Abstract
The Euonymus lectin (EUL) family is a unique group of carbohydrate-binding proteins that is omnipresent in plants. Sequences encoding EUL-related lectins have been retrieved from all completely sequenced plant genomes. The rice (Oryza sativa) genome contains 5 functional EUL genes referred to as OsEULS2, OsEULS3, OsEULD1a, OsEULD1b, and OsEULD2. In this study we focused on the tissue specific expression, stress inducibility and subcellular localization of the rice EULs. Even though the EUL domain sequence is highly conserved among the rice EULs (at least 80% sequence similarity) different biotic and abiotic stress treatments yielded unique responses for the different EULs. Transcript levels for OsEULs were differentially affected by drought and salt stress, ABA treatment, pathogen infection or insect infestation. Analysis of promoter activity revealed differential expression and tissue specificity for the 5 OsEUL genes, with most expression observed in the vascular system of roots and shoots, as well as in the root tips and seeds. At cell level, all OsEULs are located in the nucleus whereas OsEULD1b and OsEULD2 also locate to the cytoplasm. This paper contributes to the functional characterization of the EULs and provides insight in the biological importance of this family of proteins for rice.
Keywords: abscisic acid, Euonymus, lectin, nucleocytoplasmic localization, rice, stress regulation
Introduction
Lectins are defined as proteins containing one or more lectin domains that bind carbohydrate structures in a reversible, specific and non-enzymatic way. These carbohydrate structures can occur as monosaccharides, polysaccharides or as glycan structures attached to proteins or lipids (Van Holle and Van Damme, 2018). Although lectins are present in all kingdoms of life, most carbohydrate-binding proteins have been characterized from plants. The whole group of plant lectins is highly diverse in sequence, molecular structures, biochemical properties and biological activities. Nevertheless, lectins can be classified in different families based on the presence of particular lectin motifs. In the 1980s and 90s plant lectin research focused on vacuolar lectins that were abundant in storage tissues and could easily be purified using affinity chromatography. Breakthroughs in molecular biology, genomics, transcriptomics and bio-informatics resulted in the discovery of a new class of low abundant, nucleocytoplasmic lectins that are specifically upregulated after plant exposure to biotic and/or abiotic stimuli, implying the involvement of these lectins in stress signaling. For example, the tobacco lectin, referred to as Nictaba, is only detectable after treatment of tobacco leaves with methyl jasmonate (MeJA), cold stress and herbivory by caterpillars (Delporte et al., 2015). Overexpression of Nictaba-like lectin genes from Glycine max confers tolerance toward Pseudomonas syringae infection, aphid infestation and salt stress in transgenic Arabidopsis plants (Van Holle et al., 2016). Another well studied lectin is Orysata, a rice lectin that is up-regulated after several abiotic stresses such as salinity, drought stress and abscisic acid (ABA) treatment (De Souza Filho et al., 2003). Overexpression of the Orysata gene resulted in rice plants with higher salt tolerance (De Souza Filho et al., 2003; He et al., 2017).
Recent studies aiming to make an inventory of the occurrence of lectin motifs in a few model species with a fully sequenced genome revealed that most lectin sequences encode multi-domain proteins in which one or more lectin domains are linked to other protein domains such as a protein kinase domain, an F-box domain or a glycosyl hydrolase domain. However, some lectin motifs, such as the Euonymus lectin domain have not been found in association with other known protein domains (Van Holle et al., 2017). The family of Euonymus-related lectins (EUL) is unique in that it is ubiquitous in the plant kingdom. EUL sequences have been retrieved from all annotated genomes of land plants. EUL sequences are composed of one or two EUL lectin domains, further referred to as S (single) type EULs and D (double) type EULs (Fouquaert et al., 2009). The Arabidopsis thaliana genome contains only one member of the EUL family, named ArathEULS3, which interacts preferentially with galactosylated glycans. Transcript levels for ArathEULS3 are enhanced when plants are subjected to drought, salt stress, ABA treatment or after infection with P. syringae (Van Hove et al., 2015).
In contrast to many dicot species the monocots contain a mixture of S- and D-type EUL sequences. The rice genome contains two S-type EULs (called OsEULS2 and OsEULS3) and three D-type EULs (called OsEULD1a, OsEULD1b and OsEULD2). The EUL domain sequence is highly conserved among the rice EULs (at least 80% sequence similarity) (De Schutter et al., 2017). Despite the high sequence similarity between OsEULs the proteins have very different carbohydrate-binding activities. For instance, OsEULS2 has a preference for high-mannose N-glycans (Al Atalah et al., 2012) while OsEULD1a rather binds to galactose related sugars (Al Atalah et al., 2014). Analyses of the promoter region of the five rice EUL genes revealed a variety of stress responsive elements (De Schutter et al., 2017). RNA sequencing data and transcriptomics studies also indicate that transcript levels for EULs change after exposure of the plant to stress conditions. At present only a few scattered reports of proteomics data suggest that at least some of the OsEULs are stress-inducible proteins. For instance, a proteomics study for rice plants reported enhanced levels for some of the rice EULs after salt, ABA and drought treatment (Moons et al., 1995; Rabello et al., 2008).
In the past two decades several stress inducible plant lectins have been reported in literature. However, only few stress inducible lectins have been studied and functionally characterized. In this paper we focused on the tissue-specific expression, the subcellular localization and the stress inducibility for a family of closely related EULs from rice. Despite the high sequence similarity between the lectin domains important differences are observed for the five OsEULs. The experimental data confirm the stress dependent regulation of these lectins and provide insight in the biological importance and possible functions for rice EULs.
Materials and Methods
Plant Material
Oryza sativa japonica cv Nipponbare seeds were dehusked and sterilized for 5 min in 70% ethanol followed by incubation in 5% NaOCl containing 2–3 drops of Tween 20 under constant shaking at 150-180 rpm for 45 min. Afterwards the seeds were rinsed thoroughly with sterile water and incubated overnight with constant shaking at 150–180 rpm to synchronize the germination process.
Abiotic Stress Treatment
For all abiotic stress experiments, seed germination was performed on a sterile filter paper soaked in half strength liquid (MS) medium (Murashige and Skoog, 1962) (Duchefa, Haarlem, Netherlands), pH 5.8 for 4 days at 30°C in the dark. Subsequently the seedlings were transferred to 96-well tip boxes (48 plants per box) and grown at 28°C, 16h light/8h dark, in half strength Hoagland solution, pH 5.8. The solution was refreshed daily. After nine days in the 96-well tip boxes, the stress was applied to 13-day old seedlings. Stressed and non-treated samples were collected at 1, 3, 6, 10, 24, and 48 h after stress application. Shoots and roots were separated, frozen in liquid N2 and stored at −80°C until further analysis. At least three biological replications were performed for each experiment. Rice seedlings were treated with the following abiotic stress factors: salt (150 mM), drought and plant hormones (ABA, MeJA, SA) (100 µM). Drought stress was performed by air drying the roots.
Biotic Stress Experiments
For the biotic stress experiments sterilized seeds were germinated on MS medium, pH 5.8 supplemented with 8 g/L agar and 112 mg/L B5 vitamin (Duchefa) in a plant incubator (28°C, complete darkness, 70–75% relative humidity). Unless mentioned otherwise the seedlings were grown in perforated plastic trays (22 x 15 x 6 cm) containing autoclaved potting soil. Plants were fertilized weekly with iron solution (0.2% iron sulfate and 0.1% ammonium sulfate). The plants were kept in the greenhouse at 28°C under 16h/8h photoperiod and a relative humidity of 70 to 75% until treatment with pathogens.
Rice blast fungus (Magnaporthe oryzae strain VT5M1) was grown at 28°C on half-strength oatmeal agar (Difco, New Jersey, USA). Seven-day-old mycelium was placed onto the medium under blue light (mix of Philips TLD 18W/08 and Philips TLD 18W/33) for seven days to induce sporulation. At the five-leaf stage (4–5 weeks old) rice plants were inoculated with a final concentration of 4 x 104 spores per milliliter in 0.5% gelatin (type B bovine skin) and mock inoculated with a solution of 0.5% gelatin by spraying until drain off. Inoculated plants were placed in a dew chamber at 28 ± 2°C under 16/8 h photoperiod and a relative humidity of more than 90% for 24 h, and then transferred to the growth chamber for further disease development for four days under the same growth conditions. Experiments were performed in three biological replicates. The fourth leaf of each plant was collected for RNA extraction at four days after inoculation. Three leaves were pooled as one biological replicate.
Rice bacterial blight (Xanthomonas oryzae pv. oryzae, Philippine race 6, strain PXO99) was grown on sugar peptone agar in the incubator at 28°C in the dark for 3 days. Afterwards, a single bacterial colony was inoculated into 30 mL sugar peptone broth and incubated overnight on an orbital shaker with 130 rpm at 28°C. The fully expanded and lengthy leaves of four-week-old plants were inoculated with Xanthomonas bacterial suspension (OD600 was 0.8) using the leaf clipping method as described by Kauffman et al. (1973). Mock infected plants were treated with sterile water instead of bacterial solution. The Xanthomonas infected and mock infected plants were allowed to grow for 2 weeks in a quarantined greenhouse set at 30°C under 16h/8h photoperiod with a relative humidity of 75%. Experiments were performed in three biological replicates. Inoculated and mock plants were collected two weeks after inoculation for RNA extraction. Twelve plants were pooled as one biological replicate.
