Abstract
Introduction
Antiretroviral therapy (ART) is recommended for all people who are living with HIV to suppress viral load and to stop the progression and transmission of HIV-1. Fixed-dose combinations of antiretrovirals largely reduce pill burden.
Areas covered
The authors first provide an overview of the use of non-nucleoside reverse transcriptase inhibitor (NNRTI) based therapy in HIV care. They then summarize the properties of each drug in the fixed-dose combination of tenofovir alafenamide /emtricitabine/rilpivirine/ (TAF/FTC/RPV). The efficacy and safety of each component and the combination as a whole are reviewed: FTC is non-inferior to lamivudine (3TC) at assessed dosages; TAF was non-inferior to tenofovir disoproxil fumarate (TDF); the viral efficacy of RPV is non-inferior with EFV at the assessed dosage; TAF/FTC/RPV is non-inferior in efficacy but shows less of a decline in bone mineral density and renal function compared to TDF/FTC/RPV. Finally, adverse effects and drug-drug interaction data with FTC/RPV/TAF are discussed.
Expert opinion
TAF/FTC/RPV can be used as an initial regimen for people living with HIV whose HIV RNA<100,000 copies/ml and CD4 cell count > 200 cells/mm3 when INSTI-based regimens are not a treatment option. Future antiretroviral therapy development may focus on dual therapy-based regimens containing RPV, particularly as long-acting formulations.
Keywords: Antiretroviral therapy, Emtricitabine, Rilpivirine, Tenofovir alafenamide
1.0. Introduction
As of 2017, approximately 36.9 million people are living with HIV/AIDS worldwide. Among them, an estimated 21.7 million people (59%) had access to antiretroviral therapy (ART). ART significantly reduces mortality, morbidity and improves the quality of life for people living with HIV. [1, 2]Approximately a 63% reduction in mortality rate has been observed in people living with HIV who received ART within 30 days compared to no ART treatment. [3] Many people living with HIV who take their ART daily as prescribed are able to live healthy lives with an undetectable plasma viral load, substantially reducing the risk of transmitting the virus.[4, 5]
Fixed-dose combinations of antiretrovirals are designed to co-formulate antiretroviral drugs into a single tablet, largely simplifying ART, leading to maximal and durable suppression of viral load. Fixed-dose combinations of antiretrovirals contain two or more drugs, from one or more drug classes. Currently, seven classes of antiretroviral drugs which work on various stages of the viral life cycle have been approved by the US Food and Drug Administration (FDA): HIV protease inhibitors (PIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), fusion inhibitors (FIs), CCR5 antagonists, integrase strand transfer inhibitors (INSTIs) and CD4 post-attachment inhibitors. [6] ART with three drugs is now the standard of care and is recommended for most people living with HIV [7, 8]
The use of tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC), plus a third agent has been recommended by various guidelines: the WHO suggests TDF+ lamivudine (3TC) or FTC containing regimens as preferred first-line regimen for ART naïve adults and adolescents.[6] The US Department of Health and Human Services (DHHS) guidelines suggested that Complera®, a fixed dose combination of rilpivirine (RPV), FTC and TDF, may be used as initial therapy for ART naïve patients with HIV-1 RNA ≤100,000 copies/ml or to serve as a substitute regimen for patients who are virologically suppressed (HIV-1 RNA <50 copies/ml, CD 4 cell count>200) with other regimens for at least 6 month. [9]
Both TDF and tenofovir alafenamide (TAF) are prodrugs of tenofovir (TFV) which are further converted to the active metabolite tenofovir diphosphate (TFV-DP) intracellularly. Compared to TDF, TAF has considerable merits to replace TDF in fixed dose formulations: A 90% decrease in TFV plasma concentration and 2.41 fold increase in cell associated TFV-DP concentration were observed after switching to TAF (TAF 10mg/FTC 200mg/EVG 150mg/COBI 150mg) containing regimen from TDF ( TDF 300mg/FTC 200mg/EVG 150mg/COBI 150mg).[10] Higher intracellular accumulation of tenofovir diphosphate allows for a smaller dose of TAF (25mg) compared with TDF (300mg). Additionally, the reduced systemic exposure of tenofovir observed with TAF administration decreases the risk of renal toxicity since tenofovir is primarily eliminated via the kidney. A lower dose of TAF also translates into smaller tablets and easier coformulation with other antiretrovirals drugs which may improve drug adherence. Odefsey® which differs from Complera by substituting TAF for TDF (TAF/FTC/RPV) has been evaluated for efficacy and safety in HIV infected adults who switched from TDF/FTC/RPV treatment to TAF/FTC/RPV.[11]
2.0. Overview of the Market
Although NNRTI-based ART regimens were preferred first-line standard of HIV care for nearly 20 years after their first FDA approval in 1996, their use and place in care continues to decline. Current recommendations by the DHHS guidelines for the use of antiretroviral agents in adults and adolescents living with HIV restrict “recommended initial regimens for most people with HIV” to INSTI based therapies.[7] However, NNRTI-based regimens continue to have a place as first-line treatment of naïve individuals under “recommended initial regimens in certain clinical situations”. For example, doravirine, efavirenz and rilpivirine based regimens, in combination with two nucleosides are all first-line treatment options in “certain clinical situations” where INSTI based regimens cannot be used. Additionally, NNRTI’s continue to hold a place in the treatment of ART-experienced individuals, particularly those individuals who have developed virologic failure to a previous ART regimen.[7] International guidelines have varied in their approach to recommendations for first-line agents, and often consider the in-country availability of particular ARV agents, particularly as generic formulations, when making treatment recommendations. The current WHO HIV treatment guidelines continue to hold low dose efavirenz as the sole NNRTI-based first-line ART option, while RPV based options are not listed as either first or second line ART regimens. [2]
Rilpivirine-based regimens place in HIV treatment in the United States remains largely in individuals requiring an NNRTI option who cannot tolerate efavirenz.[12, 13] Rilpivirine-based treatments are advantageous in many aspects including single tablet formulation with tenofovir (either as TAForTDF) and emtricitabine, while the TAF/FTC/RPV fixed-dose combination tablet is the smallest single-tablet triple-drug regimen currently available for HIV treatment. However, the TAF/FTC/RPV combination still suffers from many of the similar issues that have plagued earlier generation NNRTIs including, a relatively low barrier to resistance, a less favorable AE profile as compared to INSTIs and a more cumbersome drug-drug interaction profile as RPV metabolism is largely CYP3A4 mediated. Additionally, there are limitations to the use of RPV-based regimens, particularly in individuals with high viral loads and low CD4 cell counts, as well as individuals receiving concurrent antacid therapy, as RPV requires an acidic gastric environment for absorption.
