Abstract
Background
Access to combination antiretroviral therapy has turned HIV into a chronic and manageable disease for many. This increased chronicity has been mirrored by increased prevalence of health‐related challenges experienced by people living with HIV (Rusch 2004). Exercise is a key strategy for people living with HIV and by rehabilitation professionals to address these disablements; however, knowledge about the effects of exercise among adults living with HIV still is emerging.
Objectives
To examine the safety and effectiveness of aerobic exercise interventions on immunologic and virologic, cardiopulmonary, psychologic outcomes and strength, weight, and body composition in adults living with HIV.
Search methods
Searches of MEDLINE, EMBASE, SCIENCE CITATION INDEX, CINAHL, HEALTHSTAR, PsycINFO, SPORTDISCUS and Cochrane Review Group Databases were conducted between 1980 and June 2009. Searches of published and unpublished abstracts and proceedings from major international and national HIV/AIDS conferences were conducted, as well as a handsearch of reference lists and tables of contents of relevant journals and books.
Selection criteria
We included studies of randomised controlled trials (RCTs) comparing aerobic exercise interventions with no aerobic exercise interventions or another exercise or treatment modality, performed at least three times per week for at least four weeks among adults (18 years of age or older) living with HIV.
Data collection and analysis
Data on study design, participants, interventions, outcomes, and methodological quality were abstracted from included studies by two reviewers. Meta‐analyses, using RevMan 5 computer software, were performed on outcomes when possible.
Main results
A total of 14 studies met inclusion criteria for this review and 30 meta‐analyses over several updates were performed. Main results indicated that performing constant or interval aerobic exercise, or a combination of constant aerobic exercise and progressive resistive exercise for at least 20 minutes at least three times per week for at least five weeks appears to be safe and may lead to significant improvements in selected outcomes of cardiopulmonary fitness (maximum oxygen consumption), body composition (leg muscle area, percent body fat), and psychological status (depression‐dejection symptoms). These findings are limited to participants who continued to exercise and for whom there were adequate follow‐up data.
Authors' conclusions
Aerobic exercise appears to be safe and may be beneficial for adults living with HIV. These findings are limited by the small sample sizes and large withdrawal rates described in the studies. Future research would benefit from participant follow‐up and intention‐to‐treat analysis. Further research is required to determine the optimal parameters in which aerobic exercise may be most beneficial for adults living with HIV.
Plain language summary
Aerobic exercise for adults living with HIV/AIDS
Performing aerobic exercise or a combination of aerobic exercise and resistive exercise for at least 20 minutes, at least three times per week for at least five weeks appears to be safe and may improve fitness, body composition, and well‐being for adults living with HIV.
Exercise is used by many people living with HIV to improve fitness, well‐being, and body image. Exercise also is used as a strategy to diminish the health‐related consequences of HIV and associated treatments. This review of 14 trials found that performing constant or interval aerobic exercise, or a combination of constant aerobic exercise and progressive resistive exercise, for at least 20 minutes, three times per week for at least five weeks appears to be safe and may be able to improve fitness, body composition, and well‐being for adults living with HIV. More high‐quality studies are needed to better evaluate the evidence on under‐represented groups, such as women and older adults living with HIV, and those who discontinue their exercise programs.
Background
The profile of HIV infection has changed over time. Although HIV infection once was viewed as an illness progressing in a predictable way toward death, access to combination antiretroviral therapy has turned it into a chronic and manageable disease for many. This increased chronicity has been mirrored by increased prevalence of disablement among people living with HIV (Rusch 2004), such as impairments (problems with body function or structure, such as pain or fatigue), activity limitations (difficulties in executing activities, such as inability to walk) and participation restrictions (problems in life situations, such as inability to work) due to HIV, its sequelae, and potential side effects of combination antiretroviral therapy (World Health Organization 2001). Exercise is a key strategy employed by people living with HIV and by rehabilitation professionals to address these issues.
Exercise has been shown to improve strength, cardiovascular function, and psychological status in general populations (Bouchard 1993), but knowledge about the benefits and risks of exercise for adults living with HIV is still emerging. What are the appropriate parameters for frequency, intensity, duration, and type of exercise? At which stage of HIV infection will exercise be most beneficial, least beneficial, or even harmful? What is the spectrum of outcomes that may result from exercise in this population?
If the risks and benefits of exercise for people living with HIV are better understood, appropriate exercise may be undertaken by people in this population and appropriate exercise prescription may be practiced by healthcare providers. Effective and safe exercise may enhance the effectiveness of HIV management, thus improving overall health outcomes for adults living with HIV.
Objectives
To examine the safety and effectiveness of aerobic exercise interventions on immunologic and virologic, cardiopulmonary, strength, weight, body composition, and psychological parameters in adults living with HIV.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing aerobic exercise with no aerobic exercise or another exercise or treatment modality performed at least three times per week for at least four weeks (American College of Sports Medicine 2010; Pollock 1998).
Types of participants
We included studies of adults (18 years of age and older) living with HIV. Studies of men only, women only, or both at all stages of infection, with and without lipodystrophy, were included. Although the title of the review refers to "adults living with HIV/AIDS" as the target population, we used the terminology "adults living with HIV" in this update to collectively represent any adult living with HIV at any stage of illness (including AIDS). This terminology increasingly is preferred because AIDS has become less common among people living with HIV (Managing Your Health 2009;UNAIDS 2008).
Types of interventions
We defined aerobic exercise as a regimen containing aerobic interventions performed at least three times per week for at least four weeks. Aerobic interventions included but were not limited to walking, jogging, cycling, rowing, stair stepping, and swimming. Interventions may or may not have been supervised. Our parameters for aerobic exercise inclusion were based on the American College of Sports Medicine Guidelines (American College of Sports Medicine 2010; Pollock 1998).
Types of outcome measures
During the original planning of this review, we met with experts, such as physical therapists, physicians, and methodologists, and people living with HIV. Our discussions concluded that the three best outcome parameters for this review were immunologic and virologic indicators, cardiopulmonary measures and psychologic measures. In this most recent update, we expanded our outcomes to include strength, weight, and body composition, which have become increasingly important with the advent of combination antiretroviral therapy and the emergence of lipodystrophy. The recent literature has increasingly considered these outcomes when evaluating the combination of aerobic and resistive exercise.
Immunologic and virologic indicators considered in this review included but were not limited to CD4 count (cells/mm3) and viral load (log10copies).
Cardiopulmonary measures considered in this review included but were not limited to maximal oxygen consumption (V02max) (mL/kg/min), oxygen pulse (02pulse), maximum heart rate (HRmax, beats/min), maximum tidal volume (maxTv), forced expiratory volume (FEV), minute ventilation (VE), lactic acid threshold (LAT), maximum work rate (Work Rate Max), fatigue (time on treadmill), and dyspnea (rate of perceived exertion).
Strength measures considered for this review included but were not limited to strength (maximum amount of weight able to resist in kilograms).
Weight measures considered for this review included but were not limited to change in body weight (kg).
Body composition measures considered for this review included but were not limited to body mass index (kg/m2), lean body mass (kg), girth (cm), percent body fat (%), skin folds (subcutaneous fat), and cross‐sectional muscle area (mm2). For the purpose of this review, we defined body composition broadly as any outcome that contributes to the direct or indirect measurement of muscle, fat, bone, or other tissues of the body.
Psychological measures considered in this review included general measures of psychological status and health‐related quality of life (HRQL) used in studies with people living with HIV. These included but were not limited to the Montgomery‐Asberg Scale for Depression, General Health Questionnaire, Quality of Life Questionnaire, SF‐20, Medical Outcomes Study‐HIV (MOS‐HIV), Short Form 36 (SF‐36), Short Form 12 (SF‐12), Short Form 21 (SF‐21), Quality of Well‐Being Scale, Q‐TWIST, Quality of Life Index, EuroQol questionnaire, Sickness Impact Profile, Profile of Mood State Scale (POMS), Beck Depression Inventory (BDI), and health utility assessment.
Safety measures considered in this review included but were not limited to injuries, immune suppression (decline in CD4 count), hospitalizations, and mortality.
Search methods for identification of studies
In our three updates of this review, we searched the following databases from 1980 ‐ June 2009: MEDLINE, EMBASE, SCIENCE CITATION INDEX, CINAHL, HEALTHSTAR, PsycINFO and SPORTDISCUS. We reviewed both published and unpublished abstracts and proceedings from major international and national HIV/AIDS conferences, such as the Intersciences Conference on Antimicrobial Agents and Chemotherapy (ICAAC), the Conference on Retroviruses and Opportunistic Infections (CROI), the Infectious Diseases Society of America Conference (IDSA), and the International AIDS Conference (IAC). Reference lists from pertinent articles and books were reviewed and personal contacts with authors were also used, as well as CochraneReview Group databases. Targeted journals were also handsearched for relevant articles. All languages were included.
Three arms of the search strategy were developed and intersected using the Boolean term 'AND': i) HIV subject headings (exploded): HIV, HIV Infections, HIV long term survivors Text words: HIV, human immunodeficiency virus, aids, acquired immune deficiency syndrome, acquired immunodeficiency syndrome. ii) Exercise subject headings (exploded): exercise, exertion, physical fitness, sports, physical education, aerobic exercises, cycling, walking, aerobics, Tae‐Bo, water aerobics, running, and training Text words: aerobic, exercise iii) Study search criteria subject headings: randomised controlled trials, random allocation, double‐blind method, single blind method, clinical trials (explode), placebos, research design, comparative study, evaluation studies (explode), follow‐up studies, prospective studies, cross‐over studies, intervention studies. Publication types: randomised controlled trial, controlled clinical trial, clinical trial. Text words: (clinic‐ trial‐), ((singl‐ or doubl‐ or tripl‐ or trebl) adj (mask‐ or blind‐)), placebo‐, random‐, latin square, control‐, prospectiv‐, volunteer‐. For some of the databases and updates, slight modification of this strategy was required.
Data collection and analysis
All abstracts retrieved from the search were reviewed independently by two reviewers (KO and AMT) who applied the following four inclusion criteria to determine if the abstract warranted further investigation:
a) Did the study include human participants who were HIV positive? b) Did the study include adults 18 years of age or older? c) Did the study include an aerobic exercise intervention performed at least three times/week for at least four weeks? d) Was there a randomised controlled comparison group?
When the review based on the abstract alone (or title and keywords if no abstract was available from the electronic search) indicated that one or both raters believed the study met eligibility criteria (i.e. if reviewers answered "yes" or "unsure" to the four questions) then hard copies of entire papers were independently reviewed by the two reviewers. In instances where there was a lack of agreement by the two reviewers, a third reviewer was asked to review the full paper to determine final inclusion. Full text was not examined in instances where both raters agreed the study did not meet the inclusion criteria. From the final group of studies that met inclusion criteria, at least two reviewers reviewed each article independently to determine final article inclusion.
From the final group of included studies, data were abstracted onto standard data abstraction forms independently by at least two reviewers (KO and AMT). Abstracted data included the study citation, study objectives, study design, length of study, time at which participants were assessed, inclusion and exclusion criteria for participants, characteristics of included participants (i.e. age, gender, stage of disease), description of intervention(s) (i.e. frequency, intensity, duration, type, level of supervision), types of outcome variables assessed and their values at baseline and study completion, and number of participants at baseline and study completion (including number of withdrawals). Methodological quality of the studies was also abstracted using criteria suggested by Jadad 1996, along with whether groups were similar at baseline. Instead of generating a formal methodological score, reviewers provided a description of the methodological quality of the studies as follows:
a) Was the study described as randomised? If yes, describe the method of randomization. b) Was the study described as double‐blinded? If yes, describe how double‐blinding was obtained. c) Was there a description of withdrawals or drop‐outs? d) Were the groups similar at baseline?
The reviewers met to achieve consensus regarding any difference in data interpretation or abstraction from included studies that arose during the review process.
Outcomes were analyzed both as continuous outcomes and as dichotomous/binary outcomes whenever possible. Where there were sufficient data available in the studies, when similar or comparable outcome measures were used, and when participant comparison groups were similar, meta‐analyses were performed using the random‐effects model for outcomes whenever possible. In the case of missing data, authors were contacted in an attempt to obtain further information when required.
Standard statistical techniques included: (1) For continuous outcomes the weighted mean difference (WMD) and 95% confidence intervals for the means were calculated whenever possible. (2) For dichotomous/binary outcomes the odds ratio, absolute difference in odds, relative risk (RR), risk difference (RD), and the number needed to treat (NNT) and 95% confidence intervals were calculated whenever possible.
A P value of less than 0.05 indicated statistical significance for overall effect and a P value of less than 0.1 indicated statistical significance for heterogeneity between studies (Lau 1997). In instances of lack of statistical significance for an overall effect, confidence intervals were assessed for potential trends that may suggest movement towards an increase or decrease in overall effect. In instances of statistical significance for heterogeneity, we performed sensitivity analyses and explained potential reasons for heterogeneity.
Subgroup analyses Subgroup analyses were performed whenever possible to estimate whether aerobic exercise interventions were associated with differences among groups using identified outcome measures. Possible subgroup analyses included supervised versus non‐supervised exercise, interval versus constant exercise, males versus females, older versus younger participants, participants with HIV wasting versus participants without HIV wasting, and participants on antiretroviral therapy versus participants not on antiretroviral therapy.
Sensitivity analyses were performed to determine whether particular studies skewed results. In situations where outliers were omitted or inclusion criteria for studies varied, sensitivity analyses were performed as needed to determine the robustness of findings. Exploratory analyses were performed based on the results of the review. Individual study design and procedures were taken into account to determine whether biases manifested. Potential biases in studies may have included selection bias (systematic differences in the comparison groups), performance bias (systematic differences in the care provided apart from the intervention being evaluated), exclusion bias (systematic differences in withdrawals from the trial), and detection bias (systematic differences in outcome assessment). Reviewers also assessed whether or not an "intention‐to‐treat" analysis was performed for participants lost to follow‐up.
Results
Description of studies
Searches of all sources of the original review retrieved a total of 487 citations, eight of which were judged to merit scrutiny of the full article. Of the eight full studies reviewed, six met inclusion criteria LaPerriere 1990, MacArthur 1993, Perna 1999, Rigsby 1992, Stringer 1998, Terry 1999.) (Table 6). One citation reported the long‐term data for the same study participants (LaPerriere 1991) and thus was excluded from the review and considered a duplication of an earlier study. Another study was not a randomised controlled trial and was of such poor quality that it had to be excluded (Mustafa 1999). (See table of characteristics of excluded studies and table of characteristics of duplicate studies).
1. Characteristics of Duplicate Studies.
| Study | Methods | Participants | Interventions | Outcomes | Notes |
| LaPerriere 1991 | Randomized exercise and control groups | 39 gay males age 18‐40, not previously diagnosed with HIV. EXCLUSION CRITERIA: if routinely active with regular aerobic exercise. | INTERVENTION GROUP: Stationary bike 45 minutes total @ 70‐80% HRmax x 3 minutes then @ <70% HRmax X 2 min (2 min warm‐up @ <70% HRmax prior to intervention.) 3 x /week frequency for 10 weeks. INTERVAL AEROBIC | IMMUNE INDICES: Seropositive exercisers showed an increase of CD4 count of 115 cells/mm3. CARDIOPULMONARY OUTCOMES: Both seropositive and seronegative exercisers showed an increase in level of aerobic capacity. No significant changes detected in V02 max values. PSYCHOLOGICAL OUTCOMES: Anxiety and depression associated with notification of HIV status appeared attenuated by aerobic exercise training (seropositive and seronegative exerciser's scores were similar post‐notification of HIV status AUTHOR'S CONCLUSIONS:aerobic exercise training is beneficial as a stress management tool in HIV disease. Moderate aerobic exercise increases CD4 cells in a 10 week period. | Article identified in initial review. Need to further investigate possibility of acute aerobic exercise‐associated 'strain' on immune function. Need to explore effects of different exercise intensities to determine an intensity of maximum efficiency. Need to explore alternative modes of exercise that may provide additional benefits. Need to investigate aerobic exercise training concurrently with the use of medications. SPECIAL NOTE: LaPerriere 1991 is a continuation of the study reported in LaPerriere 1990. Thus, results are used in combination to avoid skewed results. In particular, data are drawn from LaPerriere 1990 for meta‐analysis. |
| Lox 1996 | Randomized to two exercise groups (PRE and aerobic) and one control group | 34 participants at baseline (all males). At baseline: 12 participants in PRE group, 12 participants in the aerobic exercise group, and 10 participants in the non‐exercising control group. At study completion: 12 participants in the PRE group, 11 participants in the aerobic group, and 10 in the non‐exercising control group. | INTERVENTION GROUP (AEROBIC): Stationary bike, 45 minutes total: 5 minutes warm‐up (stretching), 24 minutes cycle ergometer at 50‐60% heart rate reserve (HRR), 15 minutes cool‐down, frequency 3X per week for 12 weeks. Supervised exercise CONSTANT AEROBIC | CARDIOPULMONARY OUTCOMES: Significant improvements in VO2max among exercisers compared to non‐exercisers with greater improvements in the aerobic compared to .
BODY COMPOSITION AND WEIGHT: Significant increase in body weight and girth compared to the non‐exercising control group. No significant differences in body fat, fat weight and mean body mass index between groups. PSYCHOLOGICAL OUTCOMES: Significant improvements in mood and life satisfaction in the aerobic exercise group compared to non‐exercising controls.