Nematode (Meloidogyne graminicola) infection experiments were performed using rice seedlings grown in polyvinyl-chloride tubes containing a mixture of fine sand and synthetic absorbent polymer substrate (AquaperlaR, DCM, Belgium). Each tube contained two seedlings. The plants were grown in the plant growth room at 28°C, and supplemented with 15 mL Hoagland solution (pH 5.8) for each tube twice a week and 15 mL tap water for each tube once a week. The nematodes were cultured on Echinochloa crus-galli grown on potting soil. Second stage juveniles of the nematodes were extracted from 3-month-old infected roots. Fifteen-day-old rice plants were inoculated with 350 second stage juveniles or mock inoculated with autoclaved water. Both inoculated and mock plants were collected two weeks after inoculation for RNA extraction. The experiment was performed in three biological replicates, 5 plants were pooled as one biological replicate.
Insect infestation assays were performed with the brown planthopper (Nilaparvata lugens). The insect colony was maintained on 4 to 8 weeks old rice plants in a growth chamber at 28°C. Low infestation was achieved by incubating 36 third instar nymphs per pot of 12 rice plants, whereas for the high infestation 100 third instar nymphs were added per pot of 12 rice plants. In the low infestation experiment, shoots of 10 infested and mock plants were collected at 5 and 13-day-after infestation for RNA extraction and pooled as one biological replicate. In the high infestation experiment, shoots of 10 infested and mock plants were collected at 3, 6 and 9 day-after infestation for RNA extraction and pooled as one biological replicate. Both infestation experiments were performed in three biological replicates.
Pythium graminicola (strain PB 912 132) infection experiments were done with rice seedlings grown in vitro. Therefore, six surface sterilized seeds were placed (2 cm apart from each other) and germinated on Gamborg B5 (Duchefa) medium supplemented with 8 g/L agar in square Petri dishes (120 × 120 mm). Plates were arranged in upstanding position (60°C angle) in the plant growth room at 28°C. Plants were grown in the dark for 3 days followed by growth in shielded light by covering the plates partially with aluminum foil on the root side. PB 912 132 was maintained on Potato Dextrose Agar medium at 28°C in the dark. Seven-day-old seedlings were inoculated by placing three mycelial plugs (5 mm in diameter) taken from the edge of a three-day-old P. graminicola mycelium between the roots. In mock plates, Potato Dextrose Agar medium plugs without mycelium were placed between the seedlings. Inoculated and mock plants were collected for RNA extraction 3 days after inoculation. The experiment was performed with three biological replicates, at least 12 plants were pooled as one biological replicate.
Real-Time Quantitative PCR
The plant material was ground in liquid nitrogen and total RNA was isolated using the Spectrum™ Plant Total RNA Kit (Sigma-Aldrich, Saint Louis, USA) according to the manufacturer’s instructions. Samples were treated with RNase-free DNase I enzyme (DNase kit, Fermentas, St. Leon-Roth, Germany) to remove residual genomic DNA. Reverse transcription reactions were performed with Maxima Reverse Transcriptase Kit (Thermo Fisher Scientific, Waltham, USA) using 0.5 µg RNA and the following PCR conditions: 10 min at 25°C, 20 min at 55°C and 5 min at 85°C. qRT-PCRs were performed using SYBR Green I Mix (Bio-Rad) and following conditions: 10 min initial denaturation step at 95°C, 42 cycles (15 s at 95°C, 25 s at 60°C, 20 s at 72°C) and detected with Bio-Rad CFX Connect™ Real-Time PCR Detection System. The expression of the genes was normalized against three reference genes: EXP (LOC_Os03g27010.1), EIF5C (LOC_Os011g21990.1), and EXP Narsai (LOC_Os07g02340.1) (Narsai et al., 2010; Kyndt et al., 2012; Al Atalah et al., 2014). The stability of the reference genes was checked by the qbase+ software (Biogazelle, Zwijnaarde, Belgium). The software addresses minimal acceptable reference target stability by defining a threshold value for two indicators of expression stability: the geNorm expression stability value of the reference gene (M ˂ 1) and the coefficient of variation of the normalized reference gene relative quantities (CV ˂ 0.5). If a reference gene was not stabile between samples, it was excluded from the qPCR analyses. The three references genes were stabile for all the qPCR analyses, except for the SA treatment where EIF5C reference was excluded and the qPCR analyses were performed with the two remaining reference genes. All primers used for qPCR are described in Supplementary Table 1 and control experiments are shown in Supplementary Figure 1. Data analysis was performed using the Bio-Rad CFX Manager software.
Vector Construction, Generation of GUS Lines and GUS Staining
Constructs used in the GUS reporter system contained the 2-kb promoter region upstream of the translation start of each OsEUL gene. All primers used for the construction of the GUS lines are described in Supplementary Table 2. The promoter fragment was inserted between the KpnI and XhoI site of the pENTL4R1 plasmid by classical cloning. The transcriptional fusion of the promoter fragment with the GUS coding sequence was obtained by Multi Gateway Cloning (Thermo Fisher Scientific) using the entry vectors pENTL4R1, pEN-L1-S-L2 and the destination vector pBb7m24GW (obtained from Plant Systems Biology, Vlaams Instituut voor Biotechnologie, Ghent, Belgium). The expression clones were introduced into the A. tumefaciens strain EHA 105 by electroporation. These strains were used to transform O. sativa cv. Nipponbare and create promotor::GUS lines.
For the establishment of an in vitro callus culture for rice transformation, surface sterilized rice seeds were inoculated on callus induction medium (N6 salts and vitamins, 30 g/L sucrose, 1 g/L casein hydrolysate, 1.5 g/L L-proline, 2 mg/L 2,4 dichlorophenoxyacetic acid and 0.8% agarose; pH 5.8). Actively proliferating pre-cultured callus material was washed with liquid infection medium [MS salts (Murashige and Skoog, 1962), 68.5 g/L sucrose, 36 g/L glucose, 1 g/L casein hydrolysate, 0.87 g/L L-glutamine, 0.26 g/L L-aspartic acid, 0.17 g/L L-arginine, and 7.5 mg/L glycine; pH 5.2] and subsequently inoculated in 35 mL of Agrobacterium suspended in infection medium at an OD of 1.2). After 10 min, the callus material was removed from the Agrobacterium suspension and incubated for 4 days at 26 ± 1°C under complete darkness on sterile Whatman filter paper placed on R2–COMAS medium [MS salts, B5 Vitamins (Gamborg et al., 1968), 20 g/L sucrose, 10 g/L glucose, 1 g/L casein hydrolysate, 2 mg/L 2,4-D, 100 µM acetosyringone, and 0.8% agarose SPI; pH 5.2]. Following co-cultivation, calli were washed with sterile distilled water containing 500 mg/L timentin (Duchefa) blotted dry on sterile Whatman filter paper and subsequently placed on selection medium (CIM containing 500 mg/L timentin and 50 mg/L phosphinothricin (Duchefa). The calli were passed through 2 selection cycles of 15 days each. At the end of selection, the surviving and actively proliferating calli were inoculated on pre-regeneration medium (N6 salts and vitamins, 30 g/L sucrose, 1 g/L casein hydrolysate, 1.5 g/L L-proline, 1 mg/L 2,4-D, and 0.8% agarose; pH 5.8) supplemented with 300 mg/L timentin and 30 mg/L phosphinothricin for 4 weeks. Well proliferated calli were then selected and inoculated onto regeneration medium (0.5 x MS salts and modified vitamins (Duchefa), 20 g/L sucrose, 1 g/L casein hydrolysate, 40 µM copper sulfate, 0.87 g/L L-glutamine, 0.26 g/L L-aspartic acid, 0.17 g/L L-arginine, and 7.5 mg/L glycine, 1 mg/L zeatin riboside, and 0.8% agarose; pH 5.8) and incubated for 6 weeks. After 6 weeks, induced shoots (2-3 cm) were individually excised and transferred onto rooting medium (0.5x MS basal salts, 0.5x B5 vitamins, 10 g/L sucrose, 0.5g/L MES, and 0.8% agar; pH 5.8). After 3–4 weeks, well rooted plantlets were transplanted to soil and grown in a growth room at 28°C. The (T0) plants were examined on genomic level for the GUS gene and allowed to undergo self-pollination. T1 and T2 seeds were used for the experiments.
Two independent reporter lines were tested for each OsEUL gene. The GUS assay was performed according to Jefferson (1987). One week-old plants were incubated for 30 min in 90% acetone at 4°C followed by washing with phosphate buffer (0.1 M NaH2PO4.2H2O and 0.1 M Na2HPO4; pH 7.2). After 30 min treatment with GUS pre-incubation buffer (0.1 M phosphate buffer, 0.5 mM potassium-ferricyanide and 0.5 mM potassium-ferrocyanide) staining was performed overnight at 37°C in GUS staining buffer (2 mM X-gluc added to the preincubation buffer). After staining the plants were washed and preserved in 70% ethanol at 4°C. Microscopic slides were made with lactic acid (90%) and analyzed under the Widefield microscope Nikon eclipse Ti (Nikon instruments, Badhoevedorp, Netherlands) using a 5x dry objective lens.
