Abstract
Objective: To provide a snapshot of the profile of adults and youth with type 1 diabetes (T1D) in the United States and assessment of longitudinal changes in T1D management and clinical outcomes in the T1D Exchange registry.
Research Design and Methods: Data on diabetes management and outcomes from 22,697 registry participants (age 1–93 years) were collected between 2016 and 2018 and compared with data collected in 2010–2012 for 25,529 registry participants.
Results: Mean HbA1c in 2016–2018 increased from 65 mmol/mol at the age of 5 years to 78 mmol/mol between ages 15 and 18, with a decrease to 64 mmol/mol by age 28 and 58–63 mmol/mol beyond age 30. The American Diabetes Association (ADA) HbA1c goal of <58 mmol/mol for youth was achieved by only 17% and the goal of <53 mmol/mol for adults by only 21%. Mean HbA1c levels changed little between 2010–2012 and 2016–2018, except in adolescents who had a higher mean HbA1c in 2016–2018. Insulin pump use increased from 57% in 2010–2012 to 63% in 2016–2018. Continuous glucose monitoring (CGM) increased from 7% in 2010–2012 to 30% in 2016–2018, rising >10-fold in children <12 years old. HbA1c levels were lower in CGM users than nonusers. Severe hypoglycemia was most frequent in participants ≥50 years old and diabetic ketoacidosis was most common in adolescents and young adults. Racial differences were evident in use of pumps and CGM and HbA1c levels.
Conclusions: Data from the T1D Exchange registry demonstrate that only a minority of adults and youth with T1D in the United States achieve ADA goals for HbA1c.
Keywords: T1D Exchange registry, Continuous glucose monitor use, Insulin pump use.
Introduction
In 2010, the type 1 diabetes (T1D) Exchange clinic registry initiated the first large database of clinical characteristics and clinical outcomes of children and adults with T1D throughout the United States. The data have provided an overview of the state of metabolic control, acute complications, and diabetes management of T1D in the United States.1,2 and the opportunity to compare U.S. data with other registries from Europe and Australia.3–12
In this study, we present an updated snapshot of the state of T1D in the United States and an assessment of changes over time.
Methods
The T1D Exchange clinic registry data collection was performed by 81 U.S.-based pediatric and adult endocrinology practices in 35 states. Nineteen and 38 centers primarily care for adult and pediatric patients, respectively, and 24 centers care for both. Sixty-three are institution based, 17 are community based, and 1 is in a managed care setting. Details on eligibility criteria, the informed consent process, and baseline data collection have been published previously.1 During the initial enrollment period (September 2010 through August 2012), 25,833 individuals with T1D (14,593 < 18 years old and 11,240 ≥ 18 years old) were enrolled. Subsequently, an additional 8544 participants were enrolled through August 2017. Core data were updated annually from medical records.
This report includes data from 22,697 participants collected between January 1, 2016, and March 31, 2018 (N = 3,536 in 2016, N = 15,955 in 2017, and N = 3,206 in 2018). Participants with a history of pancreas or islet cell transplantation and those pregnant at the time of data collection were excluded.
Participants who were followed for 5 years completed a detailed questionnaire regarding diabetes management and acute complications (Year 5 questionnaire), similar to the questionnaire completed at enrollment (N = 11,061).
Information on age, date of diagnosis, body mass index (height and weight), insurance status, insulin pump use, continuous glucose monitoring (CGM) use, noninsulin glucose-lowering medication use, and HbA1c levels obtained as part of usual care was collected from medical records. Frequency of self-monitoring of blood glucose (SMBG) was assessed from meter download (if available) or from participant report in the clinic chart. The occurrences of severe hypoglycemia (SH) and diabetic ketoacidosis (DKA) in the prior 3 months and aspects of diabetes management, including timing and frequency of insulin administration, duration of technology use, use of technology features, use of CGM to decide/adjust insulin dose, checking for ketones, use of glucagon, device downloading, and use of mobile medical applications, were participant reported from the subset of participants/caregivers who completed the Year 5 questionnaire. For an event to be counted as SH, required loss of consciousness or seizure, and for an event to be counted as DKA, required an overnight hospitalization.
