Abstract
In order to make effective collective decisions, ants lay pheromone trails to lead nest-mates to acceptable food sources. The strength of a trail informs other ants about the quality of a food source, allowing colonies to exploit the most profitable resources. However, recruiting too many ants to a single food source can lead to over-exploitation, queuing, and thus decreased food intake for the colony. The nonlinear nature of pheromonal recruitment can also lead colonies to become trapped in suboptimal decisions, if the environment changes. Negative feedback systems can ameliorate these problems. We investigated a potential source of negative feedback: whether the presence of nest-mates makes food sources more or less attractive. Lasius niger workers were trained to food sources of identical quality, scented with different odours. Ants fed alone at one odour. At the other odour ants fed either with other feeding nest-mates, or with dummy ants (black surface lipid-coated glass beads). Ants tended to avoid food sources at which other nest-mates were present. They also deposited less pheromone to occupied food sources, suggesting an active avoidance behaviour, and potentiating negative feedback. This effect may prevent crowding at a single food source when other profitable food sources are available elsewhere, leading to a higher collective food intake. It could also potentially protect colonies from becoming trapped in local feeding optima. However, ants did not avoid the food associated with dummy ants, suggesting that surface lipids and static visual cues alone may not be sufficient for nest-mate recognition in this context.
Keywords: negative feedback, ants, crowding, foraging behaviour, food choice, pheromone deposition
1. Introduction
Distributing labour and coordinating collective tasks is a challenge faced by both social insects and human societies. A critical challenge is to allocate effort to where it will be most productive, while avoiding crowding consequences and queuing costs. This must be achieved without centralised control in both social insect societies, and in many human endeavours (e.g. distributed computation, telecommunication networks). Social insects have developed numerous strategies to inform nest-mates about valuable food sources, allowing collective exploitation of resources in the surrounding environment. For example, honeybees can share information about the direction and distance of food sources with other nest-mates via the waggle dance [1]. Both the duration and the number of waggle runs increase when bees dance for higher food qualities, thus increasing the likelihood that foragers are recruited to better resources [2,3]. In ants, information about distance and direction is not directly shared with nest-mates. Instead, many ant species deposit pheromone trails when returning from a resource, such as a food source or new nest site. These pheromone trails lead other nest-mates to newly discovered food sources. The more pheromone ants lay when returning from a food source, the stronger the trail will be. Ants can modulate both their decision to deposit pheromone, and the intensity of pheromone deposition [4,5]. Both trail lay rates and pheromone deposition intensity increase as the perceived value of the resources increases [6–8]. Stronger trails result in more ants being recruited from the nest and a higher proportion of ants following the trail at a bifurcation [5,9,10]. This simple system results in a positive feedback mechanism, leading to colonies often collectively focusing their foraging effort on the most valuable resources [4,11–14].
However, recruiting too many nest-mates to a food source can lead to crowding and an overexploitation of food sources, and thus decrease colony food intake [15,16]. For example, although crowding increases foraging efficiency in leaf-cutter ants [17], it decreases walking speed of ants affected by head-on collisions [18] and can have negative effects in other species as well. Many natural food sources are limited by quantity and replenishment time. For example, honeydew-producing aphids or extrafloral nectaries slowly produce a variable amount of food over the course of a day [19–21]. Depending on an ant's crop capacity, even a single individual may have to spend around 40 min at an extrafloral nectary in order to fill its crop [22], and ants were shown to invest much time in patrolling these food sources [20,23]. Due to long replenishment times, small groups of ants may be capable of fully exploiting even larger patches of aphid colonies or extrafloral nectaries. If a resource is fully exploited, recruiting more individuals will lead to increased waiting times and foragers returning to the nest without food. An optimal distribution of foragers would allocate foragers to a resource until the efforts of any additional forager would be better focused on a different resource.
Decentralised decision-making in non-limited situations also poses challenges for positive-feedback based coordination systems. If recruitment feedback is nonlinear, as is the case in mass-recruiting ants which deposit pheromone trails [7,11,24,25], recruitment can rapidly become extremely strong to one option. If the environment then changes, colonies may not be able to break out of their previous decision and become trapped in exploiting a temporal local optimum [11,12,26,27], but also see [28].
The problems of overexploitation and crowding, and of trapping in local optima, can be ameliorated or overcome by building negative feedback into the collective decision-making system. Social insects have developed a number of negative feedback systems which decrease the number of recruited nest-mates as recruitment progresses. These systems include both active and passive processes [29,30]. Honeybees, for example, use an acoustic signal as an active inhibitory stop signal, stopping returning foragers from recruiting [29,31–35]. Ants reduce pheromone deposition when walking on a pheromone laden path and when encountering other nest-mates on the trail [30,36].
In addition to active recruitment signals, social insects also rely on cues when deciding where to forage. A very important cue for a wide variety of animals is the presence of fellow foragers, both con- and heterospecific [37,38]. In ants, as in other social insects, cuticular hydrocarbons (CHCs) are used to identify and distinguish nest-mates from non-nest-mates [39–41]. The presence of nest-mates has successfully been mimicked by presenting glass beads coated with nest-mate CHCs in ants [42–45]. The presence of conspecifics provides information about the safety and productivity of a foraging patch. Naive bumblebees, for example, prefer to visit food sources at which conspecifics are already present [46–49]. Ants show a similar behavioural pattern, preferentially choosing to follow paths on which other nest-mates are present [50]. This is somewhat at odds with the finding that ants reduce recruitment in the presence of others ants [30,36]. This highlights a trade-off foragers have to make: well-used patches imply productivity and safety, but also competition for resources and potential over-exploitation.
The aim of this study was twofold: firstly, we ask whether the presence of nest-mates at a food source (as opposed to on a trail [30]) triggers a negative-feedback effect by reducing recruitment. Secondly, we ask whether unoccupied food sources are more attractive than otherwise equally profitable occupied food sources. We trained individual ants to two alternating food sources associated with different odours. At one food source, ants fed alone. At the other, either live nest-mates or black glass beads coated with surface lipids (containing CHCs and any other lipids soluble in dichloromethane) were present at the food source. After training, odour preference was tested. If nest-mate presence has an inhibitory effect in this context, the ants should follow the odour associated with feeding alone, and deposit less pheromone when returning from occupied food sources. By contrast, if nest-mate presence enhances the attractiveness of a food source, ants should prefer the odour associated with the presence of nest-mates or lipid-coated glass beads, and deposit more pheromone when returning from occupied feeders. Several studies have considered the effect of crowding on trails or at a food source on individual and collective path and food source choice [51,52], and shown that massive overcrowding can lead to individual ants being physically ‘pushed' towards alternative options. Other studies have shown that the presence of nest-mates on paths can affect individual and collective path use by decreasing U-turning [50] and reduce pheromone deposition [30]. The presence of pheromone on paths has also been shown to reduce further pheromone deposition [36] Our study is the first to investigate the effect of nest-mate presence (not massive crowding) at the food source on the evaluation of food sources by individual ants. It is also the first to investigate the effect of nest-mate presence on food source evaluation using associative learning and binary choice assays, as opposed to simply considering pheromone deposition.
2. Methods
2.1. Study animals
Eight stock colonies of the black garden ant Lasius niger were collected on the University of Regensburg campus. Lasius niger derive much of their carbohydrate intake from tending honeydew-producing insects [54], but do not show task specialisation within aphid-tenders (e.g. to guards, shepherds, and transporters, [55]. The colonies were housed in 30 × 30 × 10 cm foraging boxes with a layer of plaster covering the bottom. Each box contained a circular plaster nest-box (14 cm diameter, 2 cm height). The colonies were queenless with around 1000–2000 workers and small amounts of brood. Queenless colonies forage and lay pheromone trails, and are frequently used in foraging experiments [51,56]. As foragers rarely interact with the queen [57], a lack of queen (but not brood, see [58]) should have little effect on the details of forager behaviour. The colonies were fed with 0.5 M sucrose solution and received Drosophila fruit flies once a week. Water was available ad libitum. Colonies were starved for 4 days prior to the experiments in order to achieve a uniform and high motivation for foraging [59,60]. During starvation, water was available ad libitum.
