Abstract
Introduction
Decubitus ulcers of the sacral region are common conditions in bedridden patients. Deep lesions (Stages III and IV) often require surgical treatment for closure. Flaps of the region are the first choice for treatment. We present our experience in the treatment of these lesions and compare two different approaches: local fasciocutaneous flap and gluteus maximus myocutaneous flap with V-Y advancement.
Method
From March 2009 to May 2014, 32 patients underwent closure of sacral pressure ulcers by flaps, 17 of them with rotational local fasciocutaneous flaps and 15 with myocutaneous flaps of the gluteus maximus muscle with V-Y advancement. Evolution regarding complications and rate of success after two months was compared between the groups.
Results
Out of the 32 operated patients we obtained resolution of lesions after two months in 23 (71.8%), 10 patients in the fasciocutaneous flap group (58.8%) and 13 cases in the myocutaneous flap group (86.6%). The most common complication was partial dehiscence of sutures in 12 patients (37.5%), 8 patients in the fasciocutaneous flap group (47%) and 4 patients in the myocutaneous flap group (26.6%). The group of patients reconstructed with local fasciocutaneous flaps presented 3 cases with seroma, one with hematoma and 6 with partial cutaneous necrosis; these patients also required more drainage time.
Conclusions
Both the local rotational fasciocutaneous flap and the myocutaneous flap of the gluteus maximus muscle in V-Y flap can be used in the surgical treatment of sacral ulcers. In our experience, a reduced success rate and more complications were found in the local fasciocutaneous reconstructive method.
Keywords: Pressure ulcer, Fasciocutaneous flap, Myocutaneous flap, Gluteus maximus muscle
Introduction
Pressure ulcers commonly affect patients who are bedridden, suffering from acute or chronic diseases.1 Among patients who are subject to a long hospital stay, the prevalence rates of these lesions reach 27.7% in the United States,2 and 10.95% in a study conducted in the city of São Paulo.3 Recent data indicate a 63% increase in the number of hospital admissions related to decubitus ulcers during the last 10 years, and the main diagnosis at admission was sepsis.4
Pressure ulcers are caused by various pathogenic mechanisms. Factors such as direct pressure, tissue slippage, friction and local humidity are directly related to the onset of these lesions.5, 6, 7, 8 In addition, factors directly related to the patient are also influential, such as age, decreased mobility, loss of awareness, mental deficiency, fecal or urinary incontinence, infection, anemia, hypoproteinemia and diseases that impair microcirculation.6, 7 However, molecular mechanisms responsible for its emergence have not yet been fully understood.9
Recently, an update of the classification of decubitus ulcers by degree of depth and tissue involvement was published10:
-
•
Suspicion of deep tissue injury: localized purplish area of intact skin or bullous lesion filled with blood because of underlying soft tissue damage due to pressure or shear.
-
•
Stage I: intact skin with hyperemia in an area normally located over bone prominence.
-
•
Stage II: loss of partial thickness of the dermis presenting as a shallow ulcer with pink bed, or bullous lesion with exudate.
-
•
Stage III: total loss of tissue thickness, with visible subcutaneous but without muscular or tendinous exposure, with possible presence of detachment and tunneling.
-
•
Stage IV: total loss of tissue thickness with bone, tendon or muscle exposure.
-
•
Unclassifiable: Total tissue loss in which the base of the lesion is covered by necrosis or fibrin.
In patients with stage I and stage II lesions, treatment is aimed at removing the causal factor to avoid progression and local care to promote healing. In patients with stage III and IV lesions, the treatment is focused on preventing secondary complications such as infection, and on promoting the closure of the lesion.11 Treatment of lesions at these stages is often surgical, since conservative treatment is associated with a high failure rate and recurrence.12, 13, 14 The treatment consists of adequate surgical debridement of the lesion, including the affected bone tissue, and subsequent tissue transfer to fill the dead spaces and provide adequate skin cover.15, 16
Surgical debridement of the lesion removes the necrotic tissue, reducing the bacterial load at the site and preparing the wound bed for subsequent surgical closure.11, 17
Regarding surgical closure, in general it is advisable to wait until the lesion has no devitalized tissue, and without any signs of infection. The primary closure of the lesion presents high rates of recurrence,18 which leads most surgeons to avoid this approach. That is local flaps in the region are the first choice for reconstruction of sacral pressure ulcers, and several designs of previously described fasciocutaneous and myocutaneous flaps have been used.5, 19 The myocutaneous flap of the gluteus maximus muscle with advancement in V-Y, and the rotational fasciocutaneous flap have been those we elected for reconstruction of the site.
