Abstract
BACKGROUND:
Intra-peritoneal adhesions (IPAs) common occurre in post abdominal surgical. Athough many methods have been developed for controlling IPAs, including mesenchymal stem cells (MSCs) application, however, there is none completely preventing in due to the mesothelial structure may promote the prolonged inflammations leading. Nevertheless hypoxia-MSCs (H-MSCs) have more potent in controlling the inflammation than normoxia-MSCs (N-MSCs) by releasing several anti-inflamation particularly IL-10, however the H-MSCs application to inhibit IPAs remain unclear.
AIM:
The aim of this study was to investigate the effectiveness of H-MSCs in preventing the AIPs event by releasing IL-10 on the ileum abrasion sutured omental patch as the animal model of peritoneal adhesion.
METHODS:
Using 24 IPAs animal model were randomly divided into 4 groups: Sham (Sh), Control (C), H-MSCs at high dose (T1) and H-MSCs at low dose (T2). H-MSCs were incubated under hypoxic conditions (5% O2), 37°C and 5% CO2 for 24 hours. The expression level of IL-10 was performed using RT-PCR analysis. The macroscopic appearance of IPAs was evaluated using Nair’s scale base on the absence/presence of adhesion, whereas the microscopic by Zuhlke’s scale at Hematoxylin and eosin (H&E) staining.
RESULTS:
This study showed a significanly increase in IL-10 expression (p < 0.05) at all T groups. In line with this, we also found a significant difference in IPAs between T groups and Control as well as a Sham (p < 0.05) either in the macroscopic or microscopic analysis.
CONCLUSION:
H-MSCs has a robust ability in inhibiting severe IPAs characterized by the decreased of adhesion formation and the enhanced expression of IL-10.
Keywords: Hypoxic, Mesenchymal stem cell, IL-10, Peritoneal adhesion
Introduction
Intra-peritoneal adhesions (IPAs) are the most common scar tissue occurring in post abdominal surgical, in addition to as a consequence of prolonged intra-abdominal inflammation [1], [2]. The incidence of post-surgical IPAs is from 67 to 97%, whereas their complications, such as the small-bowel obstructions, chronic abdominal, pelvic pain and female infertility may induce severe health problems [3]. Several methods have been developed for controlling IPAs, such as laparoscopy and surgical barriers [4], [5], however, there is none completely preventing in adhesion development. Dysregulation of the serious healing process of mesothelial structural are the basis of peritoneal adhesion formations due to the unique characteristic of mesothelial that have immune-like capacity. The post-injury mesothelial denudation may promote the prolonged inflammations leading to the impairment of fibrinolytic regulation and the extension of extracellular matrix (ECM) deposition [5].
Mesenchymal stem cells (MSCs) play a key role in completely accelerating wound healing by responding injury signals and migrating toward the injury site to control the inflammation process. These immunoregulatory properties of MSCs are due to their ability to suppress the inflammatory milieu by releasing several anti-inflammatory cytokines such as IL-10, IDO, PGE2, TGF-b, HGF and nitric oxide [6]. Several studies of MSCs have been performed to prevent IPAs, such as decreasing the inflammatory response and fibrin formation as well as increasing fibrin lysis [7], [8]. However, intraperitoneal MSCs are yet ineffective in preventing IPAs although they can control the inflammatory process [9]. Another study also reported that the topical MSCs are not effective to prevent the IPAs event. The reason behind the absence of MSCs into injured peritoneum is the incompetence of MSCs to counter phagocytosis of monocyte-macrophage system [10]. On the other side, the recent study revealed that hypoxia-MSCs (H-MSCs) have superior effect to accelerate wound healing than normoxia-MSC (N-MSCs) [11], [12].
Theoretically, MSCs may express a number of markers, including CD105, CD73, CD90, CD166, CD44 and CD29 dan lack of the expression of CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA class II. In addition to multilineage differentiation capacity, MSCs also have immune regulatory properties by secreting anti-inflammatory cytokines [13], [14]. MSCs under hypoxia condition can mimic the physiological niche including in their in-vivo environment that potentially offer the benefit of clinical case. H-MSCs are more potent in controlling inflammation than N-MSCs by increasing more anti-inflammatory cytokine [15]. Specifically, IL-10 as a pleiotropic anti-inflammatory cytokine released by MSCs can inhibit macrophage activation, T cell and NK cell proliferation. On the other side, MSCs can control pro-inflammatory cytokines release, particularly IFN-g, IL-2, TNF-a. The release of IL 10 and TGF-b of MSCs post-TNF-α exposure may suppress inflammation, thus accelerate the wound healing process [16].
