Abstract
BACKGROUND:
Inflammation that occurred in the tumor microenvironment was characterized by abundant macrophage infiltration, playing role in innate immunity. Multinucleate giant cells (MGCs) occur in a variety of inflammatory, hyperplastic, and neoplastic thyroid disorders. They also have been recognized as a feature of papillary thyroid carcinoma (PTC).
AIM:
The aim of this study was to evaluate cases of PTC for the presence of macrophages, and estimate CD68+ TAMs density in tumor stroma, margin and the surrounding tissue. We assessed also MGCs.
METHODS:
Macrophages and MGCs densities were correlated with clinicopathologic parameters to assess the possible prognostic significance. We investigated 56 patients immunohistochemically and immunofluorescence with antibodies against CD68 and IL-17.
RESULTS:
A statistically significant correlation was established between PTC patients in III stage, containing many MGCs, and PTC in I and II stage, with many MGCs. Eighty Percent of patients in III stage showed many MGCs in comparison with patients in I and II stage, where many MGCs were found only in 21,1% (χ2 = 6.189, p = 0.013).
CONCLUSION:
Our study demonstrates that the increased density of MGCs is associated with advanced stage of PTC, and therefore with tumor progression and that cases of PTC should be carefully screened for their presence.
Keywords: Classical dendritic cells, Plasmacytoid dendritic cells, MHC I and II antigen presentation, Cross presentation, Th polarization, Review
Introduction
Papillary thyroid carcinoma (PTC) accounts for 80% of the thyroid malignancy that is characterized by slow growth and an excellent prognosis. However 10-15% of cases exhibit aggressive behavior treatment resistance, and mobility [1], [2]. PTC is frequently associated with immune cell infiltration including lymphocyte dendritic cell and macrophage recruitment [3], [4], [5]. Macrophages have been categorized, into two subtypes – M1 and M2 – depending on their distinctive roles [6], [7]. Cancer cells recruit monocytes/macrophages from circulation. The main function of M1 macrophages is phagocytosis in response to LPS and/or T helper (Th1) cytokines such as IFN-γ. M1 macrophages are characterized by the production of pro-inflammatory cytokines (TNF-α, IL-6 and IL-12) and the induction of NOS [8]. In contrast the main function of M2 macrophages is immunosuppression and trophic activity in response to Th2 cytokines (after exposure to IL-4 or IL-13), [6], [8], [9]. M2 macrophages or TAMs have anti- inflammatory properties. In addition M2 TAMs produce anti- inflammatory cytokines such as IL-10 and TGFβ1 [10], [11]. TGFβ1 regulates M2 phenotype of macrophages and modulates their connections with other immune cell, like T cells [8] to tumor that dampen the immune response [11], [12]. Ryder et al., 2008 [13], investigated TAMs in 90 patients with WDTC and PDTC and found that increased macrophages number was associated with extrathyroidal extension, capsular invasion and decreased cancer-related survival for PDTC. The same authors experimentally demonstrated that increased TAMs number promote PTC progression [13]. Other investigations [14] showed that in WDTC (mostly PTC) increased TAMs matched more aggressive case with metastases at diagnosis; however paradoxically macrophage infiltration was associated with improved disease –free survival[14]. The morphology of TAMs in PTC, e.g., their canopy structure and their high density were connected with larger tumor size [4], suggesting a tumorigenic role of macrophages. That was associated with tumor dissemination in the regional lymph nodes [15].
Interleukin (IL)-17A, a signature cytokine of the T helper cell subset, Th17, has been indicated in the development of numerous inflammatory, autoimmune diseases, tumors, and in host defense against bacterial and fungal infection [16]. Besides Th17 cells, γδ T cells and macrophages are capable to produce IL-17 [21], [22], [23], [24], [25]. IL-17 can promote recruitment of macrophage and induce their cytokine/chemokine production, and thus capable of mediating a link between acquired and innate immunity, specifically, T cell and macrophage functions [17], [18].
