Abstract
This study aimed to determine the correlation of human epidermal growth factor receptor 2 (HER2) codon 655 A>G polymorphism with cardiotoxicity risk in HER2-positive breast cancer patients undergoing epirubicin/cyclophosphamide followed by docetaxel plus trastuzumab (EC-D-T) adjuvant chemotherapy. Peripheral blood from 91 HER2-positive breast cancer patients was collected for HER2 codon 655 A>G genotyping before initiation of EC-D-T adjuvant chemotherapy (M0). Left ventricular ejection fraction (LVEF), cardiac troponin I (cTnI), and N terminal pro B type natriuretic peptide (NT-proBNP) levels were measured at M0, M3, M6, M9, M12 and M15. Cardiotoxicity was assessed at each time point after initiation of adjuvant chemotherapy. There were 77 cumulative cardiotoxicity events, and totally 26 patients had cardiotoxicity with incidence of 28.6% during the study. LVEF was decreased, cTnI was increased but NT-proBNP was similar in cardiotoxicity patients compared to non-cardiotoxicity patients at each time point. Additionally, the prevalences of HER2 codon 655 AA, AG, GG genotypes were 70.3%, 26.4% and 3.3% respectively. LVEF was lower at each time point after initiation of adjuvant chemotherapy and incidence of cardiotoxicity was increased in patients with HER2 codon 655 AG/GG genotypes compared to those with HER2 codon 655 AA genotype. Logistic regression analysis further revealed that HER2 codon 655 A>G, smoking and baseline cTnI were independent predictive factors for increased cardiotoxicity risk. In conclusion, HER2 codon 655 A>G was an independent predictive factor for increased cardiotoxicity risk in HER2-positive breast cancer patients undergoing EC-D-T adjuvant chemotherapy.
Keywords: HER2, single nucleotide polymorphism, anthracycline, trastuzumab, cardiotoxicity
Introduction
Human epidermal growth factor receptor 2 (HER2)-positive breast cancer is one of the main subclassifications that accounts for 15-20% of all breast cancer cases, as well as a relatively aggressive breast cancer subtype that results in poor survival [1,2]. Inclusion of HER2-targeted agents, especially trastuzumab in anthracycline/cyclophosphamide + taxane (e.g. Epirubicin/cyclophosphamide followed by docetaxel plus trastuzumab, EC-D-T) regimens is the preferred adjuvant chemotherapy schedule for HER2-positive breast cancer patients, and this provides a notable increase in overall survival and disease-free survival [3]. However, anthracyclines are well-known for their cardiotoxic nature, and mounting evidence has also suggested that trastuzumab is a potential contributor to cardiotoxicity in HER2-positive breast cancer patients, which increases damage to cardiac function and hampers the scheduled administration of adjuvant chemotherapy for HER2 breast cancer patients [4-8]. In addition, there is currently no validated biomarker for detection of chemotherapy-induced cardiotoxicity, which presents no sign or symptoms at initiation [9]. Therefore, investigation of factors predicting the occurrence of cardiotoxicity is necessary for developing a personalized drug administration schedule and improving prognosis in HER2-positive breast cancer patients.
It is proposed that HER2 signaling induced by trastuzumab is responsible for cardiotoxicity in HER2-positive breast cancer patients, and polymorphisms in the HER2 gene have been illuminated to influence the risk of HER2 breast cancer [4]. HER2 codon 655 A>G is a common polymorphism in HER2 gene which encodes either isoleucine (Ile, ATC) or valine (Val, GTC), and HER2 codon 655 AG carriers are under higher risk of trastuzumab-induced cardiotoxicity compared to HER2 codon 655 AA carriers in Spain, French and Canadian HER2-positive breast cancer patients [10-12]. However, due to genetic variation among different populations, the existing evidence cannot be equally applied in Chinese population. With the upgrading of the preferred adjuvant chemotherapy regimens for HER2-positive breast cancer, the correlation of HER2 codon 655 polymorphism with cardiotoxicity risk in HER2-positive breast cancer patients receiving EC-D-T adjuvant chemotherapy is still unknown. Thus, our study aimed to determine the role of HER2 codon 655 A>G polymorphism in predicting cardiotoxicity risk in HER2-positive breast cancer patients undergoing EC-D-T adjuvant chemotherapy.
Methods
Patients
Ninety-one patients with HER2-positive breast cancer who underwent surgery in the Central Hospital of Wuhan from July 2013 to June 2017 were consecutively enrolled in the present study. The inclusion criteria were: (1) diagnosed as breast cancer confirmed by clinicopathologic examination; (2) HER2-positivity confirmed by immunohistochemistry (IHC) or amplification of fluorescence in situ hybridization; (3) underwent surgery and scheduled to receive EC-D-T adjuvant chemotherapy; (4) age older than 18 years; (5) left ventricular ejection fraction (LVEF) ≥55%; (6) had no history of chemotherapy or neoadjuvant therapy. Patients were excluded if they had the following conditions: (1) contraindications to chemotherapy; (2) impaired cardiac function (e.g., uncontrolled or symptomatic angina, clinically significant arrhythmias, congestive heart failure and transmural myocardial infarction); (3) serious infection; (4) other concurrent tumors; (5) life expectancy less than 12 months; (6) unable to be regularly followed up; (7) pregnant. This study was approved by the Institutional Review Board of Central Hospital of Wuhan and was performed in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent.
