Abstract
The P2X7 receptor (P2X7R) is an exclusive member of the purinergic receptor family that plays a key role in tumor progression, including colorectal cancer (CRC). P2X7R supports the tumor cells to resist unfavorable conditions by stimulating GLUT-1 expression. GLUT1 is the major glucose transporter in CRC cells and is indicated to be a poor prognostic indicator in patients with CRC. Recently, P2X7R and GLUT-1 are being investigated as prognostic biomarkers in the development of new treatment options. In this study, we aimed to investigate the prognostic value of P2X7R and GLUT-1 expression in CRC. We examined P2X7R and GLUT-1 expression in specimens of 196 CRC patients, immunohistochemically. P2X7R expression was higher in patients with poorly differentiated tumors than in those with well differentiated ones (P = 0.001). P2X7R and GLUT-1 overexpression were correlated to TILs (P<0.001; P = 0.028, respectively), depth of invasion (P<0.001; P = 014, repectively), distant metastasis (P<0.001), and advanced TNM stage (P<0.001). Moreover, multivariate Cox regression analysis showed that P2X7R overexpression clearly correlated with worsened overall survival (HR 4.69; 95% CI 1.77-12.41; P = 0.002). Similarly, patients with GLUT-1 overexpression showed shorter overall and disease-free survival than those with low expression. Our data support that P2X7R and GLUT-1 may be used as an independent prognostic markers and may present new options in terms of targeted therapies for CRC patients.
Keywords: P2X7R, colorectal cancer, GLUT-1, TILs, prognosis
Introduction
Colorectal cancer (CRC), a common malignant tumor in the gastrointestinal tract, is the third most common malignant neoplasm around the world and the second cause of cancer-related deaths [1-3]. The incidence of CRC is increasing day by day. Such that, while 1.4 million new cases were reported in 2012, this number increased to 1.8 million in 2018 [4]. Many advanced methods have been used to defeat the aggressive biologic features of CRC, including surgical approaches, chemotherapy, and targeted therapies, but the results are still unsatisfactory [3,5,6]. The prognosis of CRC is based on traditional histopathological examination, particularly evaluating the depth of tumor invasion (pT) and the status of lymph node metastasis (pN) [5]. However, the cause of different biological tumor behavior and the details of the molecular mechanisms underlying the pathogenesis of CRC are not fully understood [5,6]. For this reason, new biomarkers are needed to obtain more information about the pathogenesis of CRCs as well as to determine more reliable prognostic parameters and perhaps to establish new treatment modalities.
P2X7R is a member of the purinergic receptor family, which has attracted attention in recent years in relation to various malignant neoplasms [7,8]. P2X7R plays an important role in regulating inflammatory events. This exclusive molecule that contributes both adaptive and innate immunity is expressed from almost all inflammatory cell types, including lymphocytes, macrophages, dendritic cells, monocytes, neutrophils, basophils, eosinophils, and mast cells [8-10]. In addition, the P2X7 receptor has key regulatory abilities on aerobic glycolysis. The cells that express P2X7R upregulate glucose transporter 1 (GLUT-1) and various glycolytic enzymes. Moreover, expression of P2X7R inhibits pyruvate dehydrogenase activity, whereas increases hypoxia inducible factor 1α (HIF-1α) expression, and augments intracellular glycogen stores. These data demonstrate that the P2X7R has an unique ability to reorganize cell metabolism to compensate for the needs caused by adverse environmental conditions [11]. P2X7R is considered to be an important mediator for tumor invasion and metastasis as well as its role in inflammation and aerobic glycolysis [12]. In a few recent studies, it is claimed that P2X7R is associated with aggressive clinical features and poor prognosis in patients with CRC [5,6]. A recent study revealed that P2X7R was significantly associated with tumor progression, migration, and invasion [7]. In another study, it was reported that P2X7R regulate the cell survival and cell migration in ductal pancreatic adenocarcinoma cells and P2X7R allosteric inhibitor reduced cell proliferation [13]. Therefore, P2X7R may be a new biomarker for various cancers and a new option for targeted therapies [7,12,13].
Tumor cells have enhanced glucose uptake compared to healthy cells, thanks to a number of facilitating glucose transporter molecules located in the cytoplasmic membrane. GLUT-1 is one of these molecules that is facilitating glucose up-take [14]. It is a key molecule that plays an important role in the transport and metabolism of glucose in cancer cells as well as in normal cells [15]. GLUT-1 expression increases in hypoxic conditions due to reduced oxidative phosphorylation and HIF-1 induction [16]. Recent studies suggest that increased GLUT-1 expression in various types of cancer [17,18] can be observed. Therefore, it is assumed that high GLUT1 expression in human cancer cells may be an indicator of increased metabolic activity, increased energy usage, and aggressive biological behavior [15].
There are very few studies on the prognostic significance of P2X7R expression in CRC [5,6]. In this study, we aimed to investigate the prognostic significance of P2X7R and GLUT-1 expression in patients with CRC. In addition, we tried to analyze the effect of P2X7R expression on GLUT-1 overexpression and the role of these two molecules in the aggressive biological behavior of CRC.
Materials and methods
Patients and pathological materials
Present study was approved by the Ethical Committee of Fırat University on 17 September 2019 with the approval number 13-08. The study population consisted of 196 patients with CRC treated by surgical resection between 2009-2013 at a single institution. Patients were defined retrospectively by review of a pathological database. The cases that underwent preoperative chemotherapy and radiotherapy were not included to this study. The general clinical and pathological data were acquired from hospital medical archives and pathologic reports. The pathological materials of these patients were evaluated retrospectively by two pathologists (Calik I and Türken G) at different times. Histological tumor types were determined according to the criteria of the World Health Organisation (WHO) classification system. The TNM stages of the cases were specified according to the American Joint Committee on Cancer (AJCC), 7th edition. Survival data of the patients were collected from hospital medical data-processing record. Overall survival (OS) was determined as the interval between the dates of surgery and death.
Tissue microarray and immunohistochemistry
Immunohistochemistry (IHC) was performed using 5-µm thick histological tissue microarray slides. The following antibodies were used: anti-P2X7R (ab48871, ABCAM, Cambridge CB2 0AX, UK), anti-GLUT-1 (Ep141, ZETA, Arcadia CA91006, USA) and anti-CD8 (SP57, Ventana, Arizona, USA). The sections were stained using the Ventana Bench Mark Ultra autostainer (Ventana, Tucson, AZ-85755, USA) and the ultraView Universal DAB kit (Ventana, Tucson, AZ-85755, USA), following the manufacturer’s instructions. P2X7R and GLUT-1 expressions in tumor cells were evaluated by IHC. In addition, tumor infiltrating lymphocytes (TILs) were assessed by staining CD8. The immunostaining of each sample was examined by two objective researchers unaware of the clinical features, survival data, and pathological characteristics of the patients.
