Abstract
Presynaptic boutons support neurotransmitter release with nanoscale precision at sub-millisecond timescales. Studies over the last two decades have revealed a rich tapestry of molecular players governing synaptic vesicle fusion at highly specialized release sites in the active zone. However, the spatiotemporal organization of release in active synapses remain elusive, in part due to the extremely small size of the active zone, and limited resolution of conventional approaches. Recent advances in fluorescence nanoscopy have revolutionized direct investigation of presynaptic release organization and dynamics. Here we discuss recent nanoscopy-based studies of the molecular architecture, spatial organization and dynamic regulation of release sites, and the mechanisms of release site replenishment. These findings uncovered previously unknown levels of structural and functional organization at central synapses with important implications for synaptic transmission and plasticity.
Keywords: Neurotransmitter release, Synaptic transmission, Vesicle recycling, Release sites, Nanoscopy, Superresolution microscopy
Fluorescence nanoscopy permits direct investigation of synaptic vesicle release in central synapses
Transmission of information in the brain is governed by the release of neurotransmitter-containing vesicles at the synaptic active zone (AZ) (see Glossary). The extremely small size of the AZ, typically at or under ~250nm, is below the limit of spatial resolution of most conventional experimental techniques and represents a great challenge to studying organization and regulation of vesicle release. Thus, while we know a great deal about the molecular components of the AZ that orchestrate vesicle release, direct investigation of the composition, spatial organization, and dynamics of the release sites within the AZ has, until recently, been very limited. The difficulty of directly accessing the interior of central synapses has also limited understanding of how the number, properties and refilling of the release sites are regulated, particularly during neural activity. Similarly, until recently, our understanding of synaptic vesicle dynamics during recycling and translocation to the AZ has been based primarily on extrapolations from indirect biochemical and electrophysiological assays.
The recent advances in nanoscale imaging tools (i.e., nanoscopy, Box 1) have provided unprecedented access to the interior of individual central synapses. Here, we present an overview of recent advances in our understanding of vesicle release mechanisms in central synapses made possible by nanoscopy tools. First, we discuss how release machinery is spatially organized at a nanoscale within the AZs. We then present recent findings on the spatiotemporal organization of vesicle release sites at individual AZs. Finally, we describe mechanisms of release site refilling. Throughout the review, we highlight emerging findings on dynamic regulation of these vesicle release mechanisms during neural activity. These studies have revealed new levels of organization of release within the AZ with important implications for synaptic transmission and plasticity. We note that given the breadth of the literature on the topic and because of the primary focus of this review on central synapses, we were able to include only a subset of many excellent studies performed in PC12 cells and the NMJ.
Box 1. Nanoscopy techniques.
SMLM:
SMLM is widely used to study the dynamics of individual proteins or vesicles. This family of techniques, as well as most other nanoscopy tools, use a mathematical theory developed ~20 years ago [1] to precisely localize an individual light source: the PSF-like image of the light source is fitted to a two-dimensional Gaussian to determine its location, with a typical precision (for SMLM) of ~20nm and temporal resolution of ~10–30ms in live cells. The main limitation of SMLM tools in application to vesicle release studies is in its ability to image only a single or a very small number of light sources (proteins/vesicles) within the typical dimensions of a central synapse.
STORM:
STORM takes advantage of fluorophore blinking characteristics to temporally isolate individual fluorophores by driving the majority of fluorescent molecules into a dark state. A small number of individual fluorophores randomly returns to fluorescent state at any given time, generating sparse fluorescence signal that can be localized with nanometer precision using SMLM-like mathematical tools. The temporal separation in activation of different molecules permits a population of densely overlapping fluorophores to be localized with nanometer precision, resulting in a high-resolution structural data. STORM has been extended to 3D microscopy in fixed preparations [2], and live cells [3]. This approach has an intrinsically limited temporal resolution because a very large number of frames (~30,000 in neurons [4]) is required to obtain a high-resolution image.
STED:
This technique utilizes a pair of geometrically controlled and spatially separated laser beams to direct the excitation of fluorophores—isolating them spatially rather than temporally. The first sub-picosecond excitation laser pulse produces the typical diffraction-limited spot of excited fluorophores. A second, redshifted stimulation-depletion pulse immediately returns the fluorophores to their ground state without emitting a photon. This second pulse is focused in a toroid-like shape that surrounds the excitation beam, effectively allowing only the fluorophores in the center of the toroid to emit light. The coaxial configuration reduces the effective size of the PSF to ~50nm in the imaging plane in live tissue. STED permits image acquisition with video rates (~24frames/sec) [7] and has been extended to 3D imaging [8–10]. Its only notable limitation is a relatively small field of view imposed at high acquisition rates [8, 11], which limits the number of synapses that can be imaged simultaneously.
Spatial Organization of Release Machinery at the Active Zone
Scaffolding and Tethering
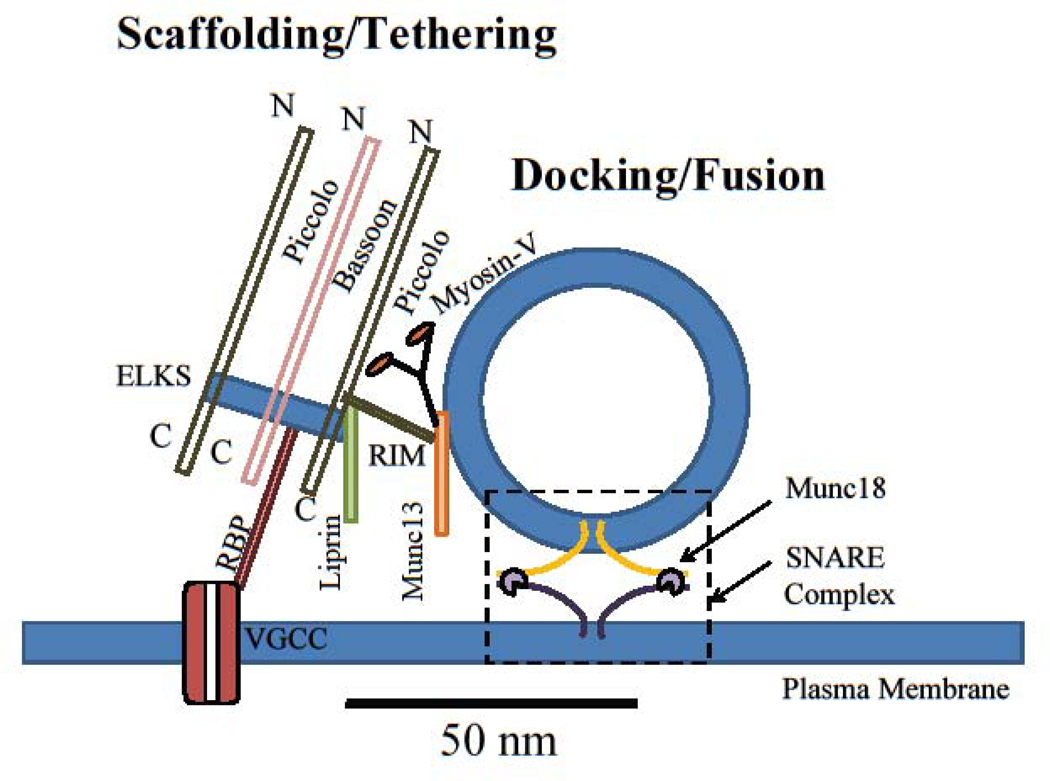
Recent applications of nanoscopy techniques have provided insights into the organization of all major scaffolding proteins such as Piccolo, Bassoon, ELKS and Liprin-α, at the AZ, as well as of tethering proteins, such as RIM1/2 and Munc13, which position synaptic vesicles at the release sites for subsequent exocytosis (Figure 1). Multicolor 3D STORM data revealed the two major AZ scaffolding proteins, Piccolo and Bassoon, have similar axial distribution (i.e. perpendicular to AZ plane) in mammalian central synapses, with their N-terminal domains 50–90nm from the presynaptic membrane [5]. These data combined with subsequent STED measurements [6] showed that Piccolo and Bassoon are oriented in respect to the AZ plane with their C-termini about 30nm closer to the plasma membrane than their N-termini. 3D STORM also revealed that the tethering protein RIM1 is even closer to the presynaptic membrane, ~35nm, consistent with its function in synaptic vesicle attachment [5]. Other scaffolding and tethering proteins, including Munc13, ELKS2, Liprin-α3, and RIM binding protein RBP2 were all localized within the area between Bassoon’s C- and N-termini, thus forming a protein complex at the AZ, within 50–100 nm of plasma membrane [5, 6, 12].
