Abstract
Circulating microRNAs (miRNAs) are attracting major interest as novel non-invasive biomarkers for human autoimmune diseases including lupus nephritis (LN). A previous study showed that altered miR-203 expression may provide highly diagnostic for systemic lupus erythematosus. However, whether miR-203 is a diagnostic biomarker for LN is still unknown. In the present research, serum samples from 35 cases of active LN patients, 58 cases of inactive LN patients, and 74 cases of healthy volunteers were collected to analyze the expression profiles of miR-203 by qRT-PCR. The serum concentration of complement component 3 (C3) and complement component 4 (C4) was detected using nephelometry method. The expression of inflammatory cytokines, including interleukin 1 beta (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α), were analyzed using enzyme-linked immunosorbent assay (ELISA). The effect of miR-203 overexpression on the TNF receptor associated factor 6 (TRAF6)-induced inflammation of human renal mesangial cells (HRMCs) and human renal tubular epithelial cell line (HK-2) were evaluated. Results showed that miR-203 in serum of active LN patients was significantly down-regulated when compared with serum from inactive LN patients and healthy volunteers. Receiver operating curve (ROC) showed that decreased circulating miR-203 was a significant diagnostic biomarker for active LN patients, with an area under curve (AUC) of 0.974; sensitivity was 85.79%, and specificity was 89.40%. Significant downregulation of C3 and C4, and obvious upregulation of IL-β, IL-6, and TNF-α, was observed in serum of active LN patients. Furthermore, circulating miR-203 expression was positively correlated with the serum concentrations of C3 and C4, and negatively correlated with the serum expression of IL-1β, IL-6, and TNF-α in active LN patients. In addition, transfection of HRMCs and HK-2 cells with miR-203 mimics could suppress TRAF6-induced IL-β, IL-6, or TNF-α expression compared to cells treated with the mimics control group. In summary, decreased circulating miR-203 might be a candidate diagnostic biomarker for human active LN, and it attenuated IL-β, IL-6, and TNF-α activation in TRAF6-treated HRMCs and HK-2 cells.
Keywords: Circulating, miR-203, active LN, biomarker, inflammation
Introduction
Human lupus nephritis (LN) is defined as a complicated autoimmune and progressive glomerulonephritis with a variety of pathologic disorders, including proteinuria, glomerular damage, hematuria, and leucopenia [1]. Due to the unpredictable serious complications progressing to end-stage renal disease, LN has become a major cause of substantial morbidity and mortality worldwide. Based on kidney involvement using the 2003 ISN/RPS classification [2], LN was divided into two subgroups, including active and inactive LN. The active LN patients often have poor long-term prognosis and about 30% will progress to end-stage renal failure [3,4]. Renal biopsy is crucial to confirm the diagnosis, and assess disease activity and/or chronicity and guide treatment of LN, but some LN patients are not willing to undergo the procedure due to its invasiveness with several complications, including pain, infection, and hemorrhage. Conventional clinical biomarkers such as proteinuria, anti-dsDNA, and complement levels are not reliable and specific enough for detecting ongoing disease activity in LN [5,6]. Hence, it is essential to explore novel biomarkers that will contribute to better diagnosis and disease severity management of LN patients.
MicroRNAs (miRNAs) are a great family of endogenous, non-coding small RNA molecules with important roles in regulating gene expression at the post-transcriptional level [7]. Circulating miRNAs are stable molecules in blood and can be easily isolated and detected. Emerging evidence shows that circulating miRNAs can serve as novel non-invasive biomarkers and have clinical significance in diagnosis and/or prognosis of cancer and cerebrovascular diseases [8]. Circulating miR-1290 is a novel diagnostic and prognostic biomarker in human colorectal cancer [9]. Circulating miR-92b-3p is a novel biomarker for monitoring of synovial sarcoma [10]. Circulating miR-451 is a biomarker of ischemic stroke [11]. Indeed, circulating miR-93 is an indicator for diagnosis and prediction of functional recovery of acute stroke patients [12]. Evaluation of miRNAs profiles using microarray and qRT-PCR may be helpful in predicting kidney involvement. Recent studies have reported that aberrant circulating miRNAs expression is involved in the pathogenesis and progression of autoimmune diseases [13]. Altered miR-203 expression is found in serum from systemic lupus erythematosus and is correlated with erythrocyte sedimentation rate, C reactive protein, anti-dsDNA antibody, complements, and SLEDAI score [14,15]. However, no study has been performed for the correlation of circulating miR-203 expression with diagnosis of LN in clinical practice.
