Abstract
Aims
This study examined the mechanisms through which discrimination influences diabetes self-care and glycemic control in patients with diabetes by using structured equation modeling.
Methods
615 patients were recruited from two adult primary care clinics in the southeastern United States. Measures were based on a theoretical model and included perceived discrimination, social support, social cohesion, and perceived stress. Structured equation modeling examined the relationship with diabetes self-care and glycemic control.
Results
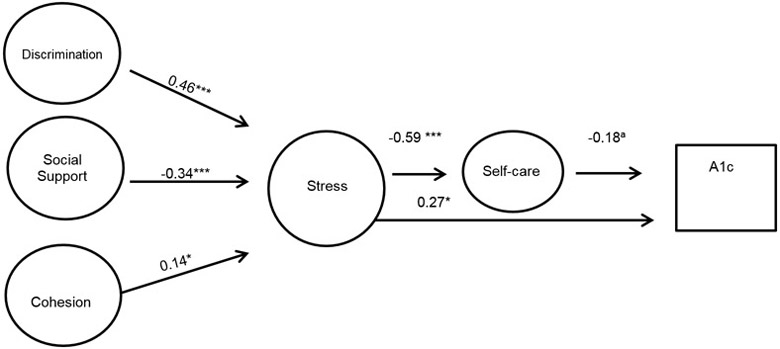
The final model (chi2(211)=328.82, p<0.0001, R2 = 0.99, RMSEA=0.03 and CFI=0.98) shows that higher stress is directly significantly related to a decreased self-care (r= −0.59, p <0.001) and increased HbA1c (r= 0.27, p<0.05). There was no significant direct association between discrimination, social support or social cohesion, and glycemic control or self-care. There was, however, a direct significant association between increased discrimination (r=0.46, p<0.001), decreased social support (r= −0.34, p<0.001), increased social cohesion (r=0.14, p<0.05) and increased stress.
Conclusions
These results support the hypothesized pathway of discrimination on health outcomes, showing both a direct and indirect influence through stress on HbA1c in adults with diabetes. Understanding the pathways through which discrimination influences diabetes outcomes is important for providing more comprehensive and effective care. These results suggest future interventions targeting patients with diabetes should take discrimination-induced stress into account.
Keywords: discrimination, glycemic control, self-care, pathways, stress
1. Introduction
Diabetes is the 7th leading cause of death in the United States, affecting 29.1 million people, or 9.3% of the population. [1] Individuals with diabetes are at an increased risk of blindness, kidney failure, heart disease, stroke, and amputation, as well as, at a 50% higher risk of death than those without diabetes. [1] In addition, medical expenditures of those with diabetes are 2.3 times higher than those without diabetes, and totaled $245 billion in the United States in 2012. [1]
Research indicates discrimination is an important possible risk factor for health outcomes, including trajectory for chronic diseases such as diabetes. [2-4] Discrimination refers to differential treatment of certain members of a society by either individuals or social institutions. [3] Those experiencing discrimination are aware of the discriminatory behavior, and their perception of this discrimination can generate stress. [3,5] While discrimination research often focuses on racial/ethnic discrimination, studies have found perceptions of non-race based discrimination similarly influences health; and in a study of patients with diabetes, discrimination based on education level was shown most significant. [3,6] The stress literature suggests the ability to manage new stressors is reduced by existing stressors. [7] Therefore, given the high psychological and behavioral burden of diabetes, it is important to understand how perceived discrimination relates to other stressors and/or combines with them to influence outcomes in diabetes. [3-4].
Studies show a consistent inverse relationship between perceived discrimination and health, including self-rated health, physical functioning, and hemoglobin A1c [2-4, 8-12] It has been hypothesized that potential pathways for this relationship include psychological and physiological stress responses, and health behaviors. [2, 4-5, 13] For example, Chen and Yang found that an indirect association between discrimination and health status existed through health behaviors (physical activity, sleep quality, fruit and vegetable intake, and smoking intensity) and the presence of chronic disease. [14] In patients with diabetes, it has been suggested that discrimination leads to unhealthy behaviors, such as increased screen time, cigarette smoking, alcohol use, drug use, and lack of seeking preventative services such as A1c testing or eye exams for diabetes. [3-4, 15-18] My physiologically based hypothesis suggest that stress can accelerate cellular aging, and the experience of chronic stress can lead to dysregulation in multiple biological systems, creating premature illness and increasing risk of mortality. [3-4, 19] In addition, acute experiences of stress can lead to cardiovascular reactivity, as seen by increased in blood pressure, and increased stress hormones are related to blood glucose levels. [3, 20]
Little research has been done to fully understand the pathway through which discrimination influences outcomes in adults with type 2 diabetes. While theoretical pathways exist, these mechanisms have not been extensively tested through either cross-sectional or interventional work. The aim of this paper is to understand the mechanisms through which discrimination influences diabetes self-care and glycemic control in patients with diabetes by using structured equation modeling to test theoretical pathways.