STRENGTH: Significant increases in upper and lower extremities in the PRE group compared to the non‐exercising control AUTHOR'S CONCLUSIONS: Exercise results in improvements in body composition, strength, cardiopulmonary fitness, and mood and life satisfaction for HIV‐infected individuals. CARDIOPULMONARY OUTCOMES: Significant improvements in VO2max among exercisers compared to non‐exercisers. PSYCHOLOGICAL OUTCOMES: Significant improvements in mood and life satisfaction in the aerobic exercise group compared to non‐exercising controls. AUTHOR'S CONCLUSIONS: Exercise results in improvements in body composition, strength, cardiopulmonary fitness, and mood and life satisfaction for HIV‐infected individuals. |
Article identified in second update of review. Identical article to Lox 1995 but looked at different outcomes and included 3 comparison groups. For the purposes of this review, only the exercise and control group were included in meta‐analyses. |
| Neidig 2003 | Randomized exercise and control groups | 60 HIV infected adults (52 males, 8 females) INCLUSION CRITERIA: Stable ARV therapy. EXCLUSION CRITERIA: Those taking steroids, GH, or appetite stimulants, presence of an AIDS‐defining illness, fever, active wasting or weight less than 85% of ideal body weight and pregnancy. INTERVENTION GROUP: 30 participants at baseline (19 participants at 12 weeks). NON‐ EXERCISING CONTROL GROUP: 30 participants at baseline and 12 weeks. Non‐Exercising Control participants were offered exercise intervention at 12‐24 weeks. At study completion: 18 participants in the exercise group and 30 in the non‐exercising control group. (12 withdrawals all from the exercise group). Note that this is one additional participant reported who withdrew compared to Smith 2001. | INTERVENTION GROUP: Minimum of 30 minutes constant aerobic exercise at 60‐80% V02 max consisting of mandatory 20 minutes walking/jogging on treadmill and remaining time spent either on stationary bicycle, stair stepper or cross‐country machine. Exercise 3x per week frequency for 12 weeks. Supervised exercise. CONSTANT AEROBIC | PSYCHOLOGICAL OUTCOMES: Significant improvements in Centre for Epidemiological Studies Depression Scale (CED‐DS), Profile of Mood State and Depression‐dejection subscale of POMS scale, and non‐significant trend to improvement in Beck Depression Inventory in the exercise group compared to non‐exercising control group. AUTHOR'S CONCLUSIONS: Exercise results in improvements in body composition, strength, cardiopulmonary fitness, and mood and life satisfaction for HIV‐infected individuals. | Article identified in second update of review. Identical to Smith 2001 article but looked at psychological outcomes. |
| Fairfield 2001 | Randomized exercise and control groups | 54 HIV infected men with AIDS‐related wasting at baseline. INTERVENTION GROUP: (exercise + placebo): 10 participants at study completion NON‐EXERCISING CONTROL GROUP: (no exercise + placebo): 12 participants at study completion | INTERVENTION GROUP: Supervised progressive strength training and constant aerobic conditioning consisting of 20 minutes aerobic exercise on stationary cycle at 60‐70% HRmax, 15min cool‐down followed by resistance training. 3X per week frequency for 12 weeks. CONSTANT AEROBIC + PRE | BODY COMPOSITION: Increase in thigh muscle attenuation with PRE along and testosterone alone. The greatest increase was found in the combined PRE and testosterone group. ADVERSE EVENTS: No deaths were reported among participants during the study. AUTHOR'S CONCLUSIONS: PRE and testosterone increase thigh muscle attenuation in people living with HIV/AIDS. | Article identified in second update of review. Identical article to Grinspoon 2000. |
| Driscoll 2004b | Randomized combined exercise and metformin and metformin‐only group | 37 HIV infected positive men and women on stable antiretroviral therapy for more than 3 months with evidence of fat redistribution. INTERVENTION GROUP: (exercise + metformin): 11 participants at study completion NON‐EXERCISING METFORMIN ONLY GROUP: (no exercise + metformin): 14 participants at study completion. | INTERVENTION GROUP: Constant aerobic followed by resistive training consisting of 20 minutes aerobic exercise on stationary cycle at 60% HRmax (week 1‐2) and progressing to 30 minutes at 75% HRmax (week 3‐12), 5min warm‐up on stationary bike, standard flexibility routine, followed by resistance training. Frequency 3X per week frequency for 12 weeks. CONSTANT AEROBIC + PRE | BODY COMPOSITION: Significant increases in cross‐sectional muscle area among the exercise and metformin group compared with the metformin only group. Significant decrease in waist‐to‐hip ratio and abdominal fat area in the exercise and metformin group compared with the metformin only group. No significant changes in weight or body mass index. AUTHOR'S CONCLUSIONS: Combined aerobic and PRE training with metformin improves muscle cross‐sectional area compared with metformin only suggesting reduction in muscle adiposity. | Article identified in third update of review. Identical article to Driscoll 2004a. This study reported primarily on body composition outcomes. |
| Mutimura 2008b | Randomized exercise and control groups | 100 HIV positive men and women between 21‐50 years of age, with moderate to severe body fat redistribution (BFR), on a stable World Health Organizations HAART regimen for > 6 months. INTERVENTION GROUP: (aerobic exercise): 50 participants at baseline and 48 participants at study completion. NON‐EXERCISING CONTROL GROUP: 50 participants at baseline and 49 participants at study completion. | INTERVENTION GROUP (Aerobic Exercise): Six month supervised exercise programme at a fitness club in Kigali, Rwanda. Aerobic Exercise: 'proper warm up', stretching, and 15 minutes of brisk walking, followed by 45‐60 minutes of jogging, running, stair climbing, low‐back and abdominal stabilization and strengthening exercises, followed by a 15 minute cool down and stretching exercises. Intensity: Gradual progression to encourage participants to perform jogging and running with the goal of achieving at least 45% maximum heart rate (Weeks 1‐3), 60% maximum heart rate (Weeks 3‐8), and 75% maximum heart rate (Weeks 8‐24). Duration and Frequency: 3X per week, 1.5 hour per session, alternating days for 24 weeks (6 months). NON‐EXERCISING CONTROL GROUP: No intervention. CONSTANT AEROBIC | IMMUNE INDICES: No significant changes in CD4 count between groups (no measure of viral load). CARDIOPULMONARY OUTCOMES: Exercise group achieved a higher heart rate and rate of perceived exertion (RPE) at the end of the 20 metre multi‐stage shuttle run test (20mMST). Significant improvements in VO2max among exercisers compared with non‐exercisers as measured by the 20mMST. STRENGTH OUTCOMES: Not measured. BODY COMPOSITION OUTCOMES: Significant decrease in body mass index (BMI), percent body fat mas (BFM), waist circumference, and waist‐to‐hip ratio among exercisers whereas these outcomes remained unchanged or increased among non‐exercisers. Significant decrease in percent body fat mass (%) and total body fat redistribution score (BFR) among exercisers compared with non‐exercisers. Significant decrease in triceps, biceps, subscapular, suprailiac, and sum of skinfold thickness decreased more in the exercisers compared with non‐exercisers. No change in hip circumference in either group. ADVERSE EVENTS: Adverse events within the study were not reported. AUTHOR'S CONCLUSIONS: Exercise training positively improves body composition, cardiorespiratory fitness in HAART‐treated HIV+ African participants with Body Fat Redistribution. Results imply that exercise training is a safe, inexpensive, practical and effective treatment for evolving metabolic and cardiovascular syndromes associated with HIV and HAART exposure in resource‐limited settings such as Su‐Saharan Africa. | Article identified in third update of review. Identical article to Mutimura 2008a. This study reported primarily on body composition outcomes. |
In the first update of the review, the literature search from July 1999 to January 2001 retrieved a total of 459 citations, 17 of which were judged to merit scrutiny of the full article. Of the 17 studies, two met the inclusion criteria (Grinspoon 2000 and Smith 2001) and were included in the first update of the original review (see table of characteristics of included studies Table 6 ).
In the second update, the literature search from February 2001 to August 2003 retrieved a total of 219 citations, 13 of which were judged to merit scrutiny of the full article. Of the 13 studies reviewed, five met the inclusion criteria (Lox 1995, Lox 1996, Fairfield 2001, Baigis 2002, Neidig 2003) for this review. Of these, three were duplicate studies (Fairfield 2001 used the same participants as Grinspoon 2000, Lox 1996 used the same participants as Lox 1995, and Neidig 2003 used the same participants as Smith 2001). In these instances, earlier published studies were included in the review, and any additional outcomes reported in the later studies were incorporated into the review (see table of characteristics of duplicate studies).
For this third update, the literature search from September 2003 to June 2009 retrieved a total of 1611 citations, 49 of which were judged to merit scrutiny of the full article. Of the 49 studies reviewed, six met the inclusion criteria (Driscoll 2004a and 2004b, Terry 2006, Dolan 2006, Mutimura 2008a and 2008b) for this review. Of these, two were duplicate studies (Driscoll 2004b used the same participants as Driscoll 2004a, and Mutimura 2008b used the same participants as Mutimura 2008a). In these instances, earlier published studies were included in the review, and any additional outcomes reported in the later studies were incorporated into the review (see table of characteristics of duplicate studies).Thus, a total of 14 studies were included in this overall systematic review update. Studies excluded were review articles, letters, or editorials which therefore did not meet the inclusion criteria. Other studies were excluded because they did not include aerobic exercise interventions, did not include a comparison group, or, as mentioned above, were duplications of previously published studies (see table of characteristics of excluded studies).
DESIGN OF INCLUDED STUDIES All 14 included studies were randomised controlled trials. Nine studies included a non‐exercising control group LaPerriere 1990Lox 1995, Perna 1999, Grinspoon 2000, Smith 2001, Stringer 1998, Baigis 2002, Dolan 2006, Mutimura 2008a). One of the studies included two additional study groups: exercise plus injection of 200 mg of testosterone enanthate per week, and a testosterone‐only group (Grinspoon 2000). These two comparison groups were not included in the analysis for this review. One study included a non‐exercising counselling group (exercise vs. counselling group) (Rigsby 1992); one study included a progressive resistive exercise (PRE) group (Lox 1995 and Lox 1996); one study compared combined aerobic exercise and low lipid diet with low lipid diet only (Terry 2006); one study compared combined aerobic and PRE and metformin with metformin only (Driscoll 2004a); and two studies had comparison groups that compared heavy with moderate exercise (MacArthur 1993 and Terry 1999). In 10 studies (Perna 1999; Rigsby 1992, Grinspoon 2000, Smith 2001, Lox 1995, Baigis 2002, Dolan 2006, Driscoll 2004a, Terry 2006, Mutimura 2008a), exercise was described as supervised and in the remaining four studies, the level of supervision was not stated.
PARTICIPANTS OF INCLUDED STUDIES A total of 454 participants were included in the review. Participants included adults living with HIV at various stages of HIV infection, with CD4 counts ranging from <100 cells/mm3 to greater than 1000 cells/mm3. Studies included both men and women, with women comprising approximately 30% of the total number of participants. The age of the participants ranged from 18 to 58 years. Six studies included participants who were on a combination antiretroviral regimen, defined as a combination of three or more different HIV medications. (Grinspoon 2000, Smith 2001, Terry, 2006, Driscoll 2004a, Dolan 2006, Mutimura 2008a). Five studies included participants who were not on combination antiretroviral therapy; however, most if not all participants were taking some form of antiretroviral medications (Lox 1995, Perna 1999, Terry 1999, Grinspoon 2000 and Baigis 2002), and three studies did not report on whether participants were taking antiretroviral therapy (LaPerriere 1990, Rigsby 1992 and Terry 1999). Four studies specifically included participants with evidence of lipodystrophy or body fat redistribution (Driscoll 2004a, Dolan 2006, Terry 2006, Mutimura 2008a); one study included participants with AIDS‐related wasting (Grinspoon 2000); two studies specifically excluded participants with active wasting or weight loss (LaPerriere 1990, Smith 2001); and the other seven studies did not specify weight or body composition within their inclusion or exclusion criteria (Baigis 2002, Lox 1995, MacArthur 1993, Perna 1999, Rigsby 1992, Stringer 1998, Terry 1999). Other personal characteristics were reported inconsistently across studies.
OUTCOMES OF INCLUDED STUDIES All 14 of the included studies assessed immunologic or virologic outcomes, or both, in the form of CD4 count (all 14 studies) or viral load (Stringer 1998, Grinspoon 2000, Smith 2001, Dolan 2006, Driscoll 2004a, Terry 2006). Thirteen of the 14 included studies assessed cardiopulmonary outcomes (LaPerriere 1990, Rigsby 1992, MacArthur 1993, Stringer 1998, Perna 1999, Terry 1999, Smith 2001, Lox 1995, Baigis 2002, Dolan 2006, Driscoll 2004a, Terry 2006, Mutimura 2008a). Six of the 14 included studies assessed strength outcomes (Dolan 2006, Driscoll 2004a, Grinspoon 2000, Lox 1995, Perna 1999, Rigsby 1992). Nine of the 14 included studies assessed weight and body composition outcomes (Dolan 2006, Driscoll 2004a, Grinspoon 2000, Lox 1995, Perna 1999, Smith 2001, Terry 1999, Terry 2006, Mutimura 2008a). Nine of the 14 included studies assessed psychologic outcomes in the form of anxiety and depression, health status, depression, mood and life satisfaction, and health‐related quality of life (LaPerriere 1990, MacArthur 1993, Stringer 1998, Perna 1999, Terry 1999, Smith 2001, Lox 1995, Baigis 2002, Mutimura 2008a). Adverse events were reported in three of the 14 studies (Rigsby 1992, Perna 1999, Dolan 2006).
Other reported outcomes, which were not the focus of this review included the Ohio State University HIV symptoms checklist (Smith 2001), four‐day food diary (Smith 2001), dietary intake (Terry 2006, Dolan 2006), metabolic outcomes (e.g. triglycerides and cholesterol) (Terry 2006, Mutimura 2008a), insulin (Driscoll 2004a, Mutimura 2008a), and hormone and lipid levels (Grinspoon 2000).
Risk of bias in included studies
Each reviewer assessed the methodologic quality of the included studies based on the following criteria derived from the Jadad 1996 checklist (#1‐3) and assessed whether the groups were similar at baseline (#4). This criterion is used by the Cochrane Review Group on HIV Infection and AIDS. Instead of a formal score, a description of the methodologic quality based on the four criteria is provided below.
a) Was the study randomized? If yes, was the randomization process described?
All 14 included studies reported that they used randomization to allocate participants to a comparison group. However, only five of the studies described the process (Terry 2006, Driscoll 2004a, Stringer 1998, Grinspoon 2000, Baigis 2002). Stringer 1998 reported that they used a computer‐generated randomization process. Grinspoon 2000 used a permuted‐block algorithm with blocks of eight. Baigis 2002 reported using block randomization while stratifying for gender, and study identifier numbers were randomized and placed in sequentially numbered opaque envelopes. Terry 2006 reported using randomized blocks of four participants. Driscoll 2004a reported using a permuted‐block algorithm.
b) Was the study double‐blinded? If yes, how was the double‐blinding obtained?
Allocation to a study group was single‐blind for elements of two studies (MacArthur 1993, Smith 2001). In MacArthur 1993, participants were not told whether they had been randomized to the low‐intensity group or the high‐intensity group. In Smith 2001, all assessors of the graded exercise test were blinded to the participants' group assignment except for the principal investigator. For all other included studies, blinding was not specified. c) Was there a description of withdrawals and/or drop‐outs?
Thirteen of the 14 included studies made reference to participants who withdrew from or were non‐adherent (or non‐compliant) with the study (Terry 1999; Stringer 1998; Rigsby 1992; Perna 1999; MacArthur 1993; Smith 2001; Grinspoon 2000, Lox 1995, Baigis 2002, Driscoll 2004a, Terry 2006, Dolan 2006, Mutimura 2008a). Withdrawal rates were not reported by LaPerriere 1990. Rates of withdrawal and a description of reasons for withdrawal (when provided) are described below. The withdrawal rate in Terry 1999 was 32% (10/31). The reasons for withdrawal were described as lack of interest, lack of time, and economic and family issues.
The withdrawal rate in Stringer 1998 was 24% (8/34). Reasons for withdrawal included transportation difficulties, conflicting work schedules, lack of motivation to exercise, and parole violation. No subjects reported dropping out due to illness. Participants who withdrew were equally distributed among the comparison groups (Stringer 1998).
The withdrawal rate in Rigsby 1992 was 35% (13/37). Reasons for withdrawal included "health reasons" among both exercise and non‐exercising counselling groups. Withdrawals reported within the exercise group were due to relocation of residence and withdrawals from the non‐exercising counselling group were due to lack of interest. One participant in the counselling group died during this study; however, the death was not attributed to exercise.
The withdrawal or lack of adherence rate in Perna 1999 was 51% (22/43). Reasons included geographic relocation, time limitations, starting school or employment, hospitalization, loss of interest, and dissatisfaction in being in the non‐exercising control group. The report of illness as a reason for missing exercise sessions occurred with similar frequency among both compliant and non‐compliant exercisers. There was no difference in withdrawal rates with respect to age, body composition, race, fitness level, or CD4 count at baseline for compliant and non‐compliant exercisers.
The withdrawal and lack of adherence rate in MacArthur 1993 was 76% (19/25). Reasons for non‐adherence included illness, transportation difficulties, and reports that the exercise interventions were too difficult.
The withdrawal rate in Smith 2001 was 18% (11/60). All withdrawals were from the exercise group and reasons for withdrawal included employment, changes in schedule, transportation difficulties, family crises, and the time required for participation. No participant withdrew from this study due to illness.
The withdrawal rate in Grinspoon 2000 was 15% (4/26). No participants withdrew from this study due to an adverse event, and the rates of withdrawal were similar among groups.
The withdrawal rate in Lox 1995 was 4% (1/22) (note that the withdrawal rate was calculated based on the aerobic and non‐exercising control groups only, and not the PRE group). The one participant withdrew due to persistent infection.
The withdrawal rate in Baigis 2002 was 44% (54/123). Reasons for withdrawal included work schedules, hospitalizations, geographical move from area, or loss of interest. There were significantly more women and African Americans who withdrew from the study and significantly more smokers than non‐smokers who completed the exercise program.
The withdrawal rate in Driscoll 2004a was 32% (12/37). Reasons for withdrawal included elevations in resting lactic acid above prespecified safety parameters, personal issues, living too far away, and unwillingness to comply with exercise.
The withdrawal rate in Dolan 2006 was 5% (2/40). Reasons for withdrawal included unwillingness to complete the study.
The withdrawal rate in Terry 2006 was 28% (12/42). Reasons for withdrawal included lack of interest, lack of time, and economic or family problems. One of the participants was not included in the analysis because he/she did not adhere to the antiretroviral regimen.
The withdrawal rate in Mutimura 2008a was 3% (3/100). Reasons for withdrawal included changing jobs and moving to a distant location, death following surgery, and loss to follow‐up. Overall compliance with the exercise program (attendance to >50% of the sessions) was 82.2%.
Adherence to the exercise intervention was reported in four of the 14 included studies (Dolan 2006, MacArthur 1993, Mutimurra 2008a, Perna 1999). Mutimura 2008a reported 82.2% adherence rate and Perna (1999) reported 61% of participants were adherent with the exercise intervention. Both studies defined adherence as attending >50% of the exercise sessions. Dolan 2006 reported that participants in the exercise group completed 96% of the total exercise interventions. These three studies also supervised the exercise intervention (Dolan 2006, Mutimura 2008a, Perna 1999). MacArthur 1993 reported six out of 25 of the participants were compliant with the exercise program (attended >80% of the sessions), seven were somewhat compliant (attended 30%‐80% of sessions) and 12 were not compliant (attended <30% of sessions). Supervision of the exercise intervention was not reported in this study.
d) Were the groups similar at baseline?
Nine of the 14 included studies reported that comparison groups were similar at baseline (LaPerriere 1990, Perna 1999, Rigsby 1992, Smith 2001, Stringer 1998, Terry 1999, Baigis 2002, Driscoll 2004a, Mutimura 2008a). MacArthur 1993 and Grinspoon 2000 did not report on group similarity at baseline. Lox 1995 indicated significant differences between comparison groups for "most" participant characteristics, but variables that were different between the groups were not specified. Dolan 2006 indicated that the exercise group had more endurance and knee strength at baseline. Terry 2006 indicated that the low lipid diet group had a small but significantly higher haemoglobin level compared with the combined exercise and low lipid diet group.
Effects of interventions
CORRESPONDENCE WITH AUTHORS
We wrote to, and received clarification and information from six authors of studies included in the initial systematic review. The additional information was used in the first update of this review and was reflected within the analyses and results. Terry 1999 provided clarification regarding the number of withdrawals from the moderate‐ versus high‐intensity group that confirmed the sample size in which analyses were conducted. Stringer 1998 provided VO2max data in mL/kg/min that allowed the incorporation of VO2max as an outcome in the meta‐analysis update. MacArthur 1993 provided clarification regarding the distribution of the number of participants between the low‐ and high‐intensity intervention groups at baseline. Rigsby 1992 provided confirmation that analyses in this study were conducted on HIV‐positive participants only (groups included HIV‐positive and ‐negative participants). Perna 1999 provided results from intention‐to‐treat analysis of exercise versus non‐exercising control group. Lox 1995 provided additional data on mean and standard deviation for all three comparison groups. Grinspoon 2000 confirmed that the participants in their study were the same participants as in Fairfield 2001.
For this third update, we wrote to Dolan 2006 who provided clarification / information on weight and standard error of the mean for the intervention and non‐exercising control groups. We wrote to Mutimura 2008a twice requesting additional data on cardiopulmonary outcomes (rate of perceived exertion and heart rate maximum) and clarification of standard deviations for the change in outcomes, but did not receive a response.
META‐ANALYSES
Thirty meta‐analyses were completed in this review for immunologic and virologic outcomes (CD4 count, CD4 percentage, and viral load), cardiopulmonary outcomes (VO2max, HRmax, exercise time), weight and body composition outcomes (body weight, body mass index, waist circumference, hip circumference, waist‐to‐hip ratio, percent body fat, fat mass, leg muscle area), and psychological outcomes (depression‐dejection subscale of the POMS). No meta‐analyses for strength outcomes were possible. Subgroup comparisons of the meta‐analyses include 1) constant or interval aerobic exercise versus non‐exercising control; 2) constant aerobic exercise versus non‐exercising control; 3) interval aerobic exercise versus non‐exercising control; 4) moderate‐intensity aerobic exercise versus heavy‐intensity aerobic exercise; and 5) constant or interval aerobic exercise combined with PRE versus non‐exercising control.
Seven of the 14 included studies compared constant or interval aerobic exercise with a non‐exercising control group (Perna 1999, Stringer 1998, LaPerriere 1990, Smith 2001, Baigis 2002, Lox 1995, Mutimura 2008a). Two studies did not include a non‐exercising control group (MacArthur 1993, Terry 1999). Driscoll 2004a included aerobic and PRE combined with metformin and Terry 2006 included aerobic exercise combined with a low lipid diet and were unable to be combined in meta‐analyses. Interventions in Rigsby 1992, Grinspoon 2000 and Dolan 2006 included aerobic exercise as well as PRE.
The number of meta‐analyses was limited due to variability in types of exercise interventions (aerobic exercise vs. combined aerobic and PRE exercise), level of exercise supervision, types of outcomes reported, and methodological quality (see table of characteristics of included studies). Aerobic interventions in the trials varied according to constant compared to interval exercise and moderate compared to heavy intensity exercise, and combined aerobic and resistive exercise compared to aerobic exercise alone. For the purposes of this review, constant exercise is defined as exercise at a constant intensity for a period of time. Interval exercise is defined as exercise conducted at a varied intensity for a total period of time. See the table of characteristics of included studies for specific descriptions of constant and interval exercise in individual studies.
For this systematic review, a priori, we considered: Immunuological and virological outcomes: 50 cells/mm3 to indicate a clinically important change in CD4 count, 5% to indicate a clinically important change in CD4 percentage, 0.5 log10copies to indicate a clinically important change in viral load; Cardiorespiratory outcomes: 2 mL/kg/min to indicate a clinically important change in VO2max, 10 beats/min to indicate a clinically important change in HRmax, 5 minutes to indicate a clinically important change in exercise time; Weight and Body Composition outcomes: 3 kg to indicate a clinically important change in body weight (which equals approximately 5% of the average baseline body weight of the participants), 5 cm to indicate a clinically important change in girth (waist and hip circumference), 3 cm to indicate a clinically important change in waist‐to‐hip ratio, 5 kg/cm2 to indicate a clinically important change in body mass index, 5 kg to indicate a clinically important change in fat mass, 5% to indicate a clinically important change in percent body fat, and 5 cm2 to indicate a clinically important change in leg muscle area; Strength outcomes: 5 kg to indicate a clinically important change in strength for lower extremities, 2 kg to indicate a clinically significant change in strength for upper extremities and; Psychological outcomes: 5.6 to indicate a clinically important change in the subscales of the POMS.
