Abstract
Placenta, the organ on which great attention is concentrated during pregnancy, represents an ineffective barrier to the transfer of hazardous heavy metals, mainly lead, into the foetus. The presence of lead in the placenta is an environmental hazard for a person's future. Due to hormonal changes, lead is released during pregnancy into the bloodstream of the mother from deposits in the bones and in the teeth, where it has accumulated for years as a result of a contaminated environment. Since lead is a neurotoxic metal, exposure to lead during prenatal and postnatal development can cause serious neurocognitive damage and hence the development of an Attention Deficit Hyperactivity Disorder (ADHD) in a developing human. Our work provides an overall picture of the “toxic pathway“ of lead through the mother's body, the risks arising from its transplacental transfer and its accumulation in the developing foetus as well as effective prevention to protect all newborns.
Keywords: human placenta, lead, calcium, neurotoxicity, ADHD
INTRODUCTION
Sources of lead
Lead is the oldest cumulative toxic metal that seriously contaminates the environment. Because of its malleability, resistance to corrosion, and low melting point humans have used lead since prehistoric times to fabricate statues, jewelry, water pipes, and drinking vessels. The Romans used lead to sweeten wine (Eisinger, 1982). Lead solder was used as a welding material in the automotive industry and lead was also a part of some pesticides. At present, most of the world’s lead production is used to produce lead accumulators in the automotive industry. Gasoline with lead additive has been used since 1923. 50–70% of lead from gasoline was received into the atmosphere as lead chloride and lead bromide by exhaust gases and lead was geo-accumulated in the soil. It is estimated that up to 4 million tons of lead was released into the air and soil by the combustion of leaded petrol which represents 90% of the lead in the atmosphere. Lead emissions from the combustion of leaded fuels and metals smelting have contributed significantly to the accumulation of atmospheric and soil Pb²+ (lead ion). From 1923 to 1986, the combustion of leaded gasoline in the United States is estimated to have dispersed around 4 million metric tons of Pb²+ into the atmosphere and eventually to the soil (Mielke, 1999). It is estimated by the Agency for Toxic Substances and Disease Registry (ATSDR) that the combustion of leaded gasoline has accounted for 90% of the Pb²+ deposited in the atmosphere (Mielke, 1999). Pb²+ contamination of soil is so extensive that it has been estimated that current soil Pb²+ levels are 1000 times higher in contaminated areas than in certain pristine forest areas (Renberg et al., 2000). Food is also one of the main sources of lead exposure (Hanning et al., 2003). Drinking water can contain lead and thus be a source of poisoning. It may be contaminated from the environment or from pipes containing lead particles. Lead reaches the body mainly through inhalation, orally and exceptionally through resorption via broken skin. After absorption from the gastrointestinal tract or lungs, lead is distributed by blood to the tissues and organs of the human body. In blood, 99% of lead is transported on the surface of erythrocytes and 1% via plasma. Lead in the liver, where it causes hepatotoxicity, penetrates the bloodstream and transports into the kidneys, spleen, lungs, brain and mineralized tissues: bones and teeth. It has been shown that nutritional status is a significant biological factor of susceptibility for elevated Pb²+ in body. Iron, zinc and calcium deficiencies increase the retention of ingested Pb²+ and may also increase the gastrointestinal absorption of Pb²+ (Goyer, 1996; Ruff et al., 1996). Age at exposure is also considered a significant risk factor for Pb²+ intoxication and its effects. Children absorb significantly more Pb²+ in the intestine than adults. Pre- and perinatal exposure results in higher brain Pb2+ levels than postnatal exposure due to an under-developed blood-brain barrier in early life (Goyer, 1996; Ruff et al., 1996). Social environment has also been shown to alter susceptibility to the cognitive effects of Pb²+ exposure. Poverty, residence in the inner city and minority status have all been demonstrated to result in the creation of a high-risk situation for Pb²+ intoxication (Koller et al., 2004; Ruff & Bijur, 1989; Ruff et al., 1996). For example, the number of non-Hispanic black children who have increased blood Pb²+ levels in the United States is from 3 to 13.5 times higher than in non-Hispanic white children with increased blood Pb²+ levels (Bernard & McGeehin, 2003).
