Abstract
Mucinous ovarian carcinoma (mEOC) represents a rare subtype of epithelial ovarian cancer, accounting for 3-4% of all ovarian carcinomas. The rarity of this tumor type renders both the preclinical and clinical research compelling. Very few preclinical in vitro and in vivo models exist. We here report the molecular, metabolic and pharmacological characterization of two patient derived xenografts (PDXs) from mEOC, recently obtained in our laboratory. These PDXs maintain the histological and molecular characteristics of the patient’s tumors they derived from, including a wild type TP53. Gene expression analysis and metabolomics profile suggest that they differ from high grade serous/endometrioid ovarian carcinoma PDXs. The pharmacological characterization was undertaken testing the in vivo antitumor activity of both cytotoxic agents (cisplatin, paclitaxel, yondelis, oxaliplatin and 5-fluorouracile) and targeted agents (bevacizumab and lapatinib). These newly established mucinous PDXs do recapitulate mEOC and will be of value in the preclinical development of possible new therapeutic strategies for this tumor type.
Keywords: Patient-derived xenografts, mucinous ovarian cancer, chemotherapy
Introduction
Mucinous ovarian carcinoma (mEOC) represents a rare subtype of epithelial ovarian cancer, accounting for 3-4% of all ovarian carcinomas [1-4]. These tumors represent a distinct entity in the plethora of epithelial ovarian carcinomas with different epidemiologic and genetic risk factors, somatic alterations, clinical presentation and therapeutic response [5]. As recently reported, they represent “both a diagnostic and therapeutic conundrum for clinical oncologists” [6]. The histological diagnosis of mEOC can be very challenging, and a correct differential diagnosis from metastases originating from the colon rectum is mandatory as standard clinical therapeutic protocol are tailored to the primary organ sites [7].
Most of mEOCs are diagnosed at early stage, have a low histologic grade and are generally associated with a good prognosis [8]. Those cases presenting at late stage have a poor prognosis for their resistance to the platinum-taxane doublet, the gold standard front-line therapy in ovarian cancer. In fact, it has been reported that overall survival (OS) is lower for women with advanced mEOC than women with other advanced non-mucinous histological types (hazard ratio 2.81; 95% CI 2.47-3.21) [9-11]. This is probably due to its lower therapeutic response to first-line based platinum therapy, reported to be 13-60% versus 64-87% in serous ovarian carcinoma patients [3,6].
At a genetic level, mEOC is rarely associated with BRCA1/BRCA2 mutations [12,13], while an activation of the RAS/MEK pathway is quite common [12] with RAS mutations reported in 65% of the cases [12,13]. TP53 mutations and HER2 amplification have been shown to be acquired later in tumor development [12,14,15].
The rarity of this tumor type renders both the preclinical and clinical research compelling. Very few preclinical in vitro and in vivo models exist [16,17]. Specifically, only immortalized cell lines from established tumor samples and at the best to our knowledge no PDXs and no transgenic mice giving rise to mEOC have been reported. Recently, organoids obtained from mucinous ovarian tumor samples have been established, but their contribution to the biology and therapy of mEOC is still lacking [18]. The availability of robust preclinical models will certainly help not only in a better understanding of the biological behaviour and the therapeutic response of this tumor type, but also to find new active tailored treatments. In the last twenty years, our laboratory has been involved in the establishment of ovarian carcinoma xenobank transplanting fresh patient’ tumor samples both orthotopically and/or subcutaneously in immune-compromised animals [19,20]. We here report the biological, molecular and pharmacological characterization of two mEOC PDXs we have available in our xenobank.
Materials and methods
Specimen collection and clinical data
Clinical specimens (primary ovarian tumors) were obtained from patients undergoing surgery for ovarian tumor by laparotomy at San Gerardo Hospital in Monza (Italy). Tumor specimens were engrafted in nude mice within 24 hr, as already reported [19]. The study protocol for tissue collection and clinical information was approved by the institutional review boards and patients provided written informed consent authorizing the collection and use of the tissue for study purposes.
Animals
Female NCr-nu/nu mice obtained from Envigo Laboratories (The Netherlands) were used when six to eight weeks old. Mice were maintained under specific pathogen-free conditions, housed in isolated vented cages, and handled using aseptic procedures. The Istituto di Ricerche Farmacologiche Mario Negri IRCCS, adheres to the principles set out in the following laws, regulations, and policies governing the care and use of laboratory animals: Italian Governing Law (D. lg 26/2014; authorization no.19/2008-A issued 6 March 2008 by the Ministry of Health); Mario Negri Institutional Regulations and Policies providing internal authorization for persons conducting animal experiments (Quality Management System Certificate: UNI EN ISO 9001:2008, reg. no. 6121); the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals (2011 edition) and EU directive and guidelines (European Economic Community [EEC] Council Directive 2010/63/UE) [21].
