Abstract
Introduction
Acinetobacter baumannii has emerged as a significant multidrug-resistant (MDR) nosocomial pathogen worldwide and is responsible for various healthcare-associated infections. The MDR strains have been reported increasingly during the last decades in hospitalized patients. They have developed resistance to most of the available antibiotics and are known to produce various acquired β-lactamases. The β-lactamase producing strains have a potential for rapid dissemination in hospital settings, as it is often plasmid-mediated. The Infectious Diseases Society of America (ISDA) stated A. baumannii as one of the “red alert” pathogens that greatly threatens the utility of our current antibacterial armamentarium. The study attempted to investigate the spectrum and antimicrobial resistance among MDR A. baumannii and their potential implications in hospitalized patients in a tertiary care hospital of Nepal.
Methods
This study was conducted at Tribhuvan University Teaching Hospital (TUTH), Nepal from January 2017 to December 2017. A total of 177 A. baumannii isolated from hospitalized patients were included in the study. The AST was performed by disc diffusion method. The MDR strains were identified by the criteria of Magiorakos et al, ESBL production by CLSI guidelines, and AmpC β-lactamase production by the AmpC disc test. MBL and KPC production were detected as per the method of Tsakris et al.
Results
Out of 177 A. baumannii, 91.0% were MDR isolates. Among the MDR isolates, the majority were isolated from respiratory tract specimens and were isolated from ICU patients. Most of the MDR isolates were resistant to all first-line antibiotics and all were completely sensitive to only polymyxin B and colistin sulfate. MBL (67.7%) was the common β-lactamase production among MDR isolates.
Conclusion
Acinetobacter baumannii can cause a vast variety of infections in hospitalized patients. The highly resistant MDR strains are common in tertiary care hospitals. This bacteria lead to high morbidity and mortality as we are left with the only option of treating them by potentially toxic antibiotics like colistin sulfate and polymyxin B. Detection of drug resistance and rational use of antibiotics play a crucial role in the fight against this MDR pathogen.
Keywords: Acinetobacter baumannii, hospitalized patients, multidrug resistant, metallo-β-lactamase
Introduction
Acinetobacter baumannii is an aerobic, non-fermentative, gram-negative, nonmotile, cocco-bacilli harboring a number of effective virulence factors.1 The organism is able to survive under a wide range of environmental conditions and persists for extended periods of time on surfaces, which makes them a frequent cause of infection outbreak and healthcare-associated infection.2 The main problem caused by A. baumannii in the hospital setting mostly concerns critically ill patients in intensive care units (ICUs), particularly those requiring mechanical ventilation, and patients with the wound or burn injuries. Infections associated with A. baumannii include ventilator-associated pneumonia, skin and soft tissue infections, wound infections, urinary tract infections, secondary meningitis, and blood-stream infections.3
Acinetobacter baumannii has emerged as a significant MDR nosocomial pathogen worldwide and has been reported increasingly during the last decade, probably due to the increasing use of broad-spectrum antibiotics in hospitalized patients.4 The Infectious Diseases Society of America (ISDA) stated A. baumannii as one of the “red alert” pathogens that greatly threaten the utility of our current antibacterial armamentarium.5 Numerous studies have indicated an upward trend in the prevalence of MDR A. baumannii, but resistance rates can vary widely according to the individual hospital, city, or country involved. Because MDR Acinetobacter infection usually occurs in severely ill patients, the associated crude mortality rate is high, ranging from 26% to 68%.6
Multidrug-resistant A. baumannii has developed resistance to most of the available antibiotics including carbapenems, which are the drugs of choice in the treatment of severe infections.7 The main mechanism for β-lactam resistance in A. baumannii corresponds to efflux pumps, porin mutations, and the production of acquired β-lactam hydrolyzing enzymes, ie, Class A (extended-spectrum β-lactamases, ESBLs), class B (metallo-β-lactamases, MBLs), Class C Ampicillinase (AmpC) as well as class D β-lactamases. Carbapenem resistance due to MBL and other carbapenemases production has a potential for rapid dissemination in hospital settings, as it is often plasmid-mediated and early detection of drug resistance is necessary for proper selection of antibiotics to treat A. baumannii infections in hospitalized patients and to initiate effective infection control measures to prevent their dissemination in hospital settings.8,9
Keeping the above views in mind, the study was carried out on A. baumannii isolated from hospitalized patients to determine their antibiotic susceptibility patterns, to identify MDR strains and to detect various β-lactamases among MDR isolates.
