Abstract
In women with human epidermal growth factor 2 (HER2)-positive breast cancer, the improved control of systemic disease with new therapies has unmasked brain metastases that historically would have remained clinically silent. The efficacy of therapeutic agents against brain metastases is limited by their inability to permeate the blood–brain and blood–tumor barriers (BBB and BTB) in therapeutic amounts. Here, we investigate the potential of mucic acid-based, targeted nanoparticles designed to transcytose the BBB/BTB to deliver a small molecule drug, camptothecin (CPT), and therapeutic antibody, Herceptin, to brain metastases in mice. Treatment with BBB-targeted combination CPT/Herceptin nanoparticles significantly inhibits tumor growth compared to free CPT/Herceptin and BBB-targeted nanoparticles carrying CPT alone. Though not as efficacious, BBB-targeted nanoparticles carrying only Herceptin also elicit considerable antitumor activity. These results demonstrate the potential of the targeted nanoparticle system for the delivery of an antibody alone or in combination with other drugs across the BBB/BTB to improve the therapeutic outcome.
Keywords: polymeric nanoparticle, blood–brain barrier, systemic delivery, Herceptin, camptothecin
Brain metastases of breast cancer are presenting an increasing challenge in the clinic. Historically, brain metastases were not a major problem for most breast cancer patients because they usually developed late in the course of the disease, and a lack of systemic control limited survival.1,2 However, new therapies have improved clinical outcomes in some subsets of patients, and brain progression has become a more significant threat to long-term survival.3,4
The risk of brain metastasis varies considerably with the breast cancer subtype.5,6 Human epidermal growth factor 2 (HER2)-positive breast cancers have been shown to metastasize to the brain at higher rates than other breast cancer subtypes (ca. 25–50%).5,7,8 Although HER2-targeted therapies can effectively control extracranial disease, they have a limited distribution to brain metastases and demonstrate a poor efficacy in this setting.9-11 Current therapeutic options such as surgery, radiation, and chemotherapy are considered palliative and rarely provide a significant increase in survival.6,12-14
The delivery of HER2-inhibitors and most chemotherapeutics to brain metastases is limited by poor drug penetration across the blood–brain barrier (BBB),13,14 a selective cellular barrier that acts as a regulator for the movement of molecules into and out of the brain.15 The tumor microvasculature associated with brain metastases, often referred to as the blood–tumor barrier (BTB), has increased passive permeability relative to the intact BBB;16 however, the loss in barrier integrity is limited and highly variable from tumor to tumor and even within the metastatic lesion.16-18 Many drugs commonly used to treat HER2-positive breast cancer are unable to reach therapeutic concentrations in the brain,10,16 and circumventing the BBB and BTB remains a major obstacle in the effective treatment of brain metastases.
