Abstract
Background
Inflammation is known to be associated with posttraumatic stress disorder. It is not known if total white blood cell count, a routinely checked inflammatory marker, is associated with posttraumatic stress disorder symptom trajectories using medical record data.
Methods
We used latent class growth analysis to identify three-year posttraumatic stress disorder symptom trajectories using posttraumatic stress disorder (PTSD) Checklist (PCL) scores. The outcome for each patient was maximum white blood cell count from index posttraumatic stress disorder diagnosis to last PCL. Using linear regression analysis, we then calculated and compared the average white blood cell count for each trajectory before and after controlling for age, gender, race, obesity, smoking, diabetes, hypertension, cardiovascular disease, depression, and other comorbid inflammatory conditions.
Results
Patients were 40.2 (SD ± 13.5) years of age, 83.7% males and 67.9% white. We identified three PCL trajectory groups based on symptom severity over time: “moderate-large decrease,” “moderate-severe-slight decrease,” and “severe-persistent.” In adjusted analyses, “severe-persistent” versus “moderate-large decrease” had significantly higher white blood cell count (B = 0.64; 95%CI = 0.18, 1.09; p = .006). Although non-significant, “moderate-severe-slight decrease” versus “moderate-large decrease” also had a higher white blood cell count (B = 0.42; 95% CI: −0.02, 0.86; p = .061).
Conclusion
Persistently severe posttraumatic stress disorder is associated with a higher white blood cell count than improving posttraumatic stress disorder. White blood cell appears to have utility for measuring the association between psychiatric disorders and inflammation in retrospective cohort studies involving large administrative medical record data bases.
Keywords: Posttraumatic stress disorder, inflammation, white blood cell, PTSD Checklist scores, symptom trajectories
Introduction
The pathogenesis of posttraumatic stress disorder (PTSD) includes a disrupted autonomic nervous system and dysfunction in the hypothalamic–pituitary–adrenal axis, both of which are associated with increased inflammation.1,2 However, while several studies have shown an association of PTSD severity with increased inflammation3,4 others have been equivocal.5,6 Low-grade, chronic inflammation is also postulated as a mechanism behind the association between PTSD and development of chronic health conditions, including cardiovascular disease (CVD).1,7 While other inflammatory conditions including major depression often co-exist with PTSD, the association of PTSD with inflammation remains even after adjustment for depression6,8 or excluding patients with depression.9
Studies of inflammation in patients with PTSD have mostly been prospective and have measured inflammation using biomarkers that are not collected as part of routine medical care. Unlike more specific biomarkers, such as C-reactive protein or interleukins, total blood white blood cell (WBC) count is routinely checked in clinical practice. WBC count has been used as a biomarker of chronic low-grade inflammation in PTSD where higher versus lower mean WBC count (within the normal range) is associated with high levels of other inflammatory biomarkers.10 In a study of Vietnam veterans, those with PTSD versus those without had a higher mean WBC count and a higher likelihood of having levels above the normal range (odds ratio (OR) = 1.83, p = .04)11 In a recent study (n = 163) of male combat-exposed veterans, Lindqvist et al. reported a higher mean WBC count in those with PTSD versus without PTSD10 that was associated with other inflammatory biomarker elevation but not with PTSD severity. These studies were limited by their cross-sectional nature. In a recent prospective study of veterans, Eswarappa et al. studied the association of inflammatory biomarkers with PTSD trajectories over a 4-year follow-up period.12 They reported that elevated mean WBC count (OR = 1.27; 95% confidence interval (CI): 1.10–1.47) was a significant predictor of persistent versus improving PTSD after adjustment for age, sex, physical activity, diabetes, body mass index (BMI), and smoking.12 This study was limited by using only baseline WBC and lack of adjustment of other inflammatory conditions.
A meta-analysis including 20 studies indicates PTSD is associated with chronic low-grade inflammation13; however, the existing studies linking PTSD with measures of chronic inflammation have been limited by small sample sizes, inconsistent methods of measuring inflammation and measuring it only at baseline, and short follow-up duration. One of the best resources for research on PTSD using “real-world” clinical data is the United States Veterans Health Affairs (VHA) administrative medical record data. VHA data provide the largest, national cohort of PTSD patients, a longer observation period to evaluate PTSD severity and ability to control for multiple comorbidities including inflammatory conditions. To assess the feasibility of using WBC count from VHA data and to measure the association between PTSD severity and inflammation, we compared the average of individual patient’s maximum WBC counts in three distinct PTSD symptom trajectories. This study leveraged the strengths of a large retrospective cohort to study the association between PTSD and inflammation over a long observation time to determine whether a PTSD severity and chronicity were associated with a marker of chronic inflammation routinely collected from patients, i.e. total WBC count.