Histopathological analysis
The morphology of patient’s tumor tissues was compared with their corresponding xenografts using paraffin-embedded sections and standard protocols as detailed in [22].
Drugs and treatments
Paclitaxel (Indena s.p.a., Milan, Italy) was dissolved in 50% CremophorEL (Sigma-Aldrich) and 50% ethanol and further diluted with saline before use. Cisplatin (CDDP, Sigma-Aldrich, Milan, Italy) and bevacizumab (Roche, Milan, Italy) were dissolved in 0.9% NaCl. Oxaliplatin (Sigma-Aldrich, Milan, Italy), 5-fluorouracile (5FU) (Sigma-Aldrich, Milan, Italy) were dissolved in sterile H2O. Yondelis, kindly supplied by PharmaMar, S.A. (Colmenar Viejo, Spain), was dissolved in water and further diluted in saline immediately before use. Lapatinib (Sigma-Aldrich, Milan, Italy) was dissolved in methylcellulose 0.5% and 0.1% Tween-80®.
After subcutaneous transplantation of PDXs, mice were randomized to treatment at approximately 150 mg of tumor weight (8-10 mice per group). Mice were monitored twice a week; tumor growth was measured with a Vernier caliper, and tumor weight (mg = mm3) calculated as follows: (length [mm] × width2 [mm2])/2 and body weight was registered as indirect measure of drug toxicity. Treatment efficacy was expressed as best tumor growth inhibition [%T/C = (median weight of treated tumors/median weight of control tumors) × 100]. Animals were euthanized when primary tumor volume exceeded 15% of body weight. Drug activity was interpreted as follows: subcutaneous tumors were considered resistant with T/C ≥ 50%, responsive with 10% < T/C < 50% and very responsive with T/C ≤ 10%, according to published criteria [23].
Genome-wide gene expression
Microarray data analysis deposited into the NCBI (National Center for Biotechnical Information) database Gene Expression Omnibus (GEO accession no.GSE56920) of patient and xenograft samples have already been reported [19]. Deregulated genes in mucinous PDXs (PDX#164 and PDX#182) as compared to seven high grade serous/endometrioid PDXs were analyzed for enrichment in cancer hallmarks using the web-based tool of the Molecular Signaling Database (MsigDB, http://software.broadinstitute.org/gsea/msigdb) filtering for a false discovery rate (FDR) < 0.05.
Genome-wide DNA profiling
Copy number variation data were obtained using the HumanCytoSNP-12 (Illumina, San Diego, CA, USA), following the manufacturer’s protocol. Raw data were processed as previously described [24].
Metabolomic profiling of tumor tissue
Metabolite extraction
For each xenograft, 20-50 mg of each tumor was homogenized using an Ultra Turex with 10 µl/mg of extraction solvent (85:15 MeOH/H2O). The homogenized sample were stored at -80°C for 20 minute and subsequently centrifuged for 15 minute at 13000 × g. Supernatants were collected and used for targeted and untargeted metabolomics analysis.
Untargeted metabolomics analysis (FIA-QTOF-MS/MS)
FIA-QTOF-MS/MS analysis was performed on an Agilent 1290 infinity Series coupled to an Agilent 6550 iFunnel Q-TOF mass spectrometer as reported in Ricci et al. (manuscript accepted, Therapeutic advances in Molecular Oncology).
Targeted metabolomics analysis
A targeted quantitative approach using a combined direct flow injection and liquid chromatography (LC) tandem mass spectrometry (MS/MS) assay (AbsoluteIDQ 180 kit, Biocrates, Innsbruck, Austria) was applied as previously published [25].
Statistical analysis
One-way ANOVA followed by Mann-Whitney-Wilcoxon test (JMP Pro13) was used to select the metabolites whose abundance was statistically significant altered between high grade serous and mucinous PDXs. Hierarchical clustering was done using the MeV module (http://mev.tm4.org).
Results
PDX establishment
Two mucinous ovarian carcinomas were established from freshly transplanted mucinous ovarian cancer samples transplanted in immune-deficient mice (see Material and Methods) (MNHOC164 and MNHOC182, from here in PDX#164 and PDX#182). Supplementary Table 1 shows patient’s characteristics. Patient #164 was a 56 year woman who was diagnosed with an ovarian mass of malignant origin, who underwent six cycles of neo-adjuvant chemotherapy (carboplatin-paclitaxel) obtaining a stable disease, followed by cyto-reductive surgery (from which we obtained the fresh sample) and histological diagnosis of ovary mucinous adenocarcinoma of intestinal type. On the contrary, patient #182 was a 44 year old woman diagnosed with a Stage I ovary mucinous adenocarcinoma of intestinal type, grade 1 with borderline areas, who underwent pelvic surgery with complete debulking of the tumor mass, point at which we obtained the tumor sample to be inoculated in nude mice. This patient was not treated with any adjuvant chemotherapy, but unfortunately, she relapsed after 42 months.
