Abstract
Background:
Few data exist on the predictors of asthma remission by early adulthood in North America.
Objective:
The predictors of adult asthma remission were determined in a multiethnic population of patients with mild-to-moderate persistent childhood asthma.
Methods:
Asthma remission in early adulthood was measured by using 2 definitions: a clinical and a strict definition. Both included normal lung function and the absence of symptoms, exacerbations, and medication use. The strict definition also included normal airways responsiveness. Predictors were identified from 23 baseline measures by using multivariate logistic regression. The probability of remission was modeled by using decision tree analysis.
Results:
In 879 subjects the mean ± SD baseline age was 8.8 ± 2.1 years, 59.4% were male, and 68.7% were white. By adulthood, 229 (26.0%) of 879 participants were in clinical remission, and 111 (15.0%) of 741 participants were in strict remission. The degree of FEV1/forced vital capacity (FVC) ratio impairment was the largest predictor of asthma remission. More than half of boys and two thirds of girls with baseline FEV1/FVC ratios of 90% or greater were in remission at adulthood. Decreased airways responsiveness was also a predictor for both remission definitions (clinical remission odds ratio, 1.23 [95% CI, 1.09–1.39]; strict remission odds ratio, 1.52 [95% CI, 1.26–1.84]). The combination of normal FEV1/FVC ratio, airways responsiveness, and serum eosinophil count at baseline yielded greater than 80% probability of remission by adulthood.
Conclusion:
A considerable minority of patients with persistent childhood asthma will have disease remission by adulthood. Clinical prognostic indicators of asthma remission, including baseline lung function, can be seen from an early age.
Keywords: Pediatric asthma, asthma remission, prognostication
Graphical Abstract
Predicting the clinical trajectory of childhood asthma has important prognostic implications for patients, parents, clinicians, and researchers. Prior longitudinal studies have estimated between 15% and 64% of patients with childhood asthma go into asthma remission by early adulthood.1,2 In those studies asthma remission rates have been noted to vary widely by population, and remission has been associated with less allergic sensitization, milder initial asthma severity, and male sex.2–4 Asthma remission occurs most commonly between the ages of 14 to 21 years.2 To date, studies characterizing asthma remission have emphasized epidemiologic associations rather than clinical translation and prognostication. Furthermore, limited data exist on asthma remission in multiethnic cohorts representative of the North American population and in cohorts with mild-to-moderate persistent asthma, whose remission rates would be expected to differ from those with either intermittent or severe disease.
In this study we determined which baseline characteristics in early childhood can predict asthma remission by adulthood in a multiethnic cohort of patients with mild-to-moderate persistent asthma. Cross-sectional baseline characteristics representative of a snapshot obtained at a physician’s visit were examined that can be used for individualized prognostication.
The Childhood Asthma Management Program (CAMP) was a 4-year, randomized, placebo-controlled trial of inhaled anti-inflammatory treatments for mild-to-moderate persistent asthma in children followed by an additional 13 years of detailed longitudinal follow-up.5 As such, this cohort is well suited to evaluate the natural history of childhood asthma. After the first 8 years of CAMP, 6% of patients had clinical asthma remission in adolescence.6 We now study asthma remission at the onset of adulthood in CAMP by using definitions for asthma remission that include both clinical and pulmonary function measurements. Asthma remission was measured at the first CAMP continuation study visit after the age of 18 years, with an average follow-up of 12 years. In addition to determining the prevalence of asthma remission in early adulthood, we specifically investigated early childhood factors to prognosticate future asthma outcomes.
METHODS
A longitudinal study was performed with 879 participants from CAMP who had asthma remission outcomes available at their first follow-up visit on or after age 18 years (range, 18–23 years). The details of CAMP have been described elsewhere.5,7 Briefly, 1041 children with mild-to-moderate persistent asthma and significant airways responsiveness to methacholine between the ages of 5 and 12 years were enrolled from December 1993 to September 1995. Participants were randomized to receive inhaled budesonide, nedocromil, or placebo for a mean 4.3 years, and observational follow-up continued for an additional 13 years. Parental informed consent with subject assent was obtained, and the study was approved by each CAMP study center institutional review board. More than 80% of subjects participated through the entire duration of observational follow-up.7
Asthma remission at early adulthood was defined in 2 ways: a clinical primary definition and a strict secondary definition. Clinical asthma remission was defined as follows: (1) no evidence of airflow obstruction with a ratio of FEV1 to forced vital capacity (FVC) of 80% or greater, (2) no asthma exacerbations in the prior year, (3) no use of asthma medications in the prior year, and (4) no reported asthma symptoms in the prior year. Strict asthma remission was defined as the above 4 criteria in addition to having negative bronchoprovocation test results by means of methacholine challenge with a provocative concentration of methacholine causing a 20% drop in FEV1 (PC20) greater than or equal to 25 mg/mL. The absence of bronchial hyperresponsiveness (BHR) in CAMP was defined at a PC20 of 25 mg/mL or greater, and thus this cutoff was used for the strict asthma remission definition. FEV1/FVC ratio was included in the remission definitions to include both objective and subjective criteria from the impairment and risk domains of National Asthma Education and Prevention Program guidelines, and the use of the absolute ratio was chosen according to American Thoracic Society guidelines.8,9 The clinical remission definition was considered the primary definition because PC20 is not typically measured longitudinally in the clinical setting.
