Abstract
While thermosensation from external environment has been extensively studied, physiological responses to temperature changes inside the body and the underlying regulatory mechanisms are less understood. As a critical link between body and brain that relays visceral organ information and regulates numerous physiological functions, the vagus nerve has been proposed to mediate diverse visceral thermal reflexes and indirectly regulate body temperature. However, the precise role of the vagus nerve in body thermal responses or visceral organ-related thermoregulation is still under debate due to extensive contradictory results. This data discrepancy is likely due to the high cell heterogeneity in the vagus nerve, as diverse vagal neuron types mediate numerous and sometimes opposite physiological functions. Here, we will review evidences that support and against the role of the vagus nerve in body thermosensation and thermoregulation and discuss potential future approaches for better understanding of this critical issue.
1. Introduction
In mammals, core body temperature is precisely controlled within a narrow optimal range primarily through the nervous system. Key neural circuits for body temperature control have been extensively investigated and thoroughly reviewed [70]. Although it is well documented that temperature changes within external environment are sensed through thermosensitive sensory fibers within the spinal and trigeminal nerves, how thermal information from visceral organs is conveyed to the brain is less clear. Most sensory afferents innervating visceral organs travel through the vagus and spinal nerves, some of which may detect temperature related cues. In this review, we will summarize the potential contributions of the vagus nerve in thermoregulation.
The vagus nerve is the 10th cranial nerve, characterized by its wondering trajectory that provides extensive innervation of tissues in the neck, chest, and abdomen (Figure 1). The vagus nerve is a critical link between body and brain that relays diverse sensory information from visceral tissues important for metabolism, respiration, inflammation, and cardiovascular functions, including stomach, duodenum, ileum, heart, aorta, liver, lung, trachea, and pancreas [6, 7, 20, 67, 116], and regulates numerous basic autonomic functions of the respiratory, cardiovascular, digestive and immune systems. The vagus nerve is highly heterogeneous, containing a diversity of sensory afferent fibers and motor efferent fibers that have distinct morphological, pharmacological, electrical, and genetic properties. Sensory neurons in the vagus nerve are pseudo-unipolar, with long projections to visceral organs and brainstem and cell bodies that reside in pairs of adjacent vagal ganglia at the base of the skull, the larger nodose and the smaller jugular ganglia (nodose/jugular complex). Centrally, vagal afferent axons target the nucleus of solitary tract (NTS) in the brainstem, which in turn transmits sensory information to deeper brain structures and descending motor nuclei. Vagal motor neurons originate from the dorsal motor nucleus of the vagus (DMV) and the nucleus of ambiguous (NA) in the brainstem and provide parasympathetic regulation to body organs mainly through acetylcholine release. Based on electrophysiological properties, fibers within the vagus nerve are categorized to slow conducting C type and fast conducting A type [67]. Similar to spinal sensory nerves, vagal C fibers are largely nonmyelinated and capsaicin responsive while vagal A fibers are mainly myelinated and capsaicin unresponsive. Like spinal sensory neurons in the dorsal root ganglia (DRG neurons), vagal neurons express diverse cell surface receptors, ion channels, neuropeptides, and neurotransmitters, and have been traditionally divided into different groups based on such markers. In addition, anatomical studies using retrograde neural tracers suggested that vagal neurons can be classified based on their peripheral targets. Surgical, electrical, or pharmacological control of vagus nerve activity impacts numerous diseases, including epilepsy, inflammation, and cardiac disorders. Nevertheless, these classical procedures treat the vagus as a single entity, and thus impact all co-fasciculating sensory and motor neuron subtypes.
Figure 1. Cartoon anatomy of the vagus nerve.
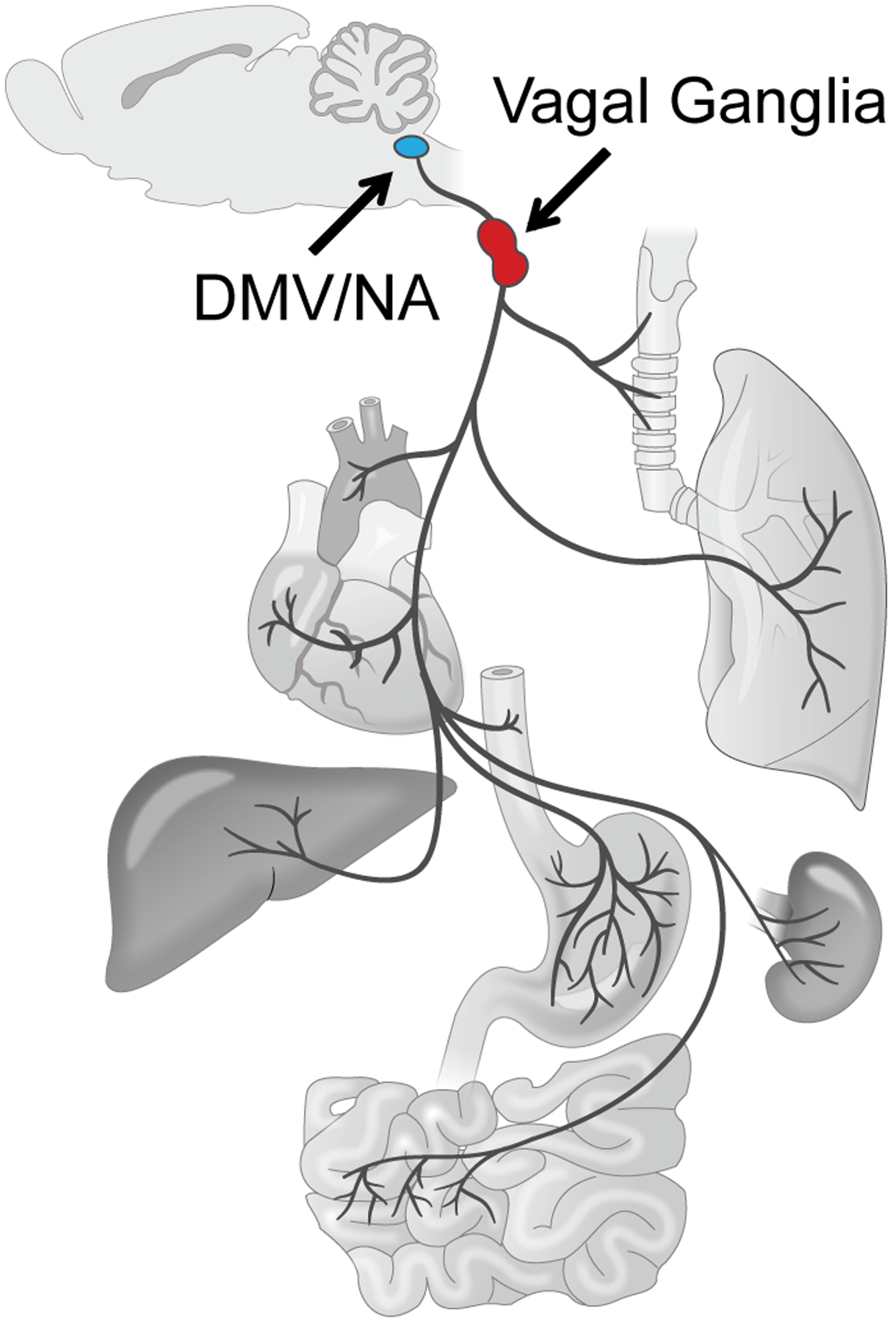
The ‘wondering’ nerve that projects to a variety of internal organs including lung, trachea, heart, aorta, esophagus, stomach, intestine, liver, and kidney provides both sensory afferent and motor efferent innervation. Cell bodies of sensory neurons reside in a pair of vagal ganglia (red) while motor neurons are located in the dorsal motor nucleus of the vagus (DMV) and nucleus ambiguus (NA) in the brainstem (blue). Figure is adapted from Chang et al., 2015.
The role of the vagus nerve in thermoregulation has been extensively investigated [95]. While vagal afferents might directly sense visceral tissue temperature changes and mediate diverse visceral thermal reflexes, metabolic and immune signals carried by the vagus nerve may also play a role in thermoregulation. In this review, we will first focus on thermosensitive vagal afferents and visceral thermal reflexes. We will then discuss the potential roles of the vagus nerve in inflammation and metabolism-related thermoregulation. Finally, we will briefly comment on recent genetic approaches in the vagus nerve and their potential applications in better understanding vagus nerve-mediated thermoregulation.
