Abstract
Idiopathic intracranial hypertension (IIH) is defined as a syndrome of raised intracranial pressure with normal imaging of the brain and cerebrospinal fluid (CSF) composition. There are many controversies and myths that surround IIH. Although patients of IIH may present “typical” symptoms and signs of raised intracranial pressure, clinical scenarios often vary. A typical clinical and radiological finding poses significant problems in diagnosis and management of patients with IIH. We have tried to resolve these controversies and provide a comprehensive update on different aspects of IIH. In this article, we review the common problems encountered while dealing with patients of IIH.
Keywords: Benign intracranial hypertension, idiopathic intracranial hypertension, headache, pseudotumor cerebri syndrome, visual loss
INTRODUCTION
The term “benign intracranial hypertension” (BIH) was first introduced by Foley.[1] Several decades later the “not so benign” nature of the entity was recognized by Corbett and Thompson, changing its name from BIH to “idiopathic intracranial hypertension” (IIH)” in 1989.[2] The diagnostic criteria for IIH were first formulated in 1937 by Dandy and were later modified by Smith in 1985.[3,4] In 2013, Friedman et al. further refined the diagnostic criteria and proposed the condition best described under the umbrella term of pseudotumor cerebri syndrome (PTCS) classifying it into primary or secondary (IIH) depending on the absence or presence of an identifiable cause[5] [Table 1a]. As a result, IIH acts as a subset within the primary PTCS category. The International Headache Society”s International Classification of Headache Disorders 3rd ed.ition (ICHD-3), 2018 defines IIH under “Headaches attributed to non vascular intracranial disorders”/Headache attributed to increased CSF pressure (ICHD-3, 7.1.1). As per ICHD-3, IIH is described as a new-onset headache or significant worsening of a preexisting headache accompanied by clinical symptoms/signs, and/or neuroimaging signs of raised increased intracranial pressure (ICP) [Table 1b].[6]
Table 1.
Diagnosis of IIH
| (a) Diagnostic criteria for pseudo tumor cerebri syndrome (PTCS)[5] |
|---|
| Diagnostic criteria for pseudo tumor cerebri syndrome (PTCS)[5] 1. Required for diagnosis: pseudo tumor cerebri syndrome (Definite if criteria A-E are fulfilled; Probable if criteria A-D are met but the opening CSF pressure is lower than described for making a definite diagnosis) A. Presence of papilledema B. Neurological examination is normal (except abnormal cranial nerve examination) C. Neuroimaging is normal with normal brain parenchyma (without hydrocephalus, space occupying lesion, meningeal enhancement) for typical patients D. CSF composition is normal E. Opening CSF pressure is elevated (>250 mm CSF in adults; >280 mm CSF in children [250 mm CSF in a non sedated, non obese child]) 2. Diagnosis of pseudo tumor cerebri syndrome without papilledema If papilledema is absent; diagnosis of pseudotumorcerebri syndrome should be considered if B-E from above are satisfied, and the patient has abducens nerve palsy (unilateral or bilateral) in addition If both papilledema and abducens nerve palsy are absent a diagnosis of pseudo tumor cerebri syndrome can only be suggested, if in addition to presence of criteria B-E from above at least 3 of the following neuroimaging criteria are present: i. Presence of an Empty sella ii. Posterior globe flattening or indentation iii. Perioptic nerve sheath prominence or distention with or without presence of tortuous optic nerves. iv. Stenosis of transverse venous sinus. |
| (b) Diagnostic criteria for IIH- ICHD-3 |
| A. New headache, or a significant worsening of a pre-existing headache, fulfilling criterion C B. Both of the following: 1. Idiopathic intracranial hypertension (IIH) has been diagnosed 2. CSF pressure exceeds 250 mm CSF (or 280 mm CSF in obese children) C. Either or both of the following: 1. Headache has developed or significantly worsened in temporal relation to the IIH, or led to its discovery 2. Headache is accompanied by either or both of the following: a) Pulsatile tinnitus b) Papilloedema D. Not better accounted |
It is clinically relevant to note that documentation of an elevated CSF pressure (≥250 mm in adults and ≥280 mm in children) is mandatory to establish the diagnosis of “definite” PTCS but the diagnosis of “probable” PTCS may be kept in patients with strongly suggestive clinical history, bilateral papilledema, supportive neuroimaging and “ normal” CSF opening pressure [Table 1a]. As CSF pressure may vary in a given individual at varied times of the day, this definition may enable to diagnose such patients of IIH with higher certainty.[5]
PATHOPHYSIOLOGY OF IIH: THE QUEST BEGINS
Myth: IIH occurs only in obese women
For long it has been believed that IIH occurs exclusively in overweight women of childbearing age group. A meta-analysis and systematic review identified 15 studies and depicted a pooled incidence rate of 1.20/100,000. Various studies have predicted that women are eight times more prone to develop IIH as compared to men.[7,8,9,10] Digre et al. in their study including 29 male patients of IIH found that clinical characteristics of men with IIH were similar to age-matched female patients.[11] Similar findings were noted by Kesler et al. in their retrospective review involving 141 IIH patients.[12]
Bruce et al. studied clinical and radiological characteristics of IIH in 721 consecutive patients (9% males) and noted that males with IIH have different symptom expression and/or different symptom threshold. Males were twice as likely as females to develop severe visual loss, probably because of lack of reporting of nonvisual symptoms like headache, Transient visual obscurtions (TVOS), and tinnitus.[13]
There is no denying that obesity and IIH are related. Several epidemiological studies have noted that more than 80 to 90% patients of IIH are overweight.[10] Nonobese patients with a history of a recent weight gain are more inclined to develop IIH.
