Abstract
BACKGROUND:
Weaning from invasive mechanical ventilation (MV) is considered as a daily challenging practice in the management of critically ill patients. The use of lung ultrasound and change in haemoglobin and hematocrit during weaning may help to predict weaning outcomes.
AIM:
We aim to study the impact of weaning induced pulmonary edema on outcomes of weaning from mechanical ventilation.
PATIENTS AND METHODS:
Sixty patients who fulfilled readiness criteria for weaning from MV. Spontaneous breathing trial (SBT) on T-piece for 120 minutes was performed under close hemodynamic monitoring. Lung ultrasound was performed using eight lung zones protocol to detect both the presence and the trend of change in B lines before and after SBT. For all the studied patients, haemoglobin and hematocrit values were checked just before and at the end of SBT.
RESULTS:
Patient who failed to pass SBT showed significant increase in lung segments showing B pattern, haemoglobin and hematocrit levels (p-value < 0.001 for all) also those patients had significantly higher duration of ICU stay (p-value < 0.001) Despite mortality rate was higher among patients who failed SBT yet it was statistically insignificant (p-value 0.104).
CONCLUSION:
lung ultrasound and both haemoglobin and hematocrit levels correlate with weaning outcomes.
Keywords: Weaning, Mechanical ventilation, Spontaneous breathing trials, Lung ultrasound, B lines, Haemoglobin, Hematocrit
Introduction
Weaning from mechanical ventilation is a daily challenging practice in intensive care units that require a thorough understanding of the factors which contribute to weaning outcomes [1]. Failure of weaning from mechanical ventilation increases the length of mechanical ventilation and length of stay in the intensive care unit, and it is associated with poor outcome [2].
Mechanical ventilation generally exerts negative hemodynamic effects in patients with normal cardiac function mainly because of the reduction in venous return induced by positive intra-thoracic pressure at each insufflation. In contrast, positive pressure ventilation exerts beneficial effects in patients with cardiogenic pulmonary oedema such that it is routinely used as a therapy in this category of patients [3].
The mechanisms that contribute to the development of cardiogenic pulmonary oedema during weaning are complex and mainly include the inspiratory fall in intrathoracic pressure, the increase in work of breathing, and the catecholamine discharge that occur during the abrupt transfer from mechanical ventilation to spontaneous breathing [4]. Inspiratory fall in intrathoracic pressure tends to increase the systemic venous return pressure gradient and the central blood volume and to decrease the left ventricular (LV) ejection pressure gradient with a resulting increase in LV afterload [5], [6].
In addition to the simultaneous increase in systemic venous return, the increased RV afterload may lead to a marked RV enlargement during weaning, thus impeding the diastolic filling of the left ventricle through a biventricular interdependence mechanism [7], [8].
Lung ultrasound is a simple, non-invasive technique that is very helpful in the detection of extravascular lung water [9]. In patients who are intubated and mechanically ventilated, during the liberation from mechanical ventilation the occurrence of weaning induced pulmonary oedema can be detected by the increase in B lines which is a simple and sensitive sonographic finding using lung ultrasound [10].
During the development of hydrostatic pulmonary oedema, the transfer of hypo-oncotic fluids from the pulmonary circulation toward the interstitial space often concerns an important volume so that it may result in a significant blood volume contraction hence the measurement of haemoglobin and hematocrit concentrations may be valuable easy and costless method for diagnosing weaning induced pulmonary oedema [11].
Methods
Design: This prospective cohort observational study to assess the accuracy of lung ultrasound and change in haemoglobin and hematocrit levels for predicting weaning outcomes.
Population: this study was conducted on sixty patients admitted to the Department of Critical Care Medicine, Faculty of Medicine, Cairo University, from April 2017 to September 2018. Patients included in the study were intubated and mechanically ventilated for more than 24 hours and fulfilled criteria of readiness to start spontaneous breathing trials (SBT) [12]. 1) Adult patients > 18 years; 2) Improvement of the underlying cause of acute respiratory failure; 3) Patients conscious, alert and can protect airways; 4) Inspired oxygen fraction below 0.5; 5) Positive end-expiratory pressure (PEEP) below 5 cm H2O, and 6) Absence of vasopressor and/or inotropic drugs. Exclusion criteria were: 1) Patients with impaired consciousness and cannot protect airways; 2) Patients on vasopressor and /or inotropic support; 3) Patients diagnosed with neuromuscular disorders, and 4) Inappropriate acoustic windows.
Ethical aspects: The study was approved by the Ethical Committee of Cairo University, and informed consent was obtained from the first degree relative of all enrolled patients.