2. Methods
2.1. Sample
Following institutional review board approval, 615 patients were recruited from two adult primary care clinics in the southeastern United States. Eligibility included ages 18 years or older, diagnosis of type 2 diabetes in their medical record, and ability to communicate in English. Patients were ineligible if through interaction or chart documentation they were determined to be cognitively impaired as a result of significant dementia or active psychosis. Patients who expressed interest after receiving letters of invitation or being approached in the clinic waiting room were provided a detailed explanation of the study and consented. Participants completed validated questionnaires that captured social determinants of health factors along with demographic and self-care information. Most recent HbA1c was abstracted from the medical record to serve as diabetes outcome measure. Validated questionnaires were included based on a modified version of the conceptual framework by Brown et al. relating social determinant of health factors to diabetes processes and outcomes [21].
Measures included in this analysis were based on the theoretical model described by Pascoe and Richman for the pathways by which perceived discrimination influence health outcomes. [4] As hypothesized based on a meta-analysis of available research on discrimination influences on health outcomes, Pascoe and Richman suggested a direct pathway connecting perceived discrimination with mental and physical health, as well as indirect pathways through both stress, and health behaviors. [4] They further hypothesized that positive influences such as social support, stigma identification, and coping style could influence these indirect pathways. [4]
2.2. Demographic Information
Previously validated items from the 2002 National Health Interview Survey were used to capture age, race, gender, marital status, number of hours worked, household income, years of education and employment status. [22]
2.3. Perceived Discrimination
Perceived discrimination was measured using questions from the Diabetes Study of Northern California (DISTANCE) survey: a 4-question measure where patients reported how often in the past 12 months they were made to feel inferior because of their race/ethnicity, education level, gender, and language. [23]. Response options were never, sometimes, usually, and often.
2.4. Social Support
Social Support was measured with the Medical Outcomes Study (MOS) Social Support Survey: a 19-item scale measuring tangible support, affection, positive social interaction, and emotional or informational support. [24] The total scale (α = 0.97) has high internal consistency, good criterion and discriminant validity, and one-year test-retest reliability (0.72-0.76). [24]
2.5. Social Cohesion
Social Cohesion was measured using the 5-item Sampson Scale. The scale measures the patient’s ability to trust and relate to individuals in their neighborhood. Answer choices range from 1 – strongly agree to 5- strongly disagree [25].
2.6. Perceived Stress
Stress was measured with the Perceived Stress Scale (PSS); a 4-item scale that assesses the frequency with which the patient finds situations stressful during the previous month [26]. The Cronbach alpha value is 0.69 and scores are highly correlated with stress, depression and anxiety. [27]
2.7. Diabetes Self-care
Medication Adherence was measured with the Morisky Medication Adherence Scale (MMAS); an 8-item scale with higher values signifying greater adherence [28].
Diabetes behavior was measured with the Summary of Diabetes Self-Care Activities (SDSCA) scale; an 11-item scale measuring frequency of self-care activity in the last 7 days for general diet (followed a healthy diet), specific diet (ate fruits/vegetables), exercise, blood glucose testing, and foot care [29].
2.8. Glycemic Control
Glycemic control was measured by extracting Hemoglobin A1C (HbA1c) from patients’ medical records. The most recent HbA1C value within the past six months was used.
2.9. Statistical Analysis
The sample size of 615 adults provides the recommended 20:1 ratio of subjects to variables needed to maintain 80% power, given the number of variables included in the model [30,31]. With a sample size of 615, parameter estimates and standard errors can be estimated without over-saturating the model [30,31].
