Abstract
Background
Carnitine, a quaternary amino acid, plays an important role in the oxidation of long chain fatty acids. Both breast milk and infant formulas contain carnitine. However, it is not routinely provided in parenteral nutrition solutions. Non supplemented parenterally fed infants have very low tissue carnitine levels. The clinical significance of this is uncertain. Carnitine deficiency may be an etiological factor in the limited ability of premature babies to utilize parenteral lipid. In vitro studies have suggested that fatty acid oxidation is impaired when the tissue carnitine levels fall below 10% of normal. Therefore relative carnitine deficiency may impair fatty acid oxidation, thus reducing the available energy and impairing growth.
Objectives
The primary aim of this review is to determine whether carnitine supplementation of parenterally fed neonates will improve weight gain. The secondary aims are to determine the effect on lipid tolerance and ketogenesis.
Search methods
Computerised searches were carried out by both reviewers. Searches were made of Medline, Embase, The National Research Register (UK), the Cochrane Controlled Trials Register and expert informants. The MeSH headings used were carnitine and parenteral nutrition.
Selection criteria
Only randomised trials were considered. Trials were included if they involved carnitine supplementation alone, parenterally fed newborn infants, and measured at least one outcome of interest (weight gain, plasma fatty acids, plasma triglycerides, quantity of lipid tolerated, respiratory quotient or beta hydroxybutyrate levels).
Data collection and analysis
The two reviewers searched the literature separately and reached a consensus for inclusion of trials. Data were extracted and evaluated by the two reviewers independently of each other. Authors were contacted if possible to clarify or provide missing data.
Main results
Fourteen studies were identified, six met the selection criteria. The results of the review are limited by the fact that the studies were generally short term and studied different outcomes. One study examined short term and long term weight gain, three reported only short term weight gain, three reported biochemical results in response to a short lipid challenge, and two reported results obtained during normal parenteral nutrition.
Among infants supplemented with carnitine, there was no evidence of effect on weight gain, lipid utilization or ketogenesis.
Authors' conclusions
We found no evidence to support the routine supplementation of parenterally fed neonates with carnitine.
Plain language summary
Carnitine supplementation of parenterally fed neonates
Not enough evidence that carnitine supplements improve weight gain in parenterally fed newborns. Preterm newborns (born before 37 weeks) frequently need extra nutritional supplements parenterally (given other ways than by the mouth). Carnitine is an amino acid found in both breast milk and infant formulas but is not routinely given parenterally. It helps fatty acids to convert into energy and helps in growth. The review of trials found not enough evidence to show any benefit of parenteral carnitine supplements on weight gain or lipid tolerance in preterm newborns. More research is needed.
Background
Preterm neonates are frequently dependent on parenteral nutritional support in the first few weeks of life. The use of intravenous lipid emulsion, usually in the form of long chain triglycerides, provides essential fatty acids and a concentrated, isosmolar source of energy. Unfortunately, limited ability to metabolize lipids may limit the quantity infused. Carnitine, a quaternary amino acid, plays an important role in long chain fatty acid oxidation. It is essential for the transport of fatty acids across the inner mitochondrial membrane into the mitochondrial matrix where the enzymes for beta oxidation are located. This is achieved via a three‐enzyme cycle with carnitine palmityl transferases on the outer and inner aspects of the inner mitochondrial membranes and carnitine translocase as a transport protein in between. Carnitine is formed in the liver from the hydroxylation of gamma butyrobetaine, which is synthesized in the kidney from lysine and methionine. The activity of gamma butyrobetaine is age dependent, being much lower in newborns than in older children and adults (Borum 1983).
Carnitine is concentrated within cells, the intracellular level being 10 ‐ 50 times the extracellular level. The total body stores are regulated by renal excretion with carnitine excreted intact. The tissue stores in newborn term and preterm infants are approximately 25‐50% of adult levels (Shenai 1984) with those of preterm being lower than term (Penn 1985). Both breast milk and infant formulas contain carnitine. However, it is not routinely provided in parenteral nutrition solutions.
Neonates therefore have both reduced synthesis and reduced stores of carnitine. Given that they are also likely to have increased demand due to rapid growth, it is perhaps not surprising that nonsupplemented parenterally fed infants have very low tissue carnitine levels within two weeks (Penn 1981). The clinical significance of this is uncertain. In vitro studies have suggested that fatty acid oxidation is impaired only when the tissue levels fall below 10% of normal (Long 1982). Clinical symptoms of carnitine deficiency in infants receiving prolonged nonsupplemented parenteral nutrition have not been described. Despite this, it is possible that relative carnitine deficiency impairs fatty acid oxidation. It would be expected that carnitine deficiency would limit the metabolism of lipid leading to a rise in plasma triglycerides and free fatty acids, and impaired ketogenesis. The available non protein energy would be impaired, amino acids would be increasingly used to meet endogenous energy needs and thus tissue growth would be impaired.
