Abstract
Background
Cerebral ischemia/reperfusion (I/R) injury contributes to mortality and morbidity in preterm infants. Curcumin has been shown to exert neuro-protective effects in the central nervous system (CNS). The aim of this study was to investigate the neuro-protective activity of curcumin and the possible underlying molecular mechanisms.
Material/Methods
A hypoxia/reoxygenation (H/R) protocol was used to simulate I/R injury in vitro. Isolated neonatal neurons were pre-treated with curcumin at serially diluted concentrations and exposed to H/R injury. Cell viability and apoptosis were assessed by MTT and flow cytometry, respectively. Contents of TNFα and IL6 in supernatant of cell culture medium were detected by ELISA. Protein expression, phosphorylation, and nuclear translocation levels were studied by Western blotting.
Results
H/R reduced cell viability and increased apoptosis of neurons. H/R significantly increased Wnt5a expression, JNK1 phosphorylation, and NF-κB nuclear translocation. Moreover, expression levels of cleaved caspase3, TNFα, and IL6 were elevated in H/R-exposed neurons. Curcumin pre-treatment significantly increased cell viability and inhibited apoptosis of neurons exposed to H/R, in a concentration-dependent manner. Moreover, curcumin pre-treatment significantly decreased expression levels of Wnt5a, IL6, TNFα, and phosphorylation level of JNK1, as well as the nuclear translocation level of NF-κB in H/R-exposed neurons, in a concentration-dependent manner.
Conclusions
Curcumin exerted neuro-protective effects against H/R-induced neuron apoptosis and inflammation by inhibiting activation of the Wnt/JNK1 signaling pathway.
MeSH Keywords: Apoptosis, Curcumin, Inflammation, Reperfusion Injury
Background
Although the survival rate of very preterm infants has been increasing along with the development of obstetrics, neonatal neural ischemia/reperfusion (I/R) injury is still a major cause of newborn morbidity and mortality [1]. I/R injury damages the central nervous system (CNS), resulting in severe neurological sequelae such as cerebral palsy in surviving newborns [2]. Neuronal inflammation and apoptosis are believed to be the underlying mechanisms of I/R-induced brain injury [3,4]. Discovering novel neuro-protective agents is of great clinical value because current therapies are limited.
According to previous investigations, apoptotic cascade and pro-inflammatory events are the consequences of activation of the c-jun N-terminal kinase (JNK) signaling pathway [4,5]. JNK activation leads to nuclear translocation of NF-κB, further initiating transcription of targeted pro-apoptotic and pro-inflammatory genes [6]. Wnt signaling plays a role in regulating several fundamental intra-cellular events such as cell survival, movement, adhesion, and secretion [7]. Previous studies proved that Wnt5a is activates the JNK pathway by phosphorylating JNK1 [8].
Curcumin is an active polyphenol agent obtained from the root of a Traditional Chinese Medical herb Curcuma longa. Various effects of curcumin have been reported previously, such as anti-cancer, anti-oxidative, anti-fibrotic, anti-inflammatory, and anti-apoptotic activities [9,10]. Notably, the inhibitory effect of curcumin on the Wnt pathway was also reported [11]. Moreover, curcumin was reported to have neural-protective effects [12]. Thus, it is reasonable to propose that curcumin could exert anti-inflammatory and anti-apoptotic effects via down-regulating Wnt/JNK signaling-induced inflammation and apoptosis in hypoxia/ischemia-induced neural injury. In the present study, curcumin pre-treated cultured neurons were exposed to hypoxia/reoxygenation (H/R). Wnt5a signaling-mediated inflammation and apoptosis were investigated. Our results add to understanding of I/R-induced neural damage and provide a novel basis for the potential clinical application of curcumin in treatment of neonatal neural I/R injury.