Another modification of TAMs was the multinucleated giant cells (MGCs) which were often associated with PTC. There existed relatively few studies of MGCs in histology sections of PTCs. Probably these cells are formed in response to degenerative tumor areas and to abnormal colloid productions in PTC [19]. The prognostic significance of MGCs in PTC is uncertain. MGCs can be rarely found in follicular or anaplastic TC or in some inflammatory thyroid diseases as Hashimoto’s, De Quervain thyreoiditis [20], [21]. In the current study, we evaluated cases of PTC for the presence of macrophages, and estimated CD68+ TAMs density in tumor stroma, margin and the surrounding thyroid tissue. We assessed also MGCs. Macrophages and MGCs density were correlated with clinicopathologic parameters including patient’s age at diagnosis, tumor stage, grade of differentiation, extrathyroidal extension, capsule and vascular invasion to assess the possible prognostic significance of TAMs and MGCs in PTC.
Material and Methods
Tumour samples
A series of 56 PTC cases has been retrieved from the Archives of the University Hospital in Stara Zagora, Bulgaria. The group of patients with PTC, include cases with lymph node metastases (n = 2 (2.9%)) and without metastases (n = 0). The mean follow-up of 152.58 months; range from 1.64 to 197.07 has been done. PTC tumors are presented as 1 cm/1 cm or less in diameter and larger than 1 cm. There are 9 (16.7%) men and 47 (83.3%) women with age ranging from 22 to 81 years (mean 53.28 ± 13.756). Among the 56 PTC studied, 6 are ≤ 1 cm in diameter, and 50 tumors are larger. According to AJCC classification [22], 30 of the patients (53.6%) are in stage I, 18 of the patients (32%) are in stage II, 8 of the patients (14.4%) are in stage III, and no patients are in stage IV of the disease. For the statistical analysis cases were united I-II stage (Table 1).
Table 1.
Correlation between clinic-pathological factors and density of multinucleated giant cells MGCs in tumor stroma
| Parameters | № | Mgcs N (%) Non/few | Mgcs N (%) Many | P value |
|---|---|---|---|---|
| Age | ||||
| < 53,28 | 30 | 21 (70.00) | 9 (30.00) | 0.213 |
| > 53.28 | 26 | 14 (53.8) | 12 (46.2) | |
| Gender | ||||
| Male | 9 | 7 (77.8) | 2 (22.2) | 0.301 |
| Female | 47 | 28 (59.6) | 19 (40.4) | |
| Pt classification | ||||
| T1-T2 | 50 | 34 (68.0) | 16 (32.0) | 0.014 |
| T3-T4 | 6 | 1 (16.7) | 5 (83.3) | |
| Lymph node metastases | ||||
| Yes | 2 | 1 (50.00) | 1 (50.00) | 0.710 |
| No | 54 | 34 (63.00) | 20 (37.00) | |
| Ptnm stage | ||||
| I = II | 48 | 33 (68.8) | 15 (31.3) | 0.018 |
| III | 8 | 2 (25.00) | 6 (75.0) | |
| Capsule invasion | ||||
| No | 8 | 5 (62.5) | 3 (37.5) | 0.656 |
| Presence | 34 | 24 (70.6) | 10 (29.4) | |
| Presence of capsule invasion but without mgcs | 14 | - | - | |
*X2-test; ** Values in bold italic shown (border) importance.
The study protocol has been approved by the Research Ethics Committee of University Hospital Stara Zagora.
All samples has been fixed in formalin and embedded in paraffin. Clinical data has been collected from the pathology reports, clinical files and from Oncology Dispensary. H & E slides are retrieved from the archives and they have been reviewed independently by two pathologists (MG and KI) and the tumors are classified using the WHO criteria [23]. Cases with doubtful PTC features are excluded.
Immunohistochemistry
Immunohistochemical staining was performed using streptavidin-biotin technique as previously described [24]. Briefly endogenous peroxidase was blocked was blocked with 3% hydrogen peroxidase in methanol for 10 min. Slides were incubated over night at room temperature with primary antibodies as followed: Anti-CD68 antibody Clone KP11 M0814 were ready-to-use produced from DAKO, Glostrub, Denmark. After washing three times in PBS, the slides were incubated with DAKO-REALTM En-VisionTM detection system (DAKO) for 60 min, then visualized with diaminobenzidine and counterstained with Mayer’s hematoxylin.
