Abstract
Background
Epilepsy is a chronic neurological disorder which affects millions of people around the world. Antiepileptic drugs (AED) are the main interventions used to prevent seizures and control epilepsy. Although effective in most cases, AEDs are related to long‐term adverse effects, such as cognitive and behavioural alterations. Thus when epilepsy is in remission, it may be in the individual’s best interest to discontinue medication. However, the optimal timing of AED discontinuation is still unknown.
This is an updated version of the original Cochrane review published in Issue 3, 2001.
Objectives
(1) To quantify and compare risk of seizure recurrence, status epilepticus and mortality after early and late AED discontinuation in adult and pediatric epilepsy patients.
(2) To assess which variables modify the risk of seizure recurrence.
(3) To define a subpopulation in which early AED discontinuation is safe.
Search methods
We searched the Cochrane Epilepsy Group Specialised Register (June 2014); CENTRAL (Issue 5, The Cochrane Library, May 2014); MEDLINE (1946 to June 2014); CINAHL (23 June 2014); Scopus (1823 to June 2014); ClinicalTrials.gov (23 June 2014); and WHO International Clinical Trials Registry Platform (23 June 2014). We also checked the reference lists of studies found through the electronic searches.
Selection criteria
Randomised controlled trials that evaluate withdrawal of AEDs after varying periods of seizure remission in adults and children with epilepsy. Included studies compared an early AED discontinuation time (defined as a period of remission of seizures of less than two years) versus a late AED discontinuation time (defined as a period of remission of seizures of more than two years).
Data collection and analysis
Two authors independently extracted data and assessed trial quality. Risk ratio (RR) with 95% confidence interval (CI) was calculated for each trial. Summary RRs and 95% CIs for dichotomous data were calculated using a fixed‐effect model. A test of statistical heterogeneity was conducted for each pooled risk ratio calculation. Each included study underwent a 'Risk of bias' assessment, based on the Cochrane Handbook recommendations, and we examined the overall quality of information through the GRADE system, presented in two 'Summary of Findings' tables.
Main results
Five trials were included in this review, representing 924 randomised children with epilepsy, all under 16 years of age at randomisation, with a median follow‐up of 5.6 years. No eligible trial evaluated adults or assessed mortality or status epilepticus as outcomes. The pooled risk ratio for seizure relapse after AED withdrawal was 1.34 (95% CI 1.13 to 1.59, P = 0.0007). Conforming to this estimate, the number needed to harm, that is expose an individual to a higher risk of seizure relapse because of early withdrawal of AED, is 8 (95% CI 5 to 20). Early discontinuation was associated with greater relapse rates in people with partial seizures with a pooled risk ratio of 1.51 (95% CI 0.97 to 2.35, P = 0.07). Absence type epilepsy showed a lower risk of relapse. Variables associated with higher risk of seizure relapse were abnormal EEG findings (pooled RR 1.44, 95% CI 1.13 to 1.83, P = 0.003), especially epileptiform activity (RR 2.58, 95% CI 2.03 to 3.28, P < 0.0001); epilepsy onset before 2 years or after 10 years of age; history of status epilepticus; intellectual disability (IQ < 70); and high seizure frequency before and during treatment. Gender and family history did not show any significant influence over seizure relapse. Overall, the included trials were classified as low or unclear risk of bias where methodological information was not reported and could not be provided by original study authors.
Authors' conclusions
There is evidence to support waiting for at least two seizure‐free years before discontinuing AEDs in children, particularly if individuals have an abnormal EEG or partial seizures, or both. There is insufficient evidence to establish when to withdraw AEDs in children with generalised seizures. There is no evidence to guide the timing of withdrawal of AEDs in seizure‐free adults. Further high‐quality randomised controlled trials are needed, particularly recruiting adults and recruiting those with generalised seizure types, to identify the optimal timing of AED withdrawal and risk factors predictive of relapse.
Plain language summary
Early versus late antiepileptic drug withdrawal for people with epilepsy in remission
Epilepsy is a disorder where recurrent seizures are caused by abnormal electrical discharges in the brain. Antiepileptic drugs (AEDs) are commonly used to prevent these seizures but have long‐term side effects. When in remission (seizure free), it may be best to stop using the drugs but the right time to withdraw them is still unclear.
We searched electronic databases in June 2014, adding to the research done in a previous version of this review. The same five trials were included in our analysis, comprising 924 epileptic children (all below 16 years old) who were randomly assigned to either early removal of AEDs (before completing two years without seizures); or late withdrawal of AEDs (after completing two years without seizures). Considering all evidence, we found that stopping AED intake before completing two years without seizures increases the risk of seizure relapse by around 34%. This percentage is increased if the child has partial seizures (if the electrical burst only involves a part of the brain, resulting mainly in localized symptoms); or an abnormal electroencephalogram (EEG) record (unusual patterns of electrical activity in the brain). Other factors that might be related to a higher relapse rate are: age below two years or above 10 years when epilepsy started; history of status epilepticus (convulsions longer than 30 minutes); an IQ lower than 70; and high frequency of seizures before and during treatment. Overall, the included trials provided a moderate quality of evidence.
The review of trials found that there is evidence to support waiting at least two years or more seizure free before discontinuing AEDs in children, especially if they had partial seizures or abnormal EEG.
There is not enough evidence to show the best time to withdraw antiepileptic drugs in adults with epilepsy who are free of seizures.
There is not enough evidence that demonstrates the optimal time to remove antiepileptic drugs in people (children or adults) with generalised seizures (if the electrical discharges affect the whole brain, causing global symptoms).
More research is needed, particularly involving adults and those with generalised seizure types.
Summary of findings
Summary of findings for the main comparison. Early versus late AED withdrawal for people with epilepsy in remission.
| Early versus late withdrawal for people with epilepsy in remission | ||||||
| Patient or population: individuals with epilepsy in remission Settings: Outpatients Intervention: Early (< 2 years in remission) versus late withdrawal (2 or more years in remission) of antiepileptic drugs. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Late withdrawal | Early withdrawal | |||||
| Seizure relapse rate by time of withdrawal Follow‐up: median 5.6 years | 343 per 1000 | 460 per 1000 (388 to 545) | RR 1.34 (1.13 to 1.59) | 924 (5 studies) | ⊕⊕⊝⊝ low1,2 | RR > 1 indicates that seizure recurrence is more likely to occur following Early withdrawal of AED |
| Seizure relapse rate in people with partial epilepsy by time of withdrawal Follow‐up: median 4.4 years | 250 per 1000 | 378 per 1000 (243 to 587) | RR 1.51 (0.97 to 2.35) | 180 (2 studies) | ⊕⊕⊝⊝ low1,2,3 | RR > 1 indicates that seizure recurrence is more likely to occur following Early withdrawal of AED |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the event rate in the control group (late withdrawal of AED after two or more years of seizure freedom). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group (early withdrawal of AED after less than two years of seizure freedom) and the relative effect (pooled RR) of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 In four out of five of the studies included in the analysis (Braathen 1996; Peters 1998; Todt 1984; Verrotti 2000), no information was given on generation of the random sequence or concealment of allocation of the randomisation. 2 Results apply only to children under the age of 16; no studies recruited adults therefore evidence is not applicable to this population. 3 This comparison was considered as indirect because one of the studies (Verrotti 2000) did not include children with generalised seizures and therefore could not compare results adequately with partial seizure epilepsy participants.
Summary of findings 2. Abnormal versus normal EEG for people with epilepsy in remission.
| Abnormal versus normal EEG for people with epilepsy in remission | ||||||
| Patient or population: people with epilepsy in remission Settings: Outpatients Intervention: Abnormal versus normal EEG | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Normal EEG | Abnormal EEG | |||||
| Seizure relapse rate by EEG findings Follow‐up: median 6.85 years | 289 per 1000 | 415 per 1000 (326 to 528) | RR 1.44 (1.13 to 1.83) | 618 (2 studies) | ⊕⊕⊝⊝ low1,2,3 | RR > 1 indicates that seizure recurrence is more likely to occur among those with abnormal EEG results. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the event rate in the control group (normal EEG before discontinuation of AEDs) . The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group (abnormal EEG before discontinuation of AEDs) and the relative effect (pooled RR) of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 In four out of five of the studies included in the analysis (Braathen 1996; Peters 1998; Todt 1984; Verrotti 2000), no information was given on generation of the random sequence or concealment of allocation of the randomisation. 2 Results apply only to children under the age of 16, no studies recruited adults therefore evidence is not applicable to this population.
3Four studies recruiting 835 children investigated the relationship between EEG results and seizure recurrence (Braathen 1996; Gebremariam 1999; Peters 1998; Todt 1984), however two studies recruiting 217 children (Gebremariam 1999; Peters 1998) did not present results in a manner which we could use in analysis, therefore we could not include 26% of the relevant evidence in our pooled analysis.
Background
Description of the condition
Affecting about 50 million people worldwide or 6 to 10 per 1000 individuals (WHO 2012), epilepsy is a chronic neurological disease characterised by recurrent, synchronous and exaggerated abnormal neuronal discharges, called seizures, which can be of focal or generalised onset. The disease definition also encompasses the neurological, cognitive and psychosocial sequelae of epilepsy, according to guidelines of the International League Against Epilepsy (ILAE), as shown in Fisher 2014. To confirm the diagnosis, two or more unprovoked seizures must occur 24 hours apart; or one seizure occurs with very high risk of recurrence (e.g. after stroke); or the individual must have a diagnosis of epilepsy syndrome (Fisher 2014). Treatment should be initiated after diagnosis only, because merely one third of people with a single provoked seizure have further ones (Hauser 1998), and individuals with single provoked seizures will most likely recover without any medical intervention.