All analyses were conducted using Stata version 13. First, we performed descriptive statistics to ensure normality and linearity. Analyses used the maximum likelihood estimation procedure with ‘mlmv option’ so variables were retained rather than using listwise deletion. A series of confirmatory factor analysis (CFA) models were estimated prior to conducting structured equation modeling (SEM), as is recommended by best practices. [31,32] For CFA, alpha statistic and factor analysis using principal component factor analysis were used to examine loading, along with goodness of fit statistics. For SEM, all analyses were conducted using standardized estimates, which can be interpreted as the change in standard deviation of the outcome due to one standard deviation increase in the predictor. Since the chi2 statistic is sensitive to sample size, we evaluated the direction and magnitude of path coefficients, along with multiple fit statistics, including root mean square error of approximation (RSMEA) and comparative fit index (CFI). [33] Lower RSMEA values indicate better fit, with 0.05 indicating good fit, and 0.08 indicating reasonable fit. [33] Higher CFI indicates good fit, with 1 indicating perfect fit, 0.9 indicating adequate fit, and 0.8 indicating marginal fit. [33]
3.0. Results
Demographic characteristics are shown in Table 1. The mean age was 61, with the majority being males (61.6%) and non-Hispanic Black (64.9%). Mean duration of diabetes was 12.2 years, 49.7% were married, and 20.2% earned less than $10,000 annually.
Table 1.
Sample Demographic Characteristics (n=615)
| % or mean±
standard deviation |
|
|---|---|
| Age | 61.3±10.9 |
| Diabetes Duration | 12.3±9.1 |
| Education | 13.4±2.8 |
| Employment Hours | 12.4±19 |
| Charlson Comorbidity Score | 25.7±2.2 |
| Race | |
| White | 33.0 |
| Black | 64.9 |
| Other | 2.1 |
| Site | |
| MUSC | 51.2 |
| VA | 48.8 |
| Gender | |
| Women | 38.4 |
| Men | 61.3 |
| Marital Status | |
| Never married | 11.2 |
| Married | 49.7 |
| Separated/Divorced | 28.2 |
| Widowed | 10.9 |
| Income | |
| <$10,000 | 20.2 |
| $10,000-$14,999 | 11.9 |
| $15,000-$19,999 | 10.1 |
| $20,000-$24,999 | 10.4 |
| $25,000-$34,999 | 14.7 |
| $35,000-$49,999 | 13.8 |
| $50,000-$74,999 | 10.1 |
| $75,000 or more | 9.4 |
Descriptive information for variables included in latent variables are presented in Table 2. Correlations among the study variables is shown in Table 3.
Table 2.
Descriptive Characteristics of Structural Equation Model Factors
| Measures | Means±
standard deviation |
|---|---|
| A1c | 7.9±1.8 |
| Discrimination | |
| Race | 1.3±0.6 |
| Education | 1.2±0.5 |
| Language | 1.1±0.4 |
| Gender | 1.1±0.4 |
| Self-Care | |
| General Diet | 4.7±2.0 |
| Special Diet | 4.0±1.6 |
| Exercise | 2.6±2.2 |
| Blood sugar testing | 4.6±2.5 |
| Footcare | 4.3±2.5 |
| Medication adherence | 5.9±2.0 |
| Social Support | |
| MOS-16 | 4.0±1.2 |
| MOS-17 | 4.0±1.3 |
| MOS-18 | 4.0±1.3 |
| Serious Psychological Distress | |
| SPD-1 | 1.2±1.1 |
| SPD-2 | 1.4±1.3 |
| SPD-3 | 1.5±1.1 |
| SPD-4 | 1.3±1.2 |
| Social Cohesion | |
| SOCIALCOH-1 | 2.6±1.0 |
| SOCIALCOH-2 | 2.3±0.9 |
| SOCIALCOH-3 | 2.3±1.0 |
| SOCIALCOH-4 | 2.5±0.9 |
| SOCIALCOH-5 | 2.8±1.0 |
Table 3.
