Abstract
This review approaches the topic of childbirth and mental illness using a model of perinatal health which takes into consideration the multiple determinants of health, approached from a lifespan perspective. The paper seeks to answer four broad questions using this model and available literature: (1) What is the relationship between childbirth and mental disorders? (2) How common are mental disorders during childbearing, and what is the perinatal course of illness? (3) What are the effects of mental illness during childbearing on foetal and infant developmental outcomes? (4) How do you approach the detection and treatment of mental disorders during the perinatal period?
Introduction and a framework
What is the relationship between childbirth and mental disorders?
Childbearing is a unique biopsychosocial occurrence that profoundly affects a woman physically, socially and emotionally. Like puberty or menopause, the reproductive life event of bearing a child involves significant somatic changes. Pregnancy dramatically transforms the physical landscape of a woman’s body, its size and shape, as well as the internal hormonal milieu. The woman’s transition to motherhood has implications for all of her relationships and for her societal role. Furthermore, pregnancy and the postpartum period involve significant psychological adjustments, and the childbearing process has been noted to be a ‘psychological stress test’ (Frank, Tuber, Slade, & Garrod, 1994).
How does childbearing affect the vulnerability to, development of, and expression of mental illness, and how does mental illness transform the experience of childbearing?
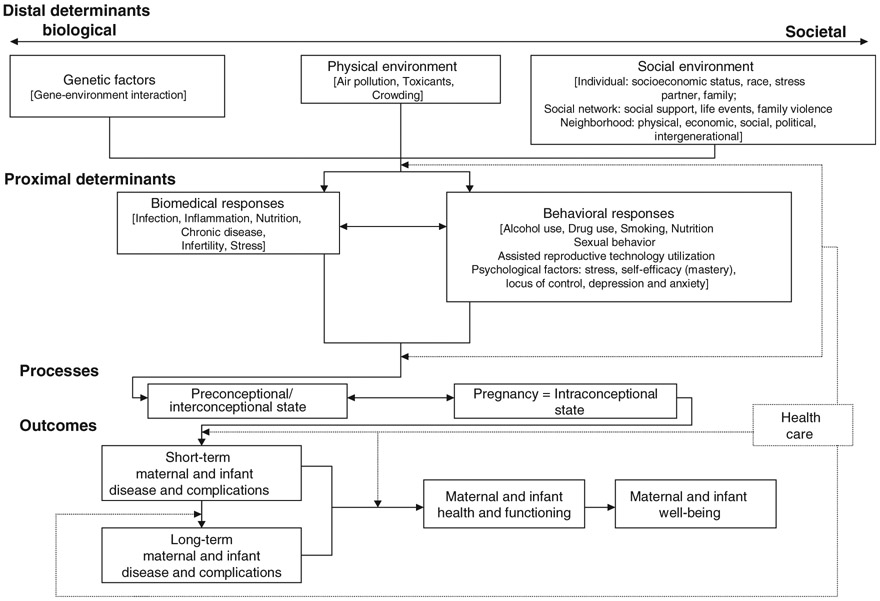
Misra, Guyer and Allston (2003) developed a model of perinatal health that encompasses the life span and incorporates multiple determinants of a woman’s health - biological, environmental, social, behavioural and psychological (Figure 1). In this life span approach, a woman is in a ‘preconception’ phase from childhood until she becomes pregnant or reaches menopause. Within the childbearing period, there are prenatal, intrapartum, postpartum, and subsequently interconception periods. Most perinatal health models attend to the prenatal-postpartum phase, whereas Misra and colleagues advocate consideration of a woman’s full life course in conceptualizing perinatal health. Distal (in time) determinants are those genetic, physical-environmental and social factors that influence a woman throughout the reproductive life cycle long before her pregnancy. For example, a woman who was sexually abused in childhood may bring different distal risks to adulthood than a woman from a family with psychologically healthy interpersonal boundaries. Distal factors increase or decrease an individual’s likelihood of developing health problems during childbearing. Proximal determinants, categorized as biomedical responses (e.g. nutrition, infection) and behavioural responses (e.g. substance use), have a direct impact on maternal perinatal health status. These proximal determinants, in turn, influence pregnancy outcomes, which can be understood as maternal and infant disease, complications, health, functioning and well-being. When the woman with a history of sexual abuse (distal determinant) becomes pregnant, does she participate in regular obstetrical care or does she avoid such care (proximal determinant) because genital exams reactivate traumatic memories? Has she avoided routine gynaecological care preconception, thus entering childbearing not knowing her HIV status or risk of cervical dysplasia? Or, has she responded to her distal trauma by proactively taking control of her health, engaging in healthy nutrition and activity, and utilizing social supports and health care access to maximize her own sense of well-being and the safety of her offspring? What determines the differential interaction between distal and proximal factors, and how do these interactions influence perinatal health outcomes?
Figure 1.
Integrated perinatal health and mental health framework: a multiple determinants model with a life span approach. Adapted from Misra et al., 2003. Included here (with adaptations) with permission from Dawn P. Misra, PhD, 4/27/2010. (Illustrative examples are in brackets.)
The lifespan model conceptualizes perinatal health comprehensively and interactively and does not distinguish between ‘physical’ and ‘mental’ health. However, for the mental health provider confronted with a childbearing woman, or for the obstetrical provider confronted with a patient with a psychiatric disorder, it is useful to apply the multiple determinants life course model to mental illness and childbearing, as it yields potential for new views. Each woman can be understood as coming to pregnancy with a set of risks and assets, mental and physical, which influence pregnancy outcome. History of mental illness can be thought of as a distal determinant, which can be either mitigated or exacerbated by other distal and proximal factors. The degree of expression of mental illness during the perinatal period can also be seen as a maternal outcome. A woman’s psychological composition, including her attachment style, her capacity for relatedness, her primary coping skills and defences, also represent distal determinants that influence her orientation to pregnancy. New-onset mental illness during childbearing may be regarded as both a proximal risk factor, as well as a maternal disease outcome with implications for foetal/infant outcome. Application of this model to psychiatric illnesses also holds potential for interventions at key impact points in the evolution of episodes. The role of the health care professional is to view the woman within this life context and improve her short and long-term health trajectories by supporting positive and reducing negative health behaviours. Such intervention requires an understanding of the role of mental illness both proximally and distally and the integration of physical and mental perinatal health into a comprehensive whole. In this paper, we will review the epidemiology and course of mental disorders in childbearing, the potential effects of mental illness on foetal and infant developmental outcomes, and finally, opportunities for detection and models for intervention.
How common are mental disorders during childbearing, and what is the perinatal course of illness?
Childbearing is a challenging biological and psychosocial event, and it confers an elevated risk for psychiatric episodes. The strongest predictor of mental illness during the perinatal period is a history of previous psychiatric illness, particularly affective illness (Coble et al., 1994; Kendell, Chalmers, & Platz, 1987). There are few epidemiologic studies of psychiatric episodes related to childbearing. In early studies examining the hospitalized population of women of childbearing age, Pugh, Jerath, Schmidt, and Reed (1960) found 21% of these women had illness related to childbirth, whereas Pfaffenbarger and McCabe (1966) found that 9.2% of psychiatric hospitalizations were perinatally related. Kendell, Wainwright, Hailey, and Shannon (1976), by linking birth and psychiatric registries, determined that rate of contact with psychiatric services peaked in the 3-month period after childbirth. In a similar record linkage study, they found rates of hospital admissions for psychiatric reasons rose dramatically in the 3 months postpartum, and 80% of the women admitted postpartum received diagnoses of affective disorders (Kendell, Rennie, Clarke, & Dean, 1981, 1987). In contrast, Cooper, Campbell, Day, Kennerley and Bond (1988), in one of the few large outpatient studies, found similar rates of affective and anxiety disorders in childbearing women compared to non-childbearing controls.
Wisner, Peindl and Hanusa (1993) utilized a psychiatric record database to divide all female patients who presented within a two-year period for evaluation to a university psychiatric hospital walk-in clinic into two groups based on whether onset of illness was during pregnancy or in the three months postpartum, or at other times in the lifecycle. Although the groups were similar in diagnostic categories, women with childbearing-related onset of illness more commonly had anxiety disorders and were more frequently given a diagnosis of adjustment disorder with depressed mood. Symptom onset began more frequently in the postpartum rather than during pregnancy. A follow-up to this study (Wisner, Peindl, & Hanusa, 1995), evaluating a subset of women in this group at 5 years from index episode, found that half of the women had psychiatric illness related to childbearing, and this group had almost exclusively affective illness (95%). Munk-Olsen et al. (2006), in a large register-based cohort study, identified the first three months postpartum as a time of particular vulnerability for all mental illness. They found that primiparous women, compared to women who had given birth 11–12 months prior, had an increased risk of incident hospital admission, as well as psychiatric outpatient contact, for the first 90 days after childbirth as compared to women who had given birth 11–12 months prior. The disease-specific risk was elevated for the first 5 months postpartum for major depression; 2 months for bipolar disorder; 1 month for schizophrenia; and 2 months for adjustment disorders. In a subsequent population-based cohort study evaluating rates of psychiatric readmission during the first 12 months postpartum, Munk-Olsen et al. (2009) identified the first month postpartum, and in particular days 10–19 postpartum, as a time of increased risk for psychiatric readmission. Following the first month, rates of psychiatric readmission were higher for non-mothers than mothers. Patients with a diagnosis of bipolar disorder were found to be at particularly high risk for readmission postpartum (Munk-Olsen et al., 2009). One recent large-scale epidemiological study noted that the prevalence of all non-psychotic postpartum psychiatric disorders at 6 weeks postpartum was 18.1%, and mood disorders comprised the majority (9.8%), followed by adjustment disorder (4.3%) and anxiety disorders (4%) (Navarro et al., 2008). At least 2% of women fulfilled DSM-IV criteria for more than one disorder, and comorbidity was associated with postpartum depression. Vesga-Lopez et al. (2008), in an epidemiological survey, concluded that there were not significant differences in the 12-month prevalence rates of all DSM-IV Axis I disorders among women pregnant in the past year (25.3%), postpartum (27.5%) and non-pregnant women of childbearing age (30.1%), with the exception of higher prevalence of major depression in postpartum women (9.3%) as compared to non-pregnant women (8.1%).
For all psychiatric illness, the postpartum period, and the first three months in particular, is a time of vulnerability to episodes of psychiatric illness. In addition, affective disorders are most prevalent in the perinatal period.
Affective disorders in pregnancy and the postpartum
Pregnant women remain at risk for depressive episodes (Bixo, 2001; Sundstrom, 2001). Gaynes and colleagues (2005) conducted a meta-analysis of different estimates of the prevalence and incidence of perinatal depression, using data from prospective and retrospective studies meeting rigorous inclusion criteria. They estimated point prevalence of 3.1–4.9% for major depression alone at different times during pregnancy, and 8.5–11.0% for major and minor depression. Other studies have estimated the prevalence of depression during pregnancy to range from 10–16% (Andersson et al., 2003; Heron, O’Connor, Evans, Golding, & Glover, 2004; Kitamura, Shima, & Sugawara, 1993). Bennett and colleagues (2004) performed a systematic evaluation of studies examining prevalence of depression during pregnancy and provided estimates by trimester: 7.4% in the first trimester, 12.8% second trimester, and 12% third trimester. Gaynes et al. (2005) estimated that as many as 14.5% of women have a new episode of major or minor depression during pregnancy, and 7.5% have a new episode of major depression, rates not significantly different for women of similar age who were not pregnant or in the immediate postpartum. Risk factors for antenatal depression include history of previous depressive episode or premenstrual dysphoric disorder, history of prior loss, particularly of a parent, poor social support and high levels of stress (Affonso et al., 1991; Barnet, Joffe, Duggan, Wilson, & Repke, 1996; Coble et al., 1994; Kitamura et al., 1993, 1994; Kumar, & Robson, 1984; O’Hara, 1986). Women with a history of prior major depressive disorder are at risk of relapse during pregnancy, particularly if antidepressant treatment is stopped (Cohen et al., 2006).
Estimates of prevalence of postpartum depression in the US, UK and Australia range from 7–20%, with most studies suggesting rates between 10–15% (Gavin et al., 2005; O’Hara, & Swain, 1996). Gaynes and colleagues (2005), in the aforementioned study, found prevalence rates of 6.5–12.9% at different times during the first year postpartum, and incidence of 14.5% in the first three months postpartum. Depression during pregnancy has been found to be the strongest predictor of postnatal depression (Llewellyn, Stowe, & Nemeroff, 1997; O’Hara, & Swain, 1996). Significant risk factors for postpartum depression also include anxiety during pregnancy, experiencing stressful life events during pregnancy or early puerperium, low levels of social support or partner support, low socioeconomic status, and obstetric complications (Milgrom et al., 2008; Robertson, Grace, Wallington, & Steward, 2004). Three meta-analyses have supported the idea that antenatal anxiety symptoms predict postpartum depression (Beck, 2001; O’Hara, & Swain, 1996, Robertson et al., 2004). Heron and colleagues found in a large longitudinal study that antenatal anxiety predicts postpartum depression at 8 weeks and 8 months, even after controlling for antenatal depression. In one recent study, depressive symptoms in middle pregnancy predicted higher anxiety in late pregnancy, and late pregnancy anxiety predicted postnatal depression (Skouteris, Wertheim, Rallis, Milgrom, & Paxton, 2009). Researchers concluded that there may be a bi-directional relationship between depression and anxiety in pregnancy and the postpartum.
