Abstract
Purpose
To review the current therapeutic options for the management of diabetic retinopathy (DR) and diabetic macular edema (DME) and examine the evidence for integration of laser and pharmacotherapy.
Methods
A review of the PubMed database was performed using the search terms diabetic retinopathy, diabetic macular edema, neovascularization, laser photocoagulation, intravitreal injection, vascular endothelial growth factor (VEGF), vitrectomy, pars plana vitreous surgery, antiangiogenic therapy. With additional cross-referencing, this yielded 835 publications of which 301 were selected based on content and relevance.
Results
Many recent studies have evaluated the pharmacological, laser and surgical therapeutic strategies for the treatment and prevention of DR and DME. Several newer diagnostic systems such as optical coherence tomography (OCT), microperimetry, and multifocal electroretinography (mfERG) are also assisting in further refinements in the staging and classification of DR and DME. Pharmacological therapies for both DR and DME include both systemic and ocular agents. Systemic agents that promote intensive glycemic control, control of dyslipidemia and antagonists of the renin-angiotensin system demonstrate beneficial effects for both DR and DME. Ocular therapies include anti-VEGF agents, corticosteroids and nonsteroidal anti-inflammatory drugs. Laser therapy, both as panretinal and focal or grid applications continue to be employed in management of DR and DME. Refinements in laser devices have yielded more tissue-sparing (subthreshold) modes in which many of the benefits of conventional continuous wave (CW) lasers can be obtained without the adverse side effects. Recent attempts to lessen the burden of anti-VEGF injections by integrating laser therapy have met with mixed results. Increasingly, vitreoretinal surgical techniques are employed for less advanced stages of DR and DME. The development and use of smaller gauge instrumentation and advanced anesthesia agents have been associated with a trend toward earlier surgical intervention for diabetic retinopathy. Several novel drug delivery strategies are currently being examined with the goal of decreasing the therapeutic burden of monthly intravitreal injections. These fall into one of the five categories: non-biodegradable polymeric drug delivery systems, biodegradable polymeric drug delivery systems, nanoparticle-based drug delivery systems, ocular injection devices and with sustained release refillable devices. At present, there remains no one single strategy for the management of the particular stages of DR and DME as there are many options that have not been rigorously tested through large, randomized, controlled clinical trials.
Conclusion
Pharmacotherapy, both ocular and systemic, will be the primary mode of intervention in the management of DR and DME in many cases when cost and treatment burden are less constrained. Conventional laser therapy has become a secondary intervention in these instances, but remains a first-line option when cost and treatment burden are more constrained. Results with subthreshold laser appear promising but will require more rigorous study to establish its role as adjunctive therapy. Evidence to support an optimal integration of the various treatment options is lacking. Central to the widespread adoption of any therapeutic regimen for DR and DME is substantiation of safety, efficacy, and cost-effectiveness by a body of sound clinical trials.
Keywords: diabetes, retina, diabetic retinopathy, diabetic macular edema, neovascularization, laser photocoagulation, intravitreal injection, vascular endothelial growth factor, vitrectomy pars plana vitreous surgery, antiangiogenic therapy
Plain Language Summary
With the recent expansion of management options for diabetic retinopathy, optimal sequences of treatment application and combination in specific clinical situations are under investigation. A review and synthesis of the ophthalmologic literature on treatment of diabetic retinopathy was performed to provide perspective on the relative prioritization of the various treatments in the contexts seen in clinical practice. In general, pharmacotherapy is ascendant, particularly with the anti-VEGF class, while laser treatment continues to have lesser roles in specific situations and under certain economic constraints. Surgical intervention continues to be reserved for those situations which fail to respond to pharmacotherapy, laser or combination therapy. Ongoing refinements in the systemic management of both hyperglycemia and hyperlipidemia continue to demonstrate significant benefits for both diabetic retinopathy and diabetic macular edema. Recent developments involving newer retinal diagnostics are proving beneficial in optimizing both initiation and maintenance of therapy. As well, recent advances in novel pharmaceutical agents and ocular drug delivery methods show promise in better controlling the disease as well as reducing the burden of treatment.
Introduction
With the increasing global incidence of diabetes mellitus (DM) in both developed and developing countries, diabetic retinopathy (DR) has likewise increased in prevalence. Recent estimates suggest that approximately 486 million people worldwide have DM and that roughly one-third demonstrate evidence of DR, including diabetic macular edema (DME).1–5 In the working adult population, DR remains a major cause of blindness in the US, causing 12,000–24,000 new cases each year. Approximately 30 million people or 9.4% of the US population in 2017 had DM.6 With DR consuming roughly 40% of the total cost of DM care in the US, this translates approximately to $120 billion annually in economic burden, not only from direct disease management costs but also from lost worker productivity.7
Methods
A systematic search of English-language articles in the PubMed database was performed using the medical subject headings (MeSH) search terms “diabetic retinopathy”, “diabetic macular edema”, “retinal neovascularization”, “laser photocoagulation”, “intravitreal injection”, “vascular endothelial growth factor” (VEGF), “vitrectomy”, “pars plana vitreous surgery”, “antiangiogenic therapy”. The date range of the search was restricted to a period from January 1st, 1965 to May 1st 2019. The initial retrieved search was followed by a manual search of reference lists of selected major review articles. Randomized controlled trials (RCTs) with more than 6 months of follow-up and meta-analyses were included. Case reports and “grey literature” articles were excluded. Only indexed, peer-reviewed articles were included and with additional cross-referencing, this yielded 839 publications of which 305 were selected based on content and relevance to the main search term “diabetic retinopathy”.
Historical Background
In the 1950s DR was the leading cause of blindness and visual disability in the United States. Perhaps because of a dearth of alternative therapies, photocoagulation had gained widespread use in clinical practice in spite of inadequate evidence as to its benefit. Begun in 1971 and completed in 1975, the landmark Diabetic Retinopathy Study (DRS) demonstrated that scatter laser photocoagulation was beneficial in reducing the risk of progression to “severe visual loss” (SVL) in eyes with “high-risk characteristics (HRC).” Reflecting the dismal prognosis of the era, the primary outcome was not VA gain but rather the reduction of SVL (<5/200 (VA) at two consecutive 4-month visits).8
The minimum degree of retinopathy for patients enrolled in the DRS was “severe nonproliferative diabetic retinopathy (NPDR).” As no patients with “mild to moderate NPDR” were studied, the results did not guide clinicians as to when to apply scatter photocoagulation in such eyes. The clinical question arose as to whether earlier application of scatter photocoagulation prior to the development of proliferative diabetic retinopathy (PDR) would help to reduce the risk of progressing to PDR and thus help reduce the risk of progressing to SVL. To answer this question, the Early Treatment Diabetic Retinopathy Study (ETDRS) was formulated. Patients were randomized to either a pattern of full panretinal laser photocoagulation (PRP) (1200–1600 burns of 500µm spot size) or mild PRP (400–650 burns of 500µm spot size).9,10 Although full PRP reduced the risk of developing HRC by 50% and mild PRP reduced the risk of developing HRC by 25%, the rates of developing SVL were low as long as patients could be followed closely with laser applied after HRC developed.11 As such, the timing of the application of scatter photocoagulation the ETDRS did not significantly alter the recommendations of the DRS.
A second major question posed by the ETDRS was whether photocoagulation was effective in the treatment of DME. When patients with DME, defined initially as thickening of the retina within one disc diameter of the center of the macula, were treated with focal laser, there was no significant VA benefit.12 However, when a more restrictive definition of “clinically significant macular edema” (CSME) was used as a treatment criteria, focal photocoagulation was shown to reduce the risk of progression to “moderate visual loss” (defined as loss of 15 or more ETDRS letters).9 The protocol laser parameters were to treat microaneurysms from 500–3000µm from the center of the fovea directly with burns of 50–100µm spot size; 0.05–0.1 seconds duration; with power sufficient to whiten or darken the microaneurysm. Either color change indicates that the microaneurysm has been changed by the absorption of laser energy, and usually leads to involution and reduction or cessation of leakage. A grid treatment of <200µm spot size with mild intensity and a 0.05–0.1 second duration was applied to areas of diffuse leakage and non-perfusion.
CSME was defined as 1) retinal thickening at or within 500µ of the center of the macula 2) hard exudates at or within 500µ of the center of the macula if associated with thickening of the adjacent retina 3) retinal thickening of at least one disc area in size if at least part of that retinal thickening was within one disc diameter of the center of the macula.12 If CSME persisted at the 4 month follow-up visits then treatable lesions received additional direct and grid photocoagulation. As with the DRS, the ETDRS goals of laser therapy were to help prevent visual loss. As the authors commented, “treatment is less effective at improving vision than in preventing further visual loss.”11
In 1991 the American Academy of Ophthalmology established a long-term educational project, “Elimination of Preventable Blindness from Diabetes by the Year 2000.”13 Somewhat akin to John F. Kennedy’s pledge to place a man on the moon by the end of the decade of the 1960s, this program was termed “Diabetes 2000” with the goal of disseminating the results of the DRS and the ETDRS such that early recognition and timely treatment of diabetic retinopathy could prevent visual loss. Experience with the Diabetes 2000 program illustrated the need for new strategies capable of improving accessibility to high-quality eye care, increasing involvement of primary care physicians in DR screening and encouraging at-risk individuals to seek testing.14
In 2002, the collaborative Diabetic Retinopathy Clinical Research Network (DRCR Network) was formed to facilitate multicenter clinical research of diabetic retinopathy, diabetic macular edema and associated conditions. Whereas the DRS and ETDRS involved primarily academic institutions, the DRCR.net has a majority of community-based sites participating in their trials. Simultaneous with the formation of the DRCR Network in the last decade, was the increasing use of intravitreal pharmacotherapy for the management of DR and DME. The DRCR Network has conducted multiple clinical trials addressing various diagnostic and therapeutic approaches to the management of DR and more recently DME.15–21
Along with advances in retinal diagnostics, the last two decades have also seen advances in vitreoretinal surgical techniques and instrumentation. When first introduced in 1970, vitrectomy for proliferative diabetic retinopathy was reserved for severe vitreous hemorrhage which had not cleared by one year or for traction retinal detachment involving the macula. Increasingly, surgical techniques are employed for less advanced stages of the disease. The development and widespread use of smaller gauge instrumentation along with intraoperative wide-field viewing systems and advanced anesthesia agents have been associated with a trend toward earlier intervention for diabetic retinopathy. There is need for a large, prospective trial to determine if the threshold for surgical intervention in both DR and DME can be reduced in comparison to the criteria established by the Diabetic Vitrectomy Study thirty years ago. The Diabetic Retinopathy Vitrectomy Study (DRVS) was done to evaluate vitrectomy in the setting of proliferative diabetic retinopathy and enrolled patients from 1976 to 1978. Group N was designed to yield information on the conventional management of eyes with very severe PDR. This information was then used to define eligibility criteria for Group NR which included eyes with retinopathy severe enough to justify randomization to either early vitrectomy or conventional management. Group H included eyes with severe vitreous hemorrhage for less than 5 months combined with reduction in visual acuity to 5/200 or worse which were randomized to either early vitrectomy or deferral of vitrectomy for one year. Early vitrectomy resulted in visual acuity of 10/20 or better in 25% of eyes versus deferral of vitrectomy which resulted in visual acuity of 10/20 or better in 15% of eyes. In Type I diabetes, early vitrectomy resulted in visual acuity of 10/20 or better in 36% of eyes whereas deferral of vitrectomy resulted in 10/20 or better visual acuity in 12% of eyes. There was no advantage of early vitrectomy found in Type 2 diabetes patients.22,23
The dramatic increase in diagnostic and therapeutic options available to the clinician managing DR and DME has made more pressing the question of the optimal integration of these treatment modalities for specific situations.
Recent Developments in Systemic Management of DM
The basis for the medical management of diabetic retinopathy consists of intensive medical control of blood glucose, blood pressure and blood lipids. The Diabetes Control and Complications Trial (DCCT) demonstrated that intensive insulin therapy over an average of 6.5 years in Type I diabetes resulted in a reduction of clinically important retinopathy (34–76%), need for laser photocoagulation (34%) and first appearance of retinopathy (27%) over four years.24 The Epidemiology of Diabetes Intervention and Complications Study, an extension of the DCCT showed that the beneficial effects persist for an additional 4–10 years and more recently out to 23 years.25,26 The relative risk reduction at 10 years was 53–56% (95% CI 45–66, p=0.001) and at 23 years the risk reduction of any diabetes-related eye surgery was 48% (95% CI 29–63, P<0.001).26 The UK Prospective Diabetes Study in Type II diabetes also showed similar results and for every 1% decrease in hemoglobin A1C, there was a 35% reduction in the risk of microvascular complications.27–29 Similarly, results from the DRCR Network’s Protocol T trial demonstrated a correlation of visual acuity (VA) improvement in patients receiving anti-VEGF therapy with lower hemoglobinA1c levels.30
The Actions to Control Cardiovascular Risk in Diabetes (ACCORD) Study was designed to test three separate strategies to reduce cardiovascular disease in those with Type II diabetes including intensive glycemic control vs standard, intensive blood pressure control vs standard and intensive therapy of blood lipids/lipoproteins vs placebo and simvastatin.31 The study examined the effect of adding fenofibrate 160 mg/d in diabetic patients with normal glomerular filtration rate (GFR) or 54 mg/d in patients with reduced GFR to simvastatin. Over four years, the progression of retinopathy was reduced by 40% in the fenofibrate group.32 The study showed that intensive glycemic, blood pressure and lipid control did not significantly reduce cardiovascular disease.32,33 Tight glycemic control increased mortality (5% vs 4%) and tight blood pressure control reduced strokes.32,34 The ACCORD Eye Study showed that intensive glycemic control, control of dyslipidemia with fenofibrate and simvastatin reduced the proportion of eyes that had progression of retinopathy by one-third. Intensive blood pressure control did not have a statistically significant effect.32,33,35 The results of the optical coherence tomography substudy of ACCORD, which will illuminate the effects on DME, have not been published yet. In order to determine whether there is a “memory imprint” for intensive glucose or lipid control, the 8 year follow-up data was recently published and demonstrated that prior intensive glycemic control continued to reduce DR progression, despite similar A1C levels; however, the benefit of fenofibrate did not persist. Likewise, intensive blood pressure control had no effect on DR progression.36
Simultaneous with the ACCORD study, the fenofibrate intervention and event lowering in diabetes (FIELD) study concluded that a potential therapeutic role existed for fenofibrate in the prevention of retinopathy alongside intensive management of hyperglycemia and high blood pressure.37,38 The FIELD study showed that oral fenofibrate 200 mg/d was associated with a statistically significant reduction in a composite endpoint of 2-step progression of retinopathy grade, macular edema, or laser treatment for either DME or proliferative retinopathy. The hazard ratio for the fenofibrate group compared to placebo was 0.66, 95% confidence interval 0.47–0.94, P=0.022. It is not known how the DME component of the composite outcome was affected by the fibrate therapy. Newer fibrates such as the selective peroxisome proliferator-activated receptor alpha modulator (SPPARM-α), pemafibrate, may have even more significant impact on ameliorating DR than older agents in this class.39 In general, studies with fibrates have been shown to have a protective effect on DR progression and possibly reduction in risk of DME development; however, their effect on preserving vision as well as retinal hard exudate formation is marginal.38
Studies with statins and their role in delaying the onset and severity of DR have yielded similar results to those obtained with the fibrates.40,41 The recent results from the prevención con dieta Mediterránea (PREDIMED) trial have demonstrated that dietary modification involving consumption of long-chain omega-3 polyunsaturated fatty acids (LCω3PUFAs) decreases the risk of DR development.42 Further support for dietary supplementation benefit in DR was provided by the diabetes visual function supplement study (DiVFuSS). The DiVFuSS was a 6 month randomized trial involving subjects with Type 1 and 2 DM for at least 5 years and a VA of 20/30 or better with no retinopathy to mild or moderate NPDR. Subjects were randomized to placebo or a twice-daily oral supplement containing xanthophyll pigments, antioxidants and botanical extracts, specifically: vitamins C, D3 and E (d-α tocopherol), zinc oxide, eicosapentaenoic acid, docosahexaenoic acid, α-lipoic acid (racemic mixture), coenzyme Q10, mixed tocotrienols/tocopherols, zeaxanthin, lutein, benfotiamine, N-acetyl cysteine, grape seed extract, resveratrol, turmeric root extract, green tea leaf and Pycnogenol. Visual function tests including contrast sensitivity, color error score and visual field mean sensitivity and macular pigment optical density showed statistically significant improvements in the treatment group compared to placebo.43
Newer adjunctive therapies such as Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are increasingly being employed to better regulate serum glucose fluctuations in DM and also appear to confer some benefit in severity reduction of DR.44,45
Ultimately, effective resolution of DR will depend on permanent correction of the underlying systemic abnormality for both type 1 and 2 DM. Although there are encouraging results from recent and limited trials of diabetic cell therapy, particularly stem cell transplantation, it is still too early for these new therapies to be applied in large-scale trials.46–49 Likewise, recent advances in artificial pancreas development hold promise for better control of both DM and DR progression.50–54
Current Role of Laser Therapy in the Management of Diabetic Retinopathy
In the past decade, new approaches in pharmacotherapeutic management of DME have overshadowed developments in laser therapy. The initial clinical trial of the DRCR Network published in 2007 illustrates the modest evolution of laser techniques for management of diabetic macular edema.55 The “modified-ETDRS” pattern involved less intense power such that a change in the microaneurysm color was not required for direct treatment and for the grid treatment the burn intensity was to be “barely visible.” The “mild macular grid photocoagulation” varied from the ETDRS pattern of grid treatment in that the intensity was “barely visible” and also that the area of treatment was to both thickened and un-thickened retina within the macula.
