Abstract
Arhinia (congenital absence of the nose) is a congenital rare disease, which has been reported in less than 60 cases in the literature. It consists of the absence of external nose, nasal cavities and olfactory apparatus and is generally associated with midline defects, microphthalmia, blepharophimosis and hypotelorism. Aesthetic problems as well as associated functional anomalies can potentially impact on the development and interpersonal relationships of the child at a later stage in life.
Arhinia requires extensive management in early life in order to ensure airway patency and protection by means of tracheostomy, and to allow adequate pharyngeal and feeding function to the child. Aesthetic issues are managed with reconstructive surgery or an external prosthesis. There is no previous description in Literature of internal prosthetic devices used to sequentially shape soft tissues in complex reconstruction.
We present an example of design and manufacturing of a bespoke nose implant produced by means of 3D printing and directly assessed on-table by means of 3D surface scanning.
Keywords: Congenital arhinia, 3D priting, 3D scanning, Patient specific implant
Introduction
Congenital arhinia is a potentially life-threatening congenital condition characterised by total absence of the external nose, nasal cavities and olfactory apparatus, which is often also associated with other facial abnormalities (hypotelorism, microphthalmia, blepharophimosis, facial cleft, encephaloceles and other midline defects).1, 2, 5
Corrective surgery includes surgical airway reconstruction6, 7 and nasal reconstruction. The choice of functional4 versus aesthetic-only8 reconstruction is based on presence of sinuses and the complexity of the underlying bony hypoplasia. In practise, it is rarely possible to create a functional nasal airway and reconstruction generally focuses on producing a balanced appearance. Traditional reconstruction generally involves the creation of a skeletal framework with soft tissue cover provided by a staged pedicled forehead flap. This form of definitive reconstruction has to be delayed until towards the end of skeletal growth (20 years old for females and after 22 years of age for males).
The use of non – specific serial solid shapes was reported in the reconstruction of the anophthalmic orbit (Krastinova et al.13). We have adopted this principle with the use of contemporary technology to present a case report utilising serial staged expansion of midfacial skin to provide age appropriate nasal reconstruction with the use of bespoke custom-made nasal implants. This approach provides skin for definitive reconstruction in adolescence and offers a satisfactory temporary reconstruction throughout childhood.
Methodology
A female patient, aged 1, presented at Great Ormond Street Hospital in 2014 with congenital arhinia. A Computed Tomography (CT) scan at the age of 2 revealed sufficient midfacial bony development to support a custom made PEEK (Poly Ether Ketone) implant in the nasal region. Implant design was undertaken based on CT data using a combination of physical and in-silico modelling using the following methodology.
-
1.
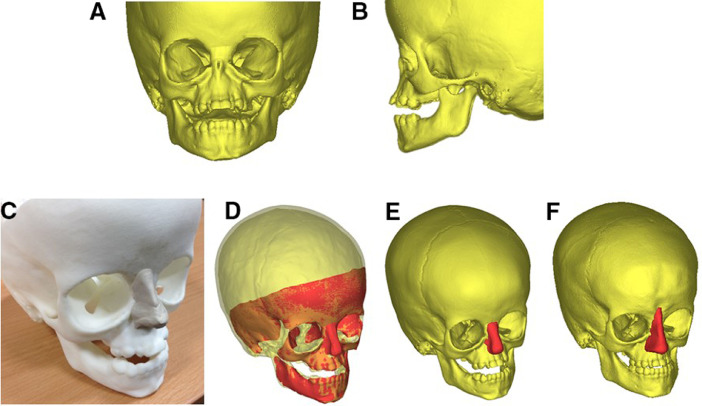
A real size 3D rapid prototyping model of the facial skeleton of the patient was produced using CT data (Cavendish Imaging, London, UK) (Figure 1A and B)
-
2.
The implant shape was designed by the surgeon using modelling clay (Plasticine) applied to the rapid prototype (RP) model. The implant shape was designed to expand the overlying skin envelope and achieve a cosmetic improvement in midfacial form (Figure 1C).
-
3.
The surface of the RP model bearing the modelling clay construct was afterwards acquired by means of 3D scanner technology (RODIN4D, Pessac, France) and imported into a dedicated 3D modelling software (MIMICS - Materialise, Leuven, Belgium) for processing.
-
4.
The original CT data was aligned with scanned surface containing the RP and nasal construct (Figure 1D).
-
5.
The volume of the Plasticine nose was extracted by means of Boolean difference (i.e. the 3D virtual RP model volume was subtracted from the RP model and nose one): such volume was afterwards exported as STL (stereolitographic) file and processed in MeshMixer (Autodesk, California). The processed model was afterwards reimported into MIMICS to assess overall quality and ensure perfect anatomical fit (Figure 1E).
-
6.
