Abstract
Background
Incontinence can have a devastating effect on the lives of sufferers with significant economic implications. Non‐surgical treatments such as pelvic floor muscle training and the use of mechanical devices are usually the first line of management, particularly when a woman does not want surgery or when she is considered unfit for surgery. Mechanical devices are inexpensive and do not compromise future surgical treatment.
Objectives
To determine whether mechanical devices are useful in the management of adult female urinary incontinence.
Search methods
For this second update we searched the Cochrane Incontinence Group Specialised Register, which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in process, ClinicalTrials.gov, WHO ICTRP and handsearching of journals and conference proceedings (searched 21 August 2014), EMBASE (January 1947 to 2014 Week 34), CINAHL (January 1982 to 25 August 2014), and the reference lists of relevant articles.
Selection criteria
All randomised or quasi‐randomised controlled trials of mechanical devices in the management of adult female urinary incontinence determined by symptom, sign or urodynamic diagnosis.
Data collection and analysis
The reviewers assessed the identified studies for eligibility and risk of bias and independently extracted data from the included studies. Data analysis was performed using RevMan software (version 5.3).
Main results
One new trial was identified and included in this update bringing the total to eight trials involving 787 women. Three small trials compared a mechanical device with no treatment and although they suggested that use of a mechanical device might be better than no treatment, the evidence for this was inconclusive. Four trials compared one mechanical device with another. Quantitative synthesis of data from these trials was not possible because different mechanical devices were compared in each trial using different outcome measures. Data from the individual trials showed no clear difference between devices, but with wide confidence intervals. One trial compared three groups: a mechanical device alone, behavioural therapy (pelvic floor muscle training) alone and behavioural therapy combined with a mechanical device. While at three months there were more withdrawals from the device‐only group, at 12 months differences between the groups were not sustained on any measure.
Authors' conclusions
The place of mechanical devices in the management of urinary incontinence remains in question. Currently there is little evidence from controlled trials on which to judge whether their use is better than no treatment and large well‐conducted trials are required for clarification. There was also insufficient evidence in favour of one device over another and little evidence to compare mechanical devices with other forms of treatment.
Keywords: Adult; Aged; Female; Humans; Middle Aged; Prostheses and Implants; Exercise Therapy; Exercise Therapy/methods; Muscle Contraction; Muscle Contraction/physiology; Pelvic Floor; Pessaries; Randomized Controlled Trials as Topic; Tampons, Surgical; Urinary Incontinence; Urinary Incontinence/rehabilitation; Urinary Sphincter, Artificial
Plain language summary
Mechanical devices for urinary incontinence in women
Urinary incontinence is involuntary loss of urine. The common types are stress and urge incontinence. Mechanical devices are made of plastic or other materials. They are placed within the urethra or vagina in order to stop or control the leakage of urine. This review of trials found that using mechanical devices might be better than no treatment but the evidence is weak. There was not enough evidence to recommend any specific type of device or to show whether mechanical devices are better than other forms of treatment such as pelvic floor muscle training.
Background
Description of the condition
Efficient urinary control depends on normal functioning detrusor (bladder) muscles, nerves, proximal urethral support, bladder neck closure and a normal urethra (Bourcier 1995).
Stress urinary incontinence is the most common type of incontinence, occurring in about half of incontinent women when lack of support at the bladder neck inhibits urethral closure. As a result, activities that increase intra‐abdominal pressure can cause involuntary leakage during effort, exertion, sneezing or coughing. Urgency urinary incontinence accounts for around 10% of incontinence and occurs when involuntary detrusor muscle contraction causes a rise in intravesical (bladder) pressure, a condition known as detrusor overactivity. In another 30% of cases, both stress and urgency urinary incontinence are present, with either type being predominant, known as mixed urinary incontinence (Hannestad 2000, Hay‐Smith 2009,)
It is widely believed that the most effective treatment for severe or persistent stress urinary incontinence is surgery (Downs 1996). Nevertheless, to avoid surgical risk, non‐surgical measures are usually the first line of management for stress urinary incontinence. Non‐surgical treatments include lifestyle interventions (such as weight reduction), pelvic floor muscle training (PFMT; Dumoulin 2014), vaginal cones (Herbison 2013), electrical stimulation devices (Berghmans 2013), oral medication (for example alpha‐adrenergic agonists (Alhasso 2005) or selective noradrenaline reuptake inhibitors (Mariappan 2005)), scheduled voiding regimens (Ostaszkiewicz 2004), local or systemic oestrogen treatment (Cody 2012) and mechanical devices within the urethra or the vagina (the subject of the current review). These modalities, which might be able to provide some extrinsic support for the bladder neck and urethra, are relatively inexpensive and do not compromise future surgical treatment.
Description of the intervention
The use of mechanical devices for urinary incontinence in women has been said to date back to Egyptian times (Edwards 1970). Despite this long tradition, and perhaps because of the lack of evidence, mechanical devices are not often used in the management of incontinence today.
Over the past three decades efforts have been made to develop devices with evidence‐based designs to control urinary incontinence. The devices that have been used include:
standard contraceptive diaphragm (Realini 1990; Suarez 1991);
Hodge vaginal pessary (Nygaard 1995);
bladder neck support prosthesis (Davila 1994; Davila 1997; Kondo 1997; Moore 1997; Moore 1999);
Ladycon intravaginal sponge (Glavind 1997a);
disposable vaginal device (Bidmead 2000),(Cornu 2012);
urethral plug (Nielsen 1993);
urethral device with a self‐inflatable balloon, for example Reliance (Staskin 1996); and
external urethral occlusive device (Prashar 1997a).
New devices periodically appear on the commercial market, initially espoused enthusiastically by the manufacturers, only to vanish within a short span of time. In most cases the clinical data supporting the use of these devices appears to be of poor quality.
This review addresses the role of various mechanical devices in the management of urinary incontinence,including those designed to control urinary leakage by being inserted into the urethra or vagina or applied to the external surface of the urethra.
We excluded weighted vaginal cones (Herbison 2013) and electrical devices (Berghmans 2013) since these treatment modalities aim to improve the function of pelvic floor musculature and have been examined in other Cochrane reviews. We have also excluded pads, catheters and other collecting devices since their function is to collect or divert the urine rather than controlling the incontinence. Urinary incontinence in men may be managed by mechanical devices (for example penile clamps) and are also the subject of a separate Cochrane review (Campbell 2012).
How the intervention might work
Women suffering from stress incontinence commonly have excessive mobility of the urethrovesical junction and proximal urethra. During increases in intra‐abdominal pressure, normal spatial relationships between the urethra, bladder and pelvis are disrupted. This results in deficient transmission of pressure to the proximal urethra, causing inadequate closure pressure and allowing leakage. Many intravaginal devices aim to restore the position of the upper urethra to above the level of the pelvic floor. The devices are thought not to be effective in patients with a fibrotic urethra, in which the fibrosis limits the transmission of pressure. In women with intrinsic sphincter deficiency, intraurethral devices may have a role in controlling incontinence by applying external pressure to the bladder base to keep it closed.
Although mechanical devices are most commonly used in stress incontinence, urethral plugs or external meatal devices, which act by blocking the leakage of urine, may occasionally be used in other types of urinary incontinence such as mixed and urgency.
Why it is important to do this review
Incontinence can have a devastating effect on the lives of sufferers and their families. Women suffer the physical discomfort associated with incontinence as well as potential social isolation and psychological distress. Incontinence also results in substantial cost to service providers and society. The aim of this review is to address the use of mechanical devices by systematically bringing together the best evidence available.
Objectives
To determine the effects of mechanical devices in the management of adult female urinary incontinence, particularly stress incontinence.
The following comparisons were made:
1. A specific mechanical device to control urinary leakage versus no treatment (placebo studies are not possible); 2. One mechanical device versus another mechanical device; 3. A mechanical device versus other treatments (for example conservative therapies such as PFMT, drug therapies such as alpha‐adrenergic agonists or selective noradrenaline reuptake inhibitors, and surgery such as sling operations).
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi‐randomised controlled trials of mechanical devices (intravaginal and intraurethral) in the management of adult female urinary incontinence.
Types of participants
All adult women with urinary incontinence diagnosed as having stress, urgency or other incontinence either by symptom classification or by urodynamic diagnosis, as defined by the trialists.
Types of interventions
Interventions using mechanical devices designed to control urinary leakage by being inserted:
within the vagina; or
within the urethra; or
applied to the external surface of the urethra.
Standard urethral catheters and simple collecting devices were excluded from the definition of 'mechanical device' because their main roles are diversion and hygienic collection of the urinary stream rather than to control incontinence. Vaginal cones and electrical stimulation devices were also excluded as they have been evaluated in separate Cochrane reviews (Berghmans 2013; Herbison 2013).
Types of outcome measures
Purely clinical assessments were accepted since the value of these devices may be more obvious within a 'low‐tech' community environment. The primary outcomes were improvement in patient symptoms, health status and quality of life.
Patient symptoms (self‐reported) 1. Numbers cured. 2. Numbers improved. 3. Number requiring alternative management. 4. Frequency of daytime micturition (mean and standard deviation (SD)). 5. Frequency of nocturia (mean and SD). 6. Incontinence episodes in 24 hours (mean and SD). 7. Pad changes over 24 hours (mean and SD).
Objective clinical measures 8. Formal pad weighing tests (comparison of two interventions or change per intervention). 9. Differences in the results of urodynamic tests (such as urethral profilometry and pressure voiding studies).
Tolerability of device and side‐effects 10. Number of participants unable to use device. 11. Number with discomfort or pain. 12. Number with infections. 13. Number where sexual function is compromised. 14. Number of times device change required.
Validated questionnaire measures of symptoms and/or quality of life 15. Use of various available questionnaires, for example Bristol Lower Urinary Tract Symptoms Questionnaire (Jackson 1996), Kings Health Questionnaire or SF 36 (Ware 1993) before and during use of the intervention.
Health economic data 16. Costs of interventions. 17. Resource implications of differences in outcomes, including possible savings to patients in numbers of pads used to control urinary leakage. 18. Economic analysis of cost effectiveness and cost utility.
Other outcomes 19. Other outcomes not pre‐specified but judged important during review.
Search methods for identification of studies
We did not impose any language restriction or other limits on the searches.
Electronic searches
This review has drawn on the search strategy developed for the Incontinence Group as a whole. Relevant trials have been primarily identified from the Cochrane Incontinence Group Specialised Register. The methods used to derive this, including the search strategy, are described under the Group's module in The Cochrane Library. The register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in process, ClinicalTrials.gov, WHO ICTRP and handsearching of journals and conference proceedings. Most of the trials in the Incontinence Group Specialised Register are also contained in CENTRAL.
We searched the Incontinence Group Register using the Group's own keyword system; the search terms used are given in Appendix 1. The date of the most recent search of the Register for this review was 21 August 2014.
We also searched other electronic databases for this review:
EMBASE on OvidSP (1 January 1947 to 2014 Week 34 inclusive). Search date: 26 August 2014.
CINAHL on EBSCO (January 1982 to 25 Augsut 2014 inclusive). Search date: 26 Augsut 2014.
Details of the strategies used to search these databases can be found in Appendix 1.
Searching other resources
We also searched the reference lists of relevant articles for other possible relevant trials.
Data collection and analysis
Selection of studies
The title and abstract of all references identified by the search strategy were assessed by both update review authors. The reports of all possibly eligible studies were evaluated for appropriateness for inclusion by the review authors without prior consideration of the results. Any disagreements were resolved by discussion and, where this was not possible, a final decision was made by a third person. Studies were excluded from the review if they were not randomised or quasi‐randomised trials or if they made comparisons other than those specified. Excluded studies were listed with reasons for their exclusion.
Data extraction and management
Data extraction was undertaken independently by the two update review authors using a standard data extraction form. Where data were possibly collected but not reported, clarification was sought from the trialists. Included trial data were processed as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Trial data were considered according to the type of management against which the devices were being compared. Trials were also grouped by type of incontinence: either stress, urge or mixed incontinence based on symptom classification or urodynamic criteria. Any differences of opinion were resolved by discussion with a third party.
The review was conducted using the standard Cochrane RevMan software. Quantitative synthesis was planned if more than one eligible study was identified. Where appropriate, a pooled estimate of treatment effect across similar studies was to be calculated for each prespecified outcome, using standard statistics such as odds ratio for dichotomous data or weighted mean differences for continuous outcomes. The 95% confidence intervals were generated, where possible. For categorical (dichotomous) outcomes, the risk ratios (RR) were calculated. For continuous variables, means and standard deviations were used aiming to derive a mean difference. A fixed‐effect approach to the analysis was planned unless there was evidence of heterogeneity across studies.
A narrative review of eligible studies was undertaken where statistical synthesis of data from more than one study was not possible or not considered appropriate. Data from cross‐over trials were analysed using the generic inverse variance approach, if possible. When insufficient information was reported and no estimates were available from the other studies, a value of 0.5 was imputed for the correlation between repeat outcomes on the same patient. Sensitivity analyses were undertaken to investigate the robustness of results to imputed correlation values. Data on the number of women with each outcome event, by allocated treated group (irrespective of compliance and whether or not the patient was later deemed ineligible or otherwise excluded from treatment or follow‐up) were sought to allow intention‐to‐treat analysis.
Assessment of risk of bias in included studies
Assessment of risk of bias for each included study was undertaken individually by each review author using the Cochrane Collaboration's 'Risk of bias' tool. This tool is used to determine the quality of random allocation and allocation concealment, appropriate handling of of dropouts and withdrawals, whether intention to treat analysis was used, and whether blinding was employed during treatment and at outcome assessment. It was appreciated that blinding is not possible for women in studies involving the use of mechanical devices, but it might be possible for those assessing some outcomes. Any disagreements were resolved as above.
Assessment of heterogeneity
Differences between trials were to be investigated when heterogeneity was apparent from either visual inspection of the results or when statistically significant heterogeneity was demonstrated by using the C2 test at the 10% probability level or assessment of the I2 statistic (Higgins 2003). If there was no obvious reason for the heterogeneity (after consideration of populations, interventions, outcomes and settings of the individual trials), a random‐effects model was to be used.
Subgroup analysis and investigation of heterogeneity
Where applicable, subgroup analysis was to be used as a means of investigating heterogeneous results.
Sensitivity analysis
Sensitivity analysis was planned to assess the effects of including studies of poorer methodological quality, for example quasi‐randomised studies.