The course of bipolar disorder in the perinatal period is less well-studied than that of unipolar depression in the perinatal period. Although some earlier studies suggested that the course of bipolar disorder might be unaffected or even impacted favourably by pregnancy (Grof et al., 2000), more recent studies support the idea that pregnancy is not protective against illness recurrence, with estimates of recurrence rates as high as 50% during pregnancy (Akdeniz et al., 2003; Freeman et al., 2002; Jones & Craddock, 2005). Viguera et al. (2000) retrospectively compared recurrence rates of illness following lithium discontinuation in childbearing versus non-childbearing women. The rates of recurrence in the first 40 weeks after lithium discontinuation were similar for pregnant (52%) and non-pregnant (58%) women, and for both groups recurrence rates were significantly lower in the year prior to lithium discontinuation (21%). In a naturalistic prospective study following women bipolar I and II women during pregnancy, Viguera, Whitfield, Baldessarini and Newport (2007) found that 70.8% of all patients experienced at least one mood episode during pregnancy, predominantly depression or a mixed episode. Risk of recurrence was significantly higher in women who discontinued their mood stabilizers (85.5%) than those who continued treatment (37%).
There appears to be a particularly elevated risk of recurrence for women with bipolar disorder in the immediate postpartum (Wisner, Hanusa, Peindl, & Perel, 2004; Yonkers et al., 2004). Kendell et al. (1987) found that the risk of first hospitalization for a bipolar episode was seven times higher among women postpartum as compared to non-pregnant/non-postpartum women, whereas Munk-Olsen et al. (2006) found a greater than 20 times higher risk of first hospitalization for bipolar women in the first month postpartum. Similarly, in their 2009 study using Danish registries to compare readmission rates between mothers and non-mothers, Munk-Olsen et al. (2009) identified the first month postpartum, and days 10–19 in particular, as the greatest period of vulnerability for psychiatric readmission. Further, they determined that a previous diagnosis of bipolar disorder was the greatest predictor of psychiatric readmission from days 10–19 postpartum (relative risk 37.22). The cumulative incidence of readmission for women with bipolar illness was 22% from 0–3 months postpartum. Furthermore, 26.9% of women with a diagnosis of bipolar disorder had psychiatric readmission in the first year postpartum, compared to 15.7% for women with schizophrenia-like disorders (Munk-Olsen et al., 2009). In the Viguera et al. study (2000), even women who maintained mood stability in the first 40 weeks after lithium discontinuation had rates of postpartum recurrence (70%) approximately three times greater than non-pregnant women in the same period of time (24%). Out of 9 women who remained on lithium in this study, all 9 maintained euthymic mood during pregnancy, but 3 had rapid recurrence of illness in the postpartum on continued lithium treatment.
Postpartum psychosis presents in approximately 1–2 per 1000 live births within two weeks of delivery in the general population, and the rate is significantly higher in patients with a history of bipolar disorder or prior postpartum psychosis (Kendell et al., 1987; Stewart, Raskin, Garfinkel, MacDonald, & Robinson, 1991). Jones and Craddock (2001), in a family study of bipolar patients, found that postpartum psychosis followed 26% of deliveries women with bipolar disorder or schizoaffective disorder, bipolar type, and followed 57% of deliveries of such patients with a family history of postpartum psychosis. Because postpartum psychosis is typically a manifestation of bipolar disorder (Kendell et al., 1987; Terp & Mortensen, 1998; Whalley, Roberts, Wentzel, & Wright, 1982; Wisner, Peindl, & Hanusa, 1994), it is generally treated as affective illness unless history suggests otherwise (Wisner et al., 1995). Kendell et al. (1987), found that among patients who developed postpartum psychosis after childbirth, 72–80% had bipolar disorder or schizoaffective disorder, and 12% had schizophrenia. Postpartum psychosis usually develops within the first two weeks postpartum, but risk remains relatively high for the first 3 months postpartum (Weissman & Olofson, 1995). Psychosis in the postpartum differs in phenomenology from psychosis at other times by the prominence of cognitive symptoms (disorganization, confusion, impaired sensorium/orientation, distractability) (Wisner, Peindl, & Hanusa, 1994). Postpartum psychosis is considered a psychiatric emergency, and all women must be screened for thoughts of harming the newborn as well as themselves (Chandra, Bhargavaraman, Raghunandan, & Shaligram, 2006; Spinelli, 2009; Viguera, Cohen, Baldessarini, & Nonacs, 2002).
Rates of affective illness are high in pregnancy and postpartum, and affective illness in general is associated with increased risk of suicide. How does childbearing affect risk of suicide? Several UK studies by Appleby and colleagues have evaluated the risk of suicide in the perinatal population (Appleby, 1991, 1996; Appleby & Turnbull, 1995; Appleby, Mortensen, & Faraghar, 1998). Using population data from England and Wales from the years 1973–1984, they calculated the expected number of postnatal suicides based on the rates of suicide in women per age group multiplied by number of births. The number of actual suicides were approximately six times less than expected (standardized mortality ratio of 0.17). For pregnancy, the number of actual suicides were one-twentieth the expected rate (standardized mortality ratio of 0.05) (Appleby, 1996). They concluded that, despite significant psychiatric morbidity in pregnancy and the postpartum, there is a lower than expected rate of fatal and non-fatal self-harm (Appleby, 1996), and suggest that pregnancy and recent motherhood are protective factors against suicidal behaviour. The preponderance of the suicides, they estimated, were conducted by women with postpartum psychosis. However, in a later study using 21 years of data from the Danish registries, they determined that for women with severe postpartum psychiatric illness, defined as admission to a psychiatric hospital within the first year after childbirth, risk of suicide is increased 70-fold within the first year and 17-fold in the long-term (Appleby et al., 1998). In their review of suicide in women, Chaudron and Caine (2004) found that pregnancy itself and having young children in the home had a protective effect against maternal suicide; however, an increased risk was noted among parents who experienced the death of a child or whose child had a psychiatric illness.
Anxiety disorders in pregnancy and the postpartum
While the reproductive time periods of pregnancy and the postpartum tend to heighten risk for development or recurrence of mood disorders, the interaction between the perinatal period and anxiety disorders remains poorly studied. It stands to reason that because pregnancy, parturition and lactation all affect neurohormonal systems that modulate anxiety, these reproductive events would have an effect on anxiety disorders; however, the impact remains to be elucidated. There are no published reports directly comparing ratings of anxiety disorders or symptoms in perinatal versus non-perinatal women. In a study of over 8000 women, Heron et al. (2004) found that anxiety symptoms were relatively common in pregnancy and that more women scored above threshold on an anxiety scale during weeks 18 and 32 of pregnancy than 8 weeks and 8 months postpartum. Regarding specific anxiety disorders, there are case series that suggest panic and anxiety symptoms are reduced in pregnancy but worsen in the postpartum (Cowley & Roy-Byrne, 1989; George & Ladenheim, 1987; Klein, Skrobola, & Garfinkel, 1995; Sholomskas et al., 1993; Cohen et al., 1996; Villeponteaux et al., 1992). In contrast, there is some evidence indicating that obsessive compulsive symptoms may worsen during pregnancy (Altemus et al., 2001; Labad et al., 2005; Williams & Koran, 1997). There is no data about the effects of pregnancy on the course of generalized anxiety disorder, specific phobias, or post-traumatic stress disorder.
Postpartum anxiety disorders were found to be as common as postpartum major depression in two studies administering interviews to establish DSM-IV diagnoses in perinatal women (Mathey et al., 2003; Wenzel, Haugen, Jackson, & Robinson, 2003).The postpartum seems to be a high-risk time for the initial presentation of anxiety disorders such as panic disorder (Sholomskas et al., 1993; Wisner et al., 1999) and obsessive compulsive disorder (Sichel, Cohen, Rosenbaum, & Driscoll, 1993; Williams & Koran, 1997). It has been suggested that up to 30% of childbearing-age women with OCD have onset in the postpartum (Labad et al., 2005). Obsessional thoughts about accidentally or intentionally harming the infant, which are ego dystonic, occur in postpartum OCD (Labad et al., 2005) but also in women with postpartum depression (Jennings et al., 1999; Wisner et al., 1999).
Lactation may be associated with the suppression of autonomic arousal, responsivity to stress, and thus anxiety. Cross-sectional studies (Abou-Saleh, Ghubash, Karim, Krymski, & Bhai, 1998; Astbury, Brown, Lumley, & Small, 1994; Hannah, Adams, Lee, Glover, & Sandler, 1992; Lane et al., 1997; Mezzacappa, Guethlein, Vaz, & Bagiella, 2000; Virden, 1988) and several studies involving non-clinical samples, observed that women experienced a reduction in anxiety, as well as depressive symptoms, immediately after a breastfeeding episode, but not after bottle-feeding (Heck & de Castro, 1993; Mezzacappa & Katlin, 2002) or after holding their infant (Heinrichs et al., 2001). Further, several studies point to a relationship between weaning and increased panic symptoms (Cowley & Roy-Byrne, 1989; Klein, Skrobola, & Garfinkel, 1994; Northcott & Stein, 1994; Villeponteaux et al., 1992).
Some women experience childbirth itself as a trauma, and the estimated prevalence of childbirth-associated post-traumatic stress disorder ranges from 1.5–6% (Beck, 2004). Women with history of sexual abuse or assault may be more vulnerable to activation of anxiety symptoms by obstetric procedures and childbearing itself (Cromptom, 1996; van der Kolk et al., 1996). It is estimated that up to 10–20% of pregnant women have intense fear of childbirth (Areskog, Uddenberg, & Kiessler, 1981; Melender, 2002; Sjogren, 1997), and some even request surgical delivery in order to avoid the process of labour (Atiba, Adeghe, Murphy, Felmingham, & Scott, 1993; Nielsen, Olausson, Ingemarsson, 1994; Ryding, 1991). Risk factors for this fear, which may be considered a specific phobia, include not only prior complicated deliveries, but also poor social support, unemployment, relationship difficulties, as well as previous emotional difficulties (Melender, 2002; Ryding, Wijma, Wijma, & Rydhstrom, 1998; Sjostrom, Valentin, Thelin, & Marsål, 1997; Zar, Wijma, & Wijma, 2002).
Psychotic disorders in pregnancy and the postpartum
Few studies have evaluated the relationship between the perinatal period and schizophrenia. In a study comparing pregnant women with psychotic illnesses to pregnant controls, McNeil and Malmquist-Larsson (1984b) found that of mentally ill women, those with schizophrenia were most likely to report mental health deterioration; 59% reported worsening mental health during pregnancy, and only 29% reported improvement. On the other hand, some researchers have described no increased risk for exacerbations of acute psychosis during pregnancy (Trixler, Gati, & Tenyi, 1995) or the postpartum, except in the presence of comorbid mood disorder (Davies, McIvor, & Kumar, 1995). A more recent study found that 27% of women with past psychosis have a recurrence of psychosis and 38% develop a non-psychotic depression in the first postpartum year (Howard, Leese, Goss, Appleby, & Thornicroft, 2004). The period of greatest vulnerability for psychiatric recurrence occurred in the first three months after delivery. Munk-Olsen et al. (2006), noted the first month postpartum to be the highest risk for psychiatric admission for women with schizophrenia-like disorders.
Women with psychotic disorders tend to receive less prenatal care, have poorer nutrition, and use more tobacco, alcohol, and illicit drugs during pregnancy than women without such illnesses. In a chart review of women with psychotic disorders hospitalized during pregnancy, Rudolph and colleagues (1990) noted high rates of substance use during pregnancy. Similarly, in a study of women with schizophrenia, 78.1% of the sample reported substance abuse during pregnancies (Miller & Finnerty, 1996). Compared to demographically equivalent women without mental illness, women with schizophrenia are less likely to receive prenatal care (Miller & Finnerty, 1996; Sacker, Done, & Crow, 1996). For women who do receive prenatal care, there seems to be an underreporting of psychiatric symptoms, which may be related to fear of child custody loss (Krener, Simmons, Hansen, & Treat, 1989).
Psychosis during pregnancy is associated with increased risk of certain adverse neonatal outcomes, including stillbirth, prematurity, and small size for gestational age. (Nilsson et al., 2002). Mothers with schizophrenia also have a higher rate of obstetric complications (Sacker et al., 1996) and are at greater risk for interventions during labour and delivery (Seeman, 2004). A record linkage study of Australian women with schizophrenia from 1980–1992 showed increased rates of placental abruption, offspring with cardiovascular congenital anomalies, and neonatal complications compared to the women without a psychiatric diagnosis (Jablensky, Morgan, Zubrick, Bower, & Yellachich, 2005). Women with schizophrenia have twice the risk for foetal death or a newborn with congenital anomalies compared to women in the general population (Altshuler et al., 1996). This association between schizophrenia and higher rates of obstetric and neonatal complications remains to be clarified. Concurrent maternal smoking, substance use, as well as socioeconomic problems increase the risk for less optimal outcomes (Bennedsen, 1998; Nilsson et al., 2002; Walker & Emory, 1983). Additionally, psychosis itself may contribute to a misinterpretation of somatic changes of pregnancy and delayed recognition of pregnancy, as well as lack of recognition of labour and even attempts at premature self-delivery (Miller, 1993; Muqtadir, Hamann, & Molnar, 1986; Spielvogel & Wile, 1986; Stewart, 1984).