Methods
Study Population and Data Sources
VHA administrative medical record data were obtained from a random sample of 5916 patients aged 18 to 70 years with a PTSD diagnosis who used one of five PTSD specialty outpatient clinics between fiscal years (FY) 2008 to 2012. Additional details regarding the cohort creation have been previously reported.14 The cohort observation period continued through 2015. Data elements included International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes, vital signs, laboratory results, medications, and demographic data. The study was approved by the institutional review board of participating institutions. A waiver of informed consent was obtained because this study used de-identified data.
PTSD Diagnosis and Severity
PTSD was defined as two outpatient visits within a 12-month period or one inpatient stay for ICD-9 code 309.81 in FY08–12.15,16 The date of initial PTSD diagnosis in FY08-12 was the index date. We used the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, of the PTSD Checklist (PCL), a validated 17-item self-report questionnaire, to compute PTSD symptom trajectories, with scores ranging from 17 to 85.17 Medical record data do not capture all PCL scores (e.g., those stored in physician notes); therefore, PCL data from administrative medical record abstraction were supplemented with manual chart abstraction conducted by Abt Associates’ (www.abtassociates.com). Abt associates abstracted 22,287 valid PCL scores for encounters between FY2008 and FY2015. Administrative and manual chart abstraction data were combined, and after removing duplicates, there were 26,631 valid PCL scores for 4441 PTSD patients. Details about chart abstraction have been previously reported.18
WBC Count Outcome
WBC counts measured from PTSD index date to the last PCL score in the three years following index date were extracted from administrative medical record laboratory result data. Extreme WBC count values (<2 or >20 × 109/L) were excluded due to high likelihood of the presence of a confounding illness involving the immune system. If a patient had multiple WBC counts, the maximum (i.e. worst) was chosen as his or her outcome.
Covariates
Covariates were selected if they were potential confounders in the relationship of exposure group (PCL trajectory) and WBC count. Sociodemographic covariates included age, gender, and race. If a sociodemographic included missing data, a category for “unknown” was created so that cases would not be excluded from analyses.
Comorbid conditions were measured from PTSD index date to the last PCL date in the three-year period and chosen on their known association with WBC count and inflammation. These included other coexistent inflammatory conditions, depression,12 obesity,19 type 2 diabetes,20 CVD,21 hypertension,22 and smoking.23 Other inflammatory condition was based on the presence of any of the following diagnostic codes: infections/parasitic diseases (001.x–139.x), disorders involving immune mechanisms (279.x), disorders of WBCs (288.x), other blood diseases (289.x), kidney infections (590.x), urinary tract infection and related (595.x, 597.x, 599.0), orchitis/epididymitis (604.x), skin/subcutaneous infections (680.x–686.x), or pneumonia (480.x–488.x). Depression was defined by at least two outpatient diagnostic codes within a 12-month period or a single inpatient code for 296.2x, 296.3x, or 311.24 Obesity was defined by the presence of a BMI ≥ 30 or an ICD-9 diagnostic code (278.00 and 278.01). Type 2 diabetes was defined as the presence of at least two diagnostic codes on separate days (250.x0, 250.x2, 357.2, 362.0x, 366.51). CVD was defined by the presence of an ICD-9 code for: hypertensive heart disease (402.x to 405.x), myocardial infarction (410.x, 411.x), ischemic heart disease (412.x to 414.x), disease of pulmonary circulation (415.x to 417.x), or other heart disease (420.x to 429.x). Hypertension was defined by the presence of ICD-9 code 401.x. Current smoking was identified with an ICD-9 code for nicotine dependence (V15.82, 305.1) or from patient-reported smoking status in VHA health factor data.
Sensitivity analysis allowed influenza diagnosis (ICD-9 codes 487.x and 488.x), and all ICD-9 codes related to injury (800.x to 959.x) to the list of co-existent inflammatory conditions which could occur anytime between index and last PCL value.
Analytic Approach
The first phase of the analysis conducted a latent class growth analysis (LCGA) using PROC TRAJ and maximum likelihood estimation in SAS v9.2 (SAS Institute, Cary, NC)25–28 to create three-year PTSD trajectories to differentiate PTSD patients with persistent symptoms versus those with symptom improvement over time based on similar methodology reported by Sripada et al.29 The date of initial PTSD diagnosis was considered the “index date,” the outcome was PCL score, and time was measured as months since PTSD index. For this analysis, patients were required to have ≥3 PCL scores in the three years after index (n = 1807), with one having to occur in the first year (n = 1603). If there were multiple PCL scores in a month, the first score was used.