Objectives
The primary aim of this review is to determine whether carnitine supplementation of parenterally fed neonates will improve weight gain.
The secondary aim is to determine whether carnitine supplementation of parenterally fed neonates will improve lipid tolerance. This will be assessed in terms of fatty acid levels, triglyceride clearance and amount of lipid tolerated.
Subgroup analysis will be conducted to determine whether the results differ for infants requiring more than 14 days of exclusive parenteral nutrition.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials in which carnitine supplementation was compared to placebo.
Types of participants
Infants 28 days postnatal age or less receiving more than 50% of their daily calorie intake from parenteral nutrition.
Types of interventions
Intravenous or enteral carnitine supplementation compared to no supplementation. This includes any dose, duration or starting age. Studies reporting response to a singe dose of carnitine will be excluded.
Studies using nutritional co‐interventions will be excluded.
Types of outcome measures
Weight gain, both short term (during the intervention period) and long term (after the intervention period)
Fatty acid levels
Triglyceride levels
Quantity of lipid tolerated
Respiratory quotient
Ketone body levels
Search methods for identification of studies
Computerised searches were carried out by both reviewers. Searches were made of MEDLINE (1966 to January 2000), EMBASE (1974‐2000), The National Research Register (UK), the Cochrane Controlled Trials Register (2000 issue 1) and expert informants. The MeSH headings used were carnitine and parenteral nutrition.
Data collection and analysis
The criteria and standard methods of the Cochrane Collaboration and its Neonatal Review Group were used. Data extraction was performed independently by each reviewer using a structured proforma; differences were resolved by discussion. We transformed graphical data into numerical data using a millimetric ruler. Two of the studies stratified randomisation by birth weight or gestation and reported outcomes within each stratum. Each stratum was entered independently in this review. Therefore Bonner 1995 and Bonner 1995 represent two birthweight strata from the same trial. Schmidt‐S. 1983a and Schmidt‐S. 1983b represent two gestational age strata from the same trial.
Statistical analysis was carried out using the standard method of the Cochrane Neonatal Review Group, using weighted mean difference and its 95% confidence interval, and a fixed effect model for meta analysis.
Results
Description of studies
Fourteen trials looking at the effect of carnitine supplementation were identified. Five trials were excluded on the basis of non randomisation. Two were excluded because of the presence of a nutritional co‐intervention. One trial was excluded because the main route of nutrition was enteral.
Excluded studies
Magnusson 1997 performed a double blind randomised trial of a standard long chain triglyceride emulsion compared to a new fat emulsion containing carnitine and gamma‐linolenic acid. The study group consisted of 20 neonates undergoing surgery; the intervention took place over five days and the nutrition was 100% parenteral. This study was excluded as a nutritional co‐intervention was present.
Melegh 1986 performed a trial of enteral carnitine supplementation in five neonates versus five controls. The intervention took place over seven days and nutrition was more than 50% enteral. This trial was excluded as it was unclear whether or not randomisation had taken place and the nutrition was more than 50% enteral throughout the study.
Orzali 1983 studied the effect of a single dose of carnitine (total 200mg/kg over six hours) on lipid tolerance in 11 newborns compared to 10 controls. This trial was excluded as it did not appear to be randomised and it examined the acute effect of a single dose of carnitine.
Orzali 1984 studied the effect of a single dose of carnitine (total 200mg/kg over six hours) on lipid tolerance in 11 newborns. A lipid tolerance test was carried out after seven days of carnitine free parenteral nutrition and was repeated with supplemental carnitine. This trial was excluded as it was a single group, nonrandomised study which examined the acute effect of a single dose of carnitine.
Rubecz 1984 studied the clearance of plasma triglycerides in five infants following a lipid infusion. This was repeated after carnitine supplementation for three days. This trial was excluded as it was a single group, nonrandomised study.
Rubecz 1985 studied the effect of a four hour carnitine infusion on resting heat production and respiratory quotient in 10 enterally fed preterm infants. This study was excluded as it was a single group, nonrandomised study which examined the acute effect of a single dose of carnitine.
Rubin 1995 performed a double blind randomised study in 49 neonates. They were randomised to receive long chain triglyceride emulsion, a 50:50 medium chain:long chain triglyceride emulsion or a new fat emulsion containing carnitine and gamma‐linolenic acid for six days. The study was excluded as a nutritional co‐intervention was present.
Sulkers 1990 compared 12 infants receiving carnitine supplemented parenteral nutrition with 12 receiving parenteral nutrition alone. Metabolic rate, protein, glucose and fat oxidation were calculated. This study was excluded as the groups were not randomised.
Included studies
Details of the included studies are presented in the table "Characteristics of included studies". All studies, with the exception of Shortland 1998, were very short term.
Population The inclusion criteria for each study varied slightly but were largely homogeneous. All studies involved newborns requiring parenteral nutrition. Helms 1995 included term post surgical neonates as did Coran 1985; the other studies included only premature neonates (27‐37 weeks). Extremely premature infants were not included in any study. Bonner 1995 used very low birth weight as the entry criterion (750‐1500g).
