Abstract
Objectives:
To evaluate clinical effectiveness of pharmacologic stress myocardial perfusion imaging (MPI) with Positron Emission Tomography (PET) compared to Single Photon Emission Computed Tomography (SPECT) in patients with known CAD presenting with symptoms suggestive of ischemia.
Background:
Although PET MPI has been shown to have higher diagnostic accuracy in detecting hemodynamically significant CAD compared to SPECT MPI, whether this impacts downstream management has not been formally evaluated in randomized trials.
Methods:
We conducted a single center trial where patients with known CAD and suspected ischemia were randomized to undergo PET or attenuation-corrected SPECT MPI between 6/09 and 9/13. Post-test management was at the discretion of the referring physician, and patients were followed for 12 months. The primary end-point was diagnostic failure, defined as unnecessary angiography (absence of ≥ 50% stenosis in ≥1 vessels) or additional non-invasive testing within 60 days of the MPI. Secondary endpoints were post-test escalation in anti-anginal therapy, referral to angiography, coronary revascularization and health status at 3, 6 and 12 months.
Results:
A total of 322 patients with an evaluable MPI were randomized (n=161 in each group). At baseline, 88.8% patients were on aspirin, 76.7% on beta-blocker and 77.3% on statin. Diagnostic failure within 60 days occurred in only 7 (2.2%) patients (3 [1.9%] in the PET and 4 [2.5%] in SPECT group; p=0.7). There were no significant differences between the 2 groups in subsequent rates of coronary angiography, coronary revascularization or health status at 3, 6 and 12 months of follow-up (all p values ≥0.2), however when stratified by findings on MPI in a post-hoc analysis, those with high-risk MPI on PET testing had higher rates of angiography and revascularization on follow up as compared with SPECT MPI whereas those with low-risk PET studies had lower rates of both procedures as compared with SPECT (interaction of randomized modality * high risk MPI for 12 month catheterization p=0.001, 12 month revascularization p=0.09).
Conclusions:
In this contemporary cohort of optimally medically managed but symptomatic CAD patients, there was no discernible difference in rates of diagnostic failure at 60 days, subsequent coronary angiography or revascularization, or patient health status at 1 year between patients evaluated with PET compared to SPECT MPI. Downstream invasive testing rates with PET MPI were more consistent with high-risk features as compared to SPECT MPI.
Clinical Trial Registration:
Keywords: myocardial perfusion imaging, positron emission tomography, single photon emission computed tomography, effectiveness
INTRODUCTION
The use of stress myocardial perfusion imaging (MPI) for diagnosing coronary artery disease (CAD) has been supported by guidelines.(1,2) Stress perfusion findings on MPI also help in the identification of a higher-risk cohort, and refining the risk of future cardiac events and death.(3,4) In addition to aiding diagnosis and providing prognostic information, the utility of a test lies, in part, on its influence in guiding post-test management to most efficiently manage patients while optimizing their outcomes. Traditionally, Single Photon Emission Computed Tomography (SPECT) MPI has been used as a diagnostic and prognostic tool for patients presenting with symptoms of possible coronary ischemia. However, PET MPI offers superior image resolution, greater diagnostic accuracy, shorter acquisition times with lower radiation exposure and improved prognostic value over SPECT MPI.(3) Accordingly, it is increasingly being used in patients who are unable to exercise.(5) In a recent study, PET MPI demonstrated the highest diagnostic accuracy in a prospective head-to-head comparison of coronary computed tomography angiography, SPECT and PET in patients with suspected CAD when compared with coronary angiography and fractional flow reserve measurements.(6) However, whether this advantage in diagnostic accuracy with PET MPI translates to change in post-test management and increased clinical effectiveness, as compared with SPECT MPI, has not been studied in a randomized trial.(7)
Patients with known CAD presenting with new symptoms concerning for ischemia are both likely to have worsening disease and are often unable to exercise.(8) Hence, pharmacologic stress MPI testing is often indicated for diagnosis and risk-stratification. To date, there has been no randomized comparison of the clinical effectiveness of SPECT and PET MPI in this higher-risk group of patients. The goal of the current study is to examine post-test clinical effectiveness in patients with a history of known CAD presenting with new symptoms concerning for suspected ischemia where patients were randomized to SPECT vs. PET MPI imaging.
METHODS
Effectiveness Study of Single Photon Emission Computed Tomography (SPECT) Versus Positron Emission Tomography (PET) Myocardial Perfusion Imaging ( NCT00976053) is a prospective randomized trial of patients with known stable CAD presenting with symptoms of suspected ischemia referred for clinically indicated MPI testing.(9) The study was funded by Blue Cross Blue Shield of Kansas City. The funding agencies had no involvement in the conduct or reporting of the study.
Study population
The study enrolled 330 patients with history of CAD presenting with new or worsening symptoms for whom an MPI test was ordered by the referring physicians, and who required pharmacologic stress MPI from June 2009 to August 2013. History of CAD was defined as presence of prior MI or prior coronary revascularization. All patients were enrolled at 4 nuclear laboratories within the Saint Luke’s Health System, which are located at 4 metro hospitals in the greater Kansas City area. Enrollment criteria included men or non-pregnant women between ages of 30-90 years with history of CAD presenting with chest pain and/or dyspnea in either the office or hospital. Enrollment was stratified by presence of diabetes and inpatient vs. outpatient status of the patient. Exclusion criteria included renal dysfunction (serum creatinine greater than 2.5 mg/dl), myocardial infarction or coronary revascularization within past 6 months, significant valvular disease, prior transplant, morbid obesity (body mass index ≥ 38 kg/m2), LV Ejection Fraction < 40%, pregnant patients and patients who were unwilling to undergo angiography if indicated.
The study protocol was approved by the Institutional Review Board at Saint Luke’s Hospital of Kansas City. All patients provided written informed consent prior to enrollment into the study.
