Abstract
BACKGROUND:
Nowadays, spinal anaesthesia is a suitable choice for most elective and emergency cesarean section (C-section) deliveries.
AIM:
This study aimed to determine the effect of adding low-dose naloxone to intrathecal morphine on postoperative pain and morphine related side effects after C-section.
MATERIAL AND METHODS:
In the present double-blind, randomised clinical trial, 70 women aged over 18 years, who were candidates for elective medical C-section under spinal anaesthesia were selected and randomly assigned to either the study group or the control group. For spinal anaesthesia, 10 mg of Bupivacaine plus 100 μg of morphine was administered for all patients. However, patients in the study group received 20 µg of naloxone intrathecally; but the patients in the control group only received normal saline as a placebo. After surgery, patient-controlled analgesia (PCA) pump with paracetamol (Apotel®) was connected to each patient. The intensity of postoperative pain in the patients was evaluated and recorded using Visual Acuity Screening (VAS) at 2, 4, 6 and 24 hours after the surgery. The patients were also examined for postoperative nausea and pruritus.
RESULTS:
Regardless of the groups to which the patients were assigned, a significant difference in pain intensity was observed during the study period (time effect; p < 0.001). Although the intensity of pain was lower in the study group, the difference was not statistically significant (group effect; p = 0.84). Also, there was no group time interaction between pain intensity and the times studied (p = 0.61). The incidence rates of postoperative nausea and pruritus were significantly lower in the study group compared to the control group (p < 0.001).
CONCLUSION:
According to the results of this study, adding low dose naloxone to intrathecal morphine did not significantly change postoperative pain intensity in the patients undergone elective C-section using spinal anaesthesia; however, significantly decreased the severity of postoperative nausea and pruritus.
Keywords: Naloxone, Morphine, Cesarean Section, Anesthesia, Spinal, Pain, Postoperative
Introduction
Cesarean section (C-section) is one of the major obstetric and gynecologic surgeries. Following C-section, patients experience different degrees of pain due to complex physiological issues in response to tissue damage and uterine contractions [1], [2]. Postoperative pain can lead to patient’s failure to discharge respiratory secretions, ileus and prolonged bed rest, which increases the risk of deep vein thrombosis and other complications. Also, it can cause delayed patient discharge from hospital, prolonged recovery time, lack of patient satisfaction with hospital care and increased recovery costs. Moreover, acute pain after C-section can delay the onset of breastfeeding [3], [4]. Therefore, finding a way to provide a maximum pain relief feeling and calmness, with the minimum complications, is one of the most important issues after C-section [5], [6].
Various studies have been conducted so far on reducing patients’ pain intensity after C-section. The growing number of studies indicates that no clear and reliable method exists yet for reducing post-cesarean pain; in other words, relieving pain in the early post-cesarean stages is still a challenge [7]. Nowadays, spinal anaesthesia is a suitable choice for most elective and emergency cesarean deliveries [8], [9]. So far, various drugs have been used intrathecally for spinal anaesthesia, and among these drugs, local anaesthetics are the most important ones. Opioids are another group of drugs that are widely used for neuraxial analgesia, especially in combination with other drugs. Using opioids in spinal anaesthesia increases its quality, reduces its complications and has advantages in postoperative analgesia [10], [11].
Additionally, it may minimize the risk of hypotension following spinal anesthesia, especially in patients undergoing C-section. Morphine is one of the opioids commonly used for spinal anesthesia in patients undergoing C-section. Despite the benefits of using intrathecal morphine in spinal anesthesia, it may cause complications such as postoperative nausea, vomiting, pruritus, sedation, and respiratory depression. Furthermore, the quality of analgesia and the incidence of intrathecal morphine side effects are dose-dependent and usually increase with increasing dose [12], [13]. Therefore, it seems reasonable to use compounds enhancing the effectiveness of opioids and reducing their dosage, side effects and pain intensity in patients.