The results of some clinical trials have demonstrated that supplementing pharmacotherapy with laser therapy both as focal and grid application for macular edema and as PRP application for proliferative disease may provide a more durable response.56–59 However, conventional, continuous wave (CW) or photocoagulative laser destroys some retinal cells and often results in permanent scotomas in the visual field.12,60–62 PRP can worsen night vision and delay light-to-dark adaptation.63–66
A newer mode of laser, termed subthreshold, involves grid type application of non-photocoagulative laser spots to “photo-stimulate” outer retinal tissues, primarily the retinal pigment epithelium (RPE), to either increase production of metabolites that inhibit neovascularization and reduce vascular permeability activity or to downregulate production of mediators that increase vascular permeability and neovascularization.67–76 In subthreshold laser, many of the benefits of CW lasers can be obtained without the adverse side effects. There are now several laser manufacturers providing subthreshold lasers (STL) systems such as the Micropulse laser™ (Iridex Corp.; Quantel Medical), Endpoint Management™ (Topcon), Microsecond Pulse (Navilas OD OS) and 2RT ® (Ellex), all of which can be safely applied over the fovea without visible damage. Several small and often uncontrolled studies have shown the benefits of subthreshold lasers in treating diabetic macular edema either alone or in combination with pharmacotherapy, yielding results comparable to those obtained with conventional laser but with no tissue damage or scotomas.77–85 To date however, a definitive advantage of these alternative laser therapies over the ETDRS established delivery method has not been borne out for PDR.86
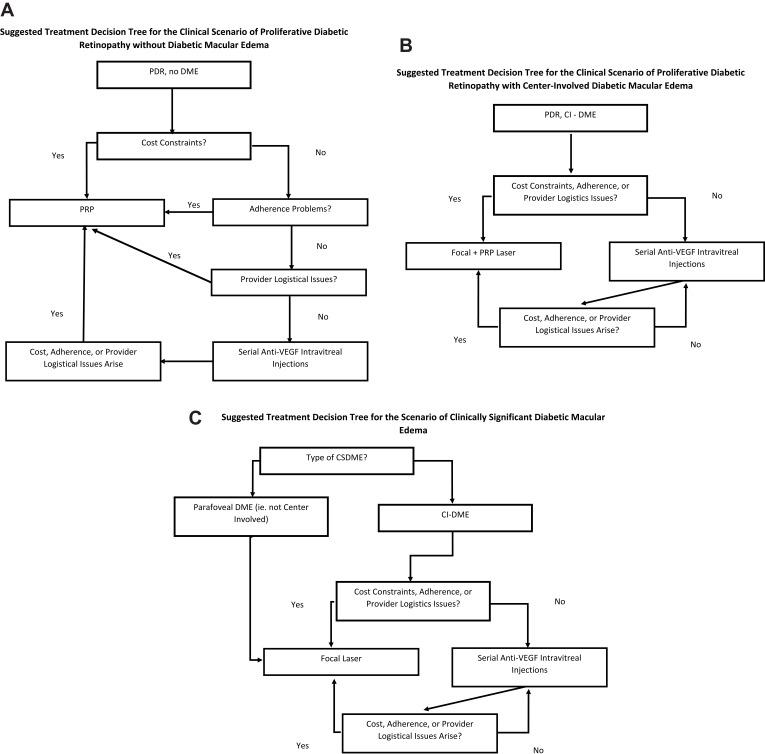
Another new development in retinal laser therapy is a fundus camera-based photocoagulation system that is integrated with retinal eye-tracking technology (NAVILAS).87–89 This technique allows the ophthalmologist to take an image of the retina of a patient with DR or DME, digitally encircle the areas requiring treatment and have the device automatically deliver the laser spots to the specified areas. Higher accuracy of laser delivery compared with conventional, manually operated lasers can be achieved.90 In managing patients with proliferative disease, this system is able to deliver a “navigated” pattern PRP which is selectively applied to areas of ischemia identified by wide-field fluorescein angiography. Fewer, more uniform laser burns are delivered in shorter time and with less discomfort. Although all treatments ultimately depend on the specifics of the clinical situation, Figure 1 shows suggested guidelines from the authors for the relationship of laser treatment and pharmacologic treatment for DME, PDR, and combined situations that are commonly encountered.
Figure 1.
(A) Suggested treatment decision tree for the clinical scenario of proliferative diabetic retinopathy without diabetic macular edema. (B) Suggested treatment decision tree for the clinical scenario of proliferative diabetic retinopathy with center-involved diabetic macular edema. (C) Suggested treatment decision tree for the scenario of clinically significant diabetic macular edema. CSDME=clinically significant diabetic macular edema, which falls into 3 subcategories: edema of 1 disc area or more within 1 disc diameter of the fovea; foveal or parafoveal hard lipid with adjacent macular thickening; or foveal thickening. Focal laser means modified focal-grid laser in the manner outlined by the diabetic retinopathy clinical research network. Subthreshold laser may eventually gain a place in this block, but currently, the evidence is not strong enough to merit commensurate standing with focal laser.
Abbreviations: PDR, proliferative diabetic retinopathy; DME, diabetic macular edema; PRP, panretinal photocoagulation; VEGF, vascular endothelial growth factor; CI-DME, center-involved diabetic macular edema.
Recent, uncontrolled studies have demonstrated that when used in combination with pharmacotherapy, the patients receiving NAVILAS guided focal laser required fewer injections of anti-VEGF agents than would otherwise have been required in order to maintain remission of the macular edema.91,92 Analogous attempts to lessen the burden of anti-VEGF injections in the setting of DME by integrating peripheral PRP have met with mixed results.93–95 Recent data from protocol V of the DRCR Network examining timing of therapy initiation in patients with center-involved DME and good VA demonstrated that focal macular laser fared equally well as anti-VEGF (aflibercept) therapy at 2 years.96–98 In addition, the same protocol validated a “watchful wait” approach to managing patients with mild DME.
In summary, the use of laser therapy for managing diabetic-related retinal disease will continue to play a role. Situations which preclude the use of pharmacotherapy in DME & DR, such as unreliable patient visit compliance, pregnancy or anti-VEGF non-responders, would be best managed by laser therapy. Large, prospective comparative trials are needed to determine if the newer subthreshold and navigated laser techniques are superior to conventional laser methods.
Pharmacotherapy in Diabetic Retinopathy and Diabetic Macular Edema
When the results of the ETDRS were published in 1985, focal laser for DME became established as the standard of care for the next 30 years.12 However, it was evident during this era that more effective therapy was needed.8 Pharmacological and surgical therapies were subsequently investigated.99–101
Pharmacotherapy for both DR and DME can be subdivided by class of drugs and methods of delivery as shown in Table 1. The most important class of drugs is the anti-vascular endothelial growth factor (anti-VEGF) agents, followed by the corticosteroids. Much less important are systemic angiotensin receptor blockers and fibrates. Topical nonsteroidal anti-inflammatory drugs have so far proven futile in long-term DME management.
Table 1.
Pharmacotherapy of Diabetic Retinopathy and Macular Edema
| Administration Route | Class of Drugs | |||||
|---|---|---|---|---|---|---|
| Anti-VEGFa | Corticosteroids | ACE Inhibitorsb | Angiotensin Receptor Blockers | Fibrates | NSAIDsc | |
| Intravitreal | Bevacizumab, Aflibercept Ranibizumab, Pegaptanib, Conbercept |
Dexamethasone, Fluocinolone, Triamcinolone | N/Ad | N/A | N/A | Diclofenac |
| Periocular | N/A | Triamcinolone | N/A | N/A | N/A | N/A |
| Topical | N/A | N/A | N/A | N/A | N/A | Nepafenac Ketorolac |
| Oral | N/A | N/A | Enalapril | Losartan Candesartan |
Fenofibrate | N/A |
Notes: aVascular endothelial growth factor; bAngiotensin-Converting Enzyme Inhibitors; cNonsteroidal anti-inflammatory drugs; dNot applicable.
Anti-VEGF Therapy
The first anti-VEGF drug used to treat DME was pegaptanib, which selectively blocks the 165 isoform of VEGF.102 Its promise was superseded by superior results obtained with anti-VEGF drugs that blocked all isoforms of VEGF. The efficacy of bevacizumab and ranibizumab was proven in randomized-controlled clinical trials in 2010 and that of aflibercept in 2014.6,7,18,103–105 Conbercept may prove to be a fifth effective anti-VEGF agent, but a level I randomized clinical trial has not yet been published. A prospective, randomized, comparative effectiveness trial of bevacizumab, ranibizumab, and aflibercept showed no difference in efficacy of the three drugs in eyes with center-involved DME and VA of 20/40 or better at one or two years of follow-up. However, in eyes with VA of 20/50 or worse, aflibercept was superior to ranibizumab and bevacizumab at one year (Table 2), whereas at two years aflibercept was no longer superior to ranibizumab, but remained superior to bevacizumab.21,106
Table 2.
Anti-VEGF Therapy for Diabetic Macular Edema: Selected Studies
| Study | na | Duration (Years) | Subgroup | Mean BLb BCVAc | Mean BL CSTd | # Injections | ∆BCVA | ∆CST | Persistent Edema (%) |
|---|---|---|---|---|---|---|---|---|---|
| RISEe | 377 | 2 | Sham | 20/80 | 467 | 0 | 2.6 | −133 | |
| 0.3R monthly | 20/80 | 475 | 24 | 12.5 | −251 | 26 | |||
| 0.5 R monthly | 20/80 | 464 | 24 | 11.9 | −253 | 24 | |||
| BOLTf | 80 | 2 | 1.25B 6 weekly | 20/80 | 501 | 13 | 8.6 | −146 | NGj |
| Focal | 20/80 | 478 | 0 | −0.5 | −118 | NG | |||
| VIVIDg and VISTAh | 872 (pooled) | 1 | 2A monthly | 20/63 | 485/502 | 11.8/12.2 | 12.5/10.5 | −186/–195 | NG |
| 2A q2 months | 20/63 | 479/518 | 8.4/8.7 | 10.7/10.7 | −183/–192 | NG | |||
| Focal | 20/63 | 483/540 | 0.1/1.2 | −73/–66 | NG | ||||
| DRCRi Protocol T | 660 | 1 | 2A monthly | 20/32 | 373 | 9 | 8.0 | −210 | 38 |
| 1.25B monthly | 20/40 | 363 | 10 | 7.5 | −135 | 66 | |||
| 0.3R monthly | 20/40 | 384 | 10 | 8.3 | −176 | 40 | |||
| 2A monthly | 20/80 | 452 | 9 | 18.9 | −129 | 30 | |||
| 1.25B monthly | 20/80 | 467 | 10 | 11.8 | −67 | 61 | |||
| 0.3R monthly | 20/80 | 431 | 10 | 14.2 | −119 | 44 |
Notes: aSample size; bBaseline; cBest corrected visual acuity; dCentral subfield thickness; eRanibizumab Injection in Subjects with clinically significant macular Edema; fBevacizumab or Laser Therapy in the management of diabetic macular edema; gVEGF Trap-Eye in Vision Impairment due to DME; hStudy of Intravitreal Administration of VEGF Trap-Eye in Patients with Diabetic Macular Edema; iDiabetic Retinopathy Clinical Research Network; jNot given.
Approaches aimed at increasing the intravitreal concentration of anti-VEGF agents have not proved beneficial. The READ-3 clinical trial examining two doses of ranibizumab (0.5 and 2.0 mg) in DME showed that at 1 year there were no significant differences between the two groups.107–109 Focal laser added from the outset to anti-VEGF does not improve VA outcomes relative to its use in a deferred manner if incomplete drying of the macula occurs with anti-VEGF therapy.110 As a result, in 2019, serial anti-VEGF intravitreal injection monotherapy has become the standard of care. Unlike clinical trials, real-world data have demonstrated that a significant portion of patients in clinical practice are undertreated with anti-VEGF and have subsequently lower best corrected visual acuity (BCVA).111 In DME patients, post hoc analysis of data from Protocol I of the DRCR Network demonstrated that the initial macular response to three injections of a particular anti-VEGF agent was predictive of long-term outcome.112 Accordingly, poor responders might potentially benefit from a switch in therapeutic agents.
Intravitreal ranibizumab injections given monthly for DME increase the proportion of 2 or 3 step improvement in the severity of diabetic retinopathy, reduce the proportion of eyes with 2 or 3 step worsening in severity of diabetic retinopathy, and reduce the proportion of eyes progressing to proliferative diabetic retinopathy.113,114 More recently, preliminary results from the Phase III PANORAMA trial demonstrated significant regression of DR severity with intravitreal aflibercept in comparison to sham injections.115 Also, recent subgroup analysis from both the RIDE and RISE trials demonstrated significant benefit in improvement of DR severity with ranibizumab use in mild and moderate NPDR.116
Despite safety concerns that intravitreal anti-VEGF drugs could raise the risk of cardiovascular complications in patients with diabetes, there is no consistent evidence that this is the case. Recent meta-analysis of anti-VEGF therapy in DME found that aflibercept, ranibizumab and bevacizumab did not differ regarding the occurrence of systemic serious adverse events.117 Likewise, the concern that in already ischemic vascular beds, additional anti-VEGF therapy could further compromise the macula has not been borne out by recent data from the RESTORE study.59 In fact, post hoc analysis of data from the VIVID and VISTA trials involving aflibercept in DME demonstrated that patients with macular nonperfusion had improvement in macular perfusion status as well as visual and anatomic improvements following treatment.118,119 In 2019, the data consensus suggests that for eyes with mild DME in terms of both retinal thickness and VA loss, treatment with either aflibercept, bevacizumab or ranibizumab will be equally efficacious. When there is moderate or worse VA loss, aflibercept is more efficacious.120 Bevacizumab remains more cost-effective than ranibizumab or aflibercept.121
For PDR management, the role of anti-VEGF and laser therapy is different than that for DME. Protocol S of the DRCR Network was a randomized prospective clinical trial, comparing standard PRP with intravitreal ranibizumab 0.5mg for eyes with proliferative diabetic retinopathy.122 In this multicenter randomized, non-inferiority trial, 305 patients with PDR were enrolled and randomly assigned to treatment requiring follow-up for 2 years. The results of this study showed that patients in the non-laser ranibizumab group gained 10 or more letters in approximately 42% of eyes compared to approximately 35% in the laser-treated arms at 2 years follow-up. Similarly, the rates of 10 letter score worsening were higher in the laser group at approximately 13% compared to 10% in the laser-treated arms at 10 years. In addition, the VA change area under the curve analysis favored the ranibizumab eyes over the 2-year course, which may have artificial been partially explained by relative undertreatment of DME with intravitreal ranibizumab injections compared to expectations set by the results of protocol I. Specifically, the patients in the baseline DME subgroup of the PRP arm of the trial received a median of 9 ranibizumab injections over 2 years compared to 13 injections over two years for patients in the ranibizumab plus deferred focal laser arm of protocol I.19 A further limitation of the study was the fact that 53% of the PRP group also received intravitreal ranibizumab for DME at baseline or newly developed DME during the follow-up period. Therefore, the PRP group may have been assisted by the application of ranibizumab in half of the study eyes. In terms of complications, there was a higher rate of vitrectomy and any vitreous hemorrhage in the PRP group compared to the ranibizumab group. Interestingly, the recently published 5-year data from this protocol demonstrated equivalent VA outcomes between both groups at 5 years and progressive visual field reduction was also present in both groups.123
The CLARITY study compared three monthly intravitreal aflibercept injections followed by as needed injections with PRP in the treatment of PDR. With a primary outcome at 52 weeks and 116 patients in each arm of the study, aflibercept was superior with a mean best corrected VA difference of 3.9 letters [95% CI 2.3–5.6], p<0.0001, fewer vitreous hemorrhages, better visual fields, and higher patient satisfaction.124 In light of the RISE, RIDE and other studies, the traditional view of PRP being a truly “one and done” therapy for PDR is inaccurate.125 For now, management of PDR will more likely be guided by both cost of therapy as well as patient-specific factors such as visit compliance. Over the coming two years, data analysis from protocol W of the DRCR Network, examining the role of aflibercept in the prevention of PDR and center-involving DME will shed more light on the optimal timing of anti-VEGF therapy in DR and DME.
