Abstract
Background
The adoption of robot-assisted laparoscopic radical prostatectomy (RALP) has increased rapidly, despite lack of conclusive evidence of its superiority regarding long-term outcomes over open retropubic RP (RRP). In 2015, we reported on 12-mo follow-up from the LAPPRO trial showing a moderate advantage of RALP regarding erectile dysfunction. No significant differences were seen for urinary incontinence or surgical margin status.
Objective
To present patient-reported functional outcomes and recurrent and residual disease at 24-mo follow-up from the prospective multicenter LAPPRO trial.
Design, setting, and participants
A total of 4003 patients with clinically localized prostate cancer were recruited from 14 Swedish centers, seven performing RALP and seven RRP.
Outcome measurements and statistical analysis
Data were only analyzed for patients operated on by surgeons with >100 prior RPs. Adjusted odds ratios (AORs) were calculated using logistic regression, with adjustment for differences in patient mix.
Results and limitations
At 24 mo, there was a significant difference in erectile dysfunction in favor of RALP (68% vs 74%; AOR 0.72, 95% confidence interval [CI] 0.57–0.91; p = 0.006. No significant difference was observed for incontinence (19% vs 16%; AOR 1.29, 95% CI 1.00–1.67; p = 0.053) or recurrent or residual disease (13% vs 13%; AOR 0.79, 95% CI 0.59–1.07; p = 0.13). We did not adjust for individual surgeon volume and experience, which is a potential limitation.
Conclusions
Extended follow-up corroborated our previous report at 12 mo of a persistent RALP benefit regarding potency.
Patient summary
LAPPRO is a Swedish trial comparing two different prostate cancer surgical techniques (robotic compared to open). At 24-mo follow-up after surgery there was a moderate advantage for the robotic technique regarding erectile dysfunction (potency), while there was a small but not significant difference in urinary leakage in favor of open surgery. We did not find any difference regarding cancer relapse.
Keywords: Prostate cancer, Open radical prostatectomy, Robot-assisted laparoscopic radical prostatectomy, Urinary incontinence, Erectile dysfunction, Biochemical recurrence
1. Introduction
Surgical practice patterns for localized prostate cancer have changed rapidly in recent decades. Robot-assisted laparoscopic prostatectomy (RALP) has challenged traditional open retropubic RP (RRP) as the most common surgical technique. Many hospitals have invested in equipment for RALP, despite a lack of conclusive evidence of its superiority regarding long-term outcomes. Advantages of RALP compared to RRP for short-term outcomes such as blood loss and length of hospital stay have been reported [1,2]. However, prior studies have shown diverging results regarding long-term functional outcomes [3–7].
In 2015, our group reported on the 12-mo follow-up for the Swedish multicenter trial LAPPRO (ISRCTN 06393679), a prospective, controlled, nonrandomized trial comparing RALP and RRP. At 12 mo after surgery, no statistically significant differences were found regarding urinary incontinence, but a statistically significant advantage was seen for RALP regarding erectile dysfunction (ED). Surgical margin rates did not differ significantly between the procedures: the positive margin rate was 21% for RRP and 22% for RALP [4]. This report presents the 24-mo follow-up results for incontinence, ED, and recurrent or residual disease from the LAPPRO trial.