Description of the intervention
Antiepileptic drugs (AED) are the main interventions used to prevent seizures and control epilepsy. Although effective in 50% to 70% of cases when used as a single or a combination therapy (Kwan 2001; Britton 2002), AEDs are related to long‐term adverse effects, such as cognitive and behavioural alterations (Eddy 2011); and withdrawal of AEDs might be advantageous for individuals with long‐term remission of seizures if the benefits of withdrawal surpass the harm caused by AED use. Furthermore some AEDs, particularly those most recently licensed, are expensive and might be a continuous financial burden to individual participants as well as public health systems around the world if taken needlessly (WHO 2012). Therefore when epilepsy is in remission, discontinuation of antiepileptic medication may be favorable to quality of life. However, removal of the drug in an inappropriate manner can lead to relapse of seizures, even in individuals with prolonged seizure freedom, therefore the relative risks and benefits of drug withdrawal should be carefully considered (Lossius 2008). Unfortunately, there are still no conclusive studies that determine when this discontinuation can safely occur. In 1994, the American Academy of Neurology published a guideline for suspending AEDs in seizure‐free participants, recommending discontinuation if the individual meets all of the following criteria: free from seizures following two to five years of treatment, has a single type of epilepsy, a normal neurological examination and a normalized EEG (AAN 1996). Similarly, randomised prospective studies in the UK, demonstrated enhanced favorable results from waiting for two or more seizure‐free years before initiating withdrawal (MRC 1991; MRC 1993). Still, the time limit of two years was chosen arbitrarily, since most studies regarding AED withdrawal have confounding variables (specific types of epilepsy, age of study participants, normal or abnormal EEG etc.), representing a sample which is not representative of the general population, and can be contradictory.
How the intervention might work
Comparing participants who withdrew with the ones still in treatment, an increased risk of seizure relapse in the initial six months was reported; however, if no seizure occurred in this initial six‐month period then after a year the seizure risk for the two groups was almost equal (Lossius 2008). Moreover, other studies show that this risk drops significantly at 3 months after withdrawal and even more as time progresses, drawing attention to driving restrictions specifically in this period (Bonnett 2011). This pattern of risk differences is confirmed by the MRC 1991 and MRC 1993 studies.
Also, there are numerous advantages to be gained from the interruption of AED. Not having to take pills regularly and a perception of health can improve one’s self‐esteem, particularly with regard to a disease such as epilepsy which is known to cause social stigma (Siqueira 2011). Also, some cognitive and behavioural long‐term effects inherent to AED treatment might only be demonstrated after years of pharmacotherapy and withdrawal may be greatly beneficial to motor coordination, rapid cognition, concentration and memory (Lossius 2008). Furthermore, some AEDs can interfere defectively with coexisting medical conditions and drugs, such as oral contraceptives, antibiotics, antipsychotics, immunosuppressants, antineoplastics and other antiepileptic drugs (Johannessen 2010).
Why it is important to do this review
In an attempt to define the optimal time of AED discontinuation, this review intended to analyse and synthesise randomised controlled trials (RCTs) in which participants, children or adults, had been allocated to groups undergoing early or late withdrawal of AEDs. For the purpose of this review, early withdrawal of AED is determined as suspension of medication after less than two continuous seizure‐free years while in treatment, whereas late withdrawal is considered as two or more seizure‐free years before discontinuation.
This systematic review’s purpose was firstly to summarize evidence from RCTs that compare early and late withdrawal of AEDs regarding risk relapse, status epilepticus occurrence, and mortality. Secondly, we assessed variables such as age at seizure onset, seizure types, EEG findings at different time points, intelligence quotient, and neurological examination. Finally, we attempted to narratively define a subpopulation of adults with epilepsy who may safely have AEDs withdrawn after remission of less than two years.
This review is an update of the previously published review in theCochrane Database of Systematic Reviews (Issue 3, 2001) titled 'Early versus late antiepileptic drug withdrawal for people with epilepsy in remission'.
Objectives
(1) To quantify and compare risk of seizure recurrence, status epilepticus and mortality after early AED discontinuation (withdrawal following period of remission of seizures of less than two years) and late AED discontinuation (withdrawal following period of remission of seizures of two or more years) in adults and children.
(2) To assess which variables and characteristics modify the risk of seizure recurrence.
(3) To narratively define a subpopulation in which early AED discontinuation is safe.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials that evaluate withdrawal of AEDs after varying periods of seizure remission in adults and children with epilepsy were eligible for inclusion. If the studies were in abstract form or published without any clear description of the methodology employed, we contacted the original trialists for clarification.
Studies were excluded if they were not randomised trials or if they involved highly specific participant samples, such as neonates, because the objective of this review is to guide clinicians with regard to epilepsies found routinely, and neonatal seizures have quite different characteristics than the ones seen in children and adults.
Types of participants
Participants must have had a diagnosis of epilepsy and have been seizure free for a described period of time (remission). Epilepsy onset may have occurred at any age and study participants could be any age, with the exception of neonates. Epilepsy and seizure types could be of any kind.
Types of interventions
Studies involving withdrawal of any antiepileptic drug and randomising people to differing durations of AED treatment while seizure‐free were included. Included studies compared an early versus late antiepileptic drug discontinuation, following two distinctly different periods of remission of seizures. Exact definitions of 'early' or 'late' withdrawal varied amongst the individual studies: for the purpose of this review, we defined early withdrawal of AED as the suspension of medication after less than two continuous seizure‐free years while in treatment, whereas late withdrawal is considered as two or more seizure‐free years before discontinuation. This definition was chosen because two years is the time limit already used in clinical practice (AAN 1996).
Types of outcome measures
Primary outcomes
The primary outcome is the risk ratio of seizure relapse as a consequence of early AED discontinuation in contrast to late withdrawal.
Secondary outcomes
The risk ratio of seizure relapse was according to seizure type (partial versus generalised) and EEG results before discontinuation of the AED (normal versus abnormal EEG)
We also considered other risk factors for seizure relapse as reported in the individual studies
Mortality risk following early or late AED discontinuation
Occurrence of status epilepticus following early or late AED discontinuation
Search methods for identification of studies
Electronic searches
Searches for the latest update were run in June 2014 through the following databases:
Cochrane Epilepsy Group Specialized Register (CRS), on 23/06/2014, using the search strategy set out in Appendix 1;
The Cochrane Central Register of Controlled Trials (CENTRAL Issue 5, The Cochrane Library, May 2014), on 23/06/2014, using the search strategy set out in Appendix 2.
MEDLINE (Ovid) from 1946 to 19/06/2014, on 19/06/2014, using the search strategy set out in Appendix 3.
CINAHL Plus (EBSCOhost) from 1937 to 23/06/2014, on 23/06/2014, using the search strategy set out in Appendix 4.
Scopus from 1823 to 23/06/2014, on 23/06/2014, using the search strategy set out in Appendix 5.
ClinicalTrials.gov, on 23/06/2014, using the search terms "epilepsy and remission and withdrawal", received on or after 04/01/2013.
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), on 23/06/2014, using the search terms "epilepsy and remission and withdrawal".
Searching other resources
The references lists in the retrieved trials were reviewed to search for any supplementary information that could strengthen our study. Authors were contacted to identify any additional published or unpublished information.
Data collection and analysis
Selection of studies
For the original review, two authors independently screened titles and abstracts of the previously‐mentioned search results, from which they selected randomised trials that met the inclusion criteria. In this update, two review authors (IS and SJN) independently assessed trials identified in the updated search for inclusion (screening titles, abstracts and full text), and any disagreements were resolved by dialogue.
Data extraction and management
Both authors (IS and SJN) independently extracted information from the studies onto a prearranged data collection form. The final results were reached by consensus amongst the authors. Data were checked and entered into a database by one author. Data were extracted according to the following:
Methodological/trial design
Method of randomisation.
Country of study.
Certainty of the diagnosis of epilepsy.
Duration of trial.
Follow‐up period.
Inclusion and exclusion criteria of individual trials.
Definition of early and late withdrawal and units of time (e.g. withdrawal following 12 months of seizure remission).
Discontinuation regimen.
Number of participants randomised to early and late withdrawal arms.
Participant/demographic information
Gender/ethnicity.
Age at seizure onset.
Age at randomisation.
Seizure types.
Antiepileptic drugs used.
Epilepsy duration.
Duration of AED treatment.
EEG results before discontinuation of treatment.
Variables and outcomes
Definition of outcome (e.g. seizure relapse, remission of seizures, occurrence of status epilepticus etc.)
Time points outcomes were measured and reported.
Number of participants from early and late withdrawal groups experiencing each outcome (seizure relapse, mortality and status epilepticus).
Number of participants experiencing each outcome according to type of seizure and results of EEG before discontinuation.
Number of participants experiencing each outcome according to other variables or characteristics (as reported).
If we could not extract the number of participants experiencing each outcome by treatment group or according to the variables of interest, we extracted any narrative information reported (statistical or otherwise) relating to the outcome.
Assessment of risk of bias in included studies
Both review authors (IS and SJN) evaluated the risk of bias for each trial independently. This assessment was made using the domain‐based evaluation tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The included studies were classified as having a low, high or unclear risk of bias in each one of the six domains investigated: randomisation, allocation concealment, blinding methods, incomplete outcome data, reporting bias, and other types of bias. Any disagreements were resolved through discussion and the results were incorporated in two 'Summary of findings' tables (Table 1; 'Table 2), using the GRADE tool.
Measures of treatment effect
The primary and secondary outcomes were presented in the form of risk ratios in this review, where possible. Where data could not be extracted for analysis in this review, we reported results of individual studies narratively.
Unit of analysis issues
No issues regarding unit of analysis were present; all studies were of a parallel randomised design and analysed participants individually.
Dealing with missing data
For any missing data, we sought responses from the authors, but no replies were received. We did not attempt to complete or impute any missing data.
Assessment of heterogeneity
Heterogeneity was assessed by comparing the distribution of data from trials by demographic and clinical factors, such as age at randomisation or at onset, seizure type, duration of epilepsy and EEG results . We assessed statistical heterogeneity using the I² statistic, with a value of greater than 75% indicating significant heterogeneity; and Chi² tests for heterogeneity with P < 0.1 indicating significant heterogeneity. Heterogeneous samples were reanalysed using a random‐effects model, and demographic and clinical factors as described above were explored. Samples with no significant heterogeneity found were analysed through fixed‐effect models.