Material and Methods
Primary neonatal neurons isolation and culturing
Pregnant Sprague-Dawley rats were provided by the Animal Experimental Centre of Zhejiang University. Rats were maintained in an artificial environment and had free access to water and standard chow. Rats were euthanized by isoflurane on the 20th day of pregnancy and the fetuses were collected. Neurons were isolated and cultured in accordance with protocols of previous investigations but with several modifications [13]. Briefly, collected fetus hippocampi were dissected and digested with 0.25% trypsin-ethylene diamine tetraacetic acid (EDTA) for 15 min at 37°C. Then, neurons were suspended in DMEM supplemented with 10% horse serum, fetal bovine serum (FBS, 10%) and 1L-glutamine (1%). We added 2.5 μmol/L β-D-arabinofuranoside to the medium to prevent proliferation of non-neuronal cells. A hemocytometer was used to count the cells, which were maintained at a density of 3×104/plate in an atmosphere containing 5% CO2 at 37°C in a humidified incubator. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Zhejiang University (ref. number 20190301-01). At over 80% confluence, cells were used for subsequent experiments.
H/R protocol and cell treatments
The H/R protocol was performed according to the descriptions from previous studies [14]. Briefly, cultured neurons were transferred to a modular chamber providing an atmosphere of 1% O2, 5% CO2, and 94% N2 and incubated at 37°C for 4 h. After that, cells were transferred to an incubator providing an atmosphere of 95% fresh air and 5% CO2 and incubated at 37°C for 4 h. Curcumin at 0.5, 1.0, 2.0, 4.0, and 8.0 μmol/L was used to pre-treat neurons for 48 h prior to H/R. The concentrations of curcumin were as determined by our pilot study.
Cell viability assessment
MTT assay was carried out to assess cell viability. According to the results of our pilot study, 1×104 neurons per well were seeded into cell culturing plates. Cells were pre-treated with curcumin at 0.0, 0.5, 1.0, 2.0, 4.0, and 8.0 μmol/L for 48 h. Then, the H/R protocol was carried out. After washing, MTT was added to each well at a final concentration of 5 mg/mL and incubated for 4 h. DMSO was added to dissolve the resulting crystals. A plate reader was used to read absorbance at 490 nm.
Cell apoptosis determination
Apoptosis of neurons was detected by flow cytometry. After washing in PBS, cells were then trypsinized. After washing, propidium iodide (PI, Invitrogen) and Annexin-V (Invitrogen) were used to incubate the cells for 30 min. Apoptosis was detected by a flow cytometry (Beckman Coulter). The experimental protocols were carried out according to the manufacturer’s instructions.
Western blot analysis
A RIPA buffering system was used to acquire the cell lysate neurons. Cytosol protein and nuclear protein were extracted using the Cytosol Protein Extraction kit (Beyotime) and Nuclear Protein Extraction kit (Invitrogen), respectively. Bicinchoninic acid (BCA) method was carried out to determine the concentrations of protein samples. SDS-PAGE was employed to separate the proteins, which were then electronically transferred to polyvinylidene fluoride (PVDF) membranes. After blocking, primary antibodies against Wnt5a, phosphorylated JNK1 (p-JNK1), JNK1, cleaved caspase3 (c-caspase3), IL6, TNFα, and GAPDH were used to incubated the membranes loaded with cytosol protein samples at 4°C for 10 h. Specific antibodies against p65 and histone H3 were used to incubate the membranes loaded with nuclear proteins. Membranes were washed by TBST (0.02%) and then incubated with corresponding secondary antibodies at room temperature for 20 min. The immunoblots were visualized by ImageQuant LAS 4000 after being developed by ECL reagents.
ELISA
The cell culture supernatants were collected after centrifugation and stored at −80°C. Concentrations of TNFα and IL6 in cell culture supernatants were measured using the TNFα Rat ELISA Kit (Invitrogen, USA) and IL6 Rat ELISA Kit (Invitrogen, USA), respectively. The protocols were performed according to instructions provided by the manufacturers.
Statistics
Data are presented as mean±standard deviation. The SPSS (version 17.0) was used to analyze the data. One-way analysis of variance (ANOVA) was used to compare differences between groups. The LSD test was used as the post hoc test. The compared differences were considered statistically significant at P<0.05.
Results
H/R reduced viabilities of neurons but was reversed by curcumin
The results are demonstrated in Figure 1. Compared with control, H/R significantly reduced the viability of cultured neurons. However, the curcumin pre-treatment significantly increased the viability of neurons exposed to H/R, in a concentration-dependent manner.
Figure 1.