Corticosteroid Therapy
Corticosteroids were first used to treat DME in 2001.100 Triamcinolone, dexamethasone, and fluocinolone have been used in many forms, including particulate suspensions, viscoelastic mixtures, and solid slow-release devices.100,126–128 Topical difluprednate for persistent DME has demonstrated short-term improvement in both VA and reduction in macular thickness, but this has been accompanied by an incidence of approximately 20% increase in intraocular pressure.129–131 Many dosages and intervals between injections have also been tried.132 Although enthusiasm for serial intravitreal triamcinolone injection was initially high, protocol B of the DRCR Network proved that focal laser led to superior VA outcomes at 3 years relative to either triamcinolone 1 mg or 4mg.16,133 Since the results of that large, prospective, randomized-controlled clinical trial were published, therapy with corticosteroids has taken a secondary role to anti-VEGF therapy, often used in cases refractory to, or with incomplete responses to, first-line anti-VEGF therapy.134,135
The availability of corticosteroids in the form of sustained release implants has potential benefits in terms of durability of therapy in vitrectomized eyes.136 Results from protocol U from the DRCR Network demonstrated that in the short term, combination intraocular steroid in the form of a dexamethasone implant plus anti-VEGF therapy (ranibizumab) in comparison with that of continued anti-VEGF therapy alone in eyes with persistent center-involved DME and VA impairment despite previous anti-VEGF treatment, had modest improvement in visual gain despite significant reductions in retinal thickness on OCT.137 In phakic eyes receiving continuous anti-VEGF therapy for DME, the addition of intravitreous corticosteroids did not result in significant visual improvements.138,139 Recent reports summarizing observational studies investigating dexamethasone implants in DME have reported similar final VA outcomes when compared to anti-VEGF monotherapy, but superior visual gains in real-life practice.140–142 There appears to be a predictive correlation between the early response to anti-VEGF therapy with the visual and anatomical outcomes following a switch to intravitreal corticosteroids. Those with poor responses to anti-VEGF demonstrated a more robust increase in BCVA.143 Side effects of cataract in phakic eyes and intraocular pressure elevation without regard to lens status have accompanied all steroids studied, although to varying degrees.144–149 However, there may be a role for long-acting intraocular corticosteroids in reducing overall treatment burden in DME as early data from both the TYBEE and PALADIN studies have recently demonstrated.150–152 Likewise, intravitreal triamcinolone 4 mg injections for DME reduced 2 step progression in severity of diabetic retinopathy compared to focal/grid laser at 3 years.110 Similar data obtained from the DR-Pro-DEX Study and others demonstrated that the dexamethasone and fluocinolone implants significantly delayed the progression and reduced the severity of DR over a 24-month study period.153,154
Nonsteroidal Anti-Inflammatory Drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) for DME have not been studied in depth, but available investigations suggest that they have little role in its management. Protocol R of the DRCR Network was a prospective, masked, randomized clinical trial of topical nepafenac 0.1% three times per day versus placebo over 12 months in eyes with non-center-involved DME and good VA.155 No differences in VA outcomes were found. Meta-analyses examining the role of NSAIDs in the prevention of post-cataract extraction cystoid macular edema in patients without or with diabetes have come to opposite conclusions.156,157 Topical bromfenac in short-term trials has similarly yielded moderate macular thickness reductions with no significant gain in VA.158 One small randomized trial used intravitreal diclofenac 500 µg in one of the treatment arms for patients with DME. DME improved, but VA did not.159 In another small case series, there was no effect on macular edema or VA.160 No further testing has been undertaken. As such, there is currently scant evidence for a benefit of NSAIDs in the treatment or prevention of DME.
Systemic Drug Therapy
Systemic drug therapy for DME and DR has also been relatively under-investigated. Drugs that block the rennin-angiotensin pathway have been one focus. The RASS study showed that the odds of retinopathy progression by two steps or more after 5 years of follow-up in patients with type 1 diabetes was reduced by 65% with enalapril, an angiotensin-converting enzyme inhibitor, and by 70% with losartan, an angiotensin receptor blocker, independently of changes in blood pressure.161 The DIRECT-Prevent 1 trial compared candesartan to placebo in type 1 diabetics without retinopathy with a median follow-up of 4.7 years. A post hoc analysis showed that the adjusted hazard ratio for a three-step increase in incidence of retinopathy was 0·71, 95% CI 0·53–0·95, p=0·046.162 Angiotensin-converting enzyme inhibition with captopril or lisinopril did not reduce the risk of incident diabetic retinopathy in patients with type 1 diabetes, but did retard progression of diabetic retinopathy.163–165 Conversely, in patients with type 1 diabetes, angiotensin II receptor antagonists reduced the risk of incident diabetic retinopathy but did not reduce diabetic retinopathy progression.162,165 High levels of diacylglycerol seen in DM patients have been known to promote activation of protein kinase C (PKC) leading to increased levels of VEGF in retinal vascular tissues. Subsequently, compounds such as Ruboxistaurin have been developed to inhibit the beta isoform of PKC and have shown some efficacy in reduction of vision loss in several large, multicenter, randomized clinical trials.166–168
The US Food and Drug short-term studies of lipoprotein-associated phospholipase A2 inhibitors such as Darapladib have demonstrated only modest improvements in DME reduction and VA gains.169
To summarize, pharmacotherapy for DME has produced the most significant progress in the treatment of the condition compared to laser or surgical therapy. Newer approaches based on other metabolic pathways involved in the pathogenesis of DME and combination approaches targeting multiple pathways simultaneously or sequentially hold promise. Controlling the cost of applying these treatments is a challenge as the burden of DME is increasing with the global rise in obesity and type 2 diabetes mellitus. In situations of incomplete or no response of DME to first-line treatment, recourse is often made to focal laser, intravitreal corticosteroids, and vitrectomy.
Integration of Laser Therapy & Pharmacotherapy
To date, there have been very few clinical trials examining whether integration of laser therapy, either conventional, targeted or in subthreshold mode, with pharmacotherapy can result in a reduced treatment burden for the patient while achieving optimal clinical efficacy. In PDR, some reports have suggested that combination treatment with anti-VEGF and PRP may be superior to monotherapy in terms of NV regression and treatment burden.170–172 As indicated earlier, recent data from protocol S of the DRCR Network demonstrated that both PRP and intravitreal ranibizumab were similar in the prevention of severe visual loss and other complications in PDR suggesting that patient-specific factors such as compliance and financial impact be considered primarily in management decisions.123,173 Protocol I of the DRCR Network demonstrated that there was little short-term benefit in combining prompt macular laser with anti-VEGF therapy for center-involved DME.174 However in that same protocol, patients who were treated with deferred laser therapy achieved the best outcome in terms of sustained visual improvement.
Protocol T of the DRCR Network was a comparative effectiveness trial utilizing bevacizumab, ranibizumab or aflibercept.21 As part of the protocol, laser treatment was mandated for persistent centrally involved macular edema at the 24-weeks follow-up examination following monthly initial treatment by 1 of the 3 agents. At the 1-year follow-up examination, 50% of eyes in the study had received laser treatment because of persistent macular edema. There was a slightly higher percentage of patients receiving laser in the bevacizumab group and a lower percentage in the aflibercept-treated group compared to the 2 other agents. In addition, deferred macular laser was still required in over 30% of study eyes with center-involved DME receiving ranibizumab in the RISE and RIDE studies.174 These studies emphasize the beneficial influence of specific targeted focal and grid laser treatment in eyes not responding initially to anti-VEGF agents alone. In addition, the same trial demonstrated that regardless of the anti-VEGF agent used, there was nearly a 50% reduction in the frequency of needed injections in the subsequent year.106 It remains to be seen if supplemental laser treatment might reduce this treatment burden further. Preliminary data from the DAVE trial examining widefield targeted PRP in conjunction with intravitreal ranibizumab for DME, have demonstrated no significant reduction in the frequency of PRN injections.93,94 Combination therapy with intravitreal corticosteroids has likewise yielded mixed results in terms of both VA stabilization or improvement and reduction in overall treatment burden.175–178 Further studies to explore potential benefits of combination treatment are planned, perhaps involving widefield imaging-guided peripheral laser to ischemic retina and subthreshold technique. Notwithstanding, there are ongoing attempts at publishing guidelines for the integration of laser and pharmacotherapy in DME management in particular.179–181
Surgical Management of Diabetic Retinopathy and Diabetic Macular Edema
Currently, vitrectomy continues to play a critical role in the management of certain scenarios in DR. These include non-clearing vitreous hemorrhages, tractional retinal detachment in PDR, and vitreoretinal interface abnormalities impeding macular edema resolution. Numerous reports over the past 40 years have clearly established the beneficial effect of vitrectomy in these settings.182–191 In theory, the removal of the majority of the vitreous body along with the hyaloid membrane during surgical vitrectomy has been shown to improve retinal oxygenation, increase intraocular cytokine turnover and remove mechanical barriers to the egress of fluid and metabolites as well as removing impediments to the intraretinal penetration of intravitreal administered medications. Debate still exists as to the necessity of ILM removal during vitrectomy for DME. In theory, the removal of the diabetic ILM with its altered histology would be beneficial to the bidirectional flow of chemokines and pharmacological agents within the retina. However, studies to date have not definitively supported that conclusion.192–194 With regards to the use of preoperative anti-VEGF therapy to minimize intraoperative and postoperative hemorrhages, the majority of studies do demonstrate a benefit.195–202 In addition, reduction in operating time and a trend toward better postoperative VA have also been demonstrated in smaller case series.181,203–205 Initial concerns regarding the potential adverse effect of vitrectomy on the durability of intravitreally administered anti-VEGF agents have not been borne out by recent studies.206–209
The exact role of vitrectomy in the management of DME, however, remains incompletely defined at present. Several studies over the past 3 decades have established the anatomical improvements following vitrectomy in recalcitrant DME cases.101,209–216 VA improvements however have not been as consistent and as significant as the reduction in retinal thickness following the procedure.192,203,217–234 This discrepancy between anatomical and functional results of vitrectomy for DME may be due to the inherent postoperative ocular sequelae, such as cataract formation that can confound VA interpretations. Also, surgical intervention continues to be reserved for those cases that have had chronic and severe forms of DME when retinal damage is irreversible thereby biasing the results. Despite these limitations, vitrectomy for DR and DME may be beneficial for certain diabetic patients with specific systemic risk factors.235–237 In addition, vitrectomy for DR and DME is widely used in regions of the world where economic resources are more constrained and even in relatively affluent nations for underinsured patients. The procedure improves VA in certain cases, but its broader role relative to serial anti-VEGF injections has never been established in a randomized-controlled clinical trial.
Future Trends
Diagnostics
New treatments often arise from insights gained with new imaging techniques. Ultra-widefield imaging has allowed clinicians to assess the severity of peripheral ischemia and new software in development is aimed at automatically quantifying and monitoring retinopathy progression in affected areas. As a result, there is an increasing need for revising the existing DR classification system.238 Newer developments in OCT imaging including swept source OCT (SS-OCT) enhanced depth imaging OCT (EDI-OCT), adaptive optics (AO) and OCT angiography (OCTA) are increasingly being used in the management of DR.239–241 These allow for more detailed and rapid imaging of both the retinal and choroidal vasculature. Increasing application of artificial intelligence (AI) techniques such as “Deep Learning” for fundus and OCT images facilitates cost-effective, widespread, diabetic eye screenings via telemedicine.242–245
Newer fundus imaging techniques, such as flavoprotein fluorescence (FPF) may allow the detection of metabolic improvements that precede structural improvements in DME patients receiving anti-VEGF injections.246 Functional testing of macular sensitivity utilizing microperimetry and electroretinography is also being increasingly used in both DR and DME to assess both disease severity and response to therapy.247–262 These provide new indices to explore, seeking prognostic value for response to therapy. In light of recent data demonstrating the incomplete correlation of VA and macular thickness on OCT imaging, non-anatomic diagnostics of macular function will play an increasing role in management of DME.263,264 These new diagnostic modalities will necessitate a redefining of universal DR and DME severity classification beyond the DRS and ETDRS definitions established nearly three decades ago. To that end, several investigators have proposed the inclusion of both temporal and spatial factors, as well as integration of multimodal biomarkers towards the formation of a more comprehensive, and clinically useful classification of DR.265
Therapeutics
Concomitant with the progress in diagnostics has been progressing in therapeutics for DR and DME. Despite the effectiveness of the current anti-VEGF agents in reducing progression of diabetic retinopathy and DME, protocol T of the DRCR Network demonstrated the incidence of persistent DME at 24 weeks to be 65.6% for bevacizumab, 31.6% for aflibercept, and 41.5% for ranibizumab.266 In clinical practice, an analysis of Medicare claims data indicates that approximately 50% of DME patients will have persistent edema after 1 year of anti-VEGF treatment.267 These rates of persistent DME in conjunction with the concomitant need for continuous monitoring call into question the long-term sustainability of such strategies. As such, there is currently a robust amount of research conducted on developing new therapies to deal with these recalcitrant cases. Newer pharmacological agents of potentially increased efficacy and durability are in clinical trials for DR and DME (Table 3).268–270 Also emerging are novel gene therapies for DR management that are in early clinical trial phases.271 Novel anti-VEGF therapies that may be more effective, durable and cheaper than current agents such as conbercept are currently in trial for DME.272 Other agents involving combination therapy, such as Genentech’s anti-VEGF/ANG-2 (RG7716), have yielded favorable efficacy and durability data in recent Phase II studies.273 In addition, optimal strategies for combining anti-VEGF and corticosteroid treatment are still undergoing investigation despite the recent marginal results provided by protocol U of the DRCR network.
Table 3.
Current Investigational Pharmacotherapy for Diabetic Retinopathy and Diabetic Macular Edema(1–4)
| Pharmacological Agent | Mechanism Category | Administration Route | Trial Phase | Sponsor |
|---|---|---|---|---|
| Abicipar pegol | DARPin | IVit | II | Allergan |
| AKB-9778 | Tie2 agonist | SC | II | Aerpio Therapeutics |
| ALG-1001 | Integrin receptor antagonist | IVit | II | Allegro Ophthalmics |
| Alpha lipoic acid | Antioxidant | PO | III | Ludwig-Maximilians University of Munich |
| Aminoguanidine | AGE Inhibitor | PO | I | University of Minnesota |
| AR-13503 | Rho kinase & Protein kinase C inhibitor | IVit | I | Aerie Pharmaceuticals |
| ASP8232 | Vascular adhesion protein 1 inhibitor | PO | II | Astellas Pharma |
| Bevasiranib (Cand5) | siRNA silencing of VEGF mRNA | IVit | II | Opko Health, Inc. |
| Betamethasone (DE-102) | Corticosteroid | ST | III | Santen Pharmaceutical Co. |
| BI 1026706 | Bradykinin 1 Antagonist | PO | II | Boehringer Ingelheim |
| BI 1467335 | SSAO (VAP-1) Inhibitor | PO | IIa | Boehringer Ingelheim |
| Brimonidine | Neuroprotection | Top | III | European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR) |
| Brolucizumab | Anti-VEGF | IVit | III | Novartis Pharmaceuticals |
| Bromocriptine, | Dopaminergic | PO | I/II | University of Southern California |
| Candesartan | Angiotensin receptor blocker | PO | III | AstraZeneca |
| Celecoxib | COX-2 inhibitor | Top | I | University of Coimbra |
| Choline fenofibrate | Triglyceride reduction | PO | II | Abbott |
| Conbercept | Anti-VEGF Anti-PlGF |
IVit | III | Chengdu Kanghong Biotech Co |
| Danazol | Androgenic vascular permeability modulator | PO | II/III | Ampio Pharmaceuticals |
| Darapladib | Phospholipase CA2 inhibitor | PO | II | GlaxoSmithKline |
| Dextromethorphan | NMDA receptor antagonism, insulinogenic | PO | II | NEI |
| Diclofenac | NSAID | IVit | IIa | Shahid Beheshti University of Medical Sciences |
| Doxycycline | Anti-inflammatory | PO | I/II | NEI |
| DS-7080a | Anti-angiogenic mAb | IVit | I/II | Daiichi Sankyo, Inc. |
| EBI-031 | Anti-interleukin-6 antibody | IVit | I | Eleven Biotherapeutics (Sesen Bio) |
| Emixustat | RPE65 inhibition | PO | II | Acucela Inc. |
| Empagliflozin | SGLT2 inhibitor | PO | IV | Hannover Medical School |
| Faricimab | Anti-VEGF & Ang-2 inhibitor | IVit | II | Roche/Genentech |
| Fasudil | Rho-kinase inhibitor | IVit | II | Shahid Beheshti University of Medical Sciences |
| Fenofibrate | PPARalpha agonist | PO | IV | University of Padova |
| Folic acid, B6, B12 | Antioxidant | PO | IV | University of Catania |
| FOV2304 | Kinin β1 receptor Antagonist (anti-angiogenic) | Top | II | Fovea Pharmaceuticals |
| GB-102 | pan-VEGF inhibitor | IVit | IIa | Graybug Vision |
| iCo-007 | Anti-sense c-RAFkinase | IVit | II | iCo Therapeutics |
| Infliximab | Anti-TNF alpha mAb | IVit | I | Retina Research Foundation |
| Ketorolac | NSAID (coxib) | Top | I | Roche |
| KP-121 | Corticosteroid | Top | II | Kala Pharmaceuticals |
| KSI-301 | Anti-VEGF Biopolymer | IVit | Ib | Kodiak Sciences |
| KVD001 | Plasma kallikrein inhibitor | IVit | II | Kalvista Pharmaceuticals |
| Levosulpiride | Dopamine D2 receptor blocker | PO | III | National University of Mexico (UNAM) |
| LKA651 | Anti-erythropoietin | IVit | I | Novartis |
| Luminate (Risuteganib) | Integrin receptor antagonist | IVit | III | Allegro Ophthalmics |
| Mecamylamine | nACh antagonist | Top | II | CoMentis |
| Minocycline | Anti-microglial (anti-inflammatory) | PO | III | NEI |
| MTP-131 | Mitochondrial cardiolipin promoter | Top | I/II | Stealth Biotherapeutics |
| Nutritional supplements | Anti-oxidative stress | PO | II | Mid-Atlantic Retinal Consultants |
| Ocriplasmin | PVD Induction | IVit | I | ThromboGenics |
| Octreotide | GH inhibitor | SC | III | Novartis |
| OC-10X | Tubulin inhibitor | Top | I | OcuCure Therapeutics |
| Opt-302 | VEGF-r/Fc-fusion | IVit | II | Opthea |
| PAN-90806 | VEGF2R tyrosine kinase inhibitor | Top | II | PanOptica |
| Pemafibrate | PPARalpha agonist | PO | III | Jaeb Center for Health Research |
| PF-04523655 | siRNA against RTP801 (antiangiogenic) | IVit | II | Quark Pharmaceuticals |
| REGN910-3 (nesvacumab) | Anti-angiopoietin 2 mAb | IVit | II | Regeneron/Bayer |
| RO6867461 | bi-specific anti-VEGF/antiangiopoietin 2 | IVit | II | Hoffman-LaRoche |
| Ruboxistaurin | PKC-β inhibitor | PO | III | Chromaderm, Inc. & Eli Lilly & Co |
| SF0166 | alphaVbeta3 integrin inhibitor | Top | I/II | SciFluor |
| Sirolimus | Anti-IL-2 (mTOR inhibitor) |
IVit/SConj | II | Santen Pharmaceutical & NEI |
| Somatostatin | Neuroprotection | Top | III | European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR) |
| Sorbinil | Aldose Reductase inhibitor |
PO | III | NEI |
| Squalamine | Anti-angiogenic | Top | II | Elman Retina Group |
| Sulodexide | Glycosaminoglycan analogue | PO | II | DRESS Research Group |
| Teprotumumab | IGF-1 receptor antagonist | IV | I | River Vision Development Corporation |
| TG100801 | Anti-angiogenic | Top | I | TargeGen |
| THR-149 | Plasma kallikrein inhibitor | IVit | I | Oxurion (ThromboGenics) |
| THR-317 | Anti-PIGF mAb | IVit | II | Oxurion (ThromboGenics) |
| Tocilizumab | Anti-IL-6 | IVit | II | University of Nebraska |
| Ubiquinone | Antioxidant | PO | IIa | University of Guadalajara, Mexico |
| Ziv-aflibercept | Anti-VEGF | IVit | II | Shahid Beheshti University of Medical Sciences |
Abbreviations: IV, Intravenous; IVit, Intravitreal; PO, Oral; SC, Subcutaneous; SConj, Subconjunctival; ST, Sub-tenons; Top, Topical.
