Abstract
Abstract: Mutations in isocitrate dehydrogenase (IDH) and telomerase reverse transcriptase promoter (TERTp) exert a far-reaching influence on clinicopathologic diagnosis and prognosis of glioma. Traditional approaches, such as Sanger sequencing and ARMS, lack sensitivity due to tumor heterogeneity and low tumor purity of glioma samples. Therefore, we propose a highly sensitive detection method for IDH1 and TERTp mutations based on ddPCR technology, named IDH1-TERT-mutation ddPCR (IT-ddPCR). We determined the IDH1 and TERTp mutations of 80 patients by Sanger sequencing, ARMS, and IT-ddPCR in parallel. We detected the TERTp mutations of 8 patients with probes by IT-ddPCR and Bio-Rad. IDH1-positive singles were detected in 56 cases by IT-ddPCR. TERTp-positive singles were detected in 50 cases by IT-ddPCR. There was a slight difference in total events, occupancy events, and C228T/C250T droplets between these two different probes. Regression analysis of the TERTp variant frequencies detected by probes of IT-ddPCR and Bio-Rad produced a slope of 1.0425 and a coefficient (R2) of 0.9231. We found that IT-ddPCR showed a higher sensitivity compared with Sanger sequencing and ARMS in the detection of IDH1 and TERTp mutations. There were no significant differences in variant frequencies of TERTp mutations between the two probes of IT-ddPCR and Bio-Rad. Thus, IT-ddPCR can be used to detect low-frequency mutation of IDH1 and TERTp in glioma.
Keywords: IDH1, TERTp, ddPCR, glioma
Introduction
Glioma, including glioblastoma (GBM), oligodendroglioma, astrocytoma, ependymoma, and a few rare histologies, arises from glial or precursor cells and accounts for about 24% of brain tumors. It is the most common primary malignant tumor of the central nervous system (CNS), GBM accounts for 56.6% of gliomas with an incidence rate of 3.21 per 100000 population among those in the USA [1]. Glioma is characterized by infiltrating growth without obvious boundaries in normal brain tissue. Despite therapy combined with surgery, radio-chemotherapy and other adjuvant therapy, the prognosis of patients for high grade glioma is still dismal, and the median survival is only between 10 and 15 months [2,3]. There has been remarkable development in the molecular characterization of glioma in the past decade. Several molecular markers are new components of the classification of CNS tumors by the 2016 World Health Organization (WHO), which exert a far-reaching influence on clinicopathologic diagnosis and prognosis of glioma [4].
One of the most important changes of CNS tumors in the 2016 WHO classification is the addition of the IDH mutation. Since the isocitrate dehydrogenase 1 (IDH1) gene mutation was identified in a large cluster of patients with glioma by whole genome sequencing [5], there has been an increasing interest in researching consequences of IDH1 mutations and their roles in glioma progression. It has been demonstrated that IDH1 mutations that occur early in the development of glioma from a stem cell frequently can give rise to both astrocytes and oligodendrocytes [6]. Numerous studies have reported that patients with IDH1 mutations have a better overall survival (OS) and progression-free survival (PFS) compared to those without mutations in lower grade glioma [7-9]. More than 90% of IDH1 mutations are IDH1 R132H (c.395G>A) [13]. Another important mutation in glioma is the TERTp mutation. The mutations in TERTp, which encodes telomerase, have been found in most GBM patients [10] and confer a dismal prognosis [11,12]. There are two types of TERTp mutations, named C228T and C250T, occur at -124bp and -146 bp upstream of the TERT transcription start site, respectively [14].
IDH1 mutations occur in low-grade glioma (WHO II and III) mostly, and TERTp mutations in GMB frequently. Therefore, the combined testing of IDH and TERTp can classify over 80% of glioma into objective subgroups [11,15], and is very significant for the diagnosis of glioma. Currently, IDH and TERTp status are mainly detected by Sanger-sequencing and ARMS-PCR (amplification refractory mutation system PCR), which are limited by low sensitivity [16]. However, glioma has a high tumor heterogeneity and is often accompanied by necrosis, resulting in a lower proportion of tumor cells than the detection threshold. In recent years, droplet digital polymerase chain reaction (ddPCR) has gotten attention because of the realization of ultra-sensitive detection and absolute quantification. The mechanism of ddPCR is to prepare thousands of water-oil emulsion droplets containing single DNA molecules and amplify each DNA fragment, enabling droplet analysis by different fluorescent markers one by one [17,18]. Here we propose a high sensitivity detection method of IDH1 and TERTp base on ddPCR technology, named IT-ddPCR, which include R132H, R132S, R132G, R132C, R132L of IDH1 and C228T, C250T of TERTp. It is more sensitive than Sanger sequencing and ARMS. The IT-ddPCR can be used to detect a low mutation frequency of IDH1 and TERTp in glioma.