2. Patients and methods
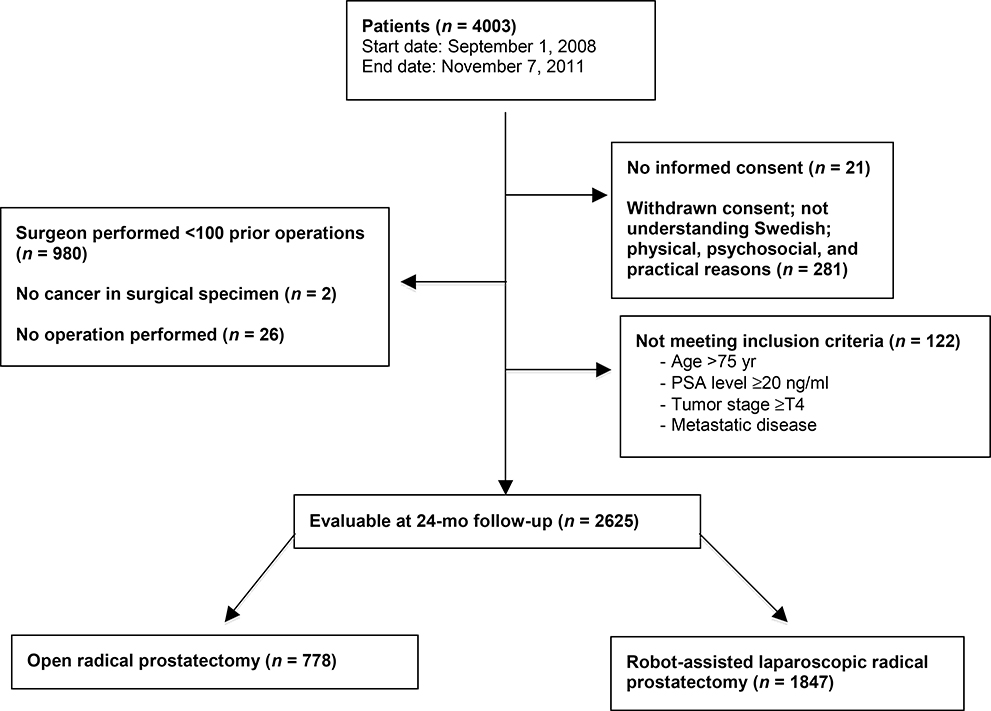
The study design has previously been published in detail [1,4,8]. In brief, 14 Swedish centers participated, of which seven performed RALP and seven RRP. Patients assigned for surgery between September 2008 and November 2011 were registered and eligible for inclusion to minimize selection bias. Inclusion criteria were: age <75 yr, prostate-specific antigen (PSA) < 20 ng/ml, tumor stage <T4, no metastatic disease, and informed consent. Clinical record forms and validated patient questionnaires were collected before and 3, 12, and 24 mo after surgery. Of the 4003 men registered in the LAPPRO trial, 3676 were eligible and were enrolled in the study, and underwent RALP or RRP. To avoid unwanted bias from inexperienced surgeons who might still be learning the surgeries, we only analyzed data from surgeons who had performed a minimum of 100 cases of RALP or RRP (n = 2625), similar to the 12-mo follow-up report. Among the 2625 patients analyzed, 778 underwent RRP and 1847 RALP. Patient characteristics are listed in Table 1 and details of the enrollment are shown in Figure 1.
Table 1 –
Patient, tumor, and surgical characteristics a
| Characteristic | RALP | RRP | p value |
|---|---|---|---|
| Patient characteristics | |||
| Patients, n (%) | 1847 (70) | 778 (30) | |
| Age at surgery (yr) | 63 (58–66) | 63 (59–67) | 0.035 |
| Smoking history | 0.2 | ||
| Never | 642 (35) | 300 (39) | |
| Former | 817 (44) | 332 (43) | |
| Current | 157 (8.5) | 57 (7.3) | |
| Unknown | 231 (13) | 88 (11) | |
| Body mass index (kg/m2) | 26 (24–28) | 26 (24–28) | 0.030 |
| <30 kg/m2 | 1424 (77) | 608 (78) | |
| ≥30 kg/m2 | 174 (9.4) | 74 (10) | |
| Unknown | 249 (13) | 95 (12) | |
| Partner at 24 mo | 1504 (81) | 635 (82) | 0.4 |
| Unknown | 172 (9.3) | 78 (10) | |
| History of cardiovascular disease | 570 (31) | 240 (31) | 0.9 |
| Unknown | 230 (12) | 91 (12) | |
| History of inguinal hernia | 266 (14) | 125 (16) | 0.3 |
| Unknown | 275 (15) | 110 (14) | |
| History of abdominal surgery | 362 (20) | 166 (21) | 0.3 |
| Unknown | 273 (15) | 109 (14) | |
| Diabetes | 102 (5.5) | 52 (6.7) | 0.3 |
| Unknown | 230 (12) | 88 (11) | |
| History of lung disease | 34 (1.8) | 19 (2.4) | 0.3 |
| Unknown | 232 (13) | 89 (11) | |
| History of mental disease | 57 (3.1) | 28 (3.6) | 0.5 |
| Unknown | 236 (13) | 88 (11) | |
| Continent at baseline | 1606 (87) | 674 (87) | 0.2 |
| Unknown | 224 (12) | 91 (12) | |
| Baseline erection sufficient for penetration | 1166 (63) | 488 (63) | 0.8 |