Statistical methods
Results were tabulated according to age group. Cross-sectional comparisons of data collected during 2010–2012 were made with data collected during 2016–2018; 12,705 participants had information available from both the 2010–2012 and 2016–2018 time periods. Cross-sectional comparisons of use of pump and CGM included participants with at least 1 year of diabetes duration. To assess mean HbA1c over the life span, participants were grouped by year of age at the time of measurement. To minimize the impact of potential cohort effects and duration effects, cross-sectional comparisons of HbA1c included 9657 participants contained in both time cohorts with at least 3 years of duration at the time of the 2010–2012 data collection.
Multivariable linear regression models were used to assess the association between HbA1c and time period (2010–2012 and 2016–2018), and to assess the association between 2016–2018 HbA1c and participant characteristics adjusting for age, diabetes duration, and clinic site. Multivariable logistic regression models were used to assess the association between reported SH and DKA (separately) in 2016–2018 and the following: insulin pump use, CGM use, and HbA1c. To account for possible confounding, the following covariates were assessed for associations with each outcome through bivariate analysis and selection models: age, diabetes duration, race/ethnicity, sex, SMBG, insurance status, pump status (when not covariate of interest), CGM status (when not covariate of interest), HbA1c (when not covariate of interest), and clinic.
Results are expressed as means ± standard deviations for normally distributed variables or medians (interquartile range) for non-normally distributed variables. Data analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, NC). All P-values are two sided.
Results
The 22,697 participants with data from 2016 to 2018 ranged in age from 1 to 93 years; duration of diabetes ranged from <1 to 80 years, 50% were female, 82% were non-Hispanic white, and 74% had private health insurance. About half (10,249 [49%]) of participants were overweight or obese. Additional participant and clinical characteristics are described, stratified by age, in Supplementary Table S1 (Supplementary Data are available online at https://www.liebertpub.com/suppl/doi/10.1089/dia.2018.0384). Participant and clinical characteristics generally were similar between the 2010–2012 time cohort and the 2016–2018 time cohort, although, as expected, participants in the 2016–2018 cohort were 4–5 years older with about 4–5 years longer diabetes duration (Supplementary Table S2).
Utilization of diabetes technology and aspects of diabetes management
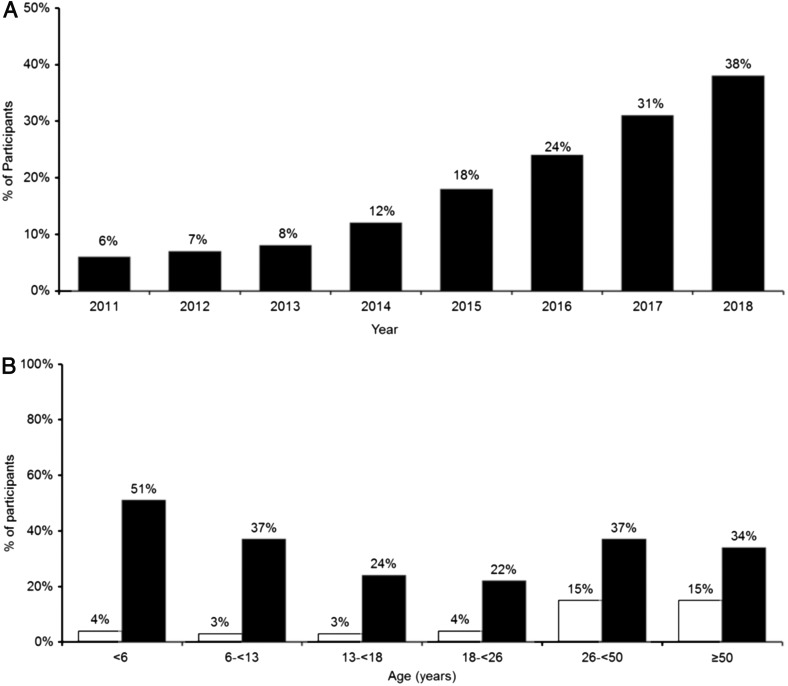
Use of an insulin pump increased from 57% in 2010–2012 to 63% in 2016–2018, with the largest increases in children (50% to 60% in children <6 years old and 58% to 68% in children 6–12 years old) (Supplementary Fig. S1). More than half of participants using an insulin pump in 2016–2018 were using a Medtronic pump (53%); 18% were using an Insulet pump, 18% Animas, and 12% Tandem. Use of CGM increased from 7% in 2010–2012 to 30% in 2016–2018, with an exponential increase in use beginning between years 2013 and 2014 (Fig. 1A, B). Children had a >10-fold increase in CGM use (4%–51% in children <6 years old and 3%–37% in children 6–12 years old) (Fig. 1B). Most participants using CGM in 2016–2018 were using a Dexcom CGM system (77%). Racial disparities were present in frequency of pump and CGM use across all age groups (Supplementary Table S3).