There has been significant interest in engineering nanoparticles and other nanoscale or polymeric drug formulations to enhance the delivery of therapeutic agents to the brain following systemic administration.13,19-22 The use of endogenous transport mechanisms at the BBB such as receptor-mediated transcytosis (RMT) has emerged as a promising approach to shuttle a variety of payloads across the BBB.23-25 In particular, transferrin receptor (TfR) has been one of the primary targets investigated for RMT because of its high expression on the blood side of the BBB endothelium.26 Recently, we investigated the brain uptake and efficacy of TfR-targeted therapeutic nanoparticles designed to transcytose the BBB/BTB. Transferrin (Tf) was attached to nanoparticles consisting of a mucic acid polymer (MAP) conjugate of camptothecin (CPT), denoted MAP-CPT, through a pH-dependent, boronic acid-diol complexation to the vicinal diols contained within the mucic acid portions of the polymer.27 With this acid-cleavable targeting strategy,28 nanoparticles retain high avidity to TfR on the blood side of the BBB to enable practical, systemic dosing, yet release the targeting agents upon acidification during transcytosis to allow their release into the brain. We demonstrated that these targeted nanoparticles, administered systemically, were capable of delivering CPT to HER2-positive breast cancer brain metastases in mice and eliciting a considerable antitumor response.27
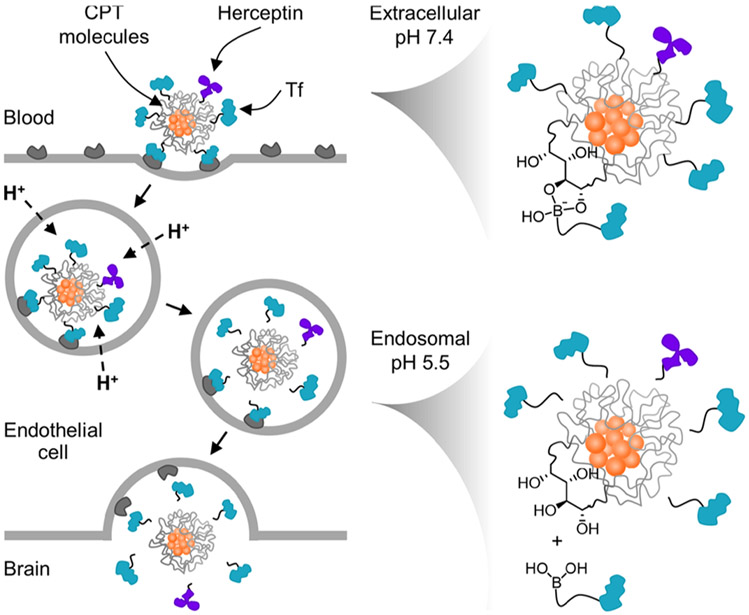
We hypothesized that TfR-targeted nanoparticles carrying more potent therapeutic agents would reveal even greater tumor size reductions.27 Here, we assess whether this delivery system can be used to shuttle an anti-HER2 monoclonal antibody, Herceptin, alone or in combination with a CPT payload, across the BBB to achieve enhanced antitumor activity over the previously reported efficacy of CPT alone (Figure 1).
Figure 1.
Proposed mechanism for the delivery of the drug and antibody combination to brain metastases using acid-cleavable targeting ligands. At extracellular pH 7.4, Tf ligands and Herceptin remain bound to the diols on the nanoparticle surface. After endocytosis, rapid acidification of the endosome to pH 5.5 triggers their dissociation from the nanoparticle core, allowing free diffusion into the brain once transcytosis is complete.
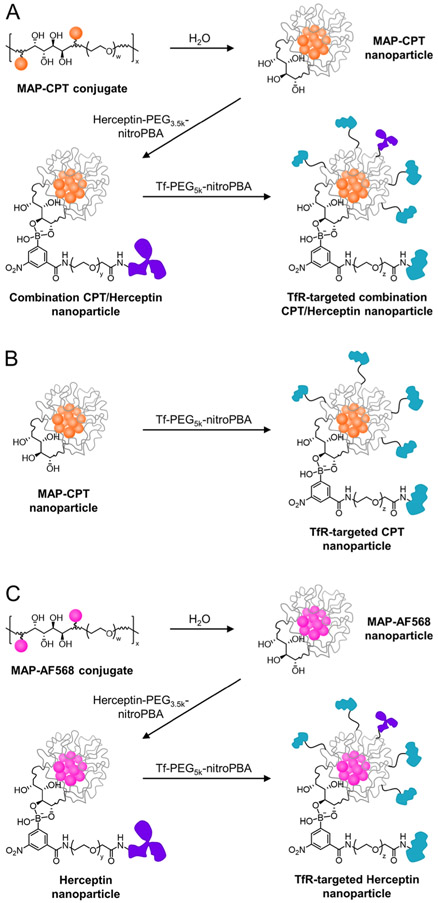
The MAP-CPT polymer–drug conjugate was prepared as previously described (Figure S1).27 Properties of the material used in this study are provided in Table S1. The MAP-CPT conjugate was then dialyzed against water to form nanoparticles with hydrophobic CPT molecules preferentially clustered in the core and mucic acid diols on the surface (Figure 2A).
Figure 2.
Single-agent or combination drug and antibody nanoparticle delivery systems. Preparation of the TfR-targeted combination CPT/Herceptin nanoparticle (A), TfR-targeted CPT nanoparticle (B), and TfR-targeted Herceptin nanoparticle (C) formulations. w ~ 82 for 3.4 kDa PEG; x ~ 20 for material used in this study; y ~ 84 for 3.5 kDa PEG; z ~ 120 for 5 kDa PEG.
