Abstract
Cardiac toxicity is one of the major advese effect associated with thoracic irradiation. Breast cancer patients with human epidermal factor receptor-2 (Her-2) overexpression could be indicated for both radiation and anti-Her2 target therapy. We aimed to investigate the early detection of radiation and Trastuzumab (TRZ) induced acute cardiotoxicity in mice. In the present study, the heart of animal was subjected to irradiation (IR, 14 Gy/1 Fx), TRZ was intraperitonealy (i.p.) administrated to mice in 2 weeks (6 fractions). The IR plus TRZ group received heart IR after TRZ. We found that body weight of mouse in treatment groups reduced significantly as compared with that of mouse in control group (P<0.05). At day 21, the diastolic function of mice decreased significantly in IR plus TRZ group compared with control group measured by E/E’ parameter using echocardiography (57.72 vs 40.82, P<0.05). The left ventricular posterior wall (LVPW) and interventricular septum (IVS) were also increased significantly in diastolic phase at day 21 in the combined group compared with TRZ alone (LVPW: 0.95 mm vs 0.70 mm, P<0.05; IVS: 0.94 mm vs 0.65 mm P<0.05). Moreover, hematoxylin and eosin (HE) staining of cardiac tissue showed that the arrangement of myocardial cell was disordered in the combined group with vacuolar and adipocyte changes, as well as the loose of structure of myocardial cells and the pyknosis of the nucleus. Moderate damage was observed in irradiation-treated group and TRZ-treated group. The expressions of γ-H2AX, vascular cell adhesion molecule-1 (VCAM-1) and von Willebrand Factor (vWF) were remarkedly appeared in co-treatment group. Heart irradiation combined with TRZ treatment simultaneously might cause acute cardiac toxicity in terms of the parameter of E/E’, LVPW and IVS. Our results suggest that the diastolic function could detect the early stage of acute cardiotoxicity in heart exposed to irradiation and TRZ co-treatment in mice. The DNA injury and microangiopathy might involve in cardiac injury that aggravated by radiation and Trastuzumab treatments.
Keywords: Trastuzumab, radiation, cardiotoxcity, echocardiography
Introduction
Radiotherapy is an important part of comprehensive treatment of thoracic malignant tumors [1]. Cardiovascular complications remain one of major treatment-related morbidity, and offset some of clinical benefits to certain extent. Darby’s report suggests that there exists no threshold for radiation-induced cardiotoxicity. For every 1 Gy increase in the average cardiac dose, the risk of injury increases by 7.4% [2]. Whereas, overexpression of Her-2 was found in 20%-25% of breast cancer patients, the prognosis of Her-2 positive breast cancer patients was significantly worse than that of Her-2 negative patients [3]. TRZ can specifically bind to Her-2 receptor, blocking the over-activation of Her-2 pathway, thus fundamentally alter the prognosis of this molecular subtype patients [4]. The activation of Her-2 signaling pathway plays an important role in the development and normal function of myocardium, which explains its cardiac injury [5]. Moreover, a significant proportion of these patients require radiotherapy and anti-Her-2 target therapy simultaneously.
Cardiac injury induced by antineoplastic therapy can be classified into type I injury and type II injury [6]. The damage caused by ionizing radiation and anthracycline drugs is type I injury, which is characterized by positive dose-effect correlation and irreversibility. The cardiac injury induced by TRZ is type II injury, which is dose-independent and reversible. Current studies suggest that the combination of anthracycline and TRZ has a synergistic effect on cardiac injury [7]. Therefore, it is recommended to avoid the simultaneous use of these two drugs in clinic. Nevertheless, clinical guidelines do not explicitly limit radiotherapy combined with TRZ, even though whether concurrent application of radiotherapy and TRZ would superimpose cardiovascular injury remains largely unknown. Our previous study found that 37.9% of patients with left breast cancer who received TRZ and radiotherapy exposed to a risk of early diastolic dysfunction, as compared with 19% in radiotherapy alone [8]. As clinical observed toxicity is documented with cardiac dysfunction as whole organ, in vivo study to reveal the functional change of heart, its correlation with serum biomarker and histopathology change will be important to study the mechanism of effects of TRZ for cardiac injury with ionizing radiation. Therefore, the aim of this study is to establish an effective model of cardiac injury in mice receiving irradiation concurred with TRZ administration. We further examine the characteristics and molecular changes of cardiac function in animal model.
