Abstract
Background
Postpartum depression is a common complication of childbirth, affecting approximately 13% of women. A hormonal aetiology has long been hypothesised due to the sudden and substantial fluctuations in concentrations of steroid hormones associated with pregnancy and the immediate postpartum period. There is also convincing evidence that oestrogens, progestins, and related compounds have important central nervous system activity at physiological concentrations.
Objectives
The primary objective of this review was to assess the effects of oestrogens and progestins, including natural progesterone and synthetic progestogens, compared with placebo or usual antepartum, intrapartum, or postpartum care in the prevention and treatment of postpartum depression.
Search methods
We searched The Cochrane Pregnancy and Childbirth Group's Trials Register (March 2010), scanned secondary references and contacted experts in the field.
Selection criteria
All published and unpublished randomised controlled trials comparing an oestrogen and progestin intervention with a placebo or usual antepartum, intrapartum, or postpartum care among pregnant women or new mothers recruited within the first year postpartum.
Data collection and analysis
Two review authors participated in the evaluation of methodological quality, data extraction, and data analysis. Results are presented using relative risk for categorical data and weighted mean difference for continuous data.
Main results
Two trials, involving 229 women, met the selection criteria. Norethisterone enanthate, a synthetic progestogen, administered within 48 hours of delivery was associated with a significantly higher risk of developing postpartum depression. Oestrogen therapy was associated with a greater improvement in depression scores than placebo among women with severe depression.
Authors' conclusions
Synthetic progestogens should be used with significant caution in the postpartum period. The role of natural progesterone in the prevention and treatment of postpartum depression has yet to be evaluated in a randomised, placebo‐controlled trial. Oestrogen therapy may be of modest value for the treatment of severe postpartum depression. Its role in the prevention of recurrent postpartum depression has not been rigorously evaluated. Further research is warranted.
Plain language summary
Oestrogens and progestins for preventing and treating postpartum depression
Additional research needed to evaluate the effect of oestrogens for the prevention and treatment of postpartum depression but synthetic progesterones should not be administered.
Postpartum depression is a common complication of childbirth, affecting approximately 13% of women. A hormonal aetiology has long been hypothesised due to the sudden and substantial fluctuations in concentrations of steroid hormones associated with pregnancy and the immediate postpartum period. This review of two trials, involving 229 women, found synthetic progestogens do not prevent the development of postpartum depression and, due to their significant negative effect on maternal mood, their administration in the postpartum period for other clinical indications (e.g., contraception) is questionable. The prophylactic effect of natural progesterone remains unknown. Despite the promising preliminary findings, additional research is also needed before oestrogens can be recommended for the routine treatment of postpartum depression. Its role in the prevention of recurrent postpartum depression has not been rigorously evaluated. Further research is warranted.
Background
Postpartum depression is a common complication of childbirth, affecting approximately 13% of women from diverse cultures (O'Hara 1996). Criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition require that symptoms of major depression with postpartum onset begin within four weeks of childbirth; however, research evidence indicates that episodes of depression are common up to one year postdelivery (Stuart 1998). The presentation of symptoms in postpartum depression is generally considered to be the same as for episodes of major depression at other times, although the symptoms may focus upon elements relevant to the childbearing context (Sugawara 1999). Postpartum depression has well documented health consequences for the mother, child, and family. In particular, women with a history of postpartum depression are at increased risk for future depressive episodes (Bell 1994), and disruptions in cognitive, social, and emotional development have been reported in their children (Essex 2001; Hay 2001; Murray 1999; Murray 2001).
The aetiology of postpartum depression is much debated as biochemical (Steiner 2003), sociocultural (Stern 1983), and biopsychosocial (Ross 2004) mechanisms have all been proposed. A hormonal basis for postpartum depression has long been hypothesised (Nott 1976), due to the sudden and substantial fluctuations in concentrations of steroid hormones associated with pregnancy and the immediate postpartum period. During pregnancy, plasma progesterone concentrations increase 10 to 18‐fold, while certain oestrogens increase up to 1000‐fold, with a return to near pre‐pregnancy levels generally within the first few hours postpartum (Russell 2001). For example, postpartum women experience approximately a 10‐fold drop in circulating levels of oestradiol with the removal of the placenta at delivery (Albrecht 1990). Significant changes are also observed in several other steroid and peptide hormones, including corticotrophin‐releasing hormone, prolactin, and oxytocin (Russell 2001). Despite this, no consistent endocrine differences have been found between women who develop postpartum depression and those who do not, perhaps due to a lack of correlation between central and peripheral concentrations of hormones (Bloch 2003). Other research has indicated that postpartum depression may develop in a subgroup of women as a pathological reaction to normal endocrine changes associated with pregnancy and the postnatal period (Bloch 2000).
There is now convincing evidence that oestrogens, progestins, and related compounds have important central nervous system activity at physiological concentrations (Russell 2001). In an animal model of postpartum depression, administration of oestrogen has been associated with an antidepressant response (Galea 2001). Further, recent research suggests that oestrogen therapy, particularly in combination with conventional antidepressant therapy, may be effective in treating depression during the perimenopause (Soares 2003). This, taken together with the limited evidence for a potential role for endocrine factors in postpartum depression (Bloch 2000), has led some researchers to propose the use of oestrogen, natural progesterone (a cholesterol derivative made from Mexican yam, soybean products, and occasionally animal sources) or their synthetic analogues (i.e., progestogens which are chemically formulated from progesterone) in the treatment or prophylaxis of postpartum depression.
Objectives
The primary objective of this review was to assess the effects of oestrogens and progestins, including natural progesterone and synthetic progestogens, compared with placebo or usual antepartum, intrapartum, or postpartum care in the prevention and treatment of postpartum depression.