Figure 1: The spatial organization of proteins at release sites of the active zone.
Scaffolding proteins (Piccolo, Bassoon, ELKS, Liprin-α, etc) organize individual release sites. Tethering and docking proteins (RIM1/2, Munc13, myosin V, Munc18, etc) orchestrate assembly of release machinery and synaptic vesicle docking at the release sites. Release machinery (SNAREs, synaptotagmins, etc) mediate synaptic vesicle fusion with the plasma membrane to release neurotransmitter. Dashed box represents the vesicle docking/fusion area at which the SNARE complex formation occurs. VGCC: voltage-gated calcium channels; RBP: RIM binding protein; N: N terminus; C: C terminus.
The complex lateral distribution patterns of scaffolding and tethering proteins along the AZ surface is also beginning to emerge at a nanoscale. Understanding the positioning of these proteins relative to the voltage-gated calcium channels (VGCCs) is particularly important because it determines calcium diffusional distances and thus the timescale by which calcium reaches the release machinery [13]. Dual-color STED studies of mouse NMJ indicate that clusters of Bassoon are localized axially above P/Q-type VGCCs and sandwiched, laterally, by clusters of Piccolo [14]. Interestingly, although clusters of Bassoon and P/Q-type VGCCs are also localized in close proximity in the hippocampal synapses, STORM measurements found limited spatial overlap between these proteins at nanoscale, suggesting there is at least some spatial segregation (~25–50nm) [12, 15]. However, these data should be interpreted with caution, as the differences in size and shape of the two protein clusters could result in measurable nearest-neighbor distances, even if the two are highly co-localized. In hippocampal synapses, RIM1/2 has also been found to form nanoclusters that are more likely to be located near the center of the AZ than in the periphery [16]. The restricted lateral organization of RIM proteins could be related to their preference for PIP2-membrane lipids [17]. Dual-color STORM and STED measurements have shown that RIM1/2 is within 40–50 nm of VGCCs [12, 15], while the distance between Bassoon and VGCCs is somewhat larger, ~75 nm in hippocampal synapses [12]. Nanoscopy approaches are thus instrumental in defining the precise spatial organization and interrelationships of the scaffolding and tethering machinery at the AZ.
Nanoscopy techniques have even proven useful in identifying molecules not previously known to contribute to AZ scaffolding and tethering. For example, SMLM data helped identify the synaptic vesicle-associated motor protein, myosin V as a vesicle tethering protein [18]. Myosin V has long been known to function as an actin-dependent, cargo-transporting motor [19], but it can also interact with SNARE proteins in a Ca2+-dependent manner [20], although the role of this interaction in vesicle release remained elusive. SMLM-based single-vesicle tracking showed that myosin V controls vesicle retention at release sites in an activity-dependent manner [18].
Docking and Fusion
Vesicle docking and subsequent calcium-driven fusion are mediated by the SNARE complex, which consists of the vesicular protein synaptobrevin, and plasma membrane proteins SNAP25 and syntaxin-1. Vesicle fusion also relies on interactions between SNARE proteins and Ca2+ sensors in the synaptotagmin family, as well as Sec1/Munc18-like (SM) proteins, such as Munc18. The total lateral interaction distance of the SNARE complex is less than 50nm [21]. Thus, direct studies of SNARE complex formation and function have historically been limited by intrinsic spatial resolution constraints of even the most advanced microscopy approaches.
Nanoscopy studies in neuroendocrine cells (excellent reviews available elsewhere, see for example [22, 23]) and more recently in the NMJ and central neurons reveal clusters of several SNARE proteins, including syntaxin-1 and SNAP-25, as well as single, freely-diffusing copies of syntaxin-1 and Munc18 [4, 24–27]. Achieving higher photon counts enabled Pertsinidis et al. to increase localization precision to ~10nm, revealing that syntaxin-1, SNAP-25, and Munc18 colocalize in ~50–100nm clusters on the plasma membrane in mouse hippocampal neurons [24]. Interestingly, this study found that genetic deletion of syntaxin-1 caused a loss of Munc18/SNAP-25 co-localization and dispersal of Munc18 into the cytoplasm, but SNAP-25 remained clustered at the membrane, suggesting that its localization is independent of syntaxin-1 [24]. This notion is further supported by STORM imaging showing that the two SNARE proteins exhibit distinct clustering structures; SNAP-25 clusters are significantly larger, less dense, and take on a shape distinct from clusters of syntaxin-1 [28].
Munc13 is another SNARE-interacting protein that is critical in the late stages of exocytosis, yet its precise function in vesicle docking/priming remains unclear. Recent nanoscopy studies have demonstrated that Munc13 molecules form multiple discrete clusters that serve to recruit the syntaxin-1/Munc18 assembly, thus enabling open syntaxin-1 molecules to form active SNARE complexes [4]. The number of Munc13 clusters per AZ (~5 on average, range [2–18]) closely correlates with the number of docked vesicles, as well as the estimated number of release sites [4]. The finding that manipulation of the Munc13 levels strongly correlated with changes in functionally estimated number of release sites suggested that Munc13 plays an important role in regulating synaptic strength. This notion is further supported by studies in C. elegans showing a correlation between unc-13 (Munc-13) location within the AZ and release properties, including release probability and kinetics [29, 30].
Despite the significant progress described above, more work is needed to establish the mechanisms governing the spatial organization and assembly of release machinery at the AZ and the role of this organization in setting the mode and kinetics of release. For example, functional studies suggest that different forms of release (i.e. spontaneous vs evoked) operate at distinct locales within the synapse [31, 32], but it remains to be elucidated whether and how the spatial organization of different forms of release is determined by the properties of docking and release machinery.