Accumulation of lead in tissues
Lead as well as other heavy metals can be accumulated in the body. The preservation of lead in human tissue creates the long-term exposure indictor of this element, as well as image of lead pollution sources. In chronic exposure, bone serves as the primary storage organ from which lead can be released during pregnancy, lactation, osteoporosis and developing conditions (Silbergeld, 1991). The half-life of the deposited lead in the bones is 32 years, the time in the trabecular bones being shorter. It is released from soft tissues in 20–30 days. Dentin, as we know, also accumulates lead from blood during early childhood (Gulson & Wilson, 1994).
Mobilization of lead from the mother’s skeleton
The skeleton is a potential endogenous source of lead during pregnancy and lactation. There are number of papers that describe the mobilization of lead in women during physiological stress such as pregnancy and lactation (Thomson et al., 1985; Ernhart & Greene, 1992; Manton, 1992). The mobilization of lead from the skeleton in the postnatal period is more extensive than in pregnancy. Such increased release of skeletal lead into blood during the postnatal period is attributable to increased lead mobilization from maternal skeletal reserves during lactation, associated with increased bone resorption and may be associated with inadequate calcium intake in the diet. The maternal gestational age affects the metabolism of minerals in mother and child. The foetus produces 1,25-dihydroxyvitamin D, which controls the transport of calcium through the placenta. This activity is the highest in the last trimester of pregnancy which is a critical period due to the development of neuronal structures, synaptogenesis, bursitis and brain growth. Inhibition of synaptic structures and the blocking of neurotransmitter connections by lead may occur during this period (Silbergeld, 1991).
Calcium – an important carrier of lead when transported by the placenta
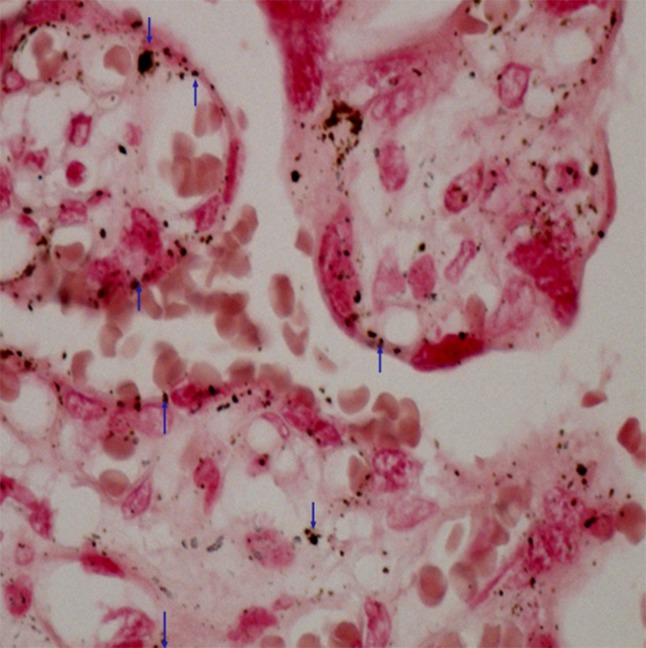
The calcium content in the foetus is potentially increasing during pregnancy. The transfer of calcium from the mother to the foetus occurs via an active mechanism, the calcium concentration being higher in foetal blood than in maternal. Heavy metals are known to act on calcium homeostasis through the perturbation of calcium channels and pumps as well as interference with the protein kinase C and calcium binding protein. Our histochemical finding points out that calcium plays a role of lead carrier (Figure1).
Figure 1.
Placenta – proof of calcium. Method after Koss was used. Cumulation of calcium deposits in the chorium villus (↓) and in the syncytiotrophoblast (↑). Magnified 400×.
Lead transport is thought to be closely related to the movement of calcium ions through the syncytiotrophoblast. The presence of lead in maternal and umbilical blood modifies calcium transport into the syncytiotrophroblast (Lafond et al., 2004). All cells contain similar systems for the permanent and temporary regulation of calcium ions and for calcium receptors. Poisons that affect mobilization and homeostasis of calcium will become a place for regulation of these processes. Lead, cadmium, zinc and other metals are transported across cell membranes via calcium transporters (Pounds, 1984; Cooper et al., 1984; Atchison et al., 1986; Koop, 1986; Simons, 1986; Hinkle et al., 1987). Placental calcium transport is dependent on a series of transplacental proteins located in the syncytiotrophoblast, forming a barrier between the mother and the foetus. These proteins include 4 plasma membrane ATPase isoforms (PMCA 1–4), which are a step towards placental calcium transport. Placental calcium transport occurs in syncytiotrophroblasts (Belkacemi et al., 2005). It continues through a well-controlled sequence of events consisting of an apical entry through the calcium channel, cytosolic calcium diffusion bound to calcium transport proteins, and basolateral calcium ion extravasation via the plasma membrane calcium-dependent ATPase (Stauffer et al., 1993; Zylinska et al., 2002).