Materials and methods
Animal model and irradiation
All animal experiments and procedures were performed with the approval of the Shanghai Jiaotong University School of Medicine Institutional Animal Care and Use Committee. Cardiac injury was conducted on male C57/BL6 mice (20-25 g, 6-8 weeks old) as described. Twenty mice were randomly assigned for four study groups: Control, IR, TRZ and IR+TRZ, respectively. The heart of animal was subjected to irradiation with 14 Gy/1 Fx, 6 MV X ray, and the exposed field was localized at 1×1 cm2, dose rate was 300 cGy/min, and source skin distance (SSD) was 100 cm. TRZ was administrated intraperitonealy (i.p.) to mice in two weeks (6 fractions) with a total dose at 10 mg/kg. The irradiation and TRZ group received heart irradiation next day with TRZ i.p. injection. The animal echocardiography was performed on day 21, and serum and heart tissue were collected accordingly.
Mouse echocardiography
Using animal visual ultrasound imaging system with mouse probe (Sonic Vevo2100 and MS-400 probe), the detection rate was set at 30 MHz. 2.2% isoflurane gas was used to breathe anesthetized mice. The mice were fixed on a thermostat in supine position and on electrodes coated with conductive agents in limbs. Superficial anesthesia was maintained with 1% isoflurane and oxygen. M-motion curves of left ventricular wall were collected, and at least 3 continuous and stable cardiac cycle images were collected and saved. Left ventricular M-mode motion curves were used to measure the following parameters: left ventricular posterior wall thickness (LVPW), interventricular septal thickness (IVST), ejection fraction (EF), short axis systolic rate (SF). All data were averaged for three cardiac cycles. The position of mitral valve orifice was judged by B-mode ultrasound, the flow of mitral valve orifice was observed by color Doppler ultrasound module, and the flow spectrum of mitral valve orifice was recorded by pulse spectrum Doppler module. The peak value of early diastolic blood flow (E peak) and late diastolic blood flow (A peak) were recorded. In Tissue Doppler Module, the myocardial motion spectrogram of the mitral annulus of the interventricular septum was collected, and the early diastolic velocity (E’) and late diastolic velocity (A’) of the mitral annulus were measured.
Hematological bioassay
After anesthesia, blood was collected and kept at room temperature for 2 hours. The samples were centrifuged at 4 degrees and 3000 rpm and superficial serum was carefully extracted. The level of cTn-I in serum was analyzed by using the cTn-I kit of (Life Diagnostics, CTNI-1-US). The procedure was carried out according to the operation manual.
Histological analysis
After the mice were sacrificed and perfused with PBS systemic circulation, the heart was quickly removed and fixed in 4% polyformaldehyde. The short-axis paraffin sections with 4 μm thickness were prepared by routine method for subsequent analysis. The sections were stained by hematoxylin-eosin to analyze changes in general cardiac structure. DNA damage in heart tissue was labled by a γ-H2AX (CY6572, Abways, 1:100) antibody. The changes of microvessels in heart slices were detected by anti-VCAM1 (CY5427, Abways, 1:100) and anti-VwF antibody (11778-1-AP, Proteintech, 1:100). At the same time, DAPI staining was used for counter stain of nuclear localization. All experiments are performed by standard laboratory protocol.
Statistical analysis
The data are represented as the means ± SEM. Results were analyzed by one-way ANOVA and P<0.05 was defined as significant difference.