Alternate, non-VEGF strategies for DR and DME management are also being developed at a rapid pace. Among these are drugs targeting a central regulator, such as Raf kinase, mammalian target of rapamycin (mTOR) and the RTP 801 gene.274–276 A Phase I/II study of an ankyrin repeat protein that binds VEGF reduced DME when injected intravitreally in several patients with a duration of effect of 8–12 weeks but had a tendency to cause iritis.277 A modified version designed to eliminate the problem of iritis is undergoing further clinical testing.
Platelet-derived growth factor (PDGF) inhibitors are also being currently investigated as potential DR therapies.278 A monoclonal antibody directed against the receptor-binding site of human placental growth factor (PLGF) developed by Thrombogenics (THR-317) is currently being investigated for DME treatment in the THR-001 study. Recent results from this Phase I/II study appear promising. Stealth Biotherapeutics has also explored subcutaneous injection, among other methods of delivery, of elamipretide, a mitochondrial therapy.
Anti-integrin therapeutic agents, such as Allegro Ophthalmics peptide, risuteganib (Luminate) and SciFluor Life Sciences’s SF0166, inhibit the oxidative stress process that is responsible for initiating DME and are also undergoing clinical trials in this setting.
Anti-Inflammatory Agents
Chronic inflammation contributes to the pathophysiology of both DR and DME. To that end, several novel therapeutic targets have been identified for this disease targeting those processes that release cytokines and chemokines. These include direct and indirect antagonism of interleukins, proteases, chemokines, tumor necrosis factor (TNF), angiopoietin-2 (ANGPT-2) and kallikrein. Currently, there are no active clinical trials for DR and DME involving interleukins or proteases. One of the potent mediators of both inflammation and breakdown of the blood retinal barrier is the chemokine ligand, CCL2.279 A CCR2/5 receptor antagonist (Pf-04634817) (Pfizer) was recently tested in patients with DME.280 Another compound implicated in many systemic inflammatory diseases as well as DR is TNF. A clinical trial with infliximab, the monoclonal antibody antagonist of alpha (TNF-α), in patients with persistent DME demonstrated significant improvement in both VA and overall reduction in retinal thickening.281,282
Angiopoietin-2 is another potent mediator of increased vascular permeability in DR. This growth factor achieves most of its biological effect by binding to the endothelial cell receptor tyrosine kinase Tie-2. A Phase I investigation of a competitive inhibitor of vascular endothelial-protein tyrosine phosphatase that promotes Tie2 activation and reduces vascular leakage in animal models showed no safety signal of concern and led to reduction in DME in a few cases.283 A Phase II trial is planned. A recent study of the Tie-2 activator AKB 9778 (Aerpio Therapeutics) in conjunction with ranibizumab demonstrated significantly greater reduction in retinal thickening in patients with DME than that seen with suppression of VEGF alone.284 Another important mediator of increased vascular permeability in DR and DME is activation of the plasma kallikrein–kinin pathway.285 Recent results from a Phase I study of the plasma kallikrein inhibitor KVD001 (KalVista Pharmaceuticals) for the treatment of central involved DME showed that not only was the compound well tolerated but also it had significant effects on VA improvement and retinal thickening reduction.286
Drug Delivery Strategies
In an attempt to decrease the therapeutic burden of monthly intravitreal injections, several novel strategies are currently being examined. These mainly fall into one of five categories: non-biodegradable polymeric drug delivery systems, biodegradable polymeric drug delivery systems, nanoparticle-based drug delivery systems, ocular injection devices and sustained release refillable devices. Non-biodegradable polymeric drug delivery systems include such devices as the Iluvien® and Retisert® implants which can release fluocinolone acetonide in the vitreous cavity for up to 3 years.287 Also in this category is the encapsulated cell technology (ECT) Renexus™ device, which utilizes recombinant RPE (NTC-200) cell lines to continuously secrete antibodies, fusion proteins and growth factors in the vitreous cavity. Recently, an ECT system utilizing genetically engineered ARPE-19 cells secreting soluble vascular endothelial growth factor receptor 1 (sVEGFR1) was developed with the hope of reducing intravitreal VEGF levels for an extended period.288 Another novel device in development in this category is the ODTx™ (On Demand Therapeutics) implant which is an intravitreally injected rod containing multiple reservoirs of drugs that are each able to be separately activated using a laser beam. This allows for a long-term, noninvasive, control of drug delivery within the eye for a period of several months.
Biodegradable polymeric drug delivery systems such as the dexamethasone containing Ozurdex™ (Allergan) implant have now been in use for several years in the management of DME. An increasingly popular approach for sustained drug delivery is the use of drug-laden microspheres. Currently, the betamethasone containing DE-102 (Santen) and the ranibizumab (Genentech) containing microspheres are in various stages of clinical trials for DME.
Nanoparticle-based drug delivery systems include entities such as liposomes, microspheres, nanospheres, emulsions and dendrimers. Bevacizumab encapsulated liposomes have been demonstrated to have significant sustained drug release at nearly 2 months following injection. Micro- and nanospheres are synthetic biodegradable polymers that allow for a slow, controlled release of the bound medication. Microparticles incorporating ranibizumab have been developed allowing therapeutic levels of the drug to be present for up to 6 months following a single administration.289–291 Emulsion-based drug delivery involves the admixture of a lipid-soluble agent with a drug of interest to extend its duration within the intravitreal space. One example of this approach is the Verisome drug-delivery platform (Icon Bioscience) which involves mixtures of triamcinolone acetate or ranibizumab injected intravitreally. So far this has only been tested in CME from retinal vein occlusions and as adjunctive therapy in ARMD. The Cortiject™ (Santen Pharmaceutical) system involves an injectable dexamethasone prodrug emulsion. Studies of both these agents for DR and DME are still pending.
Another interesting emulsion based, drug delivery system, is the topically applied, antisense oligonucleotide, aganirsen (GS-101, GeneSignal International SA). This inhibitor of insulin receptor substrate-1 expression is able to achieve effective VEGF inhibition at the retinal level for as long as 8 hrs following a single application. Dendrimers, so named because of their unique “hub and spoke” structure of a central core with radiating side chain moieties “dendrons” are very effective hydrophobic drug carriers. So far, they are still being tested at the in vitro stage for inhibition of VEGF expression with intravitreally administered antisense oligonucleotide compounds.292–296
Novel ocular injection devices, such as the iTrack microcatheter (iScience Interventional), allow the administration of various medications in the suprachoroidal space to allow for a sequestered, sustained release of drug. Recently, a combination of bevacizumab and triamcinolone was delivered via the iTrack device to the submacular area in a pilot study of patients with chronic ME and HEs.297 There was a significant and sustained improvement in both VA and in resolution of both the ME and HEs in the majority of subjects, with no significant complications for at least 1 year of the start of the study. Another sustained delivery, nonbiodegradable device for triamcinolone is the I-vation intravitreal implant (Surmodics Inc.) with a duration of 2 years. Other approaches to injecting medications into the suprachoroidal space involve devices employing 0.5–1 mm length microneedles such as the CLS1001 (Clearside Biomedical Inc.). An ongoing clinical trial of this device utilizing triamcinolone acetonide is being conducted in patients with noninfectious, uveitic, retinal vein occlusion related ME as well as DME.298,299
Sustained release, refillable devices hold the promise of a more steady and sustained intravitreal drug delivery via an infrequently replenished reservoir. Currently, there are 2 systems being investigated for retinal disease, the Posterior MicroPump™ (Replenish Inc.) and the Port Delivery System (PDS) (ForSight Vision 4 Inc.). Although these devices are currently being investigated for treatment of ARMD, as of yet, there are no ongoing preclinical trials for DR or DME.300,301
Conclusion
New imaging techniques and the ability to identify and quantitate aspects of DR will influence when to initiate treatment and when to re-treat. Pharmacotherapy, both ocular and systemic, has become the primary mode of intervention in the management of DR and DME. Conventional laser therapy has become a secondary intervention in DME, and perhaps will take that role for PDR. Subthreshold laser treatment has promising characteristics, but requires more rigorous investigation. What remains lacking is the optimal integration strategy for these treatment modalities. The goal remains to achieve the greatest reduction in clinical disease, in the shortest time, with the least amount of side effects, with the greatest duration and in the most cost-effective fashion. Testing hypothetical strategies will require sound clinical trials conducted by a consortium of large, independent entities such as the DRCR network.
Disclosure
Dr Sam Mansour reports personal fees from Iridex Corporation, Alimera Sciences, Inc., from Chengdu Kanghong Biotech Co., Novartis International AG, and personal non-financial support from Diopsys, Inc. during the conduct of the study, and personal fees from Alcon, outside the submitted work. Dr David Browning reports grants from regeneron, drcr network, novartis, and other from springer and zeiss outside the submitted work. Dr Abdhish Raman Bhavsar reports grants from DRCR and other from Allergay, outside the submitted work. No funding for this work has been received from any public or private entities, including: National Institutes of Health (NIH); Wellcome Trust; Howard and Hughes Medical Institute (HHMI). No author of the present work has any proprietary interest in the subject matter of this manuscript and the authors report no other conflicts of interest in this work.
References
- 1.International Diabetes Federation. Diabetes Atlas. 9th ed; 2019. [Google Scholar]
- 2.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17. doi: 10.1186/s40662-015-0026-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Global Report on Diabetes; 2016:88 [Google Scholar]
- 5.CDC. Centers for Disease Control and Prevention. Vision Health Initiatives (VHI). Economic Studies. Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 6.National Center for Chronic Disease Prevention and Health Promotion. National Diabetes Statistics Report, 2017: Estimates of Diabetes and Its Burden in the United States Prevention; 2017. [Google Scholar]
- 7.American Diabetes A. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol. 1976;81:383–396. doi: 10.1016/0002-9394(76)90292-0 [DOI] [PubMed] [Google Scholar]
- 9.The Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema: early treatment diabetic retinopathy study report. Int Ophthalmol Clin. 1987;27(4):265–272. doi: 10.1097/00004397-198702740-00006 [DOI] [PubMed] [Google Scholar]
- 10.Early Treatment Diabetic Retinopathy Study Research Group. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early treatment diabetic retinopathy study report number 2. Ophthalmology. 1987;94(7):761–774. doi: 10.1016/S0161-6420(87)33527-4 [DOI] [PubMed] [Google Scholar]
- 11.Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991;98(5Suppl):766–785. doi: 10.1016/S0161-6420(13)38011-7 [DOI] [PubMed] [Google Scholar]
- 12.Early Treatment Diabetic Retinopathy Study research group. Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Arch Ophthalmol. 1985;103(12):1796–1806. doi: 10.1001/archopht.1985.01050120030015 [DOI] [PubMed] [Google Scholar]
- 13.7Smith RE, Patz A. Diabetes 2000 – closing the gap. Ophthalmology. 1990;97(2):153–154. doi: 10.1016/S0161-6420(90)32612-X [DOI] [PubMed] [Google Scholar]
- 14.Hazin R, Colyer M, Lum F, Barazi MK. Revisiting diabetes 2000: challenges in establishing nationwide diabetic retinopathy prevention programs. Am J Ophthalmol. 2011;152(5):723–729. doi: 10.1016/j.ajo.2011.06.022 [DOI] [PubMed] [Google Scholar]
- 15.Bressler SB, Qin H, Melia M, et al. Diabetic retinopathy clinical research network: exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial. JAMA Ophthalmol. 2013;131(8):1033–1040. doi: 10.1001/jamaophthalmol.2013.4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115(9):1447–1449, 1449 e1441–1410. doi: 10.1016/j.ophtha.2008.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bressler SB, Glassman AR, Almukhtar T, et al. Diabetic retinopathy clinical research network: five year outcomes of ranibizumab with prompt or deferred laser versus laser or triamcinolone plus deferred ranibizumab for diabetic macular edema. Am J Ophthalmol. 2016;164:57–68. doi: 10.1016/j.ajo.2015.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elman MJ, Aiello LP, Beck RW, et al; Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077 e1035. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elman MJ, Qin H, Aiello LP, et al; Diabetic Retinopathy Clinical Research Network. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119(11):2312–2318. doi: 10.1016/j.ophtha.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott IU, Edwards AR, Beck RW, et al.; Diabetic Retinopathy Clinical Research Network. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114(10):1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–1203. doi: 10.1056/NEJMoa1414264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The DRVS Research Group. Two-year course of visual acuity in severe proliferative diabetic retinopathy with conventional management. Diabetic Retinopathy Vitrectomy Study (DRVS) report #1. Ophthalmology. 1985;92(4):492–502. [DOI] [PubMed] [Google Scholar]
- 23.The Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Two-year results of a randomized trial. Diabetic retinopathy vitrectomy study report 2. Arch Ophthalmol. 1985;103(11):1644–1652. doi: 10.1001/archopht.1985.01050110038020 [DOI] [PubMed] [Google Scholar]
- 24.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 25.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Group DER, et al. Intensive diabetes therapy and ocular surgery in type 1 diabetes. N Engl J Med. 2015;372:1722–1733. doi: 10.1056/NEJMoa1409463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853. doi: 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 28.Kohner EM, Stratton IM, Aldington SJ, Holman RR, Matthews DR. Relationship between the severity of retinopathy and progression to photocoagulation in patients with Type 2 diabetes mellitus in the UKPDS (UKPDS 52). Diabet Med. 2001;18(3):178–184. doi: 10.1046/j.1464-5491.2001.00458.x [DOI] [PubMed] [Google Scholar]
- 29.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bressler SB, Odia I, Maguire MG, et al. Factors associated with visual acuity and central subfield thickness changes when treating diabetic macular edema with anti-vascular endothelial growth factor therapy: an exploratory analysis of the protocol T randomized clinical trial. JAMA Ophthalmol. 2019;137(4):382–389. doi: 10.1001/jamaophthalmol.2018.6786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chew EY, Ambrosius WT, Howard LT, et al. Rationale, design, and methods of the Action to Control Cardiovascular Risk in Diabetes Eye study (ACCORD-EYE). Am J Cardiol. 2007;99(12A):103i–111i. doi: 10.1016/j.amjcard.2007.03.028 [DOI] [PubMed] [Google Scholar]
