Abstract
Background
A previous meta‐analysis of interferon therapy in naive patients with chronic hepatitis C has documented its efficacy in achieving virologic clearance, and improving liver biochemistry and histology; however, since its publication additional trials have been reported.
Objectives
To evaluate the response to interferon in interferon naive patients with chronic hepatitis C. The effect of treatment dose and duration, and the response in patients with cirrhosis and those with normal aminotransferases was also investigated.
Search methods
The Cochrane Controlled Trials Register (Cochrane Library Issue 1, 1999), MEDLINE (January 1966 to December 1999), and reference lists were searched, and pharmaceutical companies were contacted for unpublished trials.
Selection criteria
Randomised clinical trials comparing interferon with placebo, no treatment, or different regimens of interferon were selected. Abstracts were excluded.
Data collection and analysis
The primary outcome measure was sustained disappearance of serum HCV RNA (virologic sustained response (SR)). Biochemical and end of treatment responses, liver histology, and adverse events were also recorded. Assessment of drug efficacy used the methods of Peto and Der Simonian and Laird.
Main results
Fifty‐four trials enrolling 6545 patients were included. Compared with no treatment, interferon 3 MU thrice weekly for 12 months increased the probability of a virologic SR (Peto odds ratio (OR) 4.60; 95% confidence interval (CI) 1.53 to 13.85). At this dosage and duration of therapy, the rate of virologic SR was 17% (95% CI 10 to 28%) in interferon‐treated patients versus 3% (95% CI 1 to 10%) in controls. A dose of 6 MU was more effective than 3 MU thrice weekly (OR for 12 months treatment, 2.21; 95% CI 1.10 to 4.45), as were durations of 12 months or greater versus six months (OR 1.87; 95% CI 1.30 to 2.67). Liver biochemistry responses were alike. Adverse events were more common with higher doses and prolonged durations of treatment. Compared with no therapy, interferon increased the probability of histologic improvement (OR 9.22; 95% CI 5.69 to 14.94). The response to interferon in cirrhotic patients (virologic SR, 17%; 95% CI 11 to 26%) was similar to that in non‐cirrhotic patients. However, interferon was no more effective than control in patients with normal aminotransferases.
Authors' conclusions
Interferon is effective in achieving viral clearance and improving liver biochemistry and histology in interferon naive patients with chronic hepatitis C. Higher doses and prolonged durations are more effective, but associated with more frequent adverse events. Interferon is associated with similar benefits in patients with cirrhosis, but the efficacy in patients with normal aminotransferases is unproven.
Plain language summary
Interferons show efficacy on virologic, biochemical, and histological outcomes in interferon naive patients with chronic hepatitis C
Hepatitis C virus infection can progress to chronic hepatitis, cirrhosis, and hepatocellular carcinoma. The goal of this systematic review was to examine the effects of interferon treatment for interferon naive (previously untreated) patients with chronic hepatitis C. This review confirmed the efficacy of interferon on surrogate outcomes as well as a favourable effect of higher treatment doses and prolonged durations. However, these effects were associated with more adverse events. Compared with non‐cirrhotic patients, cirrhotic patients respond similarly, but the efficacy of interferon in patients with normal liver biochemistry is not substantiated by the data. Although interferon monotherapy is no longer considered the standard therapy for chronic hepatitis C, this review defines the optimal dose and duration of interferon monotherapy, which may be useful for patients who cannot tolerate combination therapy including interferon and ribavirin, the most effective therapy currently available.
Background
Since the first meta‐analysis of interferon therapy in chronic hepatitis C in which 18 randomised clinical trials (RCTs) were analysed (Tine 1991), at least 88 new references of interferon therapy and several other meta‐analyses in the treatment of acute or chronic hepatitis C have been published. These studies have confirmed the efficacy of interferon in achieving viral clearance and improving liver biochemistry and histology. Several questions, however, remain to be answered including defining the optimal dosage and duration of interferon monotherapy, and determining the response in subgroups including those with cirrhosis and those with normal liver biochemistry. This systematic review serves to update these previous meta‐analyses, and to address these unresolved issues.
Objectives
The a priori objectives of this review were to address the following questions: 1) What is the efficacy of interferon at a standard dose and duration (3 million international units (MU) injected thrice weekly (TIW) for six months) versus a control group (placebo or no intervention) in terms of alanine aminotransferase (ALT), normalisation, virologic response, and histological improvement? 2) Is an increase in the dose or duration of interferon treatment associated with an improved response in comparison to the standard regimen? 3) Is the response to interferon therapy different in the following subgroups: 1) patients with normal aminotransferases; and 2) patients with cirrhosis?
Methods
Criteria for considering studies for this review
Types of studies
RCTs comparing interferon with placebo, no treatment, or different doses of interferon were included. Studies employing randomisation after an initial run‐in period of interferon treatment were excluded as were those published as interim reports or abstracts only. There were no language limitations.
Types of participants
Chronic hepatitis C was defined as: 1) a positive serological test for HCV and detectable serum HCV ribonucleic acid (RNA) by polymerase chain reaction (PCR) assay; and 2) chronic hepatitis documented on liver biopsy. Only treatment‐naive patients were included in the core analysis; trials involving previously treated patients (non‐responders and relapsers) were excluded. Patients post liver transplantation or those coinfected with the hepatitis B virus and/or human immunodeficiency virus were also excluded.
Types of interventions
RCTs assessing interferon versus placebo, no treatment, or different regimens of interferon were included. There were no exclusions based on type, dose, or duration of interferon therapy.
Types of outcome measures
In order to be included, at least one of the following outcome measures had to be reported: normalisation of ALT activity at the end of treatment (biochemical ETR); sustained ALT normalisation at the end follow‐up (biochemical SR); disappearance of serum HCV RNA by PCR at the end of the treatment (virologic ETR) and at the end of follow‐up (virologic SR; the primary outcome measure). On examination of the included trials, we defined (post hoc) the time point following the end of treatment necessary for consideration of a sustained response as three months. The impact of interferon therapy on the rate of histologic improvement was assessed in patients with pre‐ and post‐treatment liver biopsies. We did not plan an analysis of the effect of treatment on clinical outcomes because, in our previous experience, no such data was available. However, when such information was discovered on reviewing the RCTs, it was recorded.
Search methods for identification of studies
We searched The Cochrane Controlled Trials Register (Cochrane Library Issue 1, 1999) and MEDLINE (January 1966 to December 1999). All publications describing (or which might describe) RCTs of interferon treatment in patients with chronic hepatitis C were obtained using the search strategy developed by the Cochrane Hepato‐Biliary Group (see Review Group details for more information). The following terms were included in the electronic search strategy of The Cochrane Controlled Trials Register and MEDLINE: 'hepatitis non‐A non‐B, ‐C' and 'clinical trial'. Furthermore, we searched the reference lists of the retrieved articles and published reviews, and contacted pharmaceutical companies to obtain unpublished RCTs.
Data collection and analysis
Meta‐analyses were conducted according to a predetermined protocol following the recommendations of Sacks et al. (Sacks 1987). Methodological quality assessment was performed by two observers independently using a previously validated questionnaire (Poynard 1988). In this questionnaire, 14 items were analysed: description of the primary outcome measure; criteria of inclusion; number of patients seen and excluded; number of subjects randomised in each group; number of participants excluded during the follow‐up period; blinding of the doctors, patients, and those responsible for the assessment criteria; a priori sample size calculation; method of randomisation and its concealment; analysis and discussion of covariables; statistical tests used; the number of drop‐outs; and the power of trials with non‐significant results. The concealment of allocation, important in reducing bias, was also analysed according to the following scale (Kjaergard 2001): A) adequate (central randomisation, sealed envelopes, or similar); B) unclear (not described); C) inadequate (open table of random numbers or similar); and D) not used (see the table 'Characteristics of included studies').
Range of patient characteristics, diagnoses, and treatments The following items were recorded as potentially useful in assessing clinical heterogeneity between RCTs: mean age, gender ratio, mean duration of hepatitis when known, mode of infection, and type of interferon administered (alfa‐2a, alfa‐2b, lymphoblastoid, other).
Criteria of combinability RCTs were combined only if at least one of the main outcome measures was assessed. For each RCT, the exact drug dosage was given as well as the duration of treatment. In the analysis of different interferon formulations, the following methods were used to assess combinability for each drug: 1) comparison of the improvement in each outcome measure in the control groups with the Chi‐square test; and 2) heterogeneity tests by the methods of Peto et al. (DeMets 1987) and Der Simonian and Laird (Der Simonian 1986).
Selection and data‐extraction bias All RCTs considered for inclusion were analysed independently by two observers (TP, TT, or VL), who conferred with one another in case of disagreement. The decision as to inclusion or exclusion of studies was independent of the study results.
Statistical methods RevMan 4.1 (The Cochrane Collaboration) and NCSS 2001 (Number Cruncher Statistical Systems, Kaysville, Utah) statistical software were used for all analyses. For the biochemical and virologic outcome measures, all analyses were performed according to the intention‐to‐treat method. Because of a high percentage of patients without a post‐treatment liver biopsy, the percentage of patients with histological improvement was estimated according to a per‐protocol method, that is, after exclusion of missing data which were not considered treatment failures. For each outcome measure, heterogeneity of results between the control groups was assessed using the Peto et al. method (DeMets 1987). Depending on the presence or absence of significant heterogeneity (P < 0.1), drug efficacy was assessed using a random effects model (Der Simonian 1986) or fixed effects model (DeMets 1987). Where a random effects model was used, the word 'Random' appears after '95% confidence interval (CI)'. Comparisons between strata were performed using Peto odds ratios (OR); each estimate was reported with its 95% CI. A P‐value of 0.05 or less was considered significant.
The following sensitivity analyses were performed after the inclusion or exclusion of appropriate RCTs from the core group of trials: 1) exclusion of RCTs with quality < median score; 2) exclusion of RCTs including patients with a prevalence of cirrhosis >30%; 3) exclusion of RCTs with a mean age > 47 years; 4) exclusion of RCTs with a mean disease duration > five years; 5) exclusion of RCTs with a follow‐up of < 12 months (and therefore a definition of sustained response of < 12 months); and 6) exclusion of RCTs assessing non‐alfa‐2b interferons.
Results
Description of studies
RCTs were described separately according to their control groups as either: 1) comparisons of interferon versus a control group receiving placebo or no treatment; or 2) comparisons of different regimens of interferon. The following clinical characteristics were described in the table 'Characteristics of included studies': first author, allocation concealment, blinding, intention‐to‐treat analysis, methodological score, interferon type, interferon schedule, follow‐up, number of patients excluded, mean age, percentage of males, transfusion history, intravenous drug use, prevalence of cirrhosis, and mean disease duration.
A total of 54 RCTs including 6545 patients met the inclusion criteria for this meta‐analysis, 21 trials more than the previous analysis (Poynard 1996). No unpublished RCTs were identified. Eight newly identified RCTs studied the effect of interferon therapy versus control, bringing the total number of eligible RCTs to 24 (Camps 1993; Capra 1993; Causse 1991; Cimino 1991; Davis 1989; Diodati 1994; Fernandez 1997; Furusyo 1997; Giudici 1991; Gomez‐Rubio 1990; Ikeda 1998; Makris 1991; Marcellin 1991; Mazella 1994; Nishiguchi 1995; Reichen 1996; Rossini 1997; Rumi 1995; Saez‐Royuela 1991; Saito 1994; Sangiovanni 1998; Saracco 1990; Valla 1999; Weiland 1990). Among the eight newly identified RCTs, one compared interferon administered for 12 months versus a tailored interferon and a separate untreated control arm (Reichen 1996), two RCTs were performed in patients with normal aminotransferases (Rossini 1997; Sangiovanni 1998), one in haemodialysis patients (Fernandez 1997), and four in cirrhotic patients only (Furusyo 1997; Ikeda 1998; Nishiguchi 1995; Valla 1999).
In comparison to our previous meta‐analysis (Poynard 1996), 15 new RCTs comparing different doses or durations of interferon were identified bringing the total number of eligible RCTs to 24 (Alberti 1993; Chemello 1995; Craxi 1996; Degos 1998; Enriquez 1995; Garson 1997; Hagiwara 1993; Hakozaki 1995; Imai 1997; Jouet 1994; Kasahara 1995; Komatsu 1997; Laghi 1997; Lin 1995; Marcellin 1995; Matsumoto 1994; McHutchison 1998; Ouzan 1998; Poynard 1995; Saracco 1993; Shiratori 1997; Simon 1997; Tassopoulos 1996). There were 23 references for 24 RCTs because the Alberti et al. publication (Alberti 1993) included two RCTs. A total of 18 RCTs published in 17 full papers (Alberti 1993; Brouwer 1998; Chemello 1995; Enriquez 1995; Garson 1997; Hagiwara 1993; Hakozaki 1995; Imai 1997; Komatsu 1997; Laghi 1997; Lin 1995; Marcellin 1995; Matsumoto 1994; Ouzan 1998; Shiratori 1997; Simon 1997; Tassopoulos 1996) compared different doses of interferon, including nine comparisons of 'high' dose versus the 'standard' dose of 3 MU (for six months in six trials; for 12 months in four trials) (Alberti 1993; Chemello 1995; Garson 1997; Hagiwara 1993; Hakozaki 1995; Laghi 1997; Lin 1995; Simon 1997), and nine other comparisons (Brouwer 1998; Enriquez 1995; Imai 1997; Komatsu 1997; Ouzan 1998; Marcellin 1995; Matsumoto 1994; Tassopoulos 1996; Shiratori 1997). The randomisation process of one trial is suspect (Shiratori 1997); nevertheless, this trial has been included and the effect of its inclusion analysed via a sensitivity analysis. A total of ten RCTs assessing the effect of treatment duration were identified (Chemello 1995; Craxi 1996; Degos 1998; Garson 1997; Jouet 1994; Kasahara 1995; Lin 1995; McHutchison 1998, Poynard 1995, Saracco 1993).
A total of four RCTs were designed assessing therapy tailored to the response (biochemical or virologic) to treatment (Brouwer 1998; Fernandez 1997; Reichen 1996; Rumi 1996). Two of these trials, enrolling a total of 401 patients, included a control arm consisting of standard, fixed‐dose interferon therapy in naive patients and were subjected to meta‐analysis (Brouwer 1998; Reichen 1996). The remaining trials were excluded because they lacked a control arm treated with fixed‐dose therapy (Fernandez 1997; Rumi 1996).
Four RCTs directly comparing different interferon preparations (beta interferon, consensus interferon, leukocyte interferon alfa, and lymphoblastoid interferon) with interferon alfa‐2a or ‐2b in a total of 2330 patients were identified (Farrell 1998; Rumi 1996; Tong 1997; Villa 1996).