Secondary objectives were to examine:
the effectiveness of specific preparations;
the effectiveness of natural versus synthetic compounds;
the effectiveness of a hormonal preparation given alone versus in combination with a non‐hormonal agent or a psychosocial/psychological intervention.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished, and ongoing randomised controlled trials that address the primary objective. Quasi‐randomised trials (e.g., those randomised by delivery date, odd versus even numbers) were excluded from the analysis.
Types of participants
Pregnant women and new mothers recruited within the first year postpartum, including those at no known risk and those identified as at‐risk to develop postpartum depression.
Types of interventions
A variety of oestrogen and progestin interventions were considered, alone or in combination with other strategies. Preventive interventions were provided antenatally or within the first month postpartum and all interventions were administered by a health professional (e.g., psychiatrist, general practitioner, obstetrician).
Types of outcome measures
A. Maternal outcomes
(1) Postpartum depression (as variously defined and measured by trialists) (2) Postpartum psychosis (3) Postpartum anxiety (4) Obstetrical or gynaecological complications (vaginal bleeding, endometrial changes)
B. Infant outcomes
(5) Breastfeeding duration (variously defined) (6) Breastfeeding level (exclusive, almost exclusive, high, partial, token, bottle‐feeding)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group trials register by contacting the Trials Search Co‐ordinator (March 2010).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
For details of searches carried out in the previous version of the review, please see:Appendix 1
Searching other resources
We scanned secondary references and obtained promising studies and made contacts with experts in the field to identify other published or unpublished trials.
We did not apply any language restrictions.
Data collection and analysis
Selection of trials
Two review authors reviewed the titles and abstracts of the electronic searches. Both review authors independently evaluated trials under consideration for methodological quality and appropriateness for inclusion, without consideration of their results. In the case of uncertainties regarding the appropriateness for inclusion, we established resolution through discussion and consensus.
Methodological quality assessment
Both review authors assessed the quality of the trials that met the eligibility criteria using the following criteria:
generation of random allocation sequence: adequate, inadequate, unclear;
allocation concealment: A = adequate, B = unclear, C = inadequate;
blinding of participants: yes, no, inadequate, no information;
blinding of caregivers: yes, no, inadequate, no information;
blinding of outcome assessment: yes, no, inadequate or no information;
completeness of follow‐up data (including any differential loss of participants from each group): A = less than 3% of participants excluded, B = 3% to 9.9% of participants excluded, C = 10% to 19.9% excluded, D = 20% or more excluded, E = unclear;
analysis of participants in randomised groups.
Both review authors assigned a rating to each trial; results were compared and differences discussed until agreement was obtained. Reasons for exclusion of any apparently eligible trial have been clearly described.
Data extraction
Both review authors independently extracted the data from the trial reports. Wherever necessary, we requested unpublished or missing data from the trial contact author. In addition, we sought data to allow an 'intent‐to‐treat' analysis. One review author entered data into the Review Manager software (RevMan 2004) and double data entry was completed by the other review author.
Data synthesis
We analysed trials using different strategies separately. While the primary meta‐analysis was based on the occurrence of postpartum depression or not (however measured by trialists), several depression rating scales or cut‐off points were incorporated and examined separately. We performed meta‐analyses using relative risks as the measure of effect size for binary outcomes and weighted mean differences for continuous outcome measures, both with 95% confidence intervals. If trials used different ways of measuring the same continuous outcome, we used standardised mean differences.
Subgroup analyses
We planned three a priori subgroup analyses. They were as follows:
trials related to the effectiveness of specific preparations (e.g., transdermal, sublingual, etc);
trials related to the effectiveness of natural versus synthetic compounds (e.g., natural progesterone versus synthetic progestogens);
trials that evaluated the effectiveness of a hormonal preparation given alone versus in combination with a non‐hormonal agent (e.g., antidepressant medication).
Results
Description of studies
Two studies met the inclusion criteria for this review. Please see table of 'Characteristics of included studies'.
Definition of postpartum depression
In both studies, the prevalence of postpartum depression included a score above a specific cut‐off point on the Edinburgh Postnatal Depression Scale (EPDS). This scale is a 10‐item self‐report instrument where items are rated on a four‐point scale to produce a summative score ranging from 0 to 30, with higher scores indicating lower maternal mood. For the Gregoire 1996 study, an EPDS score greater than 13 indicated postpartum depression while a cut‐off score of greater than nine was used in the Lawrie 1998 study to indicate minor/major depression. Both studies also reported mean EPDS scores. Lawrie 1998 also included a clinician‐rated measure, the Montgomery‐Asberg Depression Rating Scale (MADRS), to determine postpartum depression. The MADRS consists of 10 items that are primarily concerned with psychological symptoms of depression and include global ratings of disturbance and social functioning. Each item is rated in severity from zero to six with a total score ranging from 0 to 60; scores between seven and 18 indicate mild depression, although some studies have used a cut‐off level of 11. While Gregoire 1996 administered a clinical psychiatric interview, the Schedule for Affective Disorders and Schizophrenia, no data were reported. In the Lawrie 1998 prevention trial, postpartum depression was assessed at six and 12 weeks postpartum. In contrast, the Gregoire 1996 treatment trial assessed for postpartum depression at four and 12 weeks post‐treatment.
Types of interventions
The Lawrie 1998 trial evaluated the effect of a synthetic progestogen (norethisterone enanthate) administered within 48 hours of delivery on the prevention of postpartum depression among South African women using a non‐hormonal contraception. The Gregoire 1996 trial evaluated the effect of transdermal oestrogen therapy for the treatment of postpartum depression among UK women experiencing major depression that began within 12 weeks postpartum. No trials were found evaluating the preventive effect of oestrogen therapy or the treatment effect of progestins.
