Abstract
Background
Epilepsy is an important neurological condition and drug resistance in epilepsy is particularly common in individuals with focal seizures. In this review, we summarise the current evidence regarding a new antiepileptic drug, levetiracetam, when used as add‐on treatment for controlling drug‐resistant focal epilepsy. This is an update to a Cochrane Review that was originally published in 2001.
Objectives
To evaluate the effectiveness of levetiracetam, added on to usual care, in treating drug‐resistant focal epilepsy.
Search methods
We searched the Cochrane Epilepsy Group's Specialized Register (August 2012), the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library Issue 7, 2012), and MEDLINE (1946 to August week 1, 2012). We also contacted the manufacturers of levetiracetam and researchers in the field to seek any ongoing or unpublished trials.
Selection criteria
Randomised, placebo‐controlled trials of add‐on levetiracetam treatment in people with drug‐resistant focal epilepsy.
Data collection and analysis
Two review authors independently selected trials for inclusion, assessed trials for bias, extracted data, and evaluated the overall quality of evidence. Outcomes investigated included 50% or greater reduction in focal seizure frequency (response); less than 50% reduction in focal seizure frequency (non‐response); treatment withdrawal; adverse effects (including a specific analysis of changes in behaviour); cognitive effects and quality of life (QoL). Risk ratios (RR) with 95% confidence intervals (CIs) were used as measures of effect (99% CIs for adverse effects). Primary analyses were Intention‐to‐Treat (ITT). Dose response and inter‐trial heterogeneity were evaluated in regression models.
Main results
Eleven trials (1861 participants) were included. They predominantly possessed low risks of bias. Participants were adults in nine trials (1565 participants) and children in the remaining two trials (296 participants). The dose of levetiracetam tested was 1000 to 4000 mg/day in adults, and 60 mg/kg/day in children. Treatment ranged from 12 to 24 weeks. For the 50% or greater reduction in focal seizure frequency outcome, the RR was significantly in favour of levetiracetam at all doses. The naive estimates, ignoring dose, showed children (52% responded) as better responders than adults (39% responded) on levetiracetam. 25% of children and 16% of adults responded to placebo. The Number Needed to Treat for an additional beneficial outcome for children and adults was four (95% CI three to seven) and five (95% CI four to six), respectively. The significant levels of statistical heterogeneity between trials on adults precluded valid provision of an overall RR (ignoring dose). Results for the two trials that tested levetiracetam 2000 mg on adults were sufficiently similar to be combined to give an RR for 50% or greater reduction in focal seizure frequency of 4.91 (95% CI 2.75 to 8.77), with an RR of 0.68 (95% CI 0.60 to 0.77) for non‐response. At this dose, 37% and 8% of adults were responders in the levetiracetam and placebo groups, respectively. Regression analysis demonstrated that much of the heterogeneity between adult trials was likely to be explained by different doses of levetiracetam tested and different years of trial publication. There was no evidence of statistical heterogeneity between trials on children. For these trials, the RR for 50% or greater reduction in focal seizure frequency was 1.91 (95% CI 1.38 to 2.63), with an RR of 0.68 (95% CI 0.56 to 0.81) for non‐response. 27% of children responded. Participants were not significantly more likely to have levetiracetam withdrawn (RR 0.98; 95% CI 0.73 to 1.32 and RR 0.80; 95% CI 0.43 to 1.46 for adults and children, respectively). For adults, somnolence (RR 1.51; 99% CI 1.06 to 2.17) and infection (RR 1.76; 99% CI 1.03 to 3.02) were significantly associated with levetiracetam. Accidental injury was significantly associated with placebo (RR 0.60; 99% CI 0.39 to 0.92). No individual adverse effect was significantly associated with levetiracetam in children. Changes in behaviour were negligible in adults (1% affected; RR 1.79; 99% CI 0.59 to 5.41) but significant in children (23% affected; RR 1.90; 99% CI 1.16 to 3.11). Cognitive effect and QoL outcomes suggested that levetiracetam had a positive effect on cognition and some aspects of QoL in adults. In children, levetiracetam did not appear to alter cognitive function but there was evidence of worsening in certain aspects of child behaviour. The overall quality of evidence used was high.
Authors' conclusions
This update adds seven more trials to the original review, which contained four trials. At every dose analysed, levetiracetam significantly reduced focal seizure frequency relative to placebo. This indicates that levetiracetam can significantly reduce focal seizure frequency when it is used as an add‐on treatment for both adults and children with drug‐resistant focal epilepsy. As there was evidence of significant levels of statistical heterogeneity within this positive effect it is difficult to be precise about the relative magnitude of the effect. At a dose of 2000 mg, levetiracetam may be expected to be 3.9 times more effective than placebo; with 30% of adults being responders at this dose. At a dose of 60 mg/kg/day, levetiracetam may be expected to be 0.9 times more effective than placebo; with 25% of children being responders at this dose. When dose was ignored, children were better responders than adults by around 4% to 13%. The results grossly suggest that one child or adult may respond to levetiracetam for every four or five children or adults, respectively, that have received levetiracetam rather than placebo. The drug seems to be well tolerated in both adults and children although non‐specific changes in behaviour may be experienced in as high as 20% of children. This aspect of the adverse‐effect profile of levetiracetam was analysed crudely and requires further investigation and validation. It seems reasonable to continue the use of levetiracetam in both adults and children with drug‐resistant focal epilepsy. The results cannot be used to confirm longer‐term or monotherapy effects of levetiracetam or its effects on generalised seizures. The conclusions are largely unchanged from those in the original review. The most significant contribution of this update is the addition of paediatric data into the analysis.
Plain language summary
Levetiracetam add‐on for drug‐resistant focal epilepsy
Levetiracetam is one of a new cohort of antiepileptic drugs currently available. In this review, we summarise the current evidence regarding its effectiveness when used as an add‐on treatment to usual care in people suffering from epilepsy that consists of drug‐resistant focal seizures. At every dose that we analysed, levetiracetam significantly reduced the frequency of seizures as compared to placebo. However, because the size of that positive effect varies somewhat from trial to trial, it is difficult of us to provide a summary estimate of just how large or small an effect levetiracetam will have overall. At a dose of 2000 mg, levetiracetam was roughly four times more effective than placebo and approximately 30% of adults may be expected to have significant reduction in the frequency of their seizures. Children took 60 mg/kg/day of levetiracetam and this was roughly once more effective than placebo. Approximately a quarter of children may have significant reduction in seizures at this dose. The overall finding was that levetiracetam can be effective at reducing focal seizure frequency and it can also be well tolerated in both adults and children. A possibility of changes in behaviour in children on levetiracetam was highlighted and this finding requires validation. This review is an update to a review published in 2001 and we have found seven additional trials to those in the original review. The conclusions are largely unchanged between the two reviews. The most significant contribution of this update is the inclusion of data from children. The results are not relevant to the use of levetiracetam in generalised seizures or to its use as a single agent.
Background
Description of the condition
Epilepsy is a common and serious neurological condition, affecting between 260,000 and 416,000 people in England and Wales, and 1% to 2% of the global population across all ages (Crepeau 2010; NICE 2012). In the developed world, the annual incidence of epilepsy is between 24 per 100,000 and 56 per 100,000 (Hauser 1993; Forsgren 2005), and its prevalence is from five per 1000 to 10 per 1000 (Sander 1996). In the developing world, the incidence and prevalence estimates rise up to 158/100,000 and 74/1000, respectively (Burneo 2005; Preux 2005; Mac 2007). This increase among developing countries may not only be because of poorer standards of health care, but also a higher proportion of children among these populations (Shorvon 1996). The incidence of epilepsy peaks in early childhood before falling to low levels in early adult life and then rising again among elderly people (Shorvon 1996). The UK National General Practice Study of Epilepsy found that of the 60% of people with epilepsy who have convulsive seizures, focal epilepsy is more common than general epilepsy, affecting two‐thirds and one third of the people, respectively (NICE 2012). The goal of epilepsy treatment is to achieve sustained seizure freedom and to achieve this using a tolerated antiepileptic drug (AED) schedule. Various combinations of older and newer AEDs can be used to try and achieve this, with varying success rates. The prognosis in newly diagnosed epilepsy can be favourable, with up to 50% of patients entering remission (seizure‐freedom for five years on or off treatment) either without treatment or on their first AED (Brodie 2010; Maguire 2011). An additional 10% achieve remission on a second or third drug (Brodie 2010). For the remainder, AEDs may fail to provide remission from seizures. Pharmacoresistance or intolerable treatment‐emergent adverse effects, or both, are major contributors to this.
When describing epilepsy, the term 'drug‐resistant' is set to identify patients for whom there is sufficient information to predict that they will have a substantially poorer prognosis for seizure remission with AEDs when compared with the population as a whole (Kwan 2010). It does not mean that there is no chance at all of remission, which is never the case (Kwan 2010). For this reason, the term 'drug‐resistant' is now preferred to terms such as 'refractory' or 'intractable' by the International League Against Epilepsy (ILAE) (Kwan 2010). From a research point of view, a unifying definition of 'drug‐resistant' epilepsy is yet to be agreed upon (French 2006). Diverse criteria or even a lack of explicit criteria have previously been employed by different groups to describe drug‐resistance (Kwan 2010). In clinical trials set to involve patients with drug‐resistant epilepsy, the criterion of inclusion is usually failure to achieve seizure freedom (for a set time period) on one to three AEDs. The ILAE have proposed a consensus definition of drug‐resistant epilepsy as that for which there has been "failure of adequate trials of two tolerated and appropriately chosen and used AED schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom" (Kwan 2010). This definition identifies that adults and children rarely achieve sustained seizure freedom once two agents have failed to control seizures (Krauss 2011). No seizure frequency requirement is necessary to meet this ILAE definition. This allows for those patients with infrequent seizures (e.g. occurring once a year) to still be regarded as drug‐resistant, which is relevant to the impact seizures have on lifestyle factors such as driving.
Drug resistance is particularly prevalent among patients with focal seizures (Chaisewikul 2001). According to revised ILAE classifications, focal seizures can be divided into: i) those without impairment of consciousness or awareness, ii) those with impairment of consciousness or awareness, and (within i or ii) iii) those evolving to a bilateral, convulsive seizure (involving tonic, clonic, or tonic and clonic components). This term 'focal' is now preferred to 'partial' when describing seizures, and i and ii (above) are now preferred to using the terms 'simple' and 'complex' when describing focal seizures, respectively (Berg 2010). The term 'secondary generalised' is replaced by (iii) (Berg 2010).
Description of the intervention
Since the 2000s there has been the introduction of around 13 new AEDs globally, commonly termed second‐generation AEDs (Brodie 2010). In general, these newer drugs have been better tolerated by patients than the standard AEDs, such as carbamazepine, valproate, and phenytoin (Crepeau 2010). They have shown good clinical efficacy individually, and they are largely regarded as non‐inferior to the standard AEDs; although there is very little in the way of direct head‐to‐head comparisons between standard and newer AEDs (French 2004). Levetiracetam [(S)‐α‐ethyl‐2‐oxo‐1‐pyrrolidine acetamide] is one of the new AEDs, and is the subject of this review.
Levetiracetam was first introduced onto the market in April 2000, and is now marketed in over 50 countries (Tsai 2006; Crepeau 2010). It has been available as a generic brand in the US since 2008 and in the UK since 2011. Levetiracetam monotherapy has been shown to provide effective seizure control in adults with newly diagnosed epilepsy consisting of focal seizures or generalised seizures. The effect was found to be non‐inferior to carbamazepine (Brodie 2007). Intravenous (IV) levetiracetam has been tried in the treatment of status epilepticus in several open case series with reports of success in as high as 70% of cases (707 participants) (Trinka 2011). Indications for levetiracetam as add‐on treatment include focal seizures with or without evolution to bilateral convulsive seizures in adults and in children (from one month of age); primary generalised tonic‐clonic seizures in adults and in children aged six years and above with idiopathic generalised epilepsy; and myoclonic seizures in adults and adolescents above 12 years of age with juvenile myoclonic epilepsy (Crepeau 2010). Independent systematic reviews of levetiracetam use for each of these indications are ongoing and will continue to form an important part of the evidence base behind use of this second‐generation AED.
Levetiracetam can be administered orally as a tablet (either an immediate or extended‐release preparation), as an oral solution, or as an IV concentrate for infusion. Based on current evidence, it is started at an effective dose of 1000 mg/day in adults and up‐titrated in increments of 1000 mg/day every two weeks to a maximum dose of 3000 mg/day, depending on clinical response (Cereghino & Cramer 2000). In children, dose is up‐titrated to 60 mg/kg/day.
Levetiracetam possesses both antiepileptic and anti‐epileptogenic properties (Betts 2000). Its exact mode of action is not completely understood (Xiao 2009). It binds to, and modulates, the synaptic vesicle protein 2A (SV2A); a protein that has some controlling effect on neurotransmitter release from presynaptic vesicles (Lynch 2004; Gillard 2006). It also selectively inhibits N‐type Ca2+ channels and decreases intracellular calcium‐ion increase (both of which negatively impact neurotransmitter release) (Niespodziany 2001; Lukyanetz 2002). There is evidence that it releases γ‐aminobutyric acid (GABA) activity and glycine‐gated currents by acting on their negative allosteric modulators, namely zinc and the beta‐carbolines (Rigo 2002). Neuroprotective effects have also been described (Gibbs 2006). The proposed mechanisms of action of levetiracetam have been largely derived from animal‐model studies, and the results remain to be validated in humans.
With regard to pharmacokinetics, levetiracetam generally demonstrates a favourable profile. Bioavailability is the fraction of a drug's administered dose that reaches the systemic circulation. When a drug is administered orally, bioavailability can be reduced by factors such as the rates of absorption and first‐pass gut and hepatic metabolism. Oral levetiracetam provides close to 100% bioavailability, making it largely bioequivalent to IV levetiracetam (Trinka 2011). A drug's susceptibility to oxidative hepatic metabolism and its influence on cytochrome P450 enzyme function in the liver can largely determine the duration and intensity of the pharmacological action of that drug, and its interaction with other drugs. Levetiracetam is advantaged by a lack of oxidative hepatic metabolism or influence on cytochrome P450 enzyme function. Dosing is thus simplified in both adults and children by linear, dose‐proportional kinetics. Plasma concentrations of levetiracetam peak at one hour, and a steady‐state concentration is reached by 48 hours with repeated dosing (usually twice daily). The drug shows no significant pharmacokinetic interactions with other AEDs or with drugs such as warfarin, digoxin, and the oral contraceptive pill; which all interact with the aforementioned hepatic systems. Clearance is exclusively renal: 66% unchanged and 24% as an inactive metabolite following hydrolysis of its acetamide group in the blood. Clearance is 30% to 40% higher in children and it is impaired in elderly people or in patients with renal impairment (Pellock 2001; Glauser 2006; Crepeau 2010).
Description of the review
This is a Cochrane Review that takes the form of a systematic review and meta‐analysis. In this review, we assess the effectiveness of levetiracetam when used as adjuvant (add‐on) therapy in epilepsy patients suffering from focal seizures. Data are extracted from randomised, placebo‐controlled trials. This is an update to a review first published in 2001 (Chaisewikul 2001) as part of an ongoing series of reviews investigating second‐generation AEDs.
Objectives
To evaluate the effects of levetiracetam when used as an add‐on treatment for people with drug‐resistant focal epilepsy.
Methods
Criteria for considering studies for this review
Types of studies
All trials included had to meet the following criteria (mutually inclusively):
be randomised controlled trials (RCTs): included trials were those for which the study author had described the trial as 'a randomised controlled trial' (or words to that effect). A judgement was then made on the risk of selection bias of the included trials, based on the reported methods of random list generation and allocation concealment (see 'Risk of bias' assessment for details on which methods were considered to confer a low risk of selection bias);
be placebo‐controlled;
be double, single or unblinded: a judgement was then made on the risk of performance and detection biases being present in the trial (see 'Risk of bias' assessment);
be of parallel or crossover design: for crossover trials, the first treatment period was treated as a parallel trial (i.e. only data from the first treatment period were used);
consist of a treatment period of at least eight weeks in duration.
Types of participants
Participants had to meet all of the following criteria:
any age, any gender, any ethnic background;
experiencing drug‐resistant focal epilepsy: that is experiencing focal seizures with or without impairment of consciousness or awareness, with or without evolution to bilateral, convulsive seizures (involving tonic, clonic, or tonic and clonic components). There has been lack of consensus between studies when defining drug resistance. Therefore, in order to allow a fair and inclusive evaluation of all trials that have been said to involve drug‐resistant participants, a specific cut‐off for number of background AEDs and the time period on these was not set. Instead, the requirement was for trials to have described participants on AEDs as having 'failed to respond' or having 'refractory', 'drug‐resistant', or 'uncontrolled' epilepsy (or words to that effect). Information was then collected on the duration of epilepsy, the number of AEDs tried and the length of time during which seizures had not responded to those AEDs, and the minimum number of seizures required during that time for participants to have been included in the trial. Where relevant, a subgroup analysis was conducted to compare primary outcomes between studies where the mean duration of epilepsy was shorter (< 12 months) and longer (≥ 12 months).
Types of interventions
The active treatment group received treatment with levetiracetam in addition to conventional AED treatment.
The control group received matched placebo in addition to conventional AED treatment.
Types of outcome measures
(1) 50% or greater reduction in focal seizure frequency
The proportion of people with a 50% or greater reduction in focal seizure frequency in the treatment period compared to the pre‐randomisation baseline period was chosen as the primary outcome. It was chosen as it is commonly reported in this type of study, and can be calculated for studies that do not report this outcome provided that baseline seizure data were reported. For the purposes of this review, people who achieved 50% or greater reduction in focal seizure frequency were termed 'responders'.
Also provided was the proportion of people who did not achieve 50% or greater reduction in focal seizure frequency, termed 'non‐responders'.
(2) Treatment withdrawal
The proportion of people having treatment withdrawn during the course of the treatment period was used as a measure of global effectiveness. Treatment is likely to be withdrawn due to adverse effects, lack of efficacy or a combination of both, and this is an outcome to which the individuals make a direct contribution. In trials of short duration, it is likely that adverse effects will be the most common reason for withdrawal.
(3) Adverse effects
(a) Five most common adverse effects
The proportion of people experiencing the five most common adverse effects was reported for participants of any age and then for adults and children separately.
(b) General adverse effects
The proportion of people experiencing the following five adverse effects was also reported (where available and if different from the five most common adverse effects):
ataxia;
dizziness;
fatigue;
nausea;
somnolence.