Lag times for the patient derived xenografts (PDX) to appear were about 30 and 60 days for PDX#164 and PDX#182, respectively. No modification of the tumor lag times was observed with subsequent passages and the tumor take was 100% for PDX#164 and 60% for PDX#182. The pathological diagnosis of both PDXs was ovarian mucinous adenocarcinoma of intestinal type. In addition, as shown in Figure 1, both PDXs resembled the original tumors as the morphology and tissue architecture were similarly preserved. Scanty positivity to cytokeratin 7 and CA125 was similar in patient original tumor and the corresponding PDX in the case of PDX#164; while no materials was available for patient #182, PDX#182 was positive for both CA125 and cytokeratin pool; both PDXs were Ki67 positive (Supplementary Figure 1).
Figure 1.
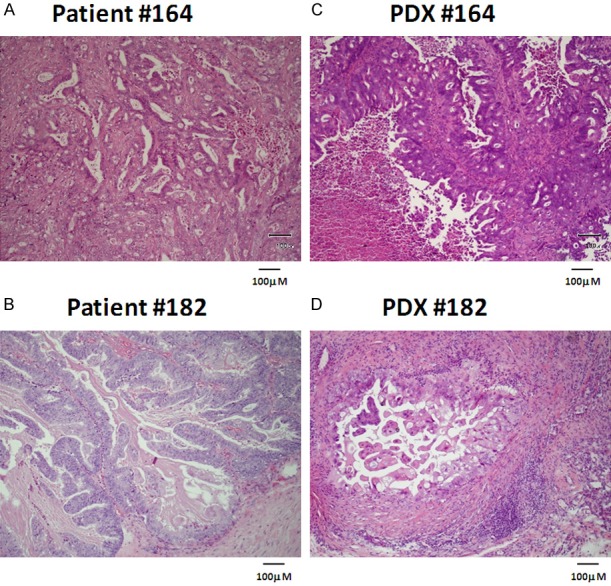
Mucinous ovarian PDX#164 and PDX#182 (C, D) are representative of the primary tumors of origin (A, B). Immunohistochemical analysis of patient’s tumors and the corresponding PDXs.
From fresh tumor samples, we tried to obtain stem cell enriched cultures as already reported [22]; however, for cells derived from patients #164 and #182 cultured in low adherence condition, we could obtained spheroids that could be maintained for three and four passages respectively, but eventually exhausted their capacity to sustain spheroid formation. Again, up to now even using different cell culture conditions we were unsuccessful in obtaining either spheroids and primary cultures from both PDX samples.
Molecular and metabolic characterization
As already reported, both PDXs were TP53, RAS, BRAF, PIK3CA wild type and have an amplification of ERBB2, resembling the original tumors [19]. We performed genome wide DNA profiling (Supplementary Table 2) and similar profiles were observed in PDX#182 as compared to the original patient’s tumor, while PDX#164 revealed an increased number of heterozygous and homozygous deletions as compared to the corresponding original tumor (Supplementary Table 2) In particular, PDX#164 had acquired different homozygous losses, including the ones affecting the CDKN2A and CDKN2B (9p21) (heterozygous in the primary) and DCC (18q21) loci (Supplementary Figure 2).
We have already reported a high correlation between the expression profile of PDX and the corresponding primary tumors, suggesting that no major molecular drift has occurred in the in vivo establishment of the mucinous ovarian PDXs [20]. We then compared the expression profile of high grade-serous and -endometrioid PDXs (number of samples 7) and the two mucinous PDXs we had available. Supplementary Table 3 shows the pathways differentially down and upregulated; among the most upregulated, there is the one involved in metastases, while among the down regulated ones there are the TNFA signalling via NFkB and the apoptosis signalling via caspase activation (Supplementary Table 3).
We investigated the metabolomics profile of PDX#164 and PDX#182 using an integrative mass spectrometry-based approach in which we combined targeted (T) and untargeted (UT) strategies, to compare the metabolic profiles between the two high grade serous PDXs of our xenobank (PDX#124, PDX#239) and the two mucinous PDXs. We observed similar central cellular metabolic profile (glycolysis, TCA cycle) between the high-grade and mucinous PDXs (Figure 2A). Interestingly, PDX#164 displayed striking differences in the abundance of lysophosphatidylcholines, phosphatidylcholines and sphingomyelins species compared to PDX#182 and high-grade PDXs (Figure 2B and Supplementary Table 4).
Figure 2.