In a retrospective cohort of 407 consecutive adult patients of IIH, Bruce et al. noted that 84% of patients had a BMI above 30 kg/m2,[14] while patients with BMI >40 kg/m2 were noted to have a worse prognosis in another retrospective review by Szweka et al. (2013).[15] Several weight-loss studies suggest that any amount of weight loss is beneficial in these patients and should be encouraged. Visual field improvement was also noted in the Longitudinal Idiopathic Intracranial Hypertension Treatment Trial (LIIHTT) subjects who never received pharmacological therapy but were only advised lifestyle modification and weight-loss.[16]
It is important to recognize that though obesity is one of the major contributory factors; it is not the sole factor responsible in the causation of IIH. There is an interplay between various factors and hence patients of either gender with varied BMI may develop IIH.[17]
DECEPTIONS AND EXCEPTIONS: CONTROVERSIES IN CLINICAL PROFILE- WHY WE MISS IT?
Myth: IIH cannot occur without headache
Headache is one of the most common symptoms with highly variable severity.
Headache due to increased intracranial pressure is often described as throbbing or bursting and is precipitated by factors that increase ICP such as bending, coughing, sneezing, or exertion. Classically, such headache has an early morning worsening (attributed to raised ICP at night as a consequence of recumbent position, raise PCO2 during sleep due to respiratory depression, and probably decrease CSF absorption).[18] Early diagnosis of IIH is often based upon the characteristics of headache (e.g. illustrative case). Symptoms such as nausea and vomiting are common and usually occur after waking, thereby frequently accompanying morning headaches.
It is often noted that the pattern of headache changes over time and depends upon the stage of IIH. Headache can be because of increased intracranial pressure, migraine, medication overuse, tension-type headache, low-pressure headache after a lumbar puncture, or iatrogenic Chiari malformation post shunting procedure. Headache was also found to be a significantly disabling symptom and was associated with a poorer quality of life in the neuro-ophthalmology research disease investigator consortium (NORDIC)-IIHTT. About 84% of patients complained of headache in IIHTT cohort, of which 52% described their headaches as migrainous, 22% had tension-type headaches (TTH), 16% had probable migraines and 4% had probable TTH.[19,20]
Freidman et al. in 2002 reviewed medical records of 82 patients with IIH and noted that these patients frequently have a headache due to causes other than IIH.[19] A small fraction of patients may be even present without any headache.
Other symptoms of IIH
Unilateral or bilateral TVOs occur in around 70% of patients with IIH.[8] The TVOs are attributed to an increase in CSF pressure around the optic nerve causing disturbances in the microcirculation of optic nerve.[21]
Around 60% patients may be present with unilateral or bilateral pulsatile or pulse-synchronous tinnitus, which occurs due to the flow turbulence in venous sinuses, due to the heightened transmission of normal vascular pulsations, or due to increased CSF around the cochlear organs.[22]
Cranial nerve paresis may occur commonly, secondary to increased intracranial pressure. Abducens nerve paresis is commonly manifesting as diplopia in these patients.
Importantly, either of the clinical symptoms may be present (or absent) in any combination. A high index of suspicion in an appropriate clinical setting is necessary to clinch the diagnosis.
Neuro-ophthalmological examination
Myth- IIH cannot occur without papilledema
Papilledema is usually bilateral and is considered as a hallmark sign of IIH [Figure 2]. A validated scale (Modified Frisens Scale) has been used to grade papilledema[23] [Table 2]. Figure 2 shows representative fundus photographs depicting various stages of papilledema. Rarely, papilledema may be asymmetric, unilateral, or absent. This might be related to complete, partial, or “compartmentalized” obliteration of the subarachnoid space around the optic nerve, which does not allow raised ICP to be transmitted to subarachnoid space around the optic nerve. Further, in patients with recurrent IIH fibrosis of the nerve fiber layer or optic atrophy, it may preclude the development of papilledema underscoring the documentation of raised CSF pressure in such cases.
Figure 2.
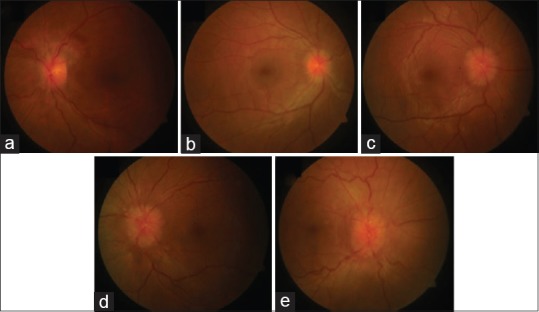
Showing- a: Grade 1 papilledema; b: Grade 2 papilledema, c: Grade 3 papilledema; d: Grade 4 papilledema and e: Grade 5 papilledema
Table 2.