Dynamics of release machinery at the AZ
Whereas some of proteins at the AZ remain relatively stable, such as unc13 (Munc13) [21], other proteins are highly dynamic. For example, the number of Bassoon molecules at the AZ changes over the course of minutes [33]. Moreover, many other critical components of release machinery, including VGCCs, Syntaxin-1, and Munc18, are highly mobile within the AZ [15, 21, 27, 34, 35]. This presents an additional experimental challenge. Snapshot techniques, even those based on nanoscopy technology, are likely to return variable results across timepoints. The spatial distribution of VGCCs is a prime example of this. Several groups have suggested that VGCCs form presynaptic clusters at the nanometer scale [36, 37], yet the distribution of P/Q-type VGCCs at mouse inner hair cell ribbon synapses resembled that of Bassoon, i.e., broad ~500nm clusters within the AZ [38]. This discrepancy may be at least partially explained by the SMLM-based findings that a large proportion (>50%) of P/Q- and N-type VGCCs are mobile within the AZ [34] (surface area explored by individual channels is ~200–250 nm, comparable to AZ dimensions [39]).
In support of the highly dynamic organization of release machinery, SMLM also showed that a substantial fraction of Syntaxin-1a and Munc18 molecules are mobile in the plasma membrane with molecular speeds of 1–10μm/s [25, 27, 34, 35]. SMLM also found that Syntaxin1a switches between periods of rapid lateral diffusion and pausing in precise locations within synaptic boutons, with pauses corresponding to the formation of the exocytotic complex with two kinetically distinct binding reactions [27]. Moreover, neural activity altered the enrichment of many scaffold proteins within their clusters, including RIM, Bassoon, and Munc13 [15, 16, 25, 40]. Nano-column formation of RIM/PSD-95 clusters is also regulated by synaptic activity [16]. Inactivity can also cause AZ restructuring, with effects generally opposite to those of increased activity. For example, prolonged neuronal silencing can dissociate Bassoon clusters from the AZ, and levels of VGCCs and Picolo increase with synaptic inactivity [15]. These observations suggest that ongoing activity constantly shapes the AZ cytomatrix architecture and can influence how essential release machinery is recruited.
Taken together, recent nanoscopy data reveals a rich landscape of precisely regulated structural organization and activity-dependent dynamics among presynaptic proteins that ultimately determines synaptic vesicle release processes.
Number and Organization of Release Sites in the Active Zone
Release site number
The number and availability of vesicle release sites have been hypothesized to play critical roles in regulating synaptic transmission [41]. Initial estimates of release site number (Nsite) were generated from fluctuation analysis of release in electrophysiological studies. These data led to the conclusion that each AZ represents a structural correlate of a quantal release site [41–43]. However, observations of multivesicular release [44, 45] suggested that synapses have multiple release sites. While ultrastructural analyses have established that over 90% of central synapses have a single AZ [39], whether the individual AZs contain a single or multiple release sites and how their properties are regulated have remained largely unknown. Nanoscopy techniques have recently made it possible to directly visualize single release events at individual AZs and determine release site number and distribution.
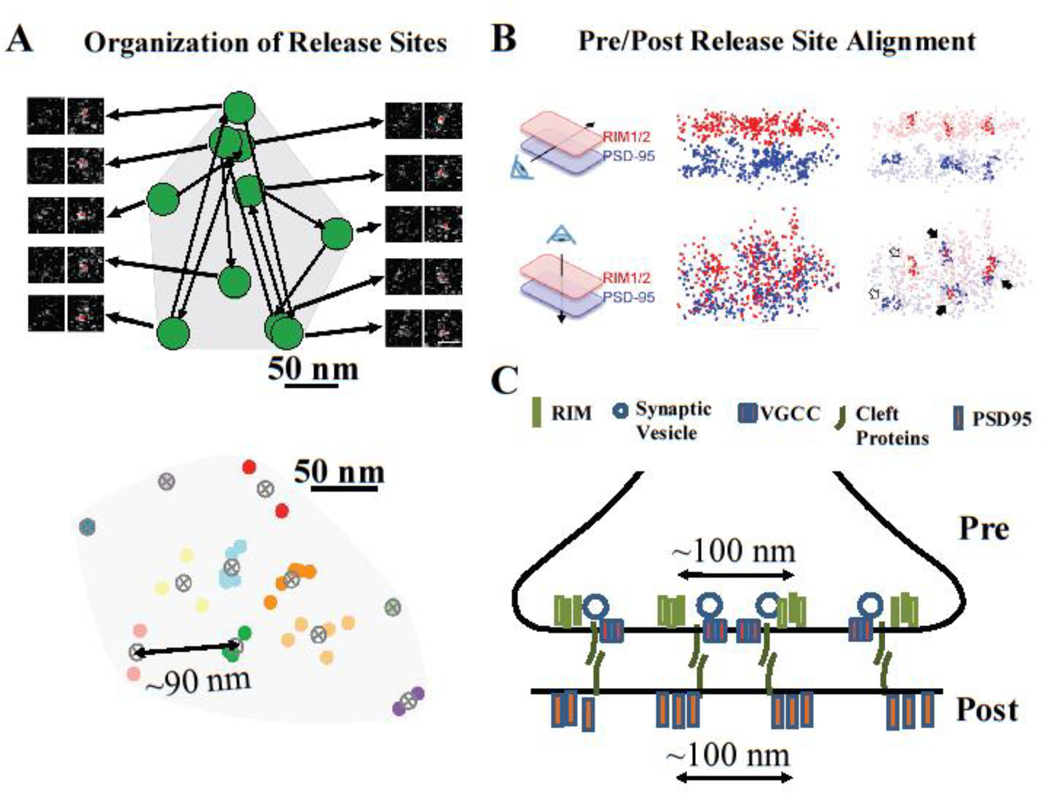
The most direct way to estimate Nsite is to label vesicles and count fusion events. Maschi et al. developed an SMLM–based approach that, in combination with the pH-sensitive indicator vGlut1-pHluorin, can robustly detect individual vesicle release events with a ~27nm localization accuracy in cultured hippocampal synapses [46]. This analysis revealed the presence of multiple discrete release sites within the individual AZs (Figure 2A); these release sites were widely distributed across the AZ without preference for the AZ center vs its periphery, and underwent repeated rounds of reuse. The number of release sites per AZ (Nsite) in these measurements varied across the synapse population in a range of 5–14 (average ~9). It is important to note that these measurements likely slightly overestimate the average Nsite because only synapses with at least 10 release events were included in these analyses (to ensure that only AZs nearly parallel to the imaging plane were analyzed), thus excluding a population of synapses with a very low release probability and small Nsite. Another recent estimate of Nsite was based on a quantal analysis of single synapses using imaging with an enhanced glutamate optical sensor (eEOS) [4]. This work produced an estimate of an average Nsite = 5 also with large variability among the AZs (range 1:18). The functional estimates of Nsite demonstrating the presence of multiple release sites per AZ are consistent with estimates based on structural data, i.e., the number of clusters of presynaptic proteins required for vesicle tethering/docking. In one study, PALM was used to localize RIM1/2 molecules and SMLM was used to track vesicle fusion, all in the same boutons [16]. The findings indicate that 1–6 nanoclusters of RIM1/2 (average ~2 clusters) colocalize with vesicle fusion events, as defined by the vGlut1-pHluorin signal (Figure 2B). These measurements likely somewhat underestimate the true Nsite values because (i) the density threshold for detecting RIM clusters may exclude some clusters, and (ii) a short acquisition paradigm used for functional definition of release sites underrepresents synapses with large Nsite. Thus, both functional and structural nanoscopy approaches indicate the presence of multiple discrete release sites in central synapses with a wide range of Nsite values across the synapse population.
Figure 2: Spatial organization of release sites in the active zone.