Materials and Methods
The laboratory-based study was conducted at the Department of Clinical Microbiology, Tribhuvan University Teaching Hospital (TUTH), a tertiary care center of Nepal from January 2017 to December 2017 (over a period of 12 months). All clinical specimens collected from the hospitalized patients suspected with infections representing different body sites (sputum, bronchoalveolar lavage, endotracheal aspirate, pus and swab specimens, different body fluids, urine, blood, catheter tips, etc.) were processed according to standard microbiological methods recommended by American Society for Microbiology (ASM) for isolation and identification of A. baumannii.10
Antibiotic Susceptibility Testing (AST)
The susceptibility of A. baumannii isolates against different antibiotics was determined by the modified Kirby–Bauer disk diffusion method on Mueller-Hinton agar and interpreted following standard procedures recommended by the Clinical and Laboratory Standards Institute (CLSI), Wayne, USA.11 The antibiotic sensitivity profile of all the isolates of A. baumannii were determined by testing against ampicillin-sulbactam (10/10 μg), ceftazidime (30 μg), gentamicin (10 μg), ciprofloxacin (5 μg), levofloxacin (5 μg), meropenem (10 μg), and imipenem (10 μg). The isolates that were resistant to at least one antimicrobial from three different groups of above-mentioned antibiotics (ie, MDR isolates) were also tested against piperacillin (100 μg), piperacillin-tazobactam (100/10 μg), cefotaxime (30 μg), cefepime (30 μg), cotrimoxazole (25 μg), amikacin (30 μg), doxycycline (30 μg), polymyxin B (300 units), and colistin sulfate (10 μg) from HiMedia Laboratories, India.
Identification of MDR Isolates
Multidrug-resistant A. baumannii isolates were identified as per guidelines recommended by the European Centre for Disease Prevention and Control (ECDC). The isolates non-susceptible to at least one antimicrobial agent in three or more antimicrobial classes were identified as MDR.12
Detection of Extended-Spectrum β-Lactamase (ESBL) Producers
The initial screening test for the production of ESBL was performed by testing with ceftazidime (CAZ, 30 μg) and cefotaxime (CTX, 30 μg) disks. The isolates were considered as potential ESBL producers when the zone of inhibition (ZOI) was either <18 mm for CAZ or <23 mm for CTX. Isolates that were suspected as ESBL producer by screen test were tested further by combination disk (CD) method for confirmation of ESBL production, in which CAZ and CTX alone and in combination with clavulanic acid [(CAZ+CA, 30/10 μg), (CTX+CA, 30/10 μg)] were used. After incubation for 16–18 hrs at 35±2°C, an increase in ZOI of ≥5 mm for either antimicrobial agent in combination with clavulanic acid than its inhibition zone when tested alone, was confirmed as positive ESBL producers.11
Detection of AmpC β-Lactamase Producers
Acinetobacter baumannii producing a zone of inhibition <18 mm for cefoxitin (CX, 30 μg) disk was tested for AmpC β-lactamase production. AmpC β-lactamase was detected by the AmpC disk test. In this method, cefoxitin susceptible Escherichia coli indicator strain (ATCC 25922) was inoculated on standard MHA plate to form a lawn culture and a cefoxitin disk was placed. A blank disk of 6 mm diameter moistened with Tris-EDTA buffer was inoculated with few colonies of test strain and placed next to the cefoxitin disk. The plates were incubated at 37°C overnight. After overnight incubation, an indentation in the cefoxitin inhibition zone adjacent to the disk containing test strain was considered positive for AmpC β-lactamase production.13
Detection of Metallo β-Lactamase (MBL) and Klebsiella pneumoniae Carbapenemase (KPC) Producers