Results
Description of studies
Results of the search
The search strategy for the review and updates resulted in 557 records which were identified by the literature search and screened for this review. Out of 33 articles initially identified (including one abstract and one thesis), 20 reports of 8 studies were potentially eligible and 13 studies were excluded. Reasons for exclusion of the papers are outlined in the 'Characteristics of excluded studies' table. Two trials, one published before and one published since the original review, were identified in the first update; one was included and one was excluded (Lipp 2011). In the current update, three additional articles were identified. One reported results of a new trial and was included; the other two articles reported data from a previously included trial, one included and the other excluded. The flow of literature through the review process is shown in Figure 1.
1.
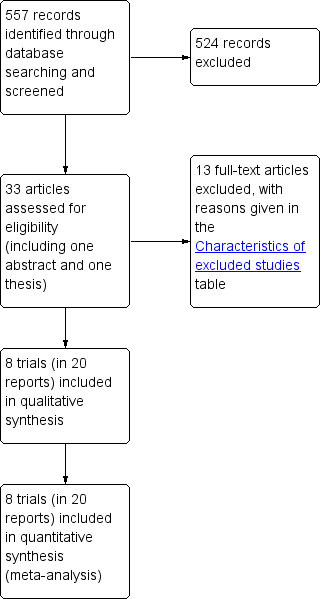
PRISMA study flow diagram.
Included studies
Eight randomised controlled trials published in 20 papers were included in this second update. Six trials were published as both abstract and either one (Glavind 1997b; Robinson 2003; Cornu 2012) or two full text articles (Nielsen 1995; Nygaard 1995; Thyssen 2001). One trial was published as full text, primarily with clinical outcomes (Richter 2010) and secondarily with quality of life outcomes (Kenton 2012), as well as once as a trial protocol and once as an abstract. The remaining trial was published as an abstract and PhD thesis (Boos 1998). All papers were written in English and one trial was also published in Danish (Nielsen 1995).
Design
Two trials were randomised and compared an intervention with a control group (Glavind 1997b; Cornu 2012). Three trials compared two interventions (Boos 1998; Nielsen 1995; Robinson 2003). One three‐arm trial contained an intervention group, a behavioural therapy group and a combined intervention‐and‐behavioural therapy group (Richter 2010). Four out of the eight trials were cross‐over trials (Glavind 1997b; Nielsen 1995; Nygaard 1995; Thyssen 2001). Two of these (Nygaard 1995; Thyssen 2001) were analysed using the generic inverse variance ratio. One trial had a three‐period, three‐treatment cross‐over design for pessary, tampon and no device (Nygaard 1995). Three trials used a two‐period, two‐treatment cross‐over design (Glavind 1997b; Thyssen 2001; Nielson 1995) but for one (Nielsen 1995), only data from period one could be included in this meta‐analysis as there was a substantial number of dropouts in period two. Four patients dropped out because they considered their incontinence to be a minor problem; no specific reasons were given for the remaining 18 dropouts. We analysed these data as if they were from a parallel‐group trial.
Types of devices
Five trials investigated intravaginal devices. One trial compared an intravaginal sponge with no treatment (Glavind 1997b), and one trial compared one vaginal loop (with thread for removal) with no treatment (Cornu 2012). One trial investigated the use of a vaginal continence dish or ring (Richter 2010), two trials compared two versions of an intravaginal device:Hodge pessary versus super tampon (Nygaard 1995) and Contrelle Continence Tampon versus Conveen Continence Guard (Thyssen 2001). The three remaining trials investigated intraurethral devices. One of these compared two versions of a urethral plug (Nielsen 1995) and two trials compared different types of intraurethral device:Reliance Urinary Control Device versus FemAssist (Boos 1998) and New Expandible Tip (NEAT) device versus Reliance Urinary Control Device (Robinson 2003).
Sample size
The number of women in the included trials was small apart from one (Richter 2010) in which 446 pre‐ and post‐menopausal women participated. Boos 1998 included 102 pre‐ and post‐menopausal participants ; Thyssen 2001 included 94 participants (43 pre‐menopausal and 51 post‐menopausal, of whom 39 received oestrogen replacement therapy); Cornu 2012 included 55 pre and post‐menopausal women; there were six participants in the Glavind trial (Glavind 1997b) of whom three were menopausal, three were peri‐menopausal and one of the six women had a hysterectomy.. Three trials did not include menopausal status, but the age range would suggest pre, peri and post‐menopausal participants. The 40 participants in the Nielsen trial were 32‐47 years of age (Nielsen 1995), there were 20 participants in the Nygaard trial (33‐73 years old) (Nygaard 1995) and 24 participants in the Robinson trial (30‐75 years old) (Robinson 2003).
Intervention
Three trials compared a mechanical device with no treatment (Glavind 1997b; Nygaard 1995; Cornu 2012).
Five of the included trials compared one mechanical device with another (Boos 1998; Nielsen 1995; Nygaard 1995; Robinson 2003; Thyssen 2001).
One trial compared a mechanical device plus behavioural therapy, with a mechanical device alone and with behavioural therapy alone (Richter 2010).
The devices were used for five weeks in the Thyssen 2001 trial. Participants in the Glavind trial were asked to perform 30 minutes of aerobic exercises on two consecutive days and were randomised (using sealed envelopes) on day one either to plus/minus or minus/plus the device (Glavind 1997b).
In the Nygaard 1995 trial, block randomisation was used; participants were randomised to the different devices and performed a 40 minute aerobic session. The Boos 1998 trial involved randomisation of participants to two devices which were used for six months with assessments at the beginning, one month, three months and six months. Similarly, the Robinson 2003 trial involved use of the randomly allocated devices for four months with assessments at zero and four months. Participants in the Richter trial remained in the trial for 12 months with primary outcomes measured at three months (Richter 2010). Period one of the Neilson trial lasted for two weeks and this is the only period taken into consideration in this review (Nielsen 1995). Similarly, the randomisation period of the Cornu 2012 trial (period two) was 14 days.
The details of the included studies are further outlined in the table 'Characteristics of included studies'.
Excluded studies
Details of excluded studies are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
All included studies were assessed separately for risk of bias (Higgins 2011). A summary of those assessments is represented by Figure 2 and Figure 3.
2.
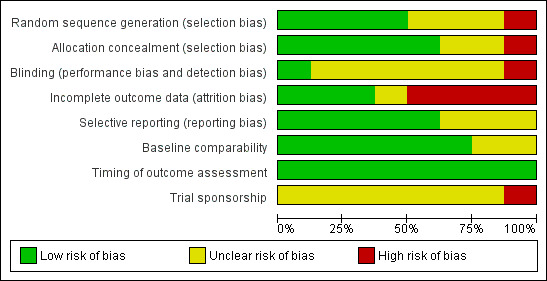
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
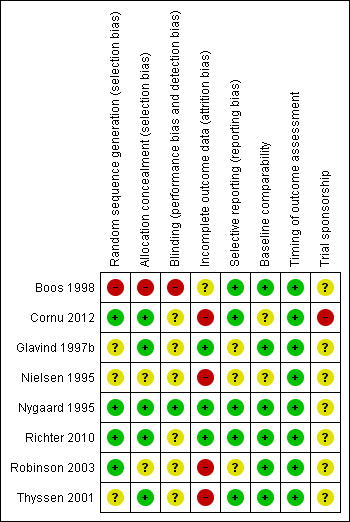
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Randomisation
All included studies were randomised trials and details of randomisation were clear in two trials (Cornu 2012; Richter 2010). In three trials, the women were randomly allocated by blocked randomisation (Nygaard 1995; Richter 2010; Thyssen 2001). In another trial, randomisation was carried out using a randomisation card (Robinson 2003). Sealed envelopes were used in the Glavind trial (Glavind 1997b).
The randomisation method in one study was deemed high risk (quasi‐randomised) as participants were allocated to groups alternately (Boos 1998).
Blinding to intervention
In trials which involved using a mechanical device, blinding can be difficult if not impossible. Only two trials confirmed that participants (Nygaard 1995; Robinson 2003) or outcome assessors (Nygaard 1995; Richter 2010) were blinded.
Withdrawal
The number of withdrawals were reported in all except one (Robinson 2003) trial and all of these stated the reasons for withdrawal (Boos 1998; Nielsen 1995; Nygaard 1995; Richter 2010; Thyssen 2001; Cornu 2012). There were no withdrawals in one trial (Glavind 1997b).
Effects of interventions
Comparison 1: mechanical device versus no treatment (Glavind 1997b; Nygaard 1995; Cornu 2012)
Three trials compared the use of a mechanical intravaginal device versus no treatment. Two trials were cross‐over and the comparison with no treatment was during exercise (Glavind 1997b; Nygaard 1995). In one trial the comparison with no treatment was over 14 days (Cornu 2012).
Nygaard's three‐arm cross‐over trial (Nygaard 1995) compared a pessary versus a tampon versus no device or treatment: eighteen women were randomised but fourteen were included in the analysis as four were stated to be continent during the trial. Although the pad weight was highest in the no‐device group, the confidence intervals around the difference were wide and the results were not statistically significant for tampon compared with no treatment (Mean difference in grams (MD) ‐14.30, 95% CI ‐38.41 to 9.81, Analysis 1.1.1). The results were statistically significant for pessary compared with no treatment (MD ‐6.55, 95% CI ‐11.20 to ‐1.90, p = 0.006, Analysis 1.1.2) when data from Glavind 1997b were included in the analysis although the small sample size (n = 26) must be taken into consideration.
1.1. Analysis.
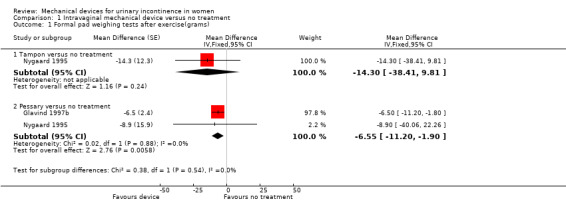
Comparison 1 Intravaginal mechanical device versus no treatment, Outcome 1 Formal pad weighing tests after exercise(grams).
The published report of the Nygaard 1995 trial showed a statistically significant difference between the tampon and pessary treatment groups and the control group. This could be due to the imputed correlation being too low though a very high value was required to show a significant difference (approximately 0.9). Alternatively, it could be due to the skewness in the data, for which the analysis used in the paper compensated. Nygaard dichotomised the data for the pad weight test and reported zero out of 14 had low weights (less than 4 g) with no device compared with eight out of 14 with the tampon and five out of 14 with a pessary.
Women in the Nygaard trial were asked about discomfort. Four of the 18 women found the pessary uncomfortable (RR 9.00, 95% CI 0.52 to 155.86, p= 0.13, Analysis 1.2.1). Two additional women reported discomfort with the tampon (RR 5.00, 95% CI 0.26, 97.37, p = 0.29,Analysis 1.2.2). One in each of the groups found the device useful.
1.2. Analysis.
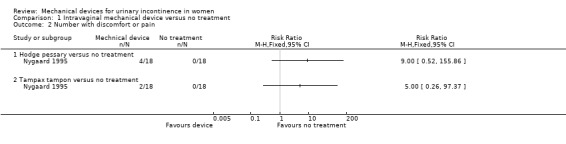
Comparison 1 Intravaginal mechanical device versus no treatment, Outcome 2 Number with discomfort or pain.
The Cornu 2012 trial measured the change in incontinence episodes from baseline and then compared the change score between groups (rather than using the absolute score at outcome) in 55 women. The incontinence episode frequency (IEF) per week was not significantly reduced (MD ‐24.10, 95% CI ‐49.60 to 1.40, Analysis 1.3). In the results of a 24 hour pad test the confidence intervals were wide and the results did not show a statistically significant improvement in incontinence (MD ‐32.90, 95% CI ‐109.41 to 43.61, Analysis 1.4).
1.3. Analysis.
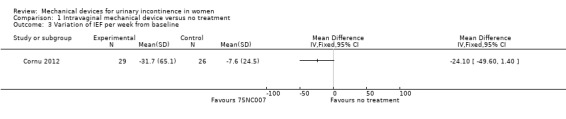
Comparison 1 Intravaginal mechanical device versus no treatment, Outcome 3 Variation of IEF per week from baseline.
1.4. Analysis.
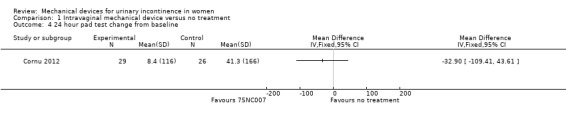
Comparison 1 Intravaginal mechanical device versus no treatment, Outcome 4 24 hour pad test change from baseline.
There was a significant reduction in urinary symptoms on the Urinary Symptom Profile questionnaire (SUI sub‐score;MD ‐2.20, 95% CI ‐3.47 to ‐0.93, Analysis 1.5). There was also a significant reduction of symptoms according to the OAB sub‐score and dysuria sub‐score (MD ‐1.67, 95% CI ‐2.78 to ‐0.56, Analysis 1.6 andMD ‐0.50, 95% CI ‐0.92 to ‐0.08, Analysis 1.7 respectively). The CONTILIFE quality of life questionnaire results did not differ significantly between treatments (MD ‐10.30, CI 95% ‐20.77 to 0.17, Analysis 1.8).
1.5. Analysis.
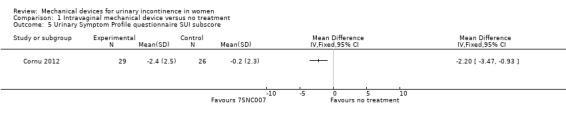
Comparison 1 Intravaginal mechanical device versus no treatment, Outcome 5 Urinary Symptom Profile questionnaire SUI subscore.
1.6. Analysis.
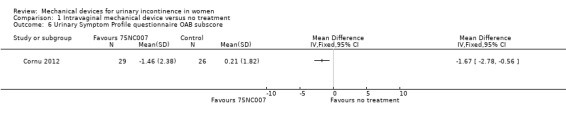
Comparison 1 Intravaginal mechanical device versus no treatment, Outcome 6 Urinary Symptom Profile questionnaire OAB subscore.
1.7. Analysis.
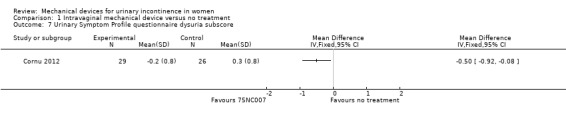
Comparison 1 Intravaginal mechanical device versus no treatment, Outcome 7 Urinary Symptom Profile questionnaire dysuria subscore.