2. Vagal afferents and visceral thermal sensation
Cutaneous thermal sensation has been studied extensively while thermal sensation from visceral organs is less understood. Physiological reflexes in response to temperature changes from visceral organs have been clearly demonstrated in a variety of species including human. In addition, visceral hyperalgesia and allodynia in response to temperature challenges have been well documented. Although it is generally believed that temperature induced visceral pain is predominantly mediated by spinal sensory nerves, the roles of the vagus nerve in thermal reflexes are often implicated. Thermosensitive vagal afferents have been identified to interact with multiple visceral organs, and a variety of thermosensory transduction mechanisms involving thermosensitive TRP channels have been proposed. In this section, we will review the role of vagal afferents in visceral thermal sensation.
Thermal reflexes from visceral organs along the respiratory and digestive tract help coordinate interaction with external environments. For example, exercise in cold weather would result in airway cooling, which will trigger defensive respiratory reflexes such as cough, bronchomotor response, changes in airway resistance, and mucosal secretion. Respiratory thermal reflexes have been characterized in a variety of animal species including cats, dogs, rabbits, and guinea pigs [49, 50, 79, 90], that cooling the inspired air would result in an increase of airway resistance and bronchoconstriction. Such respiratory changes are likely mediated through neural mechanisms [83, 90]. In particular, defensive respiratory responses to menthol, a cold receptor specific agonist, were observed in guinea pigs, suggesting that thermosensitive afferent fibers are involved [79]. Airway cooling is a well-documented factor in triggering bronchoconstriction in human and may exaggerate asthma [28, 35, 41, 94]. In other animal species, cold-induced bronchospasm has been characterized as well, and such effect was enhanced after antigen challenges [50]. In digestive organs that have direct contact with incoming external materials, hot or cold ingested food along the gastrointestinal tract triggers thermal reflexes to regulate digestive processes. Consistent with the observation that warm drinks are more filling, initial phase of gastric emptying is significantly faster when cold drinks were given in human subjects [4, 85], suggesting that gastric adaptive relaxation is regulated by meal temperature. Similar conclusions were drawn when gastric volume was evaluated using pressure changes in human subjects, that warm stimuli induced gastric relaxation while cold stimuli induced gastric contraction [106, 107]. Interestingly, thermal reflexes along the digestive tract seem to be site specific, as only warm but not cold stimuli in the small intestine can induce gastric changes, although both warm and cold stimuli within the small intestine can be perceived [107].
A large portion of vagal afferents that innervate visceral organs are thermosensitive [80]. Some vagal afferents are directly activated by temperature changes. For example, hyperthermia in the trachea or thoracic chamber was able to activate pulmonary slow conducting C afferents in the vagus nerve in dos and rats [23, 89]. In guinea pigs, thermosensitive vagal afferents in the hepatic branch that innervate the liver were different from the ones that are mechanosensitive and had different sensitivities to liver temperature increase [1, 2], suggesting that thermosensitivity is neuron specific but not cell antonymous. In cats, three types of thermosensitive nonmyelinated vagal afferents were revealed along the gastrointestinal tract using electrophysiology [27]. They responded to warm, cold, or both stimuli but were not mechanosensitive or chemosensitive. Interestingly, electrical stimulation of such thermosensitive afferents changed gut motility, suggesting that they are involved in the regulation of digestive functions. In a similar study using rats, thermosensitive vagal afferents that innervate the oesophagus were investigated and a similar conclusion was drawn that cold, hot, and mixed vagal afferents exist, although cold-sensitive fibers were all mechanosensitive in this study, and some may belong to fast conducting Aδ fibers [57]. Unlike the nerves in the gastrointestinal tract, cold-activated vagal afferents were not described in the respiratory system. In one study, pulmonary C fibers were shown to be stimulated by small decrease in temperature by a few degrees but inhibited by further temperature drop [23]. Whether cold-sensing fibers exist in the respiratory system is not clear, but instead, a number of studies have described that cold air challenge could effectively inhibit airway mechanosensory slowly adapting receptors (SARs) [12, 80, 90], suggesting that neuronal inhibition may also contribute to visceral thermal reflexes.
Thermal responses have been observed in acutely isolated vagal sensory neurons using Fura-2 based calcium imaging [118]. Subsets of vagal sensory neurons exhibited an increased intracellular calcium level in response to cooling, moderate heating, and noxious heating, while most neurons responded to warming. Calcium responses were abolished when calcium was removed from extracellular solutions, indicating that such thermal responses are mediated by calcium influx. Numerous thermoreceptors have been discovered over the past decade. In particular, thermosensitive Transient Receptor Potential (TRP) channels which are six-transmembrane cation channels have different temperature sensitivities ranging from noxious cold to noxious hot (Figure 2) [24, 96]. TRP channels are highly expressed in the cutaneous sensory fibers with cell bodies located in the trigeminal ganglion or dorsal root ganglia. Extensive studies have demonstrated their contributions to cutaneous temperature sensation. A number of thermosensitive TRP channels were discovered in the sensory vagus nerve, including TRPV1–4, TRPM8, and TRPA1 using a variety of techniques. First, isolated vagal sensory neurons that responded to temperature stimuli as revealed by calcium imaging also responded to TRP channel specific agonists [29, 101, 112, 118]. For example, in one study, most neurons (80%) that were activated by cooling also responded to icilin, a TRPM8 specific agonist while 90% neurons that responded to moderate heating also responded to capsaicin, a TRPV1 specific agonist [118]. Similar findings were reported in other studies, although percentages of neurons that responded to temperature challenges were different [29, 112]. Second, expressions of thermosensitive TRP channels in vagal sensory neurons were demonstrated using RT-PCR [118, 119]. Third, distributions of TRP channels in vagal ganglia were determined using RNA in situ hybridization, immunocytochemistry, and transgenic animal models. In particular, TRPV1 is expressed in majority of vagal sensory neurons, and is a neuronal marker for slowly conducting C-fibers [67], which is consistent with previous findings that (1) most vagal sensory neurons are sensitive to warm temperatures in vitro, and (2) warm-sensitive vagal afferents are C-fibers. Furthermore, other thermosensitive ion channels such as two-pore potassium channels were also identified from vagal sensory neurons [119], suggesting additional signaling mechanisms for thermal responses in such neurons. There is no question that similar to somatosensory DRG neurons, subsets of vagal sensory neurons are also thermosensitive; however, whether the in vivo and in vitro thermal responses aforementioned from vagal sensory neurons are physiologically relevant to visceral thermal reflexes is not clear.
Figure 2. Thermal sensitivity of TRP channels.
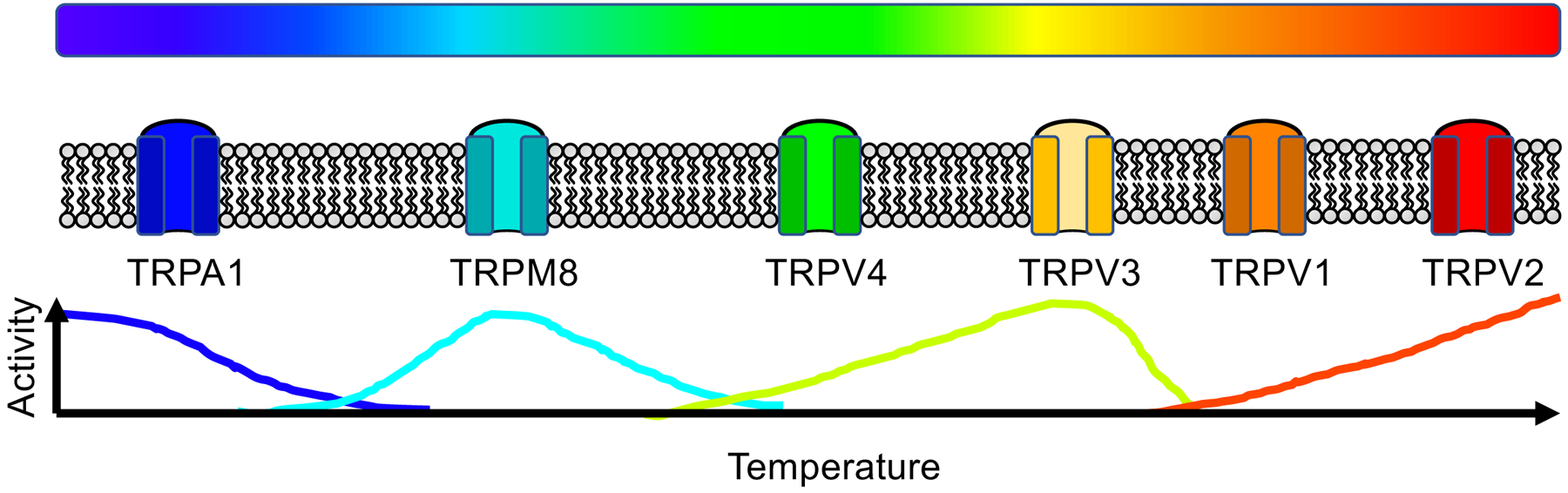
Thermosensitive TRP channels are gated by different temperatures ranging from noxious cold to noxious hot. Different channels have different temperature sensitivities. Figure is adapted from Dhaka et al., 2006.
