAcy and safety of Trimetazidine in patients with angina pectoris having been treated by percutaneous coronary intervention (ATPCI study): rationale, design, and baseline characteristics. Am Heart J 2019;210:98–107. [DOI] [PubMed] [Google Scholar]
- 103. Jadhav S, Ferrell W, Greer IA, Petrie JR, Cobbe SM, Sattar N.. Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol 2006;48:956–963. [DOI] [PubMed] [Google Scholar]
- 104. Vastesaeger M, Gillot P, Rasson G.. Etude clinique d’une nouvelle medication antiangoreuse. Acta Cardiol 1967;22:483–500. [Google Scholar]
- 105. Bertholet M, Hastir F, Renier J, Demoulin JC, Kulbertus HE.. The value of amiodarone for the treatment of unstable angina. Acta Cardiol 1983;38:503–511. [PubMed] [Google Scholar]
- 106. Rutitzky B, Girotti AL, Rosenbaum MB.. Efficacy of chronic amiodarone therapy in patients with variant angina pectoris and inhibition of ergonovine coronary constriction. Am Heart J 1982;103:38–43. [DOI] [PubMed] [Google Scholar]
- 107. Cote P, Bourassa MG, Delaye J, Janin A, Froment R, David P.. Effects of amiodarone on cardiac and coronary hemodynamics and on myocardial metabolism in patients with coronary artery disease. Circulation 1979;59:1165–1172. [DOI] [PubMed] [Google Scholar]
- 108. Meyer BJ, Amann FW.. Additional antianginal efficacy of amiodarone in patients with limiting angina pectoris. Am Heart J 1993;125:996–1001. [DOI] [PubMed] [Google Scholar]
- 109. Quintana-Villamandos B, Delgado-Martos MJ, Delgado-Baeza E.. Impact of a multichannel blocker in attenuating intramyocardial artery remodeling in hypertensive rats through increased nitric oxide bioavailability. BioMed Res Int 2019;2019:6374582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lucas AR, Korol R, Pepine CJ.. Inflammation in atherosclerosis: some thoughts about acute coronary syndromes. Circulation 2006;113:e728–e732. [DOI] [PubMed] [Google Scholar]
- 111. Ikonomidis I, Lekakis JP, Nikolaou M, Paraskevaidis I, Andreadou I, Kaplanoglou T, Katsimbri P, Skarantavos G, Soucacos PN, Kremastinos DT.. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation 2008;117:2662–2669. [DOI] [PubMed] [Google Scholar]
- 112. Protogerou AD, Zampeli E, Fragiadaki K, Stamatelopoulos K, Papamichael C, Sfikakis PP.. A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis 2011;219:734–736. [DOI] [PubMed] [Google Scholar]
- 113. Holte E, Kleveland O, Ueland T, Kunszt G, Bratlie M, Broch K, Michelsen AE, Bendz B, Amundsen BH, Aakhus S, Damås JK, Gullestad L, Aukrust P, Wiseth R.. Effect of interleukin-6 inhibition on coronary microvascular and endothelial function in myocardial infarction. Heart (British Cardiac Society) 2017;103:1521–1527. [DOI] [PubMed] [Google Scholar]
- 114. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ.. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 115. Eliseev M, Zhelyabina O, Vladimirov S, Markelova E, Novikova D, Korsakova O, Nasonov E.. Analysis of interleukin-1β inhibitor (Canakinumab) impact on endothelial dysfunction. Ann Rheum Dis 2016;75(Suppl. 2):1183. [Google Scholar]
- 116. Nowak KL, Chonchol M, Ikizler TA, Farmer-Bailey H, Salas N, Chaudhry R, Wang W, Smits G, Tengesdal I, Dinarello CA, Hung AM.. IL-1 inhibition and vascular function in CKD. J Am Soc Nephrol 2017;28:971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ursini F, Leporini C, Bene F, D’Angelo S, Mauro D, Russo E, De Sarro G, Olivieri I, Pitzalis C, Lewis M, Grembiale RD.. Anti-TNF-alpha agents and endothelial function in rheumatoid arthritis: a systematic review and meta-analysis. Sci Rep 2017;7:5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Brezinski EA, Follansbee MR, Armstrong EJ, Armstrong AW.. Endothelial dysfunction and the effects of TNF inhibitors on the endothelium in psoriasis and psoriatic arthritis: a systematic review. Curr Pharm Des 2014;20:513–528. [DOI] [PubMed] [Google Scholar]