Potential baseline predictors of asthma remission were selected based on previously published associations with asthma susceptibility, remission, or both.10 Twenty-three baseline measures collected at the time of enrollment in CAMP were selected: age at asthma diagnosis, sex, ethnicity, household income, parental education, history of parental asthma, body mass index, history of wheezing (with and without colds), asthma exacerbations after exercise, physician-diagnosed atopic dermatitis or allergies, aeroallergen skin test positivity (as an indicator of allergic sensitization with or without symptoms), exposure to home tobacco smoke, history of maternal smoking during pregnancy, use of a wood stove, any pet ownership, asthma severity classified by a physician, PC20, FEV1 percent predicted, FEV1/FVC ratio, serum IgE level, and serum eosinophil count.
Statistical analyses
The characteristics of the 162 subjects missing the primary remission outcome were compared with those of the 879 subjects who had the outcome. We used t tests to compare continuous parametric variables, Wilcoxon rank-sum tests for continuous nonparametric variables, and χ2 and Fisher exact tests for categorical variables. Most variables in the loss to follow-up analysis were not statistically significant (P >.05), and significant variables were adjusted for in multivariate models (see Table E1 in this article’s Online Repository at www.jacionline.org). R version 3.3.2 was used.
Cross-validated stepwise logistic regression was performed to determine predictors for each asthma remission outcome. Variables that were statistically significant on univariate analyses were placed in a 5-fold cross-validated stepwise logistic regression model with 100 cross-validation repeats by using the caret package.11 Cross-validation was used to reduce overfitting and improve precision and accuracy. In addition to the statistically significant variables from the loss to follow-up analysis, age, sex, CAMP treatment group, and clinic site were controlled for in all models. Before model building, continuous variables that had a nonlinear relationship with the log odds of remission were log transformed. FEV1/FVC ratio and PC20 were modeled as continuous variables in the main clinical and strict asthma remission models.
Model selection was determined by using the Akaike information criterion, and Wald tests were used to evaluate individual variables. Analyses were restricted to complete cases. Models were then stratified by sex, and the statistically significant variables that differed across sexes were tested as interaction terms.
Sex-stratified analyses for the primary clinical asthma remission definition were planned a priori because asthma persistence has been shown to vary by sex.1,2,4,12 Sex-stratified models were built by using cross-validated stepwise logistic regression. Baseline FEV1/FVC ratio was divided into 4 categories to reflect spirometric categorizations used in the Global Initiative for Asthma Report (ie, <70%, 70% to 80%, 80% to 90%, and >90%) and baseline PC20 was divided into 3 categories (ie, moderate-to-severe BHR, <1 mg/mL; mild BHR, 1–4 mg/mL; and borderline BHR, >4 mg/mL) per American Thoracic Society guidelines to increase clinical interpretability in the sex-stratified models.13,14 Receiver operating characteristic curves and area under the curve (AUC) were obtained by using the pROC, plotROC, and ggplot2 packages.15–17 The probability of asthma remission was modeled by using conditional inference trees with case weights from the party package.18
A sensitivity analysis of the primary clinical asthma remission definition was performed to examine the effect of the FEV1/FVC criterion. The FEV1/FVC ratio was removed from the definition, and a cross-validated stepwise logistic regression model was built, as specified above (see Table E2 in this article’s Online Repository at www.jacionline.org). Because airflow obstruction is one of the hallmarks of asthma, the FEV1/FVC ratio was retained in the primary clinical asthma remission definition.
RESULTS
Of the 879 participants, 229 (26.0%) subjects were in asthma remission and 650 (73.9%) were not, as determined by using the primary clinical definition (Table I). The mean baseline age at study entry was 8.8 years (SD ± 2.1 years), 59.4% were male, and 68.7% were white. Within the 879 participants, 741 (84.3%) subjects had PC20 measurements, permitting analysis of the secondary strict asthma remission definition. Of these, 111 (15.0%) subjects were in strict asthma remission, and 630 (85.0%) subjects were not (see Table E3 in this article’s Online Repository at www.jacionline.org).
TABLE I.
Baseline characteristics for all participants in the study stratified by clinical asthma remission status (n = 879)
