Abstract
Background
Acute cough due to upper respiratory tract infection (URTI) is a common symptom. Non‐prescription, over‐the‐counter (OTC) medicines are frequently recommended as a first‐line treatment, but there is little evidence as to whether these drugs are effective.
Objectives
To assess the effects of oral OTC cough preparations for acute cough in children and adults in community settings.
Search methods
We searched CENTRAL (2014, Issue 1), MEDLINE (January 1966 to March week 3 2014), EMBASE (January 1974 to March 2014), CINAHL (January 2010 to March 2014), LILACS (January 2010 to March 2014), Web of Science (January 2010 to March 2014) and the UK Department of Health National Research Register (March 2010).
Selection criteria
Randomised controlled trials (RCTs) comparing oral OTC cough preparations with placebo in children and adults suffering from acute cough in community settings. We considered all cough outcomes; secondary outcomes of interest were adverse effects.
Data collection and analysis
Two review authors independently screened potentially relevant citations, extracted data and assessed study quality. We performed quantitative analysis where appropriate.
Main results
Due to the small numbers of trials in each category, the limited quantitative data available and the marked differences between trials in terms of participants, interventions and outcome measurement, we felt that pooling of the results was inappropriate.
We included 29 trials (19 in adults, 10 in children) involving 4835 people (3799 adults and 1036 children). All studies were placebo‐controlled RCTs. However, assessment of the risk of bias of the included studies was limited by poor reporting, particularly for the earlier studies.
In the adult studies, six trials compared antitussives with placebo and had variable results. Three trials compared the expectorant guaifenesin with placebo; one indicated significant benefit, whereas the other two did not. One trial found that a mucolytic reduced cough frequency and symptom scores. Two studies examined antihistamine‐decongestant combinations and found conflicting results. Four studies compared other combinations of drugs with placebo and indicated some benefit in reducing cough symptoms. Three trials found that antihistamines were no more effective than placebo in relieving cough symptoms.
In the child studies, antitussives (data from three studies), antihistamines (data from three studies), antihistamine‐decongestants (two studies) and antitussive/bronchodilator combinations (one study) were no more effective than placebo. No studies using expectorants met our inclusion criteria. The results of one trial favoured active treatment with mucolytics over placebo. One trial tested two paediatric cough syrups and both preparations showed a 'satisfactory response' in 46% and 56% of children compared to 21% of children in the placebo group. One new trial indicated that three types of honey were more effective than placebo over a three‐day period.
Twenty‐one studies reported adverse effects. There was a wide range across studies, with higher numbers of adverse effects in participants taking preparations containing antihistamines and dextromethorphan.
Authors' conclusions
The results of this review have to be interpreted with caution because the number of studies in each category of cough preparations was small. Availability, dosing and duration of use of over‐the‐counter cough medicines vary significantly in different countries. Many studies were poorly reported making assessment of risk of bias difficult and studies were also very different from each other, making evaluation of overall efficacy difficult. There is no good evidence for or against the effectiveness of OTC medicines in acute cough. This should be taken into account when considering prescribing antihistamines and centrally active antitussive agents in children; drugs that are known to have the potential to cause serious harm.
Keywords: Adult; Child; Humans; Acute Disease; Administration, Oral; Ambulatory Care; Antitussive Agents; Antitussive Agents/administration & dosage; Antitussive Agents/adverse effects; Cough; Cough/drug therapy; Drug Therapy, Combination; Drug Therapy, Combination/methods; Expectorants; Expectorants/administration & dosage; Expectorants/adverse effects; Expectorants/therapeutic use; Histamine H1 Antagonists; Histamine H1 Antagonists/administration & dosage; Nonprescription Drugs; Nonprescription Drugs/administration & dosage; Nonprescription Drugs/adverse effects; Randomized Controlled Trials as Topic
Plain language summary
Over‐the‐counter (OTC) medications for acute cough in children and adults in community settings
Review question
We undertook a review to determine the effectiveness of over‐the‐counter cough medicines in reducing cough in children and adults in community settings. We found 29 trials involving 4835 people.
Background
Acute cough is a common and troublesome symptom in children and adults suffering from acute upper respiratory tract infection (URTI). Many people self prescribe over‐the‐counter (OTC) cough preparations, and health practitioners often recommend their use for the initial treatment of cough. There is substantial variation between countries in the availability and guidelines for use of many of these preparations.
Study characteristics
We identified a broad range of studies of different types of preparations used at different dosages in both adults and children.
Key results
The evidence is current up to March 2014. We found no good evidence for or against the effectiveness of OTC medications in acute cough. Nineteen studies reported adverse effects of these medications and described infrequent, mainly minor side effects such as nausea, vomiting, headache and drowsiness.
Quality of the evidence
The results of this review have to be interpreted with caution because the number of studies in each category of cough preparations was small. Many studies were poorly reported making assessment of risk of bias difficult. While all studies were placebo‐controlled randomised controlled trials only a minority reported their methods of allocation and randomisation and there was lack of reporting of blinding of outcome assessors and whether cough outcome measures were validated. In addition, studies supported by pharmaceutical companies or other providers were more likely to have positive results. Studies were very different from each other in terms of treatment types, treatment duration and outcomes measured, making evaluation of overall effectiveness of OTC cough medicines difficult.
Background
Description of the condition
Acute cough due to upper respiratory tract infection (URTI) is one of the most common symptoms worldwide. A large number of people self prescribe non‐prescription over‐the‐counter (OTC) cough medicines for themselves or their children, and many health professionals in primary care settings recommend them to their patients as a first‐line treatment (PAGB 2000). OTC medicines are available to the public from pharmacies, chemists and shops without medical or dental prescription in most countries, as opposed to prescription‐only medicines (POM). A national telephone survey of medication use in the US indicated that in a given week, 10% of children are given an OTC cough preparation by their carers (Vernacchio 2008). Numerous OTC cough preparations are available, but evidence regarding their efficacy is inconclusive. Some studies of cough preparations have been shown to reduce cough symptoms, whereas others found no effect compared with placebo (Banderali 1995; Freestone 1997; Kurth 1978; Smith 1993). In recent years there have been safety concerns that led to the withdrawal of OTC cough medicines containing antihistamines and antitussives in children under six years. These regulatory changes were based on rare fatalities identified by the Food and Drug Administration (FDA) in the US, highlighting the potential of these agents to cause harm, particularly if there is accidental overdosage. However, these medicines are still available for children over the age of six years. In addition, following concerns about QT prolongation and cardiac arrhythmias, the non‐narcotic antitussive clobutinol was withdrawn in the European Union in 2008.
Description of the intervention
Many studies have involved patients from different populations that have included participants with chronic cough due to underlying disease such as asthma or chronic obstructive pulmonary disease, or that were carried out on healthy volunteers in whom cough had been induced by chemical irritants (Gastpar 1984; Irwin 1993; Smith 1993). Other randomised controlled trials (RCTs) compared active agents and did not include a placebo. Cough preparations may contain different drugs with a variety of modes of action, which can make them difficult to compare (Morice 1998).
How the intervention might work
Non‐prescription oral OTC medicines for cough have different modes of action based on their active ingredients as follows.
Antitussives, for example centrally acting opioid derivatives (Irwin 1993), or other peripherally active agents, act by reducing the cough reflex.
Expectorants, i.e. drugs leading to increased bronchial mucus production, make secretions easier to remove by cough or ciliary transport (Ziment 1976).
Mucolytics, i.e. drugs aiming to decrease the viscosity of bronchial secretions, act to make secretions easier to clear through coughing (Reynolds 1993).
Antihistamine‐decongestant combinations, i.e. drugs that are combined antihistamine H1‐receptor antagonists and alpha‐adrenoceptor agonists, act by causing vasoconstriction of mucosal blood vessels thus reducing congestion (Morice 1998).
Antihistamines, i.e. antihistamine H1‐receptor agonists, act by reducing histamine release and thus reducing local congestion and production of secretions.
Other drug combinations, i.e. fixed drug combinations using different ingredients, have mechanisms of action based on individual ingredients.
Honey is regarded as having bactericidal properties and there has been recent interest in its potential effectiveness in relieving the symptoms of URTI, including cough. In studies to date it has been administered as a single dose before bedtime or diluted in a non‐caffeinated beverage. An existing Cochrane review of honey for acute cough has concluded that there is no strong evidence for or against the use of honey in acute cough in children (Oduwole 2012).
Why it is important to do this review
Previous systematic reviews of OTC cough and cold preparations revealed that there is insufficient evidence for or against an effect of OTC cough preparations compared to placebo (Anonymous 1999; Smith 1993). However, these reviews did either not use a systematic search for RCTs (Anonymous 1999), or performed searches that were limited to the MEDLINE database (Smith 1993). By using a more extensive search strategy, this systematic review aims to answer the question of whether OTC medications used for the treatment of acute cough associated with URTI are effective.
Objectives
To assess the effects of oral OTC cough preparations for acute cough in children and adults in community settings.
Many different groups of OTC medicines are available, therefore we aimed to make comparisons only within groups of preparations with a similar mode of action or other similar features.
Methods
Criteria for considering studies for this review
Types of studies
All placebo‐controlled RCTs of oral OTC cough preparations for acute cough.
Types of participants
Community or ambulatory settings in primary care and hospital outpatients.
Children and adults with acute onset of cough (less than three weeks' duration).
We excluded studies testing OTC medicines for chronic cough (more than three weeks' duration), cough due to underlying respiratory disease (such as asthma, chronic obstructive pulmonary disease, pneumonia, tuberculosis, lung malignancy). We also excluded studies where cough was induced artificially (through inhalation of chemicals) in healthy volunteers.
Types of interventions
Non‐prescription oral OTC medicines for cough are classified according to their mode of action, as outlined above, and we have grouped them as follows.
Antitussives, for example, centrally acting opioid derivatives (Irwin 1993).
Expectorants, i.e. drugs leading to increased bronchial mucus production (Ziment 1976).
Mucolytics, i.e. drugs aiming to decrease the viscosity of bronchial secretions (Reynolds 1993).
Antihistamine‐decongestant combinations, i.e. drugs that are combined antihistamine H1‐receptor antagonists and alpha‐adrenoceptor agonists, which cause vasoconstriction of mucosal blood vessels (Morice 1998).
Antihistamines, i.e. antihistamine H1‐receptor agonists.
Other drug combinations, i.e. fixed drug combinations using different ingredients.
Honey.
We excluded studies that used non‐oral preparations (for example, nasal sprays, inhalers, nebulised solutions) or that tested ingredients other than those accepted in Western (allopathic) medicine (for example, herbal or homeopathic medicines) because we felt that this review would have become too broad.
Types of outcome measures
Primary outcomes
All cough outcomes (such as frequency, severity, amount of sputum, improvement in cough symptoms using continuous and categorical data and different ways of measurement including cough sound pressure levels, cough counts, patient questionnaires, physician assessment, etc). We did not consider global patient or physician ratings of wellness or recovery as outcomes, unless these were directly related to cough symptoms.
Secondary outcomes
Significant adverse effects.
Search methods for identification of studies
Electronic searches
For this 2014 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 1) (accessed 26 March 2014), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (January 2012 to March week 3, 2014); EMBASE (March 2012 to March 2014); CINAHL (2012 to March 2014); LILACS (2012 to March 2014) and Web of Science (2012 to March 2014). We used the search strategy in Appendix 1 to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) Ovid format (Lefebvre 2011). We adapted the search string to search EMBASE (see Appendix 2), CINAHL (see Appendix 3), LILACS (see Appendix 4) and Web of Science (see Appendix 5).
For our previous 2012 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2012, Issue 3) (accessed 22 March 2012), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (February 2010 to March week 1, 2012); EMBASE (March 2010 to March 2012); CINAHL (January 2010 to March 2012); LILACS (2010 to March 2012) and Web of Science (2010 to March 2012). Details of earlier searches are in Appendix 6.
Searching other resources
We searched personal collections of references and reference lists of articles, and wrote to authors of original studies, pharmaceutical companies and the Proprietary Association of Great Britain about information on unpublished studies. We did not impose any constraints based on language or publication status.
Data collection and analysis
Selection of studies
Two review authors (SS, TF) independently screened potentially relevant citations and applied the selection criteria using an in/out/pending sheet. We resolved any differences at any stage of the review by discussion.
Data extraction and management
Two review authors (SS, TF) independently extracted data and assessed the quality of studies. We contacted investigators for additional information if necessary and obtained translations of abstracts or papers if they were written in languages other than English or German. We resolved any disagreements by discussion.