1.8. Analysis.
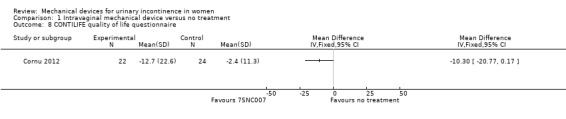
Comparison 1 Intravaginal mechanical device versus no treatment, Outcome 8 CONTILIFE quality of life questionnaire.
No data were reported for any other outcomes.
Comparison 2: intravaginal tampon versus intravaginal Hodge pessary (Nygaard 1995)
The only data available came from one trial (Nygaard 1995) which also contributed data to Comparison 1. Data from the pad weight tests showed no statistically significant difference for leakage in grams (MD ‐5.40, 95% CI ‐36.56 to 25.76, Analysis 2.1). There was no significant difference in discomfort while wearing the tampon (two out of 18) versus those wearing the pessary (four out of 18) (RR 0.50, 95% CI 0.10 to 2.40, Analysis 2.2).
2.1. Analysis.
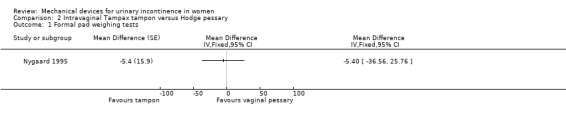
Comparison 2 Intravaginal Tampax tampon versus Hodge pessary, Outcome 1 Formal pad weighing tests.
2.2. Analysis.
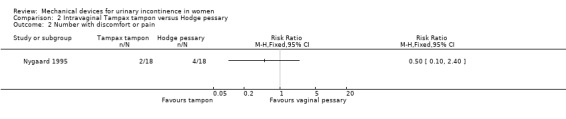
Comparison 2 Intravaginal Tampax tampon versus Hodge pessary, Outcome 2 Number with discomfort or pain.
Comparison 3: intravaginal Contrelle continence tampon (CCT) device versus intravaginal Conveen continence guard (CCG) device (Thyssen 2001)
In a single trial (Thyssen 2001), data were only available for the 24 hour pad test. These showed no statistically significant difference in grams of leakage (MD ‐9.40, 95% CI ‐20.38 to 1.58, Analysis 3.1), although the published report of the trial showed a statistically significant difference between groups. This could be due to the imputed correlation being too low,owever, a high value was required to change the conclusion (approximately 0.8).
3.1. Analysis.
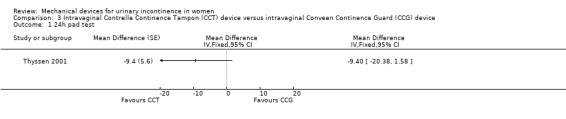
Comparison 3 Intravaginal Contrelle Continence Tampon (CCT) device versus intravaginal Conveen Continence Guard (CCG) device, Outcome 1 24h pad test.
Comparison 4: intraurethral 1‐sphere versus 2‐sphere plug (Nielsen 1995)
Data were only available for the pad weight test in one trial (Nielsen 1995). There was no evidence of a difference between groups from this small trial at the end of period one. Data could not be converted from median. There was a median of three grams of leakage (n=16;range 0 to 123) with the two‐sphere plug and six grams of leakage (n=17;range 0 to 121) with the one‐sphere plug.
Comparison 5: intraurethral new expandable tip (NEAT) device versus intraurethral Reliance device (Robinson 2003)
In one small trial (Robinson 2003) there was no statistically significant difference in the number of women improved (six out of eight versus five out of eight; RR 1.20, 95% CI 0.61 to 2.34, p = 0.59, Analysis 5.1). There was no difference in the mean reduction in urine loss (67.6% for NEAT and 59.3% for Reliance; p = 0.72). Instead of using the number of pad changes to grade incontinence, the trial used leakage score. At four months, the NEAT group had a higher leakage score than the Reliance group but this was not statistically significant (2.25 versus 1.50; p > 0.05). The ease of use was also assessed using a score and there was no difference between the devices (2.18 for NEAT versus 2.14 for Reliance; p > 0.05). The two groups were combined to assess side effects and adverse symptoms because of the small sample size and, therefore, no comparison can be made. HRT and menopausal status were also assessed and only menopausal status influenced device success; post‐menopausal participants tended to have a more successful outcome in terms of reduction of their pad weight, but this difference was not statistically significant.
5.1. Analysis.
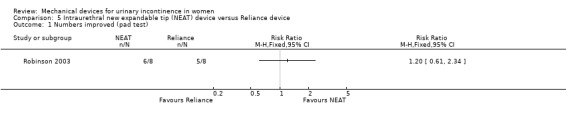
Comparison 5 Intraurethral new expandable tip (NEAT) device versus Reliance device, Outcome 1 Numbers improved (pad test).
No other outcomes were reported.
Comparison 6: intraurethral Reliance device versus intraurethral FemAssist device (Boos 1998)
In one trial (Boos 1998), based on an intention to treat analysis, nineteen of 48 women (39%) were reported as subjectively dry at six months with a Reliance device compared to 20 of 53 (38%) with a FemAssist device, but this difference was not statistically significant (RR 1.05, 95% CI 0.64 to 1.72, Analysis 6.1). Numbers improved (excluding those subjectively dry) at six months: 12 out of 48 women using a Reliance device reported improvement of incontinence. This difference was not statistically significant when compared with 13 out of 53 using a FemAssist device (RR 1.02; 95% CI 0.52 to 2.01, Analysis 6.2).
6.1. Analysis.
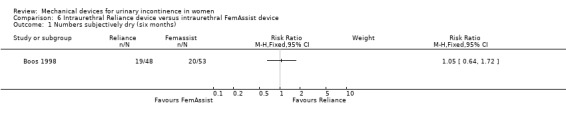
Comparison 6 Intraurethral Reliance device versus intraurethral FemAssist device, Outcome 1 Numbers subjectively dry (six months).
6.2. Analysis.
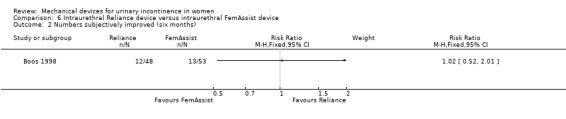
Comparison 6 Intraurethral Reliance device versus intraurethral FemAssist device, Outcome 2 Numbers subjectively improved (six months).
At the six month assessment, pad test weights decreased significantly in both groups from baseline: the Reliance group had a mean pad weight of 1.3 g compared to a baseline of 33.8 g and those using FemAssist had a mean value of 3.6 g compared to a baseline of 36 g. The difference between the devices at 6 months was statistically significant favouring Reliance (RR ‐2.30, 95% CI ‐3.52 to ‐1.08, Analysis 6.3). Pad changes over five days at six months also significantly favoured the Reliance device (MD ‐0.92, 95% CI ‐1.20 to ‐0.64, Analysis 6.4).
6.3. Analysis.
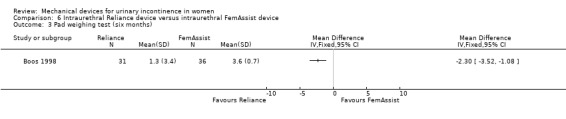
Comparison 6 Intraurethral Reliance device versus intraurethral FemAssist device, Outcome 3 Pad weighing test (six months).
6.4. Analysis.
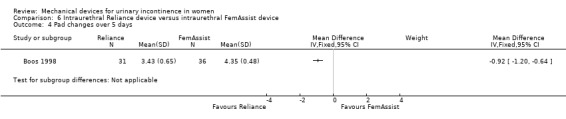
Comparison 6 Intraurethral Reliance device versus intraurethral FemAssist device, Outcome 4 Pad changes over 5 days.
There was no statistically significant difference in urinary tract infection rates (UTI) at six months (six out of 40 versus five out of 31;RR 1.08, 95% CI 0.36 to 3.20, Analysis 6.5). However, there were significantly fewer incontinence episodes in the FemAssist group at six months (MD 0.33 per day, 95% CI 0.07 to 0.59, Analysis 6.6).
6.5. Analysis.
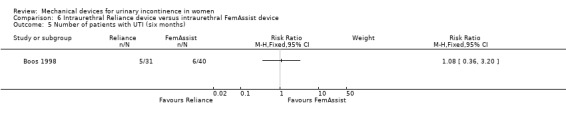
Comparison 6 Intraurethral Reliance device versus intraurethral FemAssist device, Outcome 5 Number of patients with UTI (six months).
6.6. Analysis.
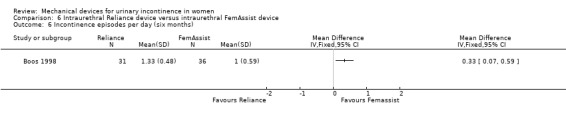
Comparison 6 Intraurethral Reliance device versus intraurethral FemAssist device, Outcome 6 Incontinence episodes per day (six months).
There were no incidents of migration of the Reliance device into the urethra and on three occasions a smaller size was required at one‐month follow‐up. Subjectively, the FemAssist device was more comfortable although it required a greater degree of user skill for appropriate insertion.
Several quality of life measures were taken and both the King's Health Questionnaire and SF‐36 were relevant to this review. There was no significant difference between the two devices on any of the domains of the King's Health Questionnaire:
general health (MD ‐1.00, 95% CI ‐12.64 to 10.64 Analysis 6.7.1),
incontinence impact (MD ‐6.90, 95% CI ‐15.55 to 1.75 Analysis 6.7.2),
role limitation (MD ‐7.40, 95% CI ‐16.68 to 1.88 Analysis 6.7.3),
sleep energy disturbance (MD 5.20, 95% CI ‐5.04 to 15.44 Analysis 6.7.4),
personal limitation (MD ‐1.00, 95% CI ‐12.64 to 10.64 Analysis 6.7.5),
social limitation (MD 1.10, 95% CI ‐9.56 to 11.76 Analysis 6.7.6), or
severity measure (MD 1.90, 95% CI ‐6.94 to 10.74 Analysis 6.7.7).
6.7. Analysis.
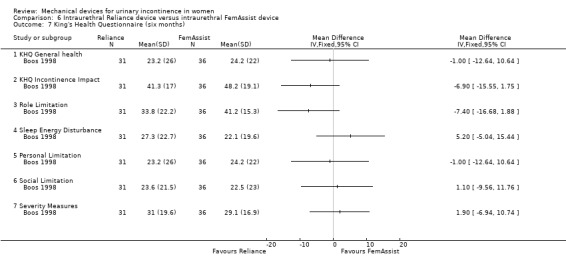
Comparison 6 Intraurethral Reliance device versus intraurethral FemAssist device, Outcome 7 King's Health Questionnaire (six months).
The domains of the SF‐36 quality of life questionnaire showed that the FemAssist device improved quality of life significantly more than the Reliance device on two domains:
mental health (MD ‐2.60, 95% CI ‐4.80 to ‐0.40 Analysis 6.8.5) and
energy/vitality (MD ‐23.60, 95% CI ‐27.68 to ‐19.52 Analysis 6.8.6).
6.8. Analysis.
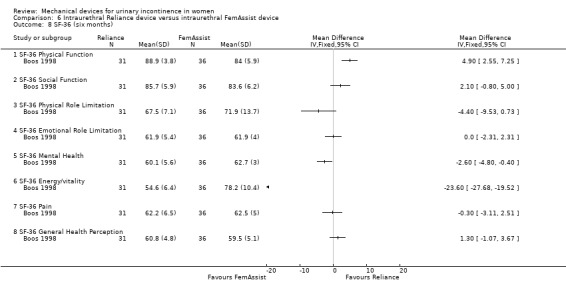
Comparison 6 Intraurethral Reliance device versus intraurethral FemAssist device, Outcome 8 SF‐36 (six months).
In one domain (physical function), Reliance improved quality of life significantly more than FemAssist (MD 4.90, 95% CI 2.55 to 7.25 Analysis 6.8.1).
In the remaining domains there was no significant difference between the two devices:
social function (MD 2.10, 95% CI ‐0.80 to 5.00 Analysis 6.8.2),
physical role limitation (MD ‐4.40, 95% CI ‐9.53 to 0.73 Analysis 6.8.3),
emotional role limitation (MD 0.00, 95% CI ‐2.31 to 2.31 Analysis 6.8.4),
pain (MD ‐0.30, 95% CI ‐3.11 to 2.51 Analysis 6.8.7), or
general health perception (MD 1.30, 95% CI ‐1.07 to 3.67 Analysis 6.8.8).
No data were available for other pre‐specified outcomes.
Comparison 7: intravaginal pessary versus behavioural therapy (PFMT) alone (Richter 2010)
In this comparison and Comparison 8, data were given for outcomes at three and 12 months.
Using an intention to treat analysis, after three months fewer women (59/149, 40%) reported continence as being much or very much better with the pessary compared with those using behavioural therapy (PFMT; 72/146, 49%) on the Patient Global Impression of Improvement rating, although this was not statistically significant (RR 0.80, 95% CI 0.62 to 1.04, Analysis 7.1.1). At 12 months the difference between the groups was less: 47/149 (32%) reported their continence as being much or very much better with the pessary compared with 48/146 (33%) with behavioural therapy (RR 0.96. 95% CI 0.69 to 1.34, Analysis 7.1.4).
7.1. Analysis.
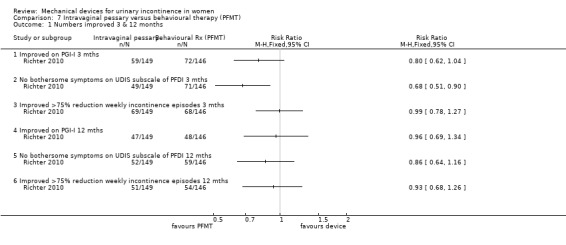
Comparison 7 Intravaginal pessary versus behavioural therapy (PFMT), Outcome 1 Numbers improved 3 & 12 months.
Women were asked about bothersome symptoms using the Urinary Distress Inventory sub‐scale (UDIS) of the Pelvic Floor Distress Inventory (PFDI). At three months the data favoured the PFMT‐only group, withno bothersome symptoms of stress incontinence in 49/149 (33%) of the pessary group versus 71/146 (49%) of the behavioural group which was statistically significant (RR 0.68, 95% CI 0.51 to 0.90, p=0.007, Analysis 7.1.2). This difference narrowed at 12 months to 35% and 40% respectively (RR 0.86, 95% CI 0.64 to 1.16, Analysis 7.1.5).