Double immunofluorescence for detection of CD68 and IL-17
For double immunofluorescent procedure, sections were incubated in a primary antibody cocktail against ghrelin (Monoclonal mouse anti-human antibody CD68 macrophagesClone KP11 M0814 DAKO, Denmark 1: 50 and rabbit anti-human antibody IL-17 Clone sc-7927 Santa Cruz Biotechnology, USA 1: 100) in a humidified chamber for 1 h at room temperature. Then the sections were washed three times in PBS, pH 7.4. After that the sections were put in a mixture of secondary antibodies: anti-rabbit IgG (WHOLE MOLECULE) TRITC conjugate antibody (T5268, Sigma-Aldrich Inc.) and anti-mouse IgG (WHOLE MOLECULE) FITC conjugate antibody (F 9006 Sigma-Aldrich, Inc.) for 1 h at room temperature in a dark chamber, sealed with coverslip with PBS/glycerol solution was used.
Control sections were processed as described above. Sections were examined under fluorescence microscope (Leica DM2500, Germany). Images were analyzed using LAS Leica Microsystems CMS GmbH software. CD68 were colored in green and IL-17 in red color (Figure 1 and Figure 2).
Figure 1.
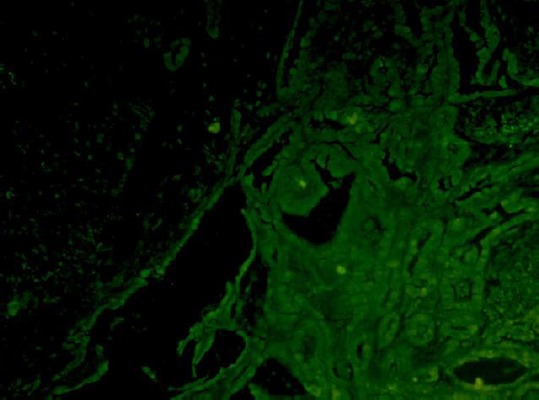
CD68 positive macrophages – MGCs, marked in green color- co-localization (x 200)
Figure 2.
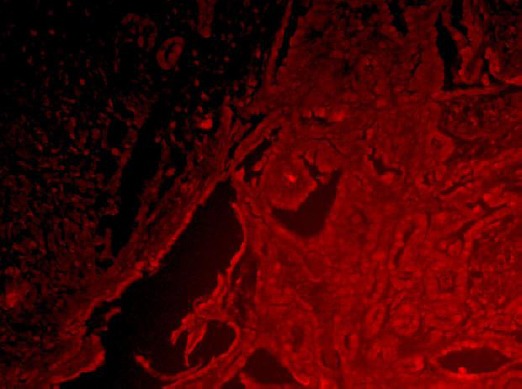
IL-17 positive macrophages – MGCs, marked in red color- co-localization (x 200)
Macrophage and MGCs counting
A single pathologist (MG), who was blinded to the clinical assessments of each case, scored the cases by counting, the number of CD68 TAMs in five independent fields in the tumor and in the invasive front under a 400 x magnification. CD68+ cell counts were expressed as the mean ± standard deviation.
Slides for the presence of MGCs [18] certain strict histological criteria were employed (Figure 3):
Figure 3.
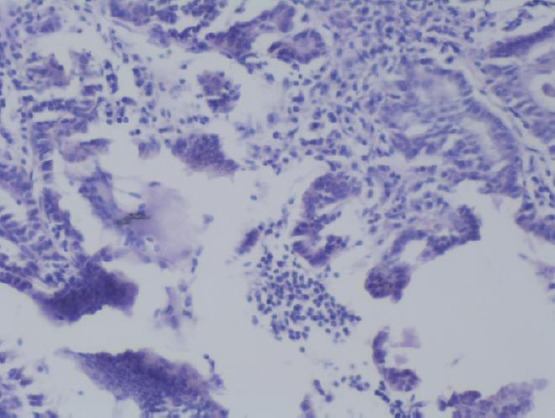
MGCs situated between papillae and next to the colloid (H&E × 100)
MGCs were situated in the lumina of tumor cystic spaces
MGCs have glossy dense eosinophilic cytoplasm
MCGs have at least three nuclei
A semi quantitative assessment was as followed: 0 MGCs per slide = none; 1-2 MGCs per slide = few; ≥ 3 MGCs per slide = many.
