Abstract
Food branding is ubiquitous, however, not all children are equally susceptible to its effects. The objectives of this study were to 1) determine whether food brands evoke differential response than non-food brands in brain areas related to motivation and inhibitory control using blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) and 2) determine the association between brain response and energy intake at test-meals presented with or without brands. Twenty-eight 7–10 year-old children completed four visits as part of a within-subjects design where they consumed three multi-item test-meals presented with familiar food brands, novel food brand, and no brand. On the fourth visit an fMRI was performed where children passively viewed food brands, non-food brands and control images. A whole-brain analysis was conducted to compare BOLD response between conditions. Pearson’s correlations were calculated to determine the association between brain response and meal intake. Relative to non-food brands, food brand images were associated with increased activity in the right lingual gyrus. Relative to control, food and non-food brand images were associated with greater response in bilateral fusiform gyri and decreased response in the cuneus, precuneus, lingual gyrus, and supramarginal gyrus. Less activation in the bilateral fusiform gyrus to both food and non-food brands was associated with greater energy intake of the branded vs unbranded meal. These findings may help explain differences in the susceptibility to the intake-promoting effects of food advertising in children.
Keywords: Branding, Food intake, Brain response, fMRI, Children
Background
In the United States, childhood obesity rates have continued to rise over the past decade and have reached an alarming 18.5% in 2016 (Hales et al. 2017). A major contributor to increasing rates of childhood obesity is an excess intake of high-fat/high-sugar foods, drinks, and snacks (Fox et al. 2004; Nicklas et al. 2003). These foods are aggressively marketed to youth with recent reports showing that food companies spend over $7.4 billion a year to increase purchase and consumption of cereals, snack foods, beverages, and fast foods (Harris et al. 2014; Harris et al. 2010, 2012, 2013, 2015). This type of targeted food marketing has been specifically implicated as a contributor to the rise in childhood obesity (Harris et al. 2009).
The majority of child-targeted food advertisements focus on establishing brand loyalty early in life (Connor 2006; Escalante de Cruz et al. 2004). Brand loyalty involves developing a unique, emotional attachment to a company through use of names, symbols, logos, characters, and slogans in attempts to increase purchases of products over the consumer’s lifetime (Connor 2006). Companies specifically target children in attempts to establish brand loyalty early in life (Escalante de Cruz et al. 2004). Brand loyalty has been associated with an increase in children’s preferences (Coon and Tucker 2002; Robinson et al. 2007), requests for (Valkenburg and Buijzen 2002; McDermott et al. 2006), and consumption of (Cravener et al. 2015; Halford et al. 2004, 2007; Keller et al. 2012b) the advertised products. This is of concern considering that a recent evaluation of television commercials promoting food brands showed that 98% of the advertised foods were high in sugar, fat, and sodium (Powell et al. 2013).
Although branding and advertising are ubiquitous, there is growing evidence of individual differences in the responsiveness to their effects. For example, in two recent studies overweight children recognized more food brands than their healthy weight counterparts in a brand awareness test (Halford et al. 2004, 2007). In addition, Forman and colleagues showed that overweight children were more responsive to the intake-promoting effects of food branding than healthy weight children (Forman et al. 2009). Identifying individuals who have increased vulnerability to the effects of branding and advertising may assist researchers in phenotyping children who are at risk for obesity (Forman et al. 2009).
Tools such as functional magnetic resonance imaging (fMRI) have been used to characterize differences in brain response to a variety of stimuli related to obesity. Functional MRI provides an indirect evaluation of metabolic changes in the brain during stimulus processing (McGonigle 2012). For example, a recent fMRI study in 10–14 year old children demonstrated increased response to food brands compared to non-food brands and control images in areas related to motivational value and cognitive control (Bruce et al. 2014). A study by this same group showed that compared to healthy weight youth, children with overweight, specifically, showed reduced response to food relative to non-food brands in brain regions implicated in cognitive control (Bruce et al. 2013). It is unknown if similar differences in brain response would be apparent in a younger population (e.g., 7–10 year-old children). Children are especially vulnerable to food marketing because they lack the cognitive ability to comprehend or evaluate its purpose compared to adolescents (McClure et al. 2013; Story and French 2004). Furthermore, research suggests that reward areas of the brain develop more rapidly than control areas, which may bias children toward selection of immediate rewards (Somerville and Casey 2010) and make them more susceptible to poor food choices following food marketing exposures (Somerville et al. 2010). More importantly, no study to date has shown whether differences in brain responses to food brands correlate with eating behaviors.
The primary aim of the present study was to determine whether food brands evoke differential response compared to non-food brands or control images in a cohort of children in middle childhood (ages 7–10 years-old). We hypothesized that brain response would be greater in areas related to reward and food motivation (e.g., ventral tegmental area, caudate, hypothalamus, etc.) and decreased in areas related to inhibitory control and executive function (e.g., prefrontal cortex, etc.) during exposure to food brands relative to non-food brands and control images. A secondary aim was to determine whether child brain responses to food brands compared to non-food brands are associated with laboratory energy intake at ad libitum test-meals presented with familiar food brands (compared to novel brand and unbranded controls). We hypothesized that increased brain response in areas related to reward and decreased response in areas related to inhibitory control and executive function would be associated with greater food intake from foods presented with brands relative to control (unbranded) foods.
Methods
Participants
Twenty-eight (N = 28) children between the ages of 7–10 years (mean ± SD = 8.56 ± 1.12 years) were tested. Participant characteristics are summarized in Table 2. Three children were excluded due to excessive motion, defined as >3 mm of motion during all fMRI runs. This lead to a final sample of 25 participants with complete data. In order to participate, children were required to be in overall good health, with no metabolic diseases, eating disorders, or learning disabilities. Furthermore, children were screened for participation in the MRI portion of the study and were excluded if they were left handed or had other characteristics that would impact fMRI performance (e.g., braces, claustrophobia, red/green color blindness). This study was approved by the Institutional Review Board of The Pennsylvania State University. All parents provided written informed consent and children provided written assent before participating.
Table 2.
Test-meal foods and beverage
| Meal Item | Serving Size | Weight (g) | Energy (kcal) | Energy Density (kcal/g) |
|---|---|---|---|---|
| Kraft® Macaroni and Cheese | 1 cup | 189 | 302.4 | 1.3 |
| Green Giant® Mixed Vegetables | 1/2 cup | 85 | 50.6 | 0.6 |
| Mott’s® Original Applesauce | 1/2 cup | 141 | 121.2 | 0.9 |
| Pringles® Original Potato Chips | 16 chips | 29 | 155.4 | 5.4 |
| Keebler® Chips Deluxe Chocolate Chip Cookies | 3 Cookies | 49 | 261.3 | 5.3 |
| Kool-Aid® Bursts Tropical Punch | 6.75 oz. | 211 | 21.1 | 0.1 |
| Nesquick® Low-Fat Chocolate Milk | 8 oz. | 251 | 159.5 | 0.6 |
Study design and overview
Using a within-subjects, cross-over design with repeated measures, participants completed four visits, each scheduled one week apart. The first three visits were conducted primarily in the Children’s Eating Behavior Laboratory, while the last visit, an fMRI, was conducted in the Social Life and Engineering Imaging Center (see Table 1). During the first three visits, children were presented with test-meals in a randomized, counter-balanced order using three conditions of food pack-aging: familiar food brands, unbranded, and a condition using a novel food brand, referred to as “novel” herein. In the branded condition, all foods were presented in plastic containers labeled with logos from familiar food brands (e.g., Kraft®, Pringles®). In the unbranded condition, food containers were presented with plain, white labels. Although we compared children’s response to popular food versus non-food brands (e.g., Nike®, Gap®) and scrambled images during the fMRI, we opted not to repeat this condition in the test-meals (i.e., by labeling applesauce with a scrambled or non-food brand logo such as Nike®) to avoid creating an unrealistic presentation that could confuse children. Therefore, the plain white label was used for the unbranded condition at the test-meals to simulate the “generic” brand condition. In the novel branding condition, a cartoon brand image was developed called “Kaiyo” that included a cartoon coyote on roller skates. The novel brand was used to test an exploratory aim of the study, determining whether children’s food intake would vary depending on brand familiarity. On the fourth visit, children completed an MRI scan where they passively viewed food brands (e.g., Kraft®, Motts®, etc.), non-food brands (e.g., Nike®, Tide®, etc.), and control images (scrambled versions of the food and non-food brands).
Table 1.