Women with psychotic denial of pregnancy are at especially high risk for poor obstetric and neonatal outcomes. In this subgroup of women, intermittent delusional denial can result in lack of prenatal care, inability to recognize labour and thus precipitous delivery, and rarely, with neonaticide (Miller, 1990). Miller noted that pregnancy denial is more likely to occur in women who have already lost custody of an infant or anticipate losing it.
Eating disorders in pregnancy and the postpartum
During pregnancy and lactation, a woman’s nutritional requirements change, and the increased demand for a healthy diet to support the development of the growing foetus or child significantly affects women with eating disorders. Some women report an improvement in their eating disorder symptomatology during pregnancy, which may lead to permanent improvements (Bulik et al., 2007; Lacey & Smith, 1987; Lemberg & Phillips, 1989; Morgan, 1999; Namir, Melman, & Yager, 1986). However, pregnancy can also be the stimulus that activates body image preoccupations and unfavourable eating habits and moves a woman from subthreshold eating disorder symptoms to a frank eating disorder (Mitchell-Gieleghem, Mittelstaedt, & Bulik, 2002). There are few systematic data on the course of eating disorders in the perinatal period. In a recent pregnancy cohort study utilizing the Norwegian Medical Birth Registry, Bulik et al. (2008) investigated the prevalence and course of eating disorders in a sample of 41,157 pregnant women. Their pre-pregnancy prevalence estimates of eating disorders were as follows: 0.1% for anorexia nervosa (AN), 0.7% for bulimia nervosa (BN), 3.5% for binge eating disorder (BED), and 0.1% for an eating disorder not otherwise specified (EDNOS-P). Bulik found that there was partial remission of both BED and EDNOS-P during pregnancy (prevalence 0.2% BN, <0.1% EDNOS-P), but worsening of preexisting and new development of BED during pregnancy (prevalence 4.8%). In was not possible to assess the prevalence of AN due to weight changes of pregnancy. Crow, Keel, Thuras and Mitchell (2004) examined the course specifically for bulimia nervosa (BN) and substance abuse during pregnancy. Body dissatisfaction was rated as better during pregnancy by 21.4%, worse by 43.8%, and unchanged by 34.8%. Symptoms of binge eating and purging improved during pregnancy; however, the number of women completely abstinent from bulimic symptoms did not change significantly with pregnancy.
In general, study data to date suggest that a history of an eating disorder or a current eating disorder may put a woman and her foetus at risk for problems during pregnancy, and that both anorexia nervosa and bulimia nervosa may negatively affect foetal outcome (Franko et al., 2001). Population-based case-control data suggests that maternal first-trimester dieting behaviours, including eating disorders, as well as fasting diets, have been associated with increased neural tube defect (NTD) risk among offspring (Carmichael, Shaw, Schaffer, Laurent, & Selvin, 2003), possibly because of effects on intake, absorption, and metabolism of micronutrients, such as folic acid. Stewart (1984) found that infants of women with active anorexia or bulimia during pregnancy had lower infant birth weights and lower Apgar scores compared to women in remission. Likewise, in a follow-up study with women who were hospitalized with an eating disorder before pregnancy compared to control subjects and their infants, Sollid and colleagues (2004) found that the risk of a low-birthweight infant was twice as high in women with a history of eating disorder compared to women without a history. The risk for preterm delivery and a small-for-gestational-age infant was also increased. In contrast, a prospective study of women with active eating disorders found that the majority had uncomplicated pregnancies and healthy infants, however were at greater risk for caesarean section and postpartum depression (Franko et al., 2001). In a longitudinal cohort study, Micali, Siminoff and Treasure (2007) noted that women with a history of bulimia nervosa had an increased rate of lifetime miscarriages, and women with a history of anorexia nervosa were more likely to deliver babies of lower birth weight than control women.
Other investigators have also found that patients with eating disorders have a higher likelihood of surgical deliveries (Mitchell-Gieleghem et al., 2002). Interestingly, evidence from a Norwegian pregnancy cohort study (Bulik et al., 2008) suggests that maternal eating disorders may have an influence on sex of offspring. Women with anorexia and bulimia were found to have a lower proportion of male live births, whereas those with binge eating disorder and EDNOS had a higher proportion of male births.
In a population-based study examining associations between eating disorders and perinatal depression, Mazzeo et al. (2006) found that both bulimia nervosa and binge eating disorder were associated with development of postpartum depression.
Substance abuse in pregnancy and postpartum
Despite ongoing public health efforts to educate about the risks inherent to substance use in pregnancy, rates among pregnant women remain relatively high. In a large epidemiological study, Vesga-Lopez et al. (2008) noted that the prevalence of substance use disorders was lower in past-year pregnant and postpartum women than in non-pregnant women of childbearing age. However, data from the most recent National Pregnancy and Health Survey (National Institute on Drug Abuse, 1996) revealed that approximately 15% to 20% of women acknowledged alcohol consumption during pregnancy, 10% of women used cocaine and marijuana during pregnancy, and 0.1% used heroin during pregnancy. Combined 2002 to 2007 data from the National Survey on Drug Use and Health found that past-month alcohol use during pregnancy was as follows: 19% in the first trimester of pregnancy, 7.8% in the second trimester, and 6.2% in the third trimester, with similar rates for cigarette and marijuana use. In the Canadian Community Health Survey of 2000/2001, 13.7% of Canadian women of childbearing years reported that they had used alcohol during their last pregnancy, and approximately 10% of women who were pregnant at the time of the survey reported consuming more than 5 drinks on one occasion. Approximately 7% of pregnant women said they regularly drank heavily (more than 12 drinks per week) in the past year. Utilizing data from the National Household Survey on Drug Abuse from 1996 to 1998, Ebrahim and Gfroerer (2003), found that 6.4% of non-pregnant women of childbearing age and 2.8% of pregnant women reported that they used illicit drugs. They estimated that of women who used drugs, those who stopped during pregnancy increased from 28% during the first trimester of pregnancy to 93% by the third trimester. Three-fourths of the illicit drug use was marijuana, and one-tenth was cocaine, and over half of the pregnant women who used illicit drugs also used alcohol and cigarettes. There was a high rate of relapse postpartum. Similarly, 2002–2007 data from the National Survey on Drug Use and Health suggests resumption of substance use in the first three months postpartum. Compared to women in their third trimester, women in the first three months postpartum had significantly higher rates of past-month use of alcohol (6.2% versus 31.9%), binge alcohol use (1% versus 10%), cigarette use (13.9% versus 20.4%), and marijuana use (1.4% versus 3.8%). Navarro et al. (2008), evaluating the prevalence of postpartum psychiatric disorders in a community sample of Spanish women, noted approximately 0.9% met criteria for substance abuse or dependence.
The majority of research regarding prenatal exposure to substances has focused on the consequences to the offspring, and there has been comparatively little attention given to factors associated with substance use and abuse during pregnancy. It has been found that rates of substance use during pregnancy vary little among socioeconomic groups, although women from higher strata use predominantly alcohol and marijuana compared to cocaine and other illicit substance use in women from lower socioeconomic groups (Chasnoff, Landress, & Barnett, 1990; Hans, 1999). Other studies have examined the relationship between substance use and desire for pregnancy and intentionality of pregnancy. In a sample of approximately 300 women from Chicago area hospitals, Altfed, Handler, Burton and Berman (1997) found that women who desired pregnancy were less likely to smoke and drink during pregnancy than those who did not desire to be pregnant. Similarly, using a sample of over 18,000 women from the National Survey of Family Growth (Kost, Landry, & Darroch, 1998) found that women with intended pregnancy, as well as women with unplanned but desired pregnancies, were more likely to reduce or stop alcohol consumption in pregnancy as compared to women with unintended pregnancies. In contrast, Poole, Klerman, Flowers, Goldenberg and Cliver (1997) reported that in a sample of 1223 low-income, high-risk pregnant women, smoking and use of illegal drugs during pregnancy was not significantly different between intended versus unintended pregnancies; women with unintended pregnancies had higher rates of alcohol use in the first trimester, as well as later onset of prenatal care. Coleman, Reardon, Rue and Cougle (2002), in a study using a nationally representative sample, found that women with prior history of abortion as compared to women with history of live birth, had greater likelihood of using alcohol, marijuana, and other illicit drugs during pregnancy.
What are the effects of mental illness during childbearing on foetal and infant developmental outcomes?
Untreated depression during pregnancy may result in poor maternal self-care and nutrition, disturbed sleep, lack of prenatal care, increased exposure to alcohol and drugs, and a higher risk of suicide by the mother (Weissman & Olfson, 1995). In addition, depression in the third trimester is related to an increase in negative pregnancy outcomes, including an increased risk of low birth-weight newborns, preterm delivery, and small-for-gestational-age newborns (Steer et al., 1992). Several studies in humans have also correlated childhood affective and anxiety disorders with exposure to antenatal depression (Allen, Lewinsohn, & Seeley, 1998: Luoma, 2001; O’Connor, Heron, & Glover, 2002; Van den Bergh, 2005). Notably, a recent study showed that children who were exposed to maternal depression during pregnancy were almost four times as likely as those not exposed to become depressed at 16 years (Pawlby, 2009). These studies must be interpreted with an acknowledgement of the fact that as the mother creates not only an intrauterine and postpartum environment, which can contribute psychopathology in offspring, but typically plays a crucial role in shaping the emotional, social and cognitive development of a child and thereby can profoundly affect the child’s mental health. Certainly, there exists a complex interaction between genetics, the intrauterine environment, the early postpartum environment, and the effects of nurture, in the development of mental illness.
Psychopathology during pregnancy has physiological consequence for the foetus. However, the mechanism through which maternal depression affects foetal brain development and function is unknown. In the absence of direct neural connections between the mother and foetus, it is thought to be mediated by hormones; specifically, stress hormones (Cosmi, Luzi, Gori, & Chiodi, 1990; O’Donnell, O’Connor, & Glover, 2009). Depression causes abnormal stress hormone responses via dysregulation of the hypothalamic-pituitary-adrenocortical (HPA) system and leads to increased cortisol levels (Carroll, Curtis, & Mendels, 1976a, 1976b, 1976c; Dinan, 1994). In adult animal studies, long-term exposure to exogenous corticosterone induces anxiety and depression-like changes in behaviour (Emack, 2008; Gourley, 2008; Johnson, Fournier, & Kalynchuk, 2006; Murray, Smith, & Hutson, 2008) and enhanced fear (Corodimas, 1994). In addition to the effects on behaviour, a variety of changes in neurochemistry and brain morphology have been found in response to exposure to glucocorticoids. In animal studies, exposure to corticosterone has been shown to decrease hippocampal neurogenesis (Fuchs & Gould, 2000; Pham, 2003) and volume (Cerquiera, 2005), and to have effects on arrangement or connectivity in the prefrontal cortex (Seib & Wellman, 2003), the hippocampus (Magarinos, Orchinik, & McEwen, 1998) the amygdala, and the nucleus accumbens (Morales-Medina, Sanchez, Flores, Dumont, & Quirion, 2009).
Prenatal stress exposure has been shown in animals and humans to have deleterious effects on development (Carmichael & Shaw, 2000; Gould, 1997; Peacock, Bland, & Anderson, 1995). Maternal stress and glucocorticoid exposure have been shown to increase maternal glucocorticoid secretion (Cadet et al., 1986; Dean, & Matthews, 1999), a proportion of which passes through the placenta and reaches the foetus. In humans, there is a strong correlation between maternal and foetal plasma cortisol levels (Gitau, 1998, 2001). While glucocorticoids are important for normal development of many brain regions (Korte, 2001; Meaney, 1996; Meyer, 1983), excessive prenatal glucocorticoid exposure has been shown to retard brain weight at birth in sheep (Huang, 1999), delay maturation of neurons (Huang, 2001) and alter synapse formation (Antonow-Schloreke, 2001), some of which appears to be permanent (Matthews, 2000). For example, in rhesus monkeys, treatment with antenatal dexamethasone causes a dose-dependent neuronal degeneration of hippocampal neurons and reduced hippocampal volume which persists until at least 20 months of age (Uno, 1990). Finally, in humans, microarray analysis has shown that the exposure to increased cortisol prenatally has widespread effects on gene expression in foetal brain cells (Salaria, 2006).
While there is increasing evidence that points to an association between prenatal stress and neurodevelopmental outcomes, there is conflicting data regarding the gestational age most sensitive to such stress (Sarkar, 2008; Talge, Neal, & Glover, 2007). During pregnancy, depression has been linked to higher basal cortisol levels in both the second and third trimesters (Field, 2004). Maternal plasma and amniotic fluid cortisol have been shown to be strongly positively correlated after 18 weeks gestation in highly anxious women (Glover, 2009). In addition, elevated levels of ACTH and cortisol have been found in women who had higher ratings of self-reported stress at 28 weeks (Wadhwa, Sandman, & Garite, 2001). In contrast, in one study, a modest association between maternal anxiety and plasma cortisol was found, but was no longer detectable after 17 weeks gestation (Sarkar, 2008). In addition, exposure to emotional stress during the first trimester, but not later in gestation, has been shown to increase the risk of a variety of birth defects in humans (Carmichael & Shaw, 2000) and to induce motor impairments and low birth weights in non-human primates (Schneider, 1999). One study in mice showed that male offspring exposed to stress in early gestation displayed maladaptive behavioural stress responsivity and anhedonia as adults (Mueller & Bale, 2008).