Study design
At the time of enrollment, patients were randomized to either SPECT or PET MPI. Post-test management was left at the discretion of the ordering physician. All patients received both SPECT and PET MPI tests as a part of the study protocol, however only the randomized study was read and reported during the duration of the trial. All patients were followed for a year and patients were queried on their medications, whether they underwent coronary angiography, coronary revascularization or other testing, vital status, and health status at 1, 3, 6 and 12 months. Records of testing and coronary angiography and revascularization were obtained, and adjudicated by members of the study team, blinded to randomized assignment.
Randomization
After patient eligibility was verified and informed consent was obtained, patients were randomized in a 1:1 fashion using permuted blocks of 4, stratified by hospital status and diabetes. Identical, tamper-evident, opaque and well-sealed envelopes containing randomization group and trial identification number were used within each stratum. Envelopes were opened sequentially and randomization information was transcribed onto a randomization form. The randomization determined the MPI test modality for which the result would be made available to the ordering physician.
Stress MPI Study Protocol
All PET studies were performed using a Siemens ECAT ACCEL PET camera or Siemens Biograph 16 or 64 PET/CT cameras. All SPECT studies were performed using a conventional small field of view camera (CardioMD, Philips Medical Systems, Milpitas, CA), a large field of view Anger SPECT cameras (Cardio60™; and CardioEPIC™) with CT Attenuation correction or D-SPECT Solid State Detector-SPECT camera (Spectrum Dynamics, Sarasota, FL). PET and SPECT studies were performed according to standard ASNC protocol guidelines.
All patients had to be fasting for at least 6 hours, and were asked to withhold caffeine containing beverages for 24 hours prior to the test. Beta-blockers, calcium channel blockers, and nitrates were withheld on the morning of the test.
First, all patients received an intravenous injection of a weight-based dose of Tc-99m sestamibi (7.8-11.0 mCi). ECG-gated rest SPECT image acquisition started approximately 60 minutes post-radioisotope injection. This was followed by PET MPI imaging approximately 90 minutes later. For the PET MPI study, patients received an intravenous injection of Rb-82 (mean rest dose=42.9 +/− 9.0 mCi (23.7-60.1 mCi)) followed by list mode ECG-gated rest emission image acquisition for 7 minutes for the PET/CT systems and a 5 minutes ECG gated perfusion study on the dedicated PET system. All patients then underwent pharmacological stress testing using regadenoson (n=36; 0.4 mg rapid iv push) or weight-based dose of dipyridamole (n=286; 0.56 mg/kg iv over 4 minutes). At peak stress, another dose of Rb-82 (mean stress dose=42.4 +/− 9.1 mCi (21.1-64.9 mCi)) was injected, and stress PET images were acquired in a similar manner to rest PET images. Stress dose of iv Tc-99m sestamibi (19.2-38.1 mCi) was also injected at peak stress, prior to the stress dose of Rb-82. Stress SPECT images were acquired approximately 60 minutes post-stress Tc-99m injection in similar fashion to rest SPECT image acquisition. All SPECT systems except D-SPECT employed line source or CT attenuation correction. Dedicated PET camera employed line-source attenuation while PET-CT cameras employed CT attenuation correction.
Post-acquisition, list mode PET studies were reconstructed by rebinning into an eight binned ECG-gate perfusion study (90-330 seconds) using commercial software (Imagen Pro, Kansas City, MO). Studies were all inspected for misregistration and corrected when necessary.
SPECT studies from the line source SPECT system were processed using iterative reconstruction and Astonish attenuation, scatter and distance dependent blur correction. SPECT/CT studies were processed and corrected for misregistration and then reconstructed using iterative reconstruction and Flash-3D attenuation, scatter and distance dependent blur correction.
Analysis of myocardial perfusion and LVEF
All studies for the trial were read by 2 experienced nuclear cardiologists (A.I.M and T.M.B). Perfusion images were displayed using commercial software (Cedars Sinai Cardiac Suite, QPS for SPECT, QPET for PET; Los Angeles, CA) and interpreted using a 17-segment model and standard 5-point scoring system (0=normal, 1=mild reduction in counts; 2= moderate reduction in counts, 3=severe reduction in counts and 4=absent counts).(10) Global Summed Rest Score (SRS), Summed Stress Score (SSS) and Summed Difference Score (SDS) were calculated from stress and rest SPECT and PET scintigraphic images. Studies were classified into non-ischemic, mild, moderate or severe ischemia using SDS cut-offs of 0, 1-2, 3-6, ≥7 respectively for both SPECT and PET MPI. Transient ischemic dilation (TID) ratio was calculated using commercial software, and a TID ratio >1.2 was considered abnormal.(11) Rest and stress LVEF were calculated from gated myocardial perfusion images acquired with 8-frame gating using the Cedars Sinai QGS software and verified visually for accuracy. LVEF reserve was calculated as the difference between Stress LVEF and Rest LVEF, and a reserve <0% was considered abnormal. (12,13) High risk MPI findings were defined as the presence of moderate or severe ischemia on MPI, a TID ratio of >1.2, or a LVEF reserve of <0%. Of note, myocardial blood flow reserve information was not available on the PET MPI patients during the study, and this was not included in the definition of high-risk patients. Patients without any high risk MPI findings were classified as having low risk MPI.
Study Endpoints
All patients with a complete evaluable MPI study at baseline were followed for a period of one year. The primary end-point was the rate of diagnostic failure, defined as unnecessary coronary angiography (i.e. absence of ≥ 50% stenosis in ≥1 vessels should the patient have undergone angiography) or additional confirmatory non-invasive testing such as repeat MPI, stress echo, of CCTA within 60 days of the baseline MPI. Secondary endpoints included post-MPI intensification of anti-anginal therapy at 3 months; and referral to coronary angiography, presence of obstructive disease on angiography and coronary revascularization (PCI/CABG) at 3, 6 and 12 months. Patient health status measured at 1, 3, 6 and 12 months of follow-up was also assessed as a secondary end-points, to assess if the lower rates of subsequent testing/treatment with PET vs. SPECT MPI if observed, would affect patients’ symptoms, function and quality of life.