Study visits timeline
| Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|
| Meal conditions randomized and counterbalanced: unbranded, familiar branded, or novel branded | |||
| Anthropometries | Ad Libitum Laboratory Meal | Ad Libitum Laboratory Meal | Mock MRI |
| Questionnaires | Questionnaires | Mock MRI | fMRI scan |
| Ad Libitum Laboratory Meal | |||
Procedures
Children’s visits were scheduled at the same time each week for four consecutive weeks. Study visits took place during lunchtime (11:30 AM to 2:00 PM) or dinnertime (4:30 PM to 7:30 PM), depending on the family’s availability. Parents were instructed to have their child fast for at least two hours prior to all appointments. Fasting compliance was confirmed verbally with parents and child fullness level was assessed to ensure they were in a neutral appetitive state at the start of each visit (Keller et al. 2006). Children were instructed to wear comfortable clothes without metal fasteners for both their mock-MRI and MRI scan.
On the first visit, participants and their parents reported to the Children’s Eating Behavior Laboratory. Each research participant and his/her parent(s) were given an overview of the study and provided informed consent/assent to participate. Parents completed questionnaires to assess demographics and general child eating behaviors while anthropometrics were performed on children. On the first, second, and third visits, children consumed ad libitum test-meals and completed fullness measures before and after meal consumption using a validated pictorial visual analog scale (Keller et al. 2006). The scale consists of an image of a doll with a 150-mm rectangular stomach and a slider that children can move up and down to indicate level of fullness. The third visit also included a mock MRI scan to expose the children to the MRI environment and help reduce movement during the actual MRI scan. On the fourth visit, participants reported to the imaging facility for a 5-min mock refresher scan and their MRI scan. Children also rated perceived fullness immediately before and after the scan.
Measurements
Anthropometrics
Duplicate measurements of children’s height and weight were collected in light clothing without jackets, socks, or shoes. Height was measured to the nearest tenth of a centimeter using a portable stadiometer (seca®, Chino, CA). Weight was measured to the nearest tenth of a pound using a digital scale (Tanita®, Arlington Heights, IL). Children’s percent body fat was measured to the nearest tenth percent using a standing bioelectrical impendence body composition analyzer (Tanita®, Arlington Heights, IL).
Pilot testing to develop food brand stimuli
Brand stimuli used in the test-meals and fMRI were pilot tested to evaluate their familiarity, emotional valence, and excitability. The purpose of the pilot test was to ensure that food brands and non-food brands selected for both the test-meal and fMRI had similar ratings for familiarity, emotional valance, and excitability. Twenty healthy, 7–10 year-old children of similar demographics (e.g., age, weight status, ethnicity, and socioeconomic status.) to those enrolled in the main study were recruited to participate. Children from the pilot study were not allowed to participate in the main study. Familiarity of brands was assessed by children reporting if they recognized a brand and if they could correctly recall the brand name. Excitability and emotional valence were assessed on pictorial, 5-point likert scales adapted from the International Affective Picture System (IAPS) (Lang 2005).
Results of the pilot study were used to determine which food brands to use in the test meal. We selected food brands that attained the highest scores for familiarity, emotional valance and excitability within each category (i.e., entree, vegetables, fruits, snacks, and beverages). These categories were selected because they provided a variety of flavors and textures and have previously been used in studies with children (Fisher et al. 2007; Forman et al. 2009; Keller et al. 2012a). The final brands selected for the test meals included: Kraft® Macaroni and Cheese (Kraft®, Chicago, IL), Green Giant® Mixed Vegetables (B&G Foods, Parsippany-Troy Hills, NJ), Mott’s® Original Applesauce (Mott’s®, Plano, TX), Pringles® Original Potato Chips (Kellogg’s, Battle Creek, MI), Keebler® Chips Deluxe Chocolate Chip Cookies (Kellogg’s, Battle Creek, MI), Kool-Aid® Bursts Tropical Punch (Kraft®, Chicago, IL), and Nesquick® Low-Fat Chocolate Milk (Nestle, Vevey, Switzerland). The results also confirmed that our novel logo was not familiar to children, as only 1 child reported recognizing the logo. In addition, the novel logo had similar emotional valence (3.0/5.0), but lower excitability (2.3/5.0) than other more well-known brands used in the study.
Results of the pilot study were also used to determine the 60 food brand and 60 non-food brand images used in the MRI paradigm. Brands were only included if they were recognized by at least 75% of children (a rating of 2 or above) and if they had mean emotional valence and excitability scores above a “neutral” rating of 3.0 on the 5-point scale. Average ratings of the food brands were compared to ratings of the non-food brands with paired T-tests. There were no significant differences between familiarity for food brands (M = 2.44; SD = 0.33) and non-food brands (M = 2.48; SD = 0.35) (T = −1.02; P = .32). Food brands scored higher in average emotional valence (M = 3.70; SD = 0.50) compared to non-food brands (M = 3.5; SD = 0.49) (T = 5.15; P = .0001). Similarly food brands scored higher for excitability (M = 3.34; SD = 0.65) compared to non-food brands (M = 3.04; SD = 0.69) (T = 5.64; P = .0001). Images were displayed on 8 by 11 1/2 plain white backgrounds and were adjusted to control for size and proportions across images. Images were also matched for brightness and intensity. Control images were created by pixelating and scrambling the images in Matlab v. 8.0. The control images were created to control for color and other low-level visual characteristics.
Ad libitum test-meals
The foods presented at each test meal were: Kraft® macaroni and cheese, Green Giant® mixed vegetables, Mott’s® applesauce, Pringles® potato chips, Keebler® chocolate chip cookies, Kool-Aid® tropical punch, and Nesquick® low-fat chocolate milk. Serving sizes were selected based on previous studies within this age group (Fearnbach et al. 2015, 2016; Fisher et al. 2007). Detailed information on serving sizes and energy density of the meal foods and beverages can be found in Table 2.
At least two servings of each item were prepared prior to the ad libitum test-meal, with additional servings of foods and beverages readily available. All test-meal foods and beverages were served in clear plastic containers with labels corresponding to the appropriate brand condition (i.e., Food Branded, Unbranded, or Novel Branded) as shown in Fig. 1. Macaroni and cheese was served in a clear 16-oz (oz.) plastic container with matching lid, mixed vegetables and applesauce were served in clear 8-oz. plastic containers with matching lids, cookies and chips were served in clear plastic zip-top sandwich bags, and punch and chocolate milk were served in clear 9-oz. plastic cups with matching lids and straws. Prior to serving, test-meal foods and beverages were weighed to the nearest hundredth of a gram using a digital food scale. Pre-meal food weights with and without their container were recorded. Meal containers were arranged identically on a tray for all meals.
Fig. 1.

The foods presented at each test meal were: Kraft® macaroni and cheese, Green Giant® mixed vegetables, Mott’s® applesauce, Pringles® potato chips, Keebler® chocolate chip cookies, Kool-Aid® tropical punch, and Nesquick® low-fat chocolate milk. Detailed information on serving sizes and energy density of the meal foods and beverages can be found in Table 1. Macaroni and cheese was served in a clear 16-oz (oz.) plastic container with matching lid, mixed vegetables and applesauce were served in clear 8-oz. plastic containers with matching lids, cookies and chips were served in clear plastic zip-top sandwich bags, and punch and chocolate milk were served in clear 9-oz. plastic cups with matching lids and straws. Foods were presented according to condition food branded (left), unbranded (right), or novel branded (bottom)
A research assistant informed children that they had 30 min to eat as much as they wanted. Children were also told that they could ask for additional servings of any item throughout the meal. Children were asked if they would like another serving of a particular item once they finished it. While the child ate, a research assistant read an age-appropriate story (e.g., Matilda) that did not contain food references. The story served as a consistent, neutral distraction and provided a reason for the researcher to be in the room to monitor the child. If the child requested seconds, the researcher brought out another serving of the same size and in the same packaging. Children were told they could end their meal early by informing the researcher they had finished before the 30-min time limit. Immediately following the meal, leftovers were weighed in their containers and weights were recorded to the nearest hundredth of a gram.
Mock MRI procedure
Following the test-meal on the third visit, children completed a training session conducted at the MRI imaging facility mock scanner. This mock-training session was developed in-house to facilitate the quality of fMRI data collection in children (English et al. 2015). The mock scanner allows children to experience the MRI environment and practice holding still while they are observed by research staff. Children were first asked to lie down in the scanner and the various components such as the table, head coil, and mirror were explained. Next, children were given examples of movements to avoid that could cause motion artifacts during the scan. Children were also instructed on how to respond to questions without moving their heads so they could communicate during the MRI. Padding was placed around the child’s head simulating the head cushions used in the MRI scanner. While in the mock scanner children watched a 5-min presentation which contained age-appropriate images not presented during the fMRI scan (e.g., puppies, kittens). Sounds similar to those experienced during an MRI scan were played over speakers so children could become accustomed to hearing them during the procedure. On the fourth visit, participants completed a second, 5-min mock protocol as a refresher before the MRI procedure.