Pairwise Correlations for Glycemic Control, Social Support, Social Cohesion, Distress, and Perceived Discrimination
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. HbA1c | - | ||||||||||||||||||||||
| 2. Race Discrimination | 0.04 | - | |||||||||||||||||||||
| 3. Education Discrimination | 0.09* | 0.7* | - | ||||||||||||||||||||
| 4. Language Discrimination | 0.01 | 0.52* | 0.51* | - | |||||||||||||||||||
| 5. Gender Discrimination | 0.03 | 0.64* | 0.65* | 0.55* | - | ||||||||||||||||||
| 6. General diet | −0.12* | −0.14* | <0.01 | −0.08* | −0.09* | - | |||||||||||||||||
| 7. Specific diet | −0.07 | −0.14* | −0.1* | −0.1* | −0.07 | 0.36* | - | ||||||||||||||||
| 8. Exercise | −0.1* | <0.01 | 0.02 | <0.01 | −0.02 | 0.29* | 0.15* | - | |||||||||||||||
| 9. Blood glucose test | 0.09* | −0.06 | 0.02 | <0.01 | −0.04 | 0.21* | 0.19* | 0.11* | - | ||||||||||||||
| 10. Foot care | 0.03 | −0.03 | 0.02 | <0.01 | −0.04 | 0.22* | 0.22* | 0.12* | 0.28* | - | |||||||||||||
| 11. Medication adherence | −0.2* | −0.16* | −0.1* | −0.12* | −0.12* | 0.28* | 0.26* | 0.13* | 0.17* | 0.23* | - | ||||||||||||
| 12. Social Support (MOS16) | −0.08* | −0.19* | −0.2* | −0.14* | −0.11* | 0.19* | 0.15* | 0.13* | 0.07 | 0.09* | 0.17* | - | |||||||||||
| 13. Social Support (MOS17) | −0.1 | −0.19* | −0.2* | −0.14* | −0.12* | 0.22* | 0.18* | 0.14* | 0.08* | 0.12* | 0.16* | 0.91* | - | ||||||||||
| 14. Social Support (MOS18) | −0.05 | −0.19* | −0.2* | −0.14* | −0.11* | 0.22* | 0.17* | 0.15* | 0.09* | 0.12* | 0.18* | 0.92* | 0.94* | - | |||||||||
| 15. Distress 1 | 0.14* | 0.28* | 0.26* | 0.2* | 0.25* | −0.18* | −0.23* | −0.18* | −0.13* | −0.11* | −0.31* | −0.37* | −0.42* | −0.41* | - | ||||||||
| 16. Distress 2 | 0.04 | 0.02 | 0.05 | 0.03 | 0.09* | −0.07 | −0.07 | −0.03 | −0.02 | 0.03 | −0.13* | −0.14* | −0.14* | −0.14* | 0.11* | - | |||||||
| 17. Distress 3 | 0.01 | 0.17* | 0.12* | 0.08 | 0.17* | −0.18* | −0.11* | −0.06 | −0.03 | −0.04 | −0.19* | −0.29* | −0.28* | −0.3* | 0.27* | 0.56* | - | ||||||
| 18. Distress 4 | 0.15* | 0.25* | 0.22* | 0.2* | 0.21* | −0.19* | −0.21* | −0.11* | −0.13* | −0.1* | −0.29* | −0.36* | −0.37* | −0.38* | 0.64* | 0.11* | 0.26* | - | |||||
| 19. SOCIALCOH-1 | 0.04 | 0.16* | 0.13* | 0.15* | 0.16* | −0.14* | −0.07 | −0.13* | −0.13* | −0.05 | −0.06 | −0.22* | −0.23* | −0.22* | 0.2* | 0.01 | 0.11* | 0.13* | - | ||||
| 20. SOCIALCOH-2 | 0.06 | 0.19* | 0.14* | 0.11* | 0.13* | −0.13* | −0.03 | −0.09* | −0.13* | −0.02 | −0.09* | −0.24* | −0.25* | −0.27* | 0.22* | 0.04 | 0.16* | 0.18* | 0.65* | - | |||
| 21. SOCIALCOH-3 | 0.12* | 0.08* | 0.09* | 0.1* | 0.1* | −0.12* | −0.06 | −0.05 | −0.04 | 0.03 | −0.13* | −0.13* | −0.13* | −0.12* | 0.12* | 0.14* | 0.17* | 0.16* | 0.27* | 0.28* | - | ||
| 22. SOCIALCOH-4 | 0.02 | 0.19* | 0.14* | 0.09* | 0.11* | −0.15* | −0.1* | −0.1* | −0.07 | −0.02 | −0.13* | −0.25* | −0.25* | −0.26* | 0.2* | 0.06 | 0.15* | 0.18* | 0.52* | 0.6* | 0.17* | - | |
| 23. SOCIALCOH-5 | 0.08 | 0.2* | 0.11* | 0.13* | 0.11* | −0.09* | −0.09* | −0.07 | −0.04 | <0.01 | −0.14* | −0.09* | −0.11* | −0.12* | 0.15* | 0.05 | 0.11* | 0.09* | 0.27* | 0.2* | 0.4* | 0.25* | - |
Bold and P=0.05
3.1. Latent variable for perceived discrimination
CFA was used to measure properties of a latent variable for perceived discrimination using four variables: race discrimination, education discrimination, language discrimination, and gender discrimination. The alpha statistic for the four variables was 0.84. The variables loaded onto one factor explaining 70% of the variance with factor loadings ranging from 0.74 to 0.87. The fit of the final model was satisfactory chi2(2)=7.97, p=0.02; RMSEA=0.07 and CFI=0.99. Standardized loadings ranged from 0.66 to 0.84, and all four measures had significant loading at the p <0.001 level.
