Abstract
Anorectal melanoma (ARM) is rare and lethal. We report a case of a 48-year-old woman with 9 months of rectal swelling and bleeding. Physical examination revealed a mass about 5 × 6 cm on the anterior wall of the rectum, 3 cm from the anal verge, and the patient underwent abdominoperineal resection (APR). After hematoxylin-eosin staining and immunohistochemical staining, it was considered an ARM, which is an aggressive disease with a poor survival. Immunohistochemical staining showed the tumor to be positive for S-100, Melan A, Ki67 proliferative index of 70%, and negative for HMB45. The melanoma had infiltrated the adventitia and metastasized to the (intestinal) 16/16 lymph nodes with cancerous nodule formation. There were multiple organs with metastasis (liver, spleen, pancreas, lung and subcutaneous soft tissue) three months after operation. Overall, pre-operative biopsy may be insufficient to make a definite diagnosis, and immunohistochemistry is necessary. Therefore, the gold standard treatment for ARM is oncological radical surgical resection.
Keywords: Anorectal melanoma, anal pain, bleeding, surgical resection, metastasis, poor survival
Introduction
ARM is a rare malignancy. In recent years, the incidence rate of ARM has been increasing. Malignant melanoma i originates from the neural crest melanocytes. 90% of malignant melanoma is primary to the skin. It is also found in the mucous membranes of the skin, choroid, pia mater and eyes. Malignant melanoma is rare as a primary in the digestive tract, accounting for only 1% to 3% of digestive tract malignant tumors [1]. It was considered to be metastatic previously, but recent studies confirmed that melanocytes also exist in the gastrointestinal tract, which means the gastrointestinal tract can give rise to malignant melanoma. The reports of melanoma occurring in the esophagus, stomach, small intestine, anus and rectum are most common, while relatively rare in the colon [2], and ileocecal primary melanomas accounted for 33% [3]. Surgical resection is the preferred treatment for ARM, but the surgical approach remains controversial. At present, there is no definite guidance on the surgical approach to ARM.
Case report
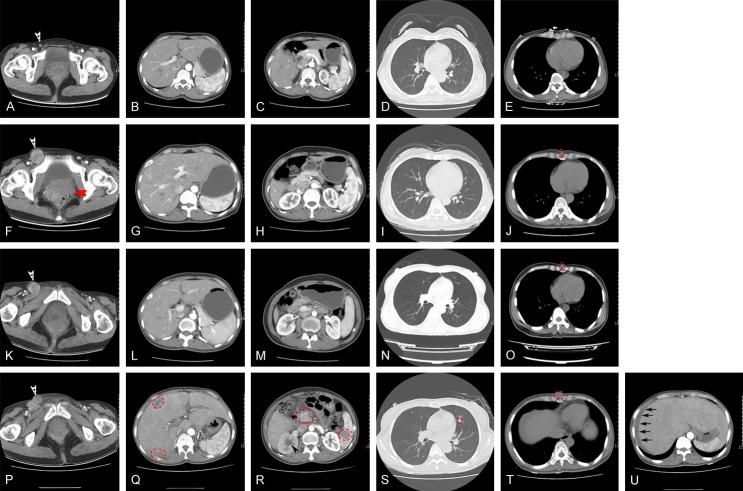
A 48-year-old female patient was admitted to Lishui People’s Hospital at 2015.08.18 for anal pain and bleeding of one month’s duration. Physical examination revealed a firm mass about 2 × 3 cm on the anterior wall of the rectum, 3 cm from the anal margin. Diagnosis was anal canal cancer. Colonoscopy showed anal canal, with undefined rectal mucosal lesions. Biopsy disclosed a spindle cell tumor, possibly malignant neurogenic tumor. The abdominal CT showed that the right inguinal area had multiple enlargement lymph nodes considered metastases (Figure 1A). No obvious abnormality was found in CT scan of liver, stomach, pancreas, spleen, lung, kidney and mediastinum (Figure 1B-E). To comfirm the diagnosis, the patient went to Shanghai Zhong Shan Hospital and underwent examination, but did not receive any special therapies. The patient came to the anorectal surgery of Lishui People’s Hospital