The purpose of this study was to compare the outcome in the reconstruction of the sacral region affected by decubitus ulcer using fasciocutaneous and myocutaneous of the gluteus maximus muscle flaps.
Material and method
From March 2009 to May 2014, 32 consecutive patients were submitted to surgical treatment for closure of pressure ulcers of the sacral region, of whom, 17 were male and 15 were female, with ages ranging from 14 to 89 years (mean of 66.4 years). The lesions had different sizes, the smallest observed diameter was 4.5 × 4.0 cm and the largest 15 × 13.5 cm. At the time of reconstruction, all patients presented without necrosis, with good granulation tissue and without clinical evidence of infection. Ten patients had intestinal diversion (colostomy) prior to surgical reconstruction.
The first 17 patients were submitted to local rotational fasciocutaneous flaps closure and the 15 subsequent patients were submitted to gluteus maximus myocutaneous flaps with V-Y advancement.
Lesions smaller than 10 cm were treated with unilateral flaps and lesions larger than 10 cm with bilateral flaps. The mean time of surgical procedures was 122 min.
The associated comorbidities included: cerebrovascular accident (10 cases), Alzheimer's disease (9 cases), spinal cord trauma (6 cases), sepsis (4 cases), idiopathic myelitis (1 case), cerebral vasculitis (1 case) and myelomeningocele (1 case). Table 1 shows patient characteristics according to the treatment group.
Table 1.
Patient characteristics according to the treatment group.
| Fasciocutaneous flap | Myocutaneous flap | |
|---|---|---|
| Vasculocerebral accident | 6 (35,3%) | 4 (26,7%) |
| Spinal cord trauma | 5 (29,4%) | 1 (6,7%) |
| Alzheimer's disease | 2 (11,8%) | 7 (46,7%) |
| Sepsis | 1 (5,88%) | 3 (20%) |
| Idiopathic myelitis | 1 (5,88%) | 0 |
| Cerebral vasculitis | 1 (5,88%) | 0 |
| Myelomeningocele | 1 (5,88%) | 0 |
| Total | 17 | 15 |
Follow-up ranged from 2 months to 2 years and 7 months postoperatively.
In order to have a clear endpoint to measure complete wound healing, we set a cut-off of 2 months after the surgery to evaluate this rate. Unfavorable events such as dehiscence, skin necrosis, seroma and hematomas were reported in each group.
Surgical procedure
Patients were positioned prone, under general anesthesia. First, the entire capsule was removed from the lesion, and rigorous hemostasis was carried out. The region was then evaluated for the presence of bone spicules, which were removed with an osteotome when necessary. In lesions smaller than 10 cm, we chose to make unilateral flaps. For lesions with a diameter greater than 10 cm, we opted for bilateral flaps to avoid excess tension in the sutures and the need for extensive detachments.
In the first 17 cases operated, local rotational fasciocutaneous flaps were performed (10 unilateral and 7 bilateral). The flap marking was performed by a line that started at the upper or lower extremity of the lesion, and extended in an arciform shape lateral-inferior (if it started on the upper extremity) or lateral-superior (if it started on the lower extremity), as shown in Figure 1.
Figure 1.
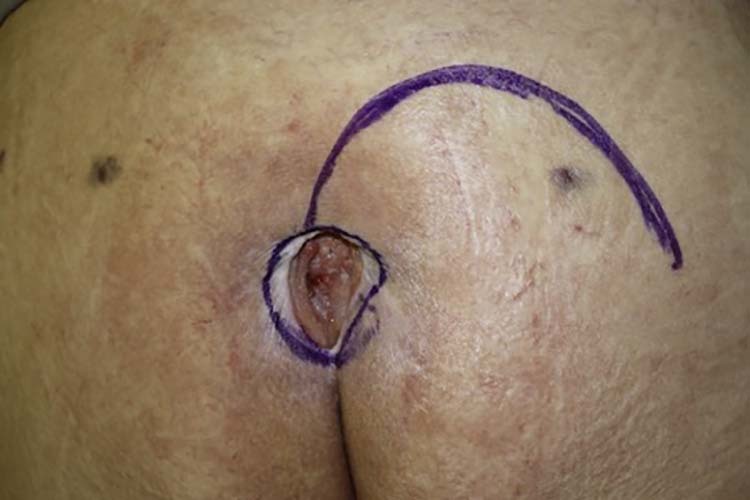
Fasciocutaneous flap markings.