3.2. Latent variables for social support
CFA was used to measure properties of a latent variable for social support using three items from the MOS Social Support Survey: Someone to have a good time with, someone to get together with for relaxation, someone to do something enjoyable with. The alpha statistic for social support was 0.97. The variables loaded onto one factor explaining 95% of the variance with factor loading ranging from 0.97 to 0.99. The final model was just fit. Standardized loadings ranged from 0.94 to 0.97, and all three measures of social support had significant loading at the p <0.001 level.
3.3. Latent variables for social cohesion
CFA was used to measure the properties of a latent variable for social cohesion using the five items from the Sampson scale. The alpha statistic for social cohesion was 0.73. One factor explained 50% of the variance with factor loading ranging from 0.53 to 0.83. The final model fit well chi2(3)=5.50, p=0.14; RMSEA=0.04 and CFI=0.99. Standardized loadings ranged from 0.32 to 0.88, and all five measures of social cohesion had significant loading at the p <0.001 level.
3.4. Latent variables for perceived stress
CFA was used to measure the properties of a latent variable for perceived stress using the four items within the perceived stress scale. The alpha statistic for perceived stress was 0.65. One factor explained 50% of the variance with factor loading ranging from 0.59 to 0.75. The final model was just fit. Standardized loadings ranged from 0.15 to 0.82, and all four measures of perceived stress had significant loading that is significant at the p<0.001 level.
3.5. Latent variables for diabetes self-care
CFA was used to measure the properties of a latent variable for diabetes self-care using six items: general diet, specific diet, exercise, blood glucose testing, foot care, and medication adherence. The alpha statistic for diabetes self-care was 0.61. The variables loaded onto one factor explaining 35% of the variance with factor loading ranging from 0.47 to 0.70. The fit of the final model was satisfactory chi2(8)=9.94, p=0.27; RMSEA=0.02 and CFI=0.99. Standardized loadings ranged from 0.33 to 0.54, and all six measures of diabetes self-care had significant loading at the p <0.001 level.
3.6. Structural model
The final model is shown in Figure 1, with direct, indirect and total effects presented in Table 4. The final model (chi2(211) = 328.82, p<0.0001, R2 = 0.99, RMSEA=0.03 and CFI=0.98) shows that higher stress is directly significantly related to a decreased self-care (r= −0.59, p <0.001) and increased HbA1c (r= 0.27, p<0.05). There was no significant direct association between discrimination, social support or social cohesion, and glycemic control or self-care. There was, however, an indirect association between increased discrimination (r= 0.17, p<0.01), decreased social support (r= −0.15, p<0.001), and increased social cohesion (r= 0.07, p<0.05) and increased glycemic control. There was direct significant association between increased discrimination (r=0.46, p<0.001), decreased social support (r= −0.34, p<0.001), increased social cohesion (r=0.14, p<0.05) and increased stress.
Figure 1.
SEM Model of Influence of Discrimination on Glycemic Control
Note: Coefficients are standardized path coefficients.
Overall model fit Chi2 (211)=328.82, p<0.001; R2=0.99, RMSEA=0.036, CFI=0.984)
a p=0.07, *p<0.05, **p<0.01, ***p<0.001
Table 4.