Anything below 20% CD4 percentage is equated to similar presentation as CD4 count <200 cells/mm3. Because 50 cells/mm3 is the clinically important change in CD4 count, using the same ratio, we can estimate the clinically important difference in CD4 percentage to be 5%. This is likely a conservative estimate because CD4 percentage is more stable than CD4 counts, which tend to fluctuate more. The minimal clinically important difference (MCID) for the fatigue subscale of the POMS is 5.6 (Schwartz 2002), making this a reasonable estimate for the MCID of other POMS subscales. No other established MCID values exist for the other outcomes. Authors based the a priori estimates based on a combination of clinical experience and interpretations in the individual included studies.
Heterogeneity (P<0.1) was evident in 16 of the 30 meta‐analyses. Reasons for heterogeneity may include differences in the types of participants in relation to antiretroviral use, body composition, or gender, as well as methods of outcome measurement. We were able to conduct sensitivity analyses on four of the 16 meta‐analyses with heterogeneity (those with greater than two studies included in the meta‐analysis). We discuss the specific sensitivity results and reasons for heterogeneity within the sub‐group analyses below.
A) IMMUNOLOGIC AND/OR VIROLOGIC OUTCOMES
All 14 included studies reported immunologic or virologic outcomes, or both. Ten of the studies included a non‐exercising control group, three of which included combined interventions of aerobic and PRE (Grinspoon 2000, Rigsby 1992, Dolan 2006). MacArthur 1993, Terry 1999, Driscoll 2004a, Terry 2006 also measured immunologic and virologic outcomes but did not include a non‐exercising control group.
A‐1) CD4 count
Five meta‐analyses were performed. Overall, no significant changes in CD4 count were found between comparison groups. Meta‐analyses demonstrated no difference in change in CD4 count for participants in the exercise intervention group compared with the non‐exercising control group (WMD: 18.08 cells/mm3, 95% confidence interval [CI]: ‐11.82, 47.99, n=306, P=0.24) (LaPerriere 1990, Perna 1999, Smith 2001, Stringer 1998, Baigis 2002, Lox 1995, Mutimura 2008a) Figure 1; no difference in change in CD4 count for participants in the constant aerobic aerobic exercise group compared with the non‐exercising control group (WMD: ‐3.11 cells/mm3, 95% CI: ‐31.06, 24.84, n=261, P=0.83) (Lox 1995, Stringer 1998, Smith 2001, Baigis 2002, Mutimura 2008a) (Figure 2); and a significant trend towards an improvement in CD4 count of 69.58 cells/mm3 for participants in the interval aerobic exercise group compared with the non‐exercising control group (95% CI: 14.08, 125.09, P=0.01, n=45) (LaPerriere 1990, Perna 1999) (Figure 3). The point estimate is above 50 cells/mm3, which suggests a potential clinically important increase in CD4 count for interval exercisers compared with non‐exercisers. Results showed no difference in change in CD4 count for participants exercising at moderate intensity compared with participants exercising at heavy intensity (WMD: ‐42.90 cells/mm3, 95% CI: ‐116.28, 30.47, n=39, P=0.25) (Stringer 1998, Terry 1999) (Figure 4) and no difference in change in CD4 count for participants in a combined aerobic and PRE group compared with the non‐exercising control group (WMD: 21.52 cells/mm3, 95% CI: ‐29.25, 72.28, n=84, P=0.41) (Rigsby 1992, Grinspoon 2000, Dolan 2006) (Figure 5).
1.
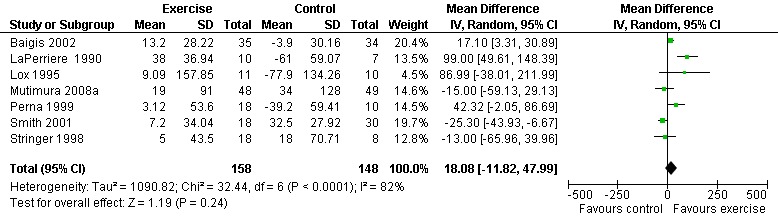
Forest plot of comparison: 1 Constant or interval aerobic exercise compared with non‐exercise, outcome: 1.1 CD4 count (cells/mm3).
2.
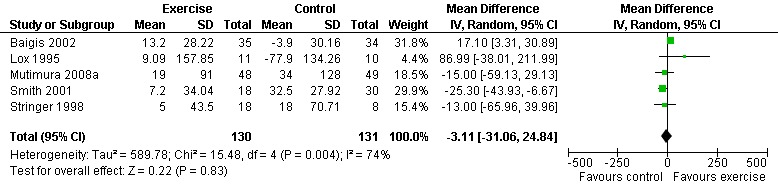
Forest plot of comparison: 2 Constant aerobic exercise compared with non‐exercise., outcome: 2.1 CD4 count (cells/mm3).
3.
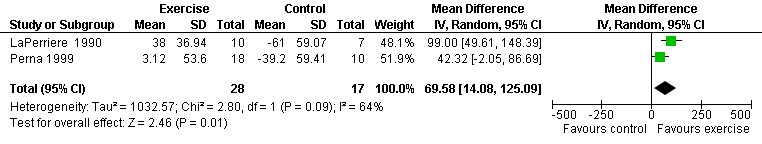
Forest plot of comparison: 3 Interval aerobic exercise compared with non‐exercise., outcome: 3.1 CD4 count (cells/mm3).
4.
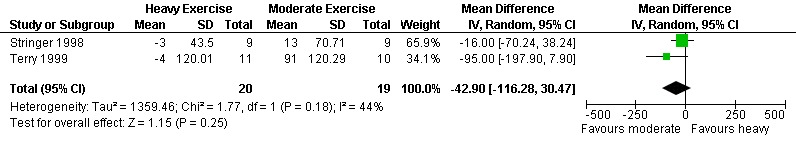
Forest plot of comparison: 4 Moderate aerobic exercise compared with heavy aerobic exercise., outcome: 4.1 CD4 count (cells/mm3).
5.
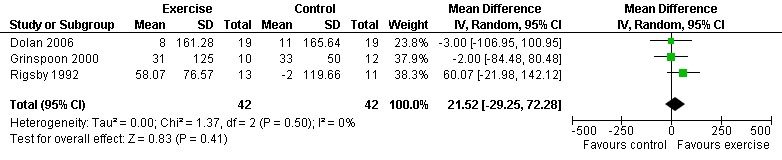
Forest plot of comparison: 5 Constant or interval aerobic exercise and progressive resistive exercise compared with no exercise, outcome: 5.1 CD4 count (cells/mm3).
Three of five meta‐analyses were statistically significant for heterogeneity (P<0.1). Sensitivity analyses indicated that removing both LaPerriere 1990 and Smith 2001 from the aerobic versus non‐exercise comparison (Figure 1) and removing Smith 2001 from the constant exercise versus non‐exercise comparison (Figure 6) successfully removed heterogeneity; however, the results for overall effect remained non‐significant. Removing Baigis 2002 from the constant exercise versus non‐exercise comparison (Figure 2) successfully removed heterogeneity (P=0.36) and the overall effect demonstrated a significant decrease in CD4 count of ‐19.70 cells/mm3 among exercisers compared with non‐exercisers (95% CI: ‐38.30, ‐1.10, n=192, P=0.04). Reasons for heterogeneity may be due to differences in characteristics of participants in the included studies.
6.
Forest plot of comparison: 1 Constant or interval aerobic exercise compared with non‐exercise, outcome: 1.2 CD4 Percentage (%).
A‐2) CD4 Percentage
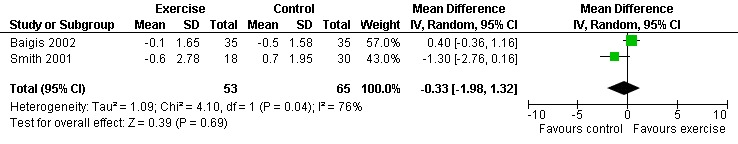
Two meta‐analyses were performed for CD4 percentage that included the same studies. Meta‐analyses demonstrated no difference in change in CD4 percentage for participants in the exercise intervention group compared with the non‐exercising control group as well as the constant aerobic exercise group compared with the non‐exercising control (WMD: ‐0.33%, 95% CI: ‐1.98, 1.32, n=118, P=0.69) (Smith 2001, Baigis 2002) (Figure 6, Figure 7).
7.
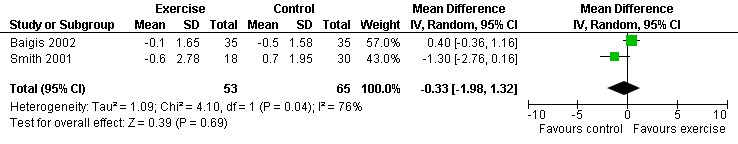
Forest plot of comparison: 2 Constant aerobic exercise compared with non‐exercise., outcome: 2.2 CD4 Percentage (%).
Individual Study Results ‐ CD4 Count
Perna 1999 found the highest increase in CD4 count of 60 cells/mm3 in the compliant exercise group and a combined increase of 3 cells/mm3 in the combined compliant and non‐compliant exercise intervention group compared to a decrease of 39 cells/mm3 in the non‐exercising control group. LaPerriere 1990 showed an average increase of 38 cells/mm3 in the interval exercise group compared to an average decrease of 61 cells/mm3 in the non‐exercising control group. Lox 1995 found an average increase of 9 cells/mm3 in the exercise group and an average decrease of 78 cells/mm3 in the non‐exercising control group. Baigis 2002 found an average increase of 13 cells/mm3 in the exercise group compared to an average decrease of 4 cells/mm3 in the non‐exercising control group. Stringer 1998 found an increase of 13 cells/mm3 in the moderate‐intensity exercise group of constant exercise and an average increase of 5 cells/mm3 in the combined moderate‐ and heavy‐intensity exercise groups compared to an increase of 18 cells/mm3 in the non‐exercising control group. Smith 2001 found an increase of 7 cells/mm3 in the exercise group compared to an increase of 32 cells/mm3 in the non‐exercising control group. Results from Stringer 1998 and Smith 2001 were statistically non‐significant. Terry 1999 and MacArther 1993 found no significant changes in CD4 count. Grinspoon 2000 found an average increase of 31 cells/mm3 in the exercise group compared to an average increase of 33 cells/mm3 in the non‐exercising control group. Rigsby 1992 found an average increase of 58 cells/mm3 in the exercise group compared to an average decrease of 2 cells/mm3 in the non‐exercising control group. These results were not statistically significant. Dolan 2006 found a non‐significant increase of 8 cells/µL and 11 cells/µL in the exercise group and non‐exercising control group, respectively. Terry 2006 reported a decrease in CD4 count in the combined exercise and low lipid diet group of 41 cells/mm3 and an increase in the low lipid diet only group by 48 cells/mm3, but these changes were not statistically significant. Driscoll 2004 reported non‐significant median increases in CD4 count of 59 and 42 cells/mm3 in the combined exercise and metformin group and the metformin only group, respectively. Mutimura 2008a found that the increase in CD4 count in the exercise group (19 cells/µL) was not significantly different from that in the non‐exercising control group (34 cells/µL).
A‐3) Viral Load
Three meta‐analyses were performed for viral load, of which two included the same studies. Meta‐analysis demonstrated no difference in change in viral load for participants in the exercise intervention group compared with the non‐exercising control group as well as the constant aerobic exercise group compared with the non‐exercising control group (WMD: 0.40 log10copies, 95% CI: ‐0.28, 1.07, n=63, P=0.25) (Smith 2001; Stringer 1998) Figure 8; Figure 9; and no difference in the combined aerobic and PRE group compared with the non‐exercising control group (WMD: 0.31 log10copies, 95% CI: ‐0.13, 0.74, n=60, P=0.17) (Grinspoon 2000, Dolan 2006) Figure 10.
8.
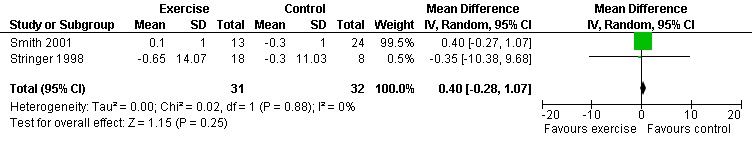
Forest plot of comparison: 1 Constant or interval aerobic exercise compared with non‐exercise, outcome: 1.3 Viral Load.
9.
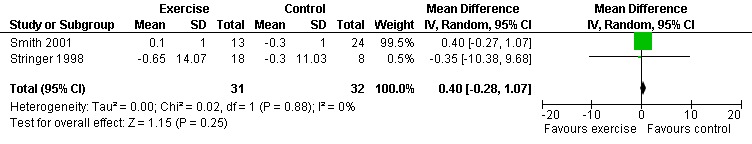
Forest plot of comparison: 2 Constant aerobic exercise compared with non‐exercise., outcome: 2.3 Viral Load (log10 copies).
10.
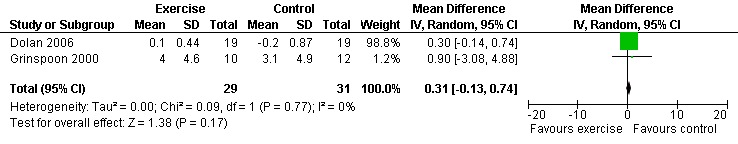
Forest plot of comparison: 5 Constant or interval aerobic exercise and progressive resistive exercise compared with no exercise, outcome: 5.2 Viral Load (log10 copies).
Individual Study Results ‐ Viral Load
Stringer 1998 found an average decrease of 0.65 log10copies in the combined moderate‐ and heavy‐intensity exercise groups versus an average decrease of 0.30 log10copies in the non‐exercising control group. The largest decrease in viral load was seen in the moderate‐exercise group with an average decrease of 0.9 log10copies. Smith 2001 found an average increase of 0.10 log10copies in the exercise group compared to an average decrease of 0.30 log10copies in the non‐exercising control group. Results from both of these studies were statistically non‐significant. Grinspoon 2000 found no significant differences in viral load. Dolan 2006 reported a non‐significant increase in viral load of 0.1 copies/µL in the exercise group and non‐significant decrease of ‐0.2 copies/µL in the non‐exercising control group. Driscoll 2004a reported no change in viral load in both the combined exercise and metformin group and the metformin only group. Terry 2006 reported that after the intervention 13 of 15 participants in both the exercise and combined exercise and low lipid diet groups had viral load values below 80 copies/mL. These results were not significant.
B) CARDIOPULMONARY OUTCOMES
Thirteen of the 14 included studies assessed cardiopulmonary outcomes, seven of which compared constant or interval aerobic exercise to non‐exercising controls (Stringer 1998, Perna 1999, LaPerriere 1990, Smith 2001, Lox 1995, Baigis 2002, Mutimura 2008a), three of which compared moderate aerobic exercise with heavy aerobic exercise (Stringer 1998, Terry 1999 and MacArthur 1993), and two of which compared constant or aerobic exercise and PRE to non‐exercising control (Rigsby 1992, Dolan 2006). Driscoll 2004a and Terry 2006 also measured cardiopulmonary outcomes but compared exercise to metformin and a low lipid diet, respectively.
B‐1) VO2max
Three meta‐analyses were performed for VO2max. Meta‐analyses showed a significant improvement in change of VO2max of 2.63 mL/kg/min for participants in the aerobic exercise intervention group compared with the non‐exercising control group (95% CI: 1.19, 4.07, n=276, P=0.0003) (Perna 1999; Smith 2001; Stringer 1998, Baigis 2002, Mutimura 2008a) (Figure 11); significant improvement in change of VO2max of 2.40 ml/kg/min for participants in the constant aerobic exercise group compared with the non‐exercising control group (95% CI: 0.82, 3.99, n=248, P=0.003) (Stringer 1998, Smith 2001, Baigis 2002, Mutimura 2008a) Figure 12; and a significant trend towards a greater improvement in VO2max of 4.30 mL/kg/min for participants in the heavy‐intensity exercise group compared with the moderate‐intensity exercise group (95% CI: 0.61, 7.98, n=24, P=0.02) (Figure 13). All point estimates are greater than 2 mL/kg/min, which suggests a potential clinically important improvement in VO2max among exercisers and a greater improvement with heavy‐ versus moderate‐intensity exercise.
11.
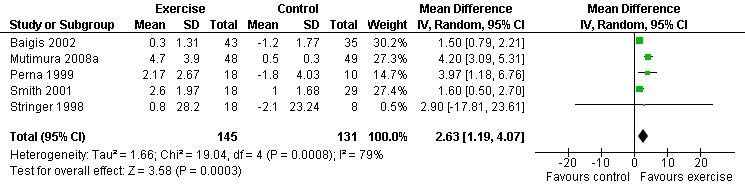
Forest plot of comparison: 1 Constant or interval aerobic exercise compared with non‐exercise, outcome: 1.4 VO2 Max (ml/kg/min).
12.
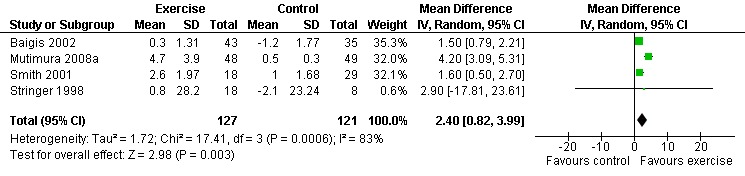
Forest plot of comparison: 2 Constant aerobic exercise compared with non‐exercise., outcome: 2.4 VO2max (ml/kg/min).
13.
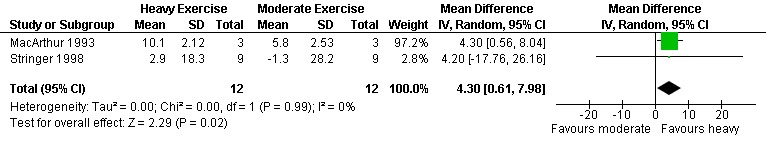
Forest plot of comparison: 4 Moderate aerobic exercise compared with heavy aerobic exercise., outcome: 4.2 VO2max (ml/kg/min).
Two of three meta‐analyses were statistically significant for heterogeneity (P<0.1). Sensitivity analyses indicated that removing Mutimura 2008a from the aerobic versus non‐exercise comparison (Figure 11) and the constant exercise versus non‐exercise comparison (Figure 12) successfully removed heterogeneity. Results for overall effect remained statistically significant favouring exercise; however, the point estimate was reduced to 1.64 mL/kg/min (95% CI: 1.06, 2.22) and 1.53 mL/kg/min (95% CI: 0.94, 2.12), respectively, which are below the threshold for clinical importance. Reasons for heterogeneity may be due to differences in characteristics of participants in the Mutimura 2008a study; they were from Rwanda and all possessed moderate to severe body fat redistribution.
B‐2) Maximum Heart Rate (HRmax)
One meta‐analysis was performed and showed a non‐significant decrease in HRmax of ‐9.81 beats/min (95% CI: ‐26.28, 6.67, n=49, P=0.24) for participants in the aerobic exercise intervention group compared with the non‐exercising control group (Lox 1995, Perna 1999) (Figure 14).
14.
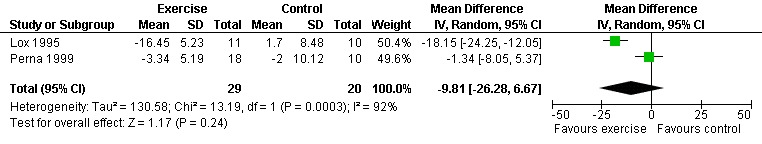
Forest plot of comparison: 1 Constant or interval aerobic exercise compared with non‐exercise, outcome: 1.5 (Maximum Heart Rate (HRmax) (beats/min).
B‐3) Exercise Time (minutes)
One meta‐analysis was performed and showed a non‐significant increase in exercise time of 3.92 minutes (95% CI: ‐0.63, 8.47, n=62, P=0.09) for participants in the combined aerobic and PRE group compared with the non‐exercising control group (Rigsby 1992, Dolan 2006) (Figure 15).
15.
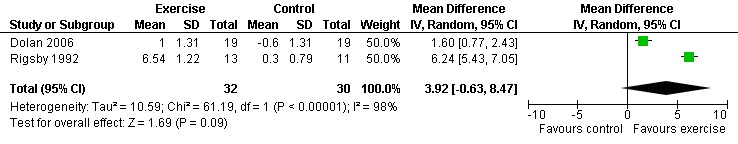
Forest plot of comparison: 5 Constant or interval aerobic exercise and progressive resistive exercise compared with no exercise, outcome: 5.6 Exercise time (min).