Assessment of risk of bias in included studies
Since the 2010 update of this review we have adapted our original quality assessment and have used the 'Risk of bias' tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions to assess the methodological quality of included studies (Higgins 2011). Two review authors (SS, TF) independently carried out these assessments. The domains considered are now described within the Characteristics of included studies table.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective reporting.
Other bias.
Measures of treatment effect
Due to the small numbers of trials in each category, the limited quantitative data available and the marked differences between trials in terms of participants, interventions and outcome measurement we felt that pooling of the results was inappropriate and we undertook no meta‐analysis. The effect of individual treatments is summarised as outlined in the original studies using mean differences in scores for continuous data where available or simple presentations of means in each group or comparison of proportions for dichotomous data.
Unit of analysis issues
All included studies were RCTs with randomisation occurring at the level of individual participants, so there was no indication to consider unit of analysis errors in this review.
Dealing with missing data
Due to the limited quantitative data available for this review, we presented simple descriptions of individual study outcomes within the pre‐specified grouping of different treatment groups. Issues relating to missing data and follow‐up are presented in the 'Risk of bias' sections in the Characteristics of included studies table.
Assessment of heterogeneity
The studies included in this review were clinically heterogeneous and provided limited data, so we undertook no meta‐analysis.
Assessment of reporting biases
There is no reason to suspect that publication bias affected the outcomes of this review. We conducted a comprehensive search of the literature with no language or publication restrictions. For the original review we also sought information from experts in the area including pharmaceutical companies and the Proprietary Association of Great Britain and Ireland. As we did not perform a meta‐analysis, we did not generate funnel plots.
Data synthesis
We undertook no meta‐analysis for this review.
Subgroup analysis and investigation of heterogeneity
Effects of treatment are presented within relevant treatment groups for both children and adults to allow comparison of related medications.
Sensitivity analysis
We undertook no meta‐analysis and limitations of the review are addressed within the Discussion section.
Results
Description of studies
Results of the search
Our initial search in 2001 identified 328 potentially relevant RCTs, which we screened for retrieval of paper copies. At that stage we excluded 235 abstracts for the following main single reasons: study not a RCT (n = 19 trials); study not placebo‐controlled (n = 39); study not testing an OTC cough medicine (n = 86); cough artificially induced (n = 26); or participants with chronic cough lasting more than three weeks (n = 65). We retrieved paper copies of 93 RCTs for more detailed evaluation. We excluded a further 72 trials because studies were not RCTs (n = 4); were not placebo‐controlled (n = 2); were not testing OTC cough medicines (n = 23); induced cough artificially (n = 3); included participants with chronic cough (n = 25); or did not report any cough outcomes (n = 15).
The search conducted for the update in 2004 identified 477 potential titles for screening and we identified three additional RCTs as eligible for inclusion, with two of these being different arms of a three‐arm RCT (Korppi 1991a; Korppi 1991b; Pavesi 2001).
The search conducted for the update in 2007 identified 799 potential titles for screening and we identified one additional RCT as eligible for inclusion (Paul 2004).
The search conducted for the 2010 update identified 112 potential titles for screening and we identified one additional RCT as eligible for inclusion (Mizoguchi 2007).
The search conducted for the update in 2012 identified 252 potential titles for screening and no additional eligible studies. We identified one potentially eligible study that we excluded as it had no placebo control group (Shadkam 2010).
The search conducted for this 2014 update identified 421 potential titles for screening and we identified three additional RCTs as eligible for inclusion (Albrecht 2012; Avner Cohen 2012; Bhattacharya 2012).
In total, 2389 potential titles have been identified for screening since the first review in 2001 and 29 RCTs were identified as eligible for inclusion in this review. See Figure 1.
1.
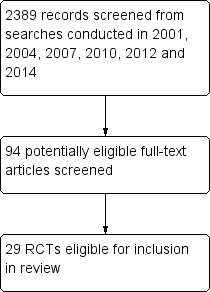
Flow diagram.
Included studies
In this 2014 update we included 29 RCTs involving 4835 participants. Nineteen of these trials were in adults (n = 3799) and 10 in children (n = 1036). The Characteristics of included studies table contains data on the number of participants randomised to the interventions, age, sex, smoking status, study setting, definition of illness, drug dosage, frequency and duration of treatment, and outcome information. Most adult trials were in young adults with mean ages ranging from 23 to 48 years. Ages in studies in children ranged from six months to 18 years. Six trials were more than 20 years old. Nearly half of the studies (13 out of 29) were carried out in the US, with the remaining trials located in the UK (five), Finland (three), Germany (two), Italy (one), India (two), South Africa (one), Thailand (one) and Israel (one). The ages of participants ranged from six months to over 70 years. Most studies were different in their definition of illness, the content of the drug preparation, drug dosage, the frequency of doses and the treatment duration (ranging from a single dose to 18 days), making comparison of trials and quantitative analysis difficult. Table 1 provides an overview of the included studies, treatment types, treatment duration and results and highlights this variation.
1. Overview of studies and outcomes.
| Group | Study ID | Number participants | Treatment and duration | Outcome and result | Notes |
| 1. Antitussives | |||||
| Antitussives Adults |
Adams | 108 | Moguisteine 3.5 days | Cough score (10‐point scale) MD 0.9 on day 4 | NS |
| Eccles | 81 | Codeine 4 days |
16‐item cough severity score MD 1.6 | NS | |
| Freestone | 82 | Codeine Single dose |
5‐point symptom rating scale MD 1.0 | NS | |
| Lee | 44 | Dextromethorphan Single dose |
Cough severity score (3‐point scale) MD at 180 minutes 0.5 | NS | |
| Parvez | 451 | Dextromethorphan Single dose |
Cough recordings at 180 minutes | Significant reduction in cough counts with treatment | |
| Pavesi | 710 | Dextromethorphan Single dose |
Cough recordings at 180 minutes | Significant reduction in various cough measures with treatment | |
| Antitussives Children |
Bhattacharya | 120 | Dextromethorphan 3 days | Composite 5‐item symptom score MD 0.4 | NS |
| Korppi | 50 | Dextromethorphan 3 days |
4‐item cough symptoms score day 3 MD 0.04 | NS | |
| Paul | 100 | Dextromethorphan Single dose |
Composite 5‐item symptom score MD 0.79 | NS | |
| Taylor | 57 | Dextromethorphan or codeine (3 arms) 3 nights |
4‐item symptom score | NS | |
| 2. Expectorants | |||||
| Expectorants Adults |
Albrecht | 378 | Guaifenesin 1600 mg bd 7 days | 8‐item composite symptom score, day 4 MD 2.6, P value = 0.04 | NS difference at day 7, reported day 4 outcomes |
| Kuhn | 65 | Guaifenesin 480 mg qid 30 hours | Cough recordings and patient‐rated improvement in cough frequency and severity | NS | |
| Robinson | 239 | Guaifenesin 200 mg qid 3 days | Patient rating as 'helpful' Int 75% versus Con 31% | P value < 0.01 | |
| Expectorants Children |
No studies | ||||
| 3. Mucolytics | |||||
| Mucolytics Adults |
Nesswetha | 99 | Bromhexine 4 days |
Cough frequency (cough every 2 to 4 minutes) Int 8.6% versus Con 15.2% |
P value < 0.02 |
| Mucolytics Children |
Nespoli | 40 | Letosteine 10 days | Composite symptom score Average 2‐point difference between intervention and control favouring intervention day 4 to 10 | P value < 0.01 |
| 4. Antihistamine/decongestants | |||||
| Antihistamine/ decongestants Adults |
Berkowitz | 283 | Loratadine and pseudoephedrine 5 days | 3‐item cough score MD 0.2 | NS |
| Curley | 73 | Dexbrompheniramine and pseudoephedrine 7 days |
4‐item cough score MD 0.6 | P value < 0.05 | |
| Antihistamine/ decongestants Children |
Clemens | 59 | Brompheniramine and phenylpropanolamine 2 days |
7‐item cough scores MD 0.1 | NS |
| Hutton | 96 | Brompheniramine and phenylephrine and propanolamine 2 days |
9‐item symptom score, % reported as improved Int 67% versus Con 58% | NS | |
| 5. Other combinations | |||||
| Other combinations Adults |
Kurth | 113 | Dextromethorphan plus salbutamol 3 days |
% improved day 3 Int 45% versus Con 27% | P value = 0.05 |
| Mizoguchi | 485 | Combination syrup Single dose | Cough score MD 0.42, day 2 |
P value < 0.0001 | |
| Thackray | 70 | 'Vicks' 2 days, cross‐over after 24 hours | Patient preference Int 66% versus Con 27% |
P value < 0.01 | |
| Tukiainen | 108 | Dextromethorphan with and without salbutamol 4 days |
5‐item symptom score MD 0.11 |
NS | |
| Other combinations Children |
Korppi | 51 | Dextromethorphan plus salbutamol 3 days |
Cough symptoms MD 0.12, day 3 |
NS |
| Reece | 43 | Combination syrups | Satisfactory antitussive response Int both 69% versus Con 57% | NS | |
| 6. Antihistamines | |||||
| Antihistamines Adults |
Berkowitz | 91 | Terfenadine 4 to 5 days |
3‐item symptom score MD 0.2 |
NS |
| Gaffey | 250 | Terfenadine 3.5 days |
Symptom score (scores not reported) | NS | |
| Antihistamines Children |
Bhattacharya | 120 | Promethazine 3 days |
5‐item symptom score MD 0.3 |
NS |
| Paul | 100 | Diphenhydramine Single dose |
5‐item symptom score MD 1.73 | NS | |
| Sakchainanont | 143 | Clemastine and chlorpheniramine 3 days |
4‐item symptom score, % reporting improvement Int 40% versus Con 28% | NS | |
| 7. Honey | |||||
| Honey Adults |
No studies | ||||
| Honey Children | Avner‐Cohen | 300 | 3 types honey (4 arms) Single dose | 5‐item composite symptom score | Significant symptom reductions in all honey groups compared to controls |
Con: control Int: intervention MD: mean difference NS: not significant qid: 4 times a day
Excluded studies
The commonest reasons for excluding studies were lack of a placebo control, that cough was artificially induced or lasted longer than three weeks, or cough outcomes were not clearly reported. See Characteristics of excluded studies table.
Risk of bias in included studies
These are summarised in Figure 2. All studies were randomised controlled trials but methods for allocation concealment and random sequence generation were reported in only five of the 29 included studies. All studies were placebo‐controlled based on inclusion criteria and the majority reported blinding of providers (24 of 29 studies), but only a minority reported on blinding of outcome assessors (9 of 29 studies). There was minimal risk of attrition bias. There was potential risk of bias related to funding sources as studies funded by pharmaceutical companies or other providers (12 of 29 studies) were more likely to have positive results.
2.
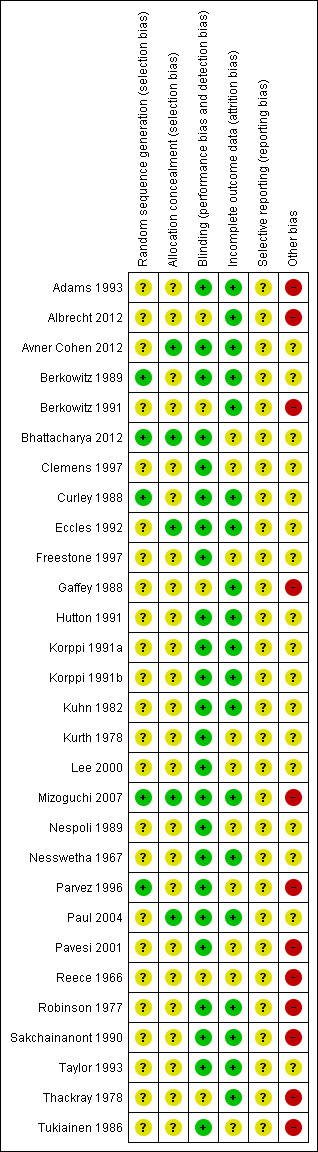
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Most studies did not report sufficient details on randomisation and allocation schedules to make meaningful conclusions about the potential for selection bias. Only five of the 29 trial reports stated the randomisation process, which was adequate in three trials.
Loss to follow‐up was well documented in 20 studies with differential loss to follow‐up in the treatment arms reported in five studies, and with the potential for attrition bias difficult to assess for the remaining studies. Only three of the studies fulfilled all the quality criteria. Only eight trials reported a power calculation.
Blinding
All studies were placebo‐controlled thus patients were blinded. In 24 of the 29 included studies providers were also blinded. Only nine of the 29 included studies reported whether outcome assessors had been blinded, which could lead to potential detection and performance bias.