The pre-marked line was then incised, down to the fascia of the gluteus maximus muscle. After rigorous hemostasis, the flap was rotated and inset with deep 2-0 Vicryl sutures. Vacuum drainage was then performed with a Blake® 15 Fr. drain, and closure was completed in layers (Figure 2). Drains were withdrawn when the flow rate was less than 30 ml in 24 consecutive hours and the sutures were removed in 3 weeks.
Figure 2.

(Incision and elevation of the fasciocutaneous flap).
The last 15 cases to be operated were closed with a V-Y gluteus maximus myocutaneous flap. Surgical procedures followed the same sequence as above until the surgical marking. At this point, flap marking was performed by means of lines that originated at the upper and lower extremities of the lesion and progressed in a latero-lower and latero-upper directions respectively, converging laterally to form a “V” (Figure 3).
Figure 3.

Bilateral miocutaneous flap markings.
The markings were then incised down to the fascia of the gluteus maximus muscle and the flap was advanced toward the defect, with closure of the donor area following a “Y” format (Figure 4).
Figure 4.

Result after bilateral miocutaneous flap advancement.
No deep sutures were required, but vacuum drainage as well as closure in layers followed the same procedure as the former.
Results
Of the patients who underwent surgery (32) completely healed wounds were obtained within 2 months in 23 cases (71.8%) and we obtained resolution of the sacral lesions in 23 cases (71,8 %), 13 cases in the myocutaneous group and 10 cases in the fasciocutaneous group. Nine patients remained unhealed after 60 days of the first treatment.
Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14 demonstrate some of the cases.
Figure 5.

(Pre-operative case 1).
Figure 6.

(Postoperative case 1).
Figure 7.

(intra-operative case 2).
Figure 8.

(postoperative case 2).
Figure 9.

(pre-operative case 3).
Figure 10.
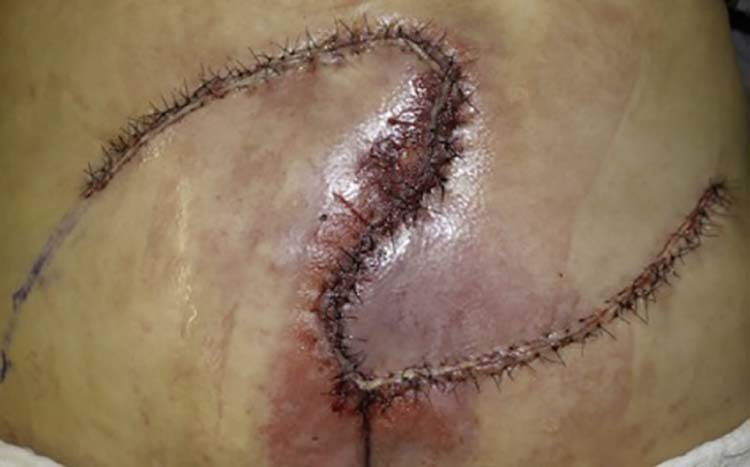
(postoperative case 3).
Figure 11.

(intra-operative case 4).
Figure 12.

(postoperative case 4).
Figure 13.

Partial necrosis of the fasciocutaneous bilaterally located flap.
Figure 14.

Postoperative with debridement of partial necrosis and resuture of the flap.
Six (6) patients had complications including partial necrosis of the flap (all of them in the fasciocutaneous flap group). Twelve (12) patients had partial dehiscence of suture line, three (3) had a seroma and one had a hematoma just after the surgery.
Partial necrosis of the flaps was approached by surgical debridement and resuturing when possible (Figure 13, Figure 14).
Cases with partial dehiscence were submitted to resuture of the margins, seromas were drained with complete resolution and the hematoma required immediate intervention.