Nicotiana benthamiana Infiltration
The coding sequence for each OsEUL sequence was cloned after the 35S promoter in the expression vectors pK7WFG2 and pK7WGF2 to create the OsEUL-EGFP and EGFP-OsEUL constructs, respectively. The cloning started by adding attB sites to the coding sequence by PCR followed by BP reaction into pDONR221 plasmid to end with the destination vector after LR reaction. All primers used for the construction of the vectors are described in Supplementary Table 3. Four week old plants were transiently transformed with Agrobacterium strain LBA4044. The culture was inoculated in YEB medium with spectinomycin (50 µg/mL) at 28°C until an OD600 of 0.8 was reached. Afterwards the cells were washed twice with infiltration buffer (50 mM MES, 2mM Na2HPO4, and 0.5% glucose, pH 5.6) and finally re-suspended in infiltration buffer containing 100 µM acetosyringone. The Agrobacterium solution was injected in the abaxial epidermal cells of the tobacco leaves. Two days after injection, the infiltrated leaf area was analyzed under the Nikon A1R confocal laser scanning microscope (Nikon instruments) mounted on a Nikon Ti-E inverted epifluorescence body with an S Plan Fluor ELWD air objective lens (NA 0.60) or a 60_ Plan APO VC water immersion lens (NA 1.20) for 4′,6-diamidino-2-phenylindole (DAPI) and propidium iodide (PI) treated slides respectively. The tobacco leaves were infiltrated with DAPI (0.01 mg/mL) or PI (0.01 mg/mL), before making the microscopic slides of the tobacco leaves in water. EGFP was excited with a 488 nm argon ion laser, PI was excited with a 560 nm laser and DAPI was excited with a 404 nm laser. Emission filters were 500–550 nm for EGFP, 570–620 nm for PI and 425–475 nm for DAPI. Image analysis and assessment of co-localization were performed in ImageJ (Schindelin et al., 2012). Plasmolysis experiments were performed by incubating leaf samples in 2 M salt solution and analysis under the microscope (Pitzschke et al., 2014).
Results
Tissue Specific Expression of the OsEUL Genes
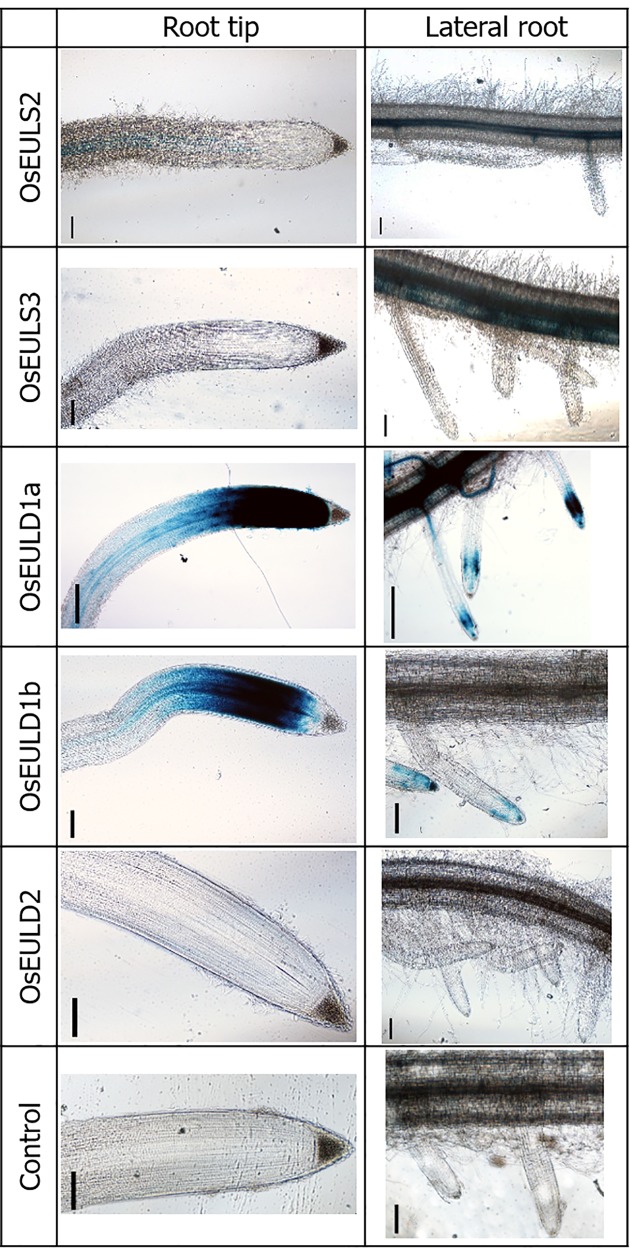
To investigate the tissue-specific expression of OsEULs, GUS reporter lines were constructed. To exclude positional effects due to insertion of the sequence in the rice genome, two independent reporter lines were tested for each OsEUL gene construct. GUS gene expression driven by the promoter of each OsEUL results in a blue histochemical staining pattern representing the tissue dependent expression for each EUL (Figure 1).
Figure 1.
OsEUL gene patterning in rice roots indicated by promoter-driven GUS expression. Scale bar represents 0.1 mm.
GUS staining in the roots yielded very different results for the different promoter lines. GUS staining was only observed in the tips of the main root as well as in the lateral roots of reporter lines for OsEULD1a and OsEULD1b. The meristematic tissue of the root tip was stained but no staining was apparent in the columella. Strong staining was observed in the vascular system for the reporter lines OsEULS3 and OsEULD1a whereas the OsEULS2 and OsEULD2 reporter lines yielded faint to no staining. Only for the OsEULS3 promoter lines the staining intensity in the vascular system increased in more mature tissues, with intense staining in the root zones with well-developed lateral roots, and fading towards the root tip, which is completely devoid of staining (Figure 1).
GUS staining in the leaf was observed in the longitudinal and transverse veins. A much stronger coloring was observed for the promoter constructs for the S-type EULs and OsEULD1a compared to the other D-type EULs. Especially the reporter lines for OsEULD2 show a very faint signal (Figure 2).
Figure 2.
OsEUL gene patterning in rice tissues indicated by promoter-driven GUS expression for shoots and seeds. Scale bar represents 0.1 mm, except for scale bars on pictures with seeds, which represent 5 mm.
Longitudinal sections of the seeds showed clear GUS staining in the embryos of the reporter lines for OsEULS2, OsEULD1a, and OsEULD1b. The GUS lines for OsEULD1a and OsEULD1b also showed staining outside the embryo, in the endosperm. The most explicit staining forms a ring following the starchy endosperm. The pericarp, outer layer of the rice seed, is not stained suggesting that the staining observed in the seeds for OsEULD1a and OsEULD1b is in the aleurone layer between the pericarp and starchy endosperm (Figure 2).
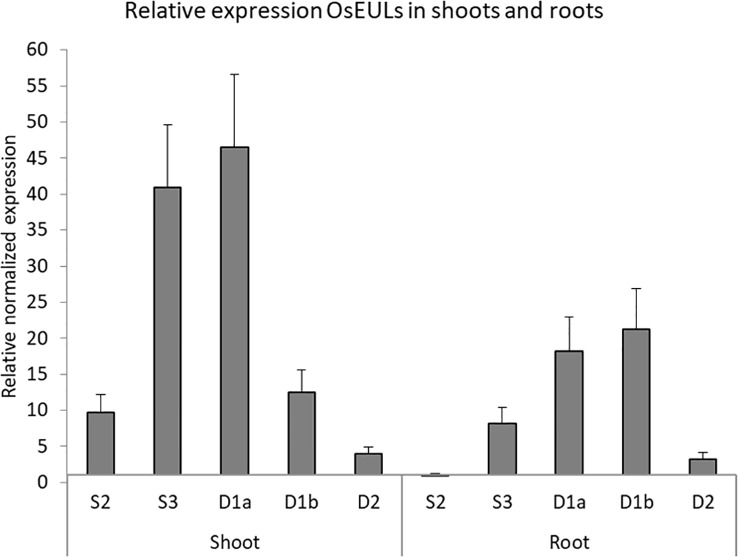
Relative Expression Profile of the OsEULs in Shoot and Root
qPCR data allowed to compare and quantify the relative expression between the EULs in roots and shoots of rice plants grown under normal (non-stressed) growth conditions (Figure 3). OsEULS3, OsEULD1a show the highest expression in shoot tissue, whereas OsEULS3, OsEULD1a, and OsEULD1b are expressed in roots. OsEULD1b is the only EUL that shows higher expression in roots compared to shoots. OsEULS2 is always present in low concentrations, but gene expression is higher in shoots compared to roots. OsEULD2 expression levels were very low in shoots and roots under non-stress conditions.
Figure 3.
Expression profile for OsEULs in shoots and roots of 13-day old non-stressed rice seedlings. The normalized transcript levels for all EUL genes are represented relative to OsEULS2 expression in roots. The data represent ten independent biological replicates, error bars indicate standard errors.