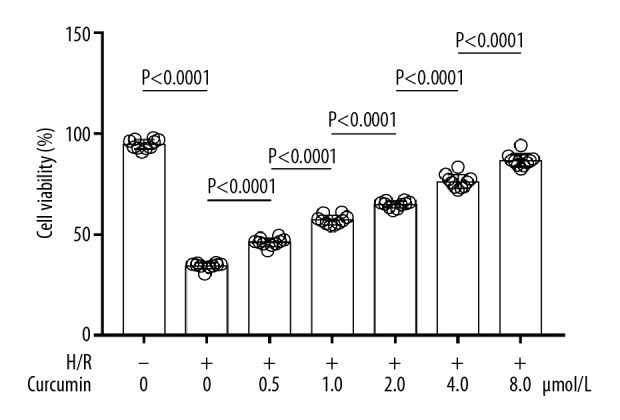
Columns show viabilities of neurons exposed to H/R and treated with curcumin at 0, 0.5, 1.0, 2.0, 4.0, and 8.0 μmol/L. H/R+ − H/R exposure; H/R− − non-H/R exposure.
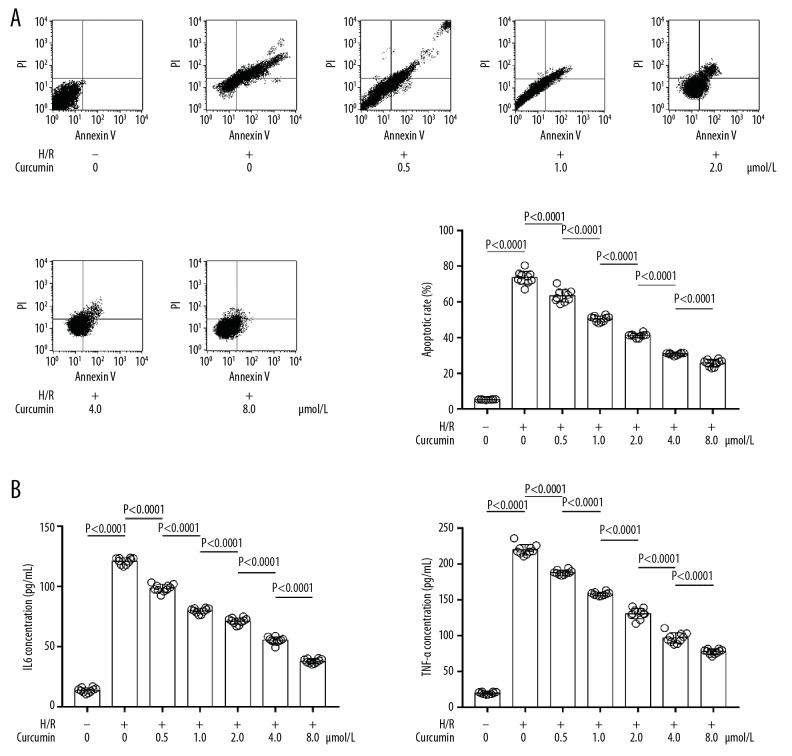
H/R induced inflammation and apoptosis of neurons, which was suppressed by curcumin
As demonstrated in Figure 2, the apoptotic rate was significantly increased after H/R exposure. The curcumin pre-treatment, however, decreased the apoptotic rate of H/R-exposed neurons in a concentration-dependent manner. Moreover, curcumin pre-treatment significantly reduced H/R exposure-induced TNF-α and IL6 production in cultured neurons, in a concentration-dependent manner.
Figure 2.
(A, B) Images of TUNEL assay. The apoptotic neurons were positively TUNEL-tagged. Columns show apoptotic rates of neurons exposed to H/R and treated with curcumin at 0, 0.5, 1.0, 2.0, 4.0, and 8.0 μmol/L. H/R+ − H/R exposure; H/R− − non-H/R exposure.