FIG. 1.
(A) CGM use over time. (B) CGM use in 2010–2012 versus 2016–2018. Solid white represents 2010–2012 (7% use of CGM overall). Solid black represents 2016–2018 (30% use of CGM overall). CGM, continuous glucose monitoring.
Use of noninsulin glucose-lowering medication in addition to insulin was uncommon across all age groups (Supplementary Table S1), although use increased slightly between 2010–2012 and 2016–2018 (Supplementary Table S2). Metformin was the most common noninsulin medication but used by only 6% of participants ≥26 years old.
Aspects of diabetes self-management in 2016–2018 are described in Supplementary Table S4. Of note, among non-CGM users, SMBG was done more frequently in younger pediatric participants. Most participants bolused insulin before the start of a meal. Checking of ketones was more common in children than adults, and blood ketones were very uncommonly checked across all ages; only about 20% reported having a blood ketone meter. Most participants never downloaded blood glucose meters, CGM devices, or insulin pumps at home. Other than using the Dexcom Share feature by Dexcom CGM users, use of mobile medical applications was very uncommon.
Metabolic control
2016–2018
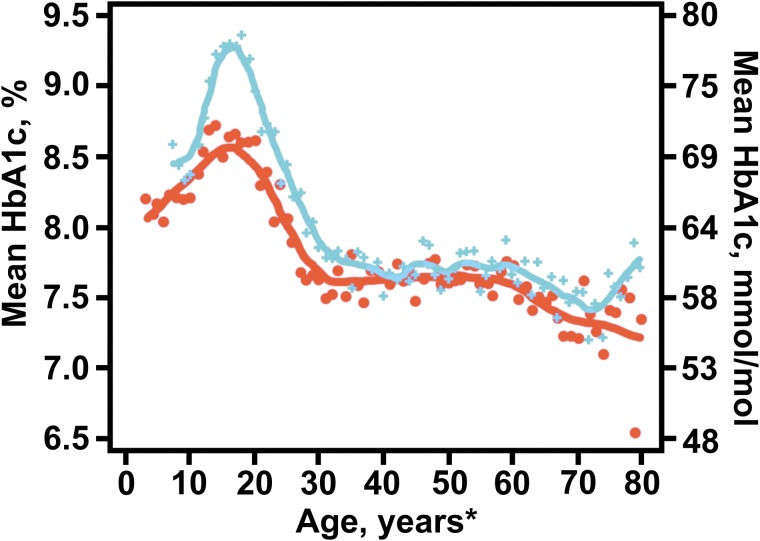
Mean HbA1c levels varied with age, race/ethnicity, and socioeconomic status (Supplementary Table S5). Mean HbA1c during childhood increased from 8.1% (65 mmol/mol) at 5 years of age to 9.3% (78 mmol/mol) between ages 15 and 18, with a steady decrease down to 8.0% (65 mmol/mol) by age 28; mean HbA1c remained fairly steady around 7.5%–7.9% (58–63 mmol/mol) beyond age 30 (Fig. 2). The American Diabetes Association (ADA) HbA1c target as of 2018 of <7.5% (<58 mmol/mol) for youth with T1D was achieved by only a small percentage of children and adolescents <18 years old (17%); only 21% of adults achieved the ADA goal of <7.0% (<53 mmol/mol) and 37% of adults had HbA1c values of <7.5% (<58 mmol/mol). Mean HbA1c was higher in African Americans than non-Hispanic whites or Hispanic whites across all age groups, even after adjusting for differences in socioeconomic status (Supplementary Table S5).
FIG. 2.
Average HbA1c by year of age: 2010–2012 versus 2016–2018. Orange line represents 2010–2012 cohort, and blue line represents 2016–2018 cohort. Participants must be contained in both cohorts with at least a 3-year duration for the 2010–2012 collection. * ≥80 years old are pooled.