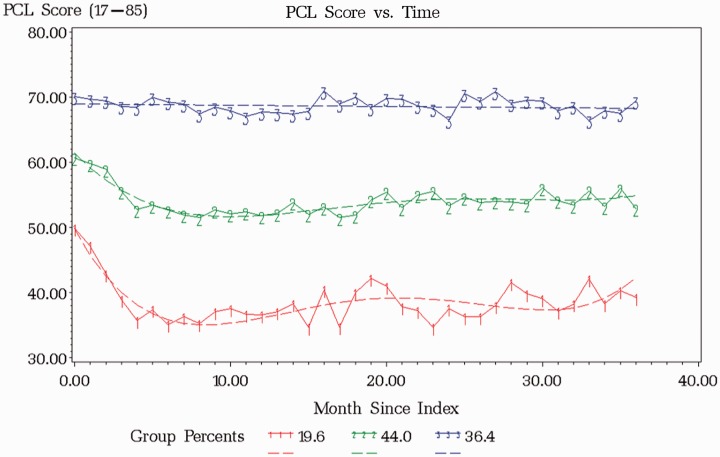
LCGA is a semiparametric technique that is a specialized application of finite mixture modeling allowing the identification of distinct subgroups in a population defined by a developmental trajectory of a particular variable over time.30–32 A censored normal distribution was used to fit the continuous data and following methods in Nagin (1999, 2005),31,33 models with the highest polynomial order (quartic) were fit and up to five classes were specified. The number of trajectories in the final model was determined with Bayesian Information Criterion (BIC), entropy, interpretability of classes (i.e., size > 5% prevalence and examination of trajectory plots), and average posterior probability > 0.80 for those assigned to each group based on the maximum probability rule.25,31–33 Once the number of groups was identified, polynomial orders were reduced until the highest order polynomial for each group was significant and BIC was minimized. The highest posterior probability of group membership was used to assign patients to a trajectory group. Supplementary Table 1 shows fit statistics and Figure 1 shows PCL score versus time using actual (solid) and model fitted (dashed) lines.
Figure 1.
Three-year PCL trajectories and predicted group membership probabilities. Group 1: moderate-large decrease; Group 2: moderate-severe-slight decrease; Group 3: severe-persistent; PCL: PTSD Checklist.
The second phase of the analysis used the PCL trajectory groups as the exposure and WBC count as the outcome. For this analysis, SAS v9.4 (SAS Institute, Cary, NC) was used with alpha set at 0.05. Eligible patients for this outcome analysis were the 1266 patients with at least one WBC count from PTSD index to last PCL score.
Descriptive analysis used chi-square tests and one-way analysis of variance with Tukey’s post hoc comparisons to compare covariates between exposure groups as well as first and last PCL score between PCL trajectory groups. Crude and adjusted linear regression models were computed to measure the association of WBC count before and after accounting for covariates, with the “moderate-large decrease” trajectory as the reference category. Unstandardized beta’s (B) and 95% CIs were calculated.
Results
Table 1 shows overall sample characteristics among the 1266 patients eligible for final analysis. Average age was 40.2 years (±13.5) and the majority of patients were White (67.9%) and male (83.7%). About half were obese (53.6%) or smokers (48.0%), 38.6% had other inflammatory conditions, 9.6% had type 2 diabetes, and about 76.9% had a diagnosis of depression. Overall average WBC count was 8.3 (±3.0) × 109/L.
Table 1.
Overall descriptive statistics, VHA patients with PTSD aged 18 to 70 years with ≥3 PCL and ≥1 WBC count in three years after PTSD (n = 1266).