Risk of bias in included studies
The methodological quality of the included studies was graded according to a previously validated method which generates a score ranging from ‐2 to 28 (Poynard 1988). The median scored of the included studies was 13 (range, 8 to 21). The mean +/‐ standard deviation was 13.5 +/‐ 3.1. Allocation concealment was considered adequate in only 14 of the included trials and outcomes were assessed by blinded reviewers in only six. Data were analysed according to the intention‐to‐treat method in 34 of the 54 trials.
Of the 54 RCTs included in the systematic review, only one (Villa 1996) reported both adequate allocation concealment and double blinding. Of the 24 RCTs comparing interferon with control, six had adequate allocation concealment (Camps 1993; Davis 1989; Furusyo 1997; Mazella 1994; Valla 1999; Weiland 1990) and two were double blinded (Causse 1991; Fernandez 1997). Of the 24 trials comparing different doses or durations of interferon, one (Garson 1997) was double blinded and five (Craxi 1996; Lin 1995; Marcellin 1995; McHutchison 1998; Poynard 1995) concealed allocation adequately. Of the four RCTs assessing therapy tailored to the response to treatment, one (Brouwer 1998) had adequate allocation concealment, and one (Fernandez 1997) was double‐blinded. Of the four trials comparing different interferon preparations, the Villa et al. trial (Villa 1996), reported adequate allocation concealment and double blinding; two others (Rumi 1996; Tong 1997) were double blinded; and another (Farrell 1998) had adequate allocation concealment.
Effects of interventions
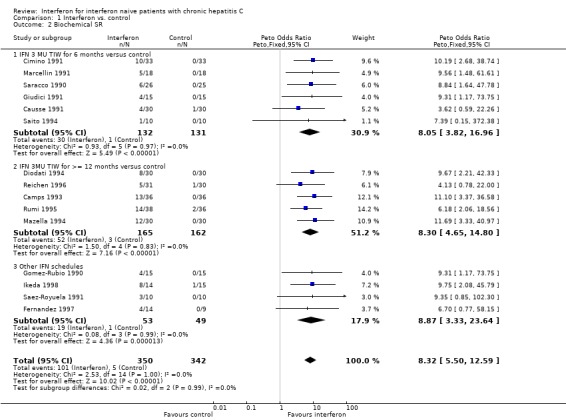
INTERFERON VERSUS PLACEBO/UNTREATED CONTROLS (Forrest plots 01.01 to 01.05) Compared with control, interferon at a dosage of 3 MU thrice weekly for six months increased the probability of achieving a biochemical ETR (OR 10.30; 95% CI 6.42 to 16.52) and SR (OR 8.05; 95% CI 3.82 to 16.96). The rates of biochemical ETR and SR were 45% (95% CI 38 to 53%) and 23% (95% CI 16 to 31%), respectively in the interferon group, versus 2% (95% CI 1 to 6%) and 1% (95% CI 0 to 4%) in untreated controls. Virologic outcome was not reported in these trials.
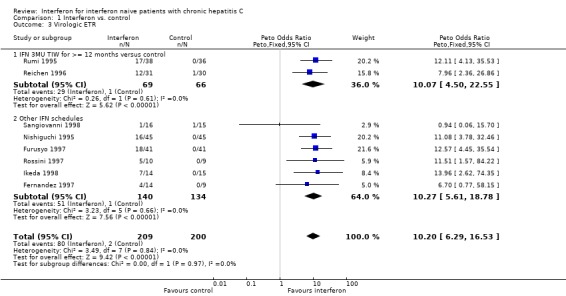
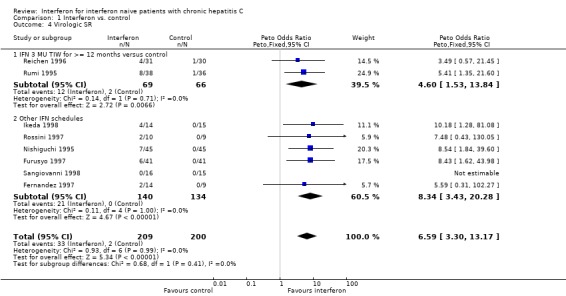
Compared with control, interferon at a dosage of 3 MU thrice weekly for at least 12 months increased the probability of achieving a virologic ETR (OR 10.07; 95% CI 4.50 to 22.55) and SR (OR 4.60; 95% CI 1.53 to 13.85). The rates of virologic ETR and SR were 42% (95% CI 31 to 54%) and 17% (95% CI 10 to 28%), respectively in the interferon group, versus 2% (95% CI 0 to 8%) and 3% (95% CI 1 to 10%) in untreated controls. Interferon treatment was also associated with an increased probability of achieving a biochemical ETR (OR 11.07; 95% CI 6.84 to 17.91) and SR (OR 8.30; 95% CI 4.65 to 14.80). The rates of biochemical ETR and SR were 49% (95% CI 41 to 56%) and 32% (95% CI 25 to 39%), respectively in the interferon group, versus 2% (95% CI 1 to 6%) and 2% (95% CI 1 to 5%) in untreated controls.
There was no significant heterogeneity in these meta‐analyses.
Liver histology (Forrest plot 01.05) Information regarding the percentage of patients with histologic improvement was not supplied in any of the eight newly identified RCTs comparing interferon with control. Therefore, the previously reported results (Poynard 1996) remain valid. In summary, after six months of treatment (Causse 1991; Saracco 1990), interferon caused a non‐significant increase in the rate of histologic improvement compared with control (OR 15.67; 95% CI Random 0.82 to 300.76). These results were heterogeneous (P = 0.057). The mean rate of improvement was 67% (33/49 patients) in the interferon 3 MU thrice weekly group versus 16% (7/45) in controls. After 12 months of treatment (Camps 1993; Makris 1991), the mean rate of histologic improvement was 91% (40/44) in the interferon group versus 30% (13/44) in the control group (OR 12.46; 95% CI 5.33 to 29.13). Six months following the end of treatment (Diodati 1994; Rumi 1995), the mean rate of histologic improvement was 55% (33/60) in the interferon group versus 17% (8/48) in controls (OR 7.74; 95% CI 3.31 to 18.08). Overall, regardless of the timing of the post‐treatment liver biopsy, interferon was associated with histologic improvement in 69% (106/153) of treated patients versus only 20% (28/137) of controls (OR 9.22; 95% CI 5.69 to 14.94).
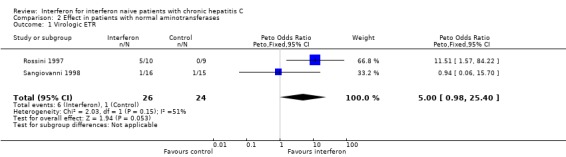
Patients with normal aminotransferases (Forrest plots 02.01 to 02.02) Two RCTs including a total of 50 patients assessed the response to interferon in patients with normal aminotransferases (Rossini 1997; Sangiovanni 1998). Only virologic outcome measures were reported in these trials. Treatment with 3 MU of interferon thrice weekly (for six months in one trial; for 12 months in the other) was associated with a virologic ETR in 23% (95% CI 9 to 44%) versus 4% (95% CI 0 to 2%) of controls (OR 5.00; 95% CI 0.98 to 25.40; P = 0.05). This difference, however, did not persist during follow‐up. Only 2/26 (8%; 95% CI 1 to 25%) of the interferon‐treated patients versus 0/24 (0%; 95% CI 0 to 14%) of the controls achieved a virologic SR (OR 7.48; 95% CI 0.43 to 130.05). There was no significant heterogeneity in the data for patients with normal aminotransferases.
Patients with cirrhosis (Forrest plots 03.01 to 03.06) Five RCTs included 330 patients with cirrhosis (Furusyo 1997; Ikeda 1998; Nishiguchi 1995; Saito 1994; Valla 1999). Two reported biochemical ETR (Saito 1994; Valla 1999) and three reported biochemical SR (Ikeda 1998; Saito 1994; Valla 1999). Compared with no treatment, interferon at a dosage of 3 MU thrice weekly (for six months in one trial; 12 months in the other) was associated with an increased probability of achieving a biochemical ETR (OR 9.75; 95% CI 2.65 to 35.90). A response was seen in 18% (10/57) of the interferon‐treated patients versus none of the controls. Interferon was also associated with an increase in the probability of achieving a biochemical SR (OR 9.17; 95% CI 2.55 to 32.95). A biochemical SR was observed in 14% (11/71) of the interferon group versus only 1% (1/77) of the controls.
Three RCTs assessed virologic responses (Furusyo 1997; Ikeda 1998; Nishiguchi 1995). HCV RNA became undetectable significantly more often in patients receiving interferon than no treatment. The virologic ETR was 41% (41/100) in interferon‐treated patients versus 0% (0/101) in controls (OR 12.16; 95% CI 6.15 to 24.05). Sustained responses were seen in 17% (17/100) of treated patients versus 0% (0/101) of controls (OR 8.84; 95% CI 3.29 to 23.77).
Three RCTs in a total of 218 cirrhotic patients supplied information regarding the occurrence of hepatocellular carcinoma (Ikeda 1998; Nishiguchi 1995; Valla 1999). In a post hoc intention‐to‐treat analysis, there was no difference in the incidence of hepatocellular carcinoma between interferon‐treated patients (46/106, 43%) and controls (52/112, 46%) (OR 0.74; 95% CI 0.38 to 1.47). The Nisghiguchi et al. trial (Nishiguchi 1995), however, which contributed 90 of the total 218 patients, had a large loss to follow‐up. After approximately seven years, 36/45 of the interferon‐treated patients and 19/45 of the controls were unavailable for assessment. Their inclusion in the meta‐analysis as treatment failures (i.e., cases of hepatocellular carcinoma using the intention‐to‐treat method) severely biased the results. In a sensitivity analysis excluding this trial (Forrest plot 03.06), there remained no significant difference in the incidence of hepatocellular carcinoma between interferon‐treated patients and controls (OR 0.50; 95% CI 0.21 to 1.21). In this revised post hoc analysis, the incidence of hepatocellular carcinoma in the interferon and control groups were 13% (8/61) and 24% (16/67), respectively (P = 0.17).
There was no significant heterogeneity in the meta‐analyses of cirrhotic patients.
COMPARISON OF DIFFERENT INTERFERON REGIMENS Dose effect: 6 MU versus 3 MU thrice weekly (Forrest plots 04.01 to 04.04) For the comparison of 6 MU versus 3 MU for six months treatment duration, the biochemical ETR was 63% (95% CI 56 to 69%) in the 6 MU group versus 51% (95% CI 44 to 58%) in the 3 MU group (OR 1.64; 95% CI 1.10 to 2.44). The rates of biochemical SR were 27% (95% CI 22 to 34%) and 15% (95% CI 11 to 21%), respectively (OR 2.03; 95% CI 1.27 to 3.23). The higher dosage of interferon also increased the probability of achieving a virologic ETR (OR 2.23; 95% CI 1.13 to 4.41) and SR (OR 2.69; 95% CI 1.24 to 5.84). The virologic ETR was 51% (95% CI 39 to 63%) in the 6 MU group versus 32% (95% CI 22 to 45%) in the 3 MU group. The corresponding rates of virologic SR were 35% (95% CI 24 to 47%) and 16% (95% CI 8 to 27%), respectively. These data were homogeneous.
For the comparison of 6 MU versus 3 MU for at least 12 months treatment duration, the biochemical ETR was 69% (95% CI 60 to 76%) in the 6 MU group versus 57% (95% CI 49 to 64%) in the 3 MU group (OR 1.63; 95% CI 1.00 to 2.67). The rates of biochemical SR were 46% (95% CI 38 to 55%) and 29% (95% CI 22 to 36%), respectively (OR 2.08; 95% CI 1.30 to 3.34). The higher dosage of interferon also increased the probability of achieving a virologic ETR (OR 2.79; 95% CI 1.53 to 5.09) and SR (OR 2.21; 95% CI 1.10 to 4.45). The virologic ETR was 59% (95% CI 48 to 69%) in the 6 MU group versus 32% (95% CI 24 to 42%) in the 3 MU group. The corresponding rates of virologic SR were 43% (95% CI 32 to 55%) and 25% (95% CI 17 to 37%), respectively. These data were homogeneous.
For the analysis of a dose effect in trials examining other treatment regimens, there was a statistically significant treatment effect in favour of higher dose therapy for a biochemical SR (OR 1.50; 95% CI 1.10 to 2.05), and a nonsignificant increase in the rate of virologic SR (OR 1.39; 95% CI 0.98 to 1.97). A sensitivity analysis excluding the Shiratori et al. trial (Shiratori 1997), which had unclear randomisation, did not significantly impact these results.
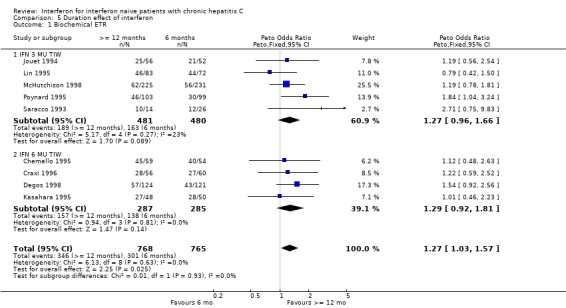
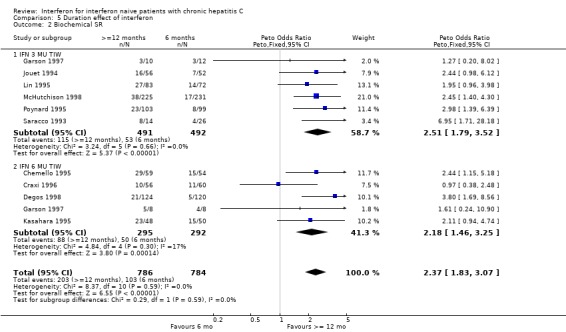
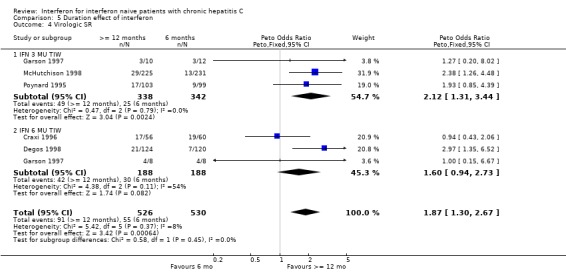
Duration effect: interferon >= 12 months versus six months (Forrest plots 05.01 to 05.04) In trials examining the impact of the duration of interferon treatment, there was no significant effect on the rates of biochemical or virologic response at the end of treatment. Sustained responses, however, were significantly more common in those receiving a prolonged duration of therapy. At a thrice weekly dosage of 3 MU, the rate of biochemical SR was 23% (95% CI 20 to 27%) in those treated for 12 months or more versus only 11% (95% CI 8 to 14%) in those receiving six months of treatment (OR 2.51; 95% CI 1.79 to 3.52). The corresponding rates of virologic SR were 14% (95% CI 11 to 19%) and 7% (95% CI 5 to 11%), respectively (OR 2.12; 95% CI 1.31 to 3.44).