| No asthma remission (n = 650) | Asthma remission (n = 229) | P value | |
|---|---|---|---|
| Baseline age (y), mean ± SD | 8.8 ± 2.1 | 8.6 ± 1.9 | .07 |
| Age at asthma diagnosis (y), mean ± SD | 3.8 ± 2.5 | 4.1 ± 2.5 | .07 |
| Sex | .001 | ||
| Male | 407 (62.6%) | 115 (50.2%) | |
| Female | 243 (37.4%) | 114 (49.8%) | |
| Ethnicity | .90 | ||
| White | 450 (69.2%) | 154 (67.2%) | |
| Black | 89 (13.7%) | 35 (15.3%) | |
| Hispanic | 57 (8.8%) | 22 (9.6%) | |
| Other | 54 (8.3%) | 18 (7.9%) | |
| Baseline household income (USD) | .95 | ||
| <$29,999 | 140 (22.3%) | 48 (22.3%) | |
| $30,000–$49,999 | 215 (34.2%) | 71 (33.0%) | |
| ≥$50,000 | 274 (43.6%) | 96 (44.7%) | |
| Parental highest education | .38 | ||
| High school or less | 116 (17.8%) | 35 (15.3%) | |
| Some college or higher | 534 (82.2%) | 194 (84.7%) | |
| CAMP treatment group | .15 | ||
| Budesonide | 178 (27.4%) | 78 (34.1%) | |
| Nedocromil | 204 (31.4%) | 63 (27.5%) | |
| Placebo | 268 (41.2%) | 88 (38.4%) | |
| Parental history of asthma | 270 (44.3%) | 94 (45.2%) | .82 |
| Body mass index (kg/m2), mean ± SD | 18.2 ± 3.6 | 17.9 ± 3.1 | .33 |
| Wheezes with colds | 601 (92.6%) | 199 (86.9%) | .009 |
| Wheezes apart from colds | 524 (80.7%) | 168 (73.4%) | .02 |
| Exacerbations after exercise | 527 (81.1%) | 164 (71.9%) | .004 |
| Aeroallergen sensitization on skin testing | 584 (89.8%) | 190 (83.0%) | .006 |
| History of physician-diagnosed allergies | 445 (68.5%) | 152 (66.4%) | .56 |
| Exposure to home tobacco smoke | 256 (39.6%) | 97 (42.7%) | .45 |
| Baseline asthma severity | .01 | ||
| Mild | 296 (45.5%) | 117 (55.5%) | |
| Moderate | 354 (54.5%) | 102 (44.5%) | |
| Baseline PC20 (mg/mL), median ± IQR | 0.9 ± 1.6 | 1.7 ± 3.6 | <.001 |
| Baseline FEV1 (% predicted), mean ± SD | 92.2 ± 14.1 | 99.0 ± 12.7 | <.001 |
| Baseline FEV1/FVC ratio (%), mean ± SD | 77.9 ± 7.9 | 85.6 ± 6.3 | <.001 |
| Baseline serum IgE level (ng/mL), median ± IQR | 516.0 ± 1166.0 | 343.0 ± 924.5 | <.001 |
| Baseline eosinophil count (cells/mL), median ± IQR | 422.0 ± 493.5 | 320.5 ± 327.3 | <.001 |
| Atopic dermatitis | 145 (76.7%) | 44 (74.6%) | .74 |
| Mother smoked while pregnant | 90 (13.9%) | 31 (13.5%) | .88 |
| Used wood stove for heating or cooking | 67 (10.3%) | 16 (7.0%) | .14 |
| Pet ownership | 51 (7.8%) | 12 (5.2%) | .19 |
| Clinic site | .06 | ||
| Albuquerque | 77 (11.8%) | 23 (10.0%) | |
| Baltimore | 75 (11.5%) | 33 (14.4%) | |
| Boston | 73 (11.2%) | 39 (17.0%) | |
| Denver | 90 (13.8%) | 19 (8.3%) | |
| San Diego | 70 (10.8%) | 27 (11.8%) | |
| Seattle | 91 (14.0%) | 32 (14.0%) | |
| St Louis | 101 (15.5%) | 25 (10.9%) | |
| Toronto | 73 (11.2%) | 31 (13.5%) |
IQR, Interquartile range.
Clinical asthma remission predictors
For the entire cohort, sex, baseline history of wheezing with and without colds, exacerbations after exercise, aeroallergen sensitization, physician-classified asthma severity, PC20, FEV1 percent predicted, FEV1/FVC ratio, serum IgE level, and serum eosinophil count were significant predictors of asthma remission on univariate analyses (Table I). In the multivariate model only FEV1/FVC ratio and PC20 remained significant. Each 10% increase in baseline FEV1/FVC ratio was associated with a 4.62 times odds of asthma remission (95% CI, 3.35–6.50; P < .001), and each 2-fold increase in PC20 was associated with a 23% increase in the odds of asthma remission (odds ratio [OR], 1.23; 95% CI, 1.09–1.39; P = .001; Table II). The AUC of the cross-validated model was 0.81 (95% CI, 0.77–0.84; see Fig E1, A, in this article’s Online Repository at www.jacionline.org).
TABLE II.
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| 10% Increase in FEV1/FVC ratio | 4.62 | 3.35–6.50 | <.001 |
| Two-fold increase in PC20 | 1.23 | 1.09–1.39 | .001 |
Covariates included in the cross-validated model: age, sex, race, CAMP treatment group, clinic site, household income level, history of wheezing with colds, history of exacerbations after exercise, and history of exposure to home tobacco smoke.
Quantitative variables were modeled continuously.
Predictors of clinical asthma remission by sex
The severity of airflow obstruction was the strongest predictor of clinical asthma remission in both male and female subjects (Fig 1, A). Within the subset of subjects with mild baseline airway obstruction, female subjects were more likely to have asthma remission than male subjects (Fig 1, B). Female subjects had 8.03 times odds (95% CI, 4.60–14.93; P < .001), whereas male subjects had 3.88 times odds (95% CI, 2.62–5.92; P < .001) of asthma remission with each 10% increase in FEV1/FVC ratio (interaction P = .04). An FEV1/FVC ratio of less than 70% was strongly indicative of asthma persistence, particularly in male subjects, in which only 1.4% of subjects had remission (Fig 1, A).
FIG 1.