Showing key features from modified Frisens scale for grading disc edema[22]
| GRADE 1. (MINIMAL DISC EDEMA) C shaped subtle grayish halo with a temporal gap obscuring the underlying retinal details, causing disruption of normal radial nerve fiber layer arrangement striations. Temporal disc margins are normal GRADE 2. (LOW DEGREE OF EDEMA) Elevation of nasal borders with circumferential halo. No major vessel obscuration is noted GRADE 3. (MODERATE DEGREE OF EDEMA) Circumferential halo with elevation of all borders causing obscuration of one segment of major blood vessels leaving disc. An irregular outer fringe halo with finger like extensions may be seen. GRADE 4 (MARKED DEGREE OF EDEMA) Complete halo and Elevation of the whole nerve head, including the cup with complete obscuration of the disc borders. There is total obscuration of a segment of a major blood vessel on the disc Grade 5 (SEVERE DEGREE OF EDEMA) Obscuration of all vessels on the disc and leaving the disc |
Figure 1.
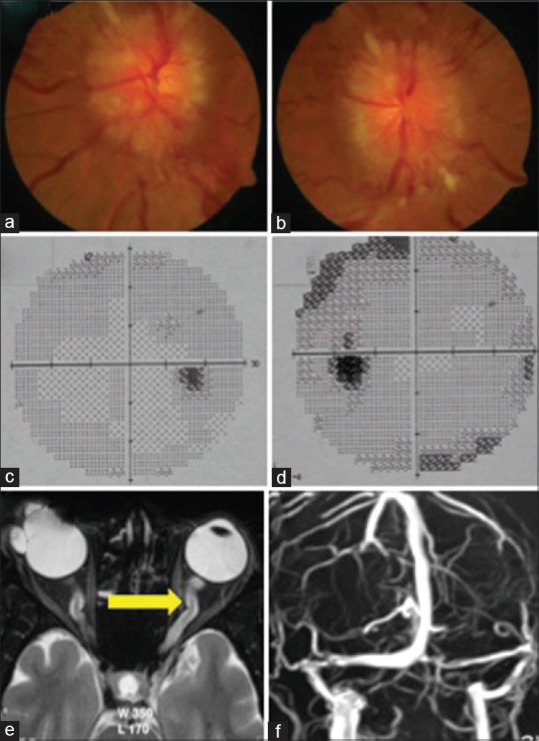
Showing bilateral papilledema (a, b) on fundus examination; Normal visual field of the right eye (c) and constriction of field and enlarged blind spot of the left eye (d). MRI Brain showing Tortuous optic nerves (e) and Normal MR Venography (f)
Digre et al. in their cross-sectional analysis of 353 IIH patients between 1990 and 2003 noted the prevalence of IIH to be 5.7% (20 out of 353 IIH patients without papilledema [IIHWOP]). Patients of IIHWOP were similar in clinical characteristics and presenting visual acuity but mean CSF opening pressure was found to be lower in these patients.[24]
In another prospective observational study, Favoni et al. reported a prevalence of 2.5% of IIHWOP in patients with chronic refractory headache.[25]
While MRI brain is generally normal in IIH, raised ICP itself can produce few MRI changes unique to raised ICP which may be helpful in settings of IIHWOP. These include empty sella turcica, flattening of the posterior portion of the globe, distension of optic nerve sheath, tortuosity of the optic nerve sheath, deformity of the pituitary, protrusion and enhancement of optic nerve head, slit-like ventricles, and tight CSF spaces [Figure 3].
Figure 3.
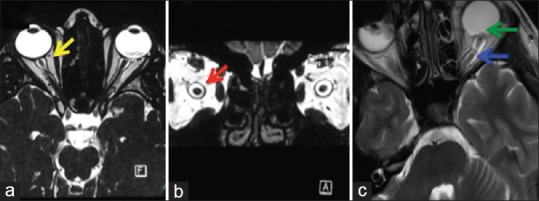
Showing MRI findings in IIH. (a) Showing Distension of Optic Nerve Sheath (T2 Weighted; axial view); (b) Showing Distension of Optic Nerve Sheath (T2 Weighted; coronal view); (c) Showing Tortuosity of Optic Nerve (Blue Arrow) and Scleral Indentation (Green Arrow)
While IIH can occur with or without asymmetrical papilledema, there is a major caveat in making the diagnosis of IIHWOP. Simply having “Headache and elevated pressure” is not enough. In the absence of an appropriate setting, an elevated CSF opening pressure may itself be nonspecific or nondiagnostic.[26,27]
Vision and IIH
Visual dysfunction in IIH is consequent to increased pressure in subarachnoid space around the optic nerve, which results in either or both of the following two processes (Flowchart 1):
Visual field (VF) defects are common in patients with IIH. The enlarged blind spot is the commonest followed by loss of infero-nasal portion of the visual field. Gradual depression of peripheral field may ensue in untreated patients with IIH.[8]
Visual field reading center (VFRC) evaluated 660 baseline VFs from 165 enrolled patients in IIHTT to characterize the VF at the baseline. The most common type of abnormality was a localized nerve fiber bundle-like defect (60%). Localized inferior hemifield loss was more common than superior hemifield loss.[29]
Visual abnormalities in IIH also occur because of retinal damage. This may be in the form of either (i) a neurosensory detachment or (ii) choroidal folds. In a subgroup analysis of IIHTT, Sibony et al. noted the frequency, patterns, associations, and biomechanical implications of retinal and choroidal folds in papilledema due to IIH. These folds are considered to be the biomechanical signs of stress/strain on the optic nerve head and load-bearing structures induced by intracranial hypertension.[30]
Visual loss in IIH
Myth: Visual loss cannot occur in IIH
Most often patients of IIH have preserved visual acuity and therefore the major brunt of visual symptoms is on the field of vision (Illustrative case). However, few patients of IIH may also develop significant visual loss which can be transient, acute, or chronic. Transient visual loss in IIH develops secondary to intermittent raised pressure in the subarachnoid sheath surrounding the optic nerve head causing axoplasmic flow stasis and ischemia.