The isolates were subjected to detection of MBL and KPC production when resistant to meropenem (MEM, 10 μg). The combination meropenem disk method was applied for detection and differentiation of MBL, KPC or co-producer of KPC/MBL as described by Tsakris et al14 In this test, four disks were used; (a) MEM = a plain MEM disk (10 μg), (b) MEM+EDTA = MEM disk (10 μg) with 292 μg of EDTA, (c) MEM+phenylboronic acid (PBA) = MEM disk (10 μg) containing 400 μg of PBA, and (d) MEM+EDTA+PBA = MEM disk (10 μg) with both 292 μg of EDTA and 400 μg of PBA. The EDTA acts as an inhibitor of MBL while PBA is a KPC inhibitor. The test was performed by inoculating a Mueller–Hinton agar with test organism as given for the standard diffusion method and four disks were applied. After incubation of overnight at 37°C, the ZOI diameter around MEM+EDTA, MEM+PBA, and MEM+EDTA+PBA disks was compared with that around the plain MEM disk. Production of MBL was considered when the ZOI diameter around the MEM+EDTA and MEM+EDTA+PBA disks was increased ≥5 mm than ZOI diameter around the MEM disk alone. Production of KPC was considered when the ZOI diameter around the MEM+PBA and MEM +EDTA +PBA disks was increased ≥5 mm than ZOI diameter around the MEM disk alone. The co-production of both KPC and MBL enzymes was considered when the ZOI diameter around the MEM+EDTA+PBA disks was increased ≥5 mm than ZOI diameter around the MEM disk alone. It should be noted that the concentration of PBA and EDTA employed in the present study did not show any detectable effect on bacterial growth.
Data Processing and Analysis
Data regarding patient demographics, specimens, wards, antibacterial profiles, resistance determinants were analyzed using SPSS 16.0 version and interpreted according to frequency distribution and percentage.
Results
During the study period, a total of 177 A. baumannii were isolated. Out of the total A. baumannii isolates, most of them (N=161, 91.0%) were identified as MDR.
Distribution of MDR Acinetobacter baumannii
Out of 161 MDR isolates, the majority (47.2%) were isolated from respiratory tract specimens (ie, sputum, bronchoalveolar lavage, and tracheal aspirate) followed by pus and swabs, body fluids, urine, blood and least were from catheter tips (1.2%) (Table 1). Among total MDR isolates, 58.3% were isolated from male and 41.7% were from female patients, with male to female ratio 1.4. The highest number of isolates were from male patients with age group ≥65 years (14.9%) and the least number was isolated from a female patient with age group 49–64 years (5.0%) (Table 2). Similarly, the higher number of MDR isolates were isolated from ICU patients (49.6%) followed by surgical wards (19.9%) and medical wards (14.3%), while the lowest number from burn wards (1.9%) (Table 3).
Table 1.
Distribution of MDR Acinetobacter baumannii in Various Clinical Specimens
| Specimens | Number | Percentage |
|---|---|---|
| Respiratory tract specimens | 76 | 47.2 |
| Pus and swabs | 44 | 27.3 |
| CSF and other body fluids | 18 | 11.1 |
| Urine | 11 | 6.8 |
| Blood | 10 | 6.2 |
| Catheter tips | 2 | 1.2 |
| Total | 161 | 100 |
Table 2.
Distribution of MDR Acinetobacter baumannii by Gender and Age Group of Patients
| Age Group | Number (%) | Total Number (%) | |
|---|---|---|---|
| Female | Male | ||
| ≤15 Years | 16 (9.9) | 16 (9.9) | 32 (19.8) |
| 16–32 Years | 18 (11.2) | 20 (12.4) | 38 (23.6) |
| 33–48 Years | 13 (8.1) | 17 (10.6) | 30 (18.7) |
| 49–64 Years | 8 (5.0) | 17 (10.5) | 25 (15.5) |
| ≥65 Years | 12 (7.5) | 24 (14.9) | 36 (22.4) |
| Total number (%) | 67 (41.7) | 94 (58.3) | 161 (100) |
Table 3.