There was little difference between the groups in incontinence episodes according to bladder diaries; more than 75% reduction in weekly incontinence episodes in 69 (46%) of the pessary group and 68 (47%) of the behavioural group at three months (RR 0.99, 95% CI 0.78 to 1.27, Analysis 7.1.3); and 34% versus 37% respectively at 12 months (RR 0.96, 99% CI 0.78 to 1.27, Analysis 7.1.6). Neither of these results were significant but the confidence intervals were wide.
Another outcome, not originally listed but judged to be important during the update of the review was patient satisfaction: 94/146 (64%) of the pessary group and 110/149 (74%) of the behavioural group were satisfied with their treatment at three months (RR 0.87, 95% CI 0.75 to 1.02, Analysis 7.2.1). By 12 months, this had reduced to 75/146 (51%) and 79/149 (53%) being satisfied (RR 0.97, 95% CI 0.78 to 1.21, Analysis 7.2.2), with wide confidence intervals; neither of these analyses were statistically significant.
7.2. Analysis.
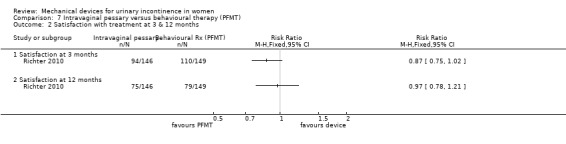
Comparison 7 Intravaginal pessary versus behavioural therapy (PFMT), Outcome 2 Satisfaction with treatment at 3 & 12 months.
Withdrawal after three months due to failure/lack of efficacy/dissatisfaction was 26% (39/149) in the pessary group and 15% (22/146) in the behavioural group (RR 1.74, 95% CI 1.09 to 2.78, Analysis 7.3.1) By 12 months this had increased to 36% (53/149) and 32% (47/146) respectively (RR 1.10, 95% CI 0.80 to 1.52, Analysis 7.3.2).
7.3. Analysis.
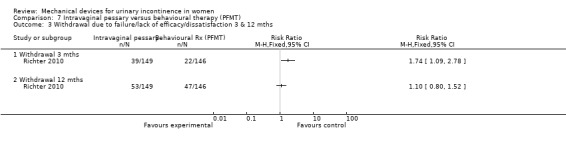
Comparison 7 Intravaginal pessary versus behavioural therapy (PFMT), Outcome 3 Withdrawal due to failure/lack of efficacy/dissatisfaction 3 & 12 mths.
Kenton 2012 reported the bother and impact of incontinence on intravaginal pessary versus behavioural therapy alone for Richter 2010. There was no significant improvement on a range of measures including the urinary impact questionnaire (UIQ), urinary distress inventory (UDI) or urinary incontinence diagnosis (QUID stress and QUID urge) scores at three months (MD 0.70, 95% CI ‐9.46 to 10.86, Analysis 7.4; MD ‐3.20, 95% CI ‐11.42 to 5.02, Analysis 7.5; MD ‐0.20, 95% CI ‐1.35 to 0.95, Analysis 7.6; MD 0.30, 95% CI ‐0.68 to 1.28, Analysis 7.7 respectively).
7.4. Analysis.
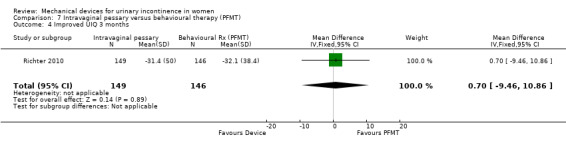
Comparison 7 Intravaginal pessary versus behavioural therapy (PFMT), Outcome 4 Improved UIQ 3 months.
7.5. Analysis.
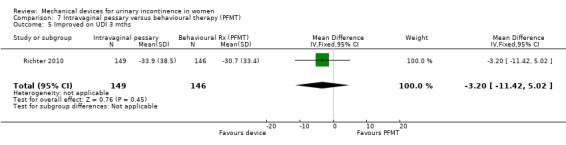
Comparison 7 Intravaginal pessary versus behavioural therapy (PFMT), Outcome 5 Improved on UDI 3 mths.
7.6. Analysis.
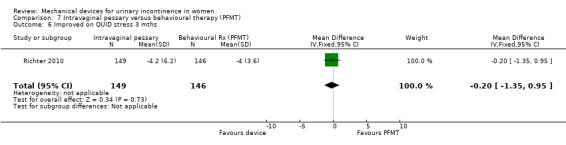
Comparison 7 Intravaginal pessary versus behavioural therapy (PFMT), Outcome 6 Improved on QUID stress 3 mths.
7.7. Analysis.
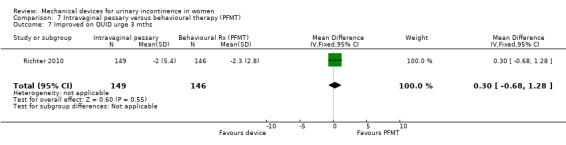
Comparison 7 Intravaginal pessary versus behavioural therapy (PFMT), Outcome 7 Improved on QUID urge 3 mths.
Comparison 8: intravaginal pessary versus combined treatment (pessary + PFMT) (Richter 2010)
One trial (Richter 2010) compared an intravaginal pessary alone with combined treatment (an intravaginal pessary plus PFMT).
In this comparison, results are given at three and 12 months. However, after the eight week treatment period women in the combined group could continue in the trial while using only one of the therapies (for example behavioural therapy alone) making the figures at 12 months in this group a less accurate reflection of the intervention. No data were available regarding numbers of women who may have dropped one element of therapy. Using an intention to treat analysis after three months, fewer women (59/149, 40%) reported their continence as being much or very much better with the pessary alone compared to combined treatment (pessary and PFMT; 80/150 (53%) on the Patient Global Impression of Improvement rating (RR 0.74, 95% CI 0.58 to 0.95, p= 0.02, Analysis 8.1.1). By 12 months, the proportions were similar (47/149 (32%) versus 49/150 (33%) respectively;RR 0.97, 95% CI 0.69 to 1.34, Analysis 8.1.4).
8.1. Analysis.
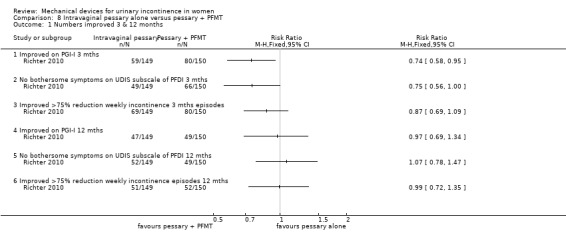
Comparison 8 Intravaginal pessary alone versus pessary + PFMT, Outcome 1 Numbers improved 3 & 12 months.
At three months no bothersome symptoms of stress incontinence were reported on the UDIS subscale of the PFDI by 49/149 (33%) of the pessary group and 66/150 (44%) of the combined group. This difference was not statistically significant (RR 0.75, 95% CI 0.56 to 1.00, Analysis 8.1.2), nor at 12 months: 52/149 (35%) and 49/150 (33%) respectively (RR 1.07, 95% CI 0.78 to1.47, Analysis 8.1.5).
When success was judged as a greater than 75% reduction in weekly incontinence episodes, 69/149 (46%) of the pessary group and 80/150 (53%) of the combined group (RR 0.87 (95% CI 0.69 to 1.09, Analysis 8.1.3) were improved at three months . This reduced to 51/149 (43%) and 52/150 (35%) respectively at 12 months (RR 0.99, 95% CI 0.72 to 1.35, Analysis 8.1.6).
The outcome patient satisfaction was not originally listed but judged to be important during the update of the review. At three months 94/146 (63%) of the pessary group and 118/150 (79%) of the combined group were satisfied with their treatment (RR 0.82, 95% CI 0.71 to 0.95, Analysis 8.2.1) which was statistically significant, however the difference was not significant at 12 months when satisfaction reduced to 75/146 (50%) and 81/150 (54%) respectively (RR 0.95, 95% CI 0.77 to 1.18, Analysis 8.2.2).
8.2. Analysis.
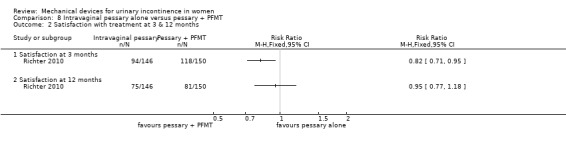
Comparison 8 Intravaginal pessary alone versus pessary + PFMT, Outcome 2 Satisfaction with treatment at 3 & 12 months.
Withdrawal after three months due to failure/lack of efficacy/dissatisfaction was 26% (39/149) in the pessary group and 12% (18/150) in the combined group (RR 2.18, 95% CI 1.31 to 3.63, Analysis 8.3.1) By 12 months this had increased to 36% (53/149) and 26% (39/150) respectively (RR 1.37, 95% CI 0.97 to 1.93, Analysis 8.3.2).
8.3. Analysis.
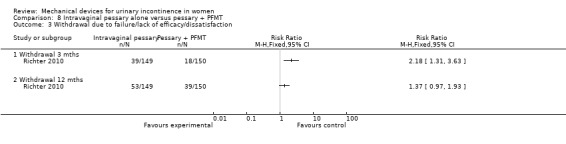
Comparison 8 Intravaginal pessary alone versus pessary + PFMT, Outcome 3 Withdrawal due to failure/lack of efficacy/dissatisfaction.
Comparison 9: combined treatment (pessary + PFMT) versus PFMT alone (Richter 2010)
In this comparison, results are given at three and 12 months. However, after the eight week treatment period women in the combined group could continue in the trial while using only one of the therapies (for example PFMT alone) making the figures at 12 months in this group a less accurate reflection of the intervention. Using an intention to treat analysis, after three months 80/150 (53%) of those using combined treatment (pessary and behavioural therapy) reported their continence as being much or very much better on the Patient Global Impression of Improvement rating, compared with 72/146 (49%) using behavioural therapy ‐ a difference that was not statistically significant (RR 1.08, 95% CI 0.87 to 1.35, p = 0.49, Analysis 9.1.1). This reduced to 49/150 (33%) and 48/146 (33%) respectively at 12 months (RR 0.99, 95% CI 0.72 to 1.38, Analysis 9.1.4).
9.1. Analysis.
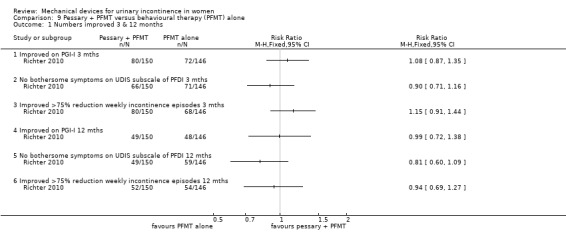
Comparison 9 Pessary + PFMT versus behavioural therapy (PFMT) alone, Outcome 1 Numbers improved 3 & 12 months.
At three months, no bothersome symptoms of stress incontinence were reported on the UDIS subscale of the PFDI by 66/150 (44%) of the combined group and 71/146 (49%) of the behavioural group. This difference was not statistically significant (RR 0.90, 95% CI 0.71 to 1.16, Analysis 9.1.2). At 12 months this reduced to 49/150 (33%) and 59/146 (40%) respectively (RR 0.81, 95% CI 0.60 to 1.09, Analysis 9.1.5).
There was greater than 75% reduction in weekly incontinence episodes in 80/150 (53%) of the combined group and 68/146 (47%) of the behavioural group at three months (RR 1.15, 95% CI 0.91 to 1.44, Analysis 9.1.3). This reduced to 52/150 (35%) and 54/146 (37%) respectively at 12 months (RR 0.94, 95% CI 0.69 to 1.27, Analysis 9.1.6); none of these comparisons had statistically significant results.
One outcome, not originally listed but judged to be important during the update of the review was patient satisfaction. 94/146 (64%) of the combined group patients, and 110/149 (74%) of the behavioural group were satisfied with their treatment (RR 0.87, 95% CI 0.75 to 1.02, Analysis 9.2.1) which was not a significant difference at three months, nor at 12 months when satisfaction reduced to 75/146 (51%) and 79/149 (54%) respectively (RR 0.97, 95% CI 0.78 to 1.21, Analysis 9.2.2).
9.2. Analysis.
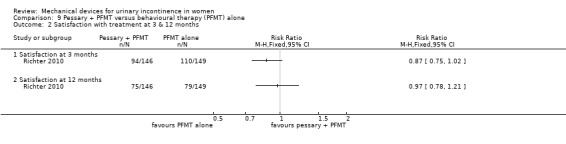
Comparison 9 Pessary + PFMT versus behavioural therapy (PFMT) alone, Outcome 2 Satisfaction with treatment at 3 & 12 months.
Withdrawal after three months due to failure/lack of efficacy/dissatisfaction was 12% (18/150) in the combined group and 15% in the behavioural group (22/146;RR 0.80, 95% CI 0.45 to 1.42, Analysis 9.3.1). By 12 months this had increased to 26% (39/150) and 32% (47/146) respectively (RR 0.81, 95% CI 0.56 to 1.16, Analysis 9.3.2).
9.3. Analysis.
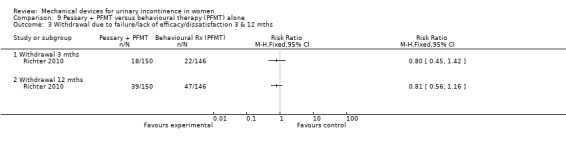
Comparison 9 Pessary + PFMT versus behavioural therapy (PFMT) alone, Outcome 3 Withdrawal due to failure/lack of efficacy/dissatisfaction 3 & 12 mths.
Discussion
The ideal mechanical device is one which can adequately control urine leakage, is easy to insert, has few adverse effects and is of low cost; the use of the device should improve health status and quality of life for users.
Two main types of mechanical device were identified: those that are placed inside the vagina; and those that are used to occlude or plug the urethra. It is possible that intravaginal tampons may perform as well as more formal mechanical devices. They are widely available and familiar to women. They are also without significant adverse effects and are the least expensive option. It would be useful to conduct a large well‐designed trial comparing the use of intravaginal tampons with no treatment in women with stress urinary incontinence. As one of the management strategies for incontinence, especially in the elderly, is use of collection devices such as pads or catheters, a useful comparison might also be mechanical device use versus use of a collecting device.