In this study, we carried out qRT-PCR for analysis of miR-203 expression profiles in serum from active LN patients, inactive LN patients, and healthy volunteers. Subsequently, the diagnostic value of miR-203 was explored, and the associations between miR-203 expression, inflammatory cytokines and complement component were also analyzed. In addition, we focused on the effect of miR-203 overexpression on the TRAF6-induced IL-β, IL-6, and TNF-α activation in HRMCs and HK-2 cells. Our study demonstrated that decreased circulating miR-203 is a candidate diagnostic biomarker for human active LN.
Methods and materials
Blood collection
The present study was carried out with the approval of the Ethics Committee of Tianjin Nankai Hospital (Tianjin, China), and all the participators were provided informed consent prior to the study. 35 cases of active LN patients (mean: 48.37±6.95 years, range: 14-73 years), 58 cases of inactive LN patients (mean: 45.60±6.18 years, range: 21-76 years), and 74 cases of healthy volunteers (mean: 49.12±7.84 years, range: 26-66 years) were enrolled in Department of Nephropathy, Tianjin Nankai Hospital from January 2010 to August 2019. 5 ml of peripheral blood was collected from all participators. All LN patients were confirmed by biopsy and fulfilled the 2003 ISN/RPS classification for LN. No patients had received immunosuppressive treatment within three months. LN patients with rheumatoid disease, acute or chronic infection within six weeks before admission, malignant tumor, severe hepatic dysfunction, and suspected drug or alcohol abuse were excluded. The clinical information of LN patients and healthy volunteers was recorded.
Sample processing and laboratory testing
The blood samples were applied for isolation of serum using centrifugation at 3,000 g for 20 min at 37°C. Then, the serum was transferred to a RNase-free tube and stored at -80°C. Blood routine and kidney function from each participator were measured by using a Fully Automated Chemistry Analyzer (AU400, Olympus, Orlando, FL, USA). The concentration of serum complement component 3 (C3) and complement component 4 (C4) was detected using nephelometry method [16].
RNA isolation and qRT-PCR assay
Total RNA was extracted using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. The quality and concentration of RNA were measured using NanoDrop™ 2000 spectrophotometer (Thermo Fisher Scientific Inc, MA, USA). Reverse transcription was performed to synthesize complementary DNA (cDNA) from the RNA using Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics, Mannheim, Germany) following the manufacturer’s instructions. qPCR was performed in a 96-well plate on an ABI 7500 system (Applied Biosystems, Foster City, CA, USA) using TB Green qPCR Master Mix with 20 μl reaction system of consisting of 1 μl cDNA, 1 μl primers, 10 μl qPCR Master Mix, and 8 μl H2O. All reactions were carried out at 95°C for 10 min, 40 cycles of amplification step at 98°C for 10 s, 58°C for 30 s, and 72°C for 30 s. The primers used in qPCR were as follows: miR-203, 5’-CAGCGGGTGAAATGTTTAGGAC-3’ (forward) and 5’-AGTGCAGGGTCCGAGGT-3’ (reverse); RNU6B (U6), 5’-CGCTTCGGCAGCACATATACTA-3’ (forward), and 5’-CGCTTCACGAATTTGCGTGTCA-3’ (reverse). U6 was chosen as housekeeping internal control for normalization of miR-203 quantification. The relative expression of miR-203 was calculated with the equation 2-ΔΔCt, in which ΔΔCt = Δ (Ct miR-203-Ct U6) sample-Δ (Ct miR-203-Ct U6) control.
Cell lines and culture conditions
Human renal mesangial cell line (HRMCs) was commercially purchased from Jennio Biological Technology Co., Ltd (Guangzhou, China), and human renal tubular epithelial cell line (HK-2) was obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were maintained in DMEM/F12 (GIBCO, Invitrogen, Carlsbad, CA, USA) at 37°C in a 5% CO2 humidified atmosphere supplemented with 10% FBS (GIBCO, Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin (Sigma, MO, USA), and 100 mg/ml streptomycin (Sigma, MO, USA).
Transfection
The miR-203 mimics and mimics control were obtained from GenePharma Co., Ltd (Shanghai, China). The sequences selected for miR-203 mimics and mimics control were 5’-GUGAAAUGUUUAGGACCACUAG-3’ and 5’-UUCUCCGAACGUGUCACGUTT-3’, respectively. HRMCs and HK-2 cells were transfected with miR-203 mimics (50 nM) or mimics control (50 nM) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocols. Cells were cultured for 48 h after transfection, and harvested to evaluate transfection efficiency by qRT-PCR.