The effect of treatment duration on sustained responses was also significant at the higher dose of 6 MU thrice weekly. Compared with a treatment duration of six months, therapy for 12 months or longer was associated with an increase in the rate of biochemical SR (OR 2.18; 95% CI 1.46 to 3.25) and a non‐significant increase in the rate of virologic SR (OR 1.60; 95% CI 0.94 to 2.73). The rates of biochemical SR were 30% (95% CI 25 to 35%) in those receiving treatment for 12 months or longer versus only 17% (95% CI 13 to 22%) in those treated for six months. The corresponding rates of virologic SR were 22% (95% CI 17 to 29%) and 16% (95% CI 11 to 22%), respectively.
There was no significant heterogeneity in the meta‐analyses assessing the effect of the duration of interferon therapy.
SENSITIVITY ANALYSES Sensitivity analyses did not reveal any significant influence upon the results of the analyses assessing interferon versus control (excluding the normal aminotransferase and cirrhotic groups) nor interferon versus different interferon regimens (including the assessments of dose and duration effects) of the following factors: exclusion of RCTs with low methodological quality; a cirrhosis prevalence > 30%; mean age > 47 years; mean disease duration > five years; a follow‐up of less than 12 months; or the exclusion of RCTs assessing non‐2b interferons (data not shown). Due to the large number of trials with inadequate or unclear allocation concealment, a post hoc sensitivity analysis was performed to determine the impact of allocation concealment on the rates of biochemical ETR and SR (Forrest plots 08.01 to 08.02). The impact of allocation concealment on the primary outcome measure (the rate of virologic SR) was not examined because only one (Furusyo 1997) of the eight trials eligible for this analysis had adequate allocation concealment. In the post hoc sensitivity analysis, the adequacy of allocation concealment did not affect the likelihood of a biochemical ETR or SR (P < 0.05 for both comparisons).
COMPARISON OF DIFFERENT INTERFERON FORMULATIONS (Forrest plots 06.01 to 06.04) There was significant heterogeneity in all of the outcome measures assessed in the comparison of different interferon formulations (P < 0.001 for all measures). Nevertheless, there appeared to be no significant differences in the rates of biochemical or virologic SR among the different formulations of interferon.
TRIALS WITH TAILORED REGIMENS (Forrest plots 07.01 to 07.04) There were no statistically significant differences between tailored and fixed‐dosed regimens for any of the outcome measures assessed, including the rate of virologic SR (OR 1.23; 95% CI 0.72 to 2.09).
ADVERSE EVENTS Compared with those treated for six months, patients treated for longer durations with standard dose therapy (3 MU thrice weekly) were more likely to require treatment discontinuation (9% versus 5%, P < 0.001) or dosage reduction (15% versus 10%, P < 0.001). Likewise, these patients were more likely to experience a variety of adverse events, including flu‐like syndromes (80% versus 64%, P < 0.001), depression (24% versus 12%, P < 0.001), and alopecia (20% versus 15%, P = 0.02). Patients receiving higher dose therapy (5‐6 MU thrice weekly) were more likely to have leukopenia than those receiving standard dose therapy (10% versus 1%, P < 0.001).
Discussion
This systematic review identified 54 published RCTs which met the inclusion criteria, compared with only 33 in a previous meta‐analysis from our group (Poynard 1996). This increase in information justified an updated review in order to examine the robustness of our previous results and to address several unresolved issues. Although interferon monotherapy has now been superseded by more effective therapy including pegylated interferon and ribavirin, this meta‐analysis provides important information for those patients ineligible for, or who cannot tolerate ribavirin, and in places where pegylated interferon is not yet available. This review confirms the efficacy of interferon in achieving virologic clearance and thereby improving hepatic biochemistry and histology in naive patients with chronic hepatitis C. In fact, a standard course of interferon 3 MU thrice weekly for 12 months was associated with a virologic SR of 17% versus only 3% in controls. This led to a 30% absolute increase in the rate of sustained biochemical response and a tripling in the probability of histologic improvement during short‐term follow‐up.
It should be emphasised, however, that no clinical outcome measures were assessed in this meta‐analysis, nor were they in any long‐term, prospective studies. Therefore, in the absence of knowledge relating to the long‐term potential of interferon to reduce important events, such as the incidence of endstage liver disease or requirement for liver transplantation, it is up to the patient and treating physician to decide whether or not to embark on interferon therapy based on surrogate evidence.
Our analysis confirmed a significant impact of dose on the response to interferon monotherapy. Treatment with 6 MU thrice weekly for 12 months was associated with a near doubling of the rate of virologic SR compared with the standard dose of 3 MU thrice weekly (43% versus 25%, P = 0.03). This rate of virologic SR is similar to that reported in recent, large‐scale trials of combination therapy with ribavirin (McHutchison 1998; Poynard 1998). Admittedly, the relative success of interferon monotherapy in our review may relate in part to the insensitivity of PCR assays available during the period of the included trials. This significant 'dose‐effect' persisted independent of treatment duration (for six months or 12 months and longer).
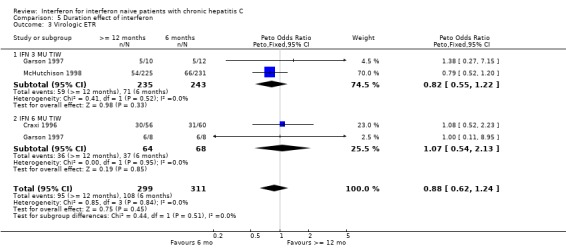
Our analysis also enabled us to determine the impact of treatment duration on the rate of biochemical and virologic response to interferon monotherapy. Treatment with 3 MU thrice weekly for 12 months or longer was associated with a doubling of the rate of virologic SR compared with a six‐month course (14% versus 7%, P = 0.003). This effect appeared to be more important with lower dose therapy than high‐dose therapy in which the increase in virologic SR according to treatment duration did not reach statistical significance (22% for 12 months or longer versus 16% for six months, P = 0.15). As previously observed, this 'duration effect' was not significant for virologic responses at the end of treatment demonstrating that the utility of long‐term therapy is the prevention of relapse in patients who have successfully cleared the virus by 24 weeks.
We also analysed the responses to different interferon formulations (including consensus, beta, and lymphoblastoid interferon) and tailored regimens of therapy. Our results revealed no clear difference between the different interferon formulations. Likewise, we could not identify a more efficacious strategy than that employing a fixed dose and duration of therapy. We found no clear advantage in terms of biochemical or virologic sustained responses with escalating or de‐escalating regimens according to the early response to therapy; although more effective than no treatment, these regimens were no more effective than standard, fixed‐dose therapy.
Our systematic review also allowed us to address the utility of interferon in two important patient subgroups: those with normal aminotransferases, and cirrhotic patients, in whom interferon treatment had generated a considerable amount of controversy. In patients with normal liver biochemistry, interferon therapy was associated with a 19% increase in the rate of virologic ETR compared with controls (23% versus 4%), but this difference did not persist during follow‐up as a virologic SR was seen in only 8% of treated patients versus 0% of controls (P = 0.17). This failure to identify a sustained difference, however, may reflect a type II (beta) error since only two trials in a total of 50 patients were assessed. A potential argument against the exclusion of these patients from treatment is the difficulty in defining a normal ALT if gender and body mass are not considered (Piton 1998). Furthermore, recent trials have failed to identify a threshold value for initial ALT which is predictive of a virologic SR to therapy (McHutchison 1998; Poynard 1998; Gordon 2000). Nevertheless, we cannot recommend interferon monotherapy in patients with normal aminotransferases based on the results of this meta‐analysis. We would, however, recommend that these patients be enrolled in randomised, controlled trials assessing pegylated interferon and ribavirin to help clarify this issue.
The effectiveness of interferon was also assessed in trials enrolling patients with compensated cirrhosis. This meta‐analysis confirms the utility of interferon therapy in this patient subgroup with respect to biochemical and virologic outcome measures. The percentage of cirrhotic patients with persistently normal ALT or undetectable serum HCV RNA after treatment (virologic SR of 17%) was significantly higher than in controls, and similar to that reported in non‐cirrhotic patients. We could not, however, demonstrate a protective effect of interferon therapy on the progression to hepatocellular carcinoma in a post hoc analysis. Using a strict intention‐to‐treat analysis in a total of 218 patients, hepatocellular carcinoma developed in 43% of interferon‐treated patients versus 46% of controls (P = 0.4) after a mean follow‐up ranging from 3.3 to 5.5 years. Only well‐designed, prospective trials with acceptable follow‐up will clarify this issue in the future.
We also attempted to analyse the frequency of adverse events attributable to interferon, but this was difficult to interpret due to heterogeneity in the quality of reporting in the included trials. Our conclusions should also be tempered by the fact that all of the outcome measures were assessed in an unblinded fashion. Nevertheless, the six‐month incidence of alopecia (15%) and depression (12%) represent meaningful information which can be supplied to patients prior to the initiation of therapy. Another clinically important point is the incidence of suicide related to interferon‐induced depression. Unfortunately, we were unable to estimate this event due to insufficient data. From the available data it was also difficult to estimate the effects of dose and duration of interferon therapy on the incidence of adverse events. We did, however, observe a larger impact of treatment duration than dose, with the exception of leukopenia, which was significantly more common with high‐dose therapy. Treatment duration had its most striking effect on the incidence of depression, which doubled from 12% in those treated for six months to 24% in patients treated for a year or longer. This result suggests that in clinical practice the risk of depression still exists in patients who have tolerated the first six months of treatment. We did not assess the impact of interferon therapy on a number of common side effects including fatigue, influenza‐like symptoms, and myalgias. However, in a recent systematic review of combination therapy with interferon and ribavirin versus interferon monotherapy (Kjaergard 2002) the incidence of these side effects in the monotherapy group was 55%, 61%, and 53%, respectively. In the same review, dosage reductions were necessary in 5% of patients and treatment was discontinued in 9%.
Our systematic review has several weaknesses. In this overview, we did not include RCTs identified only as abstracts; this may have caused a bias of publication. However, in our previous overview (Poynard 1996), the observed significant differences persisted in sensitivity analyses after the inclusion of RCTs published only as abstracts. Furthermore, the rates of virologic SR observed in our review are similar to those reported in the systematic review cited above (Kjaergard 2002). Thus, we feel that our assessment of the treatment effect of interferon is accurate. Another limitation of our systematic review is the suboptimal methodological quality of the included trials; only one of the 54 included RCTs had adequate allocation concealment and was double blinded. Nevertheless, sensitivity analyses stratifying trials according to methodological quality (defined according to a previously validated quality score) and allocation concealment revealed no significant effect on the results. Another weakness was our failure to combine individual patient data, which would have permitted a better analysis of the treatment effect by taking into account 'per‐patient' heterogeneity in multivariate analyses. In comparison to our previous overview, there are now many trials which examined virologic characteristics. However, it was impossible to perform meta‐analyses stratified by such factors as age, gender, genotype, viral load, and stage of fibrosis, which we now know are important factors in determining patients' response to interferon therapy as well as their long‐term prognosis. When we did perform such an analysis in patients treated by interferon alone in two recent trials comparing interferon monotherapy with combination therapy including ribavirin (McHutchison 1998; Poynard 1998), we did find that these five factors (younger age, female sex, infection with non‐genotype 1, low viral load, and absent or minimal fibrosis) were predictive of a sustained virologic response to interferon monotherapy (Poynard 2000).
An additional weakness of this meta‐analysis was the absence of standardisation of the outcome measures among the included trials. For example, the definition of a biochemical response ranged from one to four consecutive, normal ALT determinations. Similarly, the sensitivity of PCR testing for virologic responses was not stated in some trials, and varied from 100 to more than 3000 copies/ml in others. Furthermore, the time following the end of treatment necessary for the definition of a sustained response varied markedly in the trials from four to 36 months. It is reassuring, however, that the majority of the trials defined a sustained virologic response, undoubtedly the most important outcome assessed, at six months or more following the end of treatment. We now know that negative HCV RNA PCR tests beyond this point are highly durable (Marcellin 1997). In addition, a sensitivity analysis excluding trials with less than 12 months of follow‐up, and hence a definition of sustained response of less than 12 months, did not reveal a significant impact on the results. Finally, our histologic data is weakened by the absence of standardised histological outcome measures in most of the trials (Bedossa 1994) as well as the frequent absence of post‐treatment biopsies, which are notoriously difficult to obtain. Even in trials which focus on histological criteria, the number of available follow‐up biopsies is generally only between 50 and 60 per cent.
In comparison to our previously published meta‐analysis (Poynard 1996), the total number of patients included in this analysis was considerably higher. However, the mean number of patients included per trial was still low, with only nine RCTs having more than 100 patients per intervention arm. This limitation was most evident in the comparison of different interferon regimens since this type of comparison requires more power than comparisons versus untreated controls. By updating the previous meta‐analysis, however, we were able to increase the power of the present review, and thereby reduce the risk of false‐negative conclusions due to small trials. We now have a better estimate of the exact level of efficacy of interferon monotherapy for the biochemical, virologic, and histologic outcome measures assessed, which may serve as a benchmark for future treatments in interferon naive patients with chronic hepatitis C.
Authors' conclusions
Implications for practice.
Interferon is an effective therapy in naive patients with chronic hepatitis C (including those with cirrhosis) with respect to biochemical, virologic, and histological outcomes. There does not appear to be a significant impact of interferon formulation or the use of tailored regimens according to patient response on the sustained clearance of HCV RNA from serum. The efficacy of interferon monotherapy for achieving sustained virologic clearance is dependent on the dose and duration of therapy. The most effective regimen of interferon monotherapy for this outcome appears to be at least 6 MU thrice weekly for 12 months treatment duration but at a cost of more adverse events.
Implications for research.
These results are useful as a better assessment of the benefits of novel treatments in chronic hepatitis C. In the future, results of ongoing trials of other antiviral regimens, including pegylated interferons and ribavirin, should be compared to these results.
What's new
| Date | Event | Description |
|---|---|---|
| 9 November 2008 | Amended | Converted to new review format. |
Notes
The protocol for this systematic Review was first published in The Cochrane Library, Issue 3, 1997 with the title 'Interferon for chronic hepatitis C'. It was prepared by Poynard T, Leroy V, Cohard M, Thevenot T, Mathurin P, Opolon P, Zarski JP.
Robert P. Myers became the leading author of the Review in 2001. Due to the fact that it was only intended to include interferon naive patients, the title has been changed into 'Interferon for interferon naive patients with chronic hepatitis C'.
Investigators involved in the included trials are invited to contact the authors to clarify methodological issues (particularly the methods of generating the allocation sequence, concealing allocation, and double‐blinding) so that future versions of the review can be updated.
Acknowledgements
R.P. Myers is supported by the Dr. V. Feinman Hepatology Fellowship from the Canadian Association for the Study of the Liver and the Detweiler Traveling Fellowship from the Royal College of Physicians and Surgeons of Canada. T. Thevenot had a grant from Recherche et Partage, and C. Regimbeau from Association Française pour l'Etude du Foie and Club Francophone de l'Hypertension Portale. The authors would like to acknowledge Drs. Christian Gluud and Ronald Koretz for their helpful suggestions regarding the manuscript.