Adjuvant chemotherapy
Adjuvant chemotherapy of EC-D-T was administered to patients within one month after surgery according to the patients’ status, and the EC-D-T regimen was given as follows: Epirubicin (E) at a dose of 100 mg/m2 intravenous (IV) infusion on day 1, Cyclophosphamide (C) at a dose of 600 mg/m2 IV infusion on day 1. Both E and C were repeated every 21 days for 4 cycles (M3), then followed by docetaxel (D) 75-100 mg/m2 IV infusion on day 1 every 21 days for 4 cycles (M6), trastuzumab was administrated at a dose of 4 mg/kg in the first week concurrently with docetaxel, followed by a dose of 2 mg/kg weekly for subsequent 11 weeks, then followed by a dose of 6 mg/kg every 21 days to complete 1 year of trastuzumab therapy (M3-M15). Treatment protocol and measurements were shown in Figure 1.
Figure 1.
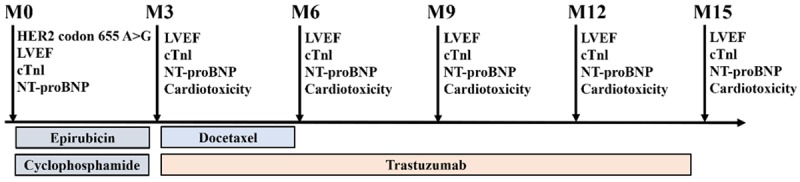
Treatment protocol and measurements. HER2 codon 655 A>G polymorphism, LVEF, cTnl, and NT-proBNP were detected at baseline (M0); LVEF, cTnI, NT-proBNP and cardiotoxicity were detected at M3, M6, M9, M12, and M15. LVEF, left ventricular ejection fraction; cTnI, cardiac troponin I; NT-proBNP, N terminal pro B type natriuretic peptide.
Sample collection and measurement
Peripheral blood samples were collected in vacutainer tubes containing EDTA before the initiation of EC-D-T adjuvant chemotherapy (M0), and at M3, M6, M9, M12 and M15, respectively. All samples were divided into two parts, one was immediately coagulated for 20 minutes at 4°C 2000 r/min to sperate plasma for the measurement of cardiac troponin I (cTnI) and N-terminal pro brain natriuretic peptide (NT-proBNP), and the other was stored at -80°C for further analysis. The plasma levels of cTnI and NT-proBNP were measured using Elecsys 2010 Automatic Electrochemiluminescence Immuno-analyzer and correlative kits (Roche, Basel, Switzerland).
HER2 codon 655 A>G genotyping
Peripheral blood leukocytes were separated from the blood samples collected at M0, and genomic DNA was isolated from the leukocytes using a phenol-chloroform assay. The HER2 codon 655 A>G was detected by a polymerase chain reaction-restriction fragment-length polymorphism (PCR-RFLP) assay. DNA was amplified in a Perkin-Elmer GeneAmp PCR System 9700 (Perkin-Elmer Corp., Norwalk City, CT, USA) according to the manufacturer’s protocol. The primers were as follows: forward: 5’-AGAGCGCCAGCCCTCTGACGTCCAT-3’, reverse: 5’-TCCGTTTCCTGCAGCAGTCTCCGCA-3’. Amplification condition was: 94°C for 5 min followed by 35 cycles of 94°C for 30 s, 62°C for 30 s, and 72°C for 30 s followed by a final extension step at 72°C for 5 min. After PCR amplification, the PCR products (148 bp) were digested with BsmAI restriction enzyme (New England Biolabs, Inc., Beverly, MA, USA) for 3 h at 55°C, and the DNA fragments were visualized on a 3.0% agarose gel. The presence of G at the codon 655 of the HER-2 gene was identified by 116 and 32 bp fragments and presence of A at this position by a single 148 bp product.
Cardiotoxicity assessment
Monitoring of ultrasound cardiogram was performed at M0, M3, M6, M9, M12 and M15, and LVEF was assessed according to the American Society of Echocardiography recommendations [13]. Cardiotoxicity was defined as the occurrence of any of the following after initiation of adjuvant chemotherapy: (1) an absolute decline of LVEF at least 10 percentage points from baseline to a value <53% identified by echocardiogram [14], (2) heart failure, acute coronary artery syndrome or fatal arrhythmia [15].