OsEUL Expression in Response to Abiotic Stresses and Hormonal Treatment
The expression of OsEULs was analyzed in 13-day old rice seedlings subjected to a range of plant hormones, abiotic and biotic stress conditions, including the most damaging and economically important stress factors influencing rice growth and yield. The plants were treated with the stressor for 1, 3, 6, 10, 24, and 48 h, and compared to non-treated plants harvested at the same time point.
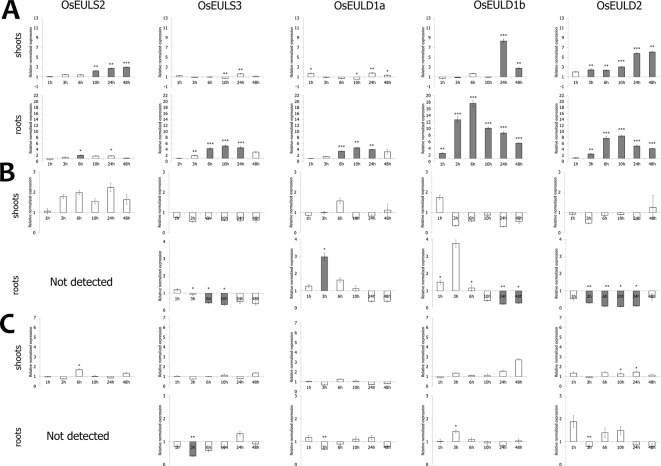
Of all abiotic and hormonal treatments tested, ABA treatment, drought and salt application showed the most prominent effect on EUL gene expression (Figures 4 and 5). ABA was the only stress factor which provoked a significant upregulation for all OsEUL transcripts, predominantly in roots and to a lesser extent in the shoots. The highest expression levels were observed for OsEULD1b (approximately 18-fold significant upregulation in roots after 6 h and 10-fold after 10 h treatment) and OsEULD2 (approximately 8-fold significant upregulation in roots after 6 h and 10 h). Transcript levels for OsEULD1a and OsEULS3 were also significantly upregulated in roots ranging from 3-5 to maximum 4-6-fold at 6-24 h after stress application (Figure 4). The highest levels of upregulation in the shoots were observed for OsEULD1b at 24 h and OsEULD2 at 48 h after the treatment reaching maximum levels of 8 and 6-fold. OsEULS2 expression was only weakly affected after ABA treatment (Figure 4).
Figure 4.
Transcript levels for OsEUL genes after different hormone treatments, including ABA (A), MeJA (B) and SA (C). Normalized expression levels, relative to the non-treated plants, set to 1, are shown for different time points. The mean values of RT-qPCR for three independent biological replicates were normalized to three reference genes and error bars indicate standard errors. Dark-gray bars represent statistically significant up-regulation of at least two-fold compared to non-treated plants and light-gray bars represent statistically significant down-regulation (*p < 0.05, **p < 0.01, ***p < 0.001).
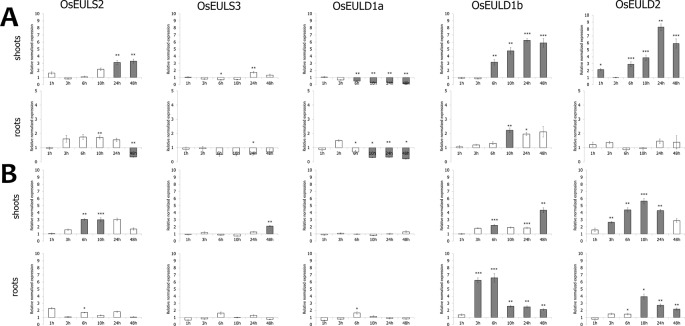
Figure 5.
Transcript levels for OsEUL genes after drought (A) and salt stress (B). Normalized expression levels, relative to the non-treated plants (set to 1), are shown for different time points. The mean values of RT-qPCR for three independent biological replicates were normalized to three reference genes and error bars indicate standard errors. Dark-gray bars represent statistically significant up-regulation of at least two-fold compared to non-treated plants and light-gray bars represent statistically significant down-regulation (*p < 0.05, **p < 0.01, ***p < 0.001).
The hormone treatments with MeJA and SA did not show a prominent up regulation on the transcript levels for any of the OsEUL genes. In contrast, MeJA treatments resulted in clear downregulation of most OsEUL levels. A slight upregulation was only observed in the early time points for OsEULD1a and OsEULD1b in the roots after MeJA treatment followed by statistically significant downregulation in the late time points for all D-type OsEUL genes (Figure 4). SA treatment yielded a significant down regulation of OsEULS3 transcript levels in roots (Figure 4). The OsEULS2 transcript was not detected in the roots for both hormone treatments (Figure 4), due to the low expression level of OsEULS2 in roots (Figure 3).
Drought stress provoked statistically significant upregulation of transcript levels for OsEULD1b, OsEULD2 and OsEULS2 in the shoot tissue (Figure 5). OsEULD1a transcripts were significantly downregulated in root and shoot tissue as a result of drought treatment, especially in the later time points. Similar to ABA treatment, the highest levels for OsEULD1b and OsEULD2 were observed for the 24h time point with a gradual increase in the transcript levels reaching up to 6-8-fold change. A similar trend was observed for OsEULS2 with expression levels that were 3-fold higher after 24 h of drought stress compared to the untreated plants (Figure 5). Drought treatment yielded a significant up regulation of OsEUL1b transcript levels in roots of approximately 2-fold after 10h (Figure 5).
Salt treatment triggered upregulation for OsEULS2, OsEULD1b and OsEULD2 transcripts starting from the 6 h time point and after 48 h for OsEULS3 in the shoots. In roots, transcript levels for OsEULD1b peaked after 6 h gradually decreased afterwards. OsEULD2 transcript levels are upregulated after 10 h salt treatment in the root (Figure 5).
OsEUL Expression in Response to Biotic Stresses
Transcript levels for OsEULs have been quantified after exposure of rice seedlings to some major pathogens, in particular P. graminicola (oomycete), M. graminicola (nematode), N. lugens (insect) and M. oryzae (fungus) and X. oryzae (bacteria).
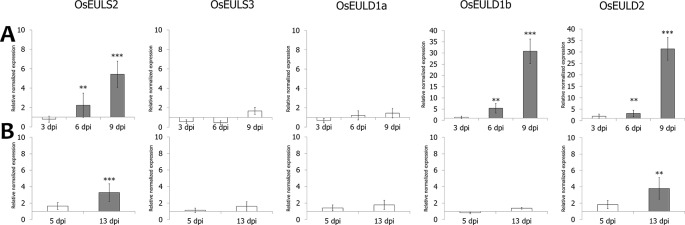
Infection of rice seedlings with typical root pathogens affected OsEUL expression mainly in roots. At 3 dpi, the transcript levels for OsEULS3 and all D-type OsEULs were decreased in roots of plants infected with the oomycete P. graminicola, whereas OsEULS2 expression was 10-fold upregulated. In rice shoots, significant upregulation was recorded for OsEULD1a, while OsEULD1b expression was slightly but significantly downregulated after Pythium infestation (Figure 6). Infection with the nematode, M. graminicola, only results in a 2-fold upregulation of OsEULS2 in the root. No changes in expression above two-fold were observed in shoot tissue after nematode infection (Figure 6).
Figure 6.
Transcript levels for OsEUL genes after infection with Pythium graminicola (A) and Meloidogyne graminicola (B). RT-qPCR analyses were performed on shoot and root material collected at 3 dpi after P. graminicola and 14 dpi after M. graminicola infection. Normalized expression levels, relative to the non-treated plants (set to 1), are indicated. The mean values of RT-qPCR from three independent biological replicates were normalized to three reference genes. Error bars indicate standard errors. Dark-gray bars represent statistically significant up-regulation of at least two-fold compared to non-treated plants and light-gray bars represent statistically significant down-regulation (*p < 0.05, **p < 0.01, ***p < 0.001).
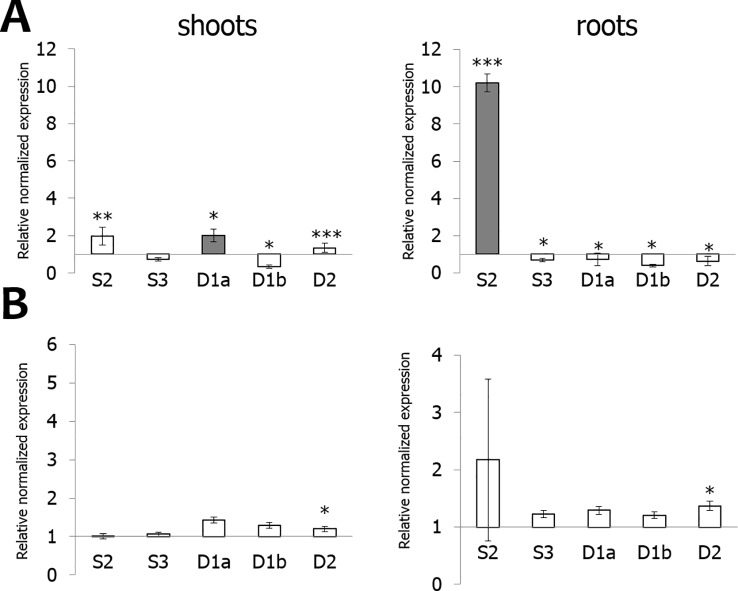
OsEUL expression was analyzed in shoot material of rice plants infested with the brown planthopper, N. lugens. A comparative analysis was made between plants subjected to low and high insect infestation. As shown in Figure 7, infestation of rice plants with a high number of nymphs yielded higher response from the plant compared to low infestation levels. Transcript levels for OsEULD2, OsEULD1b and OsEULS2 are significantly upregulated starting from 6 dpi after high infestation with N. lugens, reaching approximately 31-, 30-, 5-fold upregulation at 9 dpi (Figure 7). In plants subjected to low levels of infestation with N. lugens, transcript levels for OsEULD2 and OsEULS2 were 4-fold up at 13 dpi (Figure 7). N. lugens does not alter the expression of the OsEULS3 and OsEULD1a genes. No significant differences in expression were observed for the early time points after high and low infestation (3 dpi and 5 dpi, respectively), suggesting a late response of the plant (Figure 7).