2.2. Set-up and experimental procedure
2.2.1. Overview
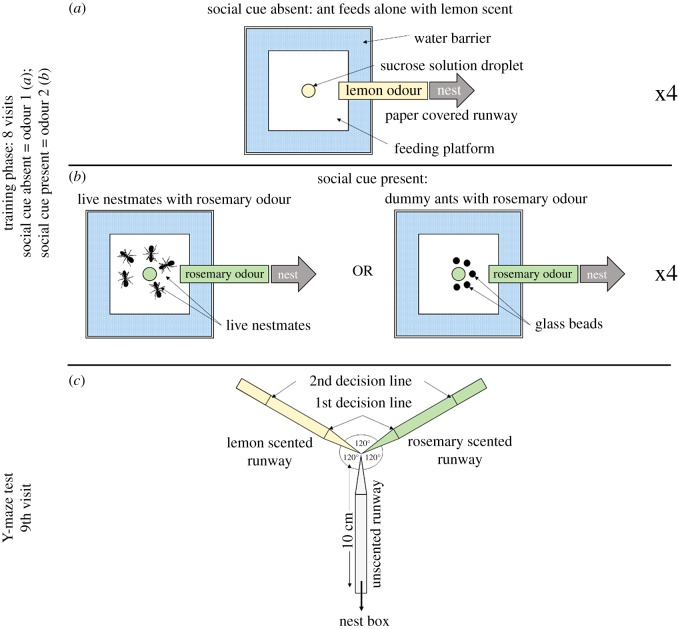
All ants underwent a similar training protocol: individual ants were allowed to make repeat visits to a food source, which was alternatingly surrounded by social cues (other nest-mates or dummy ants) and had alternating scents (figure 1). Pheromone deposition was measured on each return to the nest. After eight training visits ants were allowed to choose between social- or non-social feeding odour cues in a binary choice assay.
Figure 1.
(a,b) Experimental set-up used during training visits 1–8. A 1 M sucrose solution droplet was placed in the centre of a platform surrounded by a water barrier. The platform was connected to the nest via a paper-covered 20 cm long runway and a 40 cm drawbridge. (a) The platform for visits on which the social cue was absent. In this case, sucrose solution was presented with one odour (lemon or rosemary; lemon odour in the example used in figure 1) on the runway and in the food. (b) The platform for visits on which the social cue was present. In this case, sucrose solution was presented with another odour on the runway and in the food (lemon or rosemary; rosemary odour in the example used in figure 1). Half of the tested ants were confronted with live nest-mates as a social cue, the other half was confronted with dummy ants (black surface lipid-coated glass beads). Social cue presence (and the associated odours) alternated each visit. (c) Y-maze used on the 9th (test) visit. All arms were 10 cm long. The arm connected to the nest-box was covered with unscented paper overlays while the other two arms were covered with lemon and rosemary scented paper overlays (one scent on each side). The first decision line was located 2.5 cm from the Y-maze centre and marked the initial decision of an ant while the second decision line was placed 7.5 cm from the centre and marked the final decision.
2.2.2. Training
Two to four foragers were given access to a 20 × 1 cm long plastic runway overlaid with scented paper via a 40 cm long drawbridge. Paper overlays were scented by storing them for at least 1 day in an airtight box containing a droplet of either lemon or rosemary essential oil (rosemary: Rosmarinus officinalis; lemon: Citrus limon, Markl GbR, Grünwald) on filter paper in a Petri dish. Previous work has shown that Lasius niger foragers can form robust expectations of upcoming reward quality based on lemon or rosemary runway odour after just 1 visit to each odour/quality combination [8,61,62]. A 5 mm diameter drop of scented 1 M sucrose solution (Sigma-Aldrich) was placed in the centre of a feeding platform (4 × 4 cm; figure 1) surrounded by a water barrier (1.75 cm wide and 1.3 cm deep, platform size including the surrounding water barrier: 7.5 × 7.5 cm) at the end of the runway (60 cm from the nest). The solutions were scented using either rosemary or lemon essential oils (0.5 µl essential oil per ml sucrose solution). The first ant to reach the feeder was marked with a dot of acrylic paint (el Greco Acrylic Colors, C. Kreul, Germany) on its abdomen. The marked ant was allowed to drink to repletion at the food source, while all other ants were returned to the nest. When the ant had filled its crop, it was allowed to walk back into the nest. Inside the nest, the ant unloaded its crop to its nest-mates and was then allowed back onto the runway for another visit. The drawbridge was now used to selectively allow only the marked ant onto the runway.
The ant was allowed to make eight return visits to the feeder, with alternating odour cues on each subsequent visit: in half of the visits, ants were allowed to feed alone in the presence of one odour. In the other visits, ants fed together with either (i) five other nest-mates or (ii) five black lipid-coated glass beads (dummy ants which were placed in a semicircle around the sucrose droplet at a distance of 5 mm, allowing unlimited access to the food without disturbance) in the presence of a second odour (figure 1, see below for dummy ant creation details). Sucrose solutions and the runway overlays were scented, with the ‘nest-mate' and ‘alone' treatments each having a fixed odour. For half of the ants, lemon was associated with the ‘nest-mate' treatment and rosemary with the ‘alone' treatment, and vice versa for the other ants. Companion nest-mates were gently placed onto the feeding platform shortly before the test ant arrived by allowing them to walk onto a piece of paper, and walk off the paper onto the platform. They displayed no signs of alarm behaviour and fed calmly at the food source when they discovered it. It is thus unlikely that they emitted alarm pheromones which may have led to the test ant avoiding this food source [63]. Mimicking nest-mates with dummy ants allowed us to control for movement cues, potential local feeding cues [64], and pheromone which may be deposited on the runway or the feeding platform by foraging or returning nest-mates. In Lasius niger, black glass beads coated in nest-mate lipids were shown to have a greater effect on ant behaviour compared to clear beads [30]. In this study, we thus used black surface lipid-covered beads to mimic nest-mates. Blank beads which were not covered in nest-mate surface lipids did not affect ant behaviour in other studies simulating crowding [30,50], suggesting that ants note surface lipid-covered beads as nest-mates while blank beads are ignored.
As the ant returned to the nest from the food source, we counted the number of pheromone depositions performed. Individual pheromone deposition behaviour correlates with the (perceived) quality of a food source [4,5,8,61]. Individual ants can adapt the strength of a pheromone trail by either depositing pheromone or not, or varying the intensity of pheromone depositions [4,5]. Pheromone deposition behaviour in Lasius niger is highly stereotypic. To deposit pheromone, an ant briefly interrupts running to bend its gaster and press the tip onto the ground [6]. Pheromone depositions were measured each time the ant moved from the food source back to the nest (inward trip), and each time the ant moved from the nest towards the food source (outward trip). Because Lasius niger foragers almost never lay pheromone when they are not aware of a food source [6], we did not measure pheromone depositions for the very first outward trip (visit 1). The presence of trail pheromone on a path depresses further pheromone deposition [36]. Thus, each time an ant had passed the 20 cm runway, the paper overlay covering the runway was replaced by a fresh one.