ELISA assay
HRMCs and HK-2 cells were transfected with miR-203 mimics or mimic control for 48 h. The human IL-1β, IL-6, and TNF-α ELISA kits were performed to detect the expression of inflammatory cytokines of serum and cultured cells. Then, serum and cells were treated according to the manufacturer’s protocols, and the supernatants were added into 96-well plates coated with primary antibodies for 60 min at 37°C. After washing three times, biotinylated antibodies were added into each well for 30 min at 37°C, and detected using HRP-conjugated streptavidin and chromogen reagent. Absorbance at 570 nm was immediately observed using an ELISA reader (Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
All the data in this study are shown as mean ± SD, and analyzed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5.0 software (GraphPad Software, Inc., USA). The different expression of miR-203 between LN patients and healthy volunteers was assessed using Student’s t test. Spearman’s correlation coefficient was adopted to analyze the relationship between miR-106a-5p expression and the concentration of C3 and C4, and the expression of IL-1β, IL-6, and TNF-α. The diagnostic value of miR-203 expression was evaluated using receiver operating characteristics (ROC) analysis for patients with LN patients. P<0.05 was considered significant.
Results
Circulating miR-203 expression was down-regulated in active LN patients
The results of qRT-PCR showed that serum expression of miR-203 was significantly downregulated in 93 patients with LN patients compared to 74 healthy volunteers (P<0.05, Figure 1A). In addition, miR-203 expression was lower in serum from 35 active LN patients compared with that in serum from 58 inactive LN patients (P<0.05, Figure 1B). These data supported the notion that decreased circulating miR-203 might be associated with the pathogenesis of LN.
Figure 1.
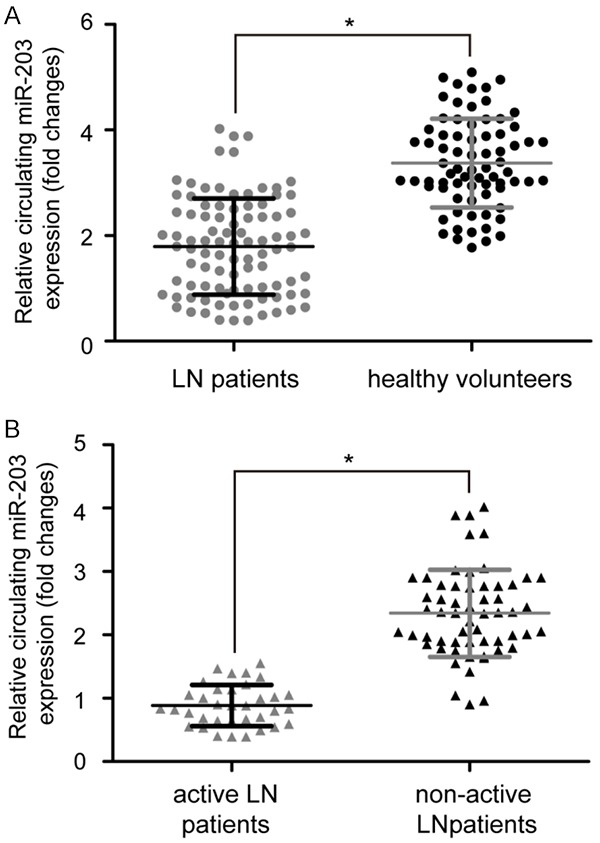
Circulating miR-203 expression levels in LN patients and healthy volunteers. A. qRT-PCR analysis of miR-203 expression in serum from 93 LN patients and 74 healthy volunteers. U6 was chosen as a housekeeping internal control for normalization of miR-203 expression. B. miR-203 expression was lower in serum from 35 active LN patients compared with that in serum from 58 inactive LN patients. Data are mean ± SD from triplicate experiments, *P<0.05. miR: microRNA, LN: lupus nephritis, U6: RNU6B.
Circulating miR-203 was a diagnostic biomarker for active LN patients
Next, in order to evaluate the clinical value of miR-203 in LN diagnosis, ROC analysis was performed. According to the results of ROC curve for differentiating LN patients from the healthy volunteers, an area under the curve (AUC) value was 0.897 (95% CI: 0.851 to 0.943), the corresponding sensitivity was 76.82% and specificity was 81.56% (P<0.001, Figure 2A). Further analysis of the diagnostic performance of miR-203 revealed that circulating miR-203 could also distinguish active LN from non-active LN patients with 85.79% sensitivity and 89.40% specificity, and the AUC of the ROC curve was 0.974 (95% CI: 0.947 to 1.00) (P<0.001, Figure 2B). The data showed that circulating miR-203 miis a candidate diagnostic biomarker for active LN.
Figure 2.