3.0. Chemistry
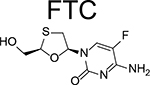
FTC is an enantiomer of a thio analog of cytidine which has a fluorine in the 5 position. The chemical name of FTC is 4-amino-5-fluoro-1-(2R-hydroxymethyl-1,3-oxathiolan-5S-yl)-(1H)-pyrimidin-2-one (Table 1). The molecular weight is 247.24 and the solubility is 112mg/ml in water at 25°C.
Table 1.
Overview of the components of Odefsey.
| Emtricitabine (FTC) | Tenofovir Alafenamide (TAF) | Rilpivirine (RPV) | ||
| Brand name | Emtriva | N/A | Edurant | |
| Route of Administration | Oral | Oral | Oral | |
| Drug Category | NRTIs | NRTIs | NNRTIs | |
| Mechanism of Action | Inhibits the activity of the HIV reverse transcriptase | Inhibits the activity of the HIV reverse transcriptase | Inhibits the activity of the HIV reverse transcriptase in a non-competitive manner | |
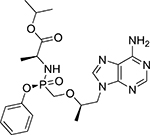
| Chemistry | Chemical name | 4-amino-5-fluoro-1-(2R-hydroxymethyl-1,3-oxathiolan-5S-yl)-(1H)-pyrimidin-2-one | L-alanine, N-[(S)-[[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]phenoxyphosphinyl]-, 1-methylethyl ester, (2E)-2-butenedioate (2:1) | 4-[[4-[4-[(E)-2-cyanoethenyl]-2,6-dimethylanilino]pyrimidin-2-yl]amino]benzonitrile |
| Molecular weight (g/mol) | 247.24 | 534.5 | 402.88 | |
| Solubility | 112mg/ml in water | 4.7 mg/ml in water | <0.1 mg/mL in water | |
| Structure | 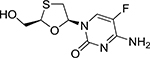 |
 |
||
| Pharmacokinetics & Metabolism | Tmax | <3 hours | <1 hour | <4 hours |
| Absorption | >90% bioavailability | 73% bioavailability | Consume with a non-protein-rich meal | |
| Plasma bound | <4% | 80% (blood to plasma ratio of 1.0) | 99% (blood to plasma ratio of 0.7) | |
| T1/2 | 10 hours; FTC-5′-TP: 10–39 hours | 0.51 hours; TFV-DP: 87–180 in PBMCs | 50 hours | |
| Elimination | 70% eliminated from urine | 85% eliminated from feces | 31% eliminated from feces | |
| Metabolism | 1. Oxidation of the thiol moiety to form the the 3′-sulfoxide diastereomers 2. Conjugation with glucuronic acid to form 2′-o-glucuronide 3. Not an inhibitor of CYP enzymes |
4. Metabolized through cathepsin A in PBMCs 5. Metabolized through CES1 in hepatocytes 6. Substrate of P-gp |
7. Metabolized through CYP3A 8. Inhibits CYP2B6 and CYP2C9 in the in vitro studies |
|
| Pivotal trials | GS-US-366-1216, GS-IS-366-1160 | |||
TAF is an analog of adenosine 5-monophosate. TAF has a molecular weight of 534.5 g/mol. The chemical name of tenofovir alafenamide is L-alanine, N-[(S)-[[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]phenoxyphosphinyl]-, 1-methylethyl ester, (2E)-2-butenedioate (2:1) (Table 1). TAF has a solubility of 4.7 mg/ml in water at 20 °C. Compared to TDF, TAF has greater intestinal and plasma stability, as well as higher systemic exposure.[14, 15]
RPV has a molecular weight of 366.42 g/mol. The chemical name of RPV is 4-[[4-[4-[(E)-2-cyanoethenyl]-2,6-dimethylanilino]pyrimidin-2-yl]amino]benzonitrile (Table 1). It is formulated as RPV hydrochloride whose molecular weight is 402.88 g/mol. RPV hydrochloride has poor solubility in water (<0.1 mg/mL).[16]
4.0. Introduction to the compounds
HIV reverse transcriptase (RT) functions to convert HIV single-stranded viral RNA into double-stranded DNA. As this is an essential step in the virus replication cycle, RT has been a major target for antiretroviral drug development. The NRTIs and NNRTIs are two classes of antiretroviral drugs used clinically to target HIV RT.