2.2.3. Choice tests
On the ninth visit (the testing phase), the linear runway was replaced with a Y-maze (figure 1c), with two 10 cm long arms and a 10 cm long stem. The Y-maze stem was covered with an unscented paper overlay while one arm was covered with the odour overlay associated with the social cue present (e.g. rosemary), and the other with the odour overlay associated with the social cue absent (e.g. lemon). The trained ant was allowed to choose between the two arms, and its decision was recorded. We used two decision lines to define arm choice—an initial decision line (figure 1c, 2.5 cm after the bifurcation) and a final decision line (7.5 cm after the bifurcation). After an ant had made a choice, it was allowed to walk onto a piece of paper at the end of the Y-maze arm and moved to the beginning of the Y-maze in order to allow it to make another choice. This was repeated until an ant had made 3–8 choices in the Y-maze (for a detailed overview of ant choices split by visit number in the Y-maze please refer to electronic supplementary material, figure S6). The number of choices made by an ant depended on its motivation to make another choice, but was limited to a maximum of eight choices.
After each experimental run, the ant was permanently removed from the colony. In addition to observations by a student sitting next to the experimental set-up, all experimental runs were recorded with a Panasonic DMC-FZ1000 camera.
While data could not be collected blind, as the presence of nest-mates or dummy ants could not be hidden, we used strict behavioural definitions. More importantly, data were collected by a naive experimenter blind to any a priori hypotheses about the data, thus protecting against unconscious bias.
2.3. Preparation of dummy ants
To simulate the presence of other nest-mates, we used black glass beads (dummy ants) coated in nest-mate surface lipids, which included CHCs. CHC profiles differ between colonies and allow ants to identify nest-mates and distinguish them from non-nest-mates [65]. CHC-coated glass beads are regularly used to mimic nest-mates [30,43,66] and non-nest-mates [42,45,67] in ants, including Lasius niger. Bead preparation followed Czaczkes et al. [30]: clean black glass beads (diameter 2.5 mm, height 1 mm; KnorrPrandell GmbH, Lichtenfels, Germany) were first washed with pentane multiple times, then baked for 1–2 h at 300°C and again washed with pentane after baking to remove any substances or odours which may interfere with nest-mate identification. To coat the beads in ant CHC-profiles, 20 foragers out of the colony to be tested were freeze-killed at −20°C for about 10 min. The ants were then placed in a 2 ml extraction vial (Sigma-Aldrich) and covered in pentane. To dissolve the surface lipids from the ants' cuticle, the vial was agitated for 5 min at 30°C. To ensure that the dummy ants were as realistic as possible, no further steps to purify the CHCs were taken, and thus the beads were coated with nest-mates CHCs and other surface lipids which may have been present on the nest-mate cuticle. In the next step, the ants of which surface lipids had been dissolved were removed from the pentane solution containing ant surface lipids and eight black glass beads (diameter 2.3 mm, height 1.5 mm) were placed into the solution instead. The solution and beads were then again agitated at 30°C until all the pentane had evaporated. This procedure left the beads coated in surface lipids.
A pilot aggression test revealed that surface lipid-coated beads elicited aggressive behaviour such as mandible opening [68] when they were coated with non-nest-mate surface lipid, while no aggressive behaviour was shown when beads were coated with nest-mate surface lipid. This suggests that beads were sufficiently coated to allow ants to recognize them as other ants and differentiate between nest-mates and non-nest-mates.
2.4. Statistical analysis
Statistical analyses were carried out in R v. 3.5.0 [69] using generalized linear mixed models (GLMMs) in the LME4 package [70]. As multiple ants were tested per colony, and we took multiple measurements from each ant, colony identity and individual ant identity nested in colony identity were added as random effects to each model. GLMMs were tested for fit, dispersion and zero inflation using the DHARMa package [71]. The model predictors and interactions were defined a priori, following Forstmeier & Schielzeth [72]. All p-values presented were corrected for multiple testing using the Benjamini–Hochberg method [73]. A total of 49 ants (3–11 ants per colony) were confronted with real nest-mates as a social cue, making a total of 278 choices. In the bead treatment, a total of 43 ants (3–10 ants per colony) was tested, of which 248 choices were made.
2.4.1. Choice tests
The initial and final choice of the ants matched in 92.4% of choices, so for simplicity, we only considered final choices in the statistical analysis. Choice preference was tested using a GLMM with a binomial distribution. We included the fixed factors social cue type (nest-mates or surface lipid-coated glass beads; SocialCueType), the odour associated with the social cue (lemon or rosemary, to test for innate odour preferences; SocialCueOdour), the side of the social cue odour in the Y-maze (right or left, to test for a side bias; SocialCueSide) and a binomial factor indicating whether ants were confronted with a social cue or fed alone on the first training visit (social cue type at first training visit; SocialCueFirst) in order to test for primacy and recency effects [74]. This resulted in the following model formula:
2.4.2. Inbound pheromone depositions during training
Inbound pheromone deposition behaviour (number of pheromone depositions on the way back to the nest) was analysed using GLMMs with a Poisson distribution. First, we attempted to predict pheromone deposition using the fixed factor social cue type in interaction with whether social cues were present that visit and a scaled visit variable included to model changes in pheromone deposition over subsequent visits. This resulted in the following model formula:
As the interaction was not significant (see Results), but social cue presence had a significant effect on ant choices and visual inspection of the data showed a clear difference between ants confronted with live nest-mates and those confronted with dummy ants (figure 3), we ran two further models in order to explore the data in more detail. We thus subsetted the data according to social cue type. This resulted in the following model formula:
Figure 3.
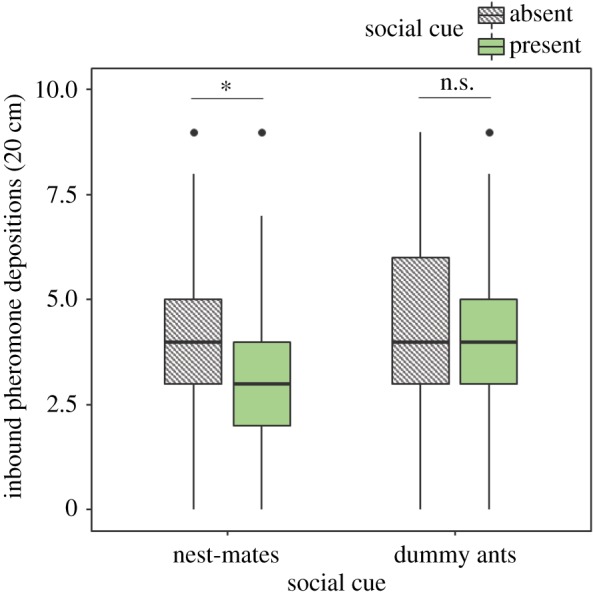
Number of pheromone depositions deposited during the way back to the nest on a 20 cm track right behind the feeding platform. Horizontal lines are medians, boxes are along with interquartile ranges, whiskers are 5%/95% ranges and dots are outliers. *p < 0.05, n.s.: p > 0.05.
3. Results
3.1. Choice tests
Only 34.8% (97 of 278) of choices in the Y-maze were made for the odour previously associated with the presence of other nest-mates, which is significantly different from chance (figure 2, GLMM: estimate = −0.68, z = −3.07, p = 0.002). By contrast, when the social cue was dummy ants, 52.8% (131 of 248) of choices were made for the arm containing the social-associated cue, which does not differ from chance (GLMM: estimate = 0.16, z = 0.79, p = 0.43). Social cue type had a significant effect on ant choices, with ants being more likely to choose the social cue side in the bead treatment compared to the nest-mate treatment (GLMM: estimate = 0.84, z = 3.13, p = 0.003). A significant effect of the first presentation of the social cue during training was also found. Ants were significantly more likely to choose the social cue side in the Y-maze when the social cue was first presented on the first training visit compared to the second training visit (electronic supplementary material, figure S5; GLMM: estimate = 0.89, z = 4.11, p < 0.001). Furthermore, ants showed side and odour biases, with significant preferences for lemon odour (electronic supplementary material, figures S1 and S2) and the left side (electronic supplementary material, figures S3 and S4) in the Y-maze (odour preference GLMM: estimate = −0.61, z = −2.71, p = 0.0083; side preference GLMM: estimate = −0.69, z = 3.19, p = 0.0029).