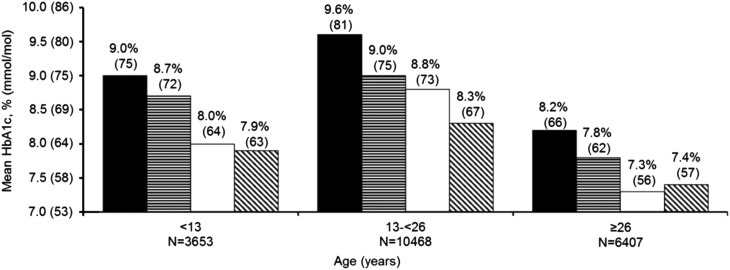
Across all age groups, HbA1c was lower in pump and CGM users (P < 0.001 adjusted for age, diabetes duration, race/ethnicity, annual income, SMBG; Fig. 3). Among CGM users, differences in HbA1c between pump and Multiple Daily Injections (MDI) users were small, except in adolescents and young adults where mean HbA1c was lower in pump users than injection users.
FIG. 3.
Mean HbA1c by technology use in 2016–2018. Solid black represents injection only. Horizontal stripes represent pump only. Solid white represents injection+CGM. Diagonal stripes represent pump+CGM.
Comparison of 2016–2018 cohort with 2010–2012 cohort
Among the 9657 participants who had data present in both 2010–2012 and 2016–2018 and at least 3 years of diabetes duration in 2010–2012, mean HbA1c was higher in 2016–2018 compared with 2010–2012. The adjusted mean HbA1c was 7.8% (62 mmol/mol) in 2010–2012 and 8.4% (68 mmol/mol) in 2016–2018 (P < 0.001 adjusted for age, diabetes duration, SMBG, and use of a CGM). The increase over time in HbA1c was predominately seen in adolescents and young adults (Fig. 2).
Acute complications in 2016–2018 cohort
Among the subset of participants with data available from the Year 5 questionnaire (N = 11,061), 6% reported experiencing seizure or loss of consciousness due to hypoglycemia in the 3 months before questionnaire completion; 3-month frequency of SH (seizure/Loss of Consciousness [LOC]) ranged from 5% in participants <18 years old to 10% in participants ≥50 years old (Table 1). Insulin pump use was associated with lower frequency of experiencing an SH event (5% vs. 9%; P < 0.001 adjusted for age, diabetes duration, sex, race/ethnicity, insurance status, and CGM status) and CGM use trended toward a lower SH frequency (5% vs. 7%; P = 0.06 adjusted for age, diabetes duration, sex, race/ethnicity, insurance status, and pump use). The frequency of SH was not associated with HbA1c level (P = 0.55 adjusted for age, diabetes duration, sex, race/ethnicity, insurance status, CGM use, and pump use; Table 1).
Table 1.
Frequency of Acute Complications in 2016–2018
| 6–12 years old | 13–17 years old | 18–25 years old | 26–49 years old | ≥50 years old | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | # ≥1 event (%) | N | # ≥1 event (%) | N | # ≥1 event (%) | N | # ≥1 event (%) | N | # ≥1 event (%) | |
| Frequency of ≥1 severe hypoglycemia event in prior 3 months | ||||||||||
| Overall | 1313 | 62 (5) | 3183 | 155 (5) | 2445 | 138 (6) | 2143 | 157 (7) | 1976 | 189 (10) |
| Insulin delivery method | ||||||||||
| Pump | 973 | 39 (4) | 2134 | 67 (3) | 1585 | 78 (5) | 1442 | 85 (6) | 1243 | 114 (9) |
| Injections | 317 | 22 (7) | 967 | 83 (9) | 817 | 58 (7) | 656 | 71 (11) | 706 | 74 (11) |
| CGM status | ||||||||||
| CGM user | 414 | 14 (3) | 584 | 15 (3) | 424 | 17 (4) | 684 | 36 (5) | 577 | 39 (7) |
| CGM nonuser | 899 | 48 (5) | 2599 | 140 (5) | 2021 | 121 (6) | 1459 | 121 (8) | 1399 | 150 (11) |
| Most recent HbA1c | ||||||||||
| <7.0% (<53 mmol/mol) | 96 | 4 (4) | 174 | 8 (5) | 257 | 11 (4) | 575 | 37 (6) | 498 | 51 (10) |
| 7.0 to <7.5% (53 to <58 mmol/mol) | 148 | 4 (3) | 268 | 9 (3) | 283 | 10 (4) | 395 | 19 (5) | 372 | 41 (11) |
| 7.5 to <8.0% (58 to <64 mmol/mol) | 214 | 9 (4) | 410 | 15 (4) | 341 | 14 (4) | 357 | 24 (7) | 375 | 30 (8) |
| 8.0 to <9.0% (64 to <75 mmol/mol) | 420 | 19 (5) | 866 | 38 (4) | 596 | 36 (6) | 398 | 27 (7) | 397 | 35 (9) |
| ≥9.0% (≥75 mmol/mol) | 380 | 25 (7) | 1370 | 79 (6) | 888 | 60 (7) | 265 | 36 (14) | 184 | 17 (9) |
