Abstract
BACKGROUND:
Extensive intracellular and extracellular formation of advanced glycation end-products (AGEs) is considered a causative factor for vascular injury triggered by hyperglycemia in diabetes. The hyperglycemia will cause accumulation of AGEs, damage to pericytes, nerve growth factor (NGF), glial acid fibrillary protein (GFAP) and increase in vascular endothelial growth factor (VEGF).
AIM:
This study aimed to assess the efficacy of RAGE inhibition in suppressing the development and progression of diabetic retinopathy through modulation of the inflammatory pathway involving NGF, GFAP, and VEGF.
METHODS:
The design was in vivo experimental study. Thirty white rats were induced with Alloxan monohydrate. Rats were divided into 5 groups, normal, negative control, groups with an anti-RAGE dose of 1 μg/uL, the dose of 10 μg/uL and 100 μg/uL. After 4 weeks of treatment, HbA1c, NGF, and GFAP levels were measured using ELISA. Quantification of VEGF expression was done using the ImageJ® application. Data was expressed with mean ± SD. Independent T-test with ANOVA and Tukey’s post hoc was done.
RESULTS:
RAGE inhibitors yielded a significant decrease in blood glucose and HbA1c levels. VEGF and RAGE expression were reduced in anti-RAGE groups in various doses. Inhibition of RAGE reduced the damage of retinal pericytes, by reducing GFAP and increasing NGF, and reduced the formation of new blood vessels, by decreasing VEGF expression, in diabetic retinopathy.
CONCLUSION:
Inhibition of receptor for advanced glycation end-products (RAGE) was effective in suppressing the development and progression of diabetic retinopathy.
Keywords: RAGEs, Diabetic retinopathy, HbA1c, Neural growth factor, Glial acidic fibrillary protein
Introduction
Diabetes is a life-threatening disease associated with deadly complications such as cardiovascular disease, stroke, and microvascular diseases. Worldwide population with diabetes is estimated to be 285 million adults in 2010 and predicted to reach 439 million adults in 2030 with a total overall prediction of a 54% increase [1], [2]. Extensive intracellular and extracellular formation of advanced glycation end-products (AGEs) is considered a causative factor for vascular injury triggered by hyperglycemia in diabetes. Mechanisms related to and without the role of receptors are known to play a role in cellular dysfunction and tissue damage caused by AGE. Receptors for AGE (RAGE) were initially found as AGE binding receptors and are now known as pro-inflammatory molecules that make up the danger signals of mediation to the body. RAGE is included in the pattern-recognition receptors associated with innate and adaptive immunity. Endogenous and exogenous ligands include AGE, high-mobility group 1 (HMGB1), calcium-binding S100 protein group, β2-integrin Mac/CD11b, amyloid β-peptide, b-sheet fibrils, advanced oxidation protein products (AOPPs), complement C3a, lipopolysaccharides (LPS), and phosphatidylserine on the surface of apoptotic cells [3]. Activation of RAGE by ligand will cause activation of nuclear factor-κB (NF-κB) and other signalling pathways through stimulation of ERK (extracellular signal-regulated kinase) 1 / 2, p38 MAPK (mitogen-activated-protein-kinase)-JNK (c-Jun N-terminal kinases), JAK (Janus-kinase)-STAT (signal transducer and activator of transcription), and Rac-Cdc42, many of which are the results and causes of reactive oxygen species (ROS) [4], [5].
Furthermore, expression of inflammatory cytokines increases, leading to an inflammatory response with cellular migration and proliferation. The hyperglycemia will cause accumulation of AGEs, which will cause the death of intramural pericyte and thinning of the basement membrane so that it will reduce the blood supply to the retina. AGEs will cause an increase in ROS, increase in damage to pericytes, nerve growth factor (NGF), glial acid fibrillary protein (GFAP) and increase in vascular endothelial growth factor (VEGF), so that blood vessels will form in the retina. The massive occurrence of this process will cause interference with the retina [6], [7].