Some evidences suggest that vagal afferents may play a role in cold induced respiratory reflexes. In anesthetized cat preparations, respiratory changes in response to cold air breathing was abolished after sectioning the superior laryngeal nerves, branches of the vagus nerve that supply the larynx and the trachea [49]. In addition, the cold-induced respiratory thermal reflex in cat was sensitive to local application of procaine, which was commonly used for selective blockade of non-myelinated C-type vagal fibers [51], suggesting that vagal afferents might be involved in such reflex. The role of the vagus nerve in airway cooling-induced bronchospasm was also confirmed using vagotomy, surgical transection of the vagus nerve, in a similar study in rabbits [50], yet whether vagal afferents are necessary for this thermal reflex is less clear. Although it is well established that vagal afferents are essential for maintaining the normal respiratory tone, and stimulation of vagal afferents that innervate the respiratory tract causes potent changes in respiratory physiology [67], evidences that support the conclusion that respiratory thermal reflexes are mediated by thermosensitive vagal afferents are weak. Whether temperature changes within the airway can be appropriately sensed by vagal afferents is still an open question. It’s been clearly demonstrated that airway stretch receptors from the vagus nerve are inhibited by cold air breathing [90, 91], but whether cold-activated respiratory vagal C-type afferents exist is under debate [34, 52, 90]. A recent study described that acutely isolated vagal sensory neurons that innervate the respiratory tract could respond to cold stimulus in vitro, and such response is likely to be TRPM8 dependent [112]. Another study also demonstrated that a subset of vagal sensory neurons was activated by cold in vitro, and in addition to TRPM8, the role of TRPA1 in cold-sensing was implicated [29]. However, whether these cold-activated neurons identified in vitro are able to respond to local airway cooling in vivo is not clear, and the roles of TRPM8, TRPA1, and other thermosensitive TRP channels in cold air-induced respiratory thermal reflex still needs to be determined.
Both cold and warm-activated vagal afferents have been identified from the gastrointestinal tract [27, 57]. While temperature changes along the digestive tract regulate gastrointestinal motility, the precise role of vagal afferents in this regulation is not clear. Temperature could modulate gut motility induced by electrical stimulation, and this modulation was vagus nerve dependent [27]; however, as vagal efferent outputs are vital for gastrointestinal movement, it is difficult to tease apart the precise contribution of thermosensitive vagal afferents in this temperature-dependent modulation. The idea that visceral thermal information can be sensed by vagal afferents is also supported by some indirect evidences. For example, cold exposure induced acute and robust c-fos expression in many brain regions including the NTS, a brainstem nucleus that receives sensory inputs from multiple sources including vagal afferents [68]. In addition, via NTS neurons, sensory inputs from the vagus nerve are further projected to the parabrachial nucleus, a region that also receives thermal information from somatic sensory fibers and is involved in body thermoregulation [72]. On the other hand, food temperature has been proposed to regulate feeding behavior, and this regulation seems to be vagus nerve independent [1]. So far, convincing direct evidences for the role of thermosensitive vagal afferents in visceral thermal reflexes are still missing. Recent advances in mouse genetics have provided an effective way to precisely dissect the physiological functions of vagal afferents, efferents, or the diverse genetically defined neuron subtypes involved [3, 19, 74, 98, 111]. Novel cell-type specific modulatory approaches such as optogentics, chemical genetics, and neuronal inactivation and ablation may be applied in the future to better assess the roles of vagal afferents in visceral thermal sensation and thermal reflexes.
In addition to the aforementioned visceral organs that have obvious contact with external environment, thermosensitive vagal afferents have also been identified from other body organs, such as the liver [1]. Whether they mediate real thermal reflexes, or they just happen to be thermosensitive because they express thermosensitive ion channels but do not contribute to temperature related functions, is not clear. A bigger question is, at least in mammals, whether the temperature change under physiological conditions in most visceral organs could be significant enough to activate described thermosensitive vagal afferents? In fact, local tissue temperature could increase dramatically during exercise, acute heat shock, hypermetabolism, or tissue inflammation [11, 13, 22, 37, 84]. For example, liver temperature may raise to over 39°C after meal in rats, which may be important for food intake regulation [22, 42]. Such temperature increase may very well activate thermosensitive afferents in the hepatic branch of the vagus nerve [2]. In the following sections, we will discuss the potential thermoregulatory roles of the vagus nerve under inflammatory and metabolic challenges.
3. The vagus nerve and inflammation
Thermoregulation is a common defense strategy in response to infectious and inflammatory diseases. While immune responses to infection or injury help eradicate invaders, over inflammation can be detrimental. Crosstalk between the immune system and the nervous system is critical for the maintenance of physiological homeostasis and protection against exogenous and endogenous threats. The vagus nerve is an essential component of the immune-to-brain axis that connects visceral organs and the central nervous system in both directions [20] (Figure 3). On one hand, vagal afferents monitor immune signals from the periphery and transmit inflammatory information to the brain. For instance, lung-innervating vagal sensory neurons can respond to Staphylococcus aureus induced pneumonia [3]. On the other hand, vagal efferents provide important regulatory signals to the spleen via postganglionic neurons to modulate immune functions. The role of the vagus nerve in immune-related thermoregulation has been extensively investigated. In this section, we will first summarize the mechanisms by which the vagus nerve controls inflammation, and discuss the potential role of vagal sensory neurons in fever.
Figure 3. The vagus nerve and the immune-to-brain axis.
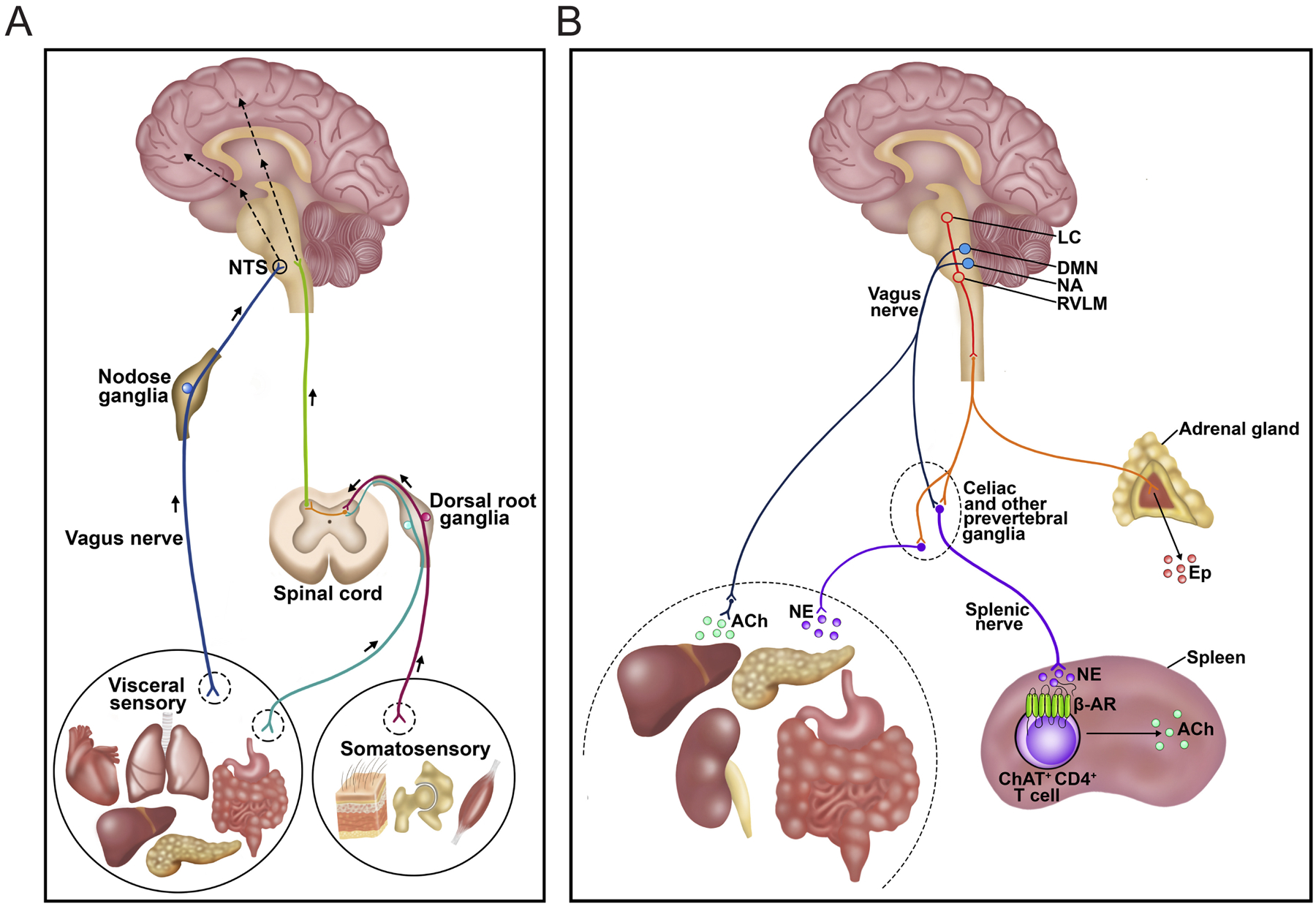
A, Vagal afferents sense and transmit immune signals from visceral organs to the brain. B, Vagal efferents originated from the brainstem control immune responses through the splenic nerve. Figure is adapted from Chavan et al., 2017.