Singling out patients according to the type of flap used (17 cases with local fasciocutaneous flaps and 15 cases of myocutaneous flaps of the gluteus maximus muscle in V-Y), we obtained resolution of the sacral pressure lesions in 10 cases (58.8%) with the local fasciocutaneous flap and 13 cases (86.6%) with the gluteus maximus flap in V-Y. The gluteus maximus muscle V-Y flap presented no cases of partial necrosis, and the local fasciocutaneous flap presented 6 cases with partial necrosis (35.29%). There were no cases of seroma or hematoma with the gluteus maximus flap in V-Y, and in 4 cases (26.6%), we had partial dehiscence near the intergluteal fold. The mean time of drain use was 12 days in the local fasciocutaneous flap and 5 days in the V-Y myocutaneous flap (Table 2).
Table 2.
Complications according to the treatment group.
| Fasciocutaneous flap | Myocutaneous flap in V-Y | |
|---|---|---|
| Partial necrosis | 6 (35.2%) | 0 |
| Dehiscence | 8 (47%) | 4 (26.6%) |
| Seroma | 3 (17.6%) | 0 |
| Hematoma | 1 (5.8%) | 0 |
| Mean drainage time | 12 days | 5 days |
| Rate of success | 10 (58.8%) | 13 (86.6%) |
Discussion
In the literature, the incidence of location of pressure ulcers varies. In some series, the most frequent location is the sacral region, reaching 82.4%.20 In others, the ischial region is the most affected.21 This difference probably reflects the variance in characteristics of the patients studied, since paraplegic and wheelchair patients have a higher prevalence of ischial lesions. On the other hand, bedridden patients commonly develop sacral pressure lesions.
Avoidance of the appearance of pressure ulcers is ideal, however once the injury has set in treatment must be undertaken. Patients who present with only local hyperemia or dermo-epidermal lesion (Stages I and II) can be treated conservatively, with local care and strict positional change.6, 13, 17, 22 However, deeper lesions (Stages III and IV), particularly necrosis, require surgical treatment.
Initially, surgical debridement should be performed to remove the devitalized tissues.11 Ulcers that present with necrosis or signs of infection are initially treated with surgical debridement, dressings and systemic antibiotics. Surgical closure is only performed when local conditions are favorable.
The advantages of performing surgical closure include improved local hygiene, lower risk of soft tissue and bone infection, decrease of water and protein loss, reduction of hospital costs, lesser need for dressings, and improved quality of the patient's life.
Flaps of the gluteal region are usually the flaps of choice when surgical closure is indicated,23 since the primary closure of the lesion presents a high rate of failure and recurrence.18 Therefore, flaps such as the myocutaneous V-Y of the gluteus maximus muscle23, 24 and local fasciocutaneous flaps have been widely used.19, 23, 24, 25, 26, 27, 28, 29, 30 The local rotational fasciocutaneous flap is an excellent option for smaller sacral lesions in patients with an adequate subcutaneous cushion. It presents several advantages, such as the preservation of the underlying muscle, reducing morbidity of the donor area, possibility of new rotation of the flap using the same incision with or without extension in case of lesion recurrence,31 and a better scar positioning avoiding the central area. Nevertheless, for larger lesions, unilateral flap preparation is often insufficient, requiring bilateral use and eventually small relaxation incisions in the flap to reduce tension in the central region, which possibly affects, to some extent its viability. As a disadvantage, these flaps require extensive detachment, with a greater chance of circulatory distress and seromas. Even with the use of deep sutures in our series with this type of flap, patients still had postoperative seromas (17%). The gluteus maximus muscle, with its vascularization coming from the upper and lower gluteal arteries, branches of the internal iliac artery, may be used in several presentations of myocutaneous flaps.23, 24, 26, 28, 29, 30, 32 The gluteus maximus myocutaneous flap is one of the most reliable and safe flaps used in the management of sacral ulcers.23, 24, 30 Originally described by Minami et al. 33 in 1977, and modified in its form of advancement to a V-Y flap by Parry and Mathes34 in 1982, it has been used with great success for the treatment of sacral decubitus ulcers, with the benefit of facilitating closure of the donor area and avoiding hip instability caused by disinsertion of the musculature.
In our series, the first 17 patients were reconstructed with local fasciocutaneous flaps and the 15 subsequent patients with the myocutaneous flap of the gluteus maximus in V-Y. Nowadays the myocutaneous flap is our first option because we believe it has fewer complications and more safety.