The previous study reported that the majority of MSCs do not survive in the hypoxia milieu that frequently occurred in most injured tissue, including in IPAs [19]. Therefore, a pre-conditioning period of MSCs under hypoxia for supporting their adaptation prior to their exogenous administration is needed. In this study, we aimed to investigate the effectiveness of H-MSCs in preventing the IPAs event by releasing IL-10 on the ileum abrasion sutured omental patch as the animal model of peritoneal adhesion.
Methods
IPAs Animal model
The study was approved by the experimental animal’s ethics committee of the medical faculty of Sultan Agung Islamic University, Semarang, Indonesia. A total of 24 healthy 8-weeks-old male Wistar-albino rats weighing between 250 and 300 g which purchased from the animal holding unit, Faculty of Veterinary Medicine, Gadjah Mada University were used. Rats were acclimatized and housed in 12 h light-dark cycle standard cages at 24°C with food and water ad-libitum. After fasting for 12 h, rats were anesthetized by intraperitoneal administration of ketamine and xylazine (90 and 10 mg per kg body weight; respectively). The surgical procedure was performed according to described previously [37]. The operations were performed under aseptic conditions and took less than 20 minutes for each rat to minimize room air tissue dying effect. Musculoperitoneal layer was incised and opened over a length of 3 cm at the linea alba after skin ventral midline incision. In order to induce IPAs, the standardized surgical injuries were applied to one side of the terminal ileum (3 cm from cecum) with 0.5 × 0.5 cm size on the right sidewall of ileum by brushing with a cytobrush (Gynobrush, Langenbrink, Emmendingen, Germany) until the punctuate red spot was observable as visual indicator for ileum trauma. Following the intervention, the abdominal incision was closed with 4 – 0 polygelatin suture and rats were reared in battery cages for 14 days.
MSCs isolation and culture
MSCs were isolated from the umbilical cord (UC) obtained from pregnant single Wistar-albino rats and expanded as described previously [28]. Briefly, UCs were chopped into smaller pieces and transferred into a T25 culture flask (Corning, Tewksbury, MA, USA) containing DMEM (Gibco™ Invitrogen, NY, USA) which supplemented with 10% FBS (Gibco™ Invitrogen, NY, USA), 1% penicillin (100 U/mL) and 0,25% streptomycin (100 µg/mL) (Gibco™ Invitrogen, NY, USA) and incubated at 37°C, 5% CO2 and ≥ 95% humidity. The medium was replaced every 3 days and harvested after reaching 80% confluenct (14 days). The 4-6th passaged MSCs-like were employed for the experiments.
H-MSCs osteogenic differentiation assay
The H-MSCs were cultured in 24 well plate (1.5 x 104 cells/well density) with standard medium containing DMEM (Sigma-Aldrich, Louis St, MO), supplemented with 10% FBS (Gibco™ Invitrogen, NY, USA), 1% penicillin (100 U/mL) and 0,25% streptomycin (100 µg/mL) (Gibco™ Invitrogen, NY, USA) at 37°C, 5% CO2, and ≥ 95% humidity. After reaching 95% confluent, standard medium were aspirated and replaced with osteogenic differentiation medium containing Human MesenCult™ Osteogenic Differentiation Basal Medium (Stem Cell Technologies, Singapore), augmented with 20% Human MesenCult™ Osteogenic Differentiation 5X Supplement (Stem Cell Technologies, Singapore) and 1% L-Glutamine (Gibco™ Invitrogen, NY, USA). The differentiation medium was renewed every 3 days. The bone matrix was formed after 15 days and can be visualized by 2% Alizarin red solution staining.
H-MSCs characterization
H-MSCs surface markers at the 4-6th passage were analyzed by flow cytometry analysis according to company protocols. Briefly, the cells were subsequently incubated in the dark room with allophycocyanin (APC) mouse anti-human CD73, fluorescein sothiocyanate (FITC) mouse anti-human CD90, perCP-Cy5.5.1 mouse anti-human CD105, and phycoerythrin (PE) mouse anti-human Lin negative (CD45/CD34/CD11b/CD19/HLA-DR) antibodies. H-MSCs cells were stained with MSC specific antibody for 30 minutes at 4°C, then examined and analyzed with a BD Accuri C6 Plus flow cytometer (BD Biosciences, San Jose, CA, USA).