Assessment of reporting biases
We initially assessed selective reporting of all included studies according to the Cochrane Risk of Bias tool for randomised trials (see Assessment of risk of bias in included studies); if we suspected studies to be at risk of reporting bias we used the Outcome Reporting Bias In Trials (ORBIT) study classification system (Kirkham 2010). Ideally we would have liked to assess selective reporting biases by comparing reported outcomes in individual studies to those specified in the study protocols; however no replies were received following contact with individual study authors to obtain protocols.
Data synthesis
We employed a fixed‐effect model meta‐analysis to find the pooled risk ratio for each outcome, using the Mantel‐Haenszel statistical method, since no significant heterogeneity was found. As seizure relapse, mortality and status epilepticus are dichotomous outcomes, we expressed the results as risk ratio (RR) with 95% confidence interval (CI). We expected to carry out comparisons between the aforementioned outcomes and variables presented in the included trials as possible prognostic factors. For trials reporting time‐to‐event (survival‐type) data, we were unable to extract the required information, as either graphs were not published or insufficient evidence was reported. As study authors did not respond to requests to provide these data for analysis, then where we could not extract data we have reported results narratively.
Sensitivity analysis
We intended to perform sensitivity analyses, if necessary, on the basis of poor methodological quality (high risk of bias for at least one domain); uncertainties or inconsistencies regarding characteristics of participants; interventions and outcomes; and to test for heterogeneity of the results, if present.
Results
Description of studies
Results of the search
Twelve full text studies were considered for inclusion in the original review. Seven met the inclusion criteria (three of them referring to the same trial), and the remaining five were excluded; see Included studies and Excluded studies for further information.
Our updated search for the updated version of this review revealed 14 articles identified from the Electronic databases listed previously (see Electronic searches section). Four duplicates were removed, leaving 10 records to be screened. From these, two coincided with studies already included from the previous review. The remaining eight abstracts were assessed for eligibility, but were found irrelevant for our review. See Figure 1 for clarification.
1.
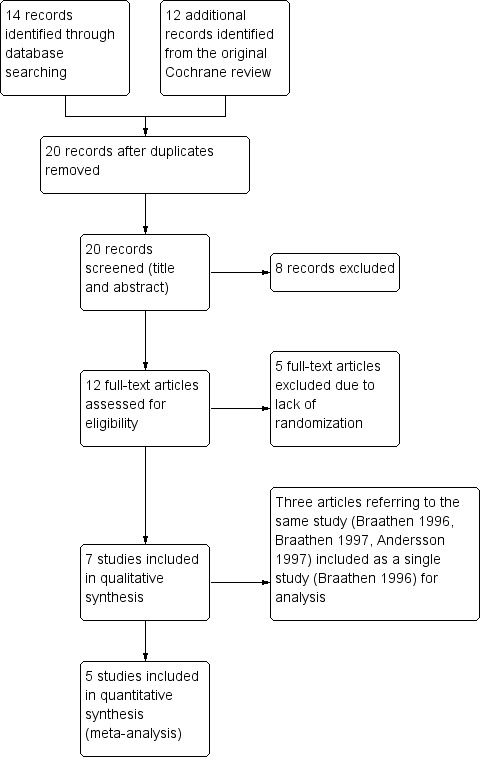
1 Study flow diagram.
Included studies
Overall, we included five trials in this analysis, representing 924 randomised children with epilepsy, all under 16 years of age at randomisation (Braathen 1996; Gebremariam 1999; Peters 1998; Todt 1984; Verrotti 2000). One trial was actually published as three different papers, which focus on various aspects of the same trial (Andersson 1997; Braathen 1996; Braathen 1997). In this case, we only considered the first two studies as one database; no additional data were presented in Braathen 1997. Data regarding EEG recordings were extracted from the Andersson 1997 article, and the rest were extracted from both papers (Andersson 1997; Braathen 1996).
Participants were only included after at least two unprovoked seizures or having previously been diagnosed as epileptic. Also, Braathen 1996, Peters 1998 and Todt 1984 included children with one episode of status epilepticus or one seizure of more than 30 minutes. However, Todt 1984 analysed these separately and their information was not included in our review together with the other participants. Children with febrile seizures, major neurological illness, neonatal seizures, infantile spasms and other specific types of epilepsy were excluded in every trial. In Verrotti 2000, only children with non‐rolandic partial epilepsy were studied. All five trials classified the participants' seizure type according to definitions of the International League Against Epilepsy (Berg 2010).
Four trials randomised individuals after a diagnosis of seizures or epilepsy had been made, treatment with AEDs had been initiated and a seizure‐free period had elapsed prior to discontinuation of drug (Gebremariam 1999; Peters 1998; Todt 1984; Verrotti 2000). Braathen 1996, however, randomised individuals after a diagnosis but prior to treatment with AEDs. Although not reported in Gebremariam 1999 and Todt 1984, participants used mainly carbamazepine, valproic acid and phenobarbital as treatment for epilepsy, except for absence types, which were medicated with ethosuximide (Braathen 1996).
In all trials, withdrawal was tested in early and late schemes. Even though definition of both groups was different in all of them, they could all be divided into before and after two seizure‐free years, with the exception of Braathen 1996, in which withdrawal was defined as 'early' or 'late' taking into consideration time of treatment with antiepileptic drugs, and not specifically seizure‐free years; and Peters 1998, where early withdrawal was considered as before six seizure‐free months and late after 1 year. Although this criterion varies slightly from ours, this trial was included after a sensitivity analysis and because the early‐withdrawal group had less than 2 years without seizures. Tapering of AEDs was conducted gradually over a period ranging from one month to up to one year.
Further summary details for all of the trials are presented in the Characteristics of included studies table.
Excluded studies
Five clinical trials (Dooley 1996; Hellström‐Westas 1995; MRC 1991; Ricci 1999; Shinnar 1994) were found ineligible because, even though they addressed antiepileptic drug withdrawal and seizure relapse, they did not randomise participants according to a seizure‐free period. Hellström‐Westas 1995 included only neonates and was therefore excluded. In MRC 1991, all participants had to be more than two years without seizures, so it could not compare the groups we were looking for. More details on each excluded study can be found in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
Each included study was classified as having a low, high or unclear risk of bias in each one of the six domains described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Refer to Figure 2 for a summary of the risk of bias of included studies and Characteristics of included studies for further details.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies stated that they were randomised. However, three trials were unclear about how randomisation was performed (Braathen 1996; Todt 1984; Verrotti 2000), while Gebremariam 1999 and Peters 1998 specified they carried it out through coin tossing and randomly permuting blocks, respectively. As to allocation concealment, the only study that stated clearly how this was achieved was Gebremariam 1999, in which a nurse who was unrelated to the study was asked to toss the coin for each participant.
Blinding
By design, we believe it is not possible or logical to blind the interrupting of AED treatment at distinctly different time points; therefore we rated the blinding of participants and personnel as 'low risk of bias'. Given that studies of this design cannot be easily or adequately blinded, we believe that the lack of blinding in these studies could not have influenced the outcome; furthermore it has been shown that the lack of blinding in studies with different interventions is not a source of bias (Wood 2008).
Incomplete outcome data
Braathen 1996 and Peters 1998 used the 'intent‐to‐treat' principle and were therefore considered as low risk of bias for this domain. Verrotti 2000 was also of low risk, but because it was made very clear that no children left the study after randomisation. Gebremariam 1999 and Todt 1984 were rated as unclear risk because they did not mention the number of participants that were excluded from analyses or dropped out of the study, nor any reason for this exclusion or drop‐out.
Selective reporting
We rated all included studies as low risk of bias for this domain as all expected outcomes were reported in each publication, whether results were statistically significant or not.
Other potential sources of bias
The trials were considered free from other types of bias and rated as low risk of bias in this domain.
Effects of interventions
In all trials, seizure activities were reported by the participants’ parents and by regular follow‐up visits made by the trialists. For our review, we used the data extracted from the last reported time point, since all of them had followed up with the participants for up to around 5 to 6 years on average. We tried to extract information regarding mortality and status epilepticus as outcomes, but none of the studies showed valid results and only one death was mentioned, in Braathen 1996.
With regard to variables which impact seizure relapse only two factors could be studied: seizure type; and EEG. All other variables were not sufficiently reported to be included with statistical significance. However, some risk factors were addressed in the review and are discussed further (see below). We contacted trialists for the additional data but none have been received at this time.
Seizure relapse
Comparison between early (fewer than two seizure‐free years) and late (more than two seizure‐free years) withdrawal regarding seizure relapse were available in all included studies, representing 924 randomised epileptic children. The pooled risk ratio for this outcome was 1.34 (95% CI 1.13 to 1.59, P = 0.0007) and four of the trials showed similar effects, with the exception of Gebremariam 1999, which demonstrated a risk ratio of 0.82 (95% CI 0.43 to 1.54) when discontinuing AEDs at 18 seizure‐free months. The Chi² test for seizure relapse showed no evidence of significant statistical heterogeneity between the trials (Chi² = 7.73, df = 4, P = 0.10, I² = 48%). The pooled risk difference was 0.12 (95% CI 0.05 to 0.19) at end of follow‐up. On the basis of the overall risk ratio, the number needed to harm is 8 (95% CI 5 to 20). In other words, for every eight individuals that are withdrawn later off AEDs, one seizure relapse is prevented compared to early withdrawal. See Analysis 1.1 and Figure 3.
1.1. Analysis.

Comparison 1 Early versus late withdrawal, Outcome 1 Seizure relapse rate by time of withdrawal.
3.

Forest plot of comparison: 1 Early versus late withdrawal, outcome: 1.1 Seizure relapse rate by time of withdrawal.