The pioneer study from Dr. Kevin Tracey’s group in 2000 described for the first time a cholinergic anti-inflammatory pathway formed by vagal efferents that transmit neuronal signals from the brain to the spleen to control systematic immune responses [10]. Electrical stimulation of the efferent vagus nerve effectively attenuated lipopolysaccharide (LPS, exdotoxin from Gram-negative microbes that induces strong inflammatory reactions in normal animals) induced cytokine release, and this anti-inflammatory effect was acetylcholine dependent. In contrast, cytokine response to LPS was exaggerated in vagotomized animals, indicating that the tonic vagal tone is contributing to inflammatory control. The full cholinergic anti-inflammatory pathway was subsequently revealed in a series of studies. In short, vagal efferent neurons reside in the DMV and NA in the brainstem use acetylcholine to communicate with parasympathetic postganglionic neurons, which further release norepinephrine (NE) to the spleen via the splenic nerve [20, 46]. NE then binds to β adrenergic receptors expressed on ChAT positive CD4 positive T cells and triggers acetylcholine release to regulate the functions of macrophages, dendritic cells, and other immune cells through α7 nicotinic acetylcholine receptor (α7nAChR). The importance of the cholinergic anti-inflammatory pathway mediated by the efferent vagus nerve has been demonstrated in a variety of visceral inflammatory conditions in addition to LPS-induced endotoxemia [82, 97]. For example, vagus nerve stimulation or cholinergic agonists attenuated ischemia-reperfusion induced renal injury [45, 46, 114]. Intriguingly, stimulating the contralateral vagus nerve while blocking the ipsilateral vagus nerve also effectively protected the kidney from acute kidney injury, suggesting that different protection mechanisms may be used by vagal afferents and efferents [45]. Similarly, elevation of serum inflammatory signals such as tumor necrosis factor (TNF) induced by myocardial ischemia-reperfusion after transverse aortic constriction procedure was reduced by vagus nerve stimulation in mice [5]. Transcutaneous vagus nerve stimulation also suppressed serum inflammatory signals and improved survival in sepsis mouse models [44]. Inflammatory bowel diseases induced by chronic intestinal inflammation including colitis were exacerbated in subdiaphragmatically vagotomized animal models [33]. Recently, the role of the cholinergic anti-inflammatory pathway in cancer was described, that subdiaphragmatic vagotomy in mouse pancreatic cancer models caused elevated tumor TNF levels and subsequent increase in tumor growth and shorter lifespan [81].
Tissue infection or inflammation may eventually lead to fever. Exogeneous pyrogens such as bacteria endotoxic LPS, or cytokines, endogenous pyrogens released by immune cells after infection or tissue injury that mediate inflammation [117], are able to trigger strong febrile responses via a neural mechanism involving the brain. Although it is clear that prostaglandin E2 (PGE2) and one of its receptors Prostaglandin EP3 receptor (EP3R) are key mediators of endotoxin or cytokine-induced fever, different routes by which endotoxins and cytokines act on the nervous system have been proposed [86]. As cytokines are peripherally released inflammatory signals, the role of vagal afferents in febrile responses has been extensively studied, yet the data are contradictory. On one hand, vagal sensory neurons express sensory machineries for peripheral immune signals that are key factors in inflammation induced fever [20]. For example, a variety of cytokine receptors that may be involved in febrile responses were identified in vagal sensory neurons, including CXCR2 (a receptor for interleukin 8 (IL8) and chemokine (C-X-C motif) ligand 1 (CXCL1/MGSA)) [108], IL1R (receptor for interleukin 1 (IL1)) [26, 93, 100], TLR4 (Toll-like receptor 4) [43], and TNFR (receptor for TNFα) [93, 100, 108], as well as EP3R [71], which is the critical PGE2 receptor for febrile response [102]. Responses to cytokine challenges have also been recorded in vagal afferents. Administration of IL-1β through intraperitoneal, intravenous or portal vein injection induced dose-dependent increase in afferent activity in the vagus nerve in anesthetized animals [26, 73, 93, 117]. IL-1β also activated isolated vagal sensory neurons in culture, as revealed using Fluo-4 based in vitro calcium imaging, and such activation was abolished in IL1R knockout animals [93]. Activation of vagal afferents by IL-1β might be PEG2 dependent [26], although PEG2 may impact vagal synaptic transmission in the NTS [66]. Alternatively, IL-1β is able to depolarize and sensitize spinal sensory DRG neurons through a sodium current dependent mechanism [8], and such mechanism may also be used by vagal sensory neurons. Similar responses of vagal afferents to TNFα were described in vivo and in vitro [93, 117]. Although receptors for IL-1β and TNFα may be expressed in the same subset of vagal sensory neurons, IL-1β and TNFα-induced in vivo responses are fundamentally different and their specific neurograms can be separated using power spectrum density (PSD) analysis [93, 117], suggesting that cytokine information may be specifically encoded by vagal afferents.
Early evidences suggested that the vagus nerve is required for fever induced by peripheral administration of IL-1β or LPS [32, 36, 40, 78, 87, 92, 109, 110]. In rats, subdiaphragmatic vagotomy attenuated or eliminated IL-1β or LPS-triggered hyperthermia [78, 109, 110]. Similar results were observed in guinea pigs [36, 92]. Later, numerous contradictory evidences that support or against the role of the vagus nerve in pyrogen-induced fever were reported. For example, in another study in rats, subdiaphragmatic vagotomy was shown to have no effect on LPS-induced hyperthermia at all doses tested [39]. However, it was also described, around the same time, that subdiaphragmatic vagotomy eliminated or attenuated IL-1β induced fever [32, 40, 109]. Likewise, although it was reported that subdiaphragmatic vagotomy suppressed LPS-induced fever in a dose-dependent manner in guinea pigs [36], IL-1 induced hyperthermia was not attenuated by vagotomy in rats at all doses examined [60, 61]. This conclusion was supported by other studies [16]. Using similar approaches, it was demonstrated that the vagus nerve is not required for PGE2 induced fever as well [77]. As subdiaphragmatic vagotomy would cause dramatic malnutrition, which may change febrile responses [47, 48, 55], data from aforementioned studies were challenged. In a subsequent study that nutrition level and health status were carefully controlled, LPS induced febrile response was abolished in vagotomized rats [87]. Contribution from the vagus nerves seems to depend on multiple factors, for example, pyrogen administration route [36], amount of pyrogen administered [40, 88], and circadian cycles [78]. As low dose of IL-1β or LPS induces a monophasic fever while high dose would instead trigger a polyphasic fever, the vagus nerve might be required only for the monophasic fever [88] but not any phases of the polyphasic fever [77]. In sum, the role of the vagus nerve in mediating pyrogen induced fever is highly controversial. It is possible that vagal afferents detect peripheral infectious and inflammatory signals and contribute to hyperthermia, however, because multiple parallel immune-to-brain pathways exist, it is difficult to assess the role of the vagus nerve in this process using conventional nerve transection-based loss-of-function approaches. Modern neuromodulatory methods such as cell-type specific stimulation may provide valuable alternative approaches to help solve this critical issue.
4. The vagus nerve and energy expenditure
Energy homeostasis is critical for survival. Energy intake and energy expenditure are tightly controlled to maintain energy balance, which is essential for normal physiological functions. Numerous factors including food intake, resting metabolic rate, body weight, and thermogenesis are involved in the process of energy homeostasis regulation. In particular, a link between body temperature and feeding status exists: body temperature decreases during food deprivation and can be restored after refeeding [95]. In this section, we will briefly review the role of the vagus nerve in food intake and metabolism, and discuss the possible reflex pathway between vagal afferents and brown adipose tissue (BAT) mediated thermogenesis and energy expenditure.
