Abstract
Background
In gender-confirming surgery of the female-to-male gender dysphoric patient, there is currently no ideal method for the creation of a neophallus. Historically, in our clinic, groin flap phalloplasty (GFP) has been the dominating method, but during the last 20 years, it has gradually been replaced with metoidioplasty (MP). The aim of this study was to investigate whether this change of method has influenced factors such as the frequency of complications and the number of operations needed to complete the reconstruction of the neophallus.
Methods
This is a retrospective, single-centre, study comprising 123 consecutive female-to-male patients receiving a neophallus by GFP or MP between 2002 and 2015 at Linköping University Hospital, Sweden.
Results
One-hundred twenty-three patients underwent 126 primary surgical procedures (39 GFPs and 87 MPs) with the intention of reconstructing a neophallus. The mean number of procedures required in the GFP group was 5.2 ± 2.7 compared with that of 2.4 ± 1.7 in the MP group (p < 0.001). In the GFP group, 18/39 (46.2%) had a documented complication compared with 30/87 (34.5%) in the MP group; however, the difference was not statistically significant (p = 0.21).
Conclusions
The present study shows that the shift in method from GFP to MP has resulted in a decreased number of complications as well as a decrease in total surgical occasions. Both methods were found to be associated with relatively high frequencies of complications, however, mostly minor.
Keywords: Groin flap phalloplasty, Metoidioplasty, Gender dysphoria, Female-to-male, Gender-confirming surgery
Introduction
Gender dysphoria (GD) is a term used to describe the condition of a person with discrepancy between one's natal sex and gender identity1. GD, formerly known as transsexualism or gender identity disorder, is highly associated with psychological comorbidity, for example, anxiety, depression and high frequency of completed suicide, suicide attempt and self-injurious behaviour2, 3. Modalities of treatment currently include psychological support, hormonal treatment and surgical interventions, also known as gender-confirming surgery (GCS)4.
The prevalence of GD is difficult to estimate. Depending on which study is cited, ranges of 1:8300–1:400,000 for female-to-male (FtM) and 1:2900–1:100,000 for male-to-female (MtF) GD have been reported1, 3. In Sweden, the number and incidence rate of applications for GCS have been vigorously increasing: from 0.16/100,000 in 1972 to 0.42/100,000 in 2010 for FtM and from 0.23/100,000 to 0.73/100,000 for MtF during the same period3. Later data suggest a continuing increase after 20105 (data not shown).
In patients with FtM GD, the mainstay of pharmacological treatment is masculinising hormone therapy with testosterone preparations, the duration of which is lifelong6. Surgical procedures may include mastectomy (“top surgery”), hysterectomy, salpingo-oophorectomy, vaginectomy, reconstruction of a neophallus and scrotoplasty (‘bottom surgery’)4.
A number of methods for reconstructing a neophallus in patients with FtM GD have been described, proving the lack of a universally ideal method of choice or ‘gold standard’. Most commonly used procedures to achieve the neophallus in patients with FtM GD are metoidioplasty (MP) and the free, or local, flap-based phalloplasty7. Both groups of surgery present advantages and disadvantages regarding the final result, such as size, ability to void while standing, ability to perform penetrative intercourse, aesthetic appearance and sexual and tactile sensation.
At our surgical centre we use several different surgical techniques to construct a neophallus, but groin flap phalloplasty (GFP), as a long-since utilised technique, has been the most used method historically. However, since the MP was introduced in our centre at approximately 20 years ago, it has gradually taken over as our most commonly used method for the reconstruction of a neophallus.
When changing surgical methods, it is of high importance to investigate the effects of the change not only regarding the end result but also regarding the surrounding factors that influence the patient's well-being as well as the total cost of the procedure. In accordance with this, our shift in method of choice has raised the question of whether it has had an impact on the frequency and panorama of complications and/or the number of surgical procedures needed to achieve a complete neophallus. Knowledge of these factors is extremely important because of an increasing consumer demand for information of factors such as risks, timeframe for the complete process and outcome of the surgery. A lack of reliable knowledge of these basic factors can leave both the patient and the caregiver in a non-satisfactory situation when providing preoperative information8. The aims of the present study were thus to compare patients with FtM GD operated by GFP and MP and to describe the need for additional surgical procedures and revisions as well as the frequency and nature of complications in a single surgical centre for GCS.