for surgery on 2016.04.22. Physical examination found the mass had grown to 5 × 6 cm. In order to observe the progress of the disease, the patient underwent a total abdominal enhanced CT examination and CT scan of the lungs again. Compared with the previous abdominal CT, the edges of tumor became coarse and the patient had pelvic cavity metastases to lymph nodes. The most obvious change was that the right inguinal area metastases lymph nodes became larger (Figure 1F). Similarly, there were no obvious abnormality found in CT scans of liver, pancreas, spleen, kidney and lung (Figure 1G-I), but a small nodule appeared in front of the xiphoid process at the mediastinal window (Figure 1J). It was not considered metastatic at that time. Abdominoperineal resection (APR) palliative resection surgery was peformed to remove the malignant tumor. As shown in Figure 2, pathologic examination of the postoperative specimens disclosed a tumor composed of epithelial and spindle shaped cells, with necrosis and no obvious pigment. Immunohistochemical staining showed S-100 +, HMB45 -, Melan A +, and Ki67 positive rate 70%. Combined with the clinical symptoms, a diagnosis of malignant melanoma was made. The malignant melanoma had infiltrated the adventitia and had metastasis to the (intestinal) (16/16) lymph nodes with cancerous nodule formation; even the intestinal lymph nodes had vascular cancer emboli. The patient had oral administration of Apatinib after leaving the hospital. In 2016.05.23, the patient returned to the hospital for re-examination. The abdomen CT indicated that the patient has no complications after operation, such as intestinal leakage or tumor recurrence (Figure 1K-O).
Figure 1.
A: Right inguinal area with multiple lymph nodes (white arrow). B-E: No obvious abnormality was found in a CT scan of liver, stomach, pancreas, spleen, lung, kidney and mediastinum. F: Right inguinal area had multiple enlarged lymph nodes (white arrow), and primary tumor (red arrow). G-I: No obvious abnormality was found in CT scan of liver, stomach, pancreas, spleen, kidney and lung. J: A small nodule appears in front of the xiphoid process (red circle). K: Right inguinal area with multiple enlargement of lymph nodes (white arrow). L-N: No obvious abnormality was found in CT scan of liver, stomach, pancreas, spleen, kidney and lung. O: A small nodule appears in front of the xiphoid process (red circle). P: Right inguinal area multiple enlargement lymph nodes (white arrow). Q: Multiple liver metastases (red circle). R: Pancreas and spleen metastases (red circle). Hydronephrosis of right kidney (black arrow). S: Multiple lung metastases (red circle). T: Soft tissue metastasis in front of the xiphoid process (red circle). U: Ascites (black arrows).
Figure 2.
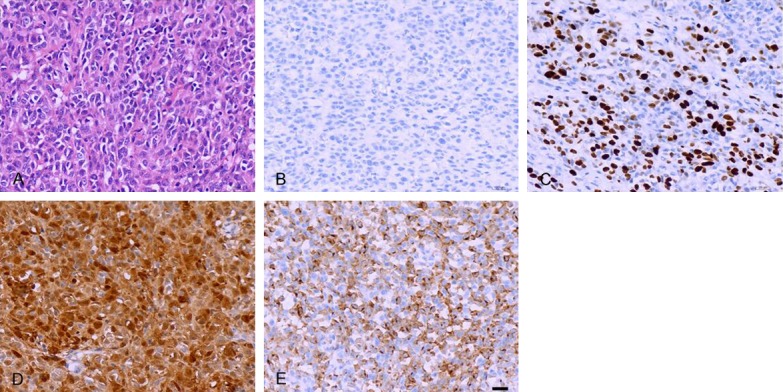
A: Epithelial and spindle-shaped cells were found by H&E stain (×400). B: HMB-45 expression was negative in the resected specimen. C: Ki67 expression positivity rate was 70% in the resected specimen. D: Melan-A expression was positive in the resected specimen. E: S-100 protein expression was positive in the resected specimen.