Ward Wise Distribution of MDR Acinetobacter baumannii
| Wards | Number of MDR Isolates | Percentage |
|---|---|---|
| ICUs | 80 | 49.6 |
| Surgical | 32 | 19.9 |
| Medical | 23 | 14.3 |
| Orthopedics | 9 | 5.6 |
| Pediatrics | 6 | 3.7 |
| Maternity | 4 | 2.5 |
| Ophthalmology | 4 | 2.5 |
| Burn | 3 | 1.9 |
| Total | 161 | 100 |
Antibiogram of MDR Acinetobacter baumannii
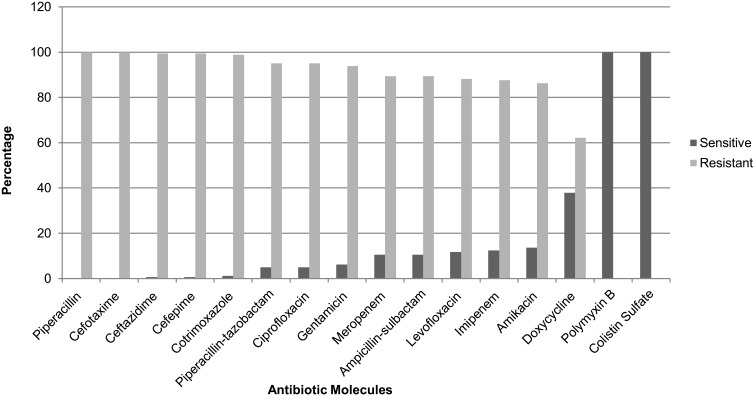
The antibiotic sensitivity profile shows that most of the MDR isolates were resistant to the majority of first-line antibiotics. Among them, all isolates were completely resistant to piperacillin and cefotaxime. Similarly, 99.4% were resistant to ceftazidime and cefepime, 98.7% to cotrimoxazole, 95% to piperacillin-tazobactam and ciprofloxacin, 93.8% to gentamicin, 89.4% resistant to ampicillin-sulbactam and meropenem. Only 11.8%, 12.4%, 13.6%, and 37.9% were sensitive to levofloxacin, imipenem, amikacin, and doxycycline, respectively. All the MDR isolates were completely sensitive to only last resort antibiotics, ie, polymyxin B and colistin sulfate (Figure 1).
Figure 1.
Percentage of antimicrobial resistance and sensitivity of MDR A. baumannii (N = 161).
ESBL, AmpC, MBL, and KPC Production in MDR Acinetobacter baumannii
In this study, the rate of β-lactamases production among MDR isolates was significantly high. MBL was the common β-lactamase detected among MDR A. baumannii (67.7%). ESBL was detected in 19.9%, AmpC in 38.5%, and KPC in 9.3% of MDR isolates. The co-production of different types of β-lactamases was also seen among some isolates. ESBL+AmpC co-producers were seen in 6.8%, ESBL+MBL co-producers in 5.0%, AmpC+MBL co-producers in 23.0% and MBL+KPC in 5.6% of MDR isolates (Table 4).
Table 4.
β-Lactamases Production Among MDR Acinetobacter baumannii
| Types of β-Lactamases | Number | Percentage |
| ESBL | 32 | 19.9 |
| AmpC | 62 | 38.5 |
| MBL | 109 | 67.7 |
| KPC | 15 | 9.3 |
| Co-Producers | ||
| ESBL + AmpC | 11 | 6.8 |
| ESBL + MBL | 8 | 5.0 |
| AmpC + MBL | 37 | 23.0 |
| MBL + KPC | 9 | 5.6 |
Discussion
Acinetobacter baumannii is an important nosocomial pathogen associated with a wide variety of illnesses in hospitalized patients especially in the intensive care units imposing a greater challenge to the patient’s management and infection control. The worldwide emergence of MDR A. baumannii isolate is of great concern.15
In our study, MDR A. baumannii was frequently isolated from respiratory tract specimens (47.2%), followed by pus and swabs (27.3%), body fluids (11.1%) and others. A study was done by Shrestha et al in 201515 from the same hospital also reported 49.18% of MDR A. baumannii from respiratory tract specimens and Samawi et al16 from Qatar reported 48.9% A. baumannii from respiratory tract infection. The demographic data in our study showed that a high prevalence of infections in male patients having age ≥65 years and a major of MDR isolates were from ICU patients because this bacteria have a predilection for higher age groups and severly ill ICU patients.