Incomplete outcome data
In general there was adequate follow‐up of participants with 19 of the 29 studies reporting adequate follow‐up and the remainder being unclear due to lack of reporting. A number of studies dated back many years, therefore it was often impossible to obtain additional trial data. As the reporting of potential causes of bias was poor in many trials, we did not formally examine the trial efficacy versus the trial quality and therefore only summarised the available data in the 'Risk of bias' section of the Characteristics of included studies. These contain summary data on randomisation processes used, blinding to treatment allocation, drop‐outs/losses to follow‐up and any additional comments.
Other potential sources of bias
Twelve of the 29 included studies were fully or partly supported by pharmaceutical or other supplier companies, which provided grants, supplied the drugs in question or gave assistance with the study (Adams 1993; Avner Cohen 2012; Berkowitz 1991; Gaffey 1988; Mizoguchi 2007; Parvez 1996; Pavesi 2001; Reece 1966; Robinson 1977; Sakchainanont 1990; Thackray 1978; Tukiainen 1986). Seven out of the 12 studies supported by industry showed positive results compared to nine out of 18 trials where no support was reported.
Effects of interventions
We grouped the trials according to drug class into antitussives, expectorants, mucolytics, antihistamine‐decongestant combinations, other combinations and antihistamines. The number of studies in each group ranged from one to a maximum of six. Cough outcomes included frequency, severity and night‐time symptoms and were measured in many different ways, for example, participant self report by symptom scores (interviews, questionnaires, diaries), physician assessment, observation by parents, cough sound pressure levels obtained by recordings via a microphone and tape recordings. Nineteen studies out of 29 reported data on adverse effects and six studies reported data on compliance with medication. Eleven out of the 29 trials reported quantitative data for the cough that could potentially have been used for meta‐analysis. Due to the small numbers of trials in each category, the limited quantitative data available and the marked differences between trials in terms of participants, interventions and outcome measurement, we felt that pooling of the results was inappropriate.
1. Antitussives
1.1 Studies in adults
We included six trials involving 1526 participants that compared antitussives with placebo.
Codeine was tested in two trials and appeared no more effective than placebo in reducing cough symptoms (Eccles 1992; Freestone 1997). One of these studies (n = 81) tested codeine in a two‐phase study (laboratory and home) at a dose of 30 mg four times daily for four days (Eccles 1992), and codeine was no more effective than placebo either as a single dose or in a total daily dose of 120 mg, reported on a five‐point cough severity score (cough severity scores were expressed as area under the curve for eight measures over five days with intervention group 17.2 versus control group 18.8, P value = 0.23). The second study of codeine (n = 82) only tested the effect of a single 50 mg dose (Freestone 1997), and cough was assessed via microphone using cough sound pressure levels 90 minutes after drug administration, cough frequency counts and subjective scores. The mean subjective score on a five‐point rating scale was reduced from 2.0 to 1.0, 90 minutes after treatment in both treatment groups (P value = 0.8). Neither study provided any data on side effects.
Dextromethorphan was tested in three of the included studies (Lee 2000; Parvez 1996; Pavesi 2001). One report on a series of three successive studies on a total of 451 adults favoured dextromethorphan 30 mg given in a single dose to placebo in terms of cough counts (measured through cough acoustic signals using a microphone on the nose) and subjective visual analogue scales (Parvez 1996). Differences in mean changes of cough counts between active treatment and placebo varied from 19% to 36% (P value < 0.05) in the three studies (up to a net difference of eight to 10 coughing bouts every 30 minutes). This study did not report on side effects.
A study involving 44 participants tested a single 30 mg dose of dextromethorphan versus placebo (Lee 2000). Both treatment groups showed a decline in cough frequency (from 50 to 19 per 10‐minute period in the active treatment arm compared with 42 to 20.5 in the placebo arm, P value = 0.38 at 180 minutes follow‐up). Mean subjective cough scores showed a decline from 2.0 to 1.0 in the active treatment group compared to a decline from 2.0 to 1.5 in the placebo group (mean difference in decline in cough scores 0.5 at 180 minutes, P value = 0.08).
Pavesi and colleagues also tested a single 30 mg dose of dextromethorphan versus placebo (Pavesi 2001). Outcomes were measured through a three‐hour continuous cough recording, measuring cough bouts, cough components, cough effort, cough intensity and cough latency. Average treatment difference was 12% to 17% in favour of dextromethorphan for cough bouts (P value = 0.004), cough components (P value = 0.003) and cough effort (P value = 0.001), with an increase in cough latency (P value = 0.002).
One trial in 108 adults comparing moguisteine at a total daily dose of 600 mg for three and a half days with placebo showed no difference apart from cough reduction in individuals with more severe night cough (Adams 1993). The overall mean cough score difference was 0.9 (intervention group score 6.4 versus control group score 5.5). There were no differences in the proportion rated as being improved by the investigators (intervention 42/48 versus control 41/48). There were more side effects in the treatment group (22%) compared to placebo (8%), which mainly included nausea, vomiting and abdominal pain. There were four withdrawals in the treatment group due to adverse effects.
1.2 Studies in children
We included four trials involving 327 participants that compared antitussives with placebo in children.
One study involving 57 children with night cough compared a single dose for three nights of dextromethorphan or codeine with placebo (Taylor 1993). Mean cough and composite scores decreased in each of the three treatment groups on each day of the study. Neither dextromethorphan (cough score reduction of 2.1, P value = 0.41) nor codeine (cough score reduction of 2.2, P value = 0.70) was more effective than placebo (cough score reduction of 2.2) on day three.
Another study involving 50 children compared dextromethorphan 1.5 mg per ml 5 ml three times a day for children under seven years and 10 ml three times daily for older children with placebo (Korppi 1991a). There were no differences between the groups in terms of parent‐recorded symptom scores (mean difference in cough symptom scores on day 3 of 0.04) or adverse effects, which were generally mild.
A third study involving 100 children compared a single nocturnal dose of dextromethorphan (dose based on child's age: age two to five, 7.5 mg; age six to 11, 15 mg; age 12 to 18, 30 mg with either a single dose of an antihistamine or with placebo) (Paul 2004). Dextromethorphan was no more effective than diphenhydramine or placebo in reducing cough frequency or impact on child or parental sleep (composite symptom score intervention 10.06 versus control 10.85, mean difference 0.79).
A new study included an arm where 40 children were given 5 mg dextromethorphan six‐ to eight‐hourly for three days and found no difference from placebo treatment (Bhattacharya 2012). There was no significant difference in composite symptom scores on day three (intervention 4.6 versus control 5.0, mean difference 0.4). Adverse events were reported in 34% of participants in the dextromethorphan group and 32% of participants in the antihistamine group compared to 5% of participants in the placebo group.
2. Expectorants
2.1 Studies in adults
Three trials with a total of 604 participants compared guaifenesin with placebo (Albrecht 2012; Kuhn 1982; Robinson 1977). In one study (n = 239), 75% of participants taking guaifenesin stated that the medicine was helpful in terms of reducing cough frequency and intensity compared to 31% in the control group (P value < 0.01) at 72 hours (Robinson 1977). Four participants (two in each group) reported side effects including nausea and hives in the active treatment group and headaches, drowsiness and excessive perspiration in the placebo group.
The second study (n = 65) evaluated an antitussive rather than an expectorant effect of guaifenesin, which is usually classified as an expectorant (Kuhn 1982). Individuals in both groups reported improvement with respect to cough frequency (100% in the active group versus 94% for placebo, P value = 0.5) and cough severity (100% in the active treatment group versus 91% in the placebo group, P value = 0.2) at 36 hours. Guaifenesin reduced sputum thickness significantly in 96% of participants compared to 54% in the placebo group (P value = 0.001). This study allowed aspirin and paracetamol for participants after inclusion in the study, and the vehicle contained 95% alcohol. Adverse effects were not reported on.
A third new study compared extended‐release guaifenesin with placebo in 378 participants aged greater than 12 years over seven days and found no difference in total spontaneous symptom severity scores on day seven but reported day four results in detail, which indicated a reduction in mean score from baseline of 7.1 in the intervention group compared to a reduction of 5.7 in the control group (mean difference (MD) 2.6, P value = 0.04) (Albrecht 2012).
2.2 Studies in children
We did not include any studies that tested expectorants in children, partly because none of the outcomes under study were reported on.
3. Mucolytics
3.1. Studies in adults
One trial involving 99 participants compared bromhexine 5 mg three times daily for an average of four days with placebo (Nesswetha 1967). Frequent cough (every two to five minutes) was more prevalent in the placebo group (15.2%) compared to active treatment (8.6%, P value < 0.02) leading to a risk ratio reduction of about 50% for frequent cough. This study did not report on any adverse effects.
3.2 Studies in children
One trial involving 40 children compared the mucolytic letosteine (preparation not available in the UK and other parts of the world) at a dose of 25 mg three times daily for 10 days with placebo (Nespoli 1989). The symptom score on a four‐point scale favoured active treatment from day four until day 10, with an average difference of about 0.2 points (P value < 0.01). No adverse effects were reported in either group.
4. Antihistamine‐decongestant combinations
4.1 Studies in adults
Two trials in adults with a total of 356 participants compared antihistamine‐decongestant combinations with placebo (Berkowitz 1989; Curley 1988). One trial comparing loratadine/pseudoephedrine (5 mg/120 mg twice daily for four days) with placebo (n = 283) did not show statistically significant differences in cough scores reported in patient diaries between both groups (Berkowitz 1989). Thirty per cent of participants in the active treatment group reported adverse effects including dry mouth, headache and insomnia compared to 21% in the control group.
The second trial (n = 73) compared dexbrompheniramine/pseudoephedrine (6 mg/120 mg twice daily for one week) with placebo. The mean severity rank of cough on a scale from zero to four obtained through a patient diary was less in the active treatment group (1.4) than in the placebo group (2.0) on days three to five (P value < 0.05) (Curley 1988). There was an increased severity of dizziness and dry mouth in the active drug group on days five to seven, and two to 10, respectively (exact figures not reported, P value = or < 0.01).
4.2 Studies in children
Two studies involving 155 children compared antihistamine‐decongestant combinations with placebo (Clemens 1997; Hutton 1991).
One study including 59 participants found that brompheniramine/phenylpropanolamine (2 mg/12.5 mg, half the dose for children from six months to one year, on a four‐hourly 'as needed' basis for 48 hours) was no more effective than placebo in reducing the number of children coughing two hours after each dose (49.0% versus 43.1%, P value = 0.66). A higher proportion of children was reported to be asleep in the active treatment group (46.6%) than in the placebo group (26.5%, P value = 0.53), and no other adverse effects were reported (Clemens 1997).
In the second study (n = 96), a combination of brompheniramine/phenylephrine/propanolamine (see Characteristics of included studies table for full dosage details) led to a non‐statistically significant improvement in cough in 67% of children (reported by their parents) compared to 58% in the placebo group and 70% in the group receiving no treatment (Hutton 1991). Side effects were rare and included one child with loose stools in the placebo group and one child reported to be hyperactive in the active drug group. A second child in the drug group was reported to be sleepier than usual.
5. Other drug combinations
For the constituent ingredients of the drug combination formulations included in the review please refer to the Characteristics of included studies table.
5.1 Studies in adults
Four studies involving 836 people compared other combinations with placebo (Kurth 1978; Mizoguchi 2007; Thackray 1978; Tukiainen 1986). These studies were very heterogeneous and used very different drug preparations and dose frequency, limiting their comparability. In one trial (n = 113) EM‐Vier (Minetten) given six times daily was more effective in reducing coughing fits (25% versus 11%, P value < 0.01) and the urge to cough (27% versus 14%, P value < 0.01) compared to placebo in the first seven days (Kurth 1978). There were no adverse effects in either group.
In a trial of Vicks Medinite syrup (n = 70) at a single dose at bedtime for two days, 57.6% of participants in the active treatment group rated the formulation as "good" or better in relieving cough compared to 32.2% in the placebo group (P value < 0.01) (Thackray 1978). Seven participants in the active treatment group reported giddiness/drowsiness compared to four participants in the placebo group.
Another study (n = 108) compared a dextromethorphan/salbutamol combination and dextromethorphan alone with placebo (Tukiainen 1986). There was spontaneous improvement of cough in all groups, and there were no statistically significant differences in cough scores between active treatments and placebo for both cough frequency and severity during the day. Dextromethorphan/salbutamol was superior to placebo or dextromethorphan alone in relieving cough at night (mean symptom score 0.19 versus 0.67 and 0.44, respectively on day four, P value < 0.01). The dextromethorphan/salbutamol combination led to more tremor than placebo (no figures given, P value < 0.05), and no serious adverse effects were reported.