3-Carboxy-5-nitrophenyl boronic acid (nitroPBA)-Herceptin and Tf conjugates were synthesized in a manner similar to that reported previously.27 Briefly, nitroPBA was added to 3.5 kDa polyethylene glycol (PEG), followed by conjugation of the polymer to Herceptin using EDC/NHS chemistry (Figure S2A). A Tf-containing analogue was prepared using 5 kDa PEG (Figure S2B). As described previously, the nitroPBA boronic acid derivative was chosen because of its high binding constant and low pKa (ca. 6.8) values with MAP.27,29 As a result, the nitroPBA conjugates form stable boronic acid esters with the nanoparticle in circulation, but quickly dissociate from the nanoparticle at pH < 6.8 to provide ligand detachment during transcytosis.
To assemble TfR-targeted combination CPT/Herceptin nanoparticles, the Herceptin-PEG3.5k-nitroPBA conjugate was added to the MAP-CPT nanoparticles at a 1:1 molar ratio, followed by Tf-PEG5k-nitroPBA at a 20 molar excess in PBS, pH 7.4 (Figure 2A). To compare the antitumor activity of nanoparticles containing only CPT, Tf-PEG5k-nitroPBA was directly added to the MAP-CPT nanoparticles at a 20 molar excess (Figure 2B). A Herceptin only nanoparticle control was prepared by conjugating a hydrophobic fluorophore (Alexa Fluor 568, AF568) lacking antitumor activity to the MAP polymer to promote the formation of nanoparticles upon dialysis in water (Figure S3). Herceptin-PEG3.5k-nitroPBA and Tf-PEG5k-nitroPBA conjugates were added as above to the MAP-AF568 nanoparticles at 1:1 and 20:1 molar ratios, respectively, to form TfR-targeted Herceptin nanoparticles (Figure 2C). Nanoparticles containing Herceptin were purposefully formulated with an average of one antibody per nanoparticle. Numerous antibodies can be added to the nanoparticles. However, adding just one allowed us to test the “worst-case scenario” for delivering an antibody to the brain. If brain delivery and antitumor activity are observed, it is likely that an even better efficacy will be achievable with nanoparticles containing multiple antibodies.
Nanoparticle diameter and zeta potential measurements were performed on the above formulations to verify that the nanoparticles had properties appropriate for transcytosis from systemic administrations28,30 as well as diffusion through brain tissue,31 namely, a sub-100 nm diameter and negative-nearneutral zeta potential. All three nanoparticle formulations had diameters between 30 and 40 nm, as measured by dynamic light scattering, and negative-near-neutral zeta potentials when measured in pH 7.4 buffer (Table S2).
The breast cancer brain metastasis model was established by intracardiac (ICD) injection of HER2-positive BT474-Gluc cells into Rag2−/−;Il2rg−/− mice. In previous work, we have shown that the method used to form brain tumors in mice can dramatically affect the efficacy of therapeutics and their brain penetration.27 We observed a marked antitumor response and brain accumulation of free CPT, a non-BBB-penetrant small molecule, and a nontargeted nanoparticle-containing CPT in tumors that were established by stereotaxic intracranial injection. In contrast, treatment with the nanoparticles lacking Tf to enable transcytosis gave no antitumor response in both the ICD model and a third model we developed involving the intravenous injection of the cancer cells that more closely replicated the metastasis process in patients. The ICD model, however, did allow CPT to penetrate and have a small antitumor effect, while our new model did not. Here, the ICD model was chosen because it appears to have an impermeable BBB/BTB to larger nanoparticle entities and will allow comparison to other studies that have employed this method of creating brain metastases.
To assess how the incorporation of the therapeutic antibody may affect the efficacy of the targeted nanoparticles, we investigated the antitumor activity of TfR-targeted combination CPT/Herceptin nanoparticles compared to TfR-targeted CPT nanoparticles, TfR-targeted Herceptin nanoparticles, and combined free CPT and Herceptin in the ICD model. A saline treatment group was used as the control. Treatment was initiated when tumors reached 2 mm3 in volume. Figure S4A shows a representative MRI image of the metastatic tumors at the start of treatment. The different formulations were systemically administered weekly for 4 weeks at a dose of 4 and/or 24 mg/kg (CPT and/or Herceptin bases, respectively), and the tumor volume was measured weekly by MRI for 8 weeks.