On August 4, 2016, the patient came back to the hospital due to production of gas and defecation, accompanied by bloating nausea. Physical examination revealed slight abdominal bulging, and both sides of the groin had multiple positive lymph nodes, clinically diagnosed as “incomplete intestinal obstruction”. The full abdominal CT scans showed that liver, stomach, spleen and pancreas had lesions considered metastatic tumor, plus hepatic hilar, pelvic, and bilateral inguinal lymph node metastasis (Figure 1P-R). Hydronephrosis of right kidney may have been caused by the pelvic mass which invaded the right ureter (Figure 1P). The patient had ascites (Figure 1U). The chest CT scans showed that the patient had lung multiple nodules considered metastatic tumor. The nodule in front of xiphoid process became larger and was considered metastatic tumor. On 2016.8.8, the patient received “abdominal paracentesis and extraction of ascites”, 1000 ml ascites were extracted. Human serum albumin was injected to maintain colloid osmotic pressure. Finally, as the patient was in the late stage of the tumor, the family asked for discharge and went back to the local hospital for further treatment.
Discussion
The incidence rate of anorectal malignant melanoma (ARM) is low. The proportion of melanoma is less than 1% in the colorectum, and less than 0.5% in anal canal tumor, thus accounting for 4% of all anal canal tumors [4,5]. Despite this, the rectum is the third most common site of malignant melanoma, second only to the skin and eyes. The median age of ARM onset is 60 years, and it is slightly more common in women. The most common site of metastasis is liver, followed by lung, brain and bone [6].
ARM often occurs in the dentate line or adjacent parts and clinical often showed no specificity. ARM is often misdiagnosed as hemorrhoids or polyps, and may not attract patients’ and clinicians’ attention. When clinicians diagnose ARM, the tumor is usually large. ARM has a high degree of malignancy, and poor prognosis, with a 6% 5-year survival rate [7]. Early diagnosis of ARM is very important. Melanoma usually has intracellular melanin granules, but there were no melanin granules in 30% of ARMs. Therefore, diagnosis depends on HMB45, and S-100 detection [8-10]. The IHC in our case showed S-100 positivity but was HMB45 negative. The patient had nonspecific symptoms such as anal pain and bleeding, thus diagnosis had to be postoperative. Based on histopathology combined with clinical symptoms, diagnosis of malignant melanoma was made.
Surgical resection is the main treatment of ARM, but the role of surgery for ARM is still controversial. According to the latest Chinese melanoma diagnosis and treatment guidelines, abdominoperineal resection (APR) was considered to be the standard treatment for a period of time [11,12], with wide local excision (WLE) getting more attention [12-14]. In Yeh et al study [15], there was no difference between patients treated with an APR or a WLE. In this background, WLE was applied in ARM due to the less aggressive treatment and better quality of life. According to the Chinese Melanoma Diagnosis and Treatment Guide (2015), surgical resection is the main treatment for ARM and inguinal lymph node dissection is not suggested currently [16]. APR can achieve better local control and negative margins. However, the large surgical area sacrifices the anal sphincter and will affect the quality of patients’ life. WLE requires a cutting edge ≥10 mm and there is no difference in the prognosis of the two surgical procedures. So if complete excision can be obtained, WLE should be the first choice [17,18]. In this case, the patient had been in the late stages of the tumor, so APR was chosen for palliative treatment [14].
Conclusions
Patients are often misdiagnosed because of the nonspecific symptoms of ARM, often at a late stage of tumor. Thus, patients may miss the best treatment period due to a misdiagnosis. Clinicians should take ARM into consideration when patients suffer from anal pain and bleeding. Early diagnosis and radical surgery are the most important treatments for ARM.
Acknowledgements
Zhejiang Medical and Health Science and Technology Program (2018KY930 and 2018KY923), and Lishui Science and Technology Project (2017GYX14 and 2018RC07).
Consent was obtained from relatives of the patient for publication of this report and any accompanying images.
Disclosure of conflict of interest
None.