H-MSCs induction and administration
For inducing H-MSCs, MSCs derived from the 4th passage were incubated under 5% O2 condition in a hypoxia incubation chamber (STEMCELL Technologies, Biopolis, Singapore) for 24 h at 37°C and 5% CO2, then collected for the following experiment. The IPAs animal model was treated by 3 x 106 as a high dose (T1) and 1,5 x 106 as a low dose of H-MSCs (T2) via submucosal injection, whereas the Sham group received NaCl only and Control group received the omental patch treatment.
Macroscopic evaluation of IPAs
Fourteen days after abdominal surgery, a necropsy was performed by opening the abdominal cavity through a reverse U-shape incision. The absence/presence of IPAs was evaluated by Nair’s scale [9] (Table 1).
Table 1.
Nair’s macroscopic adhesion grade
| Grade 0 No adhesions |
|---|
| Grade 1 One adhesion band only between the organs or one organ to the abdominal wall |
| Grade 2 Two adhesion bands between the organs or one organ to the abdominal wall |
| Grade 3 More than 2 adhesion bands between the organs or one organ to the abdominal wall. |
| Grade 4 Adhesion of all viscera to the abdominal wall |
Microscopic analysis
The terminal ileum sample was totally excised and removed from the abdomen. A single-blinded pathologist assessed the excised tissues, fixed them in 10% buffer formalin, embedded with paraffin, and then sectioned. Finally, the slide was stained by hematoxylin and eosin (H & E), whereas the histopathology appearance was evaluated using the Zühlke’s scale at high magnification [9] (Table 2).
Table 2.
Zühlke’s microscopic adhesion grade
| Grade 1 Weak connective tissue, rich cell, new and old fibrin, thin reticulin fibriles | |
|---|---|
| Grade 2 Connective tissue which has cells and capillaries and few collagen fibers | |
| Grade 3 Thicker connective tissue. Few cells and elastic and smooth muscle fibers, more vessels | |
| Grade 4 | Old and thick granulation tissue, poor cells, difficult separation of serosal surfaces |
IL-10 relative expression analysis
The RNA isolation on 100 mg of tissue sample at 14 was performed using the TRI Reagent according to the manufacturer’s instructions (Sigma-Aldrich, Dorset, UK). Reverse transcription of 1 µg of total RNA was performed using Enhanced Avian First Strand cDNA Synthesis Kit according to the instructions given (Sigma-Aldrich, Dorset, UK) and step-down PCR was performed using Eco Real-Time PCR System (Illumina Inc., San Diego CA, USA). An oligo d(T) primer was used for RT and 20 µL of reaction mix was incubated for 10 minutes at 70°C and 15 minutes at 45°C. The 2-step quantitative real-time PCR was performed using an Eco Real-Time PCR System.
Expression level analysis of beta-actin (housekeeping gene) and IL-10 were carried out using KAPA SYBR® FAST Universal Kit (Sigma-Aldrich, Dorset, UK) in combination with oligonucleotide primer (IL-10 forward 5’-GCAGGACTTTAAGGGTTACTTGG-3’ and reverse 5’-CCTTTGTCTTGGAGCTTATTAAA-3’; beta-actin forward 5’-ATTGGCAATGAGCGGTTCCGC-3’ and reverse 5’-CTCCTGCTTGCTGATCCACATC-3’).
For qPCR analysis, 10 µL of KAPA SYBR® FAST qPCR master mix, forward and reverse primers (200 nM) and 3 ng of cDNA template was added and incubated in thermocycling conditions (95°C for 3 min, followed by 45 cycles of 95°C for 10 sec and 60°C for 30 sec). Expression levels were calculated by ΔΔCt method (2−ΔΔCt formula) using Eco Study Software (Illumina Inc., San Diego CA, USA), after being normalized to the Cq-value of the beta-actin housekeeping gene.
Statistical analysis
All statistical tests were performed using the SPPS v. 20 (SPPS Inc, Chicago, IL USA). The data descriptives were presented in means ± standard errors. Both macroscopic and microscopic IPAs classification grades were presented in numbers. For analyzing all parameters, a one-way analysis of variance (ANOVA) was performed and followed by a Tukey’s multiple comparisons post-hoc test. A P-value, less than 0.05 was considered as statistically significant.