Data and analyses
Comparison 1. Interferon vs. control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Biochemical ETR | 18 | 863 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 10.42 [7.69, 14.11] |
| 1.1 IFN 3 MU TIW for 6 months versus control | 7 | 372 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 10.30 [6.42, 16.52] |
| 1.2 IFN 3 MU TIW for >= 12 months versus control | 6 | 345 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 11.07 [6.84, 17.91] |
| 1.3 Other IFN schedules | 5 | 146 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.38 [4.65, 18.90] |
| 2 Biochemical SR | 15 | 692 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.32 [5.50, 12.59] |
| 2.1 IFN 3 MU TIW for 6 months versus control | 6 | 263 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.05 [3.82, 16.96] |
| 2.2 IFN 3MU TIW for >= 12 months versus control | 5 | 327 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.30 [4.65, 14.80] |
| 2.3 Other IFN schedules | 4 | 102 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.87 [3.33, 23.64] |
| 3 Virologic ETR | 8 | 409 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 10.20 [6.29, 16.53] |
| 3.1 IFN 3MU TIW for >= 12 months versus control | 2 | 135 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 10.07 [4.50, 22.55] |
| 3.2 Other IFN schedules | 6 | 274 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 10.27 [5.61, 18.78] |
| 4 Virologic SR | 8 | 409 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.59 [3.30, 13.17] |
| 4.1 IFN 3 MU TIW for >= 12 months versus control | 2 | 135 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.60 [1.53, 13.84] |
| 4.2 Other IFN schedules | 6 | 274 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.34 [3.43, 20.28] |
| 5 Improvement in liver histology | 6 | 290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.22 [5.69, 14.94] |
| 5.1 Biopsy at the end of treatment (6 months) | 2 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.21 [3.64, 18.53] |
| 5.2 Biopsy at the end of treatment (12 months) | 2 | 88 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 12.46 [5.33, 29.13] |
| 5.3 Biopsy at the end of follow‐up (6 months following the end of treatment) | 2 | 108 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.74 [3.31, 18.08] |
1.1. Analysis.

Comparison 1 Interferon vs. control, Outcome 1 Biochemical ETR.
1.2. Analysis.
Comparison 1 Interferon vs. control, Outcome 2 Biochemical SR.
1.3. Analysis.
Comparison 1 Interferon vs. control, Outcome 3 Virologic ETR.
1.4. Analysis.
Comparison 1 Interferon vs. control, Outcome 4 Virologic SR.
1.5. Analysis.

Comparison 1 Interferon vs. control, Outcome 5 Improvement in liver histology.
Comparison 2. Effect in patients with normal aminotransferases.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Virologic ETR | 2 | 50 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.00 [0.98, 25.40] |
| 2 Virologic SR | 2 | 50 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.48 [0.43, 130.05] |
2.1. Analysis.
Comparison 2 Effect in patients with normal aminotransferases, Outcome 1 Virologic ETR.
2.2. Analysis.
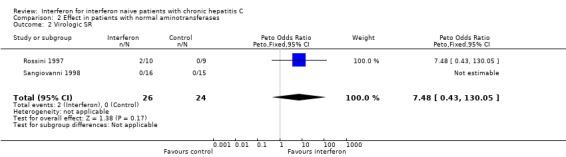
Comparison 2 Effect in patients with normal aminotransferases, Outcome 2 Virologic SR.
Comparison 3. Effect in cirrhotic patients.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Biochemical ETR | 2 | 119 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.75 [2.65, 35.90] |
| 2 Biochemical SR | 3 | 148 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.17 [2.55, 32.95] |
| 3 Virologic ETR | 3 | 201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 12.16 [6.15, 24.05] |
| 4 Virologic SR | 3 | 201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.84 [3.29, 23.77] |
| 5 Hepatocellular carcinoma | 3 | 218 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.38, 1.47] |
| 6 Hepatocellular carcinoma (excluding Nishiguchi trial) | 2 | 128 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.21, 1.21] |
3.1. Analysis.
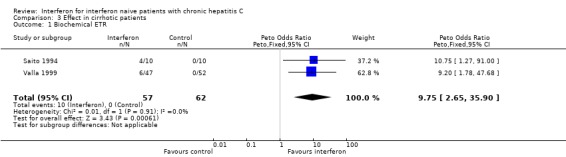
Comparison 3 Effect in cirrhotic patients, Outcome 1 Biochemical ETR.
3.2. Analysis.

Comparison 3 Effect in cirrhotic patients, Outcome 2 Biochemical SR.
3.3. Analysis.
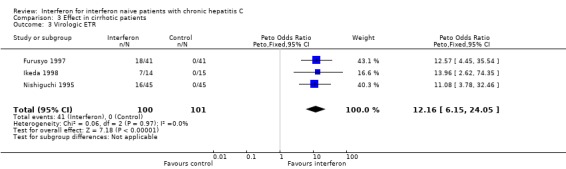
Comparison 3 Effect in cirrhotic patients, Outcome 3 Virologic ETR.
3.4. Analysis.

Comparison 3 Effect in cirrhotic patients, Outcome 4 Virologic SR.
3.5. Analysis.
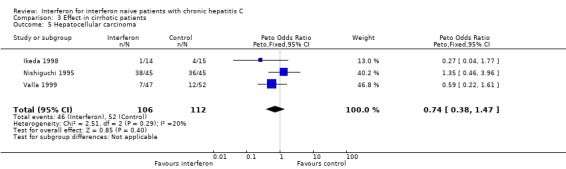
Comparison 3 Effect in cirrhotic patients, Outcome 5 Hepatocellular carcinoma.
3.6. Analysis.
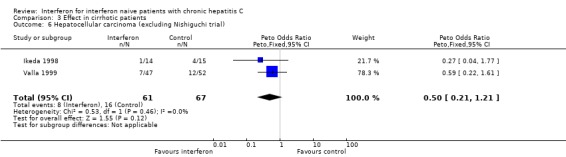
Comparison 3 Effect in cirrhotic patients, Outcome 6 Hepatocellular carcinoma (excluding Nishiguchi trial).
Comparison 4. Dose effect of interferon.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Biochemical ETR | 13 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Treatment duration: 6 months | 5 | 408 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.64 [1.10, 2.44] |
| 1.2 Treatment duration: >= 12 months | 3 | 284 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.63 [1.00, 2.67] |
| 1.3 Other interferon schedules | 6 | 938 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.74, 1.25] |
| 2 Biochemical SR | 15 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Treatment duration: 6 months | 6 | 428 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.03 [1.27, 3.23] |
| 2.2 Treatment duration: >= 12 months | 4 | 302 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.08 [1.30, 3.34] |
| 2.3 Other interferon schedules | 7 | 962 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.50 [1.10, 2.05] |
| 3 Virologic ETR | 8 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Treatment duration: 6 months | 3 | 140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.23 [1.13, 4.41] |
| 3.2 Treatment duration: >= 12 months | 3 | 183 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.79 [1.53, 5.09] |
| 3.3 Other interferon schedules | 3 | 659 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.45 [1.06, 1.98] |
| 4 Virologic SR | 8 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Treatment duration: 6 months | 3 | 140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.69 [1.24, 5.84] |
| 4.2 Treatment duration: >= 12 months | 2 | 138 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.21 [1.10, 4.45] |
| 4.3 Other interferon schedules | 4 | 747 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.39 [0.98, 1.97] |
4.1. Analysis.

Comparison 4 Dose effect of interferon, Outcome 1 Biochemical ETR.
4.2. Analysis.
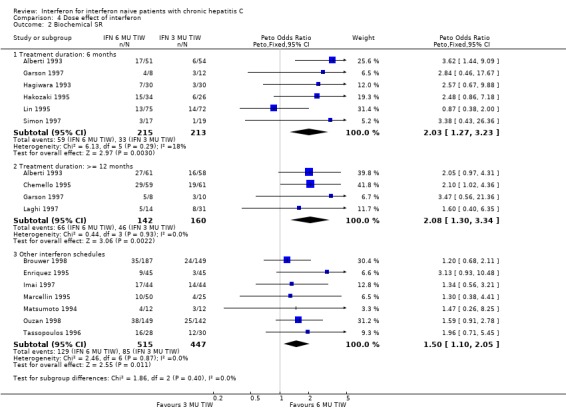
Comparison 4 Dose effect of interferon, Outcome 2 Biochemical SR.
4.3. Analysis.
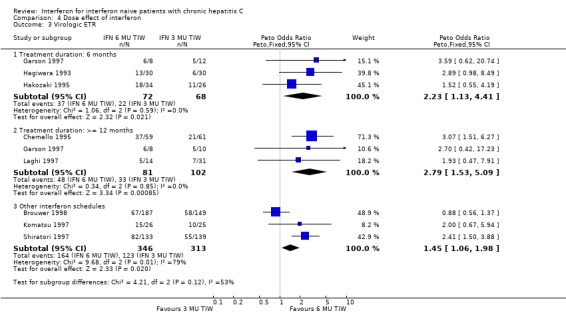
Comparison 4 Dose effect of interferon, Outcome 3 Virologic ETR.
4.4. Analysis.
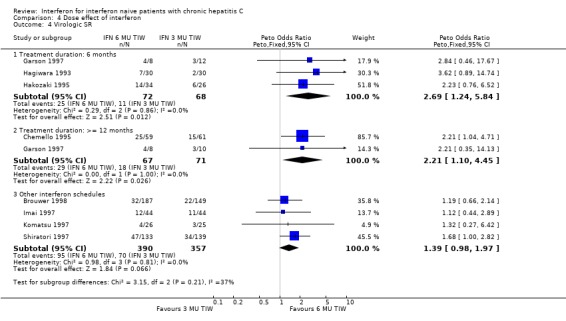
Comparison 4 Dose effect of interferon, Outcome 4 Virologic SR.
Comparison 5. Duration effect of interferon.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Biochemical ETR | 9 | 1533 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [1.03, 1.57] |
| 1.1 IFN 3 MU TIW | 5 | 961 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.96, 1.66] |
| 1.2 IFN 6 MU TIW | 4 | 572 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.29 [0.92, 1.81] |
| 2 Biochemical SR | 10 | 1570 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.37 [1.83, 3.07] |
| 2.1 IFN 3 MU TIW | 6 | 983 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.51 [1.79, 3.52] |
| 2.2 IFN 6 MU TIW | 5 | 587 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.18 [1.46, 3.25] |
| 3 Virologic ETR | 3 | 610 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.62, 1.24] |
| 3.1 IFN 3 MU TIW | 2 | 478 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.55, 1.22] |
| 3.2 IFN 6 MU TIW | 2 | 132 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.54, 2.13] |
| 4 Virologic SR | 5 | 1056 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.87 [1.30, 2.67] |
| 4.1 IFN 3 MU TIW | 3 | 680 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.12 [1.31, 3.44] |
| 4.2 IFN 6 MU TIW | 3 | 376 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.60 [0.94, 2.73] |
5.1. Analysis.
Comparison 5 Duration effect of interferon, Outcome 1 Biochemical ETR.
5.2. Analysis.
Comparison 5 Duration effect of interferon, Outcome 2 Biochemical SR.
5.3. Analysis.
Comparison 5 Duration effect of interferon, Outcome 3 Virologic ETR.
5.4. Analysis.
Comparison 5 Duration effect of interferon, Outcome 4 Virologic SR.
Comparison 6. Effect of different formulations of interferon.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Biochemical ETR | 6 | 2330 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.84, 2.30] |
| 2 Biochemical SR | 6 | 2330 | Odds Ratio (M‐H, Random, 95% CI) | 1.58 [0.81, 3.10] |
| 3 Virologic ETR | 6 | 2330 | Odds Ratio (M‐H, Random, 95% CI) | 1.43 [0.76, 2.68] |
| 4 Virologic SR | 6 | 2330 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [0.67, 2.97] |
6.1. Analysis.

Comparison 6 Effect of different formulations of interferon, Outcome 1 Biochemical ETR.
6.2. Analysis.
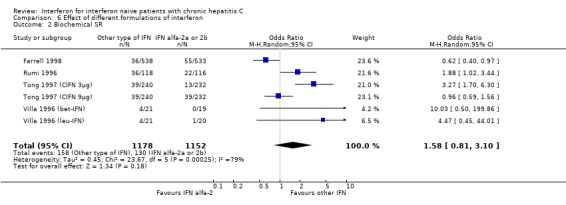
Comparison 6 Effect of different formulations of interferon, Outcome 2 Biochemical SR.
6.3. Analysis.
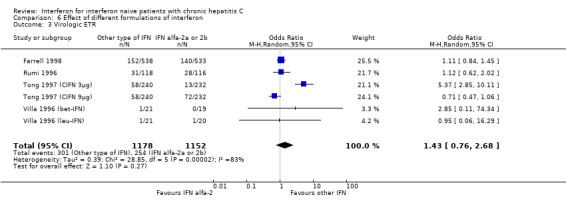
Comparison 6 Effect of different formulations of interferon, Outcome 3 Virologic ETR.
6.4. Analysis.

Comparison 6 Effect of different formulations of interferon, Outcome 4 Virologic SR.
Comparison 7. Effect of tailored regimen vs. fixed‐dose regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Biochemical ETR | 2 | 401 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.52, 1.16] |
| 2 Biochemical SR | 2 | 401 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.71, 1.98] |
| 3 Virologic ETR | 2 | 401 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.62, 1.39] |
| 4 Virologic SR | 2 | 401 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.72, 2.09] |
7.1. Analysis.
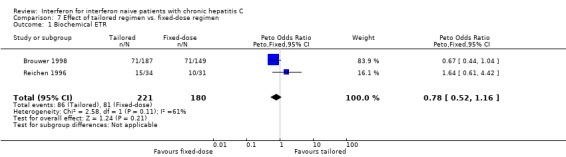
Comparison 7 Effect of tailored regimen vs. fixed‐dose regimen, Outcome 1 Biochemical ETR.
7.2. Analysis.

Comparison 7 Effect of tailored regimen vs. fixed‐dose regimen, Outcome 2 Biochemical SR.
7.3. Analysis.

Comparison 7 Effect of tailored regimen vs. fixed‐dose regimen, Outcome 3 Virologic ETR.
7.4. Analysis.
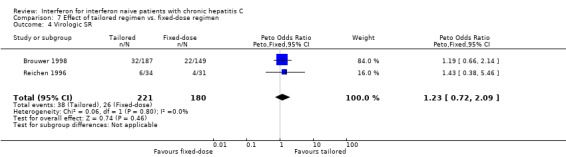
Comparison 7 Effect of tailored regimen vs. fixed‐dose regimen, Outcome 4 Virologic SR.