Intervention
Carnitine was the sole intervention in all of the included studies. In four studies supplementation was completely parenteral; Coran 1985 used the enteral route and Shortland 1998 used both. The dose used ranged from 50 micromol/kg/day (Bonner 1995) to 150 micromol/kg/day (Shortland 1998). The duration of the intervention was variable. Schmidt‐Sommerfeld 1983 commenced carnitine on day 2 of life; mean duration was 5.9 +/‐1.3 days. Patients in Larsson's study received carnitine for six to twenty one days; results were reported for the first five days. The studies by both Helms 1995 and Bonner 1995 report results for two weeks of supplementation. Shortland 1998 supplemented parenterally followed by orally once enteral feeds were established; supplementation continued until the equivalent of 40 weeks gestation. Results were reported until 6 months after the expected date of delivery. None of the studies reported the carnitine content or the actual quantity of the enteral feeds given.
Outcome measures Primary Outcome Weight gain Bonner 1995 reported weight gain (g/day) at 2 weeks. Shortland 1998 reported weight gain in g/day over two week intervals for the first eight weeks and then at three and six months after the expected date of delivery. Coran 1985 reported individual patient weights before and after two weeks of supplementation with either carnitine or placebo. Helms 1995 reported weight gain in g/kg/day over the first and second week separately.
Secondary outcomes
The outcome measures reflecting lipid metabolism varied between studies. In three of the studies blood for biochemical analysis was taken at the end of a lipid tolerance test, either 1g/kg Intralipid over 4 hours (Schmidt‐Sommerfeld 1983) or 0.5g/kg Intralipid over 2 hours (Coran 1985, Helms 1995).
Two studies (Bonner 1995, Larsson 1990) took samples during normal parenteral nutrition, Larsson 1990 after a 4 hour lipid free period. The timing of samples is not stated by Bonner 1995.
Fatty acids Free fatty acids were measured by Schmidt‐Sommerfeld 1983, Helms 1995 and Coran 1985. Helms 1995 and Coran 1985 presented the results graphically.
Triglycerides Schmidt‐Sommerfeld 1983 measured triglyceride response to a 4 hour lipid challenge. Helms 1995 and Coran 1985 measured triglyceride levels obtained in response to a 2 hour lipid tolerance test; the results were displayed graphically and the peak value at 2 hours used in this analysis. Larsson 1990 intermittently measured triglyceride levels after a 4 hour lipid free period over 20 days of the study. The results at day 5 were used for this analysis as parenteral nutrition still comprised more than 50% of the calorie intake at this stage.
Maximum amount of lipid tolerated In the study by Bonner 1995 the lipid intake was limited by the serum triglyceride levels obtained and the maximum lipid tolerated was reported.
Respiratory quotient Respiratory quotient was not measured in any study.
Ketogenesis Schmidt‐Sommerfeld 1983, Helms 1995 and Bonner 1995 measured beta hydroxybutyrate at the end of a lipid tolerance test. Larsson 1990 reported beta hydroxybutyrate levels for days 2‐5 during normal parenteral nutrition. Day five data were used for this analysis. Coran 1985 measured total ketone bodies.
Risk of bias in included studies
Three out of the six studies reported methods of randomisation. Larsson 1990 used a card system in blocks of four, Helms 1995 used a random digit table and Shortland used a predetermined random code. In two studies both the carers and assessors were blinded (Shortland 1998, Helms 1995), in two they were unblinded (Bonner 1995, Schmidt‐Sommerfeld 1983) and in two it was unclear whether either the carers or assessors were blinded (Coran 1985, Larsson 1990). As previously stated randomisation was stratified in two studies. Results were obtained for between 86% and 100% of the enrolled infants. In the Helms 1995 study it was stated that all the study participants received at least seven days parenteral nutrition. It is not clear whether this represented all patients randomized or if those receiving shorter periods of parenteral nutrition were not subsequently studied.
Effects of interventions
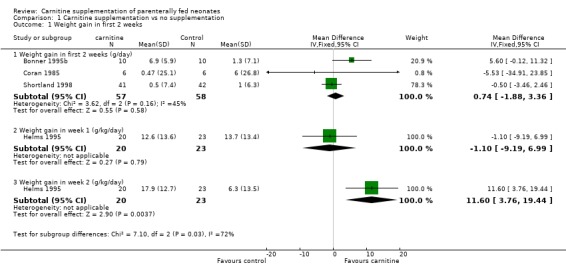
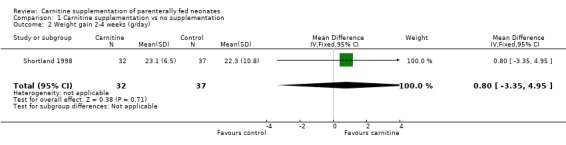
Weight gain Four trials examined early weight gain. Three of those reported weight gain over the first 2 weeks in g/day in a total of 58 treated neonates versus 57 controls. There was no difference in weight gain (WMD 0.74 g/day, 95% CI ‐1.88, 3.35). Bonner 1995 reported weight gain for the bigger infants (Bonner 1995b). They state that there was no difference between the treated and control group in the smaller infants (Bonner 1995a), but do not report the actual data. Helms 1995 reported weight gain separately for week one and week two in 20 supplemented neonates versus 23 controls. There was no difference for the first week (MD ‐1.1g/kg/day 95% CI ‐ 9.20, 7.0). A significant effect was reported in the second week (MD 11.6g/kg/day, 95% CI 3.76, 19.44). Shortland 1998 found no difference in weight gain over the subsequent two weeks (MD 0.80, 95% CI ‐0.34, 4.94).