- 32.Chew EY, Ambrosius WT, Davis MD, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–430. doi: 10.1016/S0140-6736(10)60576-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerstein HC, Ambrosius WT, Danis R, et al. Diabetic retinopathy, its progression, and incident cardiovascular events in the ACCORD trial. Diabetes Care. 2013;36(5):1266–1271. doi: 10.2337/dc12-1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chew EY, Davis MD, Danis RP, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) eye study. Ophthalmology. 2014;121(12):2443–2451. doi: 10.1016/j.ophtha.2014.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Action to Control Cardiovascular Risk in Diabetes Follow-On Eye Study G, the Action to Control Cardiovascular Risk in Diabetes Follow-On Study G. Persistent effects of intensive glycemic control on retinopathy in type 2 diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) follow-on study. Diabetes Care. 2016;39(7):1089–1100. doi: 10.2337/dc16-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370(9600):1687–1697. doi: 10.1016/S0140-6736(07)61607-9 [DOI] [PubMed] [Google Scholar]
- 38.Wong TY, Simo R, Mitchell P. Fenofibrate – a potential systemic treatment for diabetic retinopathy? Am J Ophthalmol. 2012;154(1):6–12. doi: 10.1016/j.ajo.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 39.Pradhan AD, Paynter NP, Everett BM, et al. Rationale and design of the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study. Am Heart J. 2018;206:80–93. doi: 10.1016/j.ahj.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 40.Shi R, Zhao L, Wang F, et al. Effects of lipid-lowering agents on diabetic retinopathy: a meta-analysis and systematic review. Int J Ophthalmol. 2018;11(2):287–295. doi: 10.18240/ijo.2018.02.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang EY, Chen TH, Garg SJ, et al. Association of statin therapy with prevention of vision-threatening diabetic retinopathy. JAMA Ophthalmol. 2019;137(4):363–371. doi: 10.1001/jamaophthalmol.2018.6399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sala-Vila A, Diaz-Lopez A, Valls-Pedret C, et al. Dietary marine omega-3 fatty acids and incident sight-threatening retinopathy in middle-aged and older individuals with type 2 diabetes: prospective investigation from the PREDIMED trial. JAMA Ophthalmol. 2016;134(10):1142–1149. doi: 10.1001/jamaophthalmol.2016.2906 [DOI] [PubMed] [Google Scholar]
- 43.Chous AP, Richer SP, Gerson JD, Kowluru RA. The Diabetes Visual Function Supplement Study (DiVFuSS). Br J Ophthalmol. 2016;100(2):227–234. doi: 10.1136/bjophthalmol-2014-306534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gargiulo P, Savarese G, D’Amore C, et al. Efficacy and safety of glucagon-like peptide-1 agonists on macrovascular and microvascular events in type 2 diabetes mellitus: a meta-analysis. Nutr Metab Cardiovasc Dis. 2017;27(12):1081–1088. doi: 10.1016/j.numecd.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 45.Moreira RO, Cobas R. Lopes assis Coelho RC: combination of basal insulin and GLP-1 receptor agonist: is this the end of basal insulin alone in the treatment of type 2 diabetes? Diabetol Metab Syndr. 2018;10:26. doi: 10.1186/s13098-018-0327-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang ZX, Cao JX, Li D, et al. Clinical efficacy of autologous stem cell transplantation for the treatment of patients with type 2 diabetes mellitus: a meta-analysis. Cytotherapy. 2015;17(7):956–968. doi: 10.1016/j.jcyt.2015.02.014 [DOI] [PubMed] [Google Scholar]
- 47.Yang HK, Yoon KH. Current status of encapsulated islet transplantation. J Diabetes Complications. 2015;29(5):737–743. doi: 10.1016/j.jdiacomp.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 48.Zhao Y, Jiang Z, Zhao T, et al. Reversal of type 1 diabetes via islet beta cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012;10:3. doi: 10.1186/1741-7015-10-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallner K, Shapiro AM, Senior PA, McCabe C. Cost effectiveness and value of information analyses of islet cell transplantation in the management of ‘unstable’ type 1 diabetes mellitus. BMC Endocr Disord. 2016;16:17. doi: 10.1186/s12902-016-0097-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanazaki K, Munekage M, Kitagawa H, et al. Current topics in glycemic control by wearable artificial pancreas or bedside artificial pancreas with closed-loop system. J Artif Organs. 2016;19(3):209–218. doi: 10.1007/s10047-016-0904-y [DOI] [PubMed] [Google Scholar]
- 51.Karageorgiou V, Papaioannou TG, Bellos I, et al. Effectiveness of artificial pancreas in the non-adult population: a systematic review and network meta-analysis. Metabolism. 2018. [DOI] [PubMed] [Google Scholar]
- 52.Thabit H, Hovorka R. Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia. 2016;59(9):1795–1805. doi: 10.1007/s00125-016-4022-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(7):501–512. doi: 10.1016/S2213-8587(17)30167-5 [DOI] [PubMed] [Google Scholar]
- 54.Iqbal A, Novodvorsky P, Heller SR. Recent updates on type 1 diabetes mellitus management for clinicians. Diabetes Metab J. 2018;42(1):3–18. doi: 10.4093/dmj.2018.42.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fong DS, Strauber SF, Aiello LP, et al; Writing Committee for the Diabetic Retinopathy Clinical Research N. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125(4):469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berger A, Sheidow T, Cruess AF, Arbour JD, Courseau AS, de Takacsy F. Efficacy/safety of ranibizumab monotherapy or with laser versus laser monotherapy in DME. Can J Ophthalmol. 2015;50(3):209–216. doi: 10.1016/j.jcjo.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 57.Chen G, Li W, Tzekov R, Jiang F, Mao S, Tong Y. Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema: a meta-analysis of randomized controlled trials. PLoS One. 2014;9(12):e115797. doi: 10.1371/journal.pone.0115797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishibashi T, Li X, Koh A, et al. The REVEAL study: ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian patients with diabetic macular edema. Ophthalmology. 2015;122(7):1402–1415. doi: 10.1016/j.ophtha.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 59.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–625. doi: 10.1016/j.ophtha.2011.01.031 [DOI] [PubMed] [Google Scholar]
- 60.Striph GG, Hart WM Jr., Olk RJ. Modified grid laser photocoagulation for diabetic macular edema. The effect on the central visual field. Ophthalmology. 1988;95(12):1673–1679. [DOI] [PubMed] [Google Scholar]
- 61.Sims LM, Stoessel K, Thompson JT, Hirsch J. Assessment of visual-field changes before and after focal photocoagulation for clinically significant diabetic macular edema. Ophthalmologica. 1990;200(3):133–141. doi: 10.1159/000310094 [DOI] [PubMed] [Google Scholar]
- 62.Rohrschneider K, Bultmann S, Gluck R, Kruse FE, Fendrich T, Volcker HE. Scanning laser ophthalmoscope fundus perimetry before and after laser photocoagulation for clinically significant diabetic macular edema. Am J Ophthalmol. 2000;129(1):27–32. doi: 10.1016/S0002-9394(99)00270-6 [DOI] [PubMed] [Google Scholar]
- 63.Lee HJ, Kang TS, Kwak BS, Jo YJ, Kim JY. Long-term effect of panretinal photocoagulation on spectral domain optical coherence tomography measurements in diabetic retinopathy. Curr Eye Res. 2017;42(8):1169–1173. doi: 10.1080/02713683.2017.1280510 [DOI] [PubMed] [Google Scholar]
- 64.Fong DS, Girach A, Boney A. Visual side effects of successful scatter laser photocoagulation surgery for proliferative diabetic retinopathy: a literature review. Retina. 2007;27(7):816–824. doi: 10.1097/IAE.0b013e318042d32c [DOI] [PubMed] [Google Scholar]
- 65.Seiberth V, Alexandridis E, Feng W. Function of the diabetic retina after panretinal argon laser coagulation. Graefes Arch Clin Exp Ophthalmol. 1987;225:385–390. doi: 10.1007/BF02334163 [DOI] [PubMed] [Google Scholar]
- 66.Russell PW, Sekuler R, Fetkenhour C. Visual function after pan-retinal photocoagulation: a survey. Diabetes Care. 1985;8:57–63. doi: 10.2337/diacare.8.1.57 [DOI] [PubMed] [Google Scholar]
- 67.Akduman L, Olk RJ. Subthreshold (invisible) modified grid diode laser photocoagulation in diffuse diabetic macular edema (DDME). Ophthalmic Surg Lasers. 1999;30:706–714. [PubMed] [Google Scholar]
- 68.Friberg TR. Subthreshold (invisible) modified grid diode laser photocoagulation and diffuse diabetic macular edema (DDME). Ophthalmic Surg Lasers. 1999;30:705. [PubMed] [Google Scholar]
- 69.Friberg TR. Infrared micropulsed laser treatment for diabetic macular edema–subthreshold versus threshold lesions. Semin Ophthalmol. 2001;16:19–24. doi: 10.1076/soph.16.1.19.4217 [DOI] [PubMed] [Google Scholar]
- 70.Dorin G. Subthreshold and micropulse diode laser photocoagulation. Semin Ophthalmol. 2003;18:147–153. doi: 10.1076/soph.18.3.147.29812 [DOI] [PubMed] [Google Scholar]
- 71.Laursen ML, Moeller F, Sander B, et al. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol. 2004;88:1173–1179. doi: 10.1136/bjo.2003.040949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luttrull JK, Musch DC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89:74–80. doi: 10.1136/bjo.2004.051540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iwami H, Pruessner J, Shiraki K, Brinkmann R, Miura Y. Protective effect of a laser-induced sub-lethal temperature rise on RPE cells from oxidative stress. Exp Eye Res. 2014;124:37–47. doi: 10.1016/j.exer.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 74.Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92(5):2177–2186. doi: 10.1152/japplphysiol.01267.2001 [DOI] [PubMed] [Google Scholar]
- 75.Miura Y, Treumer F, Klettner A, et al. VEGF and PEDF secretions over time following various laser irradiations on an RPE organ culture. Invest Ophthalmol Vis Sci. 2010;51:469. [Google Scholar]
- 76.Sramek C, Mackanos M, Spitler R, et al. Non-damaging retinal phototherapy: dynamic range of heat shock protein expression. Invest Ophthalmol Vis Sci. 2011;52(3):1780–1787. doi: 10.1167/iovs.10-5917 [DOI] [PubMed] [Google Scholar]
- 77.Sivaprasad S, Sandhu R, Tandon A, et al. Subthreshold micropulse diode laser photocoagulation for clinically significant diabetic macular oedema: a three-year follow up. Clin Experiment Ophthalmol. 2007;35:640–644. doi: 10.1111/ceo.2007.35.issue-7 [DOI] [PubMed] [Google Scholar]
- 78.Kumar V, Ghosh B, Raina UK, et al. Subthreshold diode micropulse panretinal photocoagulation for proliferative diabetic retinopathy. Eye. 2009;23:2122–2123;author reply 2123. doi: 10.1038/eye.2008.416 [DOI] [PubMed] [Google Scholar]
- 79.Luttrull JK, Dorin G. Subthreshold Diode Micropulse laser photocoagulation (SDM) as invisible retinal phototherapy for diabetic macular edema: a review. Curr Diabetes Rev. 2012;8:274–284. doi: 10.2174/157339912800840523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sivaprasad S, Dorin G. Subthreshold diode laser micropulse photocoagulation for the treatment of diabetic macular edema. Expert Rev Med Devices. 2012;9:189–197. doi: 10.1586/erd.12.1 [DOI] [PubMed] [Google Scholar]
- 81.Vujosevic S, Martini F, Convento E, et al. Subthreshold laser therapy for diabetic macular edema: metabolic and safety issues. Curr Med Chem. 2013;20:3267–3271. doi: 10.2174/09298673113209990030 [DOI] [PubMed] [Google Scholar]
- 82.Luttrull JK, Sinclair SH. Safety of transfoveal subthreshold diode micropulse laser for fovea-involving diabetic macular edema in eyes with good visual acuity. Retina. 2014;34:2010–2020. doi: 10.1097/IAE.0000000000000177 [DOI] [PubMed] [Google Scholar]
- 83.Parodi MB, Iacono P, Bandello F. Subthreshold grid laser versus intravitreal bevacizumab as second-line therapy for macular edema in branch retinal vein occlusion recurring after conventional grid laser treatment. Graefes Arch Clin Exp Ophthalmol. 2014. [DOI] [PubMed] [Google Scholar]
- 84.Lavinsky D, Cardillo JA, Melo LAS, et al. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Investig Ophthalmol Vis Sci. 2011;52:4314–4323. doi: 10.1167/iovs.10-6828 [DOI] [PubMed] [Google Scholar]
- 85.Lavinsky D, Sramek C, Wang J, et al. Subvisible retinal laser therapy: titration algorithm and tissue response. Retina. 2014;34:87–97. doi: 10.1097/IAE.0b013e3182993edc [DOI] [PubMed] [Google Scholar]
- 86.Moutray T, Evans JR, Lois N, Armstrong DJ, Peto T, Azuara-Blanco A. Different lasers and techniques for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2018;3:CD012314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kernt M, Cheuteu RE, Cserhati S, et al. Pain and accuracy of focal laser treatment for diabetic macular edema using a retinal navigated laser (Navilas). Clin Ophthalmol. 2012;6:289–296. doi: 10.2147/OPTH.S27859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kozak I, Kim JS, Oster SF, Chhablani J, Freeman WR. Focal navigated laser photocoagulation in retinovascular disease: clinical results in initial case series. Retina. 2012;32(5):930–935. doi: 10.1097/IAE.0b013e318227ab5b [DOI] [PubMed] [Google Scholar]
- 89.Kozak I, Oster SF, Cortes MA, et al. Clinical evaluation and treatment accuracy in diabetic macular edema using navigated laser photocoagulator NAVILAS. Ophthalmology. 2011;118(6):1119–1124. doi: 10.1016/j.ophtha.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 90.Chhablani J, Mathai A, Rani P, Gupta V, Arevalo JF, Kozak I. Comparison of conventional pattern and novel navigated panretinal photocoagulation in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2014;55(6):3432–3438. doi: 10.1167/iovs.14-13936 [DOI] [PubMed] [Google Scholar]
- 91.Barteselli G, Kozak I, El-Emam S, Chhablani J, Cortes MA, Freeman WR. 12-month results of the standardised combination therapy for diabetic macular oedema: intravitreal bevacizumab and navigated retinal photocoagulation. Br J Ophthalmol. 2014;98(8):1036–1041. doi: 10.1136/bjophthalmol-2013-304488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liegl R, Langer J, Seidensticker F, et al. Comparative evaluation of combined navigated laser photocoagulation and intravitreal ranibizumab in the treatment of diabetic macular edema. PLoS One. 2014;9(12):e113981. doi: 10.1371/journal.pone.0113981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown DM: DAVE trial (Diabetic Anti-VEGF): widefield guided panretinal photocoagulation for diabetic macular edema: eighteen-month results. American Academy of Ophthalmology Annual Meeting: Subspecialty Day. vol. Section XV: First-time Results of Clinical Trials, Part II, Las Vegas, NV; 2015: 129. [Google Scholar]
- 94.Brown DM, Ou WC, Wong TP, et al. Targeted retinal photocoagulation for diabetic macular edema with peripheral retinal nonperfusion: three-year randomized DAVE trial. Ophthalmology. 2018;125:683–690. doi: 10.1016/j.ophtha.2017.11.026 [DOI] [PubMed] [Google Scholar]
- 95.Suner IJ, Peden MC, Hammer ME, Grizzard WS, Traynom J, Cousins SW. RaScaL: a pilot study to assess the efficacy, durability, and safety of a single intervention with ranibizumab plus peripheral laser for diabetic macular edema associated with peripheral nonperfusion on ultrawide-field fluorescein angiography. Ophthalmologica. 2014. [DOI] [PubMed] [Google Scholar]
- 96.Baker CW, Glassman AR, Beaulieu WT, et al. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA. 2019;321(19):1880–1894. doi: 10.1001/jama.2019.5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peto T, Chakravarthy U. New findings from diabetic retinopathy clinical research retina network protocol V confirm a role for focal laser photocoagulation or observation for eyes with center-involved diabetic macular edema and good visual acuity: new is not always best. JAMA Ophthalmol. 2019;137(7):838–839. doi: 10.1001/jamaophthalmol.2019.1876 [DOI] [PubMed] [Google Scholar]
- 98.Chew EY. Patients with good vision and diabetic macular edema involving the center of the macula: to treat or not to treat? JAMA. 2019;321(19):1873–1875. doi: 10.1001/jama.2019.5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Browning DJ. Diabetic Retinopathy: Evidence-Based Management New York. Springer; 2010. [Google Scholar]
- 100.Jonas JB, Sofker A. Intraocular injection of crystalline cortisone as adjunctive treatment of diabetic macular edema. Am J Ophthalmol. 2001;132(3):425–427. doi: 10.1016/S0002-9394(01)01010-8 [DOI] [PubMed] [Google Scholar]
- 101.Lewis H, Abrams GW, Blumenkranz MS, Campo RV. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology. 1992;99(5):753–759. doi: 10.1016/S0161-6420(92)31901-3 [DOI] [PubMed] [Google Scholar]
- 102.Cunningham ET Jr., Adamis AP, Altaweel M, et al. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112(10):1747–1757. [DOI] [PubMed] [Google Scholar]
- 103.Schirr-Bonnans S, Costa N, Derumeaux-Burel H, et al. Cost of diabetic eye, renal and foot complications: a methodological review. Eur J Health Econ. 2016. [DOI] [PubMed] [Google Scholar]
- 104.Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247–2254. doi: 10.1016/j.ophtha.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 105.Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117(6):1078–1086 e1072. doi: 10.1016/j.ophtha.2010.03.045 [DOI] [PubMed] [Google Scholar]