Statistical analysis
The SPSS 16.0 program for Windows was used for statistical analysis. The chi-squared test and Fisher’s exact test were used to compare the immunohistochemical staining and the clinicopathological parameters. ANOVA, Student-t-test, Mann-Whitney U test and Kruskal-Wallis test were applied for comparing the continuous variables depending on the normality of the distribution. Correlations were tested by Spearmen and Person tests. The accepted level of significance was set at p < 0.05.
Results
In PTC cases a few of CD68+ TAMs were observed in cystic spaces in the tumour in close connection with the colloid. These CD68+ TAMs were oval with one nucleus (Figure 4a) (and would be interpreted as M1 in morphology) and some MGCs having many nuclei and CD68+ cytoplasm (Figure 4b). In some PTCs MGCs were few (Figure 4c) or many (Figure 4d). MGCs were situated in between papillae and next to the colloid.
Figure 4.
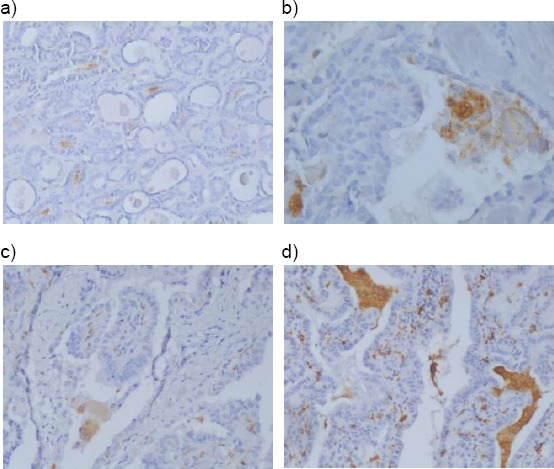
4a) CD68-positive macrophages M1 with oval form (× 100); 4b) CD68- positive macrophages - MGCs (× 200); 4c) CD68- positive macrophages – MGCs - “few” (× 200); 4d) CD68- positive macrophages – MGCs - “many” (× 100)
A statistically significant correlation was established between PTC patients in III stage, containing many MGCs, and PTC in I and II stage, with many MGCs. Eighty percent of patients in III stage showed many MGCs in comparison with patients in I and II stage, where many MGCs were found only in 21.1% (χ2 = 6.189, p = 0.013).
Discussion
Ryder at al., 2008 found that high density TAMs correlated with decrease cancer related survival in high-grade thyroid cancer (ATCs) and PDTC. Increased TAMs in PDTC were associated with capsular invasion and extrathyreoidal extension. However due the overall benign prognosis of PTC, the role of TAMs was not fully examined and understood.
Higher TAMs density was most significantly associated with lymph node metastases and with advanced tumor stage (III-IV) in PTC [25]. Other investigators had found larger PTCs had high density TAMs [4]. Fiumara et al., 1997 [3], described a negative association between neoplastic cell phagocytosis by macrophages and tumor vascular invasion and distant metastases. However these authors mainly focused on macrophage phagocytosis and on the role of immune system in thyroid cancer development. In our study we focused investigation on increased TAMs density and PTC clinicomorphological parameters, while the increased TAMs density and TGF-β1signalling pathway proteins we analyzed in previous study [26].
CD68+ TAMs were investigated in some thyroid malignancies. (Ryder et al., 2008) investigated 33 patients with WDTC (19 PTC), 13 patients with (FVPTC) and one with OC. They had found that the number of TAMs was < 5 (0.28 mm2) in most WDTC (34 patients) and > 10 (0.28 mm2) in less cases (9), and found no statistical correlation between presence of TAMs and extra thyroidal extension, capsular invasion and vascular invasion. We used CD68 antibody to identify TAMs in PTC (or M2 macrophages) since it was found that it is a universal marker for both types of macrophages (M1 and M2) [6], [9], [15]. Only the intracytoplasmic expression of IL-10 and IL-12 in TAMs could successfully differentiate two macrophage phenotypes [20]. We used CD68 staining in order to count TAMs in PTC ant to compare its number with that found by other investigators. Our TAMs numbers were that received by others in PTC [22], [26].