Naloxone, as a μ-opioid receptor antagonist, is one of the drugs that can enhance the postoperative pain-relieving effects of opioids and reduce their dosage. Releasing endogenous opioids and the regulation of opioid receptors is one the probable mechanism of naloxone in enhancing the opioids’ analgesic effect [14], [15]. Previous studies showed conflicting results regarding the effect of low-dose naloxone in combination with opioids on pain intensity, doses of opioids and side effects of opioid administration [15], [16], [17], [18], [19]. Therefore, the aim of this study was to determinate the effect of adding low-dose naloxone to intrathecal morphine on post-cesarean pain intensity and complications.
Material and Methods
In the present double-blind, randomised controlled clinical trial, patients who were scheduled for elective C-section and referred to Imam Khomeini Educational Hospital in Sari, were evaluated. Those patients who meet the inclusion criteria were initially selected. The inclusion criteria were being a candidate for elective medical C-section under spinal anaesthesia, being over 18 years of age, weight 60-80 kg, giving birth to the first or the second baby and being ASA class 1 or 2. Also, patients with known sensitivity to bupivacaine or morphine, failure of spinal anaesthesia, reluctance to continue with the study, prolonged duration of surgery (more than 1.5 hours), and in whom unpredicted adverse effects or complications occurred during surgery were excluded from the study.
After obtaining the approval of the institutional ethics committee (reference number: IR.MAZUMS.IMAMHOSPITAL.REC.13997.044) and obtaining informed consent, 70 patients included in the study. Then, the participants were randomly allocated into two equal-sized groups of study and control (n = 35). All patients received 500 ml of lactated Ringer’s solution intravenously after entering the operation room. To monitor the patients, non-invasive blood pressure measurement, pulse oximetry and electrocardiogram (ECG) monitoring were used. Spinal anaesthesia was performed in a sitting position and under aseptic condition by an anesthesiologist, who was unaware of the contents of the syringes. The anesthesiologist inserted a needle (B. Braun 25G) into the space between L3 and L4 or L4 and L5 vertebrae and entered the subarachnoid space from the midline. Needle placement in dura mater space was confirmed by the free flow of cerebrospinal fluid. The needle bevel was placed in the cephalad direction, and the anaesthetic drugs were injected. For spinal anaesthesia, 10 mg of 0.5% bupivacaine plus 100 μg of morphine were administered for every patient. Besides, 20 µg of naloxone (produced in Toliddaru Co.) was injected into the intrathecal space in the patients of the study group, but the patients in the control group only received normal saline as placebo.
After the completion of the injection process, the patient was placed in the supine position; her shoulders were raised and using nasal cannula 100% oxygen was administered. After ensuring the proper level of the sensory blockade and hemodynamic stability of the patient, the surgery started. The surgical technique (i.e., Pfannenstiel transverse incision) was the same for all patients. The patients had not received any sedatives before the surgery. After completion of the surgery, patient-controlled analgesia (PCA) pump was connected to each patient. The PCA pump included 4 gr Apotel® (Paracetamol), which had been reached to 120 ml (the total volume of the pump) with normal saline. The PCA pumps were set as follows: the bolus dose of 0.5 ml, 15 minutes lockout and a background infusion rate of 4 cc/h. Diclofenac suppository was also used if a patient’s pain had not been properly managed. The primary outcome was postoperative pain intensity, and the secondary outcomes were postoperative total morphine consumption and incidence of postoperative nausea and pruritus. The intensity of postoperative pain was evaluated using the visual analogue scale (VAS) at 2, 4, 6 and 24 hours after the surgery. The patients were also examined for postoperative nausea and pruritus. The evaluations were done by a nurse, who was unaware of the study groups; but she had already received adequate training for those evaluations. Also, patients in the two groups were not aware of the study groups. The patients’ body mass index (BMI) and characteristics such as age, dwelling place, education level, and operating room duration were also recorded at the beginning of the study.