Given the difference in definition of early and late withdrawal in Peters 1998 compared to the other studies (Peters 1998 considered 'early' as less than one seizure‐free year and 'late' as equal to, or more than, one seizure‐free years), we performed sensitivity analysis excluding the participants in Peters 1998 from meta‐analysis to investigate any potential influence of the different definition. The pooled RR for 763 children in the remaining four studies was 1.45 (95% CI 1.18 to 1.78, P = 0.0005), showing a similar pooled result and no change to the conclusions of the analysis. Therefore, following this sensitivity analysis, we conclude that our results are robust to the variation of early and late withdrawal definition in Peters 1998.
Seizure type
Partial
Only two trials reported the number of children with partial epilepsy in each group and how many of those had a seizure relapse. From Braathen 1996 and Verrotti 2000, 180 children with partial seizures were randomised in early and late withdrawal groups. There was no statistical heterogeneity between these studies (Chi² = 1.07, df = 1, P = 0.30, I² = 7%), and both showed similar effect. The pooled risk ratio was 1.51 (95% CI 0.97 to 2.35, P = 0.07), favouring late discontinuation of AEDs in children with partial epilepsies.
Braathen 1996 found that benign partial epilepsy with rolandic spikes had the lowest relapse rate in both early and late withdrawal groups, followed by simple partial epilepsies. In comparison with both these types, complex partial seizure epilepsy showed a risk ratio of 4.70 (95% CI 1.34 to 21.65).
Peters 1998 reported a risk ratio of 1.39 for partial epilepsies, regardless of time of withdrawal, but did not publish the number of participants nor send us the values when contacted. In Gebremariam 1999 and Todt 1984, numbers were also unpublished, but the authors commented in narrative that they noticed a higher rate of recurrence in children with partial seizures. See Analysis 1.2 and Figure 4.
1.2. Analysis.

Comparison 1 Early versus late withdrawal, Outcome 2 Seizure relapse rate in people with partial epilepsy by time of withdrawal.
4.

Forest plot of comparison: 1 Early versus late withdrawal, outcome: 1.2 Seizure relapse rate in people with partial epilepsy by time of withdrawal.
Generalised
Braathen 1996 was the only trial to include the number of participants with generalised seizures in each randomised group. While 50% of children with early discontinuation of AEDs relapsed, only 37% of the ones with late withdrawal had a seizure recurrence, representing a risk ratio of 1.35 (95% CI 0.73 to 2.50, P = 0.34). However, this was not considered statistically significant.
Absence
Again, only Braathen 1996 reported numbers on absence epilepsy participants. These were very few and the results were statistically insignificant, even though showing a tendency towards higher relapse rate in early discontinuation (RR 1.51 95% CI 0.48 to 4.77, P = 0.478). Peters 1998, however, showed a risk ratio of 0.31 (95% CI 0.14 to 0.68, P < 0.01) for absence epilepsy comparing to other types, independently from time of discontinuation. Unfortunately, this paper did not contain the absolute numbers and the authors did not provide unpublished information.
Electroencephalogram (EEG)
Two trials (Braathen 1996; Todt 1984) reported EEG data before withdrawal and its relationship to seizure relapse after AED discontinuation, encompassing 618 participants (see Analysis 2.1 and Figure 5). Both showed a similar effect favouring a normal EEG before discontinuing antiepileptic treatment and the overall risk ratio when EEG was abnormal was 1.44 (95% CI 1.13 to 1.83, P = 0.003). No statistical heterogeneity was found (Chi² = 0.00, df = 1, P = 0.96, I² = 0%). Todt 1984 showed that paroxysmal or epileptiform activity in EEG before discontinuation is a more specific prognostic factor than simply abnormal EEG (including non‐paroxysmal activity). In this particular study, paroxysmal activity showed a risk ratio of 2.58 (95% CI 2.03 to 3.28, P < 0.0001).
2.1. Analysis.

Comparison 2 Abnormal versus normal EEG, Outcome 1 Seizure relapse rate by EEG findings.
5.

Forest plot of comparison: 2 Abnormal versus normal EEG, outcome: 2.1 Seizure relapse rate by EEG findings.
Only Braathen 1996 (shown in Andersson 1997) correlated EEG findings with early and late withdrawal, and found that there is a higher risk ratio for early withdrawal in children with normal EEG findings before AED discontinuation (RR 2.04 95% CI 1.12 to 3.71, P = 0.0187) than children with abnormal EEG (RR 1.17 95% CI 0.63 to 2.14, P = 0.620).
Gebremariam 1999 measured EEG before treatment with AED, and before and after AED discontinuation, reporting only risk ratios, of which abnormal EEG during tapering is the most specific (RR 6.21 95% CI 5.62 to 68.50). Also, Peters 1998 showed a risk ratio of 2.02 (95% CI 1.22 to 3.34, P < 0.01) for abnormal EEG registered before discontinuation. Unfortunately, no absolute numbers were available for either study.
Other variables
Age at epilepsy onset was addressed in four of the included trials. In Braathen 1996 and Todt 1984, there was a higher risk ratio of relapse in individuals who began having seizures in puberty. Braathen 1996, who included only children with epilepsy onset above two years old, found a risk ratio of 2.24 (95% CI 1.32 to 3.74) for epilepsies after 10 years of age, and Todt 1984 showed a risk ratio of 2.36 (95% CI 1.09 to 5.10, P < 0.05) for children of 12 years of age or more. Furthermore, Gebremariam 1999 found a higher risk of recurrence when seizures started before 2 years of age (RR 1.94, 95% CI 0.98 to 6.11). Verrotti 2000 showed a slightly lower mean age of onset in children who relapsed than those that remained seizure free.
Children with intellectual disability, usually defined as having an IQ lower than 70, showed a tendency to relapse more than other infants, as reported in Braathen 1996, Gebremariam 1999 and Todt 1984. In all of the trials, the mean risk ratio was between 1 and 2, but the confidence interval extended below one.
In infants with history of status epilepticus, Braathen 1996 and Gebremariam 1999 found a tendency towards higher risk of relapse, but they had too few individuals with such history to make a precise conclusion. Todt 1984, with a larger number of participants, found a risk ratio of 2.613 (95% CI 2.00 ‐ 3.40) for seizure relapse.
Todt 1984 reported a higher seizure recurrence in children that had more than five seizures per year (62%) than those with a lower seizure rate (18%), resulting in a risk ratio of 3.42 (95% CI 2.32 to 5.03, P < 0.0001). Braathen 1996, however, stated a risk ratio of 0.96 (95% CI 0.29 to 2.38) for participants with more than four seizures each month.
Gender was found to have no significant effect regarding seizure relapse in all trials. Similarly, family history influence had a risk ratio of 0.96 (95% CI 0.93 to 1.67) and 1.64 (95% CI 0.51 to 5.26), reported by Braathen 1996 and Gebremariam 1999, respectively.
Adults, status epilepticus and mortality
There were no randomised controlled trials that studied early versus late withdrawal in adults or older adults. No trials examined the outcomes of status epilepticus or mortality between early and late withdrawal of AEDs.
Discussion
Summary of main results
We found that all trials, except Gebremariam 1999, supported waiting more than two years to withdraw AEDs in seizure‐free children, with an overall pooled risk ratio of 1.34 (95% CI 1.13 to 1.59). This preference in stalling discontinuation is accentuated in partial‐seizure epilepsies, where the pooled risk ratio increases to 1.51 (95% CI 0.97 to 2.35, p=0.07). However, Braathen 1996 suggested that when partial seizures are simple or benign with rolandic spikes, the risk of relapse is much lower than the complex kind. Overall, partial epilepsies have a higher seizure recurrence than generalised types, regardless of discontinuation scheme. Differences between early and late withdrawal were not clear when analysing generalised epilepsies, but tended towards a lower risk in late suspension of AEDs. Absence seizures probably have lower risk of relapse overall, but also follow the pattern of higher recurrence in early withdrawal.
Abnormal EEG findings, especially paroxysmal activity, indicate a higher seizure recurrence, but this may also be linked to the type of epilepsy and thus classification of seizures is the main prognostic factor, instead of simply EEG findings. Interestingly, children with normal EEG were more influenced by the seizure‐free period before AED discontinuation, having a higher risk ratio in early discontinuation than infants with abnormal EEG registrations.
Children of less than 2 or above 10 years of age at epilepsy onset have a higher risk of seizure relapse after discontinuation. Again, however, this might be associated with certain types of epilepsies that are more common in specific age groups. Other variables which may be related with seizure relapse risk are intellectual disability (IQ < 70), a history of status epilepticus, and a higher seizure frequency before or during treatment. Gender and family history of epilepsy were not shown to be associated with recurrence of seizures.
Overall completeness and applicability of evidence
Our primary outcome was difference in seizure relapse rates between early and late withdrawal of AEDs in people with epilepsy in remission, and the included studies have appropriately provided data for a conclusion regarding seizure relapse rates following early or late withdrawal of AEDs in children. However, aside investigation of the influence of EEG results and seizure types on the seizure recurrence outcome, our secondary outcomes were not addressed and we were left with no answers regarding risk of mortality or status epilepticus occurrence after withdrawal. Also, no eligible trial included adults, consequently there are currently no data to support earlier withdrawal of AEDs in adults or in elderly people.
Quality of the evidence
We included five trials (described in seven articles), which assembled a total of 924 children that could be compared regarding to seizure‐free period before AED withdrawal (early versus late). All studies were randomised but not blinded:in these studies, blinding was probably unfeasible or even unethical, since withdrawal of medication is difficult to simulate, especially in a relatively long follow‐up period and regarding a condition as serious as epilepsy. Regarding EEG analysis, 618 children were studied from two of the included trials, but Braathen 1996 and Todt 1984 included in their EEG report a different number of children than the ones used to calculate a risk ratio for early versus late withdrawal, causing a certain confusion.
As shown in Table 1, comparison between types of epilepsy was considered as indirect because one of the studies (Verrotti 2000) did not include children with generalised seizures and therefore could not compare results adequately with partial seizure epilepsy participants. This comparison, as well as the one between early and late withdrawal, was considered as imprecise because the confidence interval from one of the studies causes an appreciable risk ratio reduction (above 25%).