Functional MRI paradigm
Development of stimuli used in the fMRI was described above. Using a block design, children were shown a total of 180 images: 60 food brands, 60 non-food brands, and 60 control images over 18 min. Stimuli were presented over 12 functional runs. Each functional run lasted approximately 90 s and contained three blocks of stimuli (i.e., food brands, non-food brands, scrambled control). Block design was employed to allow comparison with previous studies (Bruce et al. 2013, 2014). Furthermore, block designs produce robust results with increased statistical power which is a benefit with smaller sample sizes (Amaro Jr and Barker 2006). Block order was randomized within and counterbalanced across each run. Each stimuli block contained 5 condition specific images displayed for 2 s each with a half second fixation cross between images. Inter-block intervals were jittered between 10 and 20 s. Participants were instructed to look at each picture as it appeared on the screen.
MRI data acquisition
MRI data were acquired using a Siemens MAGNETOM Trio 3 T MRI scanner (Siemens Medical Solutions, Erlangen, Germany) with a standard 12-channel head coil. To restrict movement, padding around the head, arms, and body was used. Furthermore, to reduce motion-induced effects during functional runs in-scan prospective movement correction (PACE) was used (Thesen et al. 2000). Stimuli were projected onto an MRI compatible screen and were controlled by a computer using Matlab v. 8.0 (The Mathworks Inc., Natick, Massachusetts).
The following parameters were used to obtain a T1-weighted MPRAGE structural scan for each subject: TR/TE = 1650/2.03 ms, flip angle = 9°, FOV = 256 mm, slice thickness = 1 mm, sagittal plane, voxel size 1×1×1 mm. Functional scans used a T2-weighted gradient single-shot echo planar imaging (EPI) sequence (TE = 25 ms, TR = 2000 ms, flip angle = 90°, matrix 64 × 64) with an in-plane resolution of 3 × 3 × 3 mm (FOV = 220 mm) to acquire 33, 3 mm (interleaved) slices along the AC-PC plane.
Data analysis
Participant’s descriptive data, food intake, and fullness scores were analyzed in SPSS 22.0 (IBM Corp., Chicago, IL, USA). Total energy intake from test meals was calculated by converting gram amounts consumed to total energy (kcal). Linear mixed models were used to test for differences in pre-meal fullness and total energy intake between the three meal conditions. Three separate models were used to assess if sex, BMI-z scores, or pre-meal fullness impacted the relationship between brand condition and test-meal intake. Covariates were tested in individual models and eliminated from the final model if they did not significantly impact the relationship between brand condition and test-meal intake.
Statistical analyses for whole-brain response from the fMRI data were conducted using BrainVoyager 20.6 (Brain Innovation, Maastricht, The Netherlands). Functional data preprocessing steps consisted of 3D motion correction (trilinear sync interpolation) using 6 vectors (3 translations, 3 rotations), temporal high-pass filtering using a GLM-Fourier basis set with 3 cycles per time course, and 3D spatial smoothing using an 6-mm3 FWHM Gaussian filter. Anatomical data were normalized to MNI space using the MNI-152 template. Functional data were co-registered to each participant’s anatomical data. Runs were excluded if there was excessive motion, defined as 3 mm of translation or 3° of rotation in any direction. Three participants did not have any successful functional runs and were excluded from final analyses. Functional data were then analyzed using a random-effects general linear model (GLM). Regressors were created coding for food brand blocks, non-food brand blocks, and control blocks. Blocks were modeled by convolving the standard hemodynamic response function with a 30-s boxcar function.
A group-level analysis was conducted using ANOVA on the whole-brain data with picture type (food brand, non-food brand, control) as fixed factors and participants as a random factor. A grey matter mask was applied to the functional data. To correct for multiple testing, results were thresholded with a voxel-wise P < 0.001 and spatial extent threshold of 17, determined by using the standard procedures of the ClusterThresh plugin of the BrainVoyager software (Forman et al. 1995; Goebel et al. 2006). Parameters were determined, as recommended by Woo and colleagues, by conducting 10,000 Monte Carlo simulations to yield an overall P-value of P < 0.05 (Forman et al. 1995; Goebel et al. 2006). To allow for comparison with previous studies that have used less stringent multiple comparison corrections (Bruce et al. 2013, 2014) we also thresholded the MRI output using an uncorrected voxel-wise P-value of P < 0.001 and an arbitrary cluster threshold of 3 voxels (3×3×3 mm) for exploratory purposes (Woo et al. 2014). Three contrasts were used for analysis, food brands > non-food brands, food brands > control images, and non-food brands > control images. Peak beta coefficients were extracted from all areas of BOLD response that survived the multiple comparison correction and were subjected to further analysis (ANOVA) in SPSS. To determine whether key variables influenced these findings, we included sex, BMI z-score, and pre-fMRI fullness ratings as covariates in general linear models predicting brain response as a function of brand condition. Again, each variable was tested in a separate model.
To determine the relationship between BOLD response and test-meal intake, we first calculated difference scores in beta coefficient values between fMRI conditions (e.g., food brands > non-food brands; food brands > control). Therefore, positive beta coefficient values indicate greater response to food brand images relative to non-food brand or control. Differences in energy intake between the food branded and unbranded meals and novel branded and unbranded meals were also calculated. Positive difference scores indicate greater intake in the presence of food brands relative to control. Pearson’s correlations were then calculated between the difference scores obtained from the fMRI data (i.e., beta values from the contrast of food brands vs. non-food brands or control) and the test-meals (i.e., energy intake in the presence of food brands vs. unbranded or novel brand) to determine the relationship between brain response and test-meal intake. Three statistical outliers (greater than 1.5 the interquartile range) were observed in the intake data. Two of these outliers were observed for differences in intake between the branded and unbranded meal (intake differences of 764.7 kcal and 558.45 kcal between meal conditions) and one outlier was observed for differences in intake between the novel branded meal and the unbranded meal (intake difference of 583.0 kcal between meal conditions). We therefore report only the correlations without the outliers present. A P-value ≤0.05 was used to determine significance in all analyses, and we corrected for false discovery rate using the Benjamini- Hochberg method (Hochberg and Benjamini 1990).
Results
Participant characteristics are summarized in Table 3. The majority of participants were Caucasian (n = 23). Participants reported no difference in pre-meal fullness between sessions (F = 1.18; P = 0.31). Furthermore, there was no difference in energy intake between conditions (familiar branded, novel branded, and unbranded containers) (F = 0.42; P = 0.65). None of the covariates tested (i.e., sex, BMI z, order of test visits, and pre-meal fullness) impacted the significance of the meal intake model.
Table 3.
Participant characteristics (n = 25)
| Age (y) | 8.56 ± 1.12 |
| Sex | Male (48%); Female (52%) |
| Height (cm) | 135.4 ± 10.02 |
| Weight (kg) | 32.95 ± 8.70 |
| BMI (kg/m2) | 17.68 ± 2.77 |
| BMI-Age-Sex-Percentile | 59.28 ± 30.46 |
| Body Fat (%) | 20.88 ± 7.90 |
| Race | White (92.9%), Black (3.6%), Hispanic (3.6%) |
Values represented are mean ± SD
BMI = Body Mass Index
fMRI results
A full list of corrected fMRI results are reported in Table 4 and visualized in Fig. 2. Regions from the uncorrected fMRI results are reported in Table 5 and visualized in Fig. 3. As regions from the uncorrected analysis are for comparison to previous studies, they will not be reported in detail.
Table 4.
Regions of interest obtained from the whole brain analysis
| Region Name | No. Voxels | X | Y | Z | F-value | P-value |
|---|---|---|---|---|---|---|
| Food Brands > Non-food Brands | ||||||
| R. Lingual Gyrus | 27 | 22 | −94 | −8 | 13.13 | 0.00002 |
| Food Brands > Control Images | ||||||
| R. Fusiform Gyrus | 338 | 39 | −62 | −14 | 20.23 | <0.00001 |
| R. Precuneus* | 21 | 13 | −60 | 28 | 8.77 | 0.0005 |
| R. Lingual Gyrus* | 36 | 1 | −87 | −9 | 10.75 | 0.0001 |
| L. Supramarginal Gyrus * | 20 | −36 | −42 | 34 | 8.58 | 0.0006 |
| L. Fusiform Gyrus | 574 | −40 | −58 | −14 | 21.91 | <0.00001 |
| Non-food Brands > Control Images | ||||||
| R. Fusiform Gyrus | 356 | 41 | −60 | −14 | 17.74 | 0.000002 |
| R. Supramarginal Gyrus* | 36 | 35 | −41 | 35 | 9.67 | 0.0002 |
| R. Lingual Gyrus* | 134 | 8 | −92 | −3 | 11.27 | 0.00009 |
| L. Cuneus* | 18 | −10 | −99 | 5 | 9.09 | 0.0004 |
| L. Fusiform Gyrus | 526 | −41 | −64 | −12 | 21.78 | <0.00001 |
Reported regions are at a voxel-wise P-value = .001 and k = 17
Greater BOLD response for control stimulus
(R = Right; L = Left)
Fig. 2.