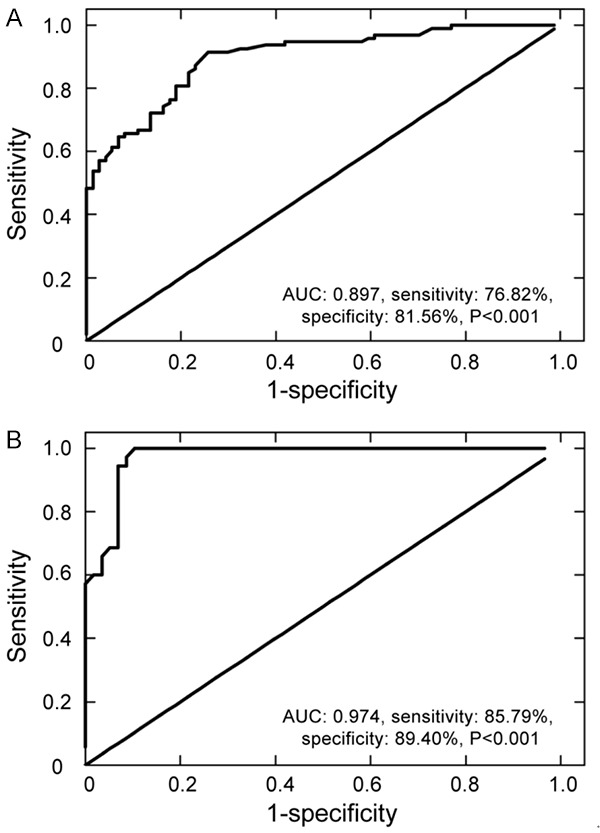
ROC curve analysis of circulating miR-203 for ability to discriminate LN patients from healthy volunteers and active LN patients from inactive LN patients. A. Diagnostic value differentiating LN patients from healthy volunteers; an AUC value was 0.897 and the corresponding sensitivity was 76.82% and specificity was 81.56%. B. Diagnostic value differentiating active LN patients from inactive LN patients; an AUC value was 0.974 and the corresponding sensitivity was 85.79% and specificity was 89.40%. Data are mean ± SD from triplicate experiments. ROC: receiver operating characteristic, AUC: area under the curve.
Circulating miR-203 expression was correlated with the serum concentration of C3, C4, IL-1β, IL-6, and TNF-α in LN patients
Results showed that the concentration of C3 and C4 was lower in serum from active LN patients compared with that in serum from with non-active LN patients (P<0.05, Figure 3A). The serum concentration of IL-1β, IL-6, and TNF-α was markedly upregulated in active LN patients compared to inactive LN patients (P<0.05, Figure 3B). As shown in Table 1, Spearman’s correlation coefficient indicated that the circulating miR-203 level was positively correlated with serum concentration of C3 (P<0.05, r=0.331) and C4 (P<0.05, r=0.284), and negatively correlated with serum concentration of IL-1β (P<0.05, r=-0.749), IL-6 (P<0.05, r=-0.305), and TNF-α (P<0.05, r=-0.416) in patients with active LN. These data suggested that circulating miR-203 is associated with inflammation in active LN patients.
Figure 3.
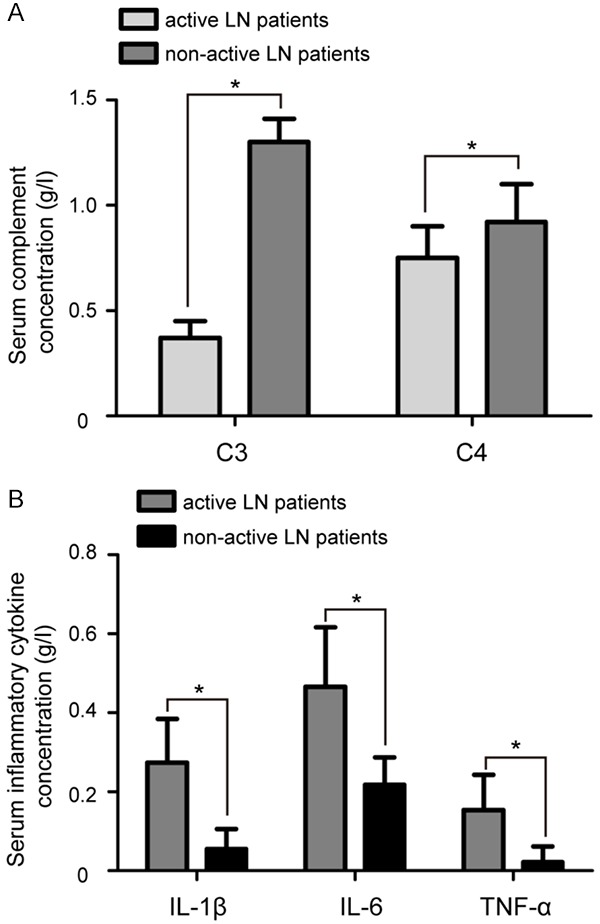
Serum concentration of C3 and C4 and expression of IL-1β, IL-6, and TNF-α in serum of LN patients. A. The concentration of C3 and C4 was lower in serum from active LN patients compared with that in serum from inactive LN patients. B. ELISA showed that serum expression of IL-1β, IL-6, and TNF-α was markedly upregulated in active LN patients compared to inactive LN patients. Data are mean ± SD from triplicate experiments, *P<0.05. C3: complement component 3, C4: complement component 4, IL-1β: interleukin 1 beta, IL-6: interleukin 10, TNF-α: tumor necrosis factor α, ELISA: enzyme-linked immunosorbent assay.