The rate of MDR A. baumannii in our study is 91.0% which is extensively high. Also in the study done by Shrestha et al and Mishra et al, around 96% and 95% of A. baumannii were MDR, respectively.17,18 This high prevalence of MDR A. baumannii may be due to the high chance of dissemination of resistance gene and their ability to present everywhere in the hospital environment. The A. baumannii infection with high MDR isolates has also alarmed us that there is further need for extensive study and application of preventive measures to reduce such a fearful threat in hospitalized patients. Multidrug-resistant A. baumannii isolates were found significantly resistant to carbapenems, aminoglycosides, and fluoroquinolone groups of antibiotics in this study. Almost all the MDR isolates were resistant to piperacillin and cephalosporins, 93.8% resistant to gentamycin and 89.4% to meropenem which is higher than that reported by Mishra et al18 from the same hospital (nearly 89% and 50% isolates were cephalosporins and carbapenem-resistant, respectively). A study conducted by Xia et al in China demonstrated carbapenem resistance in 85% of isolates which is nearly similar to this study.19 The MYSTIC (Meropenem Yearly Susceptibility Test Information Collection) program of 2007 demonstrated that 74.1% of isolates were susceptible to meropenem and 78.9% were susceptible to imipenem in Europe, compared with much lower susceptibilities of 51.3% and 52.0% in several Asian countries.20,21 Our result on the resistance rate to amikacin was 86.3% which is higher than 54% in the previous study from the same hospital.18 The increasing emergence of highly aminoglycosides resistant strains is also the cause of major concern. In this study, 95.0% and 88.2% MDR A. baumannii were resistant to ciprofloxacin and levofloxacin, respectively. The fluoroquinolone resistance is increasing rapidly in clinical isolates in recent years because of their wide use in clinical medicine as broad-spectrum antimicrobial agents. In our study, polymyxin B and colistin sulfate showed excellent effect against MDR A. baumannii as none of the isolates was found resistant to colistin sulfate and polymyxin B. But, in the study of Joseph et al22 and Al-Sweih et al,23 20% and 12% Acinetobacter spp. were resistance to colistin sulfate, respectively. However, the present study has showed a high antibiotic resistance rate against commonly used antibiotics and is a disadvantage for the health-care system in countries like Nepal as it can greatly affect patient management. This may be because of the intense use of the antimicrobial agents in the hospital, easy availability, and indiscriminate use of these drugs outside the hospitals, and many antibiotics are available over the counter for self-medication. The development of antibiotic resistance is associated with high morbidity and mortality in hospitalized patients, particularly in ICU settings.
The decreased susceptibility of A. baumannii towards the third generation and fourth-generation cephalosporins could be attributed to ESBL or AmpC β-lactamase producers or some other relevant underlying mechanisms. This study showed 19.9% of the MDR A. baumannii was ESBL-producer. A similar rate of ESBL was found in a previous study by Parajuli et al in ICU patients.24 In the study of Mishra et al,18 only 12.9% of Acinetobacter spp. were ESBL producers. In an Indian study,25 only 7% A. baumannii isolates were ESBL producers, while another study from India8 documented 29.9% of Acinetobacter spp. as ESBL producers. Studies have demonstrated that the prevalence of ESBL varies from country to country and institution to institution in both nosocomial and community isolates. This may be ascribed to antibiotic prescribing habits and the presence of pathogens harboring the genes for ESBL production. Although there are no CLSI guidelines for the detection of the AmpC β-lactamases production, we have followed AmpC disk test.13 In the present study, the prevalence of AmpC producing MDR A. baumannii was found 35.6%. Almost a similar prevalence of AmpC producing Acinetobacter spp. have been reported from the same hospital by Parajuli et al24 However, in an Indian study, the higher rate (56%) of AmpC producing A. baumannii was documented.26