Evaluating of TILs
TILs density was evaluated using CD8 staining immunohistochemically and was scored as follows: Score 0, absent; score 1, weak, rare lymphocytes; score 2, moderate focal lymphocytic infiltration; and score 3, severe diffuse lymphocytic infiltration. Cases with scores of 0 and 1 were defined as low-TILs, and those with scores of 2 and 3 were defined as high-TILs [19].
P2X7 receptor expression scoring
The expression of P2X7R was evaluated by H-score (histochemistry score) which is obtained multiplying the staining intensity (0: negative, 1: weak staining, 2: medium staining, 3: strong staining) by the extent of staining (0, <10%; 1, 10%-25%; 2, 25%-70%; 3, >70%). H-score ranged from 0 to 9. The median value of the H score was accepted as the cut-off criterion. The cases with H-score below this value were considered as low-P2X7R group and those above were characterized as high-P2X7R group. These criteria had previously been validated in various studies [5,6].
GLUT-1 expression scoring
GLUT-1 expression in tumor cells was evaluated using a semi-quantitative scoring method validated in previous studies: score 0 = no staining; score 1 = 1-10% of cells stained; score 2 = 10-50% of cells stained; and score 3 = more than 50% of the cells stained. Cases were divided into two groups as low-GLUT-1 (score 0-2) and high-GLUT-1 (score 3). When scoring, bleeding sites, containing abundant erythrocytes and ulcerated-necrotic areas were excluded [14,16].
Statistical analysis
The data were analyzed statistically using SPSS v.20 software (IBM Corporation, Armonk, NY, USA) and were expressed as percentages, means, and standard deviations. The normal distribution of the data was evaluated with the Shapiro-Wilk test. P values >0.05 were accepted as indicating a normal distribution. Kurtosis and skewness values between -2 and +2 were also considered to indicate a normal distribution. While independent sample t-test and ANOVA were used to determine the differences between the normally distributed groups, Mann-Whitney U and Kruskal-Wallis H tests were used to determine the differences between data that were not normally distributed. The relationships between survival times and prognostic parameters were evaluated using the Kaplan-Meier method (log-rank test). Cox regression analysis was applied to estimate the hazard ratio (HR) and 95% confidence interval (CI) for univariate and multivariate models. The P<0.05 threshold was considered statistically significant for all data.
Results
Some classical clinical and pathological parameters are closely related to overall survival
Of patients, 89 were women and 107 were men. The range of age was 19-90 years. The median patient age was 60.0±13.84 years, and the mean follow-up time was 51.67±0.98 months. The tumor was localized in the right colon of 74 (37.8%) patients and in the left colon those of 122 (62.2%). Sixty five (33.7%) of the cases included in the study were TNM stage I, 21 (10.7%) were stage II, 48 (24.5%) were stage III and 62 (31.6%) were stage IV. The comprehensive clinic and pathologic features are showed in Table 1. The univariate Cox regression analysis showed that age, tumor site, histological grade, tumor infiltrating lymphocytes (TILs), depth of invasion (pT), lymph node metastasis (pN), and high TNM stage were significantly correlated with poor prognosis (Table 1). Among these, tumor site (P<0.001), TILs (P<0.001), and TNM stage (P<0.001) were determined to be more associated with survival. The mean OS in cases with TNM stage II was 56.00±10.65 months, whereas it was significantly lower in those with stage IV (38.27±14.54 months). Similarly, the mean OS of patients with low-TILs density (Figure 1A) was significantly lower than those with high density of TILs (Figure 1B) (40.69±15.92; 58.67±6.19, respectively). According to our data, there were no correlation between disease-free survival (DFS) and sex, tumor size, histopathologic tumor type, and vascular invasion (Table 1).
Table 1.
The correlation of clinicopathologic characteristics with overall and disease-free survival (n = 196)
| Parameters | n (%) | OS | DFS | ||
|---|---|---|---|---|---|
|
|
|
||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender | |||||
| Male | 107 (54.6) | 0.91 (0.54-1.55) | 0.754 | 0.93 (0.58-1.50) | 0.784 |
| Female | 89 (45.4) | ||||
| Age | |||||
| 19-44 | 21 (10.7) | 1.23 (0.34-4.42) | 0.010 | 0.63 (0.26-1.48) | 0.291 |
| 45-54 | 65 (33.2) | ||||
| ≥55 | 110 (56.1) | ||||
| Tumour site | |||||
| Right site | 74 (39.7) | 3.19 (1.86-5.47) | <0.001 | 0.40 (0.24-0.64) | <0.001 |
| Left site | 122 (60.3) | ||||
| Tumour size | |||||
| <5 cm | 80 (40.8) | 1.11 (0.64-1.89) | 0.702 | 1.39 (0.84-2.30) | 0.188 |
| ≥5 cm | 116 (59.2) | ||||
| Histopatologic type | |||||
| Adenocarcinoma | 126 (64.3) | 0.56 (0.25-1.29) | 0.177 | 2.00 (0.97-4.15) | 0.060 |
| Mucinous | 51 (26.0) | ||||
| Signet-ring | 19 (9.7) | ||||
| Histologic grade | |||||
| Well | 29 (14.8) | 0.38 (0.21-0.69) | 0.001 | 2.79 (1.26-6.19) | 0.003 |
| Moderate | 134 (68.4) | ||||
| Poor | 33 (16.8) | ||||
| Vascular invasion | |||||
| Absent | 59 (30.1) | 0.83 (0.46-1.50) | 0.545 | 1.52 (0.86-2.66) | 0.143 |
| Present | 137 (69.9) | ||||
| TILs | |||||
| Low | 153 (78.1) | 0.11 (0.02-0.45) | 0.002 | 0.09 (0.05-0.16) | 0.001 |
| High | 43 (21.9) | ||||
| Depth of invasion | |||||
| pT1 | 31 (15.8) | 0.43 (0.24-0.77) | 0.005 | 6.45 (2.31-17.97) | <0.001 |
| pT2 | 75 (38.3) | ||||
| pT3 | 90 (45.9) | ||||
| Lymph node status | |||||
| Absent | 97 (49.5) | 3.39 (1.64-6.99) | 0.001 | 3.30 (1.82-5.92) | <0.001 |
| 1-3 | 52 (56.5) | ||||
| ≥4 | 47 (24.0) | ||||
| Distant Metastasis | |||||
| Absent | 132 (67.3) | 0.49 (0.02-0.14) | <0.001 | 16.97 (9.37-30.75) | 0.042 |
| Present | 64 (32.7) | ||||
| TNM staging | |||||
| Stage I | 65 (33.7) | 0.12 (0.03-0.38) | <0.001 | 3.73 (1.14-12.13) | <0.001 |
| Stage II | 21 (10.7) | ||||
| Stage III | 48 (24.5) | ||||
| Stage IV | 62 (31.6) | ||||
OS: overal survival; DFS: disease-free survival; HR: Hazard ratio; CI: confidence interval.