The role of the vagus nerve in nutrient sensing, food intake, and metabolism is well documented and extensively reviewed [18]. Vagal afferents are well poised to sense food related information along the gastrointestinal tract: sensory fibers with specialized intraganglionic laminar endings (IGLEs) or intramuscular arrays (IMAs) on the muscular wall of the esophagus, stomach, and small intestine are dedicated mechanoreceptors for monitoring food ingestion and processing while chemosensitive afferent fibers that encode nutrient information respond to diverse gut-derived hormones and contribute at least in part to feeding control [6]. A large number of gut hormone receptors are expressed in vagal sensory neurons, including GLP1R (receptor for glucogang-like peptide 1), CCKAR (A type receptor for cholecystokinin), NPY2R (receptor for neuropeptide Y and peptide YY), and many others [18]. Vagal afferents also directly sense circulating nutrient levels, such as blood glucose and amino acids, from the portal vein. In addition to satiety cues, vagal afferents carry hunger signals as well, potentially through ghrelin mediated signaling pathways [21]. Stimulation or lesion of the vagus nerve influences short-term food intake and may also regulate appetite and long-term body weight [95]. The role of vagal afferents in feeding-related thermoregulation has been suggested.
Body metabolic status is closely related to energy expenditure [30]. One of the major pathways that mediate energy expenditure is through BAT thermogenesis. BAT is a unique organ in mammals that is able to generate heat through a proton channel named uncoupling protein-1 (UCP1). In ordinary cells, a high proton gradient is maintained across the mitochondrial membrane through the electron transport chain for ATP synthesis while in BAT cells, this proton gradient will drive proton influx back into the mitochondrial matrix through UCP1, and the energy is released as heat. BAT mediated thermogenesis is tightly regulated by the nervous system via sympathetic innervation. NE released from sympathetic terminals in the BAT triggers intracellular signaling cascades that eventually lead to the generation of proton gradients across the mitochondrial membrane through binding to β3 adrenergic receptors. BAT activity is positively correlated to energy expenditure but inversely correlated with body mass index (BMI) in human subjects [103, 105, 115]. In mice, BAT activity and obesity seems to be positively correlated [38, 54, 59]. Meanwhile, thermogenesis from BAT is also important for body temperature regulation in response to cold environments[17, 75]. BAT sympathetic nerve activity is modulated by peripheral thermosensory neurons that innervate the skin as well as visceral afferents that sense organ metabolic status. As majority of visceral information is carried by vagal sensory neurons, the relationship between vagal afferents and BAT sympathetic nerve activity and BAT thermogenesis has been examined [69, 95]. Some studies suggested that vagal afferents mediate inhibition of BAT activity in response to diverse visceral changes. For example, ghrelin is a gut hormone that positively regulate energy balance by not only increasing food intake but also suppressing BAT thermogenesis mediated energy expenditure [113]. Given the fact that ghrelin receptor is expressed in vagal sensory neurons [15], and vagotomy blocked the inhibitory effect of intravenously administered ghrelin on BAT activity [65], the role of vagal afferents in regulating BAT thermogenesis has been suggested. An inhibitory liver-to-BAT circuit involving activation of vagal afferents was described to mediate overexpression of hepatic glucokinase induced BAT thermogenesis [99]. Activation of carotid arterial chemoreceptors by hypoxia also potently inhibits BAT sympathetic nerve activity, although this effect is likely mediated by the glossopharyngeal nerve [63]. Vagus nerve stimulation was also reported to decrease brain and body temperature in rats [56], although this effect was not observed in other VNS studies, and it is not clear whether a change of BAT activity was involved. In contrast, other studies argued that vagal afferents mediate BAT activation. BAT activity is potentiated by duodenal lipids or intragastric administration of capsiate, a TRP channel agonist, in a vagus nerve dependent manner [9, 76]. Moreover, in one human study in patients with refractory epilepsy, electrical stimulation of the vagus nerve significantly increased basal metabolic rate and distal skin temperature, suggesting elevated energy expenditure during vagus nerve stimulation [104]. Although the mean BAT activity revealed by 18F-fluorodeoxyglucose uptake was not significantly changed during vagus nerve stimulation, a positive correlation between the change in BAT activity and the change in energy expenditure during intervention of electrical stimulation was reported, and a conclusion that vagus nerve stimulation increases energy expenditure in part via increasing BAT activity was drawn. Consistent with this conclusion, auricular vagus nerve stimulation was shown to increase BAT weight as well as the expression level of UCP1 and β3 adrenergic receptors in the BAT [58]. Another study in anesthetized rats suggested that the effect of vagus nerve electrical stimulation on BAT activity may be frequency dependent, in that cold-induced increase in BAT sympathetic nerve activity was abolished by high frequency of vagus nerve stimulation but potentiated by low frequency stimulation potentiates [64]. As a matter of fact, this observation may explain the discrepancies among aforementioned studies. Different vagal afferent types likely detect different visceral changes and mediate diverse thermoregulatory reflexes, some of which may accelerate energy expenditure while others inhibit BAT activity. Depending on stimulation parameters (amplitude, duration, frequency, pattern, and etc.) employed, different vagal afferent and efferent types were recruited in different studies, and thus controversial results were obtained. In the following section, we will discuss some of recent advances in cell-type specific neuromodulatory approaches in the vagus nerve and their potential applications in clarifying the role of the vagus nerve in thermoregulation.
5. Cell-type specific analysis of the vagus nerve
The vagus nerve contains numerous highly heterogeneous co-faciculating sensory and motor fiber types. In fact, vagal neurons that innervate the same organ may have different properties and control distinct, even opposite physiological functions. Conventional neuromodulatory approaches, such as electrical stimulation, surgical transections, and pharmacology, impact multiple vagal fiber types and thus are not ideal tools for dissecting the diverse body-to-brain physiological circuits mediated by the vagus nerve, or deeper understanding the underlying molecular mechanisms. Recent advances in genetics-based neuromodulatory approaches provided a novel strategy for cell-type specific modulation in the vagus nerve. For example, using optogenetics, genetically distinct vagal sensory neurons that exert powerful and opposing effects on breathing have been identified, suggesting that the vagus nerve contains intermingled sensory neurons constituting genetically definable labeled lines with different anatomical connections and physiological roles [19]. On the other hand, genetically defined vagal neuron types can be selectively eliminated using a diphtheria toxin mediated cell-type specific ablation approach [14]. For instance, when TRPV1-expressing vagal afferents were ablated via ganglia injection of diphtheria toxin in TRPV1-DTR mice, both ovalbumin-sensitization triggered bronchoconstriction and Staphylococcus aureus induced immunosuppression were abolished [3, 98]. Other genetics-based neuromodulatory approaches such as chronic stimulation using chemogenetics and acute inhibition of neurotransmission using tetanus toxin light chain (TeNT) were also applicable in the vagus nerve [31, 98]. Such cell-type specific neuromodulatory approaches, together with Cre/LoxP based genetics or Cre/LoxP-Flp/Frt based intersectional genetics [25], will provide powerful tools for functional analysis of the vagus nerve at a molecular and cellular level.
In addition to aforementioned cutting-edge neuromodulatory approaches, novel imaging approaches for massively-parallel analysis of neuronal activities involving genetically encoded calcium sensors GCaMPs have been developed in the vagus nerve [111]. Hundreds of sensory neuron responses to visceral stimuli such as lung inflation, stomach stretch, and intestinal perfusion can be analyzed simultaneously at a single-cell level. Moreover, this approach is compatible with genetics so that the molecular identity of responsive neurons can be easily assessed. Applying such novel imaging technology in the vagus nerve will not only help clarify discrepancies in previous studies but also provide valuable cell-type specific information for neuronal responses. Thanks to the development of large-scale single-cell sequencing technologies, neuron heterogeneity is becoming increasingly appreciated [62]. Numerous novel neuron subpopulations within cell types previously defined using conventional markers have been revealed in a variety of brain regions as well as sensory DRG neurons, suggesting more complexity of neuronal compositions [53]. Applying microfluidics based massively-parallel single-cell RNA sequencing in the vagus nerve will help reveal diverse vagal neuron populations with specific innervation patterns, unique response properties, and distinct physiological functions at a finer resolution.
6. Conclusion
The vagus nerve is major bidirectional connection between visceral organs and the brain, and is related to numerous autonomic physiology and diseases. The role of the vagus nerve in thermoregulation has been extensively investigated, yet results are usually controversial or questionable. Thermosensitive vagal afferents may sense temperature changes from the airway and gastrointestinal tract, and mediate visceral thermal reflexes. Although the vagus nerve is essential for regulating immune responses, its role in inflammation related fever is unclear. Vagal afferents might also influence thermogenesis but data are contradictory. The discrepancy among studies is likely to be a result of non-specific targeting of multiple fiber types within the vagus nerve, as vagal neurons are extremely heterogeneous and are involved in numerous, sometimes opposite physiological functions. In future, cell-type specific analysis involving novel genetic approaches may provide a better solution for understanding the role of the vagus nerve in thermoregulation.