Figure 7.
Transcript levels for OsEUL genes after infestation with Nilaparvata lugens. RT-qPCR analyses were performed on leaf material collected at indicated dpi after high (A) and low (B) infestation with N. lugens. Normalized expression levels, relative to the non-treated plants (set to 1), are indicated. The mean values of RT-qPCR from three independent biological replicates were normalized to three reference genes. Error bars indicate standard errors Dark-gray bars represent statistically significant up-regulation of at least two-fold compared to non-treated plants and light-gray bars represent statistically significant down-regulation. (*p < 0.05, **p < 0.01, ***p < 0.001). The statistics were performed (*p < 0.05, **p < 0.01, ***p < 0.001). The data was non-significant or was significant for **p < 0.01, ***p < 0.001, but none of the data was in between meaning *p < 0.05.
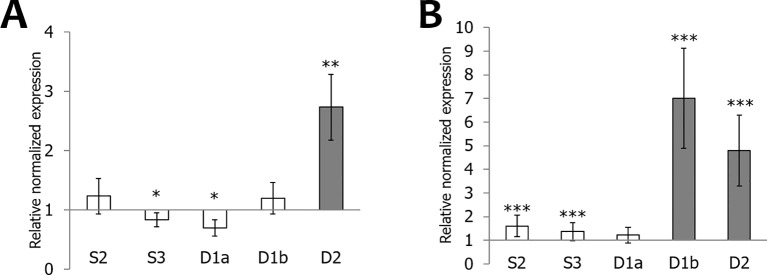
M. oryzae infection especially affects the transcript levels for OsEULD2, resulting in a significant upregulation (3-fold) at 4 dpi. In contrast, levels for OsEULD1a and OsEULS3 are significantly downregulated (Figure 8). Bacterial infection with X. oryzae results in significant upregulation of the transcript levels for OsEULD1b and OsEULD2 by 7- and 5- fold, respectively. Transcript levels for OsEULS2, OsEULS3 and OsEULD1a were not altered for more than two-fold after X. oryzae infection (Figure 8).
Figure 8.
Transcript levels for OsEUL genes after infection with Magnaporthe oryzae (A) and Xanthomonas oryzae (B). RT-qPCR analyses were performed on leaf material collected at 3 dpi after infection. Normalized expression levels, relative to the non-treated plants (set to 1), are indicated. The mean values of RT-qPCR from three independent biological replicates were normalized to three reference genes. Error bars indicate standard errors Dark-gray bars represent statistically significant up-regulation of at least two-fold compared to non-treated plants and light-gray bars represent statistically significant down-regulation (*p < 0.05, **p < 0.01, ***p < 0.001).
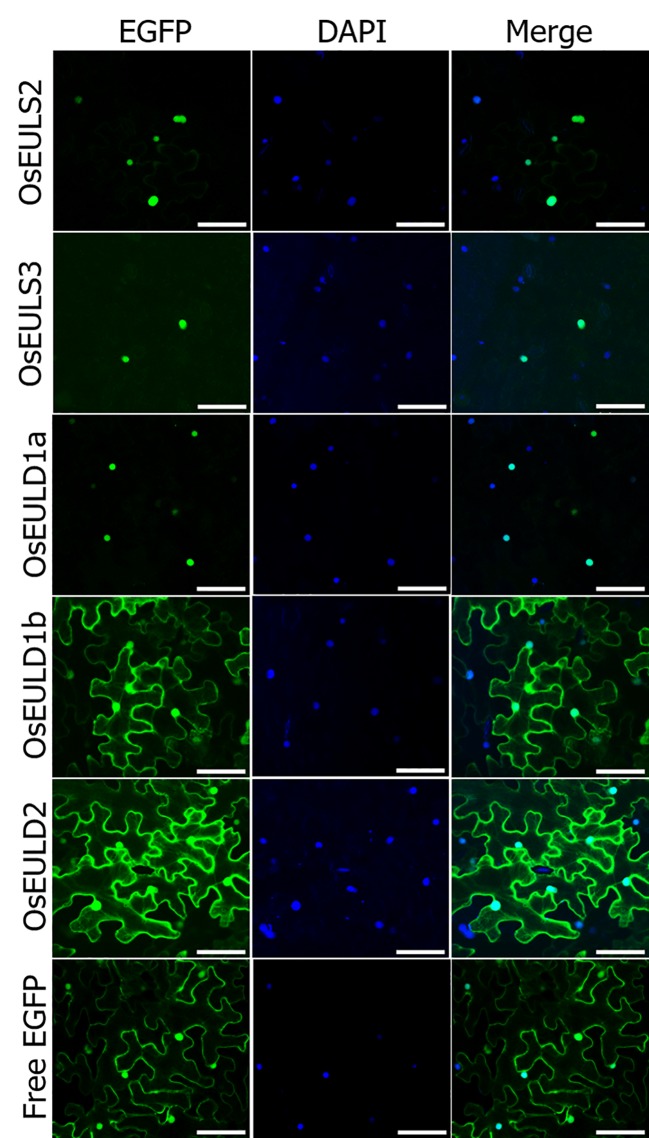
Cellular Localization of the OsEUL Proteins
All rice EUL genes are synthesized without a signal peptide suggesting that the OsEULs will be translated on the ribosomes in the cytoplasm and will not follow the secretory pathway. EGFP fusion constructs were made to investigate the subcellular localization for each OsEUL after transient transformation into Nicotiana benthamiana epidermal cells. Microscopic analysis for each OsEUL yielded fluorescence in the nucleus for the C-terminal EGFP fusion constructs (Figure 9). Similar localization patterns were observed for fusion proteins with a N-terminal EGFP tag. A construct expressing free EGFP was also infiltrated in tobacco leaves and served as a control. Fluorescence for free EGPF was observed in the nucleus and the cytoplasmic compartment (Figure 9). Co-localization experiments in which nuclei were also stained with DAPI confirmed that all OsEULs and free EGFP display expression in the nuclear compartment. Fluorescence intensity plots for DAPI and EGFP in cross sections spanning the nucleus confirmed that the EGFP fusion proteins are present in the nuclei, as shown in Supplementary Figure 2.
Figure 9.
Subcellular localization of OsEUL-EGFPs and free EGFP in Nicotiana benthamiana lower epidermal cells. Nuclei were stained with DAPI. Co-localization between DAPI and EGFP signals was assessed in overlay pictures (Merge). Microscopy settings were identical for all pictures. Scale bars represent 80 µm.
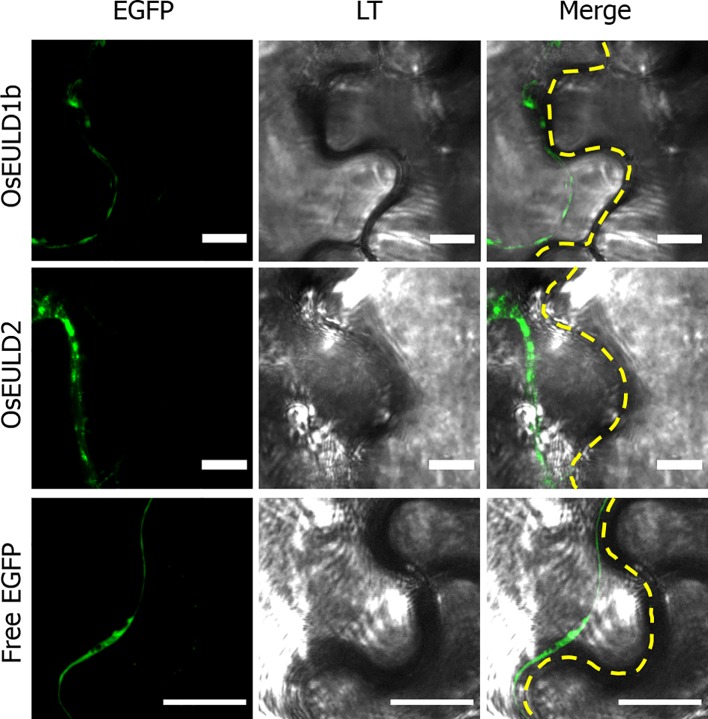
The OsEULD1b and OsEULD2 fusion constructs with EGFP also showed fluorescence in the cytoplasm. A plasmolysis experiment confirmed that fluorescence is present in the cytoplasm but is excluded from the cell wall (Figure 10). Fluorescence intensity plots for PI and EGFP cross sections spanning the cell borders of neighboring cells confirmed the fluorescence for OsEULD1b and OsEULD2 in the cytoplasm and not in the cell wall (Supplementary Figure 3).