| Frequency of ≥1 diabetic ketoacidosis event in prior 3 months | ||||||||||
| Overall | 1313 | 31 (2) | 3183 | 113 (4) | 2445 | 96 (4) | 2143 | 43 (2) | 1976 | 22 (1) |
| Insulin delivery method | ||||||||||
| Pump | 973 | 12 (1) | 2134 | 49 (2) | 1585 | 44 (3) | 1442 | 24 (2) | 1243 | 14 (1) |
| Injections | 317 | 17 (5) | 967 | 61 (6) | 817 | 51 (6) | 656 | 17 (3) | 706 | 8 (1) |
| CGM status | ||||||||||
| CGM user | 414 | 4 (1) | 584 | 9 (2) | 424 | 9 (2) | 684 | 5 (1) | 577 | 1 (<1) |
| CGM nonuser | 899 | 27 (3) | 2599 | 104 (4) | 2021 | 87 (4) | 1459 | 38 (3) | 1399 | 21 (2) |
| Most recent HbA1c | ||||||||||
| <7.0% (<53 mmol/mol) | 96 | 0 | 174 | 1 (1) | 257 | 2 (1) | 575 | 2 (<1) | 498 | 3 (1) |
| 7.0 to <7.5% (53 to <58 mmol/mol) | 148 | 1 (1) | 268 | 3 (1) | 283 | 3 (1) | 395 | 2 (1) | 372 | 2 (1) |
| 7.5 to <8.0% (58 to <64 mmol/mol) | 214 | 3 (1) | 410 | 2 (1) | 341 | 5 (1) | 357 | 2 (1) | 375 | 0 |
| 8.0 to <9.0% (64 to <75 mmol/mol) | 420 | 5 (1) | 866 | 18 (2) | 596 | 9 (2) | 398 | 9 (2) | 397 | 8 (2) |
| ≥9.0% (≥75 mmol/mol) | 380 | 20 (5) | 1370 | 83 (6) | 888 | 74 (8) | 265 | 25 (9) | 184 | 5 (3) |
CGM, continuous glucose monitoring.
At least one DKA event in the 3 months before the questionnaire was reported by 3% of participants, with the highest frequency (4%) in participants <26 years old (Table 1). Participants using an insulin pump were less likely to report experiencing a DKA event than participants using injections (2% vs. 4%; P = 0.002 adjusted for age, diabetes duration, sex, race/ethnicity, insurance status, CGM, SMBG, and HbA1c). Similarly, participants using CGM had fewer DKA events than non-CGM users (1% vs. 3%; P = 0.04 adjusted for age, diabetes duration, sex, race/ethnicity, insurance status, pump use, and HbA1c). Participants with higher HbA1c were more likely to experience a DKA event than participants with lower HbA1c (0.7% in participants with HbA1c <8.0% [<64 mmol/mol] and 7% in participants with HbA1c ≥9.0% [≥75 mmol/mol]; P < 0.001 adjusted for age, diabetes duration, sex, race/ethnicity, insurance status, SMBG, CGM status, and pump status).
Discussion
The T1D Exchange registry has provided important information about individuals with T1D and how T1D is managed in the United States, along with clinical outcomes. In the most recent data reported herein, across all age groups only a minority of individuals meet ADA HbA1c goals and HbA1c levels remain particularly high in adolescents and young adults. Indeed, mean HbA1c levels have increased from 2010–2012 to 2016–2018 in teens and emerging adults. This surprising finding remained after limiting the analysis to the participants who had T1D duration of at least 3 years at baseline (2010–2012) and after adjustment for age and duration of diabetes. Within this age range of adolescents and young adults, factors that have been associated with HbA1c levels such as race/ethnicity and socioeconomic status appeared balanced between the two time periods (data not shown). Thus, we do not have an explanation for this increase and it is possible that the finding could reflect a difference in diabetes duration between time periods even though duration was adjusted for in analysis or be due to other unmeasured confounding factors. Nevertheless, there is no indication from these data that HbA1c levels in the registry as a whole have improved over this 5-year period despite an increase in the use of insulin pumps and CGM.