Table 1.
The correlations between circulating miR-203 expression and the serum concentration of C3, C4, IL-1β, IL-6, and TNF-α in active LN patients
| Indexes | r | P |
|---|---|---|
| C3 | r=0.331 | <0.05 |
| C4 | r=0.284 | <0.05 |
| IL-1β | r=-0.749 | <0.05 |
| IL-6 | r=-0.305 | <0.05 |
| TNF-α | r=-0.416 | <0.05 |
C3: complement component 3, C4: complement component 4, IL-1β: interleukin 1 beta, IL-6: interleukin 6, TNF-α: tumor necrosis factor alpha.
Overexpression of miR-203 suppressed the TRAF6-induced IL-β, IL-6, and TNF-α activation of HRMCs and HK-2 cells
Since TRAF6 plays an important role in regulating synthesis of IL-1β, IL-6, and TNF-α in LN [17], we transfected miR-203 mimics or mimic controls into HRMCs and HK-2 cells to determine the effect of miR-203 on the TRAF6-induced inflammation in vitro. qRT-PCR results showed that the expression of miR-203 was significantly upregulated in both HRMCs and HK-2 cells transfected by miR-203 mimics compared to the cells treated with mimic control (P<0.05, Figure 4A). TRAF6 treatment significantly elevated the concentration of IL-1β, IL-6, and TNF-α compared to the control group (P<0.05, Figure 4B). Furthermore, transfection of HRMCs cells with miR-203 mimics resulted in significantly decreased TRAF6-induced expression of IL-1β, IL-6, and TNF-α compared to the control group (P<0.05, Figure 4C). Similar results were observed in HK-2 cells after treatment overexpression of miR-203 (P<0.05, Figure 4D). All these data demonstrated that miR-203 overexpression suppresses the TRAF6-induced IL-β, IL-6, and TNF-α activation of HRMCs and HK-2 cells.
Figure 4.
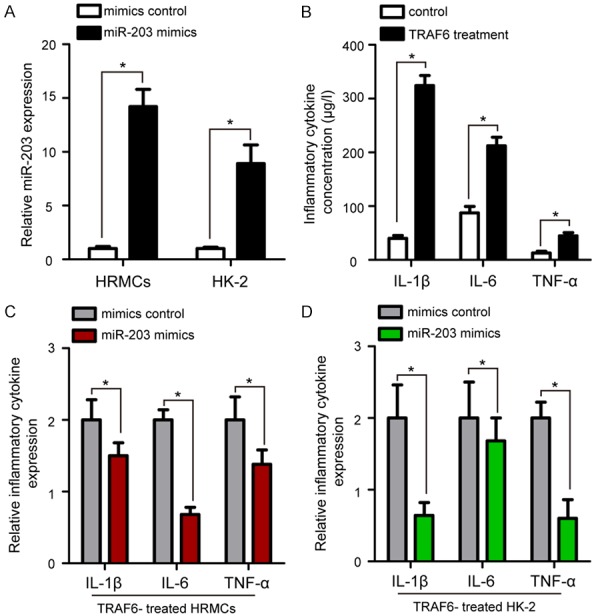
The effect of miR-203 overexpression on the TRAF6-induced IL-β, IL-6, TNF-α activation of HRMCs and HK-2 cells. A. qRT-PCR results revealed that miR-203 was efficiently overexpressed by transfection of miR-203 mimic in HRMCs and HK-2 cells, with U6 as an internal control. B. TRAF6 treatment significantly elevated the concentration of IL-1β, IL-6, and TNF-α compared to the control group. C. HRMC cells transfected with miR-203 mimics showed lower IL-1β, IL-6, and TNF-α expression than that in the mimic control group. D. Transfection of HK-2 cells with miR-203 mimics resulted in significantly decreased expression of IL-1β, IL-6, and TNF-α compared to the control group. Data are mean ± SD from triplicate experiments, *P<0.05. TRAF6: TNF receptor associated factor 6, HRMCs: human renal mesangial cell line, HK-2: human renal tubular epithelial cell line.