Figure 2.
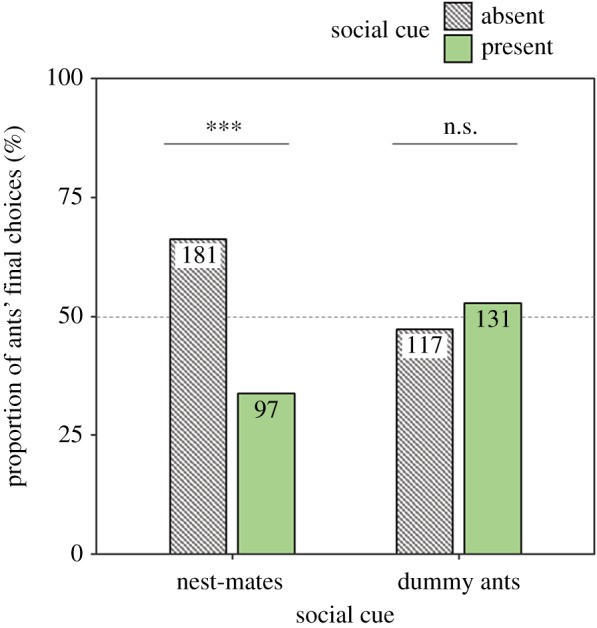
Proportions of all final choices made by ants in the Y-maze. Ants fed with either real nest-mates or with dummy ants (black surface lipid-coated glass beads) when the social cue was present and fed alone when the social cue was absent. Forty-nine ants were trained with real nest-mates and 43 with dummy ants. Numbers in the bars represent sample sizes (individual choices). ***p < 0.001, n.s.: p > 0.05.
3.2. Inbound pheromone depositions during training
The GLMM analysing the complete dataset indicated a significant effect of the social cue presence on the number of pheromone depositions: ants deposited significantly less pheromone when returning from a food source at which a social cue (live nest-mates or dummy ants) was present (GLMM: estimate = −0.13, z = −2.38, p = 0.03). Ants further deposited significantly more pheromone on later training visits (GLMM: estimate = 0.05, z = 2.76, p = 0.017).
We did not find a significant effect of social cue type (GLMM: estimate = 0.1, z = 1.75, p = 0.10) or the interaction between social cue type and social cue presence (GLMM: estimate = 0.11, z = 1.53, p = 0.13) on choice (see electronic supplementary material, figure S6). However, visual inspection of the data (figure 3) clearly showed a difference between ants confronted with live nest-mates and those confronted with dummy ants. We thus examined the data more closely by splitting it by social cue type and ran another GLMM to check for the effect of social cue presence for each of the two social cue types respectively. Ants deposited significantly less pheromone when returning from a food source at which other nest-mates were present compared to when they fed alone (GLMM: estimate = −0.13, z = −2.4, p = 0.03, figure 3). However, when beads were used as a social cue, there was no significant effect of social cue presence on the number of pheromone depositions during the ants' return to the nest (GLMM: estimate = −0.01, z = −0.18, p = 0.85, figure 3). The order of social cue presentation (first presentation of a social cue on the first or second training visit) did not have a significant effect on number of pheromone depositions in both treatments (GLMM for live nest-mates treatment: estimate = 0.03, z = 0.52, p = 0.8; GLMM for dummy ant treatment: estimate = 0.08, z = 1.29, p = 0.26).
4. Discussion
Ants showed a significant preference for food sources at which they fed alone over food sources at which other ants were feeding, and also deposited more pheromone when returning from solitary feeding (figure 2). However, surface lipid-coated beads failed to elicit this effect.
These results demonstrate that ants actively avoided feeding at already occupied food sources and recruited more heavily to unoccupied food sources. The results further suggest that the attractiveness of a food source is not solely based on direct traits such as sugar concentration, flow rate or distance to the nest [4,22,75,76], but can also be affected by the status of occupancy and most likely also by other indirect traits. The reduction of pheromone depositions on crowded trails has already been described in Lasius niger ants [30]. In addition to a similar effect from occupancy at the food source, here we report an apparent aversion to occupied food sources. Both behaviours may combine and lead to the exploitation of numerous valuable food sources in the environment rather than overexploiting only one good food source. This may counteract the tendency of the positive-feedback component of the ant recruitment system to result in only choosing one option, a phenomenon termed symmetry breaking [11,25,52,77,78]. This may be beneficial for two reasons: firstly, overexploitation can lead to queuing at the food source and slower travel speed due to crowded trails [15,16,18]. A reduction of pheromone strength on already occupied trails and preference for unoccupied food sources may lead to a more evenly distributed food exploitation and thus a higher colony-level food intake. Secondly, an aversion to occupied food sources may act as a negative feedback system, preventing colonies from becoming trapped in local foraging optima. Nonlinear positive feedback systems in general, and pheromone-mediated recruitment, particularly in ants, can result in such a strong recruitment that the system cannot react to changing environments. Thus, if an ant colony is allowed to forage extensively at a good food source, and then the quality of the food is reduced, colonies often fail to refocus their foraging effort to newly available, better food sources [11,12,25,78]. In this and other species, trails cannot realistically become strong enough to cause aversion [53]. The negative feedback system we describe may be an effective method of mitigating these effects, especially in combination with other negative feedback mechanisms, such as a simple downregulation of pheromone depositions [52,77]. Note that there is no explicit switch from positive- to negative-feedback causing behaviours—rather, both take place simultaneously. In the current study, the negative feedback is a downregulation of positive feedback behaviour (pheromone deposition), which can proceed until positive feedback is completely shut down, allowing negative feedback processes, such as pheromone decay, to take over.
However, the presence of only five nest-mates at a relatively large (ᴓ 5 mm) food source might not be reasonably considered as a crowded food source. Furthermore, the presence of nest-mates at a food patch may serve as an indicator for a safe and productive food source which is worth exploiting and should be concentrated on while it is not yet completely crowded [50]. Why then do the ants reduce exploitation of such food sources? In Czaczkes et al.'s study [50], even though ants downregulated pheromone depositions on crowded trails, colonies showed a clear preference for paths on which dummy ants were present compared to control paths. The authors argue that the presence of nest-mates and a simultaneous absence of alarm pheromones on a path inform foragers that the path is safe and productive and is thus preferred over one at which nest-mates are absent [50]. Furthermore, colonies may benefit from increased information transfer and recruitment potential on paths where nest-mates are present [17,50,79–81]. Why then do ants reduce their pheromone deposition and preference for a food source which is occupied by just a few nest-mates? Nest-mate density at a food source may be an indicator for how many foragers are already exploiting a food source and may also inform ants about whether additional nest-mates should be recruited [82]. Given the positive-feedback nature of recruitment in these ants, even the presence of a few nest-mates suggests that this food source will soon be well occupied. Foragers with experience of other, unoccupied, food sources could thus concentrate on recruiting to other food source, or on scouting for new ones. These foragers may thus accept the risk of feeding alone at a newly discovered food source until more nest-mates have been recruited. Such scouting ants have been described in various ant species [59,83–85]. A similar pattern was reported in foraging bumblebees: bees that were experienced with a food source avoided occupied food sources, but naive bees preferred them [47]. The behavioural pattern reported here and in Czaczkes et al. [50] can also be seen in this light: in the current study, we trained individual ants to food sources over the course of eight visits, allowing them to become familiar with the food, odour and nest-mate presence or absence. By contrast, Czaczkes et al. [50] investigated path preference of complete colonies in which path choice was driven by the initial decisions of the first few, naive foragers. These naive ants would be more likely to visit occupied food sources, while informed ants would rather avoid them. Importantly, individual Lasius niger workers are very flexible in their use of pheromone trails. While naive and recruited workers can follow pheromone trails with high fidelity [86], ants are not dependent on pheromone decay to maintain flexibility: knowledgeable individuals can completely ignore pheromone trails in preference for conflicting route memories [87]. However, knowledgeable individuals can again switch back to high fidelity pheromone following, even in the face of conflicting memories, when other third-source information sources become available [88]. The current results build on this picture, demonstrating how flexible individuals, changing their preferences due to perceived food crowding, may provide information used to maintain colony flexibility.