| Variable, n (%) or mean (±SD) | Overall (n = 1266) |
|---|---|
| Age (years), mean (±SD) | 40.2 (±13.5) |
| Male gender, n (%) | 1060 (83.7) |
| Race, n (%) | |
| White | 860 (67.9) |
| Black | 281 (22.2) |
| Other | 86 (6.8) |
| Unknown | 39 (3.1) |
| Other inflammatory conditions, n (%) | 489 (38.6) |
| Depression, n (%) | 973 (76.9) |
| Obese, n (%) | 679 (53.6) |
| Type 2 diabetes, n (%) | 122 (9.6) |
| Cardiovascular disease, n (%) | 180 (14.2) |
| Hypertension, n (%) | 480 (37.9) |
| Smoker, n (%) | 607 (48.0) |
| First PCL, mean (±SD) | 61.7 (±12.7) |
| Last PCL, mean (±SD) | 55.6 (±16.3) |
| WBC count, mean (±SD) | 8.3 (±3.0) |
VHA: Veterans Health Administration; PTSD: post-traumatic stress disorder; PCL: PTSD Checklist; WBC: white blood cell count (units = × 109/L)
Based on diagnostics and interpretability of classes, the LCGA identified a three-trajectory solution with predicted group membership probabilities characterized as “moderate-large decrease” (19.6%), “moderate-severe-slight decrease” (44.0%), and “severe-persistent” (36.4%) (see Figure 1 and Supplementary Table 1). Although BIC kept decreasing with added classes, additional trajectories were small and parallel to existing trajectories in the three-trajectory solution. Entropy also started to decrease dramatically after a three-trajectory solution indicating poorer prediction of trajectory membership. All average posterior probabilities for each trajectory were also > 0.80 in the three-class solution.
There was a greater proportion of White race in the “moderate-large decrease” (73.2%) and “moderate-severe-slight decrease” (72.2%) compared to the “severe-persistent” group (60.9%) (Table 2). Conversely, there was a greater proportion of Black race in “severe-persistent” (27.4%) followed by the “moderate-severe-slight decrease” (19.3%), and last by the “moderate-large decrease” (17.7%) groups. Depression was negatively associated with PTSD symptom reduction such that the highest proportion of patients with depression was in the “severe-persistent” group (84.5%), followed by the “moderate-severe-slight decrease” (75.2%) and the “moderate-large decrease” (63.2%) groups.
Table 2.
Covariate and PCL value comparisons between PCL groups, VHA patients with PTSD aged 18 to 70 years with ≥3 PCL and ≥1 WBC count in three years after PTSD (n = 1266).
| Variable, n (%) or mean (±SD) | Moderate-large decrease (n = 209) | Moderate-severe-slight decrease (n = 561) | Severe-persistent (n = 496) | pa |
|---|---|---|---|---|
| Age (years), mean (±SD) | 38.9 (±13.5) | 40.0 (±13.8) | 41.1 (±13.0) | .116 |
| Male gender, n (%) | 173 (82.8) | 461 (82.2) | 426 (85.9) | .243 |
| Race, n (%) | ||||
| White | 153 (73.2) | 405 (72.2) | 302 (60.9) | |
| Black | 37 (17.7) | 108 (19.3) | 136 (27.4) | .002 |
| Other | 11 (5.3) | 33 (5.9) | 42 (8.5) | |
| Unknown | 8 (3.8) | 15 (2.7) | 16 (3.2) | |
| Other inflammatory conditions, n (%) | 75 (35.9) | 209 (37.3) | 205 (41.3) | .267 |
| Depression, n (%) | 132 (63.2) | 422 (75.2) | 419 (84.5) | <.0001 |
| Obese, n (%) | 106 (50.7) | 298 (53.1) | 275 (55.4) | .490 |
| Type 2 diabetes, n (%) | 13 (6.2) | 54 (9.6) | 55 (11.1) | .135 |
| Cardiovascular disease, n (%) | 32 (15.3) | 81 (14.4) | 67 (13.5) | .806 |
| Hypertension, n (%) | 59 (28.2) | 218 (38.9) | 203 (40.9) | .005 |
| Smoker, n (%) | 95 (45.5) | 278 (49.6) | 234 (47.2) | .544 |
| First PCL, mean (±SD) | 48.8 (±13.1)a | 59.2 (±10.4)b | 69.9 (±8.4)c | <.0001 |
| Last PCL, mean (±SD) | 34.0 (±11.3)a | 52.3 (±12.1)b | 68.5 (±9.1)c | <.0001 |
VHA: Veterans Health Administration; PTSD: post-traumatic stress disorder; PCL: PTSD Checklist; WBC: white blood cell count (units = × 109/L); ANOVA: analysis of variance. Means with same subscripts are not significantly different between PCL groups, Tukey post-hoc p > .05.
Chi-square test or omnibus ANOVA.
Hypertension was most common in the “severe-persistent” (40.9%) and “moderate-severe-slight decrease” (38.9%) groups and least prevalent among those in the “moderate-large decrease” group (28.2%). Other inflammatory conditions, obesity, type 2 diabetes, CVD, and smoking were unrelated to PCL group (Table 2).