References
- 1.Blecker D, Abraham S, Furth EE, Kochman ML. Melanoma in the gastrointestinal tract. Am J Gastroenterol. 1999;94:3427–33. doi: 10.1111/j.1572-0241.1999.01604.x. [DOI] [PubMed] [Google Scholar]
- 2.Khalid U, Saleem T, Imam AM, Khan MR. Pathogenesis, diagnosis and management of primary melanoma of the colon. World J Surg Oncol. 2011;9:14. doi: 10.1186/1477-7819-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tessier DJ, McConnell EJ, Young-Fadok T, Wolff BG. Melanoma metastatic to the colon: case series and review of the literature with outcome analysis. Dis Colon Rectum. 2003;46:441–7. doi: 10.1097/01.DCR.0000059657.64526.B6. [DOI] [PubMed] [Google Scholar]
- 4.Klas JV, Rothenberger DA, Wong WD, Madoff RD. Malignant tumors of the anal canal: the spectrum of disease, treatment, and outcomes. Cancer. 1999;85:1686–93. doi: 10.1002/(sici)1097-0142(19990415)85:8<1686::aid-cncr7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Roumen RM. Anorectal melanoma in the netherlands: a report of 63 patients. Eur J Surg Oncol. 1996;22:598–601. doi: 10.1016/s0748-7983(96)92346-x. [DOI] [PubMed] [Google Scholar]
- 6.Falch C, Stojadinovic A, Hann-von-Weyhern C, Protic M, Nissan A, Faries MB, Daumer M, Bilchik AJ, Itzhak A, Brücher BL. Anorectal malignant melanoma: extensive 45-year review and proposal for a novel staging classification. J Am Coll Surg. 2013;217:324–35. doi: 10.1016/j.jamcollsurg.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Ross M, Pezzi C, Pezzi T, Meurer D, Hickey R, Balch C. Patterns of failure in anorectal melanoma. A guide to surgical therapy. Arch Surg. 1990;125:313–6. doi: 10.1001/archsurg.1990.01410150035007. [DOI] [PubMed] [Google Scholar]
- 8.Chute DJ, Cousar JB, Mills SE. Anorectal malignant melanoma: morphologic and immunohistochemical features. Am J Clin Pathol. 2006;126:93–100. doi: 10.1309/DVWL-TV8F-FKC3-L80H. [DOI] [PubMed] [Google Scholar]
- 9.Tomicic J, Wanebo HJ. Mucosal melanomas. Surg Clin North Am. 2003;83:237–52. doi: 10.1016/S0039-6109(02)00100-7. [DOI] [PubMed] [Google Scholar]
- 10.Somran J, Kanngurn S, Porncharoenpong S, Lertkajornsin O. Anorectal malignant melanoma: report of two cases from Buddhachinnaraj Hospital. J Med Assoc Thai. 2005;88:1128–33. [PubMed] [Google Scholar]
- 11.Brady MS, Kavolius JP, Quan SH. Anorectal melanoma. A 64-year experience at memorial sloan-kettering cancer center. Dis Colon Rectum. 1995;38:146–51. doi: 10.1007/BF02052442. [DOI] [PubMed] [Google Scholar]
- 12.Yap LB, Neary P. A comparison of wide local excision with abdominoperineal resection in anorectal melanoma. Melanoma Res. 2004;14:147–50. doi: 10.1097/00008390-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Droesch JT, Flum DR, Mann GN. Wide local excision or abdominoperineal resection as the initial treatment for anorectal melanoma? Am J Surg. 2005;189:446–9. doi: 10.1016/j.amjsurg.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Terada R, Ito S, Kobayashi M, Akama F, Tsujimura M, Ooe H. Anorectal melanoma: successful treatment by surgical excision and combination chemoimmunotherapy. Hepatogastroenterology. 2002;49:1545–8. [PubMed] [Google Scholar]
- 15.Yeh JJ, Shia J, Hwu WJ, Busam KJ, Paty PB, Guillem JG, Coit DG, Wong WD, Weiser MR. The role of abdominoperineal resection as surgical therapy for anorectal melanoma. Ann Surg. 2006;244:1012–7. doi: 10.1097/01.sla.0000225114.56565.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heeney A, Mulsow J, Hyland JM. Treatment and outcomes of anorectal melanoma. Surgeon. 2011;9:27–32. doi: 10.1016/j.surge.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Bullard KM, Tuttle TM, Rothenberger DA, Madoff RD, Baxter NN, Finne CO, Spencer MP. Surgical therapy for anorectal melanoma. J Am Coll Surg. 2003;196:206–11. doi: 10.1016/S1072-7515(02)01538-7. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda A, Miyashita M, Matsumoto S, Takahashi G, Matsutani T, Yamada T, Kishi T, Uchida E. Abdominoperineal resection provides better local control but equivalent overall survival to local excision of anorectal malignant melanoma: a systematic review. Ann Surg. 2015;261:670–7. doi: 10.1097/SLA.0000000000000862. [DOI] [PubMed] [Google Scholar]