Results
H-MSCs characterisation and differentiation
The H-MSCs incubated under hypoxic conditions showed homogeneously dense fibroblast and spindle-like appearance that meet the standard in-vitro characteristics of MSCs (Figure 1A). Osteogenic differentiation assay, the H-MSCs exhibited the calcium deposition under 2% Alizarin red staining that indicated the multipotency of H-MSCs was well-maintained as yet (Figure 1B). The flow cytometric analysis confirmed that H-MSCs expressed high levels of CD90, CD105 and CD73, and lack of Lin, according to the Internasional Society of Cellular Therapy (ISCT) as an immunophenotype characteristic of MSCs (Figure 1C).
Figure 1.
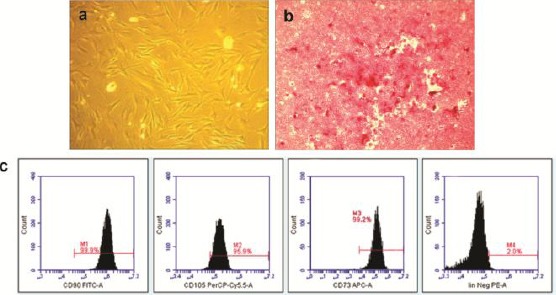
A) H-MSC showed fibroblast and spindle-like shaped characteristic (scale bar 100 μm); B) Osteogenic differentiation of H-MSCs was evidenced by calcium deposition as mineralized matrix that visualized by bright red color using Alizarin red staining (scale bar 50 μm) (magnification 10X); C) Immunophenotyping analysis of H-MSCs expressed CD90 (99.9%), CD105 (95.9%), CD73 (99.2%) and lacked the expression of Lin (2.0%)
Macroscopic and microscopic analysis of IPAs
The results of macroscopic and microscopic analysis of IPAs are presented in Table 3.
Table 3.
Microscopic and macroscopic results of the groups and intergroup analysis
| Group | Macros appearance | Micros appearance |
|---|---|---|
| Sham | 3.4 ± 0.89 | 3.4 ± 0.89 |
| Control | 3.8 ± 0.44 | 4.0 ± 0.00 |
| T1 | 0.2 ± 0.44 | 0.0 ± 0.00 |
| T2 | 0.6 ± 0.89 | 0.2 ± 0.44 |
| Sham vs Control | > 0.05 | > 0.05 |
| Sham vs H-MSCs high dose | < 0.05 | < 0.05 |
| Sham vs H-MSCs low dose | < 0.05 | < 0.05 |
| Control vs H-MSCs high dose | < 0.05 | < 0.05 |
| Control vs H-MSCs low dose | < 0.05 | < 0.05 |
| H-MSCs High dose vs H-MSCs low dose | < 0.05 | < 0.05 |
T1 = 3 x 106 H-MSCs administration, T2 = 1.5 x 106 H-MSCs administration.
In our study, the macroscopic evaluation (Nair’s scale) showed significantly difference in the IPAs between treatment groups and control as well as sham (p < 0,05). We found the severe IPAs (grade 3-4) at all control and sham groups (Figure 2), however, there was almost no adhesion in the T groups, particularly at T1 (high dose of H-MSC.
Figure 2.
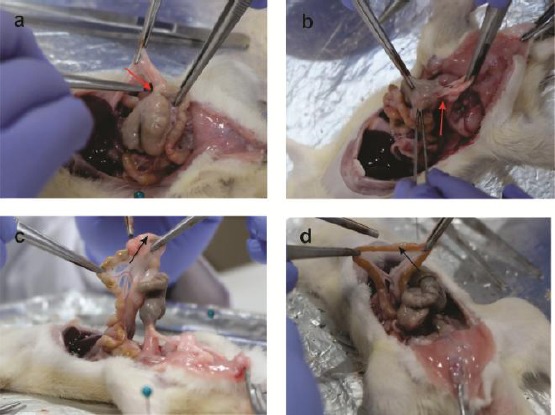
Macroscopic appearance showed the severe peritoneal adhesions in A) the Sham group (3,4 ± 0,89) (red arrow) and B) Control group (3,8 ± 0,44) (red arrow); however, treatment groups showed there were almost no adhesions at C) the T1 group (0,2 ± 0,44) (black arrow); and less peritoneal adhesions at D) the T2 group (0,6 ± 0,89) (black arrow)
In line with this finding, we also found there were significant difference of IPAs in the T group compare than control and sham at microscopic analysis (Zühlke’s scale) (p < 0.05) (Figure 3) that indicated HP-MSCs have a robust ability in preventing peritoneal adhesion.
Figure 3.