(A) SMLM-based functional measurements of individual vesicle fusion events in hippocampal synapses revealed multiple clusters or release events representing distinct release sites within individual AZs, with ~90nm between clusters (data modified with permission from ref [46]). (B) PALM and STORM measurements revealed that nanoclusters of presynaptic docking factors (RIM1/2) align trans-synaptically with nanoclusters of post-synaptic proteins (PSD95) (data reproduced with permission from ref [16]). (C) Cartoon representation of trans-synaptic alignment of functionally defined presynaptic release sites, nanoclusters of presynaptic docking factors and nanoclusters of post-synaptic proteins, such as PSD95. Protein cluster-to-cluster distances are similar to release site distances in (A).
The range of Nsite values and its variability are comparable to that of the number of docked vesicles observed in EM of hippocampal synapses (range 1–10 vesicles) [37, 47] and calyx of Held (range 3–7 vesicles) [48]. Moreover, computational 3D reconstruction of a presynaptic bouton with 5–11 release sites per AZ is able to reproduce key aspects of neurotransmitter release in the calyx of Held, including release probability and kinetics [49]. While these data support the idea that individual AZs harbor multiple release sites, further study is needed to confirm the identity between functional release sites and ultrastructurally observed docking sites.
Studies of nanoscale events within AZs revealed evidence of multiple release sites. Thus, as with studies of AZ protein organization and function, nanoscopy has led to fundamental new insights into synaptic release mechanisms.
Spatial Organization Of Release Sites
The findings of multiple release sites within AZs lead to another key question: How are release sites spatially organized within the AZ and what proteins determine their localization? Several complementary nanoscopy studies recently began to address these questions. SMLM-based functional measurements in hippocampal synapses indicate that release sites are localized throughout the AZ area with the nearest-neighbor distances of ~90nm (Figure 2A) [46]. This is consistent with STORM measurements showing cluster-to-cluster distances for both pre- and post-synaptic proteins (i.e., RIM-to-RIM, or PSD95-to-PSD95) on the order of 100nm (Figure 2B,C) [16]. Moreover, this spatial organization may reflect some optimal state, as even small changes in distance (i.e., induced via RBP2 mutations) decreased exocytosis [12]. STORM combined with sequential PALM/SMLM reveals that functionally defined release sites not only co-localized with the clusters of RIM1/2, but also align axially with clusters of postsynaptic AMPA receptors, and scaffolding proteins Homer and PSD95 (Figure 2C) [16]. These findings suggest trans-synaptic molecular nanocolumns may align pre- and postsynaptic molecular machinery for efficient signal transmission.
Although combining structural and functional nanoscopy has led to dramatic progress in understanding release site organization at the AZ, a number of challenges remain. For example, STORM showed that Bassoon clusters have little nanoscale overlap with VGCC and RIM clusters [15]. Moreover, STED revealed a significant spatial separation at a nanoscale between VGCC and RIM clusters in glutamatergic synapses [12], suggesting that these proteins may not co-localize within the same release site, although it is uncertain whether neighboring clusters in this study belong to the same AZ/synapse. In addition, STORM measurements found clusters of many pre- and postsynaptic scaffolding proteins, including RIM1/2, Munc13, Bassoon and PSD95, are localized predominately within ~50nm of the synapse center [16], while functional measurements based on release event localization using Q-dots [50] or vGlut1-pHluorin [46] observed a much wider spatial distribution of release events with distances of 50–200nm to the AZ center.
One important caveat in comparing spatial measurements across studies is different methods used in the definition of clusters, which likely lead to differences in cluster number and density, thus altering the cluster-to-cluster distances. Also, the localization of clusters within the AZ depends strongly on the definition of the AZ/bouton dimensions and center, which have been defined in a number of different ways, and thus preclude direct quantitative comparisons. Furthermore, in many studies in which antibody labeling is used to localize presynaptic tethering or docking proteins, confounds such as where an antibody binds to these large proteins, and the size of the antibody tree composed of primary and secondary antibodies may strongly affect the measured distances between the protein clusters. Thus, although the spatial organization of release sites is beginning to emerge, further studies are needed to reveal the molecular determinants of this organization within individual AZs.
Dynamics and Regulation of Release Site Use
The idea of multiple spatially distributed release sites within individual AZs also begs the question of whether release site usage is regulated. At NMJ synapses, the pattern of release site usage depends on activity level; vesicles fuse uniformly across hundreds of AZs within a single presynaptic terminal at low activity, whereas strong stimulation drives fusion more focally at a small subset of AZs [32, 51]. In contrast to the NMJ, SMLM suggests that at central synapses increased activity reduced the frequency of release site reuse [46]. Specifically, a prior event reduced the probability of release at the same site for at least 100 ms. This could reflect either an intrinsic refractory period [41], or vesicle refilling time [52]. Another difference from the NMJ is that release becomes more spatially distributed in central synapses in response to activity, with usage shifting towards more peripheral release sites during high-frequency stimulation [46]. Thus, many release sites within the same AZ may allow synapses to adjust during elevated activity. A shift in the spatial distribution of release may also effectively engage a larger population of postsynaptic receptors by spreading neurotransmitter release across the AZ, thereby minimizing receptor saturation and allowing synapses to maintain their strength during elevated activity. Interestingly, differential spatial distribution has been observed for two functionally distinct modes of release: kiss-and-run (closer to the AZ center) vs full fusion events (more peripheral) [50]. The observation of an activity-dependent shift toward increased reuse of peripheral release sites [46] is consistent with the shift from kiss-and-run to full fusion following an increase in stimulation frequency [53, 54].
Accumulating evidence suggests then that release site utilization is not random. Rather, dynamic regulation likely facilitates rapid and efficient adaptation to different levels of activity. This mechanism adds a new dimension to the landscape of spatiotemporal regulation of neurotransmitter release.