These adverse effects were chosen as they were considered by the review authors to be common and important side effects of AEDs generally.
(c) Behavioural adverse effects
The proportion of people experiencing adverse effects pertaining to changes in behaviour (e.g. aggression, agitation, anger, anxiety, apathy, depression, hostility, and irritability). Clinicians often consider changes in behaviour to be common adverse effects of levetiracetam (Asconapé 2001; Penovich 2004; NICE 2012).
(4) Cognitive effects
At present, there is no consensus as to which instruments should be used to assess the effects of AEDs on cognition, and as a result this has been approached in a heterogeneous way (Cochrane 1998). In view of this difficulty, we intended to tabulate results where a specific instrument had been used to assess the effects of levetiracetam on cognition, but made no attempt to combine the results in a meta‐analysis.
(5) Quality of Life
Once again, there is no consensus as to which instruments should be used to assess this, and Quality of life (QoL) data were also tabulated where a specific instrument had been used to assess the effects of levetiracetam on QoL, but we made no attempt to combine the results in a meta‐analysis.
Search methods for identification of studies
(1) Electronic databases
We searched the following databases. There were no language restrictions:
Cochrane Epilepsy Group Specialised Register (13 August 2012);
The Cochrane Central Register of Controlled Trials (CENTRAL,The Cochrane Library Issue 7, 2012) using the strategy set out in Appendix 1;
MEDLINE (Ovid) (1946 to August week 1, 2012) using the strategy outlined in Appendix 2.
(2) References from published studies
We reviewed the reference lists of retrieved studies to search for additional reports of relevant trials.
(3) Other sources
We contacted UCB S.A. Pharma (manufacturers of levetiracetam), and colleagues in the field for information about any unpublished or ongoing studies.
Data collection and analysis
Two review authors (GM and PD) independently assessed trials for inclusion. Any disagreements were resolved by discussion with a third review author (AM). The same two review authors extracted the information shown below from included trials, with any disagreements resolved by similar discussion. Trial authors were contacted for any information missing from the published manuscript that was deemed relevant.
(1) Publication details
Year of trials publication.
(2) Methodological/trial design
Method of random sequence generation.
Method of randomisation concealment (allocation concealment).
Method of blinding (of participants and personnel as well as investigators).
Whether any randomised participants had been excluded from reported analyses.
Duration of baseline period.
Duration of treatment period (up‐titration and maintenance phases).
Dose(s) of levetiracetam tested.
(2) Participant/demographic information
Total number of participants allocated to each treatment group.
Age and sex.
Country or continents from which the majority of participants had been recruited.
Duration of epilepsy.
Number with focal epilepsy.
Seizure classification.
Duration of time in which seizures were drug‐resistant.
Minimum seizure rate required for trial inclusion.
Seizure frequency during the baseline period.
Number of background AEDs.
All but three of the trials found were sponsored by UCB S.A. Pharma, who were asked to confirm the following information for their sponsored trials:
the method of randomisation;
the total number randomised to each group;
the number of participants in each group achieving a 50% or greater reduction in seizure frequency per treatment group;
the number of participants having treatment withdrawn post‐randomisation per treatment group;
-
for those excluded from reported analyses:
the reason for exclusion;
whether any of those excluded completed the treatment phase;
whether any of those excluded had a 50% or greater reduction in seizure frequency during the treatment phase.
Outcomes
The number of participants experiencing each outcome (see Types of outcome measures) was recorded per randomised group.
Analysis
'Risk of bias' assessment
Two review authors (GM and PD) independently assessed trials for their risks of possessing the risks of bias listed below. Any disagreements were settled by discussion with a third review author (AM). Where possible, published data were used, with unpublished data sought when details were unclear or unavailable.
Selection bias: were there adequate methods of random sequence generation and allocation concealment? Methods considered to confer a low risk of selection bias included those using random numbers tables/electronically generated random numbers for random sequence generation, and those using allocation of sequentially numbered sealed packages of medication, sealed opaque envelopes, or central/telephone randomisation for allocation concealment.
Performance bias: was knowledge of the allocated interventions by participants and personnel adequately prevented during the study? Methods considered to confer a low risk of performance bias include using packaging and tablets that were identical for levetiracetam and placebo.
Detection bias: was knowledge of the allocated interventions by outcome assessors prevented during the study? Studies were regarded as possessing low risks of this bias when it was specifically described that investigators/outcome assessors were blinded to treatment assignment.
Attrition bias: were incomplete outcome data adequately addressed? Studies were regarded as possessing low risks of this bias when sufficient data were provided to allow an intention to treat (ITT) as well as best and worst case sensitivity analysis to be conducted (see 'Analysis').
Reporting bias: were reports of the study free of suggestion of selective outcome reporting? Risks were regarded as low when the results of all outcomes measured (where the outcome was also relevant to this review) were published.
In addition to providing overall estimates, a subgroup analysis that excluded trials with unclear or high risks of any of the biases was performed for the primary outcome measure (50% or greater reduction in seizure frequency).
Efficacy and adverse effects
Statistical heterogeneity between trials was checked for each outcome using a Chi2 test for heterogeneity. Provided no significant heterogeneity was present (P < 0.05), analysis used a fixed‐effect model. Where significant heterogeneity was present, logistic regression was used to investigate the heterogeneity (see 'Regression analysis'). The preferred estimator was the Mantel‐Haenszel risk ratio (RR) (note: the Peto odds ratio was the preferred estimator in the original review). For the outcomes 50% reduction in seizure frequency and treatment withdrawal, 95% confidence intervals (CI) were quoted. For individual adverse effects, 99% CI were quoted to make allowance for multiple testing.
All analyses included all participants in the treatment groups to which they had been allocated. For the efficacy outcome (50% or greater reduction in seizure frequency) three analyses were undertaken.
Primary ITT analysis
For this, all randomised participants were analysed in the treatment group to which they had been allocated, irrespective of the treatment that they actually received. Participants randomised but excluded from analysis (e.g. for not completing follow‐up or with inadequate seizure data) were assumed non‐responders.
Worse‐case analysis
Participants randomised but excluded from analysis (e.g. for not completing follow‐up or with inadequate seizure data) were assumed non‐responders in the levetiracetam group and responders in the placebo group.
Best‐case analysis
Participants randomised but excluded from analysis (e.g. for not completing follow‐up or with inadequate seizure data) were assumed responders in the levetiracetam group and non‐responders in the placebo group.
Regression analysis to investigate heterogeneity and dose response
Reduction in seizure frequency was reduced to a binary variable, with 'success' defined as achieving 50% or greater reduction in seizure frequency, as this is the outcome usually reported in trial publications.
Logistic regression was used to investigate heterogeneity in 'treatment success', and treatment withdrawal owing to the study location, dose, year of publication, and duration of titration period and maintenance period.
Placebo was defined as a dose of zero. Dose, log(dose + 1) and dose as a factor, year of publication directly and as a factor, and duration of titration period and maintenance periods directly and as factors were considered as explanatory variables. Terms were also added for trials according to country or continents from which the majority of participants had been recruited. The best regression models for dose‐response relationships, adjusting for other factors, were chosen using AIC, the package R, which implements generalised linear models (McCullagh 1989).
Predicted probabilities of treatment success and treatment withdrawal were calculated from the fitted models, in order to provide a clear interpretation of the regression parameters.
Cognitive effects and quality of life
Data for these outcomes were summarised in tables and in the text.
Summary of findings
The GRADE Working Group grades of evidence (Schünemann 2009) were used to provide a Summary of findings (SOF) table outlining the overall quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on most important outcomes (i.e. 50% or greater reduction in seizure frequency, treatment withdrawal, and the five most common adverse effects). Within this, 'assumed risk' (also called baseline risk) is the control event rate and is therefore a measure of the typical burden of these outcomes, and 'corresponding risk' is a measure of the burden of the outcomes after the intervention is applied (i.e. the risk of an outcome in levetiracetam‐treated people based on the relative magnitude of an effect and assumed (baseline) risk). The GRADE system classifies the quality of evidence into one of four grades:
high: further research is very unlikely to change our confidence in the estimate of effect;
moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate;
low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate;
very low: any estimate of effect is very uncertain.
A judgement was made on the individual trials used to provide the pooled effect estimates and the quality of evidence was then downgraded by the presence of i) bias, ii) inconsistency, iii) indirectness, iv) imprecision, and v) publication bias; and upgraded by the presence of i) a large effect and ii) a dose‐response gradient. Only studies with no threats to validity (not downgraded for any reason) can be upgraded.
This process was independently conducted by two review authors (GM and PD) with any disagreements resolved by discussion with a third review author (AM).
Results
Description of studies
Results of the search
See Figure 1 for a flow‐diagrammatic summary of the results of database searches and records identified from other sources. Fifteen eligible trials were found. Four of these trials were excluded from the current analysis pending receipt of further information about the trials (N01221; Boon 2002; Zheng 2009; Yagi 2010) (see Characteristics of studies awaiting classification). These trials remain awaiting integration into a future update.
1.
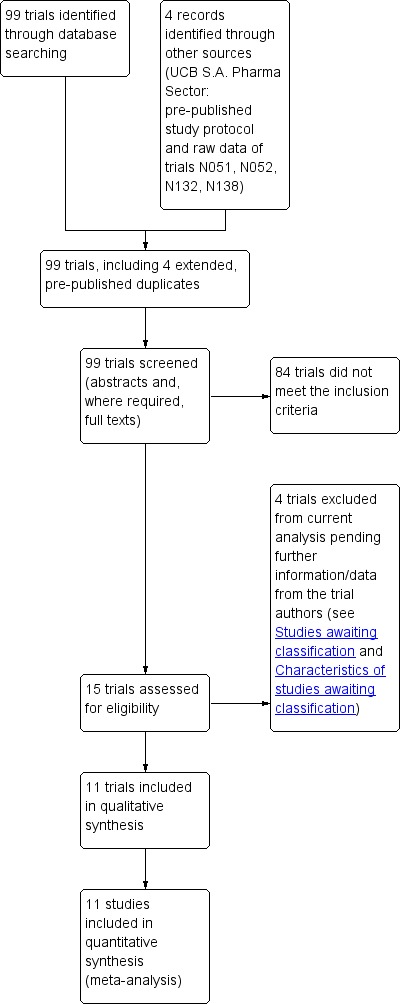
Study flow diagram.
Included studies
See Characteristics of included studies.
Eleven trials (1861 participants) were included in this update, seven of which were published subsequent to the original review (Glauser 2006; Tsai 2006; Zhou 2008; Peltola 2009; Wu 2009; Xiao 2009; Levisohn 2009 & Loge 2010) (see Characteristics of included studies). In the original review, the four included studies were analysed using both published and unpublished trial information and data (Ben‐Menachem 2000; Betts 2000; Cereghino & Cramer 2000; Shorvon 2000). The unpublished information was obtained as pre‐published study protocols provided by UCB S.A. Pharma sector. These study protocols were also available for use in this current review, in addition to their corresponding published manuscripts. The seven new trials were analysed using published data only (pre‐published study protocols were sought, where relevant, but unobtainable).
In two trials the participants were children (N = 296, age range four to 16 years) (Glauser 2006; Levisohn 2009 & Loge 2010), with the remaining trials consisting of an adult population (N = 1565). Aside from one crossover trial (Shorvon 2000), all trials were parallel design. Trials involving children (Glauser 2006; Levisohn 2009 & Loge 2010) and trials published earlier (Ben‐Menachem 2000; Betts 2000; Cereghino & Cramer 2000; Shorvon 2000: included in the original review) recruited from populations within various European countries and the US. Adult trials published since the original review largely recruited from populations within Asian countries (mostly China and Taiwan) (Tsai 2006; Zhou 2008; Wu 2009). One trial recruited from various countries (centres in Finland, India, Mexico, Russia, South Africa, and Ukraine) (Peltola 2009).
Two trials (Cereghino & Cramer 2000; Levisohn 2009 & Loge 2010) did not report the mean duration of epilepsy. For the Cereghino & Cramer 2000 trial, participants had to have experienced uncontrolled focal epilepsy for at least two years, with a minimum of 12 focal seizures within 12 weeks before study selection and two focal seizures occurring per four weeks during the 12‐week baseline period. This was on a background of at least two AEDs taken simultaneously or consecutively. For the Levisohn 2009 & Loge 2010 trial, participants had to have experienced uncontrolled focal epilepsy for a minimum of six months, with a minimum of one focal seizure during the four weeks prior to screening. This was on a background of one or two AEDs. Across the remaining trials, the overall mean duration of epilepsy was 18 years (± five years standard deviation (SD), range seven to 26 years). Within these, the Betts 2000 trial required a minimum of at least four seizures in the six months prior to study entry; the Ben‐Menachem 2000 and the Peltola 2009 trials required at least two seizures per four weeks in their 12‐ and eight‐week baseline periods, respectively; and the remaining six trials required at least four seizures per four weeks in their eight‐ or 12‐week baseline periods (Shorvon 2000; Glauser 2006; Tsai 2006; Zhou 2008; Wu 2009; Xiao 2009). This was on a background of one to three AEDs. The mean duration of epilepsy across all included trials did not range below 12 months.
Treatment periods consisted of the combination of an up‐titration and a maintenance phase in all but two trials (Betts 2000 and Peltola 2009 did not involve up‐titration). Duration of the treatment periods ranged from 12 to 24 weeks between trials (up‐titration range zero to four weeks, maintenance range eight to 24 weeks). The doses of levetiracetam tested were 60 mg/kg/day for children, and a range of 1000 mg/day to 4000 mg/day for adults. The Peltola 2009 trial was the only one in which an extended‐release preparation of levetiracetam was tested (1000 mg dose). The Betts 2000 trial was the only one in which a 4000 mg dose of levetiracetam was tested. For the Betts 2000 trial, uniform baseline seizure data were not collected for the trials participants. As a result, we were unable to calculate 50% or greater reduction in seizure frequency for this trial. For the remaining 10 trials (1446 adults, 296 children) we were able to calculate a 50% or greater reduction in seizure frequency. Data for treatment withdrawal were available for all trials, while data for adverse effects were available for all but one trial (Zhou 2008). Generally, trials published an adverse effect if 5% or more of the participants in any treatment group were affected, but in the Betts 2000 and Cereghino & Cramer 2000 trials this threshold was raised to 10%.
Four trials (Betts 2000; Cereghino & Cramer 2000; Shorvon 2000; Zhou 2008) provided data for QoL and cognitive effect outcomes in adult participants, but only 619 of the 765 participants randomised to these trials were assessed with the relevant instruments. These figures were minimally different from those in the previous review, in which 595 of the 737 participants randomised were assessed with the relevant instruments. One trial (Levisohn 2009 & Loge 2010) provided outcome data for cognitive as well as behavioural and emotional effects in children. Seventy‐three of the 99 participants randomised in this trial were assessed with the relevant instruments. A total of 18 participants (all adults) were excluded from the reported analysis, and these 18 participants contribute to the best‐ and worst‐case scenario analyses. For further details on trials, see Characteristics of included studies.
Excluded studies
See Characteristics of studies awaiting classification for reasons for the exclusion from this analysis.
Risk of bias in included studies
Figure 2 and Figure 3 summarise the risks of bias of the included trials (see also Characteristics of included studies). Eight of the 11 trials described as RCTs provided details of an adequate method of sequence generation and allocation concealment to qualify them as possessing low risk of selection bias (Ben‐Menachem 2000; Betts 2000; Cereghino & Cramer 2000; Shorvon 2000; Glauser 2006; Tsai 2006; Zhou 2008; Peltola 2009). For five trials (Betts 2000; Cereghino & Cramer 2000; Shorvon 2000; Glauser 2006; Tsai 2006) the random list was generated using random permuted blocks, and concealed by dispensing sequentially numbered sealed packages. For the Ben‐Menachem 2000 trial, randomisation was achieved using a minimisation programme, which was concealed by using 'telephone randomisation'. Participants were randomised in a 2:1 ratio to levetiracetam or placebo. For the Peltola 2009 trial, randomisation and allocation concealment were achieved by using an interactive voice response system. Participants were randomised in a 1:1 ratio to levetiracetam or placebo. A random numbers table was used for sequence generation in the Zhou 2008 trial, and participants received an exclusive random number consecutively on entry into the trial, with medication packaged by UCB S.A. Pharma.
2.
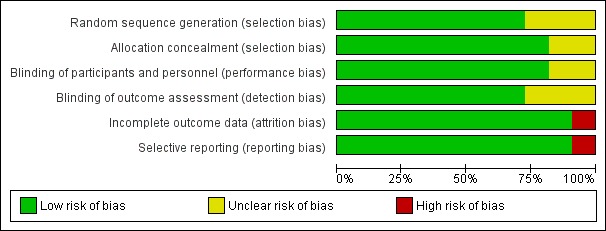
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies (shown above).
3.
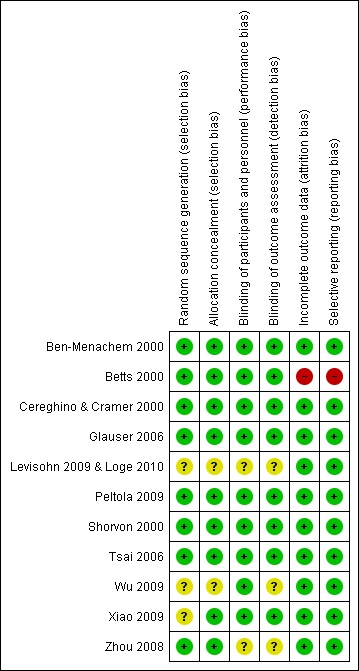
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study (shown above).
Risk of selection bias was regarded as unclear in the remaining three RCTs (Wu 2009; Xiao 2009; Levisohn 2009 & Loge 2010), for which full details on the method of random list generation or allocation concealment were not provided. In the Xiao 2009 trial, randomisation codes were generated by the study sponsor (no further specification given), with participants assigned a randomisation number and given levetiracetam or placebo accordingly. An adequate method of allocation concealment was described in this trial (concealment via the use of numbered containers). For the Wu 2009 and Levisohn 2009 & Loge 2010 trials, details on the method of random sequence generation and allocation concealment were not provided, although in the latter trial it was described that participants were randomised in a 2:1 ratio to levetiracetam or placebo, and that randomisation was stratified for age (four to seven, eight to 12, 13 to 16 years) and number of concomitant AEDs (one or two).