Anti-anginal therapy was defined as receipt of medications within the following drug classes: aspirin/other anti-platelets, beta-blockers, statins, calcium channel blockers, nitrates and ranolazine. Escalation in anti-anginal therapy at 3 months was defined as addition of another anti-anginal medication class or increase in dose or frequency of one or more of existing anti-anginal medications within 3 months post baseline MPI. Obstructive CAD was defined as stenosis of ≥ 50% of the left main coronary artery or ≥ 70% of a major epicardial or branch vessel or ≥ 70% of a graft vessel with occluded flow by native circulation for patients with history of coronary bypass graft surgery.
Patient health status was measured using Seattle Angina Questionnaire (SAQ) and Rose Dyspnea Score. The SAQ is a 19-item validated questionnaire with a 4-week recall period that measures health status in patients with coronary artery disease across the following 5 domains: Physical Limitation (PL), Anginal Stability, Angina Frequency (AF), Treatment Satisfaction and Quality of Life (QOL).(14) The SAQ Summary Score (SAQ SS) is the average of the PL, AF and QOL domains. The SAQ and its subscales are scored on a scale of 0-100, with higher scores indicating less angina and better health status. A mean between-group change of 5 points is commonly regarded as minimally clinically significant difference in the SAQ. The RDS is a 4-item questionnaire with a 1-month recall period which assesses the level of dyspnea with common activities for the patients, validated in patients with coronary artery disease.(15,16) The RDS is scored from 0-4, with 0 indicating no dyspnea and 4 indicating dyspnea with daily activities of washing and dressing; the score indicates the greatest limitation in activity that results in dyspnea for the patient.
All follow-up was conducted by a trained clinical research coordinator through telephone using a standardized form and verified against medical records for accuracy and supplemental information.
Statistical Analysis
Given the lack of prior randomized data on clinical effectiveness of SPECT vs. PET MPI, the study was powered at p <0.05 level for the primary endpoint of diagnostic failure based on retrospective data published by Merhighe et al.(17) Based on assumption that SPECT will have a diagnostic failure rate of 30%, and that PET will have a 50% lower failure rate compared to SPECT, estimated sample size was 300. Enrollment was planned for a total of 330 patients, over-sampling by 10% to compensate for drop-outs and loss to follow-up.
Study end-points were compared according to intention-to-treat analysis. Baseline patient demographic, clinical characteristics and medication data were compared between the 2 randomized groups (PET and SPECT) using t-test for continuous variables and chi-square or Fisher-exact test for categorical variables. Study end-points were also compared between the randomized groups using chi-square or Fisher-exact test. Health status measures including SAQ domain scores and RDS were compared between MPI modalities at all follow-up time points using linear repeated measures model. Follow-up was complete in 97.5%, 95.3% and 95% of all patients at 3, 6 and 12 months.
We conducted a post-hoc analysis to assess whether downstream testing rates by imaging modality corresponded to whether a patient had a stress perfusion test result which was high-risk or not high-risk. Specifically, we evaluated for differences in the rates of catheterization and revascularization at 3, 6 and 12 months for PET vs. SPECT MPI groups, stratified by low and high-risk findings on MPI studies using Fisher-exact or Chi-square tests, and tested for an interaction between randomized test modality and high risk vs. low-risk MPI on outcomes.
All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). Two-sided p-values <0.05 were considered to be statistically significant.
RESULTS
Of the 330 patients that were randomized, 8 patients (4 in each of the PET and SPECT groups) did not have an MPI test that could be evaluated (eg: technical issues in image acquisition, incomplete test, etc.) at baseline. Therefore, 322 patients constituted the analytical cohort, of which 161 were randomized to evaluation with a PET MPI and 161 with a SPECT MPI. The mean age of the cohort was 66.3 ± 9.7 years, 64.9% were men and 27.3% had diabetes mellitus. At baseline, 251 patients (78.0%) were on 3 or more anti-anginal medications, 286 (88.8%) were on aspirin, 247 (76.7%) on beta-blocker therapy, and 249 (77.3%) on a statin. Overall 39.8% of studies reported ischemia (7.8% with mild ischemia, 16.8% with moderate ischemia and 15.2% with severe ischemia), 58.7% were without ischemia and 1.6% were non-diagnostic. Baseline patient characteristics were well-balanced between the PET and SPECT groups (Table 1). Rest and Stress LVEF were higher in patients randomized to SPECT compared with those randomized to PET MPI. There were numerically higher proportions of tests read as non-diagnostic and with moderate ischemia, but fewer read as severe ischemia, in patients randomized to SPECT; however it did not reach the level of clinical significance (p=0.09; Table 1). There was also no significant difference between the 2 randomized groups in the territorial distribution of ischemia.