We chose to raise the flaps as reported by Park and Park19 and by Ohjimi et al.,26 who advocate not affecting muscle integrity. Preserving it as a unit allows for further mobilization if required, and by maintaining muscle function, avoids functional deficits. In our practice, this option proved to be beneficial, as shown by the higher number of successes and fewer complications. We observed earlier complications, such as seroma and hematoma in cases of reconstruction with a fasciocutaneous flap, probably due to the larger extension of the detachment area brought about by this technique. Furthermore, use of vacuum drain may be longer in the cases of local fasciocutaneous flap (12 days versus 5 days with V-Y myocutaneous flap). The rate of necrosis was also substantially higher in cases of fasciocutaneous flaps. We believe that this is related to vascularization by a random pedicle leading to less predictability; in addition, the poor tissue quality and general condition of patients, also inadequate nursing care with early positioning on the flap areas. There was no necrosis in any of the cases treated with a myocutaneous flap. However, there is no consensus in the literature on the benefit of using myocutaneous flaps in relation to fasciocutaneous flaps for closure of pressure ulcers in long-term follow-up. Thiessen and colleagues25 showed that the myocutaneous flap is not superior to the fasciocutaneous, and that the quality of the tissue used is more important than the quantity. This may be supported by the fact that originally all pressure points in the human body are only covered by fasciocutaneous tissue, and not by muscle,8 also the resistance of muscle tissue to pressure and ischemia is less than that of skin and fascia. Thus, we justify the option of each flap according to an individual evaluation of each case, and personal experience. Moreover, further studies are required to show the benefit of one option over the other. Several factors contribute to the appearance of pressure ulcers. Although pressure is the essential factor leading to tissue ischemia and necrosis, moisture caused by fecal and urinary incontinence reduces skin resistance and predisposes to maceration, increasing the risk of developing skin lesions.35 In patients with defined sacral ulcers, intestinal diversion through colostomy is an excellent option to facilitate local healing36 and allows adequate hygienic control, including that by the patient.36, 37 In addition, this avoids fecal contamination of the surgical incisions, which can be a determinant for surgical success. It is noteworthy that the success rate for patients with colostomy, regardless of the flap used, was 70%, and for patients without colostomy it was 50% (Table 3). Additionally, 63.63% of the patients who obtained success in treatment had previous colostomy. Besides the few number of cases and the various variables that exist, this data suggests the benefit of associating colostomy in the treatment of sacral lesions. In some cases the indication of this procedure can be very difficult due to the lack of family acceptance and even from the medical team. Despite the evident benefit to the patients when closure of the lesions is successful it must be noted that there is a high rate of relapses in the follow-up of these cases. The great difficulty in the management of these patients, associated with the severity of the underlying pathologies, leads to situations that allow new lesions. Improvement in the care of these patients associated with appropriate treatments may improve the relapse rate.
Table 3.
Relationship between colostomy and resolution.
| Resolution | No resolution | |
|---|---|---|
| Colostomy present | 7 (70%) | 3 (30%) |
| Colostomy absent | 11 (50%) | 11 (50%) |
Conclusion
Surgical treatment of sacral pressure ulcers is complex, requires multidisciplinary approach and demands special surgical care in order to achieve high success rate. Local rotational fasciocutaneous flaps or gluteus maximus myocutaneous V-Y flaps may be used. The myocutaneous flap of the gluteus maximus muscle in V-Y presents the advantages of predictable blood supply, tissue for cushioning on pressure points, reduction of dead space without great functional loss. In our series of cases, this method achieved more successful results and less risk of complications than the rotational fasciocutaneous local flap.
Conflicts of interest
None declared.
Funding
None.
Ethical approval
Not required.