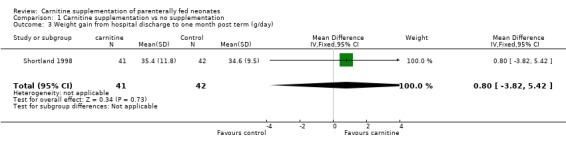
Shortland 1998 was the only study to report long term weight gain. There was no difference at one month post term (MD 0.80, 95% CI ‐3.81, 5.41) or at any stage between randomisation and three months post term.
Secondary Outcomes
Lipid tolerance was reported in response to a lipid challenge or during normal parenteral nutrition. Those studies reporting lipid tolerance in response to a lipid challenge were combined by meta analysis.
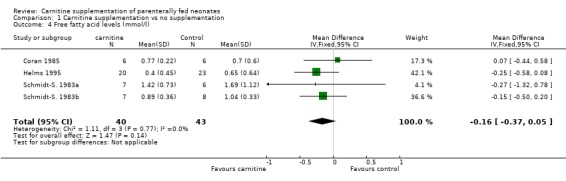
Plasma free fatty acids Three studies reported free fatty acid levels in a total of 40 supplemented neonates versus 43 controls. There was no evidence of difference between groups (WMD ‐0.16 mmol/l, 95% CI ‐0.37, 0.05).
Plasma triglycerides Three studies reported triglyceride levels in a total of 40 carnitine supplemented versus 43 controls. There was no evidence of difference between groups (WMD ‐0.69 mmol/l, 95% CI ‐1.84, 0.45).
Larsson 1990 measured plasma triglyceride and fatty acid concentrations in a further 12 neonates after a four hour lipid free period during normal parenteral nutrition (lipid infused over 20 hours) . There was no significant difference in either.
Amount of lipid tolerated Bonner 1995 increased the lipid incrementally depending on the plasma triglyceride levels in 21 carnitine supplemented neonates versus 21 controls. The mean quantity of lipid infused therefore reflected tolerance. There was no evidence of difference between groups in the amount tolerated (WMD 0.09g/kg/day, 95% CI ‐0.20, 0.38). Thus, there was no evidence of improvement in lipid tolerance in neonates supplemented with carnitine.
Ketogenesis Three studies reported beta hydroxybutyrate production in response to a lipid challenge in a total of 55 supplemented neonates versus 58 controls. There was a statistically significant improvement in beta hydroxybutyrate production (WMD 0.05mmol/l, 95% CI 0.03, 0.06). However, even a true difference of 0.06mmol/l would not be clinically significant. Larsson 1990 noted no difference in a further 12 neonates, six of which were carnitine supplemented. Coran 1985 noted no difference in ketone bodies between the supplemented and nonsupplemented groups (6 in each).
A subgroup analysis of the effect of supplementation in infants requiring prolonged parenteral nutrition (more than 14 days) was not possible as data were not available.
Discussion
The review did not demonstrate any evidence of benefit from parenteral carnitine supplementation on lipid tolerance, ketogenesis or weight gain for neonates requiring parenteral nutrition. However, the studies were all small and reported different outcome measures.
The most important clinical outcome would be that of improved weight gain. It may be expected that any effect on weight gain would be seen during the first two weeks, as parenteral nutrition plays a diminishing role with time. Bonner 1995 reported that there was no difference in weight gain in the smaller birth weight group (Bonner 1995a, 750‐1000g) and reported numerical weight gain data only for the larger infants (Bonner 1995b, birth weights 1001‐1500g). In effect this produced a publication bias. Despite this, no improvement in weight gain was demonstrated in the three studies reporting weight gain at two weeks. The only positive effect on weight gain in carnitine supplemented infants was noted by Helms 1995 for week two but not for week one. The rate of weight gain halved in the control group between week one and two, thus creating the difference. The authors state that there was no difference between the groups when the weight gain was analysed over the study period. It is possible that the carnitine status of the control group continued to fall, increasingly impeding growth, and hence the difference between weeks one and two could be real. However, this is not supported by the data obtained in the Shortland 1998 study which failed to show any difference in weight gain between the groups at any time during the study.
