Abstract
Human studies reported that the number of past-year stressors was positively related to current drinking patterns, including binge drinking. In animal models, exposure to predator odor stress (PS), considered a model of traumatic stress, consistently increased ethanol intake. Recently, we reported that repeated PS significantly increased ethanol intake and had a synergistic interaction with prior binge drinking (binge group) in male but not in female C57BL/6J mice, when compared to mice without prior binge exposure (control group). The current studies utilized plasma and dissected prefrontal cortex (PFC) and hippocampal tissue from these animals and from age-matched naïve mice (naïve group). Western blots assessed relative protein levels of P450scc (an enzyme involved in the first step of steroidogenesis), of GABAA receptor α2 and α4 subunits, and of two proteins involved in synaptic plasticity, ARC (activity-regulated cytoskeletal protein) and synaptophysin. Gas chromatography-mass spectrometry simultaneously quantified ten neurosteroid levels in plasma. A history of ethanol drinking and PS exposure produced brain regional and sex differences in the changes in proteins examined as well as in the pattern of neurosteroid levels versus (vs) values in naïve mice. For instance, P450scc levels were significantly increased only in binge and control female PFC and hippocampus vs naïve. Some neurosteroid levels were significantly altered by binge treatment in both males and females, whereas others only were significantly altered in males. These sexually divergent changes in neurosteroid and protein levels add to evidence for sex differences in the neurochemical systems influenced by traumatic stress and a history of ethanol drinking.
Keywords: predator odor, GABAA receptors, prefrontal cortex, hippocampus, neuroactive steroids, synaptic plasticity
Introduction
Epidemiological evidence documents a fairly consistent association between stress exposure and alcohol (ethanol) consumption. For instance, the number of past-year general life stressors was positively related to current drinking, incidence of binge drinking, and current alcohol use disorder (AUD), and increases in ethanol consumption also were reported after catastrophic events and disasters (see Keyes et al., 2012 and references therein). Thus, exposure to different forms of stress, as well as number of stressors, may be associated with increased ethanol consumption and AUD.
In animal models, exposure to various stressors can produce mixed effects on ethanol consumption (reviewed in Becker et al., 2011). However, a recent review highlighted animal models of stress-induced escalation in ethanol drinking (Gilpin and Weiner, 2017). Exposure to predator odor stress (PS), considered a model of traumatic stress in rodents and used as a model of post-traumatic stress disorder (PTSD), was one such model that was found to produce a consistent increase in subsequent ethanol intake in male and female mice and in male rats (female rats not tested; Cozzoli et al., 2014; Edwards et al., 2013; Manjoch et al., 2016; Roltsch et al., 2014). PS exposure also increased anxiety-related behaviors in naïve rodents (Belzung et al., 2001; Cohen and Zohar, 2004; Cohen et al., 2008; Finn et al., 2018; Gilpin and Weiner, 2017; Hebb et al., 2003; Roltsch et al., 2014; Whitaker and Gilpin, 2015), which might contribute to the PS-induced increase in subsequent ethanol intake.
We recently examined whether prior binge drinking differentially altered responsivity to intermittent PS and subsequent ethanol intake in male and female C57BL/6J mice, when compared to mice without prior binge drinking experience. This initial study (Finn et al., 2018) found sex differences in the patterns of drinking following the repeated PTSD-like stress, depending on whether the mice had prior binge drinking experience. Specifically, intermittent PS significantly increased ethanol intake and had a synergistic interaction with prior binge drinking in male but not female mice. A single or repeated PS also increased plasma corticosterone (CORT) levels in both male and female mice, consistent with a stress-induced activation of the hypothalamic-pituitary-adrenal (HPA) axis, although the CORT response was blunted in the mice that were consuming ethanol (Finn et al., 2018). Notably, the CORT response to PS was greater in female than in male mice (Cozzoli et al., 2014; Finn et al., 2018), and a history of ethanol drinking and repeated PS exposure significantly increased protein levels of the glucocorticoid receptor (GR) only in female prefrontal cortex (PFC) and hippocampus and significantly increased corticotropin releasing factor receptor 1 (CRF-R1) only in female hippocampus (Finn et al., 2018). Collectively, the results from this initial study suggested that there were significant underlying sex differences that influence behavioral responses to stress and drinking as well as neurochemical adaptations involved in stress responsivity.
The purpose of the present study was to continue to explore neurochemical adaptations that occurred in male and female C57BL/6J mice with a history of ethanol drinking and intermittent PS following prior binge drinking or no binge drinking experience. We utilized plasma and dissected PFC and hippocampus from the mice in the study by Finn et al. (2018), for which drinking data have been extensively characterized. While a number of brain areas have been implicated as contributing to responses to ethanol and stress, the PFC and hippocampus are two key areas that have been widely studied. Findings have shown that the hippocampus and PFC play an important role in stress responses (e.g., Morey et al., 2016; Wang et al., 2018; Zhu et al., 2016), and these two brain regions have been identified as targets for neuroplasticity in response to stress (reviewed in Godsil et al., 2013; McEwen et al., 2016). Additionally, the PFC and hippocampus are involved in neurochemical responses to ethanol. For example, a recent study found widespread effects of binge drinking on gene expression in the PFC and hippocampus in the adolescent brain (McClintick et al., 2018). Thus, we chose these two brain areas for the present studies as it allowed us to directly compare with and continue to build on our recent findings with traumatic stress and a history of ethanol drinking (Finn et al., 2018) as well as to compare with previous reports in dissected cortex and hippocampus from dependent rodents during withdrawal (e.g., Cagetti et al., 2003; Devaud and Alele, 2004; Matthews et al., 1998; reviewed in Kumar et al., 2009).
Gas chromatography-mass spectrometry (GC-MS) simultaneously quantified ten neurosteroid levels in plasma, many of which are potent positive modulators of γ-aminobutyric acidA receptors (GABAARs; Jensen et al., 2017; Snelling et al., 2014). Western blots assessed relative protein levels of cytochrome P450 side chain cleavage (P450scc; an enzyme involved in the first step of steroidogenesis; Mellon and Vaudry, 2001; Mellon and Griffin, 2002), of two GABAAR subunits (α2 and α4) important in addiction, additional behaviors such as anxiety, and adaptations following chronic ethanol exposure (Engin et al., 2018; Olsen and Liang, 2017; Stephens et al., 2017), and of two proteins involved in synaptic plasticity (ARC or activity-regulated cytoskeletal protein and synaptophysin; Li et al., 2002; Shephard and Bear, 2011). We predicted that prior binge ethanol drinking, repeated PS exposure and subsequent drinking would alter the pattern of changes in plasma neurosteroid levels as well as in the assayed protein levels in male and female mice and would provide insights into the neurochemical basis for sex differences in response to stress and ethanol.
Materials and Methods
Animals and general procedure.
The current studies utilized plasma and dissected brain tissue from adult male and female C57BL/6J mice that had been purchased from Jackson Laboratories West (Sacramento, CA) at 8 weeks of age and then were tested in experiments designed to determine whether exposure to prior binge drinking altered responsivity to PS and subsequent ethanol intake. The ethanol drinking data have been published (Finn et al., 2018), but Figure 1 gives an overview of the experimental timeline. All procedures that were conducted on the animals complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were conducted under Institutional Animal Care and Use Committee approved protocols. Upon euthanasia, brains were rapidly removed, quickly frozen over dry ice, and then stored at −80°C for Western blot analysis. Plasma was collected for neurosteroid analysis. Brains and plasma from age matched naïve animals were collected and used as a comparator group to the binge and control groups (Figure 1).
Figure 1. Experimental timeline.