| Unknown | 260 (14) | 107 (14) | |
| Tumor and surgical characteristics | |||
| Degree of nerve sparing | <0.0001 | ||
| None | 531 (29) | 308 (40) | |
| Unilateral | 707 (38) | 167 (21) | |
| Bilateral | 603 (33) | 284 (37) | |
| Unknown | 6 (0.3) | 18 (2.3) | |
| Preoperative PSA (ng/ml) (n = 2597) | 6.1 (4.5–8.9) | 6.2 (4.5–9.0) | 0.8 |
| 0–4.4 ng/ml | 436 (24) | 189 (24) | |
| 4.5–6.1 ng/ml | 520 (28) | 196 (25) | |
| 6.2–9.1 ng/ml | 476 (26) | 200 (26) | |
| 9.2–20 ng/ml | 407 (22) | 173 (22) | |
| Unknown | 8 (0.4) | 19 (2.4) | |
| Pathology prostate weight (g) (n = 2577) | 42 (34–53) | 44 (36–55) | <0.0001 |
| 0–19 g | 18 (1.0) | 6 (0.8) | |
| 20–39 g | 792 (43) | 281 (36) | |
| 40–59 g | 719 (39) | 320 (41) | |
| 60–79 g | 209 (11) | 97 (12) | |
| ≥80 g | 86 (4.7) | 49 (6.3) | |
| Unknown | 23 (1.2) | 24 (3.1) | |
| Clinical T stage | 0.007 | ||
| T1 | 1099 (60) | 494 (64) | |
| T2 | 652 (35) | 218 (28) | |
| T3 | 57 (3.1) | 26 (3.3) | |
| Unknown | 39 (2.1) | 39 (5.0) | |
| Biopsy Gleason score | 0.7 | ||
| ≤7 | 1732 (94) | 716 (92) | |
| ≥8 | 102 (5.5) | 45 (5.8) | |
| Unknown | 13 (0.7) | 16 (2.1) | |
| Pathology T stage | 0.2 | ||
| T2 | 1287 (70) | 562 (72) | |
| T3 | 511 (28) | 190 (24) | |
| T4 | 10 (0.5) | 3 (0.4) | |
| Unknown | 39 (2.1) | 22 (2.8) | |
| Pathology Gleason score | 0.036 | ||
| ≤7 | 1674 (91) | 718 (92) | |
| ≥8 | 140 (7.6) | 41 (5.3) | |
| Unknown | 33 (1.8) | 18 (2.3) |
PSA = prostate-specific antigen.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables.
Fig. 1 -.
Flow chart for enrollment. Numbers may not sum properly as the same participant may have fulfilled more than one exclusion criterion. PSA = prostate-specific antigen.
Definitions of functional outcomes followed those for the 12-mo report with the amendment that all patients using intracavernosal or intraurethral treatment for ED were considered impotent [4]. As in the 12-mo publication, we used six different definitions for both incontinence and ED (listed in Table 2). The primary definition for incontinence was at least one protective pad used per 24 h, and for ED an erection insufficient for intercourse more than half of the time. Recurrent disease (PSA relapse) was defined as an initial undetectable PSA value at 3 mo after surgery follow by a measurable PSA level >0.25 ng/ml at 12 or 24 mo. Residual disease was defined as a measurable PSA level >0.25 ng/ml at 3-mo follow-up and/or postoperative treatment with radiotherapy, androgen deprivation therapy, and/or chemotherapy.