Further complicating the question of the timing of stress exposure during gestation is the finding that the maternal HPA axis becomes hypo-responsive to stress as gestation increases in both rodents and humans, presumably to protect the brain from aversive consequences of increased glucocorticoids (Gunnar & Donzella, 2002; Levine, 2001). In mice, the stress hypo-responsive period lasts from about postnatal day 1 to postnatal day 12 and is characterized by low-basal corticosterone levels and a relative inability of mild stressors to induce a corticosterone response (Antonow-Schlorke, Schwab, Li, & Nathanielsz, 2003). It is notable, however, that some studies have shown that the greatest effect of prenatal stress for child development is in late pregnancy (Delarue et al., 2003; O’Connor et al., 2002, 2003; Schmidt, 2003). Therefore, it is possible that while HPA axis hyporesponsiveness buffers the foetus against mild stress insults, this hyporesponsiveness is overcome by moderate to severe stress leading to neurological injury during a vulnerable period of brain development.
How do you approach the detection and treatment of mental disorders during the perinatal period?
Misra’s model of perinatal health encourages a frame-shift in the approach to wellness during childbearing towards care during the preconception and interconception periods. While it may be argued that this approach risks defining a woman’s care by her childbearing potential alone, decisions around childbearing are faced by every woman at some point during her life cycle, even those who are unable or decide not to have children. Screening and interventions around distal determinants of perinatal health, such as nutrition, infection, and domestic violence, as well as mental health, would ideally be implemented in the preconception or interconception times. Perinatal health care in the current US system is a time of greater access, higher utilization, and relatively less fragmentation, and thus presents a unique opportunity to identify significant pathology as well as protective factors. Despite this higher frequency of contact, evidence suggests that pregnant and postpartum women with psychiatric disorders in the US are less likely than non-pregnant women to receive mental health treatment (Vesga-Lopez et al., 2008). Particularly among indigent populations, access to and utilization of health care resources are often fragmented during much of the life cycle. Gonzalez et al. (2010) found that rates of depression care relative to need were low in the general population of Americans with recent major depression, and that despite equivalent estimates of need across race and ethnicity, there are disparities in utilization of psychotherapy and medication treatments for depression, particularly among African American, Mexican American and Caribbean black individuals (Gonzalez et al., 2010). As previously discussed, in this critical time for both mother and infant, proximal factors in maternal health such as untreated mental illness and substance use become distal determinants for the children.
Screening
While many women have mental disorders that are known prior to childbearing, more still will have newly identified or episodic illness during the puerperium (Munk-Olsen et al., 2006). To try to mitigate the serious adverse outcomes associated with mental illness in mothers, there has been increasing focus on the importance of early and accurate detection and treatment of depression after or during pregnancy (Wisner, 2008). Identification of psychiatric illness during pregnancy and in the postpartum period may be complicated by some of the normal physical and emotional demands of new motherhood, including changes in energy and appetite, sleep deprivation, and heightened concern for the infant. Current recommendations for screening for postpartum depression are at the first postnatal obstetrical visit (usually 4–6 weeks after delivery), (Sit & Wisner,2009) or in the family practice (Gjerdingen & Yawn, 2007) or pediatric setting (Chaudron, Szilagyi, Campbell, Mounts, & McInerny, 2007), as these are the most widespread points of interaction with the health care system for new mothers within the first three months of delivery. Increasingly, screening has also been introduced during pregnancy (Kim et al., 2008; Stowe, Hostetter, & Newport, 2005). In the UK, the NICE guidelines on antenatal and postnatal health, which affect NHS care in England and Wales, recommend screening for depression at a woman’s first contact with primary care both antenatally, as well as postnatally (usually 4–6 weeks and 3–4 months) (NICE, 2007).
Although efforts have been made to develop assessment tools that address the potential effect that female reproductive milestones may have on diagnosis, treatment and prevention of mental disorders in women throughout the life cycle (Martini, Wittchen, Soares, Rieder, & Steiner, 2009), widespread identification of psychiatric issues in childbearing women must depend on implementation of screening measures. The most commonly used screening tool for antenatal or postpartum depression is the Edinburgh Postnatal Depression Scale (EPDS) (Cox, Holden, & Sagovsky, 1987), a 10-item self-report that emphasizes emotional and functional factors rather than somatic symptoms. Although variability in sensitivity and specificity occurs across languages and cultures (Gibson, McKenzie-McHarg, Shakespeare, Price, & Gray, 2009; Halbreich & Karkun, 2006), the recommended score to screen for probable major depression in English-speaking populations with the EPDS is 13 or more (out of a possible 30) postnatally, or 15 or more antenatally (Matthey, Henshaw, Elliott, & Barnett, 2006). Special note should be made of any positive responses to Item 10 assessing suicidal ideation. Other commonly used screening tools with some evidence of validity in the puerperium include the Postpartum Depression Screening Scale (PDSS) (Beck, 2001) as well as the 9-item Physician’s Health Questionnaire (PHQ-9), which has been validated and is widely used in primary care settings, (Gilbody, Richards, Brealey, & Hewitt, 2007; Kroenke, Spitzer, & Williams, 2001) though only preliminarily in the obstetric population (Hanusa, Scholle, Haskett, Spadaro, & Wisner, 2008; Yawn et al., 2009). The UK NICE guidelines (2007) suggest use of the following three ‘Whooley’ questions (Whooley, Avins, Miranda, & Browner, 1997) to identify women with possible depression:
During the past month, have you often been bothered by feeling down, depressed or hopeless?
During the past month, have you often been bothered by having little interest or pleasure in doing things?
A third question should be considered if the woman answers ‘yes’:
Is this something you feel you need or want help with?
These questions have not been validated for an antenatal or postnatal population (Bick & Howard, 2010), and a recent meta-analysis raised questions about their effectiveness in detecting illness (Mitchell & Coyne, 2007). It should be emphasized that the diagnosis of depression must be confirmed by clinical interview.
Screening for other psychiatric disorders in perinatal women has only rarely been studied, despite the estimated prevalence and comorbidity in this population with anxiety disorders (Ross & McLean, 2006), substance abuse (Ross & Dennis, 2009; Vesga-Lopez et al., 2008) and the high incidence of morbidity in perinatal women with bipolar disorder (Sharma, Burt, & Ritchie, 2009). Chessick and Dimidjian (2010) recently reviewed screening measures for bipolar disorder as they pertain to pregnant and postpartum women. Of the available screening tools for bipolar disorder, the highs (Glover, Liddle, Taylor, Adams, & Sandler, 1994) is the only instrument to be investigated systematically among perinatal women, whereas the Mood Disorders Questionnaire (MDQ) (Hirschfeld et al., 2000) has the most data in the general primary care setting and has been translated to multiple languages. Given low sensitivity in community samples, the authors recommend screening women who have been identified as having depressive symptoms by clinical exam or by depression screening measure, and for whom an antidepressant medication is being considered, or who have other known risk factors for bipolar disorder. Despite the prevalence of comorbid depression and anxiety, screening for anxiety disorders in the perinatal period has not received the same attention as screening for depression (Matthey et al., 2003). Measures used in studies that have looked at anxiety in pregnancy and the puerperium have included the State-Trait Anxiety Inventory Trait subscale (STAI-T) (Spielberger, Gorsuch, & Lushene, 1970; Moss, Skouteris, Wertheim, Paxton, & Milgrom, 2009), the Hamilton Rating Scale for Anxiety (Hamilton, 1969; Misri, Reebye, Corral, & Milis, 2004) the Crown–Crisp experiential index (CCEI) (Heron et al., 2004), the anxiety subset of the Physician Health Questionnaire, among others. Similarly, there are no standardized recommended screening tools for substance use disorders or eating disorders in the perinatal population. In general, because postpartum mental illness is common in the general population of new mothers, screening to identify cases for early intervention is another important public health goal. Ideally, caregivers in all settings would be able to screen for known risk factors for postpartum illness, including but not limited to personal history or family history of mental illness, particularly postpartum depression and bipolar disorder, in order for appropriate planning and interventions to be made prophylactically.
Barriers to care
While screening scales are valuable tools in the identification of psychiatric disorders, screening alone does not address the needs of the childbearing woman and does not improve clinical outcomes (Gaynes et al., 2005; Gilbody, Trevor, & House, 2008). Depression in obstetric settings remains under-recognized and undertreated (Coates, Schaefer, & Alexander, 2004; Vesga-Lopez et al., 2008). To improve outcomes, screening for psychiatric disorders must be tied to systems of care. However, traditional models of screening and referral to psychiatric care have often faltered when faced with multiple barriers to care, including the perception of stigma, social issues (Abrams, Dornig, & Curran, 2009; Dennis & Chung-Lee, 2006), child care, insurance and access limitations (Kim et al., 2010). Recommendations for screening are being implemented in a variety of settings; however, there remains a significant gap in identification of likely mental illness, and accessible, acceptable and effective treatment for perinatal mental disorders (Dennis & Chung-Lee, 2006).
A number of recent studies have looked at models of care that attempt to overcome these barriers. Sit and colleagues conducted a pilot study of integrated depression screening and treatment within a ‘Healthy Start’ centre, which is a US federally funded, community-based network that promotes maternalchild health interventions (Sit & Wisner, 2009). They found that co-located depression and maternal health care was highly acceptable and enabled evidence-based care delivery in a population of moderately to severely depressed women with numerous economic, social and psychiatric stressors. However, even in this highly supported setting, dropoff from screening to treatment remained significant. Miller and colleagues developed a model of stepped collaborative care for screening, assessment and treatment of depressed perinatal women, involving a multispecialist team based in the primary care setting (Miller, Shade, & Avasireddy, 2009). This model demonstrated improved acceptance of diagnostic assessment by patients screened for depression and facilitated initial treatment by the primary provider. A number of innovative approaches to the treatment of perinatal mental disorders have also been developed, which aim to overcome barriers of acceptability and accessibility, for example telephone screening and peer support (Dennis et al., 2009), training of non-mental health providers to conduct in-home assessment and psychologically informed approaches (Morrell et al., 2009), and partner-assisted treatment for perinatal depression (Brandon, 2009).
In the UK and Australia, the national health services have taken steps to address the gaps in recognition and treatment of maternal mental health disorders on a national scale. In the UK the National Health Service guidelines for pregnancy care include not only screening for maternal depression in the antenatal and postnatal period, but also requesting personal and family history of mental illness and history of previous mental health treatment (NICE, 2007). Maternal mental health is also prioritized in the New Horizons initiative, which lays out a public health framework for mental health and wellness for the UK (DoH, 2010). The state-funded Australian initiative ‘Beyondblue’ has developed a National Action Plan for perinatal mental health, and has assembled guidelines to inform best practice in the detection, treatment and management of depression, anxiety disorders, bipolar disorder and postpartum psychosis for expectant and new mothers. The goals of the initiative include national universal screening for perinatal depression, effective pathways to treatment, and education and prevention of perinatal mental illness (Perinatal Mental Health Consortium, 2008).
In the USA the more fragmented nature of health care delivery may present a barrier to consistent collaborative perinatal mental health care on a national scale. However, there are a number of developments in national legislation, clinical practice guidelines, and research prioritization that point to continued progress in the detection and treatment of perinatal mental illness in the USA. The President of the American College of Obstetrics and Gynecology has prioritized the diagnosis and treatment of postpartum depression as a theme of his administration (ACOG, 2009). The National Institutes of Health is funding new grants to encourage research on women’s mental health in relation to pregnancy and the postpartum period (DHHS, 2009). The health care reform legislation that was passed into law in May, 2010, includes provisions for more education and services to women suffering from postpartum depression and psychosis and their families, and will support research into the causes, diagnoses and treatments for these disorders. (PPAC, 2010).
Conclusions
Examining mental illness through the lens of the female life cycle encourages new understanding of the origins, course, and treatment of psychiatric illness. This approach can also frame an understanding of psychological resilience, which will help the field move from a reactive stance (identification and referral) to proactive engagement (education and collaboration) with patients and between medical disciplines. While there has been some literature on the importance of collaborative decision making with the patient in the context of weighing risks and benefits of somatic treatments and psychiatric illness in perinatal women (Wisner et al., 2000), there has been less discussion about such a collaborative approach in preconception care and prevention of perinatal mental illness. Current recommendations for preconception care include evaluation of psychosocial concerns such as depression or violence in women as part of routine care well before pregnancy (Johnson et al., 2006). There is a need for caregivers to consider the possibility of pregnancy at all times in the treatment of women with mental illness. A more thorough understanding of the implications of untreated mental illness in childbearing for the patient, as well as the provider, may encourage positive change and motivation for treatment in women who may otherwise hesitate to put their own needs first. For a psychiatrist treating such a patient, this life cycle model could frame for both physician and patient that the goal of treatment is recovery to full emotional, occupational and social functioning, acknowledging that part of healthy functioning for a woman of childbearing age may indeed include bearing children.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abou-Saleh MT, Ghubash R, Karim L, Krymski M, & Bhai I (1998). Hormonal aspects of postpartum depression. Psychoneuroendocrinology, 23, 465–475. [DOI] [PubMed] [Google Scholar]
- Abrams LS, Dornig K, & Curran L (2009). Barriers to service use for postpartum depression symptoms among low-income ethnic minority mothers in the United States. Qualitative Health Research, 19, 535–551. [DOI] [PubMed] [Google Scholar]
- ACOG (2009). Postpartum depression is top priority for new American College of Obstetrics and Gynecology president. Available from http://www.acog.org/from_home/publications/press_releases/nr05-06-09-1.cfm (accessed 30 November 2009).