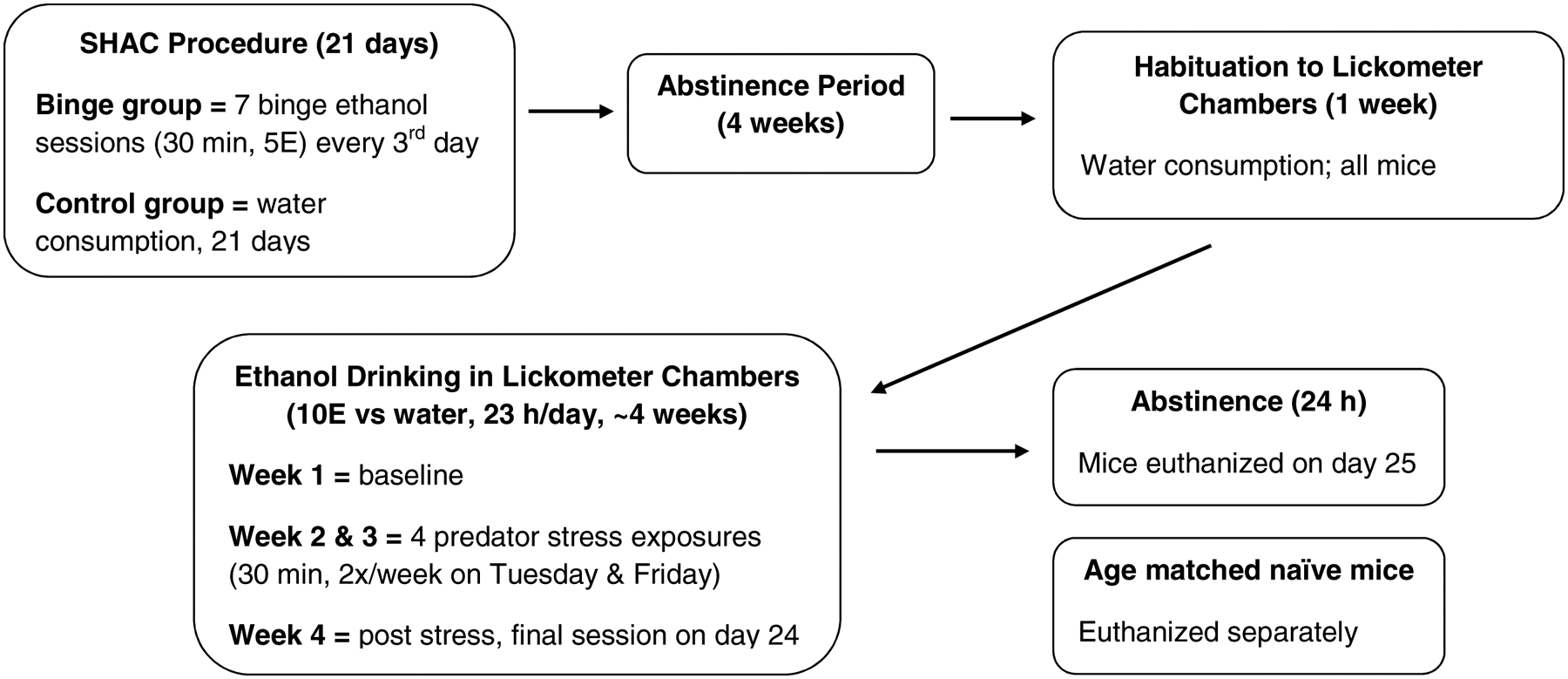
The present study utilized tissue obtained from binge and control male and female mice that were treated as described. After the initial binge or water (control) drinking sessions and a 4 week period of abstinence, binge and control mice were treated similarly. Tissue from age matched naïve mice were used as the comparator group. For predator stress, mice were exposed to dirty rat bedding for 30 min, twice per week, for 2 weeks. SHAC = Scheduled High Alcohol Consumption; 5E = 5% v/v ethanol; 10E = 10% v/v ethanol.
Neurosteroid analysis.
GC-MS was used to simultaneously quantify levels of 10 GABAAR-active steroids and estradiol. Plasma samples (300 μL) were extracted, eluted, and derivatized, as described previously (Jensen et al., 2017; Snelling et al., 2014). The samples and standards were spiked with 10 ng of 2-fluoroestradiol (internal standard) prior to the analytical work-up. The internal standard was changed to 2-fluoroestradiol instead of estradiol-d5 due to the addition of estradiol-d0 as an eleventh target analyte. We conducted a pilot study to validate the accuracy and reproducibility of the updated method. In the pilot study, deionized water (300 μL) was spiked with either 70, 300, or 700 pg of the eleven target analytes, and the samples were analyzed as if they were unknown plasma samples. Linear regression of the obtained amounts confirmed the excellent linear response for all eleven steroids, with a range in r2 values from 0.9888 – 0.9997.
Analysis of samples and calibration curves were performed on an Agilent 7890A gas chromatograph coupled to an Agilent 5975C mass spectrometer (Agilent Technologies, Santa Clara, CA). We were able to simultaneously quantify the 10 neuroactive steroids, consistent with our prior work (Jensen et al., 2017; Snelling et al., 2014), along with estradiol-d0 (the 11th steroid). Calibration curves were formed by spiking 300 μL of deionized water with 0, 50, 100, 500, 1,000, and 3,000 pg of the 11 analytes. These spiked standards were extracted, derivatized, and analyzed via GC-MS as described (Jensen et al., 2017; Snelling et al., 2014). The r2 values from the calibration curves ranged from 0.9953 – 0.9997. Every third run was a solvent wash of 5 μL acetonitrile to prevent carryover from previous runs. The calibration curves were then analyzed with linear regression, and unknown plasma samples were interpolated against these regression parameters using their individual response ratios. All neurosteroid data quantified for this study were subjected to a requirement of 5:1 signal:noise ratio. Limit of quantification for levels of the 10 neurosteroids has been published elsewhere (Jensen et al., 2017).
Western blot analysis.
Immunoblotting was performed on PFC (including prelimbic, cingulate, and overlying motor cortex) and entire hippocampal tissues that were collected via hand dissection from mouse brains obtained from the binge and control groups and from age matched naïve mice. We used a typical method of tissue harvesting and homogenization to maximize the amount of tissue from the selected brain regions, which allowed us to make comparisons across a number of proteins rather than focusing on PFC or hippocampal subregions or synaptic localization. Briefly, tissue was homogenized, and a total particulate fraction was collected by centrifugation. Protein concentrations for each tissue were determined using the Thermo Scientific (Rockport, IL) BCA procedure. Sample homogenates (20 μg protein/sample) were diluted with sample loading buffer (Bio Rad, Hercules, CA), denatured for 3 min and then separated using a 10-well 4–15% Tris-glycine gel in a Bio Rad Western Blot apparatus. Each gel had 3 naïve, 3 control and 3 binge samples from the same tissue and sex (i.e., male PFC) for within-blot comparisons. After separation, proteins were transferred to PVDF membranes (Bio Rad immuno-blot PVDF). Blots were incubated with selected antibodies: P450scc (1:500, Thermo Scientific, Rockford IL), the GABAAR α2 (1:400), GABAAR α4 subunit (1:100) and synaptophysin (1:500) from abcam (Cambridge MA), and ARC (1:100, Santa Cruz Biotechnologies, Dallas TX). β-actin (1:500, Santa Cruz Biotechnologies, Dallas TX), was used as the loading control, based on our early evidence that β-actin peptide levels did not change with chronic ethanol exposure or withdrawal (Devaud et al., 1997), which validated its use as appropriate for these types of rodent studies. After incubation with the primary antibody, blots were washed and incubated with the appropriate HRP-conjugated secondary antibody (1:8000). Blots were then incubated in chemiluminescent substrate (HyGlo, Denville Sci, Holliston MA), and signals were captured using a Bio Rad ChemiDoc MP imager. Relative density measurements were collected and quantified using the Image Lab software. Measurements were normalized to the β-actin signal for equivalent protein loading. Three – five data points were collected from each treatment group animal across independent immunoblots, converted to percent of naive values, and then the converted values were averaged for each animal for summary and statistical analysis.
Statistical analysis.
The male and female mice from Finn et al. (2018) were tested in separate studies. Because Western blots were conducted on tissue from each sex and brain region in separate analyses, these data were normalized to the respective naïve group for each sex and brain region. As a result, Western blot data for each sex and brain region were analyzed for treatment effects (naïve, control, binge) using one-way ANOVA with Tukey’s post hoc tests. For the GC-MS analysis, target ion peaks were integrated in ChemStation and interpolated using Prism6 (GraphPad Software, Inc., San Diego, CA). The interpolated values were used to calculate mean pg/mL of analyte per extracted sample. Plasma neurosteroid levels for each sex were analyzed concurrently, so data were initially analyzed by two-way ANOVAs (sex, treatment). However, based on our a priori prediction that the sexes would respond to treatment differently and the value of assessing within-sex results, levels of each analyte were compared for each sex using ANOVA (naïve, control, binge) with Tukey’s post-hoc tests. Effect size estimates also were calculated (Cohen, 1988), with d=0.2 considered a small effect, d=0.5 considered a medium effect, and d=0.8 considered a large effect. Pearson correlations analyzed relationships between the ethanol drinking following the 4th PS in Finn et al. (2018) with protein and neurosteroid levels to evaluate whether a history of ethanol drinking and intermittent PS exposure differentially altered these relationships in male and female mice. Likewise, the relationship between plasma CORT (Finn et al., 2018) and neurosteroid levels at the time of euthanasia was examined. Correlations also were conducted between PFC and hippocampal P450scc levels and plasma pregnenolone levels to determine the correspondence between enzyme and steroid levels in the first step in steroidogenesis.
All data are presented as mean ± SEM, and analysis was conducted with SYSTAT (versions 11 & 13, SYSTAT Software, Inc., Richmond, CA). The level of significance was set at p≤0.05, and p≤0.08 was considered as a non-significant trend.
Results
Analyses of protein levels in dissected PFC and hippocampus and of neurosteroid levels in plasma were conducted. Samples were obtained from male and female mice with traumatic stress exposure and differing patterns of ethanol drinking and compared to samples obtained from age-matched naïve mice. The only difference between binge and control groups was the binge ethanol versus (vs) water consumption during the first part of the study, respectively. After that, both groups were treated identically (Figure 1). In the binge groups, average ethanol intake across the seven binge sessions was 2.17 g/kg in 30 min (males) and 2.39 g/kg in 30 min (females). Average blood ethanol concentrations (BECs) after the final binge session were 1.37 mg/mL (males) and 1.42 mg/mL (females), which greatly exceeded the National Institute on Alcohol Abuse and Alcoholism (NIAAA) criteria for binge drinking (0.80 mg/mL; NIAAA, 2004). As described in Finn et al. (2018), this prior binge drinking produced a greater enhancement in 23 h ethanol intake after exposure to repeated, intermittent PS in male but not in female C57BL/6J mice. The increase in ethanol intake over baseline after the 4th PS (PS4) in males was 24% (control) vs 65% (binge), whereas the increase in females was 5% (control) vs 6% (binge). Only ethanol licks were recorded in the study by Finn et al. (2018), but an extrapolation of ethanol licks to g/kg dose revealed that averaged 23 h ethanol intake at baseline vs after PS4 increased from approximately 8 to 10 g/kg in control males, from approximately 9 to 14 g/kg in binge males, from approximately 12 to 12.5 g/kg in control females, and from 10.5 to 11 g/kg in binge females. The percent increase in ethanol intake over baseline after PS4 was similar to the calculations that were based on ethanol licks.