A further study (n = 485), identified for the 2009 update of this review, compared a single nocturnal dose of a compound containing four agents each with potential to deal with the different symptoms of the common cold, i.e. paracetamol plus dextromethorphan plus doxylamine plus ephedrine (Mizoguchi 2007). We only report the cough‐related outcomes. The outcomes in this study were measured over the following two days and included proportions who reported improvements in cough three hours after taking the treatment and mean cough scores on day one and day two. There was a significant improvement in mean cough score the morning after treatment and the following day (mean cough score 2.5 versus 2.08 on day two, MD 0.42, P value < 0.0001). There were also improvements in the proportion reporting improvement in cough three hours after taking the medication (intervention 57% and control 43%). There were 19 adverse events in the study in 14 patients with no difference between treatment and control. However, there was one serious adverse event described as a severe episode of somnolence in the active treatment group.
5.2 Studies in children
We included two trials with 94 participants examining other drug combinations.
One trial involving 43 children tested two paediatric cough syrups (Triaminicol syrup and Dorcol paediatric cough syrup) (Reece 1966). Compared to placebo, 69% of children in both active treatment groups showed a satisfactory response reported by their parents compared to 57% of children in the placebo group, which did not reach statistical significance (P value = 0.5). Adverse effects were not reported.
One trial in 51 children compared a combination of dextromethorphan 1.5 mg per ml and salbutamol 0.2 mg per ml 5 ml three times daily for children under the age of seven or 10 ml three times a day for older children with placebo (Korppi 1991b). There were no differences between the groups in terms of parent‐recorded symptom scores or adverse effects, which were generally mild.
6. Antihistamines
6.1 Studies in adults
Two trials involving 350 adult participants compared antihistamines with placebo (Berkowitz 1991; Gaffey 1988). Antihistamines were no more effective than placebo in relieving cough symptoms. Terfenadine was tested in two studies. In one of these studies (n = 100), terfenadine at a dose of 120 mg twice daily for four to five days led to a mean cough score (measured by physicians' evaluation on a scale from zero to three with higher scores meaning more coughing) of 0.8 in the active treatment group compared to 0.65 in the placebo group (MD 0.15, P value = 0.35) (Berkowitz 1991). Possible adverse effects were rare in both groups, with headache being the most common complaint (6.1% of participants in the active treatment group compared to 4% in the placebo group).
The second study (n = 250) tested terfenadine at a dose of 60 mg twice daily for three and a half days (Gaffey 1988). There were no statistically significant differences in self reported symptoms scores for cough (exact figures not reported) between groups. Side effects were uncommon in both treatment groups, with the most common complaint being excess fatigue in 12% of participants receiving terfenadine compared to 10% in the placebo group.
6.2 Studies in children
We included three trials involving 363 children comparing antihistamines with placebo.
One study compared the antihistamines clemastine (0.05 mg/kg/day) and chlorpheniramine (0.35 mg/kg/day) for three days with placebo (Sakchainanont 1990). There was spontaneous improvement in all groups. In both active treatment groups, cough scores observed by physicians and participants improved in 39.6% of individuals compared with 27.6% in the placebo group, which did not reach statistical significance (P value = 0.2). Drowsiness and sleepiness were reported in 20% of children with no difference between the groups.
The second trial included an arm in which children received diphenhydramine in a single nocturnal dose and were compared with children receiving placebo (Paul 2004). Diphenhydramine was no more effective than dextromethorphan or placebo in reducing cough frequency or impact on child or parental sleep.
The third study, added in the current update, had one arm with 40 children who received promethazine at a dose of 0.5mg/kg/dose eight‐hourly (Bhattacharya 2012). There was no difference from placebo in cough and sleep‐related outcomes. Thirteen children receiving promethazine experienced adverse effects compared to two children receiving placebo.
7 Honey
One new study compared two days of treatment with three types of honey with placebo in 300 children aged one to five years (Avner Cohen 2012). Children were administered 10 g of honey (eucalyptus honey, citrus honey or labetiae honey) or silan date extract as placebo, all provided in identical containers. Parents were told to give it as a single dose or to dilute it in a non‐caffeinated beverage 30 minutes before the child was due to go to sleep. Children receiving honey had significant improvements in total symptom scores compared to placebo with no significant differences in adverse effects between the honey and placebo groups.
Adverse effects
Twenty‐one of the 29 included studies reported on adverse effects. There was a wide variation in the range of reported adverse effects across studies from less than 1% in some studies to approximately 30% in others. No study reported adverse effects as being serious. The adverse effects were generally higher in intervention participants compared to those taking placebo and this was particularly the case for preparations containing antihistamines and dextromethorphan.
Discussion
Summary of main results
We found no good evidence for or against the effectiveness of over‐the‐counter (OTC) medications in acute cough, which confirms the findings of two previous reviews (Anonymous 1999; Smith 1993). The number of trials in each group of drugs was small, there was poor overall quality of the studies and studies showed conflicting evidence. In total, 11 of the 29 included trials showed a positive result, whereas 18 did not show active treatment to be superior to placebo. Eight out of the 12 studies that were supported by the pharmaceutical industry showed positive results compared to four positive studies out of the 15 trials that did not report any conflict of interest. The results of trials did not appear to be related to their sample size or length of follow‐up. We did not formally examine the trial efficacy versus trial quality because of the lack of reported data.
Overall completeness and applicability of evidence
The results of this systematic review have to be interpreted with caution as the number of trials in each group was small. There were marked differences between the studies even within groups of drugs with similar mode of action, making it difficult to compare trials directly. In addition, there is variation between countries in relation to medications available OTC, making international comparisons more difficult. Inclusion and exclusion criteria for participants varied, and active drugs were administered in different total daily doses. The duration of drug therapy varied from a single‐dose treatment to an 18‐day course. For example, six studies testing antitussives, either alone or in combination with other agents, used short‐term cough relief after a single dose as an outcome (Freestone 1997; Lee 2000; Mizoguchi 2007; Parvez 1996; Paul 2004; Pavesi 2001), whereas more relevant outcomes for patients would be the effect after one day, three days or a week. Outcomes were assessed and measured in many different ways, which included questionnaires, cough severity scores, acoustic signals, tape recordings, daily diaries and assessment by a physician.
Most studies failed to provide quantitative data on cough as our main outcome of interest, which made it very difficult to assess whether positive study results were clinically relevant. Quantitative data that could be combined showed wide confidence intervals, although there was no evidence of statistical heterogeneity. Many included studies failed to report adverse effects adequately, and patient compliance with the treatment was not discussed in the vast majority of study reports. Four studies carried out multiple comparisons, thereby increasing the probability of a type I error (Albrecht 2012; Berkowitz 1989; Parvez 1996; Pavesi 2001).
Quality of the evidence
Most studies failed to provide quantitative data on cough. As this was our main outcome of interest, it was very difficult to assess whether positive study results were clinically relevant. Quantitative data that could be combined showed wide confidence intervals, although there was no evidence of statistical heterogeneity. Many included studies failed to report adverse effects adequately and patient compliance with the treatment was not discussed in the majority of study reports. The overall quality of trials is dubious and there are conflicting results between trials in each medication group. The method of outcome measurement and the resulting magnitude of effect were unclear or not very well reported in some studies.
Potential biases in the review process
Eleven of the 26 included studies were funded by the pharmaceutical industry as outlined in the 'Risk of bias' section in the Results. Studies funded in this way were more likely to report positive results. However, despite this potential bias the review does not provide evidence of the effectiveness of OTC cough medicines for acute cough.
Agreements and disagreements with other studies or reviews
The findings of this review and other related published evidence were considered by an expert panel of the US Food and Drug Administration (FDA) in October 2007 and there was consensus that there is limited evidence to support the recommendation to use OTC cough medicines for acute cough in children (FDA 2007). The review findings are also supported by a recent non‐Cochrane systematic review, which found few studies that examined the effectiveness of diphenhydramine for acute cough despite its widespread use, and these studies indicated limited clinical effectiveness (Bjórnsdóttir 2001).
Authors' conclusions
Implications for practice.
There is no good evidence for or against the effectiveness of over‐the‐counter (OTC) cough medicines, and from the studies included in this review it remains unclear whether these medications are helpful for the treatment of acute cough. Although a number of randomised controlled trials (RCTs) have compared OTC cough preparations with placebo, the number of trials in each group was small. This review suggests that most preparations appear to be safe, based on those studies reporting side effects, which only described a low incidence of mainly minor adverse effects. However, more serious concerns about the safety of OTC cough medicines have arisen since this review was last updated, particularly in young children and, in general, larger numbers of patients are required in order to identify serious, though less common adverse effects (Smith 2008a). This systematic review confirms the lack of evidence for or against an effect of OTC cough preparations despite using an extensive search strategy. This lack of evidence of effectiveness also has implications for the regulatory bodies and brings into question how these products can continue to be promoted using language that implies that their effectiveness is not in doubt.
The results of this review have to be interpreted with caution because study designs, populations, interventions and outcomes varied markedly between studies, limiting the generalisability of the results. All results were based on a small number of studies. It is also questionable as to whether all of the positive results obtained with unclear outcome measures are clinically relevant.
Implications for research.
Further high‐quality RCTs of OTC cough preparations are needed as the results of this review are based on a small number of often underpowered studies. More evidence about the effectiveness of OTC cough preparations would be helpful, particularly in relation to the use of honey in adults and children, as identification of effective self care treatments may help reduce the burden of days lost at work due to acute cough as well as the number of consultations in primary care. Research should also include individuals who self medicate with OTC cough preparations, as there is likely to be a variation between countries in the proportion of individuals using these medications, with or without professional advice, particularly given the international variation in what products are available OTC or on a prescription basis. There is also a need to identify ineffective preparations in order to lower costs for consumers and healthcare providers. Studies will need to be rigorously designed and should use clinically relevant outcome measures, including cough frequency, severity and duration. It is important that future RCTs use OTC drugs in doses recommended by the manufacturers for an appropriate length of time, as drugs tested in a single and possibly too low a dose are likely to be ineffective. Trials should also report details on effect sizes and provide data on adherence and adverse effects. This review also highlights a need for an outcome measure for acute cough that is clinically relevant, valid, reliable and easy to use in RCTs.
What's new
| Date | Event | Description |
|---|---|---|
| 26 March 2014 | New citation required but conclusions have not changed | Our conclusions remain unchanged, but we added cautions in relation to potential adverse effects. |
| 26 March 2014 | New search has been performed | Searches updated. We included three new studies (Albrecht 2012; Avner Cohen 2012; Bhattacharya 2012), and excluded one new study (Vornov 2012). Title and text changed to decribe setting as 'community' rather than 'ambulatory'. |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 22 March 2012 | New search has been performed | Searches conducted. No eligible studies were identified. One new study was excluded (Shadkam 2010). |
| 22 March 2012 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 19 March 2010 | New search has been performed | One study added to this update (Mizoguchi 2007), but this did not lead to any major changes in the conclusions of this review. |
| 6 August 2009 | Amended | Contact details updated. |
| 8 May 2009 | Amended | Contact details updated. |
| 2 June 2008 | Amended | Converted to new review format. |
| 4 July 2007 | New search has been performed | Searches conducted. |
| 25 July 2004 | New search has been performed | Searches conducted. |
| 12 December 1999 | New search has been performed | Searches conducted. |
Notes
A single randomised controlled trial (RCT) was added to this review in the 2008 update (Paul 2004). Paul et al tested a single nocturnal dose of dextromethorphan, or a single nocturnal dose of diphenhydramine versus placebo. The outcomes in this study were measured the following morning and included a cough severity index and a measure of sleep difficulty both for the affected children and their parents. This study showed no significant treatment differences between the two intervention groups and the control group. Average treatment differences were between 12% and 17% in favour of dextromethorphan for cough bouts (P value = 0.004), cough components (P value = 0.003) and cough effort (P value = 0.001) with an increase in cough latency (P value = 0.002).
A further single RCT was added for the 2010 update (Mizoguchi 2007). Mizoguchi et al compared a single nocturnal dose of a combination test syrup containing dextromethorphan, doxycycline, ephedrine and paracetamol with placebo. The outcomes in this study were measured over the following two days and included proportions reporting improvements in cough three hours after taking the treatment and mean cough scores on day one and day two. This study showed significant treatment differences between the two intervention groups and the control group in terms of reduction in mean cough scores on day two.