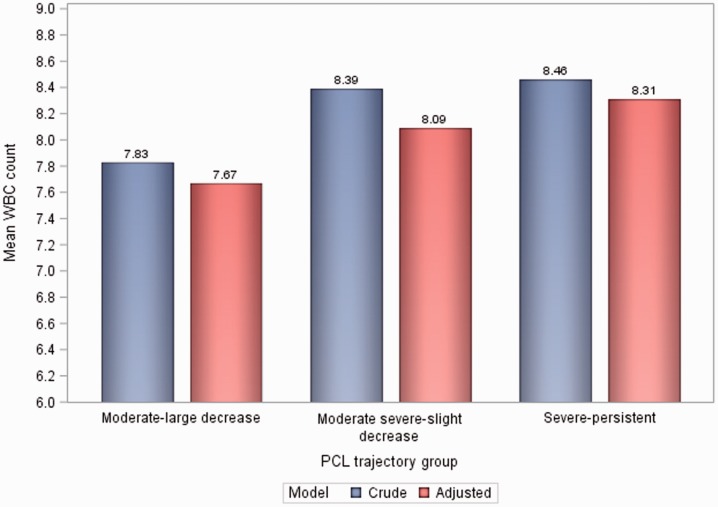
Table 3 shows results of crude and adjusted linear regression models and Figure 2 shows crude and adjusted WBC count means by group. In crude analysis, compared to “moderate-large decrease” (mean = 7.83 × 109/L), both “moderate-severe-slight decrease” (mean = 8.39 × 109/L; B = 0.56, 95% CI = 0.08, 1.04) and “severe-persistent” (mean = 8.46 × 109/L; B = 0.63, 95% CI = 0.14, 1.12) had significantly higher average WBC counts.
Table 3.
General linear models—crude and adjusted linear regression models predicting mean WBC count (n = 1266).
| Crude |
Adjusted |
|||
|---|---|---|---|---|
| Variable | B (95% CI) | p | B (95% CI) | p |
| Intercept | 7.83 (7.42, 8.24) | <.0001 | 7.93 (7.13, 8.73) | <.0001 |
| PCL group | ||||
| Moderate-large decrease | ref | ref | ref | ref |
| Moderate-severe-slight decrease | 0.56 (0.08, 1.04) | .022 | 0.42 (−0.02, 0.86) | .061 |
| Severe-persistent | 0.63 (0.14, 1.12) | .011 | 0.64 (0.18, 1.09) | .006 |
| Age (years) | −0.03 (−0.04, −0.01) | <.0001 | ||
| Male gender | −0.25 (−0.68, 0.18) | .248 | ||
| Race | ||||
| White | Ref | Ref | ||
| Black | −1.92 (−2.31, −1.53) | <.0001 | ||
| Other | 0.001 (−0.61, 0.62) | .997 | ||
| Other inflammatory conditions | 0.98 (0.66, 1.30) | <.0001 | ||
| Depression | 0.29 (−0.08, 0.66) | .123 | ||
| Obese | 0.26 (−0.06, 0.57) | .113 | ||
| Type 2 diabetes | 0.50 (−0.07, 1.06) | .085 | ||
| Cardiovascular disease | 0.80 (0.33, 1.27) | .001 | ||
| Hypertension | 0.70 (0.33, 1.07) | .0002 | ||
| Smoker | 1.18 (0.86, 1.49) | <.0001 | ||
PTSD: posttraumatic stress disorder; PCL: PTSD Checklist; WBC: white blood cell count (units = × 109/L); B: unstandardized regression coefficient; CI: confidence interval.
Figure 2.
Crude and adjusted WBC count by group. WBC: white blood cell (units = × 109/L); PCL: PTSD Checklist.
Mean differences were attenuated in the adjusted model. There was no longer a statistically significant difference in average WBC between “moderate-large decrease” and “moderate-severe-slight decrease” (B = 0.42, 95% CI = −0.02, 0.86). Yet, mean WBC count remained significantly greater in the “severe-persistent” versus the “moderate-large decrease” (B = 0.64, 95% CI = 0.18, 1.09). Black compared to White race had an average 1.92 units lower WBC count (p < .0001). Other inflammatory conditions (p < .0001), CVD (p = .001), hypertension (p = .002), and current smoking (p < .0001) were all positively associated with a higher average WBC count.
Results of sensitivity analysis which expanded the number of coexistent inflammatory conditions to include influenza and injury produced similar results and did not change our conclusions.