The longer term weight gain data are all obtained from the Shortland 1998 study. Although they did not show any difference in weight gain, data on calorie intake and feed supplementation were not provided. While the study was completely blinded it is possible that the control group was prescribed a greater calorie intake in response to poor weight gain. However, there is no evidence of even transiently impaired weight gain in the non supplemented group.
Biochemical evidence of lipid tolerance and utilisation were measured both during normal parenteral nutrition and in response to a lipid challenge. Despite the different conditions under which carnitine supplementation was compared, there was no difference between the groups. The small increase in ketogenesis in the carnitine supplemented group is unlikely to be of any clinical significance.
Authors' conclusions
Implications for practice.
Despite plausible rationale, we found no evidence from randomised trials which justifies routine carnitine supplementation of neonates receiving parenteral nutrition.
Implications for research.
Further research on carnitine supplementation should concentrate on those neonates or infants requiring long term total parenteral nutrition. Outcome measures should be robust and clinically important such as growth and lipid tolerance under conditions of normal parenteral nutrition.
What's new
| Date | Event | Description |
|---|---|---|
| 15 September 2008 | Amended | Converted to new review format. |
Data and analyses
Comparison 1. Carnitine supplementation vs no supplementation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight gain in first 2 weeks | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Weight gain in first 2 weeks (g/day) | 3 | 115 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [‐1.88, 3.36] |
| 1.2 Weight gain in week 1 (g/kg/day) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐9.19, 6.99] |
| 1.3 Weight gain in week 2 (g/kg/day) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 11.60 [3.76, 19.44] |
| 2 Weight gain 2‐4 weeks (g/day) | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐3.35, 4.95] |
| 3 Weight gain from hospital discharge to one month post term (g/day) | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐3.82, 5.42] |
| 4 Free fatty acid levels (mmol/l) | 4 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.37, 0.05] |
| 5 Triglyceride levels (mmol/l) | 4 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐1.84, 0.45] |
| 6 Lipid tolerance ‐ maximum amount of lipid tolerated (g/kg/day) | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.20, 0.38] |
| 7 Ketogenesis ‐ beta hydroxybutyrate levels (mmol/l) | 5 | 113 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [0.03, 0.07] |
1.1. Analysis.
Comparison 1 Carnitine supplementation vs no supplementation, Outcome 1 Weight gain in first 2 weeks.
1.2. Analysis.
Comparison 1 Carnitine supplementation vs no supplementation, Outcome 2 Weight gain 2‐4 weeks (g/day).
1.3. Analysis.
Comparison 1 Carnitine supplementation vs no supplementation, Outcome 3 Weight gain from hospital discharge to one month post term (g/day).
1.4. Analysis.
Comparison 1 Carnitine supplementation vs no supplementation, Outcome 4 Free fatty acid levels (mmol/l).
1.5. Analysis.
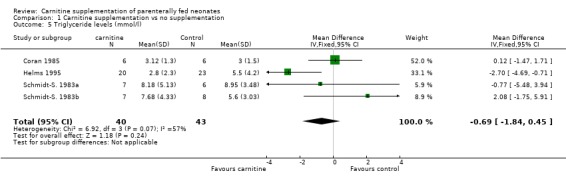
Comparison 1 Carnitine supplementation vs no supplementation, Outcome 5 Triglyceride levels (mmol/l).
1.6. Analysis.
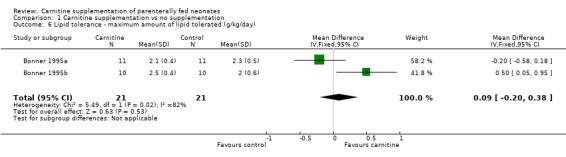
Comparison 1 Carnitine supplementation vs no supplementation, Outcome 6 Lipid tolerance ‐ maximum amount of lipid tolerated (g/kg/day).
1.7. Analysis.
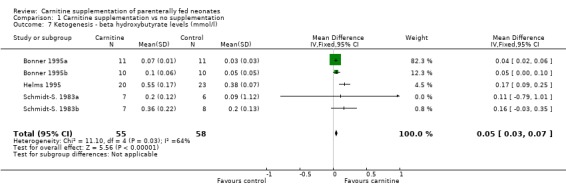
Comparison 1 Carnitine supplementation vs no supplementation, Outcome 7 Ketogenesis ‐ beta hydroxybutyrate levels (mmol/l).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bonner 1995a.