The addition of these studies did not lead to any major changes in the conclusions of this review.
Acknowledgements
We thank the editorial team of the Cochrane Acute Respiratory Infections (ARI) Group for their excellent support and help with the literature searches. Steve McDonald and Ron D'Souza from the ARI Group helped design the initial search strategy and performed additional searches. Liz Dooley, Ruth Foxlee and Sarah Thorning provided further help and support with additional searches for the 2004, 2007, 2010, 2012 and 2014 updates.
We thank Professor Debbie Sharp (Division of Primary Health Care, University of Bristol) and Professor Massimo Pignatelli (Department of Histopathology, Bristol Royal Infirmary) for their help with the French and Italian translations.
Many thanks also to Bruce Arroll, Keith Dear, Warren McIsaac and Amy Zelmer for their very helpful comments on an earlier draft of this review. Thanks to Basem Saab, Martin Schultz, Mark Jones and Chris Del Mar for commenting on the 2007 revised draft. Finally, thanks also to Ntambwe Gustav Malangu, Nina Griese, Max Bulsara, Basem Saag, Marie Kakhu and again to Sree Nair, Chris Del Mar and Martin Shulz for commenting on the 2010 and 2014 revised draft.
Appendices
Appendix 1. MEDLINE search strategy
MEDLINE (OVID)
1 Cough/ 2 cough*.mp. 3 1 or 2 4 exp Antitussive Agents/ 5 exp Expectorants/ 6 exp Cholinergic Antagonists/ 7 exp Histamine H1 Antagonists/ 8 exp Drug Combinations/ 9 exp Nonprescription Drugs/ 10 Self Medication/ 11 (cough* adj5 (suppress* or mixtur* or medicin* or remed* or relief* or formula* or syrup* or medicat*)).tw. 12 (antituss* or expectorant* or anticholinerg* or antihistamin* or anti‐histamin* or mucolytic*).tw. 13 (over‐the‐counter or otc or nonprescrip* or nonprescrib* or non‐prescrip* or non‐prescrib*).tw. 14 (drug adj2 combination*).tw. 15 or/4‐14 16 3 and 15
Appendix 2. EMBASE.com search strategy
#26 #25 AND [embase]/lim AND [1‐3‐2010]/sd NOT [22‐3‐2012]/sd163 #25 #16 AND #24 952 #24 #19 NOT #23 693139 #23 #20 NOT #22 3418939 #22 #20 AND #21 723950 #21 'human'/de AND [embase]/lim 8050790 #20 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de AND [embase]/lim 4142889 #19 #17 OR #18 776450 #18 random*:ab,ti OR placebo*:ab,ti OR allocat*:ab,ti OR trial:ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR (doubl* NEXT/1 blind*):ab,ti AND [embase]/lim733748 #17 'randomized controlled trial'/de OR 'single blind procedure'/de OR 'double blind procedure'/de OR 'crossover procedure'/de AND [embase]/lim257879 #16 #3 AND #15 5100 #15 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14188114 #14 (drug NEAR/2 combination*):ab,ti AND [embase]/lim9999 #13 'over‐the‐counter':ab,ti OR otc:ab,ti OR nonprescrip*:ab,ti OR nonprescrib*:ab,ti OR 'non‐prescription':ab,ti OR 'non‐prescribed':ab,ti AND [embase]/lim 8605 #12 antituss*:ab,ti OR expectorant*:ab,ti OR anticholinerg*:ab,ti OR antihistamin*:ab,ti OR 'anti‐histamine':ab,ti OR mucolytic*:ab,ti AND [embase]/lim 22936 #11 (cough* NEAR/5 (suppress* OR mixtur* OR medicin* OR remed* OR relief* OR formula* OR syrup* OR medicat*)):ab,ti AND [embase]/lim 1179 #10 'self medication'/de AND [embase]/lim 5809 #9 'non prescription drug'/de AND [embase]/lim 5821 #8 'drug combination'/de AND [embase]/lim 2554 #7 'histamine h1 receptor antagonist'/exp AND [embase]/lim 80640 #6 'cholinergic receptor blocking agent'/de AND [embase]/lim 17207 #5 'expectorant agent'/exp AND [embase]/lim 18166 #4 'antitussive agent'/exp AND [embase]/lim 68972 #3 #1 OR #2 52203 #2 cough*:ab,ti AND [embase]/lim 30822 #1 'coughing'/de OR 'irritative coughing'/de AND [embase]/lim 41121
Appendix 3. CINAHL (Ebsco) search strategy
S28 S16 and S26 Limiters ‐ Published Date from: 20100101‐20120331 20 S27 S16 and S26 103 S26 S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 172166 S25 (MH "Quantitative Studies") 7436 S24 TI placebo* OR AB placebo* 18646 S23 (MH "Placebos") 6225 S22 TI random* OR AB random* 91601 S21 (MH "Random Assignment") 27203 S20 TI ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) OR AB ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) 13567 S19 TI clinic* W1 trial* OR AB clinic* W1 trial* 25204 S18 PT clinical trial 49701 S17 (MH "Clinical Trials+") 101958 S16 S3 and S15 527 S15 S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 21889 S14 TI drug N2 combination* OR AB drug N2 combination* 732 S13 TI ( over‐the‐counter or otc or nonprescrip* or nonprescrib* or non‐prescrip* or non‐prescrib* ) OR AB ( over‐the‐counter or otc or nonprescrip* or nonprescrib* or non‐prescrip* or non‐prescrib* ) 2308 S12 TI ( antituss* or expectorant* or anticholinerg* or antihistamin* or anti‐histamin* or mucolytic* ) OR AB ( antituss* or expectorant* or anticholinerg* or antihistamin* or anti‐histamin* or mucolytic* ) 1695 S11 TI (cough* N5 (suppress* or mixtur* or medicin* or remed* or relief* or formula* or syrup* or medicat*) ) OR AB ( cough* N5 (suppress* or mixtur* or medicin* or remed* or relief* or formula* or syrup* or medicat*)) 279 S10 (MH "Self Medication") 690 S9 (MH "Drugs, Non‐Prescription") 2205 S8 (MH "Drug Combinations+") 9185 S7 (MH "Histamine H1 Antagonists+") 1749 S6 (MH "Cholinergic Antagonists+") 2423 S5 (MH "Expectorants+") 679 S4 (MH "Antitussive Agents+") 3355 S3 S1 or S2 4440 S2 TI cough* OR AB cough* 3755 S1 (MH "Cough") 2006
Appendix 4. LILACS (BIREME) search strategy
> Search > (MH:cough OR tos OR tosse OR cough$ OR MH:C08.618.248 OR MH:C23.888.852.293) AND (MH:"Antitussive Agents" OR MH:D27.505.954.427.153$ OR MH:D27.505.954.796.090$ OR Antitusígenos OR Antitussígenos OR antituss$ OR "Agentes Antitusígenos" OR "Agentes Antitusivos" OR Antitusivos OR "Fármacos Antitussivos" OR "Agentes Béquicos" OR "Substâncias Béquicas" OR "Medicamentos Béquicos" OR MH:Expectorants OR Expectorantes OR mucolytic$ OR MH:D27.505.954.796.250$ OR expectorant$ OR Mucolíticos OR MH:"Cholinergic Antagonists" OR "Antagonistas Colinérgicos" OR "Acetylcholine Antagonists" OR "Anticholinergic Agents" OR "Cholinergic‐Blocking Agents" OR Cholinolytics OR MH:D27.505.519.625.120.200$ OR MH:D27.505.696.577.120.200$ OR "Antagonistas de la Acetilcolina" OR "Agentes Anticolinérgicos" OR "Agentes Bloqueadores Colinérgicos" OR Colinolíticos OR MH:"Histamine H1 Antagonists" OR "Antagonistas de los Receptores Histamínicos H1" OR "Antagonistas dos Receptores Histamínicos H1" OR MH:D27.505.519.625.375.425.400$ OR MH:D27.505.696.577.375.425.400$ OR "Antihistamínicos Clásicos" OR "Antihistamínicos H1" OR "Antagonistas del Receptor H1 de Histamina" OR "Bloqueadores de los Receptores Histamínicos H1" OR "Bloqueadores de los Receptores H1 de Histamina" OR "Antihistaminas Clásicas" OR "Anti‐Histamínicos Clássicos" OR "Anti‐Histamínicos H1" OR "Antagonistas dos Receptores H1 de Histamina" OR "Bloqueadores dos Receptores Histamínicos H1" OR "Bloqueadores dos Receptores H1 de Histamina" OR "Anti‐Histaminas Clássicas" OR MH:"Drug Combinations" OR "Combinación de Medicamentos" OR "Combinação de Medicamentos" OR MH:"Multi‐Ingredient Cold, Flu, and Allergy Medications" OR "Medicamentos Compuestos contra Resfriado, Gripe y Alergia" OR "Medicamentos Compostos contra Resfriado, Influenza e Alergia" OR "Multi‐Ingredient Cold Medications" OR D26.310.500$ OR MH:"Nonprescription Drugs" OR "Medicamentos sin Prescripción" OR "Medicamentos sem Prescrição" OR "non‐prescription" OR nonprescription$ OR "over‐the‐counter" OR "behind‐the‐counter" OR otc OR "Medicamentos de Venta Libre" OR "Medicamentos de Libre Circulación" OR "Medicamentos não Prescritos" OR "Medicamentos de Venda Livre" OR "Medicamentos de Livre Circulação" OR "Medicamentos Isentos de Prescrição" OR MIPs OR MH:"Self Medication" OR Automedicación OR Automedicação OR anticholinerg$ OR antihistamin$) > clinical_trials
Appendix 5. Web of Science (Thomson Reuters) search strategy
# 968 #8 AND #7 Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=2010‐2012 Lemmatization=On # 8197,325 Topic=(random* or placebo* or (clinic* NEAR/1 trial*) or (doubl* NEAR/1 blind*) or (singl* NEAR/1 blind*)) OR Title=(trial) Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=2010‐2012 Lemmatization=On # 7299 #6 AND #1 Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=2010‐2012 Lemmatization=On # 64,980 #5 OR #4 OR #3 OR #2 Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=2010‐2012 Lemmatization=On # 5203
Topic=(cough* NEAR/5 (suppress* or mixtur* or medicin* or remed* or relief* or formula* or syrup* or medicat*)) Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=2010‐2012 Lemmatization=On # 42,027
Topic=(drug NEAR/2 combination*) Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=2010‐2012 Lemmatization=On # 31,197
Topic=(over‐the‐counter or otc or nonprescrip* or nonprescrib* or non‐prescrip* or non‐prescrib*) Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=2010‐2012 Lemmatization=On # 21,680
Topic=(antituss* or expectorant* or anticholinerg* or antihistamin* or anti‐histamin* or mucolytic*) Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=2010‐2012 Lemmatization=On # 13,195
Topic=(cough*) Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, CCR‐EXPANDED, IC Timespan=2010‐2012 Lemmatization=On
Appendix 6. Details of previous searches
This review was first published in 2001. We searched the Cochrane Controlled Trials Register (CENTRAL) (The Cochrane Library 2000, Issue 2), MEDLINE (January 1998 to December 1999), EMBASE (January 1998 to December 1999) and the UK Department of Health National Research Register (December 2000).
For the 2004 review update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2004, Issue 2), MEDLINE (January 1966 to June Week 3, 2004), EMBASE (January 1990 to March 2004) and the UK Department of Health National Research Register (December 2003).
For the 2007 review update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2006, Issue 4), MEDLINE (January 1966 to January Week 1, 2007), EMBASE (January 1990 to January 2007) and the UK Department of Health National Research Register (June 2007, http://www.update‐software.com/National/nrr‐frame.html).
For the 2010 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 1), MEDLINE (January 1966 to March, week 2, 2010), EMBASE (January 1974 to March 2010) and the UK Department of Health National Research Register (March 2010, http://www.nihr.ac.uk/Pages/NRRArchive.aspx)
We used the following search strategy to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision maximising version (2008 revision) Ovid format (Lefebvre 2011). The search string was modified slightly to search EMBASE.