Discussion
In this study, we report that patients with PTSD who experienced persistently severe PTSD symptoms and those with only a small improvement had a higher level of inflammation as measured by WBC count compared to patients who experienced large PTSD symptom decreases over time. This association remained even after adjustment of multiple comorbidities and inflammatory conditions. Our study also suggests a dose–response relationship with WBC count and PTSD severity. The highest counts were noted in those with persistent, severe PTSD followed by those with modest improvement and lowest in those who experienced largest PTSD symptom improvement. While we observed only modest differences in the magnitude of WBC count between PTSD severity trajectories and values would not warrant clinical intervention, our results demonstrate the feasibility of potentially using WBC count in large medical record databases as an indicator of PTSD severity.
Our finding of three distinct PTSD symptom trajectories (“large decrease,” “slight decrease,” and “stable high”) are similar to those reported by Sripada et al. in a study of PTSD severity in 2237 veterans, also using latent trajectory analysis.29 In our study, we note that besides higher WBC count, PTSD severity was also associated with Black race, depression, and hypertension. Similar to our findings, Sripada et al. also reported Black race and comorbid depression to be associated with PTSD severity. Black race34 and depression35 have been reported to be associated with severe, treatment-resistant PTSD in other studies as well. Unlike previous studies that focused primarily on sociodemographics and mental health comorbidities, we also included common clinical conditions such as type 2 diabetes, CVD, and hypertension in our analysis. We noted that hypertension, prevalent in 37.9% of our study sample, was also associated with PTSD severity. This interesting finding needs further investigation.
While inflammation is a potentially modifiable risk factor, there are lack of data regarding the role of inflammation in the severity of PTSD.36 WBC count is a promising biomarker of inflammation that is readily available in electronic health record databases. It also does not have issues with testing bias or the expense of conducting prospective studies with more specific biomarkers. To our knowledge, this is the first study to assess the association between three-year PTSD symptom trajectories and WBC counts using medical record data. The observations by Eswarappa et al.12 were similar to our findings where severe PTSD symptom trajectory was associated with greater level of inflammation. However, a limitation of this study was that only baseline mean WBC count was taken into consideration, while we chose the worst (i.e. maximum) WBC count per patient among all counts available after PTSD diagnosis. Second, this prior study did not adjust for an extensive array of conditions that can influence WBC count as can be done in medical record database study like ours.
To our knowledge, our study adjusted for the most comprehensive array of medical conditions associated with inflammation and elevated WBC count among all PTSD studies published to date. These conditions included those known to be associated with chronic low-grade inflammation such as depression,12 obesity,19 type 2 diabetes,20 CVD,21 hypertension,22 and smoking.23 In addition, we also adjusted for conditions directly influencing WBC count such as infections and hematological disorders that we grouped under “other inflammatory conditions.” In addition to the severity of PTSD symptoms, we also found “other” inflammatory conditions, CVD, hypertension, and current smoking to be associated with a higher WBC count. In addition, Black (compared to White) race was associated with a lower average WBC count which is consistent with previous reports.37
The mechanism behind the association of higher WBC count and severity of PTSD is not clear. Chronic stress and inflammatory cytokines are known to regulate the hematopoietic stem cell activation.38,39 Stress hormones can induce redistribution of immune cells.40 It is possible that stress and inflammation associated with severe PTSD increase the production or redistribution of WBCs leading to higher counts.
Limitations of our study include unknown generalizability towards non-VHA patients with PTSD. As with all retrospective observational cohort studies, we sacrifice fixed data collection times, only possible in a prospective study, for analysis that is relevant to the real-world patient population seeking care for PTSD. We did not control for prescription or over the counter anti-inflammatory medications given the likelihood of these being collinear with inflammatory conditions. We did not have data about specific PTSD symptoms and it is possible that some symptoms or symptom clusters could be more strongly associated with inflammation than others. Because we did not measure change in WBC, we are not able to draw conclusions about the direction of effect or causality. Lower inflammation may be associated with improvement in PTSD or patients with less inflammation may be at less risk for severe PTSD. Finally, undiagnosed comorbid conditions associated with inflammation could contribute to our results. For example, we do not have data on measures of work-related stress, fatigue, diet, or sleep deprivation.
In conclusion, we report that persistent, severe PTSD as compared to significantly improved PTSD is associated with a higher level of total WBC counts. WBC count has the potential to be used clinically as a marker of inflammation and to identify the persistently severe and treatment-resistant subgroups of PTSD patients. Our findings need to be validated in nonveteran cohorts of PTSD patients and using longer follow-up durations that will enable the study of associations of changes in WBC counts with PTSD symptoms trajectories.