Statistical analysis
Normally distributed continuous variables were presented as mean value ± standard deviation. Skewed distributed or unknown distributed continuous variables were expressed as median (25th-75th quantiles), and the comparison was determined by Wilcoxon rank sum test or Kruskal-Wallis H test. Categorized variables were described as count (percentage), and the comparison was determined by Chi-square test. Addictive model of HER2 codon 655 A>G (AA=0, AG=1, GG=2) was used in the univariate and multivariate logistic regression model analysis of factors affecting cardiotoxicity occurrence. Statistical analysis was performed by use of SPSS 22.0 software (SPSS Inc, Chicago, IL, USA), and GraphPad Prism 7.01 software (GraphPad Software, La Jolla, CA, USA) was used for making figures. P value <0.05 was considered significant.
Results
Patients’ characteristics
The mean age of 91 HER-positive breast cancer patients was 50.4±7.4 years, and the mean BMI was 21.9±2.2 kg/m2 (Table 1). The number (percentage) of patients who smoke, had hypertension, diabetes mellitus, dyslipidemia, hyperuricemia and chronic kidney disease were 20 (22.0%), 23 (25.3%), 5 (5.5%), 22 (24.2%), 18 (19.8%) and 5 (5.5%) respectively. In addition, the baseline median values of patients’ LVEF, cTnI, and NT-proBNP were 67.0 (64.0-71.0)%, 0.039 (0.012-0.079) ng/mL and 0.065 (0.052-0.087) ng/mL respectively. As for HER2 codon 655 A>G distribution, 64 (70.3%) patients had AA genotype, 24 (26.4%) had AG genotype and 3 (3.3%) had GG genotype. Other detailed baseline characteristics are listed in Table 1.
Table 1.
Baseline characteristics of HER2-positive breast cancer patients
| Characteristics | Patients (N=91) |
|---|---|
| Age (years) | 50.4±7.4 |
| BMI (kg/m2) | 21.9±2.2 |
| Smoking (n/%) | 20 (22.0) |
| Chronic comorbidities (n/%) | |
| Hypertension | 23 (25.3) |
| Diabetes mellitus | 5 (5.5) |
| Dyslipidemia | 22 (24.2) |
| Hyperuricemia | 18 (19.8) |
| Chron HER2 is a member of HER family of tyrosine kinase receptors, who regulates signaling pathways such as Ras/p42/44 MAPK and PI3K/AKT that involve in cell apoptosis, metastasis, ic kidney disease | 5 (5.5) |
| ECOG score (n/%) | |
| 0 | 64 (70.3) |
| 1 | 27 (29.7) |
| LVEF (%) | 67.0 (64.0-71.0) |
| cTnI (ng/mL) | 0.039 (0.012-0.079) |
| NT-proBNP (ng/mL) | 0.065 (0.052-0.087) |
| HER2 codon 655 A>G (n/%) | |
| AA | 64 (70.3) |
| AG | 24 (26.4) |
| GG | 3 (3.3) |
Data are presented as mean value ± standard deviation, count (percentage) or median (25th-75th quantiles). HER2: human epidermal growth factor receptor-2; BMI: body mass index; ECOG: Eastern Cooperative Oncology Group; LVEF: left ventricular ejection fraction; cTnI: cardiac troponin I; NT-proBNP: N terminal pro B type natriuretic peptide.
Cardiotoxicity events and incidences
During the study, no heart failure event, acute coronary syndrome event, or life-threatening arrhythmias event was observed in HER2-positive breast cancer patients undergoing EC-D-T adjuvant chemotherapy (Table 2). The total events of ΔLVEF≥10%, LVEF<53%, and ΔLVEF≥10%&LVEF<53% were 173, 89 and 77 cases respectively. The total cardiotoxicity events were 77 cases, and the cumulative cardiotoxicity incidences of patients were 0 (0.0%), 0 (0.0%), 12 (13.2%), 20 (22.0%), 24 (26.4%) and 26 (28.6%) at M0, M3, M6, M9, M12 and M15 respectively.
Table 2.
Cardiotoxicity events and incidence at each visit
| Items | M0 | M3 | M6 | M9 | M12 | M15 | Total events |
|---|---|---|---|---|---|---|---|
| Heart failure event (n/%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 |
| Acute coronary syndrome event (n/%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 |
| Life-threatening arrhythmia event (n/%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 |
| ΔLVEF≥10% event (n/%) | 0 (0.0) | 7 (7.7) | 30 (33.0) | 54 (59.3) | 39 (42.9) | 43 (47.3) | 173 |
| LVEF<53% event (n/%) | 0 (0.0) | 2 (2.2) | 16 (17.6) | 23 (25.3) | 24 (26.4) | 24 (26.4) | 89 |
| ΔLVEF≥10%&LVEF<53% event (n/%) | 0 (0.0) | 0 (0.0) | 12 (13.2) | 20 (22.0) | 22 (24.2) | 23 (25.3) | 77 |
| Cardiotoxicity event (n/%) | 0 (0.0) | 0 (0.0) | 12 (13.2) | 20 (22.0) | 22 (24.2) | 23 (25.3) | 77 |
| Cumulative cardiotoxicity incidence (n/%) | 0 (0.0) | 0 (0.0) | 12 (13.2) | 20 (22.0) | 24 (26.4) | 26 (28.6) | - |
Data are presented as count (percentage). LVEF: left ventricular ejection fraction; ΔLVEF: LVEF change from baseline.