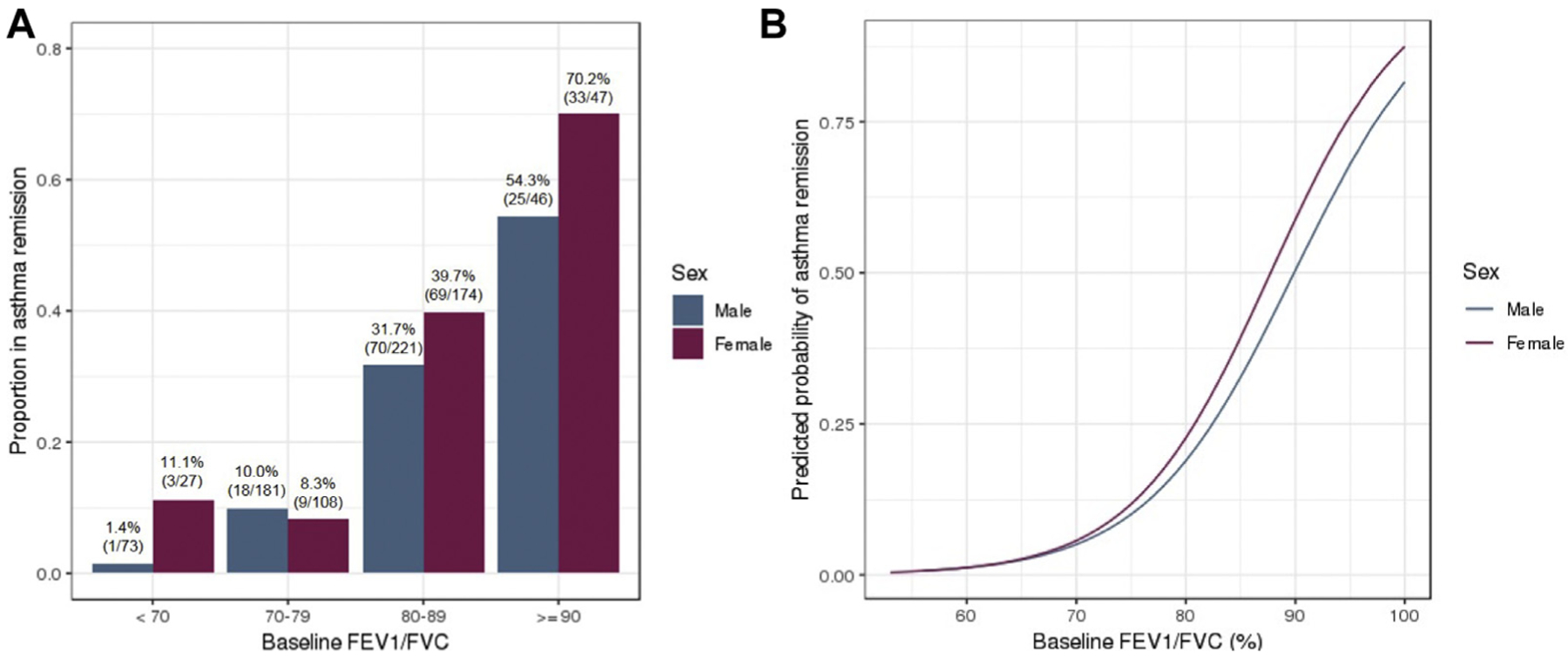
A, The proportion of patients with childhood asthma who had asthma remission in early adulthood increased as baseline FEV1/FVC ratios increased. B, Female subjects with greater baseline FEV1/FVC ratios were more likely to have remission than their male counterparts. For each 10% increase in FEV1/FVC ratio, female subjects had 8.03 times odds (95% CI, 4.60–14.93) of asthma remission, whereas male subjects had 3.88 times odds (95% CI, 2.62–5.92) of asthma remission (interaction P = .04).
In addition, in the sex-specific models of clinical asthma remission, male subjects without peripheral eosinophilia had 1.79 times odds (95% CI, 1.02–3.21; P = .045) of asthma remission compared with male subjects with peripheral eosinophilia (Table III). Treatment during the first 4 years of CAMP with inhaled budesonide was also associated with greater odds of remission in male subjects. In female subjects, Hispanic race and airway hyperresponsiveness were additional predictors of asthma remission (Table IV and see Fig E2 in this article’s Online Repository at www.jacionline.org). The odds of asthma remission decreased by 63% (OR, 0.37; 95% CI, 0.15–0.89; P = .03) in female subjects with moderate airway hyperresponsiveness and 76% (OR, 0.24; 95% CI, 0.09–0.64; P = .005) in female subjects with severe airway hyperresponsiveness. The AUC of the cross-validated models were 0.79 (95% CI, 0.75–0.84) for male subjects and 0.85 (95% CI, 0.80–0.89) for female subjects (see Fig E1, B and C).
TABLE III.
| Clinical phenotype | OR | 95% CI | P value |
|---|---|---|---|
| Serum eosinophil count <500 cells/μL | 1.79 | 1.02–3.21 | .045 |
| Inhaled budesonide treatment‡ | 2.05 | 1.11–3.83 | .02 |
| Inhaled nedocromil treatment‡ | 1.30 | 0.70–2.42 | .41 |
| FEV1/FVC ratio ≥90%§ | 73.81 | 13.07–1404.13 | <.001 |
| FEV1/FVC ratio 80% to 89%§ | 25.10 | 5.16–453.35 | .002 |
| FEV1/FVC ratio 70% to 79%§ | 6.59 | 1.28–120.93 | .07 |
Covariates included in the cross-validated model: age, race, CAMP treatment group, clinic site, household income level, history of wheezing with colds, history of exacerbations after exercise, history of exposure to home tobacco smoke, baseline asthma severity, and serum IgE level.
Quantitative variables were modeled categorically.
Reference group is placebo treatment.
Reference level is an FEV1/FVC ratio of less than 70%.
TABLE IV.
| Clinical phenotype | OR | 95% CI | P value |
|---|---|---|---|
| Hispanic race | 9.84 | 2.60–39.39 | <.001 |
| Moderate airway hyperresponsiveness (PC20 1–4 mg/mL)‡ | 0.37 | 0.15–0.89 | .03 |
| Severe airway hyperresponsiveness (PC20 <1 mg/mL)‡ | 0.24 | 0.09–0.64 | .005 |
| FEV1/FVC ≥90%§ | 54.76 | 7.56–1166.80 | <.001 |
| FEV1/FVC 80% to 89%§ | 12.13 | 2.00–239.14 | .02 |
| FEV1/FVC 70% to 79%§ | 1.44 | 0.20–29.90 | .75 |
Covariates included in the cross-validated model: age, CAMP treatment group, clinic site, household income level, history of wheezing with colds, history of exacerbations after exercise, history of exposure to home tobacco smoke, and serum IgE level.
Quantitative variables were modeled categorically.
Reference level is a PC20 of greater than 4 mg/mL.
Reference level is an FEV1/FVC ratio of less than 70%.