- Affonso D, Lovett S, Paul S, Arizmendi T, Nussbaum R, Newman L, & Johnson B (1991). Predictors of depression symptoms during pregnancy and the postpartum. Journal of Psychosomatic Obstetrics & Gynaecology, 12, 255–271. [Google Scholar]
- Akdeniz F, Vahip S, Pirildar S, Vahip I, Dogner I, & Bulut I (2003). Risk factors associated with childbearing-related episodes in women with bipolar disorder. Psychopathology, 36, 234–238. [DOI] [PubMed] [Google Scholar]
- Allen NB, Lewinsohn PM, & Seeley JR (1998). Prenatal and perinatal influences on risk for psychopathology in childhood and adolescence. Developmental Psychopathology, 10, 513–529. [DOI] [PubMed] [Google Scholar]
- Altemus M, Redwine LS, Leong YM, Frye CA, Porges SW, & Carter CS (2001). Responses to laboratory psychosocial stress in postpartum women. Psychosomatic Medicine, 63, 814–821. [DOI] [PubMed] [Google Scholar]
- Altfed S, Handler A, Burton D, & Berman L (1997). Wantedness of pregnancy and prenatal health behaviors. Women & Health, 26, 29–43. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, & Mintz J (1996). Pharmacologic management of psychiatric illness during pregnancy: Dilemmas and guidelines. American Journal of Psychiatry, 153, 592–606. [DOI] [PubMed] [Google Scholar]
- Andersson L, Sundström-Poromaa I, Bixo M, Wulff M, Bondestam K, & Åström M (2003). Point prevalence of psychiatric disorders during the second trimester of pregnancy: A population-based study. American Journal of Obstetrics & Gynecology, 189, 148–154. [DOI] [PubMed] [Google Scholar]
- Antonow-Schlorke I, Schwab M, Li C, & Nathanielsz PW (2003). Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. Journal of Physiology, 547, 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby L (1991). Suicide during pregnancy and in the first postnatal year. British Medical Journal, 302, 137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby L, & Turnbull G (1995). Parasuicide in the first postnatal year. Psychological Medicine, 25, 1087–1090. [DOI] [PubMed] [Google Scholar]
- Appleby L (1996). Suicidal behavior in childbearing women. International Review of Psychiatry, 8, 107–115. [Google Scholar]
- Appleby L, Mortensen PB, & Faraghar EB (1998). Suicide and other causes of mortality after postpartum psychiatric admission. British Journal of Psychiatry, 173, 209–211. [DOI] [PubMed] [Google Scholar]
- Areskog B, Uddenberg N, & Kiessler B (1981). Fear of childbirth in late pregnancy. Gynecological & Obstetric Investigation, 12, 262–266. [DOI] [PubMed] [Google Scholar]
- Astbury J, Brown S, Lumley J, & Small R (1994). Birth events, birth experiences and social differences in postnatal depression. Australian Journal Public Health, 18, 176–184. [DOI] [PubMed] [Google Scholar]
- Atiba EO, Adeghe AJ, Murphy PJ, Felmingham JE, & Scott GI (1993). Patients’ expectation and caesarean section rate. Lancet, 341, 246. [DOI] [PubMed] [Google Scholar]
- Austin M-P, Tully L, & Parker G (2007). Examining the relationship between antenatal anxiety and postnatal depression. Journal of Affective Disorders, 101, 169–174. [DOI] [PubMed] [Google Scholar]
- Barnet B, Joffe A, Duggan AK, Wilson MD, & Repke JT (1996). Depressive symptoms, stress, and social support in pregnant and postpartum adolescents. Archives of Pediatrics & Adolescent Medicine, 150, 64–69. [DOI] [PubMed] [Google Scholar]
- Beck CT (2001). Predictors of postnatal depression: An update. Nursing Research, 50, 275–285. [DOI] [PubMed] [Google Scholar]
- Beck CT (2004). Post-traumatic stress disorder due to childbirth: The aftermath. Nursing Research, 53, 216–224. [DOI] [PubMed] [Google Scholar]
- Beck CT, & Gable RK (2001). Comparative analysis of the performance of the postpartum depression screening scale with two other depression instruments. Nursing Research, 50, 242–250. [DOI] [PubMed] [Google Scholar]
- Bennedsen BE (1998). Adverse pregnancy outcome in schizophrenic women: Occurrence and risk factors. Schizophrenia Research, 33, 1–26. [DOI] [PubMed] [Google Scholar]
- Bennett HA, Einarson A, Taddio A, Koren G, & Einarson TR (2004). Prevalence of depression during pregnancy: Systematic review. Obstetrics & Gynecology, 103, 698–709. [DOI] [PubMed] [Google Scholar]
- Bick D, & Howard L (2010). When should women be screened for postnatal depression? Expert Review of Neurotherapeutics, 10, 151–154. [DOI] [PubMed] [Google Scholar]
- Bixo M, Sundstrom-Poromaa I, Bjorn I, & Astrom M (2001). Patients with psychiatric disorders in gynecologic practice. American Journal of Obstetrics & Gynecology, 185, 396–402. [DOI] [PubMed] [Google Scholar]
- Brandon AR (2009). Partner-assisted therapy for the treatment of depression during pregnancy. Grant 1K23MH085007-01A1 from the National Institutes of Mental Health. Available from http://www.researchgrantdatabase.com/g/1K23MH085007/ (accessed 16 May 2010). [Google Scholar]
- Bulik CM, Von Holle A, Hamer R, Knoph Berg C, Torgersen L, Magnus P, … , Reichborn-Kjennerud T (2007). Patterns of remission, continuation, and incidence of broadly defined eating disorders during early pregnancy in the Norwegian Mother and Child Cohort Study (MoBa). Psychological Medicine, 37, 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik CM, Von Holle A, Gendall K, Kyeim Lie K, Hoffman E, Mo X, … , Reichborn-Kjennerud T (2008). Maternal eating disorders influence sex ratio at birth. Acta Obstetricia et Gynecologica Scandinavica, 87, 979–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet R, Pradier P, Dalle M, & Delost P (1986). Effects of prenatal maternal stress on the pituitary adrenocortical reactivity in guinea-pig pups. Journal of Developmental Physiology, 8, 467–475. [PubMed] [Google Scholar]
- Cantwell R, & Smith S (2006). Preventing and detecting perinatal mental illness. Women’s Health Medicine, 3, 68–73. [Google Scholar]
- Carmichael SL, & Shaw GM (2000). Maternal life event stress and congenital anomalies. Epidemiology, 11, 30–35. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Schaffer DM, Laurent C, & Selvin S (2003). Dieting behaviors and risk of neural tube defects. American Journal of Epidemiology, 158, 1127–1131. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, & Mendels J (1976a). Cerebrospinal fluid and plasma free cortisol concentrations in depression. Psychology of Medicine, 6, 235–244. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, & Mendels J (1976b). Neuroendocrine regulation in depression. I. Limbic system-adrenocortical dysfunction. Archives of General Psychiatry, 33, 1039–1044. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, & Mendels J (1976c). Neuroendocrine regulation in depression. II. Discrimination of depressed from nondepressed patients. Archives of General Psychiatry, 33, 1051–1058. [DOI] [PubMed] [Google Scholar]
- Carter FA, Carter JD, Luty SE, Wilson DA, Frampton CM, & Joyce PR (2005). Screening and treatment for depression during pregnancy: A cautionary note. Australian and New Zealand Journal of Psychiatry, 39, 255–261. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Pêgo JM, Taipa R, Bessa JM, Almeida OF, & Sousa N (2005). Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. Journal of Neuroscience, 25, 7792–7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra PS, Bhargavaraman RP, Raghunandan VNGP, & Shaligram D (2006). Delusions related to infant and their association with mother-infant interactions in postpartum psychotic disorders. Archives of Women’s Mental Health, 9, 285–288. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Landress GH, & Barnett ME (1990). The prevalence of illicit drug or alcohol use during pregnancy and discrepancies in mandatory reporting in Pinellas County, Florida. New England Journal of Medicine, 322, 1202–1206. [DOI] [PubMed] [Google Scholar]
- Chaudron LH, Szilagyi PG, Campbell AT, Mounts KO, & McInerny TK (2007). Legal and ethical considerations: Risks and benefits of postpartum depression screening at well-child visits. Pediatrics, 119, 123–128. [DOI] [PubMed] [Google Scholar]
- Chaudron LH, & Caine ED (2004). Suicide among women: A critical review. Journal of the American Medical Women’s Association, 59, 125–134. [PubMed] [Google Scholar]
- Chessick CA, & Dimidjian S (2010). Screening for bipolar disorder during pregnancy and the postpartum period. Archives of Women’s Mental Health, 13, 233–248. [DOI] [PubMed] [Google Scholar]
- Coates AO, Schaefer CA, & Alexander JL (2004). Detection of postpartum depression and anxiety in a large health plan. Journal of Behavioral Health Services & Research, 31, 117–133. [DOI] [PubMed] [Google Scholar]
- Coble PA, Reynolds CF, Kupfer DJ, Houck PR, Day NL, & Giles DE (1994). Childbearing in women with and without a history of affective disorder. I. Psychiatric symptomatology. Comprehensive Psychiatry, 35, 205–214. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Sichel DA, Dimmock JA, & Rosenbaum JF (1994). Impact of pregnancy on panic disorder: A case series. Journal of Clinical Psychiatry, 55, 284–288. [PubMed] [Google Scholar]
- Cohen LS, Sichel DA, Dimmock JA, & Rosenbaum JF (1994). Postpartum course in women with preexisting panic disorder. Journal of Clinical Psychiatry, 55, 289–292. [PubMed] [Google Scholar]
- Cohen LS, Sichel DA, Faraone SV, Robertson LM, Dimmock JA, & Rosenbaum JF (1996). Course of panic disorder during pregnancy and the puerperium: A preliminary study. Biological Psychiatry, 39, 950–954. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, … , Stowe ZN (2006). Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. Journal of the American Medical Association, 295, 499–507. [DOI] [PubMed] [Google Scholar]
- Coleman PK, Reardon DC, Rue V, & Cougle J (2002). Prior history of induced abortion and substance use during pregnancy. American Journal of Obstetrics and Gynecology, 187, 1673–1678. [DOI] [PubMed] [Google Scholar]
- Coleman PK, Reardon DC, & Cougle JR (2005). Substance use among pregnant women in the context of previous reproductive loss and desire for current pregnancy. British Journal of Health Psychology, 10, 255–268. [DOI] [PubMed] [Google Scholar]
- Cooper PJ, Campbell EA, Day A, Kennerley H, & Bond AK (1988). Non-psychotic disorder after childbirth. A prospective study of prevalence, incidence, course and nature. British Journal of Psychiatry, 152, 799–806. [DOI] [PubMed] [Google Scholar]
- Corodimas KP, LeDoux JE, Gold PW, & Schulkin J (1994). Corticosterone potentiation of conditioned fear in rats. Annals of the New York Academy of Science, 746, 392–393. [DOI] [PubMed] [Google Scholar]
- Cosmi EV, Luzi G, Gori F, & Chiodi A (1990). Response of utero-placental fetal blood flow to stress situation and drugs. European Journal of Obstetrics Gynecology and Reproductive Biology, 36, 239. [DOI] [PubMed] [Google Scholar]
- Cowley DS, & Roy-Byrne PP (1989). Panic disorder during pregnancy. Journal of Psychosomatic Obstetrics & Gynecology, 10, 193–210. [Google Scholar]
- Cox J, Holden J, & Sagovsky R (1987). Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry, 150, 782–786. [DOI] [PubMed] [Google Scholar]
- Cromptom J (1996). Post-traumatic stress disorder and childbirth. British Journal of Midwifery, 4, 7–14. [Google Scholar]
- Crow SJ, Keel PK, Thuras P, & Mitchell JE (2004). Bulimia symptoms and other risk behaviors during pregnancy in women with bulimia nervosa. International Journal of Eating Disorders, 36, 220–223. [DOI] [PubMed] [Google Scholar]
- Davies A, McIvor RJ, & Kumar RC (1995). Impact of childbirth on a series of schizophrenic mothers: A comment on the possible influence of oestrogen on schizophrenia. Schizophrenia Research, 16, 25–31. [DOI] [PubMed] [Google Scholar]
- Dean F, & Matthews SG (1999). Maternal dexamethasone treatment in late gestation alters glucocorticoid and mineralocorticoid receptor mRNA in the fetal guinea pig brain. Brain Research, 846, 253–259. [DOI] [PubMed] [Google Scholar]
- Delarue C, Contesse V, Lenglet S, Sicard F, Perraudin V, Lefebvre H, … ,Vaudry H (2001). Role of neurotransmitters and neuropeptides in the regulation of the adrenal cortex. Reviews in Endocrine and Metabolic Disorders, 2, 253–267. [DOI] [PubMed] [Google Scholar]
- Dennis CL, & Chung-Lee L (2006). Postpartum depression help-seeking barriers and maternal treatment preferences: A qualitative systemic review. Birth, 33, 323–331. [DOI] [PubMed] [Google Scholar]
- Dennis CL, Hodnett E, Kenton L, Weston J, Zupancic J, Stewart DE, & Kiss A (2009). Effect of peer support on prevention of postnatal depression among high risk women: multisite randomised controlled trial. British Medical Journal, 338, a3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DoH(2010). A Framework for Developing Well Being. London: Department of Health, Mental Health Division Confident Communities, Brighter Futures; Available from www.dh.gov.uk/newhorizons (accessed 30 April 2010). [Google Scholar]