Comparison 8. Sensitivity analysis on allocation concealment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Biochemical ETR | 18 | 863 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 10.42 [7.69, 14.11] |
| 1.1 Adequate allocation concealment | 4 | 274 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.88 [5.73, 17.02] |
| 1.2 Unclear or indequate allocation concealment | 14 | 589 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 10.67 [7.40, 15.39] |
| 2 Biochemical SR | 15 | 692 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.32 [5.50, 12.59] |
| 2.1 Adequate allocation concealment | 2 | 132 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 11.37 [4.79, 26.99] |
| 2.2 Unclear or inadequate allocation concealment | 13 | 560 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.58 [4.73, 12.15] |
8.1. Analysis.
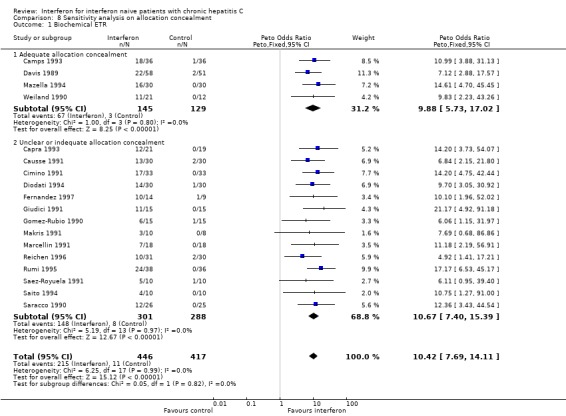
Comparison 8 Sensitivity analysis on allocation concealment, Outcome 1 Biochemical ETR.
8.2. Analysis.
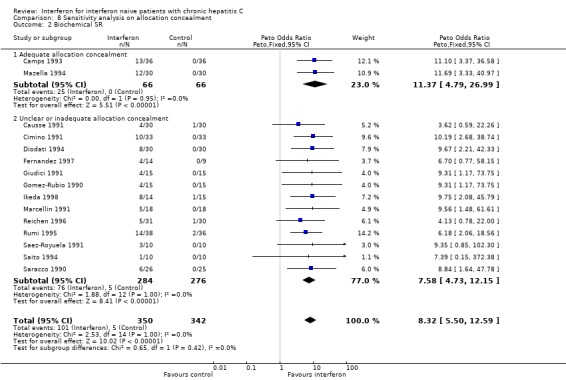
Comparison 8 Sensitivity analysis on allocation concealment, Outcome 2 Biochemical SR.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alberti 1993.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 10. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 51/54 (RCT1); 61/58 (RCT2) Excluded: No data available (NDA). Mean age: 45/48 (RCT1); 44/47(RCT2) % males: 76/66 (RCT1); 69/63(RCT2) % transfusion: NDA % drug abuse: NDA % cirrhosis: 18/26 (RCT1); 13/19(RCT2) Genotypes: NDA Disease duration: NDA | |
| Interventions | ‐ RCT 1: Alfa‐2a IFN 6 MU TIW x 6 mo vs 3 MU TIW x 6 mo ‐ RCT 2: Alfa n‐1 IFN 6 MU TIW x 6 mo; 3 MU TIW x 6 mo vs 3 MU TIW x 12 mo ‐ Follow‐up: 8 mo |
|
| Outcomes | ‐ biochemical ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Brouwer 1998.
| Methods | Allocation concealment: adequate (sealed envelopes). Blinding: no. Intention to treat: yes. Methodological score: 18. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 149/187 Excluded: NDA Mean age: 47/47 % males: 63/57 % transfusion: NDA % drug abuse: NDA % cirrhosis: 25/25 Genotypes: G1 (60/69%), G2 (11/8%), G3 (15/12%), other (13/10%) Disease duration: 14/12 | |
| Interventions | ‐ Experimental 1: Alfa‐2b IFN 3 MU TIW x 6 mo ‐ Experimental 2: Alfa‐2b IFN 6 MU TIW x 8 wk, then down‐titration until ALT normalization and RNA ‐. IFN stopped if ALT did not normalize during the 6 MU TIW period or after 52 wk if ALT normalized but RNA +. ‐ Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Camps 1993.
| Methods | Allocation concealment: adequate (sealed envelopes). Blinding: no. Intention to treat: yes. Methodological score: 14. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 36/36 Excluded: 0/0 Mean age: 44/43 % males: 67/61 % transfusion: 31/50 % drug abuse: 17/8 % cirrhosis: 28/25 Genotypes: NDA Disease duration: 5 ??? | |
| Interventions | ‐ Experimental: Alfa‐n‐1 IFN 3 MU/d x 8 weeks; 3 MU TIW x 3 mo; 1.5 MU TIW x 6mo ‐ Control: no intervention ‐ Follow‐up: 15 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ liver histology | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Capra 1993.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: no. Methodological score: 12. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 21/19 Excluded: 3/0 Mean age: 52/40 % males: 38/78 % transfusion: NDA % drug abuse: 19/42 % cirrhosis: 48/21 Genotypes: NDA Disease duration: NDA | |
| Interventions | ‐ Experimental: Alfa‐2a IFN 6 MU TIW x 6 mo ‐ Control: no intervention ‐ Follow‐up: 0 |
|
| Outcomes | ‐ biochemical ETR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Causse 1991.
| Methods | Allocation concealment: unclear. Blinding: yes. Intention to treat: yes. Methodological score: 13. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 30/30 Excluded: 2/1 Mean age: 47/50 % males: 43/37 % transfusion: 33/50 % drug abuse: 10/13 % cirrhosis: NDA Genotypes: NDA Disease duration: NDA | |
| Interventions | ‐ Experimental: Alfa‐2b IFN 3 MU TIW x 6 mo ‐ Control: placebo ‐ Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ liver histology | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chemello 1995.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 16. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 59/61/54 Excluded: 4/4/2 Mean age: 42/47/44 % males: 75/62/74 % transfusion: NDA % drug abuse: NDA % cirrhosis: 15/20/19 Genotypes: G1(52/55/47), G2 (32/32/38), G3 (16/13/13), other (0/0/2) Disease duration: 5/6/6 ??? | |
| Interventions | ‐ Experimental 1: Alfa‐2a IFN 6 MU TIW x 6 mo; 3 MU TIW x 6 mo ‐ Experimental 2: Alfa‐2a IFN 3 MU TIW x 12 mo ‐ Experimental 3: Alfa‐2a IFN 6 MU TIW x 6 mo ‐ Follow‐up: 12 mo |
|
| Outcomes | ‐ biochemical ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cimino 1991.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 12. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: n=33/33 Excluded: 0/0 Mean age: 51/48 % males: 36/46 % transfusion: NDA % drug abuse: NDA % cirrhosis: 61/48 Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental: Alfa‐2a IFN 3 MU TIW x 6 mo Control : no intervention Follow‐up: 4 mo |
|
| Outcomes | ‐ biochemical ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Craxi 1996.
| Methods | Allocation concealment: adequate (sealed envelopes). Blinding: no. Intention to treat: yes. Methodological score: 18. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 56/60 Excluded: 13/10 Mean age: 48/47 % males: 86/78 % transfusion: 100/100 % drug abuse: 0/0 % cirrhosis: 36/37 Genotypes: G1 (86/91), G2,3 (14/9) Disease duration: 4/5 | |
| Interventions | Experimental 1: Alfa‐n‐1 IFN 5 MU/m2 TIW x 2mo; 3 MU/m2 TIW x 10 mo Experimental 2: Alfa‐n‐1 IFN 5 MU/m2 TIW x 2mo; 3 MU/m2 TIW x 4 mo Follow‐up: 12 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Davis 1989.
| Methods | Allocation concealment: adequate (allocation concealed centrally). Blinding: no Intention to treat: yes. Methodological score: 15. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 58/51 Excluded: 4/1 Mean age: 54/50 % males: 52/63 % transfusion: 90/82 % drug abuse: NDA % cirrhosis: 50/43 Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental: Alfa‐2b IFN 3 MU TIW x 6 mo Control: no intervention Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Degos 1998.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 16. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 124/122 Excluded: 12/14 Mean age: 37/37 % males: 68/60 % transfusion: 33/28 % drug abuse: 31/32 % cirrhosis: 0/0 Genotypes: G1 (52/56), G2,3 (39/36), other (9/8) Disease duration: 11/12 | |
| Interventions | Experimental 1: Alfa‐2a IFN 6 MU/d x 12d; 6 MU TIW x 22 wk; 3 MU TIW x 24 wk Experimental 2: Alfa‐2a IFN 3 MU TIW x 24 wk Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Diodati 1994.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 13. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 30/30 Excluded: 1/5 Mean age: 49/55 % males: 77/43 % transfusion: 23/23 % drug abuse: NDA % cirrhosis: 50/40 Genotypes: NDA Disease duration: 8/7 | |
| Interventions | Experimental: Alfa‐2a IFN 6 MU TIW x 1mo; 3 MU x 3 mo; 1 MU TIW x 8 mo Control: no intervention Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ liver histology | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Enriquez 1995.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 13. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 45/45 Excluded: 2/0 Mean age: 49/47 % males: 64/67 % transfusion: 24/42 % drug abuse: NDA % cirrhosis: 9/11 Genotypes : NDA Disease duration: 5/4 | |
| Interventions | Experimental 1: Alfa‐n‐1 IFN 10 to 5 MU TIW x 6 mo Experimental 2: Alfa‐n‐1 IFN 5 to 3 MU TIW x 6 mo Follow‐up: 9 mo |
|
| Outcomes | ‐ biochemical ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Farrell 1998.
| Methods | Allocation concealment: adequate (centralized computer). Blinding: no. Intention to treat: yes. Methodological score: 18. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 533/538 Excluded: 26/19 Mean age: 39/39 % males: 69/69 % transfusion: NDA % drug abuse: NDA % cirrhosis: 53/59 Genotypes: G1 (60/62%), G2,3 (34/34%), other (6/4%) Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐n‐1 IFN 3 MU TIW x 6 mo Experimental 2 : Alfa‐2b IFN 3 MU TIW x 6 mo Follow‐up: 12 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Fernandez 1997.
| Methods | Allocation concealment: unclear. Blinding: yes. Intention to treat: yes. Methodological score: 17. | |
| Participants | NAIVE HEMODIALYSED PATIENTS WITH CHRONIC HEPATITIS C Arms: 14/9 Excluded: 3/2 Mean age: 44/49 % males: 36/67 % transfusion: 36/67 % drug abuse: 0/0 % cirrhosis: NDA Genotypes: G1 (54/78%), G2,3 (38/22%) Disease duration: 2/2 | |
| Interventions | Experimental: Alfa‐2b IFN 1.5 MU TIW x 3 mo; increased to 3 MU TIW x 6 mo if ALT still elevated Control: placebo Follow‐up: 18 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Furusyo 1997.
| Methods | Allocation concealment: adequate (sealed envelopes). Blinding: no. Intention to treat: no. Methodological score: 14. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C AND CIRRHOSIS Arms: 41/41 Excluded: 7/0 Mean age: 59/58 % males: 49/58 % transfusion: 41/49 % drug abuse: 0/0 % cirrhosis: 100/100 Genotypes: G1 (78/73%), G2 (22/27%) Disease duration: NDA | |
| Interventions | Experimental: Alfa‐n‐1 IFN 6 MU/day x 2 wk; 6 MU TIW x 22 wk Control: no intervention Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Garson 1997.
| Methods | Allocation concealment: unclear. Blinding: yes. Intention to treat: yes. Methodological score: 15. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 12/10/8/8 Excluded: 0/0/0/0 Mean age: 39/39/38/41 % males: 75/80/62/50 % transfusion: NDA % drug abuse: 92/90/62/88 % cirrhosis: 25/10/0/0 Genotypes: G1 (67/50/38/38%), G2,3 (33/50/62/62) Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐n‐1IFN 3 MU TIW x 6 mo Experimental 2: Alfa‐n‐1IFN 3 MU TIW x 12 mo Experimental 3: Alfa‐n‐1 IFN 5 MU TIW x 6 mo Experimental 4: Alfa‐n‐1 IFN 5 MU TIW x 12 mo Follow‐up: 12 mo |
|
| Outcomes | ‐ biochemical SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Giudici 1991.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 12. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 15/15 Excluded: 0/0 Mean age: 50/33 % males: 60/80 % transfusion: 27/13 % drug abuse: 13/7 % cirrhosis: 13/13 Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental: Alfa‐2b IFN 3 MU TIW x 6 mo Control: no intervention Follow‐up: 12 mo |
|
| Outcomes | ‐ biochemical ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gomez‐Rubio 1990.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 12. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 15/15 Excluded: 2/0 Mean age: 41/40 % males: 60/53 % transfusion: 60/73 % drug abuse: 7/7 % cirrhosis: 27/7 Genotypes: NDA Disease duration: 7/8 | |
| Interventions | Experimental: Alfa‐2b IFN 5 MU TIW x 2 mo; 1.5 MU TIW x 16 mo Control: no intervention Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hagiwara 1993.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: no. Methodological score: 10. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 30/30 Excluded: 3/4 Mean age: 47/49 % males: 82/65 % transfusion: 22/42 % drug abuse: NDA % cirrhosis: NDA Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐n‐1 IFN 6 MU/day x 2 wk; 6 MU TIW x 6 mo Experimental 2: Alfa‐n‐1 IFN 3 MU/day x 2 wk; 3 MU TIW x 6 mo Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SVR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hakozaki 1995.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: no. Methodological score: 11. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 26/35 Excluded: 0/1 Mean age: 46/46 % males: 92/94 % transfusion: 46/43 % drug abuse: NDA % cirrhosis: 31/29 Genotypes: G1 (73/71%), G2 (23/26%), G3 (4/3%) Disease duration: NDA | |
| Interventions | Experimental 1: 6 MU TIW x 6 mo Experimental 2: 3 MU TIW x 6 mo Follow‐up: 12 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ikeda 1998.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: no. Methodological score: 10. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C AND CIRRHOSIS Arms: 14/15 Excluded: 1/0 Mean age : 52/57 % males: NDA % transfusion: NDA % drug abuse: NDA % cirrhosis: 100/100 Genotypes : G2 (100/100%) Disease duration: NDA | |
| Interventions | Experimental: Alfa‐2a IFN 9 MU TIW x 6 mo Control: no intervention Follow‐up: 3 mo |
|
| Outcomes | ‐ biochemical SR ‐ virologic ETR and SR ‐ incidence of hepatocellular carcinoma | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Imai 1997.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 11. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 44/44 Excluded: 2/2 Mean age: 53/53 % males: 66/75 % transfusion: 18/20 % drug abuse: NDA % cirrhosis: 0/0 Genotypes: G1 (83/79%), G2 (14/18%), other (3/3%) Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐2a IFN 6 MU/day x 2wk; 6 MU TIW x 22 wk Experimental 2: Alfa‐2a IFN 6 MU/d x 2 wk; 3 MU TIW x 22 wk Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Jouet 1994.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 13. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 56/52 Excluded: >5 Mean age: 47/50 % males: 100/100 % transfusion: 57/79 % drug abuse: 29/19 % cirrhosis: 19/16 Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐2b IFN 3 MU TIW x 6 mo; 2 MU TIW x 3mo; 1 MU TIW x 3 mo Experimental 2: Alfa‐2b IFN 3 MU TIW x 6 mo Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kasahara 1995.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 12. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 45/48 Excluded: 2/3 Mean age: 47/47 % males: 86/78 % transfusion: 31/23 % drug abuse: NDA % cirrhosis: 0 Genotypes: G1 (65/82%), G2 (28/13%), other (7/4%) Disease duration: 9/8 | |
| Interventions | Experimental 1: Alfa‐n‐1 IFN 5 MU TIW x 6 mo; 5 MU twice weekly x 6 mo Experimental 2: Alfa‐n‐1 IFN 5 MU TIW x 6 mo Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Komatsu 1997.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: no. Methodological score: 8. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 26/25 Excluded: 2/5 Mean age : 50/50 % males: 50/72 % transfusion: NDA % drug abuse: NDA % cirrhosis: NDA Genotypes: G1(92/80%) Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐n‐3 IFN 6 MU/day 6 days/wk x 8 wk; 6 MU TIW x 16 wk Experimental 2: Alfa‐n‐3 IFN 6 MU/day 6 days/wk x 2 wk; 6 MU TIW x 22 wk Follow‐up: 6 mo |
|
| Outcomes | ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Laghi 1997.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: no. Methodological score: 8. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 14/31 Excluded: 3/0 Mean age : NDA % males: NDA % transfusion: NDA % drug abuse: NDA % cirrhosis: 0/6 Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐n‐3 IFN 6 MU TIW x 12 mo Experimental 2 : Alfa‐n‐3 IFN 3 MU TIW x 12 mo Follow‐up: 12 mo |
|
| Outcomes | ‐ biochemical ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lin 1995.