| Methods | Randomised, placebo controlled trial Blinding of randomisation:‐ Can't tell Blinding of intervention:‐ No Blinding of outcome assessors:‐No Complete follow up:‐Yes | |
| Participants | Number entered into study =22, 11 in each group Inclusion criteria= birth weight 751‐1000g requiring parenteral nutrition. Exclusion criteria=not expected to survive, recent blood transfusion, not expected to require parenteral nutrition for more than 1 week. Infants stratified by birth weight (750‐1000g and 1001‐1500g) | |
| Interventions | 50 micromol/kg/day carnitine as a continuous intravenous infusion vs placebo until >50% of caloric intake was enteral. Parenteral lipid was started at 0.5g/kg/day and increased gradually if serum triglyceride level was <1.7mmol/l | |
| Outcomes | Outcomes were reported at 2 weeks of study. Weight gain in g/day Beta hydroxybutyrate levels (weekly) Serum carnitine (weekly) Mean quantity of lipid tolerated daily. Triglyceride levels | |
| Notes | Same trial as Bonner 1995b | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bonner 1995b.
| Methods | Randomised, placebo controlled trial Blinding of randomisation:‐ Can't tell Blinding of intervention:‐ No Blinding of outcome assessors:‐No Complete follow up:‐Yes | |
| Participants | Number entered into study =20, 10 in each group Inclusion criteria= birth weight 1001‐1500g requiring parenteral nutrition. Exclusion criteria=not expected to survive, recent blood transfusion, not expected to require parenteral nutrition for more than 1 week. | |
| Interventions | 50 micromol/kg/day carnitine as a continuous intravenous infusion vs placebo until >50% of caloric intake was enteral. Parenteral lipid was started at 0.5g/kg/day and increased gradually if serum triglyceride level was <1.7mmol/l | |
| Outcomes | Outcomes were reported at 2 weeks of study. Weight gain in g/day Beta hydroxybutyrate levels (weekly) Serum carnitine (weekly) Mean quantity of lipid tolerated daily. Triglyceride levels | |
| Notes | Same trial as Bonner 1995a | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Coran 1985.
| Methods | Randomised controlled trial Blinding of randomisation:‐Can't tell Blinding of intervention:‐Yes Blinding of outcome assessors:‐Cant tell Complete follow up:‐Yes | |
| Participants | Number entered into study =12, 6 in each group Inclusion criteria Newborn undergoing major surgery in first few days of life and expected to require parenteral support for at least 1 week. No exclusions stated | |
| Interventions | 70 micromol/kg/day carnitine or placebo in divided doses every 3 hours enterally for 7 days | |
| Outcomes | Weight gain Plasma taken on days 1 and 8 for free fatty acids, triglycerides, total ketone bodies and carnitine. | |
| Notes | Biochemical results obtained after a short lipid challenge (0.5g/kg intralipid over 2 hours) at 2,4,6 and 8 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Helms 1995.
| Methods | Randomised controlled trial Blinding of randomisation:‐Yes Blinding of intervention:‐Yes Blinding of outcome assessors:‐Yes Complete follow up:‐can't tell | |
| Participants | Number entered into study = 43, 20 in the carnitine group Inclusion criteria =Neonates requiring minimum of 7 days parenteral nutrition Mean gestational age was 31 weeks Exclusion criteria=receiving drugs known to affect carnitine or lipid metabolism | |
| Interventions | 50 micromol/kg/day carnitine by continuous intravenous infusion for 7 days then 100 micromol/kg/day for 7 days. It is unclear whether the control group received placebo or if the carnitine was added to the parenteral nutrition in the pharmacy. Participants were permitted<15% enteral nutrition with formulas containing little carnitine | |
| Outcomes | Weight gain week 1 (derived from the slope of a linear regression plot of daily weight) nitrogen and carnitine balance at baseline, day 7 and day 14 of study. Plasma biochemistry on days 0,7 and 14 :‐ ketone levels, triglycerides, free fatty acids. | |
| Notes | Biochemical results obtained after a short lipid challenge (0.5g/kg Intralipid over 2 hours) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Larsson 1990.
| Methods | Randomised placebo controlled trial Blinding of randomisation:‐Yes Blinding of intervention:‐Yes Blinding of outcome assessors:‐Yes Complete follow up:‐Yes | |
| Participants | Number entering study= 14, one died, one excluded as on full feeds within 5 days, therefore results from 12 reported, 6 in each group Inclusion criteria= neonates with gestational age <33 weeks and anticipated need for at least 5 days parenteral nutrition. The mean gestational ages were 28.8 in th carnitine group compared to 30.7 weeks in the control | |
| Interventions | Carnitine 60 micromol/kg/day or normal saline added to the lipid emulsion until on 75% of enteral feeds. | |
| Outcomes | Plasma taken on days 0, 2, 5, 10, 15, 20, for ‐triglycerides, free fatty acids, betahydroxybutyrate, lactate and plasma carnitine (total, free and acyl). These were taken 4 hours after the amino acid and lipid solution had been discontinued. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Schmidt‐S. 1983a.