As described in the Introduction, Western blots were conducted on two proteins involved in synaptic plasticity (ARC, synaptophysin), two GABAAR subunits (α2, α4), and a rate-limiting enzyme in steroid biosynthesis (P450scc). Results in PFC are depicted in Figure 2; results in hippocampus are shown in Figure 3.
Figure 2. Sex dependent changes in relative density of select proteins in the prefrontal cortex (PFC) after a history of ethanol drinking with repeated intermittent predator odor stress exposure.
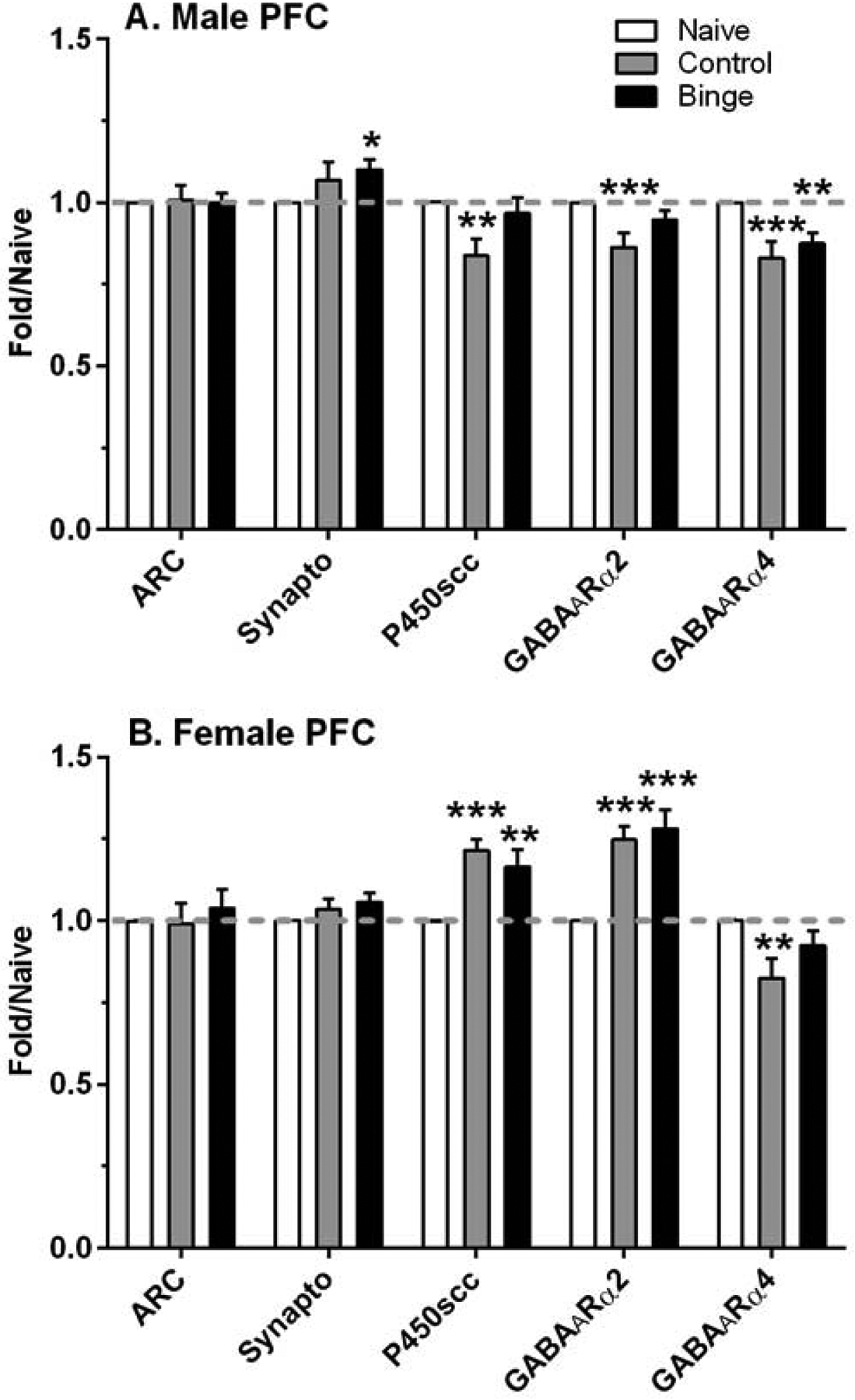
Western blot analysis was conducted on dissected PFC tissue at 24 h after the final drinking session (binge = prior binge ethanol intake; control = prior water intake) and compared to values from similarly aged naïve mice (naïve). Sexually divergent effects of treatment were observed on protein levels of synaptophysin (synapto), cytochrome P450 side chain cleavage (P450scc), and GABAA receptor (GABAAR) α2 subunit in male and female PFC, whereas treatment produced a similar suppression in GABAAR α4 subunit protein levels. Values are mean ± SEM for each group: male: n=15–18 (naïve), n=11 (control), n=12 (binge); female: n=13–17 (naïve), n=8 (control), n=10–11 (binge). All levels were initially normalized to β-actin. Fold regulation was then determined by normalizing all values to the mean of the relative expression of the respective naïve group (dashed gray line). *p<0.05, **p<0.01, ***p≤0.001 vs respective naïve group (effect size range = 1.31 – 3.41).
Figure 3. A history of ethanol drinking and intermittent predator odor stress produced changes in protein levels related to synaptic plasticity, steroid synthesis, and a GABAA receptor (GABAAR) subunit in the hippocampus.
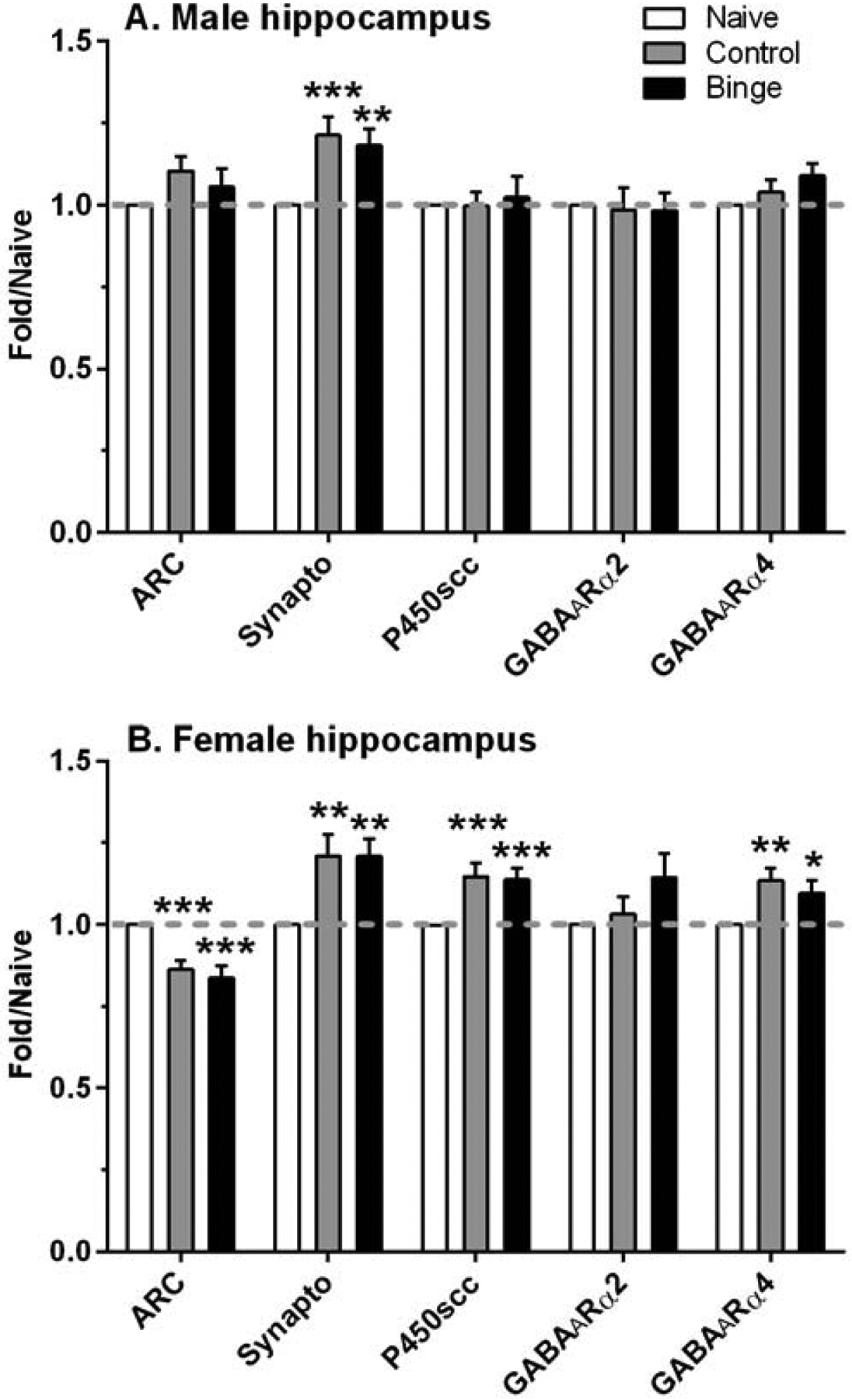
Western blot analysis was conducted on dissected hippocampal tissue at 24 h after the final drinking session (binge = prior binge ethanol intake; control = prior water intake) and compared to values from similarly aged naïve mice (naïve). Treatment produced similar significant increases in synaptophysin (synapto) levels in male and female hippocampus, whereas activity-regulated cytoskeletal protein (ARC), cytochrome P450 side chain cleavage (P450scc), and GABAAR α4 subunit protein levels were significantly altered by treatment only in female hippocampus. Values are mean ± SEM for each group: male: n=19–22 (naïve), n=12 (control), n=12 (binge); female: n=13–19 (naïve), n=11 (control), n=11 (binge). All levels were initially normalized to β-actin. Fold regulation was then determined by normalizing all values to the mean of the relative expression of the respective naïve group (dashed gray line). *p<0.05, **p<0.01, ***p≤0.001 vs respective naïve group (effect size range = 1.02 – 2.12).