Table 1:
Baseline patient characteristics in the PET and SPECT MPI groups
| PET MPI (N=161) | SPECT MPI (N=161) | p-value | |
|---|---|---|---|
| Patient characteristics | |||
| Age, years | 66.5 ± 9.4 | 66.1 ± 10.0 | 0.75 |
| Male gender | 101 (62.7%) | 108 (67.1%) | 0.41 |
| Body mass index, kg/m2 | 28.8 ± 4.5 | 29.2 ± 5.2 | 0.50 |
| Employment Status | 0.77 | ||
| Disabled | 17 (10.6%) | 17 (10.6%) | |
| Retired | 85 (53.1%) | 92 (57.5%) | |
| Full time employed | 43 (26.9%) | 41 (25.6%) | |
| Part time employed | 11 (6.9%) | 6 (3.8%) | |
| Unemployed | 4 (2.5%) | 4 (2.5%) | |
| Hypertension | 139 (86.34%) | 150 (93.17%) | 0.04 |
| Diabetes | 43 (26.7%) | 46 (28.6%) | 0.14 |
| Hyperlipidemia | 160 (99.38%) | 160 (99.38%) | 1.0 |
| Smoker | 27 (16.77%) | 32 (19.88%) | 0.47 |
| Family history of CVD | 66 (40.99%) | 74 (45.96%) | 0.37 |
| Cerebrovascular Accident | 24 (14.91%) | 25 (15.53%) | 0.88 |
| Peripheral Vascular Disease | 43 (26.71%) | 43 (26.71%) | 1.0 |
| Atrial Fibrillation | 32 (19.88%) | 19 (11.80%) | 0.05 |
| Hospital Status at time of MPI | 0.18 | ||
| Inpatient | 1 (0.62%) | 4 (2.48%) | |
| Outpatient | 160 (99.38%) | 157 (97.52%) | |
| Abnormal Baseline EKG | 72 (44.72%) | 63 (39.13%) | 0.31 |
| Symptoms | |||
| Chest Pain | 0.30 | ||
| Typical | 60 (44.1%) | 45 (36.0%) | |
| Atypical | 40 (29.4%) | 47 (37.6%) | |
| Non-anginal | 36 (26.5%) | 33 (26.4%) | |
| Dyspnea | 106 (65.8%) | 114 (70.8%) | 0.34 |
| Syncope | 5 (3.1%) | 5 (3.1%) | 1.0 |
| Baseline Medications | |||
| Aspirin | 144 (89.4%) | 142 (88.2%) | 0.72 |
| Other Antiplatelets | 58 (36.0%) | 54 (33.5%) | 0.64 |
| Beta-blockers | 122 (75.8%) | 125 (77.6%) | 0.69 |
| Calcium channel blockers | 31 (19.3%) | 30 (18.6%) | 0.89 |
| Nitrates | 74 (46.0%) | 86 (53.4%) | 0.18 |
| Statins | 127 (78.9%) | 122 (75.8%) | 0.51 |
| Ranolazine | 1 (0.6%) | 3 (1.9%) | 0.31 |
| ACEI/ARB | 98 (60.9%) | 99 (61.5%) | 0.91 |
| No of anti-anginals at baseline | 0.62 | ||
| 0 | 2 (1.2%) | 0 (0.0%) | |
| 1 | 5 (3.1%) | 7 (4.4%) | |
| 2 | 30 (18.6%) | 27 (16.8%) | |
| 3 | 66 (41.0%) | 65 (40.4%) | |
| ≥4 | 58 (36.0%) | 62 (38.5%) | |
| Stress testing characteristics | |||
| Rest Heart Rate, bpm | 64.7 ± 11.3 | 64.9 ± 11.3 | 0.90 |
| Rest Systolic Blood Pressure, mmHg | 131.9 ± 20.0 | 130.5 ± 20.5 | 0.52 |
| Stress Heart Rate, bpm | 85.8 ± 17.7 | 82.8 ± 17.8 | 0.13 |
| Stress Systolic Blood Pressure, mmHg | 122.8 ± 19.9 | 122.0 ± 20.1 | 0.74 |
| ECG Response | 0.35 | ||
| Non-ischemic | 123 (76.4%) | 117 (72.7%) | |
| Ischemic | 18 (11.2%) | 14 (8.7%) | |
| Equivocal | 0 (0.0%) | 1 (0.6%) | |
| Non-diagnostic | 20 (12.4%) | 29 (18.0%) | |
| MPI findings | |||
| Rest LVEF, % | 59 ± 12 | 65 ± 16 | <.001 |
| Stress LVEF, % | 62 ± 11 | 66 ± 14 | 0.003 |
| Summed Rest Score (median) | 0 (0, 0) | 0 (0, 1) | 0.06 |
| No ischemia | 94 (58.4%) | 95 (59.0%) | 0.09 |
| Mild ischemia | 11 (6.8%) | 14 (8.7%) | |
| Moderate ischemia | 23 (14.3%) | 31 (19.3%) | |
| Severe ischemia | 32 (19.9%) | 17 (10.6%) | |
| Non-diagnostic | 1 (0.6%) | 4 (2.5%) | |
| LAD ischemia* | 41 (25.5%) | 43 (26.7%) | 0.80 |
| LCX ischemia* | 31 (19.3%) | 32 (19.8%) | 0.89 |
| RCA ischemia* | 32 (21.1%) | 39 (24.2%) | 0.51 |
p-values obtained using chi-square test or Fisher-exact test for categorical variables and t-test or Wilcoxon Rank Sum (Summed Rest Score) for continuous variables. PET= Positron Emission Tomography, SPECT= Single Photon Emission Computed Tomography, MPI= Myocardial Perfusion Imaging, ACEI= Angiotensin Converting Enzyme Inhibitor, ARB= Angiotensin Receptor Blocker, LVEF= left ventricular ejection fraction. LAD= left anterior descending, LCX= left circumflex, RCA= right coronary artery.
there may be overlap between different vascular territories
The primary endpoint of diagnostic failure within 60 days occurred in only 7 (2.2%) patients and did not differ between the 2 imaging groups [3 (1.9%) in the PET and 4 (2.5%) in SPECT group (p=0.7); Table 2]. Anti-anginal therapy was escalated in 78 (24.2%) patients by 3 months but occurred with similar frequency in PET and SPECT evaluated patients (25.5% for PET group and 23.0% for SPECT; p=0.60). A total of 58 (18.0%) patients were referred to coronary angiography within 3 months of MPI, with no significant difference between the 2 groups [PET vs. SPECT; 20.5% vs. 15.5%, p=0.25]. Similarly, rates of coronary revascularization within 3 months were similar between the 2 groups (11.2% for PET vs. 9.9% for SPECT; p=0.72). Rates of coronary angiography or revascularization remained similar at 6 and 12 months of follow-up (see Table 2). Of the 25 patients undergoing revascularization within 12 months of follow-up in randomized PET MPI group, 16(64%) had ischemia in the left anterior descending artery territory, 8(32%) in the left circumflex and 14(56%) had ischemia in the right coronary artery distribution. Similarly, of the 24 patients undergoing revascularization within 12 months of follow up in the randomized SPECT MPI group, 14(58%), 11(46%) and 12(50%) had ischemia in the left anterior descending, left circumflex and right coronary artery territories.