MGCs
Multinucleated Giant cells or MGCs are found in thyroid diseases such as Hashimoto’s, De Quervains or in follicular or anaplastic thyroid cancer [23], [27]. In the recent years MGCs are often associated with PTC [18]. The results of our study indicated that MGCs can be found in (n= 56) of PTC cases similarly to previous reports [14]. MGCs were found in less WDTCs (27%) by Ryder M et al., 2008 and in PTCs by Qing et al., 2012. We found for the first time that there was a definite prognostic significance to having “many” MGCs in PTC. In our study ‘“many” MGCs were statistically significantly correlated to the advanced tumor stage (III). Other authors detected that many MGCs in PTC were more likely associated with extrathyreoidal extension and greater tumor size (over stage III) in PTC was reported by [17], [19], [21].
Guitar et al., examined 76 cases of PTC and found MGCs in 46% (35/76). Tabbara et al., found in all (10 cases PTC-100%). MGCs in 63% of cases of FVPTC and in 7 of follicular adenoma. Other investigators using CD68 ICH found that TAMs (or MGCs-phagocytes) correlated with high lymphocyte and DCs, these patients had significantly less vascular invasion and remote metastasis [3]. The authors supposed that TAMs might activate the host immune response to cancer cells. To our knowledge, TAMs are plastic cells and they might act as tumor suppressors [9] by secreting IL-12 and have M1 phenotype in answer to Th1 cytokines or tumor promoters by secreting IL-10 and answering to Th2 cytokines.
MGCs were considered to be of histiocytic origin [14], [18]. Our contribution to MGCs cytoplasmic staining is that MGCs are mainly CD68+, CD123+ and thyroglobulin positive (having ingested colloid) [18]. Th17 cells and M1/M2 macrophages, these immune cells potentially function as a “double-edge” sword within the tumor microenvironment to enhance both anti-tumor immunity and inflammatory responses [16]. Our study demonstrates that the increased density of MGCs is associated with advanced stage of PTC, and therefore with tumor progression and that cases of PTC should be carefully screened for their presence. Our opinion is that tumor-associated macrophages are plastic cells that after recruitment in tumor microenvironment change their phenotype from M1 to M2 and so take part in the complex regulation of tumor regression and of tumor growth and progression. They are key component inflammatory circuits that organize adaptive immune response and angiogenesis-and promote tumor development.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Gorman CA, Maurer MS, McIver B, Mullan BP. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940-1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002;26:879–885. doi: 10.1007/s00268-002-6612-1. https://doi.org/10.1007/s00268-002-6612-1 PMid:12016468. [DOI] [PubMed] [Google Scholar]
- 2.Wang NI, Jiang R, Yang J-Y, Tang C, Yang L, Xu M, Jiang Q-F, Lin Z-M. Expression of TGF-β1, SNAI1 and MMP-9 is associated with lymph node metastasis in papillary thyroid carcinoma. J Mol Histol. 2014;45:391–399. doi: 10.1007/s10735-013-9557-9. https://doi.org/10.1007/s10735-013-9557-9 PMid:24276590. [DOI] [PubMed] [Google Scholar]
- 3.Gong D, Shi W, Yi S-J, Chen H, Groffen J, Heisterkamp N. TGFβsignaling plays a critical role in promoting alternative macrophage activation. BMC Immunology. 2012;13:31–41. doi: 10.1186/1471-2172-13-31. https://doi.org/10.1186/1471-2172-13-31 PMid:22703233 PMCid:PMC3406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for papillary thyroid carcinoma:a review and comparison. Ann Surg. 2007;245:366–378. doi: 10.1097/01.sla.0000250445.92336.2a. https://doi.org/10.1097/01.sla.0000250445.92336.2a PMid:17435543 PMCid:PMC1877011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLellis RA, Lloyd RV, Heitz PU, et al. Pathology and genetics of tumors of endocrine organs World Held Organization Clasifications of tumors. Lyon: IARC press; 2004. [Google Scholar]