The fact that dummy ants (black surface lipid-coated glass beads) did not elicit a decrease in recruitment strength and food attractiveness suggests that the mere presence of a nest-mate odour may not be sufficient for nest-mate recognition in this context. Although surface lipid-coated glass beads have successfully been used in previous studies on recruitment behaviour [44], including in Lasius niger [30,66], the lack of other stimuli such as movement, home-range markings [89–91], or feeding signals (e.g. local pheromone recruitment or stridulation) [64,81,92,93] may have caused the ants to underestimate the local density of ants, or have prevented them from being perceived as nest-mates.
Ants showed a stronger preference for the odour associated with the absence of social cues when it was first experienced on the first training visit, with only 34.1% of choices for the odour at which a social cue was present. By contrast, if the social cue was first presented on the first training visit, 51.8% of choices were for the Y-maze side covered in social cue odour. This strongly suggests a primacy effect, in which memory of the first-exposed cue is stronger than memory of a cue experienced later [94–96]. Ants also chose the social cue odour more often when it was associated with lemon odour and placed on the left side of the Y-maze, suggesting an innate odour preference for lemon over rosemary odour and a side bias. Side biases especially have been widely reported in ants and other animals [97–104]. However, as our treatments were fully balanced by treatment presentation order, neither the primacy effect nor the innate biases should interfere with our interpretation.
We demonstrate that negative feedback is not only elicited by nest-mate presence on paths in Lasius niger, but also through nest-mate presence during food consumption. Moreover, ants also prefer food sources without fellow foragers. The avoidance of already occupied food sources allows ants to distribute their foraging effort and exploit multiple food sources simultaneously. This can result in a more efficient exploitation of the environment [105]. In addition, such behaviour may increase the amount of information about available food sources and may thus increase the colony-level food intake rate. Such negative-feedback systems may play a critical role in maintaining collective flexibility and preventing trapping in local optima. Taken together, the combination of passive negative feedback from reduced pheromone deposition, and active negative feedback via occupied food avoidance, may be a powerful mechanism for increasing collective foraging efficacy.
Supplementary Material
Ethics
All animal treatment guidelines applicable to ants under German law have been followed.
Data accessibility
Raw data collected in the presented experiments are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.qz612jm8x [106].
Authors' contributions
N.K. performed the experiments. S.W. supervised the experiments and analysed the data. S.W. and T.J.C. designed the study, interpreted the data and wrote the manuscript. All authors gave final approval for publication and agree to be held accountable for the content therein.
Competing interests
The authors declare that they have no conflict of interest.
Funding
We thank the DFG (Deutsche Forschungsgemeinschaft) which funded S.W. and T.J.C. with an Emmy Noether grant to T.J.C., grant no. CZ 237/1-1.
References
- 1.von Frisch K. 1965. Die Tänze der Bienen. In: Tanzsprache und Orientierung der Bienen. Berlin, Heidelberg, Germany: Springer. [Google Scholar]
- 2.Seeley T, Camazine S, Sneyd J. 1991. Collective decision-making in honey bees: how colonies choose among nectar sources. Behav. Ecol. Sociobiol. 28, 277–290. ( 10.1007/BF00175101) [DOI] [Google Scholar]
- 3.Seeley T, Mikheyev A, Pagano G. 2000. Dancing bees tune both duration and rate of waggle-run production in relation to nectar-source profitability. J. Comp. Physiol. A. 186, 813–819. ( 10.1007/s003590000134) [DOI] [PubMed] [Google Scholar]
- 4.Beckers R, Deneubourg J-L, Goss S. 1993. Modulation of trail laying in the ant Lasius niger (Hymenoptera: Formicidae) and its role in the collective selection of a food source. J. Insect Behav. 6, 751–759. ( 10.1007/BF01201674) [DOI] [Google Scholar]
- 5.Hangartner W. 1970. Control of pheromone quantity in odor trails of the ant Acanthomyops interjectus. Experientia 26, 664–665. ( 10.1007/BF01898753) [DOI] [PubMed] [Google Scholar]
- 6.Beckers R, Deneubourg J-L, Goss S. 1992. Trail laying behaviour during food recruitment in the ant Lasius niger (L.). Insectes Soc. 39, 59–72. ( 10.1007/BF01240531) [DOI] [Google Scholar]
- 7.Detrain C, Deneubourg J-L. 2008. Collective decision-making and foraging patterns in ants and honeybees. In Advances in insect physiology (ed. Simpson S.), pp. 123–173. New York, NY: Academic Press. [Google Scholar]
- 8.Wendt S, Strunk K, Heinze J, Roider A, Czaczkes T. 2019. Positive and negative incentive contrasts lead to relative value perception in ants. eLife 8, e45450 ( 10.7554/eLife.45450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Thienen W, Metzler D, Witte V.. 2015. Modeling shortest path selection of the ant Linepithema humile using psychophysical theory and realistic parameter values. J. Theor. Biol. 372, 168–178. ( 10.1016/j.jtbi.2015.02.030) [DOI] [PubMed] [Google Scholar]
- 10.Wilson E. 1962. Chemical communication among workers of the fire ant Solenopsis saevissima (Fr. Smith) 1. The organization of mass-foraging. Anim. Behav. 10, 134–147. ( 10.1016/0003-3472(62)90141-0) [DOI] [Google Scholar]
- 11.Beckers R, Deneubourg JL, Goss S, Pasteels JM. 1990. Collective decision making through food recruitment. Insect. Soc. 37, 258–267. ( 10.1007/BF02224053) [DOI] [Google Scholar]
- 12.Czaczkes T, Salmane A, Klampfleuthner F, Heinze J. 2016. Private information alone can trigger trapping of ant colonies in local feeding optima. J. Exp. Biol. 219, 744–751. ( 10.1242/jeb.131847) [DOI] [PubMed] [Google Scholar]