Table 2:
Study outcomes for patients randomized to PET and SPECT MPI groups
| PET MPI (N=161) | SPECT MPI (N=161) | p-value | |
|---|---|---|---|
| Primary Endpoint | |||
| Diagnostic failure within 60 days | 3 (1.9%) | 4 (2.5%) | 0.70 |
| Secondary Endpoints | |||
| Escalation in anti-anginal therapy (3 months) | 41 (25.5%) | 37 (23.0%) | 0.60 |
| Referral to Catheterization (3 months) | 33 (20.5%) | 25 (15.5%) | 0.25 |
| Obstructive disease on angiography (3 months) | 25 (15.5%) | 17(10.6%) | 0.19 |
| Revascularization PCI/CABG (3 months) | 18 (11.2%) | 16 (9.9%) | 0.72 |
| Referral to Catheterization (6 months) | 36 (22.4%) | 30 (18.6%) | 0.41 |
| Revascularization PCI/CABG (6 months) | 20 (12.4%) | 19 (11.8%) | 0.86 |
| Referral to Catheterization (6 months) | 46 (28.6%) | 45 (28.0%) | 0.90 |
| Revascularization PCI/CABG (6 months) | 25 (15.5%) | 24 (14.9%) | 0.88 |
| Referral to Catheterization (12 months) | 46 (28.6%) | 45 (28.0%) | 0.90 |
| Revascularization PCI/CABG (12 months) | 25 (15.5%) | 24 (14.9%) | 0.88 |
| Late revascularization after 3 months | 7 (4.3%) | 8 (5.0%) | 0.70 |
| Myocardial Infarction | 0 (0.0%) | 2 (1.2%) | 0.50 |
| Death | 1 (0.6%) | 2 (1.2%) | 1.00 |
p-values obtained using chi-square test or Fisher-exact test. Diagnostic failure= unnecessary coronary angiography (absence of ≥ 50% stenosis in ≥ 1 vessels) or additional non-invasive testing; escalation = addition/increase in dose of anti-anginal therapy. ACEI/ARB=Angiotensin Converting Enzyme Inhibitor/Angiotensin Receptor Blocker
Regarding health status outcomes, all SAQ domains for the entire study cohort increased significantly from baseline with the largest change within the first 1 month after MPI (Figure 1). However, improvements in health status, as measured by different SAQ domains, and RDS were similar between the 2 groups (SAQ angina frequency: p=0.70, SAQ physical limitation: p=0.07, SAQ quality of life: p=0.20, RDS: p=0.53).
Figure 1: Longitudinal health status outcomes for patients with known coronary artery disease and new or worsening symptoms randomized to PET vs. SPECT myocardial perfusion imaging over a year of follow-up.
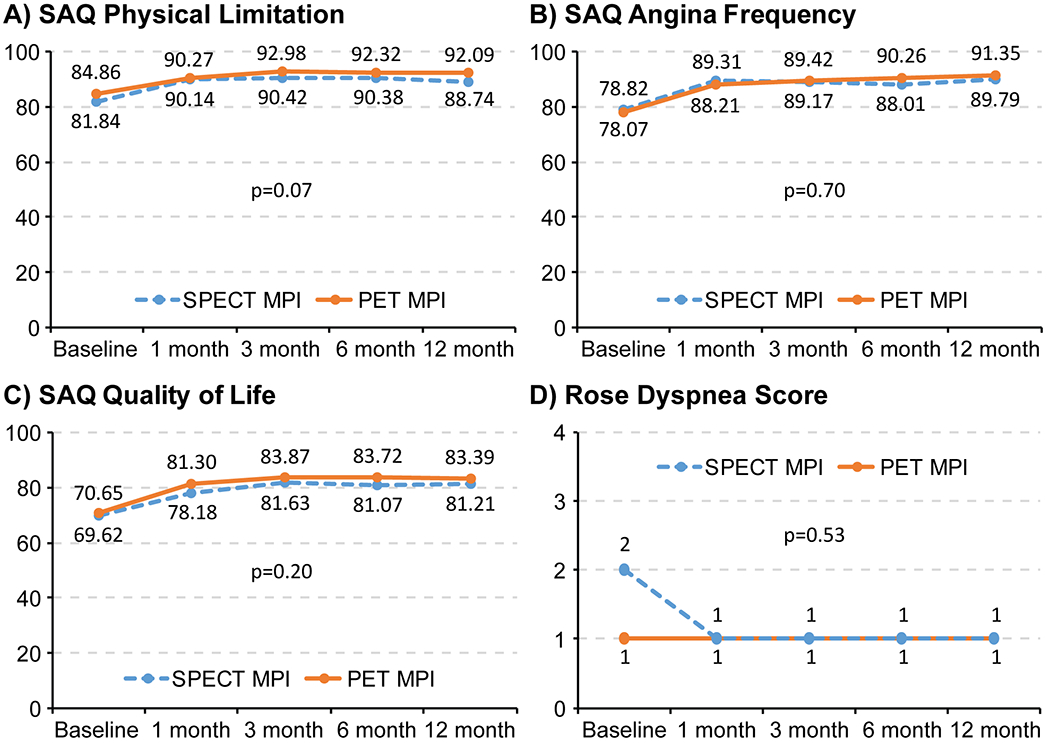
SAQ= Seattle Angina Questionnaire, RDS- Rose Dyspnea Score. Mean scores presented for SAQ and median scores presented for RDS. P values are for differences between PET and SPECT MPI over time, derived using t-test for SAQ physical limitation, angina frequency and quality of life scores, and Wilcoxon signed-rank test for RDS.
Rates of catheterization and revascularization on follow-up among patients randomized to PET and SPECT MPI with low-risk and high-risk MPI (moderate-severe ischemia or TID or LVEF reserve <0%) are shown in Table 3 and Supplemental Table 1. We found a significant interaction in post-testing treatment by testing modality as a function of high risk findings on MPI (interaction of randomized modality * high risk MPI for 12 month catheterization p=0.001, 12 month revascularization p=0.09). Among patients with low-risk findings on MPI, a lower proportion of patients in the PET group underwent catheterization and revascularization at 3, 6 and 12 months of follow-up compared to the SPECT group. Among patients with high-risk findings on MPI, greater proportion of patients in the PET underwent catheterization and revascularization at 3, 6 and 12 months of follow-up compared to SPECT group (interaction of randomized modality * high risk MPI for 12 month catheterization p=0.001, 12 month revascularization p=0.09).