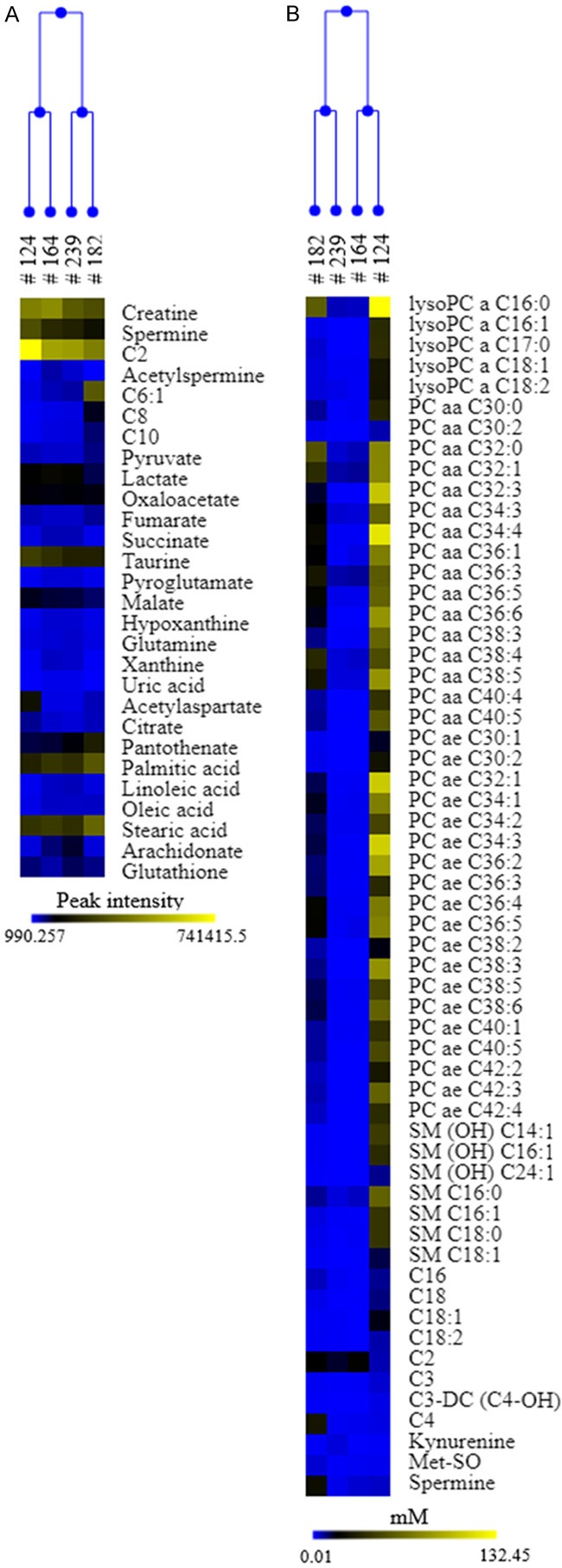
Metabolic comparison between mucinous and serous PDXs. Heat map and hierarchical clustering of the deregulated metabolites (A) untargeted (peak intensity); (B) targeted (mM) in high grade serous (#124, #239) and mucinous (#164, #182) PDXs. Each row represents a metabolite, each column the average metabolite intensity/concentration (three biological replicates) for each PDXs. Blue colour indicates lower metabolite level, yellow higher ones.
Pharmacological characterization
We pharmacologically profiled these mEOC-PDXs testing initially drugs used in first line, i.e. cisplatin and paclitaxel. As depicted in Figure 3A, paclitaxel and cisplatin, at the schedules used, were found active in PDX#164, with low T/C values, read out of effective tumor growth inhibition (Table 1); however, no tumor regressions were observed during therapy and all the treated tumors regrew. Yondelis, a DNA damaging agents, was inactive in this tumor model. We also tested bevacizumab, an antiangiogenic drug approved for maintenance setting in ovarian cancer, found to be moderately active; on the contrary, oxaliplatin and 5FU were completely inactive (Figure 3C). When the same compounds were tested in the PDX#182 model, this xenograft poorly responded to cisplatin and yondelis, while partially responded to paclitaxel (Figure 3B; Table 1). PDX#182 scarcely responded to oxaliplatin and 5FU, while again a partial response to bevacizumab could be observed (Figure 3D). We tested lapatinib, a small molecule inhibitor of the ERBB2 receptor, and no antitumor activity was observed, despite the already reported ERBB2 gene amplification in this PDX [19] and the use of an active schedule in other animal models [26].
Figure 3.