Results
In this study, 83 women who were scheduled for C-section under spinal anaesthesia were initially selected. Among them, 70 patients met the inclusion criteria and entered the study (Figure 1).
Figure 1.
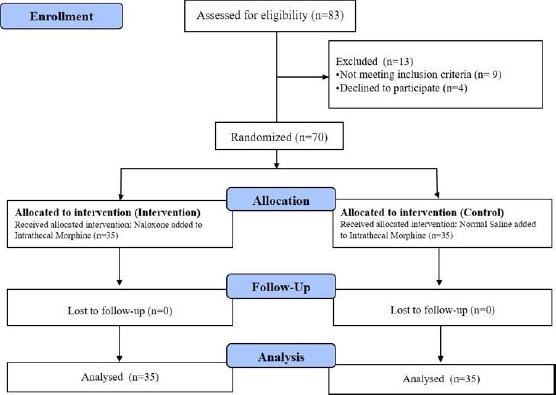
Flowchart of the study
According to the results of the study, the mean BMIs of the patients in the study and control groups were respectively 30.17 ± 2.71 and 31.73 ± 2.64 (p = 0.517). There were also no significant differences between the two groups in terms of other demographic and clinical characteristics (Table 1).
Table 1.
Demographic and clinical characteristics of the patients in both groups
| Variable | Group | p-value | ||
|---|---|---|---|---|
| Study | Control | |||
| Age (year) | 31.34 ± 6.14 | 33.23 ± 5.06 | 0.16 | |
| Dwelling place | Village | 11 (31.45) | 10 (28.6%) | 0.53 |
| City | 24 (68.7%) | 25 (70.14%) | ||
| Education level | Below high school diploma | 9 (25.7%) | 10 (28.6%) | 0.92 |
| High school diploma | 22 (62.9%) | 20 (57.1%) | ||
| Bachelor of Science or above | 4 (11.4%) | 5 (14.3%) | ||
| Duration of surgery (mean ± SD) | 45.32 ± 8.25 | 43.95 ± 9.55 | 0.35 | |
The mean and SD of postoperative pain intensity in each of the two groups are presented in Table 2. As can be seen, regardless of the groups to which the patients were assigned, there was statistically significant difference in pain intensity between the two groups (time effect; P < 0.001); regardless of the study’s timing, although the intensity of pain was lower in the study group, the difference was not statistically significant (group effect; p = 0.84). Also, there was no group time interaction between pain intensity and the times studied (p = 0.61).
Table 2.
The mean postoperative pain intensity in the two groups
| Variable | Group | ||
|---|---|---|---|
| Study (n = 35) | Control (n = 35) | ||
| VAS pain score, T1 | 1.1 (0.67) | 1.5 (0.81) | |
| VAS pain score, T2 | 1.51 (1.11) | 1.84 (1.24) | |
| VAS pain score, T3 | 3.14 (1.23) | 3.92 (1.53) | |
| VAS pain score, T4 | 2.65 (1.29) | 2.92 (1.61) | |
| F statistics (P value) | Time effect | 114.73 (< 0.001) | |
| Group effect | 0.41 (> 0.05) | ||
| Interaction effect | 0.608 (> 0.05) | ||
As shown in Table 3, the incidence rates of postoperative nausea and pruritus were significantly lower in the study group compared to the control group (p < 0.001).
Table 3.
The incidence rates of postoperative nausea and pruritus in the two groups
| Variable | Group | P-value | ||
|---|---|---|---|---|
| Study | Control | |||
| Nausea | Yes | 0 | 13 | < 0.001 |
| No | 35 | 11 | ||
| Pruritus | Yes | 1 | 34 | < 0.001 |
| No | 16 | 19 | ||
Means of paracetamol (Apotel®) use in the study and control groups were respectively 3.13 ± 0.15 gr and 3.85 ± 0.26 gr. Although the patients in the control group used Apotel® more than those in the study group, the difference was not statistically significant (p = 0.56). Also, out of the 70 patients in two groups, 11 patients needed diclofenac suppository in the first 24 hours after the surgery (4 in the study group and 7 in the control group); the difference, however, was not statistically significant (p = 0.55).