All trials were described as double‐blind trials. Nine of the trials (Ben‐Menachem 2000; Betts 2000; Cereghino & Cramer 2000; Shorvon 2000; Glauser 2006; Tsai 2006; Peltola 2009; Wu 2009; Xiao 2009) provided details that packaging and tablets were identical for levetiracetam and placebo and were therefore regarded as possessing a low risk of performance bias (blinding of participants and personnel). For the remaining two trials, in which the method used to blind participants and personnel was not described, risk of performance bias was deemed unclear (Zhou 2008; Levisohn 2009 & Loge 2010). The risk of detection bias was regarded as low in eight trials that provided details that the investigators were blinded to treatment assignment (Ben‐Menachem 2000; Betts 2000; Cereghino & Cramer 2000; Shorvon 2000; Glauser 2006; Tsai 2006; Peltola 2009; Xiao 2009), and unclear in three trials that did not provide details that the investigators were blinded to treatment assignment (Zhou 2008; Wu 2009; Levisohn 2009 & Loge 2010). All trials were viewed as having low risks of attrition and selective reporting biases aside from the Betts 2000 trial (high risk); for which uniform baseline seizure data were not reported and for which there were discrepancies in the reported number of patients per treatment group (see Characteristics of included studies).
In summary, the following six RCTs were viewed as possessing a low risk of all of five types of bias (selection bias, performance bias, detection bias, attrition bias, and reporting bias): Ben‐Menachem 2000, Cereghino & Cramer 2000, Shorvon 2000, Glauser 2006, Tsai 2006, and Peltola 2009. For the remaining RCTs, risks were largely unclear.
Effects of interventions
50% or greater reduction in seizure frequency
(1) Overall results (Mantel‐Haenszel risk ratio (RR) and percentage responders)
1. Actual risk ratio (95% CI) for individual doses versus placebo.
| Dose of Levetiracetam | Intention‐to‐treat | Best case | Worst case |
| 60mg/kg/day | 1.91 (CI 1.38 to 2.63) | 1.91 (CI 1.38 to 2.63) | 1.91 (CI 1.38 to 2.63) |
| 1000mg | 2.49 (CI 1.78 to 3.50) | 2.63 (CI 1.88 to 3.67) | 2.37 (CI 1.70 to 3.29) |
| 2000mg | 4.91 (CI 2.75 to 8.77) | 5.09 (CI 2.85 to 9.06) | 4.54 (CI 2.60 to 7.94) |
| 3000mg | 2.59 (CI 2.01 to 3.33) | 2.63 (CI 2.05 to 3.38) | 2.33 (CI 1.84 to 2.96] |
2. Actual response rates (percentage): at the different doses of Levetiracetam.
| Dose of Levetiracetam | Intention‐to‐treat responder rate | Best case responder rate | Worst case responder rate |
| 60mg/kg/day [placebo response] | 52 [25] | 52 [25] | 52 [25] |
| 1000mg [placebo response] | 33 [13] | 34 [13] | 33 [14] |
| 2000mg [placebo response] | 37 [8] | 39 [8] | 37 [8] |
| 3000mg [placebo response] | 44 [18] | 45 [18] | 44 [19] |
| All adult doses (1000, 2000, 3000 mg) [placebo response] | 39 [16] | 40 [16] | 39 [18] |
(a) ITT analysis
See Analysis 1.1, Analysis 1.2.
1.1. Analysis.
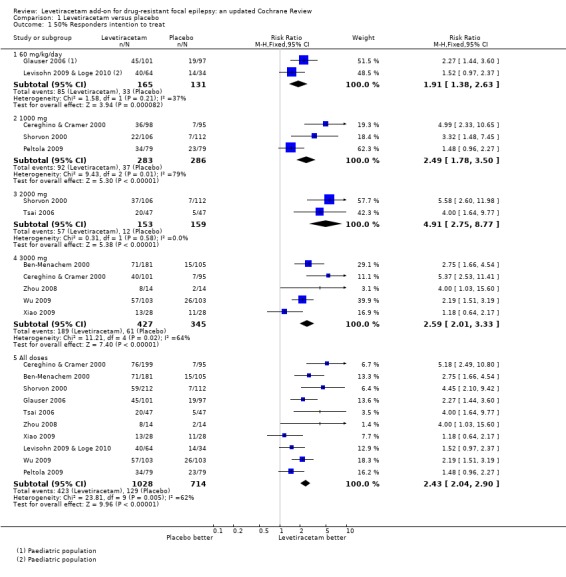
Comparison 1 Levetiracetam versus placebo, Outcome 1 50% Responders intention to treat.
1.2. Analysis.
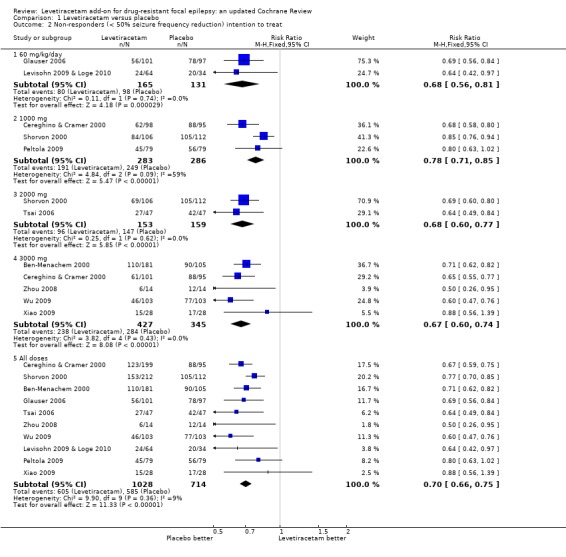
Comparison 1 Levetiracetam versus placebo, Outcome 2 Non‐responders (< 50% seizure frequency reduction) intention to treat.
Empirical data summary
At every dose analysed (adults and children combined), levetiracetam statistically significantly reduced focal seizure frequency relative to placebo (Table 2). This can be viewed as strong evidence that levetiracetam has a positive effect on this outcome. The naive estimates, ignoring dose, show children as better responders than adults, with 52% and 39% of children and adults on levetiracetam responding, respectively. A quarter of children and 16% of adults responded on placebo (Table 3). The Number of participants Needed to Treat for an additional Beneficial effect (NNTB) to get a responder with a 50% or greater reduction in seizure frequency during treatment on levetiracetam was four (95% CI three to seven) for children and five (95% CI four to six) for adults. In other words, one additional child or adult may respond for every four or five children or adults, respectively, that have received levetiracetam rather than placebo.
Heterogeneity summary
A Chi2 test for heterogeneity for a response to levetiracetam indicates significant levels of statistical heterogeneity between trials (Chi2 = 23.17, degrees of freedom (df) = 9, P = 0.006). This signifies that there was significant variation (inconsistency) in the magnitude of the positive effect of levetiracetam. This means that while we can be confident that there was likely to be a positive effect from levetiracetam, we cannot be confident about the size of that positive effect because it was extremely variable from trial to trial. This is illustrated by the observations that the proportion of adults responding varied from 6% to 57%, with a median 36%, and the proportion of children responding was 20% in one trial and 62% in the other trial. It is difficult to be precise about where the true estimate lies. In view of this, results by factors including dose and year of trial publication are given below in order to try and help explain the heterogeneity (see 'Regression models').
Adult trials
The above conclusions remained unchanged when analysis was limited to the trials involving adults (Chi2 = 20.83, df = 7, P = 0.004). Levetiracetam statistically significantly reduced focal seizure frequency relative to placebo in adults, but there was also significant statistical heterogeneity between adult trials. Heterogeneity did not exist between the early adult trials (Ben‐Menachem 2000; Cereghino & Cramer 2000; Shorvon 2000), which were included for this analysis in the original review (Chi2 = 0.76, df = 2, P = 0.68). The overall RR for 50% responders across these trials was 3.78 (95% CI 2.62 to 5.44) and 39% of adults in these trials responded to levetiracetam and 9% to placebo (implying a 30% 'real' response rate ‐ i.e. not attributable to placebo). Heterogeneity did not exist between the later adult trials (Tsai 2006; Zhou 2008; Peltola 2009; Wu 2009; Xiao 2009), published since the original review (Chi2 = 8.21, df = 4, P = 0.08). The overall RR for 50% responders across these trials was 1.97 (95% CI 1.55 to 2.51) and 49% of adults in these trials responded to levetiracetam and 25% to placebo (implying a 24% real response rate).
Paediatric trials
For the two trials that tested levetiracetam on children, the results were sufficiently similar (Chi2 = 1.58, df = 1, P = 0.21) to be combined to give an estimated RR for 50% or greater reduction in seizure frequency of 1.91 (95% CI 1.38 to 2.63). The implied real response rate for children was 27% (52% and 25% levetiracetam and placebo responses, respectively).
Dose‐response
Chi2 tests for heterogeneity for a response to levetiracetam at doses 1000 mg and 3000 mg indicate significant statistical heterogeneity between trials at these doses (1000 mg: Chi2 = 9.43, df = 2, P = 0.009; 3000 mg: Chi2 = 11.21, df = 4, P = 0.02). For the two trials that tested levetiracetam on adults at a dose of 2000 mg, the results were sufficiently similar (Chi2 = 0.31, df = 1, P = 0.58) to be combined to give an estimated RR for 50% or greater reduction in seizure frequency of 4.91 (95% CI 2.75 to 8.77) and 37% of participants in these trials responded to levetiracetam and 8% to placebo (implying a 29% real response rate). The NNTB was four (95% CI three to five).
Summary RRs for individual doses did not clearly suggest increasing efficacy with dose when analysis was conducted on the three less heterogeneous adult trials included in the previous review for this analysis (Ben‐Menachem 2000; Cereghino & Cramer 2000; Shorvon 2000). RRs for individual doses across these three trials are outlined below:
1000 mg: 4.17 (95% CI 2.40 to 7.24);
2000 mg: 5.58 (95% CI 2.60 to 11.98);
3000 mg: 3.47 (95% CI 2.29 to 5.25).
Summary RRs for individual doses did not clearly suggest increasing efficacy with dose when analysis was conducted on the five less heterogeneous adult trials published since the original review (Tsai 2006; Zhou 2008; Peltola 2009; Wu 2009; Xiao 2009). RRs for individual doses across these five trials are outlined below:
1000 mg: 1.48 (95% CI 0.96 to 2.27);
2000 mg: 4.00 (95% CI 1.64 to 9.77);
3000 mg: 2.00 (95% CI 1.47 to 2.72).
The only observable pattern would be that the strongest responses accompanied a 2000 mg dose of levetiracetam in both groups.
50% or less reduction in seizure frequency (see Analysis 1.2).
RRs for the less than 50% reduction in seizure frequency outcome are shown below. Chi2 tests for heterogeneity demonstrated no heterogeneity between trials for this outcome:
60 mg/kg/day: 0.68 (95% CI 0.56 to 0.81);
1000 mg: 0.78 (95% CI 0.71 to 0.85);
2000 mg: 0.68 (95% CI 0.60 to 0.77);
3000 mg: 0.67 (95% CI 0.60 to 0.74);
All doses: 0.71 (95% CI 0.66 to 0.75).
(b) Best‐case and worse‐case scenarios
See Analysis 1.3,. Analysis 1.4.
1.3. Analysis.
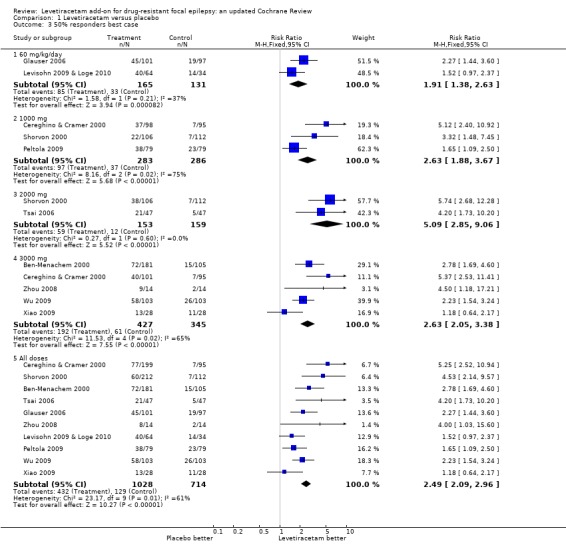
Comparison 1 Levetiracetam versus placebo, Outcome 3 50% responders best case.
1.4. Analysis.
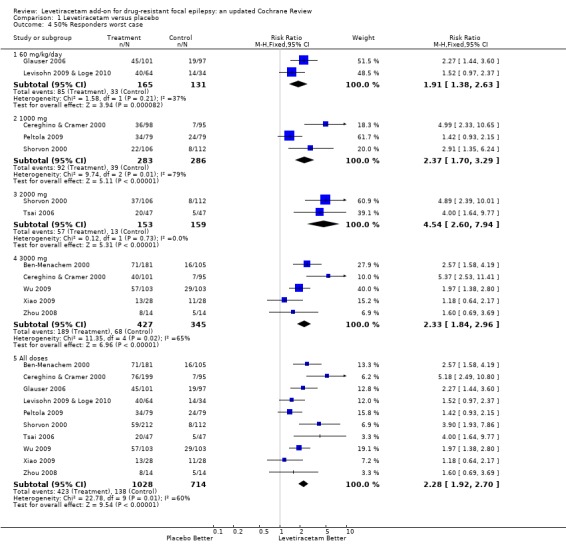
Comparison 1 Levetiracetam versus placebo, Outcome 4 50% Responders worst case.
Chi2 tests for heterogeneity for best‐case and worst‐case responses to levetiracetam indicate similar trends to those found between trials in the ITT analysis with regard to the existence of heterogeneity. Overall (adults and children combined) there was evidence of a treatment effect from levetiracetam but it was not valid to provide overall estimates given the existence of significant heterogeneity between trials (best case: Chi2 = 23.17, df = 9, P = 0.006; worst case: Chi2 = 22.78, df = 9, P = 0.007). In both best‐ and worst‐case scenarios such heterogeneity only became negligible when analysis was limited to the trials involving children (where ITT data were identical to the best‐ and worst‐case data) or the adult trials involving doses of 2000 mg levetiracetam (best case: Chi2 = 0.27, df = 1, P = 0.60, worst case: Chi2 = 0.12, df = 1, P = 0.73). The overall RRs for ≥ 50% response across adult trials involving doses of 2000 mg levetiracetam were 5.09 (95% CI 2.85 to 9.06) and 4.54 (95% CI 2.60 to 7.94) in best‐ and worst‐case scenarios, respectively.
In summary, the results suggest a significant treatment effect in children and adults for all three analyses. There is a relatively consistent existence of statistical heterogeneity between trials on adults (but not children), and this makes it difficult to provide overall estimates for adults.
(c) Subgroup analysis across trials with low risk of bias
When subgroup analysis was conducted on the six trials possessing a globally low risk of bias (Ben‐Menachem 2000; Cereghino & Cramer 2000; Shorvon 2000; Glauser 2006; Tsai 2006; Peltola 2009) the above conclusions were not changed for all three analysis:
ITT: Chi2 = 14.31, df = 5, P = 0.01; RR 2.82 (95% CI 2.24 to 3.57);
best case: Chi2 = 12.81, df = 5, P = 0.03; RR 2.91 (95% CI 2.31 to 3.67);
worst case: Chi2 = 14.56, df = 5, P = 0.01; RR 2.71 (95% CI 2.16 to 3.41).
(2) Regression models for dose
(a) ITT analysis
3. Actual and estimated treatment response rates (percentage): adults.
| Trial | Year of publication | Dose of Levetiracetam (mg) | Actual responder rate: intention‐to‐treat | Fitted responder rate: Intention‐to‐treat | Actual responder rate: best case | Fitted responder rate: best case | Actual responder rate: worst case | Fitted responder rate: worst case |
| Shorvon 2000 & Ben‐Menachem 2000 & Cereghino & Cramer 2000 | 2000 | Placebo | 9.3 | 10.7 | 9.3 | 8.6 | 9.9 | 9.2 |
| Shorvon 2000 & Cereghino & Cramer 2000 | 2000 | 1000 | 28.4 | 22.7 | 28.9 | 32 | 28.4 | 31.7 |
| Shorvon 2000 | 2000 | 2000 | 34.9 | 33.2 | 35.8 | 35.6 | 34.9 | 35.1 |
| Ben‐Menachem 2000 & Cereghino & Cramer 2000 | 2000 | 3000 | 39.4 | 41.9 | 39.7 | 37.8 | 39.4 | 37.2 |
| Tsai 2006 | 2006 | Placebo | 10.6 | 20.3 | 10.6 | 18.8 | 10.6 | 20.6 |
| Tsai 2006 | 2006 | 2000 | 42.6 | 43.2 | 44.7 | 46.7 | 42.6 | 44.8 |
| Zhou 2008 | 2008 | Placebo | 14.3 | 24.7 | 14.3 | 23.8 | 35.7 | 26.2 |
| Zhou 2008 | 2008 | 3000 | 57.1 | 53.2 | 57.1 | 52.1 | 57.1 | 49.5 |
| Peltola 2009 & Wu 2009 & Xiao 2009 | 2009 | Placebo | 28.6 | 27.1 | 28.6 | 26.7 | 30.5 | 29.3 |
| Peltola 2009 | 2009 | 1000 | 43 | 40 | 48.1 | 49.9 | 43 | 47.9 |
| Wu 2009 & Xiao 2009 | 2009 | 3000 | 53.4 | 54.6 | 54.2 | 53.9 | 55.3 | 51.1 |
For adults, the empirical response rate at 2000 mg of levetiracetam (29%, taking into account placebo response) was marginally larger than that at 1000 mg and 3000 mg (20% and 26%, respectively) (Table 3). The response rates for children were higher than for adults: the RR was 1.33 (95% CI 1.06 to 1.64); the odds were 1.7 times higher for both placebo and treated groups. A quarter of children responded on placebo, and just over half (52%) on treatment with 60 mg/kg/day. As there was only 1 df, fitted response rates were not calculated for children.
The dose levels for adults were confounded with trial, so it was not possible to separate the trial effects and dose effects fully. Only two trials, Cereghino & Cramer 2000 and Shorvon 2000, had two dose levels in addition to placebo. Models with the log RR for success, that is achieving 50% or greater response, increasing with dose or log(dose + 1) fitted considerably better than models that attributed heterogeneity to trials. Dose on the log scale fitted well, as was confirmed by the estimated coefficients from a model with dose as a factor. The year of publication was strongly associated with response rates, after allowing for log(dose + 1) (Table 4), and there was a significant interaction. The fitted placebo response rate increased from 11% in 2000 to 27% in 2009. The fitted response rates on 3000 mg of levetiracetam increased from 42% in 2000 to 55% in 2009.