Procedures/Measurements
Spontaneous breathing trial (SBT): All patients who met the readiness criteria to start SBT for weaning were connected to T-piece with continuous oxygen source. Continuous hemodynamic monitoring during SBT to the patients. SBT was continued for 120 minutes, and patients who succeeded to pass SBT were then extubated with continuous monitoring and observation for signs of failure of weaning for 48 hours.
Ultrasound technique: Lung ultrasound was performed using TOSHIBA ACUSON X 300 with 2 to 4-MHz convex probe. The sonographic examinations were performed while the patients in the supine position. Four quadrants were examined in each hemithorax: anterior–superior, anterior-basal (between the parasternal line and the anterior axillary line), lateral-superior and lateral-basal (between the anterior axillary line and the posterior axillary line) (Figure 1) [13]. The examination was performed before starting and at the end of SBT searching for B lines.
Figure 1.
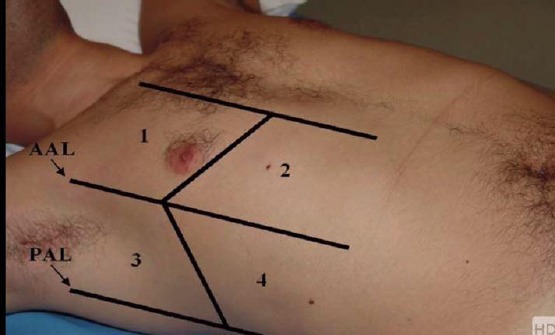
Four lung zones for LUS examination [13]
The B-lines are an “echoic, coherent bundle with a narrow basis, spreading from the pleural line to the edge of the screen without fading”. B pattern positive region is defined by the presence of three or more B-lines in a longitudinal plane between two ribs and Two or more positive regions bilaterally is consistent with the diagnosis of the interstitial syndrome [14].
Haemoglobin and hematocrit measurements: two blood samples were withdrawn from every patient just before the start and at the end of SBT to measure haemoglobin and hematocrit concentration.
Statistical Methods
Data were coded and entered using the statistical package SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 22. Data were summarised using mean and standard deviation in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data. Comparisons between quantitative variables were made using the non-parametric Mann-Whitney tests. For comparison of serial measurements within each patient in each group, the non-parametric Wilcoxon signed-rank test was used. For comparing categorical data, Chi-square (χ2) test was performed. Exact test was used instead when the expected frequency is less than 5. ROC curve was constructed with the area under curve analysis performed to detect the best cutoff value for detection of success of 1st SBT. P-values less than 0.05 were considered as statistically significant.
Results
According to the outcome of SBT, the study population were divided into two groups Group I (n=36): patients who passed SBT successfully Group II (n=24): patients who failed to pass SBT.
The baseline characteristics of the studied patients were shown in Table 1.
Table 1.
The baseline characteristics of the 60 patients included in the study
| Variables | All patients | Group 1 | Group II | P-value |
|---|---|---|---|---|
| Age | 57.25 ± 18.05 | 50.81 ± 18.18 | 67.58 ± 12.55 | < 0.001 |
| Gender | 33 males | 16 (44.4%) | 17 (70.8%) | 0.044 |
| 27 females | 20 (55.6%) | 7 (29.2%) | ||
| Body mass index (BMI) | 28.04 ± 6.57 | 27.34 ± 5.39 | 29.08 ± 8.04 | 0.850 |
| Numbers of days before SBT | 5.23 ± 2.54 | 4.00 ± 1.69 | 7.08 ± 2.50 | < 0.001 |
| Comorbidities: | ||||
| Smoking | 28 | 11 (30.6%) | 17 (70.8%) | 0.002 |
| Diabetes mellitus (DM) | 22 | 12 (33.3%) | 10 (41.7%) | 0.512 |
| Hypertension (HTN) | 29 | 10 (27.8%) | 19 (79.2%) | < 0.001 |
| Systolic heart failure | 16 | 5 (13.9) | 11 (45.8) | 0.006 |
| Renal impairment | 22 | 12 (32.2%) | 10 (41.2) | 0.512 |
| Chronic obstructive pulmonary disease (COPD) | 19 | 5 (13.9%) | 14 (58.3%) | < 0.001 |
| Chronic liver disease | 6 | 6 (16.7%) | 0 | 0.072 |
| Ischemic heart disease (IHD) | 20 | 8 (22.2%) | 12 (50%) | 0.025 |
| Malignancy | 8 | 2 (5.6%) | 6 (25%) | 0.05 |
| Cause of mechanical ventilation | ||||
| Disturbed conscious level (DCL) | 15 | 13 (36.1%) | 2 (8.3%) | 0.001 |
| Pneumonia | 15 | 7 (19.4%) | 8 (33.3%) | |
| COPD excerbation | 12 | 2 (5.6%) | 10 (41.7%) | |
| Hemodynamic instability | 9 | 8 (22.2%) | 1 (4.2%) | |
| Acute pulmonary odema | 8 | 5 (13.9%) | 3 (12.5v) | |
| Pulmonary embolism | 1 | 1 (2.8%) | 0 | |
| Cardiac functions: | ||||
| Left ventricular ejection fraction | 56.35 ±10.44 | 59.5 | 51.5 | .009 |
| Outcomes: | ||||
| Duration of ICU stay | 13.10 ± 4.55 | 10.69 | 16.71 | < 0.001 |
| In hospital mortality | 7 | 2 | 5 | 0.104 |
Data derived from Lung ultrasound
Before SBT, there was no statistically significant difference between both groups regarding several lung segments showing B pattern, Table 2.