Table 2 –
Comparison of urinary incontinence, erectile dysfunction, and recurrence rates for various definitions at 24 mo after robot-assisted and open radical prostatectomy
| Outcome and adjustment a | Patients, n/N (%) |
OR (95% CI) | p value | ||
|---|---|---|---|---|---|
| Total | RRP | RALP | |||
| Urinary incontinence | |||||
| Change of pad at least once per 24 h | 2235 | 99/627 (16) | 305/1608 (19) | ||
| A | 1.32 (1.02–1.70) | 0.034 | |||
| A + B | 1.29 (1.00–1.67) | 0.053 | |||
| A + B + C | 1.33 (1.03–1.73) | 0.030 | |||
| Not pad-free or not leakage-free (night or day) | 2180 | 294/615 (48) | 806/1565 (52) | ||
| A | 1.21 (1.00–1.46) | 0.053 | |||
| A + B | 1.20 (0.99–1.46) | 0.060 | |||
| A + B + C | 1.22 (1.00–1.48) | 0.046 | |||
| More than 2 daytime leakage in last month | 2207 | 184/619 (30) | 512/1588 (32) | ||
| A | 1.16 (0.95–1.43) | 0.15 | |||
| A + B | 1.14 (0.93–1.41) | 0.2 | |||
| A + B + C | 1.18 (0.96–1.46) | 0.12 | |||
| Any daytime leakage in last month | 2186 | 279/618 (45) | 760/1568 (48) | ||
| A | 1.17 (0.97–1.42) | 0.10 | |||
| A + B | 1.17 (0.97–1.42) | 0.10 | |||
| A + B + C | 1.19 (0.98–1.45) | 0.075 | |||
| Do you have urinary leakage? | |||||
| A | 2242 | 87/628 (14) | 267/1614 (17) | 1.28 (0.98–1.68) | 0.066 |
| A + B | 1.24 (0.95–1.62) | 0.12 | |||
| A + B + C | 1.28 (0.97–1.68) | 0.079 | |||
| Urinary discomfort | 2212 | 185/621 (30) | 495/1591 (31) | ||
| A | 1.09 (0.89–1.33) | 0.4 | |||
| A + B | 1.07 (0.87–1.32) | 0.5 | |||
| A + B + C | 1.09 (0.89–1.34) | 0.4 | |||
| Erectile dysfunction | |||||
| Erection not sufficient for penetration b | 2168 | 446/603 (74) | 1072/1565 (68) | ||
| A | 0.75 (0.59–0.94) | 0.013 | |||
| A + B | 0.72 (0.57–0.91) | 0.006 | |||
| A + B + C | 0.75 (0.59–0.96) | 0.023 | |||
| IIEF-5 score c at 24 mo <17 | 2163 | 482/605 (80) | 1163/1558 (75) | ||
| A | 0.73 (0.57–0.94) | 0.014 | |||
| A + B | 0.69 (0.54–0.89) | 0.004 | |||
| A + B + C | 0.72 (0.56–0.94) | 0.015 | |||
| IIEF-5 score c at 24 mo <22 | 2152 | 541/601 (90) | 1314/1551 (85) | ||
| A | 0.60 (0.44–0.83) | 0.002 | |||
| A + B | 0.54 (0.39–0.75) | 0.0002 | |||
| A + B + C | 0.56 (0.40–0.78) | 0.001 | |||
| Penile stiffness <50% of the time | 2169 | 477/608 (78) | 1139/1561 (73) | ||
| A | 0.74 (0.58–0.94) | 0.014 | |||
| A + B | 0.70 (0.55–0.89) | 0.004 | |||
| A + B + C | 0.71 (0.55–0.92) | 0.010 | |||
| No spontaneous morning erection | 2152 | 530/602 (88) | 1272/1550 (82) | ||
| A | 0.62 (0.47–0.83) | 0.001 | |||
| A + B | 0.57 (0.43–0.77) | 0.0002 | |||
| A + B + C | 0.58 (0.43–0.78) | 0.0004 | |||
| No spontaneous morning erections and penile stiffness <50% of the time | 2172 | 461/609 (76) | 1085/1563 (69) | ||
| A | 0.72 (0.57–0.91) | 0.005 | |||
| A + B | 0.67 (0.53–0.85) | 0.001 | |||
| A + B + C | 0.69 (0.54–0.88) | 0.003 | |||
| Recurrent or residual disease A | 2157 | 83/633 (13) | 191/1524 (13) | 0.79 (0.59–1.07) | 0.13 |
RALP = robot-assisted laparoscopic prostatectomy; RRP = open retropubic radical prostatectomy; CI = confidence interval; OR = odds ratio; IIEF = International Index of Erectile Function.
Adjustment A for urinary incontinence: age, preoperative continence, body mass index, history of inguinal hernia, prior abdominal surgery, diabetes, history of pulmonary disease, history of mental disorder, and pathology prostate weight; for erectile dysfunction: age, preoperative potency, diabetes, history of inguinal hernia, smoking status, relationship status, and history of cardiovascular disease; and for recurrent or residual disease: pathology Gleason grade, pathology T stage, preoperative prostate-specific antigen, and pathology prostate weight. Adjustment B: clinical T stage, preoperative prostate-specific antigen, biopsy Gleason grade, and length of cancer in biopsy cores. Adjustment C: degree of neurovascular bundle preservation.