Strict asthma remission predictors
Similar to the primary remission analysis, a history of wheezing with and without colds, exacerbations after exercise, aeroallergen sensitization, physician-classified asthma severity, PC20, FEV1 percent predicted, FEV1/FVC ratio, serum IgE level, and serum eosinophil count were significant predictors of strict asthma remission status on univariate analyses, along with baseline age at study entry and physician diagnosed allergies (see Table III in this article’s Online Repository at www.jacionline. org). In the multivariate model each 10% increase in baseline FEV1/FVC ratio was associated with 5.71 times odds (95% CI, 3.58–9.45; P < .001) of strict asthma remission, and each 2-fold increase in baseline PC20 was associated with a 1.52 times odds (95% CI, 1.26–1.84: P < .001) of strict asthma remission (see Table E4 in this article’s Online Repository at www.jacionline.org). Furthermore, each 2-fold increase in serum IgE level was associated with a 12% decrease in the odds of strict remission (OR, 0.88; 95% CI, 0.78–1.00; P = .047), and aeroallergen sensitization was associated with a 52% decrease in the odds of strict remission (OR, 0.48; 95% CI, 0.24–0.97; P = .04). The AUC of the cross-validated model was 0.86 (95% CI, 0.83–0.90; see Fig E1, D). Predictors of strict asthma remission did not differ between sexes.
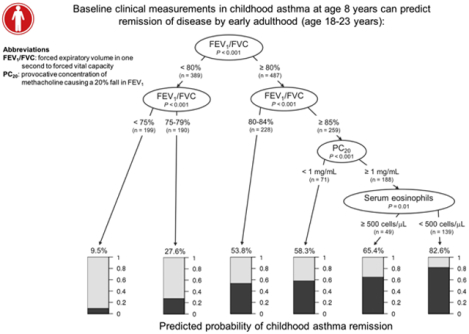
Asthma remission decision tree
A decision tree classifying the predicted probability of clinical asthma remission was constructed (Fig 2). The probability of asthma remission was lowest (9.5%) in subjects with baseline FEV1/FVC ratios of less than 75%. Conversely, subjects with the combination of a baseline FEV1/FVC ratio greater than or equal to 85%, PC20 of at least 1 mg/mL, and absence of peripheral eosinophilia had 82.6% probability of asthma remission.
FIG 2.
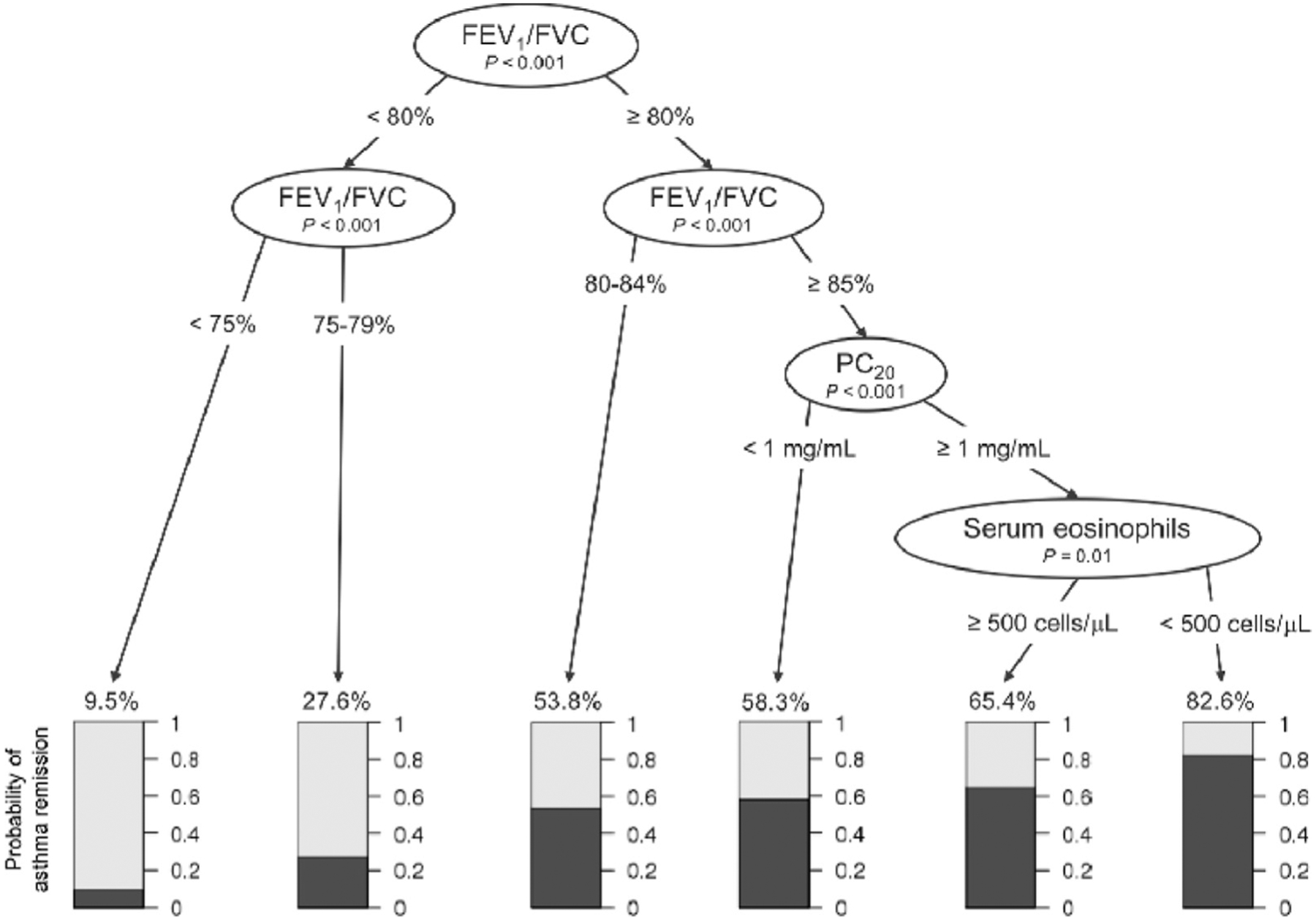
The probability of asthma remission by adulthood increases as baseline FEV1/FVC ratio increases. When the baseline FEV1/FVC ratio was at least 85%, the probability of asthma remission is dependent on baseline PC20 and serum eosinophil counts. In this subgroup the probability of asthma remission was 58.3% when the baseline PC20 was less than 1 mg/mL. When the baseline PC20 was greater than or equal to 1 mg/mL, the probability of asthma remission was 65.4% in those with peripheral eosinophilia and 82.6% in those without peripheral eosinophilia.