The drug zidovudine (AZT) which belongs to NRTI category of antiretroviral drugs was the first discovered and FDA approved antiretroviral drug. NRTIs are nucleoside analogs without a 3’-hydroxyl group. They reduce viral replication by competing with the analogous deoxy-nucleotides-tri-phosphate (dNTPs) to incorporate into DNA. This results in preventing the 3’−5’ phosphodiester bond formation and thus DNA synthesis.[17] TAF is administered as prodrugs and metabolized to active metabolites intracellularly. FTC is a cytosine analog which is also active against hepatitis B virus (HBV). [18] FTC is primarily excreted in the urine and improved efficacy of FTC is achieved with combination treatment.[19]
TAF is first metabolized into TFV, a nucleotide adenosine 5’-monophosphate derivative. A more than 600 fold enhanced antiviral activity was observed with TAF in in vitro experiments compared to the parent TFV.[20] TAF has better oral bioavailability and increased plasma stability compared to TDF.[21] TAF exhibited a higher magnitude of viral suppression at a lower dose than TDF in a phase 1b 10-day monotherapy study: the median decrease of HIV RNA in the plasma were −1.46 log10 copies/mL for 25mg TAF and −1.73 log10 copies/mL for 40mg TAF compared to −0.97 log10 copies/mL for 300mg TDF.[21, 22]
NNRTIs are a class of small (<600 Da) hydrophobic compounds with diverse structures. RPV, nevirapine (NVP), delavirdine (DLV), efavirenz (EFV), doravirine (DOR) and etravirine (ETR) are the 6 NNRTIs currently approved by the US Food and Drug Administration (FDA). NVP, DLV and EFV are the first generations while DOR, ETR, and RPV are the second-generation of NNRTIs. Due to the low genetic barrier for resistance, a single mutation can lead to drug resistance to the first-generation NNRTIs Three clusters of single mutations with two clusters occur on two opposite sides of reverse transcriptase subunit p66 and one occurs on p51were defined: L100I, K103N, V106A, and V108I in the first cluster;Y181C, Y188L/C/H, and G190A/S in the second cluster; P225H, M230L, and P236L in the third cluster.[23] However, the genetic barrier of the second-generation NNRTIs is relatively higher: ETR, the second-generation of NNRTI is sensitive to 90% of resistance mutations of the first-generation NNRTIs. [24]Cross-resistance is also common in the first-generation of NNRTIs. Second-generation of NNRTIs were used in patients who failed the first-generation of NNRTIs treatment. Study has shown that among 17 mutations, at least three mutations were required to reduce susceptibility of ETR.[25] Unlike the NRTIs, intracellular metabolism is not required for NNRTIs to be active against HIV. By binding to a specific HIV-1 reverse transcriptase binding pocket in a non-competitive manner, RPV induces conformational changes in the RT enzyme, limiting RT activity, and thus reducing viral load. RPV is highly plasma bound (99%).[16] Different dosages (25 mg to 150 mg) lead to similar anti-HIV activity with plasma levels above 12 ng/ml.[26]
5.0. Pharmacokinetics and Metabolism
When taken orally, FTC exhibits bioavailability over 90%. Linear pharmacokinetics has been observed for FTC. Peak plasma concentrations (Cmax) are reached within 3 hours post-dose. Less than 4% of FTC is bound to plasma proteins with a blood-to-plasma ratio of 0.6. [27]FTC is activated through intracellular phosphorylation to FTC-5’-triphosphate (FTC-TP). Compared to healthy adults, HIV-infected patients demonstrate similar Cmax and exposure as measured by area under the concentration-time curve (AUC). [28] FTC is predominantly eliminated in urine (70%). The median elimination half-life of FTC and FTC-5’-TP are 10 and 39 hours, respectively.[27] Co-administration of food does not have effects on the AUC of FTC. The AUC ratio of a moderate fat meal (600kcal, 27% fat) relative to fasting is 0.91(90% confidence interval [CI] 0.89, 0.93). The AUC ratio 0.88(0.85, 0.90) of high fat meal (800kcal, 50% fat) relative to fasting was observed.[29] FTC is metabolized through oxidation of the sulfur moiety to form the 3’-sulfoxide diastereomers and conjugation with glucuronic acid to form 2’-O-glucuronide. FTC is not an inhibitor of CYP enzymes according to the in vitro studies.[30]
With an oral dose of TAF, the Cmax is achieved within 1 hour. About 80% of the drug exhibits plasma protein binding demonstrating a blood to plasma ratio of 1.0. TAF is absorbed rapidly. Approximately 73% of the parent compound was observed after a single dose.[31, 32] The metabolism of TAF has been shown to occur through cathepsin A in peripheral blood mononuclear cells (PMBCs) and carboxylesterase 1 (CES1) in hepatocytes. TAF is also minimally metabolized through CYP3A. TAF is a substrate of P-glycoprotein (P-gp) which makes it vulnerable to drug interactions with drugs that strongly affect P-gp and could alter TAF absorption.[27] Compared to 300mg TDF, 25 mg TAF shows 94% decrease in mean TFV Cmax and 86% lower mean TFV AUC.[21] TAF has a median terminal plasma half-life of 0.51 hours, while the active metabolite, TFV-DP, was shown to have a half-life of 87–180 hours in PBMCs.[33] About 31% of TAF is excreted in feces. [27]
After oral administration of RPV, peak plasma concentration is reached within 4 hours. RPV is highly bound to plasma proteins (99%) with a blood-to-plasma ratio of 0.7. A lag time followed by a linear increase in plasma was observed for RPV absorption. Cmax and AUC can be reduced by up to 46% and 43% respectively under fasting conditions. A protein-rich drink can decrease the Cmax and AUC by 50%. It is therefore recommended to consume RPV with a non-protein-rich meal. [34]RPV is primarily metabolized by CYP3A. Therefore, drugs that induce or inhibit CYP3A may also effect RPV exposure. RPV was also suggested to inhibit CYP2B6 and CYP2C9 in the in vitro studies. RPV pharmacokinetics are altered when co-administered with gastric acid lowering drugs, such as famotidine and omeprazole. When a 40 mg dose of famotidine is administered two hours prior to a dose of RPV, RPV Cmax and AUC are decreased by 85% and 76%, respectively. With once daily omeprazole at a dose of 20mg, RPV Cmax and AUC demonstrate a 70% and 65% decrease, respectively. Drug interactions are also seen with strong CYP enzyme inducers, such as the tuberculosis antibiotic, rifampin, in which Cmax and AUC are reduced by 69% and 80%, respectively. RPV exhibits a median terminal elimination half-life of 50 hours and is primarily excreted in feces (85%).[27]
6.0. Pharmacodynamics
At doses from 50 to 400 mg FTC significantly suppresses the HIV viral load; a maximum reduction occurs with FTC ≥200 mg/day. [35]The 50% inhibitory concentration (IC50) of FTC has been reported to be between 0.0013 to 0.64 μM.[35] FTC has a weak affinity towards human cellular DNA polymerases α, β, γ and Ɛ. It has been shown in a study of 48 healthy subjects that TAF did not interfere with the QT/QTc interval at the recommended dose or at a dose which is 5 times of the current recommended dose.[4]