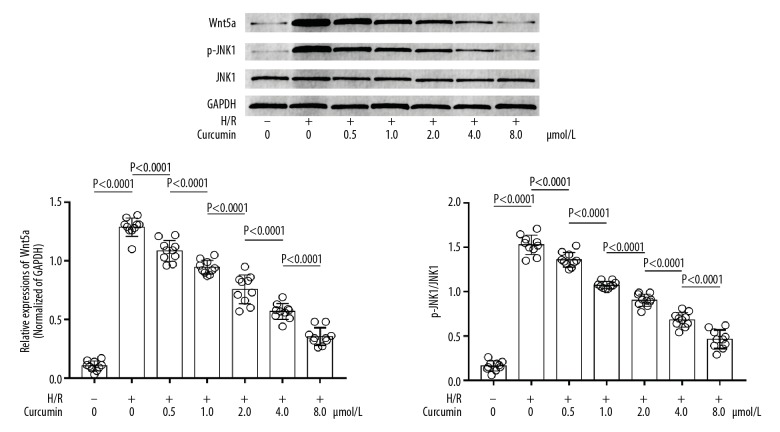
H/R activated Wnt/JNK signaling, which was inhibited by curcumin
As shown in Figure 3, H/R exposure significantly increased the expression level of Wnt5a and the phosphorylation level of JNK1 compared with control. Pre-treatment with curcumin decreased the expression level of Wnt5a and the phosphorylation level of JNK1 in H/R-exposed neurons, in a concentration-dependent manner.
Figure 3.
Immunoblots of Wnt5a, p-JNK1, JNK1, and GAPDH. Columns show relative expression level of Wnt5a (left side) and relative phosphorylation level of JNK1 (right side) in neurons exposed to H/R and treated with curcumin at 0, 0.5, 1.0, 2.0, 4.0, and 8.0 μmol/L. H/R+ − H/R exposure; H/R− − non-H/R exposure.
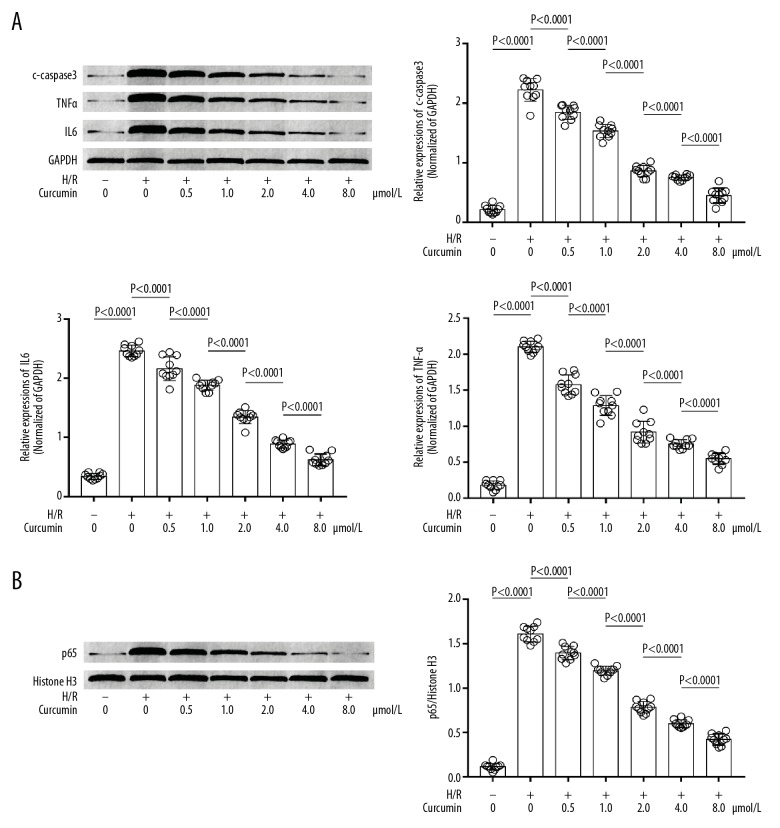
H/R increased pro-apoptotic and pro-inflammatory signaling, which was inhibited by curcumin
These results are demonstrated Figure 4. H/R exposure increased expression levels of cleaved caspase3 in H/R-exposed neurons. Moreover, the H/R exposure also increased levels of TNFα and IL6 in supernatant of culture media compared with control. The expression levels of caspase3 in H/R-exposed neurons and the contents of TNFα and IL6 in supernatant of culture media were reduced by pre-treatment with curcumin, in a concentration-dependent manner.
Figure 4.