Materials and methods
Patients
For this study, 80 patients who were diagnosed with glioma were enrolled at the Southwest Hospital, the Third Military Medical University (TMMU) in Chongqing (China), from 2009 to 2018 (Supplementary Table 1). This study was approved by the Institutional Review Board/Ethics Committee of the hospital. All patients or their guardians signed an informed consent before the experiment. Histopathologic diagnoses were made by two neuropathologists based on the tumor classification of the central nervous system by 2016 WHO. Histologic classification was determined by hematoxylin-eosin staining (H&E staining), Sanger sequencing, and fluorescence in situ hybridization (FISH).
DNA extraction
Genomic DNA was extracted from formalin-fixed paraffin-embedded (FFPE) tumor samples of these patients using the TIANamp FFPE DNA Kit (TIANGEN). The experiment was implemented according to the protocol offered by the manufacturer. The concentration of DNA was measured by the SMA4000 and the purity was evaluated through the measurement of the OD260/OD280 ratio. Extracted DNA was stored at -20°C until it was used.
ARMS-PCR and Sanger sequencing
R132H and TERTp C228T, C250T mutations were detected by ARMS-PCR using Human IDH1 Mutations Detection Kit (Fluorescent PCR) and Human TERTp Mutations Detection Kit (Genetron Health, Beijing, China) on a Stratagene MX3000P Real Time PCR system (Agilent Technologies Inc.), respectively. PCR purification and sequencing reactions were performed by Tumor Related Gene Mutation Detection Kit (Yuanqi Bio, Shanghai, China) by 3500 Dx Genetic Analyzer (Applied Biosystems Inc.). Experimental operation and result interpretation were all performed according to the protocols provided by the manufacturer.
Droplet digital PCR
Digital droplet PCR (ddPCR) assays were performed on QX200 AutoDG Droplet Digital PCR system (Bio-Rad). IDH1 and TERTp mutations were detected by IT-ddPCR kit. Meanwhile, TERTp C228T, C250T mutations were detected by Bio-Rad’s probes according to the manufacturer’s instructions. The abridged general view of the workflow of IT-ddPCR is described in Figure 1A. The PCR program of ddPCR after droplet generation was as follows: 95°C for 10 min; 40 cycles of 94°C for 15 s and 58°C for 60 s; 98°C for 10 min; 4°C for 5 min. The reaction temperature was changed at a rate of 2°C/s. DNA input for standard ddPCR analysis was 60 ng.
Figure 1.

Workflow and quantitative performances of the ddPCR panels for IDH1 and TERT promoter mutation analysis. A. First, DNA templates and primer pairs were mixed with ddPCR Supermix. Then, generating droplets using Automated Droplet Generator. Next, the PCR mixture for each assay was compartmentalized into about 20000 droplets for independent PCR reactions. Finally, droplets were scanned and analyzed using QX200 Droplet Reader one by one. B. The test in reference-standard plasmid with serial variant IDH1 mutants (R132H, R132S, R132G, R132C, R132L) and TERT promoter mutants (C228T and C250T) using IT-ddPCR.
Results
Quantitative performance of the ddPCR panels for IDH1 and TERTp mutation analysis
To investigate the detection sensitivity of IT-ddPCR method, we performed the test in reference-standard plasmid with serial variant and different IDH1 mutants (R132H, R132S, R132G, R132C, R132L), respectively. As shown in Figure 1B, when the IDH1 mutant ratio was larger than 0.1%, more than three droplets could be observed. Occasionally, under the circumstance that the IDH1 mutant ratio were lower (<0.1%), it is still determined as negative although there were one or two positive droplets. Meanwhile, the TERTp mutations (C228T and C250T) were done by the same experiments and the results were the same as above. Therefore, it can be demonstrated that the sensitivity of IT-ddPCR is at least 0.1%.