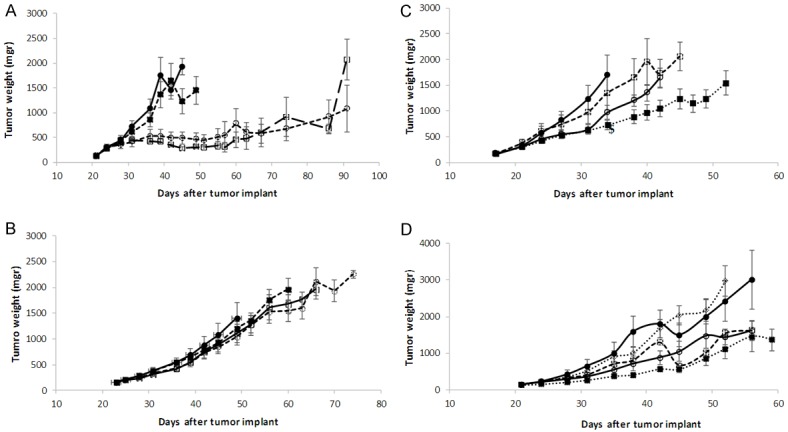
Drugs antitumor activity in mucinous ovarian cancer PDXs. Tumor bearing nude mice #164 (A) and #182 (B) were treated or not (control mice) -●-; with DDP (cisplatin, i.v., 5 mk/Kg, q7dx3) - -○- -; with PTX (paclitaxel, i.v., 20 mg/Kg, q7dx3) -□-; and yondelis (i.v., 0, 15 mg/Kg, q7dx3) - -■- -. Tumor bearing nude mice #164 (C) and #182 (D) were treated or not (control mice) -●-; with oxaliplatin (i.v., 10 mk/Kg, q7dx3) - - □ -, with 5FU (i.v., 75 mg/Kg in #164, 50 mg/Kg in #182, q7dx3) --○--; with bevacizumab (i.p., 5 mg/Kg, q7dx4) …■…, and with lapatinib (p.o., 100 mg/Kg, 5dx4) …○…. The graphs represent the mean ± SE of each group (8 mices per group).
Table 1.
Antitumor activity in PDX#164 and PDX#182
| Drugs | PDX#164 | PDX#182 |
|---|---|---|
| DDP | 30.4 (36) | 50 (56) |
| Paclitaxel | 24 (36) | 39.2 (63) |
| Yondelis | 78.4 (36) | 55.9 (46) |
| Bevacizumab | 42.7 (34) | 26.1 (38) |
| Oxaliplatin | 79.3 (34) | 51.2 (38) |
| 5FU | 52.1 (31) | 44.9 (38) |
| Lapatinib | - | 63.7 (38) |
The best T/C% values (day) are reported for every type of treatment.
Discussion
Preclinical models of mEOC are limited, reflecting the fact that this tumor type is quite rare [3,6]. As for all rare diseases, the lack of preclinical validated model greatly delays the understanding of their pathogenesis and consequently the development of possible new therapeutic strategies.
We here report the molecular and pharmacological characterization of two mucinous PDXs recently obtained in our laboratory: PDX#164 and PDX#182, both obtained from fresh tumor samples of patients diagnosed with mEOC. While PDX#164 derived from a Stage IV tumor, PDX#182 derived from a Stage I tumor; however patient #182 relapsed after 42 months, suggesting that the original tumor has some aggressive features responsible also for its tumorigenicity in immune-compromised mice. Histological and gene expression profile as compared to the original patient tumors highlight a high degree of similarity. CGH analysis suggest that PDX#182 was very similar to the tumor of origin, while higher number of deletions could be observed in PDX#164 as regards corresponding primary tumor. Loss of heterozygous and homozygous deletions in 9pand 9p21.3 have been reported to be early events in mucinous ovarian cancers, occurring in 60% of benign tumors and with higher percentages in borderlines tumors, suggesting that the silencing of p16INK4A, ARF and p15INK4B, protein coded by genes located in 9p21.3, offers a selective growth advantage [27]. The homozygous deletion we observed in PDX#164 as compared to the corresponding patients sample would confirm this hypothesis. Both patient and tumor samples harbor a wild type TP53 [19]. While TP53 has been reported to be usually mutated in high grade serous ovarian carcinoma [28], it is less frequently mutated in mEOC [6,12,29]. The fact that we were able to obtain PDXs from wild type TP53 fresh tumor samples and that these could be successfully maintained through multiple rounds of serial transplantations, suggest that mutation in TP53 is not a tumor driver for this ovarian histotype. RAS mutations, frequently reported in mEOC, characterized our PDXs. The transcriptomic profile of mucinous PDXs resemble the origin patient’ samples [19]. When their gene expression profiles were compared to the ones of 7 high grade serous/endometrial PDXs, a downregulation of the apoptosis signalling via caspase activation, supporting the fact that mucinous carcinomas are much less responsive to chemotherapy [6].
The metabolomics study, performed with the idea to characterize this rare tumor type and compared it with high grade PDXs, suggests a comparable central cellular metabolic asset among these histotypes. We found a divergence in the lipid content (phosphatidylcholines and sphingomyelin species) in PDX#164, that could be due to the induction of specific phospholipase C (PC-PLC) and/or de novo sphingolipid biosynthetic pathways already reported in some tumors [30,31].