IIEF questionnaire, question 3: “When you had erections with sexual stimulation, how often was your erection hard enough for penetration during the last 3 months?” with cutoff between response 2 and 3. The following responses were available: 0, no sexual activity; 1, almost never or never; 2, a few times (much less than half the time); 3, sometimes (about half the time); 4, most times (much more than half the time); and 5, almost always or always.
IIEF questionnaire modified version with five questions, six answer categories, 0–5 points per question; score <17 = erectile dysfunction; score <22 = some erectile function.
Logistic regression was used to compare RALP and RRP with type of surgery as a covariate. For functional outcomes, adjustments for confounders were identical to those used in the 12-mo follow-up publication [4].
For urinary incontinence, the regression model was adjusted for age at surgery, preoperative continence, body mass index (BMI), history of inguinal hernia, prior abdominal surgery, diabetes, history of pulmonary disease, history of mental disorder, and pathology prostate weight; this is referred to as adjustment set A in Table 2. The adjusted odds ratio (AOR) associated with surgery type is reported as the estimate of the difference between surgical modalities. We also reported the results of a model additionally adjusted for tumor factors (clinical T stage, preoperative PSA, biopsy Gleason grade, and length of cancer in the biopsy cores), referred to as adjustment set A + B in Table 2. Finally, we reported the results of a third regression model that was additionally adjusted for nerve-sparing status, referred to as adjustment set A + B + C in Table 2.
Differences in ED rates between RALP and RRP were also assessed via logistic regression. Adjustment set A consisted of age at surgery, preoperative potency, diabetes, history of inguinal hernia, smoking status, relationship status at 2 yr, and history of cardiovascular disease. Adjustment sets B and C (ie, tumor factors and nerve-sparing status) are the same as for urinary incontinence.
Recurrent or residual disease within 24 mo of RP was assessed via Cox proportional hazards regression. The regression model included type of surgery and was adjusted for Gleason grade in the prostatectomy specimens, pathological T stage, preoperative PSA, and prostate weight from the pathology report.
Missing covariate data unrelated to tumor characteristics and surgical decisions were imputed 50 times using multiple chained equations. Results across the 50 imputed data sets were combined using Rubin’s method. Owing to missing data in the outcome and non-imputed covariate data, analyses involved 2186–2242 patients for urinary incontinence, 2152–2169 patients for ED, and 2157 patients for assessing differences in recurrent or residual disease. All analyses were conducted using Stata 15.0 (Stata Corp., College Station, TX, USA).
3. Results
A total of 2625 men were eligible for analyses: 1847 after RALP and 778 after RRP. The response rate to self-reported patient questionnaires at 24 mo after RP was high (96%). Patient and tumor characteristics are presented in Table 1. Patient characteristics were generally well matched between the groups, except that patient undergoing RRP had higher BMI. There were significant differences between the groups for clinical T stage, pathology prostate weight, and the degree of nerve-sparing surgery, which was frequent in the RALP group, as previously reported.
3.1. Incontinence rate
Using our primary definition of incontinence, after adjustment for patient and preoperative tumor factors (A + B) there was a small, although not statistically significant, difference in the incontinence rate between RALP and RRP (19% vs 16%, AOR 1.29, 95% confidence interval [CI] 1.00–1.67; p = 0.053). When adjusting only for patient factors (A) or adding the degree of nerve sparing to the model (A + B + C) the results did not substantially change but the difference between the groups reached statistical significance (adjustment A: AOR 1.32, 95% CI 1.02–1.70; p = 0.034; adjustment A + B + C: AOR 1.33, 95% CI 1.03–1.73; p = 0.030; Table 2).
When using other definitions of incontinence and adjusting for the same parameters as mentioned above, a small but not significant benefit for RRP was observed (Table 2).