Braathen 1996, differently from the other trials, did not divide groups into different seizure‐free periods, but in different treatment schedules (one or three years) with the last six months being seizure‐free. One trial (Peters 1998) randomised participants to either six seizure‐free months or one seizure‐free year, both groups withdrawing AEDs in less than two seizure‐free years. However, we elected to include both trials because they did randomise individuals to early and late AED discontinuation. See 'Table 2'.
Potential biases in the review process
The main factor that could serve as a source of bias is the manner in which the authors of the included studies chose to report their results: only in risk ratio form instead of absolute numbers or survival curves that we could use to perform a meta‐analysis, leaving us with less information than we would ideally have had.
Agreements and disagreements with other studies or reviews
Previous trials and meta‐analyses, not randomised between early and late withdrawal of AEDs, have suggested that at least two years of seizure freedom are needed before attempting withdrawal of AEDs (AAN 1996; Berg 1994; Shinnar 1985; Su 2013). Also, other studies have concluded that the long‐term relapse rate after withdrawal is not much different from participants who continued their treatment, and the contrast is much accentuated in the first years of comparison (Aktekin 2006; MRC 1991). The purpose of this review was to quantify the relapse risk associated with an even earlier AED discontinuation (less than two years). All of the included trials pertained only to children, however, revealing a complete absence of trials which have studied earlier withdrawal of AEDs in adults.
Children who were seizure‐free for more than two years before discontinuation had a 34.3% relapse rate, comparable to incidences found in previous studies (Callaghan 1988; Gherpelli 1992; Shinnar 1994), and this rate was reported also in Sillanpää 2006, which had a follow‐up period of 37 years. Li 2014, who included adults, showed a lower relapse rate; however they explain that their population had a smaller proportion of partial seizures and most participants were seizure free for more than three years.
Partial epilepsies were found in our review to have a higher risk of relapse after withdrawal, even more so when this is too early. Overall, other studies confirm that partial seizure epilepsy is a risk factor for seizure relapse (Bouma 1987; Callaghan 1988; Dooley 1996), pointing out that complex partial seizures have a worse prognosis than benign rolandic and simple partial seizures (Dooley 1996; Ramos‐Lizana 2010), just as we inferred. However, Gherpelli 1992, Ramos‐Lizana 2010, MRC 1991, Shinnar 1985 and Sillanpää 2006 concluded that generalised tonic‐clonic seizures were a greater risk factor. Remote symptomatic or organic epilepsy had higher recurrence risk in the studies by Berg 1994, Matricardi 1989, Ramos‐Lizana 2010, Sillanpää 2006 and Shinnar 1994.
Our included studies showed that abnormal EEG findings before withdrawal (and during treatment) damaged the prognosis, especially if paroxysmal activity was registered. One included study found also a higher risk if EEG during tapering was irregular. Most of the literature seen corroborated these affirmations, stating that EEG is a good prognostic tool prior to discontinuation in neonates, children or adults (Berg 1994; Gherpelli 1992; Hellström‐Westas 1995; Matricardi 1989; Overweg 1995; Shinnar 1994; Tennison 1994), specifying that 'slowing' (Shinnar 1985; Shinnar 1994) or 'spikes' (Tennison 1994) are risk factors. Su 2013, however, found that abnormal EEG during and after tapering AEDs was more specific than before withdrawal attempt. It is interesting to point out that EEG findings are also used for epilepsy type classification, and the type of epilepsy may be true predictor of prognosis following AED withdrawal.
From the five trials we included, we inferred that both below 2 year old and over 10 year old children had a higher risk for seizure recurrence. This matches with the conclusions of Berg 1994, Dooley 1996 and Shinnar 1994, but, while Bouma 1987 contradicted such relation, Gherpelli 1992 and Li 2014 found no significant association between age at onset and relapse. We can also speculate that age at onset is associated with epilepsy types that have different kinds of prognosis.
Authors' conclusions
Implications for practice.
There is evidence that discontinuing medications prior to at least two seizure‐free years is associated with a higher recurrence risk than waiting for two or more seizure‐free years in children, particularly if individuals have an abnormal EEG and partial seizures. However, the optimal time of withdrawal is not clear because two years was chosen arbitrarily. There is insufficient evidence to establish when to withdraw AEDs in children with generalised seizures. Decisions to continue or discontinue medications prior to two years in individual children need to be based on the child's underlying epilepsy syndrome. There is no evidence to guide the timing of withdrawal of AEDs in (less than two years) seizure‐free adults.
Implications for research.
Further randomised controlled trials are needed to identify individuals who can be safely withdrawn from AEDs earlier than two seizure‐free years. Future trials should include adults, older adults and all clinically relevant outcomes such as mortality, status epilepticus as well as seizure endpoints at various seizure‐free intervals. New studies should address variables which have been shown to be related to seizure relapse such as epilepsy types, type of AED, age at seizure onset and seizure frequency. Future research should aim to identify what specific characteristics make possible a safe early withdrawal of AEDs in individuals of all ages.
What's new
| Date | Event | Description |
|---|---|---|
| 29 April 2015 | Amended | Minor edits have been made to the Plain Language Summary in order to improve clarity. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 23 June 2014 | New citation required but conclusions have not changed | No new studies identified. |
| 23 June 2014 | New search has been performed | Searches updated 23 June 2014. |
| 10 October 2011 | New search has been performed | Searches updated 10 October 2011; no new studies identified. |
| 20 May 2007 | New search has been performed | Searches updated 20 May 2007; no new studies identified. |
Acknowledgements
We would like to acknowledge the authors from the original version of this review: Joseph I. Sirven, M.D., Michael R. Sperling, M.D., and Dean M. Wingerchuk, M.D. We would also like to thank the Trials Search Coordinator, Graham Chan, who carried out the electronic searches for our review.
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Cochrane Epilepsy Group Specialised Register search strategy
#1 MeSH DESCRIPTOR Anticonvulsants Explode All WITH AD AE AG AN AI BL CF CS CH CL CT DU EC HI IM IP ME PK PD PO RE ST SD TU TO UR
#2 anticonvulsant* or antiepilep*
#3 discontinu* or withdraw* or taper*
#4 (#1 OR #2) AND #3
#5 relapse or recurrence or remission or prognosis
#6 (#4 AND #5) AND >22/04/2013:CRSCREATED
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Epilepsy] explode all trees
#2 MeSH descriptor: [Seizures] explode all trees
#3 epilep* or seizure* or convulsion*
#4 (relapse or recurrence or remission or prognosis):ti,ab,kw
#5 (#1 or #2 or #3) and #4
#6 MeSH descriptor: [Anticonvulsants] explode all trees
#7 (anticonvulsant* or antiepilep*)
#8 (discontinu* or withdraw* or taper*):ti,ab,kw
#9 (#6 or #7) and #8
#10 (#5 and #9) Publication Year from 2012, in Trials
Appendix 3. MEDLINE search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomised trials published in Lefebvre 2009.
1. (randomized controlled trial or controlled clinical trial).pt. or (randomized or placebo or randomly).ab.
2. clinical trials as topic.sh.
3. trial.ti.
4. 1 or 2 or 3
5. exp animals/ not humans.sh.
6. 4 not 5
7. exp Epilepsy/
8. exp Seizures/
9. (epilep$ or seizure$ or convuls$).tw.
10. 7 or 8 or 9
11. (relapse or recurrence or remission or prognosis).ti,ab.
12. 10 and 11
13. exp Anticonvulsants/
14. (anticonvulsant$ or antiepilep$).tw.
15. 13 or 14
16. (discontinu$ or withdraw$ or taper$).ti,ab.