Patients demographics
A total of 80 patients who underwent intracranial tumor surgery were enrolled in this study. The clinical features of these patients are shown in Table 1. For these patients, 63.75% (51/80) were male, the median age at initial diagnosis was 44 years (age ranged from 10 to 74). We assayed a cohort of glioma tumor tissues (N=80) representing the major subtypes of glioma, including various histologic types (astrocytoma, glioblastoma, oligodendroglioma, et al.), WHO grades (II-IV). The tumor percentage of all samples was more than 30%.
Table 1.
Clinical characteristics of 80 patients
| Features | No. of patients (%) |
|---|---|
| Sex | |
| Male | 51 (63.75) |
| Female | 29 (36.25) |
| Histologic type | |
| Astrocytoma | 26 (32.50) |
| Glioblastoma | 23 (28.75) |
| Oligodendroglioma | 28 (35.00) |
| Others | 3 (3.75%) |
| Grade | |
| II | 28 (35.00) |
| III | 28 (35.00) |
| IV | 24 (30.00) |
| Location | |
| Left hemisphere | 40 (50.00) |
| Right hemisphere | 36 (45.00) |
| Others | 4 (5.00) |
More sensitive detection of IDH1 and TERTp mutations by IT-ddPCR than Sanger sequencing and ARMS
IT-ddPCR was a more sensitive detection method for IDH1 and TERTp mutations than Sanger sequencing and ARMS
We determined the IDH1 and TERTp mutations of 80 patients by Sanger sequencing, ARMS, and IT-ddPCR in parallel. IDH1-positive singles were detected in 43 cases by Sanger sequencing, and in 44 and 56 cases by ARMS and IT-ddPCR, respectively. TERTp-positive singles were detected in 44 cases by Sanger sequencing, and in 44 and 50 cases by ARMS and IT-ddPCR, respectively (Figure 2A). IT-ddPCR showed higher sensitivity compared with Sanger sequencing and ARMS. Remarkably, there was a case of anaplastic astrocytoma, in which IDH1 mutation was not detected by Sanger sequencing but only by IT-ddPCR and ARMS. We found that the variant frequencies of IDH1 mutation were 9.23% in this case. It can be concluded that the sensitivity of Sanger sequencing is the lowest among the three methods.
Figure 2.
A cohort of glioma tumor samples was assayed for IDH and TERT promoter mutations by IT-ddPCR, Sanger-sequencing, and qPCR (N=80). A. More sensitive detection of IDH1 and TERT promoter mutations by IT-ddPCR. Green and white bars indicate IDH1 R132H positive and R132H negative, respectively. Red, purple, and white bars indicate C228T positive, C250T positive, and C228T/C250T negative, respectively. B. Clinical information of these 80 patients, including histopathology and WHO grade based on immunohistochemistry, Sanger sequencing and FISH. Mutation results of IDH1 and TERT promoter based on IT-ddPCR.
As shown in Figure 2B, IT-ddPCR can identify IDH1 mutations in 61.54% (16/26) of astrocytoma, 47.83% (11/23) of GBMs and 100% (28/28) of oligodendroglioma, and IDH1 mutations in greater than 70% (56/80) of all grades II-III glioma. IT-ddPCR identified TERTp mutations in 25% (7/28) of astrocytomas, 73.91% (17/23) of GBMs and 85.71% (24/28) of oligodendrogliomas.