(b) Best‐case and worse‐case scenarios
The majority of the best‐ and worst‐case response rates were similar. The one large difference was for the Zhou 2008 trial, which was a small trial. The conclusions are not changed.
(3) Regression models for heterogeneity
Although the response rates were significantly higher for trials with no titration period, and lower for US and European trials, after adjusting for dose, these factors explained less of the heterogeneity than was associated with the year of the trial publication. Titration was more strongly associated with response rates than either the maintenance period or the total period. After adjusting for both dose and year effects, no additional information was provided by titration or trial country. The secular change requires a different explanation.
Treatment withdrawal
Mantel‐Haenszel risk ratio
See Analysis 1.5.
1.5. Analysis.
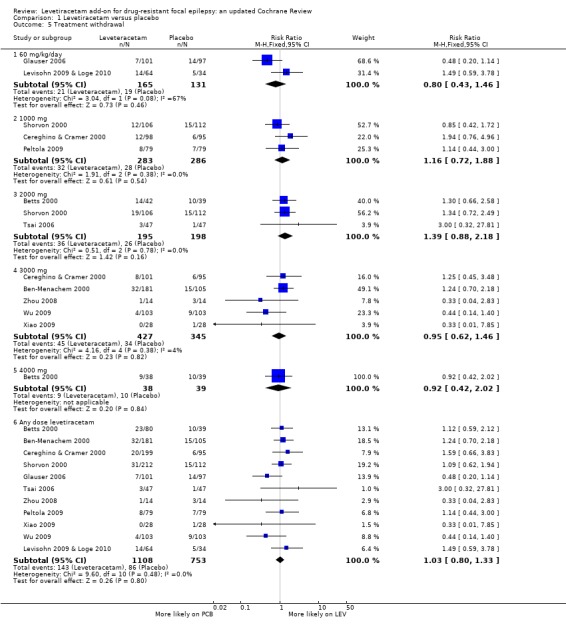
Comparison 1 Levetiracetam versus placebo, Outcome 5 Treatment withdrawal.
A Chi2 test for heterogeneity for withdrawal from levetiracetam treatment in adults and children indicates no significant statistical heterogeneity between trials (Chi2 = 9.60, df = 10, P = 0.48). The overall RR for discontinuation for any reason, at any age, was 1.03 (95% CI 0.80 to 1.33); thus there was insufficient evidence to conclude that participants were more likely to discontinue levetiracetam than placebo. This conclusion was unchanged when analysis was limited to trials involving children (Chi2 = 3.04, df = 1, P = 0.08; RR for treatment withdrawal 0.80; 95% CI 0.43 to 1.46) and trials involving adults (Chi2 = 5.79, df = 8, P = 0.67; RR for treatment withdrawal 1.09; 95% CI 0.83 to 1.45).
Regression‐modelled treatment withdrawal
See Table 5.
4. Actual and estimated treatment withdrawal rates (percentage): adults.
| Trial | Year | Study location | Maintanence period (weeks) | Levetiracetam dose (mg) | Actual withdrawal rate | Withdrawal rate fitted with trial | Withdrawal rate fitted with year of publication | Withdrawal rate fitted with year of publication and length of maintenance period |
| Shorvon 2000 | 2000 | 2 | 12 | Placebo | 13.4 | 14.2 | 13.2 | 15.0 |
| 1000 | 11.3 | |||||||
| 2000 | 17.9 | |||||||
| Cereghino & Cramer 2000 | 2000 | 4 | 14 | Placebo | 6.3 | 8.8 | 13.2 | 8.8 |
| 1000 | 12.2 | |||||||
| 3000 | 7.9 | |||||||
| Ben‐Menachem 2000 | 2000 | 2 | 12 | Placebo | 14.3 | 16.4 | 13.2 | 15.0 |
| 3000 | 17.7 | |||||||
| Tsai 2006 | 2006 | 1 | 12 | Placebo | 2.1 | 4.3 | 4.3 | 8.8 |
| 2000 | 6.4 | |||||||
| Zhou 2008 | 2008 | 1 | 12 | Placebo | 21.4 | 14.3 | 14.3 | 7.3 |
| 3000 | 7.1 | |||||||
| Wu 2009 | 2009 | 1 | 12 | Placebo | 8.7 | 6.3 | 6.9 | 6.6 |
| 3000 | 3.9 | |||||||
| Peltola 2009 | 2009 | 3 | 12 | Placebo | 8.9 | 9.5 | 6.9 | 6.6 |
| 1000 | 10.1 | |||||||
| Xiao 2009 | 2009 | 1 | 12 | Placebo | 3.6 | 1.8 | 6.9 | 6.6 |
| 3000 | 3.9 | |||||||
|
Note: Study location: 1) = China/Taiwan region; 2) Europe region; 3) Multiregional (Europe, South America, Africa, Asia); 4) USA region | ||||||||
An empirical logistic plot did not show any obvious dose‐response relationship for withdrawal.
For children, there was no difference in withdrawal rates among those treated with levetiracetam and those on placebo.
When withdrawal data for adults were examined in regression models, it became apparent that there was significant between‐trial heterogeneity; most concisely explained by a factor for the length of the maintenance period and the year of publication. These factors give a residual deviance of 21.6 on 17 df. Table 5 shows the actual and fitted treatment withdrawal rates for adult trials (excluding the Betts 2000 trial, for which the maintenance period used (24 weeks) was much longer than in the other trials). Doses of 2000 mg and 4000 mg of levetiracetam were associated with higher withdrawal rates. The withdrawal rates were higher for trials carried out in Europe, but the differences between doses and between Europe, US and China were minimal after allowing for the maintenance period and year of publication. The main differences were that the one trial (Betts 2000) with 24 weeks' maintenance and levetiracetam doses 2000 mg and 4000 mg had high withdrawal rates (fitted rate 28%). There was also only one trial with 14 weeks' maintenance (Cereghino & Cramer 2000), and the fitted rate was 9%. The remainder of the trials had a 12‐week maintenance period, with fitted withdrawal rates ranging from 7% to 15% between them.
The placebo withdrawal rates decreased from 15% in 2000 to 7% in 2009 for most trials when a logistic regression was fitted to the trials excluding the Betts 2000 trial. As 24 weeks' maintenance was twice as long as the majority, it is not unreasonable that the withdrawal rate was roughly twice as high (28% compared to 15%).
Adverse effects
(a) Five most common adverse effects
See Analysis 1.6, Analysis 1.7, Analysis 1.8.
1.6. Analysis.
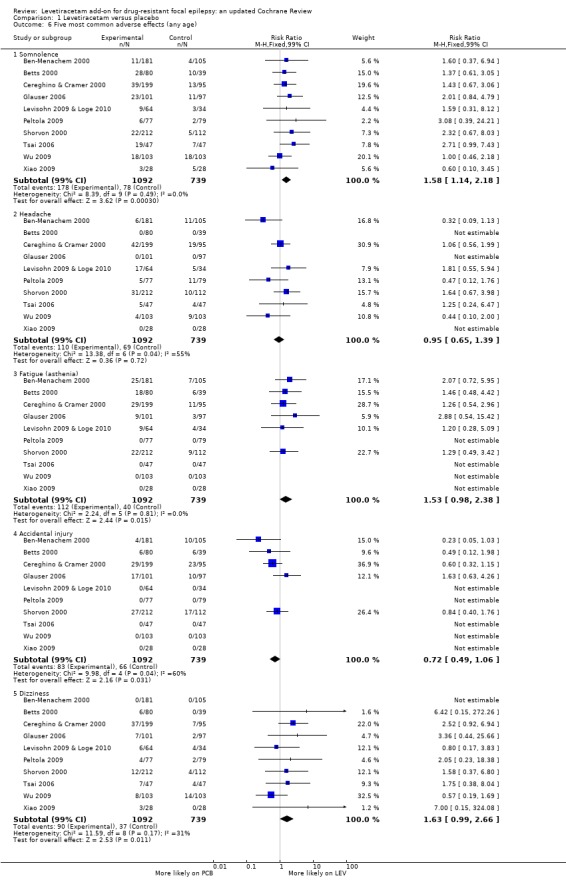
Comparison 1 Levetiracetam versus placebo, Outcome 6 Five most common adverse effects (any age).
1.7. Analysis.
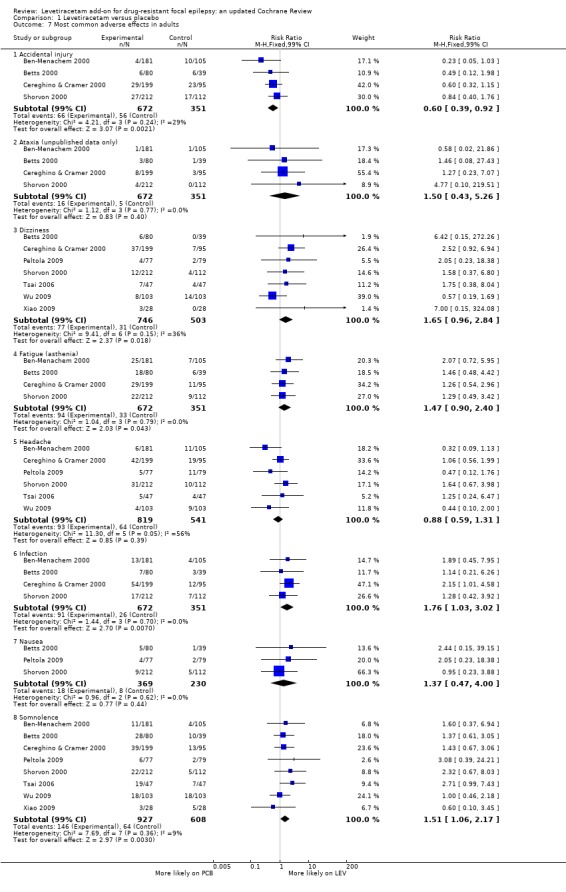
Comparison 1 Levetiracetam versus placebo, Outcome 7 Most common adverse effects in adults.
1.8. Analysis.
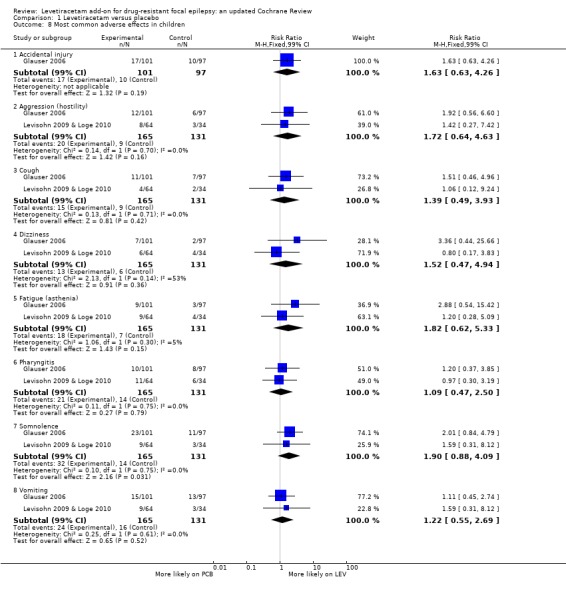
Comparison 1 Levetiracetam versus placebo, Outcome 8 Most common adverse effects in children.
Not all trials reported the same adverse effects, which altered the denominators representing number of participants from which RRs were calculated. To give a pooled summary of the five most common adverse effects across trials (1831 safety population), trials where an adverse effect was not reported (i.e. less than 5% or 10% of participants affected: see 'Description of studies') were assigned zero events for that adverse effect. With this analysis, the five most common adverse effects (any age) were as follows:
somnolence: affected 14% of participants (RR 1.58; 99% CI 1.14 to 2.18);
headache: affected 10% of participants (RR 0.95; 99% CI 0.65 to 1.39);
fatigue (asthenia): affected 8% of participants (RR 1.53; 99% CI 0.98 to 2.38);
accidental injury: affected 8% of participants (RR 0.72; 99% CI 0.49 to 1.06);
dizziness: affected 7% of participants (RR 1.63; 99% CI 0.99 to 2.66).
Only somnolence retained statistically significant risk over placebo.
The relative commonality of individual adverse effects did not largely alter when analysis was limited to adults (Analysis 1.7), aside from the introduction of infection (RR 1.76; 99% CI 1.03 to 3.02) over dizziness. Only the RRs for somnolence (RR 1.51; 99% CI 1.06 to 2.17) and infection (RR 1.76; 99% CI 1.03 to 3.02) remained statistically significant with levetiracetam over placebo. Accidental injury was statistically significantly associated with placebo (RR 0.60; 99% CI 0.39 to 0.92).
In children, somnolence remained the most common adverse effect, although it was not statistically significant over placebo (RR 1.90; 99% CI 0.88 to 4.09). This was a wide CI. The next most common adverse effects in children were vomiting (RR 1.22; 99% CI 0.55 to 2.69), pharyngitis (RR 1.09; 99% CI 0.47 to 2.50), aggression (hostility) (RR 1.72; 99% CI 0.64 to 4.63), and accidental injury (RR 1.63; 99% CI 0.63 to 4.26).
(b) General adverse effects
RRs for other general adverse effects (where available) were: ataxia (adults, unpublished data; 1.50; 99% CI 0.43 to 5.26), nausea (adults; 1.37; 99% CI 0.47 to 4.00), dizziness (children; 1.52; 99% CI 0.47 to 4.94), and fatigue ((asthenia), children; 1.82; 99% CI 0.62 to 5.33).
(c) Behavioural adverse effects
See Analysis 1.9.
1.9. Analysis.
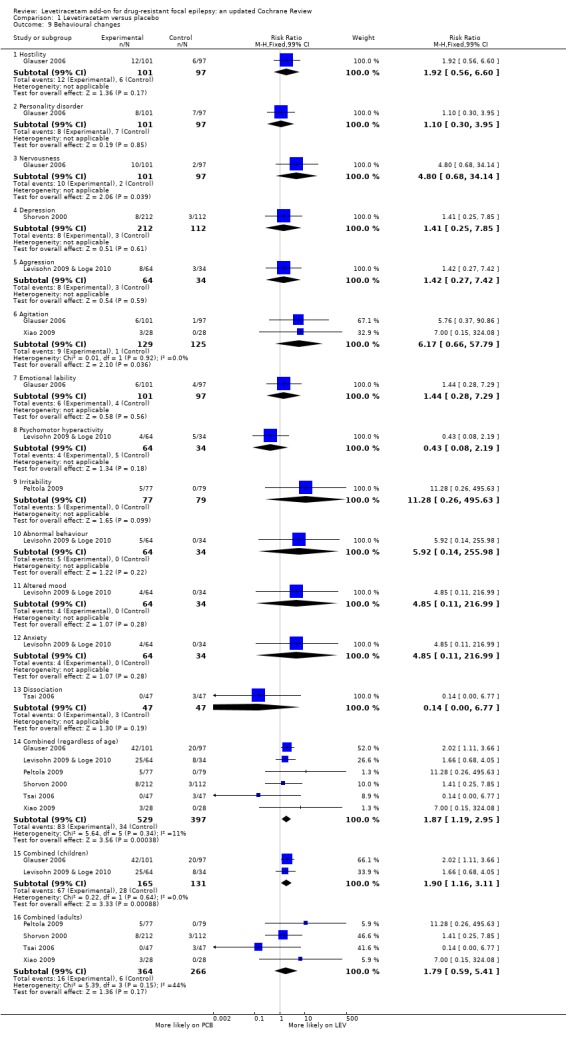
Comparison 1 Levetiracetam versus placebo, Outcome 9 Behavioural changes.
Adverse effects pertaining to changes in behaviour were described as follows:
"Hostility": affected 0.98% of participants (RR 1.92; 99% CI 0.56 to 6.60);
"Personality disorder": affected 0.82% (RR 1.10; 99% CI 0.30 to 3.95);
"Nervousness": affected 0.66% (RR 4.80; 99% CI 0.68 to 34.14);
"Depression": affected 0.60% of participants (RR 1.41; 99% CI 0.25 to 7.85);
"Aggression": affected 0.60% of participants (RR 1.42; 99% CI 0.27 to 7.42;
"Agitation": affected 0.55% of participants (RR 6.17; 99% CI 0.66 to 57.79;
"Emotional lability": affected 0.55% of participants (RR 1.44; 99% CI 0.28 to 7.29);
"Psychomotor hyperactivity": affected 0.49% of participants (RR 0.42; 99% CI 0.08 to 2.19);
"Irritability": affected 0.27% of participants (RR 11.28; 99% CI 0.26 to 495.63);
"Abnormal behaviour": affected 0.27% (RR 5.92; 99% CI 0.14 to 255.98);
"Altered mood": affected 0.22% of participants (RR 4.85; 99% CI 0.11 to 216.99);
"Anxiety": affected 0.22% of participants (RR 4.85; 99% CI 0.11 to 216.99);
"Dissociation": affected 0.16% of participants (RR 0.14; 99% CI 0.00 to 6.77).
In summary, no individual behavioural adverse effect affected more than 1% of participants or was significantly associated with levetiracetam over placebo. When behavioural adverse effects were combined, 4.53% of participants were affected (RR 1.87; 99% CI 1.19 to 2.95). In this, 22.64% of children were affected (RR 1.90; 99% CI 1.16 to 3.11) and 1.04% of adults were affected (RR 1.79; 99% CI 0.59 to 5.41).
Cognitive effects and QoL
See Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, and Table 12.