Table 3:
Rates of downstream resource utilization among patients with low and high risk MPI findings based on multi-parametric assessment of ischemia, transient ischemic dilation and LVEF Reserve
| PET MPI | SPECT MPI | p-value | |
|---|---|---|---|
| Low-risk MPI | |||
| Referral to catheterization | |||
| 3-month catheterization | 2/96 (2.1%) | 6/67 (9.0%) | 0.07* |
| 6-month catheterization | 3/96 (3.1%) | 7/67 (10.4%) | 0.09* |
| 12-month catheterization | 8/96 (8.3%) | 13/67 (19.4%) | 0.04* |
| Rate of revascularization | |||
| 3-month revascularization | 0/96 (0%) | 3/67 (4.5%) | 0.07* |
| 6-month revascularization | 0/96 (0%) | 4/67 (6.0%) | 0.03* |
| 12-month revascularization | 4/96 (4.2%) | 5/67 (7.5%) | 0.01* |
| High-risk MPI | |||
| Referral to catheterization | |||
| 3-month catheterization | 31/65 (47.7%) | 19/94 (20.2%) | <0.01 |
| 6-month catheterization | 33/65 (50.8%) | 23/94 (24.5%) | <0.01 |
| 12-month catheterization | 37/65 (56.9%) | 32/94 (34.1%) | <0.01 |
| Rate of revascularization | |||
| 3-month revascularization | 18/65 (27.7%) | 13/94 (13.8%) | 0.03 |
| 6-month revascularization | 20/65 (30.8%) | 15/94 (16.0%) | 0.03 |
| 12-month revascularization | 21/65 (32.3%) | 18/94 (19.1%) | 0.06 |
High Risk MPI was defined as patients with moderate or severe ischemia or transient ischemic dilation (TID >1.20) or LVEF Reserve <0%. Patients not meeting these criteria were considered having low-risk MPI in both PET and SPECT.
p-values calculated using chi-square or *Fisher Exact test
DISCUSSION
Our study is the first randomized comparison of the clinical effectiveness of pharmacologic stress PET or SPECT MPI in symptomatic patients undergoing risk stratification for CAD. In this cohort of patients with known CAD, who were well managed medically prior to MPI testing, there were no differences in the rates of change in medical management or angiography and revascularization up to 12 months after MPI testing. Event rates for the primary end-point of diagnostic failure were much lower than previously reported in the medical literature and did not differ between the 2 strategies. Collectively, these findings show similar post-test management and clinical outcomes with SPECT and PET stress MPI.
In addition to a lower radiation exposure and shorter time for acquisition, PET MPI offers greater diagnostic accuracy over SPECT MPI.(6,18,19) Prior comparisons of the 2 modalities have been largely done in observational settings. Large meta-analyses have demonstrated higher sensitivity(18) and greater diagnostic accuracy(19) of PET, compared with SPECT. Recently, in a prospective head-to-head clinical comparative study in 208 patients with suspected CAD, PET had greater sensitivity, similar specificity, and increased diagnostic accuracy (85%) compared to SPECT (77%) in detecting hemodynamically significant coronary stenoses defined by fractional flow reserve ≤ 0.80.(6) Whether the improvement in diagnostic accuracy leads to better post-test management in patients with PET MPI compared to SPECT MPI is not well-studied. In a case-control comparison, PET MPI was associated with lower subsequent angiography and revascularization rates compared with SPECT MPI.(17) The observational SPARC study found that the use of PET was associated with higher 90-day angiography rates compared with SPECT in patients without prior CAD, however rates of angiography and revascularization were similar between mild, moderate or severely abnormal SPECT and PET MPI. Rates of 90-day aspirin use was greater in patients undergoing PET MPI compared with SPECT MPI, but there was no difference in 90-day beta-blocker or lipid lowering therapy use by test modality.(20) To date, there has been no prospective randomized comparison of post-test patient management and clinical decision making between PET and SPECT MPI.
Our study is the first to examine clinical decision making and clinical outcomes after PET and SPECT MPI in a rigorous randomized controlled trial setting. In the current value-based care landscape, there is an urgent need to look beyond the diagnostic and prognostic benefits of an imaging modality, and study the impact of imaging on clinical decision making. While PET MPI may offer better diagnostic accuracy over SPECT MPI, our study shows that it might not be enough to change downstream patient management and resource utilization that would mediate outcomes. While there were very few cardiac events on our study follow-up, we did not see a difference in health status outcomes between the 2 modalities through a year of follow-up. However, when stratified by low and high risk findings on MPI in a post-hoc analysis, downstream invasive testing rates among patients in the PET group was more consistent with the results of their stress perfusion testing. We found that a higher proportion of patients with high risk findings on PET MPI were referred to cardiac catheterization and underwent coronary revascularization on follow-up compared to SPECT, and a lower proportion of patients with low-risk findings on PET underwent follow-up catheterization and revascularization compared to SPECT MPI. This suggests that PET MPI supports better patient risk assessment than SPECT MPI, resulting in improved downstream utilization. Improving communication of risk associated with the MPI result with the patient and the referring physician, and providing recommendations for patient management with the MPI report could potentially optimize downstream resource utilization with both modalities and help identify a difference in outcomes, if one truly exists. Our trial was designed in a pragmatic manner, to simulate real-world response by referring physicians to the MPI results they receive, and no direction was provided in addition to the standard reporting for all MPI studies that was employed at our laboratory at the time of the study. Our center routinely employs attenuation correction with SPECT MPI, as such all the SPECT MPI studies were corrected for attenuation using low-dose CT or done using newer generation CZT D-SPECT cameras with supine and upright protocols which offer better diagnostic quality images with SPECT. This might be a potential reason for low diagnostic failure rates in the SPECT comparator group. Myocardial blood flow values on PET MPI were also not taken into account while reading the PET MPI for the trial, as it was predominantly a research tool during the study period, and the provision of these data should be tested in future studies.