- 106.Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351–1359. doi: 10.1016/j.ophtha.2016.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Do DV, Sepah YJ, Boyer D, et al. Month-6 primary outcomes of the READ-3 study (Ranibizumab for edema of the mAcula in diabetes-protocol 3 with high dose). Eye. 2015;29(12):1538–1544. doi: 10.1038/eye.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sadiq MA, Hassan M, Soliman MK, et al.: Effects of two different doses of ranibizumab on diabetic retinopathy severity and progression in the ranibizumab for edema of the macula in diabetes (READ-3) study. ARVO 2016; IOVS, Seattle; 2016: 160. [Google Scholar]
- 109.Afridi R, Agarwal A, Sadiq MA, et al.: Effects of two different doses of ranibizumab on the resolution and recurrence of diabetic macular edema in the ranibizumab for edema of the macula in diabetes (READ-3) study. ARVO 2016; IOVS; 2016; Seattle. [Google Scholar]
- 110.Bressler NM, Edwards AR, Beck RW, et al. Exploratory analysis of diabetic retinopathy progression through 3 years in a randomized clinical trial that compares intravitreal triamcinolone acetonide with focal/grid photocoagulation. Arch Ophthalmol. 2009;127:1566–1571. doi: 10.1001/archophthalmol.2009.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blinder KJ, Dugel PU, Chen S, et al. Anti-VEGF treatment of diabetic macular edema in clinical practice: effectiveness and patterns of use (ECHO study report 1). Clin Ophthalmol. 2017;11:393–401. doi: 10.2147/OPTH.S128509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dugel PU, Campbell JH, Kiss S, et al. Association between early anatomic response to anti-vascular endothelial growth factor therapy and long-term outcome in diabetic macular edema: an independent analysis of protocol I study data. Retina. 2019;39(1):88–97. doi: 10.1097/IAE.0000000000002110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130(9):1145–1152. doi: 10.1001/archophthalmol.2012.1043 [DOI] [PubMed] [Google Scholar]
- 114.Ip MS, Domalpally A, Sun JK, Ehrlich JS. Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology. 2015;122(2):367–374. doi: 10.1016/j.ophtha.2014.08.048 [DOI] [PubMed] [Google Scholar]
- 115.Wykoff CC: Intravitreal aflibercept for moderately severe to severe nonproliferative diabetic retinopathy (NPDR): the Phase III PANORAMA Study. American Society of Retina Specialists Annual Meeting; 2018, Vancouver, Canada. [Google Scholar]
- 116.MJ E: To treat or not to treat: are we sacrificing treatment outcomes by allowing diabetic retinopathy (DR) to enter the proliferative stage? American Society of Retina Specialists Annual Meeting; 2018, Vancouver, Canada. [Google Scholar]
- 117.Virgili G, Parravano M, Menchini F, et al. Anti‐vascular endothelial growth factor for diabetic macular oedema: a network meta‐analysis. Cochrane Database Syst Rev. 2018;2018(10):CD007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sugimoto M, Ichio A, Mochida D, et al. Multiple effects of intravitreal aflibercept on microvascular regression in eyes with diabetic macular edema. Ophthalmol Retina. 2019;3:1067–1075. doi: 10.1016/j.oret.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 119.Wykoff CC, Shah C, Dhoot D, et al. Longitudinal retinal perfusion status in eyes with diabetic macular edema receiving intravitreal aflibercept or laser in VISTA study. Ophthalmology. 2019;126(8):1171–1180. doi: 10.1016/j.ophtha.2019.03.040 [DOI] [PubMed] [Google Scholar]
- 120.Cai S, Bressler NM. Aflibercept, bevacizumab or ranibizumab for diabetic macular oedema: recent clinically relevant findings from DRCR.net protocol T. Curr Opin Ophthalmol. 2017;28(6):636–643. doi: 10.1097/ICU.0000000000000424 [DOI] [PubMed] [Google Scholar]
- 121.Pershing S, Enns EA, Matesic B, Owens DK, Goldhaber-Fiebert JD. Cost-effectiveness of treatment of diabetic macular edema. Ann Intern Med. 2014;160(1):18–29. doi: 10.7326/M13-0768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gross JG, Glassman AR, Jampol LM, et al; Writing Committee for the Diabetic Retinopathy Clinical Research N. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137–2146. doi: 10.1001/jama.2015.15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gross JG, Glassman AR, Liu D, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138–1148. doi: 10.1001/jamaophthalmol.2018.3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sivaprasad S, Prevost AT, Vasconcelos JC, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389(10085):2193–2203. doi: 10.1016/S0140-6736(17)31193-5 [DOI] [PubMed] [Google Scholar]
- 125.Gonzalez VH, Wang P, Ruiz CQ. Panretinal photocoagulation for diabetic retinopathy in the RIDE and RISE trials: not “One and done”. Ophthalmology. 2019;126. [DOI] [PubMed] [Google Scholar]
- 126.Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626–635 e622. doi: 10.1016/j.ophtha.2010.12.028 [DOI] [PubMed] [Google Scholar]
- 127.Jonas JB, Kreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003;121(1):57–61. doi: 10.1001/archopht.121.1.57 [DOI] [PubMed] [Google Scholar]
- 128.Kuppermann BD, Blumenkranz MS, Haller JA, et al. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. 2007;125(3):309–317. doi: 10.1001/archopht.125.3.309 [DOI] [PubMed] [Google Scholar]
- 129.Kaur S, Yangzes S, Singh S, Sachdev N. Efficacy and safety of topical difluprednate in persistent diabetic macular edema. Int Ophthalmol. 2016;36(3):335–340. doi: 10.1007/s10792-015-0121-3 [DOI] [PubMed] [Google Scholar]
- 130.Nakano Goto S, Yamamoto T, Kirii E, Abe S, Yamashita H. Treatment of diffuse diabetic macular oedema using steroid eye drops. Acta Ophthalmol (Copenh). 2012;90(7):628–632. doi: 10.1111/aos.2012.90.issue-7 [DOI] [PubMed] [Google Scholar]
- 131.Nakano S, Yamamoto T, Kirii E, Abe S, Yamashita H. Steroid eye drop treatment (difluprednate ophthalmic emulsion) is effective in reducing refractory diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2010;248(6):805–810. doi: 10.1007/s00417-010-1316-y [DOI] [PubMed] [Google Scholar]
- 132.Lam DS, Chan CK, Mohamed S, et al. A prospective randomised trial of different doses of intravitreal triamcinolone for diabetic macular oedema. Br J Ophthalmol. 2007;91(2):199–203. doi: 10.1136/bjo.2006.102848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113(9):1533–1538. doi: 10.1016/j.ophtha.2006.02.065 [DOI] [PubMed] [Google Scholar]
- 134.Khan Z, Kuriakose RK, Khan M, Chin EK, Almeida DR. Efficacy of the intravitreal sustained-release dexamethasone implant for diabetic macular edema refractory to anti-vascular endothelial growth factor therapy: meta-analysis and clinical implications. Ophthalmic Surg Lasers Imaging Retina. 2017;48(2):160–166. doi: 10.3928/23258160-20170130-10 [DOI] [PubMed] [Google Scholar]
- 135.Pacella F, Romano MR, Turchetti P, et al. An eighteen-month follow-up study on the effects of Intravitreal Dexamethasone Implant in diabetic macular edema refractory to anti-VEGF therapy. Int J Ophthalmol. 2016;9(10):1427–1432. doi: 10.18240/ijo.2016.10.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pessoa B, Coelho J, Correia N, Ferreira N, Beirao M, Meireles A. Fluocinolone acetonide intravitreal implant 190 mug (ILUVIEN(R)) in vitrectomized versus nonvitrectomized eyes for the treatment of chronic diabetic macular edema. Ophthalmic Res. 2018;59(2):68–75. doi: 10.1159/000484091 [DOI] [PubMed] [Google Scholar]
- 137.Maturi RK, Glassman AR, Liu D, et al. Effect of adding dexamethasone to continued ranibizumab treatment in patients with persistent diabetic macular edema: a DRCR network phase 2 randomized clinical trial. JAMA Ophthalmol. 2018;136(1):29–38. doi: 10.1001/jamaophthalmol.2017.4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Krick TW, Bressler NM. Recent clinically relevant highlights from the diabetic retinopathy clinical research network. Curr Opin Ophthalmol. 2018;29(3):199–205. doi: 10.1097/ICU.0000000000000472 [DOI] [PubMed] [Google Scholar]
- 139.Koc I, Kadayifcilar S, Eldem B. Real-world results of intravitreal ranibizumab, bevacizumab, or triamcinolone for diabetic macular edema. Ophthalmologica. 2018;239(2–3):85–93. doi: 10.1159/000481180 [DOI] [PubMed] [Google Scholar]
- 140.Kodjikian L, Bellocq D, Mathis T. Pharmacological management of diabetic macular edema in real-life observational studies. Biomed Res Int. 2018;2018:8289253. doi: 10.1155/2018/8289253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Callanan DG, Loewenstein A, Patel SS, et al. A multicenter, 12-month randomized study comparing dexamethasone intravitreal implant with ranibizumab in patients with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):463–473. doi: 10.1007/s00417-016-3472-1 [DOI] [PubMed] [Google Scholar]
- 142.Malcles A, Dot C, Voirin N, et al. Real-life study in diabetic macular edema treated with dexamethasone implant: the reldex study. Retina. 2017;37(4):753–760. doi: 10.1097/IAE.0000000000001234 [DOI] [PubMed] [Google Scholar]
- 143.Cicinelli MV, Cavalleri M, Querques L, Rabiolo A, Bandello F, Querques G. Early response to ranibizumab predictive of functional outcome after dexamethasone for unresponsive diabetic macular oedema. Br J Ophthalmol. 2017;101(12):1689–1693. doi: 10.1136/bjophthalmol-2017-310242 [DOI] [PubMed] [Google Scholar]
- 144.Gillies MC, Simpson JM, Billson FA, et al. Safety of intravitreal injection of triamcinolone: results of a randomized clinical trial. Arch Ophthalmol. 2004;122(3):336–340. doi: 10.1001/archopht.122.3.336 [DOI] [PubMed] [Google Scholar]
- 145.Storey PP, Obeid A, Pancholy M, et al. Ocular hypertension after intravitreal injection of 2-mg triamcinolone. Retina. 2020;40(1):66–74. doi: 10.1097/IAE.0000000000002361 [DOI] [PubMed] [Google Scholar]
- 146.Smithen LM, Ober MD, Maranan L, et al. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004;138:740–743. doi: 10.1016/j.ajo.2004.06.067 [DOI] [PubMed] [Google Scholar]
- 147.Bakri SJ, Beer PM. Intravitreal triamcinolone injection for diabetic macular edema: a clinical and fluorescein angiographic case series. Can J Ophthalmol. 2004;39:755–760. doi: 10.1016/S0008-4182(04)80069-3 [DOI] [PubMed] [Google Scholar]
- 148.Haller JA, Bandello F, Belfort R Jr, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453–2460. doi: 10.1016/j.ophtha.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 149.Boyer DS, Yoon YH, Belfort R Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–1914. doi: 10.1016/j.ophtha.2014.04.024 [DOI] [PubMed] [Google Scholar]
- 150.Helzner J. Clearside study shows durable response in DME. Retin Physician. 2018;15. [Google Scholar]
- 151.Habib MS. ILUVIEN((R)) technology in the treatment of center-involving diabetic macular edema: a review of the literature. Ther Deliv. 2018;9(8):547–556. doi: 10.4155/tde-2018-0006 [DOI] [PubMed] [Google Scholar]
- 152.Fusi-Rubiano W, Mukherjee C, Lane M, et al. Treating Diabetic Macular Oedema (DMO): real world UK clinical outcomes for the 0.19mg Fluocinolone Acetonide intravitreal implant (Iluvien) at 2 years. BMC Ophthalmol. 2018;18(1):62. doi: 10.1186/s12886-018-0726-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Iglicki M, Zur D, Busch C, Okada M, Loewenstein A. Progression of diabetic retinopathy severity after treatment with dexamethasone implant: a 24-month cohort study the ‘DR-Pro-DEX Study’. Acta Diabetol. 2018;55:541–547. doi: 10.1007/s00592-018-1117-z [DOI] [PubMed] [Google Scholar]
- 154.Wykoff CC, Chakravarthy U, Campochiaro PA, Bailey C, Green K, Cunha-Vaz J. Long-term effects of intravitreal 0.19 mg fluocinolone acetonide implant on progression and regression of diabetic retinopathy. Ophthalmology. 2017;124(4):440–449. doi: 10.1016/j.ophtha.2016.11.034 [DOI] [PubMed] [Google Scholar]
- 155.Friedman SM, Almukhtar TH, Baker CW, et al. Diabetic retinopathy clinical research N: topical nepafenec in eyes with noncentral diabetic macular edema. Retina. 2015;35(5):944–956. doi: 10.1097/IAE.0000000000000403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim SJ, Schoenberger SD, Thorne JE, Ehlers JP, Yeh S, Bakri SJ. Topical nonsteroidal anti-inflammatory drugs and cataract surgery: a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122(11):2159–2168. doi: 10.1016/j.ophtha.2015.05.014 [DOI] [PubMed] [Google Scholar]
- 157.Wielders LH, Lambermont VA, Schouten JS, et al. Prevention of cystoid macular edema after cataract surgery in nondiabetic and diabetic patients: a systematic review and meta-analysis. Am J Ophthalmol. 2015;160(5):968–981 e933. doi: 10.1016/j.ajo.2015.07.032 [DOI] [PubMed] [Google Scholar]
- 158.Pinna A, Blasetti F, Ricci GD, Boscia F. Bromfenac eyedrops in the treatment of diabetic macular edema: a pilot study. Eur J Ophthalmol. 2017;27(3):326–330. doi: 10.5301/ejo.5000888 [DOI] [PubMed] [Google Scholar]
- 159.Elbendary AM, Shahin MM. Intravitreal diclofenac versus intravitreal triamcinolone acetonide in the treatment of diabetic macular edema. Retina. 2011;31(10):2058–2064. doi: 10.1097/IAE.0b013e31822a042a [DOI] [PubMed] [Google Scholar]
- 160.Soheilian M, Karimi S, Ramezani A, Peyman GA. Pilot study of intravitreal injection of diclofenac for treatment of macular edema of various etiologies. Retina. 2010;30(3):509–515. doi: 10.1097/IAE.0b013e3181bdfa43 [DOI] [PubMed] [Google Scholar]
- 161.Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361(1):40–51. doi: 10.1056/NEJMoa0808400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chaturvedi N, Porta M, Klein R, et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet. 2008;372(9647):1394–1402. doi: 10.1016/S0140-6736(08)61412-9 [DOI] [PubMed] [Google Scholar]
- 163.Chase HP, Garg SK, Harris S, Hoops S, Jackson WE, Holmes DL. Angiotensin-converting enzyme inhibitor treatment for young normotensive diabetic subjects: a two-year trial. Ann Ophthalmol. 1993;25(8):284–289. [PubMed] [Google Scholar]
- 164.Chaturvedi N, Sjolie AK, Stephenson JM, et al. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID study group. EURODIAB controlled trial of lisinopril in insulin-dependent diabetes mellitus. Lancet. 1998;351(9095):28–31. doi: 10.1016/S0140-6736(97)06209-0 [DOI] [PubMed] [Google Scholar]
- 165.Virk SA, Donaghue KC, Wong TY, Craig ME. Interventions for diabetic retinopathy in type 1 diabetes: systematic review and meta-analysis. Am J Ophthalmol. 2015;160(5):1055–1064 e1054. doi: 10.1016/j.ajo.2015.07.024 [DOI] [PubMed] [Google Scholar]
- 166.Deissler HL, Lang GE. The protein kinase C inhibitor: ruboxistaurin. Dev Ophthalmol. 2016;55:295–301. [DOI] [PubMed] [Google Scholar]
- 167.Aiello LP, Vignati L, Sheetz MJ, et al; PKC-DRS and PKC-DRS2 Study Groups. Oral protein kinase c β inhibition using ruboxistaurin: efficacy, safety, and causes of vision loss among 813 patients (1392 eyes) with diabetic retinopathy in the protein kinase C β inhibitor-diabetic retinopathy study and the protein kinase C β inhibitor-diabetic retinopathy study. Retina. 2011;31(10):2084–2094. doi: 10.1097/IAE.0b013e3182111669. [DOI] [PubMed] [Google Scholar]
- 168.Schwartz SG, Flynn HW Jr, Aiello LP. Ruboxistaurin mesilate hydrate for diabetic retinopathy. Drugs Today (Barc). 2009;45(4):269–274. doi: 10.1358/dot.2009.045.004.1354195 [DOI] [PubMed] [Google Scholar]
- 169.Staurenghi G, Ye L, Magee MH, et al. Darapladib, a lipoprotein-associated phospholipase A2 inhibitor, in diabetic macular edema: a 3-month placebo-controlled study. Ophthalmology. 2015;122(5):990–996. doi: 10.1016/j.ophtha.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 170.Cho WB, Oh SB, Moon JW, Kim HC. Panretinal photocoagulation combined with intravitreal bevacizumab in high-risk proliferative diabetic retinopathy. Retina. 2009;29(4):516–522. doi: 10.1097/IAE.0b013e31819a5fc2 [DOI] [PubMed] [Google Scholar]
- 171.Filho JA, Messias A, Almeida FP, et al. Panretinal photocoagulation (PRP) versus PRP plus intravitreal ranibizumab for high-risk proliferative diabetic retinopathy. Acta Ophthalmol (Copenh). 2011;89(7):e567–e572. doi: 10.1111/j.1755-3768.2011.02184.x [DOI] [PubMed] [Google Scholar]
- 172.Figueira J, Fletcher E, Massin P, et al. Ranibizumab plus panretinal photocoagulation versus panretinal photocoagulation alone for high-risk proliferative diabetic retinopathy (PROTEUS study). Ophthalmology. 2018;125(5):691–700. doi: 10.1016/j.ophtha.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 173.Sun JK, Glassman AR, Beaulieu WT, et al. Rationale and application of the protocol S anti-vascular endothelial growth factor algorithm for proliferative diabetic retinopathy. Ophthalmology. 2019;126(1):87–95. doi: 10.1016/j.ophtha.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mehta H, Gillies MC, Fraser-Bell S. Combination of vascular endothelial growth factor inhibitors and laser therapy for diabetic macular oedema: a review. Clin Exp Ophthalmol. 2016;44(4):335–339. doi: 10.1111/ceo.2016.44.issue-4 [DOI] [PubMed] [Google Scholar]