Transport of lead through the placenta
The lead transport mechanism is not entirely clear. Several data suggest that lead transport may be a matter of a simple diffusion from maternal circulation into the foetal circulation.
The largest amount of lead levels measured in foetal tissues at various gestation periods were described in Barltrop’s study (Barltrop, 1969). An important question is how early the lead transport takes place. In Barltrop’s studies, lead transport starts around the 13th week of gestation and is prolonged to childbirth. His study has shown that lead contained in the brain and other tissues increases with an increasing organ size (Barltrop, 1969). Barltrop (1969) noted that the concentration of lead in the femur rapidly increased in the first trimester, which resulted in an initial loss of ossification and the depositing of calcium. Calcium deficiency increases the absorption of lead and its pathological effects (Mahaffey et al., 1973). The primary mechanism for the transplacental transmission of lead is probably a simple diffusion. The concentration of lead in the developing baby’s tissue, including brain tissue, is directly dependent on lead concentration in the umbilical cord blood. The lead content increases proportionately with the growing size of the foetus organs. The blood-brain barrier is immature which facilitates the entry of lead into the brain and therefore does not prevent a direct attack on the brain structures of the developing foetus.
Human placental Syncytiotrophoblast – an important place for lead transport
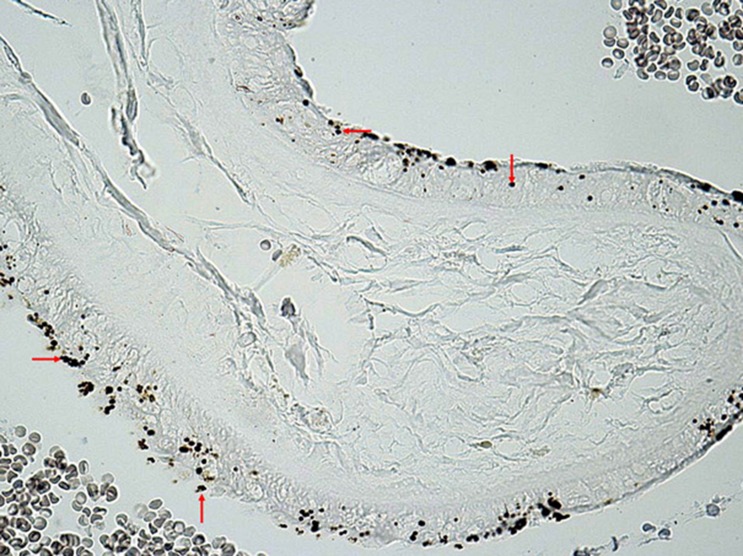
Syncytiotrophoblast is the epithelial layer separating maternal blood that flows around the villi. It is not composed of individual cells but is presented as a continuous multi-core surface layer without the separation of cellular boundaries. Syncytiotrophroblast is produced by fusing cells from the stem cells of the villous cytotrophoblast and then formed into true syncytia. The surface of the syncytia rises to irregular microvilli and the adjacent cytoplasm contains pockets formed by a smooth membrane. These pockets indicate intense pinocytosis which serves to transport substances from the mother’s circulation to the foetus (Castellucci & Kaufmann, 1995). The microvilli covering the syncytiotrophroblast surface represent the maternal-foetal contact zone (Teasdale and Jean-Jacques, 1986). The syncytiotrophoblast is the most frequent place for the accumulation of lead and nickel deposits (Figure 2).
Figure 2.
Placenta - Mallory and Parker method for proof of lead in the light microscope. Capturing of lead by resorption plasmodium of syncytiotrophoblast (←,→,↑). Available at 20 lenses using the Canon D60.