Figure 1.

Representative images showing density of CD8+ tumor infiltrating lymphocytes (TILs) (A), low density of CD8+ TILs (arrows) (B), high density of CD-8+ TILs (arrow) in colorectal cancer (×200).
High P2X7R expression is associated with aggressive clinical and pathological features
P2X7R expression was not seen in non-tumoral colonic mucosa (Figure 2A). In contrast, 77 (39.3%) of the cases had overexpression of P2X7R. We specified the interrelationship between P2X7R expression and various classic pathological parameters of patients with CRC. According to the H-scores, we divided all cases with CRC (n = 196) into low-P2X7R expression group (Figure 2B) (n = 119, 60.7%) and high-P2X7R expression group (Figure 2C) (n = 77, 39.3%). As indicated in Table 2, high-P2X7R expression was significantly related with advanced age (P<0.001), right site (P<0.001), high grade (P = 0.001), low-TILs density (P<0.001) (Figure 3A), high-pT (P<0.001) (Figure 3B), high-pN (P = 0.001) (Figure 3C), and advanced TNM stage (P<0.001) (Figure 3D), but not with other classic pathological parameters such as gender, tumor size, histological type, and vascular invasion. On the other hand, we found a significant relationship between P2X7R expression and GLUT-1 expression (P<0.001). Patients with P2X7R overexpression also had GLUT-1 up-regulation (Figure 4).
Figure 2.

Representative examples of P2X7R expression in CRC specimens: (A) No staining (normal mucosa adjacent to tumor) (×200); (B) Low-P2X7R expression (arrows) (×400) in a patient with early stage; (C) High-expression of P2X7R (arrows) (×400) in a patient with advanced TNM stage. A tissue microarray containing 196 primary CRC and adjacent non-tumoral colonic mucosa were exposed to immunohistochemical evaluation using an anti-P2X7R antibody. Cytoplasmic positivity in tumor cells was considered. Histochemical score was determined by considering the intensity of cytoplasmic staining in the cells and distributions.
Table 2.
The relationship between P2X7R and GLUT-1 expressions and classical clinicopathological parameters
| Parameters | P2X7R expression | GLUT-1 expression | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Low n (%) | High n (%) | P value | Low n (%) | High n (%) | P value | |
| Gender | ||||||
| Male | 65 (54.6) | 42 (54.5) | 0.992‡ | 87 (55.1) | 20 (52.6) | 0.787‡ |
| Female | 54 (45.4) | 35 (45.5) | 71 (44.9) | 18 (47.4) | ||
| Age | ||||||
| 19-44 | 16 (13.4) | 5 (6.5) | <0.001† | 19 (12.0) | 2 (5.3) | 0.002† |
| 45-54 | 50 (42.0) | 15 (19.5) | 60 (38.0) | 5 (13.2) | ||
| ≥55 | 53 (44.5) | 57 (74.0) | 79 (50.0) | 31 (81.6) | ||
| Tumour site | ||||||
| Right site | 33 (27.7) | 41 (53.2) | <0.001‡ | 56 (35.4) | 18 (47.4) | 0.174‡ |
| Left site | 86 (72.3) | 36 (46.8) | 102 (64.6) | 20 (52.6) | ||
| Tumour size | ||||||
| <5 cm | 53 (44.5) | 27 (35.1) | 0.189‡ | 68 (43.0) | 12 (31.8) | 0.198‡ |
| ≥5 cm | 66 (55.5) | 50 (64.9) | 90 (57.0) | 26 (68.4) | ||
| Histopatologic type | ||||||
| Adenocarcinoma | 80 (67.2) | 46 (59.7) | 0.081† | 105 (66.5) | 21 (55.3) | 0.423† |
| Mucinous | 32 (26.9) | 19 (24.7) | 39 (24.7) | 12 (31.6) | ||
| Signet-ring | 7 (5.9) | 12 (15.6) | 14 (8.9) | 5 (13.2) | ||
| Histologic grade | ||||||
| Well | 21 (17.6) | 8 (10.4) | 0.001† | 24 (15.2) | 5 (13.2) | 0.223† |
| Moderate | 87 (73.1) | 47 (61.0) | 111 (70.3) | 23 (60.5) | ||
| Poor | 11 (9.2) | 22 (28.6) | 23 (14.6) | 10 (26.3) | ||
| Vascular invasion | ||||||
| Absent | 38 (31.9) | 21 (27.3) | 0.488‡ | 44 (27.8) | 15 (39.5) | 0.162‡ |
| Present | 81 (68.1) | 56 (72.7) | 114 (72.2) | 23 (60.5) | ||
| TILs density | ||||||
| Low | 85 (71.4) | 68 (88.3) | <0.001‡ | 121 (76.6) | 32 (84.2) | 0.028‡ |
| High | 34 (28.6) | 9 (11.7) | 37 (23.4) | 6 (15.8) | ||
| Depth of invasion | ||||||
| pT1 | 26 (21.8) | 5 (6.5) | <0.001† | 29 (18.4) | 2 (5.3) | 0.014† |
| pT2 | 54 (45.4) | 21 (27.3) | 64 (40.5) | 11 (28.9) | ||
| pT3 | 39 (32.8) | 51 (66.2) | 65 (41.1) | 25 (65.8) | ||
| Lymph node status | ||||||
| Absent | 72 (60.5) | 25 (32.5) | <0.001† | 82 (51.9) | 15 (39.5) | 0.224† |
| 1-3 | 27 (22.7) | 25 (32.5) | 42 (26.6) | 10 (26.3) | ||
| ≥4 | 20 (16.8) | 27 (35.1) | 34 (21.5) | 13 (34.2) | ||
| Distant Metastasis | ||||||
| Absent | 106 (89.1) | 26 (33.8) | <0.001‡ | 118 (74.7) | 14 (36.8) | <0.001‡ |
| Present | 13 (10.9) | 51 (66.2) | 40 (25.3) | 24 (63.2) | ||
| TNM staging | ||||||
| Stage I | 57 (47.9) | 8 (10.4) | <0.001† | 59 (37.3) | 6 (15.8) | <0.001† |
| Stage II | 15 (12.6) | 6 (7.8) | 15 (9.5) | 6 (15.8) | ||
| Stage III | 34 (28.6) | 14 (18.2) | 45 (28.5) | 3 (7.9) | ||
| Stage IV | 13 (10.9) | 49 (63.6) | 39 (24.7) | 23 (60.5) | ||
ANOVA.