Risk of bias in included studies
Lawrie 1998 While generation of the random allocation sequence was via a random number table, specific details regarding allocation concealment were not provided. A power calculation was completed but the targeted sample size was not achieved. It is noteworthy that fewer than one‐quarter of the women approached agreed to participate. Both participants and outcome assessors were blinded to group allocation. However, participant blinding was compromised in one woman who complained of excessive bleeding. The trial attrition rate was 7% at 12 weeks and an intent‐to‐treat analysis was performed. While there was a significant difference in mode of delivery between the two groups (24 women in the progestogen group versus 10 women in the placebo group had a caesarean section delivery) and caesarean section was negatively associated with depression scores, the data analysis completed controlled for this potentially confounding variable using appropriate statistical methods.
Gregoire 1996 Although participants were 'randomised', the generation of random allocation sequence and method of allocation concealment were not described. A power analysis was not outlined and the researchers recruited fewer than their intended sample size of 100 women. Both participants and outcome assessors were blinded to group allocation and the overall trial attrition rate at 12 weeks post‐treatment was 27%. While the researchers noted no statistically significant differences between the two study groups, there were two clinically significant differences: (1) more women in the oestrogen group received concurrent conventional antidepressant medication (16/34 versus 10/27) and (2) those in the placebo group had been depressed longer (mean 36 weeks) than those in the oestrogen group (31 weeks). Furthermore, more women in the placebo group missed their follow‐up visits (10/27 versus 6/34); non‐compliance could indicate no response to treatment or an improvement making further treatment unnecessary. The researchers provided no details about side‐effects experienced, except endometrial changes.
Effects of interventions
Two trials, involving 229 women, met the selection criteria and were published between 1996 and 1998. One trial was conducted in South Africa (Lawrie 1998) and the other in the UK (Gregoire 1996). While both trials included the outcome postpartum depression, Lawrie 1998 reported on other variables, including: days of vaginal bleeding and breastfeeding duration.
Comparison one: Progestin versus placebo for the prevention of postpartum depression
A. Maternal outcomes
Outcome: Depressive symptoms at six weeks postpartum
Women who received a single dose of the synthetic progestogen were significantly more likely to report minor/major depressive symptoms using either self‐reported (Edinburgh Postnatal Depression Scale (EPDS) > 11; relative risk (RR) 1.75, 95% confidence interval (CI) 1.12 to 2.72) or clinician‐rated (Montgomery‐Asberg Depression Rating Scale (MADRS) > 9; RR 1.74, 95% CI 1.08 to 2.81) measures. This effect was consistent when mean scores were examined (EPDS, weighted mean difference (WMD) 3.10, 95% CI 1.02 to 5.18; MADRS, WMD 3.40, 95% CI 0.72 to 6.08). In contrast, no significant effect was found in relation to the prevention of major depressive symptoms (MADRS > 18; RR 1.97, 95% CI 0.72 to 5.42).
Outcome: Depressive symptoms at 12 weeks postpartum
At 12 weeks postpartum, there was no significant difference in minor/major depressive symptoms using either self‐reported (EPDS > 11; RR 1.09, 95% CI 0.69 to 1.71) or clinician‐rated (MADRS > 9; RR 0.97, 95% CI 0.60 to 1.58) measures. This non‐significant effect was consistent when mean scores were examined (EPDS, WMD 0.80, 95% CI ‐1.28 to 2.88; MADRS, WMD 0.50, 95% CI ‐2.14 to 3.14). No significant effect was also found in relation to the prevention of major depressive symptoms (MADRS > 18; RR 1.06, 95% CI 0.40 to 2.80).
Outcome: Mean days of vaginal bleeding
Women who received the progestin intervention had significantly more days of vaginal bleeding than those who received the placebo (WMD 13.90, 95% CI 8.19 to 19.61). The large difference in the standard deviations between the groups suggests skewness in the distribution of the variables. As such, these results should be interpreted cautiously.
B. Infant outcomes
Outcome: Breastfeeding duration
There was no significant group difference in the number of women breastfeeding (either exclusively or partially) at six weeks (RR 0.92, 95% CI 0.77 to 1.08) or 12 weeks (RR 0.90, 95% CI 0.74 to 1.09) postpartum.
Comparison two: Oestrogen versus placebo for treatment of postpartum depression
A. Maternal outcomes
Outcome: Depressive symptoms at four weeks post‐treatment
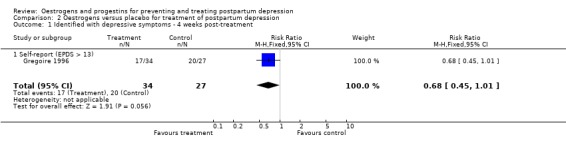
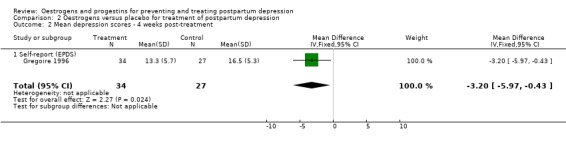
There was a significant decrease in mean EPDS scores among women in the oestrogen group in comparison to those who received a placebo (WMD ‐3.20, 95% CI ‐5.97 to ‐0.43). However, there was no significant group difference in the proportion of women scoring greater than 13 on the EPDS (RR 0.68, 95% CI 0.45 to 1.01). Only EPDS scores were reported due to high correlations between the EPDS and Schedule for Affective Disorders and Schizophrenia measures.