Similar variant frequencies of TERTp Mutations between IT-ddPCR and Bio-Rad
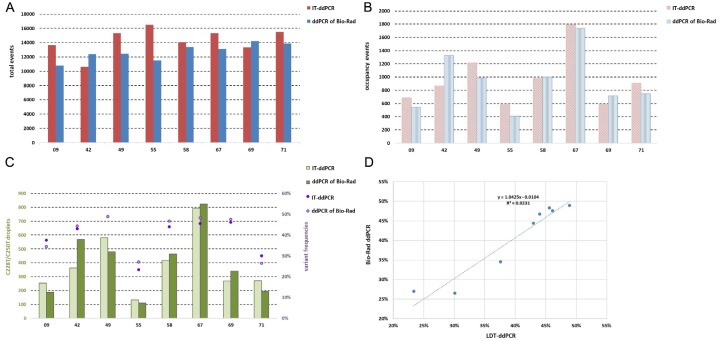
To verify the detection efficiency of IT-ddPCR probes, we detected the TERTp mutations of 8 patients using assays of Bio-Rad. The total droplets produced (total events), occupancy droplets (occupancy events), C228T/C250T droplets and TERTp variant frequencies of two different probes were evaluated for comparison (Figure 3). The results showed that there was a slight difference in the total events, occupancy events, and C228T/C250T droplets between these two methods, which was a normal phenomenon (Figure 3A). The differences of occupancy events and C228/C250T droplets between the two probes were consistent with the number of total events (Figure 3B, 3C). Regression analysis of the TERTp variant frequencies detected by two different probes produced a slope of 1.0425 and a coefficient (R2) of 0.9231 (Figure 3D). In conclusion, the detection efficiency of IT-ddPCR assays is similar to that of Bio-Rad.
Figure 3.
The assays of IT-ddPCR and Bio-Rad were performed in parallel to detect C228T and C250T in FFPE samples of 7 glioma patients: (A) total events (total droplets), (B) occupancy events (occupancy droplets), (C) C228T/C250T droplets and variant frequency of TERT promoter, (D) IT-ddPCR showing variant frequencies of TERT concordant with those from Bio-Rad ddPCR.
Discussion
Studies on genomic sequencing of diffuse glioma have identified that genetic alterations can delineate molecular subtypes of glioma [19-21]. The research from Mayo Clinic, University of California, and Memorial Sloan Kettering Cancer Center found that IDH1 or TERTp mutations occurred in 967 of 1087 (88.96%) glioma patients [11]. Coincidentally, the research from Duke University Medical Center found that IDH1 or TERTp mutations occurred in 420 of 473 (88.79%) glioma patients [15]. Similarity, it was discovered that 71 of 80 (88.75%) glioma specimens expressed IDH1 or TERTp mutations using ddPCR in this study. These gene mutations may be molecular markers for conducting glioma classification and can improve targeted therapy development because of their high frequency and location.
Sanger sequencing, as a traditional approach for detecting IDH1 mutation, lacks the sensitivity result from heterogeneity and low tumor purity of glioma samples. ddPCR, as a recently developed molecular amplification technique, has demonstrated that the lowest detectable frequency of BRAF V600E mutation is 0.001% [22]. There are some studies that showed that the sensitivity of ddPCR is 0.01% and the sensitivity of ARMS-PCR is 0.1% [23]. Based on our analyses of sensitivity, IT-ddPCR can detect mutation frequency lower than 0.1%, which is more sensitive than Sanger sequencing and ARMS.
In our study, IDH1-positive and TERTp-positive singles were detected in 56 cases and 50 cases by IT-ddPCR, respectively. Interestingly, we found that the IDH1 mutation was not detected by Sanger sequencing in a case of anaplastic astrocytoma (No. 41) while it can be detected by IT-ddPCR and ARMS with a variant frequency of 9.23%. According to the 2016 WHO classification, IDH gene status is a significant marker for lower grade glioma. Notably, there was a case of glioblastoma (No. 76), in which TERTp mutations were not detected by Sanger sequencing and ARMS but the C250T was detected by IT-ddPCR. This patient was diagnosed with astrocytoma (WHO II) in 2015 and relapsed in 2018. Six months after the second surgery, the patient relapsed again with a poor prognosis. It has been reported that diffuse glioma without any tumor mass, which was previously named as gliomatosis cerebri, mostly showed IDH-wildtype and exhibited poor prognosis [24]. Most low grade glioma patients with IDH gene mutation have a good prognosis. Therefore, ddPCR may play a vital role in disease diagnosis and facilitate treatment due to its high sensitivity and absolute quantification.