Interactions between lead and the cells of the human body
There is a considerable number of publications describing the adverse health effects of lead already at low exposure levels, with special attention paid to pregnant women and children (Carpenter, 2001; Factor-Litvak et al., 1999; Goyer, 1996; Rosen, 1995). Lead represents a significant environmental risk for pregnant women and their offspring. Exposure to high environmental levels of lead may be associated with the preterm ruptures of foetal membranes, spontaneous abortions and preterm births (Angell et al., 1982). Lead can make an impact by its toxicity even without the presence of clinical symptoms (Needleman et al., 1979) and its toxicity may be manifested several years after exposure. Animal data show that a diet highly lacking in proteins and minerals increases lead absorption (Levander, 1979). It is proven that the deficiency of iron and zinc may increase the toxic effects of lead (Levander, 1979). Animal studies have shown that lead also interferes with collagen synthesis (Vistica et al., 1977), energy metabolism and membrane structures (White & Selh, 1975) which may have an adverse effect on the integrity of the chorioamnion membranes, and may also be a predisposition for premature membrane rupture (Angell et al., 1982). Lead is a well-known human reproductive toxin. Lead exposure negatively impacts the pregnancy outcomes of mothers and their newborns (Castellino et al., 1995). Prenatal exposure to lead is manifested by toxic effects on the human foetus, including risk factors: premature birth, low birth weight and the impairment of mental development. Dawson et al. (1999), Tabacova & Balabaeva (1993) claim that exposure to lead during pregnancy may interfere with the etiology of preeclampsia. Baranowska (1995) found that high levels of lead in the placenta correlated with low Apgar scores. The effect of lead on cognitive and behavioral development is particularly critical in newborns (Goyer, 1996; Rosen, 1995). Foetal lead exposure may affect multi-organ systems in embryo development, including the retardation of cognitive development in early childhood (Banks et al., 1997). Lead causes a significant increase in the frequency of chromatin changes in maternal bone marrow cells and the reduction of nucleolar organization, causing several specific chromosomal aberrations, changes in the maternal bone marrow and foetal cells (Nayak et al., 1989). Prenatal exposure to high levels of lead causes foetal malformations, abortions, growth and mental retardation, and encephalopathy (Davis & Svendsgaard, 1987; Fahim et al., 1976). Lead carcinogenic mechanism involves direct deoxyribonucleic acid (DNA) damage, DNA inhibition and may also generate reactive oxygen forms and thus cause oxidative damage to the DNA (Silbergeld et al., 1999). Silbergeld et al. (2000) highlights the particular importance of transplacental lead exposure and the increase of cancer risk in later life.
Postnatal effects of lead on humans
The mechanisms by which lead damages the brain functions and the effects of increased sensitivity of the immature nervous system of the foetus are not yet fully understood. Lead is predominantly accumulated in the brain’s endothelial cells (Toews et al., 1978). High levels of lead exposure result in the loss of the normal blood-brain barrier function and plasma movement into interstitial brain spaces (Goldstein, 1984). The blood-brain barrier is a vulnerable place for neurotoxic lead activity. Edema, increased intracranial pressure and reduced brain perfusion may be the basis for irreversible brain damage. Exposure to already low levels of lead can change the microenvironment of the brain. Goldstein studies (1988) have confirmed that important signals in the blood-brain barrier structure arise from interactions between the endothelial cells and astrocytes. Astrocytes outweigh the amount of neurons in the brain, their protrusions encircling endothelial cells (Raine, 1988). The toxic effects of lead increase the interactions between the endothelial cells and astrocytes (Gebhart & Goldstein, 1988). It suggests that the foetal brain offers low resistance to lead toxicity because it lacks the lead-protein complexes in astrocytes that remove the lead from the mitochondria. These astrocytes are especially at high risk of lead toxicity during in utero since the immature endothelial cells that form the capillaries of the brain offer a decreased resistance to lead, and thereby easily allow fluids and ions, such as Pb²+ , to enter the brain. Lead toxicity during this development period has often been associated with cognitive impairment and learning malfunctions, as lead can accumulate in their nervous system as they develop (Gularte & McGlothan, 1998). Lead has already been shown to be a behavioral teratogen at low exposure levels. The high levels of lead exposure involve psychological harm, including mental retardation in children (Bornschein et al., 1980; Rutter, 1980). The basal ganglia are sensitive to hypoxia, a change in metabolism, which plays an important role in the pathophysiology of a hyperkinetic