Difference of LVEF, cTnl, and NT-proBNP between patients with cardiotoxicity and non-cardiotoxicity
According to the cumulative cardiotoxicity incidence of patients, there were 26 patients (28.6%) developed cardiotoxicity (cardiotoxicity group) and 65 patients (71.4%) did not (non-cardiotoxicity group). Comparisons of LVEF between patients with cardiotoxicity and non-cardiotoxicity were determined at each visit, which disclosed that at M0 (P<0.001), M3 (P<0.001), M6 (P<0.001), M9 (P<0.001), M12 (P<0.001) and M15 (P<0.001), LVEF was lower in patients with cardiotoxicity compared with patients with non-cardiotoxicity (Figure 2A). As for cTnl, it was higher in patients with cardiotoxicity compared with those with non-cardiotoxicity at M0 (P<0.001), M3 (P<0.001), M6 (P<0.001), M9 (P<0.001), M12 (P<0.001) and M15 (P<0.001) (Figure 2B). Whereas regarding NT-proBNP, its median value was higher at M0 (P=0.046) but similar at M3 (P=0.223), M6 (P=0.550), M9 (P=0.138), M12 (P=0.156) and M15 (P=0.234) in patients with cardiotoxicity compared to patients with non-cardiotoxicity (Figure 2C).
Figure 2.
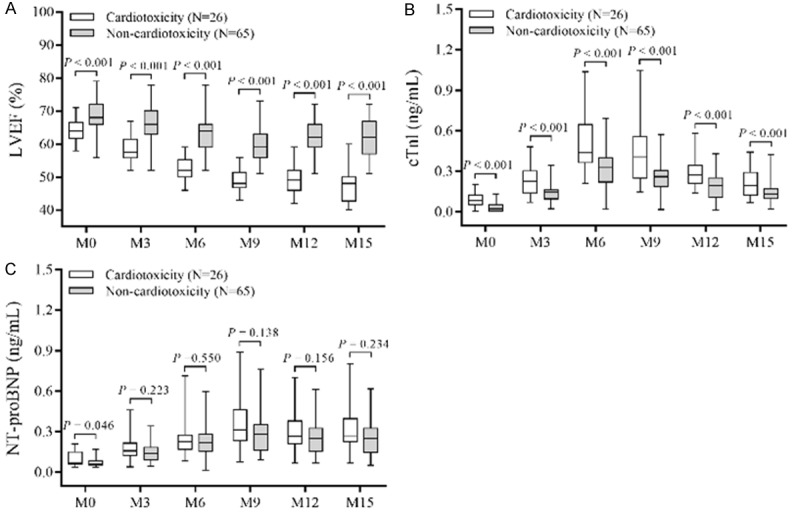
LVEF, cTnl, and NT-proBNP levels between cardiotoxicity and non-cardiotoxicity patients. LVEF was lower in patients with cardiotoxicity compared to patients with non-cardiotoxicity at each visit (A); cTnI was higher in patients with cardiotoxicity compared to patients with non-cardiotoxicity at each visit (B); NT-proBNP was higher at baseline (M0), but similar in patients with cardiotoxicity compared to patients with non-cardiotoxicity at each following visit (C). Comparison of LVEF, cTnl, and NT-proBNP levels between cardiotoxicity patients and non-cardiotoxicity patients at each visit was determined by Wilcoxon rank test. P<0.05 was considered significant. LVEF, left ventricular ejection fraction; cTnI, cardiac troponin I; NT-proBNP, N terminal pro B type natriuretic peptide.