(A) Immunoblots of c-caspase3, TNFα, IL6, and GAPDH in neurons. Columns show the relative expression levels of c-caspase3, TNFα, and IL6 in neurons exposed to H/R and treated with curcumin at 0, 0.5, 1.0, 2.0, 4.0, and 8.0 μmol/L. (B) Immunoblots of p65 and histone H3 in nuclear protein extracted from neurons. Columns show relative nuclear translocation levels of p65 in neurons exposed to H/R and treated with curcumin at 0, 0.5, 1.0, 2.0, 4.0, and 8.0 μmol/L. H/R+ − H/R exposure; H/R− − non-H/R exposure.
Discussion
The present work investigated the participation of the Wnt pathway in H/R-induced neuron apoptosis and inflammation. The neuro-protective effect of curcumin was also investigated [15]. We found that H/R induced apoptosis and inflammation of neurons by activating Wnt signaling. Specifically, Wnt signaling activation led to phosphorylation of JNK1, which further facilitated nuclear translocation of NF-κB, resulting in apoptosis and inflammation of neurons [16]. Curcumin was used to pre-treat the neurons exposed to H/R [17]. Our results showed that curcumin pre-treatment inhibited Wnt/JNK1 signaling activation, and consequently suppressed the apoptosis and inflammation of neurons. Overall, our results suggested that curcumin exerts neuro-protective effect by suppressing H/R-induced apoptosis and inflammation. The Wnt signaling pathway appears to be a pharmacological molecular target of curcumin.
The underlying molecular mechanisms of H/R-induced neurological disorders are complicated and not fully understood. Viability reduction and inflammation of neurons were thought to be the pathological basis, and it was reported that neuron apoptosis was directly associated with cerebral I/R injury [18]. Moreover, long-term learning deficits were also reported to be the secondary result of loss and dysfunctions of neurons [19]. In this study, we found that apoptosis was increased in neurons exposed to H/R. The expression of cleaved caspase3 was upregulated in H/R-exposed neurons, indicating activation of the caspase cascade. Inflammatory response is another feature characterizing brain I/R injury. Our results showed that H/R exposure dramatically increased inflammatory responses of neurons. The contents of pro-inflammatory cytokines TNFα and IL6 in neurons and cell culture media were increased after neurons were exposed H/R.
Wnt5a is an important member of the Wnt family and are associated with many cellular biological functions [20]. According to previous studies, Wnt5a plays acts as a mediator of inflammatory responses and apoptotic events via activating its downstream effectors, such as JNK1 [8]. When challenged by multiple harmful stimuli, Wnt5a/JNK1 signaling is activated [21]. In this study, in neurons exposed to H/R, the expression of Wnt5a was upregulated and the phosphorylation of JNK1 was thus increased. As a result, NF-κB performed nuclear translocation and further initiated transcription of its target genes. We found that NF-κB nuclear translocation was upregulated in H/R-exposed neurons and then induced expression of pro-apoptotic caspase3 and pro-inflammatory factors TNFα and IL6. These results suggest that H/R activated Wnt5a/JNK1/NF-κB signaling, which resulted in neuron apoptosis and inflammation.
Bio-active agents extracted from medical herbs have been attracting research attention due to their effectiveness and safety. The anti-inflammatory and anti-apoptotic effects of curcumin were reported previously [22,23]. Curcumin’s neuro-protective effects were also found in many neuro-pathological models, including cerebral I/R in vivo and in vitro models [24,25]. Many mechanisms underlying curcumin’s neuro-protective effects have been proposed. For instance, regulation of certain microRNAs, mitochondrial functions, and autophagy were reported [26–28]. In the present study, curcumin at various concentrations was used to pre-treat neurons before H/R exposure. The results show that curcumin pre-treatment significantly increased viabilities by reducing apoptosis of neurons exposed to H/R. Curcumin was also reported to be a regulator of the Wnt signaling pathway. It was suggested that curcumin plays roles in regulating the Wnt pathway by affecting microRNAs, caudal type homeobox-2 (CDX2), glypican-3, and other molecular targets [29–31]. In the present study, we found that in H/R-exposed neurons, curcumin pre-treatment decreased Wnt5a expression, JNK1 phosphorylation, and NF-κB nuclear translocation. As a result, the Wnt/JNK signaling-mediated apoptosis and inflammation were dramatically suppressed by curcumin pre-treatment.