Mann-Vitney U.
Figure 3.

Interrelation of P2X7R expression to classical clinicopathological parameters: the patients with high P2X7R expression showed low TILs density (A), advanced depth of tumor invasion (B), great number of lymph node metastasis (C), and advanced TNM stage (D).
Figure 4.

Representative diagram of the relationship between P2X7R and GLUT-1 expression: there was a positive correlation between the expression of both molecules. The cases with high P2X7R expression showed GLUT-1 overexpression.
Patients with high-P2X7R expression are correlated with low survival rates
Kaplan-Meier survival analysis was performed to compare the relationship between P2X7R expression and OS/DFS in patients with CRC (Figure 5A and 5B). By this means, we determined that the cases with high-P2X7R expression displayed clearly low mean survival time (41.51±0.71 months) than those with low-P2X7R expression (58.25±41.51 months). In addition, univariate and multivariate Cox regression analyzes were carried out to determine whether P2X7R expression and other pathological parameters could be used as independent prognostic indicators of CRC patients. As shown in Table 3, P2X7R expression together with TILs and TNM stage can be used as an independent indicator of poor prognosis in patients with CRC. In addition, when the cases were evaluated for DFS, both univariate and multivariate Cox regression analysis showed a significant correlation between high P2X7R expression and DFS (univariate: HR 9.98, 95% CI 5.89-17.82, P<0.001; multivariate: HR 3.85, 95% CI 1.93-7.65, P<0.001) (Table 4).
Figure 5.
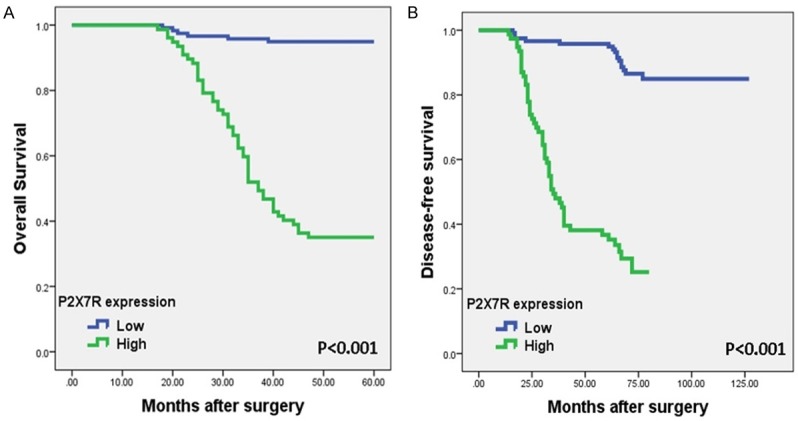
Representative Kaplan-Meier survival curves according to P2X7R expression. Patients with high-P2X7R expression had a worsened (A) overall and (B) disease-free survival rates than those with low-P2X7R expression.
Table 3.
Univariate and multivariate Cox regression analysis of P2X7R and GLUT-1 expression for correlation with overall survival
| Parameters | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex (male/female) | 0.91 (0.54-1.55) | 0.754 | 0.71 (0.40-1.26) | 0.245 |
| Age (19-44/45-54/≥55) | 1.23 (0.34-4.42) | 0.010 | 1.24 (0.78-1.97) | 0.361 |
| Location (right/left) | 3.19 (1.86-5.47) | <0.001 | 0.75 (0.41-1.38) | 0.362 |
| TILs (low/high) | 0.18 (0.10-0.32) | <0.001 | 0.48 (0.29-0.78) | 0.003 |
| pT (T1/T2/T3) | 0.43 (0.24-0.77) | 0.005 | 0.76 (0.45-1.29) | 0.316 |
| pN (absent/1-3/≥4) | 3.39 (1.64-6.99) | 0.001 | 1.14 (0.77-1.67) | 0.498 |
| TNM stage (I/II/III/IV) | 0.12 (0.03-0.38) | <0.001 | 2.56 (1.53-4.29) | <0.001 |
| P2X7R expression (low/high) | 8.86 (6.05-14.14) | <0.001 | 4.69 (1.77-12.41) | 0.002 |
| GLUT-1 expression (low/high) | 5.03 (2.96-8.53) | <0.001 | 1.84 (1.05-3.22) | 0.032 |
HR: hazard ratio, CI: confidence interval.
Table 4.
Univariate and multivariate Cox regression analysis of P2X7R and GLUT-1 expression for correlation with disease-free survival
| Parameters | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex (male/female) | 1.06 (0.66-1.72) | 0.784 | 0.71 (0.40-1.26) | 0.245 |
| Age (19-44/45-54/≥55) | 1.58 (0.67-3.73) | 0.291 | 1.24 (0.78-1.97) | 0.361 |
| Tumor site (right/left) | 0.40 (0.24-0.64) | <0.001 | 0.75 (0.41-1.38) | 0.362 |
| Grade (well/modarete/poor) | 2.79 (1.26-6.19) | 0.011 | 1.15 (0.73-1.81) | 0.529 |
| V.I. (absent/present) | 1.52 (0.86-2.66) | 0.143 | 0.77 (0.41-1.44) | 0.442 |
| TILs (low/high) | 0.18 (0.10-0.32) | <0.001 | 0.15 (0.08-0.27) | <0.001 |
| pT (T1/T2/T3) | 6.45 (2.31-17.97) | 0.001 | 0.91 (0.58-1.43) | 0.707 |
| pN (absent/1-3/≥4) | 3.96 (2.14-7.31) | <0.001 | 0.96 (0.67-1.36) | 0.830 |
| TNM stage (I/II/III/IV) | 3.73 (1.14-12.13) | 0.029 | 2.56 (1.52-3.20) | <0.001 |
| P2X7R expression (low/high) | 9.98 (5.89-17.82) | <0.001 | 3.85 (1.93-7.65) | <0.001 |
| GLUT-1 expression (low/high) | 3.89 (2.36-6.42) | <0.001 | 1.52 (0.96-2.01) | 0.043 |
HR: hazard ratio; CI: confidence interval; V.I.: vascular invasion.