Acknowledgement
Funding was provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (K01 DK113047).
References
- [1].Adachi A, Thermosensitive and osmoreceptive afferent fibers in the hepatic branch of the vagus nerve, J Auton Nerv Syst 10 (1984) 269–273. [DOI] [PubMed] [Google Scholar]
- [2].Adachi A, Niijima A, Thermosensitive afferent fibers in the hepatic branch of the vagus nerve in the guinea pig, J Auton Nerv Syst 5 (1982) 101–109. [DOI] [PubMed] [Google Scholar]
- [3].Baral P, Umans BD, Li L, Wallrapp A, Bist M, Kirschbaum T, Wei Y, Zhou Y, Kuchroo VK, Burkett PR, Yipp BG, Liberles SD, Chiu IM, Nociceptor sensory neurons suppress neutrophil and gammadelta T cell responses in bacterial lung infections and lethal pneumonia, Nat Med 24 (2018) 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bateman DN, Effects of meal temperature and volume on the emptying of liquid from the human stomach, J Physiol 331 (1982) 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, Czura CJ, Tracey KJ, Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion, J Vasc Surg 36 (2002) 1231–1236. [DOI] [PubMed] [Google Scholar]
- [6].Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D, Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract, Neurogastroenterol Motil 16 Suppl 1 (2004) 28–33. [DOI] [PubMed] [Google Scholar]
- [7].Berthoud HR, Neuhuber WL, Functional and chemical anatomy of the afferent vagal system, Auton Neurosci 85 (2000) 1–17. [DOI] [PubMed] [Google Scholar]
- [8].Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA, Nociceptors are interleukin-1beta sensors, J Neurosci 28 (2008) 14062–14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Blouet C, Schwartz GJ, Duodenal lipid sensing activates vagal afferents to regulate non-shivering brown fat thermogenesis in rats, PLoS One 7 (2012) e51898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ, Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin, Nature 405 (2000) 458–462. [DOI] [PubMed] [Google Scholar]
- [11].Bouchama A, Parhar RS, el-Yazigi A, Sheth K, al-Sedairy S, Endotoxemia and release of tumor necrosis factor and interleukin 1 alpha in acute heatstroke, J Appl Physiol (1985) 70 (1991) 2640–2644. [DOI] [PubMed] [Google Scholar]
- [12].Bradley GW, Scheurmier N, The transduction properties of tracheal stretch receptors in vitro, Respir Physiol 31 (1977) 365–375. [DOI] [PubMed] [Google Scholar]
- [13].Brooks GA, Hittelman KJ, Faulkner JA, Beyer RE, Tissue temperatures and whole-animal oxygen consumption after exercise, Am J Physiol 221 (1971) 427–431. [DOI] [PubMed] [Google Scholar]
- [14].Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A, A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration, Nat Methods 2 (2005) 419–426. [DOI] [PubMed] [Google Scholar]
- [15].Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ, Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance, Am J Physiol Gastrointest Liver Physiol 290 (2006) G1289–1297. [DOI] [PubMed] [Google Scholar]
- [16].Caldwell FT Jr., Graves DB, Wallace BH, Humoral versus neural pathways for fever production in rats after administration of lipopolysaccharide, J Trauma 47 (1999) 120–129. [DOI] [PubMed] [Google Scholar]
- [17].Cannon B, Nedergaard J, Brown adipose tissue: function and physiological significance, Physiol Rev 84 (2004) 277–359. [DOI] [PubMed] [Google Scholar]
- [18].Chambers AP, Sandoval DA, Seeley RJ, Integration of satiety signals by the central nervous system, Curr Biol 23 (2013) R379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD, Vagal Sensory Neuron Subtypes that Differentially Control Breathing, Cell 161 (2015) 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chavan SS, Pavlov VA, Tracey KJ, Mechanisms and Therapeutic Relevance of Neuro-immune Communication, Immunity 46 (2017) 927–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Date Y, Ghrelin and the vagus nerve, Methods Enzymol 514 (2012) 261–269. [DOI] [PubMed] [Google Scholar]
- [22].De Vries J, Strubbe JH, Wildering WC, Gorter JA, Prins AJ, Patterns of body temperature during feeding in rats under varying ambient temperatures, Physiol Behav 53 (1993) 229–235. [DOI] [PubMed] [Google Scholar]
- [23].Delpierre S, Grimaud C, Jammes Y, Mei N, Changes in activity of vagal bronchopulmonary C fibres by chemical and physical stimuli in the cat, J Physiol 316 (1981) 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dhaka A, Viswanath V, Patapoutian A, Trp ion channels and temperature sensation, Annu Rev Neurosci 29 (2006) 135–161. [DOI] [PubMed] [Google Scholar]
- [25].Dymecki SM, Kim JC, Molecular neuroanatomy’s “Three Gs”: a primer, Neuron 54 (2007) 17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ek M, Kurosawa M, Lundeberg T, Ericsson A, Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins, J Neurosci 18 (1998) 9471–9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].El Ouazzani T, Mei N, Electrophysiologic properties and role of the vagal thermoreceptors of lower esophagus and stomach of cat, Gastroenterology 83 (1982) 995–1001. [PubMed] [Google Scholar]
- [28].Ellis EF, Asthma in childhood, J Allergy Clin Immunol 72 (1983) 526–539. [DOI] [PubMed] [Google Scholar]
- [29].Fajardo O, Meseguer V, Belmonte C, Viana F, TRPA1 channels mediate cold temperature sensing in mammalian vagal sensory neurons: pharmacological and genetic evidence, J Neurosci 28 (2008) 7863–7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Galgani J, Ravussin E, Energy metabolism, fuel selection and body weight regulation, Int J Obes (Lond) 32 Suppl 7 (2008) S109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Garrott K, Dyavanapalli J, Cauley E, Dwyer MK, Kuzmiak-Glancy S, Wang X, Mendelowitz D, Kay MW, Chronic activation of hypothalamic oxytocin neurons improves cardiac function during left ventricular hypertrophy-induced heart failure, Cardiovasc Res 113 (2017) 1318–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gaykema RP, Goehler LE, Hansen MK, Maier SF, Watkins LR, Subdiaphragmatic vagotomy blocks interleukin-1beta-induced fever but does not reduce IL-1beta levels in the circulation, Auton Neurosci 85 (2000) 72–77. [DOI] [PubMed] [Google Scholar]
- [33].Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM, The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model, Gastroenterology 131 (2006) 1122–1130. [DOI] [PubMed] [Google Scholar]
- [34].Giesbrecht GG, Pisarri TE, Coleridge JC, Coleridge HM, Cooling the pulmonary blood in dogs alters activity of pulmonary vagal afferents, J Appl Physiol (1985) 74 (1993) 24–30. [DOI] [PubMed] [Google Scholar]
- [35].Giesbrecht GG, Younes M, Exercise- and cold-induced asthma, Can J Appl Physiol 20 (1995) 300–314. [DOI] [PubMed] [Google Scholar]
- [36].Goldbach JM, Roth J, Zeisberger E, Fever suppression by subdiaphragmatic vagotomy in guinea pigs depends on the route of pyrogen administration, Am J Physiol 272 (1997) R675–681. [DOI] [PubMed] [Google Scholar]
- [37].Greenleaf JE, Hyperthermia and exercise, Int Rev Physiol 20 (1979) 157–208. [PubMed] [Google Scholar]
- [38].Hamann A, Flier JS, Lowell BB, Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia, Endocrinology 137 (1996) 21–29. [DOI] [PubMed] [Google Scholar]
- [39].Hansen MK, Daniels S, Goehler LE, Gaykema RP, Maier SF, Watkins LR, Subdiaphragmatic vagotomy does not block intraperitoneal lipopolysaccharide-induced fever, Auton Neurosci 85 (2000) 83–87. [DOI] [PubMed] [Google Scholar]
- [40].Hansen MK, Krueger JM, Subdiaphragmatic vagotomy blocks the sleep- and fever-promoting effects of interleukin-1beta, Am J Physiol 273 (1997) R1246–1253. [DOI] [PubMed] [Google Scholar]
- [41].Heaton RW, Henderson AF, Gray BJ, Costello JF, The bronchial response to cold air challenge: evidence for different mechanisms in normal and asthmatic subjects, Thorax 38 (1983) 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Himms-Hagen J, Role of brown adipose tissue thermogenesis in control of thermoregulatory feeding in rats: a new hypothesis that links thermostatic and glucostatic hypotheses for control of food intake, Proc Soc Exp Biol Med 208 (1995) 159–169. [DOI] [PubMed] [Google Scholar]