Figure 10.
Zoom in of subcellular localization of OsEULD1b and OsEULD2 and free EGFP in plasmolyzed Nicotiana benthamiana lower epidermal cells at the cell border. Fluorescence signal (EGFP) from free EGFP, OsEULD1b:EGFP and OsEULD2:EGFP are presented. Light transmission (LT) microscopy showed the cell walls. Co-localization of LT and EGFP staining was assessed in overlay pictures (Merge). The dashed white line marks the cell wall. Scale bars represent 10 µm.
To check whether the localization patterns might change in stress conditions, localization experiments were also performed for transiently transformed tobacco leaves subjected to 50 µM ABA stress for 1h. ABA was selected as the best stress condition since the expression of all OsEULs was affected by ABA treatment (Figure 4). The cellular localization for the OsEUL proteins did not change after ABA treatment (Supplementary Figure 4).
Discussion
Rice (O. sativa) is one of the most important staple foods in the world and is cultivated in all habitable continents. According to the Food and Agricultural Organization of the United Nations (Katayama et al., 2019) more than 3.5 billion people depend on rice for more than 20% of their daily calories. Rice is the second most produced cereal and is a model organism for other important cereals, including maize and wheat, making it a highly important crop for agriculture and translational research (Matsumoto et al., 2005; Li et al., 2014; Muthayya et al., 2014). Unfortunately, the rice crop is highly susceptible to abiotic stresses, especially to drought and salinity. In addition, rice is prone to infection by bacteria, fungi, nematodes, oomycetes, and insects. It is generally accepted that plants use a whole array of proteins to cope with undesirable conditions (Bellande et al., 2017). Therefore, several rice proteins, among which lectins and ribosome-inactivating proteins, have been studied aiming to identify proteins that can help to create plants with better performance and stress tolerance.
The Euonymus-related lectins (EULs) represent a family of stress-related lectins composed of one or two EUL domains. The EUL domain is unique in that it is ubiquitous in land plants, implying that these proteins play a significant role. The rice genome contains five evolutionary related OsEUL sequences, encoding two groups of structurally diverse proteins, in particular the S-type and D-type proteins, harboring one or two EUL domains, respectively. Only limited information is available for the OsEUL proteins, probably due to the low expression levels of OsEULs in plant tissues. However, a few transcriptomic as well as proteomic studies have provided indications that OsEULs represent stress related proteins (Moons et al., 1995; Rabello et al., 2008). The aim of this study was to make a comparative analysis of the subcellular localization and tissue expression, as well as the stress responsiveness of the different OsEULs.
GUS activity was detected in the vascular system of all GUS reporter lines, but the staining pattern was different for each promotor line, suggesting that the expression of the OsEULs is tissue specific. Promoter activity for OsEULS3, OsEULD1a, and OsEULD1b was clear in roots as well as in shoots. Unlike all other OsEULs, OsEULS3 never showed GUS staining in the vascular system at the root tip. OsEULD2 has a lower basal expression compared to the other OsEULs. OsEULS2, OsEULD1a, and OsEULD1b are also expressed in the embryo pointing towards a potential role in primary growth. OsEULD1a and OsEULD1b are expressed in the seed and mainly in the endosperm. Interestingly, the endosperm has a function in seed dormancy, which is sensitive to the abiotic stress hormone ABA (Yan et al., 2014). Both OsEULD1a and OsEULD1b are expressed in the meristematic zone of the root tips from the main and the lateral roots. This meristematic zone is the location where plant cells undergo cell division. Root growth is achieved by cell division followed by cell elongation (Sanz et al., 2011). The study of the promoter elements in the promoter sequence of OsEULD1b revealed that OsEULD1b has ABA related elements, such as TRAB1 and OSBZ8 (De Schutter et al., 2017). OSBZ8 is reported in the roots and developing embryos, while TRAB1 shows expression in the embryo and seeds (Banerjee and Roychoudhury, 2017). These data are consistent with the results of the GUS staining.
ABA is considered as one of the key players in plant growth and abiotic stress response. For instance, NCED, an important enzyme in the ABA biosynthesis pathway, is upregulated by drought and salt resulting in higher levels of ABA (Huang et al., 2019). Low concentrations of ABA are required for primary root elongation and growth recovery in response to water deficit and salt stress (Sharp et al., 2004; Geng et al., 2013). Transcript levels for OsEULD1b and OsEULD2 were significantly upregulated after salt, drought and ABA treatment. Interestingly, OsEULD1a were downregulated after drought treatment, which seems contradictory since OsEULD1a levels were also upregulated by ABA in the roots. However, plants can cope with drought by utilizing both ABA dependent and ABA independent pathways (Borah et al., 2017). Salinization and drying of the soil alter the fluxes in the vascular system. The concentration of ABA in the vascular system will increase in response to drought and salt stress. Furthermore, the ABA concentration will increase much faster in the roots than in the leaf after drought stress, which explains the late response in the shoots (Pérez-Alfocea et al., 2011). Proteomics studies for ABA treated roots reported the upregulation of OsR40C1, here referred to as OsEULD1b (Moons et al., 1995; Rabello et al., 2008). In contrast to the significant increase of OsEUL expression after treatment of ABA, MeJA treatment resulted in a decrease of EUL transcript levels whereas SA treatment did not show any major effect on OsEUL levels. Crosstalk exists between MeJA and ABA signaling in plants and many genes are commonly regulated by MeJA and ABA (Kim et al., 2018). It has been shown that MeJA can stimulate stomatal closure similar to ABA, but can also act as an antagonist to ABA in rice roots through inhibition of primary root elongation resulting in reduced root length (Savchenko et al., 2014; Liu et al., 2015).
Defense responses are modulated by the induced production of a hormonal blend and are crucial to protect plants against several plant attackers. The plant hormones ABA, MeJA and SA are important regulators of induced defense mechanisms (Vos et al., 2015). MeJA plays a critical role in rice immunity, and is necessary in the defense against the nematode Hirschmanniella oryzae and M. graminicola whereas ABA suppressed host resistance against Magnaporthe by inhibiting SA signaling. A recent study showed that MeJA levels are increased after foliar application of abamine (an inhibitor of ABA biosynthesis) in the roots whereas the foliar application of ABA led to MeJA suppression. ABA acts as an antagonist to MeJA biosynthesis and its signaling pathway, making the plants more susceptible to the pests (Nahar et al., 2011; Kyndt et al., 2017). Transcript levels for all EULs except for OsEULS2 were significantly downregulated in roots by the root pathogen P. graminicola. However, this downregulation was not observed for the root nematode M. graminicola, indicating that several root pathogens affect transcript levels differently. Future experiments with transgenic lines overexpressing the OsEULs will allow to investigate the role of OsEULs in rice defense.
Generally OsEUL transcript levels are upregulated more prominently after biotic stresses compared to abiotic stresses. However, it is also well known that some pathogen infections also affect hormone levels. For instance, MeJA signaling plays a vital role in plant defense against chewing insects, but makes plants more susceptible to infestation by sucking insects, such as the brown plant hopper Nilaparvata (Tong et al., 2012). OsEULS2, OsEULD1b, and OsEULD2 were significantly upregulated after high infestation with the brown plant hopper. Moreover OsEULD2 and OsEULD1b transcript levels were also upregulated after Xanthomonas infection. OsEULD2 was significantly upregulated after Magnaporthe infection. These results are in agreement with a previous qPCR study using different time points which also showed the upregulation of OsEULD1b and OsEULD2 after Xanthomonas infection and the upregulation of OsEULD2 after disease by Magnaporthe (Al Atalah et al., 2014). Transcript levels for OsEULD2 were significantly upregulated by three different rice pathogens. OsEULD2 levels were upregulated after stress provoked by M. oryzae, X. oryzae and N. lugens, suggesting that OsEULD2 is generally more stress responsive compared to the other OsEULs.
Our results give a complete and conclusive overview of the stress responsiveness of the OsEUL genes to single stresses. However, in nature plants are exposed to multiple stresses. Recent studies showed that combined stress is regulated by overlapping cellular signaling mechanisms that are different from studies with individual stresses (Vemanna et al., 2019). However, judging from the stress treatments performed in this study we can conclude that the EUL gene family in rice is stress responsive, and reacts to a broad range of stresses, making the OsEULs good candidate genes for creating plants with durable resistance to multiple stresses. Furthermore it is clear that multiple OsEULs in rice act differently, and probably exert complementary activities (Tables 1 and 2).
Table 1.