Outcome: Depressive symptoms at 12 weeks post‐treatment
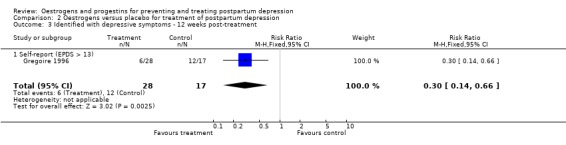
Significantly more women who received the placebo had an EPDS greater than 13 at 12 weeks post‐treatment (RR 0.30, 95% CI 0.14 to 0.66); mean scores were not reported.
Discussion
Because only two trials met the review objective and inclusion criteria, meta‐analyses were not appropriate nor could any of the proposed subgroup analyses be examined. The small number of trials also restricts the generalizability of the results. Despite these limitations, some clinically important findings were noted.
In the Lawrie trial (Lawrie 1998), a significant negative effect was found in the intervention group. In particular, women who received a single dose of the synthetic progestogen, norethisterone enanthate by injection, within 48 hours of delivery were significantly more like to report depressive symptoms at six weeks postpartum than those who received a placebo. This negative effect was not maintained to 12 weeks postpartum, quite likely because only a single dose was given. This result is noteworthy because the methodological quality of the trial was fair. It included an adequate randomisation method and intent‐to‐treat analysis. While attempts were made to blind participants, women in the progesterone group reported more days of vaginal bleeding and that this bleeding was troublesome. This side‐effect could have potentially alerted participants to study group allocation. It is also important to note that although the attrition rate in this trial was low, it was slightly uneven between the groups possibly introducing bias. Generalizability was affected by the observation that only one‐quarter of eligible women agreed to participate in the trial. Despite these methodological limitations, the results from this trial provide good evidence that the use of norethisterone and probably other synthetic progestogens should be discouraged for the prevention of postpartum depression.
In the Gregoire trial (Gregoire 1996), women who received oestrogen had significantly lower mean Edinburgh Postnatal Depression Scale (EPDS) scores at four weeks post‐treatment than those who received a placebo. However, it is important to note that the majority of women in the intervention group were still experiencing major depressive symptoms (mean EPDS score = 13.30). Although mean EPDS scores were not provided at 12 weeks post‐treatment, the proportion scoring greater than 13 on the EPDS was significantly lower for the intervention group suggesting a longer treatment effect. However, these results should be viewed cautiously due to the significant methodological limitations with this trial. Additional research is recommended. It is noteworthy that the use of oestrogens may lead to changes in the coagulation system resulting in an increased prothrombotic state (Rosendaal 2003). This potential risk needs to be considered with any oestrogen therapy.
Authors' conclusions
Implications for practice.
Results from this review suggest that synthetic progestogens should not be administered to prevent postpartum depression. Due to their significant negative effect on maternal mood, their administration for other clinical indications (e.g., contraception) is also questionable and should be examined. Despite the promising preliminary findings, additional research is also needed before oestrogens can be recommended for the treatment of postpartum depression.
Implications for research.
Despite research interest in endocrine factors in the development of postpartum depression, few methodologically strong trials have evaluated hormonal prophylaxis and treatment. Our review suggests that synthetic progestogens do not have a preventive effect, but the effect of natural progesterone is unknown. Additional research evaluating the therapeutic effect of oestrogens in the prevention and treatment of postpartum depression is also desirable. It will be particularly important to evaluate the potential adverse effects of oestrogen therapy on endometrial tissues and breastmilk production. Evaluation of maternal preferences and compliance with hormonal treatment in general is also required. A pilot trial is now being conducted in Canada to evaluate the effect of transdermal estradial patch in combination with the selective serotonin reuptake inhibitor, sertraline, among women with postpartum depression (see table of 'Characteristics of ongoing studies').
What's new
| Date | Event | Description |
|---|---|---|
| 7 June 2010 | New search has been performed | Search updated. One new trial added to Characteristics of ongoing studies (NIMH 2003). |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 3, 1999
| Date | Event | Description |
|---|---|---|
| 8 May 2008 | Amended | Converted to new review format. |
| 1 July 2004 | Amended | A new review team have taken over this review. This review was updated based upon a recent search. The background section and methodology were revised and additional studies were examined and added to the excluded studies. Revised research implications were stated. We have also changed the title from 'Oestrogens and progestins for preventing and treating postnatal depression' to 'Oestrogens and progestogens for preventing and treating postpartum depression'. |
| 30 June 2004 | New citation required but conclusions have not changed | A new review team prepared the 2004 update. |
Acknowledgements
We would like to acknowledge TA Lawrie and K Dalton for the original version of this review.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who are external to the editorial team), one or more members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Searches carried out in the previous version of the review
Authors searched Cochrane Depression, Anxiety and Neurosis Group's Trials Register (July 2004), the Cochrane Central Register of Controlled Trials (July 2004), MEDLINE (1966 to 2004), EMBASE (1980 to 2004) and CINAHL (1982 to 2004) using various combinations of the terms postpartum/postnatal depression.
Data and analyses
Comparison 1. Progestins versus placebo for prevention of postpartum depression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Identified with major depressive symptoms at 6 weeks postpartum | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.72, 5.42] |
| 1.1 Clinician‐rated (MADRS > 18) | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.72, 5.42] |
| 2 Identified with minor/major depressive symptoms at 6 weeks postpartum | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Self‐report (EPDS > 11) | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.12, 2.72] |
| 2.2 Clinician‐rated (MADRS > 9) | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.08, 2.81] |
| 3 Mean depression scores at 6 weeks postpartum | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Self‐report (EPDS) | 1 | 163 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [1.02, 5.18] |
| 3.2 Clinician‐rated (MADRS) | 1 | 163 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [0.72, 6.08] |
| 4 Identified with major depressive symptoms at 12 weeks postpartum | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.40, 2.80] |
| 4.1 Clinician‐rated (MADRS > 18) | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.40, 2.80] |
| 5 Identified with minor/major depressive symptoms at 12 weeks postpartum | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Self‐report (EPDS > 11) | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.69, 1.71] |
| 5.2 Clinician‐rated (MADRS > 9) | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.60, 1.58] |
| 6 Mean depression scores at 12 weeks postpartum | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Self‐report (EPDS) | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐1.28, 2.88] |
| 6.2 Clinician‐rated (MADRS) | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐2.14, 3.14] |
| 7 Mean days of vaginal bleeding | 1 | 164 | Mean Difference (IV, Fixed, 95% CI) | 13.90 [8.19, 19.61] |
| 7.1 At 12 weeks | 1 | 164 | Mean Difference (IV, Fixed, 95% CI) | 13.90 [8.19, 19.61] |
| 8 Identified as breastfeeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 At 6 weeks | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.77, 1.08] |