Individual Study Results ‐ Cardiopulmonary Status
Twelve of the 13 studies that measured cardiopulmonary status reported significant improvements in cardiopulmonary outcomes. Stringer 1998 found significant improvements in VO2max, Work Rate max and lactic acid threshold (LAT) in exercisers versus non‐exercising controls. Stringer 1998 reported a greater training effect of the heavy and moderate group (heavy>moderate) compared to the non‐exercising control group. VO2max and Work Rate max increased significantly in the heavy‐intensity exercise group. The LAT increased significantly in both groups. Perna 1999 found significant increases in VO2max (12% improvement), O2 pulse (13%), maxTv (8%) and VE (17%) and a non‐significant improvement in HRmax of 3.34 beats/min in the combined compliant and non‐compliant exercisers (interval aerobic exercise groups). No significant differences were found in non‐compliant exercisers and non‐exercising control groups. LaPerriere 1990 found significant improvements in VO2max and fitness level in the exercise group versus no change in the non‐exercising control group. Smith 2001 found significant decreases in fatigue in exercisers compared to non‐exercisers as measured by an increase in the time on treadmill and found significant improvements in exercisers for VO2max (2.6mL/kg/min) compared to non‐exercisers (1mL/kg/min). There was no significant effect on rate of perceived exertion (RPE) or dyspnea in either group. Lox 1995 found significant improvements in VO2max among exercisers compared to non‐exercisers. Baigis 2002 did not report significant differences in VO2max between exercisers and non‐exercisers, but attributed these results to the level of intensity and duration of the exercise intervention. Terry 1999 found significant improvements in exercise time for both the moderate‐ and high‐intensity exercise groups and a significant increase in peak systolic BP in the heavy‐exercise group. Peak HR was unchanged in both groups. Terry 1999 found significant improvements in exercise capacity demonstrated by an increase in the time on treadmill showing greater improvements in the high‐intensity versus moderate‐intensity group. MacArthur 1993 found significant increases in VO2max (29%), minute ventilation (13%), and oxygen pulse (24%) for participants in both the high‐ and low‐intensity exercise groups. Dolan 2006 reported significant improvements in exercise time as measured by submaximal exercise time (1.0 minute) and significant increases in VO2max (1.5 mL/kg/min) among exercisers compared with non‐exercisers. Driscoll 2004a reported significant increases in endurance time on the cycle ergometer in the combined exercise and metformin group compared with the metformin only group. Terry 2006 reported significant improvements in VO2max on the maximal treadmill test for the combined exercise and low lipid diet group and no change was reported in the diet only group. No significant changes were found in HRmax in either group. Mutimura 2008a reported significant improvements in VO2max (4.7 mL/kg/min) among exercisers compared with non‐exercisers (0.5 mL/kg/min).
C) STRENGTH OUTCOMES
Six of the 14 included studies assessed muscle strength (Rigsby 1992, Lox 1995, Perna 1999, Grinspoon 2000, Driscoll 2004a, Dolan 2006). Meta analysis could not be performed for strength due to differences in the types of strength outcomes assessed, types of interventions, types of comparison groups, and types of participants; however, individual studies suggested improvements in strength among exercisers compared with non‐exercisers.
Individual Study Results ‐ Strength
Five of the six individual studies reported improvements in strength among exercisers compared with non‐exercisers. Rigsby 1992 found a significant increase in strength for chest press (50.27Nm) and leg press (47.54Nm) for the combined progressive resistive and aerobic exercise intervention group compared to a non‐significant increase in strength for chest press (19.22Nm) and leg press (6.92Nm) for the non‐exercising control group. Lox 1995 found significant increases in strength in upper and lower extremities in the PRE group compared to the non‐exercising control and aerobic exercise groups. Significantly greater improvements as measured by 1‐repetition maximum were found in the PRE group (upper extremity: +31.5 lbs, lower extremity: +90.7 lbs) compared with the aerobic and non‐exercising control groups. Grinspoon 2000 found no significant differences in strength (7/7 variables) between the combined PRE and aerobic exercise group and the non‐exercising control group. The lack of statistical significance was attributed to the use of isometric methods of strength testing versus the alternative isotonic method of testing used in the other four studies. Perna 1999 reported a significant increase in leg power by 25% with adherent exercisers and no change in non‐adherent exercisers or non‐exercising controls. Dolan 2006 reported significant improvements in upper and lower extremity strength (7 measures) among exercisers compared with non‐exercisers. Driscoll 2004a reported significant increases in upper and lower extremity strength (5 of 6 indices) in the combined exercise and metformin group compared with the metformin only group.
D) WEIGHT AND BODY COMPOSITION
Nine of the 14 included studies assessed weight and body composition outcomes (Dolan 2006, Driscoll 2004a, Grinspoon 2000, Lox 1995, Perna 1999, Smith 2001, Terry 1999, Terry 2006, Mutimura 2008a).
D‐1) Weight
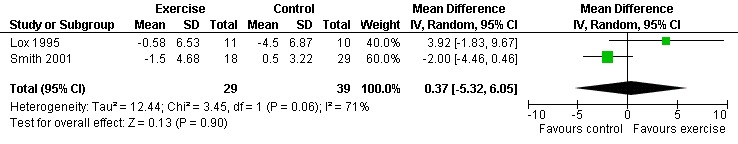
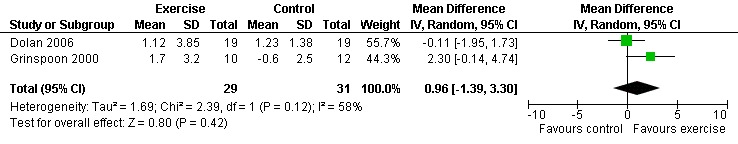
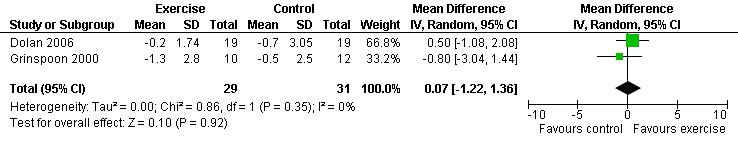
Seven studies assessed body weight (Lox 1995, Terry 1999, Grinspoon 2000, Smith 2001, Driscoll 2004a, Dolan 2006, Terry 2006). Three meta‐analyses were performed, two of which included the same studies. Meta‐analyses demonstrated no difference in change in mean body weight for participants in the exercise group compared with the non‐exercising control group as well as participants in the constant aerobic exercise group compared with the non‐exercising control group (WMD: 0.37 kg, 95% CI: ‐5.32, 6.05, n=68, P=0.90) (Smith 2001; Lox 1995) (Figure 16Figure 17); there was no difference in the combined aerobic and PRE group compared with the non‐exercising control group (WMD: 0.96 kg, 95% CI: ‐1.39, 3.30, n=60, P=0.42) (Grinspoon 2000, Dolan 2006) (Figure 18).
16.
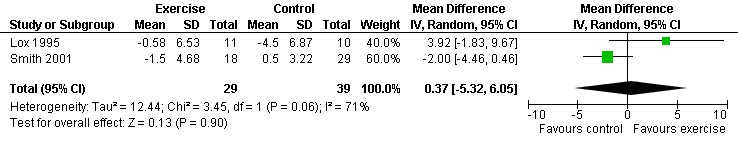
Forest plot of comparison: 1 Constant or interval aerobic exercise compared with non‐exercise, outcome: 1.6 Mean Body Weight (kg).
17.
Forest plot of comparison: 2 Constant aerobic exercise compared with non‐exercise., outcome: 2.5 Weight (kg).
18.
Forest plot of comparison: 5 Constant or interval aerobic exercise and progressive resistive exercise compared with no exercise, outcome: 5.3 Mean Body Weight (kg).
Individual Study Results ‐ Weight
Grinspoon 2000 found an increase in body weight of 1.7 kg for participants in the combined progressive resistive and aerobic exercise group compared to a decrease in body weight of 0.6 kg for participants in the non‐exercising control group. These results were not statistically significant. Lox 1995 found a significant increase of 2.12 kg in body weight for participants in the resistive exercise group compared to a decrease of 4.5 kg in the non‐exercising control group. Terry 1999 reported no significant changes in weight in either group. Smith 2001 reported a decreasing trend in weight in the exercise group. Driscoll 2004a reported no significant changes in weight in either the combined exercise and metformin group and metformin only group. Dolan 2006 reported no significant changes in weight between exercise and non‐exercise groups. Terry 2006 reported significant decreases in weight in both exercise and diet and diet only groups. Significant increases in body density were also found in both groups.
D‐2) Body Composition
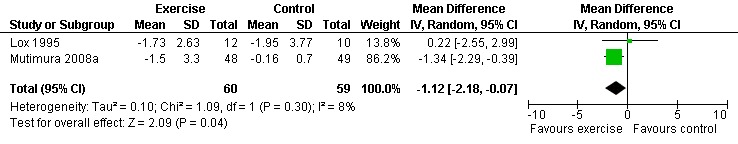
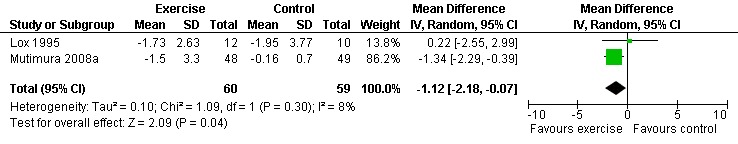
Nine studies assessed body composition (Lox 1995, Perna 1999, Terry 1999, Grinspoon 2000, Smith 2001, Driscoll 2004a, Dolan 2006, Terry 2006, Mutimura 2008a). Eleven meta‐analyses were performed, each for body mass index, hip circumference, waist circumference, waist‐to‐hip ratio, percent body fat, fat mass, and leg muscle area. Four of the eleven meta‐analyses were duplicates and included the same studies. Meta‐analyses demonstrated a significant decrease in percent body fat of 1.12% (95% CI: ‐2.18, ‐0.07,n=119, P=0.04) (Lox 1995, Mutimura 2008a) (Figure 19Figure 20) for participants in the aerobic exercise group compared with participants in the non‐exercising control group, and a significant increase in change in leg muscle area of 4.79 cm2 was found among participants in the combined aerobic and PRE group compared with the non‐exercising control group (95% CI: 2.04, 7.54, n=60, P=0.0007) (Grinspoon 2000, Dolan 2006) (Figure 21).
19.
Forest plot of comparison: 1 Constant or interval aerobic exercise compared with non‐exercise, outcome: 1.11 Percent Body Fat (%).
20.
Forest plot of comparison: 2 Constant aerobic exercise compared with non‐exercise., outcome: 2.9 Percent Body Fat (%).
21.
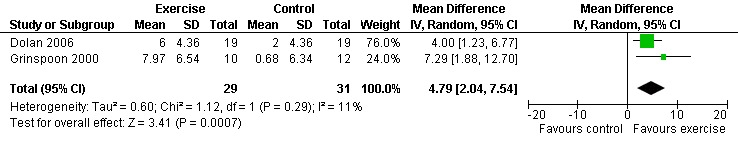
Forest plot of comparison: 5 Constant or interval aerobic exercise and progressive resistive exercise compared with no exercise, outcome: 5.5 Body Composition ‐ Mean Leg Muscle Area (cm2).
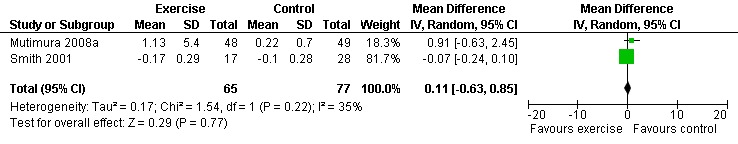
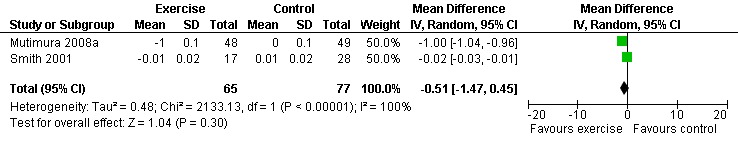
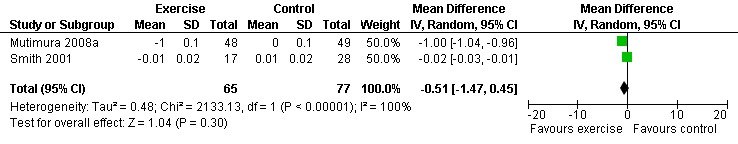
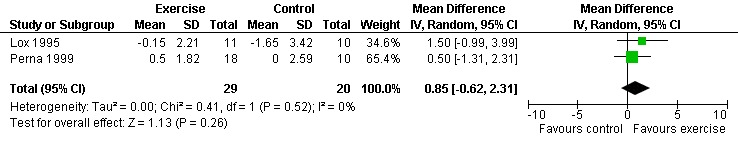
Results also demonstrated no difference in change in waist circumference (WMD: ‐3.53cm, 95% CI: ‐10.25, 3.19, n=142, P=0.30) (Smith 2001, Mutimura 2008a) (Figure 22Figure 23); hip circumference (WMD: 0.11cm, 95% CI: ‐0.63, 0.85, n=142, P=0.77) (Smith 2001, Mutimura 2008a) (Figure 24 and Figure 25) and waist‐to‐hip ratio (WMD: ‐0.51, 95% CI: ‐1.47, 0.45, n=142, P=0.30) (Smith 2001, Mutimura 2008a) (Figure 26 and Figure 27) for participants in the aerobic exercise group compared with participants in the non‐exercising control group as well as participants in the constant aerobic exercise group compared with the non‐exercising control group. Despite statistical non‐significance, the confidence interval indicates a trend towards a decrease in the waist circumference among exercisers compared with non‐exercisers. This is a favourable result given that the Mutimura 2008a participants had evidence of body fat redistribution. Results also showed no difference in the change in body mass index (WMD: 0.85kg/cm2, 95% CI: ‐0.62, 2.31, n=49, P=0.26) (Lox 1995, Perna 1999) (Figure 28) for participants in the aerobic exercise group compared with participants in the non‐exercising control group, and no difference in change in fat mass (WMD: 0.07 kg, 95% CI: ‐1.22, 1.36, n=60, P=0.92) (Grinspoon 2000, Dolan 2006) (Figure 29) for participants in the combined aerobic and PRE group compared with the non‐exercising control group.
22.
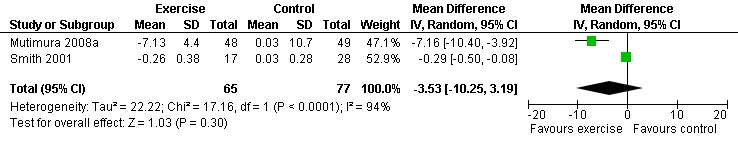
Forest plot of comparison: 1 Constant or interval aerobic exercise compared with non‐exercise, outcome: 1.8 Waist Circumference (cm).
23.
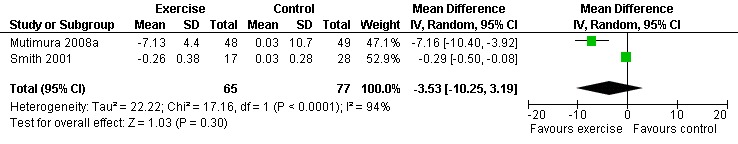
Forest plot of comparison: 2 Constant aerobic exercise compared with non‐exercise., outcome: 2.6 Waist Circumference (cm).
24.
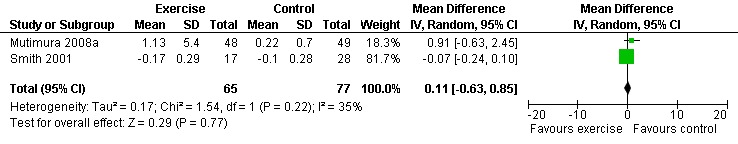
Forest plot of comparison: 1 Constant or interval aerobic exercise compared with non‐exercise, outcome: 1.9 Hip Circumference (cm).
25.
Forest plot of comparison: 2 Constant aerobic exercise compared with non‐exercise., outcome: 2.7 Hip Circumference (cm).
26.
Forest plot of comparison: 1 Constant or interval aerobic exercise compared with non‐exercise, outcome: 1.10 Waist to Hip Ratio.
27.
Forest plot of comparison: 2 Constant aerobic exercise compared with non‐exercise., outcome: 2.8 Waist to Hip Ratio.
28.
Forest plot of comparison: 1 Constant or interval aerobic exercise compared with non‐exercise, outcome: 1.7 Mean Body Mass Index (kg/cm2).
29.
Forest plot of comparison: 5 Constant or interval aerobic exercise and progressive resistive exercise compared with no exercise, outcome: 5.4 Body Composition ‐ Mean Fatt Mass (kg).
Individual Study Results ‐ Body Composition
Grinspoon 2000 found significant increases in lean body mass (2.3 kg), arm muscle area (346 mm2) and leg muscle area (797 mm2) and a non‐significant decrease in fat mass (‐1.3 kg) for participants in the combined PRE and aerobic exercise group compared to no changes in the non‐exercising control group. Lox 1995 reported no change among all three groups (PRE, aerobic, and control) for body mass index, fat mass and body fat percentage. Significant increases in lean body mass and sum of chest, arm, and thigh circumference was found in the PRE and aerobic exercise groups. Perna 1999 reported significant increases in body mass index among adherent exercisers. Terry 1999 reported no significant change in body mass, body fat percentage, and body density in both the moderate‐ and heavy‐intensity groups. Smith 2001 reported a significant decrease in waist‐to‐hip ratio, body mass index, central and peripheral skin folds, and abdominal girth among exercisers (note that many participants were above ideal body weight prior to exercise, thus decreases in weight and body composition were considered favourable outcomes). Driscoll 2004a reported no significant changes in body mass index in either the combined exercise and metformin group and metformin only group. Dolan 2006 reported no significant changes in weight between exercise and non‐exercise groups. Terry 2006 reported significant decreases in body mass index, waist‐to‐hip ratio, and percentage of body fat in both exercise and diet and diet only groups. Mutimura 2008a found significant decreases in body fat redistribution score, body mass index, body fat mass percentage, waist circumference, waist‐to‐hip ratio, triceps, biceps, subscapular, and suprailiac skin folds, and the sum of four skin folds among exercisers compared with non‐exercisers.
E) PSYCHOLOGICAL OUTCOMES
Nine of the 14 included studies assessed psychological outcomes in the form of anxiety and depression, health status, depression, mood and life satisfaction, and health‐related quality of life (LaPerriere 1990, MacArthur 1993, Stringer 1998, Perna 1999, Terry 1999, Smith 2001, Lox 1995, Baigis 2002, Mutimura 2008a).
E‐1) Depression‐dejection subscale of the Profile of Mood States Scale (POMS)
One meta‐analysis was performed and demonstrated a significant improvement in the depression‐dejection subscale of the POMS by a reduction of 7.68 points (95% CI: ‐13.47, ‐1.90, n=65, P=0.009) for participants in the aerobic exercise intervention group compared with the non‐exercising control group (LaPerriere 1990, Smith 2001) (Figure 30). This represents a clinically important improvement in depression‐dejection among exercisers compared to non‐exercisers.
30.
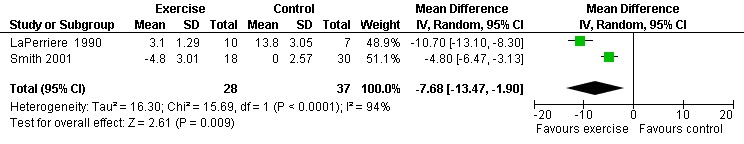
Forest plot of comparison: 1 Constant or interval aerobic exercise compared with non‐exercise, outcome: 1.12 Profile of Mood State (POMS) Depression‐Dejection subscale.
Individual Study Results ‐ Psychological Status
Stringer 1998 found significant improvements in QOL questionnaire scores for the exercise intervention groups compared to the non‐exercising control groups. LaPerriere 1990 found higher levels of anxiety and depression in non‐exercising controls compared with exercisers. Smith 2001 (or Neidig 2003) found significant improvements in depression for exercisers compared to non‐exercisers. Lox 1995 found significant improvements in mood and life satisfaction in the aerobic exercise group compared to the non‐exercising control group. Baigis 2002 found a non‐significant trend that favoured exercisers compared to non‐exercisers and found significant improvements in the overall health subscale of the MOS‐HIV among exercisers compared to non‐exercisers. Terry 1999 found no significant differences between the moderate‐ and high‐intensity exercise groups for depression. MacArther 1993 compared high‐ to low‐intensity exercise and reported improvements in the general health questionnaire in compliant exercisers. Perna 1999 found no significant differences in physician‐rated health status (note that this outcome was not considered a true measure of psychological status as it was not completed by patient self‐report). Mutimura 2008a reported significant improvements in QOL on the psychological, independence, social relationships, HIV+ HAART‐specific domains of QOL, and overall QOL score as measured by the World Health Organization Quality of Life HIV Instrument (WHOQOL‐BREF) for exercisers compared with non‐exercisers and no difference between groups on the physical QOL domain score.