Synaptic vesicle dynamics and refilling of release sites
Vesicle translocation during recycling
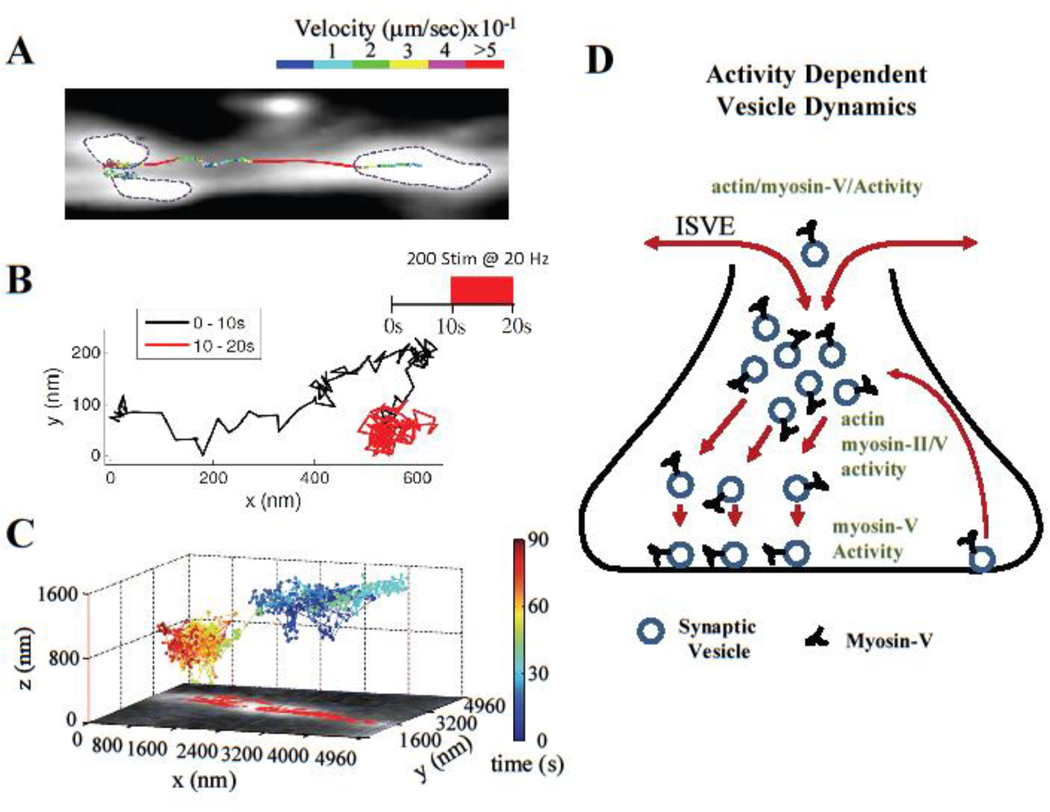
Most central synapses contain a very small pool of releasable vesicles. This necessitates a precisely controlled vesicle recycling program to sustain release, and means that vesicle mobility is likely to be a rate-limiting step in the refilling of release sites. Early studies performed at room temperature and using predominately bulk assays of vesicle dynamics have led to the conclusion that synaptic vesicles are largely immobile within the boutons, or have restricted random mobility [55–58]. These findings seem to be at odds with other studies reporting that newly endocytosed vesicles are often located hundreds of nanometers away from the AZ [47, 59], and, similarly, that sites of exo- and endocytosis are spatially separated [60, 61]. This scenario would suggest that vesicles need to travel a long-distance to reach their release sites at the AZ. The key to reconciling these two sets of results is that vesicle properties are heterogenous [62–67]. Thus, bulk measurements of vesicle dynamics and translocation behavior are likely to miss lower probability events, including perhaps subsets of vesicles that do travel to reach release sites. Indeed, several recent studies using SMLM to track single vesicles labeled with lipophilic dyes [68, 69] or Q-dots [50, 70], as well as studies applying STED [8, 71] reveal that a majority of recycling vesicles (which represent ~20% of total vesicle population) undergo large-scale translocation within small central synapses, sometimes covering several hundred nanometers (Figure 3A–C). This agrees with earlier TIRF microscopy findings of extensive vesicle mobility near the plasma membrane in ribbon synapses [72, 73]. Advanced transient motion analyses revealed that vesicle motion comprises a complex set of dynamic behaviors, including epochs of active, diffusive and stalled motion [68, 69]. Thus, more direct studies of vesicle mobility support the idea that vesicles undergo extensive translocation within synaptic boutons.
Figure 3: Synaptic vesicle dynamics during recycling and release site replenishment.
(A) Single-vesicle tracking of recycling synaptic vesicles revealed complex dynamical patterns of mobility with multiple types of motion inside synaptic boutons and along the axon (data reproduced with permission from ref [81]). (B) SMLM-based analysis of individual vesicle trajectories revealed that vesicle dynamics inside synaptic boutons is activity-dependent (data reproduced with permission from ref [69]). (C) SMLM-based tracking of single Q-dot labeled vesicles in hippocampal synapses showed that synaptic vesicle mobility toward the AZ depends on vesicle initial localization within the bouton (data reproduced with permission from ref [50]). (D) Cartoon representation of synaptic vesicle recycling and its dynamic modulation. During the recycling process, vesicle mobility inside the synaptic boutons is supported, in part, by actin and myosin motors, and is rapidly modulated by neural activity. Vesicle retention at the release sites is myosin V- and activity-dependent. Synaptic vesicles are also exchanged among synaptic boutons via an actin/myosin V-dependent inter-synaptic vesicle exchange (ISVE), which is dynamically regulated by neural activity.
The role of actin/myosins in release site refilling
Nanoscopy applications have begun to reveal the mechanisms controlling vesicle motion during recycling and refilling of release sites [74] (Figure 3D). SMLM studies indicate that acutely inhibiting actin polymerization or myosin II reduces vesicle motion in hippocampal boutons [68]. Similarly, in cerebellar synapses, vesicle refilling at release sites requires actin and myosin II [75]. These studies strongly support the idea that a component of vesicle motion is mediated by actin-based transport. However, which specific step(s) in the recycling and refilling processes is actin/myosin-dependent needs further investigation. For example, myosin II does not itself possess cargo-binding/transporting ability; instead, it acts indirectly by promoting actin dynamics, which are required for processive function of other myosin isoforms [76]. Among actin-dependent motors, only myosin V is associated with presynaptic vesicles in central neurons [77]. SMLM measurements in hippocampal synapses indicate that acutely inhibiting myosin V markedly reduces the probability of release site reuse, and causes a profound vesicle tethering/docking defect during elevated activity [18]. This is consistent with earlier EM observations that the number of docked secretory vesicles in neuroendocrine cells is reduced when myosin V is inhibited [78]. SMLM revealed that vesicles undergo cycles of docking and undocking at the AZ and that myosin V functions to control vesicle retention at release sites in an activity-dependent manner, rather than vesicle transport to release sites [18]. This function is consistent with myosin V’s ability to interact with SNARE proteins, including syntaxin 1A and synaptobrevin, and its transition from a transporting motor to a tether in a calcium-dependent manner [76]. It is important to note that these findings focused specifically on the refilling process at the AZ, and thus do not argue against an additional role of actin cytoskeleton and myosin motors in transporting vesicles earlier in the recycling process, i.e., prior to vesicle tethering/docking.
Inter-Synaptic Vesicle Exchange
In addition to local recycling within synaptic boutons, there is also a long-distance mechanism for maintaining vesicle pools through which vesicles leave the presynaptic area and travel into and along the axon from one bouton to another [79, 80] (Figure 3A,D). This process, known as inter-synaptic vesicle exchange (ISVE), is thought to represent an important mechanism for redistribution of synaptic weight across multiple boutons. However, until recently, the mechanistic details regarding how ISVE is organized and regulated remained largely unexplored.
Recent nanoscopy approaches have substantially improve our understanding of this process. SMLM experiments using Qdots or lipophilic dyes as vesicle tags indicate that a significant proportion of vesicles (from 15% to >50%, depending on experimental condition and/or model system) participate in ISVE [50, 68, 70, 81]. During ISVE, vesicles are highly mobile and directed, leading researchers to hypothesize mediation by microtubule-based transport [70, 80]. However, SMLM revealed that ISVE in hippocampal neurons relies, at least in part, on actin and myosin V [81]. This is true not only in the vicinity of synaptic boutons, but also for long-distance vesicle transport between synaptic boutons. In particular, the fastest component of directed vesicle motion in the axon is significantly reduced when actin is destabilized or myosin V is inhibited [81]. Moreover, myosin V also supports anterograde axonal transport of large dense-core vesicles [82]. These observations are consistent with recent results showing an extensive, dynamic, non-branched actin network within the axon [83], which is both close and parallel to the microtubule network. Thus, a new model of ISVE is developing in which vesicles travel via actin and microtubule networks interchangeably and myosin V plays a significant role in vesicle transport along the axons.