A few patients may develop an acute, severe, and rapidly progressive visual loss. An acute onset of symptoms and signs of intracranial hypertension (less than 4 weeks between onset of initial symptoms and severe visual loss) and rapid worsening of visual loss over a few days in patients of IIH is termed as “Fulminant IIH.”[31] As noted by Thambisetty et al. in their study including 16 patients, presentation with Fulminant IIH requires urgent surgery.[31] Acute visual loss often develops due to a sudden increase in intracranial pressure causing a vascular compromise in the optic nerve head causing massive papilledema and peripapillary retinal hemorrhages.
Most commonly, visual loss in IIH develops on a chronic basis in untreated patients of IIH who subsequently develop secondary optic atrophy.
The visual loss at presentation has been associated with worse prognosis by Takkar et al. in their study including 40 IIH patients.[9]
Thus, the usual course of IIH causes visual dysfunction in the form of slowly spreading disc edema and corresponding constricting visual fields. Awareness about “Fulminant IIH” is necessary to prevent catastrophic visual morbidity associated with it.
Controversies in diagnosis: CSF analysis
Myth- CSF pressure has to be high in IIH at all times
There are several issues concerning the method for measurement of CSF pressure as well as regarding its normal values. Reference level for CSF pressure measurement should be at the level of the left atrium whether done in a supine, prone, or sitting position. ICHD-3 also mentions that CSF pressure should be measured in the absence of ICP lowering treatment in patients of suspected IIH. No sedative must be used before monitoring and the pressure should be measured in lateral decubitus position. CSF pressure may vary during the course of a day and a single measurement may not be indicative of the average CSF pressure over the day. Repeat puncture or a prolonged lumbar/intraventricular pressure monitoring may be required in cases of diagnostic uncertainty.[6,32]
Spuriously high values can occur with Valsalva and with hypoventilation associated with sedation.[18] Moreover, the reference range for cerebrospinal fluid (CSF) opening pressure in children is higher as compared to their adult counterparts. As defined in the diagnostic criteria for the diagnosis of IIH, opening CSF pressure of more than 280 mm of CSF should be considered abnormal in obese children.[6,33]
Spuriously, low values can occur with hyperventilation (e.g. anxiety) due to reduction in carbon dioxide levels and multiple needle punctures. CSF pressure fluctuates throughout the day and for that simple reason, a single normal CSF measurement does not exclude IIH as the diagnosis.[34]
As mentioned above, the revised diagnostic criteria also suggest that in an otherwise typical patient, the diagnosis of “probable” IIH may be considered even with normal CSF pressure. The CSF pressure has to be considered in light of the patient”s clinical status.[5]
Controversies in managing IIH: Medicine or surgery!
The main aim of treatment in IIH is to preserve visual function, to provide symptomatic relief of symptoms (particularly headache) and to treat the potential etiologic factors. The treatment is guided by the “predicted threat to vision” in these patients.[35] Our protocol of managing patients of IIH is given in Flowchart 2.
CAN IIH BE PREVENTED?
Role of dietary modification
A sustained weight loss of approximately 6% from the baseline has been considered to be associated with good visual outcome.[36] The IIHTT noted an excellent and persistent response even in the placebo arm which only received low sodium, weight reduction diet, and lifestyle management.[8,37] Above all, visual field improvement was also noted in 12% of the Longitudinal IIH Treatment Trial subjects who were never treated with acetazolamide (ACZ) (and had only received lifestyle modification). Effects of gastric weight reduction surgery have been repeatedly evaluated but given the considerable complications, risks should be balanced with benefits before attempting surgery.[38]
MEDICAL THERAPY
Acetazolamide: When to use, how much to use, how long to use?