Material and methods
Approval for the study was granted by the regional ethical review board (Dnr 2018/61-31). We performed a retrospective study by including all patients with FtM GD who underwent primary genital GCS with the intention of achieving a neophallus between July 2002 and June 2015 at the Department of Plastic Surgery, Linköping University Hospital, Linköping, Sweden. Information concerning additional surgical procedures and complications was collected after at least one year of follow-up. Data collection was conducted in June 2016. The study was constructed according to guidelines provided by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for cohort studies (version 4, Oct/Nov 2017)9.
From the hospital's records, we identified the patients who underwent FtM GCS by the WHO International Statistical Classification of Diseases and Related Health Problems, 10th revision, Swedish version (ICD-10-SE) diagnosis code F64.0 (transsexualism)10 and GFP or MP surgical procedure codes. The data obtained were then cross-referenced and validated with the identified patient's medical records.
The patient's medical records were manually reviewed and cross-matched with the individual surgical notes to correct any discrepancies regarding surgical procedures and complications. Complications were defined as any deviation from the normal postoperative course causing symptoms as a direct result of the neophallus reconstruction11. Only surgical procedures and complications directly related to either the GFP or the MP were considered, that is, procedures and complications related to the anaesthesia or synchronous surgical procedures were omitted. For patients who, at some point, had undergone both procedures (GFP and MP), the complications and revisions were directly related to the corresponding technique only, that is, each complication and revision was included only once and attributed to the most recent type of neophallus reconstruction. Conversion from GFP to MP or vice versa was not considered a revision for the purpose of this study.
Selection for surgery and surgical management
All patients undergoing genital GCS in Sweden are required by law to have been approved by the Legal Counsel of the National Board of Health and Welfare. Approval can be given only if evaluation done by a multidisciplinary team specialized in determining gender identity results in the ICD-10 diagnosis F64.0 (transsexualism); this diagnosis is assessed probable to remain, a ‘real-life’ trial has been passed, and the person is >18 years old12, 13.
After referral to our centre for GCS, the patient was informed regarding the advantages and disadvantages of the different methods to achieve a neophallus and showed photographs of surgical results. Choice of the method was given to the patient, but if either method was not suitable, for example, due to an insufficiently hypertrophied clitoris in the case of MP, the other method would be recommended.
In general, patients with FtM GD underwent abdominal hysterectomy and bilateral salpingo-oophorectomy at the same operation as the neophallus was reconstructed constructed. Unless contraindicated, patients received i.v. intraoperative antibiotic prophylaxis with cloxacillin 2 g and metronidazole 1.5 g and postoperative flucloxacillin 1 g TID for 10 days. Postoperatively, low-molecular-weight heparin (LMWH) 4500 IE OD for 7 days was administered. All patients had initial postoperative pain management by intravenous morphine through a patient-controlled analgesia infusion pump and per oral paracetamol 1 g QID.
Groin flap phalloplasty (GFP)
A large, pedicled flap of size approximately 15 × 30 cm, based on the superficial circumflex iliac artery, is raised from the left inguinal area14. The flap's medial aspect is not dissected and left in situ. Commonly, the dissection can be stopped when the vessel(s) have been reached. The flap is rolled up and sutured into a tube. The lateral end is split in the middle into a ‘fish tail’, which is de-epithelialised. At the lower aspect of the os pubis (i.e. the position of the neophallus base), an Ω-shaped skin incision is made. Both de-epithelialised tail parts are anchored deep into the Ω-incision to sturdy tissues on either sides of the midline and the flap skin is sutured to the pubic skin. Thus, the flap is anchored at both ends with a resulting ‘bucket-handle’ appearance. The ‘bucket-handle’ is left for approximately 5 weeks to ascertain circulation into the tissue from both ends of the flap. At a later procedure, in local anaesthesia, the initial medial pedicle is transected and the neophallus is expected to be circulated from the base of the neophallus.
Once completely healed, a number of outpatient surgical procedures are offered, such as insertion of erectile rods and testicular prostheses, coronaplasty and tattooing of the glans.
Metoidioplasty (MP)
The procedure was mainly performed as described by Hage15 with minor variations. In brief, the hypertrophied clitoris is surgically lengthened and straightened. A tubularised full-thickness skin/mucosal pedicled flap of the anterior vaginal wall (pedicle inferior of the meatus) is sutured to the tubularised skin from the inner aspect of one labia minora to create a full-length neourethra from original meatus to the top of the clitoris/MP. The neophallus and neourethra are then covered with skin flaps from the outer aspect of the contralateral labia minora. Testicular prostheses are placed in the labia majora. If requested by the patient, a vaginectomy and a scrotoplasty could be performed at a later stage.