Supplemental Material
Supplemental material, CSS877651 Supplemental Material for Association of Severity of Posttraumatic Stress Disorder With Inflammation: Using Total White Blood Cell Count as a Marker by Farrukh M. Koraishy, Joanne Salas, Thomas C. Neylan, Beth E. Cohen, Paula P. Schnurr, Sean Clouston and Jeffrey F. Scherrer in Chronic Stress
Acknowledgments
The views expressed in this report do not necessarily reflect those of the Veterans Administration. This material is the result of work supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans' Hospital.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Heart Lung and Blood Institute (PTSD Treatment: Effects on Health Behavior, Cardiovascular and Metabolic Disease, R01HL125424).
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Speer K, Upton D, Semple S, McKune A. Systemic low-grade inflammation in post-traumatic stress disorder: a systematic review. J inflamm Res. 2018; 11: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neigh GN, Ali FF. Co-morbidity of PTSD and immune system dysfunction: opportunities for treatment. Curr Opin Pharmacol. 2016; 29: 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gola H, Engler H, Sommershof A, et al. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013; 13: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bersani FS, Wolkowitz OM, Milush JM, et al. A population of atypical CD56(−)CD16(+) natural killer cells is expanded in PTSD and is associated with symptom severity. Brain Behav Immun. 2016; 56: 264–270. [DOI] [PubMed] [Google Scholar]
- 5.Lindqvist D, Dhabhar FS, Mellon SH, et al. Increased pro-inflammatory milieu in combat related PTSD—A new cohort replication study. Brain Behav Immun. 2017; 59: 260–264. [DOI] [PubMed] [Google Scholar]
- 6.Lindqvist D, Wolkowitz OM, Mellon S, et al. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun. 2014; 42: 81–88. [DOI] [PubMed] [Google Scholar]
- 7.Black PH. The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med Hypoth. 2006; 67(4): 879–891. [DOI] [PubMed] [Google Scholar]
- 8.Heath NM, Chesney SA, Gerhart JI, et al. Interpersonal violence, PTSD, and inflammation: potential psychogenic pathways to higher C-reactive protein levels. Cytokine. 2013; 63(2): 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009; 26(5): 447–455. [DOI] [PubMed] [Google Scholar]
- 10.Lindqvist D, Mellon SH, Dhabhar FS, et al. Increased circulating blood cell counts in combat-related PTSD: associations with inflammation and PTSD severity. Psychiatry Res. Dec 2017; 258: 330–336. [DOI] [PubMed] [Google Scholar]
- 11.Boscarino JA, Chang J. Higher abnormal leukocyte and lymphocyte counts 20 years after exposure to severe stress: research and clinical implications. Psychosom Med. 1999; 61(3): 378–386. [DOI] [PubMed] [Google Scholar]
- 12.Eswarappa M, Neylan TC, Whooley MA, Metzler TJ, Cohen BE. Inflammation as a predictor of disease course in posttraumatic stress disorder and depression: a prospective analysis from the Mind Your Heart Study. Brain Behav Immun. 2019; 75: 220–227. [DOI] [PubMed] [Google Scholar]
- 13.Passos IC, Vasconcelos-Moreno MP, Costa LG, et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015; 2(11): 1002–1012. [DOI] [PubMed] [Google Scholar]
- 14.Scherrer J, Salas J, Lustman P, et al. The role of obesity in the association between posttraumatic stress disorder and incident diabetes. JAMA Psychiatry. 2018; 75(11): 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gravely AA, Cutting A, Nugent S, Grill J, Carlson K, Spoont M. Validity of PTSD diagnoses in VA administrative data: comparison of VA administrative PTSD diagnoses to self-reported PTSD Checklist scores. J Rehabil Res Dev. 2011; 48(1): 21–30. [DOI] [PubMed] [Google Scholar]
- 16.Holowka DW, Marx BP, Gates MA, et al. PTSD diagnostic validity in Veterans Affairs electronic records of Iraq and Afghanistan veterans. J Consult Clin Psychol. 2014; 82(4): 569–579. [DOI] [PubMed] [Google Scholar]
- 17.McDonald S, Calhoun P. The diagnostic accuracy of the PTSD checklist: a critical review. Clin Psychol Rev. 2010; 30(8): 976–987. [DOI] [PubMed] [Google Scholar]
- 18.Scherrer JF Salas J, Norman SB, et al. Clinically meaningful PTSD improvement and risk for type 2 diabetes [published online ahead of print August 21, 2019]. JAMA Psychiatry. doi:10.1001/jamapsychiatry.2019.2096.