3.2. Erectile dysfunction
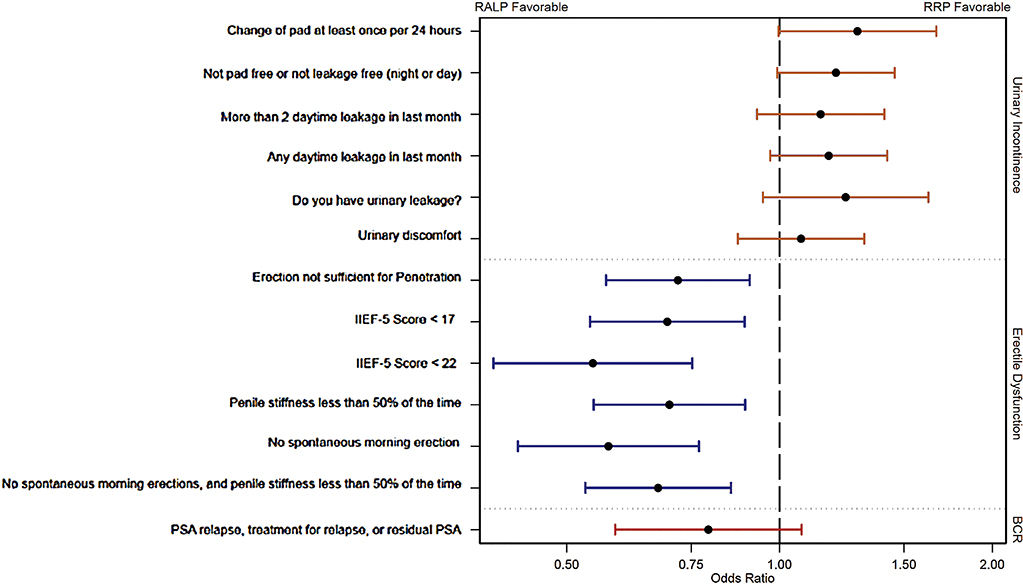
For our primary ED definition, after adjustment for patient and preoperative tumor factors (A + B) there was a significant difference between the groups in favor of RALP (68% vs 74%; AOR 0.72, 95% CI 0.57–0.91; p = 0.006). Using different adjustments (A alone or A + B + C) did not markedly change the results (adjustment A: AOR 0.75, 95% CI 0.59–0.94; p = 0.013; adjustment A + B + C: AOR 0.75, 95% CI 0.59–0.96; p = 0.023). With different definitions of ED, the statistically significant benefit for RALP remained (Table 2, Fig. 2).
Fig. 2 -.
Forest plots showing the adjusted odds ratio and 95% confidence interval for comparison between robot-assisted laparoscopic prostatectomy (RALP) and open retropubic radical prostatectomy (RRP) for each 24-mo outcome. Adjustments for urinary incontinence and erectile dysfunction are A + B as described in Table 2. Results are presented on a logarithm scale. BCR = recurrent or residual disease.
3.3. Oncological outcome
Only 88 patients (4%) experienced PSA relapse at 12- or 24-mo follow-up after an initial undetectable PSA value at 3 mo. In total, 274 of 2157 men (13%) had recurrent or residual disease according to our definition (Table 2). Among these patients, 186 (8.6%) had residual disease from a measureable PSA at 3 mo after RP and/or received adjuvant treatment with radiotherapy, androgen deprivation therapy, and/or chemotherapy. There was no significant difference between the surgical procedures for recurrent or residual disease (12.5% for RALP vs 13.1% for RRP; AOR 0.79, 95% CI 0.59–1.07; p = 0.13).
4. Discussion
With extended follow-up time of 24 mo, the results from our previously published 12-mo report remain essentially unchanged. The advantage for RALP regarding ED was confirmed, and the absolute difference increased from 5% to 6%. As illustrated in Table 2 the incontinence rate was slightly lower in the RRP group (16% vs 19%) but did not reach statistical significance when adjusting for patient- and tumor-related confounders (A + B). For both ED and incontinence outcomes, adding the degree of nerve sparing (A + B + C) as a confounder or only adjusting for patient-related confounders (A) did not markedly change the results. However, for incontinence these changes did increase the lower bound of the confidence interval beyond 1.00, resulting in a statistically significant benefit for RRP (p = 0.030 and p = 0.034).
In 2017, Sooriakumaran et al [9] reported a LAPPRO analysis for ED over time and a subgroup analysis of D’Amico risk groups. RALP was favorable in D’Amico low-risk and intermediate-risk groups, while RRP was favorable in the high-risk group. The authors also found that correlation between the degree of neurovascular bundle preservation and ED was greater for RALP. Although there were minor differences in how the cohort was analyzed compared to the present study, the results indicate that a deeper analysis after stratification into different risk groups may identify absolute differences between surgical procedures. In the present study, we did not find any overall difference in recurrent or residual disease between RALP and RRP, whereas Sooriakumaran et al reported that there was no significant difference for pT2 tumors, but for pT3 tumors the rate was higher in the RRP group in subgroup analyses.