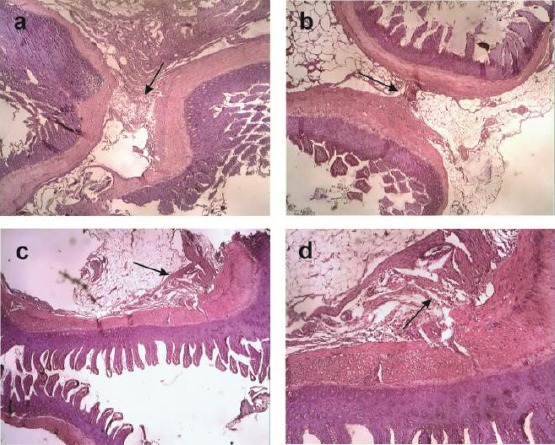
Microscopic appearance showed the severe peritoneal adhesion in A) the Sham and B) Control group respectively 3,4 ± 0,89 and 4.0 ± 0,00 with thicker connective tissue (Black arrow) while in C) the T1 groups showed no peritoneal adhesion (0,0 ± 0,00) and D) T2 groups was less peritoneal adhesion (0,2 ± 0,44) with weak connective tissue (black arrow) (magnification 10 X)
IL-10 relative expression analysis
The inflammatory status of all samples was assessed based on the existence of IL-10 expression as anti-inflammatory cytokines for controlling inflammation process. We found the significant difference in the IL-10 expression between T groups and control as well as sham (p < 0,05) using RT-PCR analysis that indicated the upregulation of IL-10 at all dose of H-MSCs. The mean fold change was recorded and presented in the graph below (Figure 4).
Figure 4.
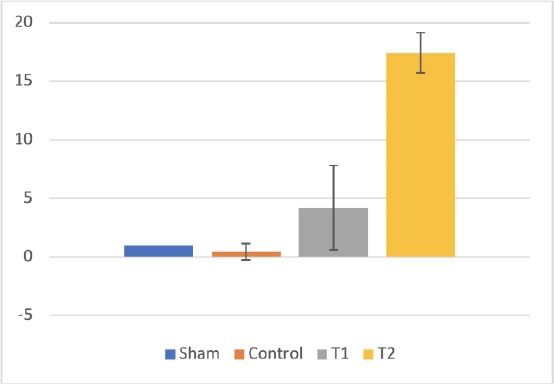
IL-10 expression in ileum tissue
Discussion
Adhesion formation following abdominal surgery may develop serious health problems ranging from severe pain up to bowel obstruction [1], [2], [3], [4], [5]. To decrease the IPAs event, MSCs administration has been performed either by topical or via systemic vein, however, the adhesion formation is occurred as yet [6], [7], [8]. The unique characteristic of mesothelial cells is considered as one of the failure factors of MSCs treatment due to the robust ability of this cell in modulating serosal inflammation than the other cells [17]. In terms of the serosa exposed to an injury or foreign agents, they may promote the development of chronic inflammation leading to peritoneal adhesion formation. Therefore, we performed the abrasion of the cecum followed by suturing a sterile absorbable thread to the omental patch as an animal model of IPAs. We used H-MSCs that have more potent in controlling the inflammation than N-MSCs. These are the first study demonstrated that H-MSCs have the ability in preventing the IPAs formation.
In this study, we found that there was a significant difference in Nair’s macroscopic adhesion of IPAs (p < 0.05) between treatment and control groups. The grade 3-4 adhesion obviously occurs in all of the control group in the form of the viscera adhesion to the abdominal wall, whereas completely no adhesion in H-MSCs at high dose (T1). We also found a little bit of adhesions (grade 1) in H-MSCs treatment group at a low dose yet. Under physiological conditions, the remodelling phase leads to complete regeneration without permanent scars. However, in this study, the abrasion of the cecum along with the surgical suture thread as foreign antigen may promotes the pathogenic remodelling process leading to adhesion [18]. The damage of the peritoneal surface, particularly mesothelial cells following injury may induce the uncontrollably mesothelialized [19] and the prolonged inflammation [20], [21]. This is in line with our microscopy finding (Zühlke’s scale) in which we found many inflammation appearance in all control and sham groups.
We found the fibrous bands of IPAs containing deposit collagen, and blood vessels in addition to inflammatory cells infiltration particularly lymphocytes in control and sham groups. However, we did not find them in the treatment group except leucocytes infiltration at the low dose of H-MSCs. These findings indicated that H-MSCs notably at high dose can optimally control inflammation [15]. Theoritically, the initial adhesion formation containing fibrin matrix occured under prolonged inflammation in which the synthesis of the ECM is more active than the degradation leading to the new endothelial layer formation connecting to other organs [22], [23]. These processes are caused by the continuous activated myofibroblast to produce an excessive amount of ECM due to the incompetence of IL-10 in suppressing the inflammation [13], [25]. Thus, to assess the controlled inflammation of treatment groups post H-MSCs administration, IL-10 expression was evaluated.