RPV does not interfere with human cellular DNA polymerase α, β and γ.[36] RPV was shown to have effects on the QTc interval at the recommended dose of 25 mg once daily, as well as the 75 mg once daily or 300 mg once daily doses. In a randomized, controlled crossover study of 60 healthy adults, the maximum mean time-matched differences in QTc interval from placebo was 2 milliseconds for 25 mg once daily, which is below the threshold of clinical concern. For supra-therapeutic doses 75 mg once daily and 300 mg once daily, the maximum mean time-matched differences in QTc interval from placebo were 10.7 and 23.3 milliseconds, respectively.[4] Compared to the recommended dose of 25 mg once daily, the 75 mg dose once daily and 300 mg once daily increased the mean steady-state Cmax approximately 2.6 fold and 6.7 fold higher, respectively. [4]
7.0. Clinical Efficacy
7.1. Phase I and Phase 2 trials
Emtricitabine
FTC potency, safety and efficacy has been evaluated in early Phase I/II studies. In studies of HIV-infected adults, daily doses of emtricitabine up to 400mg were shown to be safe and well tolerated. [37] A 10-day monotherapy trial of emtricitabine versus lamivudine (3TC) assessed relative efficacy of the two nucleoside analogues. FTC doses of 25, 100 and 200mg daily were compared with 3TC at a dose of 150mg twice daily in HIV-infected patients. Reductions in HIV viral loads were greater with FTC 200mg daily than with 3TC 150mg twice daily, as demonstrated by a 1.7 vs 1.5 log10 drop, respectively.[38]
Tenofovir Alafenamide
TAF has been studied in early clinical development in monotherapy studies of HIV-infected, treatment naïve individuals. A Phase I/II study of TAF at doses of 40mg and 120mg compared pharmacokinetics, safety and antiviral efficacy with the other prodrug of tenofovir, TDF at the clinically used dose of 300mg daily. TAF (both 40mg and 120mg) showed significantly more efficacy, as measured by HIV-1 viral load reduction at day 14 as compared to TDF. [39] A follow-up 10-day Phase Ib study assessed lower doses of TAF (8, 25 and 40mg) in comparison with the clinical dose of TDF 300mg. In this trial of both treatment-naïve and treatment-experienced individuals off therapy, the 8mg dose of TAF showed similar efficacy as the TDF 300mg dose, while a dose response relationship for HIV-1 viral load reduction was realized with TAF doses up to 25mg. Similar to other studies, the ability of TAF to load PBMC’s with TFV-DP was approximately 7-fold greater with TAF 25mg than TDF 300mg. Both TAF doses were safe and well tolerated with no premature study drug discontinuations reported during the study.[21] Two separate studies showed that TAF pharmacokinetics were significantly impacted by intestinal efflux transporter inhibitors such as cobicistat. In caco-2 cells TAF efflux was inhibited several fold (>5) when combined with cobicistat, while a clinical pharmacokinetic study affirmed these results in humans, showing a two-fold increase in plasma TAF exposures when dosed with cobicistat.[40, 41] Collectively these studies provided the clinical proof of concept to move forward with TAF at the doses of 25mg when unboosted and 10mg when used with a pharmacoenhancer such as cobicistat.
Rilpivirine
Rilpivirine (TMC-278) safety, antiviral activity and efficacy were evaluated in several Phase I/II trails. In a Phase IIa 7-day study of treatment naïve HIV-infected adults, antiviral activity of rilpivirine was assessed at doses of 25, 50, 100 or 150mg daily. Each of the doses studied were shown to be efficacious against HIV with a median viral load decline of 1.199 log10 copies per mL; however, no dose response relationship could be established. The doses studied were well tolerated and no dose-related adverse events developed.[42] A larger 96-week Phase IIb study of three hundred sixty eight patients evaluated efficacy and safety of rilpivirine in the treatment naïve HIV-infected individuals. Rilpivirine doses of 25, 75 and 150mg were compared with efavirenz 600mg, all once daily with two nucleosides. Each of the rilpivirine doses demonstrated non-statistically significant differences in viral efficacy compared with efavirenz at both 48 and 96 weeks. Again, no dose response relationship was observed for either efficacy or safety. [43]
7.2. Phase 3 trials
As TAF/FTC/RPV was originally approved based on bioequivalence trials, there have been comparatively few phase 3 trials focusing on TAF/FTC/RPV. The trials that have been completed have primarily focused on assessing antiretroviral treatment switching between other medications and this combination. In large part, these trials were designed to determine the non-inferiority of switching the NRTI backbone from TDF to TAF, due to the potential for an improved side effect profile.[44, 45]
The first trial, GS-US-366–1216, was published in March 2017 and assessed changing from a TDF/FTC/RPV combination to a TAF/FTC/RPV combination.[11] This trial was undertaken to assess substitution of TAF for TDF, due to the potential for the improved side effect profile with TAF. This trial was a randomized, double-blinded, multicenter placebo controlled trial, and the goal was to demonstrate non-inferiority for the TAF-containing regimen as compared to the TDF combination. A total of 630 subjects were enrolled across two groups. Of all the subjects who had been receiving TDF/FTC/RPV and were virally suppressed, half were randomized to continue to take TDF/FTC/RPV, while half were randomized to switch to TAF/FTC/RPV. In this study, the primary endpoint was a viral titer less than 50 copies/ml at 48 weeks after the end of the trial. Before enrollment, in addition to viral suppression, subjects needed to have a creatinine clearance of at least 50 mL/min, and have no documented resistance to TFV, FTC, or RPV. After randomization, subjects received both active tablets and placebos which matched the other treatment. Subjects were assessed at 4, 8, 12, and every 12 weeks thereafter for 96 weeks. Laboratory values, changes in bone mineral density, adverse events and other drugs which the subjects were receiving were also assessed. At 48 weeks, TAF/FTC/RPV was shown to be non-inferior to TDF/FTC/RPV in respect to viral suppression. No differences were observed for viral resistance between the two treatment groups. The authors did note, however, that as the study was powered to detect potential differences in viral suppression, it may not have had sufficient power to detect some more rare side effects. Despite this, the study supported changing patients to a TAF/FTC/RPV regimen from a TDF/FTC/RPV regimen, based on non-inferiority for viral suppression, and the improved side effect profile.