GLUT-1 overexpression is significantly correlated with aggressive pathological features
GLUT-1 expression was not observed in the non-tumoral colonic mucosa adjacent to the tumor (Figure 6A). The low expression of GLUT-1 was observed in 158 (80.6%) of all cases (Figure 6B). When compared to adjacent normal colonic epithelium, tumoral sites from only 38 (19.4%) patients showed an over-expression of the GLUT-1 (Figure 6C). The changing of clinic and pathologic parameters according to GLUT-1 expression is shown in Table 2. There was no correlation between the GLUT-1 expression and gender, tumor site, tumor size, histopathologic type, grade, vascular invasion, and pN. However, GLUT-1 expression was closely related to TILs (P = 0.001, Mann-Whitney U). TILs were significantly lower in cases with high GLUT-1 expression. Likewise, there was a significant difference between patients with low and high GLUT-1 expression in terms of distant metastasis and TNM stage (P<0.001; P<0.001, respectively, ANOVA). Distant metastasis rates were higher in patients with high GLUT-1 expression than in low ones (Figure 7). Again, patients with high GLUT-1 expression had a more advanced TNM stage than those with low expression (Figure 7).
Figure 6.

Representative images of GLUT-1 expression in tissues of patients with CRC: (A) No staining in non-tumoral mucosa (×200); (B) Low-GLUT-1 expression (arows) (×400); (C) High-expression of GLUT-1 (arrows) (×400). Samples of 196 patients were immunohistochemically stained with anti-GLUT-1 antibody. Cytoplasmic positivity in the cells was evaluated. During the examination, staining in the areas adjacent to the ulcer containing dense erythrocytes was excluded.
Figure 7.

The correlation between GLUT-1 expression and classical clinicopathological parameters: the patients with GLUT-1 overexpression showed high-distant metastasis rates and advanced TNM stage.
Patients with GLUT-1 overexpression are associated with short survival time
According to the Kaplan-Meier method, it was clear that patients with GLUT-1 overexpression had shorter OS and DFS times than patients with low GLUT-1 expression (Figure 8A and 8B). This indicates that patients with high GLUT-1 expression have worse prognosis. Moreover, Cox multivariate analysis was performed to clarify the role of the GLUT-1 expression status in the survival times. As shown in Tables 3 and 4, high GLUT-1 expression can be used as an independent prognostic parameter for both OS (multivariate: HR 1.84 95% CI 1.05-3.22, P = 0.032) and DFS (multivariate: HR 1.52 95% 0.96-2.01, P = 0.043).
Figure 8.

Kaplan-Meier curves of (A) overall and (B) disease-free survival according to GLUT-1 expression. The patients with low GLUT-1 expression had significantly longer survival time than those with high expression.
Discussion
P2X7R, a cell membrane-associated purinergic receptor for ATP, is largely expressed in various inflammatory cells and plays an essential role in the development of rapidly occurring allergic inflammatory responses by allowing the release of important cytokines that regulate inflammatory events [5,7,9]. In addition to the expression of P2X7R mainly by various inflammatory cells, recent studies have reported the presence of P2X7R overexpression in various cancers [20-22]. Prolonged activation of P2X7R may promote tumor inflammatory microenvironment (TIM) and cancer progression by facilitating chronic inflammation. Tafani et al. stated that transcription factors such as HIF 1α and nuclear factor-kappa B (NF-κB) stimulate P2X7R expression in cancer cells and then activated P2X7R stimulate aggressive biological behavior and tumor invasion by triggering NF-kB activation [23]. In addition, Ryu et al. reported that in vivo inhibition of P2X7R via P2X7R antagonist brilliant blue G (BBG) weakened inflammatory activity in TIM, leading to a decrease in tumor growth [24]. These data suggest that the effects of P2X7R on tumor progression may be mediated by tumor-associated inflammatory processes [5].
Surprisingly, activation of P2X7R may reveal contrary effects in cancer cells according to different environmental conditions and various factors. It may show a pro-tumoral effect in some conditions and an anti-tumoral effect in others [25,26]. Among the purinergic receptors, P2X7R is the most associated with cell proliferation. Basal activation of P2X7R stimulates oxidative phosphorylation. It also contributes to tumor progression as it leads to increased intracellular ATP content. According to this view, tumor cells obtain an obvious proliferative advantage when compared to normal cells by activation of several intracellular pathways such as nuclear factor of activated T cells 1 (NFACTc1), extracellular signal-regulated kinase (ERK), phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB) and HIF-1α. Coherent with the information aforementioned, many pharmaceutical molecules used as P2X7R blockers have been tested in preclinical animal models, including BBG for ovarian cancer [27], AZ10606120 for pancreatic ductal adenocarcinoma [28], and A740003 for neuroblastoma [29]. In their study by transfecting mouse-P2X7R into mouse colon cancer cell line CT26, Adinolfi et al. showed that tumors expressing P2X7R have been shown to have a faster growth rate and to form a larger tumor mass. Subsequently, significant reduction in both growth rate and tumor size was observed with the injection of P2X7 blocker oxidized-ATP (oATP). Furthermore, it was found that tumors expressing mouse-P2X7 receptor in the CT26 cell line had a thicker vascular network and showed intense positive staining with VEGF [30]. However, in a recent study supporting the anti-tumoral effect of P2X7R, P2X7R has been shown to have anti-angiogenic effects on both tumor endothelial cells (TEC) and tumor endothelial progenitor cells (TEPC) [31]. In their study, Avanzato et al. reported that benzoyl ATP (BzATP), is a P2X7R agonist, has shown to suppress the migration of TECs in human breast cancer and thus slow down angiogenesis [31]. The anti-tumor effect of P2X7R can be prevented in vivo by reducing P2X7R functionality via adverse effects of conditions such as acidosis and hypoxia that are frequently found in some solid tumors. Furthermore, elevated ATP levels typically found in tumor stroma over-stimulate P2X7R to induce tumor cell death by increasing pro-apoptotic pores. This has been excitingly stated in various types of cancer. However, clinical researches have been far from validating the anti-cancer effect of extracellular ATP stimulation [25].