Difference of LVEF and incidence of cardiotoxicity among patients with HER2 codon 655 AA, AG and GG genotypes
Comparison of LVEF among patients with HER2 codon 655 AA, AG, and GG genotypes at each visit revealed that LVEF was similar among patients with the 3 genotypes at M0 (P=0.209), M3 (P=0.053), M6 (P=0.109), M9 (P=0.061), and M12 (P=0.053) but gradually reduced in patients with HER2 codon 655 AA, AG and GG genotypes at M15 (P=0.024) (Figure 3A). Regarding the incidence of cardiotoxicity, it was higher in patients with HER2 codon 655 AG genotype compared with those with HER2 codon 655 AA genotype (P=0.017) (Figure 3B). The incidence of cardiotoxicity was numerically increased in patients with HER2 codon 655 GG genotype compared to patients with HER2 codon 655 AA genotype (P=0.060) and patients with HER2 codon 655 AG genotype (P=0.496) but with no statistical significance. Due to lack of HER2 codon 655 GG cases, patients with HER2 codon 655 AG and GG genotypes were combined together to further determine the difference of LVEF and incidence of cardiotoxicity between patients with HER2 codon 655 AA and patients with HER2 codon 655 AG/GG. It was observed that LVEF was similar at M0 (P=0.108) but reduced at M3 (P=0.021), M6 (P=0.039), M9 (P=0.018), M12 (P=0.018), and M15 (P=0.010) in patients with HER2 codon 655 AG/GG genotypes compared to those with HER2 codon 655 AA genotype (Figure 3C). The incidence of cardiotoxicity was increased in patients with HER2 codon 655 AG/GG genotypes compared to those with HER2 codon 655 AA genotype (P=0.007) (Figure 3D).
Figure 3.
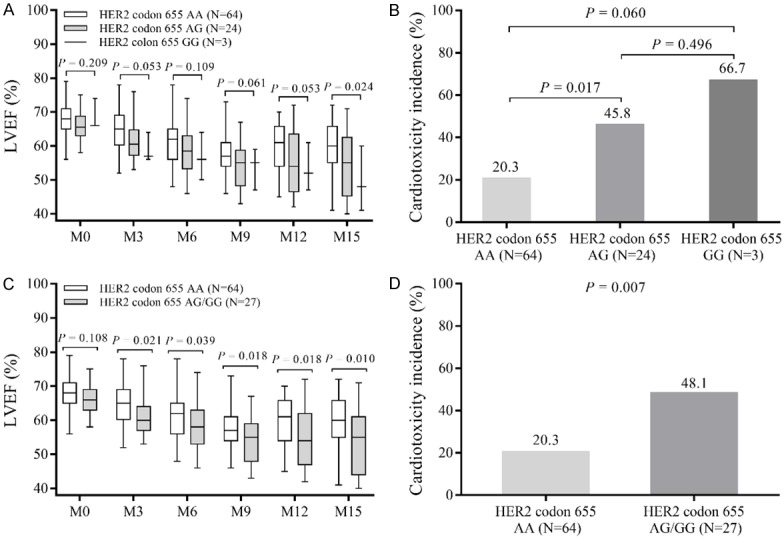
LVEF and incidence of cardiotoxicity among HER2 codon 655 AA, AG and GG carriers. LVEF among HER2 codon 655 AA, AG and GG carriers was similar at M0, M3, M6, M9, and M12, but decreased as G allele increased at M15 (A). The incidence of cardiotoxicity was higher in HER2 codon 655 AG carriers compared to AA carriers, but numerically elevated in HER2 codon 655 GG carriers compared with HER2 codon 655 AG and GG carriers (B). LVEF was similar between HER2 codon 655 AA carriers and HER2 codon 655 AG/GG carriers at baseline (M0), but higher in HER2 codon 655 AA carriers compared to HER2 codon 655 AG/GG carriers at M3, M6, M9, M12 and M15 (C). The incidence of cardiotoxicity was decreased in HER2 codon 655 AA carriers compared to HER2 codon 655 AG/GG carriers (D). LVEF level among three groups of patients was detected by Kruskal-Wallis H test, and LVEF level between two groups of patients was determined using Wilcoxon rank-sum test. Incidence of cardiotoxicity among three/two groups of patients was detected by Chi-square test. P<0.05 was considered significant. LVEF, left ventricular ejection fraction; HER2, human epidermal growth factor receptor-2; cTnI, cardiac troponin I; NT-proBNP, N terminal pro B type natriuretic peptide.
Univariate and multivariate logistic regression of factors predicting cardiotoxicity
Univariate logistic regression analysis exhibited that HER2 codon 655 A>G (OR=3.117, P=0.008), BMI (OR=1.321, P=0.013), smoking (OR=3.437, P=0.020), hypertension (OR=3.239, P=0.021), hyperuricemia (OR=3.294, P=0.029), baseline cTnI (OR=1.030, P<0.001) and baseline NT-proBNP level (OR=1.015, P=0.010) were correlated with increased incidence of cardiotoxicity in HER2-positive breast cancer patients received EC-D-T adjuvant chemotherapy (Table 3). Further multivariate logistic regression analysis disclosed that HER2 codon 655 A>G (OR=7.989, P=0.007), smoking (OR=17.200, P=0.008) and baseline cTnl (OR=1.043, P=0.001) were independent predictive factors for increased risk of cardiotoxicity in HER2-positive breast cancer patients undergoing EC-D-T adjuvant chemotherapy (Table 4).
Table 3.