- 175.Lam DS, Chan CK, Mohamed S, et al. Intravitreal triamcinolone plus sequential grid laser versus triamcinolone or laser alone for treating diabetic macular edema: six-month outcomes. Ophthalmology. 2007;114(12):2162–2167. doi: 10.1016/j.ophtha.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 176.Yilmaz T, Weaver CD, Gallagher MJ, et al. Intravitreal triamcinolone acetonide injection for treatment of refractory diabetic macular edema: a systematic review. Ophthalmology. 2009;116(5):902–911; quiz 912–903. doi: 10.1016/j.ophtha.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 177.Mirshahi A, Shenazandi H, Lashay A, Faghihi H, Alimahmoudi A, Dianat S. Intravitreal triamcinolone as an adjunct to standard laser therapy in coexisting high-risk proliferative diabetic retinopathy and clinically significant macular edema. Retina. 2010;30(2):254–259. doi: 10.1097/IAE.0b013e3181b4f125 [DOI] [PubMed] [Google Scholar]
- 178.Liu L, Wu X, Geng J, Yuan Z, Chen L. IVTA as adjunctive treatment to PRP and MPC for PDR and macular edema: a meta-analysis. PLoS One. 2012;7(9):e44683. doi: 10.1371/journal.pone.0044683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the management of diabetic macular edema by the european society of retina specialists (EURETINA). Ophthalmologica. 2017;237(4):185–222. doi: 10.1159/000458539 [DOI] [PubMed] [Google Scholar]
- 180.Regillo CD, Callanan DG, Do DV, et al. Use of corticosteroids in the treatment of patients with diabetic macular edema who have a suboptimal response to anti-VEGF: recommendations of an expert panel. Ophthalmic Surg Lasers Imaging Retina. 2017;48(4):291–301. doi: 10.3928/23258160-20170329-03 [DOI] [PubMed] [Google Scholar]
- 181.El Rami H, Barham R, Sun JK, Silva PS. Evidence-based treatment of diabetic retinopathy. Semin Ophthalmol. 2017;32(1):67–74. doi: 10.1080/08820538.2016.1228397 [DOI] [PubMed] [Google Scholar]
- 182.Machemer R. Vitrectomy in diabetic retinopathy; removal of preretinal proliferations. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol. 1975;79(2):OP394–OP395. [PubMed] [Google Scholar]
- 183.Mandelcorn M. Results after vitrectomy in diabetes. Can J Ophthalmol. 1976;11(2):130–133. [PubMed] [Google Scholar]
- 184.Mandelcorn MS, Blankenship G, Machemer R. Pars plana vitrectomy for the management of sever diabetic retinopathy. Am J Ophthalmol. 1976;81(5):561–570. doi: 10.1016/0002-9394(76)90117-3 [DOI] [PubMed] [Google Scholar]
- 185.Fassbender JM, Ozkok A, Canter H, Schaal S. A comparison of immediate and delayed vitrectomy for the management of vitreous hemorrhage due to proliferative diabetic retinopathy. Ophthalmic Surg Lasers Imaging Retina. 2016;47(1):35–41. doi: 10.3928/23258160-20151214-05 [DOI] [PubMed] [Google Scholar]
- 186.Federman JL, Boyer D, Lanning R, Breit P. An objective analysis of proliferative diabetic retinopathy before and after pars plana vitrectomy. Ophthalmology. 1979;86(2):276–282. doi: 10.1016/S0161-6420(79)35526-9 [DOI] [PubMed] [Google Scholar]
- 187.Jackson TL, Johnston RL, Donachie PH, Williamson TH, Sparrow JM, Steel DH. The Royal College of Ophthalmologists’ national ophthalmology database study of vitreoretinal surgery: report 6, diabetic vitrectomy. JAMA Ophthalmol. 2016;134(1):79–85; quiz 120. doi: 10.1001/jamaophthalmol.2015.4587 [DOI] [PubMed] [Google Scholar]
- 188.Michels RG. Vitrectomy for complications of diabetic retinopathy. Arch Ophthalmol. 1978;96(2):237–246. doi: 10.1001/archopht.1978.03910050105001 [DOI] [PubMed] [Google Scholar]
- 189.Mikhail M, Ali-Ridha A, Chorfi S, Kapusta MA. Long-term outcomes of sutureless 25-G+ pars-plana vitrectomy for the management of diabetic tractional retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2016. [DOI] [PubMed] [Google Scholar]
- 190.Sharma T, Fong A, Lai TY, Lee V, Das S, Lam D. Surgical treatment for diabetic vitreoretinal diseases: a review. Clin Experiment Ophthalmol. 2016;44(4):340–354. doi: 10.1111/ceo.2016.44.issue-4 [DOI] [PubMed] [Google Scholar]
- 191.Rice TA, Michels RG. Long-term anatomic and functional results of vitrectomy for diabetic retinopathy. Am J Ophthalmol. 1980;90(3):297–303. doi: 10.1016/S0002-9394(14)74907-4 [DOI] [PubMed] [Google Scholar]
- 192.Nakajima T, Roggia MF, Noda Y, Ueta T. Effect of internal limiting membrane peeling during vitrectomy for diabetic macular edema: systematic review and meta-analysis. Retina. 2015;35(9):1719–1725. doi: 10.1097/IAE.0000000000000622 [DOI] [PubMed] [Google Scholar]
- 193.Kumagai K, Hangai M, Ogino N, Larson E:. Effect of internal limiting membrane peeling on long-term visual outcomes for diabetic macular edema. Retina. 2015;35(7):1422–1428. doi: 10.1097/IAE.0000000000000497 [DOI] [PubMed] [Google Scholar]
- 194.Hartley KL, Smiddy WE, Flynn HW Jr., Murray TG. Pars plana vitrectomy with internal limiting membrane peeling for diabetic macular edema. Retina. 2008;28(3):410–419. doi: 10.1097/IAE.0b013e31816102f2 [DOI] [PubMed] [Google Scholar]
- 195.Arevalo JF, Lasave AF, Wu L, et al.; Pan-American Collaborative Retina Study G. Intravitreal bevacizumab for proliferative diabetic retinopathy: results From the Pan-American Collaborative Retina Study Group (PACORES) at 24 months of follow-up. Retina. 2016 [DOI] [PubMed] [Google Scholar]
- 196.Guan G, Zang J. Meta-analysis of the effect of perioperative injection of Lucentis on intraoperative bleeding in patients with proliferative diabetic retinopathy. Eye Sci. 2015;30(4):171–175. [PubMed] [Google Scholar]
- 197.Manabe A, Shimada H, Hattori T, Nakashizuka H, Yuzawa M. Randomized controlled study of intravitreal bevacizumab 0.16 mg injected one day before surgery for proliferative diabetic retinopathy. Retina. 2015;35(9):1800–1807. doi: 10.1097/IAE.0000000000000577 [DOI] [PubMed] [Google Scholar]
- 198.Pakzad-Vaezi K, Albiani DA, Kirker AW, et al. A randomized study comparing the efficacy of bevacizumab and ranibizumab as pre-treatment for pars plana vitrectomy in proliferative diabetic retinopathy. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):521–524. doi: 10.3928/23258160-20141118-06 [DOI] [PubMed] [Google Scholar]
- 199.Smith JM, Steel DH. Anti-vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2015;8:CD008214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Su L, Ren X, Wei H, et al. Intravitreal conbercept (KH902) for surgical treatment of severe proliferative diabetic retinopathy. Retina. 2016;36(5):938–943. doi: 10.1097/IAE.0000000000000900 [DOI] [PubMed] [Google Scholar]
- 201.Castillo J, Aleman I, Rush SW, Rush RB. Preoperative bevacizumab administration in proliferative diabetic retinopathy patients undergoing vitrectomy: a randomized and controlled trial comparing interval variation. Am J Ophthalmol. 2017;183:1–10. doi: 10.1016/j.ajo.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 202.Arevalo JF, Liu TYA; Pan-American Collaborative Retina Study G: Intravitreal Bevacizumab in Diabetic Retinopathy. Recommendations from the Pan-American Collaborative Retina Study Group (PACORES): the 2016 Knobloch lecture. Asia Pac J Ophthalmol (Phila). 2018;7(1):36–39. doi: 10.22608/APO.2017466 [DOI] [PubMed] [Google Scholar]
- 203.Jackson TL, Nicod E, Angelis A, Grimaccia F, Pringle E, Kanavos P. Pars plana vitrectomy for diabetic macular edema: a systematic review, meta-analysis, and synthesis of safety literature. Retina. 2017;37(5):886–895. doi: 10.1097/IAE.0000000000001280 [DOI] [PubMed] [Google Scholar]
- 204.Ahmadieh H, Shoeibi N, Entezari M, Monshizadeh R. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: a randomized clinical trial. Ophthalmology. 2009;116(10):1943–1948. doi: 10.1016/j.ophtha.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 205.Comyn O, Wickham L, Charteris DG, et al. Ranibizumab pretreatment in diabetic vitrectomy: a pilot randomised controlled trial (the RaDiVit study). Eye. 2017;31(9):1253–1258. doi: 10.1038/eye.2017.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bressler SB, Melia M, Glassman AR, et al. Diabetic retinopathy clinical research n: ranibizumab plus prompt or deferred laser for diabetic macular edema in eyes with vitrectomy before anti-vascular endothelial growth factor therapy. Retina. 2015;35(12):2516–2528. doi: 10.1097/IAE.0000000000000617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ahn SJ, Ahn J, Park S, et al. Intraocular pharmacokinetics of ranibizumab in vitrectomized versus nonvitrectomized eyes. Invest Ophthalmol Vis Sci. 2014;55(1):567–573. doi: 10.1167/iovs.13-13054 [DOI] [PubMed] [Google Scholar]
- 208.Niwa Y, Kakinoki M, Sawada T, Wang X, Ohji M. Ranibizumab and aflibercept: intraocular pharmacokinetics and their effects on aqueous VEGF level in vitrectomized and nonvitrectomized macaque eyes. Invest Ophthalmol Vis Sci. 2015;56(11):6501–6505. doi: 10.1167/iovs.15-17279 [DOI] [PubMed] [Google Scholar]
- 209.Ikeda T, Sato K, Katano T, Hayashi Y. Improved visual acuity following pars plana vitrectomy for diabetic cystoid macular edema and detached posterior hyaloid. Retina. 2000;20(2):220–222. doi: 10.1097/00006982-200002000-00023 [DOI] [PubMed] [Google Scholar]
- 210.Harbour JW, Smiddy WE, Flynn HW Jr., Rubsamen PE. Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am J Ophthalmol. 1996;121(4):405–413. doi: 10.1016/S0002-9394(14)70437-4 [DOI] [PubMed] [Google Scholar]
- 211.Ikeda T, Sato K, Katano T, Hayashi Y. Vitrectomy for cystoid macular oedema with attached posterior hyaloid membrane in patients with diabetes. Br J Ophthalmol. 1999;83(1):12–14. doi: 10.1136/bjo.83.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pendergast SD, Hassan TS, Williams GA, et al. Vitrectomy for diffuse diabetic macular edema associated with a taut premacular posterior hyaloid. Am J Ophthalmol. 2000;130(2):178–186. doi: 10.1016/S0002-9394(00)00472-4 [DOI] [PubMed] [Google Scholar]
- 213.Tachi N, Ogino N. Vitrectomy for diffuse macular edema in cases of diabetic retinopathy. Am J Ophthalmol. 1996;122(2):258–260. doi: 10.1016/S0002-9394(14)72018-5 [DOI] [PubMed] [Google Scholar]
- 214.Gandorfer A, Messmer EM, Ulbig MW, Kampik A. Resolution of diabetic macular edema after surgical removal of the posterior hyaloid and the inner limiting membrane. Retina. 2000;20(2):126–133. doi: 10.1097/00006982-200002000-00004 [DOI] [PubMed] [Google Scholar]
- 215.Uji A, Murakami T, Suzuma K, et al. Influence of vitrectomy surgery on the integrity of outer retinal layers in diabetic macular edema. Retina. 2018;38(1):163–172. doi: 10.1097/IAE.0000000000001519 [DOI] [PubMed] [Google Scholar]
- 216.Haller JA, Qin H, Apte RS, et al. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117(6):1087–1093 e1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bonnin S, Sandali O, Bonnel S, Monin C, El Sanharawi M. Vitrectomy with internal limiting membrane peeling for tractional and nontractional diabetic macular edema: long-term results of a comparative study. Retina. 2015;35(5):921–928. doi: 10.1097/IAE.0000000000000433 [DOI] [PubMed] [Google Scholar]
- 218.Browning DJ, Lee C, Stewart MW, Landers MB 3rd. Vitrectomy for center-involved diabetic macular edema. Clin Ophthalmol. 2016;10:735–742. doi: 10.2147/OPTH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Flaxel CJ, Edwards AR, Aiello LP, et al. Factors associated with visual acuity outcomes after vitrectomy for diabetic macular edema: diabetic retinopathy clinical research network. Retina. 2010;30(9):1488–1495. doi: 10.1097/IAE.0b013e3181e7974f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ichiyama Y, Sawada O, Mori T, Fujikawa M, Kawamura H, Ohji M. The effectiveness of vitrectomy for diffuse diabetic macular edema may depend on its preoperative optical coherence tomography pattern. Graefes Arch Clin Exp Ophthalmol. 2016;254(8):1545–1551. doi: 10.1007/s00417-015-3251-4 [DOI] [PubMed] [Google Scholar]
- 221.Simunovic MP, Hunyor AP, Ho IV. Vitrectomy for diabetic macular edema: a systematic review and meta-analysis. Can J Ophthalmol. 2014;49(2):188–195. doi: 10.1016/j.jcjo.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 222.Figueroa MS, Contreras I, Noval S. Surgical and anatomical outcomes of pars plana vitrectomy for diffuse nontractional diabetic macular edema. Retina. 2008;28(3):420–426. doi: 10.1097/IAE.0b013e318159e7d2 [DOI] [PubMed] [Google Scholar]
- 223.Kumagai K, Furukawa M, Ogino N, Larson E, Iwaki M, Tachi N. Long-term follow-up of vitrectomy for diffuse nontractional diabetic macular edema. Retina. 2009;29(4):464–472. doi: 10.1097/IAE.0b013e31819c632f [DOI] [PubMed] [Google Scholar]
- 224.Bahadir M, Ertan A, Mertoglu O. Visual acuity comparison of vitrectomy with and without internal limiting membrane removal in the treatment of diabetic macular edema. Int Ophthalmol. 2005;26(1–2):3–8. doi: 10.1007/s10792-006-0008-4 [DOI] [PubMed] [Google Scholar]
- 225.Jahn CE, Schopfer DC, Heinzle T, et al. Lasting resolution of diabetic macular edema and stable improvement of visual acuity after treatment with pars plana vitrectomy. Ophthalmologica. 2009;223(3):219–220. doi: 10.1159/000213642 [DOI] [PubMed] [Google Scholar]
- 226.La Heij EC, Hendrikse F, Kessels AG, Derhaag PJ. Vitrectomy results in diabetic macular oedema without evident vitreomacular traction. Graefes Arch Clin Exp Ophthalmol. 2001;239(4):264–270. doi: 10.1007/s004170000251 [DOI] [PubMed] [Google Scholar]
- 227.Otani T, Kishi S. A controlled study of vitrectomy for diabetic macular edema. Am J Ophthalmol. 2002;134(2):214–219. doi: 10.1016/S0002-9394(02)01548-9 [DOI] [PubMed] [Google Scholar]
- 228.Patel JI, Hykin PG, Schadt M, Luong V, Fitzke F, Gregor ZJ. Pars plana vitrectomy with and without peeling of the inner limiting membrane for diabetic macular edema. Retina. 2006;26(1):5–13. doi: 10.1097/00006982-200601000-00002 [DOI] [PubMed] [Google Scholar]
- 229.Recchia FM, Ruby AJ, Carvalho Recchia CA. Pars plana vitrectomy with removal of the internal limiting membrane in the treatment of persistent diabetic macular edema. Am J Ophthalmol. 2005;139(3):447–454. doi: 10.1016/j.ajo.2004.09.076 [DOI] [PubMed] [Google Scholar]
- 230.Rosenblatt BJ, Shah GK, Sharma S, Bakal J. Pars plana vitrectomy with internal limiting membranectomy for refractory diabetic macular edema without a taut posterior hyaloid. Graefes Arch Clin Exp Ophthalmol. 2005;243(1):20–25. doi: 10.1007/s00417-004-0958-z [DOI] [PubMed] [Google Scholar]
- 231.Shah SP, Patel M, Thomas D, Aldington S, Laidlaw DA. Factors predicting outcome of vitrectomy for diabetic macular oedema: results of a prospective study. Br J Ophthalmol. 2006;90(1):33–36. doi: 10.1136/bjo.2005.072934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Stolba U, Binder S, Gruber D, Krebs I, Aggermann T, Neumaier B. Vitrectomy for persistent diffuse diabetic macular edema. Am J Ophthalmol. 2005;140(2):295–301. doi: 10.1016/j.ajo.2005.03.045 [DOI] [PubMed] [Google Scholar]
- 233.Yamamoto T, Hitani K, Sato Y, Yamashita H, Takeuchi S. Vitrectomy for diabetic macular edema with and without internal limiting membrane removal. Ophthalmologica. 2005;219(4):206–213. doi: 10.1159/000085729 [DOI] [PubMed] [Google Scholar]
- 234.Yanyali A, Horozoglu F, Celik E, Nohutcu AF. Long-term outcomes of pars plana vitrectomy with internal limiting membrane removal in diabetic macular edema. Retina. 2007;27(5):557–566. doi: 10.1097/01.iae.0000249390.61854.d5 [DOI] [PubMed] [Google Scholar]
- 235.Song YS, Nagaoka T, Omae T, Yokota H, Takahashi A, Yoshida A. Systemic risk factors in bilateral proliferative diabetic retinopathy requiring vitrectomy. Retina. 2016;36(7):1309–1313. doi: 10.1097/IAE.0000000000000886 [DOI] [PubMed] [Google Scholar]
- 236.Tuuminen R, Sahanne S, Haukka J, Loukovaara S. Improved outcome after primary vitrectomy in diabetic patients treated with statins. Eur J Ophthalmol. 2016;26(2):174–181. doi: 10.5301/ejo.5000657 [DOI] [PubMed] [Google Scholar]
- 237.Yamada Y, Suzuma K, Ryu M, Tsuiki E, Fujikawa A, Kitaoka T. Systemic factors influence the prognosis of diabetic macular edema after pars plana vitrectomy with internal limiting membrane peeling. Curr Eye Res. 2013;38(12):1261–1265. doi: 10.3109/02713683.2013.820327 [DOI] [PubMed] [Google Scholar]
- 238.Sun JK, Aiello LP. The future of ultrawide field imaging for diabetic retinopathy: pondering the retinal periphery. JAMA Ophthalmol. 2016;134(3):247–248. doi: 10.1001/jamaophthalmol.2015.5384 [DOI] [PubMed] [Google Scholar]