ARC levels in the PFC were not significantly altered by treatment in males or females (Figure 2). However, synaptophysin levels were significantly altered by treatment in male PFC [F(2,38)=3.46, p<0.05], which was due to the significant 10% increase in synaptophysin levels in the binge vs naïve group. A different pattern of results was found in the hippocampus (Figure 3), where ARC levels were significantly decreased by treatment only in females [F(2,38)=19.84, p<0.001]. Post-hoc tests confirmed that ARC levels were significantly decreased by 14% and 16% vs naïve in the control and binge female mice, respectively. In contrast, treatment significantly altered hippocampus synaptophysin levels in a similar manner in both males [F(2,43)=12.23, p<0.001] and females [F(2,34)=8.43, p=0.001]. Synaptophysin levels were significantly increased by 21% vs naïve in the control and binge female mice, whereas levels were significantly increased by 21% and 18% vs naïve in the control and binge male mice, respectively.
There also were some sex and brain regional differences in the effect of treatment on levels of the two GABAAR subunits examined. In the PFC, treatment produced significant sexually divergent changes in levels of the GABAAR α2 subunit [males: F(2,38)=7.31, p=0.002; females: F(2,32)=21.59, p<0.001; Figure 2]. Post-hoc tests confirmed that GABAAR α2 subunit levels were decreased significantly by 14% vs naïve in the control males, whereas levels were increased significantly by 25% and 28% vs naïve in the control and binge females, respectively. In contrast, treatment significantly decreased PFC GABAAR α4 subunit levels in both sexes [males: F(2,38)=10.26, p<0.001; females: F(2,28)=5.55, p=0.009]. GABAAR α4 levels were significantly decreased by 18% vs naïve in the control females, and levels were decreased significantly by 17% and 13% vs naïve in the control and binge males, respectively. A different pattern of results was found in the hippocampus (Figure 3), as GABAAR α2 subunit levels were not significantly altered by treatment in male or female mice. GABAAR α4 levels were significantly increased by treatment in female hippocampus [F(2,36)=7.34, p=0.002], with post-hoc tests confirming a significant increase vs naïve of 13% in control females and of 10% in binge females. In male hippocampus, there was a strong non-statistical trend for treatment to increase GABAAR α4 levels [F(2,40)=3.09, p=0.056].
There were sexually divergent treatment effects on levels of P450scc in both PFC and hippocampus. Whereas P450scc levels in PFC were significantly altered by treatment in both sexes [males: F(2,35)=5.27, p=0.01; females: F(2,30)=12.21, p<0.001; Figure 2], post-hoc tests indicated that P450scc levels were decreased significantly vs naïve in control males (16%) and were increased significantly vs naïve in control (22%) and binge (17%) females. There was a non-significant trend for P450scc levels in the PFC to be negatively correlated with plasma pregnenolone levels only in the control male group (r = −0.57, p=0.068, n=11); correlations between P450scc and pregnenolone levels were not significant in the female mice. In hippocampus (Figure 3), treatment did not significantly alter P450scc levels in male mice, and there was a non-significant trend for P450scc levels to be negatively correlated with plasma pregnenolone in the binge group (r = −0.52, p=0.08, n=12). In contrast, and similar to the results in PFC, P450scc levels were significantly increased in female hippocampus [F(2,38)=12.34, p<0.001], which was due to the significant increase vs naïve of 15% and 14% in the control and binge females. However, hippocampal P450scc and plasma pregnenolone levels were not significantly correlated in female mice.
Correlations were conducted between the ethanol licks after the 4th PS (days 22 – 24; the final 3 days of drinking) from Finn et al. (2018) and protein levels in PFC and hippocampus that were obtained from this study and from Finn et al. (2018). In the PFC, ethanol licks from the binge male group (n=12) were significantly positively correlated with ARC (r = 0.86, p<0.001), P450scc (r = 0.69, p=0.01), and glucocorticoid receptor (r= 0.62, p<0.05) levels. Ethanol licks in the combined binge and control male groups also were significantly positively correlated with P450scc levels (r = 0.440, p<0.05, n=23). However, ethanol licks in the female groups (binge, control, or combined binge and control) were not significantly correlated with any protein levels. In the hippocampus, ARC levels were significantly positively correlated with ethanol licks in the binge female group (r = 0.82, p<0.01, n=11) and significantly negatively correlated with ethanol licks in the control male and female group (r = −0.41, p=0.05, n=23). No other correlations between hippocampal protein levels and ethanol licks reached significance.
Using gas chromatography-mass spectrometry (GC-MS), we simultaneously quantified the GABAAR-active 3α,5α-/3α,5β-reduced metabolites of progesterone, testosterone, dehydroepiandrosterone (DHEA), and deoxycorticosterone (DOC), the precursor steroids pregnenolone and DHEA, as well as estradiol (see Figure 1 in Snelling et al., 2014 for steroid pathway). Results of the 10 neuroactive steroid levels are depicted in Figure 4. The two-way ANOVA analysis of plasma levels of the two precursor steroids indicated that there only was a main effect of treatment [Fs(2,78)>4.35, p<0.05]. However, analysis of each sex revealed that there were sex differences in the effect of treatment, with significant changes in pregnenolone [F(2,39)=6.68, p=0.003; Figure 4a] and DHEA [F(2,39)=5.64, p=0.007; Figure 4b] levels only in male mice. For both steroids, levels were significantly higher in the binge vs control and naïve male mice. However, for one of the GABAAR-active derivatives of DHEA, 3α,5α-androsterone, there was a main effect of sex [F(1,78)=7.70, p=0.007], a main effect of treatment [F(2,78)=22.99, p<0.001], and a non-significant trend for a significant interaction [F(2,78)=2.92, p=0.06]. There only was a main effect of treatment [F(2,78)=8.95, p<0.001] on 3α,5β-androsterone levels. Analysis of each sex revealed that plasma 3α,5α-androsterone levels (Figure 4g) were significantly higher in the binge groups vs the control and naive groups for each sex [males: F(2,39)=7.93, p=0.001 and post-hoc tests; females: F(2,39)=15.41, p<0.001 and post-hoc tests]. Plasma 3α,5β-androsterone levels (Figure 4h) also were significantly higher in the binge vs control and naïve groups in females [F(2,39)=5.79, p=0.005 and post-hoc tests], and there was a strong non-statistical trend for treatment to increase 3α,5β-androsterone levels in males [F(2,39)=3.12, p=0.056].
Figure 4. Prior binge drinking followed by a history of ethanol drinking and intermittent predator odor stress exposure significantly alters plasma neurosteroid levels in male and female mice.