This study was the pilot study to assess the efficacy of RAGE inhibition in suppressing the development and progression of diabetic retinopathy through modulation of the inflammatory pathway involving NGF, GFAP, and VEGF in vivo.
Material and Methods
The design of the study was in vivo experimental study, using Wistar strains (Rattus norvegicus) white rats. This study was approved by the Bioethical Committee, Faculty of Medicine, Sriwijaya University-General Hospital Mohammad Hoesin (no. 198/kptfkunsri-rsmh/2018).
Subjects Preparation and Anti-RAGE Treatment
This study involved as many as 30 white rats (Rattus norvegicus) aged 2 months, bodyweight of 180-200 grams, with an equal number of animal gender, were conditioned in the housing of plastic cages, at room temperature, humidity level at 40-70%, with a dark-light cycle of 12 hours. Rats were fed with standard feed and drinking water ad libitum. Rats were induced with alloxan monohydrate (Sigma-Aldrich®, St. Louis, Missouri, USA) 120 mg/kg BW, intraperitoneally.
Blood sugar levels were examined by Glucose assay (DiaSys Diagnostic Systems GmbH, Waterbury, Connecticut, USA). If the blood sugar level was more than 200 mg/dL, then a 10% glucose drink was given to elevate the blood sugar level, once every 1 week. Alloxan monohydrate injection was performed for 12 weeks. Rats were divided into 5 groups, 1: normal, 2: negative control, 3, 4 and 5, respectively: Anti-RAGE (Abcam®, Cambridge, UK) dose of 1 μg/uL, the dose of 10 μg/uL and 100 μg/uL, in a single dose. Rats were euthanised by ketamine anaesthetic dose of 100 mg/kg BW, intraperitoneally. Blood collection from retro-orbital plexus was performed to estimate blood sugar and HbA1c levels. The serum was homogenised by centrifugation at 3000 rpm for 20 minutes. Collection of retinas was carried out, where some were put into 10% NBF and part of the tissue was processed for homogenisation by centrifugation at 10,000 rpm, 4°C, for 30 minutes.
HbA1c, NGF and GFAP examination
HbA1c, NGF (Cloud-Clone Corp®, Texas, USA), and GFAP levels (Cloud-Clone Corp®, Texas, USA) were measured using the ELISA (Enzyme-Linked Immunosorbent Assay) method. A total of 10 ml supernatant was inserted into the microplate well. Then incubation for 30 minutes at 37°C was carried out. Afterwards, 50 μL of conjugate HRP was added to the microplate well, then incubated for 30 minutes at 37°C. 50 μL of chromogen A and chromogen B was added to the microplate well, then incubated for 15 minutes, at 37°C. 50 μL stop solution was added to the microplate well. Then the microplate was inserted into the ELISA reader (Bio-rad®, Hercules, California, USA) with a wavelength of 450 nm, so that the Optical Density (OD) value was obtained. OD values were converted to levels.
VEGF and RAGE examination
The ophthalmic slices were obtained from the euthanised rats and stored at 10% NBF. The ophthalmic slices were then fixed, treated with paraffin blocks and cut into 4 um thick. Sections were deparaffinized with concentrated xylene and ethanol with various concentrations (100%, 90%, 80% and 70%).
Antigen retrieval was done by microwave using the Heat-Induced Antigen Retrieval (HIAR) method using pH 6.0 Na-citrate for 15 minutes and blocked by blocking solution (3% serum goat in 0.3% Triton X in PBS). Slides were washed with PBS 3 times and incubated with anti-VEGF (1:100), anti-RAGE (1:100) overnight at 4°C. Then counterstain and mounting were performed. Quantification was done using the ImageJ® application.
Statistical Analysis
Statistical analysis was done with SPSS for Windows (SPSS Inc., Chicago, Illinois, USA). Data was expressed with mean ± SD. Independent T-test with ANOVA Tukey’s post hoc was done. Significance was set if the p-value was less than 0.05.