- 13.Frizzi F, Talone F, Santini G. 2018. Modulation of trail laying in the ant Lasius neglectus (Hymenoptera: Formicidae) and its role in the collective selection of a food source. Ethology 124, 870–880. ( 10.1111/eth.12821) [DOI] [Google Scholar]
- 14.Latty T, Beekman M. 2013. Keeping track of changes: the performance of ant colonies in dynamic environments. Anim. Behav. 85, 637–643. ( 10.1016/j.anbehav.2012.12.027) [DOI] [Google Scholar]
- 15.Burd M. 1996. Server system and queuing models of leaf harvesting by leaf-cutting ants. Am. Nat. 148, 613–629. ( 10.1086/285943) [DOI] [Google Scholar]
- 16.Burd M. 2000. Foraging behaviour of Atta cephalotes (leaf-cutting ants): an examination of two predictions for load selection. Anim. Behav. 60, 781–788. ( 10.1006/anbe.2000.1537) [DOI] [PubMed] [Google Scholar]
- 17.Dussutour A, Beshers S, Deneubourg J-L, Fourcassié V. 2007. Crowding increases foraging efficiency in the leaf-cutting ant Atta colombica. Insect. Soc. 54, 158–165. ( 10.1007/s00040-007-0926-9) [DOI] [Google Scholar]
- 18.Burd M, Aranwela N. 2003. Head-on encounter rates and walking speed of foragers in leaf-cutting ant traffic. Insect. Soc. 50, 3–8. ( 10.1007/s000400300001) [DOI] [Google Scholar]
- 19.Fischer M, Völkl W, Hoffmann K. 2005. Honeydew production and honeydew sugar composition of polyphagous black bean aphid, Aphis fabae (Hemiptera: Aphididae) on various host plants and implications for ant-attendance. Eur. J. Entomol. 102, 155–160. ( 10.14411/eje.2005.025) [DOI] [Google Scholar]
- 20.Dreisig H. 2000. Defense by exploitation in the Florida carpenter ant, Camponotus floridanus, at an extrafloral nectar resource. Behav. Ecol. Sociobiol. 47, 274–279. ( 10.1007/s002650050666) [DOI] [Google Scholar]
- 21.Völkl W, Woodring J, Fischer M, Lorenz MW, Hoffmann KH. 1999. Ant–aphid mutualisms: the impact of honeydew production and honeydew sugar composition on ant preferences. Oecologia 118, 483–491. ( 10.1007/s004420050751) [DOI] [PubMed] [Google Scholar]
- 22.Schilman P, Roces F. 2003. Assessment of nectar flow rate and memory for patch quality in the ant Camponotus rufipes. Anim. Behav. 66, 687–693. ( 10.1006/anbe.2003.2242) [DOI] [Google Scholar]
- 23.Dreisig H. 1988. Foraging rate of ants collecting honeydew or extrafloral nectar, and some possible constraints. Ecol. Entomol. 13, 143–154. ( 10.1111/j.1365-2311.1988.tb00342.x) [DOI] [Google Scholar]
- 24.Shaffer Z, Sasaki T, Pratt SC. 2013. Linear recruitment leads to allocation and flexibility in collective foraging by ants. Anim. Behav. 86, 967–975. ( 10.1016/j.anbehav.2013.08.014) [DOI] [Google Scholar]
- 25.Sumpter D, Beekman M. 2003. From nonlinearity to optimality: pheromone trail foraging by ants. Anim. Behav. 66, 273–280. ( 10.1006/anbe.2003.2224) [DOI] [Google Scholar]
- 26.Beckers R, Deneubourg J-L, Goss S. 1992. Trails and U-turns in the selection of a path by the ant Lasius niger. J. Theor. Biol. 159, 397–415. ( 10.1016/S0022-5193(05)80686-1) [DOI] [Google Scholar]
- 27.Goss S, Aron S, Deneubourg J-L, Pasteels J. 1989. Self-organized shortcuts in the Argentine ant. Naturwissenschaften 76, 579–581. ( 10.1007/BF00462870) [DOI] [Google Scholar]
- 28.Dussutour A, Nicolis S, Shephard G, Beekman M, Sumpter D. 2009. The role of multiple pheromones in food recruitment by ants. J. Exp. Biol. 212, 2337–2348. ( 10.1242/jeb.029827) [DOI] [PubMed] [Google Scholar]
- 29.Kietzman P, Visscher P. 2015. The anti-waggle dance: use of the stop signal as negative feedback. Front. Ecol. Evol. 3, 14 ( 10.3389/fevo.2015.00014) [DOI] [Google Scholar]
- 30.Czaczkes T, Grüter C, Ratnieks F. 2013. Negative feedback in ants: crowding results in less trail pheromone deposition. J. R. Soc. Interface. 10, 20121009 ( 10.1098/rsif.2012.1009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirchner W. 1993. Vibrational signals in the tremble dance of the honeybee, Apis mellifera. Behav. Ecol. Sociobiol. 33, 169–172. ( 10.1007/BF00216597) [DOI] [Google Scholar]
- 32.Nieh J. 1993. The stop signal of honey bees: reconsidering its message. Behav. Ecol. Sociobiol. 33, 51–56. ( 10.1007/BF00164346) [DOI] [Google Scholar]
- 33.Nieh J. 2010. A negative feedback signal that is triggered by peril curbs honey bee recruitment. Curr. Biol. 20, 310–315. ( 10.1016/j.cub.2009.12.060) [DOI] [PubMed] [Google Scholar]
- 34.Pastor K, Seeley T. 2005. The brief piping signal of the honey bee: begging call or stop signal? Ethology 111, 775–784. ( 10.1111/j.1439-0310.2005.01116.x) [DOI] [Google Scholar]
- 35.Thom C, Gilley D, Tautz J. 2003. Worker piping in honey bees (Apis mellifera): the behavior of piping nectar foragers. Behav. Ecol. Sociobiol. 53, 199–205. ( 10.1007/s00265-002-0567-y) [DOI] [Google Scholar]
- 36.Czaczkes T, Grüter C, Ellis L, Wood E, Ratnieks F. 2013. Ant foraging on complex trails: route learning and the role of trail pheromones in Lasius niger. J. Exp. Biol. 216, 188–197. ( 10.1242/jeb.076570) [DOI] [PubMed] [Google Scholar]
- 37.Clark CW, Mangel M. 1984. Foraging and flocking strategies: information in an uncertain environment. Am. Nat. 123, 626–641. ( 10.1086/284228) [DOI] [Google Scholar]
- 38.Krebs J, MacRoberts M, Cullen J. 1972. Flocking and feeding in the great tit parus major—an experimental study. Ibis 114, 507–530. ( 10.1111/j.1474-919X.1972.tb00852.x) [DOI] [Google Scholar]
- 39.Lahav S, Soroker V, Hefetz A, Vander Meer R. 1999. Direct behavioral evidence for hydrocarbons as ant recognition discriminators. Naturwissenschaften 86, 246–249. ( 10.1007/s001140050609) [DOI] [Google Scholar]
- 40.Wagner D, Tissot M, Cuevas W, Gordon D. 2000. Harvester ants utilize cuticular hydrocarbons in nestmate recognition. J. Chem. Ecol. 26, 2245–2257. ( 10.1023/A:1005529224856) [DOI] [Google Scholar]
- 41.van Zweden JS, d'Ettorre P. 2010. Nestmate recognition in social insects and the role of hydrocarbons. In Insect hydrocarbons. Biology, biochemistry, and chemical ecology (eds Blomquist GJ, Bagnères A-G), pp. 222–243. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 42.Akino T, Yamamura K, Wakamura S, Yamaoka R. 2004. Direct behavioral evidence for hydrocarbons as nestmate recognition cues in Formica japonica (Hymenoptera: Formicidae). Appl. Entomol. Zool. 39, 381–387. ( 10.1303/aez.2004.381) [DOI] [Google Scholar]