HbA1c levels were higher in African Americans than in non-Hispanic or Hispanic whites as previously reported in the registry.13 A T1D Exchange study demonstrated that only about half of the HbA1c difference between whites and African Americans can be explained by higher mean glucose in African Americans, with the other half of the difference between races having a nonglycemic basis presumably due to genetic differences in red blood cell life span, differences in red blood cell glycation rates, or other, as of yet undefined, biologic or genetic factors.14 Of interest, a difference in HbA1c levels between race/ethnicities exists even among those in the highest income category.
As shown previously, SH occurs more commonly in older adults than in younger participants, particularly those with long duration of T1D. A prior T1D Exchange study demonstrated that hypoglycemia unawareness is a substantial risk factor for SH in older adults.15 In an attempt to standardize self-reporting of SH in a large registry, SH was defined by the occurrence of seizure or loss of consciousness. Of note, SH risk was not associated with HbA1c level in contrast to the Diabetes Control and Complications Trial, which found a strong association of lower HbA1c levels with a higher SH risk.16 However, the risk of DKA was strongly associated with HbA1c levels, with a substantial increase in DKA risk at HbA1c levels >9.0% (>75 mmol/mol), presumably representing more frequent missed insulin doses. As seen previously, DKA risk was highest in adolescents and young adults.
Perhaps the most notable change in diabetes management over the 5–7 years of registry data is the substantial increase in use of CGM in recent years. This increase has been most prominent in young children, presumably related to the ability of a parent to monitor the CGM glucose data remotely. It is noteworthy that there has been minimal adoption of other mobile medical applications. Pump use has increased modestly over this time period. The benefit of pump use and CGM use on HbA1c levels is apparent across age groups. Among CGM users, HbA1c levels were similar whether the participants were using MDI or an insulin pump, supporting the finding of clinical trials that have demonstrated benefit of CGM in MDI users to be comparable with that demonstrated in pump users.17,18 Pump use was associated with a lower DKA frequency compared with injection users. Although this is likely related to differences between pump users and MDI users rather than the insulin delivery modality, this finding nevertheless shows no indication that pump use poses an increased DKA risk. This finding is consistent with that of the Diabetes-Patienten-Verlaufsdokumentation (DPV) registry.19 Pump users also had a lower SH frequency than MDI users. Although CGM users would be expected to have a lower SH frequency than nonusers, the difference was relatively small, which could be reflecting the possibility that CGM was prescribed because of frequent SH. SH and DKA events were collected using different criteria in 2010–2012, precluding a comparison with the data from the earlier period. Although use of devices has increased, downloading of device data with retrospective review of the data as part of diabetes self-management has not. With recent greater emphasis on seamless transmission of data to the cloud and enhancements in reporting and decision-support tools, the integration of device data into self-management can be expected to increase.
Despite the value of the data from the registry, there are limitations to the interpretation of the results. The registry is not population based as all participants in the registry are treated at endocrinology centers that focus on the care of patients with T1D, nor are all patients at each clinic included in the registry. Thus, individuals not being seen by an endocrinologist are not represented and underinsured/uninsured individuals are likely underrepresented as well. As a result, certain reported frequencies such as use of devices likely are overestimates. The low proportion of registry participants meeting ADA HbA1c targets, particularly in adolescents and young adults, also is more likely to be an overestimate than underestimate, indicating that glycemic control in a general population of youth and adults with T1D may be even worse than what was found in the registry.
In summary, recent data from the T1D Exchange registry demonstrate that only a minority of adults and youth with T1D meet ADA goals for HbA1c. Glycemic control has not improved overall between 2010–2012 and 2016–2018 and in fact appears to have worsened particularly in adolescents. CGM use has substantially increased in recent years and CGM use is associated with lower HbA1c levels. Racial disparities remain in use of technology and in glycemic control. We hope that these data will stimulate further research and efforts to find ways to improve glucose control and bridge the gap in different racial backgrounds.