disorder. Lead transported through the transplacental barrier into the foetus also attacks dopamine pathways in the middle brain (e.g. in the striatum). Dopamine neurons in the middle brain regulate motor control and emotions thereby engage in cognitive processes and different forms of memory. In the striatum region, dopamine is responsible for the proper coordination of limb movements. The degeneration of dopamine pathways and subsequent dopamine depletion, which may be caused by the neurotoxic poisoning by lead, result in hypokinesis. Dopamine is important in influencing psychomotor and attention which are dysfunctional in ADHD. Persons with ADHD have the hypoactivity of the cortical dopamine system and the hyperactivity of the striatal system (Drtílková & Šerý, 2007). Exposure to excessive amounts of inorganic lead during the toddler years may produce permanent adverse effects on the brain functions. Maximum lead intake occurs at the age when most changes affect the density of the brain’s synaptic connections. The development of synapse reorganization is partly provided by protein kinase. This enzyme is very susceptible to lead stimulation, so lead intoxication can interrupt the development of the neural network without producing obvious change factors. Protein kinase regulates the development of brain capillaries and the blood-brain barrier. The stimulation of this enzyme by lead can interrupt the development sequence and disrupt the precise regulation of the neuronal environment which is necessary for normal brain function. These claims have shown that the sensitivity of protein kinase to lead may partially enlighten the brain dysfunctions seen in children who have been intoxicated by this poison. Defects in cognitive and behavioral functions arise in children with higher blood levels of lead (McMichael et al., 1988).
Attention deficit hyperactivity disorder (ADHD)
Recently, research has shown that lead, as a neurotoxic metal, can cause ADHD in children. The cause of this syndrome is the accumulation of lead in the striatum of the brain which contains myelin in the neurons. It is a small and diffused organic brain damage caused by various external influences in the prenatal period, during childbirth and early childhood but ADHD occurrence may also be genetically conditioned (Hudziak et al., 2005; Kuntsi et al., 2005; Sherman et al., 1997). The external factors affecting ADHD can include smoking and alcohol drinking during pregnancy, a complicated and premature birth, head trauma, and also various environmental impacts: the increased incidence of heavy metals in the air and food. The presence of metallic protozoans in blood serum disrupts the metabolism of enzymes and this can cause hyperactivity in some children. Awareness of the etiology of this disease will help in the ADHD treatment which has an increasing trend of occurrence in the world. An early, rapid, correct diagnosis is the basis for therapy and the reality of how the ADHD incidence curve in the world will progress depends on it. The open question remains the establishment of centers for the detection of this disease the consequences of which are felt by all society.
Discussion
Polluted environment can leave changes on the microscopic structure of the placenta. It has not been a long time since lead petrol has ceased being used and so the accumulation of lead and its consequences are manifesting themselves in the generation of mothers who are 25 to 30 years old. These are women who lived during the full-scale use of leaded petrol. It is characteristic for heavy metals that they are very slowly released from the body. Lead and other neurotoxic metals are believed to be transported by simple diffusion from the maternal blood through the syncytiotrophoblast to foetal blood. During a woman’s life, a pregnancy is when hormone disharmony occurs that triggers the release of lead from the bones and teeth, since the foetus needs the calcium. It is deriven from bone and teeth deposits for the development of its skeleton and the lead penetrates the bloodstream of the foetus with the calcium. Recently, attention has been paid to the importance of calcium transport in relation to lead transport. The direct exposure of the mother to lead may not always occur. The probability of lead intoxication is higher in mothers who stayed in places with an increased lead level during their lives, even during pregnancy. Lead is found in the environment almost everywhere. It can be detected in the air and dust, its levels are strictly monitored in drinking water. Therefore, as a precaution against this disease, we suggest taking an umbilical cord blood with a lead test for each newborn immediately after birth, setting up centers tracking the statistical occurrence of lead results and promptly addressing the cause behind the increase of ADHD.
Conflict of interest
The authors declare no conflicts of interest. The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
The Ethical Committee of the University Hospital Bratislava approved the study. All patients signed informed consent documents before participating in the study.