The translocation mechanisms involved in ISVE may not be limited to synaptic vesicles. Indeed, some AZ proteins also travel between synaptic boutons. For example, VGCCs traffic between synapses, perhaps as part of a synapse sharing/maintenance mechanism [34]. Interestingly, the mobility of VGCCs is an order of magnitude greater in axons than in synaptic boutons, similar to what is observed with synaptic vesicles. This suggests cytoskeleton-based mechanisms may be involved in moving VGCC along axons [34].
Activity-Dependent Regulation of Vesicle Mobility
Whether vesicle dynamics are regulated in an activity-dependent manner is still under debate. Initial STED and SMLM studies did not find any measurable activity-dependent changes in vesicle dynamics at room temperature [8, 58]. However, single-vesicle synaptopHluorin-based studies found that synaptic vesicle retrieval is both calcium- and temperature-dependent [84, 85]. Moreover, activity-dependent alterations associated with induction of LTP can increase vesicle mobility within hippocampal boutons [70]. Indeed, more recent SMLM studies, this time performed at 37°C, report that high-frequency stimulation evokes marked changes in vesicle motion (Figure 3B), but the response to stimulation is heterogeneous: a subset of vesicles “decelerates”, with a corresponding reduction in directed motion; while a second subset “accelerates” and shows an increase in directed motion; still other vesicles show no response to stimulation at all [69]. Such heterogeneous and opposing changes are likely to partially mask each other in analyses of the entire vesicle population, which may explain why these effects were not previously detected. While changes in the directed vesicle motion induced by activity suggest involvement of actin, future studies are needed to determine the complex molecular mechanisms by which activity regulates vesicle dynamics.
Nanoscopy also provided important insights into activity-dependent regulation of ISVE, which was originally thought to be a constitutive activity-independent process. SMLM observations suggest that ISVE is indeed rapidly regulated by activity, which causes a 3-fold reduction in the rate of vesicle exit from bouton to axon and also reduces vesicle mobility along the axon during high-frequency stimulation [81].
These recent nanoscopy-based studies suggest that vesicle mobility is not random, and have begun to reveal a complex set of mechanisms that control vesicle dynamics at different stages of recycling. This includes the growing recognition for the role of actin and myosin motors in vesicle translocation within and between synaptic boutons, and in vesicle tethering at the release sites.
Concluding Remarks
Advances in fluorescence nanoscopy have provided major insights into the nanoscale organization within synaptic boutons, revealing intriguing new features of vesicle transport, tethering, release, and recycling. Some of the important future directions in this area are outlined in the Outstanding Questions. Answers to these questions are likely to have major implications not only for understanding neurotransmitter release itself but also for various forms of synaptic plasticity.
OUTSTANDING QUESTIONS.
Release site organization and stability: Is the spatial organization of proteins forming release sites and/or the release sites themselves stable over time? Do scaffolding/tethering proteins dynamically change their localization/orientation to facilitate vesicle binding? What types of interactions control these dynamics?
Release site properties and use: How does the spatial organization of scaffolding /tethering/docking factors determine release site properties (e.g., site usage frequency or vesicle release probability)? Are all release sites equivalent or is there a spatial distribution of release site properties across the active zone? Are there specialized sites that support different forms of vesicle release, such as multi-vesicular, asynchronous, or spontaneous release?
The role of actin cytoskeleton and myosin motors in release site organization and re-use: Is there a structural role for actin in release site organization and/or spatial distribution? Do actin/myosins mediate or modulate scaffolding or docking processes? Are different myosin isoforms involved in modulating distinct steps in vesicle recycling, tethering, and docking? What other steps prior to release site refilling may be controlled by actin and/or myosins?
Activity-dependent modulation of release site organization and its role in synaptic plasticity: How does neuronal activity modulate the timescale of protein clustering at release sites? Does activity modulate the spatial organization or total number of release sites? If so, at what timescale? Are the association/dissociation time constants of tethering/docking factors at release sites activity-dependent? Do protein clustering dynamics, or other forms of activity-dependent modulation that impact release site properties, contribute to synaptic plasticity?
HIGHLIGHTS.
Scaffolding, tethering, and docking proteins are dynamically organized to form release sites within the synaptic active zone. Neural activity controls the strength of protein cluster organization, the enrichment of many scaffold proteins within their clusters, and protein colocalization at the nanoscale.
Multiple, non-random release sites are present within individual active zones, and their spatial and temporal usage is regulated in an activity-dependent manner.
Functional release sites co-localize with nanoclusters of presynaptic docking factors that are aligned with nanoclusters of postsynaptic receptors via trans-synaptic nanocolumns.
Synaptic vesicle mobility during release site refilling is actin/myosin- and activity-dependent.
A significant proportion of recycling vesicles are exchanged among neighboring synaptic boutons. This inter-synaptic vesicle exchange process relies, in part, on actin/myosin-dependent vesicle transport in the axon and is rapidly modulated by neural activity.
ACKNOWLEDGMENTS
This work was supported in part by grant to VAK from NINDS (RO1 NS105776).
GLOSSARY
- PSF
Point-Spread Function; PSF represents a diffraction pattern of light produced by a point light source. PSF determines the resolution of an imaging system.
- SMLM
Single Molecule Localization Microscopy; a general term for high-resolution fluorescence microscopy measurements that study the dynamics of single particles or molecules.
- GSDIM
Ground State Depletion and Individual Molecule return; a general term for a collection of high-resolution microscopy methods that bring fluorophores to a dark state before returning a small subset back to a fluorescent state to localize them individually.
- STORM
STochastic Optical Reconstruction Microscopy. A GSDIM technique that uses stochastic fluorophore blinking characteristics to isolate a small subset of fluorescent molecules temporarily from a dense population.
- PALM
Photoactivation Localization Microscopy. A GSDIM technique similar in principle to STORM wherein a small subset of photoswitchable fluorescent molecules is isolated temporarily from a dense population via stochastic photo switching and subsequent photobleaching.
- STED
STimulated Emission Depletion. A nanoscopy approach that uses geometrically controlled laser sources to reduce the effective size of the PSF.
- Active Zone
Location at the pre-synaptic membrane at which synaptic vesicles fuse to release neurotransmitter.
- Release site
Specialized areas within an active zone at which protein complexes work to tether and fuse synaptic vesicles with the plasma membrane.
- Docking
A process that mediates precise synaptic vesicle positioning at the release site, as well as SNARE complex assembly.