ACZ has been considered as the mainstay of medical management. Its carbonic anhydrase inhibitor effect impedes CSF formation at the choroid plexus. A Cochrane systematic review in 2015 identified two randomized control trials for the use of ACZ in IIH.[39,40]
In patients with any visual loss or refractory symptoms, it is advisable to initially start ACZ at a dose of 500 mg twice a day with a schedule to increase the dose by 250 mg every six days up to a maximum of 4 g daily or a maximally tolerated dose. The IIHTT has provided Class 1 evidence that ACZ is beneficial in patients with IIH with mild visual loss.
Adverse reactions like hypokalemia, allergic reactions, fatigue, paresthesia's, dysgeusia, vomiting, diarrhea, nausea, and fatigue may occur. A few patients may observe metabolic acidosis, kidney stones, transaminitis, pancreatitis, diverticulitis, and renal failure. It is contraindicated in subjects with allergies to sulfa drugs.[40]
The duration of use of ACZ is also unsettled. In the IIHTT ACZ was tapered off at 6 months in subjects with papilledema of a grade less than one unless they had persistent symptoms or deficits in their visual field. Patients who were continued on ACZ were noted to have continued improvement in the visual functions when followed until 12 months.[40]
We conclude that the duration of ACZ should be governed by the resolution of clinical symptoms, papilledema, and visual field deficits. Preferably, ACZ should be continued for a period of 6 to 12 months after resolution of papilledema to prevent recurrences.
Are other drugs helpful in IIH?
The carbonic anhydrase inhibitor activity of topiramate makes it an important alternative to ACZ (specifically in patients with contraindication to ACZ). In an open-label study, Celebisoy et al. compared ACZ to topiramate prospectively in 40 patients and concluded that topiramate is as effective as ACZ in treatment of IIH. Weight reduction and reduction of CSF formation were considered as possible mechanisms of action. A role for topiramate in IIH is established at a dose of 25 mg to 200 mg. It's potential to cause weight loss may be an advantage to obese patients. Caution is advised in patients with sulfa allergies, glaucoma, and nephrolithiasis. Other side effects include paresthesia's, depression, cognitive slowing, weight loss, and potential teratogenic risks. It may reduce the efficacy of oral contraceptive drugs.[41]
The role of other diuretics such as furosemide and amiloride is not certain hence combining diuretics may lead to severe hypokalemia and therefore is best avoided.
How to treat headache in IIH?
The type of headache should guide its treatment and an early introduction of prophylactic therapy should be considered. The patients may have varied types of headache and an adequate caution needs to be observed while selecting the drugs (e.g. propensity of weight-gain by sodium valproate, tricyclic antidepressants, beta -blockers, etc.).[19,35]
What is the role of steroids in IIH?
While the use of long-term steroids have been noted to cause/precipitate IIH, high-dose pulse steroids may be used as a temporary measure while awaiting definitive surgical procedure in patients presenting acute, severe visual loss.[42] Thambisetty et al. also considered the use of steroids in four of their 16 patients with fulminant IIH.[31]
Whence surgical measures?
The patients, who develop rapid progression of visual loss, are medically refractory or who present an acute severe visual loss require urgent CSF diversion. As already mentioned, temporary measures like high-dose pulse steroids; repeated lumbar puncture or lumbar drainage may be attempted while awaiting these definitive measures.
The controversy still remains as to which surgical procedure should be preferred.[43] The various surgical options available are (a) optic nerve sheath fenestration (ONSF) or (b) CSF diversion procedures- ventriculoperitoneal shunt (VP Shunt)/Ttheco-peritoneal shunt (TP Shunt). ONSF has been preferred when visual symptoms are predominant or the visual compromise is unilateral. Authors preferring ONSF to CSF diversion techniques have advocated low morbidity, infection rates, and mortality. Local complications may occur e.g. retrobulbar hemorrhage, orbital hematoma, orbital apex syndrome, orbital cellulitis, traumatic optic neuropathy, heterotopias, diplopia, peripapillary hemorrhages, disc hemorrhage, cyst formation, and conjunctival abscess. Formation of synechia, pupillary abnormalities, and late failure may also occur.
Authors advocating CSF diversion procedures argue that these procedures are more beneficial in patients presenting with prominent nonvisual dysfunction while providing benefit in regaining visual functions as well. A direct threat to vision may occur in patients undergoing ONSF, and in intractable cases, a CSF diversion procedure may be required after ONSF. Risk of shunt-related complications like an infection; shunt migration or obstruction, subdural hemorrhages, over drainage and tonsillar herniation need to be borne in mind while proceeding for CSF diversion.
There is a need for studies, which directly compare ONSF with CSF-shunting procedures for the treatment of IIH. As of now, the decision rests on the local preference, prompt availability and access to the procedure and surgical expertise.[43]
What is the current role of neurovascular stenting in acute IIH to prevent loss of vision?
Transverse sinus stenting in IIH is equally controversial. Starke et al. identified 17 studies including 185 patients who underwent 221 stenting procedures. A systematic review of these patients suggested that neurovascular stenting could be a safe and effective therapeutic option for medically refractory IIH.[44] Cappuzzo et al. in 2018 also concluded that transverse sinus stenosis is an effective therapeutic option in patients with IIH.[45] While recent literature claims that neurovascular stenting is a safe alternative, the available literature is based upon small or non-randomized case series and their reviews.[45] To conclude, evidence on neurovascular stenting is limited and long-term follow-up data is not available.[35]
How to follow patients of IIH?