REFERENCES
- Atchison WD, Joshi U, Thornburg JE. Irreversible suppression of calcium entry into nerve terminals by methylmercury. J Pharmacol Exp Ther. 1986;238:618–624. [PubMed] [Google Scholar]
- Banks EC, Ferretti LE, Shucard DW. Effects of low level lead exposure on cognitive function in children: a review of behavioral, neuropsychologi-cal and biological evidence. Neurotoxicology. 1997;18:237–281. [PubMed] [Google Scholar]
- Baranowska I. Lead and cadmium in human placentas and maternal and neonatal blood (in a heavily polluted area) measured by graphite fur-nace atomic absorption spectrometry. Occup Environ Med. 1995;52:229–232. doi: 10.1136/oem.52.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barltrop D. Transfer of lead to the human foetus. In: Barltrop D, Burland W, editors. Mineral metabo-lism in Paediatrics. Oxford, England: Blackwell Scientific; 1969. pp. 135–151. [Google Scholar]
- Belkacemi L, Bedard I, Simoneau L, Lafond J. Calcium channels, trans-porters and exchangers in placenta: a review. Cell Calcium. 2005;37(1):1–8. doi: 10.1016/j.ceca.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Bernard SM, McGeehin MA. Prevalence of blood lead levels >or = 5 micro g/ dL among US children 1 to 5 years of age and socioeconomic and demographic factors associated with blood of lead levels 5 to 10 micro g/ dL. Third National Health and Nutrition Examination Survey, 1988–1994. Pediatrics. 2003;112:1308–1313. doi: 10.1542/peds.112.6.1308. [DOI] [PubMed] [Google Scholar]
- Bornschein R, Pearson D, Reiter L. Behavioral effects of moderate lead exposure in children and animal models. CRC Rev Toxicology. 1980;8:43–99. doi: 10.3109/10408448009037491. [DOI] [PubMed] [Google Scholar]
- Carpenter DO. Effects of metals on the nervous system of humans and animals. Int J Occup Med Environ Health. 2001;14(3):209–218. [PubMed] [Google Scholar]
- Castellino N, Castellino P, Sannolo N. Inorganic lead exposure: metabolism and intoxication. Boca Raton, FL: Lewis Publishers; 1995. [Google Scholar]
- Castellucci M, Kaufmann P. Pathology of the Human Placenta. third edn. New York: Springer Science + Business; 1995. Basic structure of the villous trees; p. 57. [Google Scholar]
- Cooper GP, Suszkiw JB, Manalis RS. Presynaptic effects of heavy metals. In: Narahashi T, editor. Cellular and Molecular Neurotoxicology. New York: Raven Press; 1984. pp. 1–21. [Google Scholar]
- Davis JM, Svendsgaard DJ. Lead and child development. Nature. 1987;329:297–300. doi: 10.1038/329297a0. [DOI] [PubMed] [Google Scholar]
- Dawson EB, Evans DR, Nosovitch J. Third trimester amniotic fluid metal levels associated with preeclampsia. Arch Environ Health. 1999;54(6):412–415. doi: 10.1080/00039899909603372. [DOI] [PubMed] [Google Scholar]
- Drtílková I, Šerý O. Hyperkinetická porucha ADHD. Praha, Galén: 2007. p. 268. [Google Scholar]
- Eisinger J. Lead and wine: Eberhard Gockel and the Colica Pictonum. Medical History. 1982;26:279–302. doi: 10.1017/s0025727300041508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernhart CB, Greene T. Postpartum changes in maternal blood lead concentrations. Br J Ind Med. 1992;9:11–13. doi: 10.1136/oem.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor-Litvak P, Wasserman G, Kline JK, Graziano J. The Yugoslavia prospective study of environmental lead exposure. Environ Health Perspect. 1999;107(1):9–15. doi: 10.1289/ehp.991079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim MS, Fahim Z, Hall OG. Effects of subtoxic lead levels on pregnant women in the state of Missouri. Res Commun Chem Pathol Pharmacol. 1976;13:309–331. [PubMed] [Google Scholar]
- Gebhart AM, Goldstein GW. Use of an vitro system to study the effects of lead on astrocyte-endothelial cell interactions: a model for studying toxic injury to the blood-brain barrier. Toxicol Appl Pharmacol. 1988;94:191–206. doi: 10.1016/0041-008x(88)90261-x. [DOI] [PubMed] [Google Scholar]
- Goldstein GW. Brain capillaries: a target for inorganic lead poisoning. Neurotoxicology. 1984;5:167–176. [PubMed] [Google Scholar]