17. 15 and 16
18. 6 and 12 and 17
19. limit 18 to ed=20130423‐20140619
Appendix 4. CINAHL search strategy
S12 S3 AND S6 AND S10
Limiters ‐ Date from: 20130101‐20140631
S11 S3 AND S6 AND S10
S10 S7 OR S8 OR S9
S9 TI (randomised OR randomized OR randomly OR placebo) OR AB (randomised OR randomized OR randomly OR placebo)
S8 TI clin* N1 trial* or AB clin* N1 trial*
S7 MM ("Clinical Trials" OR "Random Assignment")
S6 S4 AND S5
S5 TI (discontinu* OR withdraw* OR taper*) OR AB (discontinu* OR withdraw* OR taper*)
S4 MM anticonvulsants OR TI antiepilep* OR AB antiepilep*
S3 S1 AND S2
S2 TI (relapse OR recurrence OR remission OR prognosis) OR AB (relapse OR recurrence OR remission OR prognosis)
S1 MM ("Epilepsy" OR "Seizures") OR TI (epilep* OR seizure* OR convulsi*) OR AB (epilep* OR seizure* OR convulsi*)
Appendix 5. Scopus search strategy
(((((TITLE((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") PRE/2 (trial OR method OR procedure OR study)) OR ABS((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") PRE/2 (trial OR method OR procedure OR study))) AND NOT (INDEX(medl))) AND (TITLE(relapse OR recurrence OR remission OR prognosis) OR ABS(relapse OR recurrence OR remission OR prognosis)) AND (((((TITLE‐ABS‐KEY(epilep* OR "infantile spasm" OR "ring chromosome 20" OR "R20" OR "myoclonic encephalopathy" OR "pyridoxine dependency")) OR (TITLE‐ABS‐KEY(syndrome W/2 (aicardi OR angelman OR doose OR dravet OR janz OR jeavons OR "landau kleffner" OR "lennox gastaut" OR ohtahara OR panayiotopoulos OR rasmussen OR rett OR "sturge weber" OR tassinari OR "unverricht lundborg" OR west)))) OR (TITLE(seizure OR convuls*))) OR ((TITLE‐ABS‐KEY(lafora* W/4 (disease OR epilep*))) AND NOT (TITLE(dog OR canine) OR INDEXTERMS(dog OR canine)))) AND NOT (TITLE(*eclampsia) OR INDEXTERMS(*eclampsia)) AND NOT INDEX(medl))) AND (TITLE‐ABS‐KEY((antiepilep* or anticonvulsant* or AED* or Acetazolamide or Alodorm or Arem or Ativan or Barbexaclone or Beclamide or Brivaracetam or Carbagen or Carbamazepine or Celontin or Cerebyx or Chloracon or Clobazam or Clonazepam or Clonex or Clorazepate or Convulex or Depacon or Depak* or Depamide or Desitin or Diacomit or Diamox or Diastat or Diazepam or Dilantin or Diphenin* or Diphenylhydantoin or Divalpr* or Dormicum or Ecovia or Emeside or Epanutin or Epiject or Epilim or Episenta or Epival or Eptoin or Ergenyl or Erimin or Eslicarbazepine or Ethadione or Ethosuximide or Ethotoin or Ethylphenacemide or Exalief or Excegran or Ezogabine or Fanatrex or Felbamate or Felbatol or Fosphenytoin or Frisium or Fycompa or Gabapentin or Gabarone or Gabitril or Gabrene or Gralise or Hibicon or Hypnovel or Inovelon or Insoma or Intensl or Keppra or Klonopin or Kriadex or Lacosamide or Lamict* or Lamitor or Lamitrin or Lamogine or Lamotrigine or Lamotrine or Levetiracetam or Liskantin or Loraz or Lorazepam or Luminal or Lyrica or Mebaral or Mephenytoin or Mephobarbit* or Mephyltaletten or Mesantoin or Mesuximide or Methazolamide or Methsuximide or Methylphenobarbit* or Midazolam or Mogadon or Mylepsinum or Mysoline or Neogab or Neptazane or Neurontin or Nimetazepam or Nitrados or Nitrazadon or Nitrazepam or Normison or Novo‐Clopate or Nupentin or Nydrane or Onfi or Orfiril or Orlept or Ormodon or Ospolot or Oxcarbazepine or Pacisyn or Paraldehyde or Paramethadione or Paxadorm or Paxam or Peganone or Perampanel or Petinutin or Petril or Phemiton or Phenacemide or Pheneturide or Phenobarbit* or Phensuximide or Phenytek or Phenytoin or Posedrine or Potiga or Pregabalin or Primidone or Prodilantin or Progabide or Prominal or Prysoline or Ravotril or Remacemide or Remnos or Resimatil or Restoril or Retigabine or Riv?tril or Rufinamide or Sabril or Seclar or Selenica or Seletracetam or Sertan or Somnite or Stavzor or Stedesa or Stiripentol or Sulthiam* or Sultiam* or Talampanel or Tegretol or Temazepam or Temesta or Teril or Tiagabine or Timonil or Topamax or Topiramate or Tranxene or Tridione or Trileptal or Trimethadione or Trobalt or Urbanol or Valance or Valcote or Valium or Valnoctamide or Valparin or Valpro* or Versed or Vigabatrin or Vimpat or Zalkote or Zarontin or Zebinix or Zonegran or Zonisamide) W/3 (withdraw* OR discontinu* OR taper*)))) AND NOT (TITLE(febrile OR fever* OR alcohol* or neurocysticercos*) OR ABS(febrile OR fever* OR alcohol* or neurocysticercos*))) AND (PUBYEAR > 2011)
Data and analyses
Comparison 1. Early versus late withdrawal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure relapse rate by time of withdrawal | 5 | 924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.13, 1.59] |
| 2 Seizure relapse rate in people with partial epilepsy by time of withdrawal | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.97, 2.35] |
Comparison 2. Abnormal versus normal EEG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure relapse rate by EEG findings | 2 | 618 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.13, 1.83] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Braathen 1996.
| Methods | Randomised non‐blinded controlled trial Withdrawal: 3 months Follow‐up period: mean 6.8 years for group I and 4.8 years for group II; from 2.1 to 10.7 years, overall. Study duration: January 1984 to September 1995 |
|
| Participants | Sweden 161 children with at least 2 unprovoked seizures or 1 seizure lasting more than 30 minutes, still without treatment at randomisation, and that became seizure‐free for the last 6 months of treatment, were included. Exclusion criteria: cerebral palsy, moderate to severe intellectual disability, specific epileptic syndromes with poor prognosis (infantile spasms and Lennox‐Gastaut syndrome), and previous treatment with AEDs. 85 male and 76 female participants Group I: 77 children withdrew AED after a mean 1.1 year (0.8 to 1.4 year) of treatment. Group II: 84 children withdrew AED after mean 3.2 years (2.4 to 3.7 years) of treatment. Mean age: 8.8 years (2.0 to 16.6 years) Mean age at epilepsy onset: 8.1 years (1.2 to 15.6 years) Seizure types: absence, partial (benign rolandic, partial, complex and secondarily generalised), tonic‐clonic, sylvian and grand mal at awakening. Some were unclassified. AEDs used: carbamazepine, valproate, phenytoin, ethosuximide and phenobarbital. |
|
| Interventions | Withdrawal of seizure drug after 1 versus 3 years of treatment. All drugs were tapered gradually during a 3 month period after 6 seizure‐free months. Mean duration: ‐ Group I: 2.2 months (0 to 4.8 months) ‐ Group II: 2.4 (0 to 6.6 months) |
|
| Outcomes | (1) Seizure relapse in each group. (2) Seizure/epilepsy type as risk factor. (3) EEG findings as risk factor. |
|
| Notes | (1) From the original 244 enrolled participants, 37 children (15%) were excluded after randomisation, but before treatment. (2) From the 207 left, 46 children were excluded after treatment, but before discontinuation. (3) Study represents 65% of a population of childhood epilepsy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Children were randomised to 1 (group I) or three (group II) years of treatment". Comment: there is no explanation as to how participants were randomised. |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is no explanation as to how participants were randomised. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In accordance with the intent‐to‐treat principle, all children who initiated the discontinuation phase after the allocated period of treatment were included in the analyses". Comment: a considerable number of children (30 from group I and 16 from group II) were excluded from the analysis after randomisation. |
| Selective reporting (reporting bias) | Low risk | Comment: trial protocol was unavailable. However, prespecified outcomes (seizure relapse and other prognostic factors) were reported. |
| Other bias | Low risk | Comment: the study appears to be free of other significant sources of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: since the outcome is objective, the risk of bias is minor (Wood 2008). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Parents were asked to note any seizure events in a diary". Comment: Since the outcome is objective, the risk of bias is minor (Wood 2008). |
Gebremariam 1999.
| Methods | Randomised controlled non‐blinded trial Withdrawal: 8 weeks. Follow‐up period: 38.3 months (16.5 to 51 months) for group A; 23.9 months (9.5 to 40.5 months) for group B. Study duration: April 1988 to September 1992 |
|
| Participants | Ethiopia 80 children already receiving care at a pediatric neurology clinic who were seizure free for 18 months. Exclusion criteria: children with only a single seizure, febrile seizure, neonatal seizure, infantile spasms or seizures of metabolic nature. 55 male and 25 female participants. Group A: 41 children who withdrew AEDs after 18 seizure‐free months, with a mean age of 6.67 years at the beginning of anticonvulsant treatment. Group B: 39 children who withdrew AEDs after 24 seizure‐free months, with a mean age of 5.84 years at the beginning of anticonvulsant treatment. |
|
| Interventions | Withdrawal of seizure drug after an 18 seizure‐free months versus waiting to withdraw drug until after 24 seizure‐free months. Each drug was tapered by 1/4 dose every 2 weeks until completely stopped, totaling 8 weeks. Medications tapered sequentially if more than 1 seizure drug. |
|
| Outcomes | (1) Seizure relapse in each group (2) Possible relation between seizure relapse and some variables such as: age at onset; multiple medications; neurological deficit; family history of epilepsy; EEG; and intellectual disability. |
|
| Notes | (1) This study was undertaken in a tertiary hospital. (2) About 31% of participants had intellectual disability, and 35% had behavioural problems. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The eligible children were randomly and sequentially assigned by a toss of a coin". |
| Allocation concealment (selection bias) | Low risk | "A nurse [...] who was unaware of the study hypothesis carried out the randomisation process". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no participants were reported to be excluded from the analysis until the last follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: trial protocol was unavailable. However, prespecified outcomes (seizure relapse and prognostic factors) were reported. |
| Other bias | Unclear risk | Comment: the reported information was insufficient to present a clear understanding of the outcome (e.g. was shown as risk ratios only). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: since the outcome is objective, the risk of bias is minor (Wood 2008). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: parents were guided by verbal and written instructions, and were checked up on every 2 weeks. Since the outcome is objective, the risk of bias is minor (Wood 2008). |
Peters 1998.