Table 2.
Comparison between both groups before and after SBT regarding Number of Lung segments showing B pattern
| Number of Lung segments showing B pattern | Before SBT | After SBT | ||||
| Group I | Group II | P value | Group I | Group II | P value | |
| 1.19 ± 1.01 | 1.54 ± .66 | 0.112 | 1.75 ± 1.54 | 4.71 ± 1.90 | < 0.001 | |
After SBT, despite that, both groups showed a statistically significant increase in the number of the lung segments showing B pattern, yet the increase was more evident in group II, Table 3.
Table 3.
Comparative analysis for each group before and after SBT regarding the number of Lung segments showing B pattern
| Number of Lung segments showing B pattern | Group I | Group II | ||||
| Before SBT | After SBT | P value | Before SBT | After SBT | P value | |
| 1.19 ± 1.01 | 1.75 ± 1.54 | 0.004 | 1.54 ± 66 | 4.71 ± 1.90 | < 0.001 | |
A cutoff value for the increase in lung segments showed B pattern after SBT was 1.5 segments with a p value of 0.001, AUC = 0.883, Sensitivity = 86.1% and Specificity = 83.3%.
Number of lung segments showing B pattern
Comparison between both groups before and after SBT regarding several lung segments showing B pattern is shown in Fig. 2.
Figure 2.
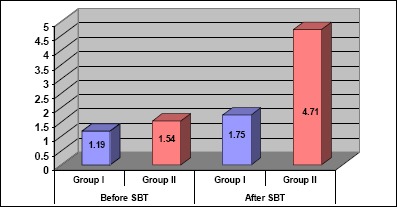
Comparison between both groups before and after SBT regarding Number of Lung segments showing B pattern
ROC curve for mean change in the number of lung segments showing B pattern is shown in Fig. 3
Figure 3.
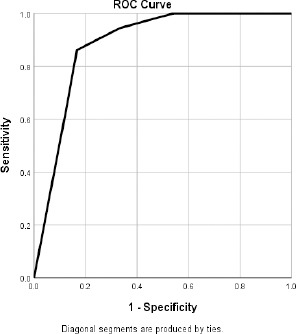
ROC curve for mean change in the number of lung segments showing B pattern
Central venous blood gases analysis
The mean PCO2 values were significantly higher in group II before, and after SBT, mean values of both PH and ScVO2 were significantly lower in the same group just after SBT (Table 4).
Table 4.
Comparison between both groups before and after SBT regarding blood gases analysis
| Parameter | Before SBT | After SBT | ||||
|---|---|---|---|---|---|---|
| Group I | Group II | P value | Group I | Group II | P value | |
| PH | 7.40 ± 04 | 7.41 ± .05 | 0.649 | 7.38 ± .03 | 7.30 ± .04 | < 0.001 |
| PCO2 | 39.71 ± 4.02 | 44.97 ± 6.13 | < 0.001 | 42.10 ± 4.30 | 58.8 ± 12.34 | < 0.001 |
| HCO3 | 24.96 ± 2.79 | 27.86 ± 5.28 | 0.036 | 24.80 ± 2.53 | 27.72 ± 6.16 | 0.116 |
| ScVO2 | 69.50 ± 5.99 | 69.52 ± 4.51 | 0.666 | 69.23 ± 7.98 | 48.04 ± 8.28 | < 0.001 |
Regarding group I mean PCO2 showed increased values but within the normal range. In contrast, group II showed a significant increase in mean PCO2 values with statistically significant lower PH values (Table 5).
Table 5.