| 8.2 At 12 weeks | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.09] |
1.1. Analysis.
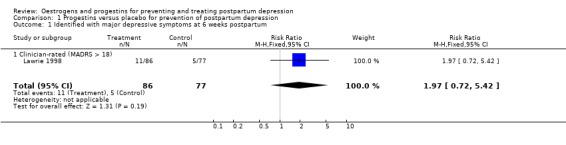
Comparison 1 Progestins versus placebo for prevention of postpartum depression, Outcome 1 Identified with major depressive symptoms at 6 weeks postpartum.
1.2. Analysis.
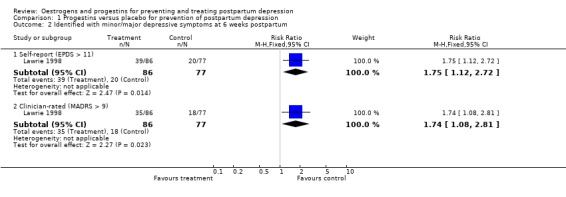
Comparison 1 Progestins versus placebo for prevention of postpartum depression, Outcome 2 Identified with minor/major depressive symptoms at 6 weeks postpartum.
1.3. Analysis.
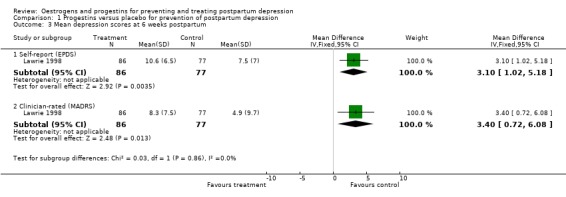
Comparison 1 Progestins versus placebo for prevention of postpartum depression, Outcome 3 Mean depression scores at 6 weeks postpartum.
1.4. Analysis.
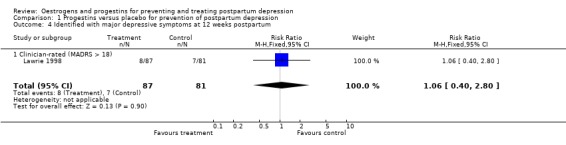
Comparison 1 Progestins versus placebo for prevention of postpartum depression, Outcome 4 Identified with major depressive symptoms at 12 weeks postpartum.
1.5. Analysis.
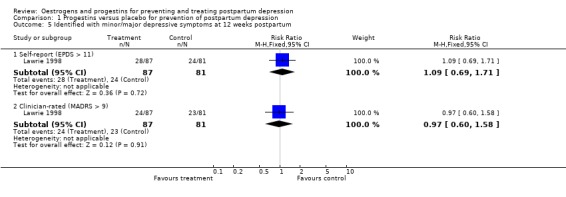
Comparison 1 Progestins versus placebo for prevention of postpartum depression, Outcome 5 Identified with minor/major depressive symptoms at 12 weeks postpartum.
1.6. Analysis.
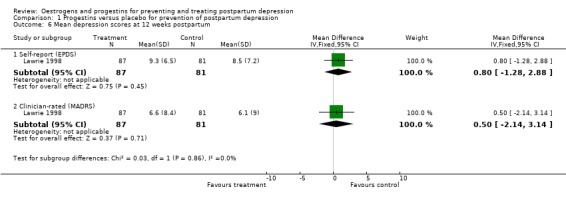
Comparison 1 Progestins versus placebo for prevention of postpartum depression, Outcome 6 Mean depression scores at 12 weeks postpartum.
1.7. Analysis.
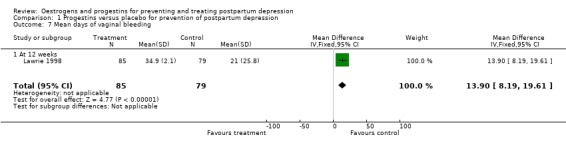
Comparison 1 Progestins versus placebo for prevention of postpartum depression, Outcome 7 Mean days of vaginal bleeding.
1.8. Analysis.
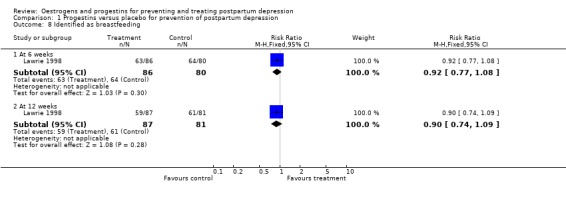
Comparison 1 Progestins versus placebo for prevention of postpartum depression, Outcome 8 Identified as breastfeeding.
Comparison 2. Oestrogens versus placebo for treatment of postpartum depression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Identified with depressive symptoms ‐ 4 weeks post‐treatment | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.45, 1.01] |
| 1.1 Self‐report (EPDS > 13) | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.45, 1.01] |
| 2 Mean depression scores ‐ 4 weeks post‐treatment | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐5.97, ‐0.43] |
| 2.1 Self‐report (EPDS) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐5.97, ‐0.43] |
| 3 Identified with depressive symptoms ‐ 12 weeks post‐treatment | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.14, 0.66] |
| 3.1 Self‐report (EPDS > 13) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.14, 0.66] |
2.1. Analysis.
Comparison 2 Oestrogens versus placebo for treatment of postpartum depression, Outcome 1 Identified with depressive symptoms ‐ 4 weeks post‐treatment.