Univariate logistic regression model analysis of factors predicting cardiotoxicity
| Items | Univariate logistic regression | |||
|---|---|---|---|---|
|
| ||||
| P value | OR | 95% CI | ||
|
| ||||
| Lower | Higher | |||
| HER2 codon 655 (GG=2, AG=1, AA=0) | 0.008 | 3.117 | 1.337 | 7.266 |
| Age | 0.155 | 1.047 | 0.983 | 1.115 |
| BMI | 0.013 | 1.321 | 1.059 | 1.647 |
| Smoking (yes vs. no) | 0.020 | 3.437 | 1.217 | 9.710 |
| Hypertension (yes vs. no) | 0.021 | 3.239 | 1.193 | 8.795 |
| Diabetes mellitus (yes vs. no) | 0.565 | 1.722 | 0.271 | 10.955 |
| Dyslipidemia (yes vs. no) | 0.146 | 2.118 | 0.770 | 5.820 |
| Hyperuricemia (yes vs. no) | 0.029 | 3.294 | 1.128 | 9.619 |
| Chronic kidney disease (yes vs. no) | 0.135 | 4.109 | 0.645 | 26.178 |
| ECOG score (1 vs. 0) | 0.248 | 1.765 | 0.673 | 4.630 |
| cTnI (M0) | <0.001 | 1.030 | 1.015 | 1.044 |
| NT-proBNP (M0) | 0.010 | 1.015 | 1.004 | 1.026 |
Factors predicting cardiotoxicity were determined by univariate logistic regression analysis. P value <0.05 was considered significant. HER2: human epidermal growth factor receptor-2; BMI: body mass index; ECOG: Eastern Cooperative Oncology Group; cTnI: cardiac troponin I; NT-proBNP: N terminal pro B type natriuretic peptide.
Table 4.
Multivariate logistic regression model analysis of factors predicting cardiotoxicity
| Items | Multivariate logistic regression | |||
|---|---|---|---|---|
|
| ||||
| P value | OR | 95% CI | ||
|
| ||||
| Lower | Higher | |||
| HER2 codon 655 (GG=2, AG=1, AA=0) | 0.007 | 7.989 | 1.785 | 35.756 |
| Age | 0.278 | 0.936 | 0.830 | 1.055 |
| BMI | 0.686 | 1.088 | 0.722 | 1.641 |
| Smoking (yes vs. no) | 0.008 | 17.200 | 2.133 | 138.680 |
| Hypertension (yes vs. no) | 0.321 | 2.443 | 0.419 | 14.251 |
| Diabetes mellitus (yes vs. no) | 0.064 | 0.030 | 0.001 | 1.221 |
| Dyslipidemia (yes vs. no) | 0.317 | 2.766 | 0.377 | 20.289 |
| Hyperuricemia (yes vs. no) | 0.302 | 2.959 | 0.377 | 23.219 |
| Chronic kidney disease (yes vs. no) | 0.766 | 0.539 | 0.009 | 31.625 |
| ECOG score (1 vs. 0) | 0.058 | 4.973 | 0.945 | 26.181 |
| cTnI (M0) | 0.001 | 1.043 | 1.017 | 1.070 |
| NT-proBNP (M0) | 0.704 | 1.004 | 0.984 | 1.024 |
Factors predicting cardiotoxicity were determined by multivariate logistic regression analysis. P value <0.05 was considered significant. HER2: human epidermal growth factor receptor-2; BMI: body mass index; ECOG: Eastern Cooperative Oncology Group; cTnI: cardiac troponin I; NT-proBNP: N terminal pro B type natriuretic peptide.
Discussion
In the present study, (1) cumulative incidence of cardiotoxicity was 28.6%; (2) the prevalence of HER2 codon 655 AA, AG, GG genotypes were 70.3%, 26.4% and 3.3% respectively in our study population. (3) HER2 codon 655 A>G, smoke and baseline cTnI were independent predictive factors for increased risk of cardiotoxicity in HER2-positive breast cancer patients undergoing EC-D-T adjuvant chemotherapy.
Cardiotoxicity induced by chemotherapy is the leading cause of morbidity and mortality in breast cancer survivors, and the use of anthracyclines or trastuzumab or the combination of the two has been reported to be a key contributor to cardiotoxicity in breast cancer patients [9]. Research discloses that at least 10-15% of all breast cancer patients experience anthracyclines-induced cardiotoxicity, and 20-33% of HER2-positive breast cancer patients suffer from trastuzumab-induced cardiotoxicity [16-20]. However, the incidence of cardiotoxicity in Chinese HER2-positive breast cancer patients receiving anthracycline and trastuzumab combined adjuvant chemotherapy (EC-D-T) has not been reported. In this study, we recorded cardiotoxicity events of Chinese HER2-positive breast cancer patients undergoing EC-D-T adjuvant chemotherapy and observed that the incidence of cardiotoxicity was 28.6%, which fell in the range reported in previous studies. This might be because anthracycline and trastuzumab generate reactive oxygen species that induced injury to cardiomyocytes, thereby impairing the normal cardiac function and reducing LVEF, which increased the risk of cardiotoxicity in HER2-positive breast cancer patients [16,17].