- ISVE
Inter-synaptic Vesicle Exchange. A process by which synaptic vesicles move among neighboring synaptic boutons via axonal transport.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Thompson RE, Larson DR, and Webb WW, Precise Nanometer Localization Analysis for Individual Fluorescent Probes. Biophys J, 2002. 82(5): p. 2775–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang B, et al. , Three-Dimensional Super-Resolution Imaging by Stochastic Optical Reconstruction Microscopy. Science, 2008. 319(5864): p. 810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones SA, et al. , Fast, three-dimensional super-resolution imaging of live cells. Nat Methods, 2011. 8: p. 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakamoto H, et al. , Synaptic weight set by Munc13–1 supramolecular assemblies. Nat Neurosci, 2018. 21(1): p. 41–49. [DOI] [PubMed] [Google Scholar]
- 5.Dani A, et al. , Superresolution Imaging of Chemical Synapses in the Brain. Neuron, 2010. 68(5): p. 843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong MY, et al. , Liprin-α3 controls vesicle docking and exocytosis at the active zone of hippocampal synapses. Proc Natl Acad Sci U S A, 2018. 115(9): p. 2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godin Antoine G., Lounis B, and Cognet L, Super-resolution Microscopy Approaches for Live Cell Imaging. Biophys J, 2014. 107(8): p. 1777–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westphal V, et al. , Video-Rate Far-Field Optical Nanoscopy Dissects Synaptic Vesicle Movement. Science, 2008. 320: p. 246–249. [DOI] [PubMed] [Google Scholar]
- 9.Wildanger D, et al. , A compact STED microscope providing 3D nanoscale resolution. J Microsc, 2009. 236(1): p. 35–43. [DOI] [PubMed] [Google Scholar]
- 10.Osseforth C, et al. , Simultaneous dual-color 3D STED microscopy. Opt Express, 2014. 22(6): p. 7028–39. [DOI] [PubMed] [Google Scholar]
- 11.Willig KI, et al. , STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature, 2006. 440(7086): p. 935–9. [DOI] [PubMed] [Google Scholar]
- 12.Grauel MK, et al. , RIM-binding protein 2 regulates release probability by fine-tuning calcium channel localization at murine hippocampal synapses. Proc Natl Acad Sci USA, 2016. 113(41): p. 11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Böhme MA, et al. , Active zone scaffolds differentially accumulate Unc13 isoforms to tune Ca2+ channel–vesicle coupling. Nat Neurosci, 2016. 19: p. 1311. [DOI] [PubMed] [Google Scholar]
- 14.Nishimune H, et al. , Dual-color STED microscopy reveals a sandwich structure of Bassoon and Piccolo in active zones of adult and aged mice. Sci Reports, 2016. 6: p. 27935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glebov OO, et al. , Nanoscale Structural Plasticity of the Active Zone Matrix Modulates Presynaptic Function. Cell Reports, 2017. 18(11): p. 2715–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang AH, et al. , A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature, 2016. 536(7615): p. 210–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong APH, et al. , RIM C2B Domains Target Presynaptic Active Zone Functions to PIP2-Containing Membranes. Neuron, 2018. 98(2): p. 335–349.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maschi D, Gramlich MW, and Klyachko VA, Myosin V functions as a vesicle tether at the plasma membrane to control neurotransmitter release in central synapses. Elife, 2018. 7: p. e39440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans LL, et al. , Vesicle-associated brain myosin-V can be activated to catalyze actin-based transport. J Cell Sci, 1998. 111:2055–66. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe M, et al. , Myosin-Va Regulates Exocytosis through the Submicromolar Ca2+-dependent Binding of Syntaxin-1A. Mol Biol Cell, 2005. 16(10): p. 4519–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy-Alla S, et al. , Stable Positioning of Unc13 Restricts Synaptic Vesicle Fusion to Defined Release Sites to Promote Synchronous Neurotransmission. Neuron, 2017. 95(6): p. 1350–1364.e12. [DOI] [PubMed] [Google Scholar]
- 22.van den Bogaart G, Lang T, and Jahn R, Microdomains of SNARE proteins in the plasma membrane. Curr Top Membr. 2013, 72: p. 193–230. [DOI] [PubMed] [Google Scholar]
- 23.Milovanovic D and Jahn R, Organization and dynamics of SNARE proteins in the presynaptic membrane. Front Physiol, 2015. 6: p. 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pertsinidis A, et al. , Ultrahigh-resolution imaging reveals formation of neuronal SNARE/Munc18 complexes in situ. Proc Natl Acad Sci U S A, 2013, 110(30): p. E2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bademosi AT, et al. , In vivo single-molecule imaging of syntaxin1A reveals polyphosphoinositide- and activity-dependent trapping in presynaptic nanoclusters. Nat Commun, 2016. 7: p. 13660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ullrich A, et al. , Dynamical Organization of Syntaxin-1A at the Presynaptic Active Zone. PLoS Comput Biol, 2015. 11(9): p. e1004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribrault C, et al. , Syntaxin1A lateral diffusion reveals transient and local SNARE interactions. J Neurosci, 2011. 31(48): p. 17590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bar-On D, et al. , Super-resolution Imaging Reveals the Internal Architecture of Nanosized Syntaxin Clusters. J Biol Chem. 2012, 287(32): p. 27158–27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou K, et al. , Position of UNC-13 in the active zone regulates synaptic vesicle release probability and release kinetics. Elife, 2013. 2: p. e01180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Z, Tong XJ, and Kaplan JM, UNC-13L, UNC-13S, and Tomosyn form a protein code for fast and slow neurotransmitter release in Caenorhabditis elegans. Elife, 2013. 2: p. e00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atasoy D, et al. , Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J Neurosci, 2008. 28(40): p. 10151–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melom JE, et al. , Spontaneous and evoked release are independently regulated at individual active zones. J Neurosci, 2013. 33(44): p. 17253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matz J, et al. , Rapid structural alterations of the active zone lead to sustained changes in neurotransmitter release. Proc Natl Acad Sci U S A, 2010. 107(19): p. 8836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider R, et al. , Mobility of Calcium Channels in the Presynaptic Membrane. Neuron. 86(3): p. 672–679. [DOI] [PubMed] [Google Scholar]
- 35.Smyth AM, et al. , Munc18–1 Protein Molecules Move between Membrane Molecular Depots Distinct from Vesicle Docking Sites. J Biol Chem. 288(7): p. 5102–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura Y, et al. , Nanoscale distribution of presynaptic Ca(2+) channels and its impact on vesicular release during development. Neuron, 2014. 85(1): p. 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holderith N, et al. , Release probability of hippocampal glutamatergic terminals scales with the size of the active zone. Nat Neurosci, 2012. 15(7): p. 988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neef J, et al. , Quantitative optical nanophysiology of Ca2+ signaling at inner hair cell active zones. Nat Commun. 9(1): p. 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schikorski T and Stevens CF, Quantitative ultrastructural analysis of hippocampal excitatory synapses. J Neurosci, 1997. 17(15): p. 5858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weyhersmüller A, et al. , Rapid Active Zone Remodeling during Synaptic Plasticity. J Neurosci, 31(16): p. 6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neher E, What is Rate-Limiting during Sustained Synaptic Activity: Vesicle Supply or the Availability of Release Sites. Front Synaptic Neurosci, 2010. 2: p. 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korn H, et al. , Fluctuating responses at a central synapse: n of binomial fit predicts number of stained presynaptic boutons. Science, 1981. 213(4510): p. 898–901. [DOI] [PubMed] [Google Scholar]