- Goldstein GW. Endothelial cell-astrocyte interactions: a cellular model of the blood-brain barrier. An N Y Acad of Sci. 1988;529:31–39. doi: 10.1111/j.1749-6632.1988.tb51417.x. [DOI] [PubMed] [Google Scholar]
- Goyer RA. Results of lead research, prenatal exposure and neurological consequences. Environ Health Perspect. 1996;104(10):1050–1054. doi: 10.1289/ehp.961041050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gularte T, McGlothan J. Hippocampal NMDA receptor mRNA undergoes subunit specific changes during developmental lead exposure. Brain Research. 1998;790:98–107. doi: 10.1016/s0006-8993(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Gulson BL, Wilson D. History of lead exposure in children revealed from isotopic analyses of teeth. Arch Environ Health. 1994;49:279–283. doi: 10.1080/00039896.1994.9937480. [DOI] [PubMed] [Google Scholar]
- Hinkle PM, Kinsella PA, Osterhoudt KC. Cadmium uptake and toxicity via voltage-sensitive calcium channels. J Biol Chem. 1987;262:16333–16337. [PubMed] [Google Scholar]
- Hudziak JJ, Derks EM, Althoffi RR, Rettew DC, Boomsma DI. The genetic and environmental contributions to attention deficit hyperactivity disorder as measured by the conners΄ rating scales-revised. American J Psychiatry. 2005;162:1614–1620. doi: 10.1176/appi.ajp.162.9.1614. [DOI] [PubMed] [Google Scholar]
- Koller K, Brown T, Spurgeon A, Levy L. Recent developments in low-level lead exposure and intellectual impairment in children. Environ Health Perspect. 2004;112:987–994. doi: 10.1289/ehp.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp SJ. Cadmium and the cardiovascular system. In: Foulkes E.C, editor. Handbook of Experimental Pharmacology. Vol. 80. Verlag: Springer; 1986. pp. 195–280. [Google Scholar]
- Kuntsi J, Rijsdijk F, Roland A, Asherson P, Plomin R. Genetic influences on the stability of attention-deficit/hyperactivity disorder symptoms from early to middle childhood. Biol Psychiatry. 2005;57:647–654. doi: 10.1016/j.biopsych.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Lafond J, Hamel A, Takser L, Vaillancourt C, Mergler D. Low environmental contamination by lead in pregnant women: effect on calcium transfer in human placental syncytiotrophoblasts. J Toxicol Environ Health. 2004;67:1069–1079. doi: 10.1080/15287390490452263. [DOI] [PubMed] [Google Scholar]
- Levander OA. Lead toxicity and nutritional deficiencies. Environ Health Perspect. 1979;29:115. doi: 10.1289/ehp.7929115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Malenka RC, Nicoll RA. Mechanisms underlying long-term potentiation of synaptic transmission. Annu Rev Neurosci. 1991;14:379–397. doi: 10.1146/annurev.ne.14.030191.002115. [DOI] [PubMed] [Google Scholar]
- Mahaffey KR, Haseman JD, Goyer RA. Dose-response to lead ingestion in rats on low dietary calcium. J Lab Clin Med. 1973;83:92–100. [PubMed] [Google Scholar]
- Manton WI. Postpartum changes in maternal blood lead concentrations. Correspondence. Br J Ind Med. 1992;49:671–672. doi: 10.1136/oem.49.9.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Baghurst PA, Wigg NR, Vimpani GV, Robertson EE, Roberts RJ. Port Pirie Cohort Study: environmental exposure to lead and children´s abilities at the age of four years. N Engl J Med. 1988;319:468–475. doi: 10.1056/NEJM198808253190803. [DOI] [PubMed] [Google Scholar]
- Mielke H. Lead in Inner cities. Am Sci. 1999;87:62–73. [Google Scholar]
- Nayak BN, Ray M, Persaud TVN, Nigli M. Relationship of embryotoxicity to genotoxicity of lead nitrate in mice. Exp Pathol. 1989;36:65–73. doi: 10.1016/s0232-1513(89)80116-1. [DOI] [PubMed] [Google Scholar]
- Needleman HL, Gunnoe C, Leviton A, Reed R, Peresie H, Maher C, Barrett P. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N Engl J Med. 1979;300:689. doi: 10.1056/NEJM197903293001301. [DOI] [PubMed] [Google Scholar]
- Pounds JG. Effect of lead intoxication on calcium homeostasis and calcium-mediated cell function: a review. Neurotoxicology. 1984;5:295–332. [PubMed] [Google Scholar]