MEDLINE (OVID)
1 exp COUGH/ 2 cough$.mp. 3 or/1‐2 4 exp Antitussive Agents/ 5 exp expectorants/ 6 exp Cholinergic antagonists/ 7 exp Histamine H1 Antagonists/ 8 exp Drug Combinations/ 9 exp Drugs, Non‐Prescription/ 10 exp Self medication/ 11 (antituss$ or expectorant$ or anticholinerg$ or antihistamin$ or (cough adj suppress$) or mucolytic$ or (drug adj combination$) or over‐the‐counter or OTC or non prescription).mp. 12 or/4‐11 13 3 AND 12
EMBASE.com
12. #8 AND #11 11. #9 OR #10 10. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR ((doubl* OR singl*) NEAR/2 (blind* OR mask)):ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti 9. 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'randomized controlled trial'/exp 8. #3 AND #7 7. #4 OR #5 OR #6 6. 'cough suppressant':ab,ti OR 'cough suppressants':ab,ti OR 'drug combination':ab,ti OR 'drug combinations':ab,ti OR 'over the counter':ab,ti OR 'over‐the‐counter':ab,ti OR otc:ab,ti OR 'behind the counter':ab,ti OR 'behind‐the‐counter':ab,ti OR 'non prescription':ab,ti OR 'non‐prescription':ab,ti OR nonprescription:ab,ti 5. antituss*:ab,ti OR expectorant*:ab,ti OR anticholinerg*:ab,ti OR antihistamin*:ab,ti OR mucolytic*:ab,ti 4. 'antitussive agent'/exp OR 'expectorant agent'/exp OR 'cholinergic receptor blocking agent'/exp OR 'histamine h1 receptor antagonist'/exp OR 'drug combination'/exp OR 'behind the counter drug'/exp OR 'non prescription drug'/exp OR 'self medication'/exp 3. #1 OR #2 2. cough*:ab,ti 1. 'coughing'/de OR 'irritative coughing'/de
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adams 1993.
| Methods | RCT | |
| Participants | 108 adults, mean age 48 years, 70% women, 60% smokers, UK primary care, acute dry or slightly productive cough | |
| Interventions | Antitussive: moguisteine 200 mg 3 times daily for 3.5 days | |
| Outcomes | Patient‐recorded cough scale from 0 to 9. Proportion rated as being improved by the investigators. |
|
| Notes | More side effects in the treatment group (22%) compared to the placebo group (8%), mainly including nausea, vomiting and abdominal pain. 4 withdrawals in the treatment group due to adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patient and provider blinded but not outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10% loss to follow‐up and reasons reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | High risk | Trial supported by pharmaceutical industry |
Albrecht 2012.
| Methods | RCT | |
| Participants | 378 patients aged 12 and over with acute upper respiratory tract infection within last 5 days, mean age 41 years, 52% male, 61% Caucasian | |
| Interventions | Guaifenesin (extended‐release) 1200 mg twice daily for 7 days | |
| Outcomes | Daily cough and phlegm diary; Spontaneous Symptom Severity Assessment and Wisconsin Upper Respiratory Symptom Survey | |
| Notes | There were no significant differences in adverse events between groups, which were reported by 8.5% of participants in the intervention group compared to 5.3% in the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants blinded but unclear if clinicians and outcome assessors blinded or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 97% follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | High risk | Trial supported by pharmaceutical industry |
Avner Cohen 2012.
| Methods | RCT | |
| Participants | 300 children aged 1 to 5 years with nocturnal cough in last 7 days, mean age 29 months, 30% male | |
| Interventions | 4 arms: eucalyptus honey; citrus honey; labetiae honey; and silan date extract as placebo | |
| Outcomes | Cough frequency; cough severity; bothersome cough; child and parental sleep quality | |
| Notes | There were no significant differences in adverse events between groups, which were reported by 2% of children taking the three types of honey compared to 1% taking placebo. Sponsored by the Israel Ambulatory Pediatric Association, Materna Infant Nutrition Research Institute and the Honey Board of Israel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | The envelopes containing the codes of the study preparations were stored off‐site and not opened until after analyses were completed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, treatment providers and outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90% follow‐up, balanced across groups |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Sponsored by the Israel Ambulatory Pediatric Association, Materna Infant Nutrition Research Institute and the Honey Board of Israel |
Berkowitz 1989.
| Methods | RCT | |
| Participants | 283 adults, mean age 30 years, mainly Caucasian, 52% women, 3 'centres', USA, common cold | |
| Interventions | Antihistamine‐decongestant combination: loratadine 5 mg and pseudoephedrine 120 mg combination twice daily for 5 days | |
| Outcomes | Patient diaries, cough score from 0 to 3 | |
| Notes | Adverse effects (dry mouth, headache and insomnia) more common in the active treatment group (30%) compared to the placebo group (21%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall 92% follow‐up and similar in both groups |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Multiple comparisons made |
Berkowitz 1991.
| Methods | RCT | |
| Participants | 100 adults, mean age 32, 56% women, non‐smokers, single centre (setting not reported), USA, common cold | |
| Interventions | Antihistamine: terfenadine 120 mg twice daily for 4 to 5 days | |
| Outcomes | Patient diary and symptom/ cough score from 0 to 3 | |
| Notes | Possible adverse effects rare in both groups. Headache most common (6.1% in active treatment group versus 4% in placebo group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding assumed but not clearly stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall 96% follow‐up and similar in both groups |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | High risk | Trial supported by pharmaceutical industry |
Bhattacharya 2012.
| Methods | RCT | |
| Participants | 120 children, aged between 1 and 22 years, with cough due to upper respiratory tract infection, mean age 5 years, 25% male | |
| Interventions | 3 arms: dextromethorphan 5 mg 6‐ to 8‐hourly; promethazine 0.5 mg/kg 8‐hourly; placebo for 3 days | |
| Outcomes | Cough frequency score; child's sleep score; parental sleep score; post‐tussive vomiting score; composite score of the above and adverse effects | |
| Notes | Adverse events were reported in 34% of participants taking dextromethorphan, 32% taking promethazine and 5% taking placebo. These included drowsiness, irritability, abdominal pain and nausea | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, treatment providers and outcomes assessors all blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 100% adherence and follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Clemens 1997.
| Methods | RCT | |
| Participants | 59 pre‐school children, mean age 2 years (6 months to 5 years), 4 paediatric offices, USA, URTI of less than 7 days' duration | |
| Interventions | Antihistamine‐decongestant combination: brompheniramine maleate 2 mg/5 ml and phenylpropanolamine‐hydrochloride 12.5 mg/5 ml (6 months to 1 year: 1.5 teaspoon and 2 to 5 years: 1 teaspoon) every 4 hours "as needed" for 48 hours | |
| Outcomes | Parent questionnaire, 7‐point Likert scale, also counted 'responses' after each dose. | |
| Notes | Higher proportion of children asleep in the active treatment group (46.6%) versus the placebo group (26.5%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as patients "randomly assigned in a double blind fashion" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients, providers and outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Curley 1988.
| Methods | RCT | |
| Participants | 73 adults, mean age 31 years, 60% women, 19% active smokers, outpatient department, USA, common cold of less than 72 hours' duration | |
| Interventions | Antihistamine‐decongestant combination: dexbrompheniramine maleate 6 mg and pseudoephedrine sulphate 120 mg combination twice daily for 1 week | |
| Outcomes | Patient diary and cough score from 0 to 4 | |
| Notes | Increased severity of dizziness and dry mouth in the active drug group compared to the placebo group (P value < 0.01, exact figures not reported) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and providers blinded; outcome assessor blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 85% follow‐up, difference between groups not reported. Overall drop‐outs due to inconvenience of study and not due to side effects |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Eccles 1992.
| Methods | RCT | |
| Participants | 81 adults, mean age 23 years (range 18 to 71), 52% men, hospital research clinic, UK, cough associated with URTI | |
| Interventions | Antitussive: codeine linctus 30 mg/10 ml 4 times daily for 4 days | |
| Outcomes | Cough severity score (5‐point scale) from diaries expressed as area under the curve for 8 measures over 5 days | |
| Notes | No data on adverse effects provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and providers blinded; outcome assessor blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90% follow‐up; no reporting of differences between groups |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Freestone 1997.
| Methods | RCT | |
| Participants | 82 university students and staff, mean age 24 years (range 18 to 46), 62% men, 'common cold centre', university department, UK, cough associated with URTI | |
| Interventions | Antitussive: codeine phosphate 50 mg as a single dose | |
| Outcomes | 5‐point subjective rating scale, cough sound pressure levels, cough frequency | |
| Notes | No data on adverse effects reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and providers blinded; outcome assessor blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | No power calculation reported |
Gaffey 1988.
| Methods | RCT | |
| Participants | 250 adults, mean age 23 years, 65% women, internal medicine clinic, USA, common cold | |
| Interventions | Antihistamine: terfenadine 60 mg twice daily for 3.5 days | |
| Outcomes | Patient diary and symptom score from 0 to 3 but no exact scores reported | |
| Notes | Side effects uncommon in both groups, with the most common complaint being excess fatigue (12% in the active treatment group versus 10% in the placebo group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "subjects received sequential admission numbers and were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding presumed but not clearly reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 94% follow‐up; difference between groups not reported. Non‐compliers were considered to be drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | High risk | Participants were "compensated for participation" Trial supported by pharmaceutical industry |
Hutton 1991.
| Methods | RCT | |
| Participants | 96 inner‐city African‐American children, 6 months to 5 years, mean age about 2 years, primary care clinic, USA, symptoms of URTI | |
| Interventions | Antihistamine‐decongestant combination: brompheniramine maleate 4 mg/5 ml, phenylephrine 5 mg/5 ml, propanolamine 5 mg/5 ml (doses calculated to achieve brompheniramine dosage of 0.5 to 0.75 mg/kg/d) 3 times daily for 2 days | |
| Outcomes | 9‐point symptom score by parents or physician, follow‐up telephone interviews | |
| Notes | Side effects were rare including 1 child with loose stools in the placebo group and 1 child reported as hyperactive in the active treatment group. A second child in the drug group was reported to be sleepier than usual | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up 86% in the active treatment group and 89% in the placebo group |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Korppi 1991a.
| Methods | RCT | |
| Participants | Arm of 3‐arm RCT, 50 children with respiratory infection, private paediatric practices, mean age 3.8 years, 53% boys, Finland | |
| Interventions | Antitussive: dextromethorphan 1.5 mg per ml 5 ml 3 times daily for children under 7 years and 10 ml 3 times daily for older children | |
| Outcomes | Daily symptom score recorded by parents including cough frequency and severity on a scale from 0 to 3 | |
| Notes | Small study with no power calculation reported. Low incidence of adverse effects with no differences between groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomly divided" into treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Reported as double‐blind; outcome assessor blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up 96% |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Korppi 1991b.
| Methods | RCT | |
| Participants | Arm of 3‐arm RCT, 51 children with respiratory infection, private paediatric practices, mean age 3.8 years, 53% boys, Finland | |
| Interventions | Other combination: dextromethorphan 1.5 mg per ml and salbutamol 0.2 mg per ml 5 ml 3 times daily for children under 7 years and 10 ml 3 times daily for older children | |
| Outcomes | Daily symptom score recorded by parents including cough frequency and severity on a scale from 0 to 3 | |
| Notes | Small study with no power calculation reported. Low incidence of adverse effects with no differences between groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomly divided" into treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Reported as double‐blind; outcome assessor blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up 96% |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Kuhn 1982.
| Methods | RCT | |
| Participants | 65 adults (mostly university students), age range 18 to 30 years, university research centre, USA, URTI with cough for less than 48 hours | |
| Interventions | Mucolytic: expectorant: guaifenesin 480 mg/30 ml every 6 hours for 30 hours | |
| Outcomes | Tape recordings of cough frequency, questionnaire on 6 symptoms. | |
| Notes | Study did not report on adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and providers blinded; outcome assessor blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | No power calculation reported |
Kurth 1978.
| Methods | RCT | |
| Participants | 113 adults, 57% men, age range from under 30 to over 70 years (no details given), primary care, Germany, cough due to URTI | |
| Interventions | Other combination: EM‐Vier (Minetten): Extr thymi aquos.sicc 5 mg, succus liquiritiae depurat. inspiss. 20 mg, menthol 3.5 mg, ephedrine hydrochloric 2 mg, ol. eucalypti 2 mg, ol. menthae piperitae 0.7 mg 6 times daily for 14 to 18 days | |
| Outcomes | Outcome measurement unclear | |
| Notes | No adverse effects in either group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients blinded; blinding of providers and outcome assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 95% follow‐up. No difference between groups |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | No power calculation reported |
Lee 2000.
| Methods | RCT | |
| Participants | 44 adults from 18 to 60 years (mean age 23 years), 70% women, university staff and students and general city population, UK | |
| Interventions | Antitussive: dextromethorphan 30 mg as a single dose | |
| Outcomes | Cough frequency recordings, cough sound pressure levels, questionnaire on cough severity (scale from 0 to 3) | |
| Notes | Side effects not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and providers blinded; outcome assessor blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 98% follow‐up; remaining 2% reported as being missing due to lack of motivation to participate in the study |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Only retrospective power calculation reported |
Mizoguchi 2007.