Carbapenem-resistant Acinetobacter baumannii (CRAB) is included in priority 1 (ie, critical) of a global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new drugs by the World Health Organization.27 Although there are different mechanisms for carbapenem resistance, the production of carbapenemases enzyme is the most effective mechanism.9 The emergence of MBLs in A. baumannii is becoming a therapeutic challenge as these enzymes possess high hydrolytic activity that leads to degradation of higher generation cephalosporins and carbapenems. Furthermore, plasmid-mediated MBL genes spread rapidly to other species of gram-negative bacilli.28 Therefore, rapid detection of MBL production is necessary to modify therapy and to initiate effective infection control to prevent their dissemination. In the current study, metallo β-lactamase (MBL) producing isolates were found common than ESBL and AmpC producers, where 67.7% MDR A. baumannii were MBL producers. In Nepal, few studies have been done on the prevalence of MBLs, Shrestha et al29 reported 47.2% and Parajuli et al24 reported 78.8% MBL producers in Acinetobacter spp. from the same hospital. In the study of Dey and Bairy,30 MBL was reported only in 21.7% of Acinetobacter spp. The coexistence of multiple MBL genes in bacteria is an alarming situation. As MBL genes are associated with integrons that can be embedded in transposons, which in turn can be accommodated on plasmids thereby resulting in a highly mobile genetic apparatus, the further spread of these genes in different pathogens is likely to occur. This study also attempted to find out the KPC-producing isolates, where 9.5% MDR A. baumannii and some of them were also co-producing MBL enzyme. Although there was no single article regarding the detection of KPC from Nepal, Parajuli et al24 recently reported KPC-producing Acinetobacter species from ICU patients. Most of the KPC-producing isolates have been reported from the USA, Greece, China, Israel, and Colombia.31 Among carbapenemases, KPC has a high frequency and has been commonly found in Klebsiella pneumonia.32 Among the β-lactamases producing isolates, some of the isolates were also co-producers of different β-lactamases and the MDR isolates producing two different types of β-lactamases showed high resistant profile. The spread of carbapenemase-producing bacteria throughout the world in recent years has been considered as a major threat to public health. After the carbapenem-resistant clones have emerged, leaving the hope of treatment of MDR A. baumannii infection is by the last resort of potentially toxic antibiotics such as polymyxin B and colistin sulfate.33
The study shows infection with MDR A. baumannii is increasing at an alarming rate in our hospital. It has now become very important to control this situation before it takes a deadly shape. Therefore, rapid detection of the resistance determinants is necessary to modify therapy and to initiate effective infection control to prevent their dissemination.
Limitations
We could not evaluate the risk factors and outcomes of MDR A. baumannii infections in hospitalized patients due to the unavailability of sufficient data from the patients. Furthermore, genetic analysis of the resistant phenotypes and drug resistance mechanisms was not determined.
Conclusion
From the present study, it is clear that infections in hospitalized patients due to MDR A. baumannii are common. Rate of MBL, ESBL, and AmpC production among MDR isolates has highly increased and this bacteria may lead to high morbidity and mortality as we are left with the only option of treating them by potentially toxic antibiotics like colistin sulfate and polymyxin B and this is the vexatious problem for hospitalized patients. Established recommendations including proper detection of drug resistance in pathogens, antimicrobial restriction policies to avoid excessive use of broad-spectrum antibiotics, improving resistance surveillance systems, and rigorous infection control measures will help to control this situation.
Acknowledgments
The authors would like to offer their sincere gratitude to all faculties and staffs of the Department of Clinical Microbiology and staffs of different wards of Tribhuvan University Teaching Hospital.
Funding Statement
No funding source was involved to complete this study.
Abbreviations
AmpC, ampicillinase C; ASM, American Society for Microbiology; CLSI, Clinical and Laboratory Standard Institute; ESBL, extended-spectrum β- lactamases; EDTA, ethylene-diamine-tetraacetic acid; ICU, intensive care unit; KPC, Klebsiella pneumoniae carbapenemase; MBL, metallo β-lactamases; MDR, multidrug resistant; PBA, phenylboronic acid; SPSS, statistical package for the social sciences; TUTH, Tribhuvan University Teaching Hospital.
Ethical Approval and Consent to Participate
Written approval (Ref No: 262(6-11-E)2/073/074) was obtained from the Institutional Review Board (IRB) of the Institute of Medicine after submitting and presenting a research proposal. In addition, written consent was taken from every patient or their guardian for participation in this study before enrollment.
Data Sharing Statement
Primary data regarding this report will be made available to the interested researchers upon written request.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Authors’ Information
Santosh Kumar Yadav: Department of Microbiology, Rajarshi Janak University, Janakpurdham, Nepal.