Despite many studies in various types of cancer, there are very few studies investigating the prognostic significance of P2X7R expression in CRCs. Qian et al. [5] in their study including 116 cases, investigated the P2X7R expression profile in CRC tissues. They found that P2X7R was significantly upregulated in human CRC tissues. Compared to peritumoral colorectal tissues, there was marked overexpression especially in highly malignant tumors. According to Qian et al. there was a statistical significance associated between high P2X7R expression and tumor size and lymph node metastasis in patients with CRC. Their results also stated that P2X7R overexpression was associated with higher tumor grade and advanced metastasis in CRC [5]. Morever, these findings may have implied that P2X7R ease the activation of the Akt/NF-κB signaling pathways in order to trigger the expression of Cyclin D1 and thus play an important role in advancing CRC cell proliferation.
P2X7R, an ATP receptor, was stated to contribute to tumor cell invasiveness. In prostate cancer cells, PI3K/AKT and ERK1/2 pathways were enhanced by the activation of P2X7R after ATP addition, with the downriver invasiveness-associated genes Claudin-1, E-cadherin, IL-8, and MMP-3 which is intended as further inductors [32]. In addition, MMP-3-dependent tumor cell invasion was also showed in mouse-CRC [33]. These data implied that an overexpression of P2X7R may also induce MMP-3-dependent tumor cell invasion in CRC. In another study investigating the effect of P2X7R on metastasis and prognosis in CRC, Zhang et al. reported that there was more prominent expression of P2X7R in patients with metastasis than in those without [6]. They determined that P2X7R expression was up-regulated in metastatic cancer cells and cell lines. In addition, Zhang et al. [6] reported that the survival time in CRC patients with P2X7R expression was significantly shorter than in those with low expression. Similar to previous studies, in our study, more intense expression of P2X7R was seen in tumoral areas compared to non-tumoral sites. The expression of P2X7R was associated with classical clinic and pathological parameters such as age, localization, and histologic grade. P2X7R overexpression was closely correlated with aggressive pathological features including advanced-pT, increased number of metastatic lymph nodes, high distant metastasis rates, and advanced TNM stage. In addition, there also were interrelationship TILs and P2X7R expression. Interestingly, the tumors with P2X7R overexpression had low TILs density. When all these data are evaluated together, it can be said that P2X7R overexpression in CRC is closely related with tumor progression, invasiveness, and ability of distant metastasis. Furthermore, the low density of TILs in cases with P2X7R overexpression suggests that P2X7R promotes the inflammatory events at the early period of tumor, but significantly suppresses the host inflammatory response to the tumor as the process progresses. Additionally, in our study, P2X7R overexpression was shown to be associated with poor prognosis and low survival rates according to both univariate and multivariate Cox regression analysis. Moreover, according to our data, some classical prognostic parameters, especially TILs density, pT, pN, and TNM stage, were closely related to both OS and DFS in univariate analysis. However, multivariate analysis showed that only TNM stage could be used as an independent prognostic marker.
P2X7R has also considerable effects on energy metabolism as well as other effects including inflammatory events and tumor progression. Basal expression of P2X7R promotes oxidative phosphorylation by moderately increasing cytoplasmic and mitochondrial Ca2+, but Ca2+ concentrations are kept below toxic levels [34]. In addition to stimulating oxidative phosphorylation, P2X7R also increases glucose uptake by stimulating GLUT-1 expression and supports aerobic glycolysis, thereby playing an important role in meeting the energy requirements of tumor cells [6,11]. Therefore, P2X7R overexpression in CRC cells may positively affect tumor metabolism by stimulating GLUT-1 and may serve as a supportive factor for CRC tumor proliferation and metastasis [6]. GLUT-1, the first identified glucose transporter molecule, provide the necessary glucose uptake for energy metabolism by mediating the transport of basal glucose in tumor cells [35]. Many studies centering the GLUT-1 molecule have shown increased GLUT1 expression in various types of cancer [15,17,18,36,37]. This suggests that it may be a marker for malignant transformation [15]. Most of these studies showed that GLUT-1 overexpression could be a prognostic indicator in CRCs. Recently, Chung et al. [38] analyzed GLUT1 mRNA in the peripheral blood of 100 patients with CRC and found out high levels in patients with stage II and III CRC compared with stage I; but, they did not further investigate whether GLUT1 is a prognostic factor in these patients. Earlier researches have demonstrated an interrelationship between Glut-1 overexpression and OS in CRC [36] and lung cancer [39], and DFS in ovarian cancer [40]. In addition, according to Airley et al., there was a significant correlation between increased Glut-1 expression and metastasis-free survival in patients with cervical carcinoma. However, it has been claimed that there is no significant relationship between P2X7R overexpression and DFS or local relapse [41]. In a later study Cooper et al. stated that the strong expression of Glut-1 was a significant prognostic factor for OS in rectal cancer patients independent of tumor stage and tumor size according to multivariate analysis. They also observed a significant association with local recurrence-free survival, but no significant correlation was found between GLUT-1 expression and metastasis-free survival [14]. Consistent with many previous studies, in our study, univariate and multivariate Cox regression analyzes clearly showed that GLUT-1 overexpression could be used as an independent poor prognostic marker for both OS and DFS in patients with CRC. The expression of GLUT-1 was stronger in advanced stage patients than in low ones and these patients showed lower survival rates. The correlation between GLUT-1 expression and lymph node metastasis has been previously reported in CRC [36,42]. In contrast to previous studies, in this study, there was no statistically significant relationship between GLUT-1 overexpression and pN. In addition, there also was no correlation between GLUT-1 expression and sex, tumor site, histologic grade, and vascular invasion in our data. In contrast, GLUT-1 overexpression was closely associated with advanced tumor depth of invasion, high TILs density, high distant metastasis rate, and advanced TNM stage. Based on these findings, it might be expressed that GLUT1 overexpression plays an important role of tumor growth and progression and is a potential biomarker for prognosis in patients with CRC.