A second, parallel trial (GS-US-366–1160) assessed a within class change (NNRTI to NNRTI) from a TDF/FTC/EFV regimen to a TAF/FTC/RPV based regimen.[46] A total of 875 subjects who were virally suppressed on TDF/FTC/EFV were randomized to either continue their current therapy or take TAF/FTC/RPV. Before enrollment, it was required that subjects had been taking TDF/FTC/EFV for at least 6 months, were virally suppressed, had a creatinine clearance > 50 mL/min, and had no documented resistance to any drugs in the potential treatment regimens. Subjects were randomized to one of the two groups. Subjects either were instructed to take TDF/FTC/EFV on an empty stomach or TAF/FTC/RPV with food. Due to the differences in food requirements, the trial was placebo controlled, with subjects receiving both an active therapy and a placebo tablet. Subjects had study visits at 4, 8, and 12 weeks, and every 12 weeks afterwards. Laboratory tests, viral RNA, and CD4 counts were monitored at clinic visits, as were potential adverse events, other drugs the subjects were receiving, and bone mineral density assessment. The study was powered for the primary outcome of participants with an HIV-1 RNA less than 50 copies/mL. Bone safety measures were the primary secondary endpoints, although other safety information was assessed as well. A switch to TAF/FTC/RPV from TDF/FTC/EFV was found to be non-inferior to remaining on TDF/FTC/EFV in terms of viral suppression at 48 weeks.
In addition to the 48 week reports, these two trials were also continued through 96 weeks. [47] TAF/FTC/RPV was still observed to be non-inferior to either TDF/FTC/EFV or TDF/FTC/RPV at 96 weeks. With all treatment options the percentage of individuals with non-detectable HIV RNA was very high. Only 11 subjects taking TAF/FTC/RPV had virologic failure in the trials. In one trial two of the subjects were virally resuppressed, and the third had pre-existing mutations. In the second trial, 8 experienced virologic failure, seven of which were subsequently resuppressed.
8.0. Safety and Tolerability
RPV-containing regimens are associated with multiple adverse effects including severe skin and hypersensitivity reactions, hepatic toxicity and depressive disorders. Constitutional symptoms, such as fever and organ dysfunction, are associated with some skin reactions.[29] Depressive disorders, such as depressed mood, dysphoria, major depression, have been reported in patients receiving RPV containing regimens.[29]
Both FTC and TAF are largely excreted via the kidney. Due to HIV associated nephropathy, people living with HIV are more prone to kidney disease which can be exacerbated by ART.[48, 49] Tenofovir has been previously reported to lead to reduced renal function, such as acute renal failure and Fanconi syndrome, an excess amount of electrolytes and other substances in the urine caused by dysfunction of proximal renal tubules of the kidney, and an increased risk of developing renal-rated adverse reactions in patients with impaired renal function or patients taking other nephrotoxic agents.[29] However, TAF-containing regimens have been shown to cause less impact on renal function compared to TDF-containing regimens in the 2 essential phase III switch studies GS-US-366–1216 and GS-US-366–116. Renal function was improved after switching to TAF/FTC/RPV from a TDF-containing regimen: median creatinine clearance was increased at week 4 and week 48 by switching to TAF/FTC/RPV treatment in both studies. At week 48, quantitative proteinuria (urine protein to creatinine ratio and urine albumin to creatinine ratio) and tubular proteinuria (Urine β−2 microglobulin to creatinine ratio and urine retinol-binding protein to creatinine ratio) was also improved with the switch to TAF/FTC/RPV in both studies. [11, 46] At week 96, kidney function was similarly improved as week 48 (creatinine clearance, quantitative proteinuria and tubular proteinuria) in the study GS-US-366–1160. However, in the study GS-US-366–1216, increases, rather than decreases were observed at week 96 in the UACR (urine protein to creatinine ratio) and RBP (urine retinol-binding protein) to creatinine ratio.[47]
TDF has been associated with bone mineral density (BMD) loss which might lead to fragility fractures among HIV-infected adults aged between 25 and 54.[50] TAF-containing regimens lead to less BMD loss compared to TDF-containing regimens in adults. Two phase III clinical studies GS-US-366–1216 and GS-US-366–116 have shown that TAF/FTC/RPV improved bone mineral density compared to the previous treatment: increases in the hip and spine bone mineral density were observed from baseline to week 24 and 48. Sixteen percent and 27% of participants had hip and spine bone mineral density increase at least 3% minimum threshold at week 48, respectively in the study GS-US-366–1216. These improvements continued up to week 96. [47] In these 2 phase III studies, no significant differences in the frequency of AEs were observed between groups. Between treatment arms, rates of premature discontinuation were also comparable. The treatment of TAF/FTC/RPV was well tolerated with only a mild or moderate severity of AEs. [46, 51] Approximately 1% participant and 3% participant in the TAF/FTC/RPV treatment group discontinued due to study drug-related adverse event in the studies GS-US-366–1216 and GS-US-366–1160, respectively.
8.1. Potential Drug-drug interaction with TAF/FTC/RPV
The most significant drug-drug interactions with TAF/FTC/RPV arise from the RPV component of the combination. Increasing concentrations of RPV were predicted upon co-administration of macrolide or ketolide antibiotics, such as clarithromycin, erythromycin, and telithromycin. When co-administering TAF/FTC/RPV with antacids drugs (such as aluminum, magnesium hydroxide) or with H2-Receptor Antagonists (such as cimetidine, famotidine) it is recommended to separate TAF/FTC/RPV at least 2 hours before or 4 hours after the antacids to avoid significant absorption related drug-drug interactions. Co-administration of TAF/FTC/RPV with antimycobacterial drugs, such as rifamycins is not recommended due to predictions of decreased RPV and TAF concentrations.[52] No dosage adjustment but clinical monitoring is required for co-administration with azole antifungal agents (such as fluconazole, itraconazole) since increased concentrations of RPV and TAF were observed. No dosage adjustment but clinical monitoring is also recommended for co-administration with narcotic analgesics.