References
- 1.Baumgarten M., Margolis D.J., Localio A.R. Pressure ulcers among elderly patients early in the hospital stay. J Gerontol A Biol Sci Med Sci. 2006;61:749–754. doi: 10.1093/gerona/61.7.749. [DOI] [PubMed] [Google Scholar]
- 2.Horn S.D., Bender S.A., Bergstrom N. Description of the national pressure ulcer long-term care study. J Am Geriatr Soc. 2002;50:1816–1825. doi: 10.1046/j.1532-5415.2002.50510.x. [DOI] [PubMed] [Google Scholar]
- 3.Chancon J.M., Blanes L., Hochman B., Ferreira L.M. Prevalence of pressure ulcers among the elderly living in long-stay institutions in São Paulo. Sao Paulo Med J. 2009;127:211–215. doi: 10.1590/S1516-31802009000400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russo A., Elixhauser A. Hospitalizations related to pressure sores. 2003. https://www.ncbi.nlm.nih.gov/books/NBK63508/ Healthcare Cost and Utilization Project. [PubMed]
- 5.Gawlitta D., Li W., Oomens C.W., Baaijens F.P., Bader D.L., Bouten C.V. The relative contributions of compression and hypoxia to development of muscle tissue damage: an in vitro study. Ann Biomed Eng. 2007;35:273–284. doi: 10.1007/s10439-006-9222-5. [DOI] [PubMed] [Google Scholar]
- 6.Enis J., Sarmiento A. The pathophysiology and management of pressure sores. Orthop Rev. 1973;2:26. [Google Scholar]
- 7.Maklebust J. Pressure ulcers: etiology and prevention. Nurs Clin North Am. 1987;22:359. [PubMed] [Google Scholar]
- 8.Daniel R.K., Faibisoff B. Muscle coverage of pressure points: the role of myocutaneous flaps. Ann Plast Surg. 1982;8:446–452. doi: 10.1097/00000637-198206000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji S., Ichioka S., Sekiya N., Nakatsuka T. Analysis of ischemia–reperfusion injury in a microcirculatory model of pressure ulcers. Wound Repair Regen. 2005;13:209–215. doi: 10.1111/j.1067-1927.2005.130213.x. [DOI] [PubMed] [Google Scholar]
- 10.NPUAP Pressure Injury Stages National Pressure Ulcer Advisory Panel 2016. Npuap.org
- 11.Schiffman J., Golinko M.S., Yan A., Flattau A., Tomic-Canic M., Brem H. Operative debridement of pressure ulcers. World J Surg. 2009;33:1396–1402. doi: 10.1007/s00268-009-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janis J.E., Kenkel J.M. Pressure sores. Sel Read Plast Surg. 2003;9:1–42. [Google Scholar]
- 13.Bauer J., Phillips L.G. MOC-PSSM CME article: pressure sores. Plast Reconstr Surg. 2008;121:1–10. doi: 10.1097/01.prs.0000294671.05159.27. [DOI] [PubMed] [Google Scholar]
- 14.Colen S.R. Pressure sores. In: McCarthy J.G., editor. Plastic Surgery. Saunders; Philadelphia: 1990. pp. 3797–3838. [Google Scholar]
- 15.Dansereau J.G., Conway H. Closure of decubiti in paraplegics. Report of 2000 cases. Plast Reconstr Surg. 1964;33:474–480. [PubMed] [Google Scholar]
- 16.Griffith B.H., Schultz R.C. The prevention and surgical treatment of recurrent decubitus ulcers in patients with paraplegia. Plast Reconstr Surg Transplant Bull. 1961;27:248–260. doi: 10.1097/00006534-196103000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Cannon B., O'Leary J.J., O'Neil J.W., Steinsieck R. An approach to the treatment of pressure sores. Ann Surg. 1950;132:760–778. doi: 10.1097/00000658-195010000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kierney P.C., Engrav L.H., Isik F.F. Results of 268 pressure sores in 158 patients managed jointly by plastic surgery and rehabilitation medicine. Plast Reconstr Surg. 1998;102:765–772. doi: 10.1097/00006534-199809030-00022. [DOI] [PubMed] [Google Scholar]
- 19.Park C., Park B.Y. Fasciocutaneous V-Y advancement flap for repair of sacral defects. Ann Plast Surg. 1988;21:23. doi: 10.1097/00000637-198807000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Soares D.A.S., Vendramin F.S., Pereira L.M.D., Proença P.K., Marques M.M. Analysis of the incidence of pressure ulcers at Hospital Metropolitano de Urgência e Emergência in Ananindeua, PA. Rev Bras Cir Plast. 2011;26:578–581. [Google Scholar]