We also investigated whether there was a correlation between P2X7R expression and GLUT-1 expression. According to our data, the expression intensities of both molecules, which were determined immunohistochemically, were statistically correlated with each other. In patients with P2X7R overexpression, GLUT-1 upregulation was also determined at the same time. This data supporting the hypotheses mentioned in previous studies [6,11] has shown that P2X7R facilitates glucose uptake in tumor cells by increasing GLUT-1 expression and thereby enable tumor cells to easily adapt to unfavorable microenvironment conditions. Moreover, it can be claimed that P2X7R and GLUT-1 exhibit complementary effects, leading to tumor progression and aggressive biological behavior.
As a summary, we determined that P2X7R overexpression was significantly correlated with aggressive clinical and pathological features. Furthermore, the patients with P2X7R overexpression showed poor OS and DFS. Similarly, it was shown that GLUT-1 overexpression is a poor prognostic indicator and can be used as an independent prognostic parameter in patients with CRC. Thus, it may be considered that P2X7R and GLUT-1 may serve as a valuable biomarker in predicting prognosis for patients with CRC in the future and may be a potential therapeutic target.
Disclosure of conflict of interest
None.
References
- 1.Yang D, Lai X, Xu F, Li Y, Jiang W, Ma D. Prognosis and clinical characteristics of colorectal cancer patients with KRAS gene mutation: a 5-year follow-up study. Int J Clin Exp Pathol. 2019;12:409–418. [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Chen G, Zheng X, Chen Y. Expression status of four mismatch repair proteins in patients with colorectal cancer: clinical significance in 1238 cases. Int J Clin Exp Pathol. 2019;12:3685–3699. [PMC free article] [PubMed] [Google Scholar]
- 3.Wang B, Wang Y, Yan Z, Sun Y, Su C. Colorectal cancer cell-derived exosomes promote proliferation and decrease apoptosis by activating the ERK pathway. Int J Clin Exp Pathol. 2019;12:2485–2495. [PMC free article] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 5.Qian F, Xiao J, Hu B, Sun N, Yin W, Zhu J. High expression of P2X7R is an independent postoperative indicator of poor prognosis in colorectal cancer. Hum Pathol. 2017;64:61–68. doi: 10.1016/j.humpath.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Ding J, Wang L. The role of P2X7 receptor in prognosis and metastasis of colorectal cancer. Adv Med Sci. 2019;64:388–394. doi: 10.1016/j.advms.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Roger S, Jelassi B, Couillin I, Pelegrin P, Besson P, Jiang LH. Understanding the roles of the P2X7 receptor in solid tumour progression and therapeutic perspectives. Biochim Biophys Acta. 2015;1848:2584–2602. doi: 10.1016/j.bbamem.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G, Knight GE. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal. 2018;14:1–18. doi: 10.1007/s11302-017-9593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity. 2017;47:15–31. doi: 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G, Boeynaems JM. Purinergic signalling and immune cells. Purinergic Signal. 2014;10:529–564. doi: 10.1007/s11302-014-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amoroso F, Falzoni S, Adinolfi E, Ferrari D, Di Virgilio F. The P2X7 receptor is a key modulator of aerobic glycolysis. Cell Death Dis. 2012;3:e370. doi: 10.1038/cddis.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JH, Williams DR, Lee JH, Lee SD, Lee JH, Ko H, Lee GE, Kim S, Lee JM, Abdelrahman A, Muller CE, Jung DW, Kim YC. Potent suppressive effects of 1-piperidinylimidazole based novel P2X7 receptor antagonists on cancer cell migration and invasion. J Med Chem. 2016;59:7410–7430. doi: 10.1021/acs.jmedchem.5b01690. [DOI] [PubMed] [Google Scholar]
- 13.Giannuzzo A, Pedersen SF, Novak I. The P2X7 receptor regulates cell survival, migration and invasion of pancreatic ductal adenocarcinoma cells. Mol Cancer. 2015;14:203. doi: 10.1186/s12943-015-0472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper R, Sarioğlu S, Sökmen S, Füzün M, Küpelioğlu A, Valentine H, Görken IB, Airley R, West C. Glucose transporter-1 (GLUT-1): a potential marker of prognosis in rectal carcinoma? Br J Cancer. 2003;89:870–876. doi: 10.1038/sj.bjc.6601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen YM, Arbman G, Olsson B, Sun XF. Overexpression of GLUT1 in colorectal cancer is independently associated with poor prognosis. Int J Biol Markers. 2011;26:166–72. doi: 10.5301/JBM.2011.8550. [DOI] [PubMed] [Google Scholar]
- 16.Brophy S, Sheehan KM, McNamara DA, Deasy J, Bouchier-Hayes DJ, Kay EW. GLUT-1 expression and response to chemoradiotherapy in rectal cancer. Int J Cancer. 2009;125:2778–2782. doi: 10.1002/ijc.24693. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki H, Shitara M, Yokota K, Hikosaka Y, Moriyama S, Yano M, Fuji Y. Overexpression of GLUT1 correlates with Kras mutations in lung carcinomas. Mol Med Rep. 2012;5:599–602. doi: 10.3892/mmr.2011.736. [DOI] [PubMed] [Google Scholar]
- 18.Maki Y, Soh J, Ichimura K, Shien K, Furukawa M, Muraoka T, Tanaka N, Ueno T, Yamamoto H, Asano H, Tsukuda K, Toyooka S, Miyoshi S. Impact of GLUT1 and Ki-67 expression on early stage lung adenocarcinoma diagnosed according to a new international multidisciplinary classification. Oncol Rep. 2013;29:133–140. doi: 10.3892/or.2012.2087. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Kong P, Wang X, Yang L, Jiang C, He W, Quan Q, Huang J, Xie Q, Xia X, Zhang B, Xia L. Tumor microenvironment classification based on T-cell infiltration and PD-L1 in patients with mismatch repair-proficient and -deficient colorectal cancer. Oncol Lett. 2019;17:2335–2343. doi: 10.3892/ol.2018.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong JH, Zheng GG, Zhu XF, Guo Y, Wang L, Ma CH, Liu SY, Xu LL, Lin YM, Wu KF. Abnormal expression of P2X family receptors in Chinese pediatric acute leukemias. Biochem Biophys Res Commun. 2010;391:498–504. doi: 10.1016/j.bbrc.2009.11.087. [DOI] [PubMed] [Google Scholar]