- DHHS (2009). Women’s Mental Health in Pregnancy and the Postpartum Period (R01). PA (program announcement)-09-174. Department of Health and Human Services; Available from http://grants.nih.gov/grants/guide/pa-files/PA-09-174.html (Accessed 9 May 2010). [Google Scholar]
- Dinan TG (1994). Glucocorticoids and the genesis of depressive illness. A psychobiological model. British Journal of Psychiatry, 164, 365–371. [DOI] [PubMed] [Google Scholar]
- Ebrahim SH, & Gfroerer J (2003). Pregnancy-related substance use in the United States during 1996–1998. Obstetrics & Gynecology, 101, 374–379. [DOI] [PubMed] [Google Scholar]
- Emack J, Kostaki A, Walker CD, & Matthews SG (2008). Chronic maternal stress affects growth, behaviour and hypothalamo-pituitary-adrenal function in juvenile offspring. Hormone Behaviour, 54, 514–520. [DOI] [PubMed] [Google Scholar]
- Etchegoyen A (2005). Psychiatric disorders in pregnancy. Women’s Health & Medicine, 2, 44–46. [Google Scholar]
- Evans J, Heron J, Francomb H, Oke S, & Golding J (2001). Cohort study of depressed mood during pregnancy and after childbirth. British Medical Journal, 323, 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Vera Y, Gil K, Schanberg S, … , Gonzalez-Garcia A (2004). Prenatal maternal biochemistry predicts neonatal biochemistry. International Journal of Neuroscience, 114, 933–945. [DOI] [PubMed] [Google Scholar]
- Frank MA, Tuber SB, Slade A, & Garrod E (1994). Mothers’ fantasy representations and infant security of attachment: A Rorschach study of first pregnancy. Psychoanalytical Psychology, 11, 475–490. [Google Scholar]
- Franko DL, Blais MA, Becker AE, Delinsky SS, Greenwood DN, Flores AT, … ,Herzog DB (2001). Pregnancy complications and neonatal outcomes in women with eating disorders. American Journal of Psychiatry, 158, 1461–1466. [DOI] [PubMed] [Google Scholar]
- Franko DL, Blais MA, Becker AE, Delinsky SS, Greenwood DN, Flores AT, … , Herzog DB (2001). Pregnancy complications and neonatal outcomes in women with eating disorders. American Journal of Psychiatry, 158, 1461–1466. [DOI] [PubMed] [Google Scholar]
- Freeman M, Smith K, Freeman S, McElroy S, Kmetz G, Wright R, & Keck P (2002). The impact of reproductive events on the course of bipolar disorder in women. Journal of Clinical Psychiatry, 63, 284–287. [DOI] [PubMed] [Google Scholar]
- Fuchs E, & Gould E (2000). Mini-review: In vivo neurogenesis in the adult brain: Regulation and functional implications. European Journal of Neuroscience, 12, 2211–2214. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T, & Miller WC. (2005). Perinatal depression: A systematic review of prevalence and incidence. Obstetrics & Gynecology, 106, 1071–1083. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, … , Miller WC (2005). Perinatal depression: Prevalence, screening, accuracy and screening outcomes. Evidence Report/Technology Assessment No. 119. AAHRQ Publication No. 05-E006-2. Rockville, MD: Agency for Healthcare Research and Quality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, & Ladenheim JA (1987). Effect of pregnancy on panic attacks. American Journal of Psychiatry, 144, 1078–1079. [DOI] [PubMed] [Google Scholar]
- Gibson J, McKenzie-McHarg K, Shakespeare J, Price J, & Gray R (2009). A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatrica Scandinavica, 119, 350–364. [DOI] [PubMed] [Google Scholar]
- Gilbody S, Richards D, Brealey S, & Hewitt C (2007). Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): A diagnostic meta-analysis. Journal of General Internal Medicine, 22, 1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbody S, Trevor S, & House A (2008). Screening and case-finding instruments for depression: A meta-analysis. Canadian Medical Association Journal, 178, 997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitau R, Cameron A, Fisk NM, & Glover V (1998). Fetal exposure to maternal cortisol. Lancet, 352, 707–708. [DOI] [PubMed] [Google Scholar]
- Gitau R, Fisk NM, Teixeira JM, Cameron A, & Glover V (2001). Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. Journal of Clinical Endocrinology & Metabolism, 86, 104–109. [DOI] [PubMed] [Google Scholar]
- Gjerdingen DK, & Yawn BP (2007). Postpartum depression screening: Importance, methods, barriers, and recommendations for practice. Journal of the American Board of Family Medicine, 20, 280–288. [DOI] [PubMed] [Google Scholar]
- Glover V, Liddle P, Taylor A, Adams D, & Sandler M (1994). Mild hypomania (the highs) can be a feature of the first postpartum week. Association with later depression. British Journal of Psychiatry, 164, 517–521. [DOI] [PubMed] [Google Scholar]
- Glover V, Bergman K, Sarkar P, & O’Connor TG (2009). Association between maternal and amniotic fluid cortisol is moderated by maternal anxiety. Psychoneuroendocrinology, 34, 430–435. [DOI] [PubMed] [Google Scholar]
- Gonzalez HM, Vega WA, Williams DR, Tarraf W, West BT, & Neighbors HW (2010). Depression care in the United States: Too little for too few. Archives of General Psychiatry, 67, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, & Fuchs E (1997). Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. Journal of Neuroscience, 17, 2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, & Taylor JR (2008). Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biological Psychiatry, 63, 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grof P, Robbins W, Alda M, Berghoefer A, Vojtechovsky M, Nilson A, & Robertson C (2000). Protective effect of pregnancy in women with lithium-responsive bipolar disorder. Journal of Affective Disorders, 61, 31–39. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Donzella B (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27, 199–220. [DOI] [PubMed] [Google Scholar]
- Halbreich U, & Karkun S (2006). Cross-cultural and social diversity of prevalence of postpartum depression and depressive symptoms. Journal of Affective Disorders, 91, 97–111. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1969). Diagnosis and rating of anxiety. British Journal of Psychiatry, 3, S76–79. [Google Scholar]
- Hannah P, Adams D, Lee A, Glover V, & Sandler M (1992). Links between early post-partum mood and postnatal depression. British Journal of Psychiatry, 160, 777–780. [DOI] [PubMed] [Google Scholar]
- Hans SL (1999). Demographic and psychosocial characteristics of substance-abusing pregnant women. Clinics in Perinatology, 25, 55–74. [PubMed] [Google Scholar]
- Hanusa BH, Scholle SH, Haskett RF, Spadaro K, & Wisner KL (2008). Screening for depression in the postpartum period: A comparison of three instruments. Journal of Women’s Health (Larchmt), 17, 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck H, & de Castro JM (1993). The caloric demand of lactation does not alter spontaneous meal patterns, nutrient intakes, or moods of women. Physiology & Behavior, 54, 641–648. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, & Hellhammer DH (2001). Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. Journal of Clinical Endocrinology & Metabolism, 86, 4798–4804. [DOI] [PubMed] [Google Scholar]
- Heron J, O’Connor TG, Evans J, Golding J, & Glover V (2004). The course of anxiety and depression through pregnancy and the postpartum in a community sample. Journal of Affective Disorders, 80, 65–73. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM, Williams JB, Spitzer RL, Calabrese JR, Flynn L, Keck PE Jr, … , Zajecka J (2000). Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. American Journal of Psychiatry, 157, 1873–1875. [DOI] [PubMed] [Google Scholar]
- Howard LM, Leese M, Goss C, Appleby L, & Thornicroft G (2004). The psychosocial outcome of pregnancy in women with psychotic disorders and for their infants in the first year post partum. Schizophrenia Research, 71, 49–60. [DOI] [PubMed] [Google Scholar]
- Huang WL, Beazley LD, Quinlivan JA, Evans SF, Newnham JP, & Dunlop SA (1999). Effect of corticosteroids on brain growth in fetal sheep. Obstetrics & Gynecology, 94, 213–218. [DOI] [PubMed] [Google Scholar]
- Jablensky AV, Morgan V, Zubrick SR, Bower C, & Yellachich L (2005). Pregnancy, delivery and neonatal complications in a population cohort of women with schizophrenia and major affective disorders. American Journal of Psychiatry, 162, 79–91. [DOI] [PubMed] [Google Scholar]
- Jennings KD, Ross S, Popper S, & Elmore M (1999). Thoughts of harming infants in depressed and non-depressed mothers. Journal of Affective Disorders, 54, 21–28. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Fournier NM, & Kalynchuk LE (2006). Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behavioural Brain Research, 168, 280–288. [DOI] [PubMed] [Google Scholar]
- Johnson K, Posner SF, Biermann J, Cordero JF, Atrash HK, Parker CS, … , Curtis MG (2006). Recommendations to improve preconception health and health care – United States: A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. Morbidity and Mortality Weekly Report, 55, (No. RR- 6), 1–23. [PubMed] [Google Scholar]
- Jomeen J (2004). The importance of assessing psychological status during pregnancy, childbirth and the postnatal period as a multidimensional construct: A literature review. Clinical Effectiveness in Nursing, 8, 143–155. [Google Scholar]
- Jones I, & Craddock N (2001). Familiality of the puerperal trigger in bipolar disorder: Results of a family study. American Journal of Psychiatry, 158, 913–917. [DOI] [PubMed] [Google Scholar]
- Jones I, & Craddock N (2005). Bipolar disorder and childbirth: The importance of recognizing risk. British Journal of Psychiatry, 186, 453–454. [DOI] [PubMed] [Google Scholar]
- Kendell RE, Wainwright S, Hailey A, & Shannon B (1976). The influence of childbirth on psychiatric morbidity. Psychological Medicine, 6, 297–302. [DOI] [PubMed] [Google Scholar]
- Kendell RE, Rennie D, Clarke JA, & Dean C (1981). The social and obstetric correlates of psychiatric admission in the puerperium. Psychological Medicine, 11, 341–350. [DOI] [PubMed] [Google Scholar]
- Kendell RE, Chalmers JC, & Platz C (1987). Epidemiology of puerperal psychoses. British Journal of Psychiatry, 150, 662–673. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Gordon TEJ, La Porte LM, Adams M, Kuendig JM, & Silver RK (2008). The utility of maternal depression screening in the third trimester. American Journal of Obstetrics and Gynecology, 199, 509.e1–509.e5. [DOI] [PubMed] [Google Scholar]
- Kim JJ, La Porte LM, Corcoran M, Magasi S, Batza J, & Silver RK (2010). Barriers to mental health treatment among obstetric patients at risk for depression. American Journal of Obstetrics & Gynecology, 202, 312.e1–5. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Shima S, Sugawara M, & Toda MA (1993). Psychological and social correlates of the onset of affective disorders among pregnant women. Psychological Medicine, 23, 967–975. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Toda MA, Shima S, & Sugawara M (1994). Early loss of parents and early rearing experience among women with antenatal depression. Journal of Psychosomatic Obstetrics & Gynecology, 15, 133–139. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Sugawara M, Sugawara K, Toda M, & Shima S (1996). Psychosocial study of depression in early pregnancy. British Journal of Psychiatry, 168, 732–738. [DOI] [PubMed] [Google Scholar]
- Klein DF, Skrobola AM, & Garfinkel RS (1994–1995). Preliminary look at the effects of pregnancy on the course of panic disorder. Anxiety, 1, 227–232. [PubMed] [Google Scholar]
- Korte SM (2001). Corticosteroids in relation to fear, anxiety and psychopathology. Neuroscience Biobehavioral Reviews, 25, 117–142. [DOI] [PubMed] [Google Scholar]
- Kost K, Landry DJ, & Darroch JE (1998). The effects of pregnancy planning status on birth outcomes and infant care. Family Planning Perspectives, 30, 223–230. [PubMed] [Google Scholar]
- Krener P, Simmons MK, Hansen RL, & Treat JN (1989). Effect of pregnancy on psychosis: Life circumstances and psychiatric symptoms. International Journal of Psychiatry in Medicine, 19, 65–84. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer R, & Williams J (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of Internal Medicine, 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, & Robson KM (1984). A prospective study of emotional disorders in childbearing women. British Journal of Psychiatry, 144, 35–47. [DOI] [PubMed] [Google Scholar]
- Labad J, Menchón JM, Alonso P, Segalàs C, Jiménez S, & Vallejo J (2005). Female reproductive cycle and obsessive compulsive disorder. Journal of Clinical Psychiatry, 66, 428–435. [DOI] [PubMed] [Google Scholar]