Bio-Rad assays of ddPCR had been used in many prior studies [25-27]. Recently, a growing number of studies have shown that ddPCR can be used for liquid biopsy in several solid cancers, such as lung and pancreatic cancers [28,29]. Circulating tumor DNA (ctDNA) has been proved to be a dependable material for diagnosis, prognostication, and monitoring in many cancers [30-32]. For example, Jiao et al. measured TERTp mutations in plasma cfDNA in 218 patients with hepatocellular carcinoma (HCC) by ddPCR using a Bio-Rad assay [26], and Juratli et al. performed TERTp detection in the cerebrospinal fluid (CSF) by ddPCR, which showed concordant results with those determined by the Ion Torrent system in all 9 cases [27]. Therefore, we wanted to verify the differences in detection efficiency between IT-ddPCR probes and Bio-Rad probes. In this study, we compared Bio-Rad analysis with our own analysis, and found no significant difference in variant frequencies of TERTp mutations between these two assays. Furthermore, our IDH1 mutant detection assay is more cost-efficient than conventional ddPCR since it includes five mutation types simultaneously. Nevertheless, the levels of ctDNA were low and discrepantly detectable in primary and metastatic brain tumors because of the blood brain barrier [33]. Over the long term, we will optimize our assay further to make it meaningful in the mutant detection of ctDNA from patients with glioma.
Acknowledgements
This research was supported by grants from National Natural Science Foundation of China (81602196), Postdoctoral Program (BX201600022, xmT2017001) and Chongqing Basic Research Project (cstc2016jcyjA2194).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(Suppl 4):iv1–iv86. doi: 10.1093/neuonc/noy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised Phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Rønning PA, Helseth E, Meling TR, Johannesen TB. A population-based study on the effect of temozolomide in the treatment of glioblastoma multiforme. Neuro Oncol. 2012;14:1178–1184. doi: 10.1093/neuonc/nos153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banan R, Hartmann C. The new WHO 2016 classification of brain tumors-what neurosurgeons need to know. Acta Neurochirurgica (Wien) 2017;159:403–418. doi: 10.1007/s00701-016-3062-3. [DOI] [PubMed] [Google Scholar]
- 5.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch T, Reifenberger G, Weller M, Loeffler M, von Deimling A. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathologica. 2010;120:707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 8.Christensen BC, Smith AA, Zheng S, Koestler DC, Houseman EA, Marsit CJ, Wiemels JL, Nelson HH, Karagas MR, Wrensch MR, Kelsey KT, Wiencke JK. DNA methylation, isocitrate dehydrogenase mutation, and survival in glioma. J Natl Cancer Inst. 2011;103:143–153. doi: 10.1093/jnci/djq497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SongTao Q, Lei Y, Si G, YanQing D, HuiXia H, XueLin Z, LanXiao W, Fei Y. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci. 2012;103:269–273. doi: 10.1111/j.1349-7006.2011.02134.x. [DOI] [PubMed] [Google Scholar]