Discussion
At present, a lack of robust and sensitive biomarkers for LN hinders the accurate diagnosis of pathogenesis toward LN. According to the related studies, circulating miRNAs are sufficient to differentiate LN patients from healthy individuals, and may be promising biomarkers for LN [18,19]. For example, circulating miR-130b-3p has been described as a biomarker and played an important role in renal damage in early stage LN patients [20]. Asimilar study by Zhang and his colleagues reported that circulating miR-200b-5p, miR-141-5p, and miR-200c-5p expression could be novel and convincing diagnostic biomarkers for LN [21]. In addition, down-regulation of circulating miR-151a-3p may play an employed for diagnosing class IV LN and evaluating renal tissue activity [22]. All these studies demonstrated the important diagnostic values of circulating miRNAs for LN. In this study, we first showed that decreased circulating miR-203 is a candidate diagnostic biomarker for active LN, and plays a suppressive role modulating TRAF6-induced inflammation.
MiR-203 is a specific miRNA first identified in the pathogenesis of psoriasis and is involved in inflammatory responses [23]. Currently, the expression levels of miR-203 in human cancers are controversial and contradictory. Lin et al [24] reported that miR-203 expression was significantly increased in the tumor tissues of pancreatic cancer compared to adjacent normal tissues, and miR-203 knockdown significantly enhanced cell apoptosis and inhibited cell proliferation. In contrast, Chi et al [25] showed that miR-203 was downregulated in during tumorigenesis, and overexpression of miR-203 inhibited the proliferation and invasion of lung cancer cells. However, the diagnostic value of circulating miR-203 has not been assessed in LN patients. Here, we found that miR-203 in serum of active LN patients was significantly down-regulated compared to inactive LN patients and healthy volunteers. ROC analysis showed that decreased circulating miR-203 was a significant diagnostic biomarker for active LN patients, with an area under curve (AUC) of 0.974, sensitivity of 85.79%, and specificity of 89.40%. Consistent with our results, several studies reported the diagnostic significance of circulating miR-203 in some human cancers. For instance, a systematic review and meta-analysis showed that circulating miR-203 is a novel biomarker for the diagnosis and prognosis of colorectal cancer [26]. Circulating miR-203 is a biomarker for monitoring of primary cutaneous T-cell lymphoma [27]. Circulating miR-203 is markedly reduced in glioblastoma cells and their culture medium, and could be used as a prognostic indicator of glioblastoma [28].
Inflammatory cytokines, including IL-1β, IL-6, and TNF-α, are produced by innate immune cells such as macrophages and dendritic cells and share similar aspects in their regulation and secretion. A previous study confirmed that IL-1β and IL-6 are closely related to the initiation and progression phase of LN [29]. TNF-α is involved in the pathogenesis of LN as it promotes the activation and differentiation of macrophages, and its levels are increased in active LN patients and correlate with disease activity. A recent study indicated that miR-203 protects microglia-mediated brain injury by regulating inflammatory responses by feedback to MyD88 [30]. We observed that serum miR-203 level was negatively correlated with serum IL-1β, IL-6, and TNF-α in patients with active LN using Spearman’s correlation coefficient. In order to determine the importance of miR-203 in TRAF6-induced inflammation, we transfected miR-203 mimics or mimic control into HRMCs and HK-2 cells. Our results showed that overexpression of miR-203 could attenuate IL-β, IL-6, and TNF-α activation in TRAF6-treated HRMCs and HK-2 cells. This finding agreed with a previous study by Wang et al [31], which showed that miR-203 inhibits inflammation to alleviate myocardial ischemia-reperfusion injury.
In conclusion, our data provide evidence that decreased circulating miR-203 might be a diagnostic biomarker for human active LN. MiR-203 played a suppressive role in TRAF6-induced IL-β, IL-6, and TNF-α activation of HRMCs and HK-2 cells, indicating miR-203 could be a therapeutic target in LN treatment.
Acknowledgements
This work was supported by the Tianjin Science Commission’s Major Project for Chronic Disease Prevention and Control (no. 16ZXMJSY00190); the Tianjin Science Commission’s Innovation Project for Internet Cross-Border Integration (no. 17ZXHLSY00060), and the Tianjin Science Commission’s Major Scientific and Technological Project for Prevention and Treatment of Major Diseases (no. 18ZXDBSY00080).
Disclosure of conflict of interest
None.