In this study, we found that there was a significant increase of IL-10 expression (p < 0,05) at all groups treatment. The lower IL-10 expression occured at the low dose (T2) of H-MSC than the high dose (T1) on day 14. This indicated that the low dose is not optimal in controlling the inflammation. This finding was in line with the microscopic appearance in which we found more inflammatory cells at low dose than high dose of H-MSCs. On the other side, we found a down-regulation of IL-10 expression on the control groups that are in accordance to the persistence infiltration of inflammatory cells. The inflammatory state may lead to the activation of myofibroblasts as the main effector cells of adhesion formations [1]. Binding of IL-10 post H-MSC administration to inflammatory cell receptor may activate tyrosine kinase-2 and Janus tyrosine kinase 1 (JAK1) pathways, then translocate into the nucleus to induce the target genes such as the suppressor of cytokine signaling 3 (SOCS3) correlated with the decreased expression of TNF-α and IL-1β [27]. IL-10 also has a gene target for effector downstream of MMP-9, iNOS, and IFN-γ as well as MHC class II, thus they may inhibit the activation of inflammatory cells [29]. Specifically, IL-10 inhibit the release of TGF-β1 of activated macrophage, that have a key role to activate myofibroblasts. The myofibroblast inactivation may decrease the procollagen I and III production leading to the adhesion formation decrease [30]. This fact indicated that IL-10 has the ability to inhibit both the innate and adaptive immune responses, in addition to inactivate the activated myofibroblast, thus may prevent IPAs formation.
This study has several limitations in which we did not analyze TGF-β as the main molecule in activating myofibroblast as well as the alpha-smooth muscle actin (α-SMA) as the marker of the activated myofibroblast. Hence, we do not have clear observation of the role of IL-10 for myofibroblast activity. We also did not analyze the other inflammatory cytokines that may involve in adhesion formation.
In conclusion, H-MSCs has a robust ability in inhibiting severe IPAs characterized by the decreased of adhesion on the terminal ileum tissues. The enhanced expression of IL-10 also indicates that there were acceleration of transition of the controlled inflammation to the completed healing process. This finding indicated that H-MSC may inhibit adhesion formation and inflamed tissue, thus may be considered as applicable agents for controlling IPAs in abdominal surgery.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Brüggmann D, Tchartchian G, Wallwiener M, Münstedt K, Tinneberg HR, Hackethal A. Intra-abdominal adhesions:definition, origin, significance in surgical practice, and treatment options. Dtsch Arztebl Int. 2010;107(44):769–75. doi: 10.3238/arztebl.2010.0769. https://doi.org/10.3238/arztebl.2010.0769 PMid:21116396 PMCid:PMC2992017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabibian N, Swehli E, Boyd A, Umbreen A, Tabibian JH. Abdominal adhesions:A practical review of an often overlooked entity. Ann Med Surg (Lond) 2017;15:9–13. doi: 10.1016/j.amsu.2017.01.021. https://doi.org/10.1016/j.amsu.2017.01.021 PMid:28203370 PMCid:PMC5295619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attard JA, MacLean AR. Adhesive small bowel obstruction:epidemiology, biology and prevention. Can J Surg. 2007;50(4):291–300. [PMC free article] [PubMed] [Google Scholar]