- 10.Brastianos PK, Nayyar N, Rosebrock D, Leshchiner I, Gill CM, Livitz D, Bertalan MS, D’Andrea M, Hoang K, Aquilanti E, Chukwueke UN, Kaneb A, Chi A, Plotkin S, Gerstner ER, Frosch MP, Suva ML, Cahill DP, Getz G, Batchelor TT. Resolving the phylogenetic origin of glioblastoma via multifocal genomic analysis of pre-treatment and treatment-resistant autopsy specimens. NPJ Precis Oncol. 2017;1:33. doi: 10.1038/s41698-017-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML, Smirnov IV, Sarkar G, Caron AA, Kollmeyer TM, Praska CE, Chada AR, Halder C, Hansen HM, McCoy LS, Bracci PM, Marshall R, Zheng S, Reis GF, Pico AR, O’Neill BP, Buckner JC, Giannini C, Huse JT, Perry A, Tihan T, Berger MS, Chang SM, Prados MD, Wiemels J, Wiencke JK, Wrensch MR, Jenkins RB. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372:2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon M, Hosen I, Gousias K, Rachakonda S, Heidenreich B, Gessi M, Schramm J, Hemminki K, Waha A, Kumar R. TERT promoter mutations: a novel independent prognostic factor in primary glioblastomas. Neuro Oncol. 2015;17:45–52. doi: 10.1093/neuonc/nou158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waitkus MS, Diplas BH, Yan H. Isocitrate dehydrogenase mutations in gliomas. Neuro Oncol. 2016;18:16–26. doi: 10.1093/neuonc/nov136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL, Giovanella BC, Grollman AP, He TC, He Y, Hruban RH, Jallo GI, Mandahl N, Meeker AK, Mertens F, Netto GJ, Rasheed BA, Riggins GJ, Rosenquist TA, Schiffman M, Shih IeM, Theodorescu D, Torbenson MS, Velculescu VE, Wang TL, Wentzensen N, Wood LD, Zhang M, McLendon RE, Bigner DD, Kinzler KW, Vogelstein B, Papadopoulos N, Yan H. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110:6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Killela PJ, Pirozzi CJ, Healy P, Reitman ZJ, Lipp E, Rasheed BA, Yang R, Diplas BH, Wang Z, Greer PK, Zhu H, Wang CY, Carpenter AB, Friedman H, Friedman AH, Keir ST, He J, He Y, McLendon RE, Herndon JE 2nd, Yan H, Bigner DD. Mutations in IDH1, IDH2, and in the TERT promoter defne clinically distinct subgroups of adult malignant gliomas. Oncotarget. 2014;5:1515–1525. doi: 10.18632/oncotarget.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorniak P, Ejduk A, Borg K, Makuch-Lasica H, Nowak G, Lech-Maranda E, Prochorec-Sobieszek M, Warzocha K, Juszczynski P. Comparison of high-resolution melting analysis with direct sequencing for the detection of recurrent mutations in DNA methyltransferase 3A and isocitrate dehydrogenase 1 and 2 genes in acute myeloid leukemia patients. Eur J Haematol. 2016;96:181–187. doi: 10.1111/ejh.12566. [DOI] [PubMed] [Google Scholar]
- 17.Sykes PJ, Neoh SH, Brisco MJ, Hughes E, Condon J, Morley AA. Quantitation of targets for PCR by use of limiting dilution. Biotechniques. 1992;13:444–449. [PubMed] [Google Scholar]
- 18.Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, Bright IJ, Lucero MY, Hiddessen AL, Legler TC, Kitano TK, Hodel MR, Petersen JF, Wyatt PW, Steenblock ER, Shah PH, Bousse LJ, Troup CB, Mellen JC, Wittmann DK, Erndt NG, Cauley TH, Koehler RT, So AP, Dube S, Rose KA, Montesclaros L, Wang S, Stumbo DP, Hodges SP, Romine S, Milanovich FP, White HE, Regan JF, Karlin-Neumann GA, Hindson CM, Saxonov S, Colston BW. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83:8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komotar RJ, Syed ON, Bruce JN, Connolly ES Jr, Dunn IF, Friedlander RM. Genomics of human glioblastoma multiforme: a glimpse of the future. Neurosurgery. 2008;63:15. doi: 10.1227/01.NEU.0000342663.21125.02. [DOI] [PubMed] [Google Scholar]