References
- 1.Yu F, Haas M, Glassock R, Zhao MH. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat Rev Nephrol. 2017;13:483–495. doi: 10.1038/nrneph.2017.85. [DOI] [PubMed] [Google Scholar]
- 2.Mubarak M, Nasri H. ISN/RPS 2003 classification of lupus nephritis: time to take a look on the achievements and limitations of the schema. J Nephropathol. 2014;3:87–90. doi: 10.12860/jnp.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yap DY, Yung S, Chan TM. Lupus nephritis: an update on treatments and pathogenesis. Nephrology (Carlton) 2018;23(Suppl 4):80–83. doi: 10.1111/nep.13469. [DOI] [PubMed] [Google Scholar]
- 4.Tang Y, Zhang W, Zhu M, Zheng L, Xie L, Yao Z, Zhang H, Cao D, Lu B. Lupus nephritis pathology prediction with clinical indices. Sci Rep. 2018;8:10231. doi: 10.1038/s41598-018-28611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi S, Chen Q, Xu D, Xie N, Dai Y. Clinical application of protein biomarkers in lupus erythematosus and lupus nephritis. Lupus. 2018;27:1582–1590. doi: 10.1177/0961203318773643. [DOI] [PubMed] [Google Scholar]
- 6.Misra R, Gupta R. Biomarkers in lupus nephritis. Int J Rheum Dis. 2015;18:219–232. doi: 10.1111/1756-185X.12602. [DOI] [PubMed] [Google Scholar]
- 7.Mukhadi S, Hull R, Mbita Z, Dlamini Z. The role of microRNAs in kidney disease. Noncoding RNA. 2015;1:192–221. doi: 10.3390/ncrna1030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Chen J, Sen S. MicroRNA as biomarkers and diagnostics. J Cell Physiol. 2016;231:25–30. doi: 10.1002/jcp.25056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imaoka H, Toiyama Y, Fujikawa H, Hiro J, Saigusa S, Tanaka K, Inoue Y, Mohri Y, Mori T, Kato T, Toden S, Goel A, Kusunoki M. Circulating microRNA-1290 as a novel diagnostic and prognostic biomarker in human colorectal cancer. Ann Oncol. 2016;27:1879–1886. doi: 10.1093/annonc/mdw279. [DOI] [PubMed] [Google Scholar]
- 10.Uotani K, Fujiwara T, Yoshida A, Iwata S, Morita T, Kiyono M, Yokoo S, Kunisada T, Takeda K, Hasei J, Numoto K, Nezu Y, Yonemoto T, Ishii T, Kawai A, Ochiya T, Ozaki T. Circulating microRNA-92b-3p as a novel biomarker for monitoring of synovial sarcoma. Sci Rep. 2017;7:14634. doi: 10.1038/s41598-017-12660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu G, Cao C, Zhu M. Peripheral blood miR-451 may serve as a biomarker of ischemic stroke. Clin Lab. 2019;65 doi: 10.7754/Clin.Lab.2019.190309. [DOI] [PubMed] [Google Scholar]
- 12.Ma Q, Li G, Tao Z, Wang J, Wang R, Liu P, Luo Y, Zhao H. Blood microRNA-93 as an indicator for diagnosis and prediction of functional recovery of acute stroke patients. J Clin Neurosci. 2019;62:121–127. doi: 10.1016/j.jocn.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Zeng L, Cui J, Wu H, Lu Q. The emerging role of circulating microRNAs as biomarkers in autoimmune diseases. Autoimmunity. 2014;47:419–429. doi: 10.3109/08916934.2014.929667. [DOI] [PubMed] [Google Scholar]
- 14.Li HS, Ning Y, Li SB, Shao PY, Chen SJ, Ye Q, Heng X. Expression and clinical significance of miR-181a and miR-203 in systemic lupus erythematosus patients. Eur Rev Med Pharmacol Sci. 2017;21:4790–4796. [PubMed] [Google Scholar]
- 15.Carlsen AL, Schetter AJ, Nielsen CT, Lood C, Knudsen S, Voss A, Harris CC, Hellmark T, Segelmark M, Jacobsen S, Bengtsson AA, Heegaard NH. Circulating microRNA expression profiles associated with systemic lupus erythematosus. Arthritis Rheum. 2013;65:1324–1334. doi: 10.1002/art.37890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin X, Lu Y, Yang X, Peng Q, Wang J, Mo C, Wu J, Sui J, Liu Y, Huang X, Zhai L, Yang S, Li R, Li S, Yang X, Gao Y, Mo Z. Determination of reference intervals for serum complement C3 and C4 levels in Chinese Han ethnic males. Clin Lab. 2014;60:775–781. doi: 10.7754/clin.lab.2013.130514. [DOI] [PubMed] [Google Scholar]