In a recently published consensus guideline on management of IIH reflecting practices from across UK, importance of documentation and serial follow up of visual acuity, pupil examination, formal visual field assessment, dilated fundus examination, and grading of papilledema and BMI calculation being stressed.[35] In general, we believe that the patient presenting with progressive or severe visual loss should be followed every two weeks until the visual functions stabilize. Patients with moderate visual loss and stable visual functions also need to be followed every 1 to 3 months depending upon the grade of papilledema and clinical signs and symptoms. Patients with mild disc edema may also be followed every 3 to 6 months if the visual functions and clinical symptoms have stabilized.
CONTROVERSIES IN IIH WITH PREGNANCY
Why IIH in pregnancy?
At several instances, IIH may first appear during pregnancy in otherwise healthy women. Sudden weight gain and hormonal fluctuations during pregnancy may be causative factors although the last word is yet to be said.
How to treat IIH in pregnancy?
For patients with minimal/stable clinical and visual functions, only follow-up is required once the diagnosis is established and secondary causes have been ruled out. The disorder may itself resolve or remit after delivery. However, in patients with mild-to-moderate visual loss or deteriorating clinical and visual symptoms, definitive treatment may be required. It is believed that the management of pregnant patients with IIH should be the same as non pregnant patients except calorie restriction or the use of diuretics. ACZ is labeled as a category C medication in pregnancy given its teratogenic potential. Use of ACZ after 20 weeks of pregnancy is often considered safe but a clear risk-benefit ratio should be considered before advising it.[46] The visual outcome is considered to be similar to non pregnant women. Topiramate should not be advised during pregnancy.[47] A clear risk-benefit assessment should be advocated while choosing agents for headache management during pregnancy. Serial lumbar punctures or ONSF may be considered in patients with imminent risks of visual loss.[48]
Does IIH have an implication on the mode of delivery/anesthesia?
No specific mode of delivery is suggested for patients with IIH. The mode of delivery should be largely governed by obstetric factors. The attempt may be made to avoid prolonged Valsalva during the second stage of labor if deemed feasible in appropriate settings. Adequate analgesia should be administered during labor. Spinal anesthesia or epidural anesthesia has been found to be safe. In operated patients of IIH with preexisting LP shunt, general anesthesia for cesarean section has been recommended over epidural anesthesia.[49]
IIH in children
None of the age group is immune to IIH. However, there is an increased incidence noted in the adolescent age group as compared to the younger population. No correlation with obesity or female gender is noted. Excluding secondary structural, endocrinal, nutritional and metabolic causes, it becomes important while dealing with this age group. The management protocol is largely the same as followed in the adult counter parts. Outcomes are usually encouraging though in rare cases devastating visual loss may occur.[5,50]
CONCLUSIONS
IIH is probably the most common “neurological” cause of preventable visual morbidity. It is important to identify subgroups of patients with “eye at risk” to salvage vision. Awareness of typical presentations and prompt recognition are of paramount importance to prevent complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Foley J. Benign forms of intracranial hypertension; toxic and otitic hydrocephalus. Brain J Neurol. 1955;78:1–41. doi: 10.1093/brain/78.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Corbett JJ, Savino PJ, Thompson HS, Kansu T, Schatz NJ, Orr LS, et al. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982;39:461–74. doi: 10.1001/archneur.1982.00510200003001. [DOI] [PubMed] [Google Scholar]
- 3.Dandy WE. Intracranial pressure without brain tumor. Ann Surg. 1937;106:492–513. doi: 10.1097/00000658-193710000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JL. Whence pseudotumor cerebri? J Clin Neuroophthalmol. 1985;5:55–6. [PubMed] [Google Scholar]
- 5.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159–65. doi: 10.1212/WNL.0b013e3182a55f17. [DOI] [PubMed] [Google Scholar]
- 6.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders. 3rd ed. vol 38. Cephalalgia: 2018. pp. 1–211. [DOI] [PubMed] [Google Scholar]
- 7.Durcan FJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana. Arch Neurol. 1988;45:875–7. doi: 10.1001/archneur.1988.00520320065016. [DOI] [PubMed] [Google Scholar]
- 8.Wall M, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain J Neurol. 1991;114:155–80. [PubMed] [Google Scholar]