Discussion
According to the result of this study, adding naloxone to intrathecal morphine, compared to intrathecal morphine alone, had no statistically significant effect on morphine-induced pain reduction during the study period. The results indicated that the incidence rates of postoperative nausea and pruritus were significantly lower in the study group than the control group. In a study by Cepeda et al., [18] which has been done on patients undergoing less than 3 hours length surgeries, showed that the addition of low-dose naloxone (6 μg/cc) to PCA morphine was significantly related to more treatment failure, greater pain intensity, higher opioid requirements, less pain relief and lower patient satisfaction. They did not report any statistically significant difference in morphine-induced complications between their two study groups. Similarly, the results of their other study [20] showed that the infusion of ultra-low-dose naloxone and morphine via PCA pump was not effective in reducing the patients’ postoperative pain intensity. However, they reported lower rates of nausea and pruritus in the patients who received naloxone plus morphine compared to those who received only morphine. Bijur et al., [21] examined the effects of adding different doses of naloxone (i.e., 0.1 ng/kg, 0.01 ng/kg and 0.001 ng/kg) to morphine on pain intensities of the patients referring to the emergency department due to their acute pain. They found that the addition of the three examined doses of naloxone to morphine did not have any significant effect on morphine-induced pain reduction and complications.
Koo et al., [22] conducted a study on the patients undergoing thyroid surgery. They reported that the addition of 0.05 μg kg/h continuous infusion of naloxone to remifentanil, compared to remifentanil alone, had no significant effect on reducing postoperative pain intensity in their examined patients. They also did not report any significant difference between the two groups in terms of postoperative complications. In a Canadian study on the patients with chronic low-back pain, Nekoui et al., [23] reported that the combination of naloxone and intrathecal morphine had no significant effect on enhancing analgesic effects of morphine and reducing morphine-induced complications. Rebel et al., [24] conducted a study on the patients undergoing radical prostatectomy; they reported that high-dose intrathecal opioid administration along with intravenous naloxone infusion significantly reduced postoperative pains and opioid-induced complication. In another study on the patients undergoing lumbar discectomy, it was shown that the infusion of ultra-low-dose naloxone (0.25 μg/kg/h) with morphine via PCA pump significantly decreased postoperative pain and morphine-induced nausea and pruritus [19]. It was also shown that addition of low-dose naloxone (1/20 to 1/10 doses of morphine) could temporarily increase analgesic effects of morphine; while higher doses (equal to morphine dose) decrease those effects [25]. The results of a study by Firouzian et al., [26] showed that adding an ultra-low dose of intrathecal naloxone (20 μg) to intrathecal morphine significantly reduce postoperative pain and pruritus and nausea in patients undergoing laminectomy with spinal fusion.
Complications of neuraxial opioids are the results of the drug being present in CSF, or systemic circulation of blood or even both. One of the suggested solutions for reducing the incidence of these complications is the use of low-dose naloxone [14], [27]. In the present study, postoperative pruritus was observed in about half of the patients in the control group, and only one patient in the experimental group. Also, almost 40% of patients receiving morphine alone experienced postoperative nausea; while none of the patients in the naloxone plus morphine group experienced it. In a systematic review and meta-analysis aimed at investigating the effect of low-dose intravenous naloxone on the incidence of postoperative nausea and vomiting and the intensity of postoperative pain, it was found that low-dose naloxone had no significant effect on the incidence rates of postoperative nausea and vomiting and also the need for opioids after the surgery. However, more studies are recommended to generalise [28]. In another meta-analysis, He et al., [29] concluded that naloxone could effectively prevent and reduce the incidence of opioid-induced nausea, vomiting, and pruritus. These findings suggest the need for further studies to evaluate the effects of low-dose naloxone on opioid-induced complications.