Rajshree Bhujel, Shyam Kumar Mishra, Sangita Sharma and Jeevan Bahadur Sherchand: Department of Clinical Microbiology, Institute of Medicine, Tribhuvan University Teaching Hospital, Kathmandu, Nepal.
Pradip Hamal: Department of Pathology, B.P. Koirala Memorial Cancer Hospital, Bharatpur, Nepal.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Young LS, Sabel AL, Price CS. Epidemiologic, clinical, and economic evaluation of an outbreak of clonal multidrug-resistant Acinetobacter baumannii infection in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2007;28(11):1247–1254. doi: 10.1086/521660 [DOI] [PubMed] [Google Scholar]
- 2.Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46(8):1254–1263. doi: 10.1086/529198 [DOI] [PubMed] [Google Scholar]
- 3.Van Looveren M, Goossens H, Baquero F, et al. Antimicrobial resistance of Acinetobacter spp. in Europe. Clin Microbiol Infect. 2004;10(8):684–704. doi: 10.1111/j.1469-0691.2004.00942.x [DOI] [PubMed] [Google Scholar]
- 4.Towner KJ. Acinetobacter: an old friend, but a new enemy. J Hosp Infect. 2009;73(4):355–363. doi: 10.1016/j.jhin.2009.03.032 [DOI] [PubMed] [Google Scholar]
- 5.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21(3):538–582. doi: 10.1128/CMR.00058-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sunenshine RH, Wright MO, Maragakis LL, et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis. 2007;13(1):97–103. doi: 10.3201/eid1301.060716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garnacho-Montero J, Amaya-Villar R. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Curr Opin Infect Dis. 2010;23(4):332–339. doi: 10.1097/QCO.0b013e32833ae38b [DOI] [PubMed] [Google Scholar]
- 8.Tripathi P, Gajbhiye S. Prevalence of multidrug resistance, ESBL and MBL production in Acinetobacter spp. Int J Recent Trends Sci Technol. 2013;6(3):139–143. [Google Scholar]
- 9.Noyal MJC, Menezes GA, Harish BN, Sujatha S, Parija SC. Simple screening tests for detection of carbapenemases in clinical isolates of nonfermentative Gram-negative bacteria. Indian J Med Res. 2009;129(6):707–712. [PubMed] [Google Scholar]
- 10.Isenberg H. Clinical Microbiology Procedure Handbook. 2nd ed. Washington, DC: American Society for Microbiology (ASM); 2007. [Google Scholar]
- 11.CLSI. Performance Standards for Antimicrobial Susceptibility Testing, Supplement M100S. 26th ed. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2016. [Google Scholar]
- 12.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pan drug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 13.Singhal S, Mathur T, Khan S, et al. Evaluation of methods for AmpC β- Lactamase in gram negative clinical isolates from tertiary care hospitals. Indian J Med Microbiol. 2005;23(2):120–124. doi: 10.4103/0255-0857.16053 [DOI] [PubMed] [Google Scholar]
- 14.Tsakris A, Poulou A, Pournaras S, et al. A simple phenotypic method for the differentiation of metallo-β-lactamases and class A KPC carbapenemases in Enterobacteriaceae clinical isolates. J Antimicrob Chemother. 2010;65(8):1664–1671. doi: 10.1093/jac/dkq210 [DOI] [PubMed] [Google Scholar]
- 15.Shrestha S, Tada T, Shrestha B, et al. Phenotypic characterization of multidrug-resistant Acinetobacter baumannii with special reference to metallo-β-lactamase production from the hospitalized patients in a tertiary care hospital in Nepal. JIOM. 2015;37(3):3–10. [Google Scholar]
- 16.Saad M, Samawi A, Khan FY, et al. Acinetobacter infections among adult patients in qatar: a 2-year hospital-based study. Canadian J Infect Dis Med Microbiol. 2016. doi: 10.1155/2016/6873689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shrestha RK, Dahal RK, Mishra SK, et al. Ventilator-associated pneumonia in tertiary care. JIOM. 2013;35(3):21–28. [Google Scholar]