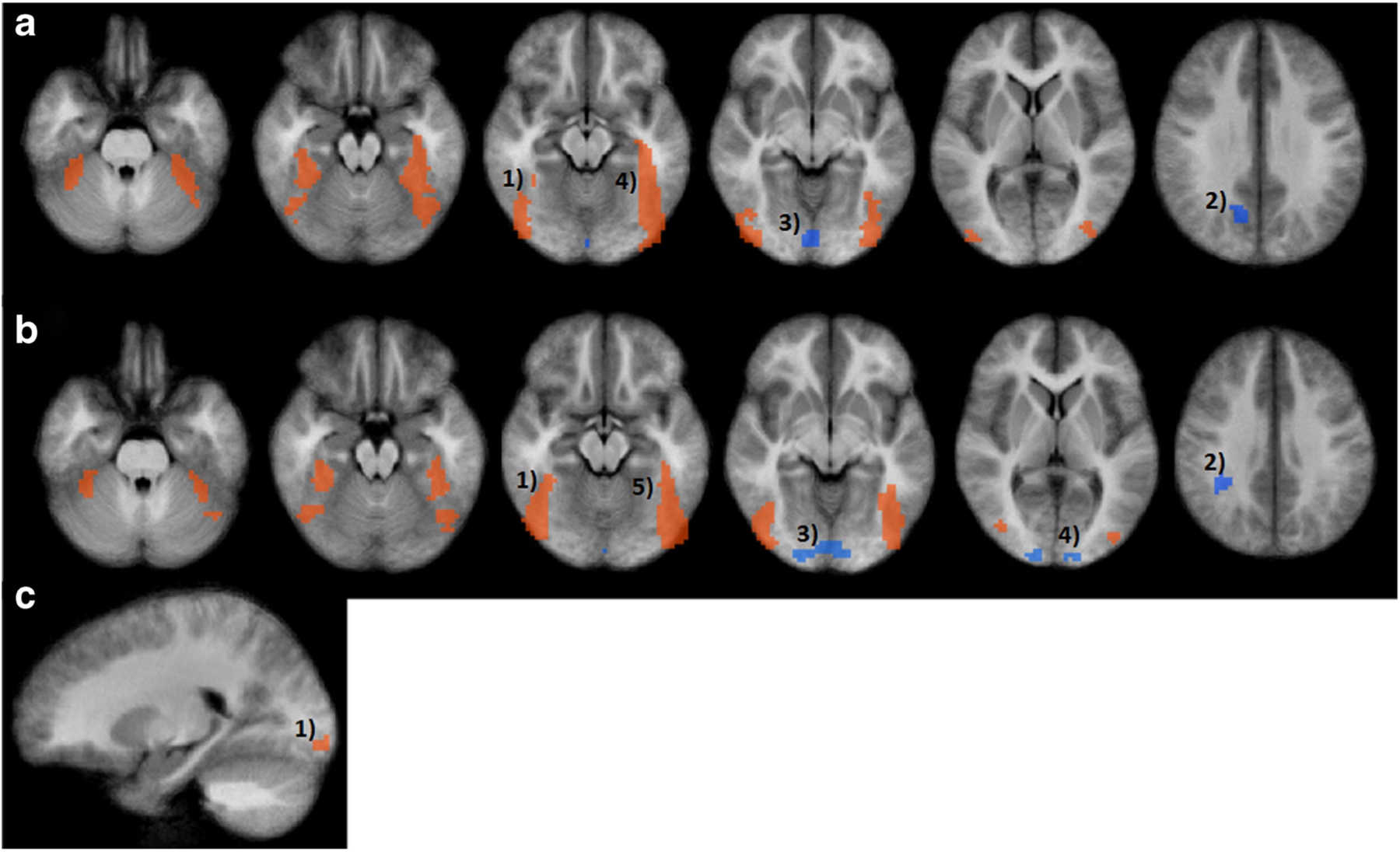
Results from the whole brain fMRI analysis (n = 28) corrected for multiple comparisons. Panel a Results from Food brand > control image contrast. Significant regions include 1) right fusiform gyrus 2) right precuneus 3) right lingual gyrus 4) left fusiform gyrus and left supramarginal gyrus (not pictured). Panel b Results from Non-food brand > control image contrast. Significant regions include 1) right fusiform gyrus 2) right supramarginal gyrus 3) right lingual gyrus 4) left cuneus 5) left fusiform gyrus. c Results from Food brand > Non-food brand contrast. Significant region 1) right lingual gyrus. Overall group-level analysis was conducted using ANOVA on the whole-brain data with picture type (food brand, non-food brand, control) as fixed factors and participants as a random factor. Results were thresholded with a voxel-wise P-value of P < 0.001 and spatial extent threshold of17. Parameters were determined by conducting 10,000 Monte Carlo simulations to yield an overall P-value of P < 0.05
Table 5.
Additional regions of interest obtained from the uncorrected whole brain analysis
| Region Name | No. Voxels | X | Y | Z | F-value | P-value |
|---|---|---|---|---|---|---|
| Food Brands > Non-food Brands | ||||||
| R. inferior Frontal Gyrus | 5 | 21 | 33 | −5 | 9.03 | 0.0004 |
| Food Brands > Control Images | ||||||
| R. Inferior Parietal Lobule* | 11 | 35 | −42 | 37 | 10.28 | 0.0001 |
| R. Cingulate Gyrus* | 7 | 8 | −33 | 41 | 6.99 | 0.002 |
| R. Posterior Cingulate* | 4 | 10 | −28 | 28 | 8.5 | 0.0006 |
| R. Medial Frontal Gyrus | 3 | 5 | 39 | −17 | 6.16 | 0.004 |
| R. Caudate | 3 | 2 | 16 | 13 | 6.85 | 0.002 |
| L. Inferior Frontal Gyrus | 4 | −36 | 34 | −16 | 7.39 | 0.001 |
| L. Middle Temporal Gyrus | 4 | −61 | −2 | −8 | 5.9 | 0.005 |
| Non-food Brands > Control Images | ||||||
| R. Inferior Frontal Gyrus* | 6 | 21 | 35 | −5 | 8.7 | 0.0005 |
| R. Caudate* | 6 | 16 | −29 | 28 | 8.16 | 0.0008 |
| R. Cingulate Gryus* | 3 | 8 | −20 | 28 | 6.8 | 0.002 |
| L. Culmen | 3 | −9 | −35 | −4 | 5.87 | 0.005 |
| L. Lingual Gyrus | 7 | −15 | −50 | −3 | 6.95 | 0.002 |
| L. Middle Temporal Gyrus | 9 | −48 | −44 | −2 | 6.6 | 0.002 |
| L. Inferior Frontal Gyrus | 9 | −55 | 28 | 11 | 8.62 | 0.0006 |
Regions are significant with a voxel-wise P-value = .001 and k = 3
Greater BOLD response for control stimulus
(R = Right; L = Left)
Fig. 3.