DISCUSSION
Prognostication of asthma remission at adulthood can be performed from an early age in childhood. We followed 879 children with mild-to-moderate persistent asthma for an average of 12 years until early adulthood and found 26% had asthma remission by focusing on clinical parameters and 15% had asthma remission by using a strict definition that additionally included normal bronchoprovocation testing. Thus a sizeable proportion of patients with childhood asthma have complete remission with no residual evidence of ongoing disease. Decreased airway obstruction and lower airways responsiveness in childhood were strongly associated with both definitions of asthma remission. Although research in other populations has demonstrated associations between greater childhood lung function, less severe airway obstruction, and absence of airway hyperresponsiveness with improved asthma prognosis, prior studies have not yielded prognostic strata related to these measures.1,4,19,20 The single greatest predictor of asthma remission was the severity of airflow obstruction. Fewer than 10% of children with a baseline FEV1/FVC ratio of less than 80% “outgrew” their asthma, whereas each 10% increase in baseline FEV1/FVC ratio was associated with a 4.62 times higher odds of asthma remission (Table II).
For asthmatic patients with well-preserved lung function at baseline (FEV1/FVC ratio, ≥90%), more than 54% of boys and 70% of girls were in remission by early adulthood (Fig 1, A). Baseline FEV1/FVC ratio is an excellent predictor of future asthma remission and lung function, which is consistent with research showing the tracking of lung function growth over time.1,21 Gains in baseline FEV1/FVC ratio beyond the threshold of 80% used in current guidelines continued to increase the probability of asthma remission, indicating that higher cutoffs within “normal” FEV1/FVC ratios continue to stratify patients with pediatric asthma (Fig 1, B).8 In the US general population the reference FEV1/FVC ratio for healthy preadolescent children approaches 90%.22 Therefore the cutoff for normal FEV1/FVC ratio in children regardless of asthma status is likely greater than indicated in current guidelines and has direct implications on future health status. Our findings further emphasize the importance of obtaining baseline spirometry in patients with pediatric asthma, which is recommended in current guidelines but not routinely obtained in all pediatric practices.8,14
From a clinical perspective, the probability of asthma remission can be estimated based on baseline FEV1/FVC ratio, PC20, and serum eosinophil count (Fig 2). Specifically, our decision tree analysis indicated that in children with mild-to-moderate persistent asthma, the predicted probability of adult clinical remission exceeds 80% when FEV1/FVC ratio is normal, PC20 is greater (lower airways responsiveness), and peripheral eosinophilia is absent at baseline. The asthma remission decision tree can be used as a clinical tool for physicians to prognosticate about patients with childhood asthma. Each of the 3 prognostic components is readily available to most pediatric care providers, and several asthma guidelines recommend measuring these components.8,14,23
In addition to these overall findings, sex-specific associations with clinical asthma remission were detected. Although sex by itself was not associated with asthma remission in our main multivariable model, we noted differences related to sex in the association of FEV1/FVC ratio with clinical remission. For each 10% increase in FEV1/FVC ratio, female subjects were more likely to have clinical asthma remission compared with male subjects (Fig 1, B). This contrasts with previous studies that have found either no association between sex and asthma remission or an association between female sex and asthma persistence.1–4,12,20,24–26
Female subjects with less severe early-onset asthma are more likely to have remission than male subjects, which suggests that early increases in FEV1/FVC ratio in female subjects that run counter to the decrements expected from dysanapsis in lung growth might disproportionately affect lung preservation.27 Within female subjects, Hispanics were more likely to have asthma remission compared with other races, which might reflect ethnic subgroup specific risks within and between races.28 In male subjects the absence of peripheral eosinophilia and use of inhaled corticosteroid treatment in early childhood (ie, during the clinical trial phase of CAMP) were associated with increased odds of asthma remission (Table III). Inhaled corticosteroid use does not alter the development of asthma in at-risk preschool children, and CAMP treatment group assignment has not been shown to affect lung growth.29–31 At this juncture, it is not clear whether early inhaled corticosteroids have the potential for a previously undescribed latent effect, and further research is needed to definitively determine the long-term effects of early inhaled corticosteroid use.
The remission of childhood asthma by adulthood has not been widely studied, and the determination of asthma remission during adolescence might not capture all events. Even fewer studies have defined asthma remission using both clinical and pulmonary function measurements.19,20,25 In The Netherlands 22% of 119 asthmatic children followed to ages 32 to 42 years were in complete remission, as defined by having no wheeze or asthma attacks in the previous 3 years, no use of inhaled corticosteroids, normal lung function (FEV1, >90% predicted), and no airway hyperresponsiveness.19 In a separate cohort from The Netherlands, Panhuysen et al20 reported 11% of 181 patients with childhood asthma to be in asthma remission at adulthood, as defined by normal lung function (FEV1, >90% predicted), absence of airway hyperresponsiveness (PC20, ≥16 mg/mL), and absence of respiratory symptoms.20 Our study had a larger multiethnic population and included more frequent follow-up and comprehensive detailing of baseline characteristics compared with prior studies.