F) ADVERSE EVENTS (SAFETY)
Six of the 14 included studies reported on adverse outcomes. Meta‐analysis was not possible due to the scarcity and variability of reporting adverse events. Adverse events were reported in three of the 14 studies, none of which were attributed to exercise (Rigsby 1992, Perna 1999, Dolan 2006). Rigsby 1992 reported one death during the study in the counselling group; however, the death was not attributed to the exercise intervention. Perna 1999 reported one hospitalization during the course of the study. Dolan 2006 reported that one participant in the exercise group had an exacerbation of asthma and one participant in the non‐exercising group experienced chest pain at baseline.
Two studies reported that no participants withdrew due to illness or infection (Smith 2001, Stringer 1998). Grinspoon 2000 reported having no reported deaths, adverse events or side effects during the course of the study. Neither were attributed to exercise. The other studies did not report on any outcomes of adverse events.
SUBGROUP ANALYSIS 0F MALES COMPARED TO FEMALES
Of the 14 included studies, 10 included female participants. Final results were not reported by gender and thus we were unable to complete a subgroup analysis for this comparison.
Discussion
The results of the review should be interpreted cautiously for a variety of reasons. This review is based on a small number of trials (n=14). Meta‐analyses were limited due to variation in outcome measures used and types of interventions, enabling only two to seven studies to be combined in meta‐analyses.
Individual studies in this review included small sample sizes and high withdrawal or non‐adherence rates (3%‐76%). Participants who withdrew from the exercise program were often excluded from the results by the authors, resulting in a per protocol approach to the analysis. Thus, the overall findings among those who continued to exercise might not reflect the general experience of exercise among adults living with HIV. In addition, publication bias may result in fewer studies with negative results being published. Furthermore, the inability to blind participants to the aerobic exercise intervention may have resulted in a Hawthorne effect, whereby participants might perceive greater benefits associated with exercise based on the expectation that exercise should be linked to positive outcomes. In addition, the lack of assessor blinding may have resulted in assessor bias whereby assessors may have measured outcomes in favour of the exercise intervention. Moreover, the majority of study participants were men between the ages of 18‐58 years. This limits the external validity and ability to generalize results to women and older adults living with HIV. Finally, the maximum duration of aerobic exercise intervention was 24 weeks. Thus the long‐term sustainable effects of aerobic exercise remain less clear.
Taking into account these limitations, we offer the following summary of our findings:
In this third update of the systematic review, four additional studies were incorporated into the results and conclusions (Driscoll 2004a, Dolan 2006, Terry 2006, Mutimura 2008a), two of which we were able to include in meta‐analyses (Dolan 2006, Mutimura 2008a). In addition, we broadened the outcomes considered in this update to include strength, weight, and body composition. This allowed 14 new meta‐analyses to be performed for outcomes of exercise time, weight, body mass index, waist circumference, hip circumference, waist‐to‐hip ratio, percent body fat, fat mass, and leg muscle area. These additional studies were also incorporated in existing meta‐analyses of CD4 count and viral load.
Results of meta‐analyses showed statistically significant improvements for some outcomes of cardiopulmonary outcomes (VO2max), body composition (leg muscle area, percent body fat), and psychological status (depression‐dejection subscale of the POMS). Cardiopulmonary status results demonstrated potentially clinical important improvements in VO2max among exercisers compared with non‐exercisers, and even greater improvements among participants doing heavy‐ compared with moderate‐intensity exercise. Body composition results demonstrated a significant increase in leg muscle area among combined progressive resistive and aerobic exercisers compared with non‐exercisers and decreases in percent body fat among exercisers compared with non‐exercisers and specifically constant exercisers compared with non‐exercisers. Psychological status results demonstrated significant and clinically important improvements in depression symptoms among exercisers compared to non‐exercisers. No significant differences were found in all but one meta‐analysis for CD4 count or viral load outcomes, suggesting that aerobic exercise has little impact on immunological or virological status.
Aside from meta‐analyses, individual studies reported significant results that demonstrated benefits of aerobic exercise interventions compared with non‐exercise. Twelve of the 13 included individual studies that measured cardiopulmonary outcomes demonstrated statistically significant improvements among exercisers. Five of the six individual studies that measured strength found significant increases in strength among exercisers. Six of the nine studies that measured weight and/or body composition found significant changes among exercisers compared with non‐exercisers. Furthermore all nine studies that measured psychological status found significant improvements in outcomes for exercisers compared with non‐exercisers.
Authors' conclusions
Implications for practice.
This review indicates that aerobic exercise plays an important role in the care of adults living with HIV. Meta‐analyses suggest that performing constant or interval aerobic exercise, or a combination of constant aerobic and PRE for at least 20 minutes three times per week for at least five weeks appears to be safe and may lead to improvements in selected outcomes of cardiopulmonary fitness (VO2max), body composition (leg muscle area, percent body fat), and psychological status (depression‐dejection symptoms). We also found a trend towards potential clinically important improvements in cardiopulmonary fitness and psychological status. Results for other meta‐analyses were not significant. Results of this review suggest that aerobic exercise appears to be safe for adults living with HIV who are medically stable. This finding is based on the absence of reports of adverse events among exercisers within individual studies and the stability of CD4 count and viral load. Findings should be interpreted cautiously because results are based on participants who completed the exercise interventions and for whom there were adequate follow‐up data.
Implications for research.
Evidence on aerobic exercise and HIV has emerged with the changing face of the disease. Evidence about the safety and effectiveness of aerobic exercise for adults living with HIV is increasing, including more recent narrative reviews that support the use of aerobic exercise interventions in the context of HIV (Scevola 2003, Dudgeon 2004, Cade 2004, Robinson 2007, Hand 2009). Prior to the advent of combination antiretroviral therapy, studies on exercise tended to include participants with AIDS‐wasting, whereas recent evidence includes participants on combination antiretroviral therapy with lipodystrophy, body fat redistribution, or hyperinsulinemia. These studies commonly assessed the impact of exercise on weight, body composition, and metabolic outcomes, and reflects our decision to broaden our outcomes to include weight and body composition in this review. Furthermore, an increasing number of studies include interventions with a combination of resistive and aerobic exercise. Many studies assessing PRE include strength as an outcome, which led us to include this outcome in this updated review, and add a subgroup analysis that specifically evaluates the effectiveness of combined PRE and aerobic exercise with non‐exercise. Next, an increasing number of studies we came across in this update assessed interventions such as tai chi (Robins 2006, Galantino 2005), diet (Engleson 2006), or co‐interventions with exercise, such as metformin (Driscoll 2004a) and low lipid diets (Terry 2006). As further research emerges that considers these co‐interventions, we expect that we will be able to carry out meta‐analyses that specifically assess the effectiveness of these co‐interventions. Finally, this update reflected an increasing number of studies with women, increasing the proportion of women in this systematic review to 30% (an increase from ˜15% in the previous update). Nevertheless, women remain largely under‐represented in this review. Finally, with combination antiretroviral therapy now increasingly reaching developing countries, this update includes the first study to assess the impact of aerobic exercise in the developing country context (Mutimura 2008a and 2008b).
Results of this review are limited by small sample sizes and high withdrawal rates. In several studies, participants who dropped out of the exercise program or were non‐adherent with exercise were not included in the final results for that study. This raises issues of effectiveness and safety of exercise among those who stop exercising. Future studies should make every effort to include all participants in an "intent‐to‐treat" analysis with follow‐up and results on those who withdraw from exercise programs.
Results are further limited by the lack of participant and assessor blinding among included studies. Given it is often difficult to achieve participant blinding with exercise interventions, future research may include blinding participants to different groups of exercise frequency, intensity, duration, and type. Future research could also ensure that assessors of outcomes are blinded to reduce the potential for assessor bias.
The long‐term effects of exercise also require attention. All studies were conducted for 15 weeks or less, except for two 24‐week studies. Further research should explore the different effects of interval versus constant exercise, as well as aerobic exercise in conjunction with other exercise treatment interventions.
In addition, if future research includes clinically meaningful and standardized outcomes of HRQL, strength, and cardiopulmonary measures (including fatigue), we may be able to incorporate additional outcomes in our review and strengthen existing meta‐analyses.
Finally, as an increasing number of individuals age with HIV, further research is needed to investigate the effects of exercise among older adults living with HIV, including those living with multiple concurrent health conditions, such as cardiovascular disease, liver disease, kidney disease, osteoporosis, and osteonecrosis, which may have implications for the future safety and effect of exercise for people living with HIV.
What's new
| Date | Event | Description |
|---|---|---|
| 7 July 2010 | New search has been performed | Third update of the review. |
| 7 July 2010 | New citation required but conclusions have not changed | Third update of the review. |
Acknowledgements
The authors would like to acknowledge the contributions of the following:
Jim Marianchuk, Sian Owen, Wayne Stump and Tracy Xavier for their role in data abstraction in the original review. Chris Sulway and Jim Marianchuk for their role in data abstraction in the first update.
Angela Eady, MLS, formerly of McMaster University, Health Information Research Unit, and Elizabeth Uleryk, Director, Hospital Library, Hospital for Sick Children, for their assistance in conducting the literature searches and refining the search strategy.
Shegalla Choy for her role in handsearching articles in the third update. Lauren Watson for her help in retrieving studies.
The following authors or co‐authors were invaluable in providing us with additional data or information about their reviews that helped with this review: William Stringer, Sheldon Levine (for R.D. MacArthur), Allen Jackson (for L.W. Rigsby), Jorge Ribeiro (for L. Terry), Frank Perna, Steven Grinspoon, Curt Lox, and Sarah Dolan.
Data and analyses
Comparison 1. Constant or interval aerobic exercise compared with non‐exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CD4 count (cells/mm3) | 7 | 306 | Mean Difference (IV, Random, 95% CI) | 18.08 [‐11.82, 47.99] |
| 2 CD4 Percentage (%) | 2 | 118 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐1.98, 1.32] |
| 3 Viral Load (copies/mL) | 2 | 63 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.28, 1.07] |
| 4 VO2 Max (ml/kg/min) | 5 | 276 | Mean Difference (IV, Random, 95% CI) | 2.63 [1.19, 4.07] |
| 5 Maximum Heart Rate (HRmax) (beats/min) | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐9.81 [‐26.28, 6.67] |
| 6 Body Weight (kg) | 2 | 68 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐5.32, 6.05] |
| 7 Body Mass Index (kg/cm2) | 2 | 49 | Mean Difference (IV, Random, 95% CI) | 0.85 [‐0.62, 2.31] |
| 8 Waist Circumference (cm) | 2 | 142 | Mean Difference (IV, Random, 95% CI) | ‐3.53 [‐10.25, 3.19] |
| 9 Hip Circumference (cm) | 2 | 142 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.63, 0.85] |
| 10 Waist to Hip Ratio | 2 | 142 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.47, 0.45] |
| 11 Percent Body Fat (%) | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐2.18, ‐0.07] |
| 12 Profile of Mood State (POMS) Depression‐Dejection Subscale | 2 | 65 | Mean Difference (IV, Random, 95% CI) | ‐7.68 [‐13.47, ‐1.90] |
1.1. Analysis.
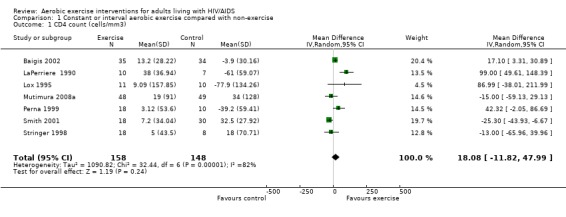
Comparison 1 Constant or interval aerobic exercise compared with non‐exercise, Outcome 1 CD4 count (cells/mm3).
1.2. Analysis.
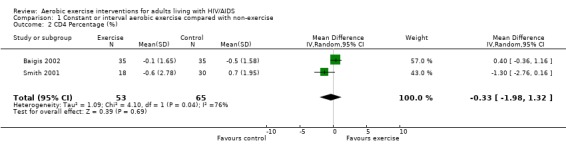
Comparison 1 Constant or interval aerobic exercise compared with non‐exercise, Outcome 2 CD4 Percentage (%).
1.3. Analysis.
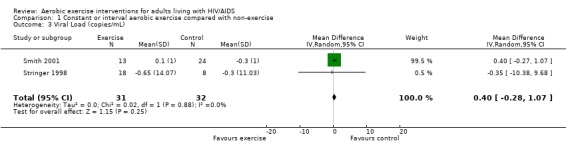
Comparison 1 Constant or interval aerobic exercise compared with non‐exercise, Outcome 3 Viral Load (copies/mL).
1.4. Analysis.
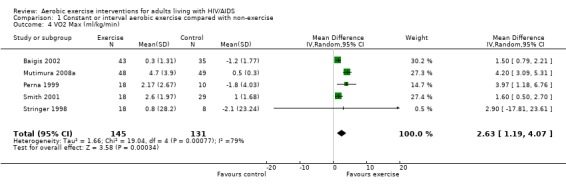
Comparison 1 Constant or interval aerobic exercise compared with non‐exercise, Outcome 4 VO2 Max (ml/kg/min).
1.5. Analysis.
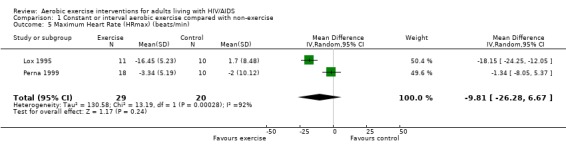
Comparison 1 Constant or interval aerobic exercise compared with non‐exercise, Outcome 5 Maximum Heart Rate (HRmax) (beats/min).
1.6. Analysis.
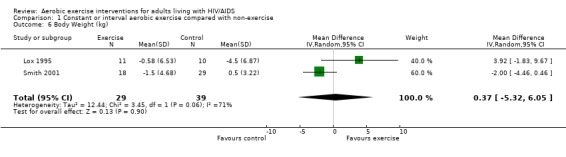
Comparison 1 Constant or interval aerobic exercise compared with non‐exercise, Outcome 6 Body Weight (kg).
1.7. Analysis.
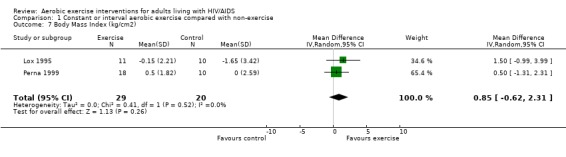
Comparison 1 Constant or interval aerobic exercise compared with non‐exercise, Outcome 7 Body Mass Index (kg/cm2).
1.8. Analysis.
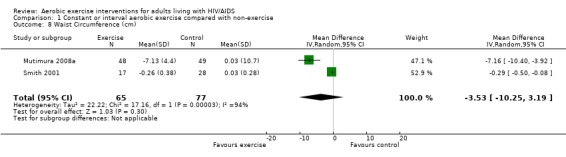
Comparison 1 Constant or interval aerobic exercise compared with non‐exercise, Outcome 8 Waist Circumference (cm).
1.9. Analysis.
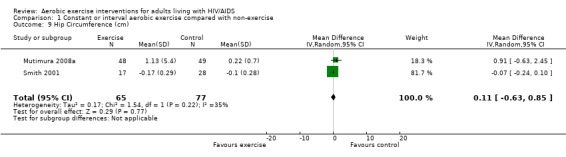
Comparison 1 Constant or interval aerobic exercise compared with non‐exercise, Outcome 9 Hip Circumference (cm).
1.10. Analysis.
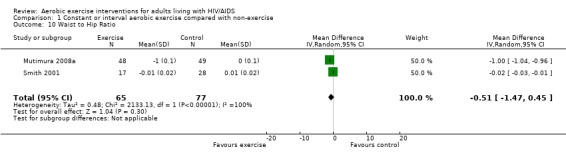
Comparison 1 Constant or interval aerobic exercise compared with non‐exercise, Outcome 10 Waist to Hip Ratio.
1.11. Analysis.
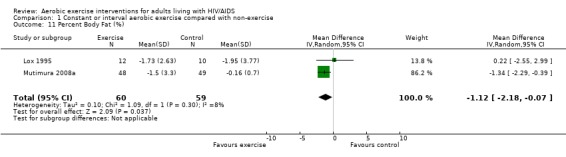
Comparison 1 Constant or interval aerobic exercise compared with non‐exercise, Outcome 11 Percent Body Fat (%).
1.12. Analysis.
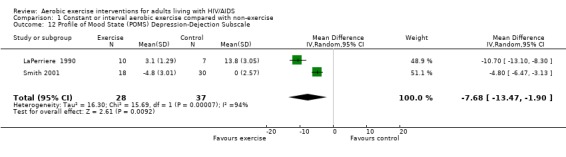
Comparison 1 Constant or interval aerobic exercise compared with non‐exercise, Outcome 12 Profile of Mood State (POMS) Depression‐Dejection Subscale.
Comparison 2. Constant aerobic exercise vs. non‐exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CD4 count (cells/mm3) | 5 | 261 | Mean Difference (IV, Random, 95% CI) | ‐3.11 [‐31.06, 24.84] |
| 2 CD4 Percentage (%) | 2 | 118 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐1.98, 1.32] |
| 3 Viral Load (log10 copies) | 2 | 63 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.28, 1.07] |
| 4 VO2max (ml/kg/min) | 4 | 248 | Mean Difference (IV, Random, 95% CI) | 2.40 [0.82, 3.99] |
| 5 Body Weight (kg) | 2 | 68 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐5.32, 6.05] |
| 6 Waist Circumference (cm) | 2 | 142 | Mean Difference (IV, Random, 95% CI) | ‐3.53 [‐10.25, 3.19] |
| 7 Hip Circumference (cm) | 2 | 142 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.63, 0.85] |
| 8 Waist to Hip Ratio | 2 | 142 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.47, 0.45] |
| 9 Percent Body Fat (%) | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐2.18, ‐0.07] |
2.1. Analysis.
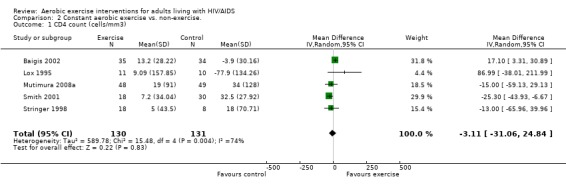
Comparison 2 Constant aerobic exercise vs. non‐exercise., Outcome 1 CD4 count (cells/mm3).
2.2. Analysis.
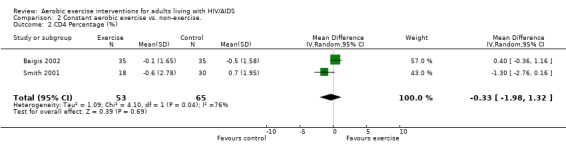
Comparison 2 Constant aerobic exercise vs. non‐exercise., Outcome 2 CD4 Percentage (%).
2.3. Analysis.
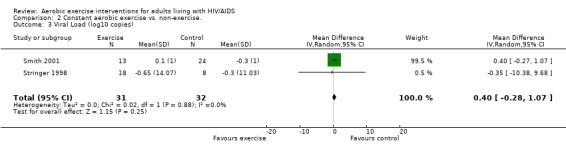
Comparison 2 Constant aerobic exercise vs. non‐exercise., Outcome 3 Viral Load (log10 copies).
2.4. Analysis.
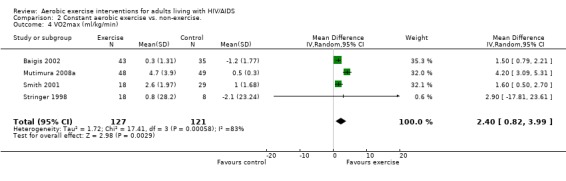
Comparison 2 Constant aerobic exercise vs. non‐exercise., Outcome 4 VO2max (ml/kg/min).
2.5. Analysis.
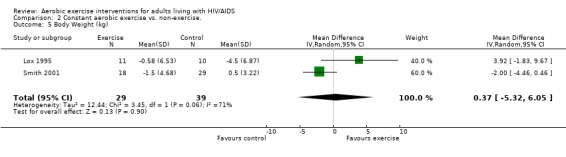
Comparison 2 Constant aerobic exercise vs. non‐exercise., Outcome 5 Body Weight (kg).
2.6. Analysis.
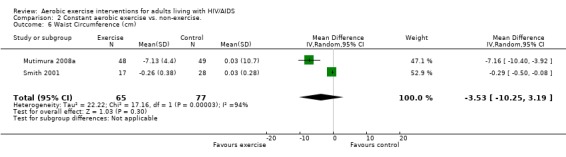
Comparison 2 Constant aerobic exercise vs. non‐exercise., Outcome 6 Waist Circumference (cm).
2.7. Analysis.
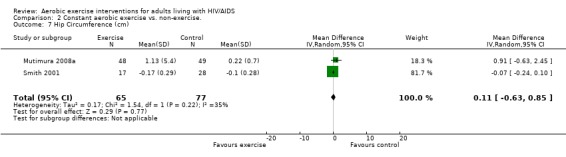
Comparison 2 Constant aerobic exercise vs. non‐exercise., Outcome 7 Hip Circumference (cm).