The results of the present study showed that adding low dose naloxone to intrathecal morphine did not significantly change postoperative pain intensity in the patients undergone elective C-section using spinal anaesthesia; however, significantly decreased the severity of postoperative nausea and pruritus.
Acknowledgement
The authors would like to express their sincere gratitude to the Clinical Research Development Unit of Imam Khomeini Hospital, Mazandaran University of Medical Sciences, Sari, Iran.
Footnotes
Funding: This research has been financially supported by the deputy of research and technology, Mazandaran University of Medical Sciences, Sari, Iran
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Lavand'homme P. Postoperative cesarean pain:real but is it preventable? Curr Opin Anaesthesiol. 2018;31(3):262–267. doi: 10.1097/ACO.0000000000000585. https://doi.org/10.1097/ACO.0000000000000585 PMid:29521684. [DOI] [PubMed] [Google Scholar]
- 2.Gholipour Baradari A, Firouzian A, Hasanzadeh Kiabi F, Emami Zeydi A, Khademloo M, Nazari Z, et al. Bolus administration of intravenous lidocaine reduces pain after an elective caesarean section:Findings from a randomised, double-blind, placebo-controlled trial. J Obstet Gynaecol. 2017;37(5):566–570. doi: 10.1080/01443615.2016.1264071. https://doi.org/10.1080/01443615.2016.1264071 PMid:28604179. [DOI] [PubMed] [Google Scholar]
- 3.Habibi A, Alipour A, Baradari AG, Gholinataj A, Habibi MR, Peivandi S. The Effect of Adding Lidocaine to Patient Controlled Analgesia with Morphine on Pain Intensity after Caesarean Section with Spinal Anesthesia:A Double-Blind, Randomized, Clinical Trial. Open Access Maced J Med Sci. 2019;7(12):1946–1950. doi: 10.3889/oamjms.2019.545. https://doi.org/10.3889/oamjms.2019.545 PMid:31406534 PMCid:PMC6684416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borges NC, Pereira LV, de Moura LA, Silva TC, Pedroso CF. Predictors for Moderate to Severe Acute Postoperative Pain after Cesarean Section. Pain Res Manag. 2016;2016:5783817. doi: 10.1155/2016/5783817. https://doi.org/10.1155/2016/5783817 PMid:27956847 PMCid:PMC5121467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kintu A, Abdulla S, Lubikire A, Nabukenya MT, Igaga E, Bulamba F, et al. Postoperative pain after cesarean section:assessment and management in a tertiary hospital in a low-income country. BMC Health Serv Res. 2019;19(1):68. doi: 10.1186/s12913-019-3911-x. https://doi.org/10.1186/s12913-019-3911-x PMid:30683083 PMCid:PMC6347795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddiqi R, Jafri SA. Maternal satisfaction after spinal anaesthesia for caesarean deliveries. J Coll Physicians Surg Pak. 2009;19(2):77–80. [PubMed] [Google Scholar]
- 7.Sutton CD, Carvalho B. Optimal Pain Management After Cesarean Delivery. Anesthesiol Clin. 2017;35(1):107–124. doi: 10.1016/j.anclin.2016.09.010. https://doi.org/10.1016/j.anclin.2016.09.010 PMid:28131114. [DOI] [PubMed] [Google Scholar]