- 6.Gulubova MV, Ananiev J, Yovchev Y, Julianov A, Karashmalakov A, Vlaykova T. The density of macrophages in colorectal cancer is inversely correlated to TGF- βexpression and patients'survival. J Mol Histol. 2013;44:679–692. doi: 10.1007/s10735-013-9520-9. https://doi.org/10.1007/s10735-013-9520-9 PMid:23801404. [DOI] [PubMed] [Google Scholar]
- 7.Jung KY, Cho SW, Kim YA, Kim D, et al. Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. J Pathol Translat Med. 2015;49:318–324. doi: 10.4132/jptm.2015.06.01. https://doi.org/10.4132/jptm.2015.06.01 PMid:26081823 PMCid:PMC4508569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward LS. Immune response in thyroid cancer:widening the boundaries. Scientifica 2014. 2014 doi: 10.1155/2014/125450. https://doi.org/10.1155/2014/125450 PMid: 25328756 PMCid:PMC4190695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, Mantovani A. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol. 2000;164(2):762–7. doi: 10.4049/jimmunol.164.2.762. https://doi.org/10.4049/jimmunol.164.2.762 PMid:10623821. [DOI] [PubMed] [Google Scholar]
- 10.Tabbara S, Acoury N, Sidawy MK. Multinucleated giant cells in thyroid neoplasms. A cytologic, histologic and immunohistochemical study. Acta Cytol. 1996;40(6):1184–8. doi: 10.1159/000333978. https://doi.org/10.1159/000333978 PMid:8960026. [DOI] [PubMed] [Google Scholar]
- 11.Barin JG, Baldeviano GC, Talor MV, Wu L, Ong S, Quader F, et al. Macrophages participate in IL-17-mediated inflammation. Eur J Immunol. 2012;42:726–36. doi: 10.1002/eji.201141737. https://doi.org/10.1002/eji.201141737 PMid:22161142 PMCid:PMC4292791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang JW, Li JC, Au KY, Yim HC, Lau AS. Interleukin-17A differentially modulates BCG induction of cytokine production in human blood macrophages. J Leukoc Biol. 2011;90:333–41. doi: 10.1189/jlb.0510311. https://doi.org/10.1189/jlb.0510311 PMid:21521755. [DOI] [PubMed] [Google Scholar]
- 13.Cunha LL, Marcello MA, Ward LS. The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocrine-Related Cancer. 2014;21(3):R85–103. doi: 10.1530/ERC-13-0431. https://doi.org/10.1530/ERC-13-0431 PMid:24302667. [DOI] [PubMed] [Google Scholar]
- 14.Nagorsen D, Voigt S, Berg E, Stein H, Thiel E, Loddenkemper C. Tumor-infiltrating macrophages and dendritic cells in human colorectal cancer:relation to local regulatory T cells, systemic T-cell response against tumor-associated antigens and survival. J Translational Medicine. 2007;5:62–70. doi: 10.1186/1479-5876-5-62. https://doi.org/10.1186/1479-5876-5-62 PMid:18047662 PMCid:PMC2212626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S, Cho SN, Min HS, Kim KM, Yeom GJ, Kim EY, Lee KE, Yun YG, Park YJ. The expression of tumor-associated macrophages in papillary thyroid carcinoma. Endocrinol Metab. 2013;28:192–198. doi: 10.3803/EnM.2013.28.3.192. https://doi.org/10.3803/EnM.2013.28.3.192 PMid:24396678 PMCid:PMC3811699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization:tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. https://doi.org/10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani A, Allavena P, Sica A. Tumor-associated magrophages as a prototypic type II polarized phagocyte population:role in tumor progression. Eur J of Cancer. 2004;40:1660–1667. doi: 10.1016/j.ejca.2004.03.016. https://doi.org/10.1016/j.ejca.2004.03.016 PMid:15251154. [DOI] [PubMed] [Google Scholar]