5. Quality of Life (QOL) assessment as mean change from baseline (QOLIE‐31): Cereghino & Cramer 2000.
| Subscale | Placebo (n=81) | Lev 1g/d (n=80) | Lev 3g/d (n=85) |
| Overall QOL | improved | improved | IMPROVED* |
| Seizure worry | worsened | IMPROVED* | IMPROVED* |
| Emotional well‐being | improved | worsened | worsened |
| Energy‐fatigue | worsened | improved | worsened |
| Cognitive functioning | WORSENED* | improved | improved |
| Medication effects | worsened | improved | improved |
| Social function | worsened | worsened | improved |
| Health status | improved | improved | improved |
| ( * ) p‐value < 0.05 |
6. Quality of Life (QOL) assessment as mean change from baseline (QOLIE‐31): Zhou 2008.
| Subscale | Placebo (n=11) | Lev 3g/d (n=13) |
| Overall QOL | Improved | Improved |
| Seizure worry | Improved | Improved |
| Emotional well‐being | Improved | Improved |
| Energy‐fatigue | Improved | worsened |
| Cognitive functioning | Worsened | IMPROVED* |
| Medication effects | Worsened | Improved |
| Social function | Improved | IMPROVED* |
| Health status | Improved | Improved |
| Note: ( * ) p‐value < 0.01 | ||
7. Quality of Life (QOL) assessment as mean change from baseline (ESI‐55): Shorvon 2000.
| QOL Domain | PCB (n=89) | LEV 1g/d (n=92) | LEV 2g/d (n=81) |
| Health status | IMPROVED* | IMPROVED* | IMPROVED* |
| Role limitation due to memory problems | improved | IMPROVED* | worsened |
| Pain | worsened | IMPROVED* | improved |
| Cognitive functioning | improved | improved | improved |
| Emotional well‐being | unchanged | improved | improved |
| Energy/fatigue | improved | IMPROVED* | improved |
| Social functioning | improved | IMPROVED* | improved |
| Role limitation due to emotional problems | improved | improved | worsened |
| Role limitation due to physical problems | improved | IMPROVED* | improved |
| Physical function | improved | worsened | improved |
| Overall quality of life | improved | improved | IMPROVED* |
| Health perceptions | improved | IMPROVED* | IMPROVED* |
| Note: Almost all patients provided information for each individual domain | |||
| Note: ( * ) p‐value < 0.05 |
8. Summary of Quality of Life (QOL): Mean change from baseline (ESI‐55 scale): Betts 2000.
| QOL composite score | Period | PCB:number | PCB:mean change | LEV2g/d:number | LEV2g/d:mean change | LEV4g/d:number | LEV4g/d:mean change |
| Mental Health | Baseline | 35 | not applicable | 40 | not applicable | 37 | not applicable |
| Mental Health | Overall Double‐Blind | 28 | ‐1.7 (worsened) | 30 | 1.7 (improved) | 28 | 3.5 (improved) |
| Physical Health | Baseline | 29 | not applicable | 37 | not applicable | 34 | not applicable |
| Physical Health | Overall Double‐Blind | 28 | 3.6 (improved) | 30 | 0.8 (improved) | 26 | 2.3 (improved) |
| Role Functioning | Baseline | 33 | not applicable | 38 | not applicable | 35 | not applicable |
| Role Functioning | Overall Double‐Blind | 28 | ‐0.5 (worsened) | 31 | 0.4 (improved) | 27 | 2.3 (improved) |
9. Cognitive assessment as mean changes from baseline in variables on neuropsychological tests: Zhou 2008.
| Test | Subscale | Placebo (n=11) | Lev 3g/d (n=13) |
| Verbal Fluency | Improved | Improved | |
| Trail Making Test | Time on Part A | Improved | Improved |
| Time on Part B | Improved | Improved | |
| Wisconsin Card Sorting Test | Number of correct responses | Improved | Improved |
| Perseverative errors | Improved | Improved | |
| Nonperseverative errors | Improved | Worsened | |
| Number of categories | Improved | Improved | |
| Performance time | Improved | IMPROVED* | |
| Digit Symbol | Worsened | Improved | |
| Digit Span | Worsened | Worsened | |
| Stroop Color–Word Interference Task | Reaction time for naming words | Worsened | Improved |
| Correct number of naming words | Worsened | Improved | |
| Reaction time for naming colours | Improved | Improved | |
| Correct number of naming colours | Improved | Worsened | |
| Logic Memory | Improved | Improved | |
| Delayed Logic Memory | Improved | IMPROVED* | |
| Visual Memory | Improved | Improved | |
| Delayed Visual Memory | Worsened | Improved | |
| Calculation | Worsened | Improved | |
| Note: ( * ) p‐value < 0.01 | |||
10. Cognitive assessment as least square mean change from baseline (Leiter‐R AM, WRAML‐2, Leiter‐R ERS): Levisohn 2009 & Loge 2010 (children).
| Test | Subscale | Placebo (n=27) | Lev 60mg/kg/day (n=46) |
| Leiter‐R AM | Composite score | Improved | Improved |
| WRAML‐2 | General memory | Improved | Improved |
| Visual memory | Improved | Improved | |
| Verbal memory | Improved | Improved | |
| Attention/concentration | Improved | Worsened | |
| Leiter‐R ERS | Cognitive/social | Improved | Improved |
| Emotions/regulations | Improved | Improved | |
| Note: ( * ) p‐value < 0.1 | |||
| Note: Results were for per protocol population | |||
11. Behavioral and emotional functioning assessment as least square mean change from baseline (CBCL and CHQ‐PF50): Levisohn 2009 & Loge 2010 (children).
| Test | Subscale | n | Placebo (n=27) | n | Lev 60mg/kg/day (n=46) |
| CBCL competence scores | Activities | 22 | WORSENED* | 41 | Worsened |
| Social | 22 | Worsened | 41 | Worsened | |
| School | 19 | Improved | 35 | Improved | |
| Total Competence | 19 | Worsened | 34 | Worsened | |
| CBCL problem scores | Anxious/Depressed | 22 | Improved | 43 | Improved |
| Withdrawn/Depressed | Improved | Worsened | |||
| Somatic Complaints | Improved | Improved | |||
| Social Problems | Improved | Worsened | |||
| Thought Problems | Improved | Worsened | |||
| Attention Problems | Improved | Improved | |||
| Rule‐Breaking Behavior | Improved | Worsened | |||
| Aggressive Behavior | IMPROVED* | WORSENED* | |||
| Internalising Syndromesa | Improved | Improved | |||
| Externalising Syndromesb | IMPROVED* | WORSENED* | |||
| Total Problems | IMPROVED* | WORSENED* | |||
| CHQ‐PF50 | Role/Social–Emotional/Behavioral | 27 | Worsened | 45 | Improved |
| Behavior | 27 | Worsened | 45 | Worsened | |
| Mental Health | 27 | Improved | 45 | Improved | |
| Psychosocial Summary | 26 | Improved | 44 | Improved | |
| Note: ( * ) p‐value < 0.05 | |||||
|
aInternalising Syndromes contain the Withdrawn/Depressed, Anxious/Depressed, and Somatic Complaints scores bExternalising Syndromes contain the Agressive Behaviour and Rule‐Breaking Behaviour scores Note: Results were for per protocol population | |||||
For adults, two trials (Cereghino & Cramer 2000; Zhou 2008) made use of the Quality of Life in Epilepsy Inventory (QOLIE‐31) as an instrument to measure QoL, while two other trials (Betts 2000; Shorvon 2000) made use of the Epilepsy Surgery Inventory Scale (ESI‐55). For one trial (Zhou 2008) cognitive effects were assessed using nine tests chosen from the Chinese version of the Wechsler Adult Intelligence Scale‐Revised (WAIS‐RC) and other tests commonly used to assess cognitive function (see Table 10). For children, one trial (Levisohn 2009 & Loge 2010) assessed cognitive effects using the following series of instruments: Leiter International Performance Scale‐Revised Attention and Memory (Leiter‐R AM), Wide Range Assessment of Memory and Learning‐2 (WRAML‐2), and Leiter International Performance Scale‐Revised, Examiner's Rating Scale (Leiter‐R ERS). In the same trial, the Achenbach Child Behavior Checklist (CBCL) and Child Health Questionnaire‐Parent Form 50 (CHQ‐PF50) were used to assess behavioural and emotional effects.
Table 6 shows results for the Cereghino & Cramer 2000 trial. This table shows mean change from baseline for each treatment group, by the subscale of QOLIE‐31. Results indicate that compared to placebo, individuals treated with levetiracetam were significantly less worried about seizures, and individuals on 3000 mg of levetiracetam had a significantly better overall QoL.
Table 7 shows results for the Zhou 2008 trial. As for the Cereghino & Cramer 2000 trial, results were for QOLIE‐31. They indicate that compared to placebo, individuals treated with levetiracetam had significantly better cognitive functioning and social function.
Table 8 shows results for the Shorvon 2000 trial. This table shows mean change from baseline for each treatment group, by domain of ESI‐55 scale. Results indicate that when compared to placebo, individuals treated with levetiracetam scored significantly better for the health perception domain. Individuals treated with 1000 mg scored significantly better for the 'role limitation due to memory problems', 'pain', 'energy', 'social functioning', and 'role limitation due to physical problems' domains. Individuals treated with 2000‐mg levetiracetam scored better but not statistically significantly for the overall QoL domain.
Table 9 shows results for the Betts 2000 trial. As for the Shorvon 2000 trial, results were for ESI‐55; however, for this trial we only had aggregate data for the three composite scores of this instrument.
Table 10 shows results for the Zhou 2008 trial. This table shows mean change from baseline for each treatment group, by way of variables within a series of neuropsychological tests. The results indicate that levetiracetam does not lessen/reduce cognitive function (no worsening in variables was statistically significant). Performance time on the Wisconsin Card Sorting Test (WCST) and Delayed Logic Memory significantly improved for patients treated with levetiracetam, but not for those treated with placebo.
Table 11 shows results for the Levisohn 2009 & Loge 2010 trial. This table shows mean change from baseline for each treatment group, by scores within the Leiter‐R AM, WRAML‐2, and Leiter‐R ERS instruments. The results indicate that levetiracetam did not lessen/reduce/impair cognitive function in children; there were no significant changes in either group of participants.
Table 12 shows more results for the Levisohn 2009 & Loge 2010 trial. This table shows mean change from baseline for each treatment group, by component of the CBCL and CHQ‐PF50. The results demonstrated statistically significant worsening of scores in aggressive behaviour, externalising syndromes (consisting of aggressive behaviour and rule‐breaking behaviour), and total problems in children treated with levetiracetam, but not those treated with placebo.
Overall, for adults, results from the Cereghino & Cramer 2000, Shorvon 2000, and Zhou 2008 trials did indicate that levetiracetam had a positive effect on some aspects of QoL, while results from the Zhou 2008 trial indicated that the drug did not negatively affect and, in a way, improved cognitive function. In children, the results from the Levisohn 2009 & Loge 2010 trial indicated that levetiracetam did not alter cognitive function but did worsen aspects of child behaviour.
Summary of findings
See Figure 4.
4.
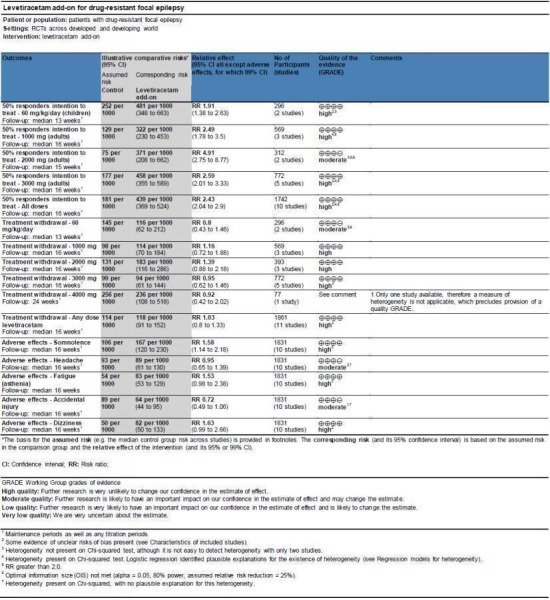
'Summary of findings (SOF)' table.
The quality of evidence (GRADE) scores are provided in an SOF table (Figure 4). The quality of evidence score was moderate for the outcome 50% or greater reduction in seizure frequency at a 2000‐mg dose of levetiracetam and also for treatment withdrawal at a levetiracetam dose of 60 mg/kg/day (each downgraded owing to the presence of an inadequate sample size). A score could not be determined for treatment withdrawal at a levetiracetam dose of 4000 mg because only one trial provided data for this dose. The quality of evidence score for headache and accidental injury (two of the five most common adverse effects) was moderate. This downgrade was as a result of unexplained inter‐trial heterogeneity, as evidenced by the Chi2 test results (with no explanatory regression modelling). The quality of evidence score was high for all other subsets of the outcomes 50% or greater reduction in seizure frequency, treatment withdrawal, and five most common adverse effects.
Discussion
In the original review of four trials (adult participants) (Chaisewikul 2001), conclusions were made that in people with drug‐resistant focal epilepsy, levetiracetam could effectively reduce seizure frequency and could be generally well tolerated as an add‐on treatment (Chaisewikul 2001). These conclusions are unchanged following an update that has added seven subsequently published trials to the meta‐analysis. The overall quality of evidence used was high (as screened by the GRADE system (Schünemann 2009)). All trials were described by their authors as double‐blind RCTs. Our analysis demonstrates that the majority of these trials possessed low risks of bias, and subgroup analysis that has excluded trials with unclear risks or high risks of bias demonstrates no substantive differences in results. Sufficient data were available to perform ITT analysis on all but one trial (Betts 2000). Substantial amounts of inter‐trial heterogeneity have largely prevented provision of overall estimates of effect (ignoring dose). The most significant contribution of this update has been the introduction of trials that tested levetiracetam in children with focal epilepsy, and it was possible to give overall estimates of effect in children.
The two paediatric trials (Glauser 2006; Levisohn 2009 & Loge 2010) tested a levetiracetam dose of 60 mg/kg/day or placebo in 296 children. Results for the outcome 50% or greater reduction in seizure frequency demonstrated that this dose of levetiracetam significantly reduced seizure frequency in children on ITT analysis. None of the children contributed to best‐ and worst‐case sensitivity analysis as all who were randomised were also analysed. The RR of 'response' (i.e. achieving 50% or greater reduction in seizure frequency) between levetiracetam and placebo was 0.91 times in favour of levetiracetam. Although this is small, the actual response rates indicated that that just over half of children achieved 'success' on levetiracetam. This suggests that at a dose of 60 mg/kg/day, levetiracetam may be expected to be effective in 25% of children (having taken into account a 25% placebo response). This prediction is based on actual response rates, and more trials will be needed if a fitted estimate is to be calculated that is meaningfully different from the actual response rates. The results demonstrate that one additional child may respond for every four children that have received add‐on levetiracetam rather than placebo. This is a favourable result given that epilepsy is particularly frequent in children, as highlighted earlier. Although it is statistically valid for us to provide these overall estimates of effect for levetiracetam in children (no significant heterogeneity present on Chi2 testing), with only two trials being included it is not easy to detect heterogeneity. Indeed, the existence of only two RCTs highlights how there is currently relatively little in the way of RCTs testing levetiracetam in children with drug‐resistant focal epilepsy. A meta‐analysis across these two trials was particularly important given that much of the clinical opinion of levetiracetam use in children with drug‐resistant focal epilepsy has come from consideration of various uncontrolled trials (Verrotti 2010). Of interest, subsequent to the publication of Glauser 2006, levetiracetam received US Food and Drug Administration (FDA) approval as an add‐on drug in the treatment of children aged four to 16 years with focal epilepsy (Verrotti 2010). This meta‐analysis provides strong evidence to back up the commonly accepted view that levetiracetam is effective in children with drug‐resistant focal epilepsy (Verrotti 2010). Interestingly, the results also suggest that the odds of being a responder to levetiracetam may well be greater for children than for adults. The response rate in children was around 4% to 13% greater than in adults. A 25% placebo response was found across paediatric trials. This is slightly larger than the expected 19% placebo response commonly described in trials involving children with drug‐resistant focal epilepsy (Guekht 2010). The explanation for this enlarged placebo response is unclear as there were no important differences between the paediatric populations recruited in these two trials from those usually recruited. However, it may have implications for the design of future trials in which levetiracetam is compared to placebo in a randomised controlled fashion.
For our global outcome treatment withdrawal, we have insufficient evidence to conclude that levetiracetam is more likely to be withdrawn than placebo in children.
No individual adverse effect was significantly associated with levetiracetam over placebo in children. The CI for the most common adverse effect (somnolence) was wide, as were the CIs for aggressive or hostile behaviour, accidental injury, and fatigue. This raises the possibility of substantial rates of these adverse effects in children on levetiracetam. Other literature concludes that the drug is safe and tolerated well in children, with many of the adverse effects being mild, transient, or reversible (Verrotti 2010). We have insufficient evidence in this review to disagree with such conclusions. In a specific analysis of the combination of adverse effects pertaining to changes in behaviour, a high proportion of children (around 20%) were affected, where these were (in combination) significantly associated with levetiracetam over placebo. In view of this, it is likely that some changes in behaviour may be common in children taking levetiracetam. It is difficult to ascertain in what form these might manifest, as the individual behavioural changes themselves were insignificant statistically, and a rather heterogeneous set of words pertaining to changes in behaviour was used across trials. Although it was concise to combine such words into one analysis of 'behavioural adverse effects', such a method means the conclusion drawn must be interpreted with caution given that the apparently increased absolute risk for children and present statistical significance could arise simply by virtue of an arbitrary combination being made. When specific tools were used to assess behavioural and emotional effects of levetiracetam in children, the results indicated that those taking levetiracetam fared worse than those taking placebo in measures of aggressive behaviour, leading to similar results for externalising syndromes and total problems. This was evidenced by one trial. As we made no attempt at a meta‐analysis of the data reviewed from neuropsychological tests, we have insufficient evidence from this to make firm conclusions about the behavioural and emotional effects of levetiracetam on children. When taken in combination, our two analyses of behavioural effects (i.e. an analysis of adverse effects pertaining to changes in behaviour and a review of data from neuropsychological tests) do seem to suggest that some adverse changes in behaviour are likely in children on levetiracetam. The general consensus in literature is that levetiracetam does demonstrate some unfavourable behavioural effects in children (Verrotti 2010), but this is yet to be validated. A future review in which the relative frequency of changes in behaviour, once they have been ranked by their level of severity, is analysed may be useful given that it is the severity of such behavioural effects that is most meaningful to clinicians and patients.
In adults, levetiracetam demonstrated statistically significant efficacy over placebo in the outcome of 50% or greater reduction in seizure frequency, at all doses. There were no substantive differences in results between our ITT analysis and the sensitivity best‐ and worst‐case analyses. Results for an overall effect (ignoring dose) indicated significant statistical heterogeneity between trials on adults, and therefore it did not seem reasonable to give overall estimates of effect. It is likely that much of the heterogeneity can be explained by different doses of levetiracetam tested and different years of publication for the trials ‐ as evidenced by the strong association of these factors with response rates during regression analysis. Response rates increased over time in both the levetiracetam and placebo groups. It was not possible to separate the dose effects and the trial year effects fully. Response rates to AEDs and placebo have been shown to increase over time in other literature as well (Guekht 2010; Rheims 2011), most notably in one review and meta‐analysis of factors determining response rates during RCTs of adjuvant‐therapy testing AEDs in adults with drug‐resistant focal epilepsy (Rheims 2011). It was suggested, in the latter review, that one of the reasons for the time‐dependent increase in response rates might be a change in population characteristics, such as an increasing proportion of patients recruited from Asian countries in later trials (Rheims 2011). The results of our regression analysis, in which we added terms for country or continents from which the majority of participants had been recruited, indicate that while response rates were lower for European trials and trials in the US, these factors were less associated with heterogeneity than were drug dose or trial publication year. As mentioned earlier, levetiracetam is not metabolised by the cytochrome P450 enzymes in the liver, therefore there is little biological reason to assume that it has differing efficacy in populations from different countries (Crepeau 2010). In view of this and the significant levels of heterogeneity found between trials, we cannot confidently say that levetiracetam demonstrates differing efficacy in different populations. The causes of the observed time‐dependent increases in response rates remain to be determined.