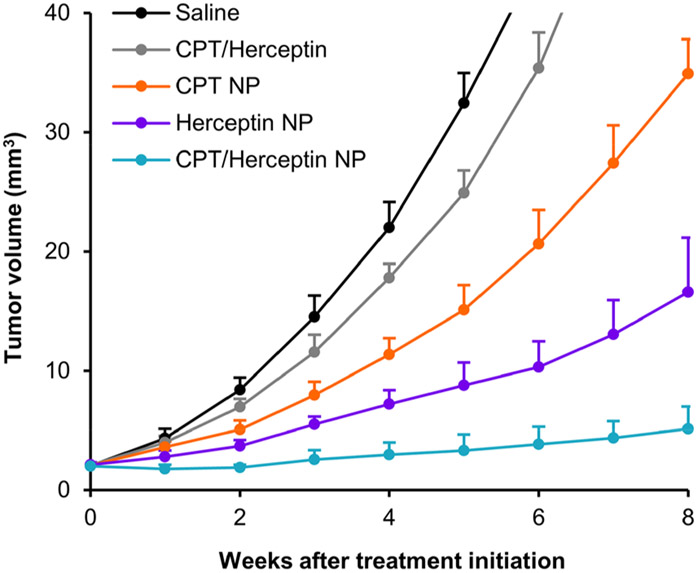
Figure 3 shows the results of treating mice bearing brain tumors with the formulations described above. Data from individual animals are provided in Figure S5. For the physical mixture of CPT and Herceptin, the tumor growth delay is not significantly different than previously observed for CPT alone.27 These results suggest that Herceptin is not penetrating the BBB/BTB to an extent to produce any antitumor activity and are consistent with data published for Herceptin alone.32
Figure 3.
Combined nanoparticle delivery of CPT and Herceptin inhibits brain metastatic tumor growth more effectively than nanoparticle delivery of either monotherapy and combined free drug. Tumor growth curves of BT474-Gluc metastatic brain tumors treated with free CPT and Herceptin (gray, 4 and 24 mg/kg, respectively), TfR-targeted CPT nanoparticles (orange, 4 mg CPT/kg), TfR-targeted Herceptin nanoparticles containing Alexa Fluor 568 (purple, 24 mg Herceptin/kg), and TfR-targeted combination CPT/Herceptin nanoparticles (blue, 4 mg CPT/kg and 24 mg Herceptin/kg) compared to saline (black). Data shown are the average of 6 mice per treatment group. Error bars indicate SE. P values for pairwise comparisons are provided in Table S3. NP, nanoparticle. Animals dosed on weeks 0, 1, 2, and 3.
The tumor growth delay from treatment with TfR-targeted CPT nanoparticles is as we observed previously27 and shows the excellent reproducibility of both the model and the nanoparticle synthesis. Compared to previous data demonstrating no tumor growth delay with nanoparticles lacking Tf, these results suggest that the antitumor effects observed for this treatment are facilitated by the targeted nanoparticle delivery of CPT alone.
TfR-targeted Herceptin nanoparticles give a greater antitumor response than those containing CPT, suggesting that the nanoparticles can deliver functional antibodies into the brain via transcytosis. It is also encouraging that significant antitumor activity can be achieved when only one antibody is on each nanoparticle. Future studies will explore variable amounts of antibody contents.
When both Herceptin and CPT are combined in a TfR-targeted nanoparticle, the best antitumor response is observed (compared to the data from Herceptin alone or CPT alone), and the antitumor effects appear to be quite durable (final dosing was on week 3). Notably, the type of formulation for the combination (free drug vs nanoparticle) greatly affected the outcome of the brain metastases, as shown in Figure 3. MRI images further illustrate the differences between the tumors after treatment with the above formulations (Figure S4). These results suggest that both the CPT and Herceptin are delivered to the brain via transcytosis of the nanoparticle and indicate that combination therapies will be possible with this type of delivery system.