We finally tested the antitumor activity of different drugs. PDX#164 was quite sensitive to both platinum and paclitaxel and these data seem to contrast with the fact that patient had a stable disease after neo-adjuvant chemotherapy. These apparently contrasting results could have different reasons: i) the experimental setting is completely different. The patient had a quite diffuse disease (stage IV), while mice have been randomized when tumor masses were little (150 mgr); ii) the clinical efficacy end-point used (stable disease) is different from the in vivo anticancer activity parameter we used. The disease stabilization observed could be similar to the tumor growth inhibition observed with both cisplatin and paclitaxel, as no treated mice underwent tumor regressions. Indeed, in the majority of high grade serous ovarian carcinoma, cisplatin and paclitaxel induced a clear regression in PDXs and response in patients [19]. On the contrary, PDX#182 was completely resistant to these drugs and this better mimics the mEOC therapeutic response to ovarian gold standard therapy. The histological similarity with metastatic mucinous colon rectal cancer has suggested that mEOC could indeed be much more sensitive to drugs currently used for colon cancer (antimetabolite and oxaliplatin) than to the platinum based/paclitaxel chemotherapy [32,33]. We tested in both xenografts, the antitumor activity of oxaliplatin and 5FU and again no activity were observed. These data parallel the preliminary data of Gynecologic Oncology Group trial (GOG) 241, an international phase 3 study, in which metastatic mucinous ovarian cancer patients were randomly assigned to receive paclitaxel and carboplatin (control group) or the combination of capecitabine and oxaliplatin [34]. Due to the slow accrual, the trial was closed; however, results on 50 patients suggest that there were no difference in progression free survival and response rates (very low) between the two arms. The failing of the randomized GOG241trial has underlined the difficulty to carry out randomized clinical trials in mEOC and has led to the suggestion to randomize these patients in trials of non-gynecological mucinous tumors [4].
We here report the molecular, metabolic and pharmacological characterization of two PDXs from ovarian mucinous carcinomas. These PDXs represent the original tumors from which they derived. The molecular and metabolic data suggest that these tumors are quite different from the more common high-grade serous/endometrioid ovarian carcinomas. In addition, the pharmacological profile partially reflects what observed in the clinical setting. We are aware of the fact that the presented data rely on only two PDXs; however, considering the rarity of this tumor types, we think that these models represent mEOC and they will be of value in the preclinical development of possible new therapeutic strategies for this tumor type.
Acknowledgements
This research was funded by the Italian Association for Cancer Research (AIRC), grant number IG 19797 to GD. The generous contributions of AIRC (The Italian Association for Cancer Research) is grateful acknowledged.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23:41–4. doi: 10.1097/01.pgp.0000101080.35393.16. [DOI] [PubMed] [Google Scholar]
- 2.Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry JP, Scolyer RA, Smith AN, Bali A, Vanden Bergh P, Baron-Hay S, Scott C, Fink D, Hacker NF, Sutherland RL, O’Brien PM. A distinct molecular profile associated with mucinous epithelial ovarian cancer. Br J Cancer. 2006;94:904–13. doi: 10.1038/sj.bjc.6603003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricci F, Affatato R, Carrassa L, Damia G. Recent insights into mucinous ovarian carcinoma. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19061569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leary AF, Quinn M, Fujiwara K, Coleman RL, Kohn E, Sugiyama T, Glasspool R, Ray-Coquard I, Colombo N, Bacon M, Zeimet A, Westermann A, Gomez-Garcia E, Provencher D, Welch S, Small W, Millan D, Okamoto A, Stuart G, Ochiai K Participants of the Fifth Ovarian Cancer Consensus Conference. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup (GCIG): clinical trial design for rare ovarian tumours. Ann Oncol. 2017;28:718–726. doi: 10.1093/annonc/mdw662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perren TJ. Mucinous epithelial ovarian carcinoma. Ann Oncol. 2016;27(Suppl 1):i53–i57. doi: 10.1093/annonc/mdw087. [DOI] [PubMed] [Google Scholar]