In summary, from the trials included in this review the value of mechanical devices in the management of urinary incontinence remains uncertain. Six of the eight included studies were of a very small sample size, not large enough to reliably detect a difference. There is a lack of evidence to suggest whether the use of mechanical devices is better than no treatment and a large well‐conducted trial is required for clarification. There was insufficient evidence to favour one device over another and the evidence to compare mechanical devices with behavioural therapy remains inconclusive.
Summary of main results
Is a mechanical device better than no treatment or a combined treatment? Of the eight trials that addressed this question, all reported data (suitable for analysis) for the outcomes of interest. None had a high risk of bias.
Unfortunately, we found only very limited evidence from controlled trials on which to judge whether or not any device was effective. One trial included in this update compared a mechanical device with behavioural therapy (Richter 2010). This trial, together with the additional trial identified for this update (Cornu 2012), and a newly released electronic PhD thesis (Boos 1998) attempted to address some of this review's broader outcomes, namely women's perception of their health status related to incontinence, and quality of life. It was interesting to note that the addition of behavioural therapy (PFMT) to the use of an incontinence device (pessary) does not appear to result in improved outcomes, apart from small but statistically significant effects for satisfaction, withdrawal and perceived improvement at three months. However, this result should be interpreted with caution as participants receiving behavioural therapy had four clinic visits whereas most of those in the device‐only group had only one clinic visit. Higher levels of clinician contact could impact on participant perceptions of satisfaction and improvement. To establish definitively whether combining PFMT with a mechanical device provides patient benefits, the clinician contact must be controlled for, and a more robust range of outcome measures would be required.
Overall completeness and applicability of evidence
Despite the addition of extra data from one trial and addition of a new trial, the review remains underpowered to assess the effectiveness of mechanical devices for female urinary incontinence.
Although there were several types of mechanical devices used in these studies, including different versions of each device, few are commercially available. Whilst it is not immediately obvious why so many devices seem to have been developed but never withstood the test of time, we can speculate that the lack of good quality supportive clinical trial data was a contributory factor. Another reason might be that women do not like using a mechanical device and would prefer to use the other available options such as PFMT or surgery.
Quality of the evidence
The quality of the evidence was generally fair. However, some trial reports were unclear regarding randomisation, allocation concealment methods and methods of blinding participants and outcome assessors.
The review was limited by few trials that were generally of small size (hence giving wide confidence intervals around estimated differences), each addressing a different comparison and with short follow‐up. Assessment was generally confined to objective measures of urine loss using a weighed pad tests. However, this latest update begins to address other parameters of a device's value to women in the form of quality of life measures, with cost effectiveness yet to be considered. The data were too few and the results too statistically imprecise to provide useful assessment of whether any device is better than no device and whether one device is better than another.
Nevertheless, we recognise that the conduct of trials involving interventions such as mechanical devices for incontinence management is fraught with difficulties. Clearly, one of the difficulties faced by trialists remains the problem of how to deal with the issue of blinding and the definition of an appropriate 'placebo' arm. Patient blinding may not be entirely feasible and blinding of care givers would not be possible in this type of trial. In one trial, a ring diaphragm was inserted into the vagina and then immediately removed when women were allocated to no treatment (Nygaard 1995). Thirteen of the 18 participants correctly identified that no mechanical device was in place.
Potential biases in the review process
We attempted to minimise bias by the following; two review authors separately assessed eligibility for inclusionand risk of bias and extracted data. Data entry into RevMan was undertaken by one author and checked by the other. These steps involve subjective assessment and may carry some risk of bias.
Agreements and disagreements with other studies or reviews
We are unaware of another systematic review on this topic. Results from one trial did not favour pelvic floor exercises in conjunction with a mechanical device, although PFMT on its own has been found to be effective in a recent systematic review (Dumoulin 2014).
Authors' conclusions
Implications for practice.
The available evidence is insufficient to clarify the current place of mechanical devices in the management of female urinary incontinence.
Implications for research.
Three broad issues need to be addressed through new research. The first is whether a device is better for selected women than not using the device. Such a trial might be particularly appropriate amongst women where incontinence is not sufficient to justify surgery. The second issue is whether one type of device is more helpful than another. It is striking how many commercially developed devices have come and gone and an appropriate comparator for a new device might be intravaginal tampons. Such a trial might be mounted amongst women who would normally have surgery but who choose not to or are unfit for surgery. The third issue is the extent to which mechanical devices are an alternative to current standard approaches to management, such as PFMT, periurethral injection therapy or minimally invasive sling surgery.
Evaluations should address all aspects of the devices that are important to women and to those providing care: do they satisfactorily control urine leakage; are they sufficiently easy to use to be practical; do they have any unwanted effects; what are their costs to women or the local health service; are they acceptable to women; and do they make women feel better? Including well‐validated quality of life measures in future studies would add to the knowledge of what is important for women who are incontinent.
As discussed in the review, there are challenges to designing rigorous trials of mechanical devices. Standard parallel‐group designs have advantages, particularly for assessing longer‐term effects. However, cross‐over designs may be more practical, acceptable and statistically 'efficient'.
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2014 | New search has been performed | Literature search updated. Data from one previous study updated (Richter 2010) and one new study included (Cornu 2012). |
| 17 December 2014 | New citation required but conclusions have not changed | Literature search updated. Data from one previous study updated (Richter 2010) and one new study included (Cornu 2012). |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 6 July 2011 | Amended | Typographical correction in Abstract |
| 13 June 2011 | New citation required but conclusions have not changed | new review authors |
| 5 March 2011 | New search has been performed | Review updated |
| 9 October 2008 | Amended | Converted to new review format. |
| 18 March 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
For this second update the authors appreciate the help provided by Sheila Wallace.
We acknowledge the work done by the previous review authors for earlier versions of this review (Frazer 1999; Shaikh 2006).
For the original review, the protocol was prepared with minor amendments from the original protocol developed by Dr Malcolm Frazer, Dr G. Lose, Dr E. Kozman, Dr K. Boos and Dr D. Tincello. The review authors are also grateful for the help provided by June Cody, Sheila Wallace and Adrian Grant.
Appendices
Appendix 1. Search strategies for literature searches
The Incontinence Group Specialised Register was searched using the Group's own keyword system, with the following search terms:
({TOPIC.URINE.INCON*}) AND ({DESIGN.CCT*} OR {DESIGN.RCT*}) AND ({INTVENT.MECH.DEVICES*}) (All searches were of the keyword field of Reference Manager 2012). The date of the most recent search of the Register for this review was: 21 August 2014.
Extra searches for this review were run by the Trials Search Co‐ordinator, details are given below:
EMBASE on OvidSP (January 1947 to 2014 Week 34 inclusive). Search date: 26 August 2014. The search strategy used is given below.
1. Randomized Controlled Trial/
2. controlled study/
3. clinical study/
4. major clinical study/
5. prospective study/
6. meta analysis/
7. exp clinical trial/
8. randomization/
9. crossover procedure/ or double blind procedure/ or parallel design/ or single blind procedure/
10. Placebo/
11. latin square design/
12. exp comparative study/
13. follow up/
14. pilot study/
15. family study/ or feasibility study/ or pilot study/ or study/
16. placebo$.tw.
17. random$.tw.
18. (clin$ adj25 trial$).tw.
19. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw.
20. factorial.tw.
21. crossover.tw.
22. latin square.tw.
23. (balance$ adj2 block$).tw.
24. factorial design/
25. parallel design/
26. triple blind procedure/
27. community trial/
28. intervention study/
29. experimental study/
30. prevention study/
31. quasi experimental study/
32. or/1‐31
33. (nonhuman not human).sh.
34. 32 not 33
35. incontinence/ or mixed incontinence/ or stress incontinence/ or urge incontinence/ or urine incontinence/
36. continence/
37. overactive bladder/
38. micturition disorder/ or lower urinary tract symptom/ or pollakisuria/
39. urinary dysfunction/ or bladder instability/ or detrusor dyssynergia/ or neurogenic bladder/ or urinary urgency/ or urine extravasation/
40. (incontinen$ or continen$).tw.
41. ((bladder or detrusor or vesic$) adj5 (instab$ or stab$ or unstab* or irritab$ or hyperreflexi$ or dys?ynerg$ or dyskinesi$ or irritat$)).tw.
42. (urin$ adj2 leak$).tw.
43. ((bladder or detrusor or vesic$) adj2 (hyper$ or overactiv$)).tw.
44. (bladder$ adj2 (neuropath$ or neurogen* or neurolog$)).tw.
45. (nervous adj pollakisur$).tw.
46. 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45
47. 34 and 46
48. female contraceptive device/ or uterine cervix cap/ or vagina pessary/
49. incontinence dish pessary/
50. mechanical device$.tw.
51. ((pubovagin$ or vagina$ or intravagina$) adj25 (pessar* or device$ or ring$ or plug$ or prosthes$ or diaphragm$ or cap or caps or sponge$ or tampon$)).tw.
52. (continen* adj25 device$).tw.
53. (continen* adj25 ring$).tw.
54. (bladder adj25 support$ adj25 (device$ or prosthes$)).tw.
55. ((urethra$ or intraurethra$) adj25 (insert or inserts)).tw.
56. (conveen or contrelle or reliance or femassist or introl).tw.
57. ((urethra$ or intraurethra$) adj25 (device$ or plug$ or prosthes$)).tw.
58. or/48‐57
59. 58 and 47
CINAHL on EBSCO (January 1982 to 25 August 2014 inclusive). Search date: 26 August 2014. The search strategy used is given below.
| S72 | S60 and S71 |
| S71 | S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S53 or S54 or S61 or S62 or S63 or S64 or S65 or S66 or S67 or S68 or S69 or S70 |
| S70 | AB urethra* N25 device* or urethra* N25 plug* or urethra N25 prosthes* or intraurethra* N25 device* or intraurethra* N25 plug* or intraurethra* N25 prosthes* |
| S69 | TI urethra* N25 device* or urethra* N25 plug* or urethra N25 prosthes* or intraurethra* N25 device* or intraurethra* N25 plug* or intraurethra* N25 prosthes* |
| S68 | AB urethra* N25 insert or urethra* N25 inserts or intraurethra* N25 insert or intraurethra* N25 inserts |
| S67 | TI urethra* N25 insert or urethra* N25 inserts or intraurethra* N25 insert or intraurethra* N25 inserts |
| S66 | AB bladder* N25 device* or bladder* N25 prosthes* |
| S65 | TI bladder* N25 device* or bladder* N25 prosthes* |
| S64 | AB intravagina* N25 pessar* or intravagina* N25 device* or intravagina* N25 ring* or intravagina* N25 plug* or intravagina* N25 prosthes* or intravagina* N25 diaphgragm* or intravagina* N25 cap or intravagina* N25 caps or intravagina* N25 sponge* or intravagina* N25 tampon* |
| S63 | TI intravagina* N25 pessar* or intravagina* N25 device* or intravagina* N25 ring* or intravagina* N25 plug* or intravagina* N25 prosthes* or intravagina* N25 diaphgragm* or intravagina* N25 cap or intravagina* N25 caps or intravagina* N25 sponge* or intravagina* N25 tampon* |
| S62 | AB vagina* N25 pessar* or vagina* N25 device* or vagina* N25 ring* or vagina* N25 plug* or vagina* N25 prosthes* or vagina* N25 diaphgragm* or vagina* N25 cap or vagina* N25 caps or vagina* N25 sponge* or vagina* N25 tampon* |
| S61 | TI vagina* N25 pessar* or vagina* N25 device* or vagina* N25 ring* or vagina* N25 plug* or vagina* N25 prosthes* or vagina* N25 diaphgragm* or vagina* N25 cap or vagina* N25 caps or vagina* N25 sponge* or vagina* N25 tampon* |
| S60 | S52 and S59 |
| S59 | S22 or S23 or S24 or S25 or S26 or S27 or S46 OR S47 OR S48 OR S49 OR S50 OR S51 or S28 or S29 or S30 or S31 or S55 or S56 or S57 or S58 |
| S58 | AB vesic* N5 instab* or vesic* N5 stab* or vesic* N5 unstab* or vesic* N5 irritab* or vesic* N5 hyperreflexi* or vesic* N5 dys?ynerg* or vesic* N5 dyskinesi* or vesic* N5 irritat* |
| S57 | TI vesic* N5 instab* or vesic* N5 stab* or vesic* N5 unstab* or vesic* N5 irritab* or vesic* N5 hyperreflexi* or vesic* N5 dys?ynerg* or vesic* N5 dyskinesi* or vesic* N5 irritat* |
| S56 | AB detrusor N5 instab* or detrusor N5 stab* or detrusor N5 unstab* or detrusor N5 irritab* or detrusor N5 hyperreflexi* or detrusor N5 dys?ynerg* or detrusor N5 dyskinesi* or detrusor N5 irritat* |
| S55 | TI detrusor N5 instab* or detrusor N5 stab* or detrusor N5 unstab* or detrusor N5 irritab* or detrusor N5 hyperreflexi* or detrusor N5 dys?ynerg* or detrusor N5 dyskinesi* or detrusor N5 irritat* |
| S54 | AB pubovagin* N25 pessar* or pubovagin* N25 device* or pubovagin* N25 ring* or pubovagin* N25 plug* or pubovagin* N25 prosthes* or pubovagin* N25 diaphgragm* or pubovagin* N25 cap or pubovagin* N25 caps or pubovagin* N25 sponge* or pubovagin* N25 tampon* |
| S53 | TI pubovagin* N25 pessar* or pubovagin* N25 device* or pubovagin* N25 ring* or pubovagin* N25 plug* or pubovagin* N25 prosthes* or pubovagin* N25 diaphgragm* or pubovagin* N25 cap or pubovagin* N25 caps or pubovagin* N25 sponge* or pubovagin* N25 tampon* |
| S52 | (S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S44 or S45) |
| S51 | AB bladder* N2 neuropath* or bladder* N2 neurogen* or bladder* N2 neurolog* |
| S50 | TI bladder* N2 neuropath* or bladder* N2 neurogen* or bladder* N2 neurolog* |
| S49 | AB bladder* N2 hyper* or bladder* N2 overactiv* or detrusor N2 hyper* or detrusor N2 overactiv* or vesic* N2 hyper* or vesic* N2 overactiv* |
| S48 | TI bladder* N2 hyper* or bladder* N2 overactiv* or detrusor N2 hyper* or detrusor N2 overactiv* or vesic* N2 hyper* or vesic* N2 overactiv* |
| S47 | AB bladder* N5 instab* or bladder* N5 stab* or bladder* N5 unstab* or bladder* N5 irritab* or bladder* N5 hyperreflexi* or bladder* N5 dys?ynerg* or bladder* N5 dyskinesi* or bladder* N5 irritat* |
| S46 | TI bladder* N5 instab* or bladder* N5 stab* or bladder* N5 unstab* or bladder* N5 irritab* or bladder* N5 hyperreflexi* or bladder* N5 dys?ynerg* or bladder* N5 dyskinesi* or bladder* N5 irritat* |
| S45 | AB singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* |
| S44 | TI singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* |
| S43 | AB conveen or contrelle or reliance or femassist or introl |
| S42 | TI conveen or contrelle or reliance or femassist or introl |
| S41 | AB continen* N25 ring* |
| S40 | TI continen* N25 ring* |
| S39 | AB continen* N25 device* |
| S38 | TI continen* N25 device* |
| S37 | AB mechanical device* |
| S36 | TI mechanical device* |
| S35 | (MH "Pessaries") |
| S34 | (MH "Tampons") |
| S33 | (MH "Incontinence Aids+") |
| S32 | (MH "Contraceptive Devices+") |
| S31 | TI urin* N2 leak* |
| S30 | AB urin* N2 leak* |
| S29 | AB nervous N2 pollakisur* |
| S28 | TI nervous N2 pollakisur* |
| S27 | AB incontinen* or continen* |
| S26 | TI incontinen* or continen* |
| S25 | (MH "Total Incontinence (NANDA)") or (MH "Altered Urinary Elimination (NANDA)+") |
| S24 | (MH "Overactive Bladder") or (MH "Bladder, Neurogenic") |
| S23 | (MH "Functional Incontinence (NANDA)") or (MH "Incontinence") |
| S22 | (MH "Urinary Incontinence+") or (MH "Functional Urinary Incontinence (Saba CCC)") or (MH "Reflex Urinary Incontinence (Saba CCC)") or (MH "Stress Urinary Incontinence (Saba CCC)") or (MH "Total Urinary Incontinence (Saba CCC)") or (MH "Urge Urinary Incontinence (Saba CCC)") or (MH "Urinary Incontinence and Frequency Comfort Questionnaire") or (MH "Urinary Incontinence Care (Saba CCC)") or (MH "Urinary Incontinence Care (Iowa NIC)") or (MH "Urinary Elimination Alteration (Saba CCC)") or (MH "Urinary Elimination Component (Saba CCC)") or (MH "Urinary Elimination Management (Iowa NIC)") or (MH "Urinary Elimination: (Iowa NOC)")(MH "Urinary Incontinence+") or (MH "Functional Urinary Incontinence (Saba CCC)") or (MH "Reflex Urinary Incontinence (Saba CCC)") or (MH "Stress Urinary Incontinence (Saba CCC)") or (MH "Total Urinary Incontinence (Saba CCC)") or (MH "Urge Urinary Incontinence (Saba CCC)") or (MH "Urinary Incontinence and Frequency Comfort Questionnaire") or (MH "Urinary Incontinence Care (Saba CCC)") or (MH "Urinary Incontinence Care (Iowa NIC)") or (MH "Urinary Elimination Alteration (Saba CCC)") or (MH "Urin ...Show Less |