In summary, TfR-targeted nanoparticles containing either the antibody Herceptin alone or in combination with the small molecule drug CPT can deliver their payloads to intracranial breast cancer tumors to provide significant antitumor activity. These results show not only that functional antibodies can be delivered to the brain but also that they can be used in combination with other drugs to provide enhanced antitumor activity. This initial study was performed with a single dose amount and a single dosing schedule. The dosing amount used here is well below what is possible with the nanoparticles and was selected in order to have a proper comparison to free CPT administered near the maximum tolerated dose. Therefore, further studies with increasing dosing amounts and alternative dosing schedules are merited. Importantly, these results also open new possibilities for delivering therapeutic combinations to treat brain metastases as well as other brain diseases.
Supplementary Material
ACKNOWLEDGMENTS
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program (DGE-1745301; to E.A.W.). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. This work was supported by the National Cancer Institute Grant (CA 151819).
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.molpharmaceut.9b01167
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.molpharmaceut.9b01167.
Detailed experimental procedures, including materials synthesis, nanoparticle formulation, antitumor efficacy, and statistical analysis (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Cairncross JG; Kim JH; Posner JB Radiation therapy for brain metastases. Ann. Neurol 1980, 7, 529–541. [DOI] [PubMed] [Google Scholar]
- (2).Barnholtz-Sloan JS; Sloan AE; Davis FG; Vigneau FD; Lai P; Sawaya RE Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol 2004, 22, 2865–2872. [DOI] [PubMed] [Google Scholar]
- (3).Crivellari D; Pagani O; Veronesi A; Lombardi D; Nolè F; Thürlimann B; Hess D; Borner M; Bauer J; Martinelli G; et al. High incidence of central nervous system involvement in patients with metastatic or locally advanced breast cancer treated with epirubicin and docetaxel. Ann. Oncol 2001, 12, 353–356. [DOI] [PubMed] [Google Scholar]
- (4).Lin NU; Amiri-Kordestani L; Palmieri D; Liewehr DJ; Steeg PS CNS metastases in breast cancer: old challenge, new frontiers. Clin. Cancer Res 2013, 19, 6404–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Kennecke H; Yerushalmi R; Woods R; Cheang MC; Voduc D; Speers CH; Nielsen TO; Gelmon K Metastatic behavior of breast cancer subtypes. J. Clin. Oncol 2010, 28, 3271–3277. [DOI] [PubMed] [Google Scholar]
- (6).Rostami R; Mittal S; Rostami P; Tavassoli F; Jabbari B Brain metastasis in breast cancer: a comprehensive literature review. J. Neuro-Oncol 2016, 127, 407–414. [DOI] [PubMed] [Google Scholar]
- (7).Lin NU; Winer EP Brain metastases: the HER2 paradigm. Clin. Cancer Res 2007, 13, 1648–1655. [DOI] [PubMed] [Google Scholar]
- (8).Aversa C; Rossi V; Geuna E; Martinello R; Milani A; Redana S; Valabrega G; Aglietta M; Montemurro F Metastatic breast cancer sybtypes and central nervous system metastases. Breast 2014, 23, 623–628. [DOI] [PubMed] [Google Scholar]
- (9).Taskar KS; Rudraraju V; Mittapali RK; Samala R; Throsheim HR; Lockman J; Gril B; Hua E; Palmieri D; Polli JW; et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm. Res 2012, 29, 770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Morikawa A; Peereboom DM; Thorsheim HR; Samala R; Baylan R; Murphy CG; Lockman PR; Simmons A; Weil RJ; Tabar V; et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: a prospective study. Neuro. Oncol 2015, 17, 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Bohn KA; Adkins CE; Mittapali RK; Terrell-Hall TB; Mohammad AS; Shah N; Dolan EL; Nounou MI; Lockman PR Semi-automated rapid quantification of brain vessel density using fluorescent microscopy. J. Neurosci. Methods 2016, 270, 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Oehrlich NE; Spineli LM; Papendorf F; Park-Simon TW Clinical outcome of brain metastases differs significantly among breast cancer subtypes. Oncol. Lett 2017, 14, 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Shah N; Mohammad AS; Saralkar P; Sprowls SA; Vickers SD; John D; Tallman RM; Lucke-Wold BP; Jarrell KE; Pinti M; et al. Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacol. Res 2018, 132, 47–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ramakrishna N; Temin S; Chandarlapaty S; Crews JR; Davidson NE; Esteva FJ; Giordano SH; Gonzalez-Angulo AM; Kirshner JJ; Krop I; et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol 2014, 32, 2100–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Pardridge WM The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lockman PR; Mittapalli RK; Taskar KS; Rudraraju V; Gril B; Bohn KA; Adkins CE; Roberts A; Thorsheim HR; Gaasch JA; et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res 2010, 16, 5664–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Mittapalli RK; Adkins CE; Bohn KA; Mohammad AS; Lockman JA; Lockman PR Quantitative fluorescence microscopy measures vascular pore size in primary and metastatic brain tumors. Cancer Res. 2017, 77, 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Osswald M; Blaes J; Liao Y; Solecki G; Gömmel M; Berghoff AS; Salphati L; Wallin JJ; Phillips HS; Wick W; et al. Impact of blood-brain barrier integrity on tumor growth and therapy response in brain metastases. Clin. Cancer Res 2016, 22, 6078–6087. [DOI] [PubMed] [Google Scholar]
- (19).Mehta AI; Brufsky AM; Sampson JH Therapeutic approaches for HER2-positive brain metastases: circumventing the blood-brain barrier. Cancer Treat. Rev 2013, 39, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Mittapalli RK; Liu X; Adkins CE; Nounou MI; Bohn KA; Terrell TB; Qhattal HS; Geldenhuys WJ; Palmieri D; Steeg PS; et al. Paclitaxel-hyaluronic nanoconjugates prolong overall survival in a preclinical brain metastases of breast cancer model. Mol. Cancer Ther 2013, 12, 2389–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Adkins CE; Nounou MI; Hye T; Mohammad AS; Terrell-Hall T; Mohan NK; Eldon MA; Hoch U; Lockman PR NKTR-102 efficacy versus irinotecan in a mouse model of brain metastases of breast cancer. BMC Cancer 2015, 15, 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Mohammad AS; Griffith JI; Adkins CE; Shah N; Sechrest E; Dolan EL; Terrell-Hall TB; Hendriks BS; Lee H; Lockman PR Liposomal irinotecan accumulates in metastatic lesions, crosses the blood-tumor barrier (BTB), and prolongs survival in an experimental model of brain metastases of triple negative breast cancer. Pharm. Res. 2018, 35 (2), 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Pardridge WM Drug targeting to the brain. Pharm. Res 2007, 24, 1733–1744. [DOI] [PubMed] [Google Scholar]
- (24).Chen Y; Liu L Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Delivery Rev 2012, 64, 640–665. [DOI] [PubMed] [Google Scholar]
- (25).Pardridge WM Delivery of biologics across the blood-brain barrier with molecular trojan horse technology. BioDrugs 2017, 31, 503–519. [DOI] [PubMed] [Google Scholar]
- (26).Uchida Y; Ohtsuki S; Katsukura Y; Ikeda C; Suzuki T; Kamiie J; Terasaki T Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J. Neurochem 2011, 117, 333–345. [DOI] [PubMed] [Google Scholar]
- (27).Wyatt EA; Davis ME Method of establishing breast cancer brain metastases affects brain uptake and efficacy of targeted, therapeutic nanoparticles. Bioeng. Tranls. Med 2019, 4, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Clark AJ; Davis ME Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc. Natl. Acad. Sci. U. S. A 2015, 112, 12486–12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Han H; Davis ME Targeted nanoparticles assembled via complexation of boronic-acid-containing targeting moieties to diol-containing polymers. Bioconjugate Chem. 2013, 24, 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Wiley DT; Webster P; Gale A; Davis ME Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc. Natl. Acad. Sci. U. S. A 2013, 110, 8662–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Nance EA; Woodworth GF; Sailor KA; Shih TY; Xu Q; Swaminathan G; Xiang D; Eberhart C; Hanes J A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci. Transl Med. 2012, 4, 149ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Terrell-Hall TB; Nounou MI; El-Amrawy F; Griffith JIG; Lockman PR Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer. Oncotarget 2017, 8, 83734–83744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.