- Lacey JH, & Smith G (1987). Bulimia nervosa. The impact of pregnancy on mother and baby. The British Journal of Psychiatry, 150, 771–781. [DOI] [PubMed] [Google Scholar]
- Lane A, Keville R, Morris M, Kinsella A, Turner M, & Barry S (1997). Postnatal depression and elation among mothers and their partners: Prevalence and predictors. British Journal of Psychiatry, 171, 550–555. [DOI] [PubMed] [Google Scholar]
- Lemberg R, & Phillips J (1989). The impact of pregnancy on anorexia nervosa and bulimia. International Journal of Eating Disorders, 8, 285–295. [Google Scholar]
- Levine S (2001). Primary social relationships influence the development of the hypothalamic-pituitary-adrenal axis in the rat. Physiology & Behavior, 73, 255–260. [DOI] [PubMed] [Google Scholar]
- Llewellyn AM, Stowe ZN, & Nemeroff CB (1997). Depression during pregnancy and the puerperium. Journal of Clinical Psychiatry, 58, S26–32. [PubMed] [Google Scholar]
- Luoma I, Tamminen T, Kaukonen P, Laippala P, Puura K, Salmelin R, & Almqvist F (2001). Longitudinal study of maternal depressive symptoms and child well-being. Journal of the American Academy of Child & Adolescent Psychiatry, 40, 1367–1374. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Orchinik M, & McEwen BS (1998). Morphological changes in the hippocampal CA3 region induced by non-invasive glucocorticoid administration: A paradox. Brain Research, 809, 314–318. [DOI] [PubMed] [Google Scholar]
- Martini J, Wittchen HU, Soares CN, Rieder A, & Steiner M (2009). New women-specific diagnostic modules: The Composite International Diagnostic Interview for Women (CIDI-VENUS). Archives of Women’s Mental Health, 12, 281–289. [DOI] [PubMed] [Google Scholar]
- Matthews SG (2000). Antenatal glucocorticoids and programming of the developing CNS. Pediatric Research, 47, 291–300. [DOI] [PubMed] [Google Scholar]
- Matthey S, Barnett B, Howie P, & Kavanagh DJ (2003). Diagnosing postpartum depression in mothers and fathers: Whatever happened to anxiety? Journal of Affective Disorders, 74, 139–147. [DOI] [PubMed] [Google Scholar]
- Matthey S, Henshaw C, Elliott S, & Barnett B (2006). Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale - Implications for clinical and research practice. Archives of Women’s Mental Health, 9, 309–315. [DOI] [PubMed] [Google Scholar]
- Mazzeo SE, Slof-Op’t Landt MC, Jones I, Mitchell K, Kendler KS, Neale MC, … , Bulik CM (2006). Associations among postpartum depression, eating disorders and perfectionism in a population-based sample of adult women. International Journal of Eating Disorders, 39, 202–211. [DOI] [PubMed] [Google Scholar]
- McNeil TF (1986). A prospective study of postpartum psychoses in a high-risk group: 1. Clinical characteristics of the current postpartum episodes. Acta Psychiatrica Scandinavica, 74, 205–216. [DOI] [PubMed] [Google Scholar]
- McNeil TF (1987). A prospective study of postpartum psychoses in a high-risk group: 2. Relationships to demographic and psychiatric history characteristics. Acta Psychiatrica Scandinavica, 75, 35–43. [DOI] [PubMed] [Google Scholar]
- McNeil TF, Kaij L, & Malmquist-Larsson A (1983). Pregnant women with nonorganic psychosis: Life situation and experience of pregnancy. Acta Psychiatrica Scandinavica, 68, 445–457. [DOI] [PubMed] [Google Scholar]
- McNeil TF, Kaij L, & Malmquist-Larsson A (1984a). Women with nonorganic psychosis: Factors associated with pregnancy’s effect on mental health. Acta Psychiatrica Scandinavica, 70, 209–219. [DOI] [PubMed] [Google Scholar]
- McNeil TF, Kaij L, & Malmquist-Larsson A (1984b). Women with nonorganic psychosis: Mental disturbance during pregnancy. Acta Psychiatrica Scandinavica, 70, 140–148. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, … ,Plotsky PM (1996). Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Developmental Neuroscience, 18, 49–72. [DOI] [PubMed] [Google Scholar]
- Melender HL (2002). Experiences of fears associated with pregnancy and childbirth: A study of 329 pregnant women. Birth, 29, 101–111. [DOI] [PubMed] [Google Scholar]
- Meyer JS (1983). Early adrenalectomy stimulates subsequent growth and development of the rat brain. Experimental Neurology, 82, 432–446. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES, Guethlein W, Vaz N, & Bagiella E (2000). A preliminary study of breast-feeding and maternal symptomatology. Annals of Behavioral Medicine, 22, 71–79. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES, & Katlin ES (2002). Breast-feeding is associated with reduced perceived stress and negative mood in mothers. Health Psychology, 21, 187–193. [PubMed] [Google Scholar]
- Micali N, Siminoff E, & Treasure J (2007). Risk of major adverse perinatal outcomes in women with eating disorders. British Journal of Psychiatry, 190, 255–259. [DOI] [PubMed] [Google Scholar]
- Milgrom J, Gemmill AW, Bilszta JL, Hayes B, Barnett B, Brooks J, … ,Buist A (2008). Antenatal risk factors for postnatal depression: A large prospective study. Journal of Affective Disorders, 108, 147–157. [DOI] [PubMed] [Google Scholar]
- Miller LJ (1990). Psychotic denial of pregnancy: Phenomenology and clinical management. Hospital and Community Psychiatry, 41, 1233–1237. [DOI] [PubMed] [Google Scholar]
- Miller LJ (1993). Psychiatric disorders during pregnancy In Stewart DE & Stotland NL (Eds.), Psychological Aspects of Women’s Health Care: The Interface Between Psychiatry and Obstetrics and Gynecology (pp. 55–70). Washington, DC: American Psychiatric Press. [Google Scholar]
- Miller LJ, & Shah A (1999). Major mental illness during pregnancy. Primary Care Update, 6, 163–168. [Google Scholar]
- Miller LJ (1997). Sexuality, reproduction and family planning in women with schizophrenia. Schizophrenia Bulletin, 23, 623–635. [DOI] [PubMed] [Google Scholar]
- Miller LJ, & Finnerty M (1996). Sexuality, pregnancy, and childrearing among women with schizophrenia-spectrum disorders. Psychiatric Services, 47, 502–505. [DOI] [PubMed] [Google Scholar]
- Miller L, Shade M, & Vasireddy V (2009). Beyond screening: Assessment of perinatal depression in a perinatal care setting. Archives Women’s Mental Health, 12, 329–334. [DOI] [PubMed] [Google Scholar]
- Misra DP, Guyer B, & Allston A (2003). Integrated perinatal health framework: a multiple determinants model with a life span approach. American Journal of Preventive Medicine, 25, 65–75. [DOI] [PubMed] [Google Scholar]
- Misri S, Reebye P, Corral M, & Milis L (2004). The use of paroxetine and cognitive-behavioral therapy in postpartum depression and anxiety: A randomized controlled trial. Journal of Clinical Psychiatry, 65, 1236–1241. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, & Coyne JC (2007). Do ultra-short screening instruments accurately detect depression in primary care? A pooled analysis and meta-analysis of 22 studies. British Journal of General Practice, 57, 144–151. [PMC free article] [PubMed] [Google Scholar]
- Mitchell-Gieleghem A, Mittelstaedt ME, & Bulik CM (2002). Eating disorders and childbearing: Concealment and consequences. Birth, 29, 182–191. [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Sanchez F, Flores G, Dumont Y, & Quirion R (2009). Morphological reorganization after repeated corticosterone administration in the hippocampus, nucleus accumbens and amygdala in the rat. Journal of Chemical Neuroanatomy, 38, 266–272. [DOI] [PubMed] [Google Scholar]
- Morgan JF (1999). Eating disorders and reproduction. Australian & New Zealand Journal of Obstetrics & Gynaecology, 39, 167–173. [DOI] [PubMed] [Google Scholar]
- Morrell CJ, Slade P, Warner R, Paley G, Dixon S, Walters SJ, … , Nicholl J (2009). Clinical effectiveness of health visitor training in psychologically informed approaches for depression in postnatal women: Pragmatic cluster randomised trial in primary care. British Medical Journal, 15, 338:a3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss KM, Skouteris H, Wertheim EH, Paxton SJ, & Milgrom J (2009). Depressive and anxiety symptoms through late pregnancy and the first year post birth: An examination of prospective relationships. Archives of Women’s Mental Health, 12, 345–349. [DOI] [PubMed] [Google Scholar]
- Mueller BR, & Bale TL (2008). Sex-specific programming of offspring emotionality after stress early in pregnancy. Journal of Neuroscience, 28, 9055–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, & Mortensen PB (2006). New parents and mental disorders: A population-based register study. Journal of the American Medical Association, 296, 2582–2589. [DOI] [PubMed] [Google Scholar]
- Munk-Olsen T, Munk Laursen T, Mendelson T, Pederson C, Mors O, & Mortenson PB (2009). Risks and predictors of readmission for a mental disorder during the postpartum period. Archives of General Psychiatry, 66, 189–195. [DOI] [PubMed] [Google Scholar]
- Muqtadir S, Hamann MW, & Molnar G (1986). Management of psychotic pregnant patients in a medical-psychiatric unit. Psychosomatics, 27, 31–33. [DOI] [PubMed] [Google Scholar]
- Murray F, Smith DW, & Hutson PH (2008). Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. European Journal of Pharmacology, 583, 115–127. [DOI] [PubMed] [Google Scholar]
- Namir S, Melman KN, & Yager J (1986). Pregnancy in restricter-type anorexia nervosa: A study of six women. International Journal of Eating Disorders, 5, 837–845. [Google Scholar]
- National Pregnancy and Health Survey (1996). Drug Abuse Among Women Delivering Live Births: 1992. NIH publication 96–3819. DHHS, NIDA, Rockville MD. [Google Scholar]
- Navarro P, Garcia-Esteve L, Acaso C, Aguado J, Gelabert E, & Martin-Santos R (2008). Non-psychotic psychiatric disorders after childbirth: Prevalence and comorbidity in a community sample. Journal of Affective Disorders, 109, 171–176. [DOI] [PubMed] [Google Scholar]
- NICE (2007). Antenatal and Postnatal Mental Health: Clinical Management and Service Guidance. London: National Institute for Health and Clinical Excellence. [PubMed] [Google Scholar]
- Nielsen TF, Olausson PO, & Ingemarsson I (1994). The cesarean section rate in Sweden: The end of the rise. Birth, 21, 34–38. [DOI] [PubMed] [Google Scholar]
- Nilsson E, Lichtenstein P, Cnattingius S, Murray RM, & Hultman CM (2002). Women with schizophrenia: Pregnancy outcome and infant death among their offspring. Schizophrenia Research, 58, 221–229. [DOI] [PubMed] [Google Scholar]
- Northcott CJ, & Stein MB (1994). Panic disorder in pregnancy. Journal of Clinical Psychiatry, 55, 539–542. [PubMed] [Google Scholar]
- O’Donnell K, O’Connor TG, & Glover V (2009). Prenatal stress and neurodevelopment of the child: Focus on the HPA axis and role of the placenta. Developmental Neuroscience, 31, 285–292. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, & Glover V (2002). Antenatal anxiety predicts child behavioral/emotional problems independently of postnatal depression. Journal of the American Academy of Child & Adolescent Psychiatry, 41, 1470–1477. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Glover V; ALSPAC Study Team. (2003). Maternal antenatal anxiety and behavioural/emotional problems in children: A test of a programming hypothesis. Journal of Child Psychology & Psychiatry & Allied Disciplines, 44, 1025–1036. [DOI] [PubMed] [Google Scholar]
- O’Hara MW (1996). Social support, life events and depression during pregnancy and the puerperium. Archives of General Psychiatry, 43, 569–573. [DOI] [PubMed] [Google Scholar]
- O’Hara M, & Swain A (1996). Rates and risk of postpartum depression: A meta-analysis. International Review of Psychiatry, 8, 37–54. [Google Scholar]
- PPAC Act (2010). Support, education, and research for postpartum depression Patient Protection and Affordable Care Act (pp. 226–228). H.R. 3590. Sec. 2952. Washington, DC: 111th Congress of the United States of America; Available from: http://democrats.senate.gov/reform/patient-protection-affordable-care-act-as-passed.pdf (accessed 9 May 2010). [Google Scholar]
- Pawlby S, Hay DF, Sharp D, Waters CS, & O’Keane V (2009). Antenatal depression predicts depression in adolescent offspring: Prospective longitudinal community-based study. Journal of Affective Disorders, 113, 236–243. [DOI] [PubMed] [Google Scholar]
- Peacock JL, Bland JM, & Anderson HR (1995). Preterm delivery: Effects of socioeconomic factors, psychological stress, smoking, alcohol, and caffeine. British Medical Journal, 311, 531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perinatal Mental Health Consortium (2008). Beyondblue Perinatal Mental Health National Action Plan (NAP) – Full Report. Available from http://www.beyondblue.org.au/ (accessed 20 April 2010).