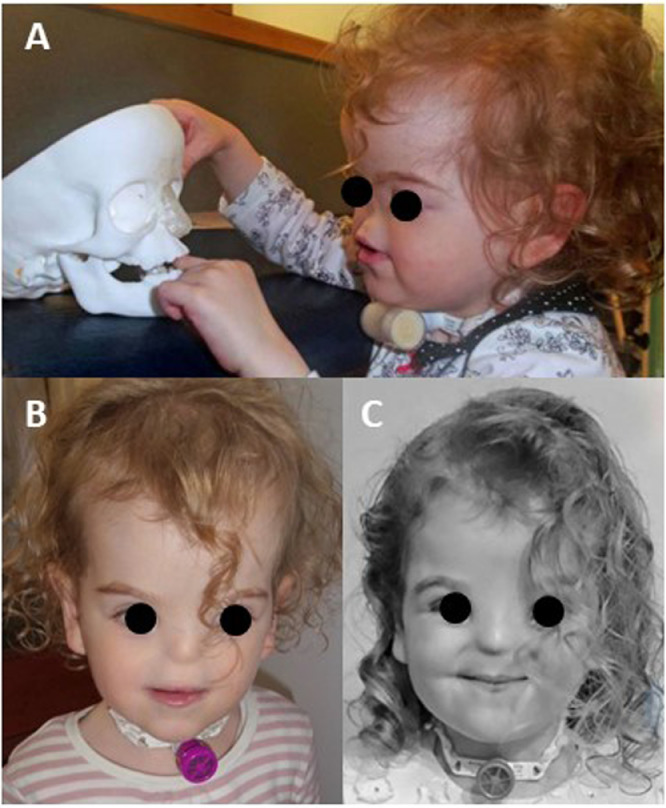
A test prototype implant was produced in polylactic acid (PLA) by means of fused deposition modelling (Makerbot Replicator II). The model was used to test fitting as well as well as for demonstrating the surgical procedure to patient and family (Figure 4A).
-
7.
An implantable custom-made PEEK implant was produced by an external company (Cavendish Implants, London, UK) with minor modifications to enable fixation.
Figure 1.
A) 3D bone reconstruction from CT scan of the patient at age 2, front view and B) side view C) 3D printed replica of the patient skull age 2; a nose implant was moulded with plasticine by an expert craniofacial surgeon; D) 3D scan of the skull with nose implant superimposed on the patient skull; E) the nose implant volume separated from the skull and ready for manufacturing; F) the second nose implant for the second procedure.
Figure 4.
A) Picture of the patient looking at the implant prototype before the first procedure aged 2; B) picture of the patient three months after the procedure aged 2; C) picture of the patient two months after the second procedure aged 4.
Insertion of the implant was undertaken under general anaesthesia via a coronal sequentially subgaleal and subperiosteal approach, to maintain the incision away from the implant pocket. The implant was then secured to the bone by a 1.5 mm miniscrew. Stability was assured with one point fixation and an exact soft tissue pocket.
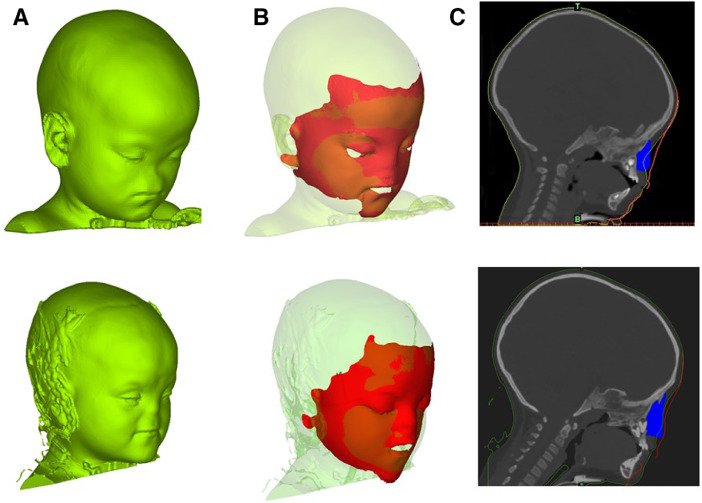
3D surface scans (RODIN4D) of the face of the patient before and after the procedure were acquired on table in theatre. The post-op scan was superimposed on the original 3D scan (Figure 2A, top) for visualization of the performance (Figure 2B, top): the change in external features matched with the site of the implants (2C, top).
Figure 2.
A) 3D reconstruction of the patient soft tissues at age 2 (top) and age 4 (bottom); B) post-op on-Table 3D scan superimposed on the 3D reconstruction at age 2 (top) and age 4 (bottom); C) sagittal cross section of the patient skull showing the soft tissue (green line), the on-Table 3D scan (red line) and the implant (blue) at age 2 (top) and age 4 (bottom). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In order to adequately expand the skin for a definitive nasal reconstruction, the insertion of serial implants of increasing size is required. To date, the patient has undergone implant insertion at the age of 2 and 4 years (Figure 1F).