- 8.Dongare PA, Nataraj MS. Anaesthetic management of obstetric emergencies. Indian J Anaesth. 2018;62(9):704–709. doi: 10.4103/ija.IJA_590_18. https://doi.org/10.4103/ija.IJA_590_18 PMid:30237596 PMCid:PMC6144558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi KA, Jain K. Management of anaesthesia for elective, low-risk (Category 4) caesarean section. Indian J Anaesth. 2018;62(9):667–674. doi: 10.4103/ija.IJA_459_18. https://doi.org/10.4103/ija.IJA_459_18 PMid:30237591 PMCid:PMC6144555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bujedo BM. Current evidence for spinal opioid selection in postoperative pain. Korean J Pain. 2014;27(3):200–9. doi: 10.3344/kjp.2014.27.3.200. https://doi.org/10.3344/kjp.2014.27.3.200 PMid:25031805 PMCid:PMC4099232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin YD, Park SH, Kim HT, Park CJ, Lee JH, Choi YJ. The effect of anaesthesia technique on caesarean section. Pak J Med Sci. 2016;32(1):147–50. doi: 10.12669/pjms.321.9028. https://doi.org/10.12669/pjms.321.9028 PMid:27022364 PMCid:PMC4795857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bujedo BM, Santos SG, Azpiazu AU. A review of epidural and intrathecal opioids used in the management of postoperative pain. J Opioid Manag. 2012;8(3):177–92. doi: 10.5055/jom.2012.0114. https://doi.org/10.5055/jom.2012.0114 PMid:22798178. [DOI] [PubMed] [Google Scholar]
- 13.Kjølhede P, Bergdahl O, Borendal Wodlin N, Nilsson L. Effect of intrathecal morphine and epidural analgesia on postoperative recovery after abdominal surgery for gynecologic malignancy:an open-label randomised trial. BMJ Open. 2019;9(3):e024484. doi: 10.1136/bmjopen-2018-024484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toljan K, Vrooman B. Low-Dose Naltrexone (LDN)-Review of Therapeutic Utilization. Med Sci (Basel) 2018;6:4. doi: 10.3390/medsci6040082. https://doi.org/10.3390/medsci6040082 PMid:30248938 PMCid:PMC6313374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Block L, Lundborg C, Bjersing J, Dahm P, Hansson E, Biber B. Ultralow Dose of Naloxone as an Adjuvant to Intrathecal Morphine Infusion Improves Perceived Quality of Sleep but Fails to Alter Persistent Pain:A Randomized, Double-blind, Controlled Study. Clin J Pain. 2015;31(11):968–75. doi: 10.1097/AJP.0000000000000200. https://doi.org/10.1097/AJP.0000000000000200 PMid:25629634 PMCid:PMC4894772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sartain JB, Barry JJ, Richardson CA, et al. Effect of combining naloxone and morphine for intravenous patient-controlled analgesia. Anesthesiology. 2003;99:148–151. doi: 10.1097/00000542-200307000-00024. https://doi.org/10.1097/00000542-200307000-00024 PMid:12826854. [DOI] [PubMed] [Google Scholar]
- 17.Yeh YC, Lin TF, Wang CH, Wang YP, Lin CJ, Sun WZ. Effect of combining ultralowdose naloxone with morphine in intravenous patient-controlled analgesia:the cut-off ratio of naloxone to morphine for antiemesis after gynecologic surgery. J Formos Med Assoc. 2008;107:478–484. doi: 10.1016/S0929-6646(08)60156-4. https://doi.org/10.1016/S0929-6646(08)60156-4. [DOI] [PubMed] [Google Scholar]
- 18.Cepeda MS, Africano JM, Manrique AM, Fragoso W, Carr DB. The combination of low dose of naloxone and morphine in PCA does not decrease opioid requirements in the postoperative period. Pain. 2002;96(1-2):73–9. doi: 10.1016/s0304-3959(01)00425-0. https://doi.org/10.1016/S0304-3959(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 19.Firouzian A, Gholipour Baradari A, Alipour A, Emami Zeydi A, Zamani Kiasari A, Emadi SA, et al. Ultra-low-dose Naloxone as an Adjuvant to Patient Controlled Analgesia (PCA) With Morphine for Postoperative Pain Relief Following Lumber Discectomy:A Double-blind, Randomized, Placebo-controlled Trial. J Neurosurg Anesthesiol. 2018;30(1):26–31. doi: 10.1097/ANA.0000000000000374. https://doi.org/10.1097/ANA.0000000000000374 PMid:27673505. [DOI] [PubMed] [Google Scholar]