| Methods | RCT | |
| Participants | 485 adult volunteers with URTI with cough, for greater than 1 day and less than 5 days, aged 18 to 65, attending 10 study centres in the USA | |
| Interventions | Single 30 ml dose of a test syrup containing 15 mg dextromethorphan; 7.5 mg doxycycline; 8 mg ephedrine and 600 mg paracetamol | |
| Outcomes | Mean cough score on day 1 and day 2 following active treatment. % with improved cough 3 hours following active treatment | |
| Notes | Other outcomes relating to URTI were also presented but we included only cough‐related outcomes Adverse events were reported in less 1% of participants and none were reprinted as serious |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants stratified by sex and overall symptom severity score and block randomised |
| Allocation concealment (selection bias) | Low risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and providers blinded; outcome assessor blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall 89% completed full follow‐up, 79% had per protocol analysis, minimal imbalance between groups |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | High risk | Interim power calculation carried out during study by independent external statistician Trial supported by pharmaceutical industry |
Nespoli 1989.
| Methods | RCT | |
| Participants | 40 children, age range 2 to 12 years (median 7.5 years), paediatric clinic, Italy, acute febrile bronchitis | |
| Interventions | Mucolytic: letosteine 25 mg 3 times daily for 10 days | |
| Outcomes | Cough score from 0 to 3, unclear how this was measured | |
| Notes | No adverse effects reported in either group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Interim power calculation carried out during study by independent external statistician |
Nesswetha 1967.
| Methods | RCT | |
| Participants | 99 factory workers in the chemical industry, age range 15 to 44 years, Germany, URTI | |
| Interventions | Mucolytic: Bisolvon linctus (N‐cyclohexyl‐N‐methyl‐(2‐amino‐3,5‐dibrombenzyl) ammonium chloride 4 mg in 5 ml 3 times daily for an average of 4 days | |
| Outcomes | Outcome measurement not clearly described; used 4‐point scale. Frequent cough (defined as cough every 2 to 4 minutes) | |
| Notes | Study did not report on adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and providers blinded; outcome assessor blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 93% follow‐up; difference between groups not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | No power calculation reported |
Parvez 1996.
| Methods | RCT | |
| Participants | 451 adults in 3 different studies, mean age 30 years, 65% men, mainly non‐smokers, corporate health centre, India, URTI | |
| Interventions | Antitussive: dextromethorphan 30 mg as a single dose | |
| Outcomes | Cough acoustic signals captured via microphone over 180 minutes. Differences in mean changes between cough counts | |
| Notes | This study did not report on adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation using a computer program |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and providers blinded; outcome assessor blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported but drop‐outs unlikely due to short period of follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | High risk | Many multiple comparisons with no corrections and high probability of type I error Trial supported by pharmaceutical industry |
Paul 2004.
| Methods | RCT | |
| Participants | 100 children (age range 2 to 18 years), cough due to URTI, university‐affiliated paediatric practices in USA | |
| Interventions | Antitussive: dextromethorphan as single dose based on age Antihistamine: diphenhydramine as single dose 1.25 mg/kg | |
| Outcomes | Cough frequency score on a 7‐point scale. Sleep disturbance in children and their parents. Composite 5‐item symptom score | |
| Notes | Adverse effects: 13/33 in dextromethorphan arm 9/33 in diphenhydramine arm 9/33 in placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomly assigned in a double‐masked manner" |
| Allocation concealment (selection bias) | Low risk | Medications were distributed in a brown paper bag to mask investigators to the volume of medication |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and providers blinded; outcome assessor blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Pavesi 2001.
| Methods | Meta‐analysis of 5 RCTs | |
| Participants | 710 adults in 5 different studies, mean age 30 years, 50% women, 90% non‐smokers, settings 'clinics' and 'in‐home' studies, South Africa and India, uncomplicated upper respiratory infection | |
| Interventions | Antitussive: dextromethorphan 30 mg as a single dose | |
| Outcomes | 3‐hour continuous cough recording, measuring cough bouts, cough components, cough effort, cough intensity and cough latency | |
| Notes | Study funded and conducted by pharmaceutical company. Results poorly reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | High risk | No power calculation reported. Medication sponsored by medical director of a laboratory who also performed the analysis |
Reece 1966.
| Methods | RCT | |
| Participants | 43 children, mean age 3.6 years (range 2 months to 12 years), 58% boys, community private practice, USA, cough due to URTI | |
| Interventions | Other combination: dextromethorphan, guaifenesin and pseudoephedrine (Triaminicol syrup) and dextromethorphan, guaifenesin and pseudoephedrine (Dorcol paediatric cough syrup), treatment frequency and duration unclear | |
| Outcomes | Parent assessment of treatment response | |
| Notes | Adverse effects were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Patients blinded; blinding of providers and outcome assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | High risk | No power calculation reported. Medication sponsored by medical director of a laboratory who also performed the analysis |
Robinson 1977.
| Methods | RCT | |
| Participants | 239 adults, mean age about 38 years, smokers and non‐smokers evenly distributed, office or clinic outpatients, USA, acute URTI | |
| Interventions | Expectorant: guaifenesin 200 mg/10 ml 4 times daily for 3 days | |
| Outcomes | Patient questionnaires, cough scores from 0 to 3 | |
| Notes | 2 participants in each group reported side effects including nausea and hives in the active treatment group and headaches, drowsiness and excessive perspiration in the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 89% follow‐up; no difference between groups |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | High risk | No power calculation reported Trial supported by pharmaceutical industry |
Sakchainanont 1990.
| Methods | RCT | |
| Participants | 143 children under 5 years, mean age 23 months (range 1.5 to 60 months), 50% girls, paediatric outpatient department, Thailand, common cold | |
| Interventions | Antihistamine: 2 groups: clemastine fumarate (0.05 mg/kg/d twice daily) and chlorpheniramine maleate syrup (0.35 mg/kg/d 3 times daily) for 3 days | |
| Outcomes | Parent assessment using 4‐level symptom score | |
| Notes | Drowsiness and sleepiness reported in 20% of children with no difference between the treatment groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 95% follow‐up; no difference between groups |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | High risk | No power calculation reported. Bonferroni correction for multiple comparisons used Trial supported by pharmaceutical industry |
Taylor 1993.
| Methods | RCT | |
| Participants | 57 children, mean age 4.7 years (range 18 months to 12 years), 53% boys, 82% white, private practices, USA, night cough due to URTI | |
| Interventions | Antitussive: dextromethorphan 15 mg/5 ml and codeine 10 mg/ 5 mg as a single dose at bedtime for 3 nights | |
| Outcomes | Parent questionnaire, cough score from 0 to 4 | |
| Notes | Both active treatments also contained guaifenesin 100 mg/5 ml. Adverse effects mainly drowsiness, diarrhoea and hyperactivity: placebo 7/13 (54%), dextromethorphan 6/19 (32%, P value = 0.2) and codeine 5/17 (29%, P value = 0.8) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and providers blinded; outcome assessor blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 86% follow‐up; difference between groups not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Post hoc power calculation demonstrates that study was powered to detect a difference of 0.9 in cough score, which is equivalent to natural resolution of cough at day 3. Authors argue that smaller reductions in cough scores are unlikely to be clinically important |
Thackray 1978.
| Methods | RCT | |
| Participants | 70 adults, mean age 34 years (range 18 to 60), 61% women, 21 general practices, UK, common cold | |
| Interventions | Other combination: Vicks Medinite syrup (dextromethorphan 15 mg, ephedrine 600 mg, doxylamine 7.5 mg, paracetamol 600 mg per dose) single dose at bedtime for 2 days | |
| Outcomes | Questionnaire, 6‐point rating scale. Cross‐over design | |
| Notes | 7 participants in the active treatment group reported giddiness/drowsiness compared to 4 participants in the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients allotted by a random number code" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Patients and providers blinded; outcome assessor blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | High risk | Main investigator was medical director of the company supplying the drug for the study. Cross‐over after 1 day, no washout period |
Tukiainen 1986.
| Methods | RCT | |
| Participants | 108 outpatients, mean age about 38 years, 55% women, 48% smokers, Finland, cough associated with URTI | |
| Interventions | Other combination: dextromethorphan (30 mg) alone and in combination with salbutamol (2 mg) 3 times daily for 4 days | |
| Outcomes | Patient diary and symptom score from 0 to 3 | |
| Notes | Dextromethorphan/salbutamol combination led to more tremor than placebo (no figures given, P value < 0.05), no serious adverse effects reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and providers blinded, outcome assessor blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | High risk | No power calculation reported Trial supported by pharmaceutical industry |
Con: control d: day Int: intervention MD: mean difference RCT: randomised controlled trial URTI: upper respiratory tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beiler 2007 | No placebo control group |
| Kim 2009 | Abstract only published and inclusion criteria unclear. Main publication awaited |
| MRC 1950 | Cough outcome not clearly reported |
| Shadkam 2010 | No placebo control group |
| Vornov 2012 | Phase 2 clinical trial of FP01 |
Differences between protocol and review
There have been no differences in review methods since the protocol was first published prior to the 2001 review.
Contributions of authors
Knut Schroeder (KS) and Tom Fahey (TF) conceived and designed the original review, undertook the searches, performed data collection, screened the search results, screened retrieved papers against the inclusion criteria, appraised the quality of the papers, extracted data from papers, interpreted the data, organised the retrieval of papers, wrote to authors of papers for additional information, managed the data, entered data into Review Manager (RevMan 2014), and wrote the review.
Susan Smith (SS) with TF and KS updated the review in 2007, 2010, 2012, 2013 and 2014, including screening updated search results, data extraction, quality appraisal and rewriting the review.
Sources of support
Internal sources
Division of Primary Health Care, University of Bristol, UK.
External sources
South & West Research and Development Directorate, UK.
NHS Primary Care Career Scientist Fund, UK.