Results
The patient received two-stage implant augmentation at the age of 2 and 4. In both cases the procedure was successful and the implant was well tolerated. An injury to the skin over the nasal tip after the insertion of the first implant was treated by surgical debridement.
After the second insertion, the patient experienced repeated implant infection associated with orbital infections. This resulted in a discharging wound at the thinned skin at the point of maximum projection of the implant. The decision was therefore taken to remove the implant, gain control of the infection, and rest the tissues before recommencing the expansion series.
We note that patients with arrhinia and blepharophimosis will have an obstruction of the nasolacrimal duct with ectasia of the lachrymal sac. Saline infusion of the lacrimal ducts intra-operatively at implant removal revealed a connection to the implant cavity, and may have been the point of entry of infection, disturbed by the previous second implant insertion. The lachrymal apparatus was therefore ablated at implant removal to reduce further infection risk.
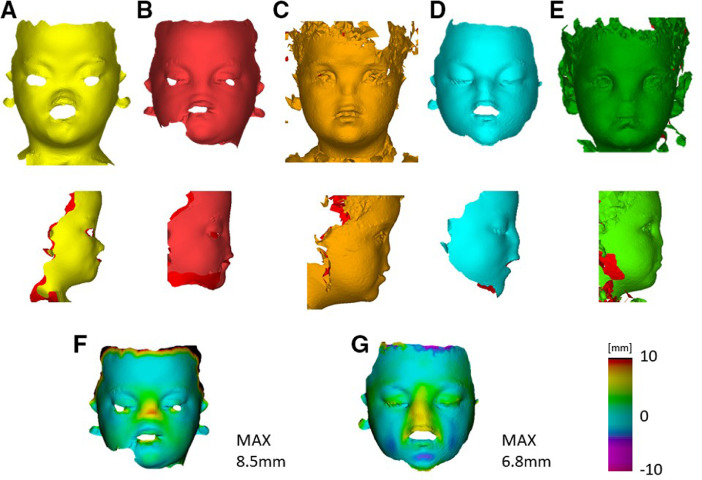
Serial 3D pictures retrieved before the first procedure (Figure 3A), after the first procedure (Figure 3B), before the second procedure (Figure 3C), after the second procedure (Figure 3D) and at 2 months follow up (Figure 3E) show a clear change in appearance in the patient, who gains a more featured silhouette (Figure 3 A–E bottom).
Figure 3.
A) Pre-op on-Table 3D scan of the patient before the first procedure: frontal (top) and lateral (bottom) view; B) Post-op on-Table 3D scan of the patient after the first procedure: frontal (top) and lateral (bottom) view C) Pre-op 3D picture of the patient before the second procedure: frontal (top) and lateral (bottom) view; D) Post-op on-Table 3D scan of the patient after the second procedure: frontal (top) and lateral (bottom) view; E) Follow up 3D picture of the patient at 2 months after the second procedure: frontal (top) and lateral (bottom) view; F) surface difference between pre-op and post-op 3D scans at the first procedure; E) surface difference between pre-op and post-op 3D scans at the second procedure.
The on-table change in face appearance was quantified: the first procedure pre-operative (Figure 3A) and postoperative scans (Figure 3B) were registered using iterative closest point algorithm (an algorithm to register two surfaces by iteratively minimizing the distance between the underlying point clouds) and further adjustment was performed by manually perform small rotation and translation of the two models. Following registration, a closest point distance map was retrieved (Cloud, Robin3D, London): the minimum distance between each point the two scans was calculated and visualised, to highlight the areas of change in soft tissue projection. A maximum surface distance of 8.5 mm, corresponding of the tip of the implant, was found (Figure 3F). In a similar way, the second procedure pre-operative (Figure 3C) and post-operative (Figure 3D) were processed: at this stage, a peak augmentation of 6.8 mm was achieved (Figure 3G). These findings demonstrate a significant increase in projection of the nasal reconstruction.
Discussion
Additive manufacturing has an important role in modern medicine: several studies in the literature have reported successful bespoke implant design,3 creation of surgical guides,9,10 surgical planning11 and patient communication and engagement.12
In the current study, the full potential of 3D printing from patient specific medical imaging was exploited: a series of life sized anatomical models were used for surgical planning, design of patient-specific implants and medical communication to the family. A combined digital and hands-on approach to the design of the prosthesis allowed precise manufacturing of an anatomically fitting nose implant and provides a mechanism for the surgeon to manipulate directly the implant shape.
The combination of medical image processing and 3D scanning allowed the direct reproduction of the shape prescribed by the operating surgeon as well a bespoke anatomical fitting to the anomalous midfacial features of the patient. The production of an accurate model allowed the use of computerised numerical control machining for the manufacturing of the implant in a stable medical grade plastic.