Results
As exhibited in Table 1, there was a decrease in body weight on diabetic rats compared to normal. RAGE inhibitors increased body weight as the dose increased. The mean blood glucose levels of diabetic rats were higher when compared to normal.
Table 1.
Body Weight, Blood Glucose and HbA1c Levels
| Parameters | Normal | Negative Control | Dose 1 μg/uL | Dose 10 μg/uL | Dose 100 μg/uL |
|---|---|---|---|---|---|
| Body weight (g) | 313 ± 12.0* | 187 ± 11.3 | 189 ± 12.5 | 195 ± 11.6* | 223 ± 15.7* |
| Blood glucose (mg/dL) | 91.4 ± 8.9* | 382 ± 21.5 | 345 ± 23.3* | 321 ± 26.9* | 307 ± 27.9* |
| HbA1c (%) | 5.89 ± 0.98* | 15.89 ± 7.81 | 13.29 ± 9.18* | 12.09 ± 7.23* | 11.89 ± 6.12* |
Data were mean ± SD;
p < 0.05 compared to negative control.
Further, diabetics rats possessed higher levels of HbA1c compared to normal. Reversely RAGE inhibitors yielded a significant decrease in blood glucose and HbA1c levels compared to the negative control, as the dose increased (Table 1).
RAGE inhibitor decreased pericyte death shown by the decreased level of GFAP and increased NGF level (Table 2). As shown in Table 2, the mean NGF levels of people with diabetes were lower compared to normal. In contrary, diabetic rats possessed higher levels of GFAP compared to normal. RAGE inhibitors increased NGF levels and decreased GFAP levels in a dose-dependent manner.
Table 2.
Level of Retinal NGF and GFAP
| Parameters | Normal | Negative Control | Dose 1 μg/uL | Dose 10 μg/uL | Dose 100 μg/uL |
|---|---|---|---|---|---|
| NGF (pg/mL) | 113.4 ± 9.7* | 67.3 ± 4.3 | 75.1 ± 9.5* | 85.3 ± 7.6* | 97.5 ± 8.7* |
| GFAP (ng/mL) | 41.4 ± 2.9* | 182.2 ± 9.5 | 165.3 ± 13.3* | 132.1 ± 11.9* | 109.2 ± 8.9* |
Data were mean ± SD;
p < 0.05 compared with the negative control.
As exhibited in Figure 1 and Figure 2, VEGF and RAGE expressions were distinctly higher in people with diabetes compared to normal. VEGF and RAGE expressions were reduced in anti-RAGE groups in various doses. Increased doses resulted in a more diminished level of VEGF and RAGE.
Figure 1.
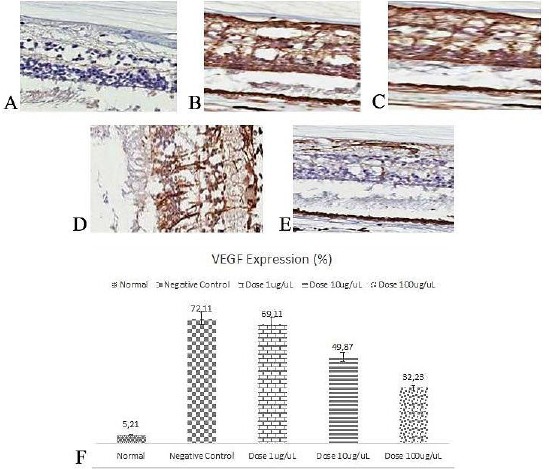
Immunohistochemistry of VEGF in Retina (400 x); A) Normal; B) Negative Control; C) Dose 1 μg/uL; D) Dose 10 μg/uL; E) Dose 100 μg/uL; F) VEGF expression (%)
Figure 2.