- 9.Takkar A, Goyal MK, Bansal R, Lal V. Clinical and neuro-ophthalmologic predictors of visual outcome in idiopathic intracranial hypertension. Neuro-Ophthalmol Aeolus Press. 2018;42:201–8. doi: 10.1080/01658107.2017.1400570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCluskey G, Doherty-Allan R, McCarron P, Loftus AM, McCarron LV, Mulholland D, et al. Meta-analysis and systematic review of population-based epidemiological studies in idiopathic intracranial hypertension. Eur J Neurol. 2018;25:1218–27. doi: 10.1111/ene.13739. [DOI] [PubMed] [Google Scholar]
- 11.Digre KB, Corbett JJ. Pseudotumor cerebri in men. Arch Neurol. 1988;45:866–72. doi: 10.1001/archneur.1988.00520320056015. [DOI] [PubMed] [Google Scholar]
- 12.Kesler A, Goldhammer Y, Gadoth N. Do men with pseudomotor cerebri share the same characteristics as women? A retrospective review of 141 cases. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2001;21:15–7. doi: 10.1097/00041327-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Bruce BB, Kedar S, Van Stavern GP, Monaghan D, Acierno MD, Braswell RA, et al. Idiopathic intracranial hypertension in men. Neurology. 2009;72:304–9. doi: 10.1212/01.wnl.0000333254.84120.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruce BB, Kedar S, Van Stavern GP, Corbett JJ, Newman NJ, Biousse V. Atypical idiopathic intracranial hypertension. Neurology. 2010;74:1827–32. doi: 10.1212/WNL.0b013e3181e0f838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szewka AJ, Bruce BB, Newman NJ, Biousse V. Idiopathic intracranial hypertension: Relation between obesity and visual outcomes. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2013;33:4–8. doi: 10.1097/WNO.0b013e31823f852d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wall M, Kupersmith MJ, Thurtell MJ, Moss HE, Moss EA, Auinger P, et al. The longitudinal idiopathic intracranial hypertension trial: Outcomes from months 6-12. Am J Ophthalmol. 2017;176:102–7. doi: 10.1016/j.ajo.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Wall M. Epidemiology and risk factors for idiopathic intracranial hypertension. Int Ophthalmol Clin. 2014;54:1–11. doi: 10.1097/IIO.0b013e3182aabf11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn LT. Raised intracranial pressure. J Neurol Neurosurg Psychiatry. 2002;73(Suppl 1):i23–7. doi: 10.1136/jnnp.73.suppl_1.i23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman DI, Quiros PA, Subramanian PS, Mejico LJ, Gao S, McDermott M, et al. Headache in idiopathic intracranial hypertension: Findings from the idiopathic intracranial hypertension treatment trial. Headache. 2017;57:1195–205. doi: 10.1111/head.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sina F, Razmeh S, Habibzadeh N, Zavari A, Nabovvati M. Migraine headache in patients with idiopathic intracranial hypertension. Neurol Int. 2017;9 doi: 10.4081/or.2017.7280. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5641834/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadun AA, Currie JN, Lessell S. Transient visual obscurations with elevated optic discs. Ann Neurol. 1984;16:489–94. doi: 10.1002/ana.410160410. [DOI] [PubMed] [Google Scholar]
- 22.Farb RI, Vanek I, Scott JN, Mikulis DJ, Willinsky RA, Tomlinson G, et al. Idiopathic intracranial hypertension: The prevalence and morphology of sinovenous stenosis. Neurology. 2003;60:1418–24. doi: 10.1212/01.wnl.0000066683.34093.e2. [DOI] [PubMed] [Google Scholar]
- 23.Frisén L. Swelling of the optic nerve head: A staging scheme. J Neurol Neurosurg Psychiatry. 1982;45:13–8. doi: 10.1136/jnnp.45.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Digre KB, Nakamoto BK, Warner JEA, Langeberg WJ, Baggaley SK, Katz BJ. A Comparison of idiopathic intracranial hypertension with and without papilledema. Headache. 2009;49:185–93. doi: 10.1111/j.1526-4610.2008.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Favoni V, Pierangeli G, Toni F, Cirillo L, La Morgia C, Abu-Rumeileh S, et al. Idiopathic Intracranial Hypertension Without Papilledema (IIHWOP) in chronic refractory headache. Front Neurol. 2018;9 doi: 10.3389/fneur.2018.00503. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6029151/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Digre KB. Not so benign intracranial hypertension. BMJ. 2003;326:613–4. doi: 10.1136/bmj.326.7390.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Digre KB. Three current controversies in idiopathic intracranial hypertension. Neuroophthal. 2009;33:93–9. [Google Scholar]
- 28.Hung HL, Kao LY, Huang C-C. Ophthalmic features of idiopathic intracranial hypertension. Eye Lond Engl. 2003;17:793–5. doi: 10.1038/sj.eye.6700443. [DOI] [PubMed] [Google Scholar]
- 29.Keltner JL, Johnson CA, Cello KE, Wall M. NORDIC Idiopathic Intracranial Hypertension Study Group. Baseline visual field findings in the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT) Invest Ophthalmol Vis Sci. 2014;55:3200–7. doi: 10.1167/iovs.14-14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sibony PA, Kupersmith MJ, Feldon SE, Wang JK, Garvin M. Retinal and choroidal folds in papilledema. Invest Ophthalmol Vis Sci. 2015;56:5670–80. doi: 10.1167/iovs.15-17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007;68:229–32. doi: 10.1212/01.wnl.0000251312.19452.ec. [DOI] [PubMed] [Google Scholar]