- [43].Hosoi T, Okuma Y, Matsuda T, Nomura Y, Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion, Auton Neurosci 120 (2005) 104–107. [DOI] [PubMed] [Google Scholar]
- [44].Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, Rosas-Ballina M, Ashok M, Goldstein RS, Chavan S, Pavlov VA, Metz CN, Yang H, Czura CJ, Wang H, Tracey KJ, Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis, Crit Care Med 35 (2007) 2762–2768. [DOI] [PubMed] [Google Scholar]
- [45].Inoue T, Abe C, Sung SS, Moscalu S, Jankowski J, Huang L, Ye H, Rosin DL, Guyenet PG, Okusa MD, Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through alpha7nAChR+ splenocytes, J Clin Invest 126 (2016) 1939–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Inoue T, Tanaka S, Okusa MD, Neuroimmune Interactions in Inflammation and Acute Kidney Injury, Front Immunol 8 (2017) 945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Inoue W, Luheshi GN, Acute starvation alters lipopolysaccharide-induced fever in leptin-dependent and -independent mechanisms in rats, Am J Physiol Regul Integr Comp Physiol 299 (2010) R1709–1719. [DOI] [PubMed] [Google Scholar]
- [48].Inoue W, Somay G, Poole S, Luheshi GN, Immune-to-brain signaling and central prostaglandin E2 synthesis in fasted rats with altered lipopolysaccharide-induced fever, Am J Physiol Regul Integr Comp Physiol 295 (2008) R133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jammes Y, Barthelemy P, Delpierre S, Respiratory effects of cold air breathing in anesthetized cats, Respir Physiol 54 (1983) 41–54. [DOI] [PubMed] [Google Scholar]
- [50].Jammes Y, Barthelemy P, Fornaris M, Grimaud C, Cold-induced bronchospasm in normal and sensitized rabbits, Respir Physiol 63 (1986) 347–360. [DOI] [PubMed] [Google Scholar]
- [51].Jammes Y, Mei N, Assessment of the pulmonary origin of bronchoconstrictor vagal tone, J Physiol 291 (1979) 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jammes Y, Nail B, Mei N, Grimaud C, Laryngeal afferents activated by phenyldiguanide and their response to cold air or helium-oxygen, Respir Physiol 67 (1987) 379–389. [DOI] [PubMed] [Google Scholar]
- [53].Kebschull JM, Garcia da Silva P, Reid AP, Peikon ID, Albeanu DF, Zador AM, High-Throughput Mapping of Single-Neuron Projections by Sequencing of Barcoded RNA, Neuron 91 (2016) 975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kopecky J, Rossmeisl M, Hodny Z, Syrovy I, Horakova M, Kolarova P, Reduction of dietary obesity in aP2-Ucp transgenic mice: mechanism and adipose tissue morphology, Am J Physiol 270 (1996) E776–786. [DOI] [PubMed] [Google Scholar]
- [55].Krall CM, Yao X, Hass MA, Feleder C, Steiner AA, Food deprivation alters thermoregulatory responses to lipopolysaccharide by enhancing cryogenic inflammatory signaling via prostaglandin D2, Am J Physiol Regul Integr Comp Physiol 298 (2010) R1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Larsen LE, Lysebettens WV, Germonpre C, Carrette S, Daelemans S, Sprengers M, Thyrion L, Wadman WJ, Carrette E, Delbeke J, Boon P, Vonck K, Raedt R, Clinical Vagus Nerve Stimulation Paradigms Induce Pronounced Brain and Body Hypothermia in Rats, Int J Neural Syst 27 (2017) 1750016. [DOI] [PubMed] [Google Scholar]
- [57].Lennerz JK, Dentsch C, Bernardini N, Hummel T, Neuhuber WL, Reeh PW, Electrophysiological characterization of vagal afferents relevant to mucosal nociception in the rat upper oesophagus, J Physiol 582 (2007) 229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Li H, Zhang JB, Xu C, Tang QQ, Shen WX, Zhou JZ, Chen JD, Wang YP, Effects and mechanisms of auricular vagus nerve stimulation on high-fat-diet--induced obese rats, Nutrition 31 (2015) 1416–1422. [DOI] [PubMed] [Google Scholar]
- [59].Lowell BB, V SS, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS, Development of obesity in transgenic mice after genetic ablation of brown adipose tissue, Nature 366 (1993) 740–742. [DOI] [PubMed] [Google Scholar]
- [60].Luheshi GN, Cytokines and fever. Mechanisms and sites of action, Ann N Y Acad Sci 856 (1998) 83–89. [DOI] [PubMed] [Google Scholar]
- [61].Luheshi GN, Bluthe RM, Rushforth D, Mulcahy N, Konsman JP, Goldbach M, Dantzer R, Vagotomy attenuates the behavioural but not the pyrogenic effects of interleukin-1 in rats, Auton Neurosci 85 (2000) 127–132. [DOI] [PubMed] [Google Scholar]
- [62].Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, Trombetta JJ, Weitz DA, Sanes JR, Shalek AK, Regev A, McCarroll SA, Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets, Cell 161 (2015) 1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Madden CJ, Morrison SF, Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats, J Physiol 566 (2005) 559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Madden CJ, Santos da Conceicao EP, Morrison SF, Vagal afferent activation decreases brown adipose tissue (BAT) sympathetic nerve activity and BAT thermogenesis, Temperature (Austin) 4 (2017) 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mano-Otagiri A, Ohata H, Iwasaki-Sekino A, Nemoto T, Shibasaki T, Ghrelin suppresses noradrenaline release in the brown adipose tissue of rats, J Endocrinol 201 (2009) 341–349. [DOI] [PubMed] [Google Scholar]
- [66].Marty V, El Hachmane M, Amedee T, Dual modulation of synaptic transmission in the nucleus tractus solitarius by prostaglandin E2 synthesized downstream of IL-1beta, Eur J Neurosci 27 (2008) 3132–3150. [DOI] [PubMed] [Google Scholar]
- [67].Mazzone SB, Undem BJ, Vagal Afferent Innervation of the Airways in Health and Disease, Physiol Rev 96 (2016) 975–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Miyata S, Ishiyama M, Shido O, Nakashima T, Shibata M, Kiyohara T, Central mechanism of neural activation with cold acclimation of rats using Fos immunohistochemistry, Neurosci Res 22 (1995) 209–218. [DOI] [PubMed] [Google Scholar]
- [69].Morrison SF, Madden CJ, Tupone D, Central neural regulation of brown adipose tissue thermogenesis and energy expenditure, Cell Metab 19 (2014) 741–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Morrison SF, Nakamura K, Central neural pathways for thermoregulation, Front Biosci (Landmark Ed) 16 (2011) 74–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Negishi M, Immunohistochemical localization of prostaglandin EP3 receptor in the rat nervous system, J Comp Neurol 421 (2000) 543–569. [DOI] [PubMed] [Google Scholar]
- [72].Nakamura K, Morrison SF, A thermosensory pathway that controls body temperature, Nat Neurosci 11 (2008) 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Niijima A, The afferent discharges from sensors for interleukin 1 beta in the hepatoportal system in the anesthetized rat, J Auton Nerv Syst 61 (1996) 287–291. [DOI] [PubMed] [Google Scholar]
- [74].Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD, Patapoutian A, Piezo2 senses airway stretch and mediates lung inflation-induced apnoea, Nature 541 (2017) 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Oelkrug R, Polymeropoulos ET, Jastroch M, Brown adipose tissue: physiological function and evolutionary significance, J Comp Physiol B 185 (2015) 587–606. [DOI] [PubMed] [Google Scholar]
- [76].Ono K, Tsukamoto-Yasui M, Hara-Kimura Y, Inoue N, Nogusa Y, Okabe Y, Nagashima K, Kato F, Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses, J Appl Physiol (1985) 110 (2011) 789–798. [DOI] [PubMed] [Google Scholar]
- [77].Ootsuka Y, Blessing WW, Steiner AA, Romanovsky AA, Fever response to intravenous prostaglandin E2 is mediated by the brain but does not require afferent vagal signaling, Am J Physiol Regul Integr Comp Physiol 294 (2008) R1294–1303. [DOI] [PubMed] [Google Scholar]
- [78].Opp MR, Toth LA, Circadian modulation of interleukin-1-induced fever in intact and vagotomized rats, Ann N Y Acad Sci 813 (1997) 435–436. [DOI] [PubMed] [Google Scholar]
- [79].Orani GP, Anderson JW, Sant’Ambrogio G, Sant’Ambrogio FB, Upper airway cooling and l-menthol reduce ventilation in the guinea pig, J Appl Physiol (1985) 70 (1991) 2080–2086. [DOI] [PubMed] [Google Scholar]
- [80].Paintal AS, Vagal sensory receptors and their reflex effects, Physiol Rev 53 (1973) 159–227. [DOI] [PubMed] [Google Scholar]