Standardized Direct, Indirect, and Total Effects for Relationship of Discrimination on Glycemic Control
| Direct Effects |
Indirect Effects |
Total Effects |
|
|---|---|---|---|
| Glycemic Control | |||
| →Self-care | −0.18a | - | −0.18a |
| →Stress | 0.27* | 0.10*** | 0.37** |
| →Discrimination | −0.01 | 0.17** | 0.16 |
| →Social Support | 0.08 | −0.15*** | −0.07 |
| →Cohesion | 0.03 | 0.07* | 0.10 |
| Self-care | |||
| →Stress | −0.59*** | −0.59*** | |
| →Discrimination | 0.01 | −0.27*** | −0.26* |
| →Social support | 0.10 | 0.20*** | 0.30*** |
| →Cohesion | −0.13 | −0.08* | −0.21* |
| Stress | |||
| →Discrimination | 0.46*** | - | 0.46*** |
| →Social support | −0.34*** | - | −0.34*** |
| →Cohesion | 0.14* | - | 0.14** |
p=0.07
p≤0.05
p≤0.01
p≤0.001
Therefore, based on Figure 1 and Table 4, an increase in perceived discrimination was associated with an increase of perceived stress. Perceived stress had both a direct and indirect association with HbA1c. The positive direct association with HbA1c, indicates that with increased stress there was an increase in HbA1c (worse glycemic control). The negative indirect association between stress and HbA1c indicates that increased stress was associated with poorer self-care practices; and a poorer self-care practices were associated with an increase in HbA1c (worse glycemic control). No direct pathway between discrimination and self-care or glycemic control existed.
4.0. DISCUSSION
4.1. Discussion
Using structured equation modeling, we tested a set of hypothesized pathways through which discrimination influences self-care and health outcomes in adults with diabetes. Based on this study, increased discrimination is associated with increased stress, and increased stress is associated with decreased self-care, and increased HbA1c (poorer glycemic control). The validated questionnaire used measured four forms of discrimination: race/ethnicity, education level, gender, and language, providing information on pathways relevant for a multifactorial measure of discrimination and broadening the utility of the findings. This study partially validates hypothesized pathways through which discrimination influences disease progression, supporting a direct pathway to stress, and both a direct pathway between stress and glycemic control, and an indirect pathway through stress and self-care. Our findings do not fully support the Pascoe and Richman’s hypothesized model for patients with diabetes, which suggested a direct pathway between discrimination and health behaviors and health outcomes themselves. [4]
This study adds to the literature by elucidating the mechanism by which experiences of discrimination impact diabetes outcomes, namely through a stress pathway. Understanding this influence on the progression of a chronic disease, especially in illnesses previously linked to a biological stress response in the neuroendocrine and cardiovascular systems is important for providing more comprehensive and effective care. [2-4] These results partially validate previously hypothesized pathways, supporting the hypothesized stress pathway, but not supporting a direct pathway between discrimination and health outcomes in patients with diabetes. Further, results illuminate both a direct and indirect influence of stress on glycemic control in adults with diabetes, with an indirect influence through self-care. Additionally, this study offers insight into the potential protective factors of social support and social cohesion that may guard against the deleterious effects of discrimination on diabetes outcomes. The literature on the stress buffering hypothesis, namely as it relates to discrimination, suggests that social support may serve to moderate and protect against the impact of discrimination on mental health, such as depression, however very little has been done to examine how these factors protect against the impact of discrimination induced stress on diabetes outcomes. [4] The results of this study suggest social support has a strong inverse relationship with stress, and could serve as a focus of healthcare system interventions to address the influence of discrimination-induced stress on diabetes outcomes. Social cohesion, however, did not show this inverse relationship, and may have additional factors that must be taken into account when considering social cohesion at a neighborhood level.
Unlike results reported by Chen and Yang, there was no direct association between discrimination and health behaviors, instead this study found an indirect association through stress. [14] The conceptual model used by Chen and Yang did not include stress as a possible variable, which may explain the different results. This study offers more specific mechanism information for patients with diabetes, suggesting the importance of stress as part of the pathway between discrimination and self-care. Stressors occurring throughout an individual’s life and impacting multiple areas of their life lead to more disruption and in turn have greater consequence on health. [3] Given the psychological and behavioral stressors attached to diagnosis and management of diabetes, these reoccurring stressors may compound discrimination-induced stress experienced by adults with diabetes.
Some limitations of this study are worth mentioning. First, this study was conducted in the southeastern U.S., and as such is not generalizable to the entire U.S. population. While there is no reason to suspect different pathways exist regionally, it is important to conduct similar studies in other regions to understand if there are regional differences in the importance of direct and indirect pathways. Secondly, research has shown severe events are better recalled than less severe events, so assessment of discrimination may not reflect all experiences and may underestimate the influence. [3] Finally, this study used cross-sectional data, which limits discussion of causation. Future studies should investigate longitudinal data to further elucidate this pathway, and stratify by factors such as gender, race, and income to understand whether dimensions of stress differ by demographic factors.