| S21 | (MH "Comparative Studies") |
| S20 | (MH "Clinical Research+") |
| S19 | (MH "Static Group Comparison") |
| S18 | (MH "Quantitative Studies") |
| S17 | (MH "Crossover Design") or (MH "Solomon Four‐Group Design") |
| S16 | (MH "Factorial Design") |
| S15 | (MH "Community Trials") |
| S14 | (MH "Random Sample") |
| S13 | TI balance* N2 block* or AB balance* N2 block* |
| S12 | TI "latin square" or AB "latin square" |
| S11 | TI factorial or AB factorial |
| S10 | TI clin* N25 trial* or AB clin* N25 trial* |
| S9 | (MH "Study Design") |
| S8 | (AB random*) OR (TI random*) |
| S7 | (AB placebo*) OR (TI placebo*) |
| S6 | (MH "Placebos") |
| S5 | (PT Clinical Trial) OR (PT "randomized controlled trial") |
| S4 | (MH "Clinical Trials+") |
| S3 | MH (random assignment) OR (crossover design) |
| S2 | cross‐over |
| S1 | crossover |
Data and analyses
Comparison 1. Intravaginal mechanical device versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formal pad weighing tests after exercise(grams) | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 Tampon versus no treatment | 1 | Mean Difference (Fixed, 95% CI) | ‐14.3 [‐38.41, 9.81] | |
| 1.2 Pessary versus no treatment | 2 | Mean Difference (Fixed, 95% CI) | ‐6.55 [‐11.20, ‐1.90] | |
| 2 Number with discomfort or pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Hodge pessary versus no treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Tampax tampon versus no treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Variation of IEF per week from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 24 hour pad test change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Urinary Symptom Profile questionnaire SUI subscore | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Urinary Symptom Profile questionnaire OAB subscore | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Urinary Symptom Profile questionnaire dysuria subscore | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 CONTILIFE quality of life questionnaire | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Intravaginal Tampax tampon versus Hodge pessary.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formal pad weighing tests | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 Number with discomfort or pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Intravaginal Contrelle Continence Tampon (CCT) device versus intravaginal Conveen Continence Guard (CCG) device.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 24h pad test | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
Comparison 5. Intraurethral new expandable tip (NEAT) device versus Reliance device.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Numbers improved (pad test) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 6. Intraurethral Reliance device versus intraurethral FemAssist device.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Numbers subjectively dry (six months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Numbers subjectively improved (six months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Pad weighing test (six months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Pad changes over 5 days | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Number of patients with UTI (six months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Incontinence episodes per day (six months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 King's Health Questionnaire (six months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 KHQ General health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 KHQ Incontinence Impact | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Role Limitation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Sleep Energy Disturbance | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 Personal Limitation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 Social Limitation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.7 Severity Measures | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 SF‐36 (six months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 SF‐36 Physical Function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 SF‐36 Social Function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 SF‐36 Physical Role Limitation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 SF‐36 Emotional Role Limitation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 SF‐36 Mental Health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.6 SF‐36 Energy/vitality | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.7 SF‐36 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.8 SF‐36 General Health Perception | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Intravaginal pessary versus behavioural therapy (PFMT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Numbers improved 3 & 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Improved on PGI‐I 3 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 No bothersome symptoms on UDIS subscale of PFDI 3 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Improved >75% reduction weekly incontinence episodes 3 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Improved on PGI‐I 12 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 No bothersome symptoms on UDIS subscale of PFDI 12 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Improved >75% reduction weekly incontinence episodes 12 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Satisfaction with treatment at 3 & 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Satisfaction at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Satisfaction at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Withdrawal due to failure/lack of efficacy/dissatisfaction 3 & 12 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Withdrawal 3 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Withdrawal 12 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Improved UIQ 3 months | 1 | 295 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐9.46, 10.86] |
| 5 Improved on UDI 3 mths | 1 | 295 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐11.42, 5.02] |
| 6 Improved on QUID stress 3 mths | 1 | 295 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.35, 0.95] |
| 7 Improved on QUID urge 3 mths | 1 | 295 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.68, 1.28] |
Comparison 8. Intravaginal pessary alone versus pessary + PFMT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Numbers improved 3 & 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Improved on PGI‐I 3 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 No bothersome symptoms on UDIS subscale of PFDI 3 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Improved >75% reduction weekly incontinence 3 mths episodes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Improved on PGI‐I 12 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 No bothersome symptoms on UDIS subscale of PFDI 12 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Improved >75% reduction weekly incontinence episodes 12 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Satisfaction with treatment at 3 & 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Satisfaction at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Satisfaction at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Withdrawal due to failure/lack of efficacy/dissatisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Withdrawal 3 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Withdrawal 12 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Pessary + PFMT versus behavioural therapy (PFMT) alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Numbers improved 3 & 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Improved on PGI‐I 3 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 No bothersome symptoms on UDIS subscale of PFDI 3 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Improved >75% reduction weekly incontinence episodes 3 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Improved on PGI‐I 12 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 No bothersome symptoms on UDIS subscale of PFDI 12 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Improved >75% reduction weekly incontinence episodes 12 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Satisfaction with treatment at 3 & 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Satisfaction at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Satisfaction at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Withdrawal due to failure/lack of efficacy/dissatisfaction 3 & 12 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Withdrawal 3 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Withdrawal 12 mths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boos 1998.
| Methods | Prospective randomised parallel‐group trial. | |
| Participants | N = 102. Withdrawal = 22. Urodynamically proven genuine stress or mixed incontinence. Mean age = 51.5 (range 28‐77). Inclusion: reasonable eyesight, mobility and manual dexterity. Exclusion: recurrent urinary tract infection (UTI), diabetes mellitus, pregnancy or undiagnosed haematuria. Single centre, Urogynaecology Unit, UK. | |
| Interventions | Use of different types of mechanical device for 3 months:
1. Use Reliance mechanical device (n = 49)
2. Use FemAssist mechanical device (n = 53) Reliance Urinary Control Device for women is a small tube inserted into the urethra using a syringe. The tip of the tube contains a balloon that is inflated against the urethra and blocks urine, with the aim of preventing leakage. FemSoft is a silicone tube insert surrounded by a liquid‐filled sleeve. When the tube is inserted into the urethra, the sleeve conforms to its shape and creates a seal at the bladder neck, with the aim of preventing leakage. Both devices can be inserted by the woman and must be discarded and replaced with new sterile devices on voiding.The Reliance applicator can be reused. |
|
| Outcomes | 1. Subjective assessment by patients (6 months)
2. Pad weighing test (6 months)
3. Urinary tract infection (6 months) 4. King's Health Questionnaire (6 months) 5. SF‐36 (6 months) |
|
| Notes | Available as an abstract and PhD thesis. FemAssist now known as FemSoft |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | '102 women... were randomised'. Women 'were selectively allocated to one or other of the devices by alternating the type of device for the next subject'. |
| Allocation concealment (selection bias) | High risk | '....were selectively allocated to one or other of the devices by alternating the type of device for the next subject'. |
| Blinding (performance bias and detection bias) All outcomes | High risk | 'The study was non‐blinded'. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Group 1: 3 lost to follow up, plus withdrawals due to 3 with inhibition and 4 with urethritis.
Group 2: 5 lost to follow up, plus 1 with urethritis and 6 with poor efficacy. ITT analysis used for subjective assessments of improvement. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported on, but the trial protocol not assessed. |
| Baseline comparability | Low risk | Age, parity, BMI, menopausal status, HRT use, severity of GSI, duration of GSI. |
| Timing of outcome assessment | Low risk | 3 months. |
| Trial sponsorship | Unclear risk | Not stated. |
Cornu 2012.
| Methods | Prospective randomised parallel‐group trial. | |
| Participants | N = 55. Withdrawal = 14.
Stress urinary incontinence (SUI) assessed by clinical examination (stress test) or mixed incontinence with predominant SUI.
Mean age = 58.7 (range 29‐88).
Inclusion: Aged 18 or more, SUI assessed by clinical examination (stress test) or mixed incontinence with predominant SUI (at least 4 incontinence episodes per week), post‐menopausal/contraception, no vaginal delivery in the past 2 months, no bladder/vaginal disease, no acute or recurrent urinary infection, no pelvic organ prolapse >stage II according to POPQ classification, no surgical intervention for SUI in the past 6 months, no drug treatment for UI in the last month, no pelvic floor muscle training underway. Exclusion: None. Multi‐centre, France. |
|
| Interventions | Use of intravaginal device (75NC007) for up to 24 hours a day for 14 days. 1. 75NC007 (n = 29) 2. wearing no mechanical device (n = 26) 75NC007 is made of thermoplastic elastomer supplied in two sizes: medium and small. Inserted into the vagina with or without an applicator. It automatically locates beneath the urethra and bladder, removed by pulling string on cylindrical part of device. The device can be inserted by the woman and must be discarded and replaced with a new device after 24 hours. |
|
| Outcomes | 1. Incontinence Episode Frequency (IEF) according to bladder diaries.
2. Urinary Symptom Profile score 3. 24 hour pad weighing test 4. CONTILIFE questionnaire |
|
| Notes | Phase two data only analysed. Change from baseline scores were used for all measures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation was centralised by the data coordinating centre. |
| Allocation concealment (selection bias) | Low risk | Computerised randomisation was centralised by the data coordinating centre. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14/55 dropouts (25%), ITT analysis performed. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported on, but the trial protocol not assessed. |
| Baseline comparability | Unclear risk | Age, pregnancies, vaginal deliveries, BMI, post‐menopausal, history of PFMT, previous surgery for SUI, pad usage, USP questionnaire SUI subgroup, dysuria subscore, leakage on 14 day diary. Differences in 24 hour pad test, hysterectomy, USP questionnaire OAB subscore were accounted for by using change from baseline. |
| Timing of outcome assessment | Low risk | on day 28 (end of phase 2). |
| Trial sponsorship | High risk | Study funded by B. Braun Medical SAS. Consultancy honoraria from B. Braun by one of the authors (S Mouly). |
Glavind 1997b.
| Methods | Prospective randomised cross‐over trial. | |
| Participants | N = 6. No withdrawal. Symptoms and urodynamically confirmed stress incontinence. Age range = 44‐68. Inclusion: Pad‐weighing test > 2g. Exclusion: Detrusor instability. Single centre, Department of Obstetric & Gynaecology, Denmark. | |
| Interventions | On 2 consecutive days, the patients performed half an hour of standardised exercise:
1. Without Ladycon intravaginal device (n = 6)
2. With Ladycon intravaginal device (n = 6) The Ladycon device is an intravaginal device composed of polyvinyl alcohol 6cm long and 3.1cm in diameter. It requires no insertion device and is soaked in warm water beforehand. It has a 'non‐wetting' string to aid removal. The device is no longer available. |
|
| Outcomes | 1. Pad weighing test 2. Subjective assessment by patients 3. Problems with insertion 4. Preference of device | |
| Notes | The study author was one of the authors of the original review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Patients were randomized'. |
| Allocation concealment (selection bias) | Low risk | 'Patients were randomized on day 1 with sealed envelopes to plus/minus or minus/plus the vaginal sponge'. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop outs reported. |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes reported on, but the trial protocol not assessed. |
| Baseline comparability | Low risk | Participants formed both intervention and control groups. |
| Timing of outcome assessment | Low risk | Following second aerobic exercise. |
| Trial sponsorship | Unclear risk | Not stated. |
Nielsen 1995.
| Methods | Prospective randomised crossover trial. | |
| Participants | N = 40. Withdrawal = 22, seven withdrew in phase one. Assumed urodynamically confirmed stress incontinence. Mean age = 51 (32‐47). Inclusion: Not listed. Exclusion: Not listed. Single centre, Department of Urology, Denmark. | |
| Interventions | Use of either type of urethral plug for 2 weeks, then cross‐over for another 2 weeks followed by preference plug for further 2 months:
1. 1‐sphere urethral plug (n = 20)
2. 2‐sphere urethral plug (n = 20) The urethral plug is composed on non‐toxic thermoplastic elastomer material with an oval meatal plate a soft stalk and 1 or 2 spheres 7mm in diameter along the stalk. Inside the stalk there is a semi‐rigid guide pin which is removed after insertion. The plug is removed and discarded on voiding and should not be worn overnight. |
|
| Outcomes | 1. Subjective assessment by patients 2. Pad weighing test 3. UTI 4. Preference of device | |
| Notes | Median rather than mean was reported for pad weighing test. Phase one data only analysed, thus treated as a parallel trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | '40 women were randomly allocated'. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22 women withdrew from the study (seven in phase one), reasons given in Table 1. No ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes reported on, but the trial protocol not assessed. |
| Baseline comparability | Unclear risk | Participants formed both intervention and control group. Stated as 'unclear' as only phase one data used in this review. |
| Timing of outcome assessment | Low risk | Follow up three months (two weeks for phase one). |
| Trial sponsorship | Unclear risk | Not stated. |
Nygaard 1995.
| Methods | Randomised controlled crossover trial. | |
| Participants | N = 20. Withdrawal = 2. Stress incontinence by stress test. Age range = 33‐73. Inclusion: History of exercise incontinence, physical ability to perform 40 minutes exercise and positive stress test. Exclusion: prolapse of uterus or vagina, stenotic vagina and pelvic mass. Single centre, Department of Obstetrics & Gynaecology, USA. | |
| Interventions | All participated in each of 3 separate standardised exercise sessions:
1. wearing a Hodge pessary with support (n= 18).