- 4.Binda MM. Humidification during laparoscopic surgery:overview of the clinical benefits of using humidified gas during laparoscopic surgery. Arch Gynecol Obstet. 2015;292(5):955–71. doi: 10.1007/s00404-015-3717-y. https://doi.org/10.1007/s00404-015-3717-y PMid:25911545 PMCid:PMC4744605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad G, O'Flynn H, Hindocha A, Watson A. Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database of Systematic Reviews. 2015;4:CD000475. doi: 10.1002/14651858.CD000475.pub3. https://doi.org/10.1002/14651858.CD000475.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang R, Liu Y, Yan K, Chen L, Chen XR, Li P, Chen FF, Jiang XD. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10:106. doi: 10.1186/1742-2094-10-106. https://doi.org/10.1186/1742-2094-10-106 PMid:23971414 PMCid:PMC3765323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang N, Li Q, Zhang L, Lin H, Hu J, Li D, Shi S, Cui S, Zhou J, Ji J, et al. Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6. PLoS One. 2012;7(8):e43768. doi: 10.1371/journal.pone.0043768. https://doi.org/10.1371/journal.pone.0043768 PMid:22912904 PMCid:PMC3422344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaires-Rosas CP, Ambriz X, Montesinos JJ, Hernández-Téllez B, Piñón-Zárate G, Herrera-Enríquez M, Hernández-Estévez É, Ambrosio JR, Castell-Rodríguez A. Differential adhesion and fibrinolytic activity of mesenchymal stem cells from human bone marrow, placenta, and Wharton's jelly cultured in a fibrin hydrogel. J Tissue Eng. 2019;10:2041731419840622. doi: 10.1177/2041731419840622. https://doi.org/10.1177/2041731419840622 PMid:31007888 PMCid:PMC6460889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karaca G, Pehlivanli F, Aydin O, Altunkaya C, Uzun H, Niyaz M, Özden H, Bulut H. The effect of mesenchymal stem cell use on intra-abdominal adhesions in a rat model. Ann Surg Treat Res. 2018;94(2):57–62. doi: 10.4174/astr.2018.94.2.57. https://doi.org/10.4174/astr.2018.94.2.57 PMid:29441333 PMCid:PMC5801328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putra A, Pertiwi D, Milla MN, Indrayani UD, Jannah D, Sahariyani M, Trisnadi S, Wibowo JW. Hypoxia-preconditioned MSCs Have Superior Effect in Ameliorating Renal Function on Acute Renal Failure Animal Model. Open Access Maced J Med Sci. 2019;7(3):305–310. doi: 10.3889/oamjms.2019.049. https://doi.org/10.3889/oamjms.2019.049 PMid:30833992 PMCid:PMC6390148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riis S, Newman R, Ipek H, Andersen JI, Kuninger D, Boucher S, Vemuri MC, Pennisi CP, Zachar V, Fink T. Hypoxia enhances the wound-healing potential of adipose-derived stem cells in a novel human primary keratinocyte-based scratch assay. Int J Mol Med. 2017;39(3):587–594. doi: 10.3892/ijmm.2017.2886. https://doi.org/10.3892/ijmm.2017.2886 PMid:28204820 PMCid:PMC5360363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antebi B, Rodriguez LA, 2nd, Walker KP, 3rd, Asher AM, Kamucheka RM, Alvarado L, Mohammadipoor A, Cancio LC. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res Ther. 2018;9(1):265. doi: 10.1186/s13287-018-1007-x. https://doi.org/10.1186/s13287-018-1007-x PMid:30305185 PMCid:PMC6180371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putra A, Ridwan FB, Putridewi AI, Kustiyah AR, Wirastuti K, Sadyah NAC, Rosdiana I, Munir D. The Role of TNF-αinduced MSCs on Suppressive Inflammation by Increasing TGF-βand IL-10. Open Access Maced J Med Sci. 2018;6(10):1779–1783. doi: 10.3889/oamjms.2018.404. https://doi.org/10.3889/oamjms.2018.404 PMid:30455748 PMCid:PMC6236029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells:Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6):667–79. doi: 10.3727/096368910X508762. https://doi.org/10.3727/096368910X508762 PMid:20525442 PMCid:PMC2957533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutsaers SE, Prêle CM, Pengelly S, Herrick SE. 2016. Mesothelial cells and peritoneal homeostasis. Fertility and Sterility. 2016;106(5):1018–1024. doi: 10.1016/j.fertnstert.2016.09.005. https://doi.org/10.1016/j.fertnstert.2016.09.005 PMid:27692285. [DOI] [PubMed] [Google Scholar]