No significant drug-drug interactions were observed or expected when co-administer TAF/FTC/RPV with the following drugs: acetaminophen, atorvastatin, buprenorphine, chlorzoxazone, digoxin, ethinyl estradiol, ledipasvir, lorazepam, metformin, midazolam,naloxone, norbuprenorphine, norethindrone, norgestimate/ethinyl estradiol, sildenafil, simeprevir, and sofosbuvir.[4]
9.0. Conclusion
TAF/FTC/RPV is well tolerated and effective for many individuals living with HIV, especially individuals started on ART with lower viral titers, or who were previously virally suppressed on other regimens. Due to concerns in individuals with high viral titers and low CD4 counts, however, this combination will likely be most commonly used as a drug to switch to in individuals who had previously been taking TDF/FTC/RPV, or who wish to take a regimen with a reduced tablet size. Antiretroviral therapy will likely continue to evolve to offer patients a wider choice of options in combination treatment. Novel formulations, new combinations of current drugs, dual therapy approaches, and long acting, reduced dosing frequency HIV treatment approaches will all continue to evolve as the field moves forward.
10.0. Expert Opinion
Since the 1990’s, the evolution of antiretroviral therapy has led to remarkable improvements in patient outcomes, transforming infection with HIV-1 from a disease with a short course to AIDS and eventual death, to, in many cases a disease which can be chronically managed. A key part of that evolution is increased options for the treatment of individuals with HIV. INSTI-based regimens remain the preferred class of drugs recommended as initial regimens in the DHHS guidelines for most people living with HIV based upon tolerability, the high barrier to resistance of the INSTIs and the favorable drug-drug interaction profile of many of the INSTI drugs. Currently, under DHHS guidelines, TAF/FTC/RPV is classified as a “Recommended Initial Regimen in Certain Clinical Situations”, with the caveat that individuals who are newly diagnosed with HIV should only start TAF/FTC/RPV if their HIV RNA is less than 100,000 copies/mL, and their CD4 cell count greater than 200 cells/mm3. [7]Clinically, TAF/FTC/RPV may be a good therapeutic option for individuals who are virally suppressed, and wish to change drug class, either due to side effects with the medications they are taking, or simply patient preference to an alternative regimen. For example, in individuals who are taking INSTI-based regimens that require multiple tablets (eg. Dolutegravir + TAF/FTC), either once daily, or multiple times of the day (eg. Raltegravir twice daily), it may be beneficial to change these patients to a more convenient regimen requiring once daily administration of a single tablet. Appealingly, among current approved single tablet regimens, TAF/FTC/RPV is the smallest coformulated triple-drug regimen tablet, which may be useful for individuals who have difficulties swallowing larger tablets.
Despite these advantages, it is likely that the main use of TAF/FTC/RPV will be in individuals previously taking TDF/FTC/RPV. The side effect profile of TAF is significantly better than the side effect profile of TDF, with individuals who take TAF observing significantly fewer effects on their kidney function and bone mineral density than individuals who are taking TDF.
With the successes that have been observed in recent years with triple therapy, the next steps in antiretroviral therapy likely reside in changes in the paradigm for the delivery of ART. One option under investigation is two drug maintenance therapy. Recently Juluca (dolutegravir/rilpivirine) has been approved as a two-drug single tablet regimen. The combination can be used in individuals who are currently virally suppressed on other regimens, and may have utility in individuals who need a NRTI sparing regimen.
Also currently under investigation is an injectable coformulation consisting of rilpivirine and cabotegravir, a novel integrase inhibitor. Previous studies assessed a long acting injectable formulation of these two drugs, injected either every 4 or 8 weeks as maintenance therapy.[53] Individuals on the injectable dual therapy were compared to individuals who took oral abacavir/lamivudine + cabotegravir. In this trial, the injectable formulation was found to be non-inferior to the oral formulation, and participants preferred both the every 4 and 8 week injections to the oral formulation. [53] More recent findings have shown that a monthly administration of this product was non-inferior to abacavir/lamivudine/dolutegravir, and well tolerated. [54]
With these new coformulations either recently approved or are currently under investigation, it seems plausible that the place in therapy of TAF/FTC/RPV will be more of a transitional combination before other dual therapy-based regimens, particularly long-active injectable formulations, become more popular.
Drug Summary Box.
Pharmaprojects - copyright to Citeline Drug Intelligence (an Informa business). Readers are referred to Informa-Pipeline (http://informa-pipeline.citeline.com) and Citeline (http://informa.citeline.com).
Acknowledgements
We wish to thank Dr. Wei Li for providing ChemDraw software, and Hanxuan Li for assistance with using the ChemDraw software.
Funding:
This work was supported by National Institutes of Health (NIH) Grants R01DA047178 (to TJ Cory), and K23AI134307 (to AT Podany)
Footnotes
Declaration of Interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures:
One referee declares that they worked on the development of tenofovir. Another referee of this manuscript is an employee and stockholder of Gilead Sciences. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
References
- 1.Basavaraj KH, Navya MA, and Rashmi R, Quality of life in HIV/AIDS. Indian J Sex Transm Dis AIDS, 2010. 31(2): p. 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO, UPDATED OF RECOMMENDATIONS ON FIRST-AND SECOND-LINE ANTIRETROVIRAL REGIMENS https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.15-eng.pdf?ua=1 2019.
- 3.Zhao Y, Wu Z, McGoogan JM, et al. , Immediate Antiretroviral Therapy Decreases Mortality Among Patients With High CD4 Counts in China: A Nationwide, Retrospective Cohort Study. Clin Infect Dis, 2018. 66(5): p. 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilead Sciences I, Application for inclusion of fixed-dose, three-drug combination COMPLERA®/EVIPLERA® (emtricitabine/rilpivirine/tenofovir disoproxil fumarate) tablets on the WHO Model List of Essential Medicines http://origin.who.int/selection_medicines/committees/expert/20/applications/EmtricitabineFDC.pdf.