2. wearing a Tampax super tampon (n= 18).
3. wearing no mechanical device (n= 18) The Hodge vaginal pessary is a ring, in this case with support, placed at the neck of the cervix and is plastic coated with wires that allow it to be shaped for different anatomies. The Tampax super tampon is placed in the vagina and is a commercially available tampon. The tampon string was 'hidden' as participants were blinded to treatment. Both devices were placed by the investigator. |
|
| Outcomes | 1. Pad weighing test. 2. Patient self reported discomfort. |
|
| Notes | 4 patients were continent during the control exercise despite positive stress test initially. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Women were randomly allocated by blocked randomization in groups of four'. |
| Allocation concealment (selection bias) | Low risk | 'blocked randomization in groups of four'. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Paticipants and outcome assessors were blinded to the intervention. It was unclear whether care providers were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 withdrew before the start of the study. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported on, but the trial protocol not assessed. |
| Baseline comparability | Low risk | Participants formed both intervention and control group. |
| Timing of outcome assessment | Low risk | Following the last of three standardised aerobics sessions. |
| Trial sponsorship | Unclear risk | Not stated. |
Richter 2010.
| Methods | Randomised controlled trial. | |
| Participants | N = 446. Withdrawal = 79. Stress only, stress predominant or mixed incontinence symptoms. Mean age = 49.8 Inclusion: At least 18 years of age, ambulatory, able to attend clinic, symptoms of SUI, SUI for at least 3 months, completed adequately at least 5 days of 7 day bladder diary with number of SUI episodes exceeding number of other types of incontinence, stable storage of oral/vaginal oestrogen for past 8 weeks if used, ability to complete bladder diary and questionnaires in English, stage 0, 1, or 2 prolapse as assessed by PoP‐Q. Exclusion: Continual leakage, UTI, pregnant or planning pregnancy, within six months postpartum, severe atrophic vaginitis, postvoid residual volume of 150ml, strongly desires surgery within 12 months, within three months of failed for SUI, current medication for incontinence, vaginal foreign body, currently using pessary or used one within last two months, neurologic conditions that may impact on bladder symptoms. Single centre, Department of Obstetric & Gynaecology, Canada. | |
| Interventions | Randomised into one of three groups for an eight week treatment period:
1. Intravaginal pessary (n = 149).
2. Behavioral therapy (PFMT; n = 146)
3. Combined (pessary and behavioral therapy, PFMT; n = 150). A physician or nurse fitted an intravaginal pessary (continence ring or dish, type not specified). Behavioural therapy (PFMT) comprised four visits at two weekly intervals which included instructions on pelvic floor muscle training and exercise as well as skills for active use of muscles to prevent stress and urge incontinence. Participants were given individualised prescriptions for daily pelvic floor muscle exercise and practice. However, after the 8 week treatment period women in the combined group could continue in the trial while using only one of the therapies (for example behavioural therapy alone or pessary alone). |
|
| Outcomes | 1. Subjective assessment by patients ('much better' or 'very much better'). 2. Validated questionnaire on symptom severity and quality of life. 3. Patient Satisfaction Survey (PSQ) 4. Incontinence episodes (over 7 days). | |
| Notes | ATLAS Trial (Ambulatory Treatments for Leakage Associated with Stress Incontinence) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Stratified by 7 day bladder diary results including incontinence type (stress only versus mixed) and severity (fewer than 14 compared with 14 or more total incontinence episodes) and then randomly assigned to one of three treatment arms... using a permuted block randomization schedule'. |
| Allocation concealment (selection bias) | Low risk | 'The data Coordinating Centre performed the randomization and provided each site with a set of sealed envelopes'. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants and care providers not stated as blinded. 'Outcome assessors were blinded to treatment group assignment'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 79 withdrew from the study at 3 months and 139 withdrew from the study in total at 12 months. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported on, but the trial protocol not assessed. Quality of life was stated as an outcome in Richter 2007. Although not included in Richter 2010, it was provided by Kenton 2012. |
| Baseline comparability | Low risk | Age, race, vaginal deliveries, menstrual status, current oestrogen therapy, prior non‐surgical UI treatment, hysterectomy, incontinence type, incontinence frequency. |
| Timing of outcome assessment | Low risk | At 3 and 12 months. |
| Trial sponsorship | Unclear risk | Not stated. |
Robinson 2003.
| Methods | Randomised controlled trial. | |
| Participants | N = 24. Withdrawal = 8. Urodynamically confirmed stress or mixed incontinence. Mean age = 51.1 Inclusion: Age 30 to 75, sound mental condition and manual dexterity, > 2 stress incontinence episodes per week, > 2 g urine loss on baseline pad weight, willing to use minimum 3 devices per week and being followed up. Exclusion: overflow incontinence, neurogenic bladder, type III incontinence, treated for pyelonephritis, interstitial cystitis or recurrent UTI, anticoagulant use, taking incontinence medications, antibiotic allergy, insulin dependent diabetic mellitus, pregnant, urethral mucosal abnormality, prosthetic heart valve or other cardiac conditions, anatomical anomalies prohibiting use of urinary catheter, started hormone replacement therapy within previous 90 days or received collagen injections or urethral bulking agents in previous 90 days. Single centre, Department of Obstetric & Gynaecology, Canada. | |
| Interventions | Use of different types of intraurethral device for 4 months:
1. Use new expandable tip (NEAT) device (n = 13)
2. Use Reliance device (n = 11) The NEAT device is inserted into the urethra it has a tip which expands with fluid following insertion into the bladder. Reliance Urinary Control Device for women is a small tube inserted into the urethra using a syringe. The tip of the tube contains a balloon that is inflated against the urethra and blocks urine, with the aim of preventing leakage. Both devices must be removed before voiding and are disposable. The Reliance applicator can be reused. |
|
| Outcomes | 1. Successful pad weight test defined by >50% reduction in urine loss 2. Subjective leakage score by patients 3. Ease of use assessments 4. UTI | |
| Notes | The trial was discontinued by Uromed Corporation the company producing both devices without an explanation. Only 16 patients had the pad weighing test completed at the time the study was discontinued. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomly assigned into device study groups by the randomization card method'. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | For care provider and outcome assessor. 'Study subjects were blinded'. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 drop outs from 24 at 4 months, no ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes reported on, but the trial protocol not assessed. |
| Baseline comparability | Low risk | Height, weight, duration of incontinence, age, pad weight, leakage score, parity, quality of life score. |
| Timing of outcome assessment | Low risk | Length of follow up 4 months. |
| Trial sponsorship | Unclear risk | The trial was sponsored by Uromed Corporation the company producing both devices. |
Thyssen 2001.
| Methods | Prospective randomised crossover trial. | |
| Participants | N = 94. Withdrawal = 32. Stress incontinence diagnosed with symptoms. Mean age = 51.1 (range 30‐75). Inclusion: 'no major uterovaginal prolapse reported'. Exclusion: criteria not listed. Multicentre, Department of Obstetrics and Gynaecology, Australia, Denmark and UK. | |
| Interventions | Use of either type of device for 5 weeks and crossover with the other type for another 5 weeks:
1. Use Conveen Continence Guard (CCG) disposable intravaginal device (n = 94)
2. Use Contrelle Continence Tampon (CCT) disposable intravaginal device (n = 94) The Conveen Continence Guard is made from soft moulded hydrophilic polyurethane with a string attached at each end. A white plastic applicator is used to insert the Guard into the vagina where the Guard unfolds. The Contrelle Continence Tampon is shaped like a tampon and is made from hydrophobic polyurethane with a disposable applicator. Both devices act to apply pressure to the bladder neck with the aim of preventing involuntary voiding, they do not require removal for voiding and can be worn up to 16 hours before being discarded. |
|
| Outcomes | 1. Number of pads used 2. Pad weighing test 3. Voiding difficulties 4. UTI 5. Vaginal irritation and culture 6. Ease of use 7. Discomfort with the device | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Block randomization was used to allocate women'. |
| Allocation concealment (selection bias) | Low risk | 'Block randomization was used to allocate women'. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | For all outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 'Thirty two (34%) withdrew from the trial, nine because of discomfort, one because of expulsion of the device and 22 did not attend for follow up or did not wish to continue the treatment'. No ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported on, but the trial protocol not assessed. |
| Baseline comparability | Low risk | Participants formed both intervention and control group. |
| Timing of outcome assessment | Low risk | Length of follow up 5 weeks for each intervention. |
| Trial sponsorship | Unclear risk | Not stated. |
BMI: Body Mass Index
CCG: Conveen Continence Guard
CCT: Contrelle Continence Tampon
GSI: Genuine Stress Incontinence
HRT: Hormone Replacement Therapy
SF‐36: Short Form 36
SUI: Stress Urinary Incontinence
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cooper 2001 | No suitable comparison. Comparison of two skin care regimes for incontinence rather than mechanical devices. |
| Dowell 1997 | No suitable comparison. Compared mechanical device plus good bladder habits and mechanical device plus pelvic floor exercise plus or minus bladder retraining programme. |
| Edwards 1973b | Not randomised controlled trial. No suitable comparison. |
| Fader 2001 | No suitable comparison. Used sheaths for urinary incontinence in men. |
| Matthews 1982 | No suitable comparison. Used urinary drainage system. |
| Medical 2000 | No suitable comparison. Used self‐adhesive sheaths in men. |
| Moore 2003 | Study assessed mechanical devices for incontinence after prostatectomy in men. |
| Prashar 1997b | No suitable comparison. Compared mechanical device plus good bladder habits and mechanical device plus pelvic floor exercise plus or minus bladder retraining programme. |
| Schaffer 2012 | Unable to use data in the form presented (data in study available in Richter 2010 and Kenton 2012) |
| Sirls 2002 | Not randomised controlled trial. |
| Staskin 1995 | Not randomised controlled trial. |
| Stelling 1996 | No suitable comparison. Used condom catheters in men. |
| Watson 1989 | No suitable comparison. Used urinary sheaths systems in men. |
Contributions of authors
For the second update both review authors (AL, CS) assessed the eligibility of potential studies.
In the first update, both review authors (AL, CS) assessed the studies, extracted the data, analysed the data from the original studies and the newly included trial and updated the review.
For the original review E. Ong and K. Glavind prepared the protocol with minor amendments from the original published protocol. E. Ong, S. Shaikh and K. Glavind assessed the studies, extracted the data, analysed the data and wrote the review. J. A. Cook extracted data and undertook the analysis of data from cross‐over studies. J.M.O. N'Dow provided critical analysis of the protocol and review.
Sources of support
Internal sources
-
Faculty of Life Sciences and Education, University of South Wales, UK.
The time and resources to complete this review update
External sources
-
The National Institute for Health Research (NIHR), UK.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Incontinence Group.
Declarations of interest
Dr K. Glavind (one of the original authors of the review) is an author of an included trial.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Boos 1998 {published data only}
- Boos K. A comparison of the FemAssist and Reliance devices in the management of urinary incontinence in women. PhD thesis 2002.
- Boos K, Anders K, Hextall A, Toozs‐Hobson P, Cardozo L. Randomised trial of reliance versus femassist devices in the management of genuine stress incontinence. Neurourology and Urodynamics 1998;17(4):455. [5678] [Google Scholar]
Cornu 2012 {published data only}
- Cornu JN, Mouly S, Amerenco G, Jacquetin B, Ciofu C, Haab F, et al. 75NC007 device for noninvasive stress urinary incontinence management in women: a randomized controlled trial. International Urogynecology Journal 2012;23(12):1727‐34. [DOI] [PubMed] [Google Scholar]
Glavind 1997b {published data only}
- Glavind K. The use of a vaginal sponge during aerobics in patients with genuine stress incontinence. Proceedings of the International Continence Society (ICS), 25th Annual Meeting; 1995 Oct 17‐20; Sydney, Australia. 1995:52.
- Glavind K. Use of a vaginal sponge during aerobic exercises in patients with stress urinary incontinence. International Urogynecology Journal and Pelvic Floor Dysfunction 1997;8:351‐3. [DOI] [PubMed] [Google Scholar]
Nielsen 1995 {published data only}
- Nielsen KK, Walter S, Maegaard E, Kromann‐Andersen B. The urethral plug II: a preliminary report of an alternative treatment for genuine urinary stress incontinence in women. Proceedings of the International Continence Society (ICS), 21st Annual Meeting; 1991 Oct 10‐12; Hanover, Federal Republic of Germany. 1991:224‐5.