- 21.Larson D.L., Hudak K.A., Waring W.P., Orr M.R., Simonelic K. Protocol management of late stage pressure ulcers: a 5-year retrospective study of 101 consecutive patients with 179 ulcers. Plast Reconstr Surg. 2012;129:897–904. doi: 10.1097/PRS.0b013e3182442197. [DOI] [PubMed] [Google Scholar]
- 22.Niezgoda J.A., Mendez-Eastman S. The effective management of pressure ulcers. Adv Skin Wound Care. 2006;19(suppl 1):3–15. doi: 10.1097/00129334-200601001-00001. [DOI] [PubMed] [Google Scholar]
- 23.Scheflan M., Nahai F., Bostwick J., III. Gluteus maximus island musculocutaneous flap for closure of sacral and ischial ulcers. Plast Reconstr Surg. 1981;68:533–538. doi: 10.1097/00006534-198110000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez O.M., Orlando J.C., Hurwitz D.J. The sliding gluteus maximus myocutaneous flap: its relevance in ambulatory patients. Plast Reconstr Surg. 1984;74:68–75. doi: 10.1097/00006534-198407000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Thiessen F.E., Andrades P., Blondeel P.N. Flap surgery for pressure sores: should the underlying muscle be transferred or not? J Plast Reconstr Aesthet Surg. 2011;64:84–90. doi: 10.1016/j.bjps.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 26.Ohjimi H., Ogata K., Setsu Y., Haraga I. Modification of the gluteus maximus V-Y advancement flap for sacral ulcers: the gluteal fasciocutaneous flap method. Plast Reconstr Surg. 1996;98:1247. doi: 10.1097/00006534-199612000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto Y., Tsutsumida A., Murazumi M., Sugihara T. Long-term outcome of pressure sores treated with flap coverage. Plast Reconstr Surg. 1997;100:1212. doi: 10.1097/00006534-199710000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Fisher J., Arnold P.G., Waldorf J., Woods J.E. The gluteus maximus musculocutaneous V-Y advancement flap for large sacral defects. Ann Plast Surg. 1983;11:517. doi: 10.1097/00000637-198312000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Rubayi S., Doyle B.S. The gluteus maximus muscle-splitting myocutaneous flap for treatment of sacral and coccygeal pressure ulcers. Plast Reconstr Surg. 1995;96:1366–1371. doi: 10.1097/00006534-199511000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Ichioka S., Okabe K., Tsuji S., Ohura N., Nakatsuka T. Distal perforator–based fasciocutaneous V-Y flap for treatment of sacral pressure ulcers. Plast Reconstr Surg. 2004;114:906–909. doi: 10.1097/01.prs.0000133167.81269.40. [DOI] [PubMed] [Google Scholar]
- 31.Wong C.H., Tan B.K., Song C. The perforator-sparing buttock rotation flap for coverage of pressure sores. Plast Reconstr Surg. 2007;119:1259–1266. doi: 10.1097/01.prs.0000254540.50381.63. [DOI] [PubMed] [Google Scholar]
- 32.Parkash S., Banerjee S. The total gluteus maximus rotation and other gluteus maximus musculocutaneous flaps in the treatment of pressure ulcers. Br J Plast Surg. 1986;39:66–71. doi: 10.1016/0007-1226(86)90006-8. [DOI] [PubMed] [Google Scholar]
- 33.Minami R.T., Mills R., Pardoe R. Gluteus maximus myocutaneous flaps for repair of pressure sores. Plast Reconstr Surg. 1977;60:242–249. doi: 10.1097/00006534-197708000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Parry S.W., Mathes S.J. Bilateral gluteus maximus myocutaneous advancement flaps: sacral coverage for ambulatory patients. Ann Plast Surg. 1982;8:443–445. doi: 10.1097/00000637-198206000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Kelly S.R., Shashidharan M., Borwell B., Tromans A.M., Finnis D., Grundy D.J. The role of intestinal stoma in patients with spinal cord injury. Spinal Cord. 1999;37:211–214. doi: 10.1038/sj.sc.3100764. [DOI] [PubMed] [Google Scholar]
- 36.Bejany D.E., Chao R., Perito P.E., Politano V.A. Continent urinary diversion and diverting colostomy in the therapy of non-healing pressure sores in paraplegic patients. Paraplegia. 1993;31:242–248. doi: 10.1038/sc.1993.43. [DOI] [PubMed] [Google Scholar]
- 37.Reuler J.B., Cooney T.G. The pressure sore: pathophysiology and principles of management. Ann Intern Med. 1981;94:661–666. doi: 10.7326/0003-4819-94-5-661. [DOI] [PubMed] [Google Scholar]