| Methods | Randomised controlled trial Blinding of randomisation:‐Can't tell Blinding of intervention:‐No Blinding of outcome assessors:‐Can't tell Complete follow up:96% | |
| Participants | Number entering study=13, 7 in the carnitine group Inclusion criteria =appropriately grown neonates, 29‐33 weeks gestational age and 1200‐2490g birth weight requiring parenteral nutrition Exclusions=severe jaundice, sepsis, metabolic disturbance or intracerebral haemorrhage | |
| Interventions | 60 micromol/kg/day IV carnitine infused over 5 hours while on parenteral nutrition. Control group not supplemented, the use of placebo is not stated. | |
| Outcomes | An intravenous lipid challenge was carried out on days 6‐10 before the start of enteral feeds Plasma was taken for ‐ Betahydroxybutyrate, triglycerides, free fatty acids and carnitine | |
| Notes | Biochemical results obtained after a lipid challenge (1g/kg Intralipid over 4 hours) Same trial as Schmidt‐S. 1983b | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Schmidt‐S. 1983b.
| Methods | Randomised, placebo controlled trial Blinding of randomisation:‐ Can't tell Blinding of intervention:‐ No Blinding of outcome assessors:‐No Complete follow up:‐Yes | |
| Participants | Number entering study=15, 7 in the carnitine group Inclusion criteria =appropriately grown neonates, 34‐37 weeks gestational age and 1200‐2490g birth weight requiring parenteral nutrition Exclusions=severe jaundice, sepsis, metabolic disturbance or intracerebral haemorrhage | |
| Interventions | 60 micromol/kg/day IV carnitine infused over 5 hours while on parenteral nutrition. Control group not supplemented, the use of placebo is not stated. | |
| Outcomes | An intravenous lipid challenge was carried out on days 6‐10 before the start of enteral feeds Plasma was taken for ‐ Betahydroxybutyrate, triglycerides, free fatty acids and carnitine | |
| Notes | Biochemical results obtained after a lipid challenge (1g/kg Intralipid over 4 hours) Same trial as Schmidt‐S. 1983a | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Shortland 1998.
| Methods | Randomised placebo controlled trial Blinding of randomisation:‐Yes Blinding of intervention:‐Yes Blinding of outcome assessors:‐Yes Complete follow up:‐94% at 6 months post term, weight data while in hospital falls to 83% at 4 weeks of the study and 25% at 8 weeks reflecting infant discharge home. | |
| Participants | Number entering study =86. 1 withdrew consent, 1 had a nasopharyngeal teratoma, 1 died from an intraventricular haemorrhage and 1 died from sudden infant death after hospital discharge. This left 83, 42 in the placebo group and 41 in the carnitine group Inclusion criteria = appropriately grown infants gestational age 28‐35 weeks and requiring parenteral nutrition. Randomisation was stratified by gestational age. The median gestational ages were 30.7 and 31.4 weeks in the carnitine and control group respectively. | |
| Interventions | Carnitine 150 micromol/kg/day or placebo intravenously until enteral feeds established and then orally until 40 weeks gestation. Infants remained in the study until 6 months after the expected date of delivery. | |
| Outcomes | Weight gain, length, head circumference and skinfold thickness. Plasma carnitine (total, free and acyl) Incidence of hypoglycaemia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Magnusson 1997 | Co‐intervention present ‐ lipid emulsion supplemented with carnitine was also supplemented with gamma linolenic acid |
| Melegh 1986 | Carnitine supplementation was enteral, unclear if the study was randomised |
| Orzali 1983 | Not randomised |
| Orzali 1984 | Crossover study Single dose of carnitine Not randomised |
| Rubecz 1984 | Not randomised |
| Rubecz 1985 | Not randomised Not on parenteral nutrition |
| Rubin 1995 | Co‐intervention present ‐ lipid emulsion supplemented with carnitine was also supplemented with gamma linolenic acid |
| Sulkers 1990 | Not randomised |
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Bonner 1995a {published data only}
- Bonner CM, DeBrie KL, Hug G, Landrigan E, Taylor BJ. Effects of parenteral L‐carnitine supplementation on fat metabolism and nutrition in premature neonates. J Pediatr 1995;126:287‐92. [DOI] [PubMed] [Google Scholar]
Bonner 1995b {published data only}
- Bonner CM, Debrie Kl, Hug G, Landrigan E, Taylor BJ. Effects of parenteral L‐carnitine supplementation on fat metabolism and nutrition in premature neonates. J Pediatr 1995;126:287‐92. [DOI] [PubMed] [Google Scholar]
Coran 1985 {published data only}