Comparative analysis for each group before and after SBT regarding blood gases
| Group I | Group II | |||||
|---|---|---|---|---|---|---|
| Before SBT | After SBT | P value | Before SBT | After SBT | P value | |
| PH | 7.40 ± .04 | 7.38 ± .03 | 0.051 | 7.41 ± .05 | 7.30 ± .04 | < 0.001 |
| PCO2 | 39.71 ± 4.02 | 42.10 ± 4.30 | 0.001 | 44.97 ± 6.13 | 58.80 ± 12.34 | < 0.001 |
| HCO3 | 24.96 ± 2.79 | 24.80 ± 2.53 | 0.489 | 27.86 ± 5.28 | 27.72 ± 6.16 | 0.360 |
| ScVO2 | 69.50 ± 5.99 | 69.23 ± 7.98 | 0.581 | 69.52 ± 4.51 | 48.04 ± 8.28 | < 0.001 |
Central venous oxygen saturation (ScVO2) values showed a significant decrease in Group II after SBT P-value < 0.001.
Change in Hb and HCT
Group II showed more increase in mean values of Hb and HCT after SBT (Table 6).
Table 6.
Change in Hb and HCT
| Mean change before and after SBT | Group I | Group II | P-value |
|---|---|---|---|
| Mean increase in HCT | 0.32 ± 1.31 | 1.48 ± 1.41 | 0.001 |
| Mean increase in Hb | 0.07 ± .32 | 0.44 ± .33 | < 0.001 |
Discussion
We aimed in our study to make a focused view over the changes occurred in lungs during weaning from MV which were detected by lung ultrasound through increase in B lines with the occurrence of weaning induced pulmonary oedema (WIPE), coinciding with this changes the occurrence of hemoconcentration was detected through increase in Hb and HCT values after SBT. The eight lung zones protocol was used to detect the prevalence of B pattern as it is informative, easy to do in the supine position with no need for change in patients positions which may cause exhaustion of the patients underwent weaning from MV.
During our evaluation of the studied patients, we focused on selection of only the data with maximum benefit, quickest to be obtained with minimal stress on the studied patients who were already under the usual vigorous physical and psychological stress occurring during weaning from mechanical ventilation. Our results revealed more increase in B pattern in patients who failed to pass SBT with a concomitant increase in mean Hb and HCT values.
Similar to our results, Soummer et al., [15] conducted a study on one hundred patients to assess whether lung derecruitment during spontaneous breathing trial assessed by lung ultrasound is predictive of post-extubation respiratory distress. Lung ultrasound, echocardiography, and plasma B-type natriuretic peptide assay were determined before and at the end of a 60-min spontaneous breathing trial and 4 hours after extubation. They concluded that the lung derecruitment (assessed by lung u/s) is greater in patients who develop post-extubation respiratory distress (irrespective of its primary cause) than in patients who are definitively weaned (p < 0.001). In concordance to our results Carolina et al., [16], studied Fifty-seven subjects eligible for ventilation liberation Lung ultrasound assessments were performed immediately before and at the end of the spontaneous breathing trial. B-predominance was defined as any profile with anterior bilateral B-pattern. Patients were followed up to 48 hours after extubation, and the- failure group showed higher B-predominance at the end of the trial (p-value 0.01). The occurrence of hemoconcentration coinciding with increased B lines proved by increase in Hb and HCT values make the presence of WIPE more likely these findings are similar to the results of Dres M, et al., [17] who studied the relation between changes in extravascular lung water, blood volume contraction indices (including haemoglobin) and B-type natriuretic peptide and tho occurrence of weaning-induced pulmonary oedema. The study concluded that spontaneous breathing trial-induced increases in extravascular lung water, plasma protein concentrations, haemoglobin concentration, and B-type natriuretic peptide are reliable alternatives to the pulmonary artery catheter for diagnosing weaning-induced pulmonary oedema.