- Nielsen KK, Walter S, Maegaard E, Kromann‐Andersen B. The urethral plug II: an alternative treatment in women with genuine urinary stress incontinence. British Journal of Urology 1993;72(4):428‐32. [DOI] [PubMed] [Google Scholar]
- Nielsen KK, Walter S, Maegaard E, Kromann‐Andersen B. [The urethral plug‐‐an alternative treatment of women with urinary stress incontinence]. [Danish]. Ugeskrift for Laeger 1995;157(22):3194‐7. [PubMed] [Google Scholar]
Nygaard 1995 {published data only}
- Nygaard I. Prevention of exercise incontinence with mechanical devices. Journal of Reproductive Medicine 1995;40(2):89‐94. [PubMed] [Google Scholar]
- Nygaard I E. Treatment of exercise incontinence with mechanical devices. Neurourology & Urodynamics 1992;11(4):367‐8. [Google Scholar]
- Nygaard I E. Treatment of exercise incontinence with mechanical devices. Proceedings of the American Urogynecology Society, 13th Annual Meeting; 1992 Sept 27‐30; Cambridge, Massachussetts. 1992:268.
Richter 2010 {published data only}
- Kenton K, Barber M, Wang L, Meikle S, Hsu Y, Rahn D, et al. Pelvic floor symptoms improve similarly after pessary and behavioral treatment for stress incontinence. Female Pelvic Medical Reconstructive Surgery 2012;18(2):118‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter HE. A randomized trial of pessary vs. behavioral therapy vs. combined therapy for treatment of stress urinary incontinence. Conference. 2009.
- Richter HE, Burgio KL, Brubaker L, Nygaard IE, Ye W, Weidner A, et al for the Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence. Obstetrics and Gynecology 2010;115(3):609‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter HE, Burgio KL, Goode PS, Borello‐France D, Bradley CS, Brubaker L, et al for the Pelvic Floor Disorders Network. Non‐surgical management of stress urinary incontinence: ambulatory treatments for leakage associated with stress (ATLAS) trial. Clinical Trials 2007;4:92‐101. [DOI] [PubMed] [Google Scholar]
Robinson 2003 {published data only}
- Robinson H, Schulz J, Flood C, Hansen L. A randomized controlled trial of the NEAT expandable tip continence device. International Urogynecology Journal and Pelvic Floor Dysfunction 2003;14(3):199‐203. [DOI] [PubMed] [Google Scholar]
- Robinson HE, Schulz JA, Flood CG, Hiltz C. A randomized controlled study of the NEAT expandable tip continence device. International Urogynecology Journal and Pelvic Floor Dysfunction 2001;12(Suppl 3):S48. [DOI] [PubMed] [Google Scholar]
Thyssen 2001 {published data only}
- Bidmead J, Lose G, Thyssen H, Dwyer P, Bek KM, Cardozo L. A new intravaginal device for stress incontinence in women. Proceedings of the International Continence Society (ICS), 30th Annual Meeting; 2000 Aug 28‐31; Tampere, Finland. 2000:A202.
- Bidmead J, Lose G, Thyssen H, Dwyer P, Moller BK, Cardozo L. A new intravaginal device for stress incontinence in women. International Urogynecology Journal and Pelvic Floor Dysfunction 2000;11(Suppl 1):S42. [Google Scholar]
- Thyssen H, Bidmead J, Lose G, Moller BK, Dwyer P, Cardozo L. A new intravaginal device for stress incontinence in women. BJU International 2001;88(9):889‐92. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cooper 2001 {published data only}
- Cooper P, Gray D. Comparison of two skin care regimes for incontinence. British Journal of Nursing 2001;10(6 Suppl):S6‐S10. [14649] [DOI] [PubMed] [Google Scholar]
Dowell 1997 {published data only}
- Dowell CJ, Bryant CM, Moore KH, Prashar S. The efficacy and user friendliness of the urethral occlusive device. Proceedings of the International Continence Society (ICS), 27th Annual Meeting; 1997 Sept 23‐26; Yokohama, Japan. 1997:295‐6. [5847]
Edwards 1973b {published data only}
- Edwards L, Malvern J. Long‐term follow‐up results with the pubo‐vaginal spring device in incontinence of urine of women: comparison with electronic methods of control. British Journal of Urology 1973;45(103):94‐6. [2631] [PubMed] [Google Scholar]
Fader 2001 {published data only}
- Fader M, Pettersson L, Dean G, Brooks R, Cottenden AM, Malone‐Lee J. Sheaths for urinary incontinence: a randomized crossover trial. BJU International 2001;88(4):367‐72. [12252] [DOI] [PubMed] [Google Scholar]
Matthews 1982 {published data only}
- Matthews HV. The development of a urinary incontinence drainage system. International Rehabilitation Medicine 1982;4(1):45‐8. [728] [DOI] [PubMed] [Google Scholar]
Medical 2000 {published data only}
- Continence Products Evaluation Network. Self‐adhesive sheaths for men using sheath systems. Report number IN6. London: Medical Devices Agency, 2000. [Google Scholar]
Moore 2003 {published data only}
- Moore KN. A study assessing the safety, efficacy, comfort, and patient satisfaction with three commonly used penile compression devices for incontinence after prostatectomy. Neurourology & Urodynamics 2003;22(5):473‐4. [17111] [Google Scholar]
Prashar 1997b {published data only}
- Prashar S, Moore K, Bryant C, Dowell C. The urethral occlusive device for the treatment of urinary incontinence: changes in quality of life. International Urogynecology Journal and Pelvic Floor Dysfunction 1997; Vol. 8, issue 1:S130. [5172]
Schaffer 2012 {published data only}
- Schaffer J, Nager CW, Xiang F, Borello‐France D, Bradley CS, Wu JM, Mueller E, Norton P, Paraiso MF, Zyczynski H, Richter HE. Predictors of success and satisfaction of nonsurgical therapy for stress urinary incontinence. Obstet Gynecol 2012;120(1):91‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sirls 2002 {published data only}
- Sirls LT, Foote JE, Kaufman JM, Lightner DJ, Miller JL, Moseley WG, et al. Long term results of the femsoft urethral insert for the management of female stress incontinence. International Urogynecology Journal 2002;13:88‐95. [DOI] [PubMed] [Google Scholar]
Staskin 1995 {published data only}
- Staskin D, Sant G, Sand P, Rappaport S, Knapp P, Bavendam T, et al. Use of an expandable urethral insert for GSI ‐ long term results of multi‐center trial. Neurourology & Urodynamics 1995; Vol. 14, issue 5:420‐2. [4568]
Stelling 1996 {published data only}
- Stelling JD, Hale AM. Protocol for changing condom catheters in males with spinal cord injury. SCI Nursing 1996;13(2):28‐34. [4847] [PubMed] [Google Scholar]
Watson 1989 {published data only}
- Watson R. A nursing trial of urinary sheath systems on male hospitalized patients. Journal of Advanced Nursing 1989;14(6):467‐70. [412] [DOI] [PubMed] [Google Scholar]
Additional references
Alhasso 2005
- Ammar A, Glazener C, Pickard R, N'Dow J. Adrenergic drugs for urinary incontinence in adults. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD001842.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berghmans 2013
- Berghmans B, Hendriks E, Bernards A, Bie R, Omar MI. Electrical stimulation with non‐implanted electrodes for urinary incontinence in men. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD001202.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bidmead 2000
- Bidmead J, Lose G, Thyssen H, Dwyer P, Bek KM, Cardozo L. A new intravaginal device for stress incontinence in women. International Urogynecology Journal and Pelvic Floor Dysfunction 2000;11(Suppl 1):S42. [11910] [Google Scholar]
Bourcier 1995
- Bourcier AP, Juras JC. Nonsurgical therapy for stress incontinence. Urological Clinics of North America 1995;22(3):613‐27. [PubMed] [Google Scholar]
Campbell 2012
- Campbell SE, Glazener CMA, Hunter KF, Cody JD, Moore KN. Conservative management for postprostatectomy urinary incontinence. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD001843.pub4] [DOI] [PubMed] [Google Scholar]
Cody 2012
- Cody JD, Jacobs ML, Richardson K, Moehrer B, Hextall A. Oestrogen therapy for urinary incontinence in post‐menopausal women. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD001405.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davila 1994
- Davila GW, Ostermann KV. The bladder neck support prosthesis: a nonsurgical approach to stress incontinence in adult women. American Journal of Obstetrics and Gynecology 1994;171(1):206‐11. [DOI] [PubMed] [Google Scholar]
Davila 1997
- Davila GW, Kondo A. Introl bladder neck support prothesis: international clinical experience. International Urogynecology Journal and Pelvic Floor Dysfunction 1997;8(5):301‐6. [DOI] [PubMed] [Google Scholar]
Downs 1996
- Downs S, Black N. Systematic review of the literature on the effectiveness of surgery for stress incontinence in women. London: Department of Public Health and Policy Publications, London School of Hygiene & Tropical Medicine, 1996. [Google Scholar]
Dumoulin 2014
- Dumoulin C, Hay‐Smith EJC, Mac Habée‐Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD005654.pub3] [DOI] [PubMed] [Google Scholar]
Edwards 1970
- Edwards L. Urinary Incontinence. Urinary Incontinence. London: Academic Press, 1970:115‐27. [Google Scholar]
Glavind 1997a
- Glavind K. Use of a vaginal sponge during aerobic exercises in patients with stress urinary incontinence. International Urogynecology Journal and Pelvic Floor Dysfunction 1997;8:351‐3. [srincont‐7882] [DOI] [PubMed] [Google Scholar]
Hannestad 2000
- Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community‐based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord‐Trondelag. Journal of Clinical Epidemiology 2000;53(11):1150‐7. [DOI] [PubMed] [Google Scholar]
Hay‐Smith 2009
- Hay‐Smith J, Berghmans B, Burgio K, Dumoulin C, Hagen S, Moore K, et al. Adult conservative management. In: Abrams P, Cardozo L, Khoury S, Wein A editor(s). Incontinence. 4th Edition. Paris: International Continence Society, 2009. [Google Scholar]
Herbison 2013
- Herbison GP, Dean N. Weighted vaginal cones for urinary incontinence. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD002114.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jackson 1996
- Jackson S, Donovan J, Brookes S, Eckford S, Swithinbank L, Abrams P. The Bristol female lower urinary tract symptoms questionnaire: development and psychometric testing. British Journal of Urology 1996;77(6):805‐12. [DOI] [PubMed] [Google Scholar]
Kondo 1997
- Kondo A, Yokoyama E, Koshiba K, Fukui J, Gotoh M, Yoshikawa Y, et al. Bladder neck support prosthesis: a nonoperative treatment for stress or mixed urinary incontinence. Journal of Urology 1997;157(3):824‐7. [DOI] [PubMed] [Google Scholar]
Mariappan 2005
- Mariappan P, Alhasso AA, Grant A, N'Dow JMO. Serotonin and noradrenaline reuptake inhibitors (SNRI) for stress urinary incontinence in adults. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004742.pub2] [DOI] [PubMed] [Google Scholar]
Moore 1997
- Moore KH, Foote A, Siva S, King J Burton G. The use of the bladder neck support prosthesis in combined genuine stress incontinence and detrusor instability. Australian and New Zealand Journal of Obstetrics and Gynaecology 1997;37(4):440‐5. [DOI] [PubMed] [Google Scholar]
Moore 1999
- Moore KH, Foote A, Burton G, King J. An open study of the bladder neck support prosthesis in genuine stress incontinence. British Journal of Obstetrics and Gynaecology 1999;106(1):42‐9. [DOI] [PubMed] [Google Scholar]
Nielsen 1993
- Nielsen KK, Walter S, Maegaard E, Kromann‐Andersen B. The urethral plug II: an alternative treatment in women with genuine urinary stress incontinence. British Journal of Urology 1993;72(4):428‐32. [72] [DOI] [PubMed] [Google Scholar]
Ostaszkiewicz 2004
- Ostaszkiewicz J, Johnston L, Roe B. Timed voiding for the management of urinary incontinence in adults. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002802.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prashar 1997a
- Prashar S, Moore K, Bryant C, Dowell C. The urethral occlusive device for the treatment of urinary incontinence: changes in quality of life. International Urogynecology Journal and Pelvic Floor Dysfunction 1997;8(1):S130. [5172] [Google Scholar]
Realini 1990
- Realini JP, Walters MD. Vaginal diaphragm rings in the treatment of stress urinary incontinence. Journal of the American Board of Family Practice 1990;3(2):99‐103. [PubMed] [Google Scholar]
Reference Manager 2012
- Reference Manager Professional Edition Version 12. New York: Thomson Reuters 2012.
Richter 2007
- Richter HE, Burgio KL, Goode PS, Borello‐France D, Bradley CS, Brubaker L, et al. Non‐surgical management of stress urinary incontinence: ambulatory treatments for leakage associated with stress (ATLAS) trial. Society for Clinical trials 2007;4:92‐101. [DOI] [PubMed] [Google Scholar]
Staskin 1996
- Staskin D, Bavendam T, Miller J, Davila GW, Diokno A, Knapp P, et al. Effectiveness of a urinary control insert in the management of stress urinary incontinence: early results of a multicentre study. Urology 1996;47(5):629‐36. [DOI] [PubMed] [Google Scholar]
Suarez 1991
- Suarez GM, Baum HN, Jacobs J. Use of standard contraceptive diaphragm in management of stress urinary incontinence. Urology 1991;37(2):119‐22. [DOI] [PubMed] [Google Scholar]
Ware 1993
- Ware JE, Snow KK, Kosinski M, Gandek B. SF‐36 Health Survey Manual and Interpretation Guide. Boston: The Health Institute, New England Medical Centre, 1993. [Google Scholar]
References to other published versions of this review
Frazer 1999
- Frazer M, Lose G, Kozman E, Boos K, Tincello D. Mechanical devices for urinary incontinence in women. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001756] [DOI] [Google Scholar]
Lipp 2011
- Lipp A, Shaw C, Glavind K. Mechanical devices for urinary incontinence in women. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD001756.pub5] [DOI] [PubMed] [Google Scholar]
Shaikh 2006
- Shaikh S, Ong EK, Glavind K, Cook J, N'Dow JMO. Mechanical devices for urinary incontinence in women. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001756.pub4] [DOI] [PubMed] [Google Scholar]