- 5.HHS.GOV, Global Statistics https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics.
- 6.DHHS, Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/11/what-to-start.
- 7.DHHS, Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
- 8.WHO, The use of antiretroviral drugs for treating and preventing HIV infection https://apps.who.int/iris/bitstream/handle/10665/208825/9789241549684_eng.pdf 2016.
- 9.NIH, Emtricitabine/Rilpivirine/Tenofovir Disoproxil Fumarate https://aidsinfo.nih.gov/drugs/441/complera/5/professional.
- 10.Podany AT, Bares SH, Havens J, et al. , Plasma and intracellular pharmacokinetics of tenofovir in patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. AIDS, 2018. 32(6): p. 761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orkin C, DeJesus E, Ramgopal M, et al. , Switching from tenofovir disoproxil fumarate to tenofovir alafenamide coformulated with rilpivirine and emtricitabine in virally suppressed adults with HIV-1 infection: a randomised, double-blind, multicentre, phase 3b, non-inferiority study. Lancet HIV, 2017. 4(5): p. e195–e204. [DOI] [PubMed] [Google Scholar]
- 12.Nelson MR, Elion RA, Cohen CJ, et al. , Rilpivirine versus efavirenz in HIV-1-infected subjects receiving emtricitabine/tenofovir DF: pooled 96-week data from ECHO and THRIVE Studies. HIV Clin Trials, 2013. 14(3): p. 81–91. [DOI] [PubMed] [Google Scholar]
- 13.Cohen CJ, Andrade-Villanueva J, Clotet B, et al. , Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet, 2011. 378(9787): p. 229–37. [DOI] [PubMed] [Google Scholar]
- 14.Birkus G, Kutty N, He GX, et al. , Activation of 9-[(R)-2-[[(S)-[[(S)-1-(Isopropoxycarbonyl)ethyl]amino] phenoxyphosphinyl]-methoxy]propyl]adenine (GS-7340) and other tenofovir phosphonoamidate prodrugs by human proteases. Mol Pharmacol, 2008. 74(1): p. 92–100. [DOI] [PubMed] [Google Scholar]
- 15.Babusis D, Phan TK, Lee WA, et al. , Mechanism for effective lymphoid cell and tissue loading following oral administration of nucleotide prodrug GS-7340. Mol Pharm, 2013. 10(2): p. 459–66. [DOI] [PubMed] [Google Scholar]
- 16.Janssen PA, Lewi PJ, Arnold E, et al. , In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2- pyrimidinyl]amino]benzonitrile (R278474, rilpivirine). J Med Chem, 2005. 48(6): p. 1901–9. [DOI] [PubMed] [Google Scholar]
- 17.Mu Y, Kodidela S, Wang Y, et al. , The dawn of precision medicine in HIV: state of the art of pharmacotherapy. Expert Opin Pharmacother, 2018. 19(14): p. 1581–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.FDA, Antiretroviral drugs used in the treatment of HIV infection. www.fda.gov/forpatients/illness/hivaids/treatment/ucm118915.htm.
- 19.Saag MS, Emtricitabine, a new antiretroviral agent with activity against HIV and hepatitis B virus. Clin Infect Dis, 2006. 42(1): p. 126–31. [DOI] [PubMed] [Google Scholar]
- 20.Lee WA, He GX, Eisenberg E, et al. , Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother, 2005. 49(5): p. 1898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruane PJ, DeJesus E, Berger D, et al. , Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr, 2013. 63(4): p. 449–55. [DOI] [PubMed] [Google Scholar]
- 22.Eisenberg EJ, He GX, and Lee WA, Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA, in blood. Nucleosides Nucleotides Nucleic Acids, 2001. 20(4–7): p. 1091–8. [DOI] [PubMed] [Google Scholar]
- 23.Ghosn J, Chaix ML, and Delaugerre C, HIV-1 resistance to first- and second-generation non-nucleoside reverse transcriptase inhibitors. AIDS Rev, 2009. 11(3): p. 165–73. [PubMed] [Google Scholar]
- 24.Andries K, Azijn H, Thielemans T, et al. , TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob Agents Chemother, 2004. 48(12): p. 4680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazzarin A, Campbell T, Clotet B, et al. , Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet, 2007. 370(9581): p. 39–48. [DOI] [PubMed] [Google Scholar]
- 26.Usach I, Melis V, and Peris JE, Non-nucleoside reverse transcriptase inhibitors: a review on pharmacokinetics, pharmacodynamics, safety and tolerability. J Int AIDS Soc, 2013. 16: p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilead Sciences I, ODEFSEY® (emtricitabine, rilpivirine, and tenofovir alafenamide) package insert. Foster City (CA): https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208351s000lbl.pdf 2018. [Google Scholar]
- 28.Wang LH, Begley J, St Claire RL 3rd, et al. , Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection. AIDS Res Hum Retroviruses, 2004. 20(11): p. 1173–82. [DOI] [PubMed] [Google Scholar]
- 29.NHIVC, https://www.hiv.uw.edu/page/treatment/drugs/rilpivirine-tenofovir-alafenamide-emtricitabine/prescribing-information#S12.4.