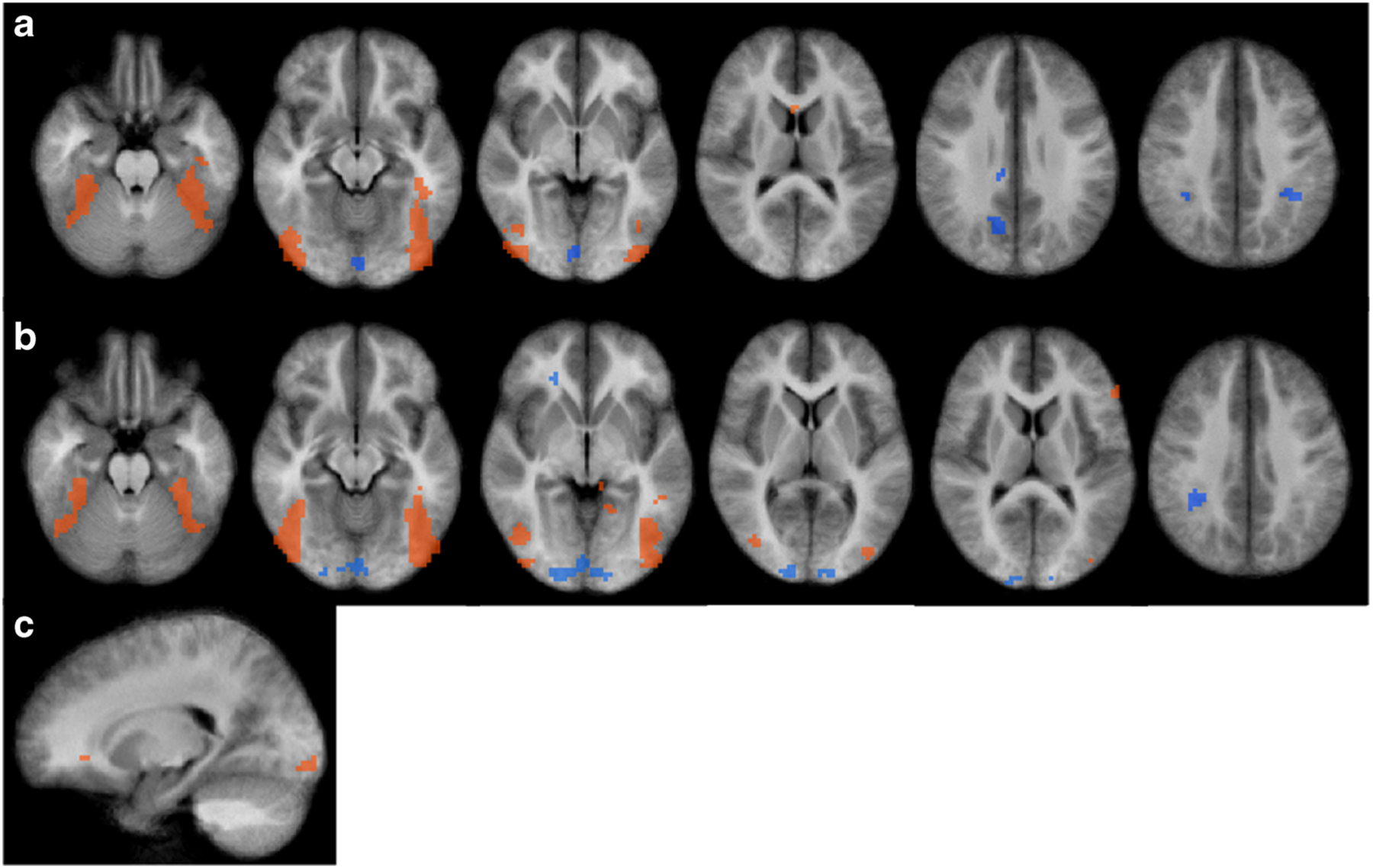
Results from the whole brain fMRI analysis (n = 28) uncorrected for multiple comparisons. a Results from Food brand > control image contrast. b Results from Non-food brand > control image contrast. c Results from Food brand > Non-food brand contrast. Overall group-level analysis was conducted using ANOVA on the whole-brain data with picture type (food brand, non-food brand, control) as fixed factors and participants as a random factor. Results were thresholded with a voxel-wise P-value of P < 0.001 and spatial extent threshold of three (3×3×3 mm) voxels
Food brands > non-food brands
In the corrected whole brain fMRI analysis, food brands were associated with increased brain response in the right lingual gyrus relative to non-food brands (P = 0.00002). Including sex, BMI z-score, and pre-fMRI fullness as covariates did not influence the significance of these results (Table 4, Fig. 2 panel C).
Food brands > control images
Relative to control images, food brand images resulted in greater response in the right and left fusiform gyrus (P < 0.00001 for both). Decreased response was observed in the right precuneus (P = 0.0005), right lingual gyrus (P = 0.001), and the left supramarginal gyrus (P = 0.0006). Including sex, BMI z-score, and pre-fMRI fullness as covariates did not influence the significance of these results (Table 4, Fig. 2 panel A).
Non-food brands > control images
Relative to control images, non-food brand images resulted in greater response in right and left fusiform gyrus (P < 0.00001 for both). Decreased response was observed in the right supramarginal gyrus (P = 0.0002), right lingual gyrus (P = 0.00009) and the left cuneus (P = 0.0004). Including sex, BMI z-score, and pre-fMRI fullness as covariates did not influence the significance of these results (Table 4, Fig. 2 panel B).
Correlation between fMRI results and test-meal intake
Pearson’s correlations between brain response to both food and non-food brands and test-meal intake are detailed in Table 6. Greater responses in the bilateral fusiform gyrus in response to both food and non-food brands relative to control images were negatively associated with intake at the branded compared to the unbranded test-meal (R-values = −0.42 to −0.52; P-values = 0.01 to 0.04; see Table 6). In other words, children who had greater BOLD response in fusiform regions to both food or non-food brands images relative to control stimuli tended to consume less from meals presented with food-brands in the laboratory (see Fig. 4). The results remained significant when including sex and pre-fMRI fullness as covariates. When controlling for child BMI-z, the correlation between BOLD response in the right and left fusiform from the food brands > control and energy intake was no longer significant. However, after applying the Benjamini Hochberg correction, only the correlation between meal intake and BOLD response in the right fusiform gyrus to non-food brands > control survived. Children’s response to food brand images in the other regions identified by the whole-brain analysis did not correlate with intake at the test-meals (P values ranging from 0.15–0.91).
Table 6.
Pearson’s correlation coefficients for the relationship between the difference in energy intake between meal conditions and the difference in brain response between brand conditions
| Meal Comparison | fMRI Contrast | Brain Region | Pearson Correlation | P-value |
|---|---|---|---|---|
| Food branded vs. Unbranded Meal | Food Brands > Non-food Brands | R. Lingual Gyrus | −0.29 | 0.18 |
| Food Brands > Control Images | R. Fusiform Gyrus | −0.47 | 0.02 | |
| R. Precuneus* | −0.11 | 0.61 | ||
| R. Lingual Gyrus* | −0.03 | 0.86 | ||
| L. Supramarginal Gyrus* | −0.17 | 0.42 | ||
| L. Fusiform Gyrus | −0.42 | 0.04 | ||
| Non-food brands > Control Images | R. Fusiform Gyrus | −0.52 | 0.01 | |
| R. Supramarginal Gyrus* | −0.07 | 0.74 | ||
| R. Lingual Gyrus* | −0.02 | 0.91 | ||
| L. Cuneus* | −0.09 | 0.68 | ||
| L. Fusiform Gyrus | −0.43 | 0.04 | ||
| Novel branded vs. Unbranded Meal | Food Brands > Non-food Brands | R. Lingual Gyrus | −0.13 | 0.54 |
| Food Brands > Control Images | R. Fusiform Gyrus | −0.23 | 0.30 | |
| R. Precuneus* | −0.18 | 0.40 | ||
| R. Lingual Gyrus* | −0.25 | 0.24 | ||
| L. Supramarginal Gyrus* | 0.02 | 0.91 | ||
| L. Fusiform Gyrus | −0.17 | 0.42 | ||
| Non-food brands > Control Images | R. Fusifonn Gyrus | −0.05 | 0.79 | |
| R. Supramarginal Gyrus* | −0.03 | 0.88 | ||
| R. Lingual Gyrus* | −0.31 | 0.15 | ||
| L. Cuneus* | −0.44 | 0.04 | ||
| L. Fusiform Gyrus | −0.09 | 0.66 |
(R = Right; L = Left)
Greater BOLD response for control stimulus
Fig. 4.