Results
Irradiation and TRZ co-treatment on physical condition of mice
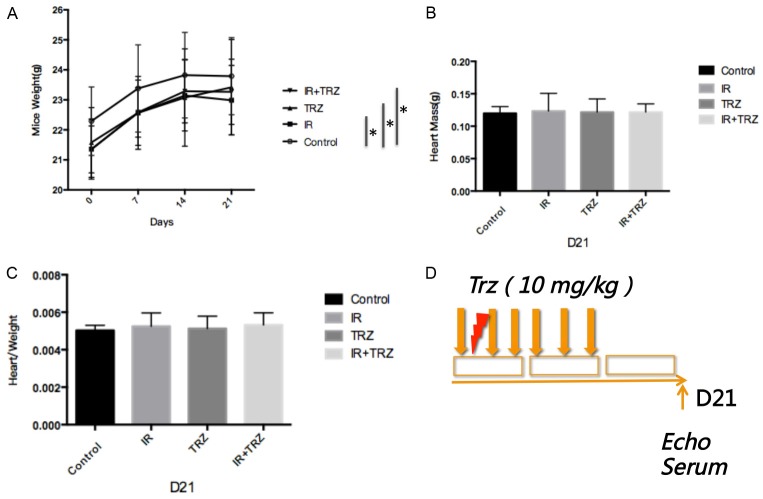
Following the treatment procedure as shown in Figure 1D, mice exposed to radiation or drug intervention showed significant weight loss compared to the control group, but no weight difference was found between the treated groups (Figure 1A). However, no significant difference was found in the heart mass of mice (Figure 1B) or in heart weight to body weight ratio (Figure 1C).
Figure 1.
Irradiation and TRZ co-treatment on physical condition of mice and the treatment procedure. A. Mice exposed to radiation or TRZ showed significant weight loss compared to the control group, but no weight difference was found between the treated groups; B and C. No significant difference was found in the heart mass of mice, or in heart weight to body weight ratio; D. The treatment procedure;In the IR+TRZ group, after the heart radiation, TRZ was intraperitonealy (i.p.) administrated to mice for two weeks (6 fractions) with a total dose at 10 mg/kg.
Effects of TRZ treatment with heart irradiation on cardiac function
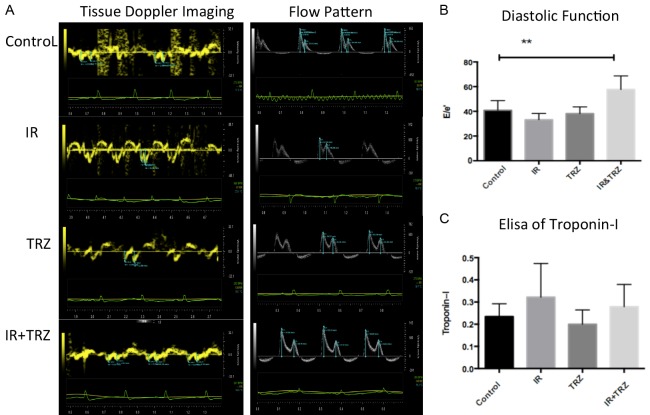
On day 21, a significant increase in E/E’ was observed only in co-treated group, suggesting that the diastolic function of the heart was impaired (Figure 2A and 2B). However, this effect was not observed in IR group or TRZ group. Furthermore, we examined the level of cTn-I (a marker of myocardial injury) release in plasma by ELISA. Unfortunately, no significant changes were found in these groups (Figure 2C).
Figure 2.
Irradiation and TRZ co-treatment induce diastolic dysfunction without CTN-I change. A. The early diastolic velocity (E’) and late diastolic velocity (A’) of the mitral annulus were measured in the tissue Doppler imaging; B. The peak value of early diastolic blood flow (E peak) and late diastolic blood flow (A peak) were recorded by flow pattern. On day 21, a significant increase in E/E’ was observed only in the combined group (57.72 vs 40.82, P<0.05; IR plus TRZ vs Control); C. The level of cTn-I shows no significant changes in these groups.
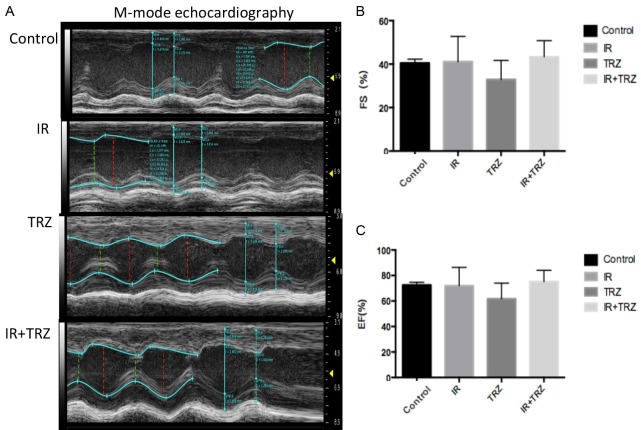
However, the systolic function parameter EF and FS showed no functional damage in the treated groups. Left ventricular ejection fraction and short axis systolic rate are commonly used indicators for evaluating cardiac systolic function. The results indicated that no abnormalities of EF (Figure 3C) or FS (Figure 3B) were found in these mice model of cardiac injury (IR, TRZ or co-treated groups).