| Methods | Allocation concealment: adequate (sealed envelopes). Blinding: no. Intention to treat: yes. Methodological score: 16. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 75/72/83 Excluded: 7/7/18 Mean age: 39/38/42 % males: 76/72/63 % transfusion: 21/19/24 % drug abuse: 47/46/37 % cirrhosis: 41/31/30 Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐2b IFN 5 MU TIW x 6 mo Experimental 2: 3 MU TIW x 6 mo Experimental 3: 3 MU TIW x 24 mo Follow‐up: 24 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Makris 1991.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 12. | |
| Participants | NAIVE HEMOPHILIACS WITH CHRONIC HEPATITIS C Arms: 10/8 Excluded: 1/0 Mean age: 38/38 % males: 100/100 % transfusion: 100/100 % drug abuse: 0/0 % cirrhosis: 10/12 Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental: Alfa‐2b IFN 1 MU TIW x 1 mo; 2 MU TIW x 1 mo; 3 MU TIW x 10 mo Control: no intervention Follow‐up: 15 mo |
|
| Outcomes | ‐ biochemical ETR ‐ liver histology | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Marcellin 1991.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: no. Methodological score: 12. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 18/18 Excluded: 2/0 Mean age: 42/41 % males: 67/50 % transfusion: 50/56 % drug abuse: 28/28 % cirrhosis: 17/11 Genotypes: NDA Disease duration: 4/4 | |
| Interventions | Experimental: Alfa‐2b IFN 3 MU TIW x 6 mo Control: no intervention Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Marcellin 1995.
| Methods | Allocation concealment: adequate (sealed envelopes). Blinding: no. Intention to treat: no. Methodological score: 15. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 50/25 Excluded: 7/1 Mean age: 43/44 % males: 53/68 % transfusion: 62/68 % drug abuse: 32/32 % cirrhosis: 8/20 Genotypes: NDA Disease duration: 12/12 | |
| Interventions | Experimental 1: AlFa‐2b IFN 3 MU TIW x 8 wk; 5 MU TIW x 8 wk; 10 MU TIW x 8 wk Experimental 2: Alfa‐2b IFN 3 MU TIW x 6 mo Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Matsumoto 1994.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 8. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 12/12 Excluded: 0/0 Mean age: 48/45 % males: 75/74 % transfusion: 25/33 % drug abuse: NDA % cirrhosis: NDA Genotypes: G1 (75/75%), G2(25/25%) Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐2a IFN 9 MU/day x 2 wk; 9 MU TIW x 6 mo Experimental 2: Alfa‐2a IFN 3 MU/day x 2 wk; 3 MU x 6 mo Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mazella 1994.
| Methods | Allocation concealment: adequate (sealed envelopes). Blinding: no. Intention to treat: no. Methodological score: 13. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 30/30 Excluded: 1/0 Mean age: 45/48 % males: 68/50 % transfusion: NDA % drug abuse: NDA % cirrhosis: NDA Genotypes: NDA Disease duration: 5/5 | |
| Interventions | Experimental: Alfa‐n‐1 IFN 3 MU TIW x 12 mo Control: no intervention Follow‐up: 16 mo |
|
| Outcomes | ‐ biochemical ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
McHutchison 1998.
| Methods | Allocation concealment: adequate (centralized computer). Blinding: no. Intention to treat: no. Methodological score: 20. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 231/225 Excluded: NDA Mean age: 45/44 % males: 75/75 % transfusion: 8/10 % drug abuse: 23/23 % cirrhosis: 3/2 Genotypes: G1(78/72%), G2,3 (21/28%), other (1/0%) Disease duration: 19/19 | |
| Interventions | Experimental 1: Alfa‐2b IFN 3MU TIW x 12 mo Experimental 2: Alfa‐2b IFN 3MU TIW x 6 mo Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Nishiguchi 1995.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: no. Methodological score: 18. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C AND CIRRHOSIS Arms: 45/45 Excluded: 0/0 Mean age: 55/57 % males: 62/51 % transfusion: NDA % drug abuse: NDA % cirrhosis: 100/100 Genotypes: G1 (73/80%), G2 (27/20%) Disease duration: 25/26 | |
| Interventions | Experimental: Alfa‐n‐1 IFN 6MU TIW x 12‐24 wk. Six patients received a second course of treatment. Control: no intervention Follow‐up: 24 mo |
|
| Outcomes | ‐ virologic ETR and SR ‐ incidence of hepatocellular carcinoma | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ouzan 1998.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 15. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 149/142 Excluded: 10/14 Mean age: 48/48 % males: 89/92 % transfusion: 42/39 % drug abuse: NDA % cirrhosis: 20/23 Genotypes : NDA Disease duration: 4/4 | |
| Interventions | Experimental 1: Alfa‐2a IFN 6MU TIW x 3mo; 3MU TIW x 3 mo Experimental 2: Alfa‐2a IFN 3MU TIW x 6 mo Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Poynard 1995.
| Methods | Allocation concealment: adequate (sealed envelopes). Blinding: no. Intention to treat: yes. Methodological score: 16. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 103/99/101 Excluded: 31/0/0 Mean age: 49/50/51 % males: 51/49/55 % transfusion: 52/47/45 % drug abuse: NDA % cirrhosis: 27/23/27 Genotypes : NDA Disease duration: 6/7/9 | |
| Interventions | Experimental 1: Alfa‐2b IFN 3MU TIW x 18 mo Experimental 2: Alfa‐2b IFN 3MU TIW x 6 mo Experimental 3: Alfa‐2b IFN 3MU TIW x 6mo; 1MU TIW x 12 mo Follow‐up: 24 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Reichen 1996.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: no. Methodological score: 13. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 34/31/30 Excluded: 5/6/6 Mean age: 45/51/47 % males: 68/71/73 % transfusion: 35/26/20 % drug abuse: NDA % cirrhosis: 26/35/27 Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐2b IFN 5MU TIW x 1 mo; decreased by 1MU each wk as long as ALT remained normal; if ALT did not normalize, a higher dose was reintroduced Experimental 2: Alfa‐2b IFN 3MU TIW x 12 mo Control: no intervention Follow‐up: 12 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rossini 1997.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 10. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C AND NORMAL ALT Arms: 10/9 Excluded: 0/0 Mean age: 51/51 % males: 60/50 % transfusion: NDA % drug abuse: 0/0 % cirrhosis: 0/0 Genotypes: G1 (20/56%), G2 (80/44%) Disease duration: NDA | |
| Interventions | Experimental: Alfa‐2b IFN 3MU TIW x 12 mo; stopped if RNA + at 6 mo Control: no intervention Follow‐up: 12 mo |
|
| Outcomes | ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rumi 1995.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: no. Methodological score: 16. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 38/36 Excluded: 3/4 Mean age: 48 % males: 63 % transfusion: NDA % drug abuse: NDA % cirrhosis: 31 Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental: Alfa‐2a IFN 6MU TIW x 2 mo; 3MU TIW x 10 mo Control: no intervention Follow‐up: 12 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic ETR and SR ‐ liver histology | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rumi 1996.
| Methods | Allocation concealment: unclear. Blinding: yes. Intention to treat: no. Methodological score: 16. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 116/118 Excluded: 13/11 Mean age : 49/48 % males: 69/67 % transfusion: 16/19 % drug abuse: 7/8 % cirrhosis: 26/31 Genotypes: G1 (63/53%), G2,3 (33/43%), other (4/3%) Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐n‐1 IFN 6MU TIW until ALT normal then 3 MU TIW x total of 12 mo Experimental 2: Alfa‐2a IFN 6MaU TIW until ALT normal at least 4 wk then 3 MU TIW x total of 12 mo In both groups, if ALT broke through, the 6MU dose was reintroduced; IFN was stopped if ALT not normal within 24 wk. Follow‐up: 12 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Saez‐Royuela 1991.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 12. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 10/10 Excluded: 2/0 Mean age : 36/39 % males: 60/60 % transfusion: 40/50 % drug abuse: NDA % cirrhosis: 30/30 Genotypes: NDA Disease duration: 4/7 | |
| Interventions | Experimental: Alfa‐2c IFN 15 MU TIW x 3 mo; 10 MU TIW x 3 mo; 5 MU TIW x 6 mo Control: no intervention Follow‐up: 6 mo |
|
| Outcomes | ‐ virologic ETR and SVR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Saito 1994.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score:10. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 10/10 Excluded: 0/0 Mean age: 59/63 % males: 20/50 % transfusion: 40/50 % drug abuse: NDA % cirrhosis: 100/100 Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental: Alfa‐n‐1 IFN 3 MU TIW x 6 mo Control: no intervention Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SVR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sangiovanni 1998.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 16. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C AND NORMAL ALT Arms: 16/15 Excluded: 0/0 Mean age: 43/46 % males: 50/47 % transfusion: 31/33 % drug abuse: NDA % cirrhosis: 0/0 Genotypes: G1 (38/47%), G2 (50/47%), other (12/6%) Disease duration: NDA | |
| Interventions | Experimental: Alfa‐2a IFN 3 MU TIW x 6 mo Control: no intervention Follow‐up: 6 mo |
|
| Outcomes | ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Saracco 1990.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: no. Methodological score: 12. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 26/25 Excluded: 3/5 Mean age: 48/47 % males: 62/60 % transfusion: 46/48 % drug abuse: 54/52 % cirrhosis: 50/24 Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental: IFN 3 MU TIW x 6 mo Control: no intervention Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ liver histology | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Saracco 1993.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 10. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 14/26 Excluded: NDA Mean age: NDA % males: NDA % transfusion: NDA % drug abuse: NDA % cirrhosis: NDA Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐2b IFN 3 MU TIW x 12 mo Experimental 2: Alfa‐2b IFN 3 MU TIW x 6 mo Follow‐up: 36 mo |
|
| Outcomes | ‐ biochemical ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Shiratori 1997.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 17. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 133/139 Excluded: 0/0 Mean age: 47/53 % males: 73/62 % transfusion: 46/51 % drug abuse: NDA % cirrhosis: 0/0 Genotypes: G1 (81/74%), G2 (19/26%) Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐nat IFN 9 MU TIW x 6 mo Experimental 2: Alfa‐nat IFN 6 MU TIW x 6 mo Follow‐up: 12 mo |
|
| Outcomes | ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Simon 1997.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: yes. Methodological score: 10 | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 18/19/17/23 Excluded: 0/5/1/5 Mean age: 47/45/44/43 % males: 83/74/71/78 % transfusion: 39/16/35/26 % drug abuse: 50/53/65/52 % cirrhosis: 28/31/24/17 Genotypes: G1 (50/47/82/52%), G2 (39/11/6/17%), other (11/42/12/31%) Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐n‐3 IFN 1 MU TIW x 6 mo Experimental 2: Alfa‐n‐3 IFN 2.5 MU TIW x 6 mo Experimental 3: Alfa‐n‐3 IFN 5 MU TIW x 6 mo Experimental 4: Alfa‐n‐3 IFN 10 MU TIW x 6 mo Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tassopoulos 1996.
| Methods | Allocation concealment: unclear. Blinding: no. Intention to treat: no. Methodological score: 10. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 28/30 Excluded: 2/2 Mean age: 42/42 % males: 71/70 % transfusion: 11/20 % drug abuse: 29/20 % cirrhosis: 32/30 Genotypes: G1 (46/31%), G2,3 (32/55%), other (21/14%) Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐2a IFN 10 MU TIW x 6 mo Experimental 2: Alfa‐2a IFN 3 MU TIW x 6 mo Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tong 1997 (CIFN 3µg).
| Methods | Allocation concealment: unclear. Blinding: yes. Intention to treat: yes. Methodological score: 21. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 232/240 Excluded: NDA Mean age: 43/43 % males: 72/72 % transfusion: 27/22 % drug abuse: 40/44 % cirrhosis: 39/24 Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental 1: C‐IFN 3 µg TIW x 6 mo Experimental 2: Alfa‐2b IFN 15 µg TIW x 6 mo Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tong 1997 (CIFN 9µg).
| Methods | Allocation concealment: unclear. Blinding: yes. Intention to treat: yes. Methodological score: 21. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 232/240 Excluded: NDA Mean age: 43/43 % males: 72/72 % transfusion: 22/22 % drug abuse: 46/44 % cirrhosis: 35/24 Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental 1: C‐IFN 9 µg TIW x 6 mo Experimental 2: Alfa‐2b IFN 15 µg TIW x 6 mo Follow‐up: 6 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Valla 1999.
| Methods | Allocation concealment: adequate (centralized randomisation; table of random numbers). Blinding: no. Intention to treat: yes. Methodological score: 16. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 47/52 Excluded: 10/13 Mean age: 57/56 % males: 70/61 % transfusion: 36/36 % drug abuse: 4/6 % cirrhosis: 100/100 Genotypes: G1 (50/63%), G2,3 (42/29), other (8/8%) Disease duration: 9/11 | |
| Interventions | Experimental: Alfa‐2b IFN 3 MU TIW x 6 mo Control: no intervention. Ten patients received IFN treatment after follow‐up wk 72. Follow‐up: 12 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ incidence of hepatocellular carcinoma | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Villa 1996 (bet‐IFN).