2.2. Analysis.
Comparison 2 Oestrogens versus placebo for treatment of postpartum depression, Outcome 2 Mean depression scores ‐ 4 weeks post‐treatment.
2.3. Analysis.
Comparison 2 Oestrogens versus placebo for treatment of postpartum depression, Outcome 3 Identified with depressive symptoms ‐ 12 weeks post‐treatment.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gregoire 1996.
| Methods | RCT, placebo‐controlled, double‐blinded. The overall trial attrition rate was 27% at 12 weeks post‐treatment. | |
| Participants | Sixty‐one UK women (34 in the intervention group; 27 in the control group) with major depression that began within 12 weeks postpartum. | |
| Interventions | Intervention group: 24 weeks of transdermal 17 beta‐oestradiol 200 micrograms daily with added cyclical dydrogesterone (10 mg daily for 12 days of each month) for the last 12 weeks. Control group: placebo patches and tablets according to the same regimen. | |
| Outcomes | Reported study outcomes included the occurrence of depression (EPDS and SADS scores) at 4 and 12 weeks post‐treatment (it is noteworthy that women were assessed monthly for 24 weeks). | |
| Notes | There was a slight mistake in the reporting of categorical data so caution should be used in interpreting this data. Endometrial changes occurred in 3 women receiving treatment. The trial was sponsored by Ciba Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lawrie 1998.
| Methods | RCT, placebo‐controlled, double‐blind trial. The overall trial attrition rate was 7% at 12 weeks postpartum. | |
| Participants | 180 South African women (90 in the intervention group; 90 in the control group) agreeable to using non‐hormonal contraception during the trial. These women were not selected based on increased risk to develop postpartum depression. | |
| Interventions | Intervention group: a single 200 mg dose of norethisterone enanthate by intramuscular injection administered within 48 hours of delivery. Control group: 1 ml normal saline by intramuscular injection administered within 48 hours of delivery. | |
| Outcomes | Reported study outcomes included the occurrence of major (MADRS score > 18) and major/minor (EPDS score > 11 and MADRS score > 9) depression, vaginal bleeding, and breastfeeding duration at 6 and 12 weeks postpartum. | |
| Notes | Sources of support included Schering Pharmaceuticals Ltd (South Africa) and the Iris Ellen Hodges Trust of the University of the Witwatersrand. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
RCT: Randomised controlled trial EPDS: Edinburgh Postnatal Depression Scale MADRS: Montgomery ‐ Åsberg Depression Rating Scale SADS: Schedule for Affective Disorders and Schizophrenia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahokas 1998 | Not an RCT: a case report study of two women (oestrogen as treatment). |
| Ahokas 1999a | Not an RCT: a case report study of two women (oestrogen as treatment). |
| Ahokas 1999b | Not an RCT: a case report study of two women suffering from puerperal psychosis (oestrogen as treatment). |
| Ahokas 2000 | Not an RCT: a pilot study that included 10 women suffering from postpartum psychosis recruited consecutively. No randomisation process was reported (oestrogen as treatment). |
| Ahokas 2001 | Not an RCT: an open label study that included 23 women (oestrogen as treatment). |
| Ball 1999 | Not an RCT: a case report study (oestrogen as treatment). |
| Cizza 1997 | A letter to the editor commenting on the Gregoire trial. No original data. |
| Dalton 1985 | Not an RCT: an open study that included 100 women who self‐selected to receive treatment and were compared with untreated women (progesterone as prophylaxis). |
| Dalton 1989 | Not an RCT: an open study that included 215 women with a previous history of postpartum depression who were encouraged to receive both injections and suppositories of progesterone for 8 weeks. Details of the intervention were unclear and the different dosage of progesterone were used by women. There was no uniform scoring system used to examine a recurrence of postpartum depression (progesterone as prophylaxis). |
| Kumar 2003 | Not an RCT: an open study that included 29 pregnant women with a history of bipolar and schizoaffective disorder. Variable oestrogen doses were used (oestrogen as prophylaxis). |
| Sichel 1995 | Not an RCT: an open label study that included 11 women with a history of either non‐psychotic major depressive episode or manic postpartum psychosis (oestrogen as prophylaxis). |
| Van Der Meer 1984 | The methodology of this study was inadequate. The sample size was only 10 women and no randomisation or blinding details were provided (progesterone as prophylaxis). |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NIMH 2003.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Steiner 2004.
| Trial name or title | Randomised, double‐blind, placebo‐controlled trial of low‐dose transdermal 17‐estradiol acceleration of sertraline in major depression with postpartum onset. |
| Methods | |
| Participants | Thirty women (15 in the intervention group; 15 in the control group), not more than 12 weeks postpartum at the onset of the depressive episode, with scores in the “severe depression” range (> 35) on the MADRS and/or > 20 on the EPDS. |
| Interventions | Intervention group: sertraline (up to 100 mg/day) plus 17 beta‐ estradiol patch (25 micrograms daily) for 12 weeks. Control group: sertraline (up to 100 mg/day) plus placebo patch for 12 weeks. |
| Outcomes | Maternal mood (EPDS, MADRS) assessed weekly for 12 weeks; secondary outcomes include functional impairment, cognitive function, and neonatal well‐ being. |
| Starting date | Spring 2004. |
| Contact information | Meir Steiner, MD, PhD, FRCPC Professor of Psychiatry & Behavioural Neurosciences and Obstetrics & Gynecology, McMaster University Director of Research, Department of Psychiatry Director, Women's Health Concerns Clinic St. Joseph's Healthcare Hamilton, ON Canada L8N 4A6 Tel: (905) 522‐1155 ext 3605 Fax: (905) 521‐6098 E‐Mail: mst@mcmaster.ca |
| Notes |
EPDS: Edinburgh Postnatal Depression Scale MADRS: Montgomery ‐ Åsberg Depression Rating Scale
Contributions of authors
Both Lori Ross and Cindy‐Lee Dennis searched for trials, extracted and analysed the data, and updated the review. Andrew Herxheimer reviewed the updated version and provided editorial suggestions.