Supplementary Material
Contributor Information
Collaborators: for the T1D Exchange Clinic Network
Authors' Contributions
N.C.F. researched data, contributed to data interpretation, and writing of the article. R.W.B. researched data, contributed to data interpretation, and writing of the article. K.M.M. researched data and contributed to data interpretation. M.A.C. researched data and contributed to data interpretation. M.R.R. researched data and contributed to data interpretation. L.A.D. researched data and contributed to data interpretation. D.M.M. researched data and contributed to data interpretation. W.V.T. researched data and contributed to data interpretation. R.B. researched data and contributed to data interpretation. E.S. researched data and contributed to data interpretation. B.A.O. researched data and contributed to data interpretation. S.K.G. researched data and contributed to data interpretation. N.C.F. is the guarantor of this work, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Beck RW, Tamborlane WV, Bergenstal RM, et al. : The T1D Exchange Clinic Registry. J Clin Endocrinol Metab 2012;97:4383–4389 [DOI] [PubMed] [Google Scholar]
- 2.Miller KM, Foster NC, Beck RW, et al. : Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 3.Charalampopoulos D, Hermann JM, Svensson J, et al. : Exploring variation in glycemic control across and within eight high-income countries: a cross-sectional analysis of 64,666 children and adolescents with type 1 diabetes. Diabetes Care 2018;41:1180–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig ME, Prinz N, Boyle CT, et al. : Prevalence of celiac disease in 52,721 youth with type 1 diabetes: international comparison across three continents. Diabetes Care 2017;40:1034–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeSalvo DJ, Miller KM, Hermann JM, et al. : Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: international comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes 2018;19:1271–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DuBose SN, Hermann JM, Tamborlane WV, et al. : Obesity in youth with type 1 diabetes in Germany, Austria, and the United States. J Pediatr 2015;167:627–632.e624. [DOI] [PubMed] [Google Scholar]
- 7.Haynes A, Hermann JM, Miller KM, et al. : Severe hypoglycemia rates are not associated with HbA1c: a cross-sectional analysis of 3 contemporary pediatric diabetes registry databases. Pediatr Diabetes 2017;18:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofer SE, Miller K, Hermann JM, et al. : International comparison of smoking and metabolic control in patients with type 1 diabetes. Diabetes Care 2016;39:e177–e178 [DOI] [PubMed] [Google Scholar]
- 9.Lyons SK, Hermann JM, Miller KM, et al. : Use of adjuvant pharmacotherapy in type 1 diabetes: international comparison of 49,996 individuals in the prospective diabetes follow-up and T1D Exchange Registries. Diabetes Care 2017;40:e139–e140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maahs DM, Hermann JM, DuBose SN, et al. : Contrasting the clinical care and outcomes of 2,622 children with type 1 diabetes less than 6 years of age in the United States T1D Exchange and German/Austrian DPV registries. Diabetologia 2014;57:1578–1585 [DOI] [PubMed] [Google Scholar]
- 11.Maahs DM, Hermann JM, Holman N, et al. : Rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the U.S., Austria, and Germany. Diabetes Care 2015;38:1876–1882 [DOI] [PubMed] [Google Scholar]
- 12.McKnight JA, Wild SH, Lamb MJ, et al. : Glycaemic control of type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med 2015;32:1036–1050 [DOI] [PubMed] [Google Scholar]
- 13.Willi SM, Miller KM, Dimeglio LA, et al. : Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics 2015;135:424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergenstal RM, Gal RL, Connor CG, et al. : Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med 2017;167:95–102 [DOI] [PubMed] [Google Scholar]
- 15.Weinstock RS, DuBose SN, Bergenstal RM, et al. : Risk factors associated with severe hypoglycemia in older adults with type 1 diabetes. Diabetes Care 2016;39:603–610 [DOI] [PubMed] [Google Scholar]
- 16.The Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 17.Beck RW, Riddlesworth T, Ruedy K, et al. : Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017;317:371–378 [DOI] [PubMed] [Google Scholar]
- 18.Lind M, Polonsky W, Hirsch IB, et al. : Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA 2017;317:379–387 [DOI] [PubMed] [Google Scholar]
- 19.Karges B, Schwandt A, Heidtmann B, et al. : Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA 2017;318:1358–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.