The strict remission criteria included normal airways responsiveness and were also associated with decreases in baseline serum IgE levels and absence of aeroallergen sensitization. These results suggest that atopic inflammation can modulate airway smooth muscle physiology, resulting in persistent asthma and airways hyperresponsiveness.32 A limited number of studies have examined atopic factors as predictors of asthma remission. One study demonstrated an inverse association with allergic sensitization to animals, and a second study did not find an association between atopy and remission.3,20 Conversely, asthma persistence has been associated with aeroallergen sensitization and history of atopy.1,2,24,26 The predictors of strict asthma remission did not differ by sex, suggesting that the baseline predictors of airways hyperresponsiveness are not sex specific.
A greater proportion of subjects went into asthma remission by early adulthood in our study compared with a prior study of asthma outcomes at adolescence in this population.6 Previously, Covar et al6 found only 6% of subjects had clinical remission of asthma at adolescence based on symptoms, health care use, and medication use after the first 4 years of observational follow-up. The difference in asthma remission rates between the 2 studies is likely due to a combination of different asthma remission definitions and the continued development of asthma remission in subjects followed from late adolescence to early adulthood across the entire 13-year observational follow-up. Covar et al included the absence of local medical provider contact and school absenteeism into their asthma remission definition, whereas we did not include these criteria because their causes are not specific to asthma. Our definitions used criteria specific for asthma remission and thus might have captured subjects who were previously labeled as patients with persistent asthma by Covar et al.
Currently, there is no consensus on the definition of asthma remission. Pulmonary function measurements are used to diagnose asthma and are obtained during follow-up for clinical monitoring per guidelines; hence pulmonary function measurements should bear some relevance in determining remission.8,14 When FEV1/FVC ratio was removed from the clinical asthma remission definition on sensitivity analysis, sex, baseline asthma severity, and serum IgE level became additional predictors of remission (see Table E2). These additional predictors are likely a proxy for future lung function because sex, disease severity, and allergic sensitization are associated with changes in FEV1/FVC ratios.33 Spirometric measurements were included in our asthma remission definitions to ensure accurate classification between persistent and remitted asthma. The primary clinical remission definition reflects current clinical guidelines and practices in which serial methacholine challenge tests are not routinely performed. However, methacholine challenge is the definitive test for airway hyperresponsiveness and was therefore included in the strict definition.
This study had several unique strengths. The CAMP population was ethnically diverse, and more than 84% of the 1041 subjects had longitudinal follow-up. The detailed long-term follow-up from the CAMP study minimized information bias, and we controlled for variables associated with loss to follow-up to reduce selection bias. Severe asthma in childhood is uncommon. This study included only patients with mild-to-moderate persistent childhood asthma, and therefore our results might not be generalizable to patients with mild intermittent or severe childhood asthma. The cutoff for normal airways responsiveness was 25 mg/mL in CAMP, and more participants would have met strict remission criteria had a less conservative cutoff been used. This study examined asthma remission at early adulthood. CAMP subjects were followed until a mean age of 26 years, and the conclusion of CAMP precluded follow-up of asthma outcomes later in life.
In summary, in a multiethnic clinical trial population from North America, a considerable minority (15% to 26%) of children with mild-to-moderate persistent asthma outgrew their disease by early adulthood. Our asthma remission definitions used clinical measurements readily available in most patients and can be applied to future studies and clinical settings. The clinical trajectory of patients with childhood asthma can be predicted by using baseline FEV1/FVC ratio, PC20, and serum eosinophil measurements. Moderate-to-severe decreases in FEV1/FVC ratio during early childhood asthma, whether because of increased disease severity or abnormal lung development, imply lifelong persistence of disease. In contrast, the majority of patients with childhood asthma with well-preserved lung function will have remission by adulthood. Our results also suggest that the increases in FEV1/FVC ratio above the current normal threshold of 80% are informative in patients with childhood asthma, and further characterization of FEV1/FVC ratio above this threshold is meaningful for clinical prognostication. Together, these findings strongly support the use of baseline spirometry in childhood asthma as an easily measurable and important prognostic component.
Supplementary Material
Clinical implications:
Lung function measurements and serum eosinophil counts obtained in early childhood predict asthma remission in early adulthood. Spirometry should be performed in all patients with childhood asthma for clinical prognostication.
Acknowledgments
Supported by National Institutes of Health (NIH) grants T32 AI007306, T32 HL007427, R01 HL127332, R01 HL129935, and U01 HL65899. The Childhood Asthma Management Program (CAMP) trial and CAMP Continuation Study were supported by contracts N01-HR-16044, 16045, 16046, 16047, 16048, 16049, 16050, 16051, and 16052 with the National Heart, Lung, and Blood Institute and General Clinical Research Center grants M01RR00051, M01RR0099718-24, M01RR02719-14, and RR00036 from the National Center for Research Resources. The CAMP Continuation Study/Phases 2 and 3 were supported by grants U01HL075232, U01HL075407, U01HL075408, U01HL075409, U01HL075415, U01HL075416, U01HL075417, U01HL075419, and U01HL075420 from the National Heart, Lung, and Blood Institute. The National Jewish Health site was also supported in part by Colorado CTSA grant UL1RR025780 from the National Center for Research Resources/NIH and UL1TR000154 from National Center for Advancing Translational Sciences/NIH.