Figure 3.
The systolic function parameter EF and FS showed no functional damage in the treated groups. A. The Left ventricular systolic function was tested by M-mode Echocardiography; B and C. No abnormalities of EF or FS were found in these mice model of cardiac injury (IR, TRZ or combined groups).
Structural changes in echocardiography and HE staining
In M-mode ultrasonography, the thickness of left ventricular posterior wall (LVPW) and interventricular septum (IVS) displayed significant abnormalities in the diastolic phase observed in the IR and TRZ co-treated group as comparied with the TRZ treated alone (Table 1).
Table 1.
The LVPW and IVS displayed significant abnormalities in the diastolic phase of IR+TRZ group comparing with the TRZ group
| Control | IR | TRZ | IR+TRZ | P | |
|---|---|---|---|---|---|
| Heart rate | 423.09±40.56 | 438.07±51.38 | 405.61±62.72 | 387.27±42.60 | |
| LVPW; S | 1.22±0.18 | 1.19±0.16 | 0.99±0.16 | 1.21±0.09 | |
| LVPW; D | 0.84±0.10 | 0.79±0.11 | 0.70±0.09* | 0.95±0.12* | <0.05 |
| IVS; S | 1.15±0.11 | 1.18±0.24 | 1.06±0.18 | 1.26±0.14 | |
| IVS; D | 0.73±0.05 | 0.78±0.12 | 0.65±0.06* | 0.94±0.10* | <0.05 |
| LVE; S | 1.84±0.20 | 1.84±0.48 | 2.29±0.36 | 1.83±0.36 | |
| LVE; D | 3.10±0.30 | 3.08±0.27 | 3.39±0.17 | 3.20±0.26 | |
| LVID; S | 2.03±0.14 | 1.87±0.45 | 2.40±0.36 | 1.88±0.33 | |
| LVID; D | 3.18±0.10 | 3.04±0.23 | 3.45±0.19 | 2.83±0.43 |
LVPW, left ventricular posterior wall; IVS, interventricular septum; LVE, left ventricular internal diameter at end phase; LVID, left ventricular internal diameter.
P<0.05 was considered as statistically significant.
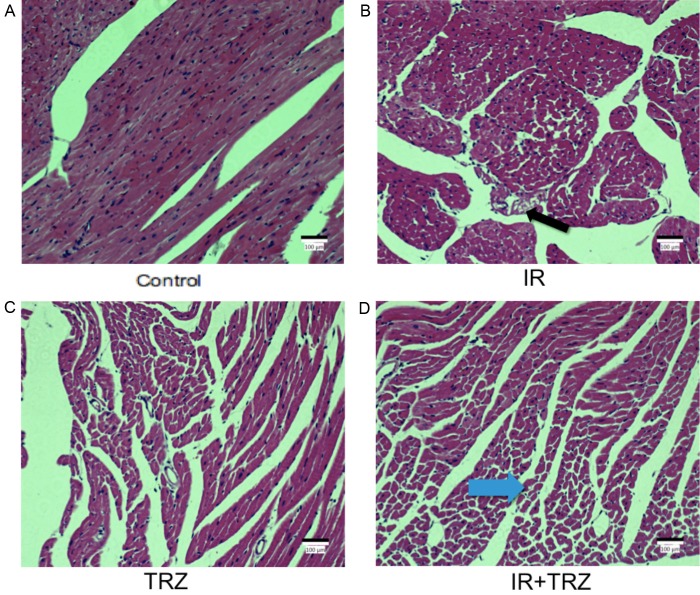
In HE staining of heart tissue (Figure 4), the tissue slices presented that the arrangement of myocardial cell was disordered in the combined group (Figure 4D) with vacuolar and adipocyte changes (blue arrow), as well as the loose of structure of myocardial cell in some areas and slight pyknosis in the nucleus. Moderate damage was observed in IR group and TRZ group. The black arrow showed the vacuolar degeneration in IR group (Figure 4B).