- 43.Greene M, Gordon D. 2003. Social insects: cuticular hydrocarbons inform task decisions. Nature 423, 32 ( 10.1038/423032a) [DOI] [PubMed] [Google Scholar]
- 44.Greene M, Gordon D. 2007. Interaction rate informs harvester ant task decisions. Behav. Ecol. 18, 451–455. ( 10.1093/beheco/arl105) [DOI] [Google Scholar]
- 45.Ozaki M, Wada-Katsumata A, Fujikawa K, Iwasaki M, Yokohari F, Satoji Y, Nisimura T, Yamaoka R. 2005. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 309, 311–314. ( 10.1126/science.1105244) [DOI] [PubMed] [Google Scholar]
- 46.Avarguès-Weber A, Chittka L. 2014. Local enhancement or stimulus enhancement? Bumblebee social learning results in a specific pattern of flower preference. Anim. Behav. 97, 185–191. ( 10.1016/j.anbehav.2014.09.020) [DOI] [Google Scholar]
- 47.Kawaguchi L, Ohashi K, Toquenaga Y. 2007. Contrasting responses of bumble bees to feeding conspecifics on their familiar and unfamiliar flowers. Proc. R. Soc. B 274, 2661–2667. ( 10.1098/rspb.2007.0860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leadbeater E, Chittka L. 2007. The dynamics of social learning in an insect model, the bumblebee (Bombus terrestris). Behav. Ecol. Sociobiol. 61, 1789–1796. ( 10.1007/s00265-007-0412-4) [DOI] [Google Scholar]
- 49.Worden B, Papaj D. 2005. Flower choice copying in bumblebees. Biol. Lett. 1, 504–507. ( 10.1098/rsbl.2005.0368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Czaczkes T, Franz S, Witte V, Heinze J. 2015. Perception of collective path use affects path selection in ants. Anim. Behav. 99, 15–24. ( 10.1016/j.anbehav.2014.10.014) [DOI] [Google Scholar]
- 51.Dussutour A, Fourcassié V, Helbing D, Deneubourg J-L. 2004. Optimal traffic organization in ants under crowded conditions. Nature 428, 70–73. ( 10.1038/nature02345) [DOI] [PubMed] [Google Scholar]
- 52.Grüter C, Schürch R, Czaczkes T, Taylor K, Durance T, Jones S, Ratnieks FL. 2012. Negative feedback enables fast and flexible collective decision-making in ants. PLoS ONE 7, e44501 ( 10.1371/journal.pone.0044501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Thienen W, Metzler D, Choe D-H, Witte V.. 2014. Pheromone communication in ants: a detailed analysis of concentration-dependent decisions in three species. Behav. Ecol. Sociobiol. 68, 1611–1627. ( 10.1007/s00265-014-1770-3) [DOI] [Google Scholar]
- 54.Eidmann H. 1927. Ameisen und Blattläuse. Biol. Centralblatt, Vol. 47. [Google Scholar]
- 55.Novgorodova TA. 2015. Organization of honeydew collection by foragers of different species of ants (Hymenoptera: Formicidae): effect of colony size and species specificity. Eur. J. Entomol. 112, 688–697. ( 10.14411/eje.2015.077) [DOI] [Google Scholar]
- 56.Detrain C, Pereira H, Fourcassié V. 2019. Differential responses to chemical cues correlate with task performance in ant foragers. Behav. Ecol. Sociobiol. 73, 107 ( 10.1007/s00265-019-2717-5) [DOI] [Google Scholar]
- 57.Stroeymeyt N, Grasse AV, Crespi A, Mersch DP, Cremer S, Keller L. 2018. Social network plasticity decreases disease transmission in a eusocial insect. Science 362, 941–945. ( 10.1126/science.aat4793) [DOI] [PubMed] [Google Scholar]
- 58.Portha S, Deneubourg J-L, Detrain C. 2004. How food type and brood influence foraging decisions of Lasius niger scouts. Anim. Behav. 68, 115–122. ( 10.1016/j.anbehav.2003.10.016) [DOI] [Google Scholar]
- 59.Mailleux A-C, Detrain C, Deneubourg J-L. 2006. Starvation drives a threshold triggering communication. J. Exp. Biol. 209, 4224–4229. ( 10.1242/jeb.02461) [DOI] [PubMed] [Google Scholar]
- 60.Josens R, Roces F. 2000. Foraging in the ant Camponotus mus: nectar-intake rate and crop filling depend on colony starvation. J. Insect. Physiol. 46, 1103–1110. ( 10.1016/S0022-1910(99)00220-6) [DOI] [PubMed] [Google Scholar]
- 61.Czaczkes T, Koch A, Fröber K, Dreisbach G. 2018. Voluntary switching in an invertebrate: the effect of cue and reward change. J. Exp. Psychol. Anim. Learn. Cognition 44, 247–257. ( 10.1037/xan0000171) [DOI] [PubMed] [Google Scholar]
- 62.Oberhauser F, Schlemm S, Wendt S, Czaczkes T. 2019. Private information conflict: Lasius niger ants prefer olfactory cues to route memory. Anim. Cogn. 22, 355–364. ( 10.1007/s10071-019-01248-3) [DOI] [PubMed] [Google Scholar]
- 63.Nonacs P. 1990. Death in the distance: mortality risk as information for foraging ants. Behaviour 112, 23–35. ( 10.1163/156853990X00662) [DOI] [Google Scholar]
- 64.Roces F, Hölldobler B. 1996. Use of stridulation in foraging leaf-cutting ants: mechanical support during cutting or short-range recruitment signal? Behav. Ecol. Sociobiol. 39, 293–299. ( 10.1007/s002650050292) [DOI] [Google Scholar]
- 65.Sturgis S, Gordon D. 2012. Nestmate recognition in ants (Hymenoptera: Formicidae): a review. Myrmecological News 16, 101–110. [Google Scholar]
- 66.Czaczkes T, Grüter C, Ratnieks F. 2014. Rapid up- and down-regulation of pheromone signalling due to trail crowding in the ant Lasius niger. Behaviour 151, 669–682. ( 10.1163/1568539X-00003157) [DOI] [Google Scholar]
- 67.Guerrieri F, Nehring V, Jørgensen C, Nielsen J, Galizia C, d'Ettorre P. 2009. Ants recognize foes and not friends. Proc. R. Soc. B 276, 2461–2468. ( 10.1098/rspb.2008.1860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guerrieri F, d'Ettorre P. 2008. The mandible opening response: quantifying aggression elicited by chemical cues in ants. J. Exp. Biol. 211, 1109–1113. ( 10.1242/jeb.008508) [DOI] [PubMed] [Google Scholar]
- 69.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org. [Google Scholar]
- 70.Bates D, Mächler M, Bolker B, Walker S.. 2014. Fitting linear mixed-effects models using lme4. arXiv. stat:1406.5823.
- 71.Hartig F. 2017. Residual diagnostics for hierarchical (multi-level/mixed) regression models. See http://florianhartig.github.io/DHARMa/.