Statistical analysis
Continuous data are presented as mean and one standard deviation (SD) or median and range. Nominal data are shown as number or frequency and per cent. Statistical analyses of comparisons were performed by Mann–Whitney U test, Pearson's chi-square test or Fisher's exact test. The significance level was set at p < 0.05. The statistical tests were two tailed and were conducted using Statistica v 13.2 (Dell Software, 5 Polaris Way, Aliso Viejo, CA, USA).
Results
One-hundred twenty-three patients were included in the study, and 126 primary surgical procedures were conducted in them. Three of the patients underwent both procedures; thus, GFP was performed in 39 (31%) cases and MP in 87 (69%) cases. All patients in both groups had received testicular implants.
The mean age of the patients at the time of primary surgery was 31.9 ± 8.8 years in the GFP group and 31.3 years ± 8.2 years in the MP group (p = 0.70).
The follow-up time after these 126 procedures was median 7.3 years (range 1–13 years) in the GFP group, which did not show statistically significant difference from median 7.5 years (range 1–13 years) in the MP group (p = 0.88).
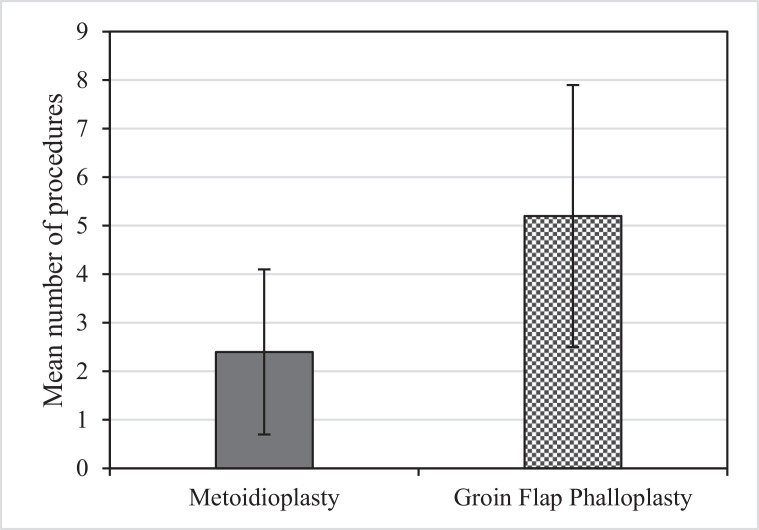
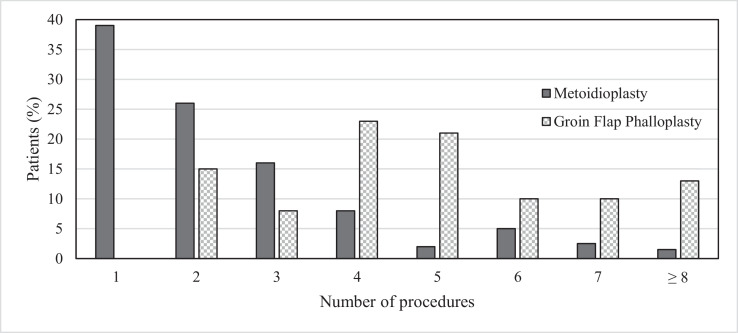
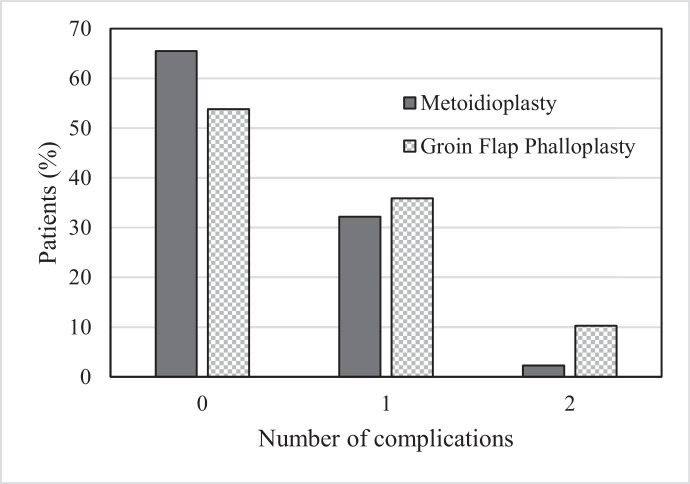
The mean number of procedures required per patient (Figure 1) was 5.2 ± 2.7 in the GFP group compared with that of 2.4 ± 1.7 in the MP group (p < 0.001) (Figure 2). The procedures were either additional stages of the neophallus reconstruction or revisions due to complication(s). Complications (Table 1) occurred more frequently in the GFP group (18 of 39 (46.2%)) than in the MP group (30 of 87 (34.5%)). However, the difference did not reach statistical significance (p = 0.21). The distribution of the number of complications per patient is shown in Figure 3.