Declarations of interest
Susan Smith: none known. Knut Schroeder: none known. Tom Fahey: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Adams 1993 {published data only}
- Adams R, Hosie J, James I, Khong T, Kohn H, Smith I, et al. Antitussive activity and tolerability of moguisteine in patients with acute cough: a randomized, double‐blind, placebo‐controlled study. Advances in Therapy 1993;10(6):263‐71. [Google Scholar]
Albrecht 2012 {published data only}
- Albrecht H, Vernon M, Solomon G. Patient‐reported outcomes to assess the efficacy of extended‐release guaifenesin for the treatment of acute respiratory tract infection symptoms. Respiratory Research 2012;13(118):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Avner Cohen 2012 {published data only}
- Avner Cohen H, Rozen J, Kristal H, Laks Y, Berkowitch M, Uziel Y, et al. Effect of honey on nocturnal cough and sleep quality: a double blind, randomized, placebo‐controlled study. Pediatrics 2012;130:465‐71. [DOI] [PubMed] [Google Scholar]
Berkowitz 1989 {published data only}
- Berkowitz RB, Connell JT, Dietz AJ, Greenstein SM, Tinkelman DG. The effectiveness of the non sedating antihistamine loratadine plus pseudoephedrine in the symptomatic management of the common cold. Annals of Allergy 1989;63(4):336‐9. [PubMed] [Google Scholar]
Berkowitz 1991 {published data only}
- Berkowitz RB, Tinkelman DG. Evaluation of oral terfenadine for treatment of the common cold. Annals of Allergy 1991;67:593‐7. [PubMed] [Google Scholar]
Bhattacharya 2012 {published data only}
- Bhattacharya M, Joshi N, Yadav S. To compare the effect of dextromethorphan, promethazine and placebo on nocturnal cough in children aged 1‐12 years with upper respiratory tract infections: a randomized controlled trial. Indian Journal of Pediatrics 2012;80(11):891‐5. [DOI] [PubMed] [Google Scholar]
Clemens 1997 {published data only}
- Clemens CJ, Taylor JA, Almquist JR, Quinn HC, Mehta A, Naylor GS. Is an antihistamine‐decongestant combination effective in temporarily relieving symptoms of the common cold in preschool children?. Journal of Pediatrics 1997;130(3):463‐6. [DOI] [PubMed] [Google Scholar]
Curley 1988 {published data only}
- Curley FJ, Irwin RS, Pratter MR, Stivers DH, Doern GV, Vernaglia PA, et al. Cough and the common cold. American Review of Respiratory Diseases 1988;138(2):305‐11. [DOI] [PubMed] [Google Scholar]
Eccles 1992 {published data only}
- Eccles R, Morris S, Jawad M. Lack of effect of codeine in the treatment of cough associated with acute upper respiratory tract infection. Journal of Clinical Pharmacy and Therapeutics 1992;17(3):175‐80. [DOI] [PubMed] [Google Scholar]
Freestone 1997 {published data only}
- Freestone C, Eccles R. Assessment of the antitussive efficacy of codeine in cough associated with common cold. Journal of Pharmacy and Pharmacology 1997;49:1045‐9. [DOI] [PubMed] [Google Scholar]
Gaffey 1988 {published data only}
- Gaffey MJ, Kaiser DL, Hayden FG. Ineffectiveness of oral terfenadine in natural colds: evidence against histamine as a mediator of common cold symptoms. Pediatric Infectious Diseases Journal 1988;7(3):215‐42. [DOI] [PubMed] [Google Scholar]
Hutton 1991 {published data only}
- Hutton N, Wilson MH, Mellits ED, Baumgartner R, Wissow LS, Bonuccelli C, et al. Effectiveness of an antihistamine‐decongestant combination for young children with the common cold: a randomized, controlled clinical trial. Journal of Pediatrics 1991;118(1):125‐30. [DOI] [PubMed] [Google Scholar]
Korppi 1991a {published data only}
- Korppi M, Laurikainen K, Pietikaenen M, Silvasti M. Antitussives in the treatment of acute transient cough in children. Acta Paediatrica Scandinavia 1991;80:969‐71. [DOI] [PubMed] [Google Scholar]
Korppi 1991b {published data only}
- Korppi M, Laurikainen K, Pietikaenen M, Silvasti M. Antitussives in the treatment of acute transient cough in children. Acta Paediatrica Scandinavia 1991;80:969‐71. [DOI] [PubMed] [Google Scholar]
Kuhn 1982 {published data only}
- Kuhn JJ, Hendley JO, Adams KF, Clark JW, Gwaltney JM Jr. Antitussive effect of guaifenesin in young adults with natural colds. Chest 1982;82(6):713‐8. [DOI] [PubMed] [Google Scholar]
Kurth 1978 {published data only}
- Kurth W. Secure therapeutic effectiveness of the traditional antitussive agent Minetten in a double‐blind study [Gesicherte therapeutische Wirksamkeit des traditionellen Antitussivums Minetten im Doppelblindversuch]. Medizinische Welt 1978;29(48):1906‐9. [PubMed] [Google Scholar]
Lee 2000 {published data only}
- Lee PCL, Jawad MSM, Eccles R. Antitussive efficacy of dextromethorphan in cough associated with acute upper respiratory infection. Journal of Pharmacy and Pharmacology 2000;52:1137‐42. [DOI] [PubMed] [Google Scholar]
Mizoguchi 2007 {published data only}
- Mizoguchi H, Wilson A, Jerdack GR, Hull JD, Goodale M, Grender JM, et al. Efficacy of a single evening dose of a syrup containing paracetamol, dextromethorphan hydrobromide, doxylamine succinate and ephedrine sulphate in subjects with multiple common cold symptoms. International Journal of Clinical Pharmacology and Therapeutics 2007;45:230‐6. [DOI] [PubMed] [Google Scholar]
Nespoli 1989 {published data only}
- Nespoli L, Monafo V, Bonetti F, Terracciano L, Savio G. Clinical evaluation of letosteine activity in the treatment of acute febrile bronchitis in paediatric age. Controlled double blind vs placebo investigation [Valutazione clinica dell'attivita della letosteina nel trattamento della bronchite acuta febbrile in eta pediatrica. Indagine controllata in doppio cieco vs placebo]. Minerva Pediatrica 1989;41(10):515‐20. [PubMed] [Google Scholar]
Nesswetha 1967 {published data only}
- Nesswetha W. Criteria of drug testing in industrial practice, demonstrated by a cough remedy [Kriterien der Arzneimittelpruefung in der werksaerztlichen Praxis, dargestellt am Beispiel eines Hustenloesers]. Arzneimittelforschung 1967;17(10):1324‐6. [PubMed] [Google Scholar]
Parvez 1996 {published data only}
- Parvez L, Vaidya M, Sakhardande A, Subburaj S, Rajagopalan TG. Evaluation of antitussive agents in man. Pulmonary Pharmacology 1996;9(5‐6):299‐308. [DOI] [PubMed] [Google Scholar]
Paul 2004 {published data only}
- Paul IM, Yoder KE, Crowell KR, Shaffer ML, McMillan HS, Carlson LC, et al. Effect of dextromethorphan, diphenhydramine, and placebo on nocturnal cough and sleep quality for coughing children and their parents. Pediatrics 2004;114(1):E85‐E90. [DOI] [PubMed] [Google Scholar]
Pavesi 2001 {published data only}
- Pavesi L, Subburaj S, Porter‐Shaw K. Application and validation of a computerized cough acquisition system for objective monitoring of acute cough: a meta‐analysis. Chest 2001;120:1121‐8. [DOI] [PubMed] [Google Scholar]
Reece 1966 {published data only}
- Reece CA, Cherry AC Jr, Reece AT, Hatcher TB, Diehl AM. Tape recorder for evaluation of coughs in children. American Journal of Diseases of Children 1966;112(2):124‐8. [DOI] [PubMed] [Google Scholar]
Robinson 1977 {published data only}
- Robinson RE, Cummings WB, Deffenbaugh ER. Effectiveness of guaifenesin as an expectorant: a cooperative double‐blind study. Current Therapeutic Research 1977;22(2):284‐96. [Google Scholar]
Sakchainanont 1990 {published data only}
- Sakchainanont B, Ruangkanchanasetr S, Chantarojanasiri T, Tapasart C, Suwanjutha S. Effectiveness of antihistamines in common cold. Journal of the Medical Association Thailand 1990;73(2):96‐101. [PubMed] [Google Scholar]
Taylor 1993 {published data only}
- Taylor JA, Novack AH, Almquist JR, Rogers JE. Efficacy of cough suppressants in children. Journal of Paediatrics 1993;122:799‐802. [DOI] [PubMed] [Google Scholar]
Thackray 1978 {published data only}
- Thackray P. A double‐blind, crossover controlled evaluation of a syrup for the night‐time relief of the symptoms of the common cold, containing paracetamol, dextromethorphan hydrobromide, doxylamine succinate and ephedrine sulphate. Journal of International Medical Research 1978;6(2):161‐5. [DOI] [PubMed] [Google Scholar]
Tukiainen 1986 {published data only}
- Tukiainen H, Karttunen P, Silvasti M, Flygare U, Korhonen R, Korhonen T, et al. The treatment of acute transient cough: a placebo‐controlled comparison of dextromethorphan and dextromethorphan‐beta 2‐sympathomimetic combination. European Journal of Respiratory Diseases 1986;69(2):95‐9. [PubMed] [Google Scholar]
References to studies excluded from this review
Beiler 2007 {published data only}
- Beiler PIM, McMonagle J, Shaffer A, Duda ML, Berlin LCM Jr. Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Archives of Pediatrics and Adolescent Medicine 2007;161(12):1140‐6. [DOI] [PubMed] [Google Scholar]
Kim 2009 {published data only}
- Kim YW, Lee SM, Lee CT, Chung HS, Chang J, Ahn CM. Clinical efficacy of theobromine for the treatment of cough in adults. Respirology 2009;14:A132. [Google Scholar]
MRC 1950 {published data only}
- Medical Research Council. Clinical trials of antihistaminic drugs in the prevention and treatment of the common cold. British Medical Journal 1950;2:425‐9. [PMC free article] [PubMed] [Google Scholar]
Shadkam 2010 {published data only}
- Shadkam MN, Mozaffari‐Khosravi H, Mozayan MR. A comparison of the effect of honey, dextromethorphan and diphenhydramine on nightly cough and sleep quality in children and their parents. Journal of Alternative and Complementary Medicine 2012;16(7):787‐93. [DOI] [PubMed] [Google Scholar]
Vornov 2012 {published data only}
- Vornov J, Birring S, Mazhari R, Fraser H, Casper R, Canning B, et al. Baseline cough frequency in a randomized, placebo‐controlled, phase 2 clinical trial in subjects with upper respiratory tract infection. Abstracts from the Fourth American Cough Conference. 2012.
Additional references
Anonymous 1999
- Anonymous. Cough medications in children. Drugs and Therapeutics Bulletin 1999;37(3):19‐21. [DOI] [PubMed] [Google Scholar]
Banderali 1995
- Banderali G, Riva E, Fiocchi A, Cordaro CI, Giovannini M. Efficacy and tolerability of levodropropizine and dropropizine in children with non‐productive cough. Journal of International Medical Research 1995;23:175‐83. [DOI] [PubMed] [Google Scholar]
Bjórnsdóttir 2001
- Bjórnsdóttir I, Einarson TR, Gudmundsson LS, Einarsdóttir RA. Efficacy of diphenhydramine against cough in humans: a review. Pharmacy World and Science 2007;29:577‐83. [DOI] [PubMed] [Google Scholar]
FDA 2007
- Food, Drug Administration. Nonprescription Drug Advisory Committee Meeting. Cold, cough, allergy, bronchodilator, antiasthmatic drug products for over‐the‐counter human use. www.fda.gov/ohrms/dockets/ac/07/briefing/2007‐4323b1‐02‐FDA.pdf 2007 (accessed 6 October 2008).
Gastpar 1984
- Gastpar H, Cricuolo D, Dieterich HA. Efficacy and tolerability of glaucine as an antitussive agent. Current Medical Research and Opinion 1984;9:21‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Irwin 1993
- Irwin RS, Curley FJ, Bennett FM. Appropriate use of antitussives and protussives. A practical review. Drugs 1993;46(1):80‐91. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Morice 1998
- Morice A, Abdul‐Manap R. Drug treatments for coughs and colds. Prescriber 1998;9(17):74‐9. [Google Scholar]
Oduwole 2012
- Oduwole O, Meremikwu MM, Oyo‐Ita A, Udoh EE. Honey for acute cough in children. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD007094.pub3] [DOI] [PubMed] [Google Scholar]
PAGB 2000
- The Proprietary Association of Great Britain. Annual Review and Report. London: PAGB, 2000. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reynolds 1993
- Reynolds JEF. Cough suppressants, expectorants and mucolytics. Martindale ‐ The Extra Pharmacopeia. 30th Edition. London: The Pharmaceutical Press, 1993:741‐53. [Google Scholar]
Smith 1993
- Smith MBH, Feldman W. Over‐the‐counter cold medications. A critical review of clinical trials between 1950 and 1991. JAMA 1993;269(17):2258‐63. [DOI] [PubMed] [Google Scholar]
Smith 2008a
- Smith SM, Henman M, Schroeder K, Fahey T. Over the counter (OTC) cough medicines in children: neither safe or efficacious?. British Journal of General Practice 2008;58(556):757‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vernacchio 2008
- Vernacchio L, Kelly JP, Kaufman DW, Mitchell AA. Cough and cold medication use by US children, 1999‐2006: Results from the Slone survey. Pediatrics 2008;122(2):e323‐e9. [DOI] [PubMed] [Google Scholar]
Ziment 1976
- Ziment I. What to expect from expectorants. JAMA 1976;236:193‐4. [PubMed] [Google Scholar]
References to other published versions of this review
Acuin 1999
- Acuin J, Smith A, Mackenzie I. Treatment of chronic suppurative otitis media. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD001831.pub3] [DOI] [Google Scholar]
Schroeder 2001
- Schroeder K, Fahey T. Over‐the‐counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD001831.pub3] [DOI] [PubMed] [Google Scholar]
Schroeder 2004
- Schroeder K, Fahey T. Over‐the‐counter (OTC) medications for acute cough in children and adults in ambulatory settings. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD001831.pub3] [DOI] [PubMed] [Google Scholar]
Smith 2008b
- Smith SM, Schroeder K, Fahey T. Over‐the‐counter (OTC) medications for acute cough in children and adults in ambulatory settings. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD001831.pub3] [DOI] [PubMed] [Google Scholar]
Smith 2010
- Smith SM, Schroeder K, Fahey T. Over‐the‐counter (OTC) medications for acute cough in children and adults in ambulatory settings. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD001831.pub3] [DOI] [PubMed] [Google Scholar]
Smith 2012
- Smith SM, Schroeder K, Fahey T. Over‐the‐counter (OTC) medications for acute cough in children and adults in ambulatory settings. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD001831.pub4] [DOI] [PubMed] [Google Scholar]