For the two trials that tested levetiracetam on adults at a dose of 2000 mg (312 participants), the results were sufficiently similar to be combined to give overall estimates of effect. Although it is valid to provide these estimates it is also important to note, as was mentioned earlier, that with only two trials available it is not easy to detect heterogeneity. At the 2000 mg dose, 37% of those treated with levetiracetam achieved 50% or greater reduction in seizure frequency on ITT analysis, as compared to 8% in the placebo group. On this analysis, the RR of 'response' between levetiracetam and placebo was 3.91 times in favour of levetiracetam. It is unclear how precise this estimate is given that the accompanying CI was wide. The upper CI was high (8.77) suggesting that the seizure‐reduction capacity of levetiracetam at this dose may well be very large. Indeed, notwithstanding heterogeneity, RRs favoured levetiracetam over placebo most strongly at 2000 mg compared to other doses, with actual responder rates also being highest at this dose (although only marginally). The NNTB at this dose was as low as that of children (four people). In view of these observations, further trials testing a 2000‐mg dose of levetiracetam are needed to improve the precision of the effect estimate and to test whether this is indeed the most effective dose. As the overall quality of evidence for this outcome was classed as moderate on the GRADE score, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
For our global outcome of treatment withdrawal, we have insufficient evidence to conclude that levetiracetam is more likely to be withdrawn than placebo in adults. When analysed using regression modelling, the data unsurprisingly suggest that longer maintenance periods are associated with greater rates of treatment withdrawal. The maintenance periods used ranged from 12 to 24 weeks. It is unlikely that these lengths of treatment time are significantly different from each other clinically, but rather all fall under the broad category of 'long‐term treatment'. Therefore, the accompanying differences in withdrawal rates found here are unlikely to be clinically meaningful. However, these differences may be helpful in planning future trial design. Withdrawal rates tended to fall with increasing year of trial publication. The explanation for this secular trend is less clear.
For adverse effects, somnolence and infection were significantly associated with levetiracetam. This slightly contrasts with results in the original review, where dizziness and infection were significantly associated with levetiracetam (Chaisewikul 2001). The likelihood is that any one of these three adverse effects will contribute to the side‐effects profile of levetiracetam. Changes in behaviour were infrequent in adults and not statistically significant. This may be surprising given that such changes (particularly aggressive behaviour) are often said to be seen frequently and advised about, or both, in clinic. The reasons behind this clinical and literary discrepancy are unclear. One possible explanation may be that clinicians frequently see patients with a complex psychiatric background that puts them at a higher risk of behavioural changes, while participants with psychiatric problems are routinely excluded from RCTs. It may also be that changes in behaviour manifest soon after starting levetiracetam, meaning that it is withdrawn before the eight‐week minimum treatment period that was set for included trials in this review.
We have insufficient data and analysis to make firm conclusions about the cognitive effects of levetiracetam and its effect on QoL. This update contributes only one more trial to the investigation of these outcomes, and this was a small trial (28 participants). We made no attempt at a meta‐analysis across data pertaining to cognitive effects and QoL. Based on the descriptive analysis conducted, the impression is that levetiracetam does not impair cognitive function, and that it confers some positive effects on QoL. It is difficult to be sure of the real life impact of these changes. The conclusions remain to be validated in a more detailed investigation of the effects of levetiracetam on cognition and QoL. These outcomes are important because they can place clinical trial evidence of clinical efficacy into the context of meaningful improvement for patients (Kerr 2011).
Limitations
The influence of a possible information bias cannot be excluded in this review. The original review (Chaisewikul 2001) had unpublished data confidentially made available for inclusion, while this update had no such data made available for new trials. To illustrate this limitation, the risks of selection, performance, and detection biases were initially regarded as 'unclear' for the Ben‐Menachem 2000 and Shorvon 2000 trials (included in the original review). This judgement was made based on the information available in the published versions of these trials. These trials were regarded as possessing a 'low risk' of these biases only after we had the opportunity to extract further data from the unpublished scripts. It stands to reason that similar discrepancies in information may exist for the other trials regarded as having an 'unclear risk' of certain biases in this review. Most RCTs implement various adequate methods of random sequence generation, allocation concealment, and investigator blinding in their protocols, but not all publish details about these methods. Future trial publications should aim to reduce this discrepancy in information in order to allow a clear interpretation of the risks of bias. The influence of this possible information bias on the conclusions of this review is likely to be small given that a predominant number of trials had low risks of bias and a subgroup analysis where trials with unclear or high risks of bias were excluded demonstrated negligible changes to the results.
The trials analysed in this review treated patients with levetiracetam for only 12 to 24 weeks. Drug‐resistant patients need even longer‐term treatment than this, and the results here are not applicable to that period. The conclusions on children are based on a sample size of fewer than 300 participants. More studies, particularly longer‐term studies and studies on children, will be needed before complete evaluation of the effectiveness of levetiracetam is possible.
Although the results of this review indicate that levetiracetam is an effective add‐on treatment for both adults and children with drug‐resistant focal epilepsy, it cannot tell us how levetiracetam compares with other AEDs in this scenario. This is an extremely important issue for clinicians who are faced with an ever increasing number of AEDs to choose from, and head‐to‐head trials are needed to provide the evidence that is needed to enable clinicians to make an evidence‐based choice between AEDs. This review focuses on the use of levetiracetam in drug‐resistant focal epilepsy, and the results cannot be generalised to add‐on treatment in people with generalised epilepsy. Likewise, no inference can be made about the efficacy and tolerability of levetiracetam when used as monotherapy.
Authors' conclusions
Implications for practice.
Levetiracetam is effective as add‐on treatment in people with drug‐resistant focal epilepsy. The most significant contribution of this update is the finding that one can expect a quarter of children to be responsive to adjuvant levetiracetam at a dose of 60 mg/kg/day. The drug is effective in adults and at a dose of 2000 mg one could expect around 30% of adults to be responsive. One additional child taking 60 mg/kg/day of levetiracetam may respond for every four children that have received levetiracetam rather than placebo. This number needed to treat is the same for adults on 2000 mg of levetiracetam. Owing to significant levels of inter‐trial heterogeneity, we are unable to provide overall estimates of effect for the doses of 1000 mg and 3000 mg of levetiracetam; although there is strong evidence that these doses are effective as well. We had insufficient data to provide details on the seizure‐reduction efficacy of levetiracetam when used at a dose of 4000 mg. All doses appear well tolerated in both adults and children although there is a possibility of adverse changes in behaviour in children, potentially affecting around 20%. It is reasonable to continue the use of adjuvant levetiracetam in clinical practice for treating adults and children with drug‐resistant focal epilepsy. The conclusions cannot be applied to levetiracetam use in generalised epilepsy or to its use as monotherapy.
Implications for research.
Further evaluation of levetiracetam add‐on for the treatment of patients suffering from drug‐resistant focal epilepsy
To evaluate further the place of levetiracetam in drug‐resistant focal epilepsy, further studies are required to address the following:
the minimum and maximum effective doses of levetiracetam;
the most effective dose of levetiracetam (2000 mg?);
the long‐term efficacy and safety of levetiracetam;
the effects of levetiracetam on behaviour;
the effects of levetiracetam on QoL and cognition;
economic aspects of levetiracetam therapy;
the influence of year of trial publication on response rates in placebo‐controlled RCTs published to date;
how levetiracetam compares with other add‐on treatments.
Further investigation is also needed on how levetiracetam compares with standard AEDs such as: a) carbamazepine as monotherapy in focal epilepsy and b) valproate as monotherapy in generalised epilepsy. The effectiveness of levetiracetam versus standard AEDs will be compared in the upcoming SANAD‐II trial (SANAD‐II).
What's new
| Date | Event | Description |
|---|---|---|
| 13 September 2012 | Amended | missing citation added Yagi 2010 |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 12 August 2012 | New citation required but conclusions have not changed | Pediatric data has been incorporated into the update. |
| 19 April 2011 | New search has been performed | Addition of seven new trials to the systematic review and meta‐analysis, published after the original 2001 review. |
| 8 November 2009 | Amended | Published notes added. |
| 23 September 2008 | Amended | Converted to new review format. |
| 1 July 2005 | New search has been performed | The date of the latest search for evidence to the review is 01/07/2005, no new studies were identified. In a previous update on 27/09/02 we found one new study which we included as published data of the study N138 (Ben‐Menachem et al. Efficacy and tolerability of levetiracetam 3000 mg/d in patients with refractory seizures: a multicenter, double‐blind, responder‐selected study evaluating monotherapy. European Levetiracetam Study Group. Epilepsia 2000;41(10):1276‐83). One study was also added to the 'Studies awaiting assessment' section (Boon P et al. Dose‐response effect of levetiracetam 1000 and 2000 mg/day in partial epilepsy. Epilepsy Research 2002;48(1‐2): 77‐89) ‐ This will be assessed for inclusion at a later date. |
Acknowledgements
We would like to thank and acknowledge two of the review authors of the original review; Rungsan Chaisewikul and Michael Privitera. We would also like to thank UCB S.A. Pharma for the provision of data, in particular Christine Spiegler and Matthew Hickling for kindly handling our queries.
Appendices
Appendix 1. CENTRAL search strategy
#1 levetiracetam OR keppra #2 MeSH descriptor Epilepsy explode all trees #3 MeSH descriptor Seizures explode all trees #4 epilep* or seizure* or convulsion* #5 (#2 OR #3 OR #4) #6 (#1 AND #5)
Appendix 2. MEDLINE search strategy
The following search strategy was used to update the searches for this review in August 2012. It is based on the Cochrane Highly Sensitive Search Strategy for identifying randomised trials published in Lefebvre 2011.
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. clinical trials as topic.sh.
6. randomly.ab.
7. trial.ti.
8. 1 or 2 or 3 or 4 or 5 or 6 or 7
9. exp animals/ not humans.sh.
10. 8 not 9
11. exp Epilepsy/
12. exp Seizures/
13. (epilep$ or seizure$ or convuls$).tw.
14. 11 or 12 or 13
15. (levetiracetam or keppra).tw.
16. 10 and 14 and 15
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
The search strategy below is the original MEDLINE strategy that was used for earlier versions of this review. It is based on the Cochrane Highly Sensitive Search Strategy for MEDLINE as set out in Appendix 5b of the Cochrane Handbook for Systematic Reviews of Interventions (version 4.2.4, updated March 2005) (Higgins 2005).
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. exp Randomized Controlled Trials/
4. exp Random Allocation/
5. exp Double‐Blind Method/
6. exp Single‐Blind Method/
7. clinical trial.pt.
8. Clinical Trial/
9. (clin$ adj trial$).ab,ti.
10. ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ab,ti.
11. exp PLACEBOS/
12. placebo$.ab,ti.
13. random$.ab,ti.
14. exp Research Design/
15. or/1‐14
16. (animals not humans).sh.
17. 15 not 16
18. levetiracetam.tw.
19. (epilep$ or seizure$ or convulsion$).tw.
20. exp Seizures/
21. exp Epilepsy/
22. 19 or 20 or 21
23. 17 and 18 and 22
Data and analyses
Comparison 1. Levetiracetam versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 50% Responders intention to treat | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 60 mg/kg/day | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.38, 2.63] |
| 1.2 1000 mg | 3 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.78, 3.50] |
| 1.3 2000 mg | 2 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.91 [2.75, 8.77] |
| 1.4 3000 mg | 5 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.59 [2.01, 3.33] |
| 1.5 All doses | 10 | 1742 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [2.04, 2.90] |
| 2 Non‐responders (< 50% seizure frequency reduction) intention to treat | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 60 mg/kg/day | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.56, 0.81] |
| 2.2 1000 mg | 3 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.71, 0.85] |
| 2.3 2000 mg | 2 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.60, 0.77] |
| 2.4 3000 mg | 5 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.60, 0.74] |
| 2.5 All doses | 10 | 1742 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.66, 0.75] |
| 3 50% responders best case | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 60 mg/kg/day | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.38, 2.63] |
| 3.2 1000 mg | 3 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.88, 3.67] |
| 3.3 2000 mg | 2 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.09 [2.85, 9.06] |
| 3.4 3000 mg | 5 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [2.05, 3.38] |
| 3.5 All doses | 10 | 1742 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [2.09, 2.96] |
| 4 50% Responders worst case | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 60 mg/kg/day | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.38, 2.63] |
| 4.2 1000 mg | 3 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.70, 3.29] |
| 4.3 2000 mg | 2 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.54 [2.60, 7.94] |
| 4.4 3000 mg | 5 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.84, 2.96] |
| 4.5 All doses | 10 | 1742 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.92, 2.70] |
| 5 Treatment withdrawal | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 60 mg/kg/day | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.43, 1.46] |
| 5.2 1000 mg | 3 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.72, 1.88] |
| 5.3 2000 mg | 3 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.88, 2.18] |
| 5.4 3000 mg | 5 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.62, 1.46] |
| 5.5 4000 mg | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.42, 2.02] |
| 5.6 Any dose levetiracetam | 11 | 1861 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.80, 1.33] |
| 6 Five most common adverse effects (any age) | 10 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 6.1 Somnolence | 10 | 1831 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.58 [1.14, 2.18] |
| 6.2 Headache | 10 | 1831 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.95 [0.65, 1.39] |
| 6.3 Fatigue (asthenia) | 10 | 1831 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.53 [0.98, 2.38] |
| 6.4 Accidental injury | 10 | 1831 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.72 [0.49, 1.06] |
| 6.5 Dizziness | 10 | 1831 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.63 [0.99, 2.66] |
| 7 Most common adverse effects in adults | 8 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 7.1 Accidental injury | 4 | 1023 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.60 [0.39, 0.92] |
| 7.2 Ataxia (unpublished data only) | 4 | 1023 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.50 [0.43, 5.26] |
| 7.3 Dizziness | 7 | 1249 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.65 [0.96, 2.84] |
| 7.4 Fatigue (asthenia) | 4 | 1023 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.47 [0.90, 2.40] |
| 7.5 Headache | 6 | 1360 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.88 [0.59, 1.31] |
| 7.6 Infection | 4 | 1023 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.76 [1.03, 3.02] |
| 7.7 Nausea | 3 | 599 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.37 [0.47, 4.00] |
| 7.8 Somnolence | 8 | 1535 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.51 [1.06, 2.17] |
| 8 Most common adverse effects in children | 2 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 8.1 Accidental injury | 1 | 198 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.63 [0.63, 4.26] |
| 8.2 Aggression (hostility) | 2 | 296 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.72 [0.64, 4.63] |
| 8.3 Cough | 2 | 296 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.39 [0.49, 3.93] |
| 8.4 Dizziness | 2 | 296 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.52 [0.47, 4.94] |
| 8.5 Fatigue (asthenia) | 2 | 296 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.82 [0.62, 5.33] |
| 8.6 Pharyngitis | 2 | 296 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.09 [0.47, 2.50] |
| 8.7 Somnolence | 2 | 296 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.90 [0.88, 4.09] |
| 8.8 Vomiting | 2 | 296 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.22 [0.55, 2.69] |
| 9 Behavioural changes | 6 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 9.1 Hostility | 1 | 198 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.92 [0.56, 6.60] |
| 9.2 Personality disorder | 1 | 198 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.10 [0.30, 3.95] |
| 9.3 Nervousness | 1 | 198 | Risk Ratio (M‐H, Fixed, 99% CI) | 4.80 [0.68, 34.14] |
| 9.4 Depression | 1 | 324 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.41 [0.25, 7.85] |
| 9.5 Aggression | 1 | 98 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.42 [0.27, 7.42] |
| 9.6 Agitation | 2 | 254 | Risk Ratio (M‐H, Fixed, 99% CI) | 6.17 [0.66, 57.79] |
| 9.7 Emotional lability | 1 | 198 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.44 [0.28, 7.29] |
| 9.8 Psychomotor hyperactivity | 1 | 98 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.43 [0.08, 2.19] |
| 9.9 Irritability | 1 | 156 | Risk Ratio (M‐H, Fixed, 99% CI) | 11.28 [0.26, 495.63] |
| 9.10 Abnormal behaviour | 1 | 98 | Risk Ratio (M‐H, Fixed, 99% CI) | 5.92 [0.14, 255.98] |
| 9.11 Altered mood | 1 | 98 | Risk Ratio (M‐H, Fixed, 99% CI) | 4.85 [0.11, 216.99] |
| 9.12 Anxiety | 1 | 98 | Risk Ratio (M‐H, Fixed, 99% CI) | 4.85 [0.11, 216.99] |
| 9.13 Dissociation | 1 | 94 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.14 [0.00, 6.77] |
| 9.14 Combined (regardless of age) | 6 | 926 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.87 [1.19, 2.95] |
| 9.15 Combined (children) | 2 | 296 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.90 [1.16, 3.11] |
| 9.16 Combined (adults) | 4 | 630 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.79 [0.59, 5.41] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ben‐Menachem 2000.
| Methods | Randomised double‐blind placebo‐controlled parallel trial 2 treatment arms: 1 PCB and 1 LEV Randomisation concealment: telephone randomisation. Random list generation: centralised minimisation procedure of an unbalanced randomisation list (1 PCB:2 LEV) Blinding: identical tablets and packages. Investigators were described as blinded to treatment assignment. If treatment code was broken, the patient had to be removed from the trial Baseline = 12 weeks. Treatment period = 16 weeks (4 weeks' titration, 12 weeks' maintenance) |
|
| Participants | All adults. Multicentre across Europe
Total randomised 286 adult; all with drug‐resistant focal epilepsy
105 adults to PCB 181 adults to LEV 3000 mg 48% male Age range 17 to 70 years Other AEDs = 1 ≥ 2 focal seizures per 4 weeks during 12‐week baseline ≥ 1‐year history of focal epilepsy Mean duration of epilepsy (± SD) (years): LEV = 19 ± 11; PCB = 19 ± 12; overall = 19 ± 11 Median baseline seizure frequency per week: 1.70; range 0.3 to 1.7 |
|
| Interventions | LEV 3000 mg/day PCB Up‐titration dosages = titrated upwards every 2 weeks from 500 mg twice daily to the target dosage of 1500 mg twice daily |
|
| Outcomes | ≥ 50% reduction in seizure frequency Treatment withdrawal Adverse effects |
|
| Notes | 2 participants excluded from 50% responder analysis: 1 from the LEV 3000‐mg, 1 from the PCB | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A ‐ Adequate |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A ‐ Adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | A ‐ Adequate |
Betts 2000.