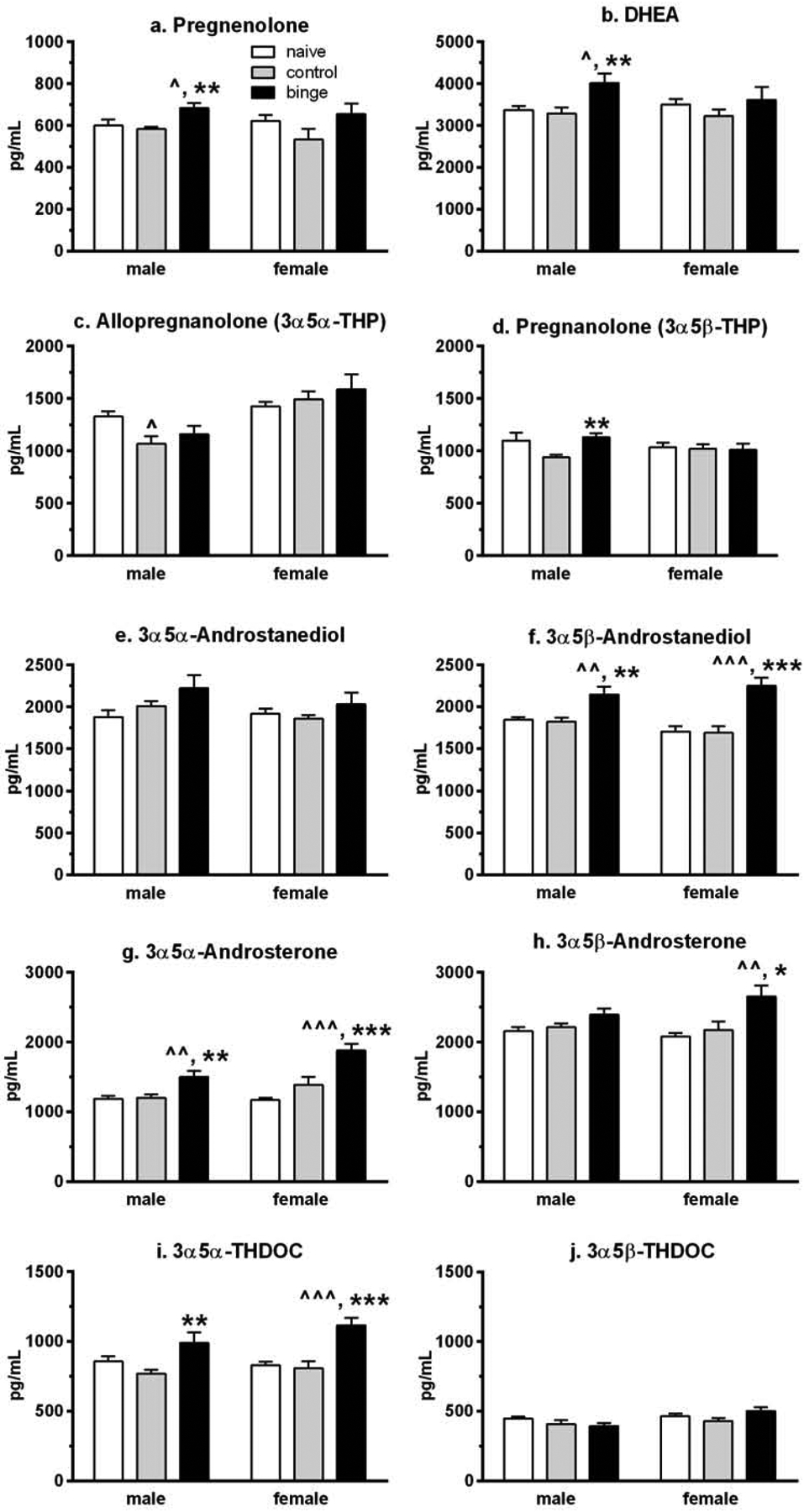
Gas chromatography-mass spectrometry was used to simultaneously quantify 10 neurosteroids from plasma that was collected at 24 h after the final drinking session (binge = prior binge ethanol intake; control = prior water intake) and compared to values from similarly aged naïve mice (naïve). With the exception of a significant suppression in allopregnanolone levels in control male mice vs naïve, the majority of neurosteroids were significantly increased in the mice with prior binge exposure and a history of ethanol drinking and stress exposure when compared to the naïve and control male and female mice. Values are mean ± SEM for each group: male: n=12 (naïve), n=15 (control), n=15 (binge); female: n=12 (naïve), n=15 (control), n=15 (binge). ^p<0.05, ^^p≤0.01, ^^^p≤0.001 vs respective naïve group (effect size range = 1.01 – 1.83); *p<0.05, **p≤0.01, ***p≤0.001 vs respective control group (effect size range = 0.87 – 1.59). DHEA = dehydroepiandrosterone; THDOC = tetrahydrodeoxycorticosterone.
For the GABAAR-active derivatives of progesterone, there only was a main effect of sex for allopregnanolone (ALLO, 3α,5α-THP, tetrahydroprogesterone) levels [F(1,78)=18.85, p<0.001]. However, analysis of each sex indicated that there were sexually divergent effects of treatment on both ALLO and pregnanolone (3α,5β-THP) levels. Plasma ALLO levels (Figure 4c) were significantly altered only in male mice [F(2,39)=3.28, p=0.05], due to the significant reduction in levels in the control males when compared to levels in naïve male mice. Plasma pregnanolone levels (Figure 4d) also were significantly altered only in male mice [F(2,39)=5.28, p=0.009], with levels in the controls significantly lower than levels in the binge male mice and a strong non-statistical trend for lower levels in the controls vs naïve male mice (p=0.053). The two-way ANOVA of the GABAAR-active derivatives of testosterone, 3α,5α-/3α,5β-androstanediol, indicated that there only was a main effect of treatment [F(2,78)=21.69, p<0.001] for 3α,5β-androstanediol levels. Subsequent one-way ANOVAs confirmed that only plasma 3α,5β-androstanediol levels (Figure 4f) were significantly altered by treatment in both sexes [males: F(2,39)=7.47, p=0.002; females: F(2,39)=14.27, p<0.001], with levels in the binge groups significantly higher than levels in the control and naïve groups for both male and female mice. And for the GABAAR-active derivatives of DOC, 3α,5α-/3α,5β-THDOC (tetrahydroDOC), there was a main effect of sex on 3α,5β-THDOC levels [F(1,78)=6.51, p=0.01], and a main effect of treatment on 3α,5α-THDOC levels [F(2,78)=16.10, p<0.001]. The analysis of each sex confirmed that only plasma 3α,5α-THDOC levels (Figure 4i) were significantly influenced by treatment in both sexes [males: F(2,39)=4.70, p=0.015; females: F(2,39)=13.44, p<0.001]. Post-hoc tests confirmed that 3α,5α-THDOC levels in male mice were significantly higher in the binge vs control group. In female mice, plasma 3α,5α-THDOC levels were significantly higher in the binge vs control and naïve groups.
Estradiol levels did not differ significantly in the three treatment groups of female mice. Mean±SEM estradiol levels in the females were 3.84±0.23 pg/mL (naïve, n=12), 2.96±0.42 pg/mL (control, n=11), and 3.50±0.31 pg/mL (binge, n=15). Levels were not quantifiable in the male mice. The minimum detectable level was 1 pg/mL.
Correlations were conducted between the ethanol licks after the 4th PS from Finn et al. (2018) and neurosteroid levels. In the combined binge and control male mice (n=24), ethanol licks after the 4th PS were significantly positively correlated with levels of the testosterone derivatives 3α,5α-androstanediol (r = 0.56, p<0.01) and 3α,5β-androstanediol (r = 0.47, p<0.05), and levels of the progesterone derivative pregnanolone (r = 0.55, p=0.005), and there was a non-significant trend for ethanol licks to be positively correlated with levels of the DOC derivative 3α,5α-THDOC (r = 0.37, p=0.07). In female mice, ethanol licks after the 4th PS were significantly positively correlated with levels of the progesterone derivative ALLO in the binge group (r = 0.71, p<0.01, n=12) and were significantly negatively correlated with pregnenolone levels in the combined binge and control groups (r = −0.44, p<0.05, n=24).
Correlations also were conducted between the plasma CORT levels from Finn et al. (2018) and the plasma neurosteroids upon euthanasia (i.e., 24 h after the last drinking session), and only a few correlations reached significance. Specifically, CORT levels in the control male and female groups (n=30) were significantly positively correlated with ALLO levels (r = 0.36, p=0.05) and significantly negatively correlated with pregnenolone levels (r = −0.43, p<0.05). CORT levels in the binge and control female groups (n=30) were significantly negatively correlated with 3α,5α-THDOC levels (r = −0.36, p=0.05).
Discussion
The current findings were generated as a follow-up to the initial study investigating the effects of prior binge-like drinking on responses to repeated, intermittent PS exposure and subsequent drinking behavior (Finn et al., 2018). Given the sex differences in the pattern of changes in ethanol drinking behavior with prior binge drinking and PS exposure, the intent of the present set of studies was to better elucidate the neurochemical contributions in male and female C57BL/6J mice. Analysis was conducted in tissue and plasma collected 24 h after the last drinking session and 6 days after the final PS exposure. Based on the time between the final PS exposure and euthanasia, it is unlikely that the changes in protein levels reflect an acute response to the traumatic stress exposure, although exposure to various PS has been reported to increase anxiety-related behavior in naïve rodents between 2 – 7 days after the PS exposure (Belzung et al., 2001; Cohen and Zohar, 2004; Cohen et al., 2008; Hebb et al., 2003; Roltsch et al., 2014; Whitaker and Gilpin, 2015). Rather, the changes in protein levels are most likely due to the long history of ethanol drinking in the male and female C57BL/6J mice that were utilized in the current study. However, it is possible that the mice were experiencing some negative affective symptoms during withdrawal at the time of euthanasia, based on recent findings that 24 h of withdrawal after 2 weeks of binge drinking or 1 week of 24 h ethanol drinking produced anxiety and hyperalgesia in male C57BL/6J mice, respectively (Lee et al., 2016; Smith et al., 2016; female mice not tested). Nonetheless, a history of ethanol drinking and intermittent PS exposure produced brain regional and sex differences in the changes in proteins examined as well as in the pattern of neurosteroid levels vs values in naïve mice.