Figure 1.
Number of surgical procedures (mean ± 1 SD) required related to the method of neophallus reconstruction construction.
Figure 2.
Number of surgical procedures required related to the mode of surgery.
Table 1.
Complications in patients who underwent female-to-male gender-confirming surgery with a follow-up period of at least one year.
| Type of complication | Metoidioplasty (n = 87) | Groin flap phalloplasty (n = 39) |
|---|---|---|
| n (%) | n (%) | |
| Neourethral fistula | 29 (33.3) | – |
| Compartment syndrome of leg | 1 (1.1) | – |
| Loss of testicular implant(s) | 2 (2.3) | 8 (20.5) |
| Loss of erectile rod(s) | – | 12 (30.8) |
| Infection of the neophallus | – | 1 (2.6) |
| Healing insufficiency | – | 1 (2.6) |
Figure 3.
Number of complications in relation to the mode of surgery.
The most common complications in the GFP group were early postoperative infection requiring testicular implants to be removed, and late complications related to the erectile rods that required removal mostly due to skin breakdown and protruding implants. In the MP group, the most common complication was neourethral fistulae. There were no cases where the groin flap was lost. Loss of testicular implants was significantly more common in the GFP group than in the MP group, eight of 39 (20.5%) versus two of 87 (2.3%), respectively (p < 0.001).
Discussion
No ideal method or ‘gold standard’ of achieving a neophallus in the patient with FtM GD seems to be currently available7. The penis can be constructed either by using the hypertrophic clitoris (MP) or by transferring autologous tissue to the genital area either as a free or a local flap (phalloplasty). The tissue needed for the phalloplasty can be obtained from a large number of different body sites, and different surgical centres have their own desirable harvest area. Two of the most common donator areas through history are the forearm15 and groin, with the latter being the most commonly used historically in our clinic even though forearm flaps are also used. When compared to others, arguably more modern methods such as radial free forearm flap phalloplasty (RFFF)16, the GFP offers, in our opinion, less donor site morbidity (scar under waistline vs. ‘shark bite’ appearance of forearm)17 as well as lower risks than microsurgical techniques. However, during the last decades, the demand from patients has resulted in the MP becoming the most common procedure when reconstructing a neophallus in our clinic. The choice of which method to be used is always ultimately left to the patient. The patient's decision must be based on the most accurate information available regarding the different methods’ advantages and disadvantages. However, there is a lack of comparative studies of different techniques even regarding basic factors such as complications and number of procedures needed for a final result7. We currently experience that the lack of scientific evidence in this field makes information to, and guidance of, the patient regarding their choice of method unsatisfactory.
This study provides evidence that may improve the information and counselling of patients with FtM GD who desires a neophallus reconstruction.
We found that reconstruction by the MP method required statistically significantly fewer surgical procedures than that by the GFP in our centre.
This may be an important issue for both the patient and the health care provider. It is, however, important to note that the GFP is a staged procedure, requiring at least 2 surgical procedures. The frequency of complications with both methods was high, being the highest for GFP, although not statistically significant in this material. We believe that in a larger study sample, the differences would most likely reach statistical significance. Moreover, this finding may be expected, as a patient with GFP is likely to undergo more surgical procedures than the one with MP and therefore may also have a higher risk of adverse events. Even though there are a substantial number of complications in both groups, it should be noted that almost all of them are to be considered as surgically minor complications that can easily be treated. The only severe complication encountered was that of a patient with an MP who developed a compartment syndrome in the lower leg, which could be managed without remaining limitations in function.
As the properties of each method's final result differ, the methods may not be interchangeable. A GFP offers a more voluminous neophallus that, with the addition of erectile rods, may be capable of penetrative sexual intercourse but lacks erogenous sensation and the ability to achieve erection without artificial aids. A MP gives the patient a small neophallus, hardly capable of performing penetrative sexual intercourse, but with a preserved erogenous and tactile sensation, it gives the ability to achieve a natural erection and to stand while voiding, the latter being a traditionally important male feature. However, the most common reason for choosing an MP among our patients is the more natural appearance than flap-based phalloplasties.
The frequent incidence of complications in the GFP group together with the need for significantly more surgical procedures could justify the more frequent use of the MP. However, other parameters such as patient satisfaction, functional and cosmetic outcome, quality of life aspects and properties of each method as mentioned above need to be taken into consideration. Although these factors have not been accounted for in this study, they should be investigated in the future, as they may be equally important for the patient's decision.