Conventional nasal reconstructive techniques generally need to be delayed until early adulthood when facial growth is almost complete. Whilst this ensures an appropriate size of reconstruction, the lack of a nose throughout childhood and adolescence may cause significant psychological challenges. Such technique allows a simple effective nasal reconstruction to be performed in infancy and childhood whilst gradually expanding skin for a definitive reconstruction. It was noticed during the second surgery (but it was also visible from the second set of CT scans) that the presence of the first implant caused the mid-face bone to grow around it. Although this posed some challenges to the implant removal, it caused no problems to the creation and implantation of the second implant, which was designed according to the shape of the underlying newly formed bone.
Figure 4B shows a picture of the patient three months after the first procedure while Figure 4C shows a picture of the patient two months after the second procedure.
Conclusion
This report presents a possible protocol for the treatment of patients affected by congenital arhinia, which combines 3D printing of patient specific anatomy, 3D scanning, and computer numeric control (CNC) machining for the production of serial bespoke nasal implants. The technique relies on accurate 3D technology to ensure interface matching between implant and cranial anatomy, whilst implant design uses physical moulding and carving techniques to provide bespoke implants in a modern application for the traditional plastic surgery technique of tissue expansion.
Acknowledgments
Conflict of interest
None declared.
Source of funding
The work has been funded by Great Ormond Street Hospital for Children Charity (grant number 12SG15). This report incorporates independent research from the National Institute for Health Research Biomedical Research Centre Funding Scheme. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research (grant number 17DS18) or the Department of Health.
Acknowledgements
We would like to thank the patient's family for kindly providing consent for the use of the medical images for the study as well as for providing up-to-date pictures of their baby.
References
- 1.McGlone L. Congenital arhinia. J Paediatr Child Health. 2003;39:474–476. doi: 10.1046/j.1440-1754.2003.00193.x. [DOI] [PubMed] [Google Scholar]
- 2.Olsen Ø.E., Gjelland K., Reigstad H., Rosendahl K. Congenital absence of the nose: a case report and literature review. Pediatr Radiol. 2001;31:225–232. doi: 10.1007/s002470000419. [DOI] [PubMed] [Google Scholar]
- 3.Fernandes N. Reconstruction of an extensive midfacial defect using additive manufacturing techniques. J Prosthodont. 2016 doi: 10.1111/jopr.12487. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes N., van den Heever J., Sykes L., Kluge H. Nasal reconstruction of a patient with complete congenital arhinia: a clinical report. J Prosthet Dent. 2016;116:924–927. doi: 10.1016/j.prosdent.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Akkuzu G., Akkuzu B., Aydin E., Derbent M., Ozluoglu L. Congenital partial arhinia: a case report. J Med Case Rep. 2007;1:97. doi: 10.1186/1752-1947-1-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mühlbauer W., Schmidt A., Fairley J. Simultaneous construction of an internal and external nose in an infant with arhinia. Plast Reconstr Surg. 1993;91:720–725. doi: 10.1097/00006534-199304000-00027. [DOI] [PubMed] [Google Scholar]
- 7.Cole R.R., Myer C.M., Bratcher G.O. Congenital absence of the nose: a case report. Int J Pediatr Otorhinolaryngol. 1989;17:171–177. doi: 10.1016/0165-5876(89)90092-x. [DOI] [PubMed] [Google Scholar]
- 8.Lütolf U. Bilateral aplasia of the nose: a case report. J Maxillofac Surg. 1976;4:245–249. doi: 10.1016/s0301-0503(76)80047-1. [DOI] [PubMed] [Google Scholar]
- 9.Shen P. Accuracy evaluation of computer-designed surgical guide template in oral implantology. J Craniomaxillofac Surg. 2015;43:2189–2194. doi: 10.1016/j.jcms.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Lu T. Cervical screw placement using rapid prototyping drill templates for navigation: a literature review. Int J Comput Assist Radiol Surg. 2016 doi: 10.1007/s11548-016-1414-3. [DOI] [PubMed] [Google Scholar]
- 11.Schievano S. Percutaneous pulmonary valve implantation based on rapid prototyping of right ventricular outflow tract and pulmonary trunk from MR data. Radiology. 2007;242:490–497. doi: 10.1148/radiol.2422051994. [DOI] [PubMed] [Google Scholar]
- 12.Biglino G. 3D-manufactured patient-specific models of congenital heart defects for communication in clinical practice: feasibility and acceptability. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krastinova D., Kelly M.B., Mihaylova M. Surgical management of the anophthalmic orbit, part 1: congenital. Plast Reconstr Surg. 2001;108:817–826. doi: 10.1097/00006534-200109150-00001. [DOI] [PubMed] [Google Scholar]