For the steroidogenic enzyme, P450scc, there were significant increases in relative protein levels in the female PFC and hippocampus in both treatment conditions (control and binge) with a significant decrease in protein levels only in the male PFC control condition, vs levels in respective naïve mice. As P450scc is the enzyme controlling the first step of steroidogenesis (converts cholesterol to pregnenolone; Mellon and Griffin, 2002; Mellon and Vaudry, 2001; Porcu et al., 2009) that plays a key role in synthesis of steroids, including CORT and neurosteroids, these data suggest that a history of ethanol drinking and traumatic stress exposure may differentially regulate neurosteroid synthesis in male and female mice. However, P450scc levels in PFC and hippocampus were not significantly correlated with plasma pregnenolone levels in female mice, yet plasma pregnenolone levels were significantly negatively correlated with ethanol licks after the 4th PS. Moreover, brain P450scc levels only tended to be negatively correlated with plasma pregnenolone levels in male mice. Previous findings indicate that basal neurosteroid levels in plasma do not simply reflect levels in cortex and hippocampus (Caruso et al., 2013), so it is possible that PFC and hippocampal pregnenolone levels would better correspond to P450scc levels in these tissues. Nonetheless, we previously observed an enhanced CORT response to PS in female vs male mice (Cozzoli et al., 2014; Finn et al., 2018), consistent with recent reports that females often show a greater response to stress than males (Moench and Wellman, 2017; Oyola and Handa, 2017).
Neurosteroid synthesis is a dynamic process. Hence, it is likely that disruptions in one or more biosynthetic steps besides P450scc could influence levels of multiple steroids with positive or negative modulatory effects at GABAARs (e.g., Belelli and Lambert, 2005). As a result, we used GC-MS to simultaneously quantify estradiol levels in females in addition to the 10 neurosteroids in males and females. Given the chronic stress or PS-induced alterations in ALLO and DHEA levels and dysregulation with CORT following PS that has been reported (Cohen et al., 2007; Guo et al., 2017; Pibri et al., 2008; Pinna and Rasmusson, 2011; Serra et al., 2000), as well as the suppression in ALLO levels in female patients with PTSD (Pineles et al., 2018; Rasmusson et al., 2006), we expected that intermittent PS would significantly decrease ALLO levels in male and female mice in the present study. However, plasma ALLO levels only were significantly suppressed in the control male mice vs naïve, and levels of the structurally related neurosteroid pregnanolone only were decreased in control vs binge and naïve male mice. Interestingly, these differences in pregnanolone levels between the control and binge groups likely contributed to the significant positive correlation with ethanol licks in the combined binge and control groups. The results in control male mice with a history of ethanol drinking and intermittent PS also are consistent with recent data suggesting that a chronic isolation stress-induced enhancement in ethanol intake in male C57BL/6J mice did not restore the stress-induced reduction in hippocampal ALLO levels (Sanna et al., 2011). It should be noted that withdrawal from various models of chronic ethanol exposure produced a consistent decrease in ALLO levels in male rodents and monkeys (see Jensen et al., 2017 and references therein), so it is not known if the current ALLO results in male mice reflect a suppression in ALLO synthesis due to traumatic stress, chronic ethanol drinking, or both. In contrast, treatment did not significantly alter plasma ALLO and pregnanolone levels in female mice, although plasma ALLO levels were significantly positively correlated with ethanol licks in the binge group. Thus, a history of ethanol drinking and exposure to intermittent PS may differently regulate production of the GABAAR-active progesterone derivatives ALLO and pregnanolone in male and female mice.
It is interesting that a different pattern of results was observed for the remaining 8 neurosteroids than what was detected for ALLO and pregnanolone. In general, neurosteroid levels were significantly increased in the binge vs control and naïve groups. This was true in male mice for levels of the precursor steroids pregnenolone and DHEA, and it also was true in both males and females for the DHEA derivatives 3α,5α-/3α,5β-androsterone, the testosterone derivative 3α,5β-androstanediol, and the DOC derivative 3α,5α-THDOC. Several points are worth mentioning about these results. First, it is possible that the increase in 3α,5α-THDOC was related to the intermittent PS exposure, as acute stress can significantly increase 3α,5α-THDOC in male rodents (Barbaccia et al., 2001; Purdy et al., 1991; Reddy and Rogawski, 2002). Considering that DOC is the precursor steroid to both 3α,5α-THDOC and CORT (see Figure 1 in Snelling et al., 2014) and that a history of ethanol drinking dampened the CORT response to PS (Finn et al, 2018), it is likely that the 5α-reduction of DOC was favored (to produce THDOC) in the mice with prior binge drinking experience. Second, the sex differences in the treatment effect on PFC and hippocampus P450scc levels did not relate to the corresponding group plasma pregnenolone levels in either the male or female mice. However, the pattern of the results suggests that synthesis of the DHEA-, DOC-, and testosterone-derived neurosteroids was increased in the male and female mice with prior binge drinking experience. Related to this point, ethanol licks in binge and control male mice were significantly positively correlated with 3α,5α-/3α,5β-androstanediol levels and tended to be positively correlated with 3α,5α-THDOC levels. Given that P450scc is the first of many enzymes that are involved in neurosteroid synthesis (Mellon and Vaudry, 2001; Porcu et al., 2009) and that activity of the neurosteroid enzymes in the brain can be regulated by neurotransmitters and neuropeptides (Do Rego et al., 2009), the present results are consistent with the idea that there can be independent regulation of neurosteroid synthesis in the periphery and in the brain at several steps in the neurosteroid pathways. We recently found that ethanol withdrawal produced a broad and complex dysregulation in neurosteroid biosynthesis, with differences in the withdrawal-induced changes in plasma vs brain tissue and altered correlations between neurosteroid levels in naïve mice vs those undergoing withdrawal (Jensen et al., 2017), providing further support for the idea that independent regulation of neurosteroid synthesis can occur. Collectively, the results suggest that prior binge drinking with a later history of ethanol drinking and exposure to intermittent traumatic stress may dysregulate neurosteroid synthesis, with some sex differences in the pattern of the effects.
In addition to the potential influence of neurosteroids on GABAAR-mediated inhibition, subtypes of GABAARs also influence GABAAR-mediated inhibition as well as addictive behaviors and behavioral responses such as anxiety (reviewed in Engin et al., 2018; Stephens et al., 2017), and they are regulated by acute and chronic ethanol exposure (reviewed in Olsen and Liang, 2017). We focused our studies on the GABAAR α2 and α4 subunits, as these two subunits are up-regulated in dependent male and female rats (reviewed in Olsen and Liang, 2017; also Devaud and Alele, 2004; Devaud et al., 1997, 1999) and are important modulators of addictive-related behaviors (reviewed in Stephens et al. 2017). Additionally, α2-containing GABAARs are important in anxiety-related behaviors and responsiveness to stress (see Engin et al., 2018). It is interesting that we again observed some divergent responses to treatment in the males and females in the present study. Relative protein levels of the GABAAR α2 subunit in the PFC were significantly increased in binge and control females, whereas levels were significantly decreased in control males, when compared to the respective naïve mice. This result in males is consistent with recent evidence for a down-regulation in the cortical GABAAR α2 subunit of adult male and female mice exposed to early life stress (Skilbeck et al., 2018). It is possible that the increased protein levels of the GABAAR α2 subunit in female PFC reflects the history of ethanol drinking in these animals, given the upregulation of this subunit that has been reported in several brain regions following chronic ethanol exposure (Devaud et al., 1999; Olsen and Liang, 2017). A different pattern of results was observed with the GABAAR α4 subunit, where treatment produced a similar significant decrease in PFC protein levels in male and female mice, but a significant increase in hippocampus protein levels only in female mice. Chronic isolation stress produced a significant decrease in hippocampus expression and peptide levels of the GABAAR α4 subunit in male C57BL/6J mice that was reversed with 6 weeks of ethanol drinking (Sanna et al., 2011), although PFC was not examined in that study. Thus, it is possible that the current PFC GABAAR α4 subunit results reflect a suppression due to the intermittent and repeated traumatic stress exposure in the binge and control mice. In the female hippocampus, the history of ethanol drinking likely influenced the significant increase in GABAAR α4 subunit levels observed in the binge and control groups, consistent with the upregulation reported in male and female rodent hippocampus after chronic ethanol exposure (Devaud and Alele, 2004; Matthews et al., 1998; Olsen and Liang, 2017). Taken together, the brain regional and sex differences in the effects of treatment on GABAAR α2 and α4 levels may reflect different contributions of repeated traumatic stress exposure and history of ethanol drinking in male and female mice.