Sources of support
Internal sources
University of Toronto, Canada.
Centre for Addiction and Mental Health, Canada.
External sources
No sources of support supplied
Declarations of interest
Lori Ross is currently a co‐investigator of an ongoing trial that is evaluating the effect of transdermal estradiol patches in women with postpartum depression.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Gregoire 1996 {published data only}
- Gregoire AJP, Kumar R, Everitt B, Henderson AF, Studd JWW. Transdermal oestrogen for the treatment of severe postnatal depression. Lancet 1996;347:930‐3. [DOI] [PubMed] [Google Scholar]
- Gregoire AJP, Kumar R, Everitt B, Henderson AF, Studd JWW. Transdermal oestrogen for treatment of severe postnatal depression. International Journal of Gynecology & Obstetrics 1996;55:88. [DOI] [PubMed] [Google Scholar]
- Henderson A, Studd JWW, Gregoire A, Kumar R. The role of oestrogen in the treatment of post‐natal depression. Proceedings of 26th British Congress of Obstetrics and Gynaecology;1992; Manchester, UK. 1992:36.
- Henderson AF, Gregoire AJP, Kumar RC, Studd JWW. Treatment of severe postnatal depression with oestradiol skin patches. Lancet 1991;338:816‐7. [DOI] [PubMed] [Google Scholar]
Lawrie 1998 {published data only}
- Lawrie T, Hofmeyr G, Jager M, Berk M. A double blind randomised placebo controlled trial of norethisterone enantate: effect on postnatal depression. XXIst Collegium Internationale Neuro‐psychopharmacologicum; 1998 July 12‐16; Glasgow, Scotland. 1998.
- Lawrie T, Hofmeyr G, Jager M, Berk M. The effect of norethisterone enantate on postnatal depression: a randomised controlled trial. Proceedings of the 17th Conference on Priorities in Perinatal Care; 1998; South Africa. 1998:68.
- Lawrie TA, Hofmeyr GJ, Jager M, Berk M. The effect of norethisterone enanthate on postnatal depression: a randomised placebo‐controlled trial. Women's Health Issues 1998;8:199‐200. [DOI] [PubMed] [Google Scholar]
- Lawrie TA, Hofmeyr GJ, Jager M, Berk M, Paiker J, Viljoen E. A double‐blind randomised placebo controlled trial of postnatal norethisterone enanthate: the effect on postnatal depression and serum hormones. British Journal of Obstetrics and Gynaecology 1998;105:1082‐90. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahokas 1998 {published data only}
- Ahokas AJ, Turtiainen S, Aito M. Sublingual oestrogen treatment of postnatal depression. Lancet 1998;351(9096):109. [DOI] [PubMed] [Google Scholar]
Ahokas 1999a {published data only}
- Ahokas A, Kaukoranta J, Aito M. Effect of oestradiol on postpartum depression. Psychopharmacology 1999;146(1):108‐10. [DOI] [PubMed] [Google Scholar]
Ahokas 1999b {published data only}
- Ahokas A, Aito M. Role of estradiol in puerperal psychosis. Psychopharmacology 1999;147:108‐10. [DOI] [PubMed] [Google Scholar]
Ahokas 2000 {published data only}
- Ahokas A, Aito M, Rimon R. Positive treatment effect of estradiol in postpartum psychosis: a pilot study. Journal of Clinical Psychiatry 2000;61(3):166‐9. [DOI] [PubMed] [Google Scholar]
Ahokas 2001 {published data only}
- Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta‐estradiol: a preliminary study. Journal of Clinical Psychiatry 2001;62(5):332‐6. [DOI] [PubMed] [Google Scholar]
Ball 1999 {published data only}
- Ball DE, Morrison P. Oestrogen transdermal patches for post partum depression in lactating mothers ‐ a case report. Central African Journal of Medicine 1999;45:68‐70. [DOI] [PubMed] [Google Scholar]
Cizza 1997 {published data only}
- Cizza G, Gold P, Chrousos G. High‐dose transdermal estrogen, corticotropin‐releasing hormone, and postnatal depression. Journal of Clinical Endocrinology and Metabolism 1997;82:704. [DOI] [PubMed] [Google Scholar]
Dalton 1985 {published data only}
- Dalton K. Progesterone prophylaxis used successfully in postnatal depression. Practitioner 1985;229:507‐8. [Google Scholar]
Dalton 1989 {published data only}
- Dalton K. Sucessful prophylactic progesterone for idiopathic postnatal depression. International Journal of Prenatal and Perinatal Studies 1989;1:322‐7. [Google Scholar]
Kumar 2003 {published data only}
- Kumar C, McIvor R, Davies T, Brown N, Papadopoulos A, Wieck A, Checkley S, Campbell I, Marks M. Estrogen adminisration does not reduce the rate of recurrence of affective psychosis after childbirth. Journal of Clinical Psychiatry 2003;64:112‐8. [DOI] [PubMed] [Google Scholar]
Sichel 1995 {published data only}
- Sichel D, Cohen L, Robertson L, Ruttenberg A, Rosenbaum J. Prophylactic estrogen in recurrent postpartum affective disorder. Biological Psychiatry 1995;38:814‐8. [DOI] [PubMed] [Google Scholar]
Van Der Meer 1984 {published data only}
- Meer YG, Loendersloot EW, Loenen AC. Effect of high‐dose progesterone in postpartum depression. Journal of Psychosomatic Obstetrics and Gynaecology 1984;3:67‐8. [Google Scholar]
References to ongoing studies
NIMH 2003 {published data only}
- National Institue of Mental Health (NIMH). Clinical trial of estrogen for postpartum depression. ClinicalTrials.gov (http://clinicaltrials.gov/ct2/show/NCT00059228) (accessed 15 September 2004) 2003.