Conclusions
Curcumin exerted neuro-protective effects against H/R-induced neuron apoptosis and inflammation by inhibiting activation of the Wnt/JNK1 signaling pathway.
Footnotes
Source of support: This study was supported by the Medical Science and Technology Project of Zhejiang Province (2019KY196)
References
- 1.Yun Q, Jiang M, Wang J, et al. Overexpression Bax interacting factor-1 protects cortical neurons against cerebral ischemia-reperfusion injury through regulation of ERK1/2 pathway. J Neurol Sci. 2015;357:183–91. doi: 10.1016/j.jns.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Schimidt HL, Vieira A, Altermann C, et al. Memory deficits and oxidative stress in cerebral ischemia-reperfusion: Neuroprotective role of physical exercise and green tea supplementation. Neurobiol Learn Memory. 2014;114:242–50. doi: 10.1016/j.nlm.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Gong L, Tang Y, An R, et al. RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death Dis. 2017;8:e3080. doi: 10.1038/cddis.2017.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bu Q, Liu X, Zhu Y, et al. w007B protects brain against ischemia-reperfusion injury in rats through inhibiting inflammation, apoptosis and autophagy. Brain Res. 2014;1558:100–8. doi: 10.1016/j.brainres.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 5.Han MS, Barrett T, Brehm MA, Davis RJ. Inflammation mediated by JNK in myeloid cells promotes the development of hepatitis and hepatocellular carcinoma. Cell Rep. 2016;15:19–26. doi: 10.1016/j.celrep.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan J, Qi Z, Shen L, et al. Crosstalk between JNK and NF-kappaB signaling pathways via HSP27 phosphorylation in HepG2 cells. Biochem Biophys Res Commun. 2015;456:122–28. doi: 10.1016/j.bbrc.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 7.Chen CM, Orefice LL, Chiu SL, et al. Wnt5a is essential for hippocampal dendritic maintenance and spatial learning and memory in adult mice. Proc Natl Acad Sci USA. 2017;114(4):E619–28. doi: 10.1073/pnas.1615792114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei W, Li H, Li N, et al. WNT5A/JNK signaling regulates pancreatic cancer cells migration by phosphorylating paxillin. Pancreatology. 2013;13:384–92. doi: 10.1016/j.pan.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi M, Al-Suhaimi EA, Wahid F, et al. Therapeutic potential of curcumin for multiple sclerosis. Neurol Sci. 2018;39:207–14. doi: 10.1007/s10072-017-3149-5. [DOI] [PubMed] [Google Scholar]
- 10.Daugherty DJ, Marquez A, Calcutt NA, Schubert D. A novel curcumin derivative for the treatment of diabetic neuropathy. Neuropharmacology. 2018;129:26–35. doi: 10.1016/j.neuropharm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu JY, Yang X, Chen Y, et al. Curcumin suppresses lung cancer stem cells via inhibiting Wnt/beta-catenin and sonic hedgehog pathways. Phytother Res. 2017;31(4):680–88. doi: 10.1002/ptr.5791. [DOI] [PubMed] [Google Scholar]
- 12.Shi LY, Zhang L, Li H, et al. Protective effects of curcumin on acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Pharmacol Rep. 2018;70:1040–46. doi: 10.1016/j.pharep.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Huang J, Zhang L, Qu Y, et al. Histone acetylation of oligodendrocytes protects against white matter injury induced by inflammation and hypoxia-ischemia through activation of BDNF-TrkB signaling pathway in neonatal rats. Brain Res. 2018;1688:33–46. doi: 10.1016/j.brainres.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Odorcyk FK, Nicola F, Duran-Carabali LE, et al. Galantamine administration reduces reactive astrogliosis and upregulates the anti-oxidant enzyme catalase in rats submitted to neonatal hypoxia-ischemia. Int J Dev Neurosci. 2017;62:15–24. doi: 10.1016/j.ijdevneu.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Vallee A, Lecarpentier Y, Guillevin R, Vallee JN. Effects of cannabidiol interactions with Wnt/beta-catenin pathway and PPARgamma on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim Biophys Sin. 2017;49:853–66. doi: 10.1093/abbs/gmx073. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Rankin SA, Zorn AM. Syndecan4 coordinates Wnt/JNK and BMP signaling to regulate foregut progenitor development. Dev Biol. 2016;416:187–99. doi: 10.1016/j.ydbio.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Ye Q, Tu J, Zhang M, Ji B. Curcumin protects against hypertension aggravated retinal ischemia/reperfusion in a rat stroke model. Clin Exp Hypertens. 2017;39:711–17. doi: 10.1080/10641963.2017.1313854. [DOI] [PubMed] [Google Scholar]