Our study results should be viewed in the context of the following limitations. This was a single-center randomized trial, conducted at a tertiary referral center with expertise in nuclear cardiology. The quality of MPI studies as well as clinical decision making patterns after MPI testing may be different in other settings. This was evidenced by how well the patients were medically managed for their coronary artery disease at baseline. All patients had known coronary artery disease prior to study enrollment, and future studies should examine the impact of alternative MPI modalities in patients with no prior CAD presenting with symptoms of suspected ischemia. We also were unable determine the appropriateness of downstream testing ordered by the referring physicians. Finally, the study appears to be under-powered for the primary endpoint of diagnostic failure as well as secondary endpoints for follow-up catheterization and revascularization rates. With the rates for diagnostic failure observed in the study, it only had 5.6% power to identify a significant difference between the 2 randomized groups in a post-hoc power calculation. Low event rates for diagnostic failure and cardiac events preclude us from making any conclusions regarding those end-points for the study. With the current sample size, it would require a 35% and a 45% relative difference in rates of 12-month catheterization and revascularization between PET and SPECT groups to detect a difference with adequate power. When stratified by high risk vs. low risk MPI findings for the 12-month catheterization outcome, it would require 72 and 151 patients per group respectively for patients with high risk and low risk MPI to identify a significant difference in 12- month catheterization rates between PET vs. SPECT with adequate power. Similarly, a sample size of 171 and 793 per group respectively for patients with high risk and low risk MPI is required to identify a difference in 12-month revascularization rates between PET and SPECT with adequate power. Future randomized efforts at evaluating effectiveness of PET vs. SPECT should be stratified by MPI findings.
In conclusion, among 322 patients with known CAD and new or worsening symptoms suggestive of ischemia, there was no difference in medical or invasive clinical management or health status over a period of 12 months after randomization to PET vs. SPECT MPI. Similarly, rates for diagnostic failure were very low and similar between the 2 modalities. However, when stratified by low and high risk MPI findings in a post-hoc analysis, downstream resource utilization was more consistent with the risk classification by PET compared to SPECT. Larger randomized studies involving multiple centers and including diagnostic as well as risk-stratification populations to allow for greater power and generalizability are needed to assess whether the PET compared with SPECT MPI testing lead to more efficient utilization of downstream invasive procedures or improved patient health status.
Supplementary Material
Central Illustration: Effectiveness Study of Single Photon Emission Computed Tomography (AC SPECT) Versus Positron Emission Tomography (PET) Myocardial Perfusion Imaging: Study design and main results.
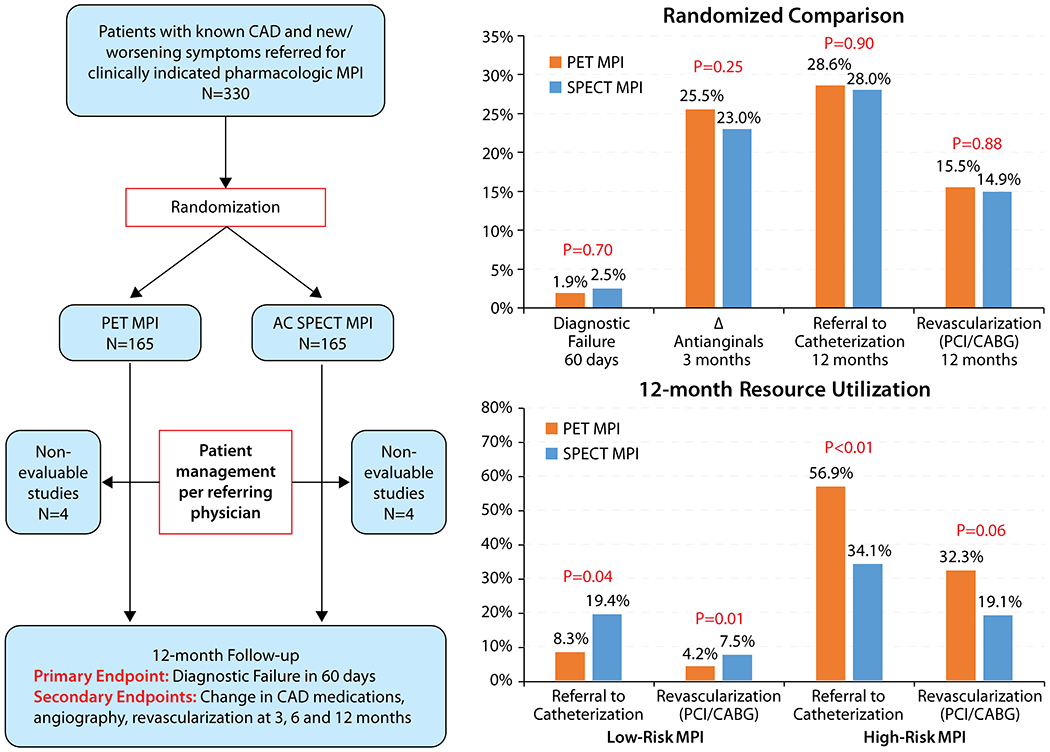
AC= Attenuation Corrected
PERSPECTIVES.
Competency in Patient Care and Procedural Skills:
In a single-center randomized controlled trial of patients with known coronary artery disease presenting with symptoms suggestive of ischemia who were well-managed medically, there was no difference in post-test management or patient health status outcomes between patients undergoing PET vs. attenuation corrected SPECT MPI over a period of 12 months. Downstream testing was more proportional to risk assessed on MPI with PET, as compared with SPECT in a post-hoc analysis.
Translational Outlook:
Larger randomized studies in a diagnostic cohort and in multiple centers are needed to assess if improved diagnostic accuracy with PET MPI translates into benefit in post-test clinical decision making compared to SPECT MPI, before its routine use is advocated. Improving communication of risk associated with MPI may optimize downstream resource utilization after MPI.
Acknowledgments
FUNDING SOURCE
This investigation was funded by a clinical research grant from Blue Cross Blue Shield of Kansas City. The funding agency had no involvement in the conduct or reporting of the study.
DISCLOSURES
Drs. Patel and Al Badarin are supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T32HL110837. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Spertus receives research grant support from Abbott Vascular, Novartis and is the PI of an analytic center for the American College of Cardiology. He serves as a consultant to United Healthcare, Bayer, Janssen, AstraZeneca and Novartis. He has an equity interest in Health Outcomes Sciences.