4.2. Practice Implications
These results suggest future interventions targeting patients with diabetes should take discrimination-induced stress into account. Research shows that stress management training helps to improve glycemic control, specifically through providing training on: 1) progressive muscle relaxation, 2) the use of cognitive and behavioral skills to recognize and reduce stress levels, and 3) education on the negative effects of stress. [37] Interventions involving patient empowerment also effectively address psychosocial components of living with diabetes such as stress and patient empowerment, and show improvement in blood glucose. [38] Similarly, resiliency training encourages the individual to take control of their well-being through coping and reintegration from adversity and stressors by drawing on one’s inherent resilience. [39-41] Taking discrimination-induced stress into account in development of diabetes education programs through previously used techniques, and incorporating social support into programs, may help patients with diabetes manage the multiple levels of stress in their lives, and result in better self-care and outcomes.
4.3. Conclusion
In conclusion, using structured equation modeling this study found increased discrimination is associated with increased stress, and increased stress is associated with decreased self-care, and increased HbA1C. These results partially validate previously hypothesized pathways and provide insight on the mechanisms through which discrimination influences health outcomes in patients with diabetes. Based on these results, diabetes education programs can provide more comprehensive support by including information on how to reduce stress through techniques such as increasing patient empowerment, social support, and resiliency.
ACKNOWLEDGEMENTS
Funding Source: This study was supported by Grant K24DK093699-01 from The National Institute of Diabetes and Digestive and Kidney Disease (PI: Leonard Egede).
Footnotes
Conflict of Interest: The authors report no potential conflicts of interest relevant to this article.
Disclaimer: This article represents the views of the authors and not those of NIH, VHA or HSR&D.
References:
- 1.Centers for Disease Control and Prevention: National Diabetes Statistics Report, 2014. Atlanta: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, 2014 [Google Scholar]
- 2.Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. Am J Public Health. 2003; 93(2): 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009; 32: 20–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pascoe EA, Richman LS. Perceived discrimination and health: a meta-analytic review. Psychol Bull. 2009;135(4):531–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans: a biopsychosocial model. The American Psychologist. 1999; 54(10): 805–516. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds DB, Walker RJ, Campbell JA, Egede LE. Differential effect of race, education, gender and language discrimination on glycemic control in adults with type 2 diabetes. Diabetes Technol Ther. 2015. April; 17(4): 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007; 298(14): 1685–1687. [DOI] [PubMed] [Google Scholar]
- 8.Krieger N Embodying inequality: a review of concepts, measures, and methods for studying health consequences of discrimination. Int J Health Services. 1999; 29(2): 295–352. [DOI] [PubMed] [Google Scholar]
- 9.Paradies Y A systematic review of empirical research on self-reported racism and health. In J Epidemiology. 2006; 35(4): 888–901. [DOI] [PubMed] [Google Scholar]
- 10.Piette JD, Bibbins-Domingo K, Schillinger D. Health care discrimination, processes of care, and diabetes patients’ health status. Patient Education and Counseling. 2006; 60(1): 41–48. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales KL, Lambert WE, Fu R, Jacob M, Harding AK. Perceived racial discrimination in health care, completion of standard diabetes services, and diabetes control among a sample of American Indian women. Diabetes Educ. 2014; 40(6): 747–755. [DOI] [PubMed] [Google Scholar]