- [81].Partecke LI, Kading A, Trung DN, Diedrich S, Sendler M, Weiss F, Kuhn JP, Mayerle J, Beyer K, von Bernstorff W, Heidecke CD, Kessler W, Subdiaphragmatic vagotomy promotes tumor growth and reduces survival via TNFalpha in a murine pancreatic cancer model, Oncotarget 8 (2017) 22501–22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Pavlov VA, Cholinergic modulation of inflammation, Int J Clin Exp Med 1 (2008) 203–212. [PMC free article] [PubMed] [Google Scholar]
- [83].Pisarri TE, Giesbrecht GG, Reflex tracheal smooth muscle contraction and bronchial vasodilation evoked by airway cooling in dogs, J Appl Physiol (1985) 82 (1997) 1566–1572. [DOI] [PubMed] [Google Scholar]
- [84].Planas ME, Rodriguez L, Sanchez S, Pol O, Puig MM, Pharmacological evidence for the involvement of the endogenous opioid system in the response to local inflammation in the rat paw, Pain 60 (1995) 67–71. [DOI] [PubMed] [Google Scholar]
- [85].Ritschel WA, Erni W, The influence of temperature of ingested fluid on stomach emptying time, Int J Clin Pharmacol Biopharm 15 (1977) 172–175. [PubMed] [Google Scholar]
- [86].Romanovsky AA, Ivanov AI, Szekely M, Neural route of pyrogen signaling to the brain, Clin Infect Dis 31 Suppl 5 (2000) S162–167. [DOI] [PubMed] [Google Scholar]
- [87].Romanovsky AA, Kulchitsky VA, Simons CT, Sugimoto N, Szekely M, Febrile responsiveness of vagotomized rats is suppressed even in the absence of malnutrition, Am J Physiol 273 (1997) R777–783. [DOI] [PubMed] [Google Scholar]
- [88].Romanovsky AA, Simons CT, Szekely M, Kulchitsky VA, The vagus nerve in the thermoregulatory response to systemic inflammation, Am J Physiol 273 (1997) R407–413. [DOI] [PubMed] [Google Scholar]
- [89].Ruan T, Gu Q, Kou YR, Lee LY, Hyperthermia increases sensitivity of pulmonary C-fibre afferents in rats, J Physiol 565 (2005) 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sant’Ambrogio FB, Sant’Ambrogio G, Mathew OP, Effects of airway cooling on tracheal stretch receptors, Respir Physiol 66 (1986) 205–214. [DOI] [PubMed] [Google Scholar]
- [91].Sant’Ambrogio G, Mathew OP, Sant’Ambrogio FB, Characteristics of laryngeal cold receptors, Respir Physiol 71 (1988) 287–297. [DOI] [PubMed] [Google Scholar]
- [92].Sehic E, Blatteis CM, Blockade of lipopolysaccharide-induced fever by subdiaphragmatic vagotomy in guinea pigs, Brain Res 726 (1996) 160–166. [PubMed] [Google Scholar]
- [93].Steinberg BE, Silverman HA, Robbiati S, Gunasekaran MK, Tsaava T, Battinelli E, Stiegler A, Bouton CE, Chavan SS, Tracey KJ, Huerta PT, Cytokine-specific Neurograms in the Sensory Vagus Nerve, Bioelectron Med 3 (2016) 7–17. [PMC free article] [PubMed] [Google Scholar]
- [94].Suzuki S, Ishii M, Sasaki J, Takishima T, Bronchial responsiveness to methacholine during airway cooling in normal subjects, Clin Allergy 16 (1986) 33–40. [DOI] [PubMed] [Google Scholar]
- [95].Szekely M, The vagus nerve in thermoregulation and energy metabolism, Auton Neurosci 85 (2000) 26–38. [DOI] [PubMed] [Google Scholar]
- [96].Tominaga M, The Role of TRP Channels in Thermosensation In: Liedtke WB, Heller S (Eds.), TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, Boca Raton (FL), 2007. [PubMed] [Google Scholar]
- [97].Tracey KJ, Physiology and immunology of the cholinergic antiinflammatory pathway, J Clin Invest 117 (2007) 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Trankner D, Hahne N, Sugino K, Hoon MA, Zuker C, Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways, Proc Natl Acad Sci U S A 111 (2014) 11515–11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Tsukita S, Yamada T, Uno K, Takahashi K, Kaneko K, Ishigaki Y, Imai J, Hasegawa Y, Sawada S, Ishihara H, Oka Y, Katagiri H, Hepatic glucokinase modulates obesity predisposition by regulating BAT thermogenesis via neural signals, Cell Metab 16 (2012) 825–832. [DOI] [PubMed] [Google Scholar]
- [100].Uceyler N, Schafers M, Sommer C, Mode of action of cytokines on nociceptive neurons, Exp Brain Res 196 (2009) 67–78. [DOI] [PubMed] [Google Scholar]
- [101].Urata T, Mori N, Fukuwatari T, Vagus nerve is involved in the changes in body temperature induced by intragastric administration of 1,8-cineole via TRPM8 in mice, Neurosci Lett 650 (2017) 65–71. [DOI] [PubMed] [Google Scholar]
- [102].Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, Hizaki H, Tuboi K, Katsuyama M, Ichikawa A, Tanaka T, Yoshida N, Narumiya S, Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3, Nature 395 (1998) 281–284. [DOI] [PubMed] [Google Scholar]
- [103].van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ, Cold-activated brown adipose tissue in healthy men, N Engl J Med 360 (2009) 1500–1508. [DOI] [PubMed] [Google Scholar]
- [104].Vijgen GH, Bouvy ND, Leenen L, Rijkers K, Cornips E, Majoie M, Brans B, van Marken Lichtenbelt WD, Vagus nerve stimulation increases energy expenditure: relation to brown adipose tissue activity, PLoS One 8 (2013) e77221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD, Brown adipose tissue in morbidly obese subjects, PLoS One 6 (2011) e17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Villanova N, Azpiroz F, Malagelada JR, Gastrogastric reflexes regulating gastric tone and their relationship to perception, Am J Physiol 273 (1997) G464–469. [DOI] [PubMed] [Google Scholar]
- [107].Villanova N, Azpiroz F, Malagelada JR, Perception and gut reflexes induced by stimulation of gastrointestinal thermoreceptors in humans, J Physiol 502 (Pt 1) (1997) 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wang J, Kollarik M, Ru F, Sun H, McNeil B, Dong X, Stephens G, Korolevich S, Brohawn P, Kolbeck R, Undem B, Distinct and common expression of receptors for inflammatory mediators in vagal nodose versus jugular capsaicin-sensitive/TRPV1-positive neurons detected by low input RNA sequencing, PLoS One 12 (2017) e0185985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, Maier SF, Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication, Neurosci Lett 183 (1995) 27–31. [DOI] [PubMed] [Google Scholar]
- [110].Watkins LR, Wiertelak EP, Goehler LE, Mooney-Heiberger K, Martinez J, Furness L, Smith KP, Maier SF, Neurocircuitry of illness-induced hyperalgesia, Brain Res 639 (1994) 283–299. [DOI] [PubMed] [Google Scholar]
- [111].Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD, Sensory Neurons that Detect Stretch and Nutrients in the Digestive System, Cell 166 (2016) 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Xing H, Ling JX, Chen M, Johnson RD, Tominaga M, Wang CY, Gu J, TRPM8 mechanism of autonomic nerve response to cold in respiratory airway, Mol Pain 4 (2008) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yasuda T, Masaki T, Kakuma T, Yoshimatsu H, Centrally administered ghrelin suppresses sympathetic nerve activity in brown adipose tissue of rats, Neurosci Lett 349 (2003) 75–78. [DOI] [PubMed] [Google Scholar]
- [114].Yeboah MM, Xue X, Duan B, Ochani M, Tracey KJ, Susin M, Metz CN, Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats, Kidney Int 74 (2008) 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y, Saito M, Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men, Obesity (Silver Spring) 19 (2011) 13–16. [DOI] [PubMed] [Google Scholar]
- [116].Yuan H, Silberstein SD, Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part I, Headache 56 (2016) 71–78. [DOI] [PubMed] [Google Scholar]
- [117].Zanos TP, Silverman HA, Levy T, Tsaava T, Battinelli E, Lorraine PW, Ashe JM, Chavan SS, Tracey KJ, Bouton CE, Identification of cytokine-specific sensory neural signals by decoding murine vagus nerve activity, Proc Natl Acad Sci U S A 115 (2018) E4843–E4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zhang L, Jones S, Brody K, Costa M, Brookes SJ, Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse, Am J Physiol Gastrointest Liver Physiol 286 (2004) G983–991. [DOI] [PubMed] [Google Scholar]
- [119].Zhao H, Sprunger LK, Simasko SM, Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat, Am J Physiol Gastrointest Liver Physiol 298 (2010) G212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]