Figure 4.
Histopathology changes in the heart stained with H&E from IR or TRZ treatment after 21 days. A. The normal heart slice was observed in the control group. C. The TRZ group showed relatively normal myocardial structure. B. The black arrow showed the vacuolar degeneration in IR group. D. The blue arrow showed the vacuolar and adipocyte changes in the combined group. The arrangement of myocardial cell was disordered in the combined group, as well as the loose of structure of myocardial cells and the pyknosis of the nucleus.
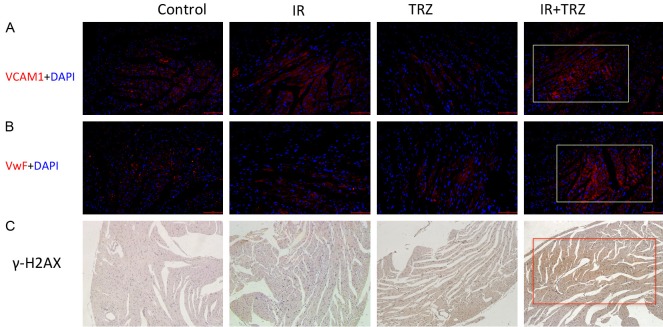
Radiation combined with TRZ caused myocardial damage and microangiopathy
The expression of VCAM-1 (Figure 5A), vWF (Figure 5B) and γ-H2AX (Figure 5C) were expressed markedly in the combine treatment group. Comparing the IR with TRZ group, the IR group showed more expression of VCAM-1 and vWF, which indicated that IR (Type I injury) might cause worse microangiopathy to TRZ (Type II injury). But no difference on DNA damage appeared between IR and TRZ group.
Figure 5.
The microvascular lesions and DNA damage in the heart tissue. (A) VCAM-1 (Red) and (B) vWF (Red) expression on the cardiac slice was detected by immunofluorescence staining. Cell nuclei were labeled by DAPI (blue). (C) The immunohistochemistry staining determines the γ-H2AX expression in the cardiac slice.
Discussion
Radiotherapy is an important part of local-regional treatment for early breast cancer after breast conserving surgery or high-risk breast cancer after mastectomy [1]. Radiation-induced heart disease (RIHD) is one of the important causes of long-term non-cancer death subsequent to thoracic irradiation. Significant cardiovascular events usually presented in 10-15 years after irradiation [9]. TRZ is the first-line treatment for Her-2 positive invasive breast cancer and provides benefit for long-term survival from several clinical studies [4]. TRZ-related cardiac injury is one of the major toxicity resulting in discontinuation of the treatment and impairment of its therapeutic outcome [5]. As the therapeutic effect of TRZ has not been fully elucidated [10], the mechanism of TRZ induced cardiac injury remains unclarified. It is believed that blocking HER-2 pathway in myocardium will affect normal cell metabolism [11] and oxidative stress repair [12], thus damaging normal function and survival of myocardium.
Current guidelines do not recommend concurrent anthracycline with TRZ, however, no restriction on combined anti-HER2 therapy with radiotherapy for breast cancer patients in the adjuvant setting as well as in the metastatic setting. In Santis’ study, TRZ was an important risk factor for late cardiotoxic events (OR=4.2, P=0.01) in patients receiving TRZ and hypofractionated whole breast radiotherapy [13]. Previous found from our team showed that, in general, patients with normal baseline cardiac function tolerate well radiotherapy and trastuzumab concurrent treatment, including left-sided patients. The exists a correlation of dose-volume of cardiac dose with the increased risk of acute left ventricular LVEF dysfunction, and diastolic dysfunction had a much higher prevalence of abnormality with cardiac irradiated dose-volume [14]. Additionally, concurrent TRZ was associated with higher prevalence of diastolic dysfunction after left-sided irradiation [8]. However, apart from these observed phenomena of additive toxicity of trastuzumab and irradiation, little was reported as to the mechanism of these combinations, especially in vivo study.
Cardiac ultrasonography, blood troponin and pro-BNP detection are currently the most widely application for monitoring cardiac safety after chemo- and/or radiotherapy [15]. Our current animal results confirmed that left ventricular diastolic function is more sensitive than systolic function in monitoring early cardiotoxicity, the results of animal experiments are consistent with our previous clinical studies.