- Raine CS. In: Neurocellular anatomy. 4th ed. Siegel G, Agranoff B, Albers R.W, Molinoff P, editors. New York: Raven Press; 1988. pp. 3–33. [Google Scholar]
- Renberg I, Brannvall M, Bindler R, Emeteryd O. Atmospheric lead pollution history during four millennia (2000BC to 2000 AD) Ambio. 2000;29:152–158. [Google Scholar]
- Rosen JF. Adverse health effects of lead at low exposure levels, trends in the management of childhood lead poisoning. Toxicology. 1995;97(1–3):11–17. doi: 10.1016/0300-483x(94)02963-u. [DOI] [PubMed] [Google Scholar]
- Ruff HA, Bijur PE. The effects of low to moderate lead levels on neurobehavioral functioning in children: toward and conceptual model. J Dev Behav Pediatr. 1989;10:103–109. [PubMed] [Google Scholar]
- Ruff HA, Markowitz ME, Bijur PE, Rosen JF. Relationships among blood lead levels, iron deficiency, and cognitive development in two-year-old children. Environ Health Perspect. 1996;104:180–185. doi: 10.1289/ehp.96104180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Raised lead level and impaired cognitive behavioral functioning: A review of the evidence. Devel Med Child Neurol. 1980;22:1–25. [PubMed] [Google Scholar]
- Sherman DK, McGue MK, Iacono WG. Twin concordace for attention deficit hyperactivity disorder: a comparison of teachers΄ and mothers΄ reports. Am J Psychiatry. 1997;154:532–535. doi: 10.1176/ajp.154.4.532. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK. Implications for toxicology during pregnancy and lactation. Environ Health Pespect. 1991;91:63–70. doi: 10.1289/ehp.919163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbergeld EK, Waalkes M, Rice JM. Lead as an carcinogen: Experimental evidence and mechanisms of action. Am J Ind Med. 1999;38:316–323. doi: 10.1002/1097-0274(200009)38:3<316::aid-ajim11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, Waalkes M, Rice JM. Lead as a carcinogen: Experimental evidence and mechanisms of action. Am J Ind Med. 2000;38:316–323. doi: 10.1002/1097-0274(200009)38:3<316::aid-ajim11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Simons TJB. Cellular interactions between lead and calcium. Br Med Bull. 1986;42:431–434. doi: 10.1093/oxfordjournals.bmb.a072162. [DOI] [PubMed] [Google Scholar]
- Stauffer TP, Hilfiker H, Carafolie E, Strehler EE. Quantitative analysis of alternative speicing options of human plasma membrane calcium pump genes. J Biol Chem. 1993;268(34):25993–26003. [PubMed] [Google Scholar]
- Tabacova S, Balabaeva L. Environmental pollutants in relation to complications of pregnancy. Environ Health Perspect. 1993;101:27–31. doi: 10.1289/ehp.93101s227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale F, Jean-Jacque G. Morphometry of the microvillous membrane of the human placenta in maternal diabetes mellitus. Placenta. 1986;7:81–88. doi: 10.1016/s0143-4004(86)80020-0. [DOI] [PubMed] [Google Scholar]
- Thompson GN, Robertson EF, Fitzgerald S. Lead mobilization during pregnancy. Med J Australia. 1985;43:131. doi: 10.5694/j.1326-5377.1985.tb122859.x. [DOI] [PubMed] [Google Scholar]
- Toews AD, Kolber A, Hayward J, Krigman MR, Morrell P. Experimental lead encephalopathy in the suckling rat: concentrations of lead in cellular fractions enriched in brain capillaries. Brain Res. 1978;147:131–138. doi: 10.1016/0006-8993(78)90777-1. [DOI] [PubMed] [Google Scholar]
- Vistica DT, Ahrens FA, Ellison WR. The effects of lead upon collagen synthesis and proline hydroxylation in the Swiss mouse 3T6 fibroblast. Arch Biochem Biophys. 1977;177:15. doi: 10.1016/0003-9861(77)90081-9. [DOI] [PubMed] [Google Scholar]
- White JM, Selh HS. Lead and the red cell. Br J Haematol. 1975;30:133. doi: 10.1111/j.1365-2141.1975.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Zylinska L, Kawecka I, Lachowicz L, Szemraj J. The isoform-and location-dependence of the functioning of the plasma membrane calcium pump. Cell Mol Biol Lett. 2002;7(4):1037–1045. [PubMed] [Google Scholar]