Overview of OsEUL expression in response to abiotic and hormone treatments.
| ABA | Salt | Drought | MeJA | SA | ||
|---|---|---|---|---|---|---|
| OsEULS2 | Shoot | ↗ | ↗ | ↗ | ||
| OsEULS3 | ||||||
| OsEULD1a | ↘ | |||||
| OsEULD1b | ↗ | ↗ | ↗ | |||
| OsEULD2 | ↗ | ↗ | ↗ | |||
| OsEULS2 | Root | ↗ | n.d. | n.d. | ||
| OsEULS3 | ↗ | ↘ | ↘ | |||
| OsEULD1a | ↗ | ↘ | ↘ | |||
| OsEULD1b | ↗ | ↗ | ↗ | ↘ | ||
| OsEULD2 | ↗ | ↗ | ↗ | ↘ |
Green and red arrows indicate significant upregulation and downregulation of expression, respectively, for at least two-fold. n.d., not detected.
Table 2.
Overview of OsEUL expression in response to biotic treatments. Green and red arrows indicate significant upregulation and downregulation of expression, respectively, for at least two-fold.
| Pathogen/Insect | Tissue | OsEULS2 | OsEULS3 | OsEULD1a | OsEULD1b | OsEULD2 |
|---|---|---|---|---|---|---|
| Pythium graminicola | Root | ↗ | ||||
| Shoot | ↗ | |||||
| Melidogyne graminicola | Root | |||||
| Shoot | ||||||
| Nilaparvata lugens | Shoot | ↗ | ↗ | ↗ | ||
| Magnaporthe oryzae | Shoot | ↗ | ||||
| Xanthomonas oryzae | Shoot | ↗ | ↗ |
The rice plant contains multiple lectins, some of which are expressed in the same tissues. For instance, next to EULs, rice also expresses Orysata, a nucleocytoplasmic jacalin-related lectin. Orysata expression is enhanced after drought and salt stress as well as SA, MeJA, ABA treatment and M. oryzae attack (Garcia et al., 1998; De Souza Filho et al., 2003; Shinjo et al., 2011; Al Atalah et al., 2014). Similar to OsEULs, Orysata shows expression in roots and shoots. Overexpression of Orysata in rice plants was reported to improve salinity tolerance (He et al., 2017). Recently, Orysata, also known as “SALT”, was identified in Saltol-1, a major quantitative trait locus related to salt stress, as the major contributor to the salt stress resistance (Patishtan et al., 2018). Orysata interacts with RING finger proteins suggesting that it is regulated by the ubiquitination pathway (Van Holle and Van Damme, 2018).
The EUL sequences are synthesized without a signal peptide and therefore EULs are presumably translated on free ribosomes in the cytosol of the plant cell. Our data show that all OsEULs fused to EGFP locate to the nucleus. Moreover, the S-type EULs and OsEULD1a are detected only in the nucleus whereas OsEULD1b and OsEULD2 also reside in the cytoplasm. None of the EUL sequences contains a classical nuclear localization site (NLS). At present, it remains unclear how the OsEUL proteins are at least partially translocated from the cytosol to the nucleus. The nuclear transport could be by passive diffusion through the nuclear pore or active nuclear transport which does not rely on the presence of a classical NLS (Wang and Brattain, 2007; Yao et al., 2014). At present the role of EULs in the nucleus is not known. It is possible that OsEULs act in similar way as described for the nucleocytoplasmic lectins Orysata and Nictaba. Nictaba is a nucleocytoplasmic lectin from tobacco, it acts as a stress-inducible modulator of gene transcription by chromatin remodeling through binding to O-GlcNAc modified core histones (Schouppe et al., 2011; Delporte et al., 2014). Overexpression of Nictaba-like lectin genes from Glycine max confers tolerance toward P. syringae infection, aphid infestation and salt stress in transgenic Arabidopsis plants (Van Holle et al., 2016), suggesting that Nictaba can regulate the plant immune response.
Only few stress inducible lectins have been studied and functionally characterized. This paper highlights the tissue-specific expression, the subcellular localization and the stress inducibility of a family of closely related lectins from rice. The experimental data confirm the stress dependent regulation of the EUL lectins for a broad range of stresses, like drought and salinity, and infections by bacteria, fungi, oomycetes and insect attack. Despite the fact that OsEUL sequences show a high degree of sequence similarity they show marked differences in subcellular localization and tissue expression, as well as in their stress responsiveness, suggesting that they play a different or a complementary role in the rice plant. More research, such as the generation of rice plants overexpressing OsEULs, is needed to experimentally prove the involvement of EULs in stress signaling and plant defense in rice. Furthermore, the identification of interaction partners for OsEULs will contribute to define the physiological role and the importance of these genes in the plant.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
JL, SD, MD, MT, PW, JZ, and ED outlined and designed the study. JL, SD, MD, MT, PW, JZ, IV, PW, and KS performed the experiments. JL, SD, MD, PW, and JZ analyzed and interpreted the data. JL, SD, MD, MT, and ED prepared the manuscript. GS assisted with the design and interpretation of the insect experiments. ED conceived and supervised the experiments and critically revised the manuscript. All authors have read, revised, and approved the final manuscript.
Funding
This research was funded by the Research Council of Ghent University (project BOF15/GOA/005) and the Fund for Scientific Research–Flanders (FWO Grant G006114N).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank Prof. Monica Höfte of the Phytopathology Lab (Ghent University, Belgium) for providing the pathogens used in this study and Prof. Tina Kyndt (Ghent University, Belgium) for the nematode culture. Rice seeds were a kind gift of Prof. Fabio Fornara, University of Milan, Department of Biosciences, Italy.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.00185/full#supplementary-material
References
- Al Atalah B., Rougé P., Smith D. F., Proost P., Lasanajak Y., Van Damme E. J. M. (2012). Expression analysis of a type S2 EUL-related lectin from rice in Pichia pastoris. Glycoconj. J. 29, 467–479. 10.1007/s10719-012-9405-2 [DOI] [PubMed] [Google Scholar]
- Al Atalah B., Vanderschaeghe D., Bloch Y., Proost P., Plas K., Callewaert N., et al. (2014). Characterization of a type D1A EUL-related lectin from rice expressed in Pichia pastoris. Biol. Chem. 395, 413–424. 10.1515/hsz-2013-0267 [DOI] [PubMed] [Google Scholar]
- Banerjee A., Roychoudhury A. (2017). Abscisic-acid-dependent basic leucine zipper (bZIP) transcription factors in plant abiotic stress. Protoplasma 254, 3–16. 10.1007/s00709-015-0920-4 [DOI] [PubMed] [Google Scholar]
- Bellande K., Bono J. J., Savelli B., Jamet E., Canut H. (2017). Plant lectins and lectin receptor-like kinases: How do they sense the outside? Int. J. Mol. Sci. 18 (6), 1164. 10.3390/ijms18061164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borah P., Sharma E., Kaur A., Chandel G., Mohapatra T., Kapoor S., et al. (2017). Analysis of drought-responsive signalling network in two contrasting rice cultivars using transcriptome-based approach. Sci. Rep. 7, 42131. 10.1038/srep42131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schutter K., Tsaneva M., Kulkarni S. R., Rougé P., Vandepoele K., Van Damme E. J. M. (2017). Evolutionary relationships and expression analysis of EUL domain proteins in rice (Oryza sativa). Rice 10, 26. 10.1186/s12284-017-0164-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza Filho G. A., Ferreira B. S., Dias J. M., Queiroz K. S., Branco A. T., Bressan-Smith R. E., et al. (2003). Accumulation of SALT protein in rice plants as a response to environmental stresses. Plant Sci. 164, 623–628. 10.1016/S0168-9452(03)00014-1 [DOI] [Google Scholar]
- Delporte A., De Zaeytijd J., De Storme N., Azmi A., Geelen D., Smagghe G., et al. (2014). Cell cycle-dependent O-GlcNAc modification of tobacco histones and their interaction with the tobacco lectin. Plant Physiol. Biochem. 83, 151–158. 10.1016/j.plaphy.2014.07.021 [DOI] [PubMed] [Google Scholar]
- Delporte A., Van Holle S., Lannoo N., Van Damme E. J. M. (2015). The tobacco lectin, prototype of the family of Nictaba-related proteins. Curr. Prot. Pept. Sci. 16, 5–16. 10.2174/1389203716666150213154107 [DOI] [PubMed] [Google Scholar]
- Fouquaert E., Peumans W. J., Vandekerckhove T. T. M., Ongenaert M., Van Damme E. J. M. (2009). Proteins with an Euonymus lectin-like domain are ubiquitous in Embryophyta. BMC Plant Biol. 9, 136. 10.1186/1471-2229-9-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. (1968). Nutrient requirements of suspension cultures of soyabean root cells. Exp. Cell Res. 50, 151–158. [DOI] [PubMed] [Google Scholar]
- Garcia A. B., De Almeida Engler J., Claes B., Villarroel R., Van Montagu M., Gerats T., et al. (1998). The expression of the salt-responsive gene salT from rice is regulated by hormonal and developmental cues. Planta 207, 172–180. 10.1007/s004250050470 [DOI] [PubMed] [Google Scholar]