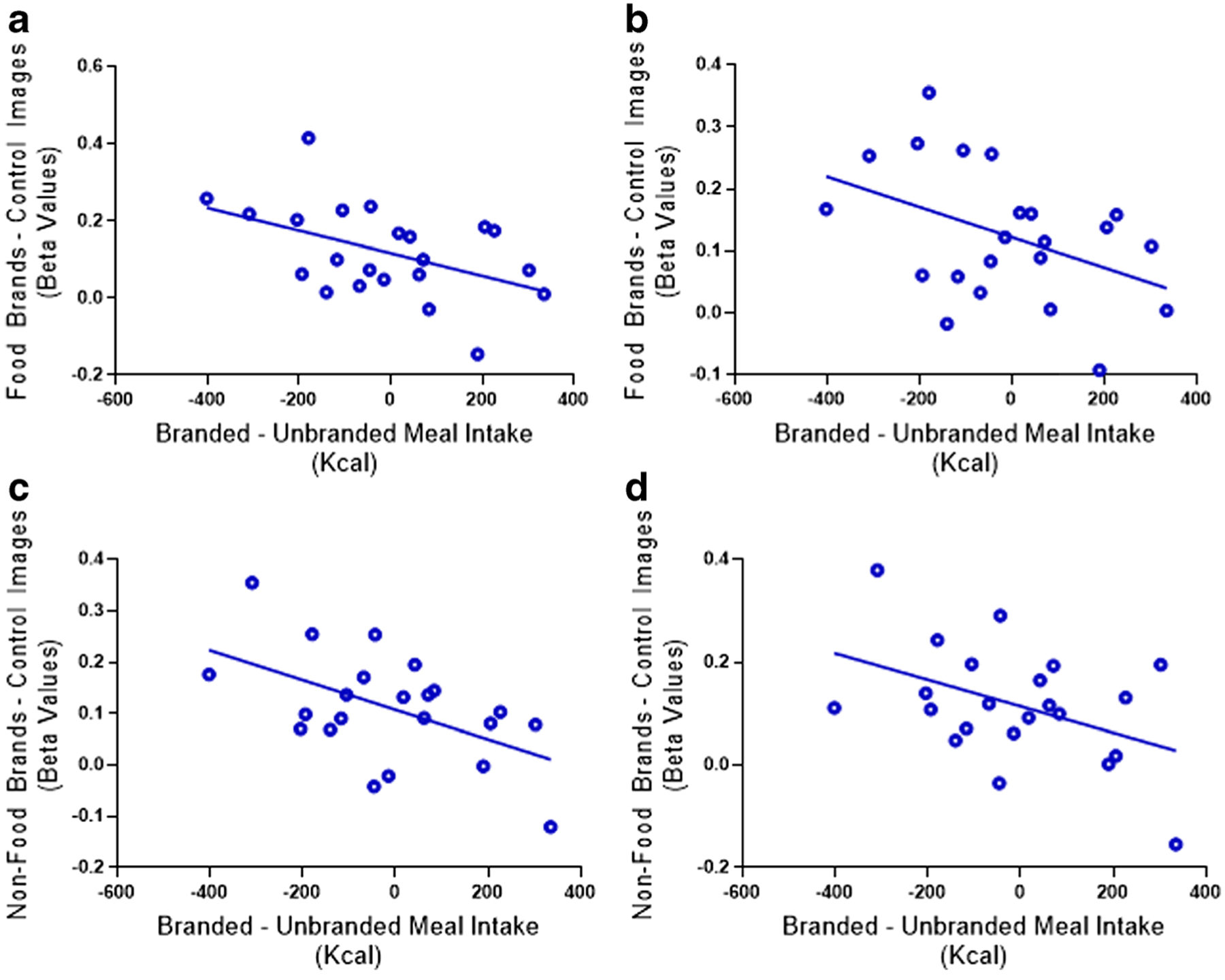
Relationships between brain response and difference between food branded and unbranded meal intakes. a R. Fusiform from Food brands > Control Images Contrast. b L. Fusiform from Food brands > Control Images Contrast. c R. Fusiform from Non-food brands > Control Images Contrast. d L. Fusiform from Non-food brands > Control Images Contrast. Differences in fMRI beta values were calculated by subtracting one condition from another. Differences in energy intake was calculated by subtracting caloric intake between meal conditions i.e., Food Branded kcal – Unbranded kcal. Pearson’s correlations were then conducted between the differences in BOLD response and meal intake to determine the relationship between brain response and test-meal intake. Lower response in all regions were negatively associated with intake at the branded compared to the unbranded test-meal
Discussion
This study makes two contributions to the literature. First, we partially confirm previous fMRI results from studies conducted with older children (Bruce et al. 2013, 2014). These studies, like ours, demonstrate that food brands compared to control images increase response in brain regions related to visual processing and attention (i.e., fusiform gyrus). Our study also observed reduced BOLD response to food and non-food brand images when compared to control images in regions related to memory, information retrieval, and spatial attention (i.e., precuneus, lingual gyrus, and supramarginal gyrus). These results confirm previous studies and extend their results to a younger cohort of pre-adolescent youth.
Second, this is the first study to our knowledge examining the association between brain response to food and non-food brands and objectively measured intake from meals presented under similar experimental conditions (i.e., food brand, non-branded, novel branded). While there were no differences in children’s intake across the three conditions, individual differences in BOLD response to food brand images were associated with laboratory food intake. Specifically, BOLD response in bilateral fusiform gyri to either food brands or non-food brands was negatively associated with children’s intake from meals presented with food brands relative to a no-brand condition. These findings provide evidence that BOLD response to food cues is associated with objective measures of food intake, whereas previous studies (Masterson et al. 2016; Rothemund et al. 2007; Yokum et al. 2011) have only been able to speculate about this relationship.
Functional MRI results
Relative to non-food brands we found increased brain response to food brands in the right lingual gyrus. The lingual gyrus is associated with visual processing, recognition, memory, and information retrieval (Badgaiyan and Posner 1997; Esch et al. 2012; Gilboa et al. 2004). It is also related to selective attention processing (Labudda et al. 2008). In this study of primarily healthy weight children, greater brain response was observed to food brands relative to non-food brands. This greater activation likely reflects stronger attentional bias to the food logos compared to non-food logos. It is important to note that food and non-food brand images used in the fMRI paradigm were matched for visual saliency characteristics (size, brightness, and intensity) and therefore observed response is likely not due to differences in visual characteristics of the images themselves. However, the pilot study results presented in this paper suggest that the food brands used in the fMRI paradigm had intrinsically higher emotional valence and excitability than the non-food brands. Although the food and non-food brand stimuli were matched for level of familiarity, we were unable to practically match for these other characteristics. However, it has been suggested that food logos may be inherently more emotional and exciting as they are related to the salience of food as a biological necessity (Bruce et al. 2014).
In both the food brands > control and the non-food brands > control contrasts, greater activation to brand logos was observed in the bilateral fusiform gyri. The fusiform gyrus is thought to be primarily involved in the retrieval of information (de Zubicaray et al. 2001; Okada et al. 2000), memory processes (Garoff et al. 2005), object recognition (Gauthier et al. 1999) and processing of negative emotions (Schienle et al. 2005). Our results corroborate other studies that have shown brand images, regardless of if they are of food or non-foods, engage this region to a greater extent than control stimuli (Bruce et al. 2013, 2014; Esch et al. 2012; Maynard et al. 2017). Greater response in the fusiform gyrus has also been implicated in response to evaluation of a variety of food stimuli (Killgore et al. 2003; Masterson et al. 2016; Pelchat et al. 2004) suggesting it may play a more general role in the evaluation of food images, specifically.
Greater BOLD response to control images relative to either food or non-food brands was observed in several brain regions implicated in memory, information retrieval, and spatial attention including the cuneus (Price 2000), precuneus (Cavanna and Trimble 2006), lingual gyrus (Esch et al. 2012), and supramarginal gyrus (Hillis et al. 2005). One possible explanation for the observed response in these regions may be due to the use of scrambled images as a control. These images were pixilated and scrambled versions of the original images. Therefore, response may be partly due to children’s attempts to unscramble and recognize the images.
We do note that while we identified many similar regions to previous studies we ultimately reported fewer regions overall than reported by others (Bruce et al. 2013, 2014). This is likely due to the utilization of a stricter voxel-wise threshold (P = .001) for multiple comparisons testing. The initial voxel-wise p-value has been suggested as the most important factor for reducing the likelihood of false positives (Eklund et al. 2016; Woo et al. 2014). Our uncorrected analysis, however, suggests that very similar regions to previous studies may be involved in the processing of food and non-food brands, particularly in frontal regions of the brain (i.e., inferior frontal gyrus, medial frontal gyrus). This speaks to the replicability of neuroimaging findings more broadly, given that this has been a concern raised in recent reports (Eklund et al. 2016). It is possible that the use of strict multiple comparison thresholding (P = .001; k = 17) within the relatively small sample size of this study may be masking these results. This has previously been noted as a concern of the use of strict multiple comparison corrections (Woo et al. 2014).