2.8. Analysis.
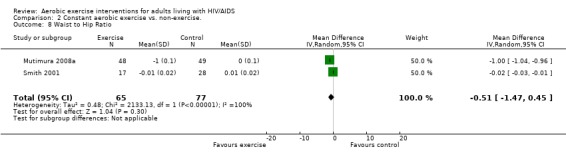
Comparison 2 Constant aerobic exercise vs. non‐exercise., Outcome 8 Waist to Hip Ratio.
2.9. Analysis.
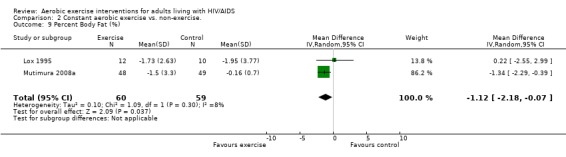
Comparison 2 Constant aerobic exercise vs. non‐exercise., Outcome 9 Percent Body Fat (%).
Comparison 3. Interval aerobic exercise vs. non‐exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CD4 count (cells/mm3) | 2 | 45 | Mean Difference (IV, Random, 95% CI) | 69.58 [14.08, 125.09] |
3.1. Analysis.
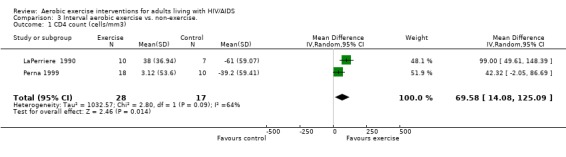
Comparison 3 Interval aerobic exercise vs. non‐exercise., Outcome 1 CD4 count (cells/mm3).
Comparison 4. Moderate aerobic exercise vs. heavy aerobic exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CD4 count (cells/mm3) | 2 | 39 | Mean Difference (IV, Random, 95% CI) | ‐42.90 [‐116.28, 30.47] |
| 2 VO2max (ml/kg/min) | 2 | 24 | Mean Difference (IV, Random, 95% CI) | 4.30 [0.61, 7.98] |
4.1. Analysis.
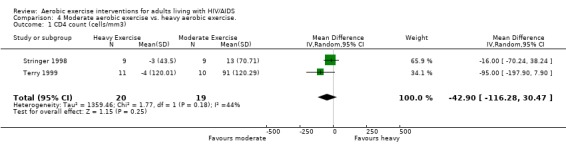
Comparison 4 Moderate aerobic exercise vs. heavy aerobic exercise., Outcome 1 CD4 count (cells/mm3).
4.2. Analysis.
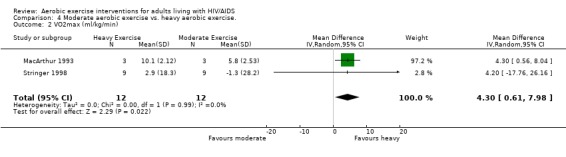
Comparison 4 Moderate aerobic exercise vs. heavy aerobic exercise., Outcome 2 VO2max (ml/kg/min).
Comparison 5. Constant or interval aerobic exercise and progressive resistive exercise compared with no exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CD4 count (cells/mm3) | 3 | 84 | Mean Difference (IV, Random, 95% CI) | 21.52 [‐29.25, 72.28] |
| 2 Viral Load (log10 copies) | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.13, 0.74] |
| 3 Exercise time (min) | 2 | 62 | Mean Difference (IV, Random, 95% CI) | 3.92 [‐0.63, 8.47] |
| 4 Body Weight (kg) | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 0.96 [‐1.39, 3.30] |
| 5 Leg Muscle Area (cm2) | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 4.79 [2.04, 7.54] |
| 6 Fat Mass (kg) | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐1.22, 1.36] |
5.1. Analysis.
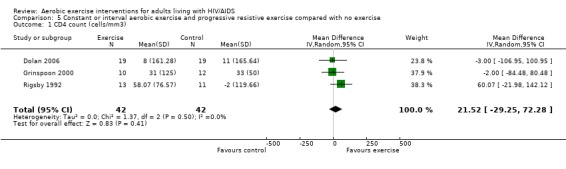
Comparison 5 Constant or interval aerobic exercise and progressive resistive exercise compared with no exercise, Outcome 1 CD4 count (cells/mm3).
5.2. Analysis.
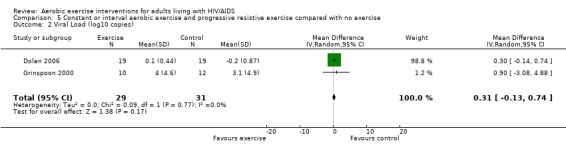
Comparison 5 Constant or interval aerobic exercise and progressive resistive exercise compared with no exercise, Outcome 2 Viral Load (log10 copies).
5.3. Analysis.
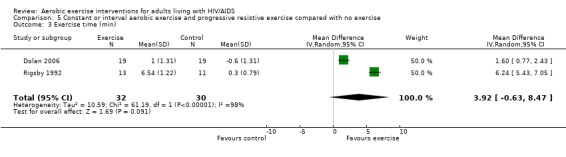
Comparison 5 Constant or interval aerobic exercise and progressive resistive exercise compared with no exercise, Outcome 3 Exercise time (min).
5.4. Analysis.
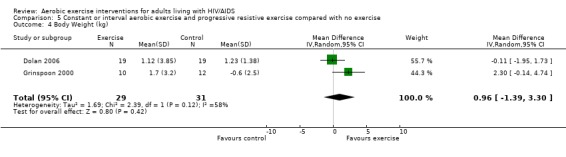
Comparison 5 Constant or interval aerobic exercise and progressive resistive exercise compared with no exercise, Outcome 4 Body Weight (kg).
5.5. Analysis.
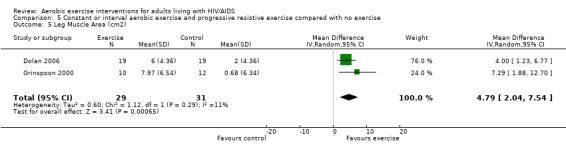
Comparison 5 Constant or interval aerobic exercise and progressive resistive exercise compared with no exercise, Outcome 5 Leg Muscle Area (cm2).
5.6. Analysis.
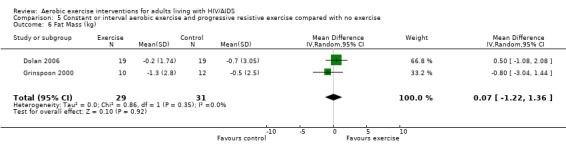
Comparison 5 Constant or interval aerobic exercise and progressive resistive exercise compared with no exercise, Outcome 6 Fat Mass (kg).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baigis 2002.
| Methods | Randomized exercise and control groups. | |
| Participants | 123 participants at baseline (80% males). EXERCISE GROUP: 68 participants at baseline and 35 at study completion. NON‐EXERCISING CONTROL GROUP: 55 participants at baseline and 34 at study completion. | |
| Interventions | INTERVENTION GROUP: Ski machine. 40 minutes total: 5 minute stretching, 5 minute warm‐up on machine, 20 minutes constant aerobic exercise at 75‐85% HRmax followed by 5 minutes cool‐down and 5 minutes stretching. Duration and Frequency: 3X per week for 15 weeks. Supervised exercise. CONSTANT AEROBIC VERSUS NON‐EXERCISING CONTROL | |
| Outcomes | IMMUNE INDICES: No significant changes in CD4 count. CARDIOPULMONARY OUTCOMES: No significant differences in VO2max between exercisers versus non‐exercisers. Results were attributed to the level of intensity and duration of exercise. STRENGTH OUTCOMES: Not assessed. WEIGHT AND BODY COMPOSITION OUTCOMES: Not assessed. PSYCHOLOGICAL OUTCOMES: Non‐significant trend favouring exercisers compared to non‐exercisers in HRQL. Significant improvement in overall health subscale of the MOS‐HIV found among exercisers compared to non‐exercisers. ADVERSE EVENTS: Not reported. AUTHOR'S CONCLUSIONS: Exercise appeared to be safe in HIV‐infected individuals. | |
| Notes | This study was added in the second update of this review (published in 2005). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Dolan 2006.
| Methods | Randomized exercise and control groups. | |
| Participants | 40 HIV positive women with self‐reported and physical evidence of changes in fat distribution, between 18‐60 years of age, with a waist‐to‐hip ratio of 0.85 or more. Women were excluded it they used megestrol acetate, growth hormone or glucocorticoid therapy who had significant liver or kidney disease, or severe anemia, who were receiving insulin, had history of diabetes, had a fasting glucose level of 126 mg/dL, were pregnant or seeking to become pregnant, or breast feeding, had an acute infection or initiated a new ARV regimen within 1 month of the study. INTERVENTION GROUP: (aerobic + PRE exercise): 20 participants at baseline and 19 participants at study completion NON‐EXERCISING CONTROL GROUP: 20 participants at baseline and 19 participants at study completion. | |
| Interventions | INTERVENTION GROUP (Aerobic + PRE Exercise): Combined PRE and aerobic exercise for 2 hours total. Aerobic Component: 5 minute warm‐up on stationary bike at 50% estimated HRmax, followed by standard flexibility routine and aerobic and PRE exercise according to ACSM guidelines followed by a cool down period. PRE Component: concentric and eccentric phases of 6 selected upper and lower body muscle groups; Week 1: 3 sets of 10 reps for each muscle group at 60% 1‐RM, 3‐5 seconds between reps rest, 2 min rest between sets, 4 min rest between muscle groups; week 3‐16: 4 sets of 8 reps for each muscle group at 70% 1‐RM (Week 2‐3), and 80% 1‐RM (week 4‐16), 2‐3 seconds between reps rest, 1 min rest between sets, 2 min rest between muscle groups. Each repetition lasted 6‐10 seconds each. Duration and Frequency: 3X per week for 16 weeks. Supervised exercise at home. NON‐EXERSCISING CONTROL GROUP: No intervention. 1‐RM made at baseline, 8 and 16 weeks. CONSTANT AEROBIC + PRE VERSUS NON‐EXERCISING CONTROL | |
| Outcomes | IMMUNE INDICES: No significant changes in CD4 count or viral load. CARDIOPULMONARY OUTCOMES: Significant improvements in exercise time as measured by submaximal exercise time and 6MWT distance among exercisers compared with non‐exercisers. Significant improvements in VO2max among exercisers compared with non‐exercisers. STRENGTH OUTCOMES: Significant improvements in upper and lower extremity strength (7 measures) among exercisers compared with non‐exercisers. WEIGHT AND BODY COMPOSITION OUTCOMES: No significant change in weight between groups. Significant increase in total cross‐sectional muscle area and muscle attenuation among exercisers compared with non‐exercisers. Significant decrease in waist circumference among exercisers compared with non‐exercisers. No significant difference between group for body mass index, abdominal visceral tissue area, subcutaneous adipose tissue area, and total fat. PSYCHOLOGICAL OUTCOMES: Not assessed. ADVERSE EVENTS: Authors reported 1 participant who had an exacerbation of asthma, and 1 participant had chest pain but neither were related to exercise. AUTHOR'S CONCLUSIONS: A 16 week supervised home based PRE and aerobic exercise program improves measures of strength, cardiorespiratory fitness, and body composition among women living with HIV. | |
| Notes | Intervention was a home‐based exercise program. Participants completed 96% of the total exercise sessions. This study was added in this third and most recent update of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Driscoll 2004a.
| Methods | Randomized combined exercise and metformin and metformin‐only group. | |
| Participants | 37 HIV infected positive men and women on stable antiretroviral therapy for more than 3 months with evidence of fat redistribution (truncal obesity with a WHR ratio >0.90 in men and >0.85 in women and lipodystrophy score >2) and hyperinsulinemia. INTERVENTION GROUP: (exercise + metformin): 19 participants at baseline and 11 participants at study completion NON‐EXERCISING METFORMIN ONLY GROUP: (no exercise + metformin): 18 participants at baseline and 14 participants at study completion. | |
| Interventions | INTERVENTION GROUP (Exercise + Metformin): Constant aerobic followed by resistive training consisting of 20 minutes aerobic exercise on stationary cycle at 60% HRmax (week 1‐2) and progressing to 30 minutes at 75% HRmax (week 3‐12) according to ACSM guidelines, 5min warm‐up on stationary bike, standard flexibility routine, followed by resistance training. PRE consisted of 3 sets of 10 repetitions for every muscle group, resting 2‐3 seconds between repetitions, 2 minutes between sets, and 4 minutes between muscle group. Week 1: initial intensity of PRE was 60% 1‐RM; week 2‐4 intensity increased to 70% 1‐RM; week 4‐12 intensity of 80% 1‐RM. 1‐RM was measured every other week and load adjusted to maintain relative intensity at 80% 1‐RM. Duration and Frequency: 3X per week for 12 weeks. Supervised exercise. METFORMIN ONLY GROUP: 500 mg of metformin twice per day, with a dose increase to 850 mg twice a day (week 2‐12). CONSTANT AEROBIC + PRE + METFORMIN VERSUS METFORMIN ONLY | |
| Outcomes | IMMUNE INDICES: No significant changes in CD4 count or viral load. CARDIOPULMONARY OUTCOMES: Significant improvements in endurance time on cycle ergometer during submaximal stress test in the exercise and metformin group compared with the metformin only group. STRENGTH OUTCOMES: Significant increases in upper and lower extremity strength (five of six indices) in the exercise and metformin group compared with the metformin only group. WEIGHT AND BODY COMPOSITION OUTCOMES: Significant increases in cross‐sectional muscle area, and significant decreases in waist‐to‐hip ratio and abdominal fat area in the exercise and metformin group compared with the metformin only group. No significant changes in weight and body mass index in either group. PSYCHOLOGICAL OUTCOMES: Not assessed. ADVERSE EVENTS: None reported. AUTHOR'S CONCLUSIONS: Exercise training and metformin significantly improve cardiovascular outcomes more than metformin alone in persons living with HIV with fat redistribution and hyperinsulinemia. Exercise training (aerobic and PRE) is well‐tolerated and improves muscle strength and size as well as aerobic fitness in persons living with HIV. | |
| Notes | This study was added in this third and most recent update of this review. Driscoll 2004a and 2004b are two studies that report on the same participants. Driscoll 2004a is reported in the table of included studies, because it is the earlier of the two studies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Grinspoon 2000.
| Methods | Randomized exercise and control groups | |
| Participants | 54 HIV‐infected men with AIDS‐related wasting (weight<90% ideal body weight or self‐reported weight loss>10%) and normal serum level of free testosterone. 4 groups included exercise + testosterone, exercise + placebo, no exercise + testosterone, control. INTERVENTION GROUP (exercise + placebo): 13 participants at baseline (10 participants analysed at week 12). NON‐EXERCISING CONTROL GROUP: 13 participants at baseline (12 participants analysed at week 12). | |
| Interventions | INTERVENTION GROUP: Supervised progressive strength training and constant aerobic conditioning consisting of 20 minutes aerobic exercise on stationary cycle at 60‐70% HRmax, 15min cool‐down followed by resistance training. Duration and Frequency: 3x per week for 12 weeks. Supervised exercise. CONSTANT AEROBIC + PRE VERSUS NON‐EXERCISING CONTROL | |
| Outcomes | IMMUNE INDICES: No significant response in CD4 count or viral load to exercise or testosterone therapy either alone or together as a co‐intervention. CARDIOPULMONARY OUTCOMES: Not assessed. STRENGTH OUTCOMES: No significant change in strength (note strength was tested isometrically, which may underestimate change in strength). WEIGHT AND BODY COMPOSITION OUTCOMES: Participants in the exercise only group showed significant increases in lean body mass, arm muscle area, leg muscle area, HDL cholesterol and significant decreases in AST level compared to non‐exercising control group. No significant changes in weight and fat mass in either the exercisers or non‐exercising control group. PSYCHOLOGICAL OUTCOMES: Not assessed. ADVERSE EVENTS: Reported no deaths or adverse events. AUTHOR'S CONCLUSIONS: Exercise has a significant effect on lean body mass and muscle area independent of testosterone. Muscle mass and strength may further increase in response to combined exercise and testosterone therapy. Exercise was associated with an increase in HDL cholesterol whereas testosterone decreased HDL cholesterol. Exercise significantly increases muscle mass and offers cardio protective effects by increasing the HDL cholesterol in eugonadal men with AIDS wasting. Exericse may be a strategy to reverse muscle loss in this population. | |
| Notes | This study was duplicated by Fairfield 2001 which reported on the same participants in Grinspoon 2000. AUTHOR'S COMMENTS: Participants in this study were men with AIDS‐related wasting. The goal of exercise was to increase body composition versus other previous studies where the goal was to decrease body composition. NOTE: For the purposes of this systematic review, we extracted results from the control group (no exercise + placebo) and exercise + placebo group to isolate the effects of exercise. [note that Fairfield 2001 is a duplicate of this study] | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
LaPerriere 1990.
| Methods | Randomized exercise and control groups. | |
| Participants | 50 gay men, not previously diagnosed HIV positive, between 18 and 40 years of age. Participants were excluded if they were routinely active with regular aerobic exercise. INTERVENTION GROUP: 30 participants (10 later found to be HIV+) NON‐EXERCISING CONTROL GROUP: 20 participants (7 later found to be HIV+). | |
| Interventions | INTERVENTION GROUP: Stationary bike 45 minutes total @ 80% HRmax for 3 minutes, then @ 60‐79% HRmax for 2 minutes. Duration and Frequency: 3x /week for five weeks. Supervision: Not reported. INTERVAL AEROBIC VERSUS NON‐EXERCISING CONTROL | |
| Outcomes | IMMUNE INDICES: Seropositive exercisers showed increase in CD4 count by 38 cells/mm3. Seropositive controls showed decrease in CD4 count by 61 cells/mm3. CARDIOPULMONARY MEASURES: No change in V02 max in non‐exercising controls. Improvements in fitness level averaged 10% change in VO2 max in both seronegative and seropositive exercisers. STRENGTH OUTCOMES: Not assessed. WEIGHT AND BODY COMPOSITION OUTCOMES: Not assessed. PSYCHOLOGICAL OUTCOMES: Seropositive non‐exercising controls showed significantly larger increases in anxiety and depression than intervention groups as measured by the tension‐anxiety subscale and depression‐dejection subscale of the profile of mood state (POMS) scale. ADVERSE EVENTS: Not reported. AUTHOR's CONCLUSIONS: Aerobic exercise is a beneficial stress management intervention which may be a useful strategy for attenuating an acute stressor such as postnotification of HIV status. | |
| Notes | AUTHOR'S COMMENTS: Measures were taken pre and post notification of HIV status (not at baseline prior to exercise intervention). Additional clarification is required to confirm the actual number of participants that were analyzed in the study after the intervention. [note that LaPerriere 1991 is a duplicate of this study] | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Lox 1995.
| Methods | Randomized to two exercise groups (PRE and aerobic) and one control group. | |
| Participants | 34 participants at baseline (all males). PRE GROUP: 12 participants at baseline and at study completion AEROBIC GROUP: 12 participants at baseline and 11 at study completion. NON‐EXERCISING CONTROL GROUP: 10 participants at baseline and study completion. | |
| Interventions | INTERVENTION GROUP (AEROBIC): Stationary bike, 45 minutes total: 5 minutes warm‐up (stretching), 24 minutes cycle ergometer at 50‐60% heart rate reserve (HRR), 15 minutes cool‐down. INTERVENTION GROUP (PRE): Isotonic resistance to major muscle groups in legs, arms and upper body. Resistance was initiated at 60% of an individual's 1‐RM and increased by either 5 or 10 pounds at a time after successful performing 3 sets of 10 reps at constant weight. Duration and Frequency: 3X per week for 12 weeks. Supervised exercise. CONSTANT AEROBIC VERSUS PRE VERSUS NON‐EXERCISING CONTROL | |
| Outcomes | IMMUNE INDICES: No significant changes in CD4 count. CARDIOPULMONARY OUTCOMES: Significant improvements in VO2max among exercisers compared to non‐exercisers with greater improvements in the aerobic compared to the PRE and non‐exercising control groups. Non‐significant decrease in submaximum HR in the PRE group compared to a non‐significant increase in the non‐exercising control group. STRENGTH OUTCOMES: Significant improvements in the PRE and aerobic exercise groups compared to the non‐exercising control groups. Significantly greater improvements as measured by 1‐RM in the PRE group (U/E: +31.5 lbs, L/E: +90.7 lbs) compared to the aerobic and non‐exercising control groups (U/E: ‐6.8 lbs, L/E: ‐14.7 lbs). WEIGHT AND BODY COMPOSITION OUTCOMES: No change among all 3 groups in average body mass index, fat mass, and body fat percentage. Significant increases in weight, lean body mass and sum of chest, arm and thigh circumference among PRE and aerobic exercise groups. PSYCHOLOGICAL OUTCOMES: Significant improvements in mood and life satisfaction in both the aerobic and PRE exercise groups compared to the non‐exercising control group. Significantly higher life satisfaction in the aerobic group compared with the PRE group. ADVERSE EVENTS: Not reported. AUTHOR'S CONCLUSIONS: Exercise results in improvements in body composition, strength, cardiopulmonary fitness, and mood and life satisfaction for people living with HIV. | |
| Notes | This study was added in the second update of this review. Lox 1995 and Lox 1996 are two studies that report on the same participants. Lox 1995 is reported in the table of included studies, because it is the earlier of the two studies. For the purpose of this review, only the aerobic and control groups were included in meta‐analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
MacArthur 1993.