| Methods | Allocation concealment: adequate (centralized computer). Blinding: yes. Intention to treat: yes. Methodological score: 16. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 20/19/21 Excluded: 1/1/1 Mean age: 46/41/45 % males: 70/79/71 % transfusion: 5/11/10 % drug abuse: 10/10/5 % cirrhosis: 7/7/3 Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐n3 IFN 3 MU TIW x 6 mo Experimental 2: Beta‐IFN 3 MU TIW x 6 mo Experimental 3: Alfa‐2b IFN 3 MU TIW x 6 mo Follow‐up: 30 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Villa 1996 (leu‐IFN).
| Methods | Allocation concealment: adequate (centralized computer). Blinding: yes. Intention to treat: yes. Methodological score: 16. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 20/19/21 Excluded: 1/1/1 Mean age: 46/41/45 % males: 70/79/71 % transfusion: 5/11/10 % drug abuse: 10/10/5 % cirrhosis: 7/7/3 Genotypes: NDA Disease duration: NDA | |
| Interventions | Experimental 1: Alfa‐n3 IFN 3 MU TIW x 6 mo Experimental 2: Beta‐IFN 3 MU TIW x 6 mo Experimental 3: Alfa‐2b IFN 3 MU TIW x 6 mo Follow‐up: 30 mo |
|
| Outcomes | ‐ biochemical ETR and SR ‐ virologic ETR and SR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Weiland 1990.
| Methods | Allocation concealment: adequate (sealed envelopes). Blinding: no. Intention to treat: no. Methodological score: 13. | |
| Participants | NAIVE PATIENTS WITH CHRONIC HEPATITIS C Arms: 21/12 Excluded: 2/0 Mean age: 54/56 % males: 91/92 % transfusion: 100/100 % drug abuse: 0/0 % cirrhosis: 0/0 Genotypes: NDA Disease duration: 3/4 | |
| Interventions | Experimental: Alfa‐2b IFN 3 MU TIW x 9 mo Control: no intervention Follow‐up: 12 mo |
|
| Outcomes | ‐ biochemical ETR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
ALT: alanine aminotransferase NDA: no data available. TIW: thrice each week. wk: weeks. mo: months. naive patients: naive to previous interferon treatment. vs: versus.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Angelini 1995 | Quasi‐randomised trial. |
| Bonkovsky 1996 | Trial in non‐responders and relapsers. |
| Bresci 1996 | Trial in non‐responders. |
| Caporaso 1993 | Quasi‐randomised trial. |
| Chemello 1997 | Trial in non‐responders and relapsers. |
| Di Marco 1997 | Randomised patients after initial course of therapy. |
| Ferenci 1996 | Trial in non‐responders. |
| Friedlander 1996 | Quasi‐randomised trial. |
| Heathcote 1998 | Trial in non‐responders and relapsers. |
| Lindsay 1996 | Randomised patients after initial course of therapy. |
| Payen 1998 | Trial in relapsers. |
| Poynard 1999 | Trial in relapsers. |
| Rolachon 1997 | Trial in non‐responders. |
| Saracco 1997 | Randomised patients after initial course of therapy. |
| Sheiner 1998 | Liver transplant recipients. |
| Shiffman 1996 | Randomised patients after initial course of therapy. |
Contributions of authors
CR, TT, VL, PM, PO, JPZ and TP wrote the first draft of the protocol and the review. TT, VL and TP performed the literature searches, and participated in the selection of trials and data extraction. TT and TP did the statistical analyses. RM verified the statistical analyses. RM, CR and TP rewrote the manuscript in light of the suggestions of the reviewers.
Sources of support
Internal sources
No sources of support supplied
External sources
Canadian Association for the Study of the Liver, Canada.
Royal College of Physicians and Surgeons of Canada, Canada.
Club Francophone de l'Hypertension Portale, France.
Association Francaise pour l'Etude du Foie, France.
Recherche et Partage, France.
Declarations of interest
The authors have no permanent financial contracts with companies producing interferon. T. Poynard, P. Opolon, and J.P. Zarski have been involved in prospective studies sponsored by Schering Plough, Roche, Glaxo Welcome, and AMGEN.
Edited (no change to conclusions)
References
References to studies included in this review
Alberti 1993 {published data only}
- Alberti A, Chemello L, Bonetti P, Casarin C, Diodati G, Cavalletto L, et al. Treatment with interferon(s) of community‐acquired chronic hepatitis and cirrhosis type C. The TVVH Study Group. J Hepatol 1993;17(Suppl 3):S123‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brouwer 1998 {published data only}
- Brouwer JT, Nevens F, Kleter B, Elewaut A, Adler M, Brenard R, Chamuleau RA, et al. Efficacy of interferon dose and prediction of response in chronic hepatitis C: Benelux study in 336 patients. J Hepatol 1998;28(6):951‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Camps 1993 {published data only}
- Camps J, Castilla A, Ruiz J, Civeira MP, Prieto J. Randomised trial of lymphoblastoid alpha interferon in chronic hepatitis C. Effects on inflammation, fibrogenesis and viremia. J Hepatol 1993;17(3):390‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Capra 1993 {published data only}
- Capra F, Casaril M, Gabrielli GB, Tognella P, Rizzi A, Dolci L, Colombari R, et al. alpha‐Interferon in the treatment of chronic viral hepatitis: effects on fibrogenesis serum markers. J Hepatol 1993;18(1):112‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Causse 1991 {published data only}
- Causse X, Godinot H, Chevallier M, Chossegros P, Zoulim F, Ouzan D, Heyraud JP, et al. Comparison of 1 or 3 MU of interferon alfa‐2b and placebo in patients with chronic non‐A, non‐B hepatitis. Gastroenterology 1991;101(2):497‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chemello 1995 {published data only}
- Chemello L, Bonetti P, Cavalletto L, Talato F, Donadon V, Casarin P, Belussi F, et al. Randomized trial comparing three different regimens of alpha‐2a‐ interferon in chronic hepatitis C. The TriVeneto Viral Hepatitis Group. Hepatology 1995;22(3):700‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cimino 1991 {published data only}
- Cimino L, Nardone G, Citarella C, Perna E, Capuano G, Budillon G. Treatment of chronic hepatitis C with recombinant interferon alfa. Ital J Gastroenterol 1991;23(7):399‐402. [MEDLINE: ] [PubMed] [Google Scholar]
Craxi 1996 {published data only}
- Craxi A, Marco V, Lo Iacono O, Almasio P, Bruno R, Camma C, Volpes R, Pagliaro L. Transfusion‐associated chronic hepatitis C: alpha‐n1 interferon for 6 vs. 12 months. J Hepatol 1996;24(5):539‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Davis 1989 {published data only}
- Davis GL, Balart LA, Schiff ER, Lindsay K, Bodenheimer HC, Perrillo RP, et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med 1989;321(22):1501‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Degos 1998 {published data only}
- Degos F, Daurat V, Chevret S, Gayno S, Bastie A, Riachi G, Bartolomei‐Portal I, et al. Reinforced regimen of interferon alfa‐2a reduces the incidence of cirrhosis in patients with chronic hepatitis C: a multicentre randomised trial. Multicentre GER‐CYT‐04 Group. J Hepatol 1998;29(2):224‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Diodati 1994 {published data only}
- Diodati G, Bonetti P, Noventa F, Casarin C, Rugge M, Scaccabarozzi S, et al. Treatment of chronic hepatitis C with recombinant human interferon‐alpha 2a: results of a randomized controlled clinical trial. Hepatology 1994;19(1):1‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Enriquez 1995 {published data only}
- Enriquez J, Torras X, Miralles F, Martinez Cerezo FJ, Sancho Poch FJ, Buenestado J, et al. Comparative study of two high doses of lymphoblastoid interferon in the treatment of chronic hepatitis C: influence on the levels of ALT, viraemia and histologic activity [published erratum appears in J Viral Hepat 1996 Mar;3(2):107]. J Viral Hepat 1995;2(4):181‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Farrell 1998 {published data only}
- Farrell GC, Bacon BR, Goldin RD. Lymphoblastoid interferon alfa‐n1 improves the long‐term response to a 6‐month course of treatment in chronic hepatitis C compared with recombinant interferon alfa‐2b: results of an international randomized controlled trial. Clinical Advisory Group for the Hepatitis C Comparative Study. Hepatology 1998;27(4):1121‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fernandez 1997 {published data only}
- Fernandez JL, Rendo P, Pino N, Viola L. A double‐blind controlled trial of recombinant interferon‐alpha 2b in haemodialysis patients with chronic hepatitis C virus infection and abnormal aminotransferase levels. Nephrologists' Group for the Study of HCV infection. J Viral Hepat 1997;4(2):113‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Furusyo 1997 {published data only}
- Furusyo N, Hayashi J, Ueno K, Sawayama Y, Kawakami Y, Kishihara Y, Kashiwagi S. Human lymphoblastoid interferon treatment for patients with hepatitis C virus‐related cirrhosis. Clin Ther 1997;19(6):1352‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garson 1997 {published data only}
- Garson JA, Uhnoo I, Whitby K, Braconier JH, Deaville R, Duberg A, et al. Virological, biochemical and histological effects of human lymphoblastoid interferon in Swedish patients with chronic hepatitis C. J Viral Hepat 1997;4(5):325‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Giudici 1991 {published data only}
- Giudici‐Cipriani A, Rainisio C, Ponassi I, Rattenni S, Imberciadori G, Cavagnaro G, et al. Therapy of chronic non‐A, non‐B hepatitis with interferon alfa‐2B. A controlled clinical study and long‐term follow‐up. Minerva Gastroenterol Dietol 1991;37(2):85‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Gomez‐Rubio 1990 {published data only}
- Gomez‐Rubio M, Porres JC, Castillo I, Quiroga JA, Moreno A, Carreno V. Prolonged treatment (18 months) of chronic hepatitis C with recombinant alpha‐interferon in comparison with a control group. J Hepatol 1990;11(Suppl 1):S63‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hagiwara 1993 {published data only}
- Hagiwara H, Hayashi N, Mita E, Takehara T, Kasahara A, Fusamoto H, Kamada T. Quantitative analysis of hepatitis C virus RNA in serum during interferon alfa therapy. Gastroenterology 1993;104(3):877‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hakozaki 1995 {published data only}
- Hakozaki Y, Shirahama T, Katou M, Nakagawa K, Oba K, Mitamura K. A controlled study to determine the optimal dose regimen of interferon‐alpha 2b in chronic hepatitis C. Am J Gastroenterol 1995;90(8):1246‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Ikeda 1998 {published data only}
- Ikeda K, Kumada H, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, et al. A randomized controlled trial of interferon‐alpha in patients with cirrhosis caused by 2a/2b genotype hepatitis C virus [letter]. J Hepatol 1998;28(5):910‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Imai 1997 {published data only}
- Imai Y, Kawata S, Tamura S, Ito N, Seki K, Nishiuchi M, et al. Recombinant interferon‐alpha‐2a for treatment of chronic hepatitis C: results of a multicenter randomized controlled dose study. Liver 1997;17(2):88‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jouet 1994 {published data only}
- Jouet P, Roudot‐Thoraval F, Dhumeaux D, Metreau JM. Comparative efficacy of interferon alfa in cirrhotic and noncirrhotic patients with non‐A, non‐B, C hepatitis. Le Groupe Francais pour l'Etude du Traitement des Hepatites Chroniques NANB/C. Gastroenterology 1994;106(3):686‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kasahara 1995 {published data only}
- Kasahara A, Hayashi N, Hiramatsu N, Oshita M, Hagiwara H, Katayama K, et al. Ability of prolonged interferon treatment to suppress relapse after cessation of therapy in patients with chronic hepatitis C: a multicenter randomized controlled trial. Hepatology 1995;21(2):291‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Komatsu 1997 {published data only}
- Komatsu M, Ishii T, Ono T, Kuramitsu T, Hoshino T, Masamune O, et al. A dose‐dependent controlled trial of human lymphoblastoid interferon‐alpha for genotype 1b chronic hepatitis C associated with high HCV‐RNA levels.. Hepatology Research 1997;7:105‐12. [Google Scholar]
Laghi 1997 {published data only}
- Laghi V, Toccaceli F, Rosati S, Monini A, Grimaldi M, Foglianti G, et al. Comparison of treatment with two different doses of leukocyte interferon alpha in patients with chronic hepatitis C. Hepatogastroenterology 1997;44(16):1182‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Lin 1995 {published data only}
- Lin R, Roach E, Zimmerman M, Strasser S, Farrell GC. Interferon alfa‐2b for chronic hepatitis C: effects of dose increment and duration of treatment on response rates. Results of the first multicentre Australian trial. Australia Hepatitis C Study Group. J Hepatol 1995;23(5):487‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Makris 1991 {published data only}
- Makris M, Preston FE, Triger DR, Underwood JC, Westlake L, Adelman MI. A randomized controlled trial of recombinant interferon‐alpha in chronic hepatitis C in hemophiliacs. Blood 1991;78(7):1672‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Marcellin 1991 {published data only}
- Marcellin P, Boyer N, Giostra E, Degott C, Courouce AM, Degos F, et al. Recombinant human alpha‐interferon in patients with chronic non‐A, non‐ B hepatitis: a multicenter randomized controlled trial from France. Hepatology 1991;13(3):393‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Marcellin 1995 {published data only}
- Marcellin P, Pouteau M, Martinot‐Peignoux M, Degos F, Duchatelle V, Boyer N, et al. Lack of benefit of escalating dosage of interferon alfa in patients with chronic hepatitis C. Gastroenterology 1995;109(1):156‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Matsumoto 1994 {published data only}
- Matsumoto A, Tanaka E, Suzuki T, Ogata H, Kiyosawa K. Viral and host factors that contribute to efficacy of interferon‐alpha 2a therapy in patients with chronic hepatitis C. Dig Dis Sci 1994;39(6):1273‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mazella 1994 {published data only}
- Mazzella G, Salzetta A, Casanova S, Morelli MC, Villanova N, Miniero R, et al. Treatment of chronic sporadic‐type non‐A, non‐B hepatitis with lymphoblastoid interferon: gamma GT levels predictive for response. Dig Dis Sci 1994;39(4):866‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McHutchison 1998 {published data only}
- McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al. Interferon alfa‐2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med 1998;339(21):1485‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nishiguchi 1995 {published data only}
- Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, et al. Randomised trial of effects of interferon‐alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet 1995;346(8982):1051‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ouzan 1998 {published data only}
- Ouzan D, Babany G, Valla D, Opolon P. Comparison of high initial and fixed‐dose regimens of interferon‐alpha2a in chronic hepatitis C: a randomized controlled trial. French Multicenter Interferon Study Group. J Viral Hepat 1998;5(1):53‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Poynard 1995 {published data only}