- Pfaffenbarger RS, & McCabe LJ (1966). The effect of obstetric and perinatal events on risk of mental illness in women of childbearing age. American Journal of Public Health, 56, 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, & McEwen BS (2003). Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. European Journal of Neuroscience, 17, 879–886. [DOI] [PubMed] [Google Scholar]
- Poole VL, Klerman LV, Flowers JS, Goldenberg RL, & Cliver SP (1997). Pregnancy intendedness and maternal behavior in a low income, high risk population. Journal of Nursing Science, 2, 133–141. [Google Scholar]
- Pugh TF, Jerath BK, Schmidt WM, & Reed RB (1960). Rates of mental disease related to childbearing. New England Journal of Medicine, 268, 1224–1228. [DOI] [PubMed] [Google Scholar]
- Robertson E, Grace S, Wallington T, & Steward MD (2004). Antenatal risk factors for postpartum depression: A synthesis of recent literature. General Hospital Psychiatry, 26, 289–295. [DOI] [PubMed] [Google Scholar]
- Ross LE, & McLean LM (2006). Anxiety disorders during pregnancy and the postpartum period: A systematic review. Journal of Clinical Psychiatry, 67, 1285–1298. [DOI] [PubMed] [Google Scholar]
- Ross LE, & Dennis CL (2009). The prevalence of postpartum depression among women with substance use, an abuse history, or chronic illness: A systematic review. Journal of Women’s Health, 18, 475–486. [DOI] [PubMed] [Google Scholar]
- Rudolph B, Larson GL, Sweeny S, Hough EE, & Arorian K (1990). Hospitalized pregnant psychotic women: Characteristics and treatment issues. Hospital and Community Psychiatry, 41, 159–163. [DOI] [PubMed] [Google Scholar]
- Ryding EL (1991). Psychosocial indications for cesarean section. A retrospective study of 43 cases. Acta Obstetricia et Gynecologica Scandinavica, 70, 47–49. [DOI] [PubMed] [Google Scholar]
- Ryding EL, Wijma B, Wijma K, & Rydhstrom H (1998). Fear of childbirth during pregnancy may increase the risk of emergency cesarean section. Acta Obstetricia et Gynecologica Scandinavica, 77, 542–547. [PubMed] [Google Scholar]
- Sacker A, Done DJ, & Crow TJ (1996). Obstetric complications in children born to parents with schizophrenia: A meta-analysis of case-control studies. Psychological Medicine, 26, 279–287. [DOI] [PubMed] [Google Scholar]
- Salaria S, Chana G, Caldara F, Feltrin E, Altieri M, Faggioni F, … , Everall IP (2006). Microarray analysis of cultured human brain aggregates following cortisol exposure: Implications for cellular functions relevant to mood disorders. Neurobiology of Disease, 23, 630–636. [DOI] [PubMed] [Google Scholar]
- Sarkar P, Bergman K, O’Connor TG, & Glover V (2008). Maternal antenatal anxiety and amniotic fluid cortisol and testosterone: Possible implications for foetal programming. Journal of Neuroendocrinology, 20, 489–496. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Enthoven L, van der Mark M, Levine S, de Kloet ER, & Oitzl MS (2003). The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. International Journal of Developmental Neuroscience, 21, 125–132. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, Koehler AJ, & Lubach GR (1999). Growth and development following prenatal stress exposure in primates: An examination of ontogenetic vulnerability. Child Development, 70, 263–274. [DOI] [PubMed] [Google Scholar]
- Seeman MV (2004). Relational ethics: When mothers suffer from psychosis. Archives of Women’s Mental Health, 7, 201–210. [DOI] [PubMed] [Google Scholar]
- Seib LM, & Wellman CL (2003). Daily injections alter spine density in rat medial prefrontal cortex. Neuroscience Letters, 337, 29–32. [DOI] [PubMed] [Google Scholar]
- Sharma V, Burt VK, & Ritchie HL (2009). Bipolar II postpartum depression: Detection, diagnosis, and treatment. American Journal of Psychiatry, 166, 1217–1221. [DOI] [PubMed] [Google Scholar]
- Sholomskas DE, Wiskamaratne PJ, Dogolo L, O’Brien DW, Leaf PJ, & Woods SW (1993). Postpartum onset of panic disorder: A coincidental event? Journal of Clinical Psychiatry, 54, 476–480. [PubMed] [Google Scholar]
- Sichel DA, Cohen LS, Rosenbaum JF, & Driscoll J (1993). Postpartum onset of obsessive-compulsive disorder. Psychosomatics, 34, 277–279. [DOI] [PubMed] [Google Scholar]
- Sit DKY, & Wisner KL (2009). Identification of postpartum depression. Clinical Obstetrics & Gynecology, 52, 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren B (1997). Reasons for anxiety about childbirth in 100 pregnant women. Journal of Psychosomatic Obstetrics & Gynaecology, 18, 266–272. [DOI] [PubMed] [Google Scholar]
- Sjostrom K, Valentin L, Thelin L, & Marsål K (1997). Maternal anxiety in late pregnancy and fetal hemodynamics. European Journal of Obstetrics & Gynecology & Reproductive Biology, 74, 149–155. [DOI] [PubMed] [Google Scholar]
- Skouteris H, Wertheim EH, Rallis S, Milgrom J, & Paxton SJ (2009). Depression and anxiety through pregnancy and the early postpartum: An examination of prospective relationships. Journal of Affective Disorders, 113, 303–308. [DOI] [PubMed] [Google Scholar]
- Sollid CP, Wisborg K, Hjort J, & Secher NJ (2004). Eating disorder that was diagnosed before pregnancy and pregnancy outcome. American Journal of Obstetrics & Gynecology, 190, 206–210. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, & Lushene RE (1970). Test Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Spielvogel AM, & Wile J (1986). Treatment of the psychotic pregnant patient. Psychosomatics, 27, 487–492. [DOI] [PubMed] [Google Scholar]
- Spinelli MG (2009). Postpartum psychosis: Detection of risk and management. American Journal of Psychiatry, 166, 405–408. [DOI] [PubMed] [Google Scholar]
- Statistics Canada (2002). Canadian Community Health Survey (CCHS), Cycle 1.1. Available from: www.statcan.ca/english/concepts/health/index.htm (accessed 30 January 2006).
- Steer RA, Scholl TO, Hediger ML, & Fischer RL (1992). Self-reported depression and negative pregnancy outcomes. Journal of Clinical Epidemiology, 45, 1093–1099. [DOI] [PubMed] [Google Scholar]
- Stewart DE (1984). Pregnancy and schizophrenia. Canadian Family Physician, 30, 1537–1541. [PMC free article] [PubMed] [Google Scholar]
- Stewart DE, Raskin J, Garfinkel PE, MacDonald OL, & Robinson GE (1987). Anorexia nervosa, bulimia, and pregnancy. American Journal of Obstetrics & Gynecology, 157, 1194–1198. [DOI] [PubMed] [Google Scholar]
- Stewart D, Klompenhouwer J, Kendell R, & Van Hulst A (1991). Prophylactic lithium in puerperal psychosis: The experience of three centres. British Journal of Psychiatry, 158, 393–397. [DOI] [PubMed] [Google Scholar]
- Stowe ZN, Hostetter AL, & Newport DJ (2005). The onset of postpartum depression: Implications for clinical screening in obstetrical and primary care. American Journal of Obstetrics & Gynecology, 192, 522–526. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2009). The NSDUH Report: Substance Use among Women During Pregnancy and Following Childbirth. Rockville, MD: Office of Applied Studies. [Google Scholar]
- Sugawara M, Toda MA, Shima S, Mukai T, Sakakura K, & Kitamura T (1997). Premenstrual mood changes and maternal mental health in pregnancy and the postpartum period. Journal of Clinical Psychology, 53, 225–232. [DOI] [PubMed] [Google Scholar]
- Sundström IM, Bixo M, Björn I, & Aström M (2001). Prevalence of psychiatric disorders in gynecologic outpatients. American Journal of Obstetrics & Gynecology, 184, 8–13. [DOI] [PubMed] [Google Scholar]
- Talge NM, Neal C, & Glover V (2007). Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? Journal of Child Psychology & Psychiatry, 48, 245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terp IM, & Mortensen PB (1998). Post-partum psychoses. Clinical diagnoses and relative risk of admission after parturition. British Journal of Psychiatry, 172, 521–526. [DOI] [PubMed] [Google Scholar]
- Trixler M, Gati A, & Tenyi T (1995). Risks associated with childbearing in schizophrenia. Acta Psychiatrica Belgica, 95, 159–162. [PubMed] [Google Scholar]
- Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, & Farrell PM (1990). Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus. Brain Research, Developmental Brain Research, 53, 157–167. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Mulder EJ, Mennes M, & Glover V (2005). Antenatal maternal anxiety and stress and the neuro-behavioural development of the fetus and child: Links and possible mechanisms. A review. Neuroscience & Biobehavioral Reviews, 29, 237–258. [DOI] [PubMed] [Google Scholar]
- Van der Kolk BA, Pelcovitz D, Roth S, Mandel FS, McFarlane A, & Herman JL (1996). Dissociation, somatization and affect dysregulation: The complexity of adaptation of trauma. American Journal of Psychiatry, 153, 83–93. [DOI] [PubMed] [Google Scholar]
- Vesga-Lopez O, Blanco C, Keyes K, Olfson M, Grant BF, & Hasin DS (2008). Psychiatric disorders in pregnant and postpartum women in the United States. Archives of General Psychiatry, 65, 805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viguera AC, Cohen LS, Baldessarini RJ, & Nonacs R (2002). Canadian Journal of Psychiatry, 47, 426–436. [DOI] [PubMed] [Google Scholar]
- Viguera AD, Nonacs R, Cohen LS, Tondo L, Murray A, & Baldessarini RJ (2000). Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. American Journal of Psychiatry, 157, 179–184. [DOI] [PubMed] [Google Scholar]
- Viguera AC, Whitfield T, Baldessarini RJ, Newport DJ, Stowe Z, Reminick A, … , Cohen LS (2007). Risk of recurrence in women with bipolar disorder during pregnancy: Prospective study of mood stabilizer discontinuation. American Journal of Psychiatry, 164, 1817–1824. [DOI] [PubMed] [Google Scholar]
- Villeponteaux VA, Lydiard RB, Laraia MT, Stuart GW, & Ballenger JC (1992). The effects of pregnancy on preexisting panic disorder. Journal of Clinical Psychiatry, 53, 201–203. [PubMed] [Google Scholar]
- Virden SF (1988). The relationship between infant feeding method and maternal role adjustment. Journal of Nurse-Midwifery, 33, 31–35. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, & Garite TJ (2001). The neurobiology of stress in human pregnancy: Implications for prematurity and development of the fetal central nervous system. Progress in Brain Research, 133, 131–142. [DOI] [PubMed] [Google Scholar]
- Walker E, & Emory E (1983). Infants at risk for psychopathology: Offspring of schizophrenic parents. Child Development, 54, 1269–1285. [PubMed] [Google Scholar]
- Weissman MM, & Olfson M (1995). Depression in women: Implications for health care research. Science, 269, 799–801. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Haugen EN, Jackson LC, & Robinson K (2003). Prevalence of generalized anxiety at eight weeks postpartum. Archives of Women’s Mental Health, 6, 43–49. [DOI] [PubMed] [Google Scholar]
- Whalley LJ, Roberts DF, Wentzel J, & Wright AF (1982). Genetic factors in puerperal affective psychoses. Acta Psychiatrica Scandinavica, 65, 180–193. [DOI] [PubMed] [Google Scholar]
- Whooley MA, Avins AL, Miranda J, & Browner WS (1997). Case-finding instruments for depression. Two questionnaires are as good as many. Journal of General Internal Medicine, 12, 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KE, & Koran LM (1997). Obsessive-compulsive disorder in pregnancy, the puerperium and the premenstruum. Journal of Clinical Psychiatry, 58, 335–336. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Hanusa BH, Peindl KS, & Perel JM (2004). Prevention of postpartum episodes in women with bipolar disorder. Biological Psychiatry, 56, 592–596. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Peindl K, & Hanusa BH (1993). Relationship of psychiatric illness to childbearing status: A hospital-based epidemiologic study. Journal of Affective Disorders, 28, 39–50. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Peindl K, & Hanusa BH (1994). Symptomatology of affective and psychotic illnesses related to childbearing. Journal of Affective Disorders, 30, 77–87. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Peindl KS, & Hanusa BH (1995). Psychiatric episodes in women with young children. Journal of Affective Disorders, 34, 1–11. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Peindl KS, & Hanusa BH (1996). Effects of childbearing on the natural history of panic disorder with comorbid mood disorder. Journal of Affective Disorders, 41, 173–180. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Peindl KS, Gigliotti T, & Hanusa BH (1999). Obsessions and compulsions in women with postpartum depression. Journal of Clinical Psychiatry, 60, 176–180. [DOI] [PubMed] [Google Scholar]
- Wisner KL (2008). Perinatal disorders: Advancing public health opportunities. Journal of Clinical Psychiatry, 69, 1602–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KL, Zarin DA, Holmboe ES, Appelbaum PS, Gelenberg AJ, Leonard HL, & Frank E (2000). Risk-benefit decision making for treatment of depression during pregnancy. American Journal of Psychiatry, 157, 1933–1940. [DOI] [PubMed] [Google Scholar]
- Yawn BP, Pace W, Wollan PC, Bertram S, Kurland M, Graham D, & Dietrich A (2009). Concordance of Edinburgh Postnatal Depression Scale (EPDS) and Patient Health Questionnaire (PHQ-9) to assess increased risk of depression among postpartum women. Journal of the American Board of Family Medicine, 22, 483–491. [DOI] [PubMed] [Google Scholar]
- Zar M, Wijma K, & Wijma B (2002). Relations between anxiety disorders and fear of childbirth during late pregnancy. Clinical Psychology & Psychotherapy, 9, 122–130. [Google Scholar]