- 32.Olesen J. International classification of headache disorders. Lancet Neurol. 2018;17:396–7. doi: 10.1016/S1474-4422(18)30085-1. [DOI] [PubMed] [Google Scholar]
- 33.Avery RA, Licht DJ, Shah SS, Huh JW, Seiden JA, Boswinkel J, et al. CSF opening pressure in children with optic nerve head edema. Neurology. 2011;76:1658–61. doi: 10.1212/WNL.0b013e318219fb80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004;75:813–21. doi: 10.1136/jnnp.2003.033126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mollan SP, Davies B, Silver NC, Shaw S, Mallucci CL, Wakerley BR, et al. Idiopathic intracranial hypertension: Consensus guidelines on management. J Neurol Neurosurg Psychiatry. 2018;89:1088–100. doi: 10.1136/jnnp-2017-317440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kupersmith MJ, Gamell L, Turbin R, Peck V, Spiegel P, Wall M. Effects of weight loss on the course of idiopathic intracranial hypertension in women. Neurology. 1998;50:1094–8. doi: 10.1212/wnl.50.4.1094. [DOI] [PubMed] [Google Scholar]
- 37.Smith SV, Friedman DI. The idiopathic intracranial hypertension treatment trial: A review of the outcomes. Headache. 2017;57:1303–10. doi: 10.1111/head.13144. [DOI] [PubMed] [Google Scholar]
- 38.Handley JD, Baruah BP, Williams DM, Horner M, Barry J, Stephens JW. Bariatric surgery as a treatment for idiopathic intracranial hypertension: A systematic review. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2015;11:1396–403. doi: 10.1016/j.soard.2015.08.497. [DOI] [PubMed] [Google Scholar]
- 39.Piper RJ, Kalyvas AV, Young AMH, Hughes MA, Jamjoom AAB, Fouyas IP. Interventions for idiopathic intracranial hypertension. Cochrane Database Syst Rev. 2015:CD003434. doi: 10.1002/14651858.CD003434.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee. Wall M, McDermott MP, Kieburtz KD, Corbett JJ, Feldon SE, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: The idiopathic intracranial hypertension treatment trial. JAMA. 2014;311:1641–51. doi: 10.1001/jama.2014.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Celebisoy N, Gökçay F, Sirin H, Akyürekli O. Treatment of idiopathic intracranial hypertension: Topiramate vs acetazolamide, an open-label study. Acta Neurol Scand. 2007;116:322–7. doi: 10.1111/j.1600-0404.2007.00905.x. [DOI] [PubMed] [Google Scholar]
- 42.Liu GT, Glaser JS, Schatz NJ. High-dose methylprednisolone and acetazolamide for visual loss in pseudotumor cerebri. Am J Ophthalmol. 1994;118:88–96. doi: 10.1016/s0002-9394(14)72847-8. [DOI] [PubMed] [Google Scholar]
- 43.Spitze A, Malik A, Al-Zubidi N, Golnik K, Lee AG. Optic nerve sheath fenestration vs cerebrospinal diversion procedures: What is the preferred surgical procedure for the treatment of idiopathic intracranial hypertension failing maximum medical therapy? J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2013;33:183–8. doi: 10.1097/WNO.0b013e318292d06f. [DOI] [PubMed] [Google Scholar]
- 44.Starke RM, Wang T, Ding D, Durst CR, Crowley RW, Chalouhi N, et al. Endovascular treatment of venous sinus stenosis in idiopathic intracranial hypertension: Complications, neurological outcomes, and radiographic results. Sci World J. 2015;2015:140408. doi: 10.1155/2015/140408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cappuzzo JM, Hess RM, Morrison JF, Davies JM, Snyder KV, Levy EI, et al. Transverse venous stenting for the treatment of idiopathic intracranial hypertension, or pseudotumor cerebri. Neurosurg Focus. 2018;45:E11. doi: 10.3171/2018.5.FOCUS18102. [DOI] [PubMed] [Google Scholar]
- 46.Falardeau J, Lobb BM, Golden S, Maxfield SD, Tanne E. The use of acetazolamide during pregnancy in intracranial hypertension patients. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2013;33:9–12. doi: 10.1097/WNO.0b013e3182594001. [DOI] [PubMed] [Google Scholar]
- 47.Weston J, Bromley R, Jackson CF, Adab N, Clayton-Smith J, Greenhalgh J, et al. Monotherapy treatment of epilepsy in pregnancy: Congenital malformation outcomes in the child. Cochrane Database Syst Rev. 2016;11:CD010224. doi: 10.1002/14651858.CD010224.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang RA. Management of idiopathic intracranial hypertension in pregnancy. Medscape Gen Med. 2005;7:40. [PMC free article] [PubMed] [Google Scholar]
- 49.Tang RA, Dorotheo EU, Schiffman JS, Bahrani HM. Medical and surgical management of idiopathic intracranial hypertension in pregnancy. Curr Neurol Neurosci Rep. 2004;4:398–409. doi: 10.1007/s11910-004-0087-4. [DOI] [PubMed] [Google Scholar]
- 50.Ko MW, Liu GT. Pediatric idiopathic intracranial hypertension (Pseudotumor Cerebri) Horm Res Paediatr. 2010;74:381–9. doi: 10.1159/000321180. [DOI] [PubMed] [Google Scholar]