- 20.Cepeda MS, Alvarez H, Morales O, Carr DB. Addition of ultralow dose naloxone to postoperative morphine PCA:unchanged analgesia and opioid requirement but decreased incidence of opioid side effects. Pain. 2004;107(1-2):41–6. doi: 10.1016/j.pain.2003.09.011. https://doi.org/10.1016/j.pain.2003.09.011 PMid:14715387. [DOI] [PubMed] [Google Scholar]
- 21.Bijur PE, Schechter C, Esses D, Chang AK, Gallagher EJ. Intravenous bolus of ultra-low-dose naloxone added to morphine does not enhance analgesia in emergency department patients. J Pain. 2006;7(2):75–81. doi: 10.1016/j.jpain.2005.08.008. https://doi.org/10.1016/j.jpain.2005.08.008 PMid:16459272. [DOI] [PubMed] [Google Scholar]
- 22.Koo CH, Yoon S, Kim BR, Cho YJ, Kim TK, Jeon Y, et al. Intraoperative naloxone reduces remifentanil-induced postoperative hyperalgesia but not pain:a randomized controlled trial. Br J Anaesth. 2017;119(6):1161–1168. doi: 10.1093/bja/aex253. https://doi.org/10.1093/bja/aex253 PMid:29029049. [DOI] [PubMed] [Google Scholar]
- 23.Nekoui A, Eldufani J, Mayer P, Blaise G. The Effect of Epidural Morphine-Bupivacaine Combined with a Low Dose of Naloxone on Respiratory Function and Analgesia in Patients with Chronic Low-Back Pain. J Health Med Informat. 2018;9(1):298. https://doi.org/10.4172/2157-7420.1000298. [Google Scholar]
- 24.Rebel A, Sloan P, Andrykowski M. Postoperative analgesia after radical prostatectomy with high-dose intrathecal morphine and intravenous naloxone:a retrospective review. J Opioid Manag. 2009;5(6):331–9. doi: 10.5055/jom.2009.0033. https://doi.org/10.5055/jom.2009.0033 PMid:20073407. [DOI] [PubMed] [Google Scholar]
- 25.Song S. The dual effects of naloxone on morphine analgesia:Dose-related different responses on formalin-induced nociception. The Journal of Pain. 2005;6(3):S21. https://doi.org/10.1016/j.jpain.2005.01.081. [Google Scholar]
- 26.Firouzian A, Gholipour Baradari A, Ehteshami S, Zamani Kiasari A, Shafizad M, Shafiei S, et al. The effect of ultra-low-dose intrathecal naloxone on pain intensity after lumbar laminectomy with spinal fusion:A randomized controlled trial. J Neurosurg Anesthesiol. 2018 doi: 10.1097/ANA.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 27.Sultan P, Halpern SH, Pushpanathan E, Patel S, Carvalho B. The Effect of Intrathecal Morphine Dose on Outcomes after Elective Cesarean Delivery:A Meta-Analysis. Anesth Analg. 2016;123(1):154–64. doi: 10.1213/ANE.0000000000001255. https://doi.org/10.1213/ANE.0000000000001255 PMid:27089000. [DOI] [PubMed] [Google Scholar]
- 28.Barrons RW, Woods JA. Low-Dose Naloxone for Prophylaxis of Postoperative Nausea and Vomiting:A Systematic Review and Meta-analysis. Pharmacotherapy. 2017;37(5):546–554. doi: 10.1002/phar.1930. https://doi.org/10.1002/phar.1930 PMid:28349575. [DOI] [PubMed] [Google Scholar]
- 29.He F, Jiang Y, Li L. The effect of naloxone treatment on opioid-induced side effects:A meta-analysis of randomized and controlled trails. Medicine (Baltimore) 2016;95(37):e4729. doi: 10.1097/MD.0000000000004729. https://doi.org/10.1097/MD.0000000000004729 PMid:27631221 PMCid:PMC5402564. [DOI] [PMC free article] [PubMed] [Google Scholar]