- Coran AG, Drongowski RA, Baker PJ. The metabolic effects of oral L‐carnintine administration in infants receiving total parenteral nutrition with fat. J Pediatr Surg 1985;20:758‐764. [DOI] [PubMed] [Google Scholar]
Helms 1995 {published data only}
- Helms RA, Mauer EC, Hay WW, Christensen ML, Storm MC. Effect of intravenous L‐carnitine on growth parameters and fat metabolism during parenteral nutrition in neonates. JPEN 1990;14:448‐453. [DOI] [PubMed] [Google Scholar]
Larsson 1990 {published data only}
- Larsson LE, Olegard R, Ljung L, Niklasson A, Rubensson A, Gederblad G. Parenteral nutrition in preterm neonates with and without carnitine supplementation. Acta Anaesth Scand 1990;34:501‐05. [DOI] [PubMed] [Google Scholar]
Schmidt‐S. 1983a {published data only}
- Schmidt‐Sommerfeld E, Penn D, Wolf H. Carnitine deficiency in premature infants receiving total parenteral nutrition: effect of L‐carnitine supplementation. J Pediatr 1983;102:931‐935. [DOI] [PubMed] [Google Scholar]
Schmidt‐S. 1983b {published data only}
- Schmidt‐Somerfeld E, Penn D, Wolf H. Carnitine deficiency in premature infants receiving total parenteral nutrition: effect of L‐carnitine supplementation. J Pediatr 1983;102:931‐935. [DOI] [PubMed] [Google Scholar]
Shortland 1998 {published data only}
- Shortland GJ, Walter JH, Stroud C, Fleming PJ, Speidel BD, Marlow N. Randomised controlled trial of L‐carnitine as a nutritional supplement in preterm infants. Arch Dis Child 1998;78:F185‐F188. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Magnusson 1997 {published data only}
- Magnusson G, Boberg M, Cederblad G, Meurling S. Plasma and tissue levels of lipids, fatty acids and plasma carnitine in neonates receiving a new fat emulsion. Acta Paediatr 1997;86:638‐44. [DOI] [PubMed] [Google Scholar]
Melegh 1986 {published data only}
- Melegh B, Kerner J, Sandor A, Vinceller M, Kispal G. Oral L‐carnitine supplementation in low birth weights: a study on neonates requiring combined parenteral and enteral nutrition. Acta Paediatr Hung 1986;27:253‐258. [PubMed] [Google Scholar]
Orzali 1983 {published data only}
- Orzali A, Donzelli F, Enzi G, Rubaltelli F. Effect of carnitine on lipid metabolism in the newborn. Biol Neonate 1983;43:186‐190. [DOI] [PubMed] [Google Scholar]
Orzali 1984 {published data only}
- Orzali A, Maetzke G, Donzelli F, Rubaltelli F. Effect of carnitine on lipid metabolism in the neonate. II. Carnitine addition to lipid infusion during prolonged total parenteral nutrition. J Pediatr 1984;104:436‐440. [DOI] [PubMed] [Google Scholar]
Rubecz 1984 {published data only}
- Rubecz I, Sandor A, Hamar A, Mestyan J. Blood levels of carnitine and lipid utilisation with and without carnitine supplementation in newborn infants. Acta Paediatr Hung 1984;25:165‐171. [PubMed] [Google Scholar]
Rubecz 1985 {published data only}
- Rubecz I, Sandor A, Hamar A, Vinceller M, Mestyan J. Absence of responses in energy metabolism and respiratory quotient to carnitine infusion in premature infants. Acta Paed Hung 1985;26:227‐231. [PubMed] [Google Scholar]
Rubin 1995 {published data only}
- Rubin M, Naor N, Sirota L, Moser A, Pakula R, Harell D, Sulkes J, Davidson S, Lichtenberg D. Are bilirubin and plasma lipid profiles of premature infants dependent on the lipid emulsion infused. J Pediatr Gastroenterol Nutr 1995;21:25‐30. [DOI] [PubMed] [Google Scholar]
Sulkers 1990 {published data only}
- Sulkers EJ, Lafeber HN, Degenhart HJ, Przyrembel H, Schlotzer E, Sauer PJJ. Effects of high carnitine supplementation on substrate utilization in low‐birth‐weight infants receiving total parenteral nutrition. Am J Clin Nutr 1990;52:889‐94. [DOI] [PubMed] [Google Scholar]
Additional references
Borum 1983
- Borum PR. Carnitine. Ann Rev Nutr 1983;3:233‐259. [DOI] [PubMed] [Google Scholar]
Long 1982
- Long CS, Haller RG, Foster DW, McGarry JD. Kinetics of carnitine‐dependent fatty acid oxidation: implications for human carnitine deficiency. Neurology 1982;32:663‐6. [DOI] [PubMed] [Google Scholar]
Penn 1981
- Penn D, Schmidt‐Sommerfeld E, Pascu F. Decreased tissue carnitine concentrations in newborn infants receiving total parenteral nutrition. J Pediatr 1981;98:976‐978. [DOI] [PubMed] [Google Scholar]
Penn 1985
- Penn D, Ludwigs B, Schmidt‐Sommerfeld, Pascu F. Effect of nutrition on tissue carnitine concentrations in infants of different gestational ages. Biol Neonate 1985;47:130‐135. [DOI] [PubMed] [Google Scholar]
Shenai 1984
- Shenai JP, Borum PR. Tissue carnitine reserves of newborn infants. Ped Res 1984;18:679‐681. [DOI] [PubMed] [Google Scholar]