- Geng Y., Wu R., Wee C. W., Xie F., Wei X., Chan P. M. Y., et al. (2013). A spatio-temporal understanding of growth regulation during the salt stress response in Arabidopsis. Plant Cell. 25, 2132–2154. 10.1105/tpc.113.112896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Li L., Xu H., Xi J., Cao X., Xu H., et al. (2017). A rice jacalin-related mannose-binding lectin gene, OsJRL, enhances Escherichia coli viability under high salinity stress and improves salinity tolerance of rice. Plant Biol. 19, 257–267. 10.1111/plb.12514 [DOI] [PubMed] [Google Scholar]
- Huang Y., Jiao Y., Xie N., Guo Y., Zhang F., Xiang Z., et al. (2019). OsNCED5, a 9-cis-epoxycarotenoid dioxygenase gene, regulates salt and water stress tolerance and leaf senescence in rice. Plant Sci. 287, 110188. 10.1016/J.PLANTSCI.2019.110188 [DOI] [PubMed] [Google Scholar]
- Jefferson R. A. (1987). Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol. Biol. Rep. 5, 387–405. 10.1007/bf02667740 [DOI] [Google Scholar]
- Katayama N., Osada Y., Mashiko M., Baba Y., Tanaka K., Kusumoto Y., et al. (2019). Organic farming and associated management practices benefit multiple wildlife taxa: a large‐scale field study in rice paddy landscapes. J. Appl. Ecol. 56, 1970–1981. 10.1111/1365-2664.13446 [DOI] [Google Scholar]
- Kauffman H. E., Reddy A. P. K., Hsieh S. P. Y., Merca S. D. (1973). An improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae. Plant Disease Rep. 57, 537–541. [Google Scholar]
- Kim J. A., Bhatnagar N., Kwon S. J., Min M. K., Moon S. J., Yoon I. S., et al. (2018). Transcriptome analysis of ABA/JA-dual responsive genes in rice shoot and root. Curr. Genomics 19, 4–11. 10.2174/1389202918666170228134205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyndt T., Nahar K., Haegeman A., De Vleesschauwer D., Höfte M., Gheysen G. (2012). Comparing systemic defence‐related gene expression changes upon migratory and sedentary nematode attack in rice. Plant Biology 14, 73–82. 10.1111/j.1438-8677.2011.00524.x [DOI] [PubMed] [Google Scholar]
- Kyndt T., Nahar K., Haeck A., Verbeek R., Demeestere K., Gheysen G. (2017). Interplay between carotenoids, abscisic acid and jasmonate guides the compatible rice-Meloidogyne graminicola interaction. Front. Plant Sci. 8, 951. 10.3389/fpls.2017.00951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Fu B. Y., Gao Y. M., Wang W. S., Xu J. L., Zhang F., et al. (2014). The 3,000 rice genomes project. Gigascience 3, 7. 10.1186/2047-217X-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Zhang S., Sun N., Liu H., Zhao Y., Liang Y., et al. (2015). Functional diversity of jasmonates in rice. Rice 8, 42. 10.1186/s12284-015-0042-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Wu J., Kanamori H., Katayose Y., Fujisawa M., Namiki N., et al. (2005). The map-based sequence of the rice genome. Nature 436, 793–800. 10.1038/nature03895 [DOI] [PubMed] [Google Scholar]
- Moons A., Bauw G., Prinsen E., Van Montagu M., Van der Straeten D. (1995). Molecular and physiological responses to abscisic acid and salts in roots of salt-sensitive and salt-tolerant Indica rice varieties. Plant Physiol. 107, 177–186. 10.1104/pp.107.1.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15, 473–497. 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- Muthayya S., Sugimoto J. D., Montgomery S., Maberly G. F. (2014). An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 1324, 7–14. 10.1111/nyas.12540 [DOI] [PubMed] [Google Scholar]
- Nahar K., Kyndt T., De Vleesschauwer D., Höfte M., Gheysen G. (2011). The jasmonate pathway is a key player in systemically induced defense against root knot nematodes in rice. Plant Physiol. 157, 305–316. 10.1104/pp.111.177576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsai R., Ivanova A., Ng S., Whelan J. (2010). Defining reference genes in Oryza sativa using organ, development, biotic and abiotic transcriptome datasets. BMC Plant Biology 10, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Alfocea F., Ghanem M. E., Gómez-Cadenas A., Dodd I. C. (2011). Omics of root-to-shoot signaling under salt stress and water deficit. Omi. A J. Integr. Biol. 15, 893–901. 10.1089/omi.2011.0092 [DOI] [PubMed] [Google Scholar]
- Patishtan J., Hartley T. N., Fonseca de Carvalho R., Maathuis F. J. M. (2018). Genome-wide association studies to identify rice salt-tolerance markers. Plant Cell Environ. 41, 970–982. 10.1111/pce.12975 [DOI] [PubMed] [Google Scholar]
- Pitzschke A., Datta S., Persak H. (2014). Salt stress in Arabidopsis: lipid transfer protein AZI1 and its control by mitogen-activated protein kinase MPK3. Mol. Plant 7 (4), 722–738. 10.1093/mp/sst157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabello A. R., Guimarães C. M., Rangel P. H. N., da Silva F. R., Seixas D., de Souza E., et al. (2008). Identification of drought-responsive genes in roots of upland rice (Oryza sativa L). BMC Genomics 9, 485. 10.1186/1471-2164-9-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L., Murray J. A. H., Dewitte W. (2011). To divide and to rule; Regulating cell division in roots during post-embryonic growth. Prog. Bot. 73, 57–80. 10.1007/978-3-642-22746-2_2 [DOI] [Google Scholar]
- Savchenko T., Kolla V. A., Wang C. Q., Nasafi Z., Hicks D. R., Phadungchob B., et al. (2014). Functional convergence of oxylipin and abscisic acid pathways controls stomatal closure in response to drought. Plant Physiol. 164, 1151–1160. 10.1104/pp.113.234310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., et al. (2012). Fiji: an open source platform for biological image analysis. Nat. Methods 9, 676–682. 10.1038/nmeth.2019.Fiji [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouppe D., Ghesquière B., Menschaert G., De Vos W. H., Bourque S., Trooskens G., et al. (2011). Interaction of the tobacco lectin with histone proteins. Plant Physiol. 155, 1091–1102. 10.1104/pp.110.170134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp R. E., Poroyko V., Hejlek L. G., Spollen W. G., Springer G. K., Bohnert H. J., et al. (2004). Root growth maintenance during water deficits: Physiology to functional genomics. J. Exp. Bot. 55, 2343–2351. 10.1093/jxb/erh276 [DOI] [PubMed] [Google Scholar]
- Shinjo A., Araki Y., Hirano K., Arie T., Ugaki M., Teraoka T. (2011). Transgenic rice plants that over-express the mannose-binding rice lectin have enhanced resistance to rice blast. J. Gen. Plant Pathol. 77, 85–92. 10.1007/s10327-010-0290-x [DOI] [Google Scholar]
- Tong X., Qi J., Zhu X., Mao B., Zeng L., Wang B., et al. (2012). The rice hydroperoxide lyase OsHPL3 functions in defense responses by modulating the oxylipin pathway. Plant J. 71, 763–775. 10.1111/j.1365-313X.2012.05027.x [DOI] [PubMed] [Google Scholar]
- Van Holle S., Van Damme E. J. M. (2018). Signaling through plant lectins: modulation of plant immunity and beyond. Biochem. Soc Trans. 46, 217–233. 10.1042/bst20170371 [DOI] [PubMed] [Google Scholar]
- Van Holle S., Smagghe G., Van Damme E. J. M. (2016). Overexpression of Nictaba-like lectin genes from Glycine max confers tolerance toward Pseudomonas syringae infection, aphid infestation and salt stress in transgenic Arabidopsis plants. Front. Plant Sci. 7, 1590. 10.3389/fpls.2016.01590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Holle S., De Schutter K., Eggermont L., Tsaneva M., Dang L., Van Damme E. J. M. (2017). Comparative study of lectin domains in model species: New insights into evolutionary dynamics. Int. J. Mol. Sci. 18, 1136. 10.3390/ijms18061136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hove J., De Jaeger G., De Winne N., Guisez Y., Van Damme E. J. M. (2015). The Arabidopsis lectin EULS3 is involved in stomatal closure. Plant Sci. 238, 312–322. 10.1016/j.plantsci.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Vemanna R. S., Bakade R., Bharti P., Kumar M. K. P., Sreeman S. M., Senthil-Kumar M., et al. (2019). Cross-talk signaling in rice during combined drought and bacterial blight stress. Front. Plant Sci. 10, 193. 10.3389/fpls.2019.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos I. A., Moritz L., Pieterse C. M. J., Van Wees S. C. M. (2015). Impact of hormonal crosstalk on plant resistance and fitness under multi-attacker conditions. Front. Plant Sci. 6, 639. 10.3389/fpls.2015.00639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Brattain M. G. (2007). The maximal size of protein to diffuse through the nuclear pore is larger than 60kDa. FEBS Lett. 581 (17), 3164–3170. 10.1016/j.febslet.2007.05.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D., Duermeyer L., Leoveanu C., Nambara E. (2014). The functions of the endosperm during seed germination. Plant Cell Physiol. 55, 1521–1533. 10.1093/pcp/pcu089 [DOI] [PubMed] [Google Scholar]
- Yao H., Wang G., Wang X. (2014). Nuclear translocation of proteins and the effect of phosphatidic acid. Plant Signal. Behav. 9 (12), e977711. 10.4161/15592324.2014.977711 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.