| Methods | Block randomised to two exercise intervention groups and one control group. | |
| Participants | 25 participants at baseline (24 male, 1 female). Analysis based on six participants defined as 'compliant with exercise program' | |
| Interventions | HIGH INTENSITY EXERCISE‐INTERVENTION #1: High intensity exercise: 24 minutes total @75‐85% V02max x 4 minutes x 6 intervals. LOW INTENSITY EXERCISE INTERVENTION #2: Low intensity exercise: 40 minutes total @50‐60% V02max x 10 minutes x 4 intervals. Exercise included walking, jogging, biking, rowing and stair‐stepping. Duration and Frequency: 3 x /week for 24 weeks. Supervision: Not reported. INTERVAL AEROBIC (HIGH VERSUS LOW INTENSITY) | |
| Outcomes | Six participants were compliant (>80% exercise program), seven somewhat compliant (30‐80%) and 12 were non‐compliant (<30%). Results based on six 'compliant' participants. IMMUNE INDICES: No significant changes in CD4 count. CARDIOPULMONARY OUTCOMES: The high intensity group may have obtained a greater training effect than the low‐intensity group (not significant.) Significant increases in compliant exercisers (n=6) for V02 max (24%), minute ventilation (13%), and oxygen pulse (24%). At 12 weeks HR rate pressure product and RPE all decreased significantly in a group of 10 subjects (comp, somcomp, noncomp). STRENGTH OUTCOMES: Not assessed. WEIGHT AND BODY COMPOSITION OUTCOMES: Not assessed. PSYCHOLOGICAL OUTCOMES: General health questionnaire scores improved for the 6 compliant participants. ADVERSE EVENTS: No detrimental hematologic or immunologic effects were noted. AUTHOR'S CONCLUSIONS: Exercise training is feasible and beneficial for moderate to severely immunocompromised HIV‐infected individuals. | |
| Notes | AUTHOR'S COMMENTS: Unable to determine differences in high vs. low intensity exercise groups due to low number of participants (6) who were compliant with exercise program. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Mutimura 2008a.
| Methods | Randomized exercise and control groups. | |
| Participants | 100 HIV positive men and women between 21‐50 years of age, with moderate to severe body fat redistribution (BFR), on a stable World Health Organizations HAART regimen for > 6 months. Moderate to severe BFR defined as score of 3 to 4 overall rating for change in body fat content and a total Body Fat Ratio score of >18. Excusion criteria included emotional distress or psychosis, unstable angina, shortness of breath with exercise, a known medical history that would contraindicate exercise training, acute infections, or AIDS defining opportunistic infections, musculoskeletal or neuromuscular disorders, unwillingness to exercise for >6 months. INTERVENTION GROUP: (aerobic exercise): 50 participants at baseline and 48 participants at study completion. NON‐EXERCISING CONTROL GROUP: 50 participants at baseline and 49 participants at study completion. | |
| Interventions | INTERVENTION GROUP (Aerobic Exercise): Six month supervised exercise programme at a fitness club in Kigali, Rwanda. Aerobic Exercise: 'proper warm up', stretching, and 15 minutes of brisk walking, followed by 45‐60 minutes of jogging, running, stair climbing, low‐back and abdominal stabilization and strengthening exercises, followed by a 15 minute cool down and stretching exercises. Intensity: Gradual progression to encourage participants to perform jogging and running with the goal of achieving at least 45% maximum heart rate (Weeks 1‐3), 60% maximum heart rate (Weeks 3‐8), and 75% maximum heart rate (Weeks 8‐24). Compliance was defined as attending >50% of the sessions. Duration and Frequency: 3X per week, 1.5 hour per session, alternating days for 24 weeks (6 months). Supervised exercise at a fitness club. NON‐EXERCISING CONTROL GROUP: No intervention. CONSTANT AEROBIC VERSUS NON‐EXERCISING CONTROL | |
| Outcomes | IMMUNE INDICES: No significant changes in CD4 count between groups (no measure of viral load). CARDIOPULMONARY OUTCOMES: Exercise group achieved a higher heart rate and rate of perceived exertion (RPE) at the end of the 20 metre multi‐stage shuttle run test (20mMST). Significant improvements in VO2max among exercisers compared with non‐exercisers as measured by the 20mMST. STRENGTH OUTCOMES: Not measured. WEIGHT AND BODY COMPOSITION OUTCOMES: Change in body weight not measured. Significant decrease in body mass index (BMI), percent body fat mas (BFM), waist circumference, and waist‐to‐hip ratio among exercisers whereas these outcomes remained unchanged or increased among non‐exercisers. Significant decrease in sum of skim folds, and percent body fat mass (%) and total body fat redistribution score (BFR) among exercisers compared with non‐exercisers. Significant decrease in triceps, biceps, subscapular, suprailiac, and sum of skinfold thickness decreased more in the exercisers compared with non‐exercisers. No change in hip circumference in either group. PSYCHOLOGICAL OUTCOMES: Significant improvements in quality of life (QOL) on the psychological, independence, social relationships, HIV+ HAART‐specific domains of QOL, and overall QOL score as measured by the World Health Organization Quality of Life HIV Instrument (WHOQOL‐BREF) for exercisers compared with non‐exercisers. No difference between groups on the physical QOL domain score. ADVERSE EVENTS: Adverse events within the study were not reported. Compliance with exercise was 82.2% AUTHOR'S CONCLUSIONS: Exercise training positively improves body composition, cardiorespiratory fitness and several components of QOL in HAART‐treated HIV+ African participants with Body Fat Redistribution. Results imply that exercise training is a safe, inexpensive, practical and effective treatment for evolving metabolic and cardiovascular syndromes associated with HIV and HAART exposure in resource‐limited settings such as Su‐Saharan Africa. | |
| Notes | SPECIAL NOTES: While the description of exercise includes 'strengthening exercises' there are no specific details provided on the types of strengthening, intensity, and progression. Given the overall intervention is described as 'Cardiorespiratory Exercise Training (CET) we classified this study as an aerobic exercise intervention only. Note, all participants had moderate to severe body fat redistribution. Overall compliance with the exercise was 82.2%. This study included two additional comparison groups: HIV positive non‐exercisers with no body fat redistribution, and HIV seronegative non‐exercisers. However, we only considered the two comparable groups of exercisers versus non‐exercisers (with BFR) in this review. This study was added in this third and most recent update of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D‐not used |
Perna 1999.
| Methods | Randomized exercise and control groups. | |
| Participants | 43 participants at baseline between 18 and 49 years old, HIV+, symptomatic, mildly progressed HIV infection (but not AIDS); diagnosed with HIV at least 6 months prior to study. INTERVENTION GROUP: 18 participants (13 men, 5 women) at study completion. NON‐EXERCISING CONTROL GROUP: 10 participants (5 men, 5 women) at study completion. 11/18 participants from intervention group were 'compliant' with exercise program (9 men, 2 women). | |
| Interventions | INTERVENTION GROUP: Stationary bike 45 minutes total @ 70‐80% HR max x 3 min then 2 min "off" (10 min stretch pre and post). Duration and Frequency: 3 x/week for 12 weeks. Supervised exercise. INTERVAL AEROBIC VERSUS NON‐EXERCISING CONTROL | |
| Outcomes | 61% of the exercisers adherent (>50% attendance) with the exercise program. IMMUNE INDICES: Adherent exercisers increased CD4 count by 13% whereas non‐adherent exercisers decreased CD4 count by 18%. Control participants showed a decreasing trend of CD4 count by 10%. CARDIOPULMONARY OUTCOMES: Significant increase in V02 max (12%), 02 pulse (13%), maximum tidal volume (8%), and minute ventilation (VE) (17%) among adherence exercisers. No significant differences were found in non‐adherent exercisers and non‐exercising control groups. STRENGTH OUTCOMES: Significant increase in leg power by 25% adherent exercisers and no change in non‐adherent exercisers or non‐exercising controls. WEIGHT AND BODY COMPOSITION OUTCOMES: Significant increase in body mass index among adherent exercisers. PSYCHOLOGICAL OUTCOMES: No significant differences were noted of physician‐rated health status (note this outcome was not considered a true measure of psychological status because it was not completed by patient self‐report). ADVERSE EVENTS: One hospitalization was reported during the course of the study. AUTHOR'S CONCLUSIONS: Aerobic exercise may significantly increase CD4 count among symptomatic HIV+ individuals. Exercise non‐adherence may be associated with a faster CD4 decline (due to several possible mechanisms). | |
| Notes | AUTHOR'S COMMENTS: Possible reason for non‐adherent exercisers showing a decrease in CD4 count is moderate intensity exercise of sporadic frequency may exert a temporary immunosuppressive effect among those already immunocompromised. Attention to factors influencing exercise adherence is needed. SPECIAL NOTE: The article showed results of adherent exercisers versus non‐adherent exercisers versus non‐exercising control group. For the meta‐analysis a weighted average was calculated to combine data of compliant and non‐adherent exercisers in order to compare results to the non‐exercising control group. Additional clarification is required to determine the standard deviation of results of intention to treat analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Rigsby 1992.
| Methods | Randomized exercise and control (counselling) groups. | |
| Participants | 45 male participants (37 HIV+, 8HIV‐), physically inactive for 6 months, CD4 count range 8‐804 cells/mm3. INTERVENTION GROUP: 22 participants (19 HIV+, 3 HIV‐) at baseline and 16 participants (13 HIV+, 3 HIV‐) study completion. NON‐EXERCISING CONTROL GROUP: 23 participants (18 HIV+, 5 HIV‐) at baseline and 15 participants (11 HIV+, 4HIV‐) at study completion. | |
| Interventions | INTERVENTION GROUP: Stationary bike 60 minutes total @60‐80% HRreserve x 20 min (2 min warm‐up and 3 min cool down at low intensity.) Stretching x 10‐15 minutes. Strengthening x 20‐25 minutes. Duration and Frequency: 3 x/ week for 12 weeks. Supervised exercise. NON‐EXERCISING CONTROL GROUP: Received 90‐120 minutes of counselling 1‐2 times per week for 12 weeks. CONSTANT AEROBIC + PRE VERSUS NON‐EXERCISING CONTROL | |
| Outcomes | IMMUNE INDICES: No significant changes in CD4 count. CARDIOPULMONARY OUTCOMES: Significant increases in aerobic capacity were shown in the exercise group with no change in non‐exercising control group. Significant decreases in HR and increases in total time exercise to voluntary exhaustion, STRENGTH OUTCOMES: Significant increases in chest press and leg extension in the exercise group. WEIGHT AND BODY COMPOSITION: Not assessed. PSYCHOLOGICAL OUTCOMES: Not assessed. ADVERSE EVENTS: One death reported in the counselling group during the course of the study and one death one month after the study. Of the 4 participants who dropped out of the exercise group, one died immediately after the study conclusion. AUTHOR'S CONCLUSIONS: HIV+ men (including those symptomatic for AIDS‐related complex) can experience significant increases in neuromuscular strength and cardiorespiratory fitness when prescribed and monitored in accordance with ACSM guidelines for healthy adults. Increased fitness may occur without negative effects on immune status. | |
| Notes | AUTHOR'S COMMENTS: This study looked at exercise across entire range of disease spectrum. Only one participant who had progressed to AIDS completed the exercise protocol. This participant demonstrated similar results to the others. Exercise could exert an immune response that is not reflected in the clinical measures taken. Also note: for the purposes of this review, 'non‐exercising control group' was defined as non‐exercise. In this study, however, the controls received 90‐120 minutes of counselling 1‐2 x/week for 12 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Smith 2001.
| Methods | Randomized exercise and control groups. | |
| Participants | 60 HIV positive adults (52 males, 8 females) at baseline on stable ARV therapy. Participants were excluded if they were taking steroids, growth hormone, or appetite stimulants, had an AIDS‐defining illness, fever, active wasting or weight less than 85% of ideal body weight and pregnancy. INTERVENTION GROUP: 30 participants at baseline (19 participants at 12 weeks). NON‐ EXERCISING CONTROL GROUP: 30 participants at baseline and 12 weeks. Non‐Exercising Control participants were offered the exercise intervention at 12‐24 weeks. | |
| Interventions | INTERVENTION GROUP: Minimum of 30 minutes constant aerobic exercise at 60‐80% V02 max consisting of mandatory 20 minutes walking/jogging on treadmill and remaining time spent either on stationary bicycle, stair stepper or cross‐country machine. Duration and Frequency: 3x per week for 12 weeks. Supervised exercise. CONSTANT AEROBIC VERSUS NON‐EXERCISING CONTROL | |
| Outcomes | IMMUNE INDICES: No significant changes in CD4 cell count, CD4+ percentage, and viral load in either group. CARDIOPULMONARY OUTCOMES: Significant decrease in fatigue in exercisers compared with non‐exercisers as exercisers were able to stay on the treadmill 1 minute longer compared to non‐exercising control group (significant decrease in fatigue). No significant effect on rate of perceived exertion (RPE) or FEV1 in either group (dyspnea). Significant improvements in V02max in the experimental group (2.6ml/kg per min) compared with the non‐exercising control group (1.0ml/kg per min). STRENGTH OUTCOMES: Not assessed. WEIGHT AND BODY COMPOSITION OUTCOMES: Significant decrease in waist‐to‐hip ratio among exercisers (note many participants were above ideal body weight prior to exercise; thus decreases in both weight and body composition were considered favourable outcomes). Exercise group showed significant decreasing trends in weight, BMI, triceps, central and peripheral skin folds, abdominal girth and waist‐to‐hip ratio. PSYCHOLOGICAL OUTCOMES: [reported in Neidig 2003]: Significant improvements in Centre for Epidemiological Studies Depression Scale (CED‐DS), Profile of Mood State and Depression‐dejection subscale of POMS scale, and non‐significant trend to improvement in Beck Depression Inventory in the exercise group compared to non‐exercising control group. ADVERSE EVENTS: No adverse events reported. AUTHOR'S CONCLUSIONS: Supervised aerobic exercise training safely decreases fatigue, weight, BMI, subcutaneous fat and central fat in HIV‐infected individuals. [Neidig 2003]: Exercisers showed reductions in depressive symptoms. | |
| Notes | AUTHOR'S COMMENTS: Only 14 of the 60 participants were on highly ARV therapy, thus more research is needed to investigate whether central fat can be preferentially reduced without a loss of peripheral fat. Many participants were above ideal body weight and reduced body composition without reducing calories consumed. [note that Neidig 2003 is a duplicate of this study]. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Stringer 1998.
| Methods | Randomized to two exercise intervention groups and one control group. | |
| Participants | 34 HIV+ participants at baseline, CD4 between 100‐500 cells/mm3, no signs of opportunistic infection (11% females ˜4/34). MODERATE INTENSITY (INTERVENTION #1): 12 participants at baseline and 9 participants at study completion. HEAVY INTENSITY (INTERVENTION #2): 11 participants at baseline and 9 participants at study completion. NON‐EXERCISING CONTROLS: 11 participants at baseline and 8 at study completion. | |
| Interventions | MODERATE INTENTSITY (INTERVENTION #1): stationary cycle ergometer @ 80% LAT x 60 min. HEAVY INTENSITY (INTERVENTION #2): stationary cycle ergometer @ 50% of difference between LAT and VO2 max x 30‐40 minutes. Duration and Frequency: 3 x/week frequency for 6 weeks. Supervision: Not reported. CONSTANT AEROBIC VERSUS NON‐EXERCISING CONTROL | |
| Outcomes | IMMUNE INDICES: No significant changes in CD4 counts or viral load in all three groups. CARDIOPULMONARY OUTCOMES: An intensity‐related aerobic training effect was seen (heavy>moderate) relative to the non‐exercising control group. Significant increases in V02 max and Work Rate max in the heavy exercise group. LAT increased significantly in both intervention groups. STRENGTH OUTCOMES: Not assessed. WEIGHT AND BODY COMPOSITION OUTCOMES: Not assessed. PSYCHOLOGICAL OUTCOMES: Significant improvements in both exercise groups on the QOL questionnaire relative to the non‐exercising control group. No significant differences in QOL between the two intervention groups. ADVERSE EVENTS: No adverse events reported. AUTHOR'S CONCLUSIONS: Exercise training resulted in a substantial improvement in aerobic function (heavy>moderate group) while immune indices were essentially unchanged. QOL markers improved significantly with exercise. Exercise training is safe and effective in this group and should be promoted for HIV+ individuals. | |
| Notes | AUTHOR'S COMMENTS: 77% study completion rate (8 drop outs). Baseline measures were taken. SPECIAL NOTE: For the meta‐analysis of exercise versus non‐exercising control group the results of the moderate and heavy exercise groups were combined. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Terry 1999.
| Methods | Randomized to two exercise intervention groups. | |
| Participants | 31 participants at baseline. Analysis based on 21 HIV+ participants (14 men, 7 women), inactive for at least 6 months, CD4>200 cells/mm3. MODERATE INTENSITY (INTERVENTION #1): 10 participants (6 males) at study completion. HEAVY INTENSITY (INTERVENTION #2): 11 participants (8 males) at study completion. | |
| Interventions | MODERATE INTENSITY (INTERVENTION #1): Moderate exercise: walking @55‐60% HRmax x 30 min (5 minutes @ target intensity, 1 minute recovery.) (15 minutes stretch pre and post) HIGH INTENSITY (INTERVENTION #2): High exercise: running @75‐85% HRmax x 30 minutes (5 minutes @ target intensity, 1 minute recovery.) (15 minute stretch pre and post) Exercise included walking, running and stretching. Duration and Frequency: 3 x/week for 12 weeks. Supervision: Not reported. CONSTANT AEROBIC (MODERATE VERSUS HEAVY INTENSITY) | |
| Outcomes | IMMUNE INDICES: No significant changes in CD4 count. CARDIOPULMONARY OUTCOMES: Peak HR was unchanged for both groups; peak systolic BP increased significantly only in high intensity group. STRENGTH OUTCOMES: Not assessed. WEIGHT AND BODY COMPOSITION OUTCOMES: No significant change in body mass, body fat percentage, and body density in either intensity exercise group. PSYCHOLOGICAL OUTCOMES: No significant changes detected in depression scores of the Montgomery‐Asberg Depression Score. ADVERSE EVENTS: Not reported. AUTHOR'S CONCLUSIONS: HIV+ individuals can increase fitness levels with aerobic exercise at a range of intensities. HIV+ individuals can obtain cardiorespiratory benefits of exercise similar to seronegative individuals. Moderate exercise was not associated with an improvement in immunologic markers. High intensity had no shown harmful effects. Short term aerobic exercise programs may be safely recommended to HIV+ individuals for improvement in functional capacity. | |
| Notes | AUTHOR'S COMMENTS: 31 participants recruited for study. 9 drop‐outs, 1 excluded. Because there was no non‐exercising control group, it cannot be determined if exercise halted immunological deterioration. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Terry 2006.