- 16.Selvi F, Cakarer S, Can T, Kirli Topcu Sİ, Palancioglu A, Keskin B, Bilgic B, Yaltirik M, Keskin C. Effects of different suture materials on tissue healing. J Istanb Univ Fac Dent. 2016;50(1):35–42. doi: 10.17096/jiufd.79438. https://doi.org/10.17096/jiufd.79438 PMid:28955553 PMCid:PMC5573451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinchuk IV, Mifflin RC, Saada JI, Powell DW. Intestinal mesenchymal cells. Curr Gastroenterol Rep. 2010;12(5):310–8. doi: 10.1007/s11894-010-0135-y. https://doi.org/10.1007/s11894-010-0135-y PMid:20690004 PMCid:PMC2975955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011;17(41):4545–53. doi: 10.3748/wjg.v17.i41.4545. https://doi.org/10.3748/wjg.v17.i41.4545 PMid:22147959 PMCid:PMC3225091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartupee J, Mann DL. Role of inflammatory cells in fibroblast activation. J Mol Cell Cardiol. 2016;93:143–8. doi: 10.1016/j.yjmcc.2015.11.016. https://doi.org/10.1016/j.yjmcc.2015.11.016 PMid:26593723 PMCid:PMC4846511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mutsaers SE, Birnie K, Lansley S, Herrick SE, Lim CB, Prêle CM. Mesothelial cells in tissue repair and fibrosis. Front Pharmacol. 2015;6:113. doi: 10.3389/fphar.2015.00113. https://doi.org/10.3389/fphar.2015.00113 PMid:26106328 PMCid:PMC4460327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwasaki K, Ahmadi AR, Qi L, Chen M, Wang W, Katsumata K, Tsuchida A, Burdick J, Cameron AM, Sun Z. Pharmacological Mobilization and Recruitment of Stem Cells in Rats Stops Abdominal Adhesions After Laparotomy. Sci Rep. 2019;9(1):7149. doi: 10.1038/s41598-019-43734-1. https://doi.org/10.1038/s41598-019-43734-1 PMid:31073167 PMCid:PMC6509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittal SK, Roche PA. Suppression of antigen presentation by IL-10. Curr Opin Immunol. 2015;34:22–7. doi: 10.1016/j.coi.2014.12.009. https://doi.org/10.1016/j.coi.2014.12.009 PMid:25597442 PMCid:PMC4444374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pakyari M, Farrokhi A, Maharlooei MK, Ghahary A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv Wound Care (New Rochelle) 2013;2(5):215–224. doi: 10.1089/wound.2012.0406. https://doi.org/10.1089/wound.2012.0406 PMid:24527344 PMCid:PMC3857353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25(1):9–18. doi: 10.1016/j.clindermatol.2006.09.007. https://doi.org/10.1016/j.clindermatol.2006.09.007 PMid:17276196. [DOI] [PubMed] [Google Scholar]
- 25.Nishikai-Yan Shen T, Kanazawa S, Kado M, Okada K, Luo L, Hayashi A, Mizuno H, Tanaka R. Interleukin-6 stimulates Akt and p38 MAPK phosphorylation and fibroblast migration in non-diabetic but not diabetic mice. PLoS One. 2017;12(5):e0178232. doi: 10.1371/journal.pone.0178232. https://doi.org/10.1371/journal.pone.0178232 PMid:28542434 PMCid:PMC5441644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang WG, Sanders AJ, Ruge F, Harding KG. Influence of interleukin-8 (IL-8) and IL-8 receptors on the migration of human keratinocytes, the role of PLC-γand potential clinical implications. Exp Ther Med. 2012;3(2):231–236. doi: 10.3892/etm.2011.402. https://doi.org/10.3892/etm.2011.402 PMid:22969874 PMCid:PMC3438606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraemer B, Wallwiener C, Rajab TK, Brochhausen C, Wallwiener M, Rothmund R. Standardised models for inducing experimental peritoneal adhesions in female rats. Biomed Res Int. 2014;2014:435056. doi: 10.1155/2014/435056. https://doi.org/10.1155/2014/435056 PMid:24809049 PMCid:PMC3997962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassan G, Kasem I, Soukkarieh C, Aljamali M. A Simple Method to Isolate and Expand Human Umbilical Cord Derived Mesenchymal Stem Cells:Using Explant Method and Umbilical Cord Blood Serum. Int J Stem Cells. 2017;10(2):184–192. doi: 10.15283/ijsc17028. https://doi.org/10.15283/ijsc17028 PMid:28844128 PMCid:PMC5741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mittal SK, Roche PA. Suppression of antigen presentation by IL-10. Curr Opin Immunol. 2015;34:22–7. doi: 10.1016/j.coi.2014.12.009. https://doi.org/10.1016/j.coi.2014.12.009 PMid:25597442 PMCid:PMC4444374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caja L, Dituri F, Mancarella S, Caballero-Diaz D, Moustakas A, Giannelli G, Fabregat I. TGF-βand the Tissue Microenvironment:Relevance in Fibrosis and Cancer. Int J Mol Sci. 2018;19(5):1294. doi: 10.3390/ijms19051294. https://doi.org/10.3390/ijms19051294 PMid:29701666 PMCid:PMC5983604. [DOI] [PMC free article] [PubMed] [Google Scholar]