- 12.Wagner JA, Tennen H, Feinn R, Osborn CY. Self-reported discrimination, diabetes distress, and continuous blood glucose in women with type 2 diabetes. J Immigr Minor Health. 2015; 17(2): 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuevas AG, Reitzel LR, Cao Y, Nguyen N, Wetter DW, Adams CE, Watkins KL, Regan SD, McNeill LH. Mediators of discrimination and self-rated health among African Americans. Am J Health Behav. 2013; 37(6): 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen D and Yang T. The pathways from perceived discrimination to self-rated health: an investigation of the roles of distrust, social capital, and health behaviors. Social Science and Medicine. 2014; 104: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Womack VY, Ning H, Lewis CE, Loucks EB, Puterman E, Rels J, Siddique J, Sternfeld B, Van Horn L, Carnethon MR. Relationship between perceived discrimination and sedentary behavior in adults. Am J Health Behav. 2014; 38(5): 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawson AZ, Walker RJ, Campbell JA, Egede LE. Effect of perceived racial discrimination on self-care behaviors, glycemic control, and quality of life in adults with type 2 diabetes. Endocrine. 2015. June; 49(2): 422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006; 63(4): 1011–1022. [DOI] [PubMed] [Google Scholar]
- 18.Trivedi AN and Ayanian JZ. Percieved discrimination and use of preventative health services. JGIM. 2006; 21(6): 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, et al. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. 2004. Soc Sci Med. 58: 1985–1997. [DOI] [PubMed] [Google Scholar]
- 20.Surwit RS, Schneider MS. Role of Stress in the Etiology and Treatment of Diabetes Mellitus. Psychosomatic Medicine. 1993; 55:380–393. [DOI] [PubMed] [Google Scholar]
- 21.Brown AF, Ettner SL, Piette J, Weinberger M, Gregg E, Shapiro MF, et al. Socioeconomic position and health among persons with diabetes mellitus: a conceptual framework and review of the literature. Epidemiol Rev 2004;26:63–77 [DOI] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics. Survey Questionnaire, National Health Interview Survey. Hyattsville, Maryland: National Center for Health Statistics;2002-2004. [Google Scholar]
- 23.Moffet Adler, Schillinger Ahmed, Laraia Selby, Neugebauer Liu, Parker, Warton, Karter. Cohort Profile: Diabetes Study of North California (DISTANCE) – objectives and design of a survey follow-up study of social health disparities in a managed care population. Int J Epidemiol. 38(1), 38–47 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherbourne CD, Stewart AL. The MOS Social Support Survey. Soc Sci Med 1991;32:705–14 [DOI] [PubMed] [Google Scholar]
- 25.Sampson, Raudenbush, Earls (1997) Neighborhood and violent crime: A multilevel study of collective efficacy. Science, 277, 918–924. [DOI] [PubMed] [Google Scholar]
- 26.Cohen S, Williamson G. Perceived stress in a probability sample of the United States In: Spacapan S, Oskamp S, editors. The social psychology of health. Newbury Park, CA; Sage; 1988. [Google Scholar]
- 27.Andreou E, Alexopoulos EC, Lionis C, Varvogli L, Gnardellis C, Crousos GP, et al. Perceived stress scale: reliability and validity study in Greece. Int J Environ Res Public Health 2011;8:3287–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morisky DE, Green LW, Levine DM. Concurent and predictive valifity of a self-reported measure of medication adherence. Med Care 1986;24:67–74. [DOI] [PubMed] [Google Scholar]
- 29.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results form 7 studies and a revised scale. Diabetes Care 2000;23:943–50. [DOI] [PubMed] [Google Scholar]
- 30.Schumacker RE, Lomax Richard G. A beginner’s guide to structural equation modeling. 3rd ed. New York, NY: Taylor and Francis Group; 2010. [Google Scholar]
- 31.Costello AB, Osborned JW. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval 2005;10. [Google Scholar]
- 32.Schreiber JB. Core reporting practices in structural equation modeling. Research in Social and Administrative Pharmacy 2008; 4: 83–97. [DOI] [PubMed] [Google Scholar]
- 33.Hooper D, Caughlan J, Mullen MR. Structural equation modeling: guidelines for determining model fit. Electronic J of Business Res Meth. 2008;6(1):53–60. [Google Scholar]
- 34.Surwit RS, van Tilburg MAL, Zucker N, McCaskill CC, Parekh P et al. Stress management improves long-term glycemic control in type 2 diabetes. Diabetes Care. 2002;25(1):30–34. [DOI] [PubMed] [Google Scholar]
- 35.Anderson RM, Funnell MM, Butler PM, Arnold MS, Fitzgerald JT, Feste CC. Patient empowerment: results of a randomized controlled trial. Diabetes Care. 1995;18(7):943–949. [DOI] [PubMed] [Google Scholar]
- 36.Richardson GE. The metatheory of resilience and resiliency. Journal of Clinical Psychology. 2002;58(3):307–321. [DOI] [PubMed] [Google Scholar]
- 37.Bradshaw BG, Richardson GE, Kulkarni K. Thriving with diabetes: an introduction to the resiliency approach for diabetes educators. The Diabetes Educator. 2007;33(4):643–649. [DOI] [PubMed] [Google Scholar]
- 38.Bradshaw BG, Richardson GE, Kumpfer K, Carlson J, Stanchfield J, et al. Determining the efficacy of a resiliency training approach in adults with type 2 diabetes. The Diabetes Educator. 2007;33(4)650–659. [DOI] [PubMed] [Google Scholar]