- 18.Mishra SK, Rijal BP, Pokhrel BM. Emerging threat of multidrug resistant bugs – acinetobacter calcoaceticus baumannii complex and Methicillin-resistant Staphylococcus aureus. BMC Res Notes. 2013;6(1):1. doi: 10.1186/1756-0500-6-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie D, Xiong W, Lai R, et al. Ventilator-associated pneumonia in intensive care units in Hubei Province, China: a multicentre prospective cohort survey. J Hosp Infect. 2011;78(4):284–288. doi: 10.1016/j.jhin.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 20.Villegas MV, Kattan JN, Quinteros MG, Casellas JM. Prevalence of extended-spectrum beta-lactamases in South America. Clin Microbiol Infect. 2008;14 Suppl 1:154–158. doi: 10.1111/j.1469-0691.2007.01869.x [DOI] [PubMed] [Google Scholar]
- 21.Mendes RE, Bell JM, Turnidge JD, Castanheira M, Jones RN. Emergence and widespread dissemination of OXA-23, −24/40 and −58 carbapenemases among Acinetobacter spp. in Asia-Pacific nations: report from the SENTRY surveillance program. J Antimicrob Chemother. 2009;63(1):55–59. doi: 10.1093/jac/dkn434 [DOI] [PubMed] [Google Scholar]
- 22.Joseph NM, Sistla S, Dutta TK, Badhe AS, Rasitha D, Parija SC. Ventilator-associated pneumonia in a tertiary care hospital in India: role of multidrug resistant pathogens. J Infect Dev Ctries. 2010;4(4):218–225. doi: 10.3855/jidc.634 [DOI] [PubMed] [Google Scholar]
- 23.Al-Sweih NA, Al-Hubail MA, Rotimi VO. Emergence of tigecycline and colistin resistance in Acinetobacter species isolated from patients in Kuwait hospitals. J Chemother. 2011;23(1):13–16. doi: 10.1179/joc.2011.23.1.13 [DOI] [PubMed] [Google Scholar]
- 24.Parajuli NP, Acharya SP, Mishra SK, Parajuli K, Rijal BP, Pokhrel BM. High burden of antimicrobial resistance among gram negative bacteria causing healthcare associated infections in a critical care unit of Nepal. Antimicrob Resist Infect Control. 2017;6(1):67. doi: 10.1186/s13756-017-0222-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safari M, Sasan A, Nejad M, Bahador A. Prevalence of ESBL and MBL encoding genes in Acinetobacter baumannii strains isolated from patients of intensive care units (ICU). Saudi J Biol Sci. 2015;22(4):424–429. doi: 10.1016/j.sjbs.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hans R, Bisht D, Agarwal R, Medical S. Phenotypic detection of MBL, AmpC beta-lactamase and carbapenemases in multi drug resistant isolates of Acinetobacter baumannii. Int J Med Res Heal Sci. 2014;4(2):311–316. doi: 10.5958/2319-5886.2015.00058.2 [DOI] [Google Scholar]
- 27.Tacconelli E, Magrini N, Kahlmeter G, Singh N. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Heal Organ. 2017;27:1–7. [Google Scholar]
- 28.Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011;11(5):355–362. doi: 10.1016/S1473-3099(11)70059-7 [DOI] [PubMed] [Google Scholar]
- 29.Shrestha S, Chaudhari R, Karmacharya S, et al. Prevalence of nosocomial lower respiratory tract infections caused by Multidrug resistance pathogens. JIOM. 2011;33(2). [Google Scholar]
- 30.Dey A, Bairy I. Incidence of multidrug-resistant organisms causing ventilator-associated pneumonia in a tertiary care hospital: a nine months’ prospective study. Ann Thorac Med. 2007;2(2):52–57. doi: 10.4103/1817-1737.32230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13(9):785–796. doi: 10.1016/S1473-3099(13)70190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villegas MV, Lolans K, Correa A, Kattan JN, Lopez JA, Quinn JP. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing β-lactamase. Antimicrob Agents Chemother. 2007;51(4):1553–1555. doi: 10.1128/AAC.01405-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poirel L, Menuteau O, Agoli N, Cattoen C, Nordmann P. Outbreak of extended-spectrum β-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a French hospital. J Clin Microbiol. 2003;41(8):3542–3547. doi: 10.1128/JCM.41.8.3542-3547.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]