- 6.Morice P, Gouy S, Leary A. Mucinous ovarian carcinoma. N Engl J Med. 2019;380:1256–1266. doi: 10.1056/NEJMra1813254. [DOI] [PubMed] [Google Scholar]
- 7.Simons M, Bolhuis T, De Haan AF, Bruggink AH, Bulten J, Massuger LF, Nagtegaal ID. A novel algorithm for better distinction of primary mucinous ovarian carcinomas and mucinous carcinomas metastatic to the ovary. Virchows Arch. 2019;474:289–296. doi: 10.1007/s00428-018-2504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobb LP, Gershenson DM. Treatment of rare epithelial ovarian tumors. Hematol Oncol Clin North Am. 2018;32:1011–1024. doi: 10.1016/j.hoc.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Hess V, A’Hern R, Nasiri N, King DM, Blake PR, Barton DP, Shepherd JH, Ind T, Bridges J, Harrington K, Kaye SB, Gore ME. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J. Clin. Oncol. 2004;22:1040–4. doi: 10.1200/JCO.2004.08.078. [DOI] [PubMed] [Google Scholar]
- 10.Winter WE 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, Markman M, Armstrong DK, Muggia F, McGuire WP Gynecologic Oncology Group Study. Prognostic factors for stage III epithelial ovarian cancer: a gynecologic oncology group study. J. Clin. Oncol. 2007;25:3621–7. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 11.Firat Cuylan Z, Karabuk E, Oz M, Turan AT, Meydanli MM, Taskin S, Sari ME, Sahin H, Ulukent SC, Akbayir O, Gungorduk K, Gungor T, Kose MF, Ayhan A. Comparison of stage III mucinous and serous ovarian cancer: a case-control study. J Ovarian Res. 2018;11:91. doi: 10.1186/s13048-018-0464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryland GL, Hunter SM, Doyle MA, Caramia F, Li J, Rowley SM, Christie M, Allan PE, Stephens AN, Bowtell DD Australian Ovarian Cancer Study Group. Campbell IG, Gorringe KL. Mutational landscape of mucinous ovarian carcinoma and its neoplastic precursors. Genome Med. 2015;7:87. doi: 10.1186/s13073-015-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackenzie R, Kommoss S, Winterhoff BJ, Kipp BR, Garcia JJ, Voss J, Halling K, Karnezis A, Senz J, Yang W, Prigge ES, Reuschenbach M, Doeberitz MV, Gilks BC, Huntsman DG, Bakkum-Gamez J, McAlpine JN, Anglesio MS. Targeted deep sequencing of mucinous ovarian tumors reveals multiple overlapping RAS-pathway activating mutations in borderline and cancerous neoplasms. BMC Cancer. 2015;15:415. doi: 10.1186/s12885-015-1421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang KL, Lee MY, Chao WR, Han CP. The status of Her2 amplification and Kras mutations in mucinous ovarian carcinoma. Hum Genomics. 2016;10:40. doi: 10.1186/s40246-016-0096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller JJ, Schlappe BA, Kumar R, Olvera N, Dao F, Abu-Rustum N, Aghajanian C, DeLair D, Hussein YR, Soslow RA, Levine DA, Weigelt B. Massively parallel sequencing analysis of mucinous ovarian carcinomas: genomic profiling and differential diagnoses. Gynecol Oncol. 2018;150:127–135. doi: 10.1016/j.ygyno.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hisamatsu T, McGuire M, Wu SY, Rupaimoole R, Pradeep S, Bayraktar E, Noh K, Hu W, Hansen JM, Lyons Y, Gharpure KM, Nagaraja AS, Mangala LS, Mitamura T, Rodriguez-Aguayo C, Eun YG, Rose J, Bartholomeusz G, Ivan C, Lee JS, Matsuo K, Frumovitz M, Wong KK, Lopez-Berestein G, Sood AK. PRKRA/PACT expression promotes chemoresistance of mucinous ovarian cancer. Mol Cancer Ther. 2019;18:162–172. doi: 10.1158/1535-7163.MCT-17-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takata A, Terauchi M, Hiramitsu S, Uno M, Wakana K, Kubota T. Dkk-3 induces apoptosis through mitochondrial and Fas death receptor pathways in human mucinous ovarian cancer cells. Int J Gynecol Cancer. 2015;25:372–9. doi: 10.1097/IGC.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 18.Kopper O, de Witte CJ, Lõhmussaar K, Valle-Inclan JE, Hami N, Kester L, Balgobind AV, Korving J, Proost N, Begthel H, van Wijk LM, Revilla SA, Theeuwsen R, van de Ven M, van Roosmalen MJ, Ponsioen B, Ho VWH, Neel BG, Bosse T, Gaarenstroom KN, Vrieling H, Vreeswijk MPG, van Diest PJ, Witteveen PO, Jonges T, Bos JL, van Oudenaarden A, Zweemer RP, Snippert HJG, Kloosterman WP, Clevers H. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med. 2019;25:838–849. doi: 10.1038/s41591-019-0422-6. [DOI] [PubMed] [Google Scholar]