Dr. Bateman receives research grant support from Astellas and GE Healthcare. He serves as a consultant to GE Healthcare. He has an equity interest in Cardiovascular Imaging Technologies. He has intellectual property rights for Imagen Pro/MD/Q/3D software.
Dr. Case has an equity interest in Cardiovascular Imaging Technologies and has intellectual property rights for Imagen Pro/MD/Q/3D software.
Dr. Heller is a medical advisor for Molecular Imaging Services and is a consultant for GE HealthCare.
The other authors report no conflicts.
ABBREVIATION LIST:
- CAD
coronary artery disease
- MPI
myocardial perfusion imaging
- SPECT
Single Photon Emission Computed Tomography
- PET
Positron Emission Tomography
- PCI
percutaneous coronary intervention
- CABG
coronary artery bypass surgery
- SAQ
Seattle Angina Questionnaire
- RDS
Rose Dyspnea Score
References:
- 1.Fihn SD, Gardin JM, Abrams J et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease: Executive Summary. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons 2012;60:2564–2603. [DOI] [PubMed] [Google Scholar]
- 2.Wolk MJ, Bailey SR, Doherty JU et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 Multimodality Appropriate Use Criteria for the Detection and Risk Assessment of Stable Ischemic Heart Disease. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons 2014;63:380–406. [DOI] [PubMed] [Google Scholar]
- 3.Dorbala S, Di Carli MF. Cardiac PET perfusion: prognosis, risk stratification, and clinical management. Semin Nucl Med 2014;44:344–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cremer P, Hachamovitch R. Assessing the prognostic implications of myocardial perfusion studies: identification of patients at risk vs patients who may benefit from intervention? Curr Cardiol Rep 2014;16:472. [DOI] [PubMed] [Google Scholar]
- 5.Bateman TM, Dilsizian V, Beanlands RS, DePuey EG, Heller GV, Wolinsky DA. American Society of Nuclear Cardiology and Society of Nuclear Medicine and Molecular Imaging Joint Position Statement on the Clinical Indications for Myocardial Perfusion PET. J Nucl Med 2016;57:1654–1656. [DOI] [PubMed] [Google Scholar]
- 6.Danad I, Raijmakers PG, Driessen RS et al. Comparison of Coronary CT Angiography, SPECT, PET, and Hybrid Imaging for Diagnosis of Ischemic Heart Disease Determined by Fractional Flow Reserve. JAMA Cardiol 2017;2:1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hachamovitch R, Nutter B, Hlatky MA et al. Patient management after noninvasive cardiac imaging results from SPARC (Study of myocardial perfusion and coronary anatomy imaging roles in coronary artery disease). J Am Coll Cardiol 2012;59:462–74. [DOI] [PubMed] [Google Scholar]
- 8.Jouni H, Askew JW, Crusan DJ, Miller TD, Gibbons RJ. Temporal Trends of Single-Photon Emission Computed Tomography Myocardial Perfusion Imaging in Patients With Coronary Artery Disease: A 22-Year Experience From a Tertiary Academic Medical Center. Circ Cardiovasc Imaging 2017;10. [DOI] [PubMed] [Google Scholar]
- 9.Effectiveness Study of Single Photon Emission Computed Tomography (SPECT) Versus Positron Emission Tomography (PET) Myocardial Perfusion Imaging. https://clinicaltrials.gov/ct2/show/NCT00976053. Last accessed April 8, 2019.
- 10.Dilsizian V, Bacharach SL, Beanlands RS et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol 2016;23:1187–1226. [DOI] [PubMed] [Google Scholar]
- 11.Abidov A, Bax JJ, Hayes SW et al. Transient ischemic dilation ratio of the left ventricle is a significant predictor of future cardiac events in patients with otherwise normal myocardial perfusion SPECT. J Am Coll Cardiol 2003;42:1818–25. [DOI] [PubMed] [Google Scholar]
- 12.Dorbala S, Vangala D, Sampson U, Limaye A, Kwong R, Di Carli MF. Value of vasodilator left ventricular ejection fraction reserve in evaluating the magnitude of myocardium at risk and the extent of angiographic coronary artery disease: a 82Rb PET/CT study. J Nucl Med 2007;48:349–58. [PubMed] [Google Scholar]
- 13.Dorbala S, Hachamovitch R, Curillova Z et al. Incremental prognostic value of gated Rb-82 positron emission tomography myocardial perfusion imaging over clinical variables and rest LVEF. JACC Cardiovasc Imaging 2009;2:846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spertus JA, Winder JA, Dewhurst TA et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333–41. [DOI] [PubMed] [Google Scholar]
- 15.Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ 1968;56:1–188. [PubMed] [Google Scholar]
- 16.Arnold SV, Spertus JA, Jones PG, Xiao L, Cohen DJ. The impact of dyspnea on health-related quality of life in patients with coronary artery disease: results from the PREMIER registry. Am Heart J 2009;157:1042–9 e1. [DOI] [PubMed] [Google Scholar]
- 17.Merhige ME, Breen WJ, Shelton V, Houston T, D’Arcy BJ, Perna AF. Impact of myocardial perfusion imaging with PET and (82)Rb on downstream invasive procedure utilization, costs, and outcomes in coronary disease management. J Nucl Med 2007;48:1069–76. [DOI] [PubMed] [Google Scholar]
- 18.Parker MW, Iskandar A, Limone B et al. Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease: a bivariate meta-analysis. Circ Cardiovasc Imaging 2012;5:700–7. [DOI] [PubMed] [Google Scholar]
- 19.Takx RA, Blomberg BA, El Aidi H et al. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging 2015;8. [DOI] [PubMed] [Google Scholar]
- 20.Hachamovitch R, Nutter B, Hlatky MA et al. Patient management after noninvasive cardiac imaging results from SPARC (Study of myocardial perfusion and coronary anatomy imaging roles in coronary artery disease). J Am Coll Cardiol 2012;59:462–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