- 18.Edin S, Wikberg ML, Dahlin AM, Rutegard J, Oberg A, Oldenborg P, Palmquist R. The Distribution of Macrophages with a M1 or M2 Phenotype in Relation to Prognosis and the Molecular Characteristics of Colorectal Cancer. Plos One. 2012;7(10):47045–47056. doi: 10.1371/journal.pone.0047045. https://doi.org/10.1371/journal.pone.0047045 PMid:23077543 PMCid:PMC3471949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryder M, Ghossein RA, Ricarte-Filho JCM, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocrine Relat Cancer. 2008;15(4):1069–1074. doi: 10.1677/ERC-08-0036. https://doi.org/10.1677/ERC-08-0036 PMid:18719091 PMCid:PMC2648614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryder M, Gild M, Hohl TM, Pamer E, Knauf J, Ghossein R, Joyce JA, Fagin JA. Genetic and Pharmacological Targeting of CSF-1/CSF-1R Inhibits Tumor-Associated Macrophages and Impairs BRAF-Induced Thyroid Cancer Progression. Plos ONE. 2013;8:e54302. doi: 10.1371/journal.pone.0054302. https://doi.org/10.1371/journal.pone.0054302 PMid:23372702 PMCid:PMC3553126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunha LL, Morari EC, Guihen AC, Razolli D, Gerhard R, Nonogaki S, Soares FA, Vassallo J, Ward LS. Infiltration of a mixture of different immune cells may be related to molecular profile of differentiated thyroid cancer. Endocrine-related cancer. 2012;19(3):L31–6. doi: 10.1530/ERC-11-0285. https://doi.org/10.1530/ERC-11-0285 PMid:22461634. [DOI] [PubMed] [Google Scholar]
- 22.Cunha LL, Marcello MA, Morari EC, Nonogaki S, Conte FF, Gerhard R, Soares FA, Vassallo J, Ward LS. Infiltration of a mixture of different immune cells may be related to molecular profile of differentiated thyroid carcinoma. Clinical Endocrinology. 2012;77:918–925. doi: 10.1111/j.1365-2265.2012.04482.x. https://doi.org/10.1111/j.1365-2265.2012.04482.x PMid:22738343. [DOI] [PubMed] [Google Scholar]
- 23.Fiumara A, Belfiore A, Russo G, Salomone E, Santonocito GM, Ippolito O, Vigneri R, Gangemi P. In situ evidence of neoplastic cell phagocytosis by macrophages in papillary thyroid cancer. 1997;82(5):1615–1620. doi: 10.1210/jcem.82.5.3909. https://doi.org/10.1210/jc.82.5.1615. [DOI] [PubMed] [Google Scholar]
- 24.Brooks E, Simmons-Arnold L, Naud S, Evans MF, Elhosseiny A. Multinucleated giant cells incidence immune markers and significance:a study of 172 cases of papillary thyroid carcinoma. Head and Neck Pathol. 2009;3:95–99. doi: 10.1007/s12105-009-0110-9. https://doi.org/10.1007/s12105-009-0110-9 PMid:19644545 PMCid:PMC2715459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qing W, Fang W-Y, Ye L, Shen L-Y, Zang X-F, Fei X-C, Chen X, Wang W-Q, Li X-Y, Xiao J-C, Ning G. Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid. 2012;22(9):905–910. doi: 10.1089/thy.2011.0452. https://doi.org/10.1089/thy.2011.0452 PMid:22870901 PMCid:PMC3429273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivanova K, Manolova I, Ignatova MM, Gulubova M. Immunohistochemical Expression of TGF-Β1, SMAD4, SMAD7, TGFβRII and CD68-Positive TAM Densities in Papillary Thyroid Cancer. Open access Macedonian journal of medical sciences. 2018;6(3):435–441. doi: 10.3889/oamjms.2018.105. https://doi.org/10.3889/oamjms.2018.105 PMid:29610597 PMCid:PMC5874362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 Cells:Biology, Pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012;181:8–18. doi: 10.1016/j.ajpath.2012.03.044. https://doi.org/10.1016/j.ajpath.2012.03.044 PMid:22640807. [DOI] [PubMed] [Google Scholar]