- 19.Yu S, Alper HE, Nguyen AM, Brackbill RM. Risk of stroke among survivors of the September 11, 2001, World Trade Center Disaster. J Occup Environ Med. 2018; 60(8): e371–e376. [DOI] [PubMed] [Google Scholar]
- 20.Mahdiani A, Kheirandish M, Bonakdaran S. Correlation between white blood cell count and insulin resistance in type 2 diabetes. Curr Diabetes Rev. 2019; 15(1): 62–66. [DOI] [PubMed] [Google Scholar]
- 21.Welsh C, Welsh P, Mark PB, et al. Association of total and differential leukocyte counts with cardiovascular disease and mortality in the UK biobank. Arterioscler Thromb Vasc Biol. Jun 2018; 38(6): 1415–1423. [DOI] [PubMed] [Google Scholar]
- 22.Xi L, Hao Y, Liu J, et al. [Relationship between leukocyte count and risk of hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi. 2015; 43(4): 312–318. [PubMed] [Google Scholar]
- 23.Luetragoon T, Rutqvist LE, Tangvarasittichai O, et al. Interaction among smoking status, single nucleotide polymorphisms and markers of systemic inflammation in healthy individuals. Immunology. 2018; 154(1): 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frayne SM, Miller DR, Sharkansky EJ, et al. Using administrative data to identify mental illness: what approach is best? Am J Med Qual. 2010; 25(1): 42–50. [DOI] [PubMed] [Google Scholar]
- 25.Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B. Latent class growth modelling: a tutorial. Tutor Quant Meth Psychol. 2009; 5(1): 11–24. [Google Scholar]
- 26.Jones B, Nagin D, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Meth Res. 2001; 29(3): 374–393. [Google Scholar]
- 27.Jones B, Nagin D. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Meth Res. 2007; 35(4): 542. [Google Scholar]
- 28.Nagin D, Jones B, Passos V, Tremblay R. Group-based multi-trajectory modeling. Stat Meth Med Res. 2018; 27(7): 2015–2023. [DOI] [PubMed] [Google Scholar]
- 29.Sripada R, Pfeiffer P, Rampton J, et al. Predictors of PTSD symptom change among outpatients in the U.S. Department of Veterans Affairs Health Care System. J Trauma Stress. 2017; 30(1): 45–53. [DOI] [PubMed] [Google Scholar]
- 30.Jung T, Wickrama K. An Introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass. 2008; 2(1): 302–317. [Google Scholar]
- 31.Nagin D. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol Meth. 1999; 4(2): 139–157. [Google Scholar]
- 32.Nagin D, Odgers C. Group-based trajectory modeling in clinical research. Ann Review Clin Psychol. 2010; 6: 109–138. [DOI] [PubMed] [Google Scholar]
- 33.Nagin D. Group-based modeling of development., Cambridge, MA: Harvard University Press, 2005. [Google Scholar]
- 34.Spoont MR, Nelson DB, Murdoch M, et al. Are there racial/ethnic disparities in VA PTSD treatment retention? Depress Anxiety. 2015; 32(6): 415–425. [DOI] [PubMed] [Google Scholar]
- 35.Murphy D, Smith KV. Treatment efficacy for veterans with posttraumatic stress disorder: latent class trajectories of treatment response and their predictors. J Trauma Stress. 2018; 31(5): 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaccarino V. An inflammatory phenotype for posttraumatic stress disorder and depression? Brain Behav Immun. 2019; 76: 5–6. [DOI] [PubMed] [Google Scholar]
- 37.Lim EM, Cembrowski G, Cembrowski M, Clarke G. Race-specific WBC and neutrophil count reference intervals. Int J Lab hematol. 2010; 32(6 Pt 2): 590–597. [DOI] [PubMed] [Google Scholar]
- 38.Zhao JL, Baltimore D. Regulation of stress-induced hematopoiesis. Curr Opin Hematol. 2015; 22(4): 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heidt T, Sager HB, Courties G, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014; 20(7): 754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells–from barracks to boulevards to battlefields: a tale of three hormones–Curt Richter Award winner. Psychoneuroendocrinology. 2012; 37(9): 1345–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, CSS877651 Supplemental Material for Association of Severity of Posttraumatic Stress Disorder With Inflammation: Using Total White Blood Cell Count as a Marker by Farrukh M. Koraishy, Joanne Salas, Thomas C. Neylan, Beth E. Cohen, Paula P. Schnurr, Sean Clouston and Jeffrey F. Scherrer in Chronic Stress