- 20.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O’Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas Research Network. Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Rheinbay E, Miller CR, Vitucci M, Morozova O, Robertson AG, Noushmehr H, Laird PW, Cherniack AD, Akbani R, Huse JT, Ciriello G, Poisson LM, Barnholtz-Sloan JS, Berger MS, Brennan C, Colen RR, Colman H, Flanders AE, Giannini C, Grifford M, Iavarone A, Jain R, Joseph I, Kim J, Kasaian K, Mikkelsen T, Murray BA, O’Neill BP, Pachter L, Parsons DW, Sougnez C, Sulman EP, Vandenberg SR, Van Meir EG, von Deimling A, Zhang H, Crain D, Lau K, Mallery D, Morris S, Paulauskis J, Penny R, Shelton T, Sherman M, Yena P, Black A, Bowen J, Dicostanzo K, Gastier-Foster J, Leraas KM, Lichtenberg TM, Pierson CR, Ramirez NC, Taylor C, Weaver S, Wise L, Zmuda E, Davidsen T, Demchok JA, Eley G, Ferguson ML, Hutter CM, Mills Shaw KR, Ozenberger BA, Sheth M, Sofia HJ, Tarnuzzer R, Wang Z, Yang L, Zenklusen JC, Ayala B, Baboud J, Chudamani S, Jensen MA, Liu J, Pihl T, Raman R, Wan Y, Wu Y, Ally A, Auman JT, Balasundaram M, Balu S, Baylin SB, Beroukhim R, Bootwalla MS, Bowlby R, Bristow CA, Brooks D, Butterfield Y, Carlsen R, Carter S, Chin L, Chu A, Chuah E, Cibulskis K, Clarke A, Coetzee SG, Dhalla N, Fennell T, Fisher S, Gabriel S, Getz G, Gibbs R, Guin R, Hadjipanayis A, Hayes DN, Hinoue T, Hoadley K, Holt RA, Hoyle AP, Jefferys SR, Jones S, Jones CD, Kucherlapati R, Lai PH, Lander E, Lee S, Lichtenstein L, Ma Y, Maglinte DT, Mahadeshwar HS, Marra MA, Mayo M, Meng S, Meyerson ML, Mieczkowski PA, Moore RA, Mose LE, Mungall AJ, Pantazi A, Parfenov M, Park PJ, Parker JS, Perou CM, Protopopov A, Ren X, Roach J, Sabedot TS, Schein J, Schumacher SE, Seidman JG, Seth S, Shen H, Simons JV, Sipahimalani P, Soloway MG, Song X, Sun H, Tabak B, Tam A, Tan D, Tang J, Thiessen N, Triche T Jr, Van Den Berg DJ, Veluvolu U, Waring S, Weisenberger DJ, Wilkerson MD, Wong T, Wu J, Xi L, Xu AW, Yang L, Zack TI, Zhang J, Aksoy BA, Arachchi H, Benz C, Bernard B, Carlin D, Cho J, DiCara D, Frazer S, Fuller GN, Gao J, Gehlenborg N, Haussler D, Heiman DI, Iype L, Jacobsen A, Ju Z, Katzman S, Kim H, Knijnenburg T, Kreisberg RB, Lawrence MS, Lee W, Leinonen K, Lin P, Ling S, Liu W, Liu Y, Liu Y, Lu Y, Mills G, Ng S, Noble MS, Paull E, Rao A, Reynolds S, Saksena G, Sanborn Z, Sander C, Schultz N, Senbabaoglu Y, Shen R, Shmulevich I, Sinha R, Stuart J, Sumer SO, Sun Y, Tasman N, Taylor BS, Voet D, Weinhold N, Weinstein JN, Yang D, Yoshihara K, Zheng S, Zhang W, Zou L, Abel T, Sadeghi S, Cohen ML, Eschbacher J, Hattab EM, Raghunathan A, Schniederjan MJ, Aziz D, Barnett G, Barrett W, Bigner DD, Boice L, Brewer C, Calatozzolo C, Campos B, Carlotti CG Jr, Chan TA, Cuppini L, Curley E, Cuzzubbo S, Devine K, DiMeco F, Duell R, Elder JB, Fehrenbach A, Finocchiaro G, Friedman W, Fulop J, Gardner J, Hermes B, Herold-Mende C, Jungk C, Kendler A, Lehman NL, Lipp E, Liu O, Mandt R, McGraw M, Mclendon R, McPherson C, Neder L, Nguyen P, Noss A, Nunziata R, Ostrom QT, Palmer C, Perin A, Pollo B, Potapov A, Potapova O, Rathmell WK, Rotin D, Scarpace L, Schilero C, Senecal K, Shimmel K, Shurkhay V, Sifri S, Singh R, Sloan AE, Smolenski K, Staugaitis SM, Steele R, Thorne L, Tirapelli DP, Unterberg A, Vallurupalli M, Wang Y, Warnick R, Williams F, Wolinsky Y, Bell S, Rosenberg M, Stewart C, Huang F, Grimsby JL, Radenbaugh AJ, Zhang J. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372:2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinheiro LB, Coleman VA, Hindson CM, Herrmann J, Hindson BJ, Bhat S, Emslie KR. Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Anal Chem. 2012;84:1003–11. doi: 10.1021/ac202578x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oxnard GR, Paweletz CP, Kuang Y, Mach SL, O’Connell A, Messineo MM, Luke JJ, Butaney M, Kirschmeier P, Jackman DM, Jänne PA. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20:1698–1705. doi: 10.1158/1078-0432.CCR-13-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seiz M, Tuettenberg J, Meyer J, Essig M, Schmieder K, Mawrin C, von Deimling A, Hartmann C. Detection of IDH1 mutations in gliomatosis cerebri, but only in tumors with additional solid component: evidence for molecular subtypes. Acta Neuropathol. 2010;120:261–267. doi: 10.1007/s00401-010-0701-2. [DOI] [PubMed] [Google Scholar]