| Methods | Randomised double‐blind placebo‐controlled parallel trial 3 treatment arms: 1 PCB and 2 LEV Randomisation concealment: allocated sequentially sealed, numbered packages containing either LEV or PCB. Random list generation: computer‐generated random permuted blocks (size 3) Blinding: identical tablets and packages. Investigators were described as blinded to treatment assignment. If treatment code was broken, the patient had to be removed from the trial Baseline = 4 weeks. No titration period. Treatment period = 24 weeks |
|
| Participants | All adults. Multicentre across Europe. Total randomised 119 adults 39 adults to PCB 42 adults to LEV 2000‐mg 38 adults to LEV 4000 mg 61% male Age range 16 to 67 years Other AEDs 1 to 3 ≥ 4 seizures in 6 months before study entry Mean duration of epilepsy (± SD) (years): LEV 2000 mg = 21.1 ± 14.4; LEV 4000 mg = 24.6 ± 15.6; PCB = 26.0 ± 13.2 Median of baseline seizure frequency per week: LEV 2000 mg = 1.21; LEV 4000 mg = 1.34; PCB = 1.24 |
|
| Interventions | LEV 2000 mg/day LEV 4000 mg/day PCB add‐on |
|
| Outcomes | Treatment withdrawal Adverse effects QoL and cognitive effects |
|
| Notes | Baseline seizure frequency data were derived from N = 34, N = 36, and N = 36 patients in the LEV 2000 mg, LEV 4000 mg groups, and PCB, respectively In the text for the trial, the number of participants in the inferential ITT population was reported as N = 27, N = 28, and N = 31, in the LEV 2000 mg, LEV 4000 mg, and PCB groups, respectively. In a graph for the trial, the number of participants in the inferential ITT population was reported as N = 26 N = 28, and N = 25, in the LEV 2000 mg, LEV 4000 mg, and PCB groups, respectively All participants had drug‐resistant epilepsy and some had generalised‐onset and unclassified seizures QoL was assessed using the ESI‐55 for 30 to 31 participants in LEV 2000 mg, 26 to 28 participants in LEV 4000 mg, and 28 participants in PCB |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A ‐ Adequate |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A ‐ Adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | C ‐ Inadequate (for outcome of ≥ 50% reduction in seizure frequency) |
| Selective reporting (reporting bias) | High risk | C ‐ Inadequate (for outcome of ≥ 50% reduction in seizure frequency) |
Cereghino & Cramer 2000.
| Methods | Randomised double‐blind placebo‐controlled parallel trial 3 treatment arms: 1 PCB and 2 LEV Randomisation concealment: allocated sequentially sealed, numbered packages containing either LEV or PCB. Random list generation: random permuted blocks Blinding: identical tablets and packages. Investigators were described as blinded to treatment assignment. If treatment code was broken, the patient had to be removed from the trial Baseline = 12 weeks. Treatment period = 18 weeks (4 weeks' titration, 14 weeks' maintenance) |
|
| Participants | All adults. Multicentre across USA Total randomised 294 adults 95 adults to PCB 98 adults to LEV 1000 mg 101 adults to LEV 3000 mg 61% male Age range 16 to 70 years Other AEDs ≥ 1 ≥ 2 focal seizures per 4 weeks during 12‐week baseline ≥ 2‐year history of uncontrolled focal epilepsy Mean duration of epilepsy (years): not given Median baseline seizure frequency per week: 2.13; range 0.15 to 163.56 |
|
| Interventions | LEV 1000 mg/day LEV 3000 mg/day PCB add‐on Up‐titration dosages = LEV dose was escalated at 2‐week intervals during the titration period. Doses of LEV were 333 mg/day for 2 weeks, then 666 mg/day for 2 weeks and 1000 mg/day started on the first visit of the observation period, or 1000 mg/day, 2000 mg/day, then 3000 mg/day |
|
| Outcomes | ≥ 50% reduction in seizure frequency Treatment withdrawal Adverse effects QoL and cognitive effects |
|
| Notes | A minority of participants also had generalised or unclassified seizures, or both, in addition to partial‐onset seizures 1 participant in LEV 1000 mg was excluded from 50% responder analysis QoL was assessed using the QOLIE‐31, for 80 participants in LEV 1000 mg, 85 participants in LEV 3000 mg, and 81 participants in PCB |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A ‐ Adequate |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A ‐ Adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | A ‐ Adequate |
Glauser 2006.
| Methods | Randomised, double‐blind, placebo‐controlled trials 2 treatment arms: 1 PCB and 1 LEV Randomisation concealment: randomisation schedule was performed by centre and patients allocated sequentially. Random list generation: computer‐generated schedule with a permuted block (size 4) Blinding: identical tablets and packages. Investigators, site personnel, study personnel from the contract research organisation responsible for the monitoring and conduct of the trial, and study sponsor personnel were described as blinded to treatment assignment Baseline: 8 weeks. Treatment period = 14 weeks (4 weeks' titration, 10 weeks' maintenance) |
|
| Participants | All children. Multicentre (60 centres) across the US and Canada
Total randomised 216 children; all with drug‐resistant focal epilepsy 97 children to PCB 101 children to LEV 60 mg/kg/day 47% male in PCB, 54% male in LEV Age range 3 to 17 years Other AEDs 1 or 2 ≥ 4 focal seizures per 4 weeks during 8‐week baseline ≥ 4 focal seizures during 4 weeks before screening Diagnosis of uncontrolled focal epilepsy made ≥ 6 months before screening Mean duration of epilepsy (years): LEV = 7.4, PCB = 6.8 Median baseline seizure frequency per week: 4.7 (range 0 to 696) in LEV, 5.3 (range 0 to 467) in PCB |
|
| Interventions | LEV 60 mg/kg/day PCB add‐on Up‐titration dosages = 20 mg/kg/day, increasing every 2 weeks |
|
| Outcomes | ≥ 50% reduction in seizure frequency Treatment withdrawal Adverse effects |
|
| Notes | Before breaking the blind, 18 patients were excluded, including all 16 patients at 1 site who were excluded because of extensive violation of the protocol and consequent unreliability of the data, and 2 patients because they discontinued before taking any study medication. It is unclear to which groups the 16 patients were assigned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A ‐ Adequate |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A ‐ Adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | A ‐ Adequate |
Levisohn 2009 & Loge 2010.
| Methods | Randomised, double‐blind, placebo‐controlled trial 2 treatment arms: 1 PCB and 1 LEV Randomisation concealment: method not stated. Random list generation: no explicit statement of sequence‐generation method, but patients were randomised either to LEV or PCB in a 2:1 ratio. Randomisation was stratified for age (4 to 7, 8 to 12, 13 to 16 years) and number of concomitant AEDs (1 or 2) Blinding: descried as double‐blind without further specification aside from stating that neurocognitive testing was carried out by the same experienced, blinded neuropsychologist Baseline: 4 weeks historical, 1 week prospective. Treatment period = 12 weeks (4 weeks' titration, 8 weeks' maintenance) |
|
| Participants | All children. Multicentre (28) across the US, Canada, and South Africa Total randomised 98 children 34 children to PCB 64 children to LEV 60 mg/kg/day 50% male in PCB, 61% male in LEV Age range 4.1 to 16.7 years Other AEDs: 1 or 2 ≥ 1 focal seizure during 4 weeks before screening Diagnosis of focal epilepsy made ≥ 6 months before screening Mean duration of epilepsy (years): not given Median baseline seizure frequency per week: LEV = 0.9 (IQR 0.4 to 1.9); PCB = 1.4 (IQR 0.4 to 5.2) |
|
| Interventions | LEV 60 mg/kg/day PCB add‐on Up‐titration dosages = 20 mg/kg/day orally twice a day as tablets or 10% solution, up‐titrated in increments of 20 mg/kg/day every 2 weeks |
|
| Outcomes | ≥ 50% reduction in seizure frequency Treatment withdrawal Adverse effects Cognitive effects Behavioural and emotional functioning |
|
| Notes | Cognitive assessment was done using Leiter‐R AM, WRAML‐2, and Leiter‐R ERS Behavioural and emotional functioning were assessed using CBCL and CHQ‐PF50 Cognitive, behavioural, and emotional function results were shown only for the per protocol population: 46 in LEV, 27 in PCB A few participants had generalised‐onset (1 in LEV, 1 in PCB) or unclassified seizures (1 in PCB), or both, in addition to partial‐onset seizures |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | B ‐ Unclear |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | B ‐ Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | A ‐ Adequate |
Peltola 2009.
| Methods | Randomised, double‐blind, placebo‐controlled trial 2 treatment arms: 1 PCB and 1 LEV XR Randomisation concealment: interactive voice response system. Random list generation: randomised 1:1 using interactive voice response system Blinding: identical tablets and packages, all study personnel and participants were described as being blinded to treatment assignment Baseline: 8 weeks. Treatment period = 12 weeks (no up‐titration took place) |
|
| Participants | All adults. Multicentre (7 centres) including centres in Finland, India, Mexico, Russia, South Africa, and Ukraine
Total randomised: 158 adults
79 adults to PCB 79 adults to LEV XR 1000 mg 59% male in PCB and 66% male in LEV XR Age range 12 to 70 years Other AEDs 1 to 3 ≥ 8 focal seizures during 8‐week baseline within which ≥ 2 focal seizures per 4 weeks Diagnosis of uncontrolled focal epilepsy made ≥ 6 months before screening Mean duration of epilepsy (± SD) (years): LEV XR 13.11 ± 10.87 (range 0.8 to 42.6), PCB 16.43 ± 11.93 (range 0.7 to 53.5) Mean baseline seizure frequency per week (mean ± SD): LEV XR 40.7 ± 66.0; PCB 30.6 ± 52.5 |
|
| Interventions | LEV XR 1000 mg PCB |
|
| Outcomes | ≥ 50% reduction in seizure frequency Treatment withdrawal Adverse effects |
|
| Notes | 5 participants excluded from 50% responder analysis: 4 in LEV XR 1000 mg, 1 in PCB 2 patients randomised to LEV XR did not receive any medication; therefore, they were excluded from the safety population, leaving 77 patients on LEV XR and 79 on PCB in the safety analysis data‐set Baseline level for determining reduction in seizure frequency was derived from 74 patients in LEV XR group and 78 in PCB A few participants had other seizure types in addition to partial‐onset seizures "Study personnel" taken to mean investigators |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A ‐ Adequate |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A ‐ Adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | A ‐ Adequate |
Shorvon 2000.
| Methods | Randomised double‐blind placebo‐controlled crossover trial 3 treatment arms: 1 PCB and 2 LEV Randomisation concealment: allocated sequentially sealed, numbered packages containing either LEV or PCB. Random list generation: random permuted blocks (size 6) Blinding: identical tablets and packages. Investigators and staff were described as blinded to treatment assignment. If treatment code was broken, the patient had to be removed from the trial Baseline = 8 to 12 weeks. Treatment period = 16 weeks (4 weeks' titration, 12 weeks' maintenance) |
|
| Participants | All adults. Multicentre across Europe
Total randomised 324 adults; all with drug‐resistant focal epilepsy but a few also had generalised‐onset or unclassified seizures, or both
112 adults to PCB 106 adults to LEV 1000 mg 106 adults to LEV 2000 mg 49% male Age range 14 to 69 years Other AEDs: 1 or 2 ≥ 4 focal seizures per 4 weeks during 8‐ or 12‐week baseline ≥ 2‐year history of uncontrolled focal epilepsy Mean duration of epilepsy (± SD) (years): LEV 1000 mg = 23.8 ± 12.3; LEV 2000 mg = 23.6 ± 13.3; PCB = 23.2 ± 11.0; overall = 23.6 ± 12.2 Mean baseline seizure frequency per week: 2.62; range 0.3 to 102.7 |
|
| Interventions | LEV 1000 mg LEV 2000 mg PCB Up‐titration dosages = LEV was titrated upwards in twice‐daily increments of 500 mg at 2‐week intervals until patients were stabilised on their assigned dosages (1000 mg/day or 2000 mg/day). The 1000‐mg LEV group received PCB for 2 weeks before initiation of active drug |
|
| Outcomes | ≥ 50% reduction in seizure frequency Treatment withdrawal Adverse effects QoL and cognitive effects |
|
| Notes | 2 participants excluded from 50% responder analysis: 1 in LEV 2000 mg, 1 in PCB A few participants had generalised‐onset or unclassified seizures, or both, in addition to partial‐onset seizures QoL was assessed using the ESI‐55 for 92 participants in LEV 1000 mg and LEV 2000 mg, and 89 participants in PCB |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A ‐ Adequate |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A ‐ Adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | A ‐ Adequate |
Tsai 2006.
| Methods | Randomised, double‐blind, placebo‐controlled trial 2 treatment arms: 1 PCB and 1 LEV Randomisation concealment: allocated sequentially sealed, numbered packages containing either LEV or PCB. Random list generation: random permuted blocks (size 4) Blinding: identical tablets and packages. Investigators were described as blinded to treatment assignment Baseline: 8 weeks. Treatment period = 14 weeks (2 weeks' titration, 12 weeks' maintenance) |
|
| Participants | All adults. Multicentre (Taiwan) Total randomised 94 adults 47 adults to PCB 47 adults to LEV 2000 mg 53% male in PCB and 36% male in LEV Age range 16 to 60 years Other AEDs 1 to 3 ≥ 4 focal seizures during 8‐week baseline Diagnosis of uncontrolled focal epilepsy made ≥ 6 months before study Mean duration of epilepsy (± SD) (years): LEV = 18.6 ± 8.5; PCB = 18.7 ± 10.7 Mean baseline seizure frequency per week LEV = 4.0 ± 14.1, PCB = 4.3 ± 7.0 |
|
| Interventions | LEV 2000 mg/day PCB Up‐titration dosages = initial LEV dose was 500 mg twice daily, which was increased to 1000 mg twice daily after 2 weeks |
|
| Outcomes | ≥ 50% reduction in seizure frequency Treatment withdrawal Adverse effects |
|
| Notes | 1 participant (LEV group) excluded from 50% responder analysis A minority of participants also had generalised or unclassified, or both, seizures in addition to partial‐onset seizures 14 participants required dose reduction (11 in LEV; 3 in PCB) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A ‐ Adequate |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A ‐ Adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | A ‐ Adequate |
Wu 2009.
| Methods | Randomised, double‐blind, placebo‐controlled trial 2 treatment arms: 1 PCB and 1 LEV Randomisation concealment: method not stated. Study medications were supplied and packaged by UCB S.A. Pharma. Method of sequence generation: not stated Blinding: "matched placebo" was used. No further specification Baseline: 8 weeks. Treatment period = 16 weeks (4 weeks' titration, 12 weeks' maintenance) |
|
| Participants | All adults. Multicentre (6 centres in China)
Total randomised 206 adults
103 adults to PCB 103 adults to LEV 3000 mg 54% male in PCB and 50% male in LEV Age range: 16 to 70 years Other AEDs: 1 or 2 ≥ 8 focal seizures during 8‐week baseline Diagnosis of focal epilepsy made ≥ 6 months before screening Mean duration of epilepsy (± SD) (years): LEV = 16.5 ± 12.7, PCB = 17.3 ± 12.1 Median baseline seizure frequency per week: LEV 1.81 (IQR = 1.13 to 3.38), PCB 1.75 (IQR = 1.13 to 4.00) |
|
| Interventions | LEV 3000 mg PCB Up‐titration dosages: started with 500 mg (1 tablet) twice daily and was up‐titrated in twice‐daily increments of 500 mg (1 tablet) at 2‐week intervals; the dose was increased to 2000 mg/day after 2 weeks and to 3000 mg/day after an additional 2 weeks |
|
| Outcomes | ≥ 50% reduction in seizure frequency Treatment withdrawal Adverse effects |
|
| Notes | 4 participants excluded from 50% responder analysis: 1 in LEV 3000 mg and 3 in PCB A few participants (1 in LEV, 2 in PCB) had primary generalised‐onset seizures in addition to partial‐onset seizures. 1 patient (1.0%) in the LEV group and 2 (1.9%) in the PCB group temporarily discontinued the study drug, while 8 (7.8%) and 2 (1.9%) patients in the LEV and PCB groups, respectively, reduced the dosage because of adverse events |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | B ‐ Unclear |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A ‐ Adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | A ‐ Adequate |
Xiao 2009.
| Methods | Randomised, double‐blind, placebo‐controlled trial 2 treatment arms: 1 PCB and 1 LEV Randomisation concealment: numbered containers containing either LEV or PCB. Random list generation: randomisation codes were generated by the study sponsor. Each patient who qualified to receive double‐blind treatment was assigned a randomisation number and given LEV or PCB accordingly Blinding: identical tablets and packages. Investigators were described as blinded to treatment assignment Baseline: 8 weeks. Treatment period = 16 weeks (4 weeks' titration, 12 weeks' maintenance) |
|
| Participants | All adults. Single centre (China) Total randomised 56 adults; all with drug‐resistant focal epilepsy 28 adults to PCB 28 adults to LEV 3000 mg 42.9% male in PCB, 42.9% male in LEV Age range 16 to 70 years Other AEDs: 1 or 2 ≥ 4 focal seizures per month over preceding 2 months ≥ 10 weeks' background AED treatment Mean duration of epilepsy (± SD) (years): LEV = 14.1 ± 9.4 (range 2 to 40), PCB = 16.1 ± 12.5 (range 2 to 48) Mean baseline seizure frequency per week: LEV 4.9; range 1 to 23.6, PCB 5.6; range 1 to 50 |
|
| Interventions | LEV 3000 mg PCB add‐on Up‐titration dosages: received LEV 1000 mg/day (administered twice a day) and increased to 2000 mg/day after 2 weeks, and to 3000 mg/day after another 2 weeks |
|
| Outcomes | ≥ 50% reduction in seizure frequency Treatment withdrawal Adverse effects |
|
| Notes | 2 LEV‐treated patients decreased dose to 2000 mg (owing to adverse effects) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | B ‐ Unclear |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A ‐ Adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | A ‐ Adequate |
Zhou 2008.
| Methods | Randomised, double‐blind, placebo‐controlled trial 2 treatment arms: 1 PCB and 1 LEV Randomisation concealment: participants received an exclusive random number consecutively on entry into the study, and received treatment on the basis of this random number. Random list generation: random numbers table Blinding: described as double‐blind with no further specification. Medications were supplied and packaged by UCB S.A|. Pharma Baseline: 8 weeks. Treatment period = 16 weeks (4 weeks' titration, 12 weeks' maintenance) |
|
| Participants | All adults. 1 centre in China. Total randomised 28 adults 14 adults to PCB 14 adults to LEV 3000 mg 55% male in PCB, 54% male in LEV Age range 16 to 70 years. Other AEDs: 1 or 2 ≥ 8 seizures during 8‐week baseline with 2 per 4 weeks Mean duration of epilepsy (± SD) (years): LEV = 8.7 ± 6.4, PCB = 16.5 ± 7.2 Mean baseline seizure frequency per week (± SD) 6.55 ± 10.79 in LEV, 6.15 ± 11.20 in PCB |
|
| Interventions | LEV 3000 mg/day PCB add‐on Up‐titration dosages: 500 mg twice daily in the first 2 weeks, 1000 mg twice daily in the third and fourth weeks) |
|
| Outcomes | ≥ 50% reduction in seizure frequency Treatment withdrawal Cognitive function QoL |
|
| Notes | Cognitive function assessment was with a battery of neuropsychological tests: Wisconsin Card Sorting Test, Verbal Fluency, Trail Making Test, Digit Symbol, Stroop Color–Word Interference Task, Logic Memory, Delayed Logic Memory, Visual Memory, Delayed Visual Memory, Calculation QoL assessment was with the use of QOLIE‐31 Drop‐outs (1 in LEV, 3 in PCB) were excluded from the study author's analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A ‐ Adequate |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | B ‐ Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | B ‐ Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | A ‐ Adequate |
AEDs: antiepileptic drugs; CBCL: Achenbach Child Behavior Checklist; CHQ‐PF50: Child Health Questionnaire‐Parent Form 50;
ESI: Epilepsy Surgery Inventory scale;
IQR: interquartile range;
ITT: intention to treat;
Leiter‐R AM: Leiter International Performance Scale‐Revised Attention and Memory; LEV: levetiracetam; Leiter‐R ERS: Leiter International Performance Scale‐Revised, Examiner’s Rating Scale;
PCB: placebo;
QOLIE: Quality of life in epilepsy inventory ;
QoL: quality of life;
WRAML‐2: Wide Range Assessment of Memory and Learning‐2;
XR: extended release.