- 21.Solini A, Cuccato S, Ferrari D, Santini E, Gulinelli S, Callegari MG, Dardano A, Faviana P, Madec S, Di Virgilio F, Monzani F. Increased P2X7 receptor expression and function in thyroid papillary cancer: a new potential marker of the disease? Endocrinology. 2008;149:389–396. doi: 10.1210/en.2007-1223. [DOI] [PubMed] [Google Scholar]
- 22.Raffaghello L, Chiozzi P, Falzoni S, Di Virgilio F, Pistoia V. The P2X7 receptor sustains the growth of human neuroblastoma cells through a substance P-dependent mechanism. Cancer Res. 2006;66:907–914. doi: 10.1158/0008-5472.CAN-05-3185. [DOI] [PubMed] [Google Scholar]
- 23.Tafani M, Russo A, Vito MD, Sale P, Pellegrini L, Schito L, Gentileschi S, Bracaglia R, Marandino F, Garaci E, Russo MA. Up-regulation of pro-inflammatory genes as adaptation to hypoxia in MCF-7 cells and in human mammary invasive carcinoma microenvironment. Cancer Sci. 2010;101:1014–1023. doi: 10.1111/j.1349-7006.2010.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu JK, Jantaratnotai N, Serrano-Perez MC, McGeer PL, Mclarnon JG. Block of purinergic P2X7R inhibits tumor growth in a C6 glioma brain tumor animal model. J Neuropathol Exp Neurol. 2011;70:13–22. doi: 10.1097/NEN.0b013e318201d4d4. [DOI] [PubMed] [Google Scholar]
- 25.Scarpellino G, Genova T, Munaron L. Purinergic P2X7 receptor: a cation channel sensitive to tumor microenvironment. Recent Pat Anticancer Drug Discov. 2019;14:32–38. doi: 10.2174/1574892814666190116122256. [DOI] [PubMed] [Google Scholar]
- 26.Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer. 2018;18:601–618. doi: 10.1038/s41568-018-0037-0. [DOI] [PubMed] [Google Scholar]
- 27.Vázquez-Cuevas FG, Martínez-Ramírez AS, Robles-Martínez L, Garay E, García-Carrancá A, Pérez-Montiel D, Castañeda-García C, Arellano RO. Paracrine stimulation of P2X7 receptor by ATP activates a proliferative pathway in ovarian carcinoma cells. J Cell Biochem. 2014;115:1955–1966. doi: 10.1002/jcb.24867. [DOI] [PubMed] [Google Scholar]
- 28.Giannuzzo A, Saccomano M, Napp J. Targeting of the P2X7 receptor in pancreatic cancer and stellate cells. Int J Cancer. 2016;139:2540–2552. doi: 10.1002/ijc.30380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amoroso F, Capece M, Rotondo A. The P2X7 receptor is a key modulator of the PI3K/GSK3β/VEGF signaling network: evidence in experimental neuroblastoma. Oncogene. 2015;34:5240–5251. doi: 10.1038/onc.2014.444. [DOI] [PubMed] [Google Scholar]
- 30.Adinolfi E, Raffaghello L, Giuliani AL. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72:2957–2969. doi: 10.1158/0008-5472.CAN-11-1947. [DOI] [PubMed] [Google Scholar]
- 31.Avanzato D, Genova T, Fiorio Pla A. Activation of P2X7 and P2Y11 purinergic receptors inhibits migration and normalizes tumor-derived endothelial cells via cAMP signaling. Sci Rep. 2016;6:32602–32608. doi: 10.1038/srep32602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu Y, Li WH, Zhang HQ, Liu Y, Tian XX, Fang WG. P2X7 mediates ATP-driven invasiveness in prostate cancer cells. PLoS One. 2014;9:e114371. doi: 10.1371/journal.pone.0114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji Y, Li J, Li P, Wang L, Yang H, Jiang G. C/EBPβ promotion of MMP3-dependent tumor cell invasion and association with metastasis in colorectal cancer. Genet Test Mol Biomarkers. 2018;22:5–10. doi: 10.1089/gtmb.2017.0113. [DOI] [PubMed] [Google Scholar]
- 34.Adinolfi E, Callegari MG, Ferrari D, Bolognesi C, Minelli M, Wieckowski MR, Pinton P, Rizzuto R, Di Virgilio F. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serumindependent growth. Mol Biol Cell. 2005;16:3260–3272. doi: 10.1091/mbc.E04-11-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J, Wen J, Tian T, Lu Z, Wang Y, Wang Z, Wang X, Yang Y. GLUT-1 overexpression as an unfavorable prognostic biomarker in patients with colorectal cancer. Oncotarget. 2017;8:11788–11796. doi: 10.18632/oncotarget.14352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haber RS, Rathan A, Weiser KR, Pritsker A, Itzkowitz SH, Bodian C, Slater G, Weiss A, Burstein DE. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer. 1998;83:34–40. doi: 10.1002/(sici)1097-0142(19980701)83:1<34::aid-cncr5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 37.Younes M, Lechago LV, Somoano JR, Mosharaf M, Lechago J. Wide expression of the human erythrocyte glucose transporter Glut1 in human cancers. Cancer Res. 1996;56:1164–1167. [PubMed] [Google Scholar]
- 38.Chung FY, Huang MY, Yeh CS, Chang HJ, Cheng TL, Yen LC, Yen LC, Wang JY, Lin SR. GLUT1 gene is a potential hypoxic marker in colorectal cancer patients. BMC Cancer. 2009;9:241. doi: 10.1186/1471-2407-9-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Younes M, Brown RS, Stephenson M, Gondo M, Cagle PT. Overexpression of Glut1 and Glut3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer. 1997;80:1046–1051. doi: 10.1002/(sici)1097-0142(19970915)80:6<1046::aid-cncr6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Cantuaria G, Fagotti A, Ferrandina G, Magalhaes A, Nadji M, Angioli R, Penalver M, Mancuso S, Scambia G. Glut-1 expression in ovarian carcinoma. Association with survival and response to chemotherapy. Cancer. 2001;92:1144–1150. doi: 10.1002/1097-0142(20010901)92:5<1144::aid-cncr1432>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 41.Airley R, Loncaster J, Davidson S, Bromley M, Roberts S, Patterson A, Hunter R, Stratford I, West C. Glucose transporter Glut-1 expression correlates with tumour hypoxia and predicts metastasis-free survival in advanced carcinoma of the cervix. Clin Cancer Res. 2001;7:928–934. [PubMed] [Google Scholar]
- 42.Younes M, Lechago LV, Lechago J. Overexpression of the human erythrocyte glucose transporter occurs as a late event in human colorectal carcinogenesis and is associated with an increased incidence of lymph node metastases. Clin Cancer Res. 1996;2:1151–1154. [PubMed] [Google Scholar]