- 43.Silver RA, et al. , High-probability uniquantal transmission at excitatory synapses in barrel cortex. Science, 2003. 302(5652): p. 1981–4. [DOI] [PubMed] [Google Scholar]
- 44.Korn H, et al. , The one-vesicle hypothesis and multivesicular release. Adv Sec Mess Phos Res, 1994. 29: p. 301–22. [DOI] [PubMed] [Google Scholar]
- 45.Tong G and Jahr CE, Multivesicular release from excitatory synapses of cultured hippocampal neurons. Neuron, 1994. 12(1): p. 51–9. [DOI] [PubMed] [Google Scholar]
- 46.Maschi D and Klyachko VA, Spatiotemporal Regulation of Synaptic Vesicle Fusion Sites in Central Synapses. Neuron. 94(1): p. 65–73 e3. [DOI] [PubMed] [Google Scholar]
- 47.Schikorski T and Stevens CF, Morphological correlates of functionally defined synaptic vesicle populations. Nat Neurosci, 2001. 4(4): p. 391–5. [DOI] [PubMed] [Google Scholar]
- 48.Taschenberger H, et al. , Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron, 2002. 36(6): p. 1127–43. [DOI] [PubMed] [Google Scholar]
- 49.Keller D, et al. , An Exclusion Zone for Ca2+ Channels around Docked Vesicles Explains Release Control by Multiple Channels at a CNS Synapse. PLoS Comput Biol, 2015. 11(5): p. e1004253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park H, Li Y, and Tsien RW, Influence of synaptic vesicle position on release probability and exocytotic fusion mode. Science, 2012. 335(6074): p. 1362–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaffield MA, Tabares L, and Betz WJ, Preferred sites of exocytosis and endocytosis colocalize during high- but not lower-frequency stimulation in mouse motor nerve terminals. J Neurosci, 2009. 29(48): p. 15308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Midorikawa M and Sakaba T, Imaging Exocytosis of Single Synaptic Vesicles at a Fast CNS Presynaptic Terminal. Neuron, 2015. 88(3): p. 492–8. [DOI] [PubMed] [Google Scholar]
- 53.Harata NC, et al. , Frequency-dependent kinetics and prevalence of kiss-and-run and reuse at hippocampal synapses studied with novel quenching methods. Neuron, 2006. 49(2): p. 243–56. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Q, Li Y, and Tsien RW, The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science, 2009. 323(5920): p. 1448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henkel AW, et al. , Synaptic vesicle movements monitored by fluorescence recovery after photobleaching in nerve terminals stained with FM1–43. J. Neurosci 1996. 16(12): p. 3960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kraszewski K, et al. , Mobility of synaptic vesicles in nerve endings monitored by recovery from photobleaching of synaptic vesicle-associated fluorescence. J. Neurosci, 1996. 16: p. 5905–5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaffield MA, Rizzoli SO, and Betz WJ, Mobility of synaptic vesicles in different pools in resting and stimulated frog motor nerve terminals. Neuron, 2006. 51(3): p. 317–25. [DOI] [PubMed] [Google Scholar]
- 58.Lemke EA and Klingauf J, Single synaptic vesicle tracking in individual hippocampal boutons at rest and during synaptic activity. J. Neurosci, 2005. 25(47): p. 11034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schikorski T, Readily releasable vesicles recycle at the active zone of hippocampal synapses. Proc Natl Acad Sci U S A, 2014. 111(14): p. 5415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watanabe S, et al. , Ultrafast endocytosis at mouse hippocampal synapses. Nature, 2013. 504(7479): p. 242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watanabe S, et al. , Ultrafast endocytosis at Caenorhabditis elegans neuromuscular junctions. Elife, 2013. 2: e00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chung C, et al. , Acute Dynamin Inhibition Dissects Synaptic Vesicle Recycling Pathways That Drive Spontaneous and Evoked Neurotransmission. J. Neurosci, 2010. 30: p. 1363–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fredj NB and Burrone J, A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci, 2009. 12: p. 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hua Z, et al. , v-SNARE composition distinguishes synaptic vesicle pools. Neuron, 2011. 71(3): p. 474–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sara Y, et al. , An Isolated Pool of Vesicles Recycles at Rest and Drives Spontaneous Neurotransmission. Neuron, 2005. 45: p. 563–573. [DOI] [PubMed] [Google Scholar]
- 66.Bal M, et al. , Reelin mobilizes a VAMP7-dependent synaptic vesicle pool and selectively augments spontaneous neurotransmission. Neuron, 2013. 80(4): p. 934–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raingo J, et al. , VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission. Nat Neurosci, 2012. 15(5): p. 738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng A, et al. , Differential motion dynamics of synaptic vesicles undergoing spontaneous and activity-evoked endocytosis. Neuron, 2012. 73(6): p. 1108–15. [DOI] [PubMed] [Google Scholar]
- 69.Forte LA, Gramlich MW, and Klyachko VA, Activity-Dependence of Synaptic Vesicle Dynamics. J Neurosci. 37(44): p. 10597–10610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee S, et al. , Dynamics of multiple trafficking behaviors of individual synaptic vesicles revealed by quantum-dot based presynaptic probe. PLoS One, 2012. 7(5): p. e38045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamin D, et al. , High- and Low-Mobility Stages in the Synaptic Vesicle Cycle. Biophys J, 2010. 99: p. 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zenisek D, Steyer JA, and Almers W, Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature, 2000. 406(6798): p. 849–54. [DOI] [PubMed] [Google Scholar]
- 73.Holt M, et al. , High mobility of vesicles supports continuous exocytosis at a ribbon synapse. Curr Biol, 2004. 14(3): p. 173–83. [DOI] [PubMed] [Google Scholar]
- 74.Maschi D and Klyachko VA, A nanoscale resolution view on synaptic vesicle dynamics. Synapse. 69(5): p. 256–67. [DOI] [PubMed] [Google Scholar]
- 75.Miki T, et al. , Actin- and Myosin-Dependent Vesicle Loading of Presynaptic Docking Sites Prior to Exocytosis. Neuron. 91(4): p. 808–823. [DOI] [PubMed] [Google Scholar]
- 76.Kneussel M and Wagner W, Myosin motors at neuronal synapses: drivers of membrane transport and actin dynamics. Nat Rev Neurosci, 2013. 14(4): p. 233–47. [DOI] [PubMed] [Google Scholar]
- 77.Takamori S, et al. , Molecular anatomy of a trafficking organelle. Cell, 2006. 127(4): p. 831–46. [DOI] [PubMed] [Google Scholar]
- 78.Desnos C, et al. , Myosin va mediates docking of secretory granules at the plasma membrane. J Neurosci, 2007. 27(39): p. 10636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Staras K, et al. , A vesicle superpool spans multiple presynaptic terminals in hippocampal neurons. Neuron, 2010. 66(1): p. 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Darcy KJ, et al. , Constitutive sharing of recycling synaptic vesicles between presynaptic boutons. Nat Neurosci, 2006. 9(3): p. 315–21. [DOI] [PubMed] [Google Scholar]
- 81.Gramlich MW and Klyachko VA, Actin/Myosin-V- and Activity-Dependent Inter-synaptic Vesicle Exchange in Central Neurons. Cell Rep. 18(9): p. 2096–2104. [DOI] [PubMed] [Google Scholar]
- 82.Bittins CM, et al. , Dominant-negative myosin Va impairs retrograde but not anterograde axonal transport of large dense core vesicles. Cell Mol Neurobiol, 2009. 30(3): p. 369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ganguly A, et al. , A dynamic formin-dependent deep F-actin network in axons. J Cell Biol. 210(3): p. 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leitz J and Kavalali ET, Ca(2)(+) influx slows single synaptic vesicle endocytosis. J Neurosci, 2011. 31(45): p. 16318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chanaday NL and Kavalali ET, Optical detection of three modes of endocytosis at hippocampal synapses. Elife, 2018. 7: e36097. [DOI] [PMC free article] [PubMed] [Google Scholar]