We also assayed relative protein levels of ARC and synaptophysin, which play a role in synaptic plasticity (Li et al., 2002; Shepherd and Bear, 2011). Earlier work in male rats found that ARC expression was altered by ethanol in a bidirectional manner that also was related to anxiety, with acute ethanol’s anxiolytic effect associated with increases in ARC in the amygdala and the anxiogenic effect of ethanol withdrawal associated with a decrease in ARC in the amygdala (Pandey et al., 2008). In contrast, ARC labelling in the motor cortex was increased during ethanol withdrawal in male and female rats (Walls et al., 2012) and was increased in the medial PFC of male rats following CORT treatment after inhibitory avoidance training that involved footshock (McReynolds et al., 2014). Despite no significant effects of treatment on ARC levels in the PFC of male or female mice in the present study, there was a significant positive correlation between ethanol licks and ARC levels in male mice from the binge group. Synaptophysin levels have been reported to be increased in the PFC from chronic alcoholics (Henriksson et al., 2008), to be increased in the hippocampus of male but not female rats during ethanol withdrawal (Alele and Devaud, 2005), and to be altered differently in male (↓) and female (↑) rats following chronic mild stress and voluntary ethanol consumption (Marco et al., 2017). Overall, the current results indicated that there were minimal effects of our behavioral paradigm on either ARC or synaptophysin levels in the PFC. In contrast, there were significant alterations in relative levels of both proteins in the hippocampus, with similar increases in synaptophysin levels in binge and control males and females and a significant decrease in ARC levels only in binge and control female mice. However, ethanol licks and ARC levels in the hippocampus were significantly positively correlated in binge females and significantly negatively correlated in control males and females, suggesting that relationships between ethanol licks and hippocampal ARC levels may differ across sex or prior treatments (as they contribute to the history of ethanol drinking). And although hippocampal synaptophysin levels and ethanol licks were not significantly correlated, the significant treatment-induced increase in synaptophysin levels in both males and females is consistent with reports of withdrawal-induced or stress- and drinking-induced changes in male and female rats, respectively (Alele and Devaud, 2005; Marco et al., 2017). Taken in conjunction with the evidence that ARC and synaptophysin have been linked to prominent roles in synaptic plasticity, particularly for glutamatergic transmission (Korb and Finkbeiner, 2011; Henriksson et al, 2008; Marco et al 2017; Shepherd and Bear, 2011), the consistent increase in hippocampus synaptophysin levels for males and females following a history of ethanol drinking and repeated traumatic stress exposure supports the suggestion of a common route for enhancing glutamatergic transmission across both sexes. Interestingly, a recent report found that environmental enrichment afforded protection against early life stress-induced behaviors by resulting in increased levels of synaptophysin and brain-derived neurotrophic factor in rat hippocampus (Dandi et al, 2018), further supporting a neurochemical contribution of this protein in adapting to ethanol and stress.
One limitation of the present findings is that protein levels were not assessed in PFC or hippocampal subregions. Evidence indicates that avoidance of a predator stress-paired context in rodents (Edwards et al., 2013; Schreiber et al., 2017) or symptoms of PTSD in humans (Gilpin and Weiner, 2017; Morey et al., 2016) are associated with alterations or structural deficits in the ventral but not dorsal subregion of the medial PFC (i.e., vmPFC). These effects are not surprising, given the distinct functional and anatomical characteristics of these two subregions (reviewed in Heidbreder and Groenewegen, 2003). Additionally, the hippocampal-to-PFC pathway is highly sensitive to stress, with the strongest projection originating in the ventral hippocampus and terminating in the mPFC (Godsil et al., 2013), and several lines of evidence confirming the necessity of the ventral hippocampal to mPFC projection in anxiety-related behavior (Padilla-Coreano et al., 2016). Thus, it is possible that the lack of treatment effect for some proteins in the present study was confounded by the assessment of protein levels in the entire brain region rather than in subregions. Future studies will explore additional brain regions and sub-regions within the PFC and hippocampus for select protein levels to better map the circuitry involved in a history of ethanol drinking and PTSD-like stress exposures in male and female mice.
In conclusion, the present findings, in conjunction with our recent work indicating increases in GR and CRF-R1 levels in females but not in males following a history of ethanol drinking and traumatic stress exposure (Finn et al., 2018), add to evidence that different biological mechanisms may underlie sex differences in responsiveness to stress and contribute to the sex differences in prevalence of psychiatric disorders (Bolea-Alamanac et al, 2018). We again noted a much greater effect of stress in conjunction with a history of ethanol drinking on changes in PFC and hippocampus for select relative protein levels, suggesting a greater influence of the traumatic stress concurrent with ethanol drinking history than of the earlier binge-like drinking experience. There were sex-selective effects for neurosteroid-related, GABAA receptor-related and synaptic plasticity-related proteins that also varied by brain area. When considered with the sex differences in pattern of enhanced ethanol drinking in response to traumatic stress exposure, the findings suggest the involvement of multiple and divergent adaptations. This adds further support to the need to address risk for harm from the interactions between stress and drinking in both men and women.
Highlights.
Sex and brain region differences in P450 levels after stress and ethanol drinking
Stress and ethanol drinking suppress allopregnanolone levels only in male mice
Traumatic stress and prior binge drinking increase many GABAergic neurosteroids
Traumatic stress and ethanol drinking increase synaptophysin levels in hippocampus
Acknowledgements
This research was supported by VA Merit grants (I01 BX001070 and I01 BX002966 to DAF) from the U.S. Department of Veterans Affairs, by RO1 grant (AA012439 to DAF and DJR, MPIs) from the National Institute on Alcohol Abuse and Alcoholism, by resources and facilities at the VA Portland Health Care System and Oregon Health & Science University (DAF), and by a Pacific University School of Pharmacy award (LLD). The contributions of Jessica Lally and Jae Bang of the Devaud Lab are greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: None. The contents do not represent the views of the United States Department of Veterans Affairs, the United States Government, or the National Institute of Health.
References
- Alele PE, Devaud LL, 2005. Differential adaptations in GABAergic and glutamatergic systems during ethanol withdrawal in male and female rats. Alcoholism: Clinical and Experimental Research, 29, 1027–1034. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G, 2001. Stress and neuroactive steroids. International Review of Neurobiology, 46, 243–272. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL, 2011. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology, 218, 131–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ, 2005. Neurosteroids: endogenous regulators of the GABAA receptor. Nature Reviews Neuroscience, 6, 565–575. [DOI] [PubMed] [Google Scholar]
- Belzung C, El Hage W, Moindrot N, Griebel G, 2001. Behavioral and neurochemical changes following predatory stress in mice. Neuropharmacology, 41, 400–408. [DOI] [PubMed] [Google Scholar]
- Bolea-Alamanac B, Bailey SJ, Lovick TA, Scheele D, Valentino R, 2018. Female psychopharmacology matters! Towards sex-specific psychopharmacology. Journal of Psychopharmacology, 32, 125–133. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW, 2003. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Molecular Pharmacology, 63, 53–64. [DOI] [PubMed] [Google Scholar]
- Caruso D, Pesaresi M, Abbiati F, Calabrese D, Giatti S, Garcia-Segura LM, Melcangi RC, 2013. Comparison of plasma and cerebrospinal fluid levels of neurosteroids with their brain, spinal cord and peripheral nerve levels in male and female rats. Psychoneuroendocrinology, 38, 2278–2290. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Cohen H, Zohar J, 2004. An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria. Annals of the New York Academy of Sciences, 1032, 167–178. [DOI] [PubMed] [Google Scholar]
- Cohen H, Maayan R, Touati-Werner D, Kaplan Z, Matar MA, Loewenthal U, Kozlovsky N, Weizman R, 2007. Decreased circulatory levels of neuroactive steroids in behaviourally more extremely affected rats subsequent to exposure to a potentially traumatic experience. International Journal of Neuropsychopharmacology, 10, 203–209. [DOI] [PubMed] [Google Scholar]
- Cohen H, Geva AB, Matar MA, Zohar J, Kaplan Z, 2008. Post-traumatic stress behavioural responses in inbred mouse strains: can genetic predisposition explain phenotypic vulnerability? International Journal of Neuropsychopharmacology, 11, 331–349. [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Tanchuck-Nipper MA, Kaufman MN, Horowitz CB, Finn DA, 2014. Environmental stressors influence limited-access ethanol consumption by C57BL/6J mice in a sex-dependent manner. Alcohol, 48, 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandi E, Kalamari A, Touloumi O, Lagoudaki R, Nousiopoulou E, Simeonidou C, Spandou E, Tata DA, 2018. Beneficial effects of environmental enrichment on behavior, stress reactivity and synaptophysin/BDNF expression in hippocampus following early life stress. International Journal of Developmental Neuroscience, 67, 19–32. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Alele P, 2004. Differential effects of chronic ethanol administration and withdrawal on γ-aminobutyric acid type A and NMDA receptor subunit proteins in male and female rat brain. Alcoholism: Clinical and Experimental Research, 28, 957–965. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Fritschy J-M, Sieghart W, Morrow AL, 1997. Bidirectional alterations of GABAA receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. Journal of Neurochemistry, 69, 126–130. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Matthews DB, Morrow AL, 1999. Gender impacts behavioral and neurochemical adaptations in ethanol-dependent rats. Pharmacology, Biochemistry and Behavior, 64, 841–849. [DOI] [PubMed] [Google Scholar]
- Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon M-C, Pelletier G, Vaudry H, 2009. Neurosteroid biosynthesis: Enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Frontiers in Neuroendocrinology, 30, 259–301. [DOI] [PubMed] [Google Scholar]
- Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, Gilpin NW, 2013. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Translational Psychiatry, 3, e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Benham RS, Rudolph U, 2018. An emerging circuit pharmacology of GABAA receptors. Trends in Pharmacological Sciences, 39, 710–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Helms ML, Nipper MA, Cohen A, Jensen JP, Devaud LL, 2018. Sex differences in the synergistic effect of prior binge drinking and traumatic stress exposure on subsequent ethanol intake and neurochemical responses in adult C57BL/6J mice. Alcohol, 71, 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Weiner JL, 2017. Neurobiology of comorbid post-traumatic stress disorders and alcohol-use disorder. Genes, Brain and Behavior, 16, 15–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsil BP, Kiss JP, Spedding M, Jay TM, 2013. The hippocampal-prefrontal pathway: The weak link in psychiatric disorders? European Neuropsychopharmacology, 23, 1165–1181. [DOI] [PubMed] [Google Scholar]
- Guo X, Qiu W, Liu Y, Zhang Y, Zhao H, Chen J, 2017. Effects of refined xiaoyaosan on depressive-like behaviors in rats with chronic unpredictable mild stress through neurosteroids, their synthesis and metabolic enzymes. Molecules, 22, 1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb ALO, Zacharko RM, Dominguez H, Laforest S, Gauthier M, Levac C, Drolet G, 2003. Changes in brain cholecystokinin and anxiety-like behavior following exposure of mice to predator odor. Neuroscience, 116, 539–551. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ, 2003. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neuroscience and Biobehavioral Reviews, 27, 555–579. [DOI] [PubMed] [Google Scholar]
- Henriksson R, Kuzman A, Okvist A, Harper C, Sheedy D, Garrick T, Yakovleva T, Bakalkin G, 2008. Elevated synaptophysin I in the prefrontal cortex of human chronic alcoholics. Synapse, 62, 829–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JP, Nipper MA, Helms ML, Ford MM, Crabbe JC, Rossi DJ, Finn DA, 2017. Ethanol withdrawal-induced dysregulation of neurosteroid levels in plasma, cortex, and hippocampus in genetic animal models of high and low withdrawal. Psychopharmacology, 234, 2793–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, Grant BF, Hasin DS, 2012. Stress and alcohol: epidemiologic evidence. Alcohol Research, 34, 391–400. [PMC free article] [PubMed] [Google Scholar]
- Korb E, Finkbeiner S, 2011. Arc in synaptic plasticity: from gene to behavior. Trends in Neuroscience, 34, 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL, 2009. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology, 205, 529–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, McGregor HA, Solton NR, Cohen M, Szumlinski KK, 2016. Adolescent mice are resilient to alcohol withdrawal-induced anxiety and changes in indices of glutamate function within the nucleus accumbens. Frontiers in Cellular Neuroscience, 10, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Reinprecht I, Fahnestock M, Racine RJ, 2002. Activity-dependent changes in synaptophysin immunoreactivity in hippocampus, piriform cortex, and entorhinal cortex of the rat. Neuroscience, 115, 1221–1229. [DOI] [PubMed] [Google Scholar]
- Manjoch H, Vainer E, Matar M, Ifergane F, Zohar J, Kaplan Z, Cohen H, 2016. Predator-scent stress, ethanol consumption and the opioid system in an animal model of PTSD. Behavioural Brain Research, 306, 91–105. [DOI] [PubMed] [Google Scholar]
- Marco EM, Ballesta JA, Irala C, Hernández M-D, Serrano ME, Mela V, LópezGallrado M, Viveros MP, 2017. Sex-dependent influence of chronic mild stress (CMS) on voluntary alcohol consumption; study of neurobiological consequences. Pharmacology, Biochemistry and Behavior, 152, 68–80. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Devaud LL, Fritschy JM, Sieghart W, Morrow AL, 1998. Differential regulation of GABAA receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. Journal of Neurochemistry, 70, 1160–1166. [DOI] [PubMed] [Google Scholar]
- McClintick JN, McBride WJ, Bell RL, Ding Z-M, Liu Y, Xuei X, Edenberg HJ, 2018. Gene expression changes in the ventral hippocampus and medial prefrontal cortex of adolescent alcohol-preferring (P) rats following binge-like alcohol drinking. Alcohol, 68, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD, 2016. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology, 41, 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds JR, Holloway-Erickson CM, Parmar TU, McIntyre CK, 2014. Corticosterone-induced enhancement of memory and synaptic Arc protein in the medial prefrontal cortex. Neurobiology of Learning and Memory, 112, 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD, 2002. Neurosteroids: biochemistry and clinical significance. Trends in Endocrinology and Metabolism, 13, 35–43. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Vaudry H, 2001. Biosynthesis of neurosteroids and regulation of their synthesis. International Review of Neurobiology, 46, 33–78. [DOI] [PubMed] [Google Scholar]
- Moench KM, Wellman CL, 2017. Differential dendritic remodeling in prelimbic cortex of male and female rats during recovery from chronic stress. Neuroscience, 357, 145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Haswell CC, Hooper SR, De Bellis MD, 2016. Amygdala, hippocampus, and ventral medial prefrontal cortex volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Neuropsychopharmacology, 41, 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA, 2004. NIAAA council approves definition of binge drinking. NIAAA Newsletter, number 3.
- Olsen RW, Liang J, 2017. Role of GABAA receptors in alcohol use disorders suggested by chronic intermittent ethanol (CIE) rodent model. Molecular Brain, 10, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyola MG, Handa RJ, 2017. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress, 20, 476–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, Spellman TJ, Gordon JA, 2016. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron, 89, 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K, 2008. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. Journal of Neuroscience, 28, 2589–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pibri F, Nelson M, Guidotti A, Costa E, Pinna G, 2008. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America, 105, 5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles SL, Nillni YI, Pinna G, Irvine J, Webb A, Hall KAA, Hauger R, Miller MW, Resick PA, Orr SP, Rasmusson AM, 2018. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology, 93, 133–141. [DOI] [PubMed] [Google Scholar]
- Pinna G, Rasmusson AM, 2011. Up-regulation of neurosteroid synthesis as a pharmacological strategy to improve behavioral deficits in a putative mouse model of posttraumatic stress disorder. Journal of Neuroendocrinology, 24, 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O’Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, Morrow AL, 2009. Simultaneous quantification of GABAergic 3α,5α/3α,5β neuroactive steroids in human and rat serum. Steroids, 74, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH Jr, Paul SM, 1991. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proceedings of the National Academy of Sciences of the United States of America, 88, 4553–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, Guidotti A, 2006. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biological Psychiatry, 60, 704–713. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA, 2002. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABAA receptor function and seizure susceptibility. Journal of Neuroscience, 22, 3795–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roltsch EA, Baynes BB, Mayeux JP, Whitaker AM, Baiamonte BA, Gilpin NW, 2014. Predator odor stress alters corticotropin-releasing factor-1 receptor (CRF1R)-dependent behaviors in rats. Neuropharmacology, 79, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Talani G, Obili N, Mascia MP, Mostallino MC, Secci PP, Pisu MG, Biggio F, Utzeri C, Olla P, Biggio G, Follesa P, 2011. Voluntary ethanol consumption induced by social isolation reverses the increase of α4δ GABAA receptor gene expression and function in the hippocampus of C57BL/6J mice. Frontiers in Neuroscience, 5, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AL, Lu Y-L, Baynes BB, Richardson HN, Gilpin NW, 2017. Corticotropinreleasing factor in ventromedial prefrontal cortex mediates avoidance of a traumatic stresspaired context. Neuropharmacology, 113, 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy RH, Biggio G, 2000. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. Journal of Neurochemistry, 75, 732–740. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF, 2011. New views of Arc, a master regulator of synaptic plasticity. Nature Neuroscience, 14, 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skilbeck KJ, Johnston GAR, Hinton T, 2018. Long-lasting effects of early-life intervention in mice on adulthood behaviour, GABAA receptor subunit expression and synaptic clustering. Pharmacological Research, 128, 179–189. [DOI] [PubMed] [Google Scholar]
- Smith ML, Hostetler CM, Heinricher MM, Ryabinin AE, 2016. Social transfer of pain in mice. Science Advances, 2, e1600855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelling C, Tanchuck-Nipper MA, Ford MM, Jensen JP, Cozzoli DK, Ramaker MJ, Helms M, Crabbe JC, Rossi DJ, Finn DA, 2014. Quantification of ten neuroactive steroids in plasma in Withdrawal Seizure–Prone and –Resistant mice during ethanol withdrawal. Psychopharmacology, 231, 3401–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, King SL, Lambert JJ, Belelli D, Duka T, 2017. GABAA receptor subtype involvement in addictive behaviour. Genes, Brain and Behavior, 16, 149–184. [DOI] [PubMed] [Google Scholar]
- Walls SA, Macklin ZL, Devaud LL, 2012. Ethanol-induced loss-of-righting response during ethanol withdrawal in male and female rats: Associations with alterations in Arc labeling. Alcoholism: Clinical and Experimental Research, 36, 234–241. [DOI] [PubMed] [Google Scholar]
- Wang R, Wang W, Zu J, Liu D, Jiang H, Pan F, 2018. Dynamic effects of early adolescent stress on depressive-like behaviors and expression of cytokines and JMJD3 in the prefrontal cortex and hippocampus of rats. Frontiers in Psychiatry, 9, 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker AM, Gilpin NW, 2015. Blunted hypothalamo-pituitary-adrenal axis response to predator odor predicts high stress reactivity. Physiology and Behavior, 147, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Sun H, Gong X, Li H, 2016. Different effects of prenatal stress on ERK2/CREB/Bcl-2 expression in the hippocampus and the prefrontal cortex of adult offspring rats. NeuroReport, 27, 600–604. [DOI] [PubMed] [Google Scholar]