- 239.Huang SS, Marcantonio C. The role of optical coherence tomography in managing diabetic maculopathy and retinopathy. Asia Pac J Ophthalmol (Phila). 2016;5(5):317–318. doi: 10.1097/APO.0000000000000231 [DOI] [PubMed] [Google Scholar]
- 240.Verma A, Nagpal M, Mehrotra N. In vivo assessment of choroid in diabetic retinopathy by enhanced depth imaging in spectral domain optical coherence tomography. Asia Pac J Ophthalmol (Phila). 2016;5(5):319–323. doi: 10.1097/APO.0000000000000204 [DOI] [PubMed] [Google Scholar]
- 241.Ruia S, Saxena S, Gemmy Cheung CM, Gilhotra JS, Lai TY. Spectral domain optical coherence tomography features and classification systems for diabetic macular edema: a review. Asia Pac J Ophthalmol (Phila). 2016;5(5):360–367. doi: 10.1097/APO.0000000000000218 [DOI] [PubMed] [Google Scholar]
- 242.Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402–2410. doi: 10.1001/jama.2016.17216 [DOI] [PubMed] [Google Scholar]
- 243.Arcadu F, Benmansour F, Maunz A, Willis J, Haskova Z, Prunotto M. Deep learning algorithm predicts diabetic retinopathy progression in individual patients. NPJ Digit Med. 2019;2:92. doi: 10.1038/s41746-019-0172-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Arcadu F, Benmansour F, Maunz A, et al. Deep learning predicts OCT measures of diabetic macular thickening from color fundus photographs. Invest Ophthalmol Vis Sci. 2019;60(4):852–857. doi: 10.1167/iovs.18-25634 [DOI] [PubMed] [Google Scholar]
- 245.Natarajan S, Jain A, Krishnan R, Rogye A, Sivaprasad S. Diagnostic accuracy of community-based diabetic retinopathy screening with an offline artificial intelligence system on a smartphone. JAMA Ophthalmol. 2019;137:1182. doi: 10.1001/jamaophthalmol.2019.2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Andrade Romo JS, Lynch G, Liu K, et al. Flavoprotein fluorescence correlation with visual acuity response in patients receiving anti-VEGF injection for diabetic macular edema. Oxid Med Cell Longev. 2018;2018:3567306. doi: 10.1155/2018/3567306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Vujosevic S, Bottega E, Casciano M, Pilotto E, Convento E, Midena E. Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina. 2010;30(6):908–916. doi: 10.1097/IAE.0b013e3181c96986 [DOI] [PubMed] [Google Scholar]
- 248.Gella L, Raman R, Kulothungan V, Saumya Pal S, Ganesan S, Sharma T. Retinal sensitivity in subjects with type 2 diabetes mellitus: Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetics study (SN-DREAMS II, Report no. 4). Br J Ophthalmol. 2016;100(6):808–813. doi: 10.1136/bjophthalmol-2015-307064 [DOI] [PubMed] [Google Scholar]
- 249.Holm K, Schroeder M, Lovestam Adrian M. Peripheral retinal function assessed with 30-Hz flicker seems to improve after treatment with lucentis in patients with diabetic macular oedema. Doc Ophthalmol. 2015;131(1):43–51. doi: 10.1007/s10633-015-9495-9 [DOI] [PubMed] [Google Scholar]
- 250.Karacorlu M, Ozdemir H, Senturk F, Arf Karacorlu S, Uysal O. Macular function by multifocal electroretinogram in diabetic macular edema after intravitreal triamcinolone acetonide injection. Eur J Ophthalmol. 2008;18(4):601–608. doi: 10.1177/112067210801800417 [DOI] [PubMed] [Google Scholar]
- 251.Kim YH, Yun C, Kim JT, Kim SW, Oh J, Huh K. The correlation between retinal sensitivity assessed by microperimetry and contrast sensitivity in diabetic macular oedema. Br J Ophthalmol. 2014;98(12):1618–1624. doi: 10.1136/bjophthalmol-2013-304765 [DOI] [PubMed] [Google Scholar]
- 252.Lecleire-Collet A, Audo I, Aout M, et al. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Invest Ophthalmol Vis Sci. 2011;52(6):2861–2867. doi: 10.1167/iovs.10-5960 [DOI] [PubMed] [Google Scholar]
- 253.Longhin E, Tormene AP, Olivato E, et al. Rod function in diabetic patients without and with early diabetic retinopathy. Eur J Ophthalmol. 2016;26(5):418–424. doi: 10.5301/ejo.5000800 [DOI] [PubMed] [Google Scholar]
- 254.Harrison WW, Bearse MA Jr., Ng JS, et al. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Vis Sci. 2011;52(2):772–777. doi: 10.1167/iovs.10-5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ng JS, Bearse MA Jr., Schneck ME, Barez S, Adams AJ. Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Invest Ophthalmol Vis Sci. 2008;49(4):1622–1628. doi: 10.1167/iovs.07-1157 [DOI] [PubMed] [Google Scholar]
- 256.Adams AJ, Bearse MA Jr. Retinal neuropathy precedes vasculopathy in diabetes: a function-based opportunity for early treatment intervention? Clin Exp Optom. 2012;95(3):256–265. doi: 10.1111/cxo.2012.95.issue-3 [DOI] [PubMed] [Google Scholar]
- 257.Hoshikawa Y, Ohkoshi K, Yamaguchi T. [Retinal sensitivity following subthreshold diode laser micropulse photocoagulation for diabetic macular edema]. Nihon Ganka Gakkai Zasshi. 2011;115(1):13–19. Japanese. [PubMed] [Google Scholar]
- 258.Nakamura Y, Mitamura Y, Ogata K, Arai M, Takatsuna Y, Yamamoto S. Functional and morphological changes of macula after subthreshold micropulse diode laser photocoagulation for diabetic macular oedema. Eye. 2010;24(5):784–788. doi: 10.1038/eye.2009.207 [DOI] [PubMed] [Google Scholar]
- 259.Andrade GC, Dias JR, Maia A, Farah ME, Meyer CH, Rodrigues EB. Intravitreal injection of ziv-aflibercept for diabetic macular edema: a pilot study. Retina. 2016;36(9):1640–1645. doi: 10.1097/IAE.0000000000001000 [DOI] [PubMed] [Google Scholar]
- 260.Kim YM, Lee SY, Koh HJ. Prediction of postoperative visual outcome after pars plana vitrectomy based on preoperative multifocal electroretinography in eyes with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2010;248(10):1387–1393. doi: 10.1007/s00417-010-1398-6 [DOI] [PubMed] [Google Scholar]
- 261.Venkatesh P, Ramanjulu R, Azad R, Vohra R, Garg S. Subthreshold micropulse diode laser and double frequency neodymium: YAG laser in treatment of diabetic macular edema: a prospective, randomized study using multifocal electroretinography. Photomed Laser Surg. 2011;29(11):727–733. doi: 10.1089/pho.2010.2830 [DOI] [PubMed] [Google Scholar]
- 262.Ye H, Yu M, Lu L, Jin C, Luo G. Electroretinogram evaluation for the treatment of proliferative diabetic retinopathy by short-pulse pattern scanning laser panretinal photocoagulation. Lasers Med Sci. 2018:33:1095–1102. [DOI] [PubMed] [Google Scholar]
- 263.Bressler NM, Odia I, Maguire M, et al. Network ftDR: association between change in visual acuity and change in central subfield thickness during treatment of diabetic macular edema in participants randomized to aflibercept, bevacizumab, or ranibizumab: a post hoc analysis of the protocol T randomized clinical trial. JAMA Ophthalmol. 2019;137(9):977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Deak GG, Schmidt-Erfurth UM, Jampol LM. Correlation of central retinal thickness and visual acuity in diabetic macular edema. JAMA Ophthalmol. 2018;136(11):1215–1216. doi: 10.1001/jamaophthalmol.2018.3848 [DOI] [PubMed] [Google Scholar]
- 265.Abramoff MD, Fort PE, Han IC, Jayasundera KT, Sohn EH, Gardner TW. Approach for a clinically useful comprehensive classification of vascular and neural aspects of diabetic retinal disease. Invest Ophthalmol Vis Sci. 2018;59(1):519–527. doi: 10.1167/iovs.17-21873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Bressler NM, Beaulieu WT, Glassman AR, et al. Persistent macular-thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for center-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;136(3):257–269. doi: 10.1001/jamaophthalmol.2017.6565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Dugel PU, Layton A, Varma RB. Diabetic macular edema diagnosis and treatment in the real world: an analysis of medicare claims data (2008 to 2010). Ophthalmic Surg Lasers Imaging Retina. 2016;47(3):258–267. doi: 10.3928/23258160-20160229-09 [DOI] [PubMed] [Google Scholar]
- 268.Bolinger MT, Antonetti DA. Moving past anti-VEGF: novel therapies for treating diabetic retinopathy. Int J Mol Sci. 2016;17:9. doi: 10.3390/ijms17091498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Spencer DB, Protopsaltis NJ, Chao DL. New pharmacotherapies for diabetic retinopathy. Ann Eye Sci. 2018;3(43):1–13. doi: 10.21037/aes [DOI] [Google Scholar]
- 270.Stewart MW. Future treatments of diabetic retinopathy: pharmacotherapeutic products under development. Eur Med J Diabetes. 2017;5(1):93–103. [Google Scholar]
- 271.Wang JH, Ling D, Tu L, van Wijngaarden P, Dusting GJ, Liu GS. Gene therapy for diabetic retinopathy: are we ready to make the leap from bench to bedside? Pharmacol Ther. 2017;173:1–18. doi: 10.1016/j.pharmthera.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 272.Xu Y, Rong A, Bi Y, Xu W. Intravitreal conbercept injection with and without grid laser photocoagulation in the treatment of diffuse diabetic macular edema in real-life clinical practice. J Ophthalmol. 2016;2016:2143082. doi: 10.1155/2016/2143082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sahni J, Patel SS, Dugel PU, et al. Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-A with faricimab in diabetic macular edema: BOULEVARD phase 2 randomized trial. Ophthalmology. 2019;126(8):1155–1170. doi: 10.1016/j.ophtha.2019.03.023 [DOI] [PubMed] [Google Scholar]
- 274.Mohammad G, Kowluru RA. The role of Raf-1 kinase in diabetic retinopathy. Expert Opin Ther Targets. 2011;15(4):357–364. doi: 10.1517/14728222.2011.553604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ozdemir G, Kilinc M, Ergun Y, Sahin E. Rapamycin inhibits oxidative and angiogenic mediators in diabetic retinopathy. Can J Ophthalmol. 2014;49(5):443–449. doi: 10.1016/j.jcjo.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 276.Nakahara T, Morita A, Yagasaki R, Mori A, Sakamoto K. Mammalian target of rapamycin (mTOR) as a potential therapeutic target in pathological ocular angiogenesis. Biol Pharm Bull. 2017;40(12):2045–2049. doi: 10.1248/bpb.b17-00475 [DOI] [PubMed] [Google Scholar]
- 277.Campochiaro PA, Channa R, Berger BB, et al. Treatment of diabetic macular edema with a designed ankyrin repeat protein that binds vascular endothelial growth factor: a phase I/II study. Am J Ophthalmol. 2013;155(4):697–704, 704. doi: 10.1016/j.ajo.2012.09.032 [DOI] [PubMed] [Google Scholar]
- 278.Sadiq MA, Hanout M, Sarwar S, et al. Platelet-derived growth factor inhibitors: a potential therapeutic approach for ocular neovascularization. Dev Ophthalmol. 2016;55:310–316. [DOI] [PubMed] [Google Scholar]
- 279.Rangasamy S, McGuire PG, Franco Nitta C, Monickaraj F, Oruganti SR, Das A. Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS One. 2014;9(10):e108508. doi: 10.1371/journal.pone.0108508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gale JD, Berger B, Gilbert S, et al. A CCR2/5 inhibitor, PF-04634817, is inferior to monthly ranibizumab in the treatment of diabetic macular edema. Invest Ophthalmol Vis Sci. 2018;59:2659–2669. doi: 10.1167/iovs.17-22731 [DOI] [PubMed] [Google Scholar]
- 281.Sfikakis PP, Grigoropoulos V, Emfietzoglou I, et al. Infliximab for diabetic macular edema refractory to laser photocoagulation: a randomized, double-blind, placebo-controlled, crossover, 32-week study. Diabetes Care. 2010;33(7):1523–1528. doi: 10.2337/dc09-2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wu L, Hernandez-Bogantes E, Roca JA, Arevalo JF, Barraza K, Lasave AF. intravitreal tumor necrosis factor inhibitors in the treatment of refractory diabetic macular edema: a pilot study from the Pan-American Collaborative Retina Study Group. Retina. 2011;31(2):298–303. doi: 10.1097/IAE.0b013e3181eac7a6 [DOI] [PubMed] [Google Scholar]
- 283.Campochiaro PA, Sophie R, Tolentino M, et al. Treatment of diabetic macular edema with an inhibitor of vascular endothelial-protein tyrosine phosphatase that activates Tie2. Ophthalmology. 2015;122(3):545–554. doi: 10.1016/j.ophtha.2014.09.023 [DOI] [PubMed] [Google Scholar]
- 284.Campochiaro PA, Khanani A, Singer M, et al. Enhanced benefit in diabetic macular edema from AKB-9778 Tie2 activation combined with vascular endothelial growth factor suppression. Ophthalmology. 2016;123(8):1722–1730. doi: 10.1016/j.ophtha.2016.04.025 [DOI] [PubMed] [Google Scholar]
- 285.Abdulaal M, Haddad NM, Sun JK, Silva PS. The role of plasma kallikrein-kinin pathway in the development of diabetic retinopathy: pathophysiology and therapeutic approaches. Semin Ophthalmol. 2016;31(1–2):19–24. doi: 10.3109/08820538.2015.1114829 [DOI] [PubMed] [Google Scholar]
- 286.Sun JK, Maturi RK, Boyer DS, et al.: Intravitreous Plasma Kallikrein (PK) inhibition for diabetic macular edema: a phase 1 study of the novel PK inhibitor KVD001 ARVO 2016 Annual Meeting Abstracts; Edited by ARVO; 2016; Seattle. [Google Scholar]
- 287.Figueira J, Henriques J, Amaro M, Rosas V, Alves D, Cunha-Vaz J. A nonrandomized, open-label, multicenter, Phase 4 pilot study on the effect and safety of ILUVIEN(R) in chronic diabetic macular edema patients considered insufficiently responsive to available therapies (RESPOND). Ophthalmic Res. 2017;57(3):166–172. doi: 10.1159/000455235 [DOI] [PubMed] [Google Scholar]
- 288.Kontturi LS, Collin EC, Murtomaki L, Pandit AS, Yliperttula M, Urtti A. Encapsulated cells for long-term secretion of soluble VEGF receptor 1: material optimization and simulation of ocular drug response. Eur J Pharm Biopharm. 2015;95(Pt B):387–397. doi: 10.1016/j.ejpb.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 289.Elsaid N, Jackson TL, Elsaid Z, Alqathama A, Somavarapu S. PLGA microparticles entrapping chitosan-based nanoparticles for the ocular delivery of ranibizumab. Mol Pharm. 2016;13(9):2923–2940. doi: 10.1021/acs.molpharmaceut.6b00335 [DOI] [PubMed] [Google Scholar]
- 290.Moreno MR, Tabitha TS, Nirmal J, et al. Study of stability and biophysical characterization of ranibizumab and aflibercept. Eur J Pharm Biopharm. 2016;108:156–167. doi: 10.1016/j.ejpb.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 291.Tanetsugu Y, Tagami T, Terukina T, Ogawa T, Ohta M, Ozeki T. Development of a sustainable release system for a ranibizumab biosimilar using poly(lactic-co-glycolic acid) biodegradable polymer-based microparticles as a platform. Biol Pharm Bull. 2017;40(2):145–150. doi: 10.1248/bpb.b16-00437 [DOI] [PubMed] [Google Scholar]
- 292.Ming X, Wu L, Carver K, Yuan A, Min Y. Dendritic nanoconjugates for intracellular delivery of neutral oligonucleotides. Nanoscale. 2015;7(29):12302–12306. doi: 10.1039/C5NR01665G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Yuan A, Hu Y, Ming X. Dendrimer conjugates for light-activated delivery of antisense oligonucleotides. RSC Adv. 2015;5:35195–35200. doi: 10.1039/C5RA04091D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kamaleddin MA. Nano-ophthalmology: applications and considerations. Nanomedicine. 2017;13(4):1459–1472. doi: 10.1016/j.nano.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 295.Xu L, Shen W, Wang B, et al. Efficient siRNA delivery using PEG-conjugated PAMAM dendrimers targeting vascular endothelial growth factor in a CoCl2-induced neovascularization model in retinal endothelial cells. Curr Drug Deliv. 2016;13(4):590–599. doi: 10.2174/1567201812666150817123049 [DOI] [PubMed] [Google Scholar]
- 296.Yavuz B, Bozdag Pehlivan S, Sumer Bolu B, Nomak Sanyal R, Vural I, Unlu N. Dexamethasone - PAMAM dendrimer conjugates for retinal delivery: preparation, characterization and in vivo evaluation. J Pharm Pharmacol. 2016;68(8):1010–1020. doi: 10.1111/jphp.12587 [DOI] [PubMed] [Google Scholar]
- 297.Chiang B, Jung JH, Prausnitz MR. The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv Drug Deliv Rev. 2018;15(126):58–66. doi: 10.1016/j.addr.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Clearside Biomedical I. Suprachoroidal injection of CLS-TA in subjects with macular edema associated with non-infectious uveitis (PEACHTREE) ClinicalTrialsgov. Bethesda (MD): National Library of Medicine (US); 2015. NCT02595398. [Google Scholar]
- 299.Ip M, Gupta M, Velaga S, Group TS: Suprachoroidal CLS-TA plus aflibercept compared with aflibercept monotherapy for DME: primary and selected secondary results of the randomized phase 2 TYBEE trial. American Society of Retina Specialists 37th Annual Scientific Meeting; 2019; Chicago, IL. [Google Scholar]
- 300.Humayun M, Santos A, Altamirano JC, et al. Implantable micropump for drug delivery in patients with diabetic macular edema. Transl Vis Sci Technol. 2014;3(6):5. doi: 10.1167/tvst.3.6.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hussain RM, Ciulla TA. Emerging vascular endothelial growth factor antagonists to treat neovascular age-related macular degeneration. Expert Opin Emerg Drugs. 2017;22(3):235–246. doi: 10.1080/14728214.2017.1362390 [DOI] [PubMed] [Google Scholar]