- 72.Forstmeier W, Schielzeth H. 2011. Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner's curse. Behav. Ecol. Sociobiol. 65, 47–55. ( 10.1007/s00265-010-1038-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B (Methodological). 57, 289–300. ( 10.1111/j.2517-6161.1995.tb02031.x) [DOI] [Google Scholar]
- 74.Lipatova O, Wheeler D, Vadillo M, Miller R. 2006. Recency-to-primacy shift in cue competition. J. Exp. Psychol. Anim. Behav. Proces. 32, 396–406. ( 10.1037/0097-7403.32.4.396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fewell J, Harrison J, Stiller T, Breed M. 1992. Distance effects on resource profitability and recruitment in the giant tropical ant, Paraponera clavata. Oecologia 92, 542–547. ( 10.1007/BF00317846) [DOI] [PubMed] [Google Scholar]
- 76.Josens R, Farina W, Roces F. 1998. Nectar feeding by the ant Camponotus mus: intake rate and crop filling as a function of sucrose concentration. J. Insect. Physiol. 44, 579–585. ( 10.1016/S0022-1910(98)00053-5) [DOI] [PubMed] [Google Scholar]
- 77.Czaczkes T. 2014. How to not get stuck—negative feedback due to crowding maintains flexibility in ant foraging. J. Theor. Biol. 360, 172–180. ( 10.1016/j.jtbi.2014.07.005) [DOI] [PubMed] [Google Scholar]
- 78.de Biseau J, Deneubourg J-L, Pasteels J.. 1991. Collective flexibility during mass recruitment in the ant Myrmica sabuleti (Hymenoptera: Formicidae). Psyche 98, 323–336. ( 10.1155/1991/38402) [DOI] [Google Scholar]
- 79.Farji-Brener A, Amador-Vargas S, Chinchilla F, Escobar S, Cabrera S, Herrera M, Sandoval C. 2010. Information transfer in head-on encounters between leaf-cutting ant workers: food, trail condition or orientation cues? Anim. Behav. 79, 343–349. ( 10.1016/j.anbehav.2009.11.009) [DOI] [Google Scholar]
- 80.Roces F. 1990. Olfactory conditioning during the recruitment process in a leaf-cutting ant. Oecologia 83, 261–262. ( 10.1007/BF00317762) [DOI] [PubMed] [Google Scholar]
- 81.Roces F. 1994. Odour learning and decision-making during food collection in the leaf-cutting ant Acromyrmex lundi. Insect. Soc. 41, 235–239. ( 10.1007/BF01242294) [DOI] [Google Scholar]
- 82.Jarau S, Hrncir M. 2009. Food exploitation by social insects: ecological, behavioral, and theoretical approaches. Boca Raton, FL: CRC Press, Taylor & Francis Group. [Google Scholar]
- 83.Breed M, Fewell J, Moore A, Williams K. 1987. Graded recruitment in a ponerine ant. Behav. Ecol. Sociobiol. 20, 407–411. ( 10.1007/BF00302983) [DOI] [Google Scholar]
- 84.Chadab R, Rettenmeyer C. 1975. Mass recruitment by army ants. Science 188, 1124–1125. ( 10.1126/science.1215991) [DOI] [PubMed] [Google Scholar]
- 85.Jaffe K, Howse P. 1979. The mass recruitment system of the leaf cutting ant, Atta cephalotes (L.). Anim. Behav. 27, 930–939. ( 10.1016/0003-3472(79)90031-9) [DOI] [Google Scholar]
- 86.Czaczkes T, Castorena M, Schürch R, Heinze J. 2017. Pheromone trail following in the ant Lasius niger: high accuracy and variability but no effect of task state. Physiol. Entomol. 42, 91–97. ( 10.1111/phen.12174) [DOI] [Google Scholar]
- 87.Grüter C, Czaczkes T, Ratnieks F. 2011. Decision making in ant foragers (Lasius niger) facing conflicting private and social information. Behav. Ecol. Sociobiol. 65, 141–148. ( 10.1007/s00265-010-1020-2) [DOI] [Google Scholar]
- 88.Czaczkes T, Beckwith J, Horsch A-L, Hartig F. 2019. The multi-dimensional nature of information drives prioritization of private over social information in ants. Proc. R. Soc. B 286, 20191136 ( 10.1098/rspb.2019.1136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Depickère S, Fresneau D, Detrain C, Deneubourg J-L. 2004. Marking as a decision factor in the choice of a new resting site in Lasius niger. Insect. Soc. 51, 243–246. [Google Scholar]
- 90.Detrain C, Deneubourg J-L. 2009. Social cues and adaptive foraging strategies in ants. In Food exploitation by social insects (eds Jarau S, Hrncir M), pp. 29–54. Boca Raton, FL: CRC Press. [Google Scholar]
- 91.Devigne C, Detrain C. 2002. Collective exploration and area marking in the ant Lasius niger. Insect. Soc. 49, 357–362. ( 10.1007/PL00012659) [DOI] [Google Scholar]
- 92.Bouchebti S, Ferrere S, Vittori K, Latil G, Dussutour A, Fourcassié V. 2015. Contact rate modulates foraging efficiency in leaf cutting ants. Sci. Rep. 5, 18650 ( 10.1038/srep18650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hölldobler B, Wilson E. 1990. The ants. Berlin, Germany: Springer. [Google Scholar]
- 94.Pineño O, Miller R. 2005. Primacy and recency effects in extinction and latent inhibition: a selective review with implications for models of learning. Behav. Process. 69, 223–235. ( 10.1016/j.beproc.2005.02.006) [DOI] [PubMed] [Google Scholar]
- 95.Wright A, Santiago H, Sands S, Kendrick D, Cook R. 1985. Memory processing of serial lists by pigeons, monkeys, and people. Science 229, 287–289. ( 10.1126/science.9304205) [DOI] [PubMed] [Google Scholar]
- 96.Wright A, Roediger H. 2003. Interference processes in monkey auditory list memory. Psychon. Bull. Rev. 10, 696–702. ( 10.3758/BF03196534) [DOI] [PubMed] [Google Scholar]
- 97.Buchanan S, Kain J, de Bivort B.. 2015. Neuronal control of locomotor handedness in Drosophila. Proc. Natl Acad. Sci. USA 112, 6700–6705. ( 10.1073/pnas.1500804112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cooper R, Nudo N, González J, Vinson S, Liang H. 2011. Side-dominance of Periplaneta americana persists through antenna amputation. J. Insect Behav. 24, 175–185. ( 10.1007/s10905-010-9246-4) [DOI] [Google Scholar]
- 99.Frasnelli E. 2013. Brain and behavioral lateralization in invertebrates. Front. Psychol. 4, 939 ( 10.3389/fpsyg.2013.00939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Glick S, Ross D. 1981. Right-sided population bias and lateralization of activity in normal rats. Brain Res. 205, 222–225. ( 10.1016/0006-8993(81)90737-X) [DOI] [PubMed] [Google Scholar]
- 101.Guo K, Meints K, Hall C, Hall S, Mills D. 2009. Left gaze bias in humans, rhesus monkeys and domestic dogs. Anim. Cogn. 12, 409–418. ( 10.1007/s10071-008-0199-3) [DOI] [PubMed] [Google Scholar]
- 102.Hunt E, O'Shea-Wheller T, Albery G, Bridger T, Gumn M, Franks N. 2014. Ants show a leftward turning bias when exploring unknown nest sites. Biol. Lett. 10, 20140945 ( 10.1098/rsbl.2014.0945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kight S, Steelman L, Coffey G, Lucente J, Castillo M. 2008. Evidence of population-level lateralized behaviour in giant water bugs, Belostoma flumineum Say (Heteroptera: Belostomatidae): T-maze turning is left biased. Behav. Processes. 79, 66–69. ( 10.1016/j.beproc.2008.04.001) [DOI] [PubMed] [Google Scholar]
- 104.Stancher G, Clara E, Regolin L, Vallortigara G. 2006. Lateralized righting behavior in the tortoise (Testudo hermanni). Behav. Brain Res. 173, 315–319. ( 10.1016/j.bbr.2006.06.023) [DOI] [PubMed] [Google Scholar]
- 105.Czaczkes T, Czaczkes B, Iglhaut C, Heinze J. 2015. Composite collective decision-making. Proc. R. Soc. B 282, 20142723 ( 10.1098/rspb.2014.2723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wendt S, Kleinhoelting N, Czaczkes TJ. 2020. Data from: Negative feedback: ants choose unoccupied over occupied food sources and lay more pheromone to them Dryad Digital Repository. ( 10.5061/dryad.qz612jm8x) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Wendt S, Kleinhoelting N, Czaczkes TJ. 2020. Data from: Negative feedback: ants choose unoccupied over occupied food sources and lay more pheromone to them Dryad Digital Repository. ( 10.5061/dryad.qz612jm8x) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Raw data collected in the presented experiments are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.qz612jm8x [106].