We first demonstrate in vivo that the functional alteration of TRZ combined with local cardiac radiation in mice, which confirms that abnormal diastolic function companied with normal systolic function may be an early manifestation of radiation-induced cardiac injury associated with TRZ. Diastolic function is necessary to be incorporated into the cardiac toxicity monitor. We are currently conducting a prospective phase 3 clinical trial (NCT02942615) evaluating cardiac toxicity throughout adjuvant systemic therapy and radiotherapy using the aforementioned examination.
Cell death subsequent to X-ray irradiation is mainly caused by single strand of DNA damage, and TRZ interfered with the self-repair of myocardium by combining to HER-2, which leads to accumulation of reactive oxygen species and cell apoptosis of myocardial cells [16]. The overexpression of γ-H2AX in radiotherapy combined with TRZ group, suggesting that DNA damage may be a common event involving in both types of radiation-induced damage, and concurrent application of TRZ and IR in clinic does have the potential to aggravate the detrimental effect of IR on myocardium. The long-term clinical outcome of cardiac injury induced by TRZ during radiotherapy is yet to be further revealed. The late histological changes are characterized by microvascular embolism and myocardial fibrosis [17].
Radiation heart injury is a complex process. The characteristic changes of microvascular lesions are also the earlier pathological changes. Irradiation could induce microvascular disease, which triggered subsequent inflammatory reactions and myocardial ischemic necrosis. The endothelium injury in cardiovascular diseases and vasculitis is associated with an enhanced von Willebrand factor vWF and VCAM-1 [18,19]. Therefore, we detected the expression of VCAM-1 and vWF by immunofluorescence to observe the microvascular lesions in the mice heart. We have observed the microvascular changes (labeled by the overexpression of VCAM-1 and vWF) in heart tissues of TRZ and IR co-treatment, suggested that microangiopathy might trigger the RIHD.
It remains unidentified whether the acute abnormalities of cardiac function in mice model is a transient or self-limiting phase, early detection of cardiac function is essential for the safety management of patients after antineoplastic therapy. The molecular mechanism and biomarker of cardiac injury needs to be further explored.
In summary, heart irradiation combined with TRZ treatment simultaneously cause acute cardiac toxicity in terms of the parameter of E/E’, LVPW and IVS of echocardiography. Our results suggest that the diastolic function is able to detect the early stage of acute cardiotoxicity in heart exposure to irradiation and TRZ treatments of mice, as compared with the systolic function. The acute cardiac injury by radiation and TRZ co-treatment may be mediated by the expressions of γ-H2AX, VCAM-1 and vWF in cell damage and microangiopathy.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (grants 81673102, 81602791, 81803164 and 81972963) and National Key Research and Development Program of China (grant 2016YFC0105409); Scientific and Technological Innovation Action Plan of Shanghai Science and Technology Committee (grants 19411950900 and 19411950901); Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (grant 20171904).
Disclosure of conflict of interest
None.