Correlations between BOLD response and food intake
The BOLD signal from both left and right fusiform gyri from both the food brands > control and non-food brands > control showed a negative correlation with energy intake between the branded compared to the unbranded meal. Reduced BOLD response in the fusiform to brands may be indicative of vulnerability to the intake promoting effects of food marketing. While our study population was primarily healthy weight previous work has observed reduced BOLD response to food logos in children with obesity when compared to healthy weight counterparts (Bruce et al. 2013). Of note is that the correlation between BOLD response and intake is present in both the food brand > control and non-food brand > control contrasts. This suggests that brain response to branding more generally, and not specifically food brands, could be predictive of a child’s vulnerability to the energy intake promoting effects of food marketing. We do note that the correlation between response in the fusiform gyri to food brands and energy intake appears to be partially explained by child BMI-z, suggesting that these results may be partly mediated by weight status. While these initial results are promising it is important to interpret them with caution, particularly since they are correlational and were only observed after the removal of statistical outliers. Therefore, future studies are needed to confirm these results.
Although these results should be interpreted cautiously and require replication in larger more diverse samples, they do hold implications for public policy and the development of novel intervention techniques. First, marketing cues, such as food branding, may affect energy intake even within healthy weight populations. Therefore, reducing children’s exposure to these cues may help in regulating energy intake, particularly for children who may have an attentional bias toward these stimuli. Second, characterizing the brain neurocircuitry that is associated with increased intake in the presence of food brands may help researchers identify neural targets for future interventions aimed at treating excess energy intake and obesity.
Strengths and limitations
This study has several strengths and limitations. Although some studies have examined the effects of food branding and advertising in youth (Bruce et al. 2013, 2014; Gearhardt et al. 2014; Yokum et al. 2014), few have focused on pre-adolescent children. Children at this age are especially vulnerable to food advertising and branding because they have limited cognitive ability to fully comprehend or evaluate its purpose (McClure et al. 2013; Story and French 2004). Furthermore, those previous studies in adolescents have lacked measures of food intake, which limits the interpretations that can be drawn. This study also had a high success rate of fMRI scanning which is likely due to the careful mock training protocol we employed. A final strength is the use of brand images that were piloted with a cohort of children from similar demographics and matched for contrast, color, and visual saliency.
It should be noted that previous studies have found differential responses to food brands in children with overweight and obesity. Although this study had a wide BMI range we may have been underpowered to detect these effects particularly since the majority of the sample was considered to be healthy weight. Additionally, our cohort was homogenous in ethnicity and socioeconomic status, which affects the generalizability of the results. Future studies should examine the effects of branding in more diverse populations as food advertising may have unique impacts on energy intake within specific sub groups of the population as food companies spend a disproportionate amount of money on advertising to racial minorities such as Hispanic and African Americans (Grier 2009; Grier and Kumanyika 2008, 2010). A final limitation to consider is the conversion of children’s MRI data to a standardized MNI space. This conversion allows for generalization of findings and for comparison to previous studies and has been shown to be feasible in populations as young as 6 years old (Muzik et al. 2000). However, no current process for spatial normalization of anatomical data perfectly matches brains across individuals (Sanchez et al. 2012). Future studies may consider the use of study specific templates to help increase spatial specificity.
Conclusion
In conclusion, relative to non-food brands we found increased brain response to food brands in the right lingual gyrus, an area related to visual processing, recognition, and attention. Furthermore, we provide preliminary evidence that brain response in the fusiform gyri to branding in general correlates with children’s eating behavior when foods are presented paired with associated brands in the laboratory. These findings may help explain differences in children’s susceptibility to the intake-promoting effects of food advertising.
Acknowledgments
We would like to thank the parents and children who participated in this project. We would like to acknowledge the support of the USDA/NIFA Childhood Obesity Prevention Training Grant #2011-67001-30117 for study funding and doctoral fellowship program support. We would also like to thank the Social, Life, and Engineering Sciences Imaging Center at Penn State for imaging support.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Amaro E Jr., & Barker GJ (2006). Study design in fMRI: Basic principles. Brain and Cognition, 60(3), 220–232. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD, & Posner MI (1997). Time course of cortical activations in implicit and explicit recall. Journal of Neuroscience, 17(12), 4904–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AS, Lepping RJ, Bruce JM, Cherry JBC, Martin LE, Davis AM, et al. (2013). Brain responses to food logos in obese and healthy weight children. Journal of Pediatrics, 162(4), 759–764.e2. 10.1016/j.jpeds.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Bruce AS, Bruce JM, Black WR, Lepping RJ, Henry JM, Cherry JB, et al. (2014). Branding and a child’s brain: An fMRI study of neural responses to logos. Social Cognitive and Affective Neuroscience, 9(1), 118–122. 10.1093/scan/nss109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, & Trimble MR (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129(3), 564–583. [DOI] [PubMed] [Google Scholar]
- Connor SM (2006). Food-related advertising on preschool television: Building brand recognition in young viewers. Pediatrics, 118(4), 1478–1485. 10.1542/peds.2005-2837. [DOI] [PubMed] [Google Scholar]
- Coon KA, & Tucker KL (2002). Television and children’s consumption patterns. A review of the literature. Minerva Pediatrica, 54(5), 423–436. [PubMed] [Google Scholar]
- Cravener TL, Schlechter H, Loeb KL, Radnitz C, Schwartz M, Zucker N, et al. (2015). Feeding strategies derived from behavioral economics and psychology can increase vegetable intake in children as part of a home-based intervention: Results of a pilot study. Journal of the Academy of Nutrition and Dietetics, 115(11), 1798–1807. 10.1016/j.jand.2015.03.024. [DOI] [PubMed] [Google Scholar]
- de Zubicaray GI, Wilson SJ, McMahon KL, & Muthiah S (2001). The semantic interference effect in the picture-word paradigm: An event-related fMRI study employing overt responses. Human Brain Mapping, 14(4), 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, 113(28), 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English L, Lasschuijt M, & Keller KL (2015). Mechanisms of the portion size effect. What is known and where do we go from here? Appetite, 88, 39–49. [DOI] [PubMed] [Google Scholar]
- Escalante de Cruz A, Phillips S, Visch M, & Bulan Saunders D (2004). The junk food generation: A multi-country survey of the influence of television advertisements on children. Kuala Lumpur: Consumers International Asia Pacific Office. [Google Scholar]
- Esch FR, Möll T, Schmitt B, Elger CE, Neuhaus C, & Weber B (2012). Brands on the brain: Do consumers use declarative information or experienced emotions to evaluate brands? Journal of Consumer Psychology, 22(1), 75–85. [Google Scholar]
- Fearnbach SN, Thivel D, Meyermann K, & Keller KL (2015). Intake at a single, palatable buffet test meal is associated with total body fat and regional fat distribution in children. Appetite, 92, 233–239. 10.1016/j.appet.2015.05.036. [DOI] [PubMed] [Google Scholar]
- Fearnbach SN, Silvert L, Keller KL, Genin PM, Morio B, Pereira B, et al. (2016). Reduced neural response to food cues following exercise is accompanied by decreased energy intake in obese adolescents. International Journal of Obesity, 40(1), 77–83. 10.1038/ijo.2015.215. [DOI] [PubMed] [Google Scholar]
- Fisher JO, Liu Y, Birch LL, & Rolls BJ (2007). Effects of portion size and energy density on young children’s intake at a meal. The American Journal of Clinical Nutrition, 86(1), 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, & Noll DC (1995). Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a clustersize threshold. Magnetic Resonance in Medicine, 33(5), 636–647. [DOI] [PubMed] [Google Scholar]
- Forman J, Halford JC, Summe H, MacDougall M, & Keller KL (2009). Food branding influences ad libitum intake differently in children depending on weight status. Results of a pilot study. Appetite, 53(1), 76–83. 10.1016/j.appet.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Fox MK, Pac S, Devaney B, & Jankowski L (2004). Feeding infants and toddlers study: What foods are infants and toddlers eating? Journal of the American Dietetic Association, 104, 22–30. [DOI] [PubMed] [Google Scholar]