Few studies have been published comparing the long-term functional outcomes of the surgical treatments for prostate cancer versus observation or radiation therapy. The SPCG-4 trial randomly assigned men to RP or watchful waiting between 1989 and 1999. Among the 182 men allocated to RP, 66% reported ED at 4 yr and 81% at 12 yr. The incontinence rate, defined as incontinence once a day or more, was 27% at 4 yr and 41% at 12 yr [10]. Direct comparison with the data we report here is difficult because the definitions of ED and incontinence differ and the cohorts are not similar in terms of age. In addition, the surgical technique may have improved during the last 20 yr.
In the PIVOT trial, 731 men with localized prostate cancer were randomly assigned to RP or observation and patient-reported functional outcomes were captured. At 2-yr follow-up after RP, the incontinence rate was 17%, similar to our data, and the ED rate was 81%, compared to 68–74% in our study [11].
The more recent ProtecT study enrolled men with localized prostate cancer for randomization to RP, radical radiotherapy, or active monitoring. Patient-reported outcome measures collected from 553 men undergoing RP showed that the incontinence rate stabilized after 2 yr and was reported as 17% at 6-yr follow-up. These results are also similar to our study. The ED rate reported also stabilized at 2-yr follow-up after surgery in the ProtecT study and at approximately the same level as in our study [12,13].
Results from a population-based study of 1141 men comparing quality of life at 3, 12 and 24 mo after RP, external beam radiotherapy and brachytherapy versus active surveillance were recently reported by Chen et al [14]. In accordance with the present study, they did not find any major difference in incontinence or ED among the 469 men who underwent RP when 12- and 24-mo results were compared.
A limited number of reports from other groups comparing long-term outcomes after RRP and minimally invasive techniques such as RALP and laparoscopic RP (LRP) have reported somewhat diverging results. In 2012, Barry et al [5] did not find any significant differences regarding incontinence or ED between groups in a retrospective population-based study with median follow-up of 14 mo. By contrast, Hu et al [3] reported a significant advantage for RRP over minimally invasive techniques (RALP and LRP in combination) for both incontinence and potency rates. Ficarra and co-authors [6,7] reviewed the current literature in 2012 and reported a small but significant benefit for both incontinence and ED in favor of RALP.
In contrast to other studies, LAPPRO is a large, prospective, multicenter study that includes patients undergoing surgery at hospitals of different size in Sweden. Detailed preoperative, perioperative, and postoperative clinical record forms, as well as patient-reported information for functional outcomes with a high response rate (96%), are another strength of our study. Surgical volume, experience, and skill are factors that have a major impact on both functional and oncological outcomes after RP [15,16]. In this study, we did not adjust for individual experience and annual RP volume among the participating surgeons, which, alongside the lack of randomization, is a limitation. Future studies using the LAPPRO cohort are planned to explore the effects of surgeon heterogeneity in terms of experience and annual RP caseload.
5. Conclusions
With longer follow-up (24 mo) in this large, prospective, multicenter study comparing RALP and RRP, functional outcomes were unchanged from those reported after 12 mo. A significant difference in potency favoring RALP remained. For continence a small, but not significant, benefit was observed for RRP. We did not find any difference in recurrent or residual disease between RALP and RRP at 24 mo.
Acknowledgments
Funding/Support and role of the sponsor: This study was supported by research grants from the Swedish Cancer Society (2008/922, 2010/593, 2013/497, 2016/362), The Swedish Research Council (2012-1770. 2015-02483), Region Västra Götaland, Sahlgrenska University Hospital (ALFBGB grants 13875, 146201, 4307771; HTA–VGR 6011; agreement concerning research and the education of doctors), the Mrs. Mary von Sydow Foundation, and the Anna and Edvin Berger Foundation. Martin Nyberg and Anders Bjartell were supported by research grants from Region Skåne, Sweden, Lund University, and The Swedish Cancer Society (2014/2783). Sigrid V. Carlsson’s role in this work was supported in part by funds from the Sidney Kimmel Center for Prostate and Urologic Cancers, a Specialized Programs of Research Excellence grant (P50-CA92629) from the National Cancer Institute to Dr. Howard Scher, a National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30-CA008748) to Memorial Sloan Kettering Cancer Center, a grant from the National Cancer Institute as part of the Cancer Intervention and Surveillance Modelling Network (U01CA199338-02), and the David H. Koch Prostate Cancer research fund. None of the sponsors had any access to the data or any influence on or access to the analysis plan, the results, or the manuscript.