Steiner 2004 {unpublished data only}
- Steiner M. Randomized, double‐blind, placebo‐controlled trial of low‐dose transdermal 17‐estradiol acceleration of sertraline in major depression with postpartum onset. Personal communication 2004.
Additional references
Albrecht 1990
- Albrecht ED, Pepe GJ. Placental steroid hormone biosynthesis in primate pregnancy. Endocrine reviews 1990;11(1):124‐50. [DOI] [PubMed] [Google Scholar]
Bell 1994
- Bell AJ, Land NM, Milne S, Hassanyeh F. Long‐term outcome of post‐partum psychiatric illness requiring admission. Journal of Affective Disorders 1994;31(1):67‐70. [DOI] [PubMed] [Google Scholar]
Bloch 2000
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. American Journal of Psychiatry 2000;157(6):924‐30. [DOI] [PubMed] [Google Scholar]
Bloch 2003
- Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Comprehensive Psychiatry 2003;44(3):234‐46. [DOI] [PubMed] [Google Scholar]
Essex 2001
- Essex MJ, Klein MH, Miech R, Smider NA. Timing of initial exposure to maternal major depression and children's mental health symptoms in kindergarten. British Journal of Psychiatry 2001;179:151‐6. [DOI] [PubMed] [Google Scholar]
Galea 2001
- Galea LA, Wide JK, Barr AM. Estradiol alleviates depressive‐like symptoms in a novel animal model of post‐partum depression. Behavioural Brain Research 2001;122(1):1‐9. [DOI] [PubMed] [Google Scholar]
Hay 2001
- Hay DF, Pawlby S, Sharp D, Asten P, Mills A, Kumar R. Intellectual problems shown by 11‐year‐old children whose mothers had postnatal depression. Journal of Child Psychology & Psychiatry & Allied Disciplines 2001;42(7):871‐89. [DOI] [PubMed] [Google Scholar]
Murray 1999
- Murray L, Sinclair D, Cooper P, Ducournau P, Turner P, Stein A. The socioemotional development of 5‐year‐old children of postnatally depressed mothers. Journal of Child Psychology & Psychiatry & Allied Disciplines 1999;40:1259‐71. [PubMed] [Google Scholar]
Murray 2001
- Murray L, Woolgar M, Cooper P, Hipwell A. Cognitive vulnerability to depression in 5‐year‐old children of depressed mothers. Journal of Child Psychology and Psychiatry 2001;42:891‐9. [DOI] [PubMed] [Google Scholar]
Nott 1976
- Nott PN, Franklin M, Armitage C, Gelder MG. Hormonal changes and mood in the puerperium. British Journal of Psychiatry 1976;128:379‐83. [DOI] [PubMed] [Google Scholar]
O'Hara 1996
- O'Hara MW, Swain AM. Rates and risk of postpartum depression‐a meta‐analysis. International Review of Psychiatry 1996;8(1):37‐54. [Google Scholar]
RevMan 2004 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
Rosendaal 2003
- Rosendaal FR, Hylckama Vlieg A, Tanis BC, Helmerhorst FM. Estrogens, progestogens and thrombosis. Journal of Thrombosis and Haemostasis 2003;1:1371‐1380. [DOI] [PubMed] [Google Scholar]
Ross 2004
- Ross LE, Sellers EM, Gilber Evans SE, Romach MK. Mood changes during pregnancy and the postpartum period: development of a biopsychosocial model. Acta Psychiatrica Scandinavica 2004;109:457‐66. [DOI] [PubMed] [Google Scholar]
Russell 2001
- Russell JA, Douglas AJ, Ingram CD. Brain preparations for maternity‐‐adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation. An overview. Progress in Brain Research 2001;133:1‐38. [DOI] [PubMed] [Google Scholar]
Soares 2003
- Soares CN, Poitras JR, Prouty J. Effect of reproductive hormones and selective estrogen receptor modulators on mood during menopause. Drugs & Aging 2003;20(2):85‐100. [DOI] [PubMed] [Google Scholar]
Steiner 2003
- Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. Journal of Affective Disorders 2003;74(1):67‐83. [DOI] [PubMed] [Google Scholar]
Stern 1983
- Stern G, Kruckman L. Multi‐disciplinary perspectives on post‐partum depression: an anthropological critique. Social Science & Medicine 1983;17(15):1027‐41. [DOI] [PubMed] [Google Scholar]
Stuart 1998
- Stuart S, Couser G, Schilder K, O'Hara M, Gorman L. Postpartum anxiety and depression: onset and comorbidity in a community sample. Journal of Nervous & Mental Disease 1998;186:420‐4. [DOI] [PubMed] [Google Scholar]
Sugawara 1999
- Sugawara M, Sakamoto S, Kitamura T, Toda MA, Shima S. Structure of depressive symptoms in pregnancy and the postpartum period. Journal of Affective Disorders 1999;54(1‐2):161‐9. [DOI] [PubMed] [Google Scholar]