References
- 1.EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M, Gray R, Mannu G, Peto R, Whelan T, Wang Y, Wang Z, Darby S. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 3.Fabi A, Malaguti P, Vari S, Cognetti F. First-line therapy in HER2 positive metastatic breast cancer: is the mosaic fully completed or are we missing additional pieces? J Exp Clin Cancer Res. 2016;35:104. doi: 10.1186/s13046-016-0380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genuino AJ, Chaikledkaew U, The DO, Reungwetwattana T, Thakkinstian A. Adjuvant trastuzumab regimen for HER2-positive early-stage breast cancer: a systematic review and meta-analysis. Expert Rev Clin Pharmacol. 2019;12:815–824. doi: 10.1080/17512433.2019.1637252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J. 2013;34:1102–1111. doi: 10.1093/eurheartj/ehs181. [DOI] [PubMed] [Google Scholar]
- 6.Chargari C, Kirov KM, Bollet MA, Magne N, Vedrine L, Cremades S, Beuzeboc P, Fourquet A, Kirova YM. Cardiac toxicity in breast cancer patients: from a fractional point of view to a global assessment. Cancer Treat Rev. 2011;37:321–330. doi: 10.1016/j.ctrv.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Di Cosimo S. Heart to heart with trastuzumab: a review on cardiac toxicity. Target Oncol. 2011;6:189–195. doi: 10.1007/s11523-011-0203-8. [DOI] [PubMed] [Google Scholar]
- 8.Cao L, Cai G, Chang C, Miao AY, Yu XL, Yang ZZ, Ma JL, Zhang Q, Wu J, Guo XM, Chen JY. Diastolic dysfunction occurs early in HER2-positive breast cancer patients treated concurrently with radiation therapy and trastuzumab. Oncologist. 2015;20:605–614. doi: 10.1634/theoncologist.2014-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams MJ, Lipshultz SE, Schwartz C, Fajardo LF, Coen V, Constine LS. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol. 2003;13:346–356. doi: 10.1016/S1053-4296(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 10.Goldhar HA, Yan AT, Ko DT, Earle CC, Tomlinson GA, Trudeau ME, Krahn MD, Krzyzanowska MK, Pal RS, Brezden-Masley C, Gavura S, Lien K, Chan KK. The temporal risk of heart failure associated with adjuvant trastuzumab in breast cancer patients: a population study. J Natl Cancer Inst. 2015;108 doi: 10.1093/jnci/djv301. [DOI] [PubMed] [Google Scholar]
- 11.Kitani T, Ong SG, Lam CK, Rhee JW, Zhang JZ, Oikonomopoulos A, Ma N, Tian L, Lee J, Telli ML, Witteles RM, Sharma A, Sayed N, Wu JC. Human-induced pluripotent stem cell model of trastuzumab-induced cardiac dysfunction in patients with breast cancer. Circulation. 2019;139:2451–2465. doi: 10.1161/CIRCULATIONAHA.118.037357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cote GM, Sawyer DB, Chabner BA. ERBB2 inhibition and heart failure. N Engl J Med. 2012;367:2150–2153. doi: 10.1056/NEJMcibr1203156. [DOI] [PubMed] [Google Scholar]
- 13.De Santis MC, Bonfantini F, Di Salvo F, Fiorentino A, Riboldi VM, Di Cosimo S, Bianchi GV, Gennaro M, Cosentino V, Sant M, Pignoli E, Valdagni R, Lozza L. Trastuzumab and hypofractionated whole breast radiotherapy: a victorious combination? Clin Breast Cancer. 2018;18:e363–e371. doi: 10.1016/j.clbc.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Cao L, Cai G, Chang C, Yang ZZ, Feng Y, Yu XL, Ma JL, Wu J, Guo XM, Chen JY. Early cardiac toxicity following adjuvant radiotherapy of left-sided breast cancer with or without concurrent trastuzumab. Oncotarget. 2016;7:1042–1054. doi: 10.18632/oncotarget.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H, Chung WB, Cho KI, Kim BJ, Seo JS, Park SM, Kim HJ, Lee JH, Kim EK, Youn HJ. Diagnosis, treatment, and prevention of cardiovascular toxicity related to anti-cancer treatment in clinical practice: an opinion paper from the working group on cardio-oncology of the Korean society of echocardiography. J Cardiovasc Ultrasound. 2018;26:1–25. doi: 10.4250/jcu.2018.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon LI, Burke MA, Singh AT, Prachand S, Lieberman ED, Sun L, Naik TJ, Prasad SV, Ardehali H. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J Biol Chem. 2009;284:2080–2087. doi: 10.1074/jbc.M804570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boerma M, Hauer-Jensen M. Potential targets for intervention in radiation-induced heart disease. Curr Drug Targets. 2010;11:1405–1412. doi: 10.2174/1389450111009011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernardo A, Ball C, Nolasco L, Moake JF, Dong JF. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104:100–106. doi: 10.1182/blood-2004-01-0107. [DOI] [PubMed] [Google Scholar]
- 19.Lino DOC, Freitas IA, Meneses GC, Martins AMC, Daher EF, Rocha JHC, Silva Junior GB. Interleukin-6 and adhesion molecules VCAM-1 and ICAM-1 as biomarkers of post-acute myocardial infarction heart failure. Braz J Med Biol Res. 2019;52:e8658. doi: 10.1590/1414-431X20198658. [DOI] [PMC free article] [PubMed] [Google Scholar]