| Methods | Randomised non‐blinded controlled trial Withdrawal: 4 weeks. Follow‐up period: 41.9 months (6.0 to 60.9 months) Follow‐up visits: every 3 months. Study duration: January 1989 to August 1994 |
|
| Participants | The Netherlands 161 children who had 2 or more unprovoked seizures or at least 1 episode of status epilepticus, and became seizure free within 2 months of AED treatment were included. Exclusion criteria: neonatal, febrile and acute symptomatic seizures, and progressive neurologic disorder, such as tuberous sclerosis, brain tumor, juvenile myoclonic epilepsy, infantile spasms and photosensitivity. 77 male and 84 female participants. Group A: 78 children that discontinued AED use after 6 seizure‐free months. Group B: 83 children that discontinued AED use after 12 seizure‐free months. Median age at epilepsy onset: 6.4 years (1 month to 16 years). Seizure types: partial, generalised tonic‐clonic, absence and other. AEDs used: valproic acid and carbamazepine. |
|
| Interventions | Withdrawal of seizure drug after 6 months of seizure freedom versus 12 months of seizure freedom. Withdrawal of drug was conducted over a period of 4 weeks in both groups. |
|
| Outcomes | (1) Seizure relapse in each group. (2) Possible relation between seizure relapse and some variables such as: age at onset; seizure type; epilepsy type; brain imaging; etiology; and EEG findings before AED discontinuation. |
|
| Notes | (1) 312 out of 494 children with epilepsy were excluded from the study because they had not become seizure free after 2 months. (2) Two children with juvenile myoclonic epilepsy (one in each group) and one with tuberous sclerosis (group A) were diagnosed as so after randomisation, and were included in the results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Balance between treatment assignments within hospitals was achieved by using randomly permuted blocks of varying size". |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is no explanation as to how participants were randomised. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome regarding seizure relapse was shown through Kaplan‐Meier curves. Children later found to have JME or tuberous sclerosis were included anyway. Quote: "'intention‐to‐treat' principle" |
| Selective reporting (reporting bias) | Low risk | Comment: trial protocol was unavailable. However, prespecified outcomes (seizure relapse and prognostic factors) were reported. |
| Other bias | Low risk | Comment: the study appears to be free of other significant sources of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: since the outcome is objective, the risk of bias is minor (Wood 2008). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: seizures were recorded by the examiners of the study during quarterly follow‐up visits (3 to 6 months). Since the outcome is objective, the risk of bias is minor (Wood 2008). |
Todt 1984.
| Methods | Randomised controlled non‐blinded trial Withdrawal: also randomised, independently from withdrawal group, between 1, 3, 6 or 12 months. Follow‐up period: from 3 to 6 years after end of therapy. Study duration: between 1971 and 1979. |
|
| Participants | German Democratic Republic 433 children with at least 2 seizures within 6 months, or 3 seizures within a year. Exclusion criteria: children with febrile seizures, and progressive cerebral illness. 215 male and 258 female participants from the original 473 children. Children were allocated into 4 groups with different numbers of seizure‐free years: ‐ 1 year: 59 children ‐ 2 years: 137 children ‐ 3 years: 104 children ‐ 4 years: 133 children Note: For our review, we considered 1 year as 'early', and 2, 3 and 4 years as 'late' withdrawal. Age range: 3 to 16 years. Seizure types: absences (simple and complex), partial (simple, complex and secondarily generalised), generalised tonic‐clonic, unilateral, bilateral massive myoclonus, Lennox‐Gastaut syndrome, West syndrome and unclassified seizures. |
|
| Interventions | Withdrawal of seizure drug after 1, 2, 3, or 4 seizure‐free years. Drugs were tapered in 3 stages by 1/3 dose over a period determined through randomisation (1, 3, 6 or 12 months). If more than 1 drug was used, then all drugs were stopped at the same time. | |
| Outcomes | (1) Seizure relapse in each group (2) Possible relation between seizure relapse and some variables such as: gender, family history, epilepsy/seizure type, duration of epilepsy, age at onset, IQ, duration of taper, and EEG data. |
|
| Notes | (1) Only children with absence seizures were discontinued after 1 year of seizure freedom ('early' group). (2) 40 participants with only 1 seizure before AED treatment were studied separately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: there is no explanation as to how participants were randomised. |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is no explanation as to how participants were randomised. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information given if participants were lost. |
| Selective reporting (reporting bias) | Low risk | Comment: trial protocol was unavailable. However, prespecified outcomes (seizure relapse and prognostic factors) were reported. |
| Other bias | Unclear risk | Comment: most data seemed to have been measured but not reported. For example, stated as 'non significant', without including the information itself. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: since the outcome is objective, the risk of bias is minor (Wood 2008). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no information given as to how outcome was assessed. Since the outcome is objective, the risk of bias is minor (Wood 2008). |
Verrotti 2000.
| Methods | Randomised controlled non‐blinded trial. Withdrawal: 6 to 12.1 months. Follow‐up period: 5.1 to 6.7 years after AED discontinuation. |
|
| Participants | Italy 89 children and adolescents with partial epilepsy with normal EEG at the time of withdrawal. Exclusion criteria: cerebral palsy, perinatal asphyxiation, abnormal CT scan, MRI or EEG, rolandic epilepsy, neonatal seizures, CNS infections, seizures from metabolic disturbances, abnormal neuropsychiatric examination, febrile seizures, and status epilepticus. 40 male and 49 female participants. Group A: 45 children that discontinued AEDs after 1.8 seizure‐free year (1.2 to 2.4 years). Group B: 44 children that discontinued AEDs after 2.7 seizure‐free years (2 to 3.4 years). Mean age: 11.2 years (7.8 to 14.6 years) Age at epilepsy onset: mean 6.1 years (1.8 to 10.4 years) Seizure types: only cryptogenic partial, simple or complex epilepsies, with or without secondary generalisation. AEDs used: carbamazepine, valproic acid and phenobarbital, all monotherapy. |
|
| Interventions | Withdrawal of seizure drug after 1 seizure‐free year versus 2 seizure‐free years. Withdrawal of drug was conducted gradually over 6 months to 1 year. ‐ Group A: 7.9 months (4.11 to 11.7 months) ‐ Group B: 8.1 months (4 to 12.2 months) Follow‐up visits were scheduled every 3 months during the first years, and at 6‐month intervals thereafter. |
|
| Outcomes | (1) Seizure relapse in each group (2) Possible relation between seizure relapse and some variables such as: gender, age at discontinuation or at onset, familial history of epilepsy, type of partial seizure and time to seizure control. |
|
| Notes | (1) Only children with partial epilepsy were included. (2) Children with abnormal EEG were excluded. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: there is no explanation as to how participants were randomised. |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is no explanation as to how participants were randomised. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no exclusions or dropouts were reported, and results presented all participants for seizure relapse. |
| Selective reporting (reporting bias) | Low risk | Comment: trial protocol was unavailable. However, prespecified outcomes (seizure relapse and prognostic factors) were reported. |
| Other bias | Low risk | Comment: the study appears to be free of other significant sources of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: since the outcome is objective, the risk of bias is minor (Wood 2008). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: it is unclear how seizure events were reported. Since the outcome is objective, the risk of bias is minor (Wood 2008). |
AED: antiepileptic drug; CNS: central nervous system; CT: computerized tomography; EEG: electroencephalogram; MRI: magnetic resonance imaging.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dooley 1996 | The study was not randomised and did not compare early and late withdrawal groups. Study analysed only risk of seizure in children who discontinued AED after one seizure‐free year. |
| Hellström‐Westas 1995 | The study was not randomised, did not compare early and late withdrawal groups, and only neonates were included. Study analysed only seizure recurrence and mortality within the first year after neonatal seizures treated with AEDs for different periods (1 to 65 days). |
| MRC 1991 | The study did not compare early and late withdrawal groups. To be included in this study, all participants had to be seizure free for at least 2 years. randomisation was made to compare treatment versus withdrawal after remission. |
| Ricci 1999 | The study was not randomised and did not compare early and late withdrawal groups. |
| Shinnar 1994 | The study was not randomised and did not compare early and late withdrawal groups. Study analysed children who were seizure‐free for at least 1 year and measured seizure relapse in all of them, without labelling groups. |
Differences between protocol and review
The review title was changed from 'When can antiepileptic drugs be withdrawn in patients with epilepsy?' in the protocol to 'Early versus late antiepileptic drug withdrawal for people with epilepsy in remission' in order to better fit the content of the paper, emphasizing the seizure‐free period in question.
Participants from the included studies were changed from 'seizure free for at least two years' to 'seizure free for a described period of time', so the review could compare participants seizure free for less or more than 2 years.
Studies were in fact analysed through a 'fixed‐effect model' instead of a 'random‐effects model' as described in the protocol, because the studies were consistent enough to be considered as homogeneous. Also, results were reported as risk ratio, instead of risk difference, for a better understanding.
Contributions of authors
Two authors (Isabella Strozzi and Sarah J. Nolan) independently abstracted data for the review, rated the quality of the trials and provided statistical support for the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Health Research (NIHR), UK.