Financial disclosures: Anders Bjartell certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Appendix A. Additional members of the LAPPRO group
Bo Anderberg, Department of Surgery, Capio St. Görans Hospital, Stockholm, Sweden
Ingela Björholt, Nordic Health Economics, Göteborg, Sweden
Thomas Jiborn, Department of Urology, Skåne University Hospital, Lund University, Malmö, Sweden
Ove Gustafsson, Department of Molecular Medicine and Surgery, Section of Urology, Karolinska Institutet, Stockholm, Sweden
Ali Khatami, Department of Urology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Sahlgrenska University Hospital, Göteborg, Sweden
Mikael Wulker-Sylmé, Department of Urology, Varberg Hospital, Varberg, Sweden
Christer Edlund, Department of Surgery, Kungsbacka Hospital, Kungsbacka, Sweden
Erik Pileblad, Capio Lundby Hospital, Göteborg, Sweden
Hans Boman, Department of Surgery, Borås Hospital, Borås, Sweden
Ola Bratt, Department of Urology, Helsingborg Hospital, Helsingborg, Sweden
Ulrika Westlund, Department of Urology, Södersjukhuset, Stockholm, Sweden
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
At 24-mo follow-up a moderate difference in favor of robot-assisted laparoscopic radical prostatectomy (RP) over open retropubic RP was observed for erectile dysfunction, and a small, but not statistically significant, difference in urinary incontinence was observed in favor of open surgery. There was no difference regarding recurrent or residual disease.
References
- 1.Wallerstedt A, Tyritzis SI, Thorsteinsdottir T, et al. Short-term results after robot-assisted laparoscopic radical prostatectomy compared to open radical prostatectomy. Eur Urol 2015;67:660–70. [DOI] [PubMed] [Google Scholar]
- 2.Yaxley JW, Coughlin GD, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet 2016;388:1057–66. [DOI] [PubMed] [Google Scholar]
- 3.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA 2009;302:1557–64. [DOI] [PubMed] [Google Scholar]
- 4.Haglind E, Carlsson S, Stranne J, et al. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. Eur Urol 2015;68:216–25. [DOI] [PubMed] [Google Scholar]
- 5.Barry MJ, Gallagher PM, Skinner JS, Fowler FJ. Adverse effects of robotic-assisted laparoscopic versus open retropubic radical prostatectomy among a nationwide random sample of Medicare-age men. J Clin Oncol 2012;30:513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ficarra V, Novara G, Ahlering TE, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol 2012;62:418–30. [DOI] [PubMed] [Google Scholar]
- 7.Ficarra V, Novara G, Rosen RC, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol 2012;62:405–17. [DOI] [PubMed] [Google Scholar]
- 8.Thorsteinsdottir T, Stranne J, Carlsson S, et al. LAPPRO: a prospective multicentre comparative study of robot-assisted laparoscopic and retropubic radical prostatectomy for prostate cancer. Scand J Urol Nephrol 2011;45:102–12. [DOI] [PubMed] [Google Scholar]
- 9.Sooriakumaran P, Pini G, Nyberg T, et al. Erectile function and oncologic outcomes following open retropubic and robot-assisted radical prostatectomy: results from the LAParoscopic Prostatectomy Robot Open trial. Eur Urol 2018;73:618–27. [DOI] [PubMed] [Google Scholar]
- 10.Johansson E, Steineck G, Holmberg L, et al. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol 2011;12:891–9. [DOI] [PubMed] [Google Scholar]
- 11.Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med 2017;377:132–42. [DOI] [PubMed] [Google Scholar]
- 12.Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamdy FC, Donovan JL, Lane JA, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016;375:1415–24. [DOI] [PubMed] [Google Scholar]
- 14.Chen RC, Basak R, Meyer AM, et al. Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. JAMA 2017;317:1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlsson S, Berglund A, Sjoberg D, et al. Effects of surgeon variability on oncologic and functional outcomes in a population-based setting. BMC Urol 2014;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vickers A, Savage C, Bianco F, et al. Cancer control and functional outcomes after radical prostatectomy as markers of surgical quality: analysis of heterogeneity between surgeons at a single cancer center. Eur Urol 2011;59:317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]