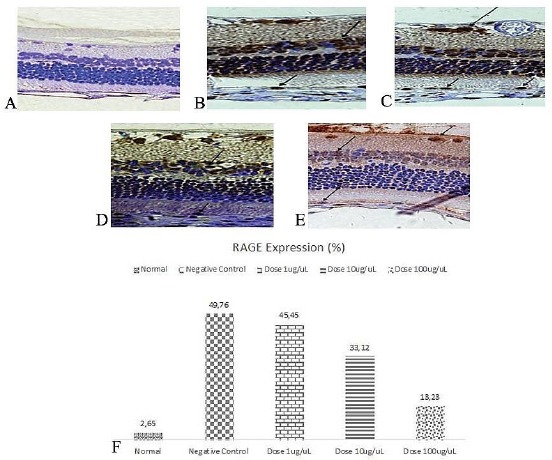
Immunohistochemistry of RAGE in Retina (400 x); A) Normal; B) Negative Control; C) Dose 1 μg/uL; D) Dose 10 μg/uL; E) Dose 100 μg/uL; F) RAGE expression (%)
Discussion
Chronic complications of diabetes mellitus are health problems that had become a global priority. AGEs are believed to play a vital role in the pathophysiology of various complications of diabetes mellitus. AGE accumulation in chronic diabetes mellitus causes pericyte death and thickening of the basement membrane, which causes incompetence of blood vessel walls. AGEs also stimulate RAGE expression which leads to activation of VEGF production which in turn triggers angiogenesis in the retina. Also, AGE induces pericytes apoptotic cell death through interaction with RAGE and VEGF induction. It is becoming clear that hypoxia plays an important role in all diabetes complications as it has been postulated that hyperglycemia induces a pseudohypoxia state. Cell adaptive response to hypoxia is mediated by the induced hypoxia-1 factor (HIF-1a) [8]. VEGF is a powerful mitogen for endothelial cells that triggers proliferation, migration and tube formation which causes the growth of new blood vessels. Also, AGE induces VEGF expression in cell culture and animal models, so it is considered to be involved in the pathogenesis of diabetic retinopathy. GFAP is an intermediate filament protein that is present in retinal glial cells (ganglion cells) and retinal astrocytes. Retinal glia (astrocytes, Muller cells, and microglia) is a sensitive indicator of retinal tissue injury and is very affected in the condition of diabetes due to the formation of AGE [9], [10], [11], [12], [13], [14].
This study showed that inhibition of RAGEs was able to reduce pericytes damage by reducing the formation of ROS due to accumulation of AGEs. The levels of NGF and GFAP that were assessed in this study showed that inhibition of RAGEs was able to reduce pericytes damage. Decreased accumulation of AGEs reduced VEGF expression so that the formation of new blood vessels in the retina could be prevented [15]. These results suggest a new treatment strategy for diabetic retinopathy conditions.
In conclusion, inhibition of RAGE reduced the damage of retinal pericytes, by reducing GFAP and increasing NGF, and reduced the formation of new blood vessels, by decreasing VEGF expression in diabetic retinopathy. Inhibition of receptor for advanced glycation end-products (RAGE) was effective in suppressing the development and progression of diabetic retinopathy.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Browning DJ. Diabetic Retinopathy:Evidence-Based Management. New York: Springer; 2010. pp. 12–105. https://doi.org/10.1007/978-0-387-85900-2_14. [Google Scholar]
- 2.Nentwich MM, Ulbig MW. Diabetic retinopathy - ocular complications of diabetes mellitus. World J Diabetes. 2015;6(3):489–99. doi: 10.4239/wjd.v6.i3.489. https://doi.org/10.4239/wjd.v6.i3.489 PMid:25897358 PMCid:PMC4398904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy:clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–34. doi: 10.1016/S1474-4422(12)70065-0. https://doi.org/10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soewondo P, Soegondo S, Suastika K, Pranoto A, Soeatmadji DW, Tjokroprawiro A. Outcomes on control and complications type 2 diabetic patients in Indonesia. The DiabCare Asia 2008 study. 2010;19(4):235–244. https://doi.org/10.13181/mji.v19i4.412. [Google Scholar]
- 5.Mavrikakis E, Khan BU, Lam W. Macular edema in diabetes. 2018. [online resource] http://emedicine.medscape.com/article/1224138-overview .