The findings in this study will, in a substantial way, improve our possibilities to inform the patients in an adequate way before their decision regarding which method to be used when reconstructing their neophallus. Furthermore, the information collected will help us to focus on our efforts to further develop the parts of the procedures that are in the greatest need for improvement.
Declaration of Competing Interest
None.
Acknowledgments
The study was supported financially by unrestricted grants from Region Östergötland and Linköping University, grant numbers LIO-701841 and LIO-810231.
References
- 1.Zucker K.J., Lawrence A.A., Kreukels B.P. Gender dysphoria in adults. Annu Rev Clin Psychol. 2016;12:217–247. doi: 10.1146/annurev-clinpsy-021815-093034. [DOI] [PubMed] [Google Scholar]
- 2.Reisner S.L., Poteat T., Keatley J. Global health burden and needs of transgender populations: a review. Lancet. 2016;388(10042):412–436. doi: 10.1016/S0140-6736(16)00684-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhejne C., Öberg K., Arver S. An analysis of all applications for sex reassignment surgery in Sweden, 1960–2010: prevalence, incidence, and regrets. Arch Sex Behav. 2014;43:1535–1545. doi: 10.1007/s10508-014-0300-8. [DOI] [PubMed] [Google Scholar]
- 4.Frisén L., Söder O., Rydenlius P.A. [Dramatic increase of gender dysphoria in youth] Läkartidningen. 2017;114 Swedish. EFMY. [PubMed] [Google Scholar]
- 5.Selvaggi G., Dhejne C., Landen M. The 2011 WPATH standards of care and penile reconstruction in female-to-male transsexual individuals. Adv Urol. 2012;2012 doi: 10.1155/2012/581712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unger C.A. Hormone therapy for transgender patients. Transl Androl Urol. 2016;5(6):877–884. doi: 10.21037/tau.2016.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey J.D., Poudrier G., Chiodo M.V. An update on genital reconstruction options for the female-to-male transgender patient: a review of the literature. Plast Reconstr Surg. 2017;139(3):728–737. doi: 10.1097/PRS.0000000000003062. [DOI] [PubMed] [Google Scholar]
- 8.Frey J.D., Poudrier G., Chiodo M.V. A systematic review of metoidioplasty and radial forearm flap phalloplasty in female-to-male transgender genital reconstruction: is the “ideal” neophallus an achievable goal? Plast Reconstr Surg Glob Open. 2016;4(12):e1131. doi: 10.1097/GOX.0000000000001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Elm E., Altman D.G., Egger M. STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 10.The National Board of Health and Welfare. [International statistical classification of diseases and related health Problems: a systematic list]. Swedish. 1997. http://www.socialstyrelsen.se/klassificeringochkoder/diagnoskodericd-10[Accessibility verified January 20, 2019].
- 11.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.[Law on determining gender identity in certain cases] (SFS 1972:119). Swedish. Stockholm: Socialdepartementet. 1972. http://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/lag-1972119-om-faststallande-av_sfs-1972-119[Accessibility verified January 20, 2019].
- 13.The National Board of Health and Welfare. [Recommendations regarding testimony in cases regarding the law on determining gender identity in certain cases] (Dnr 10.2-25178/2016). Swedish. 2016. http://www.socialstyrelsen.se/SiteCollectionDocuments/rattsliga-radet-rekommendationer-rorande-faststallande-av-konsidentitet.pdf[Accessibility verified January 20, 2019].
- 14.Knutson G. The groin flap: a new technique to repair traumatic tissue defects. Can Med Assoc J. 1977;116(6):623–625. [PMC free article] [PubMed] [Google Scholar]
- 15.Hage J.J. Metaidoioplasty: an alternative phalloplasty technique in transsexuals. Plast Reconstr Surg. 1996;97(1):161–167. doi: 10.1097/00006534-199601000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Monstrey S., Hoebeke P., Selvaggi G. Penile reconstruction: is the radial forearm flap really the standard technique? Plast Reconstr Surg. 2009;124:510–518. doi: 10.1097/PRS.0b013e3181aeeb06. [DOI] [PubMed] [Google Scholar]
- 17.Selvaggi G., Monstrey S., Hoebeke P. Donor-site morbidity of the radial forearm free flap after 125 phalloplasties in gender identity disorder. Plast Reconstr Surg. 2006;118:1171–1177. doi: 10.1097/01.prs.0000221110.43002.a0. [DOI] [PubMed] [Google Scholar]