- 18.Li N, Yuan Q, Cao XL, et al. Opposite effects of HDAC5 and p300 on MRTF-A-related neuronal apoptosis during ischemia/reperfusion injury in rats. Cell Death Dis. 2017;8:e2624. doi: 10.1038/cddis.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Veenman L, Singh S, et al. 2-Cl-MGV-1 ameliorates apoptosis in the thalamus and hippocampus and cognitive deficits after cortical infarct in rats. Stroke. 2017;48:3366–74. doi: 10.1161/STROKEAHA.117.019439. [DOI] [PubMed] [Google Scholar]
- 20.Kumawat K, Gosens R. WNT-5A: Signaling and functions in health and disease. Cell Mol Life Sci. 2016;73:567–87. doi: 10.1007/s00018-015-2076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Z, Yin S, Wu K, et al. Downregulation of Sfrp5 in insulin resistant rats promotes macrophage-mediated pulmonary inflammation through activation of Wnt5a/JNK1 signaling. Biochem Biophys Res Commun. 2018;505:498–504. doi: 10.1016/j.bbrc.2018.09.070. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Feng K, Li J, et al. Curcumin inhibits apoptosis of chondrocytes through activation ERK1/2 signaling pathways induced autophagy. Nutrients. 2017;9(4) doi: 10.3390/nu9040414. pii: E414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chin KY. The spice for joint inflammation: Anti-inflammatory role of curcumin in treating osteoarthritis. Drug Des Devel Ther. 2016;10:3029–42. doi: 10.2147/DDDT.S117432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang L, Chen C, Zhang X, et al. Neuroprotective effect of curcumin against cerebral ischemia-reperfusion via mediating autophagy and inflammation. J Mol Neurosci. 2018;64:129–39. doi: 10.1007/s12031-017-1006-x. [DOI] [PubMed] [Google Scholar]
- 25.Xie CJ, Gu AP, Cai J. Curcumin protects neural cells against ischemic injury in N2a cells and mouse brain with ischemic stroke. Brain Behav. 2018;8(2):e00921. doi: 10.1002/brb3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, Nie B, Liu L, et al. Curcumin prevents brain damage and cognitive dysfunction during ischemic-reperfusion through the regulation of miR-7-5p. Curr Neurovasc Res. 2019 doi: 10.2174/1567202616666191029113633. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Bavarsad K, Barreto GE, Hadjzadeh MA, et al. Protective effects of curcumin against ischemia-reperfusion injury in the nervous system. Mol Neurobiol. 2019;56:1391–404. doi: 10.1007/s12035-018-1169-7. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Fang M, Sun Y, et al. Curcumin attenuates cerebral ischemia injury in Sprague-Dawley rats and PC12 cells by suppressing overactivated autophagy. J Photochem Photobiol B. 2018;184:1–6. doi: 10.1016/j.jphotobiol.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Hu P, Ke C, Guo X, et al. Both glypican-3/Wnt/β-catenin signaling pathway and autophagy contributed to the inhibitory effect of curcumin on hepatocellular carcinoma. Dig Liver Dis. 2019;51:120–26. doi: 10.1016/j.dld.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Jiang X, Li S, Qiu X, et al. Curcumin inhibits cell viability and increases apoptosis of SW620 human colon adenocarcinoma cells via the caudal type homeobox-2 (CDX2)/Wnt/β-catenin pathway. Med Sci Monit. 2019;25:7451–58. doi: 10.12659/MSM.918364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel SS, Acharya A, Ray RS, et al. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit Rev Food Sci Nutr. 2019;11:1–53. doi: 10.1080/10408398.2018.1552244. [DOI] [PubMed] [Google Scholar]