HER2 is a member of the HER family of tyrosine kinase receptors, that regulates signaling pathways such as Ras/p42/44 MAPK and PI3K/AKT that are involved in cell apoptosis, metastasis, invasion, angiogenesis and differentiation. It predicts increased risk of metastasis and reduced survival in breast cancer patients [2]. Polymorphism of HER2 codon 655 A>G changes the encoded amino acid from Ile to Val, and the presence of Val is revealed to decrease the tyrosine activity and increase cell sensitivity to trastuzumab in HER2-positive breast cancer patients [10]. HER2 codon 655 A>G polymorphism is seen in nearly all the population globally, while the prevalence varies from one race to another [19]. In China, one previous study observes that the frequencies of HER2 codon 655 AA, AG, and GG genotypes were 72%, 26% and 2% in breast cancer patients [10]. However, the prevalence of HER2 codon 655 A>G in HER2-positive breast cancer patients is not frequently reported in China. Our study recruited 91 Chinese HER2-positive breast cancer patients and disclosed that the prevalence of HER2 codon 655 AA, AG, GG genotypes were 70.3%, 26.4% and 3.3% respectively, which was consistent with the previous data.
In addition, HER2 codon 655 A>G also increases the dependency of cardiomyocytes on HER2 signaling as well as their sensitivity to cytotoxic drugs [5]. This enlightens the explorations on whether HER2 codon 655 A>G influences cardiotoxicity in HER2-positive breast cancer patients and studies have validated that HER2 codon 655 A>G increases the risk of trastuzumab-induced cardiotoxicity in HER2-positive breast cancer patients among Spain, French, and Canadian populations [10-12]. However, it is also well-known that anthracycline is a critical contributor to cardiotoxicity, and for HER2-positive breast cancer patients underwent EC-D-T adjuvant chemotherapy regimen in Chinese population, the predictive value of HER2 codon 655 A>G on the risk of cardiotoxicity is still unclear. Therefore, the present study investigated the influence of HER2 codon 655 A>G on cardiotoxicity in HER2-positive patients underwent EC-D-T adjuvant chemotherapy, which displayed that HER2 codon 655 A>G, smoking and baseline cTnI were independent predictive factors for increased risk of cardiotoxicity in HER2-positive breast cancer patients received EC-D-T adjuvant chemotherapy. The possible explanations were: (1) anthracyclines and trastuzumab might elevate the oxidative stress, damage the DNA, impair mitochondrial function as well as induce senescence or death of the key cardiac and progenitor cells; and HER2 codon 655 A>G might increase the effect of cytotoxic drugs on cardiac cells, hence increasing the risk of cardiotoxicity in HER2-positive breast cancer patients. (2) cell damage and cell death initiated far before cardiotoxicity was recognized, and cell membrane rupture released the cTnI located on sarcomeres into the peripheral blood. Thus, increased baseline level of cTnI in peripheral blood could predict an increase in cardiotoxicity risk [21].
Several limitations still exist in this study. Although the sample size in our study was relatively larger compared with most single cohort studies, the number of HER2 codon 655 GG cases was still insufficient for a decent statistical analysis. Therefore, a larger-scaled study was necessary to validate our findings. The follow-up duration was relatively short, thus the long-term incidence of cardiotoxicity or the long-term influence of HER2 codon 655 A>G on cardiotoxicity risk of EC-D-T adjuvant chemotherapy was not investigated. Finally, the detailed mechanism of HER2 codon 655 A>G in HER2-positive breast cancer or its molecular correlation with EC-D-T chemotherapy-induced cardiotoxicity in HER2-positive breast cancer patients still needs further investigation.
In conclusion, cardiotoxicity incidence is 28.6% and HER2 codon 655 A>G serves as an independent predictive factor for increased cardiotoxicity risk in Chinese HER2-positive breast cancer patients undergoing EC-D-T adjuvant chemotherapy.
Disclosure of conflict of interest
None.