In conclusion, changes in B pattern detected by lung ultrasound, together with an increase in haemoglobin and hematocrit values could detect the changes in lung aeration occurring during weaning from mechanical ventilation and correlates with weaning outcomes.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.MacIntyre NR, Cook DJ, Ely EW, Epstein SK, Fink JB, Heffner JE. Evidence-based guidelines for weaning and discontinuing ventilatory support. Chest. 2001;120(6):375S–95S. doi: 10.1378/chest.120.6_suppl.375s. https://doi.org/10.1378/chest.120.6_suppl.375S PMid:11742959. [DOI] [PubMed] [Google Scholar]
- 2.Esteban A, Alia I, Gordo F, et al. Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. The Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1997;156(2 Pt 1):459–65. doi: 10.1164/ajrccm.156.2.9610109. https://doi.org/10.1164/ajrccm.156.2.9610109 PMid:9279224. [DOI] [PubMed] [Google Scholar]
- 3.Spicher JE, White DP. Outcome and function following prolonged mechanical ventilation. Arch Intern Med. 1987;147(3):421–5. https://doi.org/10.1001/archinte.1987.00370030025005. [PubMed] [Google Scholar]
- 4.Frazier SK, Stone KS, Moser D, et al. Hemodynamic changes during discontinuation of mechanical ventilation in medical intensive care unit patients. Am J Crit Care. 2006;15(6):580–93. quiz 594. [PubMed] [Google Scholar]
- 5.Heunks LM, van der Hoeven JG. Clinical review:The ABC of weaning failure-a structured approach. Critical Care. 2010;14(6):245. doi: 10.1186/cc9296. https://doi.org/10.1186/cc9296 PMid:21143773 PMCid:PMC3220047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. European Respiratory Journal. 2007;29(5):1033–1056. doi: 10.1183/09031936.00010206. https://doi.org/10.1183/09031936.00010206 PMid:17470624. [DOI] [PubMed] [Google Scholar]
- 7.Gomez H, Pinsky MR. Principles and Practice of Mechanical Ventilation. 3rd Edn. MacGrawHill; 2013. Effect of mechanical ventilation on heart-lung interactions; p. 82. [Google Scholar]
- 8.Tyberg JV, Grant DA, Kingma I, et al. Effects of positive intrathoracic pressure on pulmonary and systemic hemodynamics. Respiration physiology. 2000;119(2):171–179. doi: 10.1016/s0034-5687(99)00112-7. https://doi.org/10.1016/S0034-5687(99)00112-7. [DOI] [PubMed] [Google Scholar]
- 9.Picano E, Frassi F, Agricola E, et al. Ultrasound lung comets:a clinically useful sign of extravascular lung water. Journal of the American Society of Echocardiography. 2006;19(3):356–363. doi: 10.1016/j.echo.2005.05.019. https://doi.org/10.1016/j.echo.2005.05.019 PMid:16500505. [DOI] [PubMed] [Google Scholar]
- 10.Bouhemad B, Zhang M, Lu Q, et al. Clinical review:bedside lung ultrasound in critical care practice. Critical Care. 2007;11:1. doi: 10.1186/cc5668. https://doi.org/10.1186/cc5668 PMid:17316468 PMCid:PMC2151891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pottier V, Valette X, Seguin A, Masson R, Parienti JJ, Sauneuf B, DU DC, Terzi N. Diagnostic accuracy of hemoconcentration for pulmonary edema as the cause of weaning failure. Minerva anestesiologica. 2016;82(4):419–28. [PubMed] [Google Scholar]
- 12.Aikat Sengupta S, et al. A Review for:Evidence-Based Practice of Weaning from Ventilator. February. 2018 anaesthesia-tutorial-of-the-week. [Google Scholar]
- 13.Volpicelli G, Mussa A, Garofalo G, Cardinale L, Casoli G, Perotto F, Fava C, Frascisco M. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24(6):689–96. doi: 10.1016/j.ajem.2006.02.013. https://doi.org/10.1016/j.ajem.2006.02.013 PMid:16984837. [DOI] [PubMed] [Google Scholar]
- 14.Elbarbary M, Volpicelli G. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. https://doi.org/10.1007/s00134-012-2513-4 PMid:22392031. [DOI] [PubMed] [Google Scholar]
- 15.Soummer A, Perbet S, et al. Lung Ultrasound Study Group:Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit Care Med. 2012;40:2064–2072. doi: 10.1097/CCM.0b013e31824e68ae. https://doi.org/10.1097/CCM.0b013e31824e68ae PMid:22584759. [DOI] [PubMed] [Google Scholar]
- 16.Antonio AC, Teixeira C, Castro PS, Savi A, Maccari JG, Oliveira RP, Knorst MM. Behavior of lung ultrasound findings during spontaneous breathing trial. Rev Bras Ter Intensiva. 2017;29(3):279–86. doi: 10.5935/0103-507X.20170038. https://doi.org/10.5935/0103-507X.20170038 PMid:28832706 PMCid:PMC5632969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dres M, JL Teboul, et al. Extravascular lung water, B-type natriuretic peptide, and blood volume contraction enable diagnosis of weaning-induced pulmonary edema. Crit Care Med. 2014;42(8):1954–5. doi: 10.1097/CCM.0000000000000295. https://doi.org/10.1097/CCM.0000000000000295 PMid:24717458. [DOI] [PubMed] [Google Scholar]