- 6.Amano S, Yamagishi S, Koda Y, Tsuneoka M, Soejima M, Okamoto T, et al. Polymorphism of sorbitol dehydrogenase (SDH) gene and susceptibility to diabetic retinopathy. Med Hypotheses. 2003;60(4):550–1. doi: 10.1016/s0306-9877(03)00013-6. https://doi.org/10.1016/S0306-9877(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 7.Waisbourd M, Goldstein M, Loewenstein A. Treatment of diabetic retinopathy with anti VEGF drugs. J Acta Ophthalmol. 2011;89(3):2003–7. doi: 10.1111/j.1755-3768.2010.02010.x. https://doi.org/10.1111/j.1755-3768.2010.02010.x PMid:21044274. [DOI] [PubMed] [Google Scholar]
- 8.Gragoudas ES, Adamis AP, Cunningham ET, Feinsod M, Guyer DR VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–16. doi: 10.1056/NEJMoa042760. https://doi.org/10.1056/NEJMoa042760 PMid:15625332. [DOI] [PubMed] [Google Scholar]
- 9.Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of Diabetic Retinopathy. ISRN Ophthalmol. 2013;2013:343560. doi: 10.1155/2013/343560. https://doi.org/10.1155/2013/343560 PMid:24563789 PMCid:PMC3914226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrovic MG, Peterlin B, Hawlina M, Petrovic D. Aldose Reductase (AC) gene polymorphism and susceptibilty to diabetic retinopathy in type 2 diabetes in Caucasians. J Diabetes Complications. 2005;19(2):70–3. doi: 10.1016/j.jdiacomp.2004.08.004. https://doi.org/10.1016/j.jdiacomp.2004.08.004 PMid:15745835. [DOI] [PubMed] [Google Scholar]
- 11.Ramprasad S, Radha V, Mathias RA, Majumder PP, Rao MR, Rema M. Rege gene promoter polymorphism and diabetic retinopathy in a clinic-based population from South India. Eye (Lond) 2007;21(3):395–401. doi: 10.1038/sj.eye.6702239. https://doi.org/10.1038/sj.eye.6702239 PMid:16440015. [DOI] [PubMed] [Google Scholar]
- 12.Olmos P, Futers S, Acosta AM, Siegel S, Maiz A, Schiaffino R, et al. (AC)23 (2-2) Polymorphism of the aldose reductase gene and fast progression of Retinopathy in Chilea type 2 Diabetics. Diabetes Res Clin Pract. 2000;47(3):169–76. doi: 10.1016/s0168-8227(99)00118-7. https://doi.org/10.1016/S0168-8227(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 13.Suganthalakshmi B, Anand R, Kim R, Mahalakshmi R, Karthikprakash S, Namperumalsamy P, et al. Association of VEGF and eNOS gene polymorphism in type 2 diabetic retinopathy. Mol Vis. 2006;12:336–341. [PubMed] [Google Scholar]
- 14.Awata T, Inoue K, Kurihara S, Ohkubo T, Watanabe M, Inukai K, et al. A Common Polymorphism in the 5'-Untranslated Region of the VEGF Gene is Associated With Diabetic Retinopathy in Type 2 Diabetes. Diabetes. 2002;51(5):1635–9. doi: 10.2337/diabetes.51.5.1635. https://doi.org/10.2337/diabetes.51.5.1635 PMid:11978667. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzi M. The Polyol Pathway as a Mechanism for Diabetic Retinopathy:Attractive, Ellusive, and Resilient. Exp Diabetes Res. 2007;2007:61038. doi: 10.1155/2007/61038. https://doi.org/10.1155/2007/61038 PMid:18224243 PMCid:PMC1950230. [DOI] [PMC free article] [PubMed] [Google Scholar]