Characteristics of studies awaiting assessment [ordered by study ID]
Boon 2002.
| Methods | ‐ |
| Participants | ‐ |
| Interventions | ‐ |
| Outcomes | ‐ |
| Notes | This is a cross‐over trial where separate data pertaining to the first treatment period only has not been made available upon request. We therefore cannot analyse the first treatment period as if it were a parallel trial (see Methods) |
N01221.
| Methods | ‐ |
| Participants | ‐ |
| Interventions | ‐ |
| Outcomes | ‐ |
| Notes | The publication for this trial is currently under preparation. The amount of unpublished information and data available from www.clinicaltrials.gov on this trial is currently insufficient for use in this review |
Yagi 2010.
| Methods | ‐ |
| Participants | ‐ |
| Interventions | ‐ |
| Outcomes | ‐ |
| Notes | This trial has an English abstract showing that the trial is likely to be eligible for inclusion. The full text is awaiting translation. The authors await further data. |
Zheng 2009.
| Methods | ‐ |
| Participants | ‐ |
| Interventions | ‐ |
| Outcomes | ‐ |
| Notes | This trial has an English abstract showing that the trial is likely to be eligible for inclusion. The full text is awaiting translation. The authors await further data. |
Contributions of authors
Gashirai Mbizvo and Pete Dixon were involved in all stages of conducting and writing of this review. Gashirai Mbizvo and Pete Dixon assessed trials for inclusion, extracted data, assessed trials for bias, and evaluated the overall quality of evidence. These steps were each conducted independently before collaboration, with any disagreements were resolved by discussion with Tony Marson. Jane Hutton oversaw data analysis.
Sources of support
Internal sources
-
None, UK.
Work done independently as part of a Master of Research degree program.
External sources
No sources of support supplied
Declarations of interest
Pete Dixon is funded as part of a research programme (RP‐PG‐0606‐1062) that receives financial support from the National Institute for Health Research (NIHR) Programme Grants for Applied Research (PGfAR) funding scheme. The original review (Chaisewikul 2001) was funded by the Health Technology Assessment (HTA) programme. The views and opinions expressed within this article do not necessarily reflect those of the National Health Service (NHS), the HTA, or the Department of Health. The researchers are independent from the funders.
Edited (no change to conclusions)
References
References to studies included in this review
Ben‐Menachem 2000 {unpublished data only}
- Ben‐Menachem E, Falter U. Efficacy and tolerability of levetiracetam 3000 mg/d in patients with refractory seizures: a multicenter, double‐blind, responder‐selected study evaluating monotherapy. European Levetiracetam Study Group. Epilepsia 2000;41(10):1276‐83. [DOI] [PubMed] [Google Scholar]
- UCB. Evaluation of the efficacy and tolerability of UCB L059 (1500 mg b.i.d., 500 mg tablets) monotherapy in epileptic patients with complex partial onset seizures, having experienced improved seizure control under add‐on treatment: a 60‐week (maximum), double‐blind, multicenter, responder‐selected trial (phase III study, placebo comparator). UCB S.A. Pharma Sector.
Betts 2000 {published and unpublished data}
- Betts T, Waegemans T, Crawford P. A multicentre, double‐blind, randomized, parallel group study to evaluate the tolerability and efficacy of two oral doses of levetiracetam, 2000 mg daily and 4000 mg daily, without titration in patients with refractory epilepsy. Seizure 2000;9:80‐7. [DOI] [PubMed] [Google Scholar]
- UCB. A 24‐week multicentered, double‐blind, parallel group, add‐on study to compare the efficacy and the tolerability of 2 oral daily doses of UCB L059 (2000 mg and 4000 mg tablets) with placebo in patients with refractory epilepsy, followed by a 24‐week open‐label active treatment. UCB S.A. Pharma Sector.
Cereghino & Cramer 2000 {published and unpublished data}
- Cereghino JJ, Biton V, Abou‐Khalil B, Dreifuss F, Gauer LJ, Leppik I, and the United States Levetiracetam Study Group. Levetiracetam for partial seizures: results of a double‐blind, randomized clinical trial. Neurology 2000;55:236‐42. [DOI] [PubMed] [Google Scholar]
- Cramer JA, Arrigo C, Hammee G, Gauer LJ, Cereghino JJ. Effect of levetiracetam on epilepsy‐related quality of life: N132 study group. Epilepsia 2000;41(7):868‐74. [DOI] [PubMed] [Google Scholar]
- UCB. Evaluation of the efficacy and tolerability of UCB L059 (500 and 1500 mg bid, tablets) add‐on treatment in epileptic patients with partial onset seizures: a 38‐week, double‐blind, placebo‐controlled, parallel‐group multicenter trial. UCB S.A. Pharma Sector.
Glauser 2006 {published data only}
- Glauser TA, Ayala R, Elterman RD, Mitchell WG, Orman CB, Gauer LJ, et al. Double‐blind placebo‐controlled trial of adjunctive levetiracetam in pediatric partial seizures. Neurology 2006;66(11):1654‐60. [DOI] [PubMed] [Google Scholar]
Levisohn 2009 & Loge 2010 {published data only (unpublished sought but not used)}
- Levisohn PM, Mintz M, Hunter SJ, Yang H, Jones J. Neurocognitive effects of adjunctive levetiracetam in children with partial‐onset seizures: a randomized, double‐blind, placebo‐controlled, noninferiority trial. Epilepsia 2009;50(11):2377‐89. [DOI] [PubMed] [Google Scholar]
- Loge C, Hunter SJ, Schiemann J, Yang HC. Assessment of behavioral and emotional functioning using standardized instruments in children and adolescents with partial‐onset seizures treated with adjunctive levetiracetam in a randomized, placebo‐controlled trial. Epilepsy & Behavior 2010;18(3):291‐8. [DOI] [PubMed] [Google Scholar]
Peltola 2009 {published data only}
- Peltola J, Coetzee C, Jimenez F, Litovchenko T, Ramaratnam S, Zaslavaskiy L, et al. Once‐daily extended‐release levetiracetam as adjunctive treatment of partial‐onset seizures in patients with epilepsy: a double‐blind, randomized, placebo‐controlled trial. Epilepsia 2009;50(3):406‐14. [DOI] [PubMed] [Google Scholar]
Shorvon 2000 {published and unpublished data}
- Shorvon SD, Lowenthal A, Janz D, Bielen E, Loiseau P, for the European Levetiracetam Study Group. Multicenter double‐blind, randomized, placebo‐controlled trial of levetiracetam as add‐on therapy in patients with refractory partial seizures. Epilepsia 2000;41(9):1179‐86. [DOI] [PubMed] [Google Scholar]
- UCB. Evaluation of the efficacy and tolerability of UCB L059 (500 and 1000 mg b.i.d., tablets) add‐on treatment in refractory epileptic patients with partial onset seizures: a 32‐week double‐blind placebo‐controlled crossover multicenter trial. UCB S.A. Pharma Sector.
Tsai 2006 {published data only}
- Tsai JJ, Yen DJ, Hsih MS, Chen SS, Hiersemenzel R, Edrich P, et al. Efficacy and safety of levetiracetam (up to 2000 mg/day) in Taiwanese patients with refractory partial seizures: a multicenter, randomized, double‐blind, placebo‐controlled study. Epilepsia 2006;47(1):72‐81. [DOI] [PubMed] [Google Scholar]
Wu 2009 {published data only (unpublished sought but not used)}
- Wu XY, Hong Z, Wu X, Wu LW, Wang XF, Zhou D, et al. Multicenter double‐blind, randomized, placebo‐controlled trial of levetiracetam as add‐on therapy in Chinese patients with refractory partial‐onset seizures. Epilepsia 2009;50(3):398‐405. [DOI] [PubMed] [Google Scholar]
Xiao 2009 {published data only}
- Xiao Z, Li JM, Wang XF, Xiao F, Xi ZQ, Lv Y, et al. Efficacy and safety of levetiracetam (3,000 mg/day) as an adjunctive therapy in Chinese patients with refractory partial seizures. European Neurology 2009;61(4):233‐9. [DOI] [PubMed] [Google Scholar]
Zhou 2008 {published data only (unpublished sought but not used)}
- Zhou B, Zhang Q, Tian LY, Xiao J, Stefan H, Zhou D. Effects of levetiracetam as an add‐on therapy on cognitive function and quality of life in patients with refractory partial seizures. Epilepsy & Behavior 2008;12(2):305‐10. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Boon 2002 {published data only}
- Boon P, Chauvel P, Pohlmann‐Eden B, Otoul C, Wroe S. Dose‐response effect of levetiracetam 1000 and 2000mg/day in partial epilepsy. Epilepsy Research 2002;48(1):77‐89. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
N01221 {unpublished data only}
- UCB. A double‐blind, randomized, placebo‐controlled 5 parallel groups, confirmatory trial on the efficacy and safety of Levetiracetam used as add‐on therapy at doses of 0.5 to 3g/day in patients from 16 to 65 years with epilepsy with partial onset seizures under treatment with 1 to 3 anti‐epileptic drug(s). UCB Japan Co., Ltd. (RRCE07D0208). [CT Registry ID: NCT00280696]
Yagi 2010 {published data only}
- Yagi K, Kameyama S, Kaneko S, Murasaki M, Yamauchi T. [Multicenter, double‐blind, randomized, placebo‐controlled study of levetiracetam as add‐on therapy in Japanese patients with uncontrolled partial seizures]. Journal of the Japan Epilepsy Society. 2010;28(1):3‐16. [Google Scholar]
Zheng 2009 {published data only (unpublished sought but not used)}
- Zheng XZ, Wu SJ, Xia M, Li Q. Study on the therapeutic effect of levetiracetam as an additive therapy for refractory partial epilepsy and the relativity between levetiracetam and multidrug resistance gene: a randomised double‐blind placebo‐controlled trial. Chinese Journal of Contemporary Neurology and Neurosurgery 2009;9(2):173‐7. [Google Scholar]
Additional references
Asconapé 2001
- Asconapé JJ, Gerardot JM, Costa G. Behavioral changes associated with levetiracetam use in patients with epilepsy. Epilepsia 2001;42(Suppl 7):299. [Google Scholar]
Berg 2010
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005‐2009. Epilepsia 2010;51:676‐85. [DOI] [PubMed] [Google Scholar]
Brodie 2007
- Brodie MJ, Perucca E, Ryvlin P, Ben‐Menachem E, Meencke HJ, Levetiracetam Monotherapy Study Group. Comparison of levetiracetam and controlled‐release carbamazepine in newly diagnosed epilepsy. Neurology 2007;68(6):402‐8. [DOI] [PubMed] [Google Scholar]
Brodie 2010
Burneo 2005
Chaisewikul 2001
- Chaisewikul R, Privitera MD, Hutton JL, Marson AG. Levetiracetam add‐on for drug‐resistant localization related (partial) epilepsy. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD001901] [DOI] [PubMed] [Google Scholar]
Cochrane 1998
- Cochrane HC, Marson AG, Baker GA, Chadwick DW. Neuropsychological outcomes in randomized controlled trials of antiepileptic drugs: a systematic review of methodology and reporting standards. Epilepsia 1998;39(10):1088‐97. [DOI] [PubMed] [Google Scholar]
Crepeau 2010
- Crepeau AZ, Treiman DM. Levetiracetam: a comprehensive review. Expert Review of Neurotherapeutics 2010;10(2):159‐71. [DOI] [PubMed] [Google Scholar]
Forsgren 2005
French 2004
- French JA, Kanner AM, Bautista J, Abou‐Khalil B, Browne T, Harden CL, et al. Efficacy and tolerability of the new antiepileptic drugs I: treatment of new onset epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2004; Vol. 62, issue 8:1252‐60. [DOI] [PubMed]
French 2006
- French JA. Refractory epilepsy: one size does not fit all. Epilepsy Currents / American Epilepsy Society 2006;6(6):177‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibbs 2006
- Gibbs JE, Walker MC, Cock HR. Levetiracetam: antiepileptic properties and protective effects on mitochondrial dysfunction in experimental status epilepticus. Epilepsia 2006;47:469–78. [DOI] [PubMed] [Google Scholar]
Gillard 2006
Guekht 2010
- Guekht AB, Korczyn AD, Bondareva IB, Gusev EI. Placebo responses in randomized trials of antiepileptic drugs. Epilepsy & Behavior 2010;17(1):64‐9. [DOI] [PubMed] [Google Scholar]
Hauser 1993
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935‐1984. Epilepsia 1993;34(3):453‐68. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.4 [updated March 2005]. The Cochrane Collaboration. Chichester, UK: John Wiley & Sons, Ltd.
Kerr 2011
- Kerr C, Nixon A, Angalakuditi M. The impact of epilepsy on children and adult patients' lives: development of a conceptual model from qualitative literature. Seizure 2011;20(10):764‐74. [DOI] [PubMed] [Google Scholar]
Krauss 2011
- Krauss GL, Sperling MR. Treating patients with medically resistant epilepsy. Neurology Clinical Practice 2011 ;1:14‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwan 2010
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51(6):1069‐77. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lukyanetz 2002
Lynch 2004
- Lynch BA, Lambeng N, Nocka K, Kensel‐Hammes P, Bajjalieh SM, Matagne A, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proceedings of the National Academy of Sciences of the United States of America 2004; Vol. 101, issue 26:9861‐6. [DOI] [PMC free article] [PubMed]
Mac 2007
Maguire 2011
- Maguire M, Marson AG, Ramaratnam S. Epilepsy (partial). Clinical Evidence (Online) 2011; Vol. 2011. [PMC free article] [PubMed]
McCullagh 1989
- McCullagh P, Nelder JA. Generalized Linear Models. 2nd Edition. London: Chapman and Hall, 1989. [Google Scholar]
NICE 2012
- National Institute for Clinical Excellence. NICE Clinical Guideline 137. The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care, January 2012. www.guidance.nice.org.uk/CG137 (accessed 3 August 2012).
Niespodziany 2001
Pellock 2001
Penovich 2004
- Penovich PE. Much ado about something or nothing: behavioral problems with levetiracetam use in epilepsy patients. Epilepsy Currents / American Epilepsy Society 2004;4(4):145–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Preux 2005
Rheims 2011
- Rheims S, Perucca E, Cucherat M, Ryvlin P. Factors determining response to antiepileptic drugs in randomized controlled trials. A systematic review and meta‐analysis. Epilepsia 2011;52(2):219‐33. [DOI] [PubMed] [Google Scholar]
Rigo 2002
- Rigo JM, Hans G, Nguyen L, Rocher V, Belachew S, Malgrange B, et al. The anti‐epileptic drug levetiracetam reverses the inhibition by negative allosteric modulators of neuronal GABA‐ and glycine‐gated currents. British Journal of Pharmacology 2002; Vol. 136, issue 5:659‐72. [DOI] [PMC free article] [PubMed]
SANAD‐II
- SANAD‐II. A pragmatic randomised controlled trial comparing the effectiveness and cost effectiveness of levetiracetam and zonisamide versus standard treatments for epilepsy: a comparison of Standard And New Antiepileptic Drugs (SANAD‐II). www.hta.ac.uk/2562 (accessed 3 August 2012). [DOI] [PMC free article] [PubMed]
Sander 1996
- Sander JW, Shorvon SD. Epidemiology of the epilepsies. Journal of Neurology, Neurosurgery, and Psychiatry 1996;61(5):433‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann H, Brożek J, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2 [updated March 2009]. The GRADE Working Group, 2009. Available from www.cc‐ims.net/gradepro (accessed 3 August 2012).
Shorvon 1996
- Shorvon SD. The epidemiology and treatment of chronic and refractory epilepsy. Epilepsia 1996;37(Suppl 2):S1‐3. [DOI] [PubMed] [Google Scholar]
Trinka 2011
- Trinka E. What is the evidence to use new intravenous AEDs in status epilepticus?. Epilepsia 2011;52(Suppl 8):35‐8. [DOI] [PubMed] [Google Scholar]
Verrotti 2010
- Verrotti A, D'Adamo E, Parisi P, Chiarelli F, Curatolo P. Levetiracetam in childhood epilepsy. Paediatric Drugs 2010;12(3):177‐86. [DOI] [PubMed] [Google Scholar]