- 25.Hirano M, Ohka F, Maeda S, Chalise L, Yamamichi A, Aoki K, Kato A, Tanahashi K, Motomura K, Nishimura Y, Hara M, Shinjo K, Kondo Y, Wakabayashi T, Natsume A. A novel high-sensitivity assay to detect a small fraction of mutant IDH1 using droplet digital PCR. Brain Tumor Pathol. 2018;35:97–105. doi: 10.1007/s10014-018-0310-7. [DOI] [PubMed] [Google Scholar]
- 26.Jiao J, Watt GP, Stevenson HL, Calderone TL, Fisher-Hoch SP, Ye Y, Wu X, Vierling JM, Beretta L. Telomerase reverse transcriptase mutations in plasma DNA in patients with hepatocellular carcinoma or cirrhosis: prevalence and risk factors. Hepatol Commun. 2018;2:718–731. doi: 10.1002/hep4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juratli TA, Stasik S, Zolal A, Schuster C, Richter S, Daubner D, Juratli MA, Thowe R, Hennig S, Makina M, Meinhardt M, Lautenschlaeger T, Schackert G, Krex D, Thiede C. TERT promoter mutation detection in cell-free tumor-derived DNA in patients with IDH wild-type glioblastomas-a pilot prospective study. Clin Cancer Res. 2018;24:5282–5291. doi: 10.1158/1078-0432.CCR-17-3717. [DOI] [PubMed] [Google Scholar]
- 28.Kinugasa H, Nouso K, Miyahara K, Morimoto Y, Dohi C, Tsutsumi K, Kato H, Matsubara T, Okada H, Yamamoto K. Detection of K-ras gene mutation by liquid biopsy in patients with pancreatic cancer. Cancer. 2015;121:2271–2280. doi: 10.1002/cncr.29364. [DOI] [PubMed] [Google Scholar]
- 29.Zhu G, Ye X, Dong Z, Lu YC, Sun Y, Liu Y, McCormack R, Gu Y, Liu X. Highly sensitive droplet digital PCR method for detection of EGFR-activating mutations in plasma cell-free DNA from patients with advanced non-small cell lung cancer. J Mol Diagn. 2015;17:265–272. doi: 10.1016/j.jmoldx.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM, Parkinson C, Chin SF, Kingsbury Z, Wong AS, Marass F, Humphray S, Hadfield J, Bentley D, Chin TM, Brenton JD, Caldas C, Rosenfeld N. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497:108–112. doi: 10.1038/nature12065. [DOI] [PubMed] [Google Scholar]
- 31.Cristofanilli M, Fortina P. Circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;369:93. doi: 10.1056/NEJMc1306040. [DOI] [PubMed] [Google Scholar]
- 32.Germano G, Mauri G, Siravegna G, Dive C, Pierce J, Di Nicolantonio F, D’Incalci M, Bardelli A, Siena S, Sartore-Bianchi A. Parallel evaluation of circulating tumor DNA and circulating tumor cells in metastatic colorectal cancer. Clin Colorectal Cancer. 2018;17:80–83. doi: 10.1016/j.clcc.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, Antonarakis ES, Azad NS, Bardelli A, Brem H, Cameron JL, Lee CC, Fecher LA, Gallia GL, Gibbs P, Le D, Giuntoli RL, Goggins M, Hogarty MD, Holdhoff M, Hong SM, Jiao Y, Juhl HH, Kim JJ, Siravegna G, Laheru DA, Lauricella C, Lim M, Lipson EJ, Marie SK, Netto GJ, Oliner KS, Olivi A, Olsson L, Riggins GJ, Sartore-Bianchi A, Schmidt K, Shih lM, Oba-Shinjo SM, Siena S, Theodorescu D, Tie J, Harkins TT, Veronese S, Wang TL, Weingart JD, Wolfgang CL, Wood LD, Xing D, Hruban RH, Wu J, Allen PJ, Schmidt CM, Choti MA, Velculescu VE, Kinzler KW, Vogelstein B, Papadopoulos N, Diaz LA Jr. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.