| Methods | Randomized to two groups (aerobic exercise + low lipid diet versus low lipid diet only). | |
| Participants | 42 participants at baseline (26 males, 16 females) who were physically inactive for at least six months before study entry, had no contraindications of exercise training and currently taking ARV therapy. Participants were limited to those with hyperlipidemia associated with lipodystrophy syndrome and not on lipid‐lowering therapy and not taking any other medications (steroids). INTERVENTION GROUP (EXERCISE + LOW LIPID DIET): 21 participants at baseline (6 participants at 12 weeks). NON‐EXERCISING CONTROL GROUP (LOW LIPID DIET ONLY): 21 participants at baseline (5 participants at 12 weeks). | |
| Interventions | INTERVENTION GROUP (Exercise + Low Lipid Diet): Constant aerobic exercise consisting of running for 30 minutes at 70‐85% HRmax, with 15min stretching exercises to warm‐up and 15 min to cool‐down (total of 1 hour). NON‐EXERCISING CONTROL GROUP (Low Lipid DIet Only): 45 minutes soft stretching and relaxation routines, without significant elevation of HR. Duration and Frequency: 3X per week for 12 weeks. Supervised exercise. CONSTANT AEROBIC + LOW LIPID DIET VERSUS LOW LIPID DIET ONLY | |
| Outcomes | IMMUNE INDICES: No significant changes in CD4 count or viral load. CARDIOPULMONARY OUTCOMES: Significant improvements in exercise capacity as measured by VO2max on the maximal treadmill test for the combined exercise and diet group and no change seen in the diet only group. No significant changes in HRmax in either group. STRENGTH OUTCOMES: Not assessed. WEIGHT AND BODY COMPOSITION OUTCOMES: Significant decreases in weight, body mass index, waist‐to‐hip ratio, and percentage of body fat in both groups. Significant increases in body density in both groups. No difference between groups. PSYCHOLOGICAL OUTCOMES: Not assessed. ADVERSE EVENTS: No participants dropped out of the study due to infection or illness. AUTHOR'S CONCLUSIONS: HIV positive adults with hyperlipidemia, when engage in 3 months of aerobic exercise and a low lipid diet do not experience significant changes in triglycerides, total cholesterol, or HDL cholesterol levels (not shown here) but they do improve functional exercise capacity. | |
| Notes | This study was added in this third and most recent update of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agin 2001 | Study did not meet inclusion criteria |
| Batterham 2000 | Study did not meet inclusion criteria |
| Bhasin 2000 | Study did not meet inclusion criteria |
| Birk 2000 | Study did not meet inclusion criteria |
| Driscoll 2004b | Duplication of Driscoll 2004a. For characteristics of this study, please see table of characteristics of duplicate studies. The participants are the same, but this study may report on different outcomes. Any new outcomes that were reported in this study were included in the review. |
| Engelson 2006 | Study did not meet inclusion criteria (no comparison group). |
| Fairfield 2001 | Duplication of Grinspoon 2001. For characteristics of this study, please see table of characteristics of duplicate studies.The participants are the same, but this study may report on different outcomes. Any new outcomes that were reported in this study were included in the review. |
| Galantino 2005 | Study did not meet inclusion criteria (exercise only two times weekly). |
| Greene 1999 | Study did not meet inclusion criteria |
| Hand 2008 | Study did not meet inclusion criteria (exercise only two times weekly). |
| Henry 1999 | Study did not meet inclusion criteria |
| Lane 2000 | Study did not meet inclusion criteria |
| LaPerriere 1991 | Duplication of LaPerriere 1990. For characteristics of this study, please see table of characteristics of duplicate studies.The participants are the same, but this study may report on different outcomes. Any new outcomes that were reported in this study were included in the review. |
| Lox 1996 | Duplicate of Lox 1995. For characteristics of this study, please see table of characteristics of duplicate studies.The participants are the same, but this study may report on different outcomes. Any new outcomes that were reported in this study were included in the review. |
| McCain 2008 | Study did not meet inclusion criteria (only once weekly exercise (tai chi) sessions). |
| Melroe 1999 | Study did not meet inclusion criteria |
| Mustafa 1999 | Does not specify type of exercise, exercise was only considered at the time of entry into the study (therefore change in exercise intensity may have occurred over time), subjects at varying stages of disease on entry. Subjects followed over time, not an RCT. |
| Mutimura 2008b | Duplication of Mutimura 2008a. For characteristics of this study, please see table of characteristics of duplicate studies.The participants are the same, but this study may report on different outcomes. Any new outcomes that were reported in this study were included in the review. |
| Neidig 2003 | Duplicate of Smith 2001. For characteristics of this study, please see table of characteristics of duplicate studies.The participants are the same, but this study may report on different outcomes. Any new outcomes that were reported in this study were included in the review. |
| Nieman 1999 | Study did not meet inclusion criteria |
| Robins 2006 | Study did not meet inclusion criteria (once weekly tai chi sessions only). |
| Robinson 2007 | Study did not meet inclusion criteria (pilot study with no comparison group). |
| Romeyn 2000 | Study did not meet inclusion criteria |
| Roubenoff 1999 | Study did not meet inclusion criteria |
| Roubenoff 2000 | Study did not meet inclusion criteria |
| Samson 2006 | Study did not meet inclusion criteria (description of study design only). |
| Sattler 1999 | Study did not meet inclusion criteria |
Contributions of authors
Kelly O'Brien ‐ contributions included: protocol development, review of abstracts, consensus process leader, abstraction of study results and quality, written report, meta‐analyses in RevMan, providing a clinical perspective.
Stephanie Nixon ‐ contributions included: protocol development, review of abstracts, consensus process leader, abstraction of study results and quality, written report, providing a clinical perspective.
Anne‐Marie Tynan ‐ contributions included: literature search, organising retrieval of papers, review of abstracts, follow‐up and communication with authors of included studies, abstraction of study results and quality, written report.
Richard Glazier ‐ contributions included: protocol development, review of abstracts, consensus process participant, abstraction of study results and quality, development of meta‐analysis, written report, providing a clinical perspective, providing a methodological perspective.
Sources of support
Internal sources
Department of Family and Community Medicine, University of Toronto, Canada.
Centre for Research on Inner City Health, The Keenan Research Centre in the Li Ka Shing Knowledge Institute of St. Michael's Hospital, Toronto, Canada.
External sources
The Ontario HIV Treatment Network, Canada.
Kelly O'Brien is supported by a Fellowship from the Canadian Institutes of Health Research, HIV/AIDS Research Initiative (2004‐2011) and a Michael DeGroote Postdoctoral Fellowship (McMaster University) (2008‐2011), Canada.
Declarations of interest
None.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Baigis 2002 {published data only}
- Baigis J, Korniewicz DM, Chase G, Butz A, Jacobson D, Wu AW. Effectiveness of a home‐based exercise intervention for HIV‐infected adults: a randomized trial. JANAC 2002;13(2):33‐45. [DOI] [PubMed] [Google Scholar]
Dolan 2006 {published data only}
- Dolan SE, Frontera W, Librizzi J, Ljungquist K, Juan S, Dorman R, Cole ME, Kanter JR, Grinspoon S. Effects of a supervised home‐based aerobic and progressive resistance training regimen in women infected with human immunodeficiency virus. A randomized trial. Arch Inter Med 2006;166:1225‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Driscoll 2004a {published data only}
- Driscoll SD, Meininger GE, Lareau MT, Dolan SE, Killilea KM, Hadigan CM, Lloyd‐Jones DM, Frontera A, Walter R, Grinspoon SK. Effects of exercise training and metformin on body composition and cardiovascular indices in HIV‐infected patients. AIDS 2004;18(3):465‐73. [DOI] [PubMed] [Google Scholar]
Grinspoon 2000 {published data only}
- Grinspoon S, Corcoran C, Parlman K, Costello M, Rosenthal D, Anderson E, Stanley T, Schoenfeld D, Burrows B, Hayden D, Basgoz N, Klibanski A. Effects of testosterone and progressive resistance training in eugonadal men with AIDS wasting. Ann Intern Med 2000;133(5):348‐355. [DOI] [PubMed] [Google Scholar]
LaPerriere 1990 {published data only}
- LaPerriere AR, Antoni H, Schneiderman N, Ironson G, Klimas N, Caralis P, Fletcher MA. Exercise Intervention Attenuates Emotional Distress and Natural Killer Cell Decrements Following Notification of Positive Serologic Status for HIV‐1. Biofeedback Self‐Regul 1990;15:229‐242. [DOI] [PubMed] [Google Scholar]
Lox 1995 {published data only}
- Lox CL, McAuley E, Tucker RS. Exercise as an intervention for enhancing subjective well‐being in an HIV‐1 population. J Sport Ex Psych 1996;17:346‐62. [Google Scholar]
MacArthur 1993 {published data only}
- MacArthur R D, Levine SD, Birk TJ. Supervised exercise training improves cardiopulmonary fitness in HIV‐infected persons. Med Sci Sports Exerc 1993;25:684‐88. [PubMed] [Google Scholar]
Mutimura 2008a {published data only}
- Mutimura E, Stewart A, Crowther NJ, Yarasheski KE, Cade WT. The effects of exercise training on quality of life in HAART‐treated HIV‐positive Rwandan subjects with body fat redistribution. Qual Life Res 2008;17:377‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perna 1999 {published data only}
- Perna FM LaPerriere A, Klimas N, Ironson G, Perry A, et al. Cardiopulmonary and CD4 cell changes in response to exercise training in early symptomatic HIV infection. Med Sci Sports Exerc 1999;31:973‐79. [DOI] [PubMed] [Google Scholar]
Rigsby 1992 {published data only}
- Rigsby LW, Dishman RK, Jackson AW, Maclean GS, Raven PB. Effects of exercise training on men seropositive for the human immunodeficiency virus‐1. Med Sci Sports Exerc 1992;24:6‐12. [PubMed] [Google Scholar]
Smith 2001 {published data only}
- Smith BA, Neidig JL, Nickel JT, Mitchell GL, Para MF, Fass RJ. Aerobic exercise: effects on parameters related to fatigue, dyspnea, weight and body composition in HIV‐infected adults. AIDS 2001;15:693‐701. [DOI] [PubMed] [Google Scholar]
Stringer 1998 {published data only}
- Stringer WW, Berezovskaya M, O'Brien WA, Beck K, Casaburi R. The effect of exercise training on aerobic fitness, immune indices, and quality of life in HIV+ patients. Med Sci Sports Exerc 1998:11‐16. [DOI] [PubMed] [Google Scholar]
Terry 1999 {published data only}
- Terry L, Sprinz E, Ribeiro JP. Moderate and High Intensity Exercise Training in HIV‐1 Seropositive Individuals: a Randomized Trial. Int J Sports Med 1999;20:142‐46. [DOI] [PubMed] [Google Scholar]
Terry 2006 {published data only}
- Terry L, Sprinz E, Stein R, Medeiros NB, Oliveira J, Ribeiro JP. Exercise training in HIV‐1‐infected individuals with dyslipidemia and lipodystrophy. Med Sci Sport Exer 2006;38(3):411‐17. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agin 2001 {published data only}
- Agin D, Gallagher D, Wang J, Heymsfield SB, Pierson RN, Kotler DP. Effects of whey protein and resistance exercise on body cell mass, muscle strength, and quality of life in women with HIV. AIDS 2001;15:2431‐40. [DOI] [PubMed] [Google Scholar]
Batterham 2000 {published data only}
- Batterham MJ, Garsia R, Greenop PA. Dietary intake, serum lipids, insulin resistance and body composition in the era of highly active antiretroviral therapy 'Diet FRS Study'. AIDS 2000;14:1839‐1843. [DOI] [PubMed] [Google Scholar]
Bhasin 2000 {published data only}
- Bhasin S, Storer TW, Javanbakht M, Berman N, Yarasheski KE, et al. Testosterone replacement and resistance exercise in HIV‐infected men with weight loss and low testosterone levels. JAMA 2000;283(6):763‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Birk 2000 {published data only}
- Birk TJ, McGrady A, MacArthur R, Khuder S. The effects of massage therapy alone and in combination with other complementary therapies on immune system measures and quality of life in human immunodeficiency virus. J of Alt and Comp Medicine 2000;6(5):405‐414. [DOI] [PubMed] [Google Scholar]
Driscoll 2004b {published data only}
- Driscoll SD, Meininger GE, Ljungquist K, Hadigan C, Torriani M, Klibanski A, Frontera WR, Grinspoon S. Differential effects of metformin and exercise on muscle adiposity and metabolic indices in human immunodeficiency virus‐infected patients. J Clin Endocrinol Metab 2004;89:2171‐8. [DOI] [PubMed] [Google Scholar]
Engelson 2006 {published data only}
- Engelson ES, Agin D, Kenya S, Werber‐Zion G, Luty B, Albu JB, et al. Body composition and metabolic effects of a diet and exercise weight loss regimen on obese, HIV‐infected women. Metabolism Clinical and Experimental 2006;55:1327‐36. [DOI] [PubMed] [Google Scholar]
Fairfield 2001 {published data only}
- Fairfield WP, Treat M, Rosenthal DI, Frontera W, Stanley T, Corcoran C, et al. Effects of testosterone and exercise on muscle leanness in eugonadal men with AIDS wasting. J Appl Physiol 2001;90:2166‐71. [DOI] [PubMed] [Google Scholar]
Galantino 2005 {published data only}
- Galantino ML, Shepard K, Drafft L, LaPerriere A, Ducette J, Sorbello A, et al. The effect of group aerobic exercise and T'ai Chi on functional outcomes and quality of life for persons living with acquired immunodeficiency syndrome. Journal of Alternative and Complementary Medicine 2005;11(6):1085‐92. [DOI] [PubMed] [Google Scholar]
Greene 1999 {published data only}
- Greene KB, Berger J, Reeves C, Moffat A, Standish LJ, Calabrese C. Most frequently used alternative and complementary therapies and activities by participants in the AMCOA study. J of Assoc of Nurses in AIDS Care 1999;10(3):60‐73. [DOI] [PubMed] [Google Scholar]
Hand 2008 {published data only}
- Hand GA, Phillips KD, Dudgeon WD, Lyerly GW, Durstine JL, Burgess SE. Moderate intensity training reverses functional aerobic impairment in HIV‐infected individuals. AIDS Care 2008;20(9):1066‐74. [DOI] [PubMed] [Google Scholar]
Henry 1999 {published data only}
- Henry K, Melroe H, Huebesch J, Kopaczewski J, Simpson J. Experience with the national cholesterol education program (NCEP) guidelines for the identification and treatment of protease inhibitor related lipid abnormalities. Retroconf 1999. 1999.
Lane 2000 {published data only}
- Lane BJ, Provost‐Craig, MA. Resting energy expenditure in asymptomatic HIV‐infected females. J of Wom Health and Gender‐Based Med 2000;9(3):321‐327. [DOI] [PubMed] [Google Scholar]
LaPerriere 1991 {published data only}
- LaPerriere A, Fletcher MA, Antoni MH, Klimas NG, Schneiderman, N. Aerobic Exercise Training in an AIDS Risk Group. Int J Sports Med 1991;12:S53‐S57. [DOI] [PubMed] [Google Scholar]
Lox 1996 {published data only}
- Lox CL, McAuley E, Tucker RS. Aerobic and resistance exercise training effects on body composition, muscular strength, and cardiovascular fitness in an HIV‐1 population. Int J Behav Med 1996;3(1):55‐69. [DOI] [PubMed] [Google Scholar]
McCain 2008 {published data only}
- McCain NL, Gray DP, Elswick RK, Robins JW, Tuck I, Walter JM, et al. A randomized clinical trial of alternative stress management interventions in persons with HIV infection. Journal of Consulting and Clinical Psychology 2008;76(3):431‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Melroe 1999 {published data only}
- Melroe NH, Kopaczewski BA, Henry K, Huebsh J. Intervention for hyperlipidemia associated with protease inhibitors.. J of Ass of Nurses in Aids Care 1999;10(4):55‐69. [DOI] [PubMed] [Google Scholar]
Mustafa 1999 {published data only}
- Mustafa T, Sy FS, Macera CA, Thompson SJ, Jackson KL, Selassie A, Dean LL. Association between exercise and HIV disease progression in a cohort of homosexual men. Ann Epidemiol 1999;9:127‐31. [DOI] [PubMed] [Google Scholar]
Mutimura 2008b {published data only}
- Mutimura E, Crowther NJ, Cade TW, Yarasheski KE, Stewart A. Exercise training reduces central adiposity and improves metabolic indices in HAART‐treated HIV‐positive subjects in Rwanda: A randomized controlled trial. AIDS Res and Human Retrov 2008;24(1):15‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neidig 2003 {published data only}
- Neidig JL, Smith BA, Brashers DE. Aerobic exercise training for depressive symptom management in adults living with HIV infection. JANAC 2003;14(2):30‐40. [DOI] [PubMed] [Google Scholar]
Nieman 1999 {published data only}
- Nieman DC, Pedersen BK. Exercise and immune function. Sports Med 1999;27(2):32‐38. [DOI] [PubMed] [Google Scholar]
Robins 2006 {published data only}
- Robins JLW, McCain NL, Gray DP, Elswick RK, Walter JM, McDade E. Research on psychoneuroimmunology: tai chi as a stress management approach for individuals with HIV disease. Applied Nursing Research 2006;19:2‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 2007 {published data only}
- Robinson FP, Quinn LT, Rimmer JH. Effects of high‐intensity endurance and resistance exercise on HIV metabolic abnormalities: a pilot study. Biological Research for Nursing 2007;8(3):177‐85. [DOI] [PubMed] [Google Scholar]
Romeyn 2000 {unpublished data only}
- Romeyn M, Gunn N. Resistance exercise and oxandrolone for men with HIV‐related weight loss. JAMA 2000. [PubMed]
Roubenoff 1999 {published data only}
- Roubenoff Ronenn, et al. A pilot study of exercise training to reduce trunk fat in adults with HIV‐associated fat redistribution. AIDS 1999;13:1373‐1375. [DOI] [PubMed] [Google Scholar]
Roubenoff 2000 {published data only}
- Roubenoff, R. Acquired immunodeficiency syndrome wasting, functional performance, and quality of life. Am J Man Care 2000;6(9):1003‐1016. [PubMed] [Google Scholar]
Samson 2006 {published data only}
- Samson SL, Pownall HJ, Scott LW, Ballantyne CM, O'Brien Smith E, Sekhar RV, et al. Heart positive: design of a randomized controlled clinical trial of intensive lifestyle intervention, niacin and fenofibrate for HIV lipodystrophy / dyslipidemia. Contemporary Clinical Trials 2006;27:518‐30. [DOI] [PubMed] [Google Scholar]
Sattler 1999 {published data only}
- Sattler FR, Jaque SV, Schroeder ET, Olson C, Dube MP, et al. Effects of pharmacological doses of nandrolene decanoate and progressive resistance training in immunodeficient patients infected with HIV. J Clin End & Met 1999;84(4):1268‐1276. [DOI] [PubMed] [Google Scholar]
Additional references
American College of Sports Medicine 2010
- American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. 8th Edition. Philadelphia PA: Lippincott, Williams & Wilkins, 2010. [Google Scholar]
Bouchard 1993
- Bouchard C, Shepherd RJ, Stephens T. Physical Activity, Fitness, and Health. Champagne, IL: Human Kinetics Publishers, 1993. [Google Scholar]
Cade 2004
- Cade WT, Peralta L, Keyser RE. Aerobic exercise dysfunction in human immunodeficiency virus: a potential link to physical disability. Phys Ther 2004;84:655‐64. [PubMed] [Google Scholar]
Dudgeon 2004
- Dudgeon WD, Phillips KKD, Bopp CM, Hand GA. Physiological and psychological effects of exercise interventions in HIV disease. AIDS Patient Care and STDS 2004;18:81‐98. [DOI] [PubMed] [Google Scholar]
Fraser 1998
- Fraser C, Thomson‐O'Brien MA. Identifying non‐randomized studies in Medline. Poster presented at: 6th International Cochrane Colloquium, Oct 22‐26, 1998, Baltimore, MD.
Hand 2009
- Hand GA, Lyerly GW, Jaggers JR, Dudgeon WD. Impact of aerobic and resistance exercise on the health of HIV‐infected persons. American Journal of Lifestyle Medicine 2009;3(6):489‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports or randomized clinical trials: is blinding necessary?. Controlled Clin Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Lau 1997
- Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Annals of Internal Medicine 1997;127:820‐6. [DOI] [PubMed] [Google Scholar]
Managing Your Health 2009
- Canadian AIDS Treatment Information Exchange (CATIE). Managing Your Health. 4th Edition. http://www.catie.ca/eng/myh/toc.shtml: Canadian AIDS Treatment Information Exchange (CATIE), 2009. [Google Scholar]
Pollock 1998
- Pollock M, Gaesser GA, Butcher JD, Despres JP, Dishman RK, Franklin BA, Garber CE. ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports & Exerc 1998;30(6):975‐91. [DOI] [PubMed] [Google Scholar]
Robinson 1998
- Robinson KA, Hinegardner PG, Lansing P. Development of an optimal search strategy for the retrieval of controlled trials using PubMed. Poster presented at: 6th International Cochrane Colloquium, Oct 22‐26, 1998, Baltimore, MD.
Rusch 2004
- Rusch M, Nixon S, Schilder A, Braitstein P, Chan K, Hogg RS. Impairments, activity limitations and participation restrictions: Prevalence and associations among persons living with HIV/AIDS in British Columbia. Health and Quality of Life Outcomes 2004;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scevola 2003
- Scevola D, DiMatteo A, Lanzarini P, Uberti F, Scevola S, Bernini V, et al. Effect of exercise and strength training on cardiovascular status in HIV‐imnfected patients receiving highly active antiretroviral therapy. AIDS 2003;17 Suppl 1:S123‐9. [DOI] [PubMed] [Google Scholar]
Schwartz 2002
- Schwartz AL, Meek PM, Nail LM, Fargo J, Lundquist M, Donofrio M, et al. Measurement of fatigue determining minimally important clinical differences. J Clin Epidemiol 2002;55:239‐244. [DOI] [PubMed] [Google Scholar]
UNAIDS 2008
- UNAIDS. UNAIDS Terminology Guidelines. 2008:http://data.unaids.org/pub/Manual/2008/jc1336_unaids_terminology_guide_en.pdf.
World Health Organization 2001
- World Health Organization. International Classification of Functioning, Disability and Health. Geneva: World Health Organization, 2001. [Google Scholar]
References to other published versions of this review
O'Brien 2006
- O'Brien K, Nixon S, Tynan AM, Glazier RH. Aerobic exercise interventions for people living with HIV/AIDS: Implications for practice, education and research. Physiother Can 2006;58:114‐29. [Google Scholar]