- 19.Ricci F, Bizzaro F, Cesca M, Guffanti F, Ganzinelli M, Decio A, Ghilardi C, Perego P, Fruscio R, Buda A, Milani R, Ostano P, Chiorino G, Bani MR, Damia G, Giavazzi R. Patient-derived ovarian tumor xenografts recapitulate human clinicopathology and genetic alterations. Cancer Res. 2014;74:6980–90. doi: 10.1158/0008-5472.CAN-14-0274. [DOI] [PubMed] [Google Scholar]
- 20.Ricci F, Fratelli M, Guffanti F, Porcu L, Spriano F, Dell’Anna T, Fruscio R, Damia G. Patient-derived ovarian cancer xenografts re-growing after a cisplatinum treatment are less responsive to a second drug re-challenge: a new experimental setting to study response to therapy. Oncotarget. 2017;8:7441–7451. doi: 10.18632/oncotarget.7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA, Glennie MJ, Kelland LR, Robinson V, Stratford IJ, Tozer GM, Watson S, Wedge SR, Eccles SA Committee of the National Cancer Research Institute. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102:1555–77. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricci F, Bernasconi S, Perego P, Ganzinelli M, Russo G, Bono F, Mangioni C, Fruscio R, Signorelli M, Broggini M, Damia G. Ovarian carcinoma tumor-initiating cells have a mesenchymal phenotype. Cell Cycle. 2012;11:1966–76. doi: 10.4161/cc.20308. [DOI] [PubMed] [Google Scholar]
- 23.Massazza G, Tomasoni A, Lucchini V, Allavena P, Erba E, Colombo N, Mantovani A, D’Incalci M, Mangioni C, Giavazzi R. Intraperitoneal and subcutaneous xenografts of human ovarian carcinoma in nude mice and their potential in experimental therapy. Int J Cancer. 1989;44:494–500. doi: 10.1002/ijc.2910440320. [DOI] [PubMed] [Google Scholar]
- 24.Rinaldi A, Kwee I, Young KH, Zucca E, Gaidano G, Forconi F, Bertoni F. Genome-wide high resolution DNA profiling of hairy cell leukaemia. Br J Haematol. 2013;162:566–9. doi: 10.1111/bjh.12393. [DOI] [PubMed] [Google Scholar]
- 25.Brunelli L, Caiola E, Marabese M, Broggini M, Pastorelli R. Comparative metabolomics profiling of isogenic KRAS wild type and mutant NSCLC cells in vitro and in vivo. Sci Rep. 2016;6:28398. doi: 10.1038/srep28398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang WJ, Li Y, Wei MN, Chen Y, Qiu JG, Jiang QW, Yang Y, Zheng DW, Qin WM, Huang JR, Wang K, Zhang WJ, Wang YJ, Yang DH, Chen ZS, Shi Z. Synergistic antitumor activity of regorafenib and lapatinib in preclinical models of human colorectal cancer. Cancer Lett. 2017;386:100–109. doi: 10.1016/j.canlet.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Hunter SM, Gorringe KL, Christie M, Rowley SM, Bowtell DD Australian Ovarian Cancer Study Group. Campbell IG. Pre-invasive ovarian mucinous tumors are characterized by CDKN2A and RAS pathway aberrations. Clin Cancer Res. 2012;18:5267–77. doi: 10.1158/1078-0432.CCR-12-1103. [DOI] [PubMed] [Google Scholar]
- 28.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rechsteiner M, Zimmermann AK, Wild PJ, Caduff R, von Teichman A, Fink D, Moch H, Noske A. TP53 mutations are common in all subtypes of epithelial ovarian cancer and occur concomitantly with KRAS mutations in the mucinous type. Exp Mol Pathol. 2013;95:235–41. doi: 10.1016/j.yexmp.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Iorio E, Caramujo MJ, Cecchetti S, Spadaro F, Carpinelli G, Canese R, Podo F. Key Players in choline metabolic reprograming in triple-negative breast cancer. Front Oncol. 2016;6:205. doi: 10.3389/fonc.2016.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer. 2018;18:33–50. doi: 10.1038/nrc.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato S, Itamochi H, Kigawa J, Oishi T, Shimada M, Sato S, Naniwa J, Uegaki K, Nonaka M, Terakawa N. Combination chemotherapy of oxaliplatin and 5-fluorouracil may be an effective regimen for mucinous adenocarcinoma of the ovary: a potential treatment strategy. Cancer Sci. 2009;100:546–51. doi: 10.1111/j.1349-7006.2008.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu W, Rush J, Rickett K, Coward JI. Mucinous ovarian cancer: a therapeutic review. Crit Rev Oncol Hematol. 2016;102:26–36. doi: 10.1016/j.critrevonc.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Gore ME, Hackshaw A, Brady WE, Penson RT, Zaino RJ, McCluggage WG, et al. Multicentre trial of carboplatin/paclitaxel versus oxaliplatin/capecitabine, each with/without bevacizumab, as first line chemotherapy for patients with mucinous epithelial ovarian cancer (mEOC) J. Clin. Oncol. 2015;33(Suppl):5528–5528. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