Abbreviations used
- AUC
Area under the curve
- BHR
Bronchial hyperresponsiveness
- CAMP
Childhood Asthma Management Program
- FVC
Forced vital capacity
- OR
Odds ratio
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor R, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med 2003;349:1414–22. [DOI] [PubMed] [Google Scholar]
- 2.Tai A, Tran H, Roberts M, Clarke N, Gibson AM, Vidmar S, et al. Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol 2014;133: 1572–8.e3. [DOI] [PubMed] [Google Scholar]
- 3.Andersson M, Hedman L, Bjerg A, Forsberg B, Lundback B, Ronmark E. Remission and persistence of asthma followed from 7 to 19 years of age. Pediatrics 2013; 132:e435–42. [DOI] [PubMed] [Google Scholar]
- 4.Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet 2008;372: 1058–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long-term effects of budesonide or nedocromil in children with asthma The Childhood Asthma Management Program Research Group; N Engl J Med 2000; 343:1054–63. [DOI] [PubMed] [Google Scholar]
- 6.Covar RA, Strunk R, Zeiger RS, Wilson LA, Liu AH, Weiss S, et al. Predictors of remitting, periodic, and persistent childhood asthma. J Allergy Clin Immunol 2010; 125:359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Childhood Asthma Management Program (CAMP): design, rationale, and methods Childhood Asthma Management Program Research Group; Control Clin Trials 1999;20:91–120. [PubMed] [Google Scholar]
- 8.Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol 2007;120(suppl):S94–138. [DOI] [PubMed] [Google Scholar]
- 9.Culver BH, Graham BL, Coates AL, Wanger J, Berry CE, Clarke PK, et al. Recommendations for a standardized pulmonary function report. An Official American Thoracic Society Technical Statement. Am J Respir Crit Care Med 2017;196: 1463–72. [DOI] [PubMed] [Google Scholar]
- 10.Sears MR. Predicting asthma outcomes. J Allergy Clin Immunol 2015;136:829–36. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn M Building predictive models in R using the caret package. J Stat Softw 2008;28:1–26.27774042 [Google Scholar]
- 12.Roorda RJ, Gerritsen J, Van Aalderen WM, Schouten JP, Veltman JC, Weiss ST, et al. Risk factors for the persistence of respiratory symptoms in childhood asthma. Am Rev Respir Dis 1993;148:1490–5. [DOI] [PubMed] [Google Scholar]
- 13.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 2000;161:309–29. [DOI] [PubMed] [Google Scholar]
- 14.Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. Available at: http://ginasthma.org/. Accessed November 26, 2018. [Google Scholar]
- 15.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J- C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sachs MC. plotROC: A tool for plotting ROC curves. J Stat Softw 2017;79: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag; 2009. [Google Scholar]
- 18.Hothorn T, Hornik K, Aeileis A. Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat 2006;15:651–74. [Google Scholar]
- 19.Vonk JM, Postma DS, Boezen HM, Grol MH, Schouten JP, Koeter GH, et al. Childhood factors associated with asthma remission after 30 year follow up. Thorax 2004;59:925–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panhuysen CI, Vonk JM, Koeter GH, Schouten JP, van Altena R, Bleecker ER, et al. Adult patients may outgrow their asthma: a 25-year follow-up study. Am J Respir Crit Care Med 1997;155:1267–72. [DOI] [PubMed] [Google Scholar]
- 21.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med 2016;374:1842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hankinson JL, Odencrantz JR., Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159: 179–87. [DOI] [PubMed] [Google Scholar]
- 23.National Institute for Health and Care Exellence guideline. Asthma: diagnosis, monitoring and chronic asthma management. 2017. Available at: https://www.nice.org.uk/guidance/ng80#. Accessed January 10, 2018. [Google Scholar]
- 24.Burgess JA, Matheson MC, Gurrin LC, Byrnes GB, Adams KS, Wharton CL, et al. Factors influencing asthma remission: a longitudinal study from childhood to middle age. Thorax 2011;66:508–13. [DOI] [PubMed] [Google Scholar]
- 25.Carpaij OA, Nieuwenhuis MAE, Koppelman GH, van den Berge M, Postma DS, Vonk JM. Childhood factors associated with complete and clinical asthma remission at 25 and 49 years. Eur Respir J 2017;49. [DOI] [PubMed] [Google Scholar]
- 26.Blair H Natural history of childhood asthma. 20-year follow-up. Arch Dis Child 1977;52:613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couriel JM, Child F. Applied physiology: lung function tests in children. Curr Paediatr 2006;16:413–9. [Google Scholar]
- 28.Rosser FJ, Forno E, Cooper PJ, Celedon JC. Asthma in Hispanics. An 8-year update. Am J Respir Crit Care Med 2014;189:1316–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strunk RC, Sternberg AL, Szefler SJ, Zeiger RS, Bender B, Tonascia J. Long-term budesonide or nedocromil treatment, once discontinued, does not alter the course of mild to moderate asthma in children and adolescents. J Pediatr 2009;154:682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bisgaard H, Hermansen MN, Loland L, Halkjaer LB, Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med 2006; 354:1998–2005. [DOI] [PubMed] [Google Scholar]
- 31.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med 2006;354:1985–97. [DOI] [PubMed] [Google Scholar]
- 32.Grunstein MM, Hakonarson H, Hodinka RL, Maskeri N, Kim C, Chuang S. Mechanism of cooperative effects of rhinovirus and atopic sensitization on airway responsiveness. Am J Physiol Lung Cell Mol Physiol 2001;280:L229–38. [DOI] [PubMed] [Google Scholar]
- 33.Nagarajan S, Ahmad S, Quinn M, Agrawal S, Manilich E, Concepcion E, et al. Allergic sensitization and clinical outcomes in urban children with asthma, 2013–2016. Allergy Asthma Proc 2018;39:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