- Garoff RJ, Slotnick SD, & Schacter DL (2005). The neural origins of specific and general memory: The role of the fusiform cortex. Neuropsychologia, 43(6), 847–859. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, & Gore JC (1999). Activation of the middle fusiform’face area’increases with expertise in recognizing novel objects. Nature Neuroscience, 2(6), 568–573. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum S, Stice E, Harris JL, & Brownell KD (2014). Relation of obesity to neural activation in response to food commercials. Social Cognitive and Affective Neuroscience, 9(7), 932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, & Moscovitch M (2004). Remembering our past: Functional neuroanatomy of recollection of recent and very remote personal events. Cerebral Cortex, 14(11), 1214–1225. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, & Formisano E (2006). Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping, 27(5), 392–401. 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier S (2009). African American and Hispanic youth vulnerability to target marketing: Implications for understanding the effects of digital marketing. Paper presented at the BMSG Meeting on Digital Media and Marketing to Children. [Google Scholar]
- Grier SA, & Kumanyika SK (2008). The context for choice: Health implications of targeted food and beverage marketing to African Americans. American Journal of Public Health, 98(9), 1616–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier SA, & Kumanyika S (2010). Targeted marketing and public health. Annual Review of Public Health, 31, 349–369. [DOI] [PubMed] [Google Scholar]
- Hales CM, Carroll MD, Fryar CD, & Ogden CL (2017). Prevalence of obesity among adults and youth: United States, 2015–2016: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. [Google Scholar]
- Halford JCG, Gillespie J, Brown V, Pontin EE, & Dovey TM (2004). Effect of television advertisements for foods on food consumption in children. Appetite, 42(2), 221–225. 10.1016/j.appet.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Halford JCG, Boyland EJ, Hughes G, Oliveira LP, & Dovey TM (2007). Beyond-brand effect of television (TV) food advertisements/commercials on caloric intake and food choice of 5–7-year-old children. Appetite, 49(1), 263–267. 10.1016/j.appet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Harris JL, Bargh JA, & Brownell KD (2009). Priming effects of television food advertising on eating behavior. Health Psychology, 28(4), 404–413. 10.1037/a0014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JL, Schwartz MB, & Brownell KD (2010). Evaluating fast food nutrition and marketing to youth. New Haven: Yale Rudd Center for Food Policy & Obesity. [Google Scholar]
- Harris JL, Schwartz MB, Brownell KD, Sarda V, Dembek C, Munsell C, et al. (2012). Cereal FACTS 2012: limited progress in the nutrition quality and marketing of children’s cereals. New Haven: Rudd Center for Food Policy & Obesity. [Google Scholar]
- Harris JL, Schwartz MB, Munsell CR, Dembek C, Liu S, LoDolce M, & Kidd B (2013). Fast food FACTS 2013: Measuring progress in nutrition and marketing to children and teens. New Haven: Yale Rudd Center for Food Policy and Obesity. [Google Scholar]
- Harris JL, Schwartz MB, LoDolce M, Munsell C, Fleming-Milici F, Elsey J, et al. (2014). Sugary drink FACTS 2014: Some progress but much room for improvement in marketing to youth. New Haven: Rudd Center for Food Policy and Obesity. [Google Scholar]
- Harris JL, Schwartz M, Shehan C, Hyary M, Appel J, Haraghey K, & Li X (2015). Snack FACTS 2015: Evaluating snack food nutrition and marketing to youth. Rudd Center for Food Policy & Obesity. [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits EH, & Degaonkar M (2005). Anatomy of spatial attention: Insights from perfusion imaging and hemispatial neglect in acute stroke. Journal of Neuroscience, 25(12), 3161–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y, & Benjamini Y (1990). More powerful procedures for multiple significance testing. Statistics in Medicine, 9(7), 811–818. [DOI] [PubMed] [Google Scholar]
- Keller KL, Assur SA, Torres M, Lofink HE, Thornton JC, Faith MS, & Kissileff HR (2006). Potential of an analog scaling device for measuring fullness in children: Development and preliminary testing. Appetite, 47(2), 233–243. 10.1016/j.appet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Keller KL, Kuilema LG, Lee N, Yoon J, Mascaro B, Combes AL, et al. (2012a). The impact of food branding on children’s eating behavior and obesity. Physiology & Behavior, 106(3), 379–386. 10.1016/j.physbeh.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Keller KL, Kuilema LG, Lee N, Yoon J, Mascaro B, Combes AL, et al. (2012b). The impact of food branding on children’s eating behavior and obesity. Physiology & Behavior, 106(3), 379–386. 10.1016/j.physbeh.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, & Yurgelun-Todd DA (2003). Cortical and limbic activation during viewing of high-versus low-calorie foods. Neuroimage, 19(4), 1381–1394. [DOI] [PubMed] [Google Scholar]
- Labudda K, Woermann FG, Mertens M, Pohlmann-Eden B, Markowitsch HJ, & Brand M (2008). Neural correlates of decision making with explicit information about probabilities and incentives in elderly healthy subjects. Experimental Brain Research, 187(4), 641–650. [DOI] [PubMed] [Google Scholar]
- Lang PJ (2005). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Gainesville: Technical report. [Google Scholar]
- Masterson TD, Kirwan CB, Davidson LE, & LeCheminant JD (2016). Neural reactivity to visual food stimuli is reduced in some areas of the brain during evening hours compared to morning hours: An fMRI study in women. Brain Imaging and Behavior, 10(1), 68–78. [DOI] [PubMed] [Google Scholar]
- Maynard OM, Brooks JC, Munafò MR, & Leonards U (2017). Neural mechanisms underlying visual attention to health warnings on branded and plain cigarette packs. Addiction, 112(4), 662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure AC, Tanski SE, Gilbert-Diamond D, Adachi-Mejia AM, Li Z, Li Z, & Sargent JD (2013). Receptivity to television fast-food restaurant marketing and obesity among US youth. American Journal of Preventive Medicine, 45(5), 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott L, O’Sullivan T, Stead M, & Hastings G (2006). International food advertising, pester power and its effects. International Journal of Advertising, 25(4), 513–539. [Google Scholar]
- McGonigle DJ (2012). Test-retest reliability in fMRI: Or how I learned to stop worrying and love the variability. Neuroimage, 62(2), 1116–1120. 10.1016/j.neuroimage.2012.01.023. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Juhász C, Shen C, & Chugani HT (2000). Statistical parametric mapping: Assessment of application in children. Neuroimage, 12(5), 538–549. [DOI] [PubMed] [Google Scholar]
- Nicklas TA, Yang S-J, Baranowski T, Zakeri I, & Berenson G (2003). Eating patterns and obesity in children: The Bogalusa heart study. American Journal of Preventive Medicine, 25(1), 9–16. [DOI] [PubMed] [Google Scholar]
- Okada T, Tanaka S, Nakai T, Nishizawa S, Inui T, Sadato N, et al. (2000). Naming of animals and tools: A functional magnetic resonance imaging study of categorical differences in the human brain areas commonly used for naming visually presented objects. Neuroscience Letters, 296(1), 33–36. [DOI] [PubMed] [Google Scholar]
- Pelchat ML, Johnson A, Chan R, Valdez J, & Ragland JD (2004). Images of desire: Food-craving activation during fMRI. Neuroimage, 23(4), 1486–1493. [DOI] [PubMed] [Google Scholar]
- Powell LM, Schermbeck RM, & Chaloupka FJ (2013). Nutritional content of food and beverage products in television advertisements seen on Children’s programming. Childhood Obesity, 9(6), 524–531. 10.1089/chi.2013.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD (2000). Psychological and neural mechanisms of the affective dimension of pain. Science, 288(5472), 1769–1772. [DOI] [PubMed] [Google Scholar]
- Robinson TN, Borzekowski DL, Matheson DM, & Kraemer HC (2007). Effects of fast food branding on young children’s taste preferences. Archives of Pediatrics & Adolescent Medicine, 161(8), 792–797.17679662 [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht H-C, Klingebiel R, Flor H, & Klapp BF (2007). Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage, 37(2), 410–421. [DOI] [PubMed] [Google Scholar]
- Sanchez CE, Richards JE, & Almli CR (2012). Age-specific MRI templates for pediatric neuroimaging. Developmental Neuropsychology, 37(5), 379–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A, Schäfer A, Walter B, Stark R, & Vaitl D (2005). Brain activation of spider phobics towards disorder-relevant, generally disgust-and fear-inducing pictures. Neuroscience Letters, 388(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Somerville LH, & Casey B (2010). Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology, 20(2), 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, & Casey B (2010). A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition, 72(1), 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story M, & French S (2004). Food advertising and marketing directed at children and adolescents in the US. International Journal of Behavioral Nutrition and Physical Activity, 1(1), 3 10.1186/1479-5868-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, & Schad LR (2000). Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magnetic Resonance in Medicine, 44(3), 457–465. [DOI] [PubMed] [Google Scholar]
- Valkenburg PM, & Buijzen M (2002). Appeals in television advertising: A content analysis of commercials aimed at children and teenagers. Communications - the European Journal of Communication Research, 27(3), 349–364. [Google Scholar]
- Woo C-W, Krishnan A, & Wager TD (2014). Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage, 91, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokum S, Ng J, & Stice E (2011). Attentional bias to food images associated with elevated weight and future weight gain: An fMRI study. Obesity, 19(9), 1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokum S, Gearhardt AN, Harris JL, Brownell KD, & Stice E (2014). Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity, 22(12), 2544–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]