- 17.Zheng CZ, Shu YB, Luo YL, Luo J. The role of miR-146a in modulating TRAF6-induced inflammation during lupus nephritis. Eur Rev Med Pharmacol Sci. 2017;21:1041–1048. [PubMed] [Google Scholar]
- 18.Navarro-Quiroz E, Pacheco-Lugo L, Navarro-Quiroz R, Lorenzi H, España-Puccini P, Díaz-Olmos Y, Almendrales L, Olave V, Gonzalez-Torres H, Diaz-Perez A, Dominguez A, Iglesias A, García R, Aroca-Martinez G. Profiling analysis of circulating microRNA in peripheral blood of patients with class IV lupus nephritis. PLoS One. 2017;12:e0187973. doi: 10.1371/journal.pone.0187973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zununi Vahed S, Nakhjavani M, Etemadi J, Jamshidi H, Jadidian N, Pourlak T, Abediazar S. Altered levels of immune-regulatory microRNAs in plasma samples of patients with lupus nephritis. Bioimpacts. 2018;8:177–183. doi: 10.15171/bi.2018.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Mou S, Wang L, Zhang M, Shao X, Fang W, Lu R, Qi C, Fan Z, Cao Q, Wang Q, Fang Y, Ni Z. Up-regulation of serum miR-130b-3p level is associated with renal damage in early lupus nephritis. Sci Rep. 2015;5:12644. doi: 10.1038/srep12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Wang Y. The correlation of plasma microRNA-200 family expressions with risk and disease severity of lupus nephritis. Eur Rev Med Pharmacol Sci. 2018;22:3118–3125. doi: 10.26355/eurrev_201805_15070. [DOI] [PubMed] [Google Scholar]
- 22.Xiao H, Wei N, Su M, Xiong Z. Down-regulation of serum miR-151a-3p is associated with renal tissue activity in class IV lupus nephritis. Clin Exp Rheumatol. 2019;37:67–72. [PubMed] [Google Scholar]
- 23.Sonkoly E, Wei T, Janson PC, Sääf A, Lundeberg L, Tengvall-Linder M, Norstedt G, Alenius H, Homey B, Scheynius A, Ståhle M, Pivarcsi A. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS One. 2007;2:e610. doi: 10.1371/journal.pone.0000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin XM, Chen H, Zhan XL. MiR-203 regulates JAK-STAT pathway in affecting pancreatic cancer cells proliferation and apoptosis by targeting SOCS3. Eur Rev Med Pharmacol Sci. 2019;23:6906–6913. doi: 10.26355/eurrev_201908_18730. [DOI] [PubMed] [Google Scholar]
- 25.Chi Y, Jin Q, Liu X, Xu L, He X, Shen Y, Zhou Q, Zhang J, Jin M. miR-203 inhibits cell proliferation, invasion, and migration of non-small-cell lung cancer by downregulating RGS17. Cancer Sci. 2017;108:2366–2372. doi: 10.1111/cas.13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye H, Hao H, Wang J, Chen R, Huang Z. miR-203 as a novel biomarker for the diagnosis and prognosis of colorectal cancer: a systematic review and meta-analysis. Onco Targets Ther. 2017;10:3685–3696. doi: 10.2147/OTT.S134252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dusílková N, Bašová P, Polívka J, Kodet O, Kulvait V, Pešta M, Trněný M, Stopka T. Plasma miR-155, miR-203, and miR-205 are biomarkers for monitoring of primary cutaneous T-cell lymphomas. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18102136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Yang L, Wang X. Reduced circulating microRNA-203 predicts poor prognosis for glioblastoma. Cancer Biomark. 2017;20:521–526. doi: 10.3233/CBM-170335. [DOI] [PubMed] [Google Scholar]
- 29.Wright RD, Beresford MW. Podocytes contribute, and respond, to the inflammatory environment in lupus nephritis. Am J Physiol Renal Physiol. 2018;315:F1683–F1694. doi: 10.1152/ajprenal.00512.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Z, Zhong L, Zhong S, Xian R, Yuan B. miR-203 protects microglia mediated brain injury by regulating inflammatory responses via feedback to MyD88 in ischemia. Mol Immunol. 2015;65:293–301. doi: 10.1016/j.molimm.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Yu W, Chen J, Yao T, Deng F. LncRNA MALAT1 sponges miR-203 to promote inflammation in myocardial ischemia-reperfusion injury. Int J Cardiol. 2018;268:245. doi: 10.1016/j.ijcard.2018.03.085. [DOI] [PubMed] [Google Scholar]