References
- 1.Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med. 2015;66:111–128. doi: 10.1146/annurev-med-042513-015127. [DOI] [PubMed] [Google Scholar]
- 2.Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017;389:2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 3.Bevers TB, Helvie M, Bonaccio E, Calhoun KE, Daly MB, Farrar WB, Garber JE, Gray R, Greenberg CC, Greenup R, Hansen NM, Harris RE, Heerdt AS, Helsten T, Hodgkiss L, Hoyt TL, Huff JG, Jacobs L, Lehman CD, Monsees B, Niell BL, Parker CC, Pearlman M, Philpotts L, Shepardson LB, Smith ML, Stein M, Tumyan L, Williams C, Bergman MA, Kumar R. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362–1389. doi: 10.6004/jnccn.2018.0083. [DOI] [PubMed] [Google Scholar]
- 4.Putt M, Hahn VS, Januzzi JL, Sawaya H, Sebag IA, Plana JC, Picard MH, Carver JR, Halpern EF, Kuter I, Passeri J, Cohen V, Banchs J, Martin RP, Gerszten RE, Scherrer-Crosbie M, Ky B. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. Clin Chem. 2015;61:1164–1172. doi: 10.1373/clinchem.2015.241232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez Pena C, Davila-Fajardo CL, Martinez-Gonzalez LJ, Carmona-Saez P, Soto Pino MJ, Sanchez Ramos J, Moreno Escobar E, Blancas I, Fernandez JJ, Fernandez D, Correa C, Cabeza Barrera J. Influence of the HER2 Ile655Val polymorphism on trastuzumab-induced cardiotoxicity in HER2-positive breast cancer patients: a meta-analysis. Pharmacogenet Genomics. 2015;25:388–393. doi: 10.1097/FPC.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 6.Rochette L, Guenancia C, Gudjoncik A, Hachet O, Zeller M, Cottin Y, Vergely C. Anthracyclines/trastuzumab: new aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol Sci. 2015;36:326–348. doi: 10.1016/j.tips.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Young AM, Dhillon T, Bower M. Cardiotoxicity after liposomal anthracyclines. Lancet Oncol. 2004;5:654. doi: 10.1016/S1470-2045(04)01605-5. [DOI] [PubMed] [Google Scholar]
- 8.Brower V. Cardiotoxicity debated for anthracyclines and trastuzumab in breast cancer. J Natl Cancer Inst. 2013;105:835–836. doi: 10.1093/jnci/djt161. [DOI] [PubMed] [Google Scholar]
- 9.Arciniegas Calle MC, Sandhu NP, Xia H, Cha SS, Pellikka PA, Ye Z, Herrmann J, Villarraga HR. Two-dimensional speckle tracking echocardiography predicts early subclinical cardiotoxicity associated with anthracycline-trastuzumab chemotherapy in patients with breast cancer. BMC Cancer. 2018;18:1037. doi: 10.1186/s12885-018-4935-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han X, Diao L, Xu Y, Xue W, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, Xie Y. Association between the HER2 Ile655Val polymorphism and response to trastuzumab in women with operable primary breast cancer. Ann Oncol. 2014;25:1158–1164. doi: 10.1093/annonc/mdu111. [DOI] [PubMed] [Google Scholar]
- 11.Beauclair S, Formento P, Fischel JL, Lescaut W, Largillier R, Chamorey E, Hofman P, Ferrero JM, Pages G, Milano G. Role of the HER2 [Ile655Val] genetic polymorphism in tumorogenesis and in the risk of trastuzumab-related cardiotoxicity. Ann Oncol. 2007;18:1335–1341. doi: 10.1093/annonc/mdm181. [DOI] [PubMed] [Google Scholar]
- 12.Lemieux J, Diorio C, Cote MA, Provencher L, Barabe F, Jacob S, St-Pierre C, Demers E, Tremblay-Lemay R, Nadeau-Larochelle C, Michaud A, Laflamme C. Alcohol and HER2 polymorphisms as risk factor for cardiotoxicity in breast cancer treated with trastuzumab. Anticancer Res. 2013;33:2569–2576. [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Kitayama H, Kondo T, Sugiyama J, Kurimoto K, Nishino Y, Kawada M, Hirayama M, Tsuji Y. High-sensitive troponin T assay can predict anthracycline- and trastuzumab-induced cardiotoxicity in breast cancer patients. Breast Cancer. 2017;24:774–782. doi: 10.1007/s12282-017-0778-8. [DOI] [PubMed] [Google Scholar]
- 16.Das M. Small benefits in trastuzumab-related cardiotoxicity. Lancet Oncol. 2017;18:e5. doi: 10.1016/S1470-2045(16)30639-8. [DOI] [PubMed] [Google Scholar]
- 17.Burki TK. Trastuzumab cardiotoxicity in early-stage breast cancer. Lancet Oncol. 2016;17:e226. doi: 10.1016/S1470-2045(16)30114-0. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Hu B, Guo Z, Jiang X, Su X, Zhang X. Correlation of UGT2B7 polymorphism with cardiotoxicity in breast cancer patients undergoing epirubicin/cyclophosphamide-docetaxel adjuvant chemotherapy. Yonsei Med J. 2019;60:30–37. doi: 10.3349/ymj.2019.60.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen BC, McLeod HL. Pharmacogenomics as a risk mitigation strategy for chemotherapeutic cardiotoxicity. Pharmacogenomics. 2013;14:205–213. doi: 10.2217/pgs.12.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 21.Nebigil CG, Desaubry L. Updates in anthracycline-mediated cardiotoxicity. Front Pharmacol. 2018;9:1262. doi: 10.3389/fphar.2018.01262. [DOI] [PMC free article] [PubMed] [Google Scholar]