- 30.Masho SW, Wang CL, and Nixon DE, Review of tenofovir-emtricitabine. Ther Clin Risk Manag, 2007. 3(6): p. 1097–104. [PMC free article] [PubMed] [Google Scholar]
- 31.Aloy B, Tazi I, Bagnis C I, et al. , Is Tenofovir Alafenamide Safer than Tenofovir Disoproxil Fumarate for the Kidneys? AIDS Rev, 2016. 18(4): p. 184–192. [PubMed] [Google Scholar]
- 32.Ray AS, Fordyce MW, and Hitchcock MJ, Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus. Antiviral Res, 2016. 125: p. 63–70. [DOI] [PubMed] [Google Scholar]
- 33.Baheti G, Kiser JJ, Havens PL, et al. , Plasma and intracellular population pharmacokinetic analysis of tenofovir in HIV-1-infected patients. Antimicrob Agents Chemother, 2011. 55(11): p. 5294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford N, Lee J, Andrieux-Meyer I, et al. , Safety, efficacy, and pharmacokinetics of rilpivirine: systematic review with an emphasis on resource-limited settings. HIV AIDS (Auckl), 2011. 3: p. 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.FDA, Emtriva® (emtricitabine) Capsules and Emtriva® (emtricitabine) Oral Solution https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021896lbl.pdf.
- 36.Azijn H, Tirry I, Vingerhoets J, et al. , TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother, 2010. 54(2): p. 718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rousseau FS, Kahn JO, Thompson M, et al. , Prototype trial design for rapid dose selection of antiretroviral drugs: an example using emtricitabine (Coviracil). J Antimicrob Chemother, 2001. 48(4): p. 507–13. [DOI] [PubMed] [Google Scholar]
- 38.Rousseau FS, Wakeford C, Mommeja-Marin H, et al. , Prospective randomized trial of emtricitabine versus lamivudine short-term monotherapy in human immunodeficiency virus-infected patients. J Infect Dis, 2003. 188(11): p. 1652–8. [DOI] [PubMed] [Google Scholar]
- 39.Markowitz M, Zolopa A, Squires K, et al. , Phase I/II study of the pharmacokinetics, safety and antiretroviral activity of tenofovir alafenamide, a new prodrug of the HIV reverse transcriptase inhibitor tenofovir, in HIV-infected adults. J Antimicrob Chemother, 2014. 69(5): p. 1362–9. [DOI] [PubMed] [Google Scholar]
- 40.Lepist EI, Phan TK, Roy A, et al. , Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. Antimicrob Agents Chemother, 2012. 56(10): p. 5409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramanathan S, Di Paolo JA, Jin F, et al. , Pharmacokinetics, Pharmacodynamics, and Safety of Entospletinib, a Novel pSYK Inhibitor, Following Single and Multiple Oral Dosing in Healthy Volunteers. Clin Drug Investig, 2017. 37(2): p. 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goebel F, Yakovlev A, Pozniak AL, et al. , Short-term antiviral activity of TMC278--a novel NNRTI--in treatment-naive HIV-1-infected subjects. AIDS, 2006. 20(13): p. 1721–6. [DOI] [PubMed] [Google Scholar]
- 43.Pozniak AL, Morales-Ramirez J, Katabira E, et al. , Efficacy and safety of TMC278 in antiretroviral-naive HIV-1 patients: week 96 results of a phase IIb randomized trial. AIDS, 2010. 24(1): p. 55–65. [DOI] [PubMed] [Google Scholar]
- 44.Gupta SK, Post FA, Arribas JR, et al. , Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS, 2019. 33(9): p. 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seto WK, Asahina Y, Brown TT, et al. , Improved Bone Safety of Tenofovir Alafenamide Compared to Tenofovir Disoproxil Fumarate Over 2 Years in Patients With Chronic HBV Infection. Clin Gastroenterol Hepatol, 2018. [DOI] [PubMed] [Google Scholar]
- 46.DeJesus E, Ramgopal M, Crofoot G, et al. , Switching from efavirenz, emtricitabine, and tenofovir disoproxil fumarate to tenofovir alafenamide coformulated with rilpivirine and emtricitabine in virally suppressed adults with HIV-1 infection: a randomised, double-blind, multicentre, phase 3b, non-inferiority study. Lancet HIV, 2017. 4(5): p. e205–e213.** This is one of the essential clinical phase III trials to compare the efficacy and safety of TAF-containing regimen with a TDF-containing regimen.
- 47.Hagins D, Orkin C, Daar ES, et al. , Switching to coformulated rilpivirine (RPV), emtricitabine (FTC) and tenofovir alafenamide from either RPV, FTC and tenofovir disoproxil fumarate (TDF) or efavirenz, FTC and TDF: 96-week results from two randomized clinical trials. HIV Med, 2018. 19(10): p. 724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wyatt CM, Morgello S, Katz-Malamed R, et al. , The spectrum of kidney disease in patients with AIDS in the era of antiretroviral therapy. Kidney Int, 2009. 75(4): p. 428–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fine DM, Perazella MA, Lucas GM, et al. , Kidney biopsy in HIV: beyond HIV-associated nephropathy. Am J Kidney Dis, 2008. 51(3): p. 504–14. [DOI] [PubMed] [Google Scholar]
- 50.Battalora LA, Young B, and Overton ET, Bones, Fractures, Antiretroviral Therapy and HIV. Curr Infect Dis Rep, 2014. 16(2): p. 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ClinicalTrials.gov, Switch Study to Evaluate the Safety and Efficacy of Emtricitabine/Rilpivirine/Tenofovir Alafenamide (FTC/RPV/TAF) Fixed Dose Combination (FDC) in HIV-1 Positive Adults Who Are Virologically Suppressed on Emtricitabine/Rilpivirine/Tenofovir Disoproxil Fumarate (FTC/RPV/TDF) https://clinicaltrials.gov/ct2/show/NCT02345252.
- 52.Gilead Science I, Foster City, CA94404, Complera (FTC/RPV/TDF) US Presceibing Information. Revised February 2016. https://www.gilead.com/-/media/files/pdfs/medicines/hiv/complera/complera_pi.pdf. [Google Scholar]
- 53.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. , Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet, 2017. 390(10101): p. 1499–1510. [DOI] [PubMed] [Google Scholar]
- 54.Orkin C, Long-acting cabotegravir+ rilpivirine for HIV maintenance: FLAIR Week 48 results. Webcast presented at: Conference on Retroviruses and Opportunistic Infections (CROI); March 4–7, 2019; Seattle, WA.** This is another important clinical phase III trial to compare the efficacy and safety of TAF-containing regimen with the TDF-containing regimen.