- Poynard T, Bedossa P, Chevallier M, Mathurin P, Lemonnier C, Trepo C, et al. A comparison of three interferon alfa‐2b regimens for the long‐term treatment of chronic non‐A, non‐B hepatitis. Multicenter Study Group [published erratum appears in N Engl J Med 1996 Apr 25;334(17):1143]. N Engl J Med 1995;332(22):1457‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reichen 1996 {published data only}
- Reichen J, Bianchi L, Buhler H, Dolivo N, Gonvers JJ, Lavanchy D, et al. Fixed versus titrated interferon‐alpha 2B in chronic hepatitis C. A randomized controlled multicenter trial. The Swiss Association for the Study of the Liver. J Hepatol 1996;25(3):275‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rossini 1997 {published data only}
- Rossini A, Ravaggi A, Biasi L, Agostinelli E, Bercich L, Gazzola GB, et al. Virological response to interferon treatment in hepatitis C virus carriers with normal aminotransferase levels and chronic hepatitis. Hepatology 1997;26(4):1012‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rumi 1995 {published data only}
- Rumi MG, Ninno E, Parravicini ML, Romeo R, Soffredini R, Donato MF, et al. Long‐term titrated recombinant interferon‐alpha 2a in chronic hepatitis C: a randomized controlled trial. J Viral Hepat 1995;2(2):73‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rumi 1996 {published data only}
- Rumi M, Ninno E, Parravicini ML, Romeo R, Soffredini R, Donato MF, et al. A prospective, randomized trial comparing lymphoblastoid to recombinant interferon alfa 2a as therapy for chronic hepatitis C. Hepatology 1996;24(6):1366‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saez‐Royuela 1991 {published data only}
- Saez‐Royuela F, Porres JC, Moreno A, Castillo I, Martinez G, Galiana F, Carreno V. High doses of recombinant alpha‐interferon or gamma‐interferon for chronic hepatitis C: a randomized, controlled trial. Hepatology 1991;13(2):327‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saito 1994 {published data only}
- Saito T, Shinzawa H, Kuboki M, Ishibashi M, Toda H, Okuyama Y, et al. A randomized, controlled trial of human lymphoblastoid interferon in patients with compensated type C cirrhosis. Am J Gastroenterol 1994;89(5):681‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Sangiovanni 1998 {published data only}
- Sangiovanni A, Morales R, Spinzi G, Rumi M, Casiraghi A, Ceriani R, et al. Interferon alfa treatment of HCV RNA carriers with persistently normal transaminase levels: a pilot randomized controlled study. Hepatology 1998;27(3):853‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saracco 1990 {published data only}
- Saracco G, Rosina F, Torrani Cerenzia MR, Lattore V, Chiandussi L, Gallo V, et al. A randomized controlled trial of interferon alfa‐2b as therapy for chronic non‐A, non‐B hepatitis. J Hepatol 1990;11(Suppl 1):S43‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saracco 1993 {published data only}
- Saracco G, Rosina F, Abate ML, Chiandussi L, Gallo V, Cerutti E, et al. Long term follow‐up of patients with chronic hepatitis C treated with different doses of interferon alpha‐2b. Hepatology 1993;18(6):1300‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Shiratori 1997 {published data only}
- Shiratori Y, Kato N, Yokosuka O, Imazeki F, Hashimoto E, Hayashi N, et al. Predictors of the efficacy of interferon therapy in chronic hepatitis C virus infection. Tokyo‐Chiba Hepatitis Research Group. Gastroenterology 1997;113(2):558‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simon 1997 {published data only}
- Simon DM, Gordon SC, Kaplan MM, Koff RS, Regenstein F, Everson G, et al. Treatment of chronic hepatitis C with interferon alfa‐n3: a multicenter, randomized, open‐label trial. Hepatology 1997;25(2):445‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tassopoulos 1996 {published data only}
- Tassopoulos NC, Karvountzis G, Touloumi G, Delladetsima JK, Papatheodoridis GV, Katsoulidou A, et al. Comparative efficacy of a high or low dose of interferon alpha 2b in chronic hepatitis C: a randomized controlled trial. Am J Gastroenterol 1996;91(9):1734‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Tong 1997 (CIFN 3µg) {published data only}
- Tong MJ, Reddy KR, Lee WM, Pockros PJ, Hoefs JC, Keeffe EB, et al. Treatment of chronic hepatitis C with consensus interferon: a multicenter, randomized, controlled trial. Consensus Interferon Study Group. Hepatology 1997;26(3):747‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tong 1997 (CIFN 9µg) {published data only}
- Tong MJ, Reddy KR, Lee WM, Pockros PJ, Hoefs JC, Keeffe EB, et al. Treatment of chronic hepatitis C with consensus interferon: a multicenter, randomized, controlled trial. Consensus Interferon Study Group. Hepatology 1997;26(3):747‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Valla 1999 {published data only}
- Valla DC, Chevallier M, Marcellin P, Payen JL, Trepo C, Fonck M, et al. Treatment of hepatitis C virus‐related cirrhosis: a randomized, controlled trial of interferon alfa‐2b versus no treatment. Hepatology 1999;29(6):1870‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Villa 1996 (bet‐IFN) {published data only}
- Villa E, Trande P, Grottola A, Buttafoco P, Rebecchi AM, Stroffolini T, et al. Alpha but not beta interferon is useful in chronic active hepatitis due to hepatitis C virus. A prospective, double‐blind, randomized study. Dig Dis Sci 1996;41(6):1241‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Villa 1996 (leu‐IFN) {published data only}
- Villa E, Trande P, Grottola A, Buttafoco P, Rebecchi AM, Stroffolini T, et al. Alpha but not beta interferon is useful in chronic active hepatitis due to hepatitis C virus. A prospective, double‐blind, randomized study. Dig Dis Sci 1996;41(6):1241‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weiland 1990 {published data only}
- Weiland O, Schvarcz R, Wejstal R, Norkrans G, Fryden A. Therapy of chronic post‐transfusion non‐A, non‐B hepatitis with interferon alfa‐2b: Swedish experience. J Hepatol 1990;11(Suppl 1):S57‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Angelini 1995 {published data only}
- Angelini G, Sgarbi D, Colombari R, Bezzi A, Castagnini A, Berardinis F, Conti A, et al. Alpha‐Interferon treatment of chronic hepatitis C: a controlled, multicentre, prospective study. Digestion 1995;56(3):199‐203. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bonkovsky 1996 {published data only}
- Bonkovsky HL, Clifford BD, Smith LJ, Allan C, Banner B. High‐dose interferon‐alpha 2b for re‐treatment of nonresponders or relapsing patients with chronic hepatitis C. A controlled randomized trial. Dig Dis Sci 1996;41(1):149‐54. [DOI] [PubMed] [Google Scholar]
Bresci 1996 {published data only}
- Bresci G, Parisi G, Banti S, Scatena F, Bertoni M, Capria A. Chronic hepatitis C. What treatment for nonresponders to recombinant interferon‐alpha?. Clin Drug Invest 1996;11:224‐228. [Google Scholar]
Caporaso 1993 {published data only}
- Caporaso N, Suozzo R, Morisco F, D'antonio M, Romano M, Coltorti M. Recombinant human interferon alpha‐2a therapy for chronic hepatitis C with or without cirrhosis: comparison of 3 or 6 MU for 1 year. Ital J Gastroenterol 1993;25(9):482‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Chemello 1997 {published data only}
- Chemello L, Cavalletto L, Donada C, Bonetti P, Casarin P, Urban F, et al. Efficacy of a second cycle of interferon therapy in patients with chronic hepatitis C. Gastroenterology 1997;113(5):1654‐9. [DOI] [PubMed] [Google Scholar]
Di Marco 1997 {published data only}
- Marco V, Lo Iacono O, Camma C, Almasio PL, Vaccaro A, Fuschi P, et al. A randomized controlled trial of high‐dose maintenance interferon therapy in chronic hepatitis C. J Med Virol 1997;51(1):17‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferenci 1996 {published data only}
- Ferenci P, Stauber R, Propst A, Fiedler R, Muller C, Gschwantler M, et al. Dose increase augments response rate to interferon‐alpha in chronic hepatitis C. Dig Dis Sci 1996;41(12 Suppl):103S‐108S. [DOI] [PubMed] [Google Scholar]
Friedlander 1996 {published data only}
- Friedlander L, Thiel DH, Faruki H, Molloy PJ, Kania RJ, Hassanein T. New approach to HCV treatment. Recognition of disease process as systemic viral infection rather than as liver disease. Dig Dis Sci 1996;41(8):1678‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heathcote 1998 {published data only}
- Heathcote EJ, Keeffe EB, Lee SS, Feinman SV, Tong MJ, Reddy KR, et al. Re‐treatment of chronic hepatitis C with consensus interferon [published erratum appears in Hepatology 1998 Aug;28(2):599]. Hepatology 1998;27(4):1136‐43. [DOI] [PubMed] [Google Scholar]
Lindsay 1996 {published data only}
- Lindsay KL, Davis GL, Schiff ER, Bodenheimer HC, Balart LA, Dienstag JL, et al. Response to higher doses of interferon alfa‐2b in patients with chronic hepatitis C: a randomized multicenter trial. Hepatitis Interventional Therapy Group. Hepatology 1996;24(5):1034‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Payen 1998 {published data only}
- Payen JL, Izopet J, Galindo‐Migeot V, Lauwers‐Cances V, Zarski JP, Seigneurin JM, et al. Better efficacy of a 12‐month interferon alfa‐2b retreatment in patients with chronic hepatitis C relapsing after a 6‐month treatment: a multicenter, controlled, randomized trial. Le Groupe D'etude et De Traitement du Virus De L'hepatite C (Get.Vhc). Hepatology 1998;28(6):1680‐6. [DOI] [PubMed] [Google Scholar]
Poynard 1999 {published data only}
- Poynard T, Daurat V, Chevret S, Moussali J, Degos F, Bailly F, et al for the multicentre GER‐CYT‐01 group. A short induction regimen of interferon‐alpha is not effective for treatment of relapse in chronic hepatitis C : a randomized trial. J Viral Hepatitis 1999;6:381‐386. [DOI] [PubMed] [Google Scholar]
Rolachon 1997 {published data only}
- Rolachon A, Kezachian G, Causse X, Baud M, Leroy V, Maynard‐Muet M, et al. Value of high‐dose interferon‐alpha in chronic viral hepatitis C patients non‐responder to a 1st treatment. Pilot study prospective and randomized trial. Gastroenterol Clin Biol 1997;21(12):924‐8. [PubMed] [Google Scholar]
Saracco 1997 {published data only}
- Saracco G, Borghesio E, Mesina P, Solinas A, Spezia C, Macor F, et al. Prolonged treatment (2 years) with different doses (3 versus 6 MU) of interferon alpha‐2b for chronic hepatitis type C. Results of a multicenter randomized trial. J Hepatol 1997;27(1):56‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sheiner 1998 {published data only}
- Sheiner PA, Boros P, Klion FM, Thung SN, Schluger LK, Lau JY, et al. The efficacy of prophylactic interferon alfa‐2b in preventing recurrent hepatitis C after liver transplantation. Hepatology 1998;28(3):831‐8. [DOI] [PubMed] [Google Scholar]
Shiffman 1996 {published data only}
- Shiffman ML, Hofmann CM, Luketic VA, Sanyal AJ, Contos MJ, Mills AS. Improved sustained response following treatment of chronic hepatitis C by gradual reduction in the interferon dose. Hepatology 1996;24(1):21‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Bedossa 1994
- Bedossa P and the French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994;20:15‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Camma 1996
- Camma C, Almasio P, Craxi A. Interferon as treatment for acute hepatitis C. A meta‐analysis. Dig Dis Sci 1996;41:1248‐1255. [DOI] [PubMed] [Google Scholar]
Camma 1997
- Camma C, Giunta M, Linea C, Pagliaro L. The effect of interferon on the liver in chronic hepatitis C: a quantitative evaluation of histology by meta‐analysis. J Hepatol 1997;26(6):1187‐99. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods of combining randomized clinical trials: strengths and limitations. Stat Med 1987;6:341‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Der Simonian 1986
- Simonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clin Trials 1986;7(3):177‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gordon 2000
- Gordon SC, Fang JW, Silverman AL, McHutchison JG, Albrecht JK. The significance of baseline serum alanine aminotransferase on pretreatment disease characteristics and response to antiviral therapy in chronic hepatitis C. Hepatology 2000;32(2):400‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between small and large randomized trials in meta‐analyses. Ann Intern Med 2001;135(11):982‐989. [DOI] [PubMed] [Google Scholar]
Kjaergard 2002
- Kjaergard LL, Krogsgaard K, Gluud C. Ribavirin with or without alpha interferon versus no intervention, placebo, or alpha interferon for chronic hepatitis C (Cochrane Review). The Cochrane Library 2002, Issue 1. [DOI] [PubMed] [Google Scholar]
Marcellin 1997
- Marcellin P, Boyer N, Gervais A, Martinot M, Pouteau M, Castelnau C, et al. Long‐term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon‐alpha therapy. Ann Intern Med 1997;127(10):875‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Piton 1998
- Piton A, Poynard T, Imbert‐Bismut F, Khalil L, Delattre J, Pelissier E, et al. Factors associated with serum alanine transaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. MULTIVIRC Group. Hepatology 1998;27(5):1213‐9. [9581673] [DOI] [PubMed] [Google Scholar]
Poynard 1988
- Poynard T. Evaluation of the methodological quality of randomized therapeutic trials [Evaluation de la qualité méthodologique des essais thérapeutiques randomisés]. Presse Med 1988;17(7):315‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Poynard 1996
- Poynard T, Leroy V, Cohard M, Thevenot T, Mathurin P, Opolon P, et al. Meta‐analysis of interferon randomized trials in the treatment of viral hepatitis C : effects of dose and duration. Hepatology 1996;24(4):778‐789. [DOI] [PubMed] [Google Scholar]
Poynard 1998
- Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Gaetano I, et al. Randomised trial of interferon alfa‐2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alfa‐2b plus placebo for 48 weeks for treatment f chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet 1998;352(9138):1426‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Poynard 2000
- Poynard T, McHutchinson J, Goodman Z, Ling MH, Albrecht J. Is an "a la carte" combination interferon Alpha‐2b plus Ribavirin regimen possible for the first line treatment in patients with chronic hepatitis C ?. Hepatology 2000;31(1):211‐218. [DOI] [PubMed] [Google Scholar]
Sacks 1987
- Sacks HS, Berrier J, Reitman D, Ancona‐Berk VA, Chalmers TC. Meta‐analysis of randomized controlled trials. N Engl J Med 1987;316(8):450‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tine 1991
- Tine F, Magrin S, Craxi A, Pagliaro L. Interferon for non‐A, non‐B chronic hepatitis. A meta‐analysis of randomised clinical trials. J Hepatol 1991;13(2):192‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Thevenot 2001
- Thevenot T, Regimbeau C, Ratziu V, Leroy V, Opolon P, Poynard T. Meta‐analysis of interferon randomized trials in the treatment of viral hepatitis C in naive patients: 1999 update. J Viral Hepat 2001;8(1):48‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