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
The authors report no conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Braathen 1996 {published data only}
- Andersson T, Braathen G, Persson A, Theorell K. A comparison between one and three years of treatment in uncomplicated childhood epilepsy: a prospective study. II. The EEG as predictor of outcome after withdrawal of treatment. Epilepsia 1997;38(2):225‐32. [DOI] [PubMed] [Google Scholar]
- Braathen G, Andersson T, Gylje H, Melander H, Naglo A, Noren L, et al. Comparison between one and three years of treatment in uncomplicated childhood epilepsy: a prospective study. I. Outcome in different seizure types. Epilepsia 1996;37(9):822‐32. [DOI] [PubMed] [Google Scholar]
- Braathen G, Melander H. Early discontinuation of treatment in children with uncomplicated epilepsy: a prospective study with a model for prediction of outcome. Epilepsia 1997;38(5):561‐9. [DOI] [PubMed] [Google Scholar]
Gebremariam 1999 {published data only}
- Gebremariam A, Mengesha W, Enqusilassie F. Discontinuing anti‐epileptic medication(s) in epileptic children: 18 versus 24 months. Annals of Tropical Paediatrics 1999;19(1):93‐9. [DOI] [PubMed] [Google Scholar]
Peters 1998 {published data only}
- Peters ACB, Brouwer OF, Geerts AT, Arts WMF, Stroink H, Donselaar CA. Randomized prospective study of early discontinuation of antiepileptic drugs in children with epilepsy. Neurology 1998;50(3):724‐30. [DOI] [PubMed] [Google Scholar]
Todt 1984 {published data only}
- Todt H. The late prognosis of epilepsy in childhood: results of a prospective follow‐up study. Epilepsia 1984;25(2):137‐44. [DOI] [PubMed] [Google Scholar]
Verrotti 2000 {published data only}
- Verrotti A, Morresi S, Basciani F, Cutarella R, Morgese G, Chiarelli F. Discontinuation of anticonvulsant therapy in children with partial epilepsy. Neurology 2000;55(9):1393‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dooley 1996 {published data only}
- Dooley J, Gordon K, Camfield P, Camfield C, Smith E. Discontinuation of anticonvulsant therapy in children free of seizures for 1 year: a prospective study. Neurology 1996;46(4):969‐74. [DOI] [PubMed] [Google Scholar]
Hellström‐Westas 1995 {published data only}
- Hellström‐Westas L, Blennow G, Lindroth M, Rosén I, Svenningsen NW. Low risk of seizure recurrence after early withdrawal of antiepileptic treatment in the neonatal period. Archives of Disease in Childhood Fetal Neonatal Edition 1995;72 Suppl(2):F97‐F101. [DOI] [PMC free article] [PubMed] [Google Scholar]
MRC 1991 {published data only}
- Medical Research Council Antiepileptic Drug Withdrawal Study Group. Randomised study of antiepileptic drug withdrawal in patients in remission. Lancet 1991;337(8751):1175‐80. [PubMed] [Google Scholar]
Ricci 1999 {published data only}
- Ricci S, Picciolo M, Mari F, Egco G, Manfredi M. Drug withdrawal in adults: retrospective analysis of 125 patients [Sospensione della terapia antiepilettica nell’adulto:analisi retrospettiva di una casistica di 125 pazienti]. Bolletino Lega Italiana Contro l’Epilessia 1999;106/107:337‐41. [Google Scholar]
Shinnar 1994 {published data only}
- Shinnar S, Berg AT, Moshé SL, Kang H, O'Dell C, Alemany M, et al. Discontinuing antiepileptic drugs in children with epilepsy: a prospective study. Annals of Neurology 1994;35(5):534‐45. [DOI] [PubMed] [Google Scholar]
Additional references
AAN 1996
- American Academy of Neurology quality standards subcommittee. Practice parameter: a guideline for discontinuing antiepileptic drugs in seizure‐free patients. Neurology 1996;47:600‐2. [DOI] [PubMed] [Google Scholar]
Aktekin 2006
- Aktekin B, Dogan EA, Oguz Y, Senol Y. Withdrawal of antiepileptic drugs in adult patients free of seizures for 4 years: A prospective study. Epilepsy & Behavior 2006;8(3):616‐9. [DOI: 10.1016/j.yebeh.2006.01.018] [DOI] [PubMed] [Google Scholar]
Andersson 1997
- Andersson T, Braathen G, Persson A, Theorell K. A comparison between one and three years of treatment in uncomplicated childhood epilepsy: a prospective study II. The EEG as predictor of outcome after withdrawal of treatment. Epilepsia 1997;38(2):225‐32. [DOI] [PubMed] [Google Scholar]
Berg 1994
- Berg A, Shinnar A. Relapse following discontinuation of antiepileptic drugs: a meta‐analysis. Neurology 1994;44(4):601‐8. [DOI] [PubMed] [Google Scholar]
Berg 2010
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross H, Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE commission on classification and terminology, 2005 ‐ 2009. Epilepsia 2010;51(4):676‐85. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bonnett 2011
- Bonnett LJ, Shukralla A, Tudur‐Smith C, Williamson PR, Marson AG. Seizure recurrence after antiepileptic drug withdrawal and the implications for driving: further results from the MRC Antiepileptic Drug Withdrawal Study and a systematic review. Journal of Neurology, Neurosurgery, and Psychiatry 2011;82(12):1328‐33. [DOI: 10.1136/jnnp.2010.222885] [DOI] [PubMed] [Google Scholar]
Bouma 1987
- Bouma PAD, Peters ACB, Arts RJHM, Stijnen TH, Rossum J. Discontinuation of antiepileptic therapy: a prospective study in children. Journal of Neurology, Neurosurgery, and Psychiatry 1987;50(12):1579‐83. [PUBMED: PMC1032597] [DOI] [PMC free article] [PubMed] [Google Scholar]
Braathen 1997
- Braathen G, Melander H. Early discontinuation of treatment in children with uncomplicated epilepsy: a prospective study with a model for prediction of outcome. Epilepsia 1997;38(5):561‐9. [DOI] [PubMed] [Google Scholar]
Britton 2002
- Britton JW. Antiepileptic drug withdrawal: literature review. Mayo Clinic Proceedings 2002;77(12):1378‐88. [PUBMED: 12479528] [DOI] [PubMed] [Google Scholar]
Callaghan 1988
- Callaghan N, Garrett A, Goggin T. Withdrawal of anticonvulsant drugs in patients free of seizures for two years. A prospective study. New England Journal of Medicine 1988;318(15):942‐6. [DOI] [PubMed] [Google Scholar]
Eddy 2011
- Eddy CM, Rickards HE, Cavanna AE. The cognitive impact of antiepileptic drugs. Therapeutic Advances in Neurological Disorders 2011;4(6):385‐407. [DOI: 10.1177/1756285611417920] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2014
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross H, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55(4):475‐82. [DOI: 10.1111/epi.12550] [DOI] [PubMed] [Google Scholar]
Gherpelli 1992
- Gherpelli JLD, Kok F, dal Forno S, Elkis LC, Lefevre BH, Diament AJ. Discontinuing medication in epileptic children: a study of risk factors related to recurrence. Epilepsia 1992;33(4):681‐6. [PUBMED: 1628584] [DOI] [PubMed] [Google Scholar]
Hauser 1998
- Hauser WA, Rich SS, Lee JRJ, Annegers JF, Anderson VE. Risk of recurrent seizures after two unprovoked seizures. New England Journal of Medicine 1998;338(7):429‐34. [DOI: 10.1056/NEJM19982123380704] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, March 2011. [www.cochrane‐handbook.org] [Google Scholar]
Johannessen 2010
- Johannessen SI, Landmark CJ. Antiepileptic drug interactions ‐ principles and clinical implications. Current Neuropharmacology 2010;8(3):254‐67. [DOI: 10.2174/157015910792246254; PUBMED: 21358975] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340(2):340‐65. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Kwan 2001
- Kwan P, Brodie MJ. Effectiveness of first antiepileptic drug. Epilepsia 2001;42(10):1255‐60. [DOI: 10.1046/j.1528-1157.2001.04501.x] [DOI] [PubMed] [Google Scholar]
Lefebvre 2009
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Li 2014
- Li W, Si Y, Zou X, An D, Yang H, Zhou D. Prospective study on the withdrawal and reinstitution of antiepileptic drugs among seizure‐free patients in west China. Journal of Clinical Neuroscience 2014;21(6):997‐1001. [DOI: 10.1016/j.jocn.2013.09.019] [DOI] [PubMed] [Google Scholar]
Lossius 2008
- Lossius MI, Hessen E, Mowinckel P, Stavem K, Erikssen J, Gulbrandsen P, et al. Consequences of antiepileptic drug withdrawal: a randomized, double‐blind study (Akershus Study). Epilepsia 2008;49(3):455‐63. [DOI: 10.1111/j.1528-1167.2007.01323.x] [DOI] [PubMed] [Google Scholar]
Matricardi 1989
- Matricardi M, Brinciotti M, Benedetti P. Outcome after discontinuation of antiepileptic drug therapy in children with epilepsy. Epilepsia 1989;30(5):582‐9. [DOI] [PubMed] [Google Scholar]
MRC 1993
- Medical Research Council Antiepileptic Drug Withdrawal Study Group. Prognostic index for recurrence of seizures after remission of epilepsy. BMJ 1993;306(6889):1374‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Overweg 1995
- Overweg J. Withdrawal of antiepileptic drugs (AEDs) in seizure‐free patients, risk factors for relapse with special attention for the EEG. Seizure 1995;4(1):19‐36. [PUBMED: 7788104] [DOI] [PubMed] [Google Scholar]
Ramos‐Lizana 2010
- Ramos‐Lizana J, Aguirre‐Rodríguez J, Aguilera‐López P, Cassinello‐García E. Recurrence risk after withdrawal of antiepileptic drugs in children with epilepsy: a prospective study. European Journal Of Paedriatic Neurology 2010;14(2):116‐24. [10.1016/j.ejpn.2009.05.006. Epub 2009 Jul 9.; PUBMED: 19541516] [DOI] [PubMed] [Google Scholar]
Shinnar 1985
- Shinnar S, Vining EP, Mellits ED, D'Souza BJ, Holden K, Baumgardner RA, et al. Discontinuing antiepileptic medication in children with epilepsy after two years without seizures. A prospective study. New England Journal of Medicine 1985;313(16):976‐80. [DOI] [PubMed] [Google Scholar]
Sillanpää 2006
- Sillanpää M, Schmidt D. Prognosis of seizure recurrence after stopping antiepileptic drugs in seizure‐free patients: A long‐term population‐based study of childhood‐onset epilepsy. Epilepsy & Behavior 2006;8(4):713‐9. [PUBMED: 16616648] [DOI] [PubMed] [Google Scholar]
Siqueira 2011
- Siqueira NF, Guerreiro MM, Souza EAP. Self‐esteem, social support perception and seizure controllability perception in adolescents with epilepsy. Arquivos de Neuropsiquiatria 2011;69(5):770‐4. [http://www.scielo.br/pdf/anp/v69n5/a08v69n5.pdf] [DOI] [PubMed] [Google Scholar]
Su 2013
- Su L, Di Q, Yu N, Zhang Y. Predictors for relapse after antiepileptic drug withdrawal in seizure‐free patients with epilepsy. Journal of Clinical Neuroscience 2013;20(6):790‐4. [10.1016/j.jocn.2012.07.010. Epub 2013 Apr 28.; PUBMED: 23632288] [DOI] [PubMed] [Google Scholar]
Tennison 1994
- Tennison M, Greenwood R, Lewis D, Thorn M. Discontinuing antiepileptic drugs in children with epilepsy: A comparison of a six‐week and a nine‐month taper period. New England Journal of Medicine 1994;330:1407‐10. [DOI: 10.1056/NEJM199405193302002] [DOI] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. Fact Sheet n°999. http://www.who.int/mediacentre/factsheets/fs999/en/# 2012.
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