- 119. Pulakazhi Venu VK, El-Daly M, Saifeddine M, Hirota SA, Ding H, Triggle CR, Hollenberg MD.. Minimizing hyperglycemia-induced vascular endothelial dysfunction by inhibiting endothelial sodium-glucose cotransporter 2 and attenuating oxidative stress: implications for treating individuals with type 2 diabetes. Can J Diabetes 2019;43:510–514. [DOI] [PubMed] [Google Scholar]
- 120. English KM, Steeds RP, Jones TH, Diver MJ, Channer KS.. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study. Circulation 2000;102:1906–1911. [DOI] [PubMed] [Google Scholar]
- 121. Adamson DL, Webb CM, Collins P.. Esterified estrogens combined with methyltestosterone improve emotional well-being in postmenopausal women with chest pain and normal coronary angiograms. Menopause (New York, NY) 2001;8:233–238. [DOI] [PubMed] [Google Scholar]
- 122. Manson JE, Aragaki AK, Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Chlebowski RT, Howard BV, Thomson CA, Margolis KL, Lewis CE, Stefanick ML, Jackson RD, Johnson KC, Martin LW, Shumaker SA, Espeland MA, Wactawski-Wende J; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative Randomized Trials. JAMA 2017;318:927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hammadah M, Kim JH, Al Mheid I, Samman Tahhan A, Wilmot K, Ramadan R, Alkhoder A, Khayata M, Mekonnen G, Levantsevych O, Bouchi Y, Kaseer B, Choudhary F, Gafeer MM, Corrigan FE 3rd, Shah AJ, Ward L, Kutner M, Bremner JD, Sheps DS, Raggi P, Vaccarino V, Samad H, Mavromatis K, Quyyumi AA.. Coronary and peripheral vasomotor responses to mental stress. J Am Heart Assoc 2018;7:e008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gullestad L, Jørgensen B, Bjurø T, Pernow J, Lundberg JM, Dota C-D, Hall C, Simonsen S, ÅBlad B.. Postexercise ischemia is associated with increased neuropeptide Y in patients with coronary artery disease. Circulation 2000;102:987–993. [DOI] [PubMed] [Google Scholar]
- 125. Gullestad L, Bjuro T, Aaberge L, Apelland T, Skårdal R, Kjekshus E, Nordlander M, Ablad B, Pernow J.. The effect of a neuropeptide Y Y1 receptor antagonist in patients with angina pectoris. Eur Heart J 2003;24:1120–1127. [DOI] [PubMed] [Google Scholar]
- 126. Wang Y, Zhang D, Ashraf M, Zhao T, Huang W, Ashraf A, Balasubramaniam A.. Combining neuropeptide Y and mesenchymal stem cells reverses remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol 2010;298:H275–H286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Jiang W, Velazquez EJ, Kuchibhatla M, Samad Z, Boyle SH, Kuhn C, Becker RC, Ortel TL, Williams RB, Rogers JG, O’Connor C.. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA 2013;309:2139–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Burchfield JS, Dimmeler S.. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair 2008;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hara H, Takeda N, Kondo M, Kubota M, Saito T, Maruyama J, Fujiwara T, Maemura S, Ito M, Naito AT, Harada M, Toko H, Nomura S, Kumagai H, Ikeda Y, Ueno H, Takimoto E, Akazawa H, Morita H, Aburatani H, Hata Y, Uchiyama M, Komuro I.. Discovery of a small molecule to increase cardiomyocytes and protect the heart after ischemic injury. JACC Basic Transl Sci 2018;3:639–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sidik N, McCartney P, Corcoran D, Collison D, Rush C, McConnachie A, Touyz RM, Oldroyd KG, Berry C.. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol 2018;72:2841–2855. [DOI] [PubMed] [Google Scholar]
- 131. Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN.. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 132. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2019;41:407–477. [DOI] [PubMed] [Google Scholar]
- 133. Group J. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J 2014;78:2779–2801. [DOI] [PubMed] [Google Scholar]
- 134. Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014;64:1929–1949. [DOI] [PubMed] [Google Scholar]
- 135. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, Kligfield PD, Krumholz HM, Kwong RYK, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR, Smith SC, Spertus JA, Williams SV; Society of Thoracic Surgeons. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012;60:e44–e